ABSTRACT
Human cytomegalovirus (HCMV) is the most common congenitally transmitted pathogen worldwide, impacting an estimated 1 million newborns annually. Congenital HCMV (cCMV) infection is a major global contributor to long-term neurologic deficits, including deafness, microcephaly, and neurodevelopmental delay, as well as to fetal loss and occasional infant mortality. Accordingly, design of a maternal vaccine to prevent cCMV continues to be a top public health priority. Nevertheless, we remain without a licensed vaccine. Maternal immunity provides partial protection, as the risk of vertical HCMV transmission from chronically infected mothers is reduced compared to settings in which the mother is newly infected during pregnancy. Therefore, an understanding of the maternal immune correlates of protection against cCMV is critical to informing design of an efficacious maternal vaccine. Although vaccine development is being assiduously pursued by a large number of pharmaceutical manufacturers, biotechnology organizations, and academic researchers, some pessimism has been expressed regarding the issue of whether a vaccine to protect against cCMV is possible. This pessimism is based on observations that natural immunity is not completely protective against maternal reinfection and congenital transmission. However, we assert that optimism regarding vaccine development is indeed justified, on the basis of accruing evidence of immune correlates of protection—readily achievable by vaccination—that are associated with reduced transmission of HCMV to the fetus in seronegative women. In light of the substantial burden on society conferred by cCMV infection, even a modest reduction in the occurrence of this fetal disease is an important public health goal and justifies aggressive clinical evaluation of vaccines currently in the pipeline.
KEYWORDS: congenital infections, cytomegalovirus, vaccines
TEXT
Human cytomegalovirus (HCMV) congenital infection impacts 1 in every 150 live-born infants (0.7%) globally, making it the most common infectious cause of birth defects. Nearly 40,000 cases of congenital HCMV infection (cCMV) occur in the United States annually, resulting in up to 7,000 infants with permanent sequelae such as sensorineural hearing loss (SNHL), growth restriction, and intellectual disability (1). Moreover, recent work has linked cCMV to the risk for acute lymphoblastic leukemia (2) and has identified CMV infection as a contributor to a number of chronic diseases, including glioblastoma (3, 4). A maternal vaccine to prevent cCMV has been labeled a “tier 1 priority” of the National Academy of Medicine (NAM) for over 15 years, and, stimulated in part by a NAM report (5), recent years have seen an increased interest on the part of academic researchers, the pharmaceutical industry, and the lay public in development of a vaccine. The elimination of congenital rubella syndrome through high vaccine coverage and the recent fervor concerning congenital Zika virus infections and the rapid progress by the research community in design of a Zika vaccine to prevent such infections demonstrate that a firm societal commitment can enable rapid development of vaccines aimed at preventing infant birth defects and brain damage sustained prior to birth. And yet an HCMV vaccine should have a similarly high priority, since a successful immunization program would lead to substantial improvement of pediatric and population health.
Candidate HCMV vaccine development started in the 1970s (6) but is now truly progressing in earnest, with a number of viable candidates in clinical trials or in preclinical studies. The target populations for the vaccine are women of childbearing age (who would be immunized prior to pregnancy to prevent transmission to their fetuses) and solid-organ or hematopoietic cell transplant patients who are at risk of either primary infection (acquisition from a transplanted organ) or reactivation from latent infection in the context of immune suppression. Preliminary clinical studies of HCMV glycoprotein B (gB) subunit vaccine in postpartum (7) and adolescent (8) women, as well as in transplant patients vaccinated with the gB subunit (9) or with a DNA plasmid coding for CD8+ T cell-stimulating proteins (10), have yielded promising results and yet require improvement prior to further clinical development. Further, work delineating maternal immune correlates of protection against congenital transmission after primary and nonprimary maternal infection in both humans and animal models, combined with the identification of neutralizing epitopes on the viral proteins, have provided new leads for the HCMV vaccine field. Thus, HCMV vaccine product development designed to improve on the efficacy achieved in the prior vaccine trials, including advanced stage trials, is proceeding at a rapid pace.
In spite of this progress, vaccine development to eliminate cCMV presents unique challenges in that, unlike the responses seen in cases of rubella, natural immunity is not fully protective against maternal reinfection or congenital transmission. This recognition has led to a cloud hanging over the field, representing a point of view recently eloquently expressed in an article pointing out that cCMV infection takes place commonly in the setting of preexisting maternal immunity, raising skepticism about whether a vaccine can achieve a higher level of protection than that provided by the imperfect natural immunity induced by HCMV infection (11). In fact, the debate over whether a vaccine against cCMV infection that elicits immunity similar to natural immunity would be adequate, or whether a vaccine must provide augmented immunity over and above that conferred by natural infection, has been ongoing for decades (12–15). In this article, we consider the evidence derived from animal and human studies indicating that vaccine induction of CMV-specific immunity can protect against congenital transmission in seronegative individuals and why a better understanding of immunity in seropositive individuals could lead to progress in identifying immunologic endpoints and in targeting epitopes for vaccine development. We also summarize the status of candidate HCMV vaccines for humans.
CONGENITAL CMV TRANSMISSION IN ACUTELY AND CHRONICALLY HCMV-INFECTED WOMEN
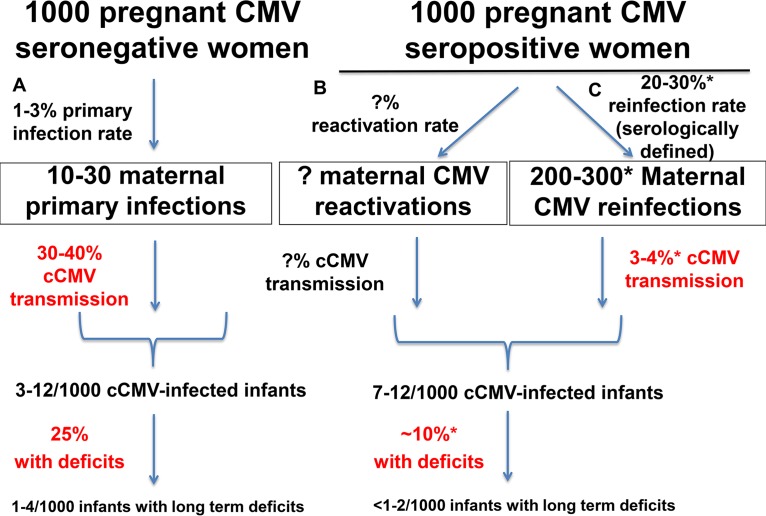
Vertical transmission of HCMV in utero is thought to begin with virus in the maternal circulation, replication of virus in decidual cells, and subsequent spread of virus leading to focal infection of cytotrophoblasts in the placenta (16–19). Thus, preexisting vaccine-elicited maternal anti-HCMV immunity that impedes this initial chain of infection events could prevent cCMV transmission, and such immunity should be the goal in designing an HCMV vaccine. While ubiquitous HCMV infections occur readily in both seronegative and seropositive women (20) and superinfection can be readily achieved in nonhuman primates (21), it has been estimated that there is a lower fetal transmission rate per maternal CMV infection in the setting of preexisting maternal immunity (1, 22, 23). Up to 30% to 40% of seronegative women who acquire primary HCMV infection during pregnancy transmit the virus to their fetus, and yet less than 1% of women with chronic CMV infection transmit to their fetus (1). However, this stark difference in transmission rates does not account for the risk of fetal transmission per maternal HCMV exposure and could be underestimating the transmission rate in seropositive women (11). Studies are now emerging that more closely compare the rates of transmission in primary versus nonprimary maternal infection and support the notion that the risk of transmission is considerably higher in the setting of primary maternal infection (22, 23). In one such study of seropositive women with serologic and/or virologic evidence of recent reinfection, Simonazzi et al. reported a fetal transmission rate of 3.4% in the setting of nonprimary maternal infection (23), considerably lower than the 30% to 40% rate of cCMV transmission in the setting of primary CMV infection (Fig. 1). Another recent retrospective study of saliva screening in newborns demonstrated that the risk of fetal transmission is 4-fold higher in primary maternal infection than in nonprimary maternal infection (22). And yet the latter study did not take into account recent reinfection status; thus, the Simonazzi study represents a better estimate of the risk of congenital transmission upon maternal reinfection (3.4%). Together, the data from those studies indicate a partially protective role of maternal immune factors against cCMV infection.
FIG 1.
Congenital HCMV transmission rates in CMV-seronegative and -seropositive women. (A) Primary HCMV infection occurs infrequently (rate, 1% to 3%) in HCMV-seronegative pregnant women, but rates of cCMV transmission (30% to 40%) and infant disease (25%) are known to be high following primary maternal infection (1, 97). (B) The rate of systemic maternal HCMV reactivation in chronically infected women is not known; nor is the rate of cCMV infection in the setting of maternal reactivation known. (C) HCMV reinfection rates, identified by detection of a serologic response against a new strain of HCMV, have suggested that nearly 1 of 3 CMV seropositive women become reinfected during pregnancy (24, 39), and yet the cCMV transmission rate is up to 10-fold lower than that in primary maternal infection (3.4%) (23) and the disease rates have been reported as 10% or less (24, 29, 33, 34). Thus, similar numbers of cCMV-infected and -impaired infants occur in HCMV-seronegative and -seropositive pregnant women populations (1). *, data points where more studies are needed to further advance our understanding of the partial protective nature of preexisting HCMV immunity.
The emerging understanding of the differing cCMV transmission rates in cases of primary and nonprimary maternal infection cannot be overlooked as the prospects for deploying prepregnancy maternal HCMV vaccines are debated (1, 23). While a number of studies have demonstrated that congenitally infected infants born to women with preexisting immunity can develop symptomatic disease (11, 24), there is also evidence that cCMV occurring in the setting of preexisting maternal immunity is less severe and less likely to result in disabilities. Placental virus pathology has been described as less severe in the setting of preexisting and robust neutralizing antibody responses (16, 25). Moreover, the major sequela of cCMV infection, hearing loss, may be less severe in the setting of nonprimary maternal infection, with a reported lower rate of severe/profound hearing loss (26). There have also been a number of studies performed with highly seroprevalent populations that indicated a low rate of symptomatic disease compared to that described in seronegative populations (24, 27–34). However, other studies have reported rates of symptomatic disease in CMV-infected infants of seropositive women that were similar to those reported for CMV-infected infants of seronegative women (35, 36), fueling the debate over whether maternal immunity modulates cCMV disease severity. While the impact of maternal immunity on the severity of disease remains ill-defined, the established high rates of congenital transmission and disease sequelae in the absence of preexisting immunity make maternal vaccine development for this setting imperative. The impact of preexisting natural immunity on reducing the rate of placental transmission and, potentially, also disease severity suggests that CMV-specific immunity should be an effective means to reduce the effects of exposure to CMV during pregnancy.
Because of the high global rate of HCMV seroprevalence, congenital transmission in mothers with preexisting natural HCMV is a contributor to cCMV cases that is equal in significance to or more significant than primary maternal infections in developed countries, despite an overall transmission rate of ∼1% (22, 23, 37). Thus, CMV vaccine development to prevent congenital transmission should include seropositive women. However, it is unknown whether reinfection by an exogenous strain of HCMV or latent viral reactivation is responsible for transmission in the setting of natural maternal immunity, as complete molecular characterizations of maternal reinfecting strains of HCMV are still lacking. The frequency of exposure to HCMV during pregnancy is likely to be higher in settings of nearly universal seropositivity or of clustering of seropositive individuals. In support of this concept, a 20% to 30% rate of reinfection in seropositive women with an antigenically distinct strain has been reported based on serologic evidence of reinfection both in areas of high HCMV seroprevalence (24, 38) and in seropositive U.S. populations (39). This high rate of reinfection of seropositive women is in contrast to the 1% to 3% infection rate seen among seronegative women in developed countries (1). This discrepancy can be explained by the knowledge that cases of HCMV seropositivity are highly clustered among populations by ethnicity, race, and socioeconomic factors (40–42). Since HCMV is typically transmitted by close contact within households, the high reinfection rate among seropositive women versus the primary infection rate in seronegative women seems plausible. Thus, even if immunity reduces the risk of abnormalities in the infants of those women, the importance of cCMV in seropositive populations remains substantial. Of note, nearly all HCMV-seropositive women reactivate virus postpartum in breast milk (43), and yet very few transmit the virus across the placenta, indicating that preexisting maternal immunity controls systemic replication of endogenous HCMV strains at a low enough level to prevent or eliminate placental infection in the majority of cases. Defining the maternal immune correlates of protection against congenital transmission in the setting of nonprimary and reactivated maternal HCMV infection, as well as in the setting of infections that occur in the setting of experimental vaccine immunity, will iteratively inform and guide maternal vaccine development. And yet protection of seronegative women remains an important goal given the equal contributions of transmissions in the seronegative and seropositive settings to cCMV infections in developed countries.
HUMORAL IMMUNITY AND PROTECTION AGAINST CONGENITAL CMV TRANSMISSION
Maternal HCMV-specific IgG responses appear to be critical for protection against congenital transmission (16, 44–47). The impact of humoral immune responses on protection against congenital infection has been examined by comparisons of IgG responses in transmitting and nontransmitting HCMV-infected women (45, 48–50). In those studies, HCMV neutralization titer, IgG avidity, and rapidity of neutralizing antibody development, but not HCMV-specific IgG binding, were correlated with protection against congenital transmission. Application of a combination of HCMV IgM and IgG avidity testing can predict congenital transmission in primary infections but may miss the identification of congenital CMV transmission cases in the setting of nonprimary maternal infection (51). Further support for the idea of a role of neutralizing antibodies in reducing the risk of placental HCMV transmission comes from studies demonstrating that neutralizing titers induced by the HCMV pentameric complex (PC; gH/gL/UL128-131A) against epithelial-cell-tropic viruses are strongly associated with reduced congenital CMV transmission in the setting of nonprimary congenital CMV transmission in HIV/CMV-coinfected pregnant women (48, 52). Whereas another study reported that high fetal cord blood neutralization titers were associated with sequelae of cCMV among infected infants from mothers with first-trimester primary infection (11), high neutralization titers in those mothers might have been a result of high levels of earlier maternal systemic virus replication, which is independently associated with symptomatic infection (53, 54). Together, these findings suggest that a high HCMV neutralization titer contributes to protection against congenital transmission, though there is a concern that after transmission has occurred, higher neutralization titers may be associated with poor outcome.
Results of studies of passive IgG protection against congenital CMV in animal models have been convincing in establishing the ability of virus-specific IgG to block placental CMV transmission. In a recent study in the rhesus monkey model of placental CMV transmission, provision of hyperimmune globulin to CD4+ T cell-depleted pregnant rhesus monkey dams prior to rhesus CMV (RhCMV) challenge provided complete protection against fetal loss and, after adjustment for dose optimization to achieve high neutralizing titers for >1 week postinfusion, completely protected against placental transmission (55). In the guinea pig cytomegalovirus (GPCMV) congenital infection model, the passive administration of antibody targeting envelope glycoproteins was first shown to modify the risk of vertical transmission in studies in the 1980s (56). Later passive transfer studies using pooled antisera generated following immunization with purified glycoprotein preparations identified the anti-gB response as the protective component in these guinea pig pregnancy/challenge studies (46, 57). Passive antibody transfer studies in the GPCMV model have also demonstrated efficacy of a monoclonal antibody targeting the gH/gL (44). Notably, unlike current human trials assessing the role of hyperimmune globulin administered after primary maternal infection (58), passive antibody was more beneficial in improving pregnancy outcomes in animal studies when it was administered prior to viral challenge during pregnancy. These observations suggest that a greater benefit is likely to be conferred by induction of maternal antibodies (via vaccination) prior to exposure to HCMV than by passive transfer of antibody after infection has already been established (58).
Complicating the role of antibody responses in protection against congenital HCMV infection, preexisting, nonneutralizing maternal IgG has been implicated in facilitation of placental transmission. IgG-CMV virion complexes can be transported across the syncytiotrophoblast by FcRn and epidermal growth factor receptor (EGFR) (59). Indirectly supporting the data concerning this mechanism of placental transmission, congenital CMV risk is only 30% during the first trimester when FcRn and EGFR are not fully expressed compared to a 72% transmission risk during the third trimester, when the placental IgG transfer peaks (59–61). To assess the role of maternal antibody function, placental transmission was further assessed in an ex vivo human placental model (16) which demonstrated that the presence of weakly neutralizing monoclonal IgG, but not potently neutralizing monoclonal IgG, resulted in placental HCMV infection. While the results of administration of CMV hyperimmune globulin to acutely CMV-infected pregnant women to protect their fetuses have been mixed, partial benefit may have been achieved, even though the antibodies were administered days to weeks after maternal infection (58, 62).
Critical to vaccine development is defining which glycoprotein targets are important to protection against placental transmission, as multiple HCMV surface glycoproteins are involved in viral entry into host cells and contain neutralizing epitopes (63). Within gB, distinct regions have been characterized as targets for neutralizing antibodies, including antigenic domain 1 (AD-1), AD-2, domain I (AD-5), and domain II (AD-4) (64–67). Additionally, the PC has been identified as the target of the most potently neutralizing antibodies (50). In sera from transmitter and nontransmitter mothers with primary infection, adsorption with the PC, but not gH/gL or gB, resulted in a dramatic reduction in neutralizing activity in epithelial cells at all postinfection time points. Importantly, anti-PC and anti-gH/gL IgG titers, but not anti-gB IgG titers, were higher in magnitude in nontransmitting mothers than in acutely infected transmitting mothers within 30 days postinfection (50). Identified immune correlates of protection against primary cCMV transmission (i.e., immune correlates of risk for cCMV [68]) were as follows: delayed antibody response to gB; higher and more-rapid antibody response to PC; broad and rapid antibody response to neutralizing epitopes on PC; rapid plaque formation-inhibiting antibody response; the presence of gamma interferon IFN-γ-producing CD4+ and CD8+ T cells; higher levels of reverted effector memory cells (TEMRA); rapid virus-specific lymphocyte proliferation; rapid virus-specific interleukin-2 (IL-2) production by CD4+ T cells; and higher levels of 1L-7R+ CD4+ T cells. (Note that all of the immune correlates listed above pertain to responses in women who did or did not transmit CMV to their fetuses.)
However, several recent studies have suggested that gB-directed antibodies remain important to prevent viral spread. Monoclonal antibodies against gB, but not the PC, have been reported to block placental trophoblast infection, and an HCMV mutant strain lacking the PC was able to infect human trophoblast progenitor cells, suggesting the PC is not essential for placental cell entry (69). Furthermore, in the setting of nonprimary cCMV transmission in HIV-infected women, a weak association between protection and maternal IgG binding to gB AD-2 was observed, but not with the PC, gH/gL, or gH/gL/gO (48). Eliciting antibody responses to multiple epitopes may be essential for vaccine-mediated protection against cCMV, with the best-established targets consisting of neutralizing epitopes within gB and the PC.
T CELL IMMUNITY AND PROTECTION AGAINST CONGENITAL CMV TRANSMISSION IN HUMANS
A major issue in the field of congenital HCMV prevention research has been whether vaccine-elicited maternal T cell responses would be required to effectively prevent congenital CMV transmission. Studies comparing cellular immune responses in transmitting and nontransmitting mothers after primary HCMV infection resulted in the observation that an early lymphoproliferative response to HCMV was associated with nontransmission (70; see also the list of immune correlates given above). Moreover, Lilleri and colleagues found that classical HCMV-specific CD4+ and CD8+ T cell responses correlated with peripheral viral clearance and that delayed CD4+ T cell responses, but not delayed CD8+ T cell responses, were associated with congenital transmission (71), a finding that was confirmed in a distinct pregnancy cohort (72). This finding was further bolstered by studies in the nonhuman primate model of cCMV transmission in which CD4+ T cell depletion prior to maternal RhCMV infection led to more-frequent transmission and a high rate of fetal loss. These studies indicated a protective role for HCMV-specific CD4+ T cell frequency and/or proliferation in preventing congenital transmission of HCMV. Thus, induction of T cell responses remains a goal for vaccine-elicited protection against placental HCMV transmission, as the T cell response would both contribute to eliminating virus-infected cells and support the B cell responses, and yet their role seems to be secondary to that of robust humoral immunity for cCMV prevention.
TIMING OF IMMUNE CONTAINMENT OF PRIMARY VIRUS REPLICATION AND CONGENITAL TRANSMISSION
As the virus that inoculates the placenta is thought to originate in the maternal blood, rapid containment of systemic virus replication after primary infection is likely key to protection against congenital transmission. In fact, in the setting of isolated preexisting humoral immunity in pregnant rhesus monkey dams, the plasma viral load in the nontransmitting dams was significantly lower than that seen in the transmitting dams. Furthermore, the peak viral load in plasma of transmitting dams predicted the peak viral load in amniotic fluid (55). Immune analysis of human primary maternal infection also suggests that rapid induction of immunity, which could predict rapid viral containment, is an important factor in protection against congenital transmission (50). As natural immunity to HCMV is not completely protective against virus acquisition or placental transmission, the goal of designing a vaccine eliciting sterilizing immunity that can always prevent virus acquisition may be unrealistic. A more achievable goal for vaccine development might be a moderate reduction of virus acquisition, as achieved in the previous gB/MF59 vaccine trials, plus the induction of responses that can rapidly contain virus replication soon after infection and reduce the chance of spread to the placenta, which may require responses to the viral PC. Importantly, the dynamics of rapid viral containment or prevention of placental infection, as opposed to maternal virus acquisition of infection assessed by indirect means (such as seroconversion), has implications for clinical vaccine trial design.
CURRENT STATUS OF CMV VACCINE DEVELOPMENT
In view of the evidence that natural immunity is at least partly protective against congenital CMV infection, the critical issue is how that immunity can be reproduced or improved on through vaccination. Currently, three CMV antigens appear to be of greatest interest for a vaccine: the gB glycoprotein, the PC, and the pp65 tegument protein. Table 1 lists the candidates according to their ability to induce responses to each of these targets. Antibodies to gB are thought to primarily prevent entry into fibroblasts (90), but note that some of the gB candidates are claimed to block epithelial cell entry as well as fibroblast cell entry (79), perhaps because those gB constructs have a structure different from those of the gB subunit vaccines that do not prevent entry into epithelial cells (91). Interestingly, a gB vaccine has been shown to be highly effective in preventing CMV disease in organ transplant patients (9).
TABLE 1.
CMV vaccines currently under development
| Vaccine | Developer | Antigena |
Reference no. | ||
|---|---|---|---|---|---|
| gB | Pentamer | pp65 | |||
| Adenovirus vector | Queensland Institute | X | 73 | ||
| Alphavirus replicons | GSK | X | X | 74 | |
| Canarypox vector | Sanofi | X | 75 | ||
| Dense bodies | Vaccine Project Management, Serum Institute, India | X | X | X | 76 |
| DNA plasmids | Astellas, Inovio | X | X | 77, 78 | |
| Lentivirus particles | Variations Bio | X | X | 79 | |
| Live attenuated | Medimmune | X | X | 80, 81 | |
| Live replication-defective | Merck | X | X | X | 82 |
| LCMV vector | Hookipa | X | X | 83 | |
| mRNA | GSK, Moderna | X | X | 84 | |
| MVA vector | City of Hope | X | X | 85 | |
| Peptides | City of Hope, University of Heidelberg | X | 86 | ||
| Soluble pentamer | Humabs, Redbiotech GSK | X | 87 | ||
| Subunit gB | Sanofi, GSK | X | 8, 9, 88 | ||
| VSV vector | Yale | X | 89 | ||
LCMV, lymphocytic choriomeningitis virus; VSV, vesicular stomatitis virus. An “X” indicates that the antigen is included in the vaccine candidate.
The role of pp65 as an important inducer of T cell responses is recognized for protection of transplant patients (78). Immediate early (IE) proteins are also important inducers of T cell responses and have been included in modified vaccinia Ankara (MVA) and alphavirus vectored vaccines (74, 85). The role of these T cell targets, and of T cell responses in general, in preventing transmission from mother to fetus requires more study (72). However, as preliminary data suggest that T cell responses to pp65 peptide epitopes can reduce reactivation (1, 22, 23) of CMV in seropositive hematogenous stem cell transplant recipients (86) and as CD4+ T cell helper function is important to antibody production, T cell responses continue to be an important goal in designing HCMV vaccine candidates. Notably, two candidates that provide all three main vaccine antigens of interest (gB, pentamer, and pp65) are currently in the clinical trial pipeline: replication-defective virus V160 and a purified dense-body vaccine (92, 93). An IE1/pp65 fusion protein-expressing alphavirus has been assessed (74), while a Triplex MVA currently in clinical testing includes pp65 IE1 and IE2 (85). Subsequent efficacy studies are required to determine whether a combination of these antigens would be effective in preventing HCMV acquisition or cCMV.
With regard to demonstration of efficacy in clinical trials, it is notable that an attenuated virus has prevented HCMV disease in renal transplant patients and that subunit gB has been found to both moderately reduce acquisition of HCMV by seronegative women and to reduce CMV disease in recipients of solid-organ transplants (7–9). As mentioned above, pp65 protein has been shown to reduce reactivation of HCMV in hematopoietic stem cell transplant recipients (10), and peptides from pp65 have also given preliminary positive results in those patients (94). The pentamer has not yet been tested for efficacy against HCMV infection or disease. Results of these trials with efficacy outcomes should continue to be reevaluated with an eye toward defining vaccine-elicited immune correlates of protection, similarly to what has been pursued with partially effective HIV vaccine trials (95). It is also important that a HCMV vaccine that does not prevent infection of women, but does prevent transmission to the fetus, would still be valuable.
The path to licensure of a HCMV vaccine is relatively clear. For transplant populations, it will be necessary to show a reduction in the levels of viremia and disease. For seronegative women of child-bearing age, the FDA has recommended a placebo-controlled vaccine study in women prior to establishment of pregnancy, with subsequent evaluation of acquisition of HCMV infection in vaccinees and of acquisition of cCMV infection in their newborn infants (96). Prevention of cCMV infection was considered to be the most relevant and practical outcome endpoint for a putative phase III efficacy study; however, it was noted that a vaccine that demonstrated high efficacy in preventing acquisition of HCMV by women in the community could achieve licensure, since, by definition, the developing fetus of uninfected women would remain uninfected. The vaccine strategies for seropositive women remain uncharted and should be informed by studies of immune deficits that permit reinfection or cCMV transmission in the setting of preexisting immunity (24). Thus, the priorities for HCMV vaccine development remain 3-fold: (i) early-phase testing of novel vaccine candidates, comparing responses to natural immunity and to that previously elicited by partially efficacious vaccines; (ii) defining the immune correlates of congenital HCMV transmission, particularly in seropositive women and vaccine recipients; and (iii) continuing to develop and utilize animal models that can provide the proof of concept that specific immune responses can block placental CMV infection. Continued progress in these areas will ensure that an effective vaccine to eliminate cCMV as a major cause of infant birth defects and brain damage is within reach.
ACKNOWLEDGMENTS
S.R.P. is a consultant with Pfizer Vaccines and collaborates with Merck on a sponsored program on CMV immunity in nonhuman primates. M.R.S. is currently a consultant for Merck vaccines. S.A.P. is a consultant to a number of developers of CMV vaccines, including Sanofi, GSK, Merck, Pfizer, and Variations Bio.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1.Kenneson A, Cannon MJ. 2007. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 17:253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 2.Francis SS, Wallace AD, Wendt GA, Li L, Liu F, Riley LW, Kogan S, Walsh KM, de Smith AJ, Dahl GV, Ma X, Delwart E, Metayer C, Wiemels JL. 2017. In utero cytomegalovirus infection and development of childhood acute lymphoblastic leukemia. Blood 129:1680–1684. doi: 10.1182/blood-2016-07-723148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joseph GP, McDermott R, Baryshnikova MA, Cobbs CS, Ulasov IV. 2017. Cytomegalovirus as an oncomodulatory agent in the progression of glioma. Cancer Lett 384:79–85. doi: 10.1016/j.canlet.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 4.Navarro D. 2016. Expanding role of cytomegalovirus as a human pathogen. J Med Virol 88:1103–1112. doi: 10.1002/jmv.24450. [DOI] [PubMed] [Google Scholar]
- 5.Stratton KR, Durch JS, Lawrence RS (ed). 2000. Vaccines for the 21st century: a tool for decisionmaking. National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- 6.Plotkin SA, Farquhar J, Horberger E. 1976. Clinical trials of immunization with the Towne 125 strain of human cytomegalovirus. J Infect Dis 134:470–475. doi: 10.1093/infdis/134.5.470. [DOI] [PubMed] [Google Scholar]
- 7.Pass RF, Zhang C, Evans A, Simpson T, Andrews W, Huang ML, Corey L, Hill J, Davis E, Flanigan C, Cloud G. 2009. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med 360:1191–1199. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernstein DI, Munoz FM, Callahan ST, Rupp R, Wootton SH, Edwards KM, Turley CB, Stanberry LR, Patel SM, McNeal MM, Pichon S, Amegashie C, Bellamy AR. 2016. Safety and efficacy of a cytomegalovirus glycoprotein B (gB) vaccine in adolescent girls: a randomized clinical trial. Vaccine 34:313–319. doi: 10.1016/j.vaccine.2015.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffiths PD, Stanton A, McCarrell E, Smith C, Osman M, Harber M, Davenport A, Jones G, Wheeler DC, O'Beirne J, Thorburn D, Patch D, Atkinson CE, Pichon S, Sweny P, Lanzman M, Woodford E, Rothwell E, Old N, Kinyanjui R, Haque T, Atabani S, Luck S, Prideaux S, Milne RS, Emery VC, Burroughs AK. 2011. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet 377:1256–1263. doi: 10.1016/S0140-6736(11)60136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kharfan-Dabaja MA, Boeckh M, Wilck MB, Langston AA, Chu AH, Wloch MK, Guterwill DF, Smith LR, Rolland AP, Kenney RT. 2012. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis 12:290–299. doi: 10.1016/S1473-3099(11)70344-9. [DOI] [PubMed] [Google Scholar]
- 11.Britt WJ. 2017. Congenital human cytomegalovirus infection and the enigma of maternal immunity. J Virol 91:e02392–16. doi: 10.1128/JVI.02392-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R; National Vaccine Advisory Committee. 2004. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin Infect Dis 39:233–239. doi: 10.1086/421999. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths P, Plotkin S, Mocarski E, Pass R, Schleiss M, Krause P, Bialek S. 2013. Desirability and feasibility of a vaccine against cytomegalovirus. Vaccine 31(Suppl 2):B197–B203. doi: 10.1016/j.vaccine.2012.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schleiss M. 2005. Progress in cytomegalovirus vaccine development. Herpes 12:66–75. [PubMed] [Google Scholar]
- 15.Schleiss MR. 2013. Developing a vaccine against congenital cytomegalovirus (CMV) infection: what have we learned from animal models? Where should we go next? Future Virol 8:1161–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maidji E, McDonagh S, Genbacev O, Tabata T, Pereira L. 2006. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am J Pathol 168:1210–1226. doi: 10.2353/ajpath.2006.050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonagh S, Maidji E, Ma W, Chang HT, Fisher S, Pereira L. 2004. Viral and bacterial pathogens at the maternal-fetal interface. J Infect Dis 190:826–834. doi: 10.1086/422330. [DOI] [PubMed] [Google Scholar]
- 18.Pereira L, Maidji E, McDonagh S, Genbacev O, Fisher S. 2003. Human cytomegalovirus transmission from the uterus to the placenta correlates with the presence of pathogenic bacteria and maternal immunity. J Virol 77:13301–13314. doi: 10.1128/JVI.77.24.13301-13314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pereira L, Maidji E, McDonagh S, Tabata T. 2005. Insights into viral transmission at the uterine-placental interface. Trends Microbiol 13:164–174. doi: 10.1016/j.tim.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. 2001. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med 344:1366–1371. doi: 10.1056/NEJM200105033441804. [DOI] [PubMed] [Google Scholar]
- 21.Hansen SG, Powers CJ, Richards R, Ventura AB, Ford JC, Siess D, Axthelm MK, Nelson JA, Jarvis MA, Picker LJ, Fruh K. 2010. Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science 328:102–106. doi: 10.1126/science.1185350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leruez-Ville M, Magny JF, Couderc S, Pichon C, Parodi M, Bussieres L, Guilleminot T, Ghout I, Ville Y. 2017. Risk factors for congenital cytomegalovirus infection following primary and nonprimary maternal infection: a prospective neonatal screening study using polymerase chain reaction in saliva. Clin Infect Dis 65:398–404. doi: 10.1093/cid/cix337. [DOI] [PubMed] [Google Scholar]
- 23.Simonazzi G, Curti A, Cervi F, Gabrielli L, Contoli M, Capretti MG, Rizzo N, Guerra B, Farina A, Lazzarotto T. 12 July 2017. Perinatal outcomes of non-primary maternal cytomegalovirus infection: a 15-year experience. Fetal Diagn Ther doi: 10.1159/000477168. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto AY, Mussi-Pinhata MM, Isaac MDL, Amaral FR, Carvalheiro CG, Aragon DC, Manfredi AK, Boppana SB, Britt WJ. 2011. Congenital cytomegalovirus infection as a cause of sensorineural hearing loss in a highly immune population. Pediatr Infect Dis J 30:1043–1046. doi: 10.1097/INF.0b013e31822d9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maidji E, Nigro G, Tabata T, McDonagh S, Nozawa N, Shiboski S, Muci S, Anceschi MM, Aziz N, Adler SP, Pereira L. 2010. Antibody treatment promotes compensation for human cytomegalovirus-induced pathogenesis and a hypoxia-like condition in placentas with congenital infection. Am J Pathol 177:1298–1310. doi: 10.2353/ajpath.2010.091210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross SA, Fowler KB, Ashrith G, Stagno S, Britt WJ, Pass RF, Boppana SB. 2006. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J Pediatr 148:332–336. doi: 10.1016/j.jpeds.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Dar L, Pati SK, Patro AR, Deorari AK, Rai S, Kant S, Broor S, Fowler KB, Britt WJ, Boppana SB. 2008. Congenital cytomegalovirus infection in a highly seropositive semi-urban population in India. Pediatr Infect Dis J 27:841–843. doi: 10.1097/INF.0b013e3181723d55. [DOI] [PubMed] [Google Scholar]
- 28.Luchsinger V, Suarez M, Schultz R, Barraza P, Guzman M, Terrada L, Mendez V, Kaltwasser G. 1996. Incidence of congenital cytomegalovirus infection in newborn infants of different socioeconomic strata. Rev Med Chil 124:403–408. (In Spanish.) [PubMed] [Google Scholar]
- 29.Mussi-Pinhata MM, Yamamoto AY, Moura Brito RM, de Lima Isaac M, de Carvalho e Oliveira PF, Boppana S, Britt WJ. 2009. Birth prevalence and natural history of congenital cytomegalovirus infection in a highly seroimmune population. Clin Infect Dis 49:522–528. doi: 10.1086/600882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noyola DE, Mejía-Elizondo AR, Canseco-Lima JM, Allende-Carrera R, Hernánsez-Salinas AE, Ramírez-Zacarías JL. 2003. Congenital cytomegalovirus infection in San Luis Potosi, Mexico. Pediatr Infect Dis J 22:89–90. [DOI] [PubMed] [Google Scholar]
- 31.Schopfer K, Lauber E, Krech U. 1978. Congenital cytomegalovirus infection in newborn infants of mothers infected before pregnancy. Arch Dis Child 53:536–539. doi: 10.1136/adc.53.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sohn YM, Park KI, Lee C, Han DG, Lee WY. 1992. Congenital cytomegalovirus infection in Korean population with very high prevalence of maternal immunity. J Korean Med Sci 7: 47–51. doi: 10.3346/jkms.1992.7.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai CH, Tsai FJ, Shih YT, Wu SF, Liu SC, Tseng YH. 1996. Detection of congenital cytomegalovirus infection in Chinese newborn infants using polymerase chain reaction. Acta Paediatr 85:1241–1243. doi: 10.1111/j.1651-2227.1996.tb18237.x. [DOI] [PubMed] [Google Scholar]
- 34.van der Sande MA, Kaye S, Miles DJ, Waight P, Jeffries DJ, Ojuola OO, Palmero M, Pinder M, Ismaili J, Flanagan KL, Aveika AA, Zaman A, Rowland-Jones S, McConkey SJ, Whittle HC, Marchant A. 2007. Risk factors for and clinical outcome of congenital cytomegalovirus infection in a peri-urban West-African birth cohort. PLoS One 2:e492. doi: 10.1371/journal.pone.0000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanzieri TM, Dollard SC, Bialek SR, Grosse SD. 2014. Systematic review of the birth prevalence of congenital cytomegalovirus infection in developing countries. Int J Infect Dis 22:44–48. doi: 10.1016/j.ijid.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang XW, Li F, Yu XW, Shi XW, Shi J, Zhang JP. 2007. Physical and intellectual development in children with asymptomatic congenital cytomegalovirus infection: a longitudinal cohort study in Qinba mountain area, China. J Clin Virol 40:180–185. doi: 10.1016/j.jcv.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 37.Wang C, Zhang X, Bialek S, Cannon MJ. 2011. Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Clin Infect Dis 52:e11–e13. doi: 10.1093/cid/ciq085. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto AY, Mussi-Pinhata MM, Boppana SB, Novak Z, Wagatsuma VM, Oliveira Pde F, Duarte G, Britt WJ. 2010. Human cytomegalovirus reinfection is associated with intrauterine transmission in a highly cytomegalovirus-immune maternal population. Am J Obstet Gynecol 202:297.e1–297.e8. doi: 10.1016/j.ajog.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross SA, Arora N, Novak Z, Fowler KB, Britt WJ, Boppana SB. 2010. Cytomegalovirus reinfections in healthy seroimmune women. J Infect Dis 201:386–389. doi: 10.1086/649903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lantos PM, Hoffman K, Permar SR, Jackson P, Hughes BL, Kind A, Swamy G. 24 August 2017. Neighborhood disadvantage is associated with high cytomegalovirus seroprevalence in pregnancy. J Racial Ethn Health Disparities doi: 10.1007/s40615-017-0423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lantos PM, Hoffman K, Permar SR, Jackson P, Hughes BL, Swamy GK. 2017. Geographic disparities in cytomegalovirus infection during pregnancy. J Pediatric Infect Dis Soc 6:e55–e61. doi: 10.1093/jpids/piw088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lantos PM, Permar SR, Hoffman K, Swamy GK. 2015. The excess burden of cytomegalovirus in African American communities: a geospatial analysis. Open Forum Infect Dis 2:ofv180. doi: 10.1093/ofid/ofv180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jim WT, Shu CH, Chiu NC, Chang JH, Hung HY, Peng CC, Kao HA, Wei TY, Chiang CL, Huang FY. 2009. High cytomegalovirus load and prolonged virus excretion in breast milk increase risk for viral acquisition by very low birth weight infants. Pediatr Infect Dis J 28:891–894. doi: 10.1097/INF.0b013e3181a55c52. [DOI] [PubMed] [Google Scholar]
- 44.Auerbach MR, Yan D, Vij R, Hongo JA, Nakamura G, Vernes JM, Meng YG, Lein S, Chan P, Ross J, Carano R, Deng R, Lewin-Koh N, Xu M, Feierbach B. 2014. A neutralizing anti-gH/gL monoclonal antibody is protective in the guinea pig model of congenital CMV infection. PLoS Pathog 10:e1004060. doi: 10.1371/journal.ppat.1004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boppana SB, Britt WJ. 1995. Antiviral antibody responses and intrauterine transmission after primary maternal cytomegalovirus infection. J Infect Dis 171:1115–1121. doi: 10.1093/infdis/171.5.1115. [DOI] [PubMed] [Google Scholar]
- 46.Chatterjee A, Harrison CJ, Britt WJ, Bewtra C. 2001. Modification of maternal and congenital cytomegalovirus infection by anti-glycoprotein b antibody transfer in guinea pigs. J Infect Dis 183:1547–1553. doi: 10.1086/320714. [DOI] [PubMed] [Google Scholar]
- 47.Fowler KB, Stagno S, Pass RF. 2003. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA 289:1008–1011. doi: 10.1001/jama.289.8.1008. [DOI] [PubMed] [Google Scholar]
- 48.Bialas KM, Westreich D, Cisneros de la Rosa E, Nelson CS, Kauvar LM, Fu TM, Permar SR. 2016. Maternal antibody responses and nonprimary congenital cytomegalovirus infection of HIV-1-exposed infants. J Infect Dis 214:1916–1923. doi: 10.1093/infdis/jiw487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furione M, Rognoni V, Sarasini A, Zavattoni M, Lilleri D, Gerna G, Revello MG. 2013. Slow increase in IgG avidity correlates with prevention of human cytomegalovirus transmission to the fetus. J Med Virol 85:1960–1967. doi: 10.1002/jmv.23691. [DOI] [PubMed] [Google Scholar]
- 50.Lilleri D, Kabanova A, Revello MG, Percivalle E, Sarasini A, Genini E, Sallusto F, Lanzavecchia A, Corti D, Gerna G. 2013. Fetal human cytomegalovirus transmission correlates with delayed maternal antibodies to gH/gL/pUL128-130-131 complex during primary infection. PLoS One 8:e59863. doi: 10.1371/journal.pone.0059863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanimura K, Tairaku S, Morioka I, Ozaki K, Nagamata S, Morizane M, Deguchi M, Ebina Y, Minematsu T, Yamada H. 2017. Universal screening with use of immunoglobulin G avidity for congenital cytomegalovirus infection. Clin Infect Dis 65:1652–1658. doi: 10.1093/cid/cix621. [DOI] [PubMed] [Google Scholar]
- 52.Grønborg HL, Jespersen S, Hønge BL, Jensen-Fangel S, Wejse C. 7 October 2017. Review of cytomegalovirus coinfection in HIV-infected individuals in Africa. Rev Med Virol doi: 10.1002/rmv.1907. [DOI] [PubMed] [Google Scholar]
- 53.Boppana SB, Miller J, Britt WJ. 1996. Transplacentally acquired antiviral antibodies and outcome in congenital human cytomegalovirus infection. Viral Immunol 9:211–218. doi: 10.1089/vim.1996.9.211. [DOI] [PubMed] [Google Scholar]
- 54.Revello MG, Gerna G. 2002. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin Microbiol Rev 15:680–715. doi: 10.1128/CMR.15.4.680-715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nelson CS, Cruz DV, Tran D, Bialas KM, Stamper L, Wu H, Gilbert M, Blair R, Alvarez X, Itell H, Chen M, Deshpande A, Chiuppesi F, Wussow F, Diamond DJ, Vandergrift N, Walter MR, Barry PA, Cohen-Wolkowiez M, Koelle K, Kaur A, Permar SR. 6 July 2017. Preexisting antibodies can protect against congenital cytomegalovirus infection in monkeys. JCI Insight doi: 10.1172/jci.insight.94002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bia FJ, Griffith BP, Tarsio M, Hsiung GD. 1980. Vaccination for the prevention of maternal and fetal infection with guinea pig cytomegalovirus. J Infect Dis 142:732–738. doi: 10.1093/infdis/142.5.732. [DOI] [PubMed] [Google Scholar]
- 57.Bratcher DF, Bourne N, Bravo FJ, Schleiss MR, Slaoui M, Myers MG, Bernstein DI. 1995. Effect of passive antibody on congenital cytomegalovirus infection in guinea pigs. J Infect Dis 172:944–950. doi: 10.1093/infdis/172.4.944. [DOI] [PubMed] [Google Scholar]
- 58.Revello MG, Lazzarotto T, Guerra B, Spinillo A, Ferrazzi E, Kustermann A, Guaschino S, Vergani P, Todros T, Frusca T, Arossa A, Furione M, Rognoni V, Rizzo N, Gabrielli L, Klersy C, Gerna G; CHIP Study Group. 2014. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med 370:1316–1326. doi: 10.1056/NEJMoa1310214. [DOI] [PubMed] [Google Scholar]
- 59.Pereira L, Maidji E. 2008. Cytomegalovirus infection in the human placenta: maternal immunity and developmentally regulated receptors on trophoblasts converge. Curr Top Microbiol Immunol 325:383–395. [DOI] [PubMed] [Google Scholar]
- 60.Enders G, Daiminger A, Bader U, Exler S, Enders M. 2011. Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestational age. J Clin Virol 52:244–246. doi: 10.1016/j.jcv.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 61.Simister NE, Story CM, Chen HL, Hunt JS. 1996. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur J Immunol 26:1527–1531. doi: 10.1002/eji.1830260718. [DOI] [PubMed] [Google Scholar]
- 62.Nigro G, Adler SP, La Torre R, Best AM; Congenital Cytomegalovirus Collaborating Group. 2005. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med 353:1350–1362. doi: 10.1056/NEJMoa043337. [DOI] [PubMed] [Google Scholar]
- 63.Compton T, Feire A. 2007. Early events in human cytomegalovirus infection, p 231–240. In Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K (ed), Human herpesviruses: biology, therapy, and immunoprophylaxis, Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- 64.Burke HG, Heldwein EE. 2015. Crystal structure of the human cytomegalovirus glycoprotein B. PLoS Pathog 11:e1005227. doi: 10.1371/journal.ppat.1005227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Potzsch S, Spindler N, Wiegers AK, Fisch T, Rucker P, Sticht H, Grieb N, Baroti T, Weisel F, Stamminger T, Martin-Parras L, Mach M, Winkler TH. 2011. B cell repertoire analysis identifies new antigenic domains on glycoprotein B of human cytomegalovirus which are target of neutralizing antibodies. PLoS Pathog 7:e1002172. doi: 10.1371/journal.ppat.1002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spindler N, Rucker P, Potzsch S, Diestel U, Sticht H, Martin-Parras L, Winkler TH, Mach M. 2013. Characterization of a discontinuous neutralizing epitope on glycoprotein B of human cytomegalovirus. J Virol 87:8927–8939. doi: 10.1128/JVI.00434-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wiegers AK, Sticht H, Winkler TH, Britt WJ, Mach M. 2015. Identification of a neutralizing epitope within antigenic domain 5 of glycoprotein B of human cytomegalovirus. J Virol 89:361–372. doi: 10.1128/JVI.02393-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lilleri D, Gerna G. 23 December 2017. Maternal immune correlates of protection from human cytomegalovirus transmission to the fetus after primary infection in pregnancy. Rev Med Virol doi: 10.1002/rmv.1921. [DOI] [PubMed] [Google Scholar]
- 69.Zydek M, Petitt M, Fang-Hoover J, Adler B, Kauvar LM, Pereira L, Tabata T. 2014. HCMV infection of human trophoblast progenitor cells of the placenta is neutralized by a human monoclonal antibody to glycoprotein B and not by antibodies to the pentamer complex. Viruses 6:1346–1364. doi: 10.3390/v6031346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Revello MG, Lilleri D, Zavattoni M, Furione M, Genini E, Comolli G, Gerna G. 2006. Lymphoproliferative response in primary human cytomegalovirus (HCMV) infection is delayed in HCMV transmitter mothers. J Infect Dis 193:269–276. doi: 10.1086/498872. [DOI] [PubMed] [Google Scholar]
- 71.Lilleri D, Fornara C, Furione M, Zavattoni M, Revello MG, Gerna G. 2007. Development of human cytomegalovirus-specific T cell immunity during primary infection of pregnant women and its correlation with virus transmission to the fetus. J Infect Dis 195:1062–1070. doi: 10.1086/512245. [DOI] [PubMed] [Google Scholar]
- 72.Fornara C, Cassaniti I, Zavattoni M, Furione M, Adzasehoun KMG, De Silvestri A, Comolli G, Baldanti F. 30 October 2017. Human cytomegalovirus-specific memory CD4+ T-cell response and its correlation with virus transmission to the fetus in pregnant women with primary infection. Clin Infect Dis doi: 10.1093/cid/cix622. [DOI] [PubMed] [Google Scholar]
- 73.Zhong J, Rist M, Cooper L, Smith C, Khanna R. 2008. Induction of pluripotent protective immunity following immunisation with a chimeric vaccine against human cytomegalovirus. PLoS One 3:e3256. doi: 10.1371/journal.pone.0003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bernstein DI, Reap EA, Katen K, Watson A, Smith K, Norberg P, Olmsted RA, Hoeper A, Morris J, Negri S, Maughan MF, Chulay JD. 2009. Randomized, double-blind, phase 1 trial of an alphavirus replicon vaccine for cytomegalovirus in CMV seronegative adult volunteers. Vaccine 28:484–493. doi: 10.1016/j.vaccine.2009.09.135. [DOI] [PubMed] [Google Scholar]
- 75.Berencsi K, Gyulai Z, Gonczol E, Pincus S, Cox WI, Michelson S, Kari L, Meric C, Cadoz M, Zahradnik J, Starr S, Plotkin S. 2001. A canarypox vector-expressing cytomegalovirus (CMV) phosphoprotein 65 induces long-lasting cytotoxic T cell responses in human CMV-seronegative subjects. J Infect Dis 183:1171–1179. doi: 10.1086/319680. [DOI] [PubMed] [Google Scholar]
