Abstract
Adenoviral (Ad) vector vaccines represent one of the most promising modern vaccine platforms, and Ad vector vaccines are currently being investigated in human clinical trials for infectious disease and cancer. Our studies have shown that specific targeting of adenovirus to dendritic cells dramatically enhanced vaccine efficacy. However, this was achieve using a molecular adapter, thereby necessitating a two component vector approach. To address the mandates of clinical translation of our strategy, we here sought to accomplish the goal of DC targeting with a single component adenovirus vector approach. To redirect the specificity of Ad vector vaccines, we replaced the Ad fiber knob with fiber-fibritin chimeras fused to DC1.8, a single domain antibody (sdAb) specific for murine immature DC. We engineered a fiber-fibritin-sdAb chimeric molecule using the coding sequence for DC1.8, and then replaced the native Ad5 fiber knob sequence by homologous recombination. The resulting Ad5 virus, Ad5FF1.8, expresses the chimeric fiber-fibritin sdAb chimera. Infection with Ad5FF1.8 dramatically enhances transgene expression in DC 2.4 dendritic cells compared to infection with native Ad5. Ad5FF1.8 infection of bone marrow derived DC demonstrates that Ad5FF1.8 selectively infects immature DC consistent with the known specificity of DC1.8. Thus, sdAb can be used to selectively redirect the tropism of Ad5 vector vaccines, providing the opportunity to engineer Ad vector vaccines that are specifically targeted to DC, or specific DC subsets.
Keywords: Adenoviral vaccine, Targeted Ad5, dendritic cell, single domain antibody
INTRODUCTION
Ad vector vaccines have emerged as one of the most promising modern vaccine platforms,1 and there are currently a number of human clinical trials ongoing in the infectious disease and cancer vaccine fields.2–9 Ad vector vaccines have several key biologic properties that make them attractive10, 11: (1) Ad vector vaccines have been deleted in E1 and are replication-defective. They have an excellent safety profile in clinical translation; (2) Ad vector vaccines can be grown to high titer in cell culture, facilitating manufacture of clinical grade material, regulatory approval and clinical translation; (3) Multiple transgenes can be inserted into Ad vector vaccines, including not only the antigen of interest but also other genes to enhance the response to vaccination, such as cytokines or danger signals; and (4) Ad vector vaccines induce potent inflammatory responses following vaccination. These inflammatory responses are associated with the induction of pro-inflammatory cytokines and stimulation of both the innate and adaptive immune systems.12–14
A relatively unique property of Ad vector vaccines is the fact that the cellular attachment and entry processes are molecularly distinct. The Ad fiber knob domain initiates viral attachment via the coxsackie and adenovirus receptor (CAR), a receptor expressed ubiquitously on epithelial cells. Internalization of the viral particle is then mediated by distinct molecular interactions between Arg-Gly-Asp (RGD) motifs in the Ad penton base capsomer and cellular αν integrins on the cell surface.15, 16 This separation of the cellular attachment and entry processes provides an opportunity to re-direct the specificity of Ad cellular attachment without compromising internalization.
A major focus in the infectious disease and cancer vaccine fields is to improve primary and memory CD8 T cell responses. Efficient delivery of antigen to specialized antigen presenting cells (APC) appears to be critical for the generation of productive CD8 T cell responses. Dendritic cells (DC) are a highly specialized subset of APC that play a critical role in regulating immune responses with the ability to steer immune responses towards immunity or tolerance depending on the activation state, location, and specific subset of DC.17 Previous studies have demonstrated that targeting antigen to DC can dramatically enhance the efficacy of vaccines, and this has proven to be true targeting multiple different DC receptors, in the context of multiple different vaccine platforms (reviewed in 18).
In our earlier studies we sought to modify adenovirus tropism to enhance gene delivery to DCs as a strategy to improve vaccine efficacy.19 These studies employed the general approach of a two component “adapter”, cross-linking the adenovirus fiber knob to a DC cell surface marker.20, 21 In various studies, we were able to show that this approach dramatically enhanced the efficiency, and specificity, of Ad-mediated gene delivery to DCs. Most importantly, we could show that these vector gains translated directly into improved vaccine outcomes in murine models of cancer immunotherapy.22, 23 Despite these efficacy gains, the two component design of this targeting strategy presented logistical challenges vis-à-vis clinical development.24–26 On this basis, we sought to develop a single component adenovirus vector capable of DC-selective gene delivery.
Single-domain antibodies (sdAb) are antigen-binding antibody fragments engineered from heavy-chain antibodies found in camelids. sdAb offer the high binding affinity and specificity of conventional antibodies, combined with the small size, stability, tissue penetration, and favorable pharmacokinetics of small molecules.27, 28 Of note, the monomeric structure and small size of sdAb offer clear advantages at the level of protein engineering in the context of redirecting the specificity of Ad vector vaccines.29 Based on the hypothesis that sdAb would allow the most flexible, precise and readily translated DC targeting strategy, we adapted our “fiber replacement” methodology 24, 30, 31 to allow incorporation of sdAb into the Ad5 capsid. We subsequently tested the ability of engineered Ad vector vaccines incorporating a sdAb specific for immature DC to specifically infect DC.
MATERIALS AND METHODS
Generation of DC-targeted Ad5
We employed a genetic fiber modification approach to ablate the native tropism of Ad5, and redirect cellular attachment.32 The knob domain of the Ad capsid fiber protein, was replaced with the C-terminal 95 amino acid long foldon domain from the T4 phage fibritin protein to maintain fiber trimerization while allowing incorporation of targeting moieties.31, 33 To selectively target DC, we employed DC 1.8, a camelid sdAb that is specific for murine immature bone marrow-derived DC (BMDC) in vitro and in vivo.34 We constructed an Ad5-based genome carrying both firefly luciferase and eGFP expression cassettes in place of the deleted early E1 and E3 regions, respectively, and encoding chimeric fiber-fibritin-DC1.8 protein in place of the endogenous fiber knob gene. This replication incompetent genome was rescued using 211B cells35 expressing both E1 and wildtype Ad5 fiber genes, allowing packaging of the Ad genome into capsid that incorporates modified fibers along with wild type fibers resulting in fiber-mosaic virus progeny as described previously.32 Of note, fiber-mosaic virus is only an intermediate step in the production of the virus. The viral progeny were subsequently upscaled in 211B cells, and then used to infect 293 cells in order to amplify viral particles containing only chimeric fiber-fibritin-DC1.8 proteins. The resultant CsCl-purified Ad5FF1.8 vector preparation was then analyzed by western blot to confirm incorporation of only chimeric FF1.8 fiber in the context of assembled Ad5FF1.8 viral particles. We also constructed Ad5FF, a non-targeted Ad5 vector with a chimeric fiber-fibritin construct to serve as an isogenic control.
Mice and cell lines
C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained under specific pathogen-free conditions in the animal facility at Washington University School of Medicine (WUSM). All experiments were carried out in 6–12 week old mice using an institutionally-approved protocol and in accordance with the guidelines established by the WUSM Animal Studies Committee. The DC2.4 cell line 36 was received from Dr. K.L. Rock (University of Massachusetts Medical School, Worcester, MA) while PEA-10 (catalogue number CRL-2215) and SVEC4-10 (catalogue number CRL-2181) cell lines were obtained from the American Type Culture Collection.
Bone marrow-derived DC cultures
Femurs were harvested from mice and soaked in 70% ethanol on ice for 5 minutes. Epiphyses were cut, and the BM was then flushed out using a sterile syringe (26–28 gauge needle) filled with 200 μL of culture media. BM cells were treated with Tris-ammonium chloride at room temperature for 5 minutes to lyse RBC. Cells were then resuspended in culture medium consisting of Iscove’s Modified Dulbecco’s Medium supplemented with 2 mM L-Glutamine, 100 I.E./mL sodium penicillin, 100 μg/mL streptomycin, 2.5 μg/mL Amphotericin B, essential and non-essential amino acids, sodium pyruvate, HEPES buffer and 10% fetal bovine serum. BM cells were plated in 6 well plates at a density of 1 × 106 cells/mL in culture media supplemented with mouse Flt3L (200 ng/mL) and/or GMCSF (300 pg/mL) at 37°C in 5% CO2. On day 6 floating cells were removed and the GM-CSF concentration was increased to 1 ng/mL. The cells growing in monolayers were incubated for an additional 48 hours before being infected with the indicated Ad5 vectors. For generation of GM-CSF and GM-CSF/IL-4 DC cultures we used media supplemented with either GM-CSF alone (20 ng/mL), or GM-CSF (20 ng/mL) and IL-4 (20 ng/mL) respectively. For activation of DC, TNFα (100 ng/mL) was added to the culture media on day 7. All the cytokines used were obtained from PeproTech (Rocky Hill, NJ). Analysis for IL-12 was performed by ELISA following the manufacturer’s instructions (IL-12p70, BioLegend, San Diego, CA).
Gene transfer assay
Monolayers of DC2.4 and BMDC were washed one time with PBS, and then infected with 3000 viral particles/cell in triplicate. After one hour, the media was removed, the tissue culture wells were washed with PBS and fresh media was added. Then the infected cells were incubated for 24 hours at 37°C and 5% CO2. GFP expression was evaluated using an inverted immunofluorescence microscope (EVOS FL Cell Imaging System, ThermoFisher Scientific).
Flow Cytometry
BMDC were stained with mAb specific for CD45RA (14.8-BV650, Cat. # 564360, BD Biosciences), B220 (RA3-6B2-PE/Cy7, Cat. # 103221, BioLegend), CD11c (N418-APC, Cat. # 117309, BioLegend), CD11b (M1/70-BV711, Cat. # 101241, BioLegend), class II MHC (M5/114.15.2-PerCP/Cy5.5, Cat. # 107625, BioLegend), CD8α (53-6.7-PE/Cy5, Cat. # 100709, BioLegend), CD24 (M1/69-Pacific Blue, Cat. # 10819, BioLegend), and SIRPα (P84-PE, Cat. # 14011, BioLegend). Cell sorting and analysis was performed on a LSRFortessa (BD Biosciences) instrument. Post acquisition analysis was performed using FlowJo software v10.1 (Tree Star Inc., Ashland, OR).
Luciferase Assay
The luciferase assay system (Promega) and ORION microplate luminometer (Berthold Detection systems, Oak Ridge, TN) were used for the evaluation of luciferase activity in transduced cells. The DC Protein assay (Bio-Rad, Hercules, CA) was used to normalize the protein concentration of the cell lysate. Activity is reported in relative light units (RLU) per 1 × 106 cells.
In vivo characterization
In vivo experiments involving mice were carried out under protocol nos. 20140289 and 20110035 approved by the Washington University Animal Studies Committee. C57BL/6 mice at 10 weeks of age, obtained from Jackson Laboratory (Bar Harbor, ME, USA), were used in the present work. For in vivo distribution, mice were injected intradermally with 1×1011 particles of virus in 200 μL of saline. Seventy-two hours post virus administration, mice were anesthetized with 2.5% 2, 2, 2-tribromoethanol (Avertin, Sigma-Aldrich, St Louis, MO, USA), left -ventricle perfused with phosphate-buffered saline (PBS) followed by 10% neutral-buffered formalin. Mouse tissues were harvested, post-fixed in formalin for 2–4 hours at room temperature, cryopreserved in 30% sucrose for 16 hours at 4°C, and cryo-embedded in NEG50 (Thermo Fisher Scientific, Waltham, MA) over 2-methylbutane chilled in liquid nitrogen. For immunization experiments, mice were injected intradermally with 1×109 vp per mouse on days 0 and 7. Control mice received three immunizations with ovalbumin (OVA) cDNA at days 0, 3, and 6, as described.37 All mice were analyzed for OVA-specific reactivity in vitro by interferon gamma (IFNγ) ELISPOT on day 11 using splenocytes. Splenocytes were tested for recognition of the OVA peptide SIINFEKL, p257–264, as described.37
Immunofluorescence Staining and Imaging
Sixteen-micrometer frozen sections were collected, air-dried briefly, rehydrated in PBS, blocked with protein block (5% donkey serum in PBS containing 0.1% Triton X-100), and incubated over night at 4 °C with primary antibodies including: chicken anti-GFP 1:400 (A10262, Life Technologies, Carlsbad, CA, USA) and anti-CD11c. After PBS washes for three times, the slides were incubated with corresponding Alexa Fluor 488 and Alexa Fluor 594 conjugated secondary antibodies, 1:400, (Jackson ImmunoResearch Laboratories, West Grove, PA) and counterstained for nuclei with SlowFade Gold Antifade mounting reagent with 49,6-diamidino-2-phenylindole (DAPI) (Life Technologies). Immunofluorescence microscope images were collected using an FVII digital camera with Extended Focal Imaging (EFI) function (Olympus America, Center Valley, PA). The camera acquisition time for EGFP fluorescence was optimized and set a priori for each tissue.
Statistics
Data are presented as mean ± standard error of mean or standard deviation, as indicated. Graphs were drawn using Prism v6.0 (GraphPad Software, La Jolla, CA). Statistical significance was accepted at p < 0.05.
RESULTS AND DISCUSSION
The Ad5FF1.8 capsid contains chimeric fiber-fibritin-DC1.8 molecules
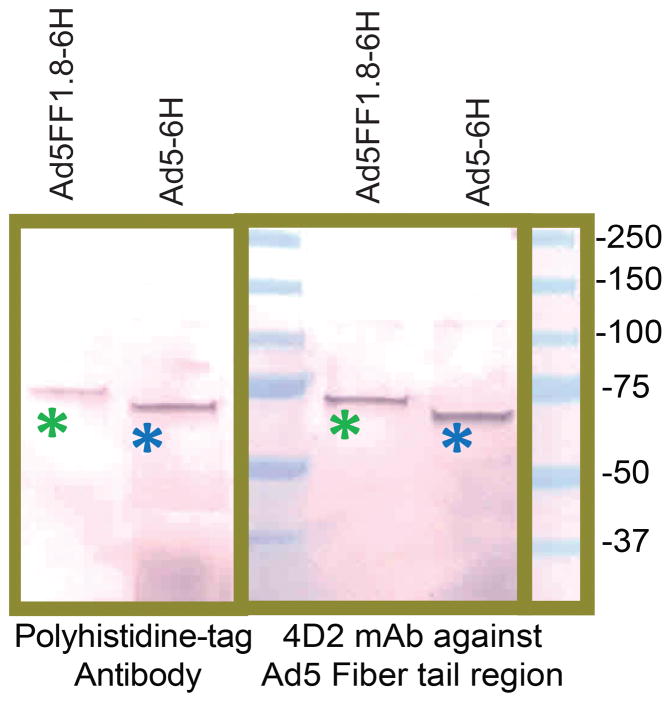
To redirect the specificity of Ad vector vaccines we created chimeric fiber-fibritin molecules incorporating the sdAb DC1.8 using methods we have previously described.38 We validated the successful incorporation of the chimeric molecules into the Ad5FF1.8 capsid by detecting similar sized protein bands using mAb against the N-terminus of the Ad fiber tail and against the polyhistidine-tag introduced into the C-terminus of DC1.8 (Fig. 1). The efficiency of chimeric fiber incorporation into the Ad5FF1.8 capsid was similar to that of the control Ad5 vector, which was constructed previously to encode wildtype fiber carrying the C-terminal polyhistidine-tag.39
Figure 1. Validation of Nb-DC1.8 incorporation into the Ad5FF1.8 capsid.
Purified samples of Ad5FF1.8 (lanes 1 and 4) and control Ad5-6H vector (lanes 2 and 5) were boiled in Laemmli sample buffer and run on 4-20% gradient SDS-PAGE. Viral proteins were transferred to PVDF membrane and incubated with either 4D2 mAb against Ad5 fiber tail region (left panel) or Penta-His mAb against poly-histidine tag (right panel). Molecular masses of Precision Plus marker proteins (MW) are indicated in kilodaltons (kDa) on the right. Protein bands corresponding to Ad5FF1.8 fiber with expected molecular mass of 66 kDa is marked with green asterisk, protein band corresponding to the control Ad5F-6H fiber of 63 kDa is marked with blue asterisk.
Ad5FF1.8 efficiently transduces the murine dendritic cell line DC2.4
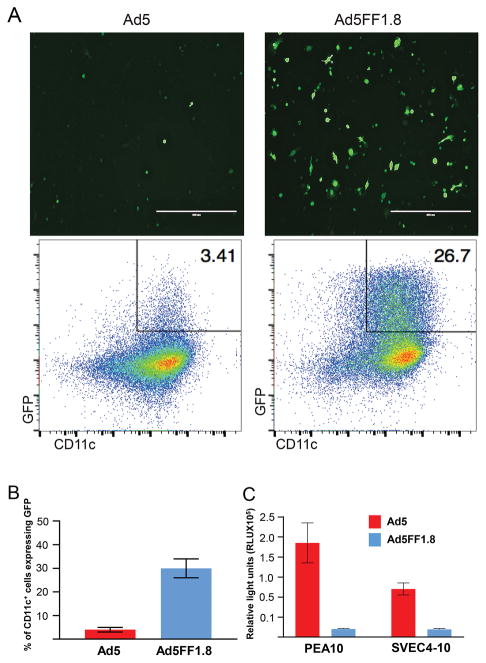
DC do not express CAR and the transduction efficiency using wildtype Ad5 vectors is very low. To assess whether Ad5FF1.8 could successfully transduce DC, we infected DC2.4, a murine DC cell line with Ad5FF1.8 and control Ad5. 30 ± 11% of the DC infected with Ad5FF1.8 expressed GFP compared to 3.5 ± 0.9 % infected with control Ad5 (Fig. 2A, B). The intensity of GFP expression was also higher in the DC infected with Ad5FF1.8 (MFI of GFP+ cells 15,986 ± 411 vs 7,702 ± 106). Ad5FF1.8 was associated with higher transduction efficiency across multiple different time points (12, 24 and 48 hours) and multiple different multiplicity of infection (600, 1000, 3000 and 5000 MOI, data not shown). Similar differences were seen when we tested for expression of luciferase (69,258 vs 11,372 relative light units, data not shown). To demonstrate that replacement of the fiber knob domain of Ad5FF1.8 with chimeric fiber-fibritin molecules eradicates the broad tropism to CAR-expressing cells, we used the CAR-expressing cell lines PEA10 (mouse fibroblast) and SVEC4-10 (mouse vascular epithelium). Infection with Ad5FF1.8 was associated with a 12 to 15-fold reduction in gene transfer compared to control Ad5 in PEA10 and SVEC4-10 cells, respectively (Fig. 2C).
Figure 2. Ad5FF1.8 efficiently transduces dendritic cells in a CAR independent manner.
(A) Comparison of GFP expression in DC2.4 cells by Ad5 and Ad5FF1.8 using immunofluorescence microscopy (20X objective) and flow cytometry. (B) Histogram representing increased transduction efficiency of Ad5FF1.8 in BMDC. (C) Deletion of fiber knob domain abrogates CAR-dependent gene transfer by Ad5FF1.8. Monolayers of SVEC4-10 (murine axillary lymph node/vascular epithelium) and PEA-10 (murine fibroblasts) cell lines were incubated with Ad5FF1.8 and Ad5 for 1 hour at increasing MOIs. Luciferase activity was measured after a further 24 hour incubation in virus free medium. Both SVEC4 and PEA-10 cells infected with Ad5FF1.8 showed a lower Luciferase expression as compared to cells infected with Ad5.
Ad5FF1.8 targeting specificity is mediated by the sdAb DC1.8
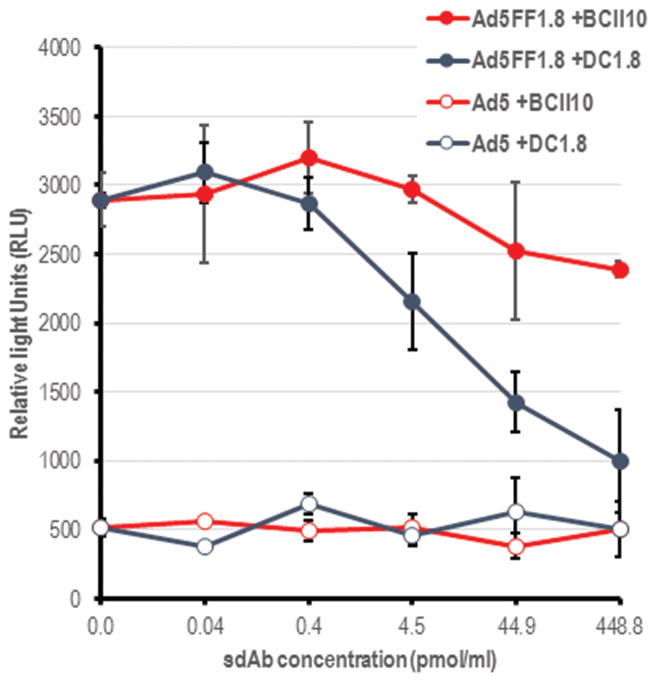
We infected DC in the presence or absence of soluble DC1.8 to determine if soluble sdAb could specifically interfere with DC infection. We used the BCII10 sdAb as a negative control (BCII10 is specific for the subunit 10 of the β-lactamase BC-II enzyme of Bacillus cereus). 40 Increasing concentrations of sdAb DC1.8 resulted in a dose-dependent inhibition of the ability of Ad5FF1.8 to infect DC, while BCII10 had no effect (Fig. 3). Control Ad5 showed poor infectivity of DC, and this was not affected by either DC1.8 or the control sdAb. Overall, these results provide additional evidence that the chimeric fiber-fibritin-sdAb mediates the targeting specificity of Ad5FF1.8.
Figure 3. sdAb DC1.8 and Ad5FF1.8 compete for binding to DC.
As the amount of DC1.8 added to the BMDC culture is increased, the expression of reporter gene Luciferase decreases in a dose dependent manner.
Ad5FF1.8 specifically transduces immature DC in murine BMDC cultures
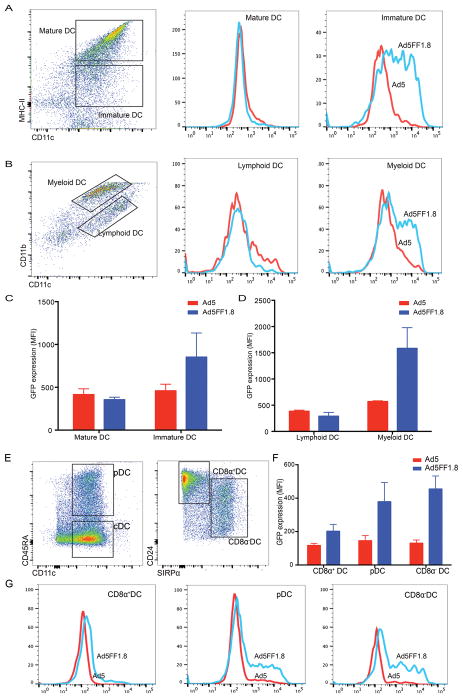
To determine if Ad5FF1.8 retains the specificity of DC1.8, we infected BMDC cultured in GM-CSF/IL-4 with Ad5FF1.8 and control Ad5 and harvested the cells after overnight incubation. We sorted live CD11c+ cells into mature (MHC-IIhigh) and immature (MHC-IIlow) subpopulations and compared GFP expression between the two (Fig. 4A). Infection with Ad5FF1.8 was associated with higher GFP expression in immature DC (MFI 852 ± 281 vs 459 ± 77), but similar levels of GFP expression in mature DC (MFI GFP 355 ± 29 vs 415 ± 67) (Fig 4C). To determine the specific subset(s) of DC infected by Ad5FF1.8, we added TNF-α to BMDC cultures to trigger maturation and infected the monolayers with Ad5FF1.8 or control Ad5. Infection with Ad5FF1.8 was associated with higher GFP expression in myeloid DC (CD11bhigh/CD11c+, MFI 1583 ± 279 vs 570 ± 9). There were similar levels of GFP expression in lymphoid DC (CD11blow/CD11c+, MFI 291 ± 51 vs 384 ± 14) (Fig 4B, D). Next, we cultured murine bone marrow cells with Flt3L and GM-CSF according to established protocols and exposed the monolayers to Ad5FF1.8 and control Ad5 on day 8 of culture. At 24 hours post-infection we found that Ad5FF1.8 infection was associated with increased GFP expression in plasmacytoid DC (MFI 379 ± 81 vs 145 ± 21.5) and CD8α− DC (MFI 455 ± 55 vs 130 ± 13.5), but there was no change in CD8α+ DC (MFI 202 ± 29 vs 117 ± 9) (Fig 4E, F, G). To assess if infectivity altered the maturation status of DC, Flt3 + GM-CSF-derived DC and GM-CSF + IL-4-derived DC were infected with either Ad5 or Ad5FF1.8, and both IL-12 production and cell surface expression of CD80 and CD86 was assessed. Unlike LPS-matured DC, Ad5 and Ad5FF1.8-infected DC did not produce detectable levels of IL-12 (data not shown). Comparison of DC maturation phenotypes by flow cytometry showed similar levels of expression of CD80 and slightly increased levels of CD86 after infection with Ad5FF1.8 (data not shown).
Figure 4. Subset specific targeting by Ad5FF1.8.
(A) Gating strategy used to segregate MHC-II high and MHC-II low CD11c+ cells in GMCSF/IL4-supplemented BMDC culture. BMDC infected with Ad5FF1.8 (in blue) have an increased eGFP expression in immature DC but not in mature DC when compared to cells infected with Ad5 (in red). (B) Gating strategy used to segregate CD11c+ cells in GMCSF/IL-4 supplemented BMDC culture activated with TNFα as myeloid DCs (CD11b+) and lymphoid (CD11b− DCs). BMDC infected with Ad5FF1.8 (in blue) have an increased eGFP expression in the myeloid DC but not in lymphoid DC when compared to cells infected with Ad5 (in red). Histograms representing differential transduction specificity of Ad5FF1.8 and Ad5 in (C) GMCSF/IL4 supplemented BMDC culture and (D) GMCSF/IL-4 supplemented BMDC activated with TNFα. (E) Gating strategy used to segregate CD11c+ cells into pDC (CD45RAhigh plasmacytoid dendritic cells), cDC (CD45RAlow conventional dendritic cells), CD8α+DC (CD24high Sirpalow) and CD8α −DC(CD24low Sirpahigh). (F, G) Ad5FF1.8 causes an increased transduction of GFP in pDC and CD8α − DC but not in CD8α+ DC.
In vivo administration of Ad5FF1.8
We performed in vivo biodistribution studies demonstrating that incorporation of the DC1.8 sdAb into the Ad5 capsid (Ad5FF1.8 expressing GFP) significantly alters the in vivo biodistribution compared to wildtype Ad5 (control Ad5 expressing GFP). Specifically, there is a dramatic decrease in GFP expression at the injection site following injection with Ad5FF1.8 compared to Ad5. These studies validate that replacement of the fiber knob with a chimeric construct integrating a sdAb (with consequent ablation of CAR recognition), alters the biodistribution of Ad5FF1.8 (Fig. 5). While we attempted detection of CD11c dendritic cells, the CD11c immunofluorescence was suboptimal to undetectable in the skin and suboptimal in retroperitoneal, axillary, and cervical lymph nodes and spleen (data not shown). We also generated Ad5 vectors expressing the model antigen ovalbumin (Ad5-OVA and Ad5FF1.8-OVA), and used these vectors to test the immune response to OVA following vaccination. Vaccination with either Ad5-OVA or Ad5FF1.8-OVA resulted in robust immune responses (Fig. 6).
Figure 5. Biodistribution of Ad5 and Ad5FF1.8.
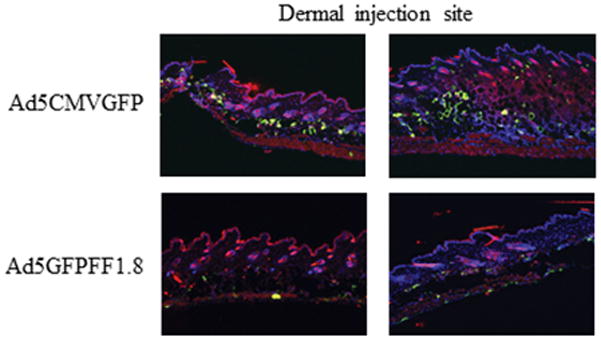
Ad5CMVGFP and Ad5GFPFF1.8 were injected intradermally with 1011 vp in PBS per mouse. After 72-hours, mice were sacrificed and the injection site skin tissue was processed for analysis of GFP (green), CD11c (red) and DAPI (blue) by immunofluorescence. Results from two representative mice for each virus construct are shown.
Figure 6. In vivo immunogenicity of Ad5 vs Ad5FF1.8.
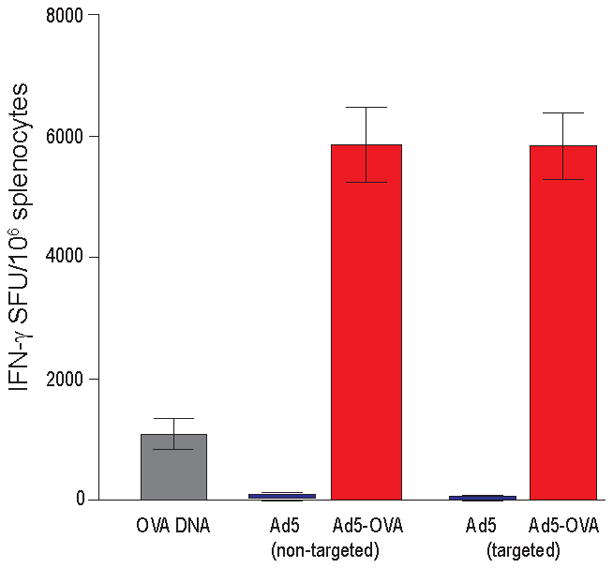
Mice were injected intradermally on days 0 and 7 with 109 vp in PBS or with OVA cDNA through gene gun on days 0, 3, and 6. Splenocytes were analyzed for recognition of the OVA peptide p257–264 on day 11 by INFγ ELISPOT assay. Data are presented as the number of Spot Forming Units (SFU) per 106 splenocytes.
DC are a highly specialized subset of APC that play a critical role in orchestrating adaptive immune responses following vaccination. Seminal studies performed by Steinman et al. documented the potential of targeting antigen to DC via the C-type lectin receptor DEC-205.41, 42 In these studies antigen was selectively delivered to DC using DEC-205-specific antibody-antigen fusion proteins. Subsequent studies confirmed the potential of targeting antigen to DC via multiple different receptors and vaccine platforms. Of note, these studies also highlighted that targeting antigen to DC is not sufficient to induce immunity; antigen must be delivered in the context of additional stimuli to induce potent and long-lived immune responses. Although initial clinical trials based on these studies are ongoing, we have sought to leverage these insights to enhance the efficacy of the Ad vector vaccine platform.
Natural ligands, antibodies and antibody mimetics have been investigated to redirect the specificity of Ad vectors, but most of these molecules are functionally incompatible with cytosolic Ad capsid synthesis and assembly. Typically, the redox state of the cytosol results in improper folding of targeting molecules, altering the structural configuration required for binding specificity and antigen recognition. Previous attempts to retarget Ad vectors with chimeras integrating natural high affinity ligands have been challenged by the limited tolerance of the Ad fiber for genetic modifications,43 and biosynthetic incompatibilities between viral capsid assembly in the nucleus and posttranslational processing of ligand molecules.44 The paucity of naturally existing molecules that can be successfully incorporated into the Ad fiber prompted the use of affibody antibody mimetics, a novel type of artificial protein ligand derived from the three-helix bundle domain Z (dZ) of Staphylococcus protein A.45 However, subsequent advances using this affibody technology have been mainly limited to targeting the human epidermal growth factor receptor type 2 (HER2).46–50
We have recently demonstrated the feasibility of Ad vector retargeting to selected cellular receptors using sdAb. sdAb are compatible with Ad vector biosynthesis and assembly and maintain high binding affinity after incorporation into fiber-fibritin chimeras.38 Recently, Poulin et al. and Kaliberov et al. demonstrated that sdAb can be successfully incorporated into the Ad capsid and redirect Ad specificity.38, 44 The compatibility of sdAb with phage panning/selection to allow exquisite target cell specificity adds to the appeal of sdAb as a candidate targeting moiety for targeting Ad vectors to DC. Indeed, Poulin et al. recently showed that expression of anti-EGFRvIII sdAb on the Ad capsid through fusion to pIX protein can be used to redirect Ad infection.44 This strategy represents a significant advance over previous attempts to redirect the specificity of Ad vectors. In addition, effective particle-to-infectivity ratios and production yields suggest the compatibility of this vector design with human clinical translation applications.
In terms of strategies to retarget Ad vector vaccines to DC, we have previously demonstrated the utility of genetic modification of Ad fiber to display the functional TNF-like domain of human CD40 ligand (hCD40L). These engineered Ad were able to achieve selective DC transduction, activation and migration in a clinically relevant human skin explant model.24 This was done using “mosaic” Ad vectors incorporating fiber proteins with a mutation in the CAR binding domain, and replacement of the fiber knob with a heterologous trimerization domain, derived from bacteriophage T4 fibritin protein and hCD40L.31 Despite the promise of this strategy in preclinical models, clinical translation has been hampered by cGMP production issues related to the mosaic nature of the viral capsids.26 Nevertheless, these data, in combination with our previous success using adapter-mediated CD40 targeting,21, 22, 25, 51–56 provide strong rationale to pursue alternative strategies of Ad vector retargeting to DC.
In the current study we used the sdAb DC1.8 for proof-of-concept studies. It should be noted, however, that we do not think that the DC subset targeted by the sdAb DC1.8 is the ideal subset to target to induce CD8 T cell responses. In fact, previous studies have used the sdAb DC1.8 to target antigen to DC.34 In this study, induction of CD8 T cell responses was not improved. The authors concluded that the inability of DC1.8 targeting to improve vaccine efficacy was likely related to the fact that DC1.8 does not target a dendritic cell subset involved in CD8 T cell priming. Second, in this manuscript we confirm the hypothesis that DC1.8 does not target the ideal DC subset for CD8 T cell priming, demonstrating that Ad5FF1.8 demonstrates superior transduction of immature DC, but not CD8α+/CD141+ DC. Given these results, we are not surprised that targeting DC with sdAb DC1.8 did not enhance the ability of Ad5FF1.8 to prime CD8 T cell responses. We were impressed, however, that despite ablation of CAR-mediated transduction, Ad5FF1.8 was still able to mediate a very robust immune response.
DC have been subcategorized into various subsets with each possessing a distinct functional, transcriptional, and surface marker profile.57, 58 Most DC can present antigen to CD4+ T cells in the context of MHC-II molecules but the capability of processing exogenous antigens and presenting them on MHC-I molecules to CD8+ T cells is restricted to only a few specialized DC subsets.59 This capability, known as cross-presentation, is key for mounting an effective cytotoxic T cell response against tumor cells and provides strong rationale for targeting specific DC subsets to enhance T cell response following vaccination.60 CD8α+ DC in mice and CD141+ DC in human are known to be proficient in antigen cross-presentation. We have recently made an important contribution to the understanding of CD8 T cell priming, demonstrating that CD8α+ DC are required to prime CD8 T cell responses to DNA and cell-based vaccines.37 XCR1 is selectively expressed on CD8α+/CD 141+ DC.61, 62 Current evidence suggests that CD8α+ DC arise from a non-myeloid thymic progenitor while CD8α − DC have a myeloid lineage.63, 64 There are functional differences as well with CD8α+ DC preferentially inducing a Th1 response whereas CD8α − DC mainly activating CD4+ cells.65, 66
The key role of DCs in regulating immunity has been exploited for therapeutic purposes. In addition to ex vivo preparation of antigen-expressing DCs followed by adoptive transfer, in vivo delivery of antigen to DCs through antigen-antibody conjugates is an attractive strategy. By targeting endocytic receptors, antigen is internalized, processed, and presented by MHC class I and/or II molecules. Endocytic receptors such as C type lectin, DEC-205 67 have been targeted and proof-of-concept studies have demonstrated protective immunity can be induced (reviewed in Lehmann et al.,).68 As our understanding of the physiological role of diverse DC subsets improves, the need for specific targeting becomes greater. For example, HIV antigen gag p24 linked with antibodies to molecules expressed by CD8α+ DC such as Langerin/CD207, DEC205/CD205 and CLEC9A receptors, along with anti-CD40 antibody induced a more robust gag-specific Th1 and CD8+T cell response than that obtained by targeting gag to CD8α − DC via DCIR.69 Thus by targeting specific DC subsets one could potentially modulate the immune response against the vaccine antigen both qualitatively and quantitatively.
The recent isolation and validation of sdAb raised against murine bone marrow-derived DC with yet unidentified target specificity,34 and integration of these sdAb into lentivirus using an envelope display technology, allowed Goyvaerts et al. to target of human and murine APC subsets, including DCs and macrophages.70, 71 Of particular interest, sdAb DC1.8, which showed specific binding to immature BMDCs in vitro, 34 mediated selective lentiviral transduction of murine cDC subsets in vivo.72 These findings contrast with our data showing sdAb DC1.8 mediated adenovirus transduction primarily of pDC and CD8α − cDc (Fig. 4). It should be noted that DC subsets were defined differently in both studies and our data were generated through transduction of bone marrow-derived DCs in vitro rather than in vivo.
We also studied the efficacy of transduction via our sdAb-targeted Ad in IL-12 release and DC expression of CD80 and CD86. In both instances there was no detectable DC activation and not a significant difference between targeted and un-targeted Ad. These findings contrast with our earlier studies whereby targeting via CD40 led to enhanced DC secretion of IL-12 and upregulation of CD86.22 In this earlier study, vector targeting also allowed an enhanced induction of anti-tumor immunity. 22 As both CD40 and Clec9a are activating receptors on DC, we anticipate that Ads targeted to Clec9a will activate DC and trigger antitumor immunity.
In summary, we have developed an innovative strategy to specifically target Ad vectors to DC. We have replaced the Ad fiber knob with fiber-fibritin chimeras fused to single domain antibodies (sdAb) specific for DC. This flexible and robust strategy ablates the native tropism of Ad, and permits selective and efficient DC targeting based on the specificity of the sdAb. We successfully generated Ad5FF1.8, a novel Ad5 vector that incorporates DC1.8, a sdAb specific for immature DC. Ad5FF1.8 selectively transduces immature myeloid BMDC in vitro, but does not transduce CAR-expressing cells. These data, establishing a versatile and robust Ad vector vaccine platform for targeting DC, pave the way for testing DC-targeted Ad vector vaccines in vivo. Of note, the use of non-human primate Ads has recently provided a technology to circumvent immunity to human Ad-based vectors. On this basis, we are currently pursuing strategies to retarget gorilla Ads. Such a vector could thus allow DC targeting, even in the context of pre-formed anti-Ad5 immunity. We are convinced that Ad vectors incorporating fiber-fibritin-sdAb chimeras have tremendous potential, leveraging the ability to induce innate and adaptive immune responses with the ability to target specific subsets of DC. We hypothesize that specific targeting of Ad vaccines to DC will ensure that the DC presenting antigen will also be activated by viral infection, minimizing the risk of nonproductive immune responses.
Substantial differences exist between humans and mice with respect to the cellular populations within the dermis. These differences have impacted our ability in this report to document vector-mediated transduction of DC targets in context of intradermal delivery. On this basis, we have employed a human skin plug system to enable study of vector properties as would predicate efficacy in clinical applications. In this regard, we have been able to show that DC-targeted Ad species can accomplish DC selective gene delivery.24 Importantly, this enhanced and selective transduction of DCs in this surrogate in vivo model achieves an enhanced cellular immune response.56 Whereas the limitations of the mouse model have not allowed a similar analysis, murine vaccine studies have also shown a direct correlation between DC targeting and improved vaccine efficacy.22, 23 On this basis, the studies in this report provide the technical basis to study these correlates in the context of the more precise DC targeting we achieve via these current methods.
Acknowledgments
We thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, MO, for the use of the Flow Cytometry Shared Resource Core. The Siteman Cancer Center is supported in part by an NCI Cancer Center Support Grant #P30 CA91842. Sam Kim was supported by an NCI training grant, T32 CA 009621.
Footnotes
CONFLICTS OF INTEREST
No conflicts to disclose.
References
- 1.Tang DC, Zhang J, Toro H, Shi Z, Van Kampen KR. Adenovirus as a carrier for the development of influenza virus-free avian influenza vaccines. Expert review of vaccines. 2009;8(4):469–81. doi: 10.1586/erv.09.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biswas S, Choudhary P, Elias SC, Miura K, Milne KH, de Cassan SC, et al. Assessment of humoral immune responses to blood-stage malaria antigens following ChAd63-MVA immunization, controlled human malaria infection and natural exposure. PloS one. 2014;9(9):e107903. doi: 10.1371/journal.pone.0107903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Barra E, Hodgson SH, Ewer KJ, Bliss CM, Hennigan K, Collins A, et al. A phase Ia study to assess the safety and immunogenicity of new malaria vaccine candidates ChAd63 CS administered alone and with MVA CS. PloS one. 2014;9(12):e115161. doi: 10.1371/journal.pone.0115161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabitzsch ES, Tsang KY, Palena C, David JM, Fantini M, Kwilas A, et al. The generation and analyses of a novel combination of recombinant adenovirus vaccines targeting three tumor antigens as an immunotherapeutic. Oncotarget. 2015 doi: 10.18632/oncotarget.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammer SM, Sobieszczyk ME, Janes H, Karuna ST, Mulligan MJ, Grove D, et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. The New England journal of medicine. 2013;369(22):2083–92. doi: 10.1056/NEJMoa1310566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodgson SH, Ewer KJ, Bliss CM, Edwards NJ, Rampling T, Anagnostou NA, et al. Evaluation of the efficacy of ChAd63-MVA vectored vaccines expressing circumsporozoite protein and ME-TRAP against controlled human malaria infection in malaria-naive individuals. The Journal of infectious diseases. 2015;211(7):1076–86. doi: 10.1093/infdis/jiu579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ledgerwood JE, DeZure AD, Stanley DA, Novik L, Enama ME, Berkowitz NM, et al. Chimpanzee Adenovirus Vector Ebola Vaccine - Preliminary Report. The New England journal of medicine. 2014 doi: 10.1056/NEJMc1505499. [DOI] [PubMed] [Google Scholar]
- 8.Rampling T, Ewer K, Bowyer G, Wright D, Imoukhuede EB, Payne R, et al. A Monovalent Chimpanzee Adenovirus Ebola Vaccine - Preliminary Report. The New England journal of medicine. 2015 doi: 10.1056/NEJMoa1411627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sedegah M, Hollingdale MR, Farooq F, Ganeshan H, Belmonte M, Kim Y, et al. Sterile immunity to malaria after DNA prime/adenovirus boost immunization is associated with effector memory CD8+T cells targeting AMA1 class I epitopes. PloS one. 2014;9(9):e106241. doi: 10.1371/journal.pone.0106241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butterfield LH, Comin-Anduix B, Vujanovic L, Lee Y, Dissette VB, Yang JQ, et al. Adenovirus MART-1-engineered autologous dendritic cell vaccine for metastatic melanoma. Journal of immunotherapy. 2008;31(3):294–309. doi: 10.1097/CJI.0b013e31816a8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santosuosso M, McCormick S, Xing Z. Adenoviral vectors for mucosal vaccination against infectious diseases. Viral immunology. 2005;18(2):283–91. doi: 10.1089/vim.2005.18.283. [DOI] [PubMed] [Google Scholar]
- 12.Cerullo V, Seiler MP, Mane V, Brunetti-Pierri N, Clarke C, Bertin TK, et al. Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors. Molecular therapy : the journal of the American Society of Gene Therapy. 2007;15(2):378–85. doi: 10.1038/sj.mt.6300031. [DOI] [PubMed] [Google Scholar]
- 13.Hartman ZC, Black EP, Amalfitano A. Adenoviral infection induces a multi-faceted innate cellular immune response that is mediated by the toll-like receptor pathway in A549 cells. Virology. 2007;358(2):357–72. doi: 10.1016/j.virol.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 14.Huang X, Yang Y. Innate immune recognition of viruses and viral vectors. Human gene therapy. 2009;20(4):293–301. doi: 10.1089/hum.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73(2):309–19. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 16.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275(5304):1320–3. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 17.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nature reviews Immunology. 2002;2(3):151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 18.Kreutz M, Tacken PJ, Figdor CG. Targeting dendritic cells--why bother? Blood. 2013;121(15):2836–44. doi: 10.1182/blood-2012-09-452078. [DOI] [PubMed] [Google Scholar]
- 19.Timares L, Douglas JT, Tillman BW, Krasnykh V, Curiel DT. Adenovirus-mediated gene delivery to dendritic cells. Methods Mol Biol. 2004;246:139–54. doi: 10.1385/1-59259-650-9:139. [DOI] [PubMed] [Google Scholar]
- 20.Brandao JG, Scheper RJ, Lougheed SM, Curiel DT, Tillman BW, Gerritsen WR, et al. CD40-targeted adenoviral gene transfer to dendritic cells through the use of a novel bispecific single-chain Fv antibody enhances cytotoxic T cell activation. Vaccine. 2003;21(19–20):2268–72. doi: 10.1016/s0264-410x(03)00050-1. [DOI] [PubMed] [Google Scholar]
- 21.Pereboev AV, Nagle JM, Shakhmatov MA, Triozzi PL, Matthews QL, Kawakami Y, et al. Enhanced gene transfer to mouse dendritic cells using adenoviral vectors coated with a novel adapter molecule. Molecular therapy : the journal of the American Society of Gene Therapy. 2004;9(5):712–20. doi: 10.1016/j.ymthe.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Hangalapura BN, Oosterhoff D, de Groot J, Boon L, Tuting T, van den Eertwegh AJ, et al. Potent antitumor immunity generated by a CD40-targeted adenoviral vaccine. Cancer research. 2011;71(17):5827–37. doi: 10.1158/0008-5472.CAN-11-0804. [DOI] [PubMed] [Google Scholar]
- 23.Williams BJ, Bhatia S, Adams LK, Boling S, Carroll JL, Li XL, et al. Dendritic cell based PSMA immunotherapy for prostate cancer using a CD40-targeted adenovirus vector. PloS one. 2012;7(10):e46981. doi: 10.1371/journal.pone.0046981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korokhov N, Noureddini SC, Curiel DT, Santegoets SJ, Scheper RJ, de Gruijl TD. A single-component CD40-targeted adenovirus vector displays highly efficient transduction and activation of dendritic cells in a human skin substrate system. Molecular pharmaceutics. 2005;2(3):218–23. doi: 10.1021/mp050002w. [DOI] [PubMed] [Google Scholar]
- 25.Hangalapura BN, Oosterhoff D, Aggarwal S, Wijnands PG, van de Ven R, Santegoets SJ, et al. Selective transduction of dendritic cells in human lymph nodes and superior induction of high-avidity melanoma-reactive cytotoxic T cells by a CD40-targeted adenovirus. Journal of immunotherapy. 2010;33(7):706–15. doi: 10.1097/CJI.0b013e3181eccbd4. [DOI] [PubMed] [Google Scholar]
- 26.Hangalapura BN, Timares L, Oosterhoff D, Scheper RJ, Curiel DT, de Gruijl TD. CD40-targeted adenoviral cancer vaccines: the long and winding road to the clinic. The journal of gene medicine. 2012;14(6):416–27. doi: 10.1002/jgm.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gainkam LO, Huang L, Caveliers V, Keyaerts M, Hernot S, Vaneycken I, et al. Comparison of the biodistribution and tumor targeting of two 99mTc-labeled anti-EGFR nanobodies in mice, using pinhole SPECT/micro-CT. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2008;49(5):788–95. doi: 10.2967/jnumed.107.048538. [DOI] [PubMed] [Google Scholar]
- 28.Huang L, Gainkam LO, Caveliers V, Vanhove C, Keyaerts M, De Baetselier P, et al. SPECT imaging with 99mTc-labeled EGFR-specific nanobody for in vivo monitoring of EGFR expression. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2008;10(3):167–75. doi: 10.1007/s11307-008-0133-8. [DOI] [PubMed] [Google Scholar]
- 29.Vincke C, Loris R, Saerens D, Martinez-Rodriguez S, Muyldermans S, Conrath K. General strategy to humanize a camelid single-domain antibody and identification of a universal humanized nanobody scaffold. The Journal of biological chemistry. 2009;284(5):3273–84. doi: 10.1074/jbc.M806889200. [DOI] [PubMed] [Google Scholar]
- 30.Noureddini SC, Krendelshchikov A, Simonenko V, Hedley SJ, Douglas JT, Curiel DT, et al. Generation and selection of targeted adenoviruses embodying optimized vector properties. Virus research. 2006;116(1–2):185–95. doi: 10.1016/j.virusres.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Belousova N, Korokhov N, Krendelshchikova V, Simonenko V, Mikheeva G, Triozzi PL, et al. Genetically targeted adenovirus vector directed to CD40-expressing cells. Journal of virology. 2003;77(21):11367–77. doi: 10.1128/JVI.77.21.11367-11377.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krasnykh V, Belousova N, Korokhov N, Mikheeva G, Curiel DT. Genetic targeting of an adenovirus vector via replacement of the fiber protein with the phage T4 fibritin. Journal of virology. 2001;75(9):4176–83. doi: 10.1128/JVI.75.9.4176-4183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alberti MO, Roth JC, Ismail M, Tsuruta Y, Abraham E, Pereboeva L, et al. Derivation of a myeloid cell-binding adenovirus for gene therapy of inflammation. PloS one. 2012;7(5):e37812. doi: 10.1371/journal.pone.0037812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Groeve K, Deschacht N, De Koninck C, Caveliers V, Lahoutte T, Devoogdt N, et al. Nanobodies as tools for in vivo imaging of specific immune cell types. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2010;51(5):782–9. doi: 10.2967/jnumed.109.070078. [DOI] [PubMed] [Google Scholar]
- 35.Von Seggern DJ, Chiu CY, Fleck SK, Stewart PL, Nemerow GR. A helper-independent adenovirus vector with E1, E3, and fiber deleted: structure and infectivity of fiberless particles. Journal of virology. 1999;73(2):1601–8. doi: 10.1128/jvi.73.2.1601-1608.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. Journal of immunology. 1997;158(6):2723–30. [PubMed] [Google Scholar]
- 37.Li L, Kim S, Herndon JM, Goedegebuure P, Belt BA, Satpathy AT, et al. Cross-dressed CD8alpha+/CD103+ dendritic cells prime CD8+ T cells following vaccination. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(31):12716–21. doi: 10.1073/pnas.1203468109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaliberov SA, Kaliberova LN, Buggio M, Tremblay JM, Shoemaker CB, Curiel DT. Adenoviral targeting using genetically incorporated camelid single variable domains. Laboratory investigation; a journal of technical methods and pathology. 2014;94(8):893–905. doi: 10.1038/labinvest.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Douglas JT, Miller CR, Kim M, Dmitriev I, Mikheeva G, Krasnykh V, et al. A system for the propagation of adenoviral vectors with genetically modified receptor specificities. Nature biotechnology. 1999;17(5):470–5. doi: 10.1038/8647. [DOI] [PubMed] [Google Scholar]
- 40.Conrath KE, Lauwereys M, Galleni M, Matagne A, Frere JM, Kinne J, et al. Beta-lactamase inhibitors derived from single-domain antibody fragments elicited in the camelidae. Antimicrobial agents and chemotherapy. 2001;45(10):2807–12. doi: 10.1128/AAC.45.10.2807-2812.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194(6):769–79. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahnke K, Guo M, Lee S, Sepulveda H, Swain SL, Nussenzweig M, et al. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J Cell Biol. 2000;151(3):673–84. doi: 10.1083/jcb.151.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magnusson MK, Hong SS, Henning P, Boulanger P, Lindholm L. Genetic retargeting of adenovirus vectors: functionality of targeting ligands and their influence on virus viability. The journal of gene medicine. 2002;4(4):356–70. doi: 10.1002/jgm.285. [DOI] [PubMed] [Google Scholar]
- 44.Poulin KL, Lanthier RM, Smith AC, Christou C, Risco Quiroz M, Powell KL, et al. Retargeting of adenovirus vectors through genetic fusion of a single-chain or single-domain antibody to capsid protein IX. Journal of virology. 2010;84(19):10074–86. doi: 10.1128/JVI.02665-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nord K, Gunneriusson E, Ringdahl J, Stahl S, Uhlen M, Nygren PA. Binding proteins selected from combinatorial libraries of an alpha-helical bacterial receptor domain. Nature biotechnology. 1997;15(8):772–7. doi: 10.1038/nbt0897-772. [DOI] [PubMed] [Google Scholar]
- 46.Henning P, Magnusson MK, Gunneriusson E, Hong SS, Boulanger P, Nygren PA, et al. Genetic modification of adenovirus 5 tropism by a novel class of ligands based on a three-helix bundle scaffold derived from staphylococcal protein A. Human gene therapy. 2002;13(12):1427–39. doi: 10.1089/10430340260185067. [DOI] [PubMed] [Google Scholar]
- 47.Magnusson MK, Henning P, Myhre S, Wikman M, Uil TG, Friedman M, et al. Adenovirus 5 vector genetically re-targeted by an Affibody molecule with specificity for tumor antigen HER2/neu. Cancer Gene Ther. 2007;14(5):468–79. doi: 10.1038/sj.cgt.7701027. [DOI] [PubMed] [Google Scholar]
- 48.Belousova N, Mikheeva G, Gelovani J, Krasnykh V. Modification of adenovirus capsid with a designed protein ligand yields a gene vector targeted to a major molecular marker of cancer. Journal of virology. 2008;82(2):630–7. doi: 10.1128/JVI.01896-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myhre S, Henning P, Friedman M, Stahl S, Lindholm L, Magnusson MK. Re-targeted adenovirus vectors with dual specificity; binding specificities conferred by two different Affibody molecules in the fiber. Gene Ther. 2009;16(2):252–61. doi: 10.1038/gt.2008.160. [DOI] [PubMed] [Google Scholar]
- 50.Magnusson MK, Kraaij R, Leadley RM, De Ridder CM, van Weerden WM, Van Schie KA, et al. A transductionally retargeted adenoviral vector for virotherapy of Her2/neu-expressing prostate cancer. Human gene therapy. 2012;23(1):70–82. doi: 10.1089/hum.2011.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang D, Pereboev AV, Korokhov N, He R, Larocque L, Gravel C, et al. Significant alterations of biodistribution and immune responses in Balb/c mice administered with adenovirus targeted to CD40(+) cells. Gene therapy. 2008;15(4):298–308. doi: 10.1038/sj.gt.3303085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thacker EE, Nakayama M, Smith BF, Bird RC, Muminova Z, Strong TV, et al. A genetically engineered adenovirus vector targeted to CD40 mediates transduction of canine dendritic cells and promotes antigen-specific immune responses in vivo. Vaccine. 2009;27(50):7116–24. doi: 10.1016/j.vaccine.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tillman BW, de Gruijl TD, Luykx-de Bakker SA, Scheper RJ, Pinedo HM, Curiel TJ, et al. Maturation of dendritic cells accompanies high-efficiency gene transfer by a CD40-targeted adenoviral vector. Journal of immunology. 1999;162(11):6378–83. [PubMed] [Google Scholar]
- 54.Tillman BW, Hayes TL, DeGruijl TD, Douglas JT, Curiel DT. Adenoviral vectors targeted to CD40 enhance the efficacy of dendritic cell-based vaccination against human papillomavirus 16-induced tumor cells in a murine model. Cancer research. 2000;60(19):5456–63. [PubMed] [Google Scholar]
- 55.Pereboev AV, Asiedu CK, Kawakami Y, Dong SS, Blackwell JL, Kashentseva EA, et al. Coxsackievirus-adenovirus receptor genetically fused to anti-human CD40 scFv enhances adenoviral transduction of dendritic cells. Gene therapy. 2002;9(17):1189–93. doi: 10.1038/sj.gt.3301767. [DOI] [PubMed] [Google Scholar]
- 56.de Gruijl TD, Luykx-de Bakker SA, Tillman BW, van den Eertwegh AJ, Buter J, Lougheed SM, et al. Prolonged maturation and enhanced transduction of dendritic cells migrated from human skin explants after in situ delivery of CD40-targeted adenoviral vectors. Journal of immunology. 2002;169(9):5322–31. doi: 10.4049/jimmunol.169.9.5322. [DOI] [PubMed] [Google Scholar]
- 57.Crozat K, Guiton R, Guilliams M, Henri S, Baranek T, Schwartz-Cornil I, et al. Comparative genomics as a tool to reveal functional equivalences between human and mouse dendritic cell subsets. Immunological reviews. 2010;234(1):177–98. doi: 10.1111/j.0105-2896.2009.00868.x. [DOI] [PubMed] [Google Scholar]
- 58.Guilliams M, Henri S, Tamoutounour S, Ardouin L, Schwartz-Cornil I, Dalod M, et al. From skin dendritic cells to a simplified classification of human and mouse dendritic cell subsets. European journal of immunology. 2010;40(8):2089–94. doi: 10.1002/eji.201040498. [DOI] [PubMed] [Google Scholar]
- 59.Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunological reviews. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y, Chen G, Liu Z, Tian S, Zhang J, Carey CD, et al. Genetic vaccines to potentiate the effective CD103+ dendritic cell-mediated cross-priming of antitumor immunity. Journal of immunology. 2015;194(12):5937–47. doi: 10.4049/jimmunol.1500089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bachem A, Hartung E, Guttler S, Mora A, Zhou X, Hegemann A, et al. Expression of XCR1 Characterizes the Batf3-Dependent Lineage of Dendritic Cells Capable of Antigen Cross-Presentation. Frontiers in immunology. 2012;3:214. doi: 10.3389/fimmu.2012.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre CA, Ventre E, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. The Journal of experimental medicine. 2010;207(6):1283–92. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu L, Li CL, Shortman K. Thymic dendritic cell precursors: relationship to the T lymphocyte lineage and phenotype of the dendritic cell progeny. J Exp Med. 1996;184(3):903–11. doi: 10.1084/jem.184.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steinman RM, Inaba K. Myeloid dendritic cells. Journal of leukocyte biology. 1999;66(2):205–8. doi: 10.1002/jlb.66.2.205. [DOI] [PubMed] [Google Scholar]
- 65.Maldonado-Lopez R, De Smedt T, Pajak B, Heirman C, Thielemans K, Leo O, et al. Role of CD8alpha+ and CD8alpha- dendritic cells in the induction of primary immune responses in vivo. Journal of leukocyte biology. 1999;66(2):242–6. doi: 10.1002/jlb.66.2.242. [DOI] [PubMed] [Google Scholar]
- 66.Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, et al. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(3):1036–41. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, et al. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375(6527):151–5. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 68.Lehmann CH, Heger L, Heidkamp GF, Baranska A, Luhr JJ, Hoffmann A, et al. Direct Delivery of Antigens to Dendritic Cells via Antibodies Specific for Endocytic Receptors as a Promising Strategy for Future Therapies. Vaccines. 2016;4(2) doi: 10.3390/vaccines4020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Idoyaga J, Lubkin A, Fiorese C, Lahoud MH, Caminschi I, Huang Y, et al. Comparable T helper 1 (Th1) and CD8 T-cell immunity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin, DEC205, and Clec9A. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(6):2384–9. doi: 10.1073/pnas.1019547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goyvaerts C, De Groeve K, Dingemans J, Van Lint S, Robays L, Heirman C, et al. Development of the Nanobody display technology to target lentiviral vectors to antigen-presenting cells. Gene Ther. 2012;19(12):1133–40. doi: 10.1038/gt.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goyvaerts C, Dingemans J, De Groeve K, Heirman C, Van Gulck E, Vanham G, et al. Targeting of human antigen-presenting cell subsets. Journal of virology. 2013 doi: 10.1128/JVI.01498-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goyvaerts C, Kurt de G, Van Lint S, Heirman C, Van Ginderachter JA, De Baetselier P, et al. Immunogenicity of targeted lentivectors. Oncotarget. 2014;5(3):704–15. doi: 10.18632/oncotarget.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]