ABSTRACT
Zika virus (ZIKV), which can cause devastating disease in fetuses of infected pregnant women, can be transmitted by mosquito inoculation and sexual routes. Little is known about immune protection against sexually transmitted ZIKV. In this study, we show that previous infection through intravaginal or subcutaneous routes with a contemporary Brazilian strain of ZIKV can protect against subsequent intravaginal challenge with a homologous strain. Both routes of inoculation induced high titers of ZIKV-specific and neutralizing antibody in serum and the vaginal lumen. Virus-specific T cells were recruited to and retained in the female reproductive tract after intravaginal and subcutaneous ZIKV infection. Studies in mice with genetic or acquired deficiencies in B and/or T cells demonstrated that both lymphocyte populations redundantly protect against intravaginal challenge in ZIKV-immune animals. Passive transfer of ZIKV-immune IgG or T cells significantly limited intravaginal infection of naive mice, although antibody more effectively prevented dissemination throughout the reproductive tract. Collectively, our experiments begin to establish the immune correlates of protection against intravaginal ZIKV infection, which should inform vaccination strategies in nonpregnant and pregnant women.
IMPORTANCE The recent ZIKV epidemic resulted in devastating outcomes in fetuses and may affect reproductive health. Unlike other flaviviruses, ZIKV can be spread by sexual contact as well as a mosquito vector. While previous studies have identified correlates of protection for mosquito-mediated infection, few have focused on immunity against sexual transmission. As exposure to ZIKV via mosquito bite has likely occurred to many living in areas where ZIKV is endemic, our study addresses whether this route of infection can protect against subsequent sexual exposure. We demonstrate that subcutaneous ZIKV infection can protect against subsequent vaginal infection by generating both local antiviral T cell and antibody responses. Our research begins to define the immune correlates of protection for ZIKV infection in the vagina and provides a foundation for testing ZIKV vaccines against sexual transmission.
KEYWORDS: Zika, sexual transmission, immunity, Zika virus
INTRODUCTION
Zika virus (ZIKV), a member of the Flaviviridae family of positive-stranded RNA viruses, has caused a recent epidemic of congenital malformations in the Americas. Historically, ZIKV infection was associated with a mild febrile illness that resolved within 1 to 2 weeks of mosquito inoculation (1, 2). However, ZIKV now is associated with adverse outcomes in pregnant women, as transplacental transmission causes a devastating fetal syndrome, including intrauterine growth restriction, microcephaly, and other neurodevelopmental abnormalities (3–8). Additionally, ZIKV infection is linked to a rise in cases of Guillain-Barré syndrome, a polyneuropathy that can result in paralysis (9, 10).
While transmission of ZIKV by Aedes species mosquitoes is well studied, human-to-human transmission can occur through a sexual route. Although most sexual transmission is male to female (11–15), male-to-male (16) and female-to-male (17) transmission has been reported. ZIKV persists in the reproductive tissues of both males (18–20) and females (21, 22) for prolonged periods of time. In mice, infected males transmitted ZIKV sexually to naive females at rates as high as 50% during the acute phase of infection and as late as 19 days postinfection (p.i.) (23). Intravaginal infection with RNA viruses elicits dampened innate immune responses in the lower female reproductive tract (FRT), which likely facilitate ZIKV replication (24). Vaginal infection of mice with ZIKV during pregnancy has teratogenic effects that are similar to those observed after subcutaneous (s.c.) infections (7). Furthermore, the contribution of sexual transmission to the spread of ZIKV during the recent epidemic may have been substantially underestimated (25). Thus, mounting evidence points to sexual acquisition of ZIKV as an important transmission route that can have consequences as severe as after mosquito inoculation.
Studies in mice and nonhuman primates have begun establishing immune correlates of protection against ZIKV infection after subcutaneous inoculation. High titers of serum neutralizing antibodies block infection, and many protective antibodies against the ZIKV envelope (E) protein have been identified (26–33). CD8+ T cells also can mediate protection against ZIKV after subcutaneous or intravenous administration both in immunocompetent mice and when there are defects in type I interferon (IFN) signaling (34–37). In rhesus macaques, primary subcutaneous infection completely protected against reinfection through a homologous route (38, 39). It remains unclear whether immune responses elicited by prior ZIKV exposure through systemic infection can protect against subsequent exposure through the genital mucosa.
To begin to address these questions, we evaluated immune protection against intravaginal ZIKV challenge after previous subcutaneous infection. We focused on understanding protection against reinfection with a homologous virus, as most ZIKV-infected patients are likely to be at risk of reexposure to the same or highly similar strain of ZIKV. Using a mouse model of infection with a ZIKV isolate from Brazil (40), we found that subcutaneous ZIKV infection conferred robust protection against secondary vaginal challenge. Genital and subcutaneous infection elicited strong neutralizing antibody responses in the serum and ZIKV-specific antibody in the lumen of the vagina. Intravaginal and subcutaneous infection led to virus-specific T cell infiltration of the FRT during the acute phase, and these cells established populations of resident memory T cells (TRM). Transfer of ZIKV-specific IgG or circulating memory T cells was sufficient to limit viral replication after intravaginal infection, with greater protection provided by the humoral immune response.
RESULTS
Suppression of type I IFN responses leads to ZIKV replication throughout the FRT after intravaginal infection.
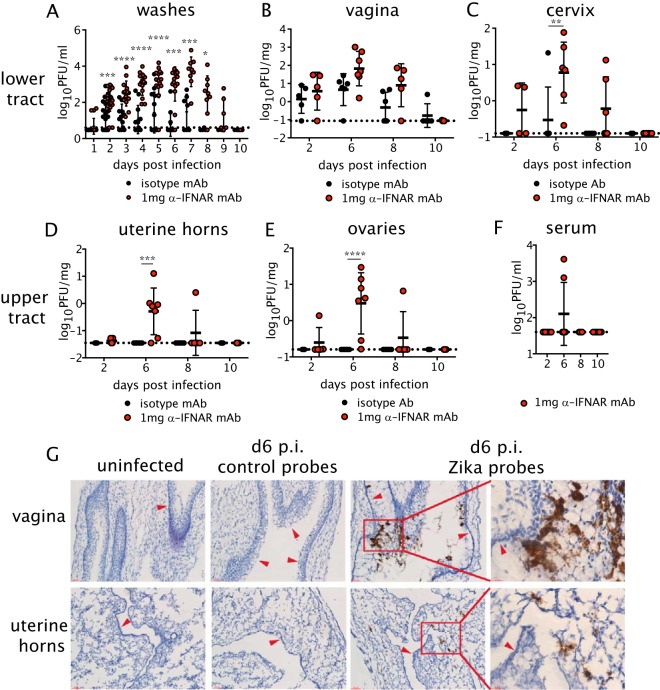
Wild-type (WT) C57BL/6 mice were first injected with depot medroxyprogesterone acetate (DMPA; Depo-Provera) to place females in the diestrous phase of the estrous cycle, as this hormonal stage is required in models of intravaginal viral infections of virgin mice, including ZIKV infection (7, 24, 41, 42). DMPA-treated mice were inoculated intravaginally (ivag) with 104 PFU of a Brazilian ZIKV isolate. Because ZIKV blocks type I IFN signaling efficiently in human but not mouse cells (43), 1 day prior to infection, mice were intraperitoneally (i.p.) treated with 1 mg of a blocking anti-IFNAR1 monoclonal antibody (MAR1-5A3, anti-IFNAR1 mAb) or with an isotype control mAb (44). In animals treated with anti-IFNAR1 mAb, infectious ZIKV was detected in the vaginal lumen beginning at day 1 postinfection (p.i.) until day 9 p.i. (Fig. 1A). Infectious ZIKV also was detected in the vaginal lumen of mice treated with the isotype control antibody, as reported previously (7), although the amount was less than in animals treated with the anti-IFNAR1 mAb and lasted for a shorter duration (Fig. 1A). Remarkably, infectious ZIKV was detected in the vaginal tissue at relatively similar levels in the isotype control and anti-IFNAR1 mAb-treated animals (Fig. 1B), consistent with the observation of dampened type I IFN responses in this tissue (24). In contrast to what was found in the vagina, we noted differences in the spread of ZIKV throughout the FRT in isotype control and anti-IFNAR1 mAb-treated mice. ZIKV was detected in both the lower (vagina and cervix) and upper (uterine horns and ovaries) FRT of most mice treated with anti-IFNAR1 mAb, whereas only a small subset of animals treated with the isotype control mAb had ZIKV in the cervix, with none in the uterine horn or ovaries (Fig. 1C to E). In all FRT tissues, infectious virus was cleared almost completely by day 10 p.i. regardless of anti-IFNAR1 mAb treatment. To determine whether treatment with anti-IFNAR1 mAb led to dissemination of infectious virus beyond the FRT, we also measured viral titers in the serum (Fig. 1F). We found that intravaginal infection led to a transient viremia in a small subset of animals that was cleared by day 8 (Fig. 1F); this suggests that hematogenous dissemination to peripheral tissues was not a strong feature of our model. We next examined the distribution of virally infected cells by in situ hybridization (ISH) with ZIKV-specific probes in the lower (vagina) and upper (uterine horns) FRT from uninfected or ZIKV-infected animals treated with anti-IFNAR1 Ab. The majority of ZIKV RNA in the vagina was localized to the lumenal edge of the epithelium, which was suggestive of the sloughing of dead cells (Fig. 1G). In the uterine horns, ZIKV RNA was detected in patches of cells throughout the tissue parenchyma (Fig. 1G). Thus, our data show that while immunocompetent C57BL/6 mice treated with isotype control antibody supported viral replication in the vagina after genital infection, dissemination to the upper FRT required suppression of the type I IFN response.
FIG 1.
Replication of ZIKV throughout the FRT after intravaginal infection. Five-week-old female C57BL/6 mice were injected subcutaneously (s.c.) with DMPA and inoculated intravaginally (ivag) with 104 PFU of ZIKV. One day prior to infection, mice were treated intraperitoneally (i.p.) with 1 mg anti-IFNAR1 mAb or an isotype control. (A) Vaginal washes were collected daily after infection, and replicating ZIKV was measured by plaque assay. Day 1, n = 15 to 17; day 2, n = 22 to 25; day 3, n = 13; days 4 to 6, n = 12; day 7, n = 7; day 8, n = 4; days 9 to 10, n = 2. (B to E) At days 2, 6, 8, and 10 p.i., mice were sacrificed, and the vagina (B), cervix (C), uterine horns (D), and ovaries (E) were harvested for virus titration by plaque assay. (F) Viremia was measured by plaque assay. Isotype, n = 5 to 6; anti-IFNAR1, n = 4 to 10. Data in panels A to F were pooled from three independent experiments. Statistical significance was measured by two-way ANOVA with Bonferroni's post hoc test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Horizontal bars indicate the means, error bars show SD, and dashed lines show the limit of detection. (G) Representative images of ZIKV RNA detected by in situ hybridization in uninfected or ZIKV-infected mice at day 6 p.i. using ZIKV-specific or negative-control probes. The first three columns on the left show ×40 magnification, and the rightmost images show the inset outlined in red at ×63. The top row shows images for the vagina, and the bottom row shows images for the uterine horns. Arrows point to the lumenal edge of epithelium. All images are counterstained with hematoxylin. Scale bars, 50 μm in 40× images and 20 μm in 63× images. Data are representative of 3 animals per group.
Primary intravaginal or subcutaneous ZIKV infection protects against subsequent intravaginal challenge.
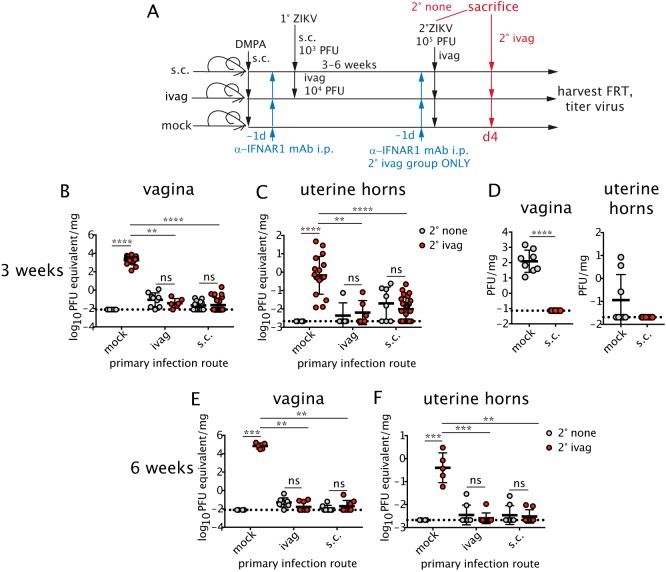
We next evaluated whether prior inoculation through a homologous (intravaginal) or heterologous (subcutaneous) route could confer protection against secondary intravaginal challenge. As our model of infection with a Brazilian strain of ZIKV does not cause overt disease with central nervous system (CNS)-related symptoms or lethality as described for intravaginal ZIKV infection of mice with more severe immunodeficiencies (7, 42), we defined protection as the rapid control of viral replication. DMPA-treated female mice were given a single dose of anti-IFNAR1 mAb i.p. and then inoculated with 103 PFU of ZIKV subcutaneously through the footpad or 104 PFU of ZIKV through the intravaginal route; different doses were used based on published studies (7, 44). Three weeks later, mice were either sacrificed or treated again with anti-IFNAR1 mAb and challenged intravaginally 1 day later with a higher, more stringent 105 PFU dose of ZIKV (Fig. 2A). At day 4 postchallenge, viral titers in the vagina, the portal of entry (Fig. 2B and D), and in the uterus, a site of dissemination (Fig. 2C and D), were analyzed after tissue perfusion. We measured viral RNA in the tissues by quantitative reverse transcription-PCR (qRT-PCR) to increase the sensitivity of detection. Due to the persistence of low levels of ZIKV RNA in FRT tissue after primary infection (22), we compared viral RNA levels in the tissues of intravaginally challenged animals (2° ivag) to a parallel cohort of unchallenged mice at day 21 after the initial infection (2° none). As expected, naive animals without primary infection (mock) were susceptible to intravaginal ZIKV challenge. However, mice that were previously infected via the intravaginal or subcutaneous route were protected from secondary challenge. No statistically significant differences in ZIKV RNA levels were found between unchallenged and intravaginally challenged cohorts in the vagina (Fig. 2B) or uterus (Fig. 2C) of mice that were initially infected via subcutaneous or intravaginal routes. Plaque assays performed after challenge did not detect any infectious virus in the vagina or uterine horns of mice previously infected subcutaneously (Fig. 2D). These data suggested that the low level of viral RNA detected in the FRT after intravaginal challenge likely was residual from the primary infection. Mice also were challenged up to 6 weeks after primary intravaginal or subcutaneous infection and remained protected, with no detectable increase in viral RNA in the vagina (Fig. 2E) or uterine horns (Fig. 2F). Thus, prior exposure to ZIKV induced protective immunity against secondary intravaginal ZIKV infection regardless of whether the primary infection was through a homologous or heterologous route.
FIG 2.
Previous intravaginal (ivag) or subcutaneous (s.c.) ZIKV infection protects against secondary intravaginal challenge. (A) Five-week-old female C57BL/6 mice were injected with DMPA and inoculated with ZIKV ivag (104 PFU) or s.c. through the footpad (103 PFU). One cohort was left uninfected (mock). One day prior to infection, mice were treated i.p. with 1 mg of anti-IFNAR1 mAb. Three to 6 weeks after infection, mice were either sacrificed (no anti-IFNAR1 mAb treatment) or again treated with 1 mg anti-IFNAR1 mAb and challenged intravaginally with 105 PFU ZIKV and then sacrificed at day 4. Viral RNA was measured by qRT-PCR in the vagina (B and E) or uterine horns (C and F). Viral titers were also measured by plaque assay in the vagina and uterine horns on day 4 after the 3-week challenge (D). (B) For no secondary (2°) challenge (2° none), n = 5 to 12; for secondary intravaginal challenge (2° ivag), n = 6 to 25. (C) For 2° none, n = 4 to 8; for 2° ivag, n = 6 to 25. (D) All groups, n = 8. (E and F) For 2° none, n = 4 to 8; for 2° ivag, n = 5 to 9. Statistical significance was analyzed by the Kruskal-Wallis test with Dunn's post hoc test. Data were pooled from two to four independent experiments. Horizontal bars show the means, error bars show SD, and dashed lines show the limit of detection. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; ns, not significant.
Local T cell responses are generated in the genital tract after intravaginal and subcutaneous ZIKV infection.
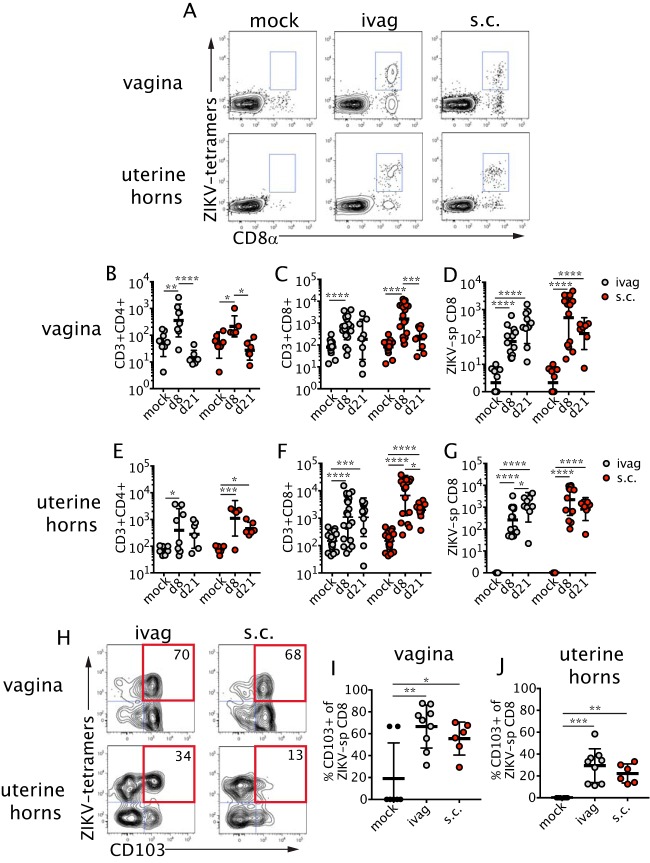
As the FRT is the portal of entry and primary site of viral replication after intravaginal infection (42), we focused on immunity within this organ. We evaluated the total and virus-specific cellular immune response in the vagina and uterine horns after ZIKV was administered via intravaginal or subcutaneous routes. Virus-specific CD8+ T cells were identified with two major histocompatibility complex (MHC) class I tetramers bound to peptides derived from ZIKV E protein (34) (Fig. 3A). Perfusion of tissues prior to collection was sufficient to remove intravascular T cells, thus limiting our analysis to tissue-specific populations (data not shown). At day 8 p.i. by either route, we detected an increased total number of CD4+ and CD8+ T cells in the vagina (Fig. 3B and C). The ZIKV-specific CD8+ T cell response also was increased at day 8 p.i. and sustained through day 21 p.i. (Fig. 3D). In the uterine horns, subcutaneous and intravaginal ZIKV infection led to a large influx of CD4+ and CD8+ T cells (Fig. 3E and F). Similar to the findings in the vagina, ZIKV-specific CD8+ T cells were detectable in the tissue at day 8 after intravaginal or subcutaneous infection and were retained through day 21 p.i. (Fig. 3G). We also examined the expression of cell surface CD103, an integrin that can be highly expressed by TRM (45), at a later time point during infection in both the vagina and uterine horns (Fig. 3H). A substantial fraction of vaginal ZIKV-specific CD8+ T cells expressed CD103 at day 21 p.i. after intravaginal or subcutaneous inoculation (Fig. 3I), indicating that virus-specific CD8+ T cells retained in the tissue likely were differentiating into TRM. Although the frequency of CD103-expressing CD8+ T cells was lower in the uterine horns (Fig. 3J) than in the vagina (Fig. 3I), there was a significant increase in CD103 expression in the virus-specific CD8+ T cell population in infected animals compared to that in uninfected controls. Thus, recruitment of effector T cells to the FRT occurs after both subcutaneous and intravaginal routes of ZIKV infection, and virus-specific CD8+ T cells that accumulate in the FRT are capable of differentiating into TRM.
FIG 3.
ZIKV-specific T cells are recruited to the FRT. Primary infection of mice was performed as described in the legend for Fig. 2A. Mice were then sacrificed at the indicated time points on the graphs. (A) Example of tetramer staining in the vagina (top) and uterine horns (bottom) at 21 days p.i. Plots are gated on live cells, and gates show the combination of two ZIKV-specific populations (two tetramers). CD4+ (B and E) and CD8+ (C and F) T cells were counted in the vagina (B and C) and uterine horns (E and F). After ivag infection, for both tissues: CD4, n = 6 to 11; CD8, n = 9 to 22. After s.c. infection, for both tissues: CD4, n = 6 to 9; CD8, n = 9 to 19. The numbers of ZIKV-specific CD8+ T cells in the vagina (D) and the uterine horns (G) were measured on the indicated days. After ivag infection, for both tissues: n = 9 to 14. After s.c. infection, for both tissues: n = 7 to 14. (H) Example of CD103 staining in the vagina (top) and uterine horns (bottom). Plots are gated on CD3+ CD8+ cells. The numbers in the plots indicate the percentages of tetramer-positive cells that are CD103+. The frequency of CD103+ ZIKV-specific CD8+ T cells was measured at day 21 p.i. in the vagina (I) and uterine horns (J) (n = 6 to 7). Statistical significance was measured by two-way ANOVA with Bonferroni's post hoc test (B to G) or by one-way ANOVA with Bonferroni's post hoc test (I and J). Horizontal bars show the means, error bars show SD. Data for panels B, C, E, and F were pooled from seven independent experiments. Data for panels D, G, I, and J were pooled from four independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Antibody responses are elicited by intravaginal and subcutaneous ZIKV infection.
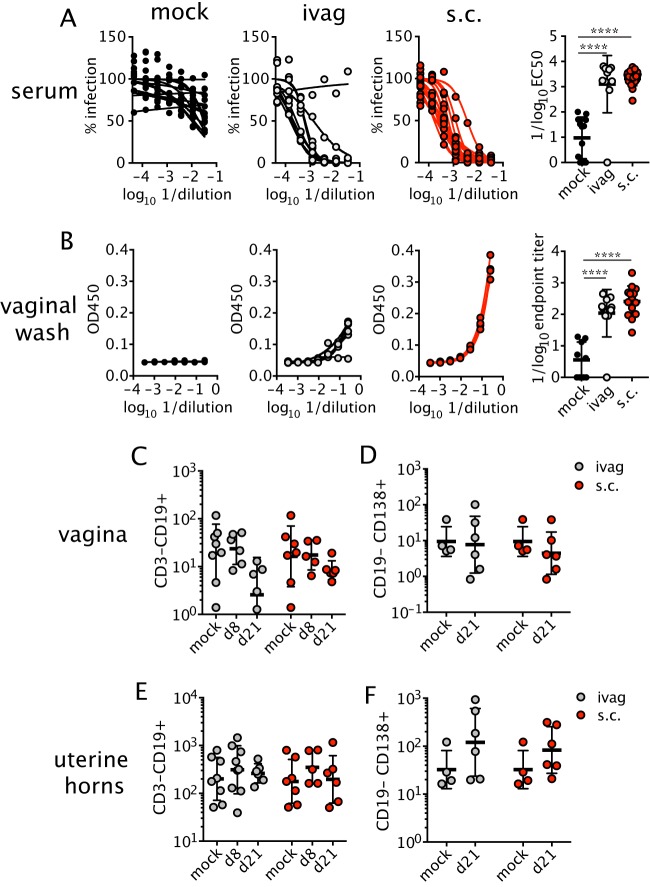
We next measured the ZIKV-specific antibody response after subcutaneous and intravaginal infection. Mice were treated with anti-IFNAR1 mAb and inoculated intravaginally or subcutaneously with ZIKV. At 3 weeks postinfection, neutralizing antibody responses in the serum were evaluated using a focus reduction neutralization test (FRNT). As expected, serum from uninfected animals had minimal levels of ZIKV neutralizing activity. Serum from animals infected through intravaginal or subcutaneous routes had strong neutralizing antibody responses (mean 50% effective concentrations [EC50s] of 1:1,270 and 1:2,128) after intravaginal and subcutaneous infection, respectively (Fig. 4A). For other sexually transmitted viruses (e.g., herpes simplex virus [HSV]), high titers of circulating antiviral antibodies are insufficient for protection (46, 47); rather, the titer of virus-specific antibodies in the vaginal lumen correlates with protection (48, 49). To determine whether neutralizing antibodies in serum correlated with ZIKV-specific antibodies in the vaginal lumen, we measured ZIKV E protein-specific IgG in vaginal washes collected from animals at 3 weeks postinfection (26), as IgG is the predominant isotype within the vaginal secretions of both mice and humans (49, 50). Due to the intrinsic antiviral activity of vaginal mucus (51), we measured ZIKV-specific IgG by enzyme-linked immunosorbent assay (ELISA) rather than neutralizing activity. As expected, uninfected animals had low levels of ZIKV-specific IgG in the lumen of the vagina (Fig. 4B). In contrast, animals inoculated intravaginally or subcutaneously developed measurable levels of ZIKV-specific IgG in the vaginal washes (mean ELISA endpoint titers of 1:108 and 1:257, respectively) (Fig. 4B). Of note, CD19+ B cells (Fig. 4C and E) and CD138+ antibody-producing plasma cells (Fig. 4D and F) were not recruited substantially to the FRT after intravaginal or subcutaneous infection, suggesting that antibody in the vaginal lumen was not produced locally. Collectively, these data show that potent neutralizing antibody responses against ZIKV can be induced after intravaginal or subcutaneous infection and that ZIKV-specific antibodies can accumulate in the vaginal lumen.
FIG 4.
Virus-specific antibodies are present in circulation and the vagina after ZIKV infection. Mice were infected ivag or s.c. with ZIKV after anti-IFNAR1 mAb injection as described in the legend for Fig. 2A. At 3 weeks p.i., neutralizing antibody was measured in the serum by FRNT (n = 10 to 21) (A), and E protein-specific IgG in the vaginal lumen was measured by ELISA (n = 9 to 14) (B). Graphs on the left were used to generate EC50s and endpoint titer dilutions. Each curve represents data from an individual animal. Total B cells (C and E) and antibody-secreting cells (D and F) were measured in the vagina (C and D) and uterine horns (E and F) on the indicated days. For CD19+ cells and both tissues and infection routes, n = 5 to 8. For CD138+ cells and both tissues and infection routes, n = 4 to 6. Horizonal bars show the means, and error bars show SD. Statistical significance was determined by one-way ANOVA with Bonferroni's post hoc test for panels A and B and by two-way ANOVA with Bonferroni's post hoc test on log-transformed data for panels C to F. Data were pooled from four independent experiments. ****, P < 0.0001.
Compensatory T cell and B cell responses protect against intravaginal ZIKV infection.
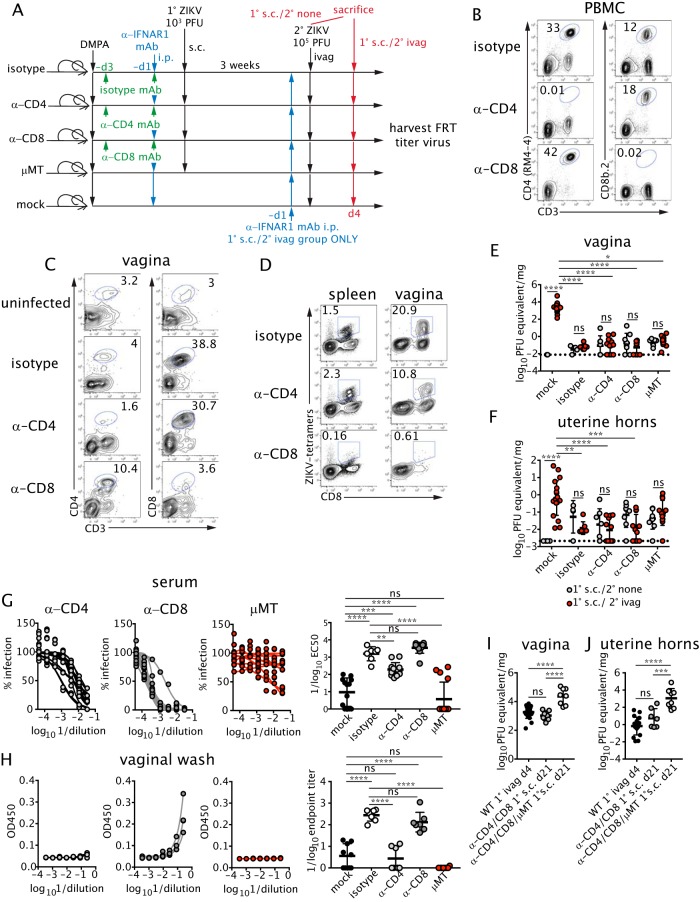
To begin to identify the immune determinants of protection against sexually transmitted ZIKV after subcutaneous inoculation, we evaluated the requirement of individual arms of the adaptive immune response. To test the role of T cells, WT mice were injected i.p. with CD4+ or CD8+ T cell-depleting antibodies prior to anti-IFNAR1 mAb treatment and primary subcutaneous infection with ZIKV (Fig. 5A and B). Since TRM populations are refractory to depletion by antibody injection (52–54), we treated animals with the depleting antibodies prior to infection, as this prevents the induction of a virus-specific T cell response and seeding of the FRT by TRM precursors (Fig. 5C and D) (54–56). To assess the role of B cells, B cell-deficient μMT mice were inoculated subcutaneously with ZIKV after treatment with a lower dose (0.75 mg) of anti-IFNAR1 mAb; this dose was used to reduce dissemination and minimize lethality in the μMT mice. At 3 weeks postinfection, T cell-depleted or B cell-deficient mice were challenged intravaginally with ZIKV, and viral titers were measured 4 days later in the vagina (Fig. 5E) and uterine horns (Fig. 5F). Unexpectedly, mice depleted of CD4+ T cells, CD8+ T cells, or B cells remained protected, with no increase in viral burden compared to that of unchallenged controls (Fig. 5E and F).
FIG 5.
T and B cells have redundant roles in protecting against intravaginal ZIKV infection. (A) DMPA-treated wild-type (WT) female mice were injected with 250 μg of isotype control or depleting anti-CD4 or anti-CD8 antibodies at day −3 and day −1 prior to s.c. inoculation with ZIKV. μMT animals also were infected. All WT mice were treated with 1 mg anti-IFNAR1 mAb prior to infection, whereas μMT mice were treated with 0.75 mg. At 3 weeks p.i., mice were challenged ivag with ZIKV as described in the legend for Fig. 2. (B) The percentages of CD4+ (left) and CD8+ (right) T cells were measured in the blood on the day of primary infection after depleting antibody treatment. At 3 weeks after primary infection, the percentages of CD4+ (left) and CD8+ (right) T cells in the vagina (C) and ZIKV-specific CD8+ T cells in the spleen (left) and vagina (right) (D) also were assessed. Plots are gated on total lymphocytes. Data are representative of two to four independent experiments. Viral titers were measured by qRT-PCR in the vagina (E) or uterine horns (F). For primary (1°) s.c. infection/no secondary (2°) challenge (1° s.c./2° none), n = 4 to 9; for primary s.c. infection/secondary intravaginal challenge (1° s.c./2° ivag), mock, n = 7 to 19. Prior to challenge, serum neutralizing antibodies were measured by FRNT (n = 7 to 15) (G), and E-protein specific IgG was measured by ELISA (n = 7 to 10) (H). μMT or WT mice were treated with DMPA and injected with 250 μg each anti-CD4 and anti-CD8 antibody at day −3 and day −1 prior to infection. Mice also were given 0.75 mg (μMT) or 1 mg (WT) of anti-IFNAR1 mAb i.p. 1 day before s.c. infection with 103 PFU ZIKV. At 3 weeks p.i., viral RNA was measured by qRT-PCR in the vagina (I) and uterine horns (J). Viral RNA from naive wild-type mice treated i.p. with 1 mg of anti-IFNAR mAb and infected ivag with 105 PFU ZIKV is shown as a control (1° ivag d4). Data were analyzed by the Kruskal-Wallis test with Dunn's post hoc test (E and F) or by one-way ANOVA with Bonferroni's post hoc test (G to J). Error bars show SD. Curves in panels G and H represent individual animals. Data were pooled from four independent experiments. Horizontal bars show the means, error bars show SD, and dashed lines show the limit of detection. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; ns, not significant.
To determine whether antibody responses were intact in the T cell-depleted animals, we evaluated serum neutralizing activity and ZIKV-specific antibody levels in the vaginal lumen of mice prior to intravaginal challenge. Mice depleted of CD8+ T cells had serum and vaginal lumen antibody levels comparable to those of isotype control-treated mice, indicating that antibody-mediated protection likely could compensate for the loss of CD8+ T cells (Fig. 5G and H). Despite full protection against intravaginal challenge, the CD4+ T cell-depleted mice had less serum neutralizing activity and ZIKV-specific vaginal antibodies than the isotype control-treated animals (Fig. 5G and H). Thus, CD4+ T cell help is required for optimal neutralizing antibody responses against ZIKV, but CD8+ T cells and the lower levels of circulating neutralizing antibody were sufficient to protect against intravaginal ZIKV challenge. To determine the protective capacity of the B cell response in the absence of any T cells, we depleted animals of both CD4+ and CD8+ T cells. These mice failed to control primary subcutaneous infection, as FRT viral titers at 3 weeks postinfection were comparable to acute viral titers measured at day 4 postinfection in naive, immunologically intact animals inoculated intravaginally with ZIKV (Fig. 5I and J). When adaptive immune responses were eliminated by depleting CD4+ and CD8+ T cells in μMT mice prior to the primary subcutaneous infection, viral titers in the FRT were even higher, suggesting that B cells in the absence of CD4+ T cell help could reduce primary subcutaneous infection (Fig. 5I and J). Together, these studies reveal the compensatory nature of cellular and humoral responses in protecting against intravaginal ZIKV challenge after primary subcutaneous infection in mice.
ZIKV-specific antibodies or T cells are sufficient to protect against intravaginal infection.
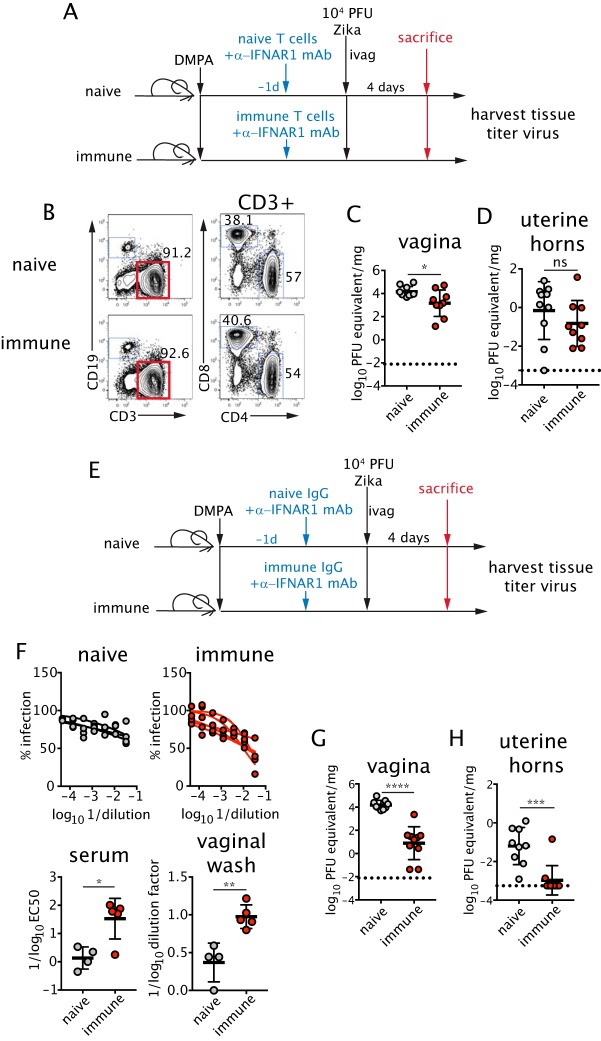
We next tested the sufficiency of immune IgG or memory T cells in protection against intravaginal infection. To examine the activity of T cells, ZIKV-experienced T cells were isolated from the spleens of mice subcutaneously infected 3 weeks earlier or from naive mice. Equal numbers of immune or naive T cells were adoptively transferred to naive, DMPA-treated C57BL/6 recipients at a ratio of 60% CD4+ to 40% CD8+ T cells (Fig. 6A and B). Recipient mice were treated with anti-IFNAR1 mAb and inoculated intravaginally with ZIKV. Four days later, viral titers were measured in the vagina and uterine horns. Notably, transfer of T cells from ZIKV-immune donors resulted in a small but significant reduction (10-fold, P < 0.05) of viral replication in the vagina compared to transfer of naive T cells (Fig. 6C). However, ZIKV-experienced T cells did not protect against viral dissemination into the uterine horns. (Fig. 6D).
FIG 6.
Transfer of memory T cells or ZIKV-immune IgG protects against intravaginal infection. (A) A total of 107 T cells isolated from naive or ZIKV-immune mice (3 weeks p.i.) were adoptively transferred into 5-week-old DMPA-treated naive female recipients. Mice then were treated with 1 mg of anti-IFNAR1 mAb, and recipients were inoculated ivag with ZIKV 6 days after DMPA injection. (B) The numbers in the plots are percentages of total cells that are CD3+ (left) and percentages of CD3+ T cells that are CD4+ or CD8+ (right) of transferred T cells. Viral RNA was measured by qRT-PCR at day 4 p.i. in the vagina (C) and uterine horns (D) (n = 9 to 10 for both tissues). (E) Serum IgG was isolated from naive or ZIKV-immune mice (3 weeks p.i.). Four hundred micrograms of antibody was injected intraperitoneally into DMPA-treated naive female recipients. Mice were infected as described for panel A. (F) Serum neutralizing titers of recipient mice were measured by FRNT (bottom left), and ZIKV-specific IgG in the vaginal lumen of recipient mice was measured by ELISA 1 day posttransfer (bottom right) (n = 4 to 5). Viral RNA was measured by qRT-PCR at day 4 p.i. in the vagina (G) and uterine horns (H) (n = 9 to 10). Data were pooled from two to four independent experiments. Statistical significance was measured by an unpaired two-tailed t test. Horizontal bars show the means, error bars show SD, and dashed lines show the limit of detection. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; ns, not significant.
To test whether antibodies could confer protection, we purified ZIKV-immune IgG from the serum of mice 21 days after subcutaneous infection. Equal amounts of immune or naive IgG were transferred passively to naive recipients that were then treated with anti-IFNAR1 mAb and inoculated intravaginally with ZIKV (Fig. 6E). Passive transfer of ZIKV-immune IgG led to an increase in the neutralizing activity of recipient serum compared to that of recipients of naive IgG as well as an increase in detection of ZIKV-specific antibodies in the vaginal lumen (Fig. 6F). Remarkably, passive transfer of ZIKV-immune IgG resulted in ∼1,000- and 10-fold reductions in ZIKV RNA levels in the vagina (Fig. 6G) and uterine horns (Fig. 6H), respectively, at day 4 postinfection. Thus, passive transfer of memory T cells or antibody alone is sufficient to confer protection against intravaginal infection with ZIKV.
DISCUSSION
We evaluated the requirements for protection against intravaginal infection of ZIKV in mice and showed that primary infection through an intravaginal or subcutaneous route induced B and T cell immunity that protected against secondary intravaginal ZIKV challenge with the homologous strain. Low-level persistence of viral RNA, but not infectious virus, was detected in the FRT after primary subcutaneous and intravaginal ZIKV infection; however, this small RNA reservoir did not appear to impede protective immunity against subsequent vaginal ZIKV infection. While adaptive immune responses are not required for controlling ZIKV infection in mice in the context of an intact type I IFN response (7), we found that both B and T cells were required to restrict ZIKV when type I IFN was suppressed. CD4+ and virus-specific CD8+ T cells were recruited to the FRT after intravaginal and subcutaneous inoculation, and CD8+ T cells began differentiating into TRM after the acute phase of infection. Both intravaginal and subcutaneous ZIKV infection elicited neutralizing serum antibody and ZIKV-specific IgG in the vaginal lumen, although B cells and antibody-secreting plasma cells were not recruited to the FRT in high numbers. Depletion of CD4+ T cells and CD8+ T cells or a genetic deficiency of B cells did not result in a significant loss of protection against secondary intravaginal ZIKV challenge, suggesting redundancy in the adaptive immune response. However, transfer of immune IgG led to control of viral replication in the vagina and diminished viral dissemination into the upper FRT. Transfer of immune T cells also led to a reduction of viral replication in the vagina, but protection was not as robust as observed with transfer of immune IgG. These results suggest that circulating T cell and/or antibody responses may protect against sexual transmission of ZIKV infection (32).
There has been great interest in understanding the pathways that establish local immunity in the FRT, and particularly TRM, as a tool to develop better vaccines against sexually transmitted viruses (57). While recruitment of virus-specific CD8+ T cells into the FRT during the effector phase of the response after ZIKV infection was high, there was no decrease in cell number at 3 weeks postinfection indicative of T cell contraction. It is most likely that our analysis did not capture the peak of the ZIKV-specific CD8+ T cell response. However, contraction of differentiating TRM does not occur in the skin after HSV-1 infection (56), and it is possible that a similar phenomenon might be occurring in the FRT after ZIKV infection. Furthermore, human CD8+ T cell responses against ZIKV infection appear to peak relatively late after onset of symptoms (58), suggesting that protracted expansion of T cells could be a natural feature of ZIKV infection. Unlike the vagina, which was populated by a large pool of CD103+ virus-specific CD8 T cells, a relatively low frequency of virus-specific CD8+ T cells in the uterus expressed CD103 at 21 days after infection. The CD103− CD8+ T cells may represent circulating T cells or bona fide early TRM, as previous studies have shown that the majority of CD8+ T cells retained in the upper FRT are tissue resident and expression of CD103 in the lower and upper FRT may not be as high as that in other mucosal tissues (59). It is unclear whether CD103+ and CD103− uterine TRM are distinct subsets with discrete functional roles, as has been observed in the gut (60). We speculate that there may be distinct requirements for differentiation of TRM in the lower and upper FRT and that the composition of the early TRM population at day 21 postinfection may reflect such requirements. The intravaginal model of ZIKV infection may reveal insight as to how TRM differentiate and are retained in different compartments.
Although passive transfer of immune IgG with neutralizing activity decreased viral replication in the vagina and prevented dissemination to the upper FRT, it remains unclear whether this protection was dependent on the inhibitory activity in blood or the local antibody in the vaginal lumen. Transfer of IgG into the vaginal lumen is mediated by the neonatal Fc receptor (FcRn) (49). As FcRn-deficient animals have a significantly decreased half-life of circulating IgG (61), selective deletion of FcRn from the vaginal epithelium would likely be required to assess the roles of lumenal versus serum antibodies. As adoptive transfer of T cells, which likely remain in circulation prior to infection, led to considerable yet incomplete protection against ZIKV infection, pharmacological induction of TRM in the FRT might improve protection (57). Furthermore, while passive transfer of immune IgG led to marked reduction of viral titers within the FRT, again protection was not complete. Additional studies are needed to determine whether there is a threshold of serum neutralizing activity above which antibodies alone can provide sterilizing immunity within the FRT after sexual transmission of ZIKV and whether augmenting the transfer of antibodies into the vaginal lumen will enhance protection (49). If a threshold does exist, our data suggest it may be relatively low (EC50, >1:100). Further study will be required to define the exact contribution of local antibody and T cells to protection against ZIKV.
Several vaccine formulations against ZIKV (e.g., purified inactivated virus [62, 63], subunit DNA [62–64], subunit mRNA [65, 66], and live attenuated virus [67]) induce neutralizing antibody and T cells in mice and nonhuman primates and have entered clinical trials (68–71). However, none of these vaccines have been evaluated for protection against sexual transmission of ZIKV in nonpregnant or pregnant female animals or humans. Such experiments will be important considering the possible increased risk of fetal malformations after intravaginal infection and the high level of susceptibility of the lower FRT to ZIKV infection. While our data suggest that current vaccine designs may protect against sexually transmitted ZIKV in nonpregnant females, it remains unclear if they will achieve sterilizing immunity in the genital tract. Whether immunological protection against sexual transmission can be maintained during pregnancy also is unknown. In animal models, vaccination does not completely protect the fetus after a subcutaneous challenge, as low levels of viral RNA can be detected in the placenta and fetal head even in the context of high titers of neutralizing antibody (72, 73). Furthermore, “asymptomatic” infection of mice with intact type I IFN signaling prior to mating achieved robust but not sterilizing immunity against a subsequent subcutaneous challenge after mating, as fetuses still harbored low levels of viral RNA (74). Whether similar outcomes would occur after intravaginal challenge is unknown, and the consequences, if any, of residual viral RNA in the placenta and fetal head remain unclear. However, as sexual transmission of ZIKV can result in adverse fetal outcomes in animal models (75), it will be important to determine the efficacy of vaccines against this route of transmission. The most recent outbreak of ZIKV has waned, likely due to high exposure rates in areas of endemicity and the acquisition of immunity (76). Our work supports the hypothesis that immunity acquired after infection with live virus provides robust protection against rechallenge with a homologous ZIKV strain. It remains unknown whether immune responses raised against one strain of ZIKV after vaccination or subcutaneous infection will protect against sexual transmission of a distantly related ZIKV strain. In summary, our study begins to reveal the requirements for immunity against sexually transmitted ZIKV infection and provides a foundation for evaluating the correlates of vaccine-mediated protection.
MATERIALS AND METHODS
Mice.
Three- to 5-week-old female C57BL/6J mice were purchased from Jackson Laboratories, rested for at least 1 week, and infected at a minimum age of 5 weeks. B cell-deficient μMT mice on a C57BL/6 background were purchased from Jackson Laboratories (B6.129S2-Ighmtm1Cgn/J) and bred at Washington University School of Medicine. This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols were approved by the Institutional Animal Care and Use Committee at the Washington University School of Medicine (assurance number A3381-01). Subcutaneous injections were performed under anesthesia induced and maintained with isoflurane, and all efforts were made to minimize animal suffering. All animal experiments were performed under biosafety level 2 (A-BSL2) containment. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Washington University.
Viruses.
ZIKV-Brazil (Paraiba 2015 strain) was provided as a gift by S. Whitehead (Bethesda, MD). Low-passage viral stocks were propagated in Vero cells as previously described (44), and titers of virus were determined by plaque assay on Vero cells (ATCC). All tissue culture experiments were performed under biosafety level 2 (BSL2) containment.
Mouse infection studies.
All mice were injected subcutaneously in the neck once with 2 mg of DMPA (Pfizer) 5 to 7 days prior to primary virus inoculation. Secondary challenges occurred 4 weeks after DMPA treatment. One day prior to primary infection or secondary challenge, mice were injected intraperitoneally with 1 mg of purified anti-IFNAR1 mAb (clone MAR1-5A3; Leinco Technologies) or mouse IgG1 isotype control mAb (Leinco Technologies). For experiments that required depletion of CD4+ or CD8+ T cells, 250 μg of anti-CD8 antibody (clone YTS-169.4; Leinco Technologies, BioXCell), anti-CD4 antibody (clone GK1.5; Leinco Technologies, BioXCell), or an isotype control mAb (Leinco Technologies, BioXCell) was injected intraperitoneally at 1 and 3 days prior to virus inoculation.
For intravaginal infection, a 10-μl volume with 104 and 105 PFU was used per mouse for primary and secondary infections, respectively. For infection, a sterile calginate swab (Puritan Medical) moistened with sterile phosphate-buffered saline (PBS) was used to gently remove mucus from the vaginal cavity. A pipette tip was inserted into the vagina to deliver the 10-μl viral inoculum. Subcutaneous inoculum was delivered in a 50-μl volume with 103 PFU administered per mouse.
T cell and antibody transfers.
T cells were recovered by negative selection from the spleens of WT mice 3 weeks after subcutaneous ZIKV infection or from naive animals using an EasySep mouse T cell isolation kit (Stem Cell Technologies). A total of 107 naive or ZIKV-experienced T cells were injected via the retro-orbital route into mice 1 day prior to infection. For passive transfer of antibodies, serum was harvested from WT mice 3 weeks after subcutaneous ZIKV infection or from naive animals. IgG was purified using NAb Protein G spin columns (Pierce), concentrated with Amicon Ultra-15 centrifugal filter units (EMD Millipore), and diluted with sterile PBS. Antibody was quantitated on a Nanodrop spectrophotometer by measuring absorption at 280 nm (Thermo Fisher). Normal mouse gamma globulin (Jackson ImmunoResearch) also was used as a control for the IgG passive transfer studies. ZIKV-immune or negative-control antibody (400 μg) was injected intraperitoneally into recipient mice 1 day prior to infection.
Virus quantification.
For titration of virus in the vaginal lumen, 50-μl washes with sterile PBS were collected using a pipette and a sterile calginate swab and diluted in Dulbecco's modified Eagle's medium (DMEM) with 2% fetal bovine serum (FBS). To measure viral burden in tissues, mice were perfused with a minimum of 15 ml of PBS after sedation with ketamine and xylazine. FRT tissues were harvested and snap-frozen on dry ice. If virus was measured by plaque assay, tissues were weighed and homogenized in 2% DMEM using sterile 1-mm zirconia/silica beads (Biospec Products). For viral RNA measurements, tissues were weighed and homogenized with beads in 2% DMEM or in RLT buffer (Qiagen). RNA was extracted using the QiAmp viral RNA minikit or the RNeasy minikit (Qiagen). Standards for qRT-PCR were made by extracting RNA from viral stocks using the QiAmp MinElute virus spin kit (Qiagen). qRT-PCR was performed with the iTaq universal probes one-step kit (Bio-Rad) and previously published ZIKV primers and probes (77): F, CCGCTGCCCAACACAAG; R, CCACTAACGTTCTTTTGCAGACAT; probe, 50-/56-FAM/AGCCTACCT/ZEN/TGACAAGCAATCAGACACTCAA/3IABkFQ/-30 (Integrated DNA Technologies). qRT-PCR was performed on a CFX Connect real-time system (Bio-Rad). Plaque assays were performed on Vero cells as previously described (44).
RNA in situ hybridization.
For viral RNA detection by in situ hybridization, tissues were perfused with PBS and then 4% paraformaldehyde (PFA) in PBS, cryoprotected in 30% sucrose, and frozen in OCT medium (Sakura) and sectioned on a cryostat. RNA ISH was performed using RNAscope 2.5 (Advanced Cell Diagnostics) according to the manufacturer's protocol, as previously described (19).
Measurement of ZIKV antibody response.
To measure levels of circulating neutralizing antibodies, an FRNT was performed as previously described (26). Serum was collected from mice prior to secondary challenge and heat inactivated at 56°C for 15 min. Serially diluted serum was incubated with 100 focus-forming units (FFU) of ZIKV-Brazil for 1 h at 37°C and then added to Vero cells for 1.5 h at 37°C. Methylcellulose (1%, wt/vol) was added to the wells, incubated at 37°C for 28 h, and fixed with 1% PFA for at least 1 h. Infected cell foci were detected using 500 ng/ml of a ZIKV-specific mouse mAb (ZV-2) (26) and a horseradish peroxidase-conjugated donkey anti-mouse antibody (Jackson ImmunoResearch). Plates were developed with TrueBlue peroxidase substrate (KPL), and foci were counted on a BioSpot analyzer (Cellular Technology Limited). EC50s were generated by a variable-slope (four-parameter) nonlinear regression analysis. To measure ZIKV E protein-specific antibodies in the vaginal lumen, ELISAs were performed as previously described (26).
Flow cytometry.
Vaginas and uterine horns were digested as previously described (78). Briefly, tissues were incubated with Dispase (Roche) for 15 min at 37°C and collagenase D (Roche) and DNase I (Roche) for 30 min and then mechanically disrupted. Cell counts were performed by adding CountBright beads (Invitrogen) to samples prior to acquisition. Dead cells were excluded using the fixable aqua dead cell stain kit (Life Technologies). Tetramers were prepared with ZIKV E protein peptides 294 to 302 and 635 to 645 (34) (ABI Peptides) by the Center of Human Immunology and Immunotherapy Programs at Washington University and conjugated to streptavidin-allophycocyanin (APC) (Invitrogen). Tetramer staining was performed at 37°C for 20 min in PBS with 2% FBS. The following antibodies were used for this study: CD3 (clone 145-2C11), CD103 (clone 2E7), CD138 (clone 281-2), CD19 (clone 6D5), CD4 (clone GK1.5 or RM4-4), CD8a (clone 53-6.7), and CD8b.2 (clone 53-5.8). All antibodies were purchased from Biolegend. Samples were acquired on an LSR Fortessa (BD Biosciences) and analyzed by FlowJo (Treestar).
Statistical analysis.
All numerical data analysis was performed on GraphPad Prism7 software. Values were transformed by log10 to normalize distribution and variances where necessary. Viral titers were analyzed by 2-way analysis of variance (ANOVA), with a Bonferroni post hoc test to correct for multiple comparisons or an unpaired two-tailed Student's t test for normally distributed data, or by Kruskal-Wallis with Dunn's multiple-comparison test for nonnormally distributed data. Cell number data were analyzed by two-way ANOVA or one-way ANOVA with Bonferroni's post hoc test to correct for multiple comparisons. EC50s and endpoint titers were analyzed by one-way ANOVA with Bonferroni's post hoc test or by an unpaired two-tailed Student's t test. All data are means ± standard deviations (SD). A P of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
We thank Akiko Iwasaki for providing insightful input for this study and critical readings of the manuscript. We also thank Jennifer Hyde, Julie Fox, and Jennifer Govero for technical advice on assays used for this study.
This work was supported by grants from the NIH NIAID (R21 AI132133 to H.S., R01 AI104972 and P01 AI106695 to M.S.D., and R01 AI073755 to M.S.D. and D.H.F.) and by NIAID contracts (HHSN272201400018C and HHSN272201200026C [CSGID] to D.H.F.).
J.M.S., T.J.L., J.M.R., and X.J. conducted experiments, J.M.S. and T.J.L. acquired data, E.F., H.Z., D.H.F., and M.S.D. aided in the design of experiments, M.S.D. aided in the interpretation of results, and H.S. designed and conducted experiments and acquired, analyzed, and interpreted data. H.S. wrote the manuscript, M.S.D. provided major editorial changes, and all authors edited the final version of the paper.
M.S.D. is a consultant for Inbios, Sanofi, and Aviana Molecular Technologies and is on the Scientific Advisory Board of Moderna.
REFERENCES
- 1.Petersen LR, Jamieson DJ, Powers AM, Honein MA. 2016. Zika virus. N Engl J Med 374:1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 2.Lazear HM, Diamond MS. 2016. Zika virus: new clinical syndromes and its emergence in the western hemisphere. J Virol 90:4864–4875. doi: 10.1128/JVI.00252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martines RB, Bhatnagar J, de Oliveira Ramos AM, Davi HPF, Iglezias SDA, Kanamura CT, Keating MK, Hale G, Silva-Flannery L, Muehlenbachs A, Ritter J, Gary J, Rollin D, Goldsmith CS, Reagan-Steiner S, Ermias Y, Suzuki T, Luz KG, de Oliveira WK, Lanciotti R, Lambert A, Shieh W-J, Zaki SR. 2016. Pathology of congenital Zika syndrome in Brazil: a case series. Lancet 388:898–904. doi: 10.1016/S0140-6736(16)30883-2. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. 2016. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med 374:1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 5.Brasil P, Pereira JPJ, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, Rabello RS, Valderramos SG, Halai U-A, Salles TS, Zin AA, Horovitz D, Daltro P, Boechat M, Raja Gabaglia C, Carvalho de Sequeira P, Pilotto JH, Medialdea-Carrera R, Cotrim da Cunha D, Abreu de Carvalho LM, Pone M, Machado Siqueira A, Calvet GA, Rodrigues Baião AE, Neves ES, Nassar de Carvalho PR, Hasue RH, Marschik PB, Einspieler C, Janzen C, Cherry JD, Bispo de Filippis AM, Nielsen-Saines K. 2016. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med 375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JLM, Guimarães KP, Benazzato C, Almeida N, Pignatari GC, Romero S, Polonio CM, Cunha I, Freitas CL, Brandão WN, Rossato C, Andrade DG, Faria DdP, Garcez AT, Buchpigel CA, Braconi CT, Mendes E, Sall AA, Zanotto PMdA, Peron JPS, Muotri AR, Beltrão-Braga PCB. 2016. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534:267–271. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yockey LJ, Varela L, Rakib T, Khoury-Hanold W, Fink SL, Stutz B, Szigeti-Buck K, Van den Pol A, Lindenbach BD, Horvath TL, Iwasaki A. 2016. Vaginal exposure to Zika virus during pregnancy leads to fetal brain infection. Cell 166:1247–1256.e4. doi: 10.1016/j.cell.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, Mysorekar IU, Diamond MS. 2016. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao-Lormeau V-M, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, Vial A-L, Decam C, Choumet V, Halstead SK, Willison HJ, Musset L, Manuguerra J-C, Despres P, Fournier E, Mallet H-P, Musso D, Fontanet A, Neil J, Ghawché F. 2016. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, Salje H, Van Kerkhove MD, Abadie V, Garel C, Fontanet A, Mallet H-P. 2016. Association between Zika virus and microcephaly in French Polynesia, 2013-15: a retrospective study. Lancet 387:2125–2132. doi: 10.1016/S0140-6736(16)00651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, Lanciotti RS, Tesh RB. 2011. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 17:880. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Ortenzio E, Matheron S, de Lamballerie X, Hubert B, Piorkowski G, Maquart M, Descamps D, Damond F, Yazdanpanah Y, Leparc-Goffart I. 2016. Evidence of sexual transmission of Zika virus. N Engl J Med 374:2195–2198. doi: 10.1056/NEJMc1604449. [DOI] [PubMed] [Google Scholar]
- 13.Turmel JM, Abgueguen P, Hubert B, Vandamme YM, Maquart M, Le Guillou-Guillemette H, Leparc-Goffart I. 2016. Late sexual transmission of Zika virus related to persistence in the semen. Lancet 387:2501. doi: 10.1016/S0140-6736(16)30775-9. [DOI] [PubMed] [Google Scholar]
- 14.Brooks RBCMP, Myers RA, White MG, Bobo-Lenoci T, Aplan D, Blythe D, Feldman KA. 2016. Likely sexual transmission of Zika virus from a man with no symptoms of infection—Maryland, 2016. MMWR Morb Mortal Wkly Rep 65:915–916. doi: 10.15585/mmwr.mm6534e2. [DOI] [PubMed] [Google Scholar]
- 15.Russell K, Hills SL, Oster AM, Porse CC, Danyluk G, Cone M, Brooks R, Scotland S, Schiffman E, Fredette C, White JL, Ellingson K, Hubbard A, Cohn A, Fischer M, Mead P, Powers AM, Brooks JT. 2017. Male-to-female sexual transmission of Zika virus—United States, January–April 2016. Clin Infect Dis 64:211–213. doi: 10.1093/cid/ciw692. [DOI] [PubMed] [Google Scholar]
- 16.Deckard DT CW, Brooks JT, Smith JC, Woldai S, Hennessey M, Kwit N, Mead P. 2016. Male-to-male sexual transmission of Zika virus—Texas, January 2016. MMWR Morb Mortal Wkly Rep 65:372–374. doi: 10.15585/mmwr.mm6514a3. [DOI] [PubMed] [Google Scholar]
- 17.Davidson ASS, Komoto K, Rakeman J, Weiss D. 2016. Suspected female-to-male sexual transmission of Zika virus—New York City, 2016. MMWR Morb Mortal Wkly Rep 65:716–717. doi: 10.15585/mmwr.mm6528e2. [DOI] [PubMed] [Google Scholar]
- 18.Mansuy JM, Suberbielle E, Chapuy-Regaud S, Mengelle C, Bujan L, Marchou B, Delobel P, Gonzalez-Dunia D, Malnou CE, Izopet J, Martin-Blondel G. 2016. Zika virus in semen and spermatozoa. Lancet Infect Dis 16:1106–1107. doi: 10.1016/S1473-3099(16)30336-X. [DOI] [PubMed] [Google Scholar]
- 19.Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, Gorman MJ, Richner JM, Caine EA, Salazar V, Moley KH, Diamond MS. 2016. Zika virus infection damages the testes in mice. Nature 540:438–442. doi: 10.1038/nature20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uraki R, Hwang J, Jurado KA, Householder S, Yockey LJ, Hastings AK, Homer RJ, Iwasaki A, Fikrig E. 2017. Zika virus causes testicular atrophy. Sci Adv 3:e1602899. doi: 10.1126/sciadv.1602899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray KO, Gorchakov R, Carlson AR, Berry R, Lai L, Natrajan M, Garcia MN, Correa A, Patel SM, Aagaard K, Mulligan MJ. 2017. Prolonged detection of Zika virus in vaginal secretions and whole blood. Emerg Infect Dis 23:99. doi: 10.3201/eid2301.161394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirsch AJ, Smith JL, Haese NN, Broeckel RM, Parkins CJ, Kreklywich C, DeFilippis VR, Denton M, Smith PP, Messer WB, Colgin LMA, Ducore RM, Grigsby PL, Hennebold JD, Swanson T, Legasse AW, Axthelm MK, MacAllister R, Wiley CA, Nelson JA, Streblow DN. 2017. Zika virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog 13:e1006219. doi: 10.1371/journal.ppat.1006219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duggal NK, Ritter JM, Pestorius SE, Zaki SR, Davis BS, Chang G-JJ, Bowen RA, Brault AC. 2017. Frequent Zika virus sexual transmission and prolonged viral RNA shedding in an immunodeficient mouse model. Cell Rep 18:1751–1760. doi: 10.1016/j.celrep.2017.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan S, Woodruff EM, Trapecar M, Fontaine KA, Ezaki A, Borbet TC, Ott M, Sanjabi S. 2016. Dampened antiviral immunity to intravaginal exposure to RNA viral pathogens allows enhanced viral replication. J Exp Med 213:2913–2929. doi: 10.1084/jem.20161289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allard A, Althouse BM, Hébert-Dufresne L, Scarpino SV. 2017. The risk of sustained sexual transmission of Zika is underestimated. PLoS Pathog 13:e1006633. doi: 10.1371/journal.ppat.1006633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao H, Fernandez E, Dowd KA, Speer SD, Platt DJ, Gorman MJ, Govero J, Nelson CA, Pierson TC, Diamond MS, Fremont DH. 2016. Structural basis of Zika virus-specific antibody protection. Cell 166:1016–1027. doi: 10.1016/j.cell.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney M-C, Medits I, Sharma A, Simon-Lorière E, Sakuntabhai A, Cao-Lormeau V-M, Haouz A, England P, Stiasny K, Mongkolsapaya J, Heinz FX, Screaton GR, Rey FA. 2016. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature 536:48–53. doi: 10.1038/nature18938. [DOI] [PubMed] [Google Scholar]
- 28.Sapparapu G, Fernandez E, Kose N, Bin C, Fox JM, Bombardi RG, Zhao H, Nelson CA, Bryan AL, Barnes T, Davidson E, Mysorekar IU, Fremont DH, Doranz BJ, Diamond MS, Crowe JE. 2016. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature 540:443–447. doi: 10.1038/nature20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, Foglierini M, Pedotti M, Simonelli L, Dowall S, Atkinson B, Percivalle E, Simmons CP, Varani L, Blum J, Baldanti F, Cameroni E, Hewson R, Harris E, Lanzavecchia A, Sallusto F, Corti D. 2016. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 353:823–826. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- 30.Wang Q, Yang H, Liu X, Dai L, Ma T, Qi J, Wong G, Peng R, Liu S, Li J, Li S, Song J, Liu J, He J, Yuan H, Xiong Y, Liao Y, Li J, Yang J, Tong Z, Griffin BD, Bi Y, Liang M, Xu X, Qin C, Cheng G, Zhang X, Wang P, Qiu X, Kobinger G, Shi Y, Yan J, Gao GF. 2016. Molecular determinants of human neutralizing antibodies isolated from a patient infected with Zika virus. Sci Transl Med 8:369ra179. doi: 10.1126/scitranslmed.aai8336. [DOI] [PubMed] [Google Scholar]
- 31.Magnani DM, Rogers TF, Beutler N, Ricciardi MJ, Bailey VK, Gonzalez-Nieto L, Briney B, Sok D, Le K, Strubel A, Gutman MJ, Pedreño-Lopez N, Grubaugh ND, Silveira CGT, Maxwell HS, Domingues A, Martins MA, Lee DE, Okwuazi EE, Jean S, Strobert EA, Chahroudi A, Silvestri G, Vanderford TH, Kallas EG, Desrosiers RC, Bonaldo MC, Whitehead SS, Burton DR, Watkins DI. 2017. Neutralizing human monoclonal antibodies prevent Zika virus infection in macaques. Sci Transl Med 9:pii:eean8184. doi: 10.1126/scitranslmed.aan8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez E, Dejnirattisai W, Cao B, Scheaffer SM, Supasa P, Wongwiwat W, Esakky P, Drury A, Mongkolsapaya J, Moley KH, Mysorekar IU, Screaton GR, Diamond MS. 2017. Human antibodies to the dengue virus E-dimer epitope have therapeutic activity against Zika virus infection. Nat Immunol 18:1261–1269. doi: 10.1038/ni.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Bardelli M, Espinosa DA, Pedotti M, Ng T-S, Bianchi S, Simonelli L, Lim EXY, Foglierini M, Zatta F, Jaconi S, Beltramello M, Cameroni E, Fibriansah G, Shi J, Barca T, Pagani I, Rubio A, Broccoli V, Vicenzi E, Graham V, Pullan S, Dowall S, Hewson R, Jurt S, Zerbe O, Stettler K, Lanzavecchia A, Sallusto F, Cavalli A, Harris E, Lok S-M, Varani L, Corti D. 2017. A human bi-specific antibody against Zika virus with high therapeutic potential. Cell 171:229–241.e15. doi: 10.1016/j.cell.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elong Ngono A, Vizcarra EA, Tang WW, Sheets N, Joo Y, Kim K, Gorman MJ, Diamond MS, Shresta S. 2017. Mapping and role of the CD8+ T cell response during primary Zika virus infection in mice. Cell Host Microbe 21:35–46. doi: 10.1016/j.chom.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen J, Tang WW, Sheets N, Ellison J, Sette A, Kim K, Shresta S. 2017. Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive CD8+ T cells. Nat Microbiol 2:17036. doi: 10.1038/nmicrobiol.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pardy RD, Rajah MM, Condotta SA, Taylor NG, Sagan SM, Richer MJ. 2017. Analysis of the T cell response to Zika virus and identification of a novel CD8+ T cell epitope in immunocompetent mice. PLoS Pathog 13:e1006184. doi: 10.1371/journal.ppat.1006184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang H, Li S, Zhang Y, Han X, Jia B, Liu H, Liu D, Tan S, Wang Q, Bi Y, Liu WJ, Hou B, Gao GF, Zhang F. 2017. CD8+ T cell immune response in immunocompetent mice during Zika virus infection. J Virol 91:e00900-. doi: 10.1128/JVI.00900-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osuna CE, Lim S-Y, Deleage C, Griffin BD, Stein D, Schroeder LT, Omange R, Best K, Luo M, Hraber PT, Andersen-Elyard H, Ojeda EFC, Huang S, Vanlandingham DL, Higgs S, Perelson AS, Estes JD, Safronetz D, Lewis MG, Whitney JB. 2016. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med 22:1448–1455. doi: 10.1038/nm.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dudley DM, Aliota MT, Mohr EL, Weiler AM, Lehrer-Brey G, Weisgrau KL, Mohns MS, Breitbach ME, Rasheed MN, Newman CM, Gellerup DD, Moncla LH, Post J, Schultz-Darken N, Schotzko ML, Hayes JM, Eudailey JA, Moody MA, Permar SR, O'Connor SL, Rakasz EG, Simmons HA, Capuano S, Golos TG, Osorio JE, Friedrich TC, O'Connor DH. 2016. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun 7:12204. doi: 10.1038/ncomms12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsetsarkin KA, Kenney H, Chen R, Liu G, Manukyan H, Whitehead SS, Laassri M, Chumakov K, Pletnev AG. 2016. A full-length infectious cDNA clone of Zika virus from the 2015 epidemic in Brazil as a genetic platform for studies of virus-host interactions and vaccine development. mBio 7:e01114-. doi: 10.1128/mBio.01114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaushic C, Ashkar AA, Reid LA, Rosenthal KL. 2003. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J Virol 77:4558–4565. doi: 10.1128/JVI.77.8.4558-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang WW, Young MP, Mamidi A, Regla-Nava JA, Kim K, Shresta S. 2016. A mouse model of Zika virus sexual transmission and vaginal viral replication. Cell Rep 17:3091–3098. doi: 10.1016/j.celrep.2016.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grant A, Ponia SS, Tripathi S, Balasubramaniam V, Miorin L, Sourisseau M, Schwarz MC, Sánchez-Seco MP, Evans MJ, Best SM, García-Sastre A. 2016. Zika virus targets human STAT2 to inhibit type I interferon signaling. Cell Host Microbe 19:882–890. doi: 10.1016/j.chom.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. 2016. A mouse model of Zika virus pathogenesis. Cell Host Microbe 19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shin H, Iwasaki A. 2013. Tissue-resident memory T cells. Immunol Rev 255:165–181. doi: 10.1111/imr.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrison LA, Zhu L, Thebeau LG. 2001. Vaccine-induced serum immunoglobin contributes to protection from herpes simplex virus type 2 genital infection in the presence of immune T cells. J Virol 75:1195–1204. doi: 10.1128/JVI.75.3.1195-1204.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDermott MR, Brais LJ, Evelegh MJ. 1990. Mucosal and systemic antiviral antibodies in mice inoculated intravaginally with herpes simplex virus type 2. J Gen Virol 71:1497–1504. doi: 10.1099/0022-1317-71-7-1497. [DOI] [PubMed] [Google Scholar]
- 48.Parr EL, Parr MB. 1997. Immunoglobulin G is the main protective antibody in mouse vaginal secretions after vaginal immunization with attenuated herpes simplex virus type 2. J Virol 71:8109–8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Palaniyandi S, Zeng R, Tuo W, Roopenian DC, Zhu X. 2011. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc Natl Acad Sci U S A 108:4388–4393. doi: 10.1073/pnas.1012861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Usala SJ, Usala FO, Haciski R, Holt JA, Schumacher GF. 1989. IgG and IgA content of vaginal fluid during the menstrual cycle. J Reprod Med 34:292–294. [PubMed] [Google Scholar]
- 51.Kumamoto Y, Iwasaki A. 2012. Unique features of antiviral immune system of the vaginal mucosa. Curr Opin Immunol 24:411–416. doi: 10.1016/j.coi.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schenkel JM, Fraser KA, Vezys V, Masopust D. 2013. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol 14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, Braun A, Masson F, Kallies A, Belz GT, Carbone FR. 2015. T-box transcription factors combine with the cytokines TGF-β and IL-15 to control tissue-resident memory T cell fate. Immunity 43:1101–1111. doi: 10.1016/j.immuni.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 54.Shin H, Kumamoto Y, Gopinath S, Iwasaki A. 2016. CD301b+ dendritic cells stimulate tissue-resident memory CD8+ T cells to protect against genital HSV-2. Nat Commun 7:13346. doi: 10.1038/ncomms13346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, Fraser KA, Webby RJ, Brinkmann V, Butcher EC, Newell KA, Ahmed R. 2010. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med 207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon M-L, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, Tscharke DC, Heath WR, Inouye M, Carbone FR, Gebhardt T. 2013. The developmental pathway for CD103+ CD8+ tissue-resident memory T cells of skin. Nat Immunol 14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 57.Shin H, Iwasaki A. 2012. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491:463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ricciardi MJ, Magnani DM, Grifoni A, Kwon Y-C, Gutman MJ, Grubaugh ND, Gangavarapu K, Sharkey M, Silveira CGT, Bailey VK, Pedreño-Lopez N, Gonzalez-Nieto L, Maxwell HS, Domingues A, Martins MA, Pham J, Weiskopf D, Altman J, Kallas EG, Andersen KG, Stevenson M, Lichtenberger P, Choe H, Whitehead SS, Sette A, Watkins DI. 2017. Ontogeny of the B- and T-cell response in a primary Zika virus infection of a dengue-naïve individual during the 2016 outbreak in Miami, FL. PLoS Negl Trop Dis 11:e0006000. doi: 10.1371/journal.pntd.0006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyártó BZ, Southern PJ, Masopust D. 2015. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell 161:737–749. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bergsbaken T, Bevan MJ. 2015. Proinflammatory microenvironments within the intestine regulate the differentiation of tissue-resident CD8+ T cells responding to infection. Nat Immunol 16:406–414. doi: 10.1038/ni.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, Petkova S, Avanessian L, Choi EY, Shaffer DJ, Eden PA, Anderson CL. 2003. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol 170:3528–3533. doi: 10.4049/jimmunol.170.7.3528. [DOI] [PubMed] [Google Scholar]
- 62.Larocca RA, Abbink P, Peron JPS, Zanotto PM, Iampietro MJ, Badamchi-Zadeh A, Boyd M, Ng'ang'a D, Kirilova M, Nityanandam R, Mercado NB, Li Z, Moseley ET, Bricault CA, Borducchi EN, Giglio PB, Jetton D, Neubauer G, Nkolola JP, Maxfield LF, De La Barrera RA, Jarman RG, Eckels KH, Michael NL, Thomas SJ, Barouch DH. 2016. Vaccine protection against Zika virus from Brazil. Nature 536:474–478. doi: 10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, Kirilova M, Li Z, Ng'ang'a D, Nanayakkara O, Nityanandam R, Mercado NB, Borducchi EN, Agarwal A, Brinkman AL, Cabral C, Chandrashekar A, Giglio PB, Jetton D, Jimenez J, Lee BC, Mojta S, Molloy K, Shetty M, Neubauer GH, Stephenson KE, Peron JPS, Zanotto PM, Misamore J, Finneyfrock B, Lewis MG, Alter G, Modjarrad K, Jarman RG, Eckels KH, Michael NL, Thomas SJ, Barouch DH. 2016. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 353:1129–1132. doi: 10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dowd KA, Ko S-Y, Morabito KM, Yang ES, Pelc RS, DeMaso CR, Castilho LR, Abbink P, Boyd M, Nityanandam R, Gordon DN, Gallagher JR, Chen X, Todd J-P, Tsybovsky Y, Harris A, Huang Y-JS, Higgs S, Vanlandingham DL, Andersen H, Lewis MG, De La Barrera R, Eckels KH, Jarman RG, Nason MC, Barouch DH, Roederer M, Kong W-P, Mascola JR, Pierson TC, Graham BS. 2016. Rapid development of a DNA vaccine for Zika virus. Science 354:237–240. doi: 10.1126/science.aai9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC, Ciaramella G, Diamond MS. 2017. Modified mRNA vaccines protect against Zika virus infection. Cell 168:1114–1125.e10. doi: 10.1016/j.cell.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, Dowd KA, Sutherland LL, Scearce RM, Parks R, Wagner W, Granados A, Greenhouse J, Walker M, Willis E, Yu J-S, McGee CE, Sempowski GD, Mui BL, Tam YK, Huang Y-J, Vanlandingham D, Holmes VM, Balachandran H, Sahu S, Lifton M, Higgs S, Hensley SE, Madden TD, Hope MJ, Karikó K, Santra S, Graham BS, Lewis MG, Pierson TC, Haynes BF, Weissman D. 2017. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 543:248–251. doi: 10.1038/nature21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shan C, Muruato AE, Nunes BTD, Luo H, Xie X, Medeiros DBA, Wakamiya M, Tesh RB, Barrett AD, Wang T, Weaver SC, Vasconcelos PFC, Rossi SL, Shi P-Y. 2017. A live-attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models. Nat Med 23:763–767. doi: 10.1038/nm.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tebas P, Roberts CC, Muthumani K, Reuschel EL, Kudchodkar SB, Zaidi FI, White S, Khan AS, Racine T, Choi H, Boyer J, Park YK, Trottier S, Remigio C, Krieger D, Spruill SE, Bagarazzi M, Kobinger GP, Weiner DB, Maslow JN. 4 October 2017. Safety and immunogenicity of an anti-Zika virus DNA vaccine—preliminary report. N Engl J Med doi: 10.1056/NEJMoa1708120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Modjarrad K, Lin L, George SL, Stephenson KE, Eckels KH, De La Barrera RA, Jarman RG, Sondergaard E, Tennant J, Ansel JL, Mills K, Koren M, Robb ML, Barrett J, Thompson J, Kosel AE, Dawson P, Hale A, Tan CS, Walsh SR, Meyer KE, Brien J, Crowell TA, Blazevic A, Mosby K, Larocca RA, Abbink P, Boyd M, Bricault CA, Seaman MS, Basil A, Walsh M, Tonwe V, Hoft DF, Thomas SJ, Barouch DH, Michael NL. 4 December 2017. Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: phase 1, randomised, double-blind, placebo-controlled clinical trials. Lancet doi: 10.1016/S0140-6736(17)33106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gaudinski MR, Houser KV, Morabito KM, Hu Z, Yamshchikov G, Rothwell RS, Berkowitz N, Mendoza F, Saunders JG, Novik L, Hendel CS, Holman LA, Gordon IJ, Cox JH, Edupuganti S, McArthur MA, Rouphael NG, Lyke KE, Cummings GE, Sitar S, Bailer RT, Foreman BM, Burgomaster K, Pelc RS, Gordon DN, DeMaso CR, Dowd KA, Laurencot C, Schwartz RM, Mascola JR, Graham BS, Pierson TC, Ledgerwood JE, Chen GL, Plummer S, Costner P, Zephir K, Casazza J, Ola A, Victorino M, Levinson C, Whalen W, Wang X, Cunningham J, Vasilenko O, Burgos Florez M, Hickman S, Pittman I, Le L, Larkin B, et al. . 4 December 2017. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: randomised, open-label, phase 1 clinical trials. Lancet doi: 10.1016/S0140-6736(17)33105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fernandez E, Diamond MS. 2017. Vaccination strategies against Zika virus. Curr Opin Virol 23:59–67. doi: 10.1016/j.coviro.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shan C, Muruato AE, Jagger BW, Richner J, Nunes BTD, Medeiros DBA, Xie X, Nunes JGC, Morabito KM, Kong W-P, Pierson TC, Barrett AD, Weaver SC, Rossi SL, Vasconcelos PFC, Graham BS, Diamond MS, Shi P-Y. 2017. A single-dose live-attenuated vaccine prevents Zika virus pregnancy transmission and testis damage. Nat Commun 8:676. doi: 10.1038/s41467-017-00737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richner JM, Jagger BW, Shan C, Fontes CR, Dowd KA, Cao B, Himansu S, Caine EA, Nunes BTD, Medeiros DBA, Muruato AE, Foreman BM, Luo H, Wang T, Barrett AD, Weaver SC, Vasconcelos PFC, Rossi SL, Ciaramella G, Mysorekar IU, Pierson TC, Shi P-Y, Diamond MS. 2017. Vaccine mediated protection against Zika virus-induced congenital disease. Cell 170:273–283.e12. doi: 10.1016/j.cell.2017.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Turner LH, Kinder JM, Wilburn A, D'Mello RJ, Braunlin MR, Jiang TT, Pham G, Way SS. 2017. Preconceptual Zika virus asymptomatic infection protects against secondary prenatal infection. PLoS Pathog 13:e1006684. doi: 10.1371/journal.ppat.1006684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Uraki R, Jurado KA, Hwang J, Szigeti-Buck K, Horvath TL, Iwasaki A, Fikrig E. 2017. Fetal growth restriction caused by sexual transmission of Zika virus in mice. The J Infect Dis 215:1720–1724. doi: 10.1093/infdis/jix204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Netto EM, Moreira-Soto A, Pedroso C, Höser C, Funk S, Kucharski AJ, Rockstroh A, Kümmerer BM, Sampaio GS, Luz E, Vaz SN, Dias JP, Bastos FA, Cabral R, Kistemann T, Ulbert S, de Lamballerie X, Jaenisch T, Brady OJ, Drosten C, Sarno M, Brites C, Drexler JF. 2017. High Zika virus seroprevalence in Salvador, Northeastern Brazil limits the potential for further outbreaks. mBio 8:e01390-17. doi: 10.1128/mBio.01390-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Sanfield SM, Duffy MR. 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iijima N, Linehan MM, Saeland S, Iwasaki A. 2007. Vaginal epithelial dendritic cells renew from bone marrow precursors. Proc Natl Acad Sci U S A 104:19061–19066. doi: 10.1073/pnas.0707179104. [DOI] [PMC free article] [PubMed] [Google Scholar]