- 76.Cayatte C, Schneider-Ohrum K, Wang Z, Irrinki A, Nguyen N, Lu J, Nelson C, Servat E, Gemmell L, Citkowicz A, Liu Y, Hayes G, Woo J, Van Nest G, Jin H, Duke G, McCormick AL. 2013. Cytomegalovirus vaccine strain Towne-derived dense bodies induce broad cellular immune responses and neutralizing antibodies that prevent infection of fibroblasts and epithelial cells. J Virol 87:11107–11120. doi: 10.1128/JVI.01554-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shedlock DJ, Talbott KT, Wu SJ, Wilson CM, Muthumani K, Boyer JD, Sardesai NY, Awasthi S, Weiner DB. 2012. Vaccination with synthetic constructs expressing cytomegalovirus immunogens is highly T cell immunogenic in mice. Hum Vaccin Immunother 8:1668–1681. doi: 10.4161/hv.22447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith LR, Wloch MK, Chaplin JA, Gerber M, Rolland AP. 2013. Clinical development of a cytomegalovirus DNA vaccine: from product concept to pivotal phase 3 trial. Vaccines (Basel) 1:398–414. doi: 10.3390/vaccines1040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kirchmeier M, Fluckiger AC, Soare C, Bozic J, Ontsouka B, Ahmed T, Diress A, Pereira L, Schodel F, Plotkin S, Dalba C, Klatzmann D, Anderson DE. 2014. Enveloped virus-like particle expression of human cytomegalovirus glycoprotein B antigen induces antibodies with potent and broad neutralizing activity. Clin Vaccine Immunol 21:174–180. doi: 10.1128/CVI.00662-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adler SP, Manganello AM, Lee R, McVoy MA, Nixon DE, Plotkin S, Mocarski E, Cox JH, Fast PE, Nesterenko PA, Murray SE, Hill AB, Kemble G. 2016. A phase 1 study of 4 live, recombinant human cytomegalovirus Towne/Toledo chimera vaccines in cytomegalovirus-seronegative men. J Infect Dis 214:1341–1348. doi: 10.1093/infdis/jiw365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Plotkin SA, Smiley ML, Friedman HM, Starr SE, Fleisher GR, Wlodaver C, Dafoe DC, Friedman AD, Grossman RA, Barker CF. 1984. Towne-vaccine-induced prevention of cytomegalovirus disease after renal transplants. Lancet i:528–530. [DOI] [PubMed] [Google Scholar]
- 82.Fu TM, An Z, Wang D. 2014. Progress on pursuit of human cytomegalovirus vaccines for prevention of congenital infection and disease. Vaccine 32:2525–2533. doi: 10.1016/j.vaccine.2014.03.057. [DOI] [PubMed] [Google Scholar]
- 83.Schleiss MR, Berka U, Watson E, Aistleithner M, Kiefmann B, Mangeat B, Swanson EC, Gillis PA, Hernandez-Alvarado N, Fernandez-Alarcon C, Zabeli JC, Pinschewer DD, Lilja AE, Schwendinger M, Guirakhoo F, Monath TP, Orlinger KK. 5 January 2017. Additive protection against congenital cytomegalovirus conferred by combined glycoprotein B/pp65 vaccination using a lymphocytic choriomeningitis virus vector. Clin Vaccine Immunol doi: 10.1128/CVI.00300-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brito LA, Chan M, Shaw CA, Hekele A, Carsillo T, Schaefer M, Archer J, Seubert A, Otten GR, Beard CW, Dey AK, Lilja A, Valiante NM, Mason PW, Mandl CW, Barnett SW, Dormitzer PR, Ulmer JB, Singh M, O'Hagan DT, Geall AJ. 2014. A cationic nanoemulsion for the delivery of next-generation RNA vaccines. Mol Ther 22:2118–2129. doi: 10.1038/mt.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.La Rosa C, Longmate J, Martinez J, Zhou Q, Kaltcheva TI, Tsai W, Drake J, Carroll M, Wussow F, Chiuppesi F, Hardwick N, Dadwal S, Aldoss I, Nakamura R, Zaia JA, Diamond DJ. 2017. MVA vaccine encoding CMV antigens safely induces durable expansion of CMV-specific T cells in healthy adults. Blood 129:114–125. doi: 10.1182/blood-2016-07-729756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakamura R, La Rosa C, Longmate J, Drake J, Slape C, Zhou Q, Lampa MG, O'Donnell M, Cai JL, Farol L, Salhotra A, Snyder DS, Aldoss I, Forman SJ, Miller JS, Zaia JA, Diamond DJ. 2016. Viraemia, immunogenicity, and survival outcomes of cytomegalovirus chimeric epitope vaccine supplemented with PF03512676 (CMVPepVax) in allogeneic haemopoietic stem-cell transplantation: randomised phase 1b trial. Lancet Haematol 3:e87–e98. doi: 10.1016/S2352-3026(15)00246-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kabanova A, Perez L, Lilleri D, Marcandalli J, Agatic G, Becattini S, Preite S, Fuschillo D, Percivalle E, Sallusto F, Gerna G, Corti D, Lanzavecchia A. 2014. Antibody-driven design of a human cytomegalovirus gHgLpUL128L subunit vaccine that selectively elicits potent neutralizing antibodies. Proc Natl Acad Sci U S A 111:17965–17970. doi: 10.1073/pnas.1415310111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pass RF. 2009. Development and evidence for efficacy of CMV glycoprotein B vaccine with MF59 adjuvant. J Clin Virol 46(Suppl 4):S73–S76. doi: 10.1016/j.jcv.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilson SR, Wilson JH, Buonocore L, Palin A, Rose JK, Reuter JD. 2008. Intranasal immunization with recombinant vesicular stomatitis virus expressing murine cytomegalovirus glycoprotein B induces humoral and cellular immunity. Comp Med 58:129–139. [PMC free article] [PubMed] [Google Scholar]
- 90.Britt WJ, Mach M. 1996. Human cytomegalovirus glycoproteins. Intervirology 39:401–412. doi: 10.1159/000150510. [DOI] [PubMed] [Google Scholar]
- 91.McVoy MA. 2013. Cytomegalovirus vaccines. Clin Infect Dis 57(Suppl 4):S196–S199. doi: 10.1093/cid/cit587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Plachter B. 2016. Prospects of a vaccine for the prevention of congenital cytomegalovirus disease. Med Microbiol Immunol 205:537–547. doi: 10.1007/s00430-016-0472-z. [DOI] [PubMed] [Google Scholar]
- 93.Wang D, Freed DC, He X, Li F, Tang A, Cox KS, Dubey SA, Cole S, Medi MB, Liu Y, Xu J, Zhang ZQ, Finnefrock AC, Song L, Espeseth AS, Shiver JW, Casimiro DR, Fu TM. 2016. A replication-defective human cytomegalovirus vaccine for prevention of congenital infection. Sci Transl Med 8: 362ra145. doi: 10.1126/scitranslmed.aaf9387. [DOI] [PubMed] [Google Scholar]
- 94.La Rosa C, Longmate J, Lacey SF, Kaltcheva T, Sharan R, Marsano D, Kwon P, Drake J, Williams B, Denison S, Broyer S, Couture L, Nakamura R, Dadwal S, Kelsey MI, Krieg AM, Diamond DJ, Zaia JA. 2012. Clinical evaluation of safety and immunogenicity of PADRE-cytomegalovirus (CMV) and tetanus-CMV fusion peptide vaccines with or without PF03512676 adjuvant. J Infect Dis 205:1294–1304. doi: 10.1093/infdis/jis107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krause PR, Bialek SR, Boppana SB, Griffiths PD, Laughlin CA, Ljungman P, Mocarski ES, Pass RF, Read JS, Schleiss MR, Plotkin SA. 2013. Priorities for CMV vaccine development. Vaccine 32:4–10. doi: 10.1016/j.vaccine.2013.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Revello MG, Gerna G. 2004. Pathogenesis and prenatal diagnosis of human cytomegalovirus infection. J Clin Virol 29:71–83. doi: 10.1016/j.jcv.2003.09.012. [DOI] [PubMed] [Google Scholar]