ABSTRACT
Highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) possesses greater replicative capacity and pathogenicity than classical PRRSV. However, the factors that lead to enhanced replication and pathogenicity remain unclear. In our study, an alignment of all available full-length sequences of North American-type PRRSVs (n = 204) revealed two consistent amino acid mutations that differed between HP-PRRSV and classical PRRSV and were located at positions 519 and 544 in nonstructural protein 9. Next, a series of mutant viruses with either single or double amino acid replacements were generated from HP-PRRSV HuN4 and classical PRRSV CH-1a infectious cDNA clones. Deletion of either of the amino acids led to a complete loss of virus viability. In both Marc-145 and porcine alveolar macrophages, the replicative efficiencies of mutant viruses based on HuN4 were reduced compared to the parent, whereas the replication level of CH-1a-derived mutant viruses was increased. Plaque growth assays showed clear differences between mutant and parental viruses. In infected piglets, the pathogenicity of HuN4-derived mutant viruses, assessed through clinical symptoms, viral load in sera, histopathology examination, and thymus atrophy, was reduced. Our results indicate that the amino acids at positions 519 and 544 in NSP9 are involved in the replication efficiency of HP-PRRSV and contribute to enhanced pathogenicity. This study is the first to identify specific amino acids involved in PRRSV replication or pathogenicity. These findings will contribute to understanding the molecular mechanisms of PRRSV replication and pathogenicity, leading to better therapeutic and prognostic options to combat the virus.
IMPORTANCE Porcine reproductive and respiratory syndrome (PRRS), caused by porcine reproductive and respiratory syndrome virus (PRRSV), is a significant threat to the global pig industry. Highly pathogenic PRRSV (HP-PRRSV) first emerged in China in 2006 and has subsequently spread across Asia, causing considerable damage to local economies. HP-PRRSV strains possess a greater replication capacity and higher pathogenicity than classical PRRSV strains, although the mechanisms that underlie these characteristics are unclear. In the present study, we identified two mutations in HP-PRRSV strains that distinguish them from classical PRRSV strains. Further experiments that swapped the two mutations in an HP-PRRSV strain and a classical PRRSV strain demonstrated that they are involved in the replication efficiency of the virus and its virulence. Our findings have important implications for understanding the molecular mechanisms of PRRSV replication and pathogenicity and also provide new avenues of research for the study of other viruses.
KEYWORDS: residue, highly pathogenic PRRSV, mutation, pathogenicity, replication
INTRODUCTION
RNA-dependent RNA polymerases (RdRps) are catalytic components of the RNA genomic replication system encoded by RNA viruses. They are ubiquitous in these viruses, regardless of genomic structure. To promote RNA virus replication, RdRp mediates the transcription of message sense viral RNAs that can act as a template for protein synthesis (1). Although the amino acid sequences of RdRps from different RNA viral families vary tremendously, they are strikingly similar in their three-dimensional organization. Most RNA virus RdRps possess a conserved structural “cupped right hand” feature that includes finger, palm, and thumb subdomains (2). A channel formed in the fingers allows entry of the template RNA and ribonucleotide triphosphates to assist the positioning of nucleotides in the active site. The active site is located in the palm domains. The thumb domain functions through interactions with the exiting nascent RNA (3). RdRp is critically important to virus replication and is a key regulator of nucleotide selectivity and fidelity.
Porcine reproductive and respiratory syndrome (PRRS) is a globally important disease that has caused significant economic losses to the pig industry since it was first recognized in the United States in 1987 (4). The etiological agent, PRRS virus (PRRSV), is an enveloped, single-stranded, positive-sense RNA virus. Its genome is approximately 15.4 kb in length and includes at least 10 open reading frames (ORFs), ORF1a, ORF1b, ORF2a, ORF2b, ORF3, ORF4, ORF5a, ORF5, ORF6, and ORF7. A −2 ribosomal frameshift has recently been identified for expression of nsp2TF in the nsp2 coding region. The nsp2TF coding sequence is conserved in PRRSV, lactate dehydrogenase elevating virus (LDV), and simian hemorrhagic fever virus (SHFV) but absent in equine arteritis virus (EAV) (5, 6). Among the ORFs, ORF1b encodes several important enzymes, including RdRp, that assemble into the viral replication and transcription complex (RTC) (7, 8). PRRSV is typically divided into two genotypes, European (EU) type (genotype I) and North American (NA) type (genotype II), represented by the LV strain and the ATCC VR-2332 strain, respectively (9–11). The first Chinese PRRSV strain (CH-1a) was isolated from an aborted porcine fetus in 1996 (12). In 2006, a highly pathogenic form of PRRS was identified in China, where infected individuals presented with high fever and experienced high morbidity and high mortality, resulting in considerable economic damage (13). The full-length genomes of North American-type PRRSVs were classified into 5 subgroups, including subgroup 1 (VR-2332-like PRRSVs), subgroup 2 (CH-1a-like PRRSVs), subgroup 4 (HP-PRRSVs), and subgroup 3 (the intermediate PRRSV, between subgroups 2 and 4). Highly pathogenic PRRSVs (HP-PRRSVs) likely originated from the Chinese subgroup 2 PRRSV and evolved through the intermediate subgroup 3 step by step (14). The newly emerged NADC30-like PRRSVs were defined as a new subgroup, as they share no more than 90% homology with the above-mentioned four subgroups and form a separate cluster in the phylogenetic tree (15–18).
In contrast to classical PRRSV, HP-PRRSV strains are characterized by certain features, such as greater replicative capacity and higher pathogenicity (19). They also share a unique discontinuous 30-amino-acid deletion in nonstructural protein 2 (NSP2). However, recent evidence has shown that this deletion is not related to the increased virulence of HP-PRRSV (20). Further experiments in which a series of chimeric viruses were constructed by swapping the corresponding regions of HP-PRRSV and classical PRRSV demonstrated that both NSP9 and NSP10 are involved in generating the increased virulence of HP-PRRSV (21). Although some factors have been investigated to establish what underlies PRRSV virulence, the mechanisms that underlie enhanced replication and fatal virulence are still unknown.
To identify possibly involved factors, we performed full-length sequence alignments of NA-type PRRSVs (n = 204), identifying two regular amino acid mutations in NSP9 that were putatively associated with virulence. A series of mutant viruses were generated based on reverse genetics by swapping either single or double amino acids between the HP-PRRSV strain rHuN4 and the classical PRRSV rCH-1a strain. In vitro and in vivo examination demonstrated that the 2 amino acids located at positions 519 and 544 in NSP9 are involved in replicative efficiency and the increased virulence of HP-PRRSV. These data will contribute to a better understanding of the molecular mechanisms of HP-PRRSV replication and pathogenicity and may lead to better measures to combat PRRSV in the pig industry.
RESULTS
Identification of consistent amino acid mutations in NSP9.
The alignment of all 204 NA-type PRRSV strains included in the study revealed two consistent amino acid mutations between HP-PRRSV and classical PRRSV strains, which were located at positions 519 and 544 of NSP9 (Fig. 1). All HP-PRRSV strains (145/145; 100%) feature a serine (S) at position 519, and the vast majority (143/145; 98.6%) have a threonine (T) at position 544. Classical PRRSV strains, including the VR-2332-related and CH-1a-related subgroups, typically possess a threonine and an alanine (A) at these two positions, respectively (Table 1). In the intermediate HB-1(sh) 2002-related subgroup, both the mutant and prototype amino acid residues can be found at positions 519 and 544. In addition, the NADC30- and MN184-related subgroups also show intermediate pathogenicity, and classical or HP-PRRSV-like amino acids are alternately found at positions 519 and 544 (i.e., S or T at position 519 and T or A at position 544). As subgroups progress from being more similar to classical PRRSV to being more similar to the HP-PRRSV subgroup, the 2 amino acids at positions 519 and 544 gradually change from a more classical to an HP-PRRSV-like distribution.
FIG 1.
Phylogenetic tree and alignment based on the full-length nucleotide sequences of 204 NA-type PRRSV strains. (Left) Red, blue, black, green, and brown represent HP-PRRSV, intermediate PRRSV, CH-1a-like PRRSV, VR-2332-like PRRSV, and NADC-30-like PRRSV strains, respectively. (Right) Alignment of partial amino acid sequences of NSP9 of 204 NA-type PRRSVs. The two conserved amino acids are highlighted in yellow.
TABLE 1.
Percentages of amino acids occurring at positions 519 and 544 in NSP9 in different subgroups
| Subgroup | Representative strain | No. of strains | No. (%) with amino acid at position: |
|||
|---|---|---|---|---|---|---|
| 519 |
544 |
|||||
| T | S | T | A | |||
| 1 | VR-2332 | 26 | 24 (92.3) | 0 | 3 (11.5) | 23 (88.5) |
| 2 | CH-1a | 9 | 9 (100) | 0 | 0 | 9 (100) |
| 3 | HB-1(sh) 2002 | 7 | 2 (28.6) | 5 (71.4) | 4 (57.1) | 3 (42.9) |
| 4 | HuN4/JXWn06 | 145 | 0 | 145 (100) | 143 (98.6) | 2 (1.4) |
| 5 | NADC30/MN184A | 7 | 1 (14.3) | 5 (71.4) | 55 (71.4) | 22 (28.6) |
| Unclassified | 10 | 10 (100) | 0 | 0 | 10 (100) | |
Structural analysis of NSP9.
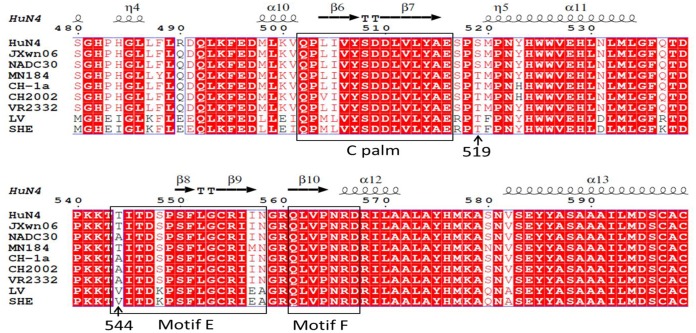
The three-dimensional structure of PRRSV NSP9 was predicted using I-TASSER (http://zhanglab.ccmb.med.umich.edu/I-TASSER/) based on the template of Protein Data Bank (PDB) identifier (ID) 2ijd1 (the crystal structure of poliovirus RdRp), as it shared the highest TM score (0.879), which is a measure of global structural similarity between the query and template proteins, with PRRSV RdRp in the known structures of proteins. This predicted structure suggested that the protein is comprised of two major sections, an N-terminal section at amino acid residues 1 to 180 and a C-terminal section formed of amino acid residues 181 to 646. This structural analysis predicted that amino acid position 519 was located in the coiling region of the C-terminal section, whereas position 544 was located in the motif E region (Fig. 2).
FIG 2.
Sequence alignment of NSP9 from representative NA-type or EU-type PRRSV strains. The locations of the amino acids refer to the HuN4 NSP9 sequence. The mutant amino acids at positions 519 and 544 in NSP9 are labeled with arrows. C palm, motif E, and motif F are boxed in black. Fully conserved residues are shaded in red.
Construction of infectious cDNA clones of HuN4 and CH-1a and their mutant viruses.
Two infectious cDNA clones of pHuN4 and pCH-1a were generated and validated. Viral N protein was detectable by immunofluorescent staining in cells transfected with pHuN4 and pCH-1a. N protein expression was also detected by Western blotting using an anti-N protein antibody. Using these rescued (r) rHuN4 and rCH-1a cDNAs as templates, PCR products were digested with MluI, confirming the presence of two bands (314 bp and 118 bp) in the rescued strains due to the addition of a novel MluI restriction site, whereas only one band (432 bp) was visible in the wild-type strains. Three mutant viruses based on rHuN4 (rHuN4-S519T, rHuN4-T544A, and rHuN4-S519T-T544A) and three mutant viruses based on rCH-1a (rCH-1a-T519S, rCH-1a-A544T, and rCH-1a-T519S-A544T) were generated by swapping single or double amino acids at positions 519 and 544 of NSP9, and the mutant viruses were rescued. However, mutant clones that had amino acid deletions at positions 519 and 544 were unable to be rescued (Fig. 3), suggesting that the two amino acid residues are essential for PRRSV rescue.
FIG 3.
Schematic representation of the construction of mutant viruses. Amino acids at positions 519 and 544 were swapped between rHuN4 and rCH-1a sequences. The triangles and dots represent the mutant amino acids present in HuN4 and CH-1a, respectively. +, mutant viruses could be rescued; −, viruses could not be rescued, and there was total loss of viability.
The identified amino acid mutations affect the in vitro replicative capacity of PRRSV.
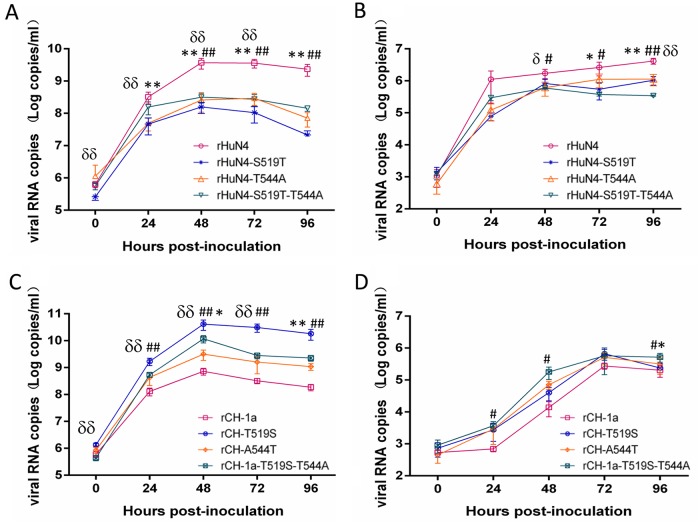
Six mutant viruses and their parental strains were used to compare viral replicative properties in either Marc-145 cells or porcine alveolar macrophages (PAMs). In Marc-145 cells, all of the mutant viruses and their parental strains reached their peak replication rates at 48 h postinfection (hpi) (Fig. 4A). However, rHuN4-S519T-T544A, rHuN4-S519T, and rHuN4-T544A, which had been modified to be more classical PRRSV-like, each had a lower viral RNA copy number than rHuN4, with an approximately 1.0- to 1.5-log-unit decrease in the viral RNA copy number that was significantly different at 24 to 96 hpi (P < 0.01) (Fig. 4A). Conversely, the mutant viruses based on CH-1a (rCH-1a-T519S-A544T, rCH-1a-T519S, and rCH-1a-A544T) that were more HP-PRRSV-like had a higher growth rate than their parent virus. Moreover, rCH-1a-T519S-A544T, rCH-1a-T519S, and rCH-1a-A544T each had significantly higher viral RNA copy numbers than rCH-1a at several time points (P < 0.01) (Fig. 4C). As PAMs are the primary in vivo target of PRRSV, we next evaluated the replication levels of the mutant viruses in that cell type. Analysis of growth kinetics revealed that, at earlier time points, the mutant viruses based on the rHuN4 backbone had lower viral copy numbers than rHuN4, although without any significant differences (Fig. 4B). However, there was a significant difference in the viral RNA copy numbers of rHuN4-S519T-T544A-infected cells, which were lower than those in rHuN4-infected cells, with an approximate 1.0-log-unit decrease in viral RNA copy numbers from 24 to 72 hpi (P < 0.01) (Fig. 4B). Similarly, rHuN4-S519T-infected cells had significantly lower viral RNA copy numbers than rHuN4 at 48 and 72 hpi (P < 0.05 and P < 0.01, respectively). rHuN4-T544A-infected cells also had significantly lower viral RNA copy numbers at 36 and 72 hpi (P < 0.05 and P < 0.01, respectively) (Fig. 4B). The number of viral copies in PAMs of mutants based on rCH-1a was higher than that of the parental virus (Fig. 4D). At 24, 36, and 96 hpi, the viral RNA copy number of rCH-1a-T519S-A544T-infected cells was significantly higher than that of rCH-1a (P < 0.05). At 24 hpi, the viral RNA copy numbers of rCH-1a-infected cells were significantly lower than those of rCH-1a-A544T-infected cells (P < 0.05). Finally, the viral RNA copy numbers of rCH-1a-T519S-infected cells were higher than those of the rCH-1a parent, although the differences were not significant. Regardless, we found that the rCH-1a-T519S mutant virus had a higher replicative capacity than the rCH-1a parent.
FIG 4.
Virus RNA copy numbers in Marc-145 cells and PAMs at different time points after infection with each mutant virus. (A and C) RNA copies of mutant viruses based on HuN4 (rHuN4-S519T, rHuN4-T544A, and rHuN4-S519T-T544A) (A) and CH-1a (rCH-1a-T519S, rCH-1a-A544T, and rCH-1a-T519S-A554T) (C) in Marc-145 cells relative to their parental viruses. (B and D) Viral copy numbers of mutant viruses based on HuN4 (B) and CH-1a (D) in PAM cells. The data are presented as means ± standard deviations from the results three independent experiments. δ, significant difference between rHuN4 and rHuN4-S519T or between rCH-1a and rCH-1a-T519S (δ, P < 0.05; δδ, P < 0.01); *, significant difference between rHuN4 and rHuN4-T544A or between rCH-1a and rCH-1a-A544T (*, P < 0.05; **, P < 0.01); #, significant difference between rHuN4 and rHuN4-S519T-T544A or between rCH-1a and rCH-1a-T519S-A544T (#, P < 0.05; ##, P < 0.01).
We also determined the titers of the mutant viruses and parent viruses harvested at 0, 24, 48, 72, and 96 h after infection of Marc-145 cells and PAMs. The growth kinetics of the viruses harvested from Marc-145 cells showed that the mutant virus based on HuN4 had lower titers than rHuN4. rHuN4-S519T and rHuN4-T544A showed obviously lower titers than rHuN4, with significant differences at 24 to 96 hpi, and rHuN4-S519T-T544A also showed lower virus titers than rHuN4, with significant differences at 72 to 96 hpi (P < 0.05 and P < 0.01) (Fig. 5A). In contrast, the mutant virus based on CH-1a harvested from Marc-145 cells showed the opposite trend. rCH-1a-T519S, rCH-1a-A544T, and rCH-1a-T519S-A544T showed a higher growth rate than rCH-1a at most times (Fig. 5C). Moreover, rCH-T519S-A544T displayed significantly higher virus titers than rCH-1a at 24 to 96 hpi. rCH-1a-T519S and rCH-A544T also showed a significantly higher virus titer than rCH-1a at 24, 48, and 72 hpi (Fig. 5C). The mutant viruses harvested from PAMs showed a trend similar to that of the viruses harvested from Marc-145 cells (Fig. 5B and D). Therefore, the virus RNA copy numbers and virus titers showed the same trends.
FIG 5.
Growth kinetics of rescued viruses with amino acid mutations. The growth kinetics of the mutant viruses based on HuN4 (rHuN4-S519T-T544A, rHuN4-S519T, and rHuN4-T544A) and CH-1a (rCH-1a-T519S-A554T, rCH-1a-T519S, and rCH-1a-A544T) relative to their parental viruses in Marc-145 cells (A and C) and in PAMs (B and D) are shown. Virus titers from 12 hpi to 96 hpi were determined by microtitration infectivity assays. The data are presented as means ± standard deviations from the results of three independent experiments. δ, significant difference between rHuN4 and rHuN4-S519T or between rCH-1a and rCH-1a-T519S (δ, P < 0.05; δδ, P < 0.01); *, significant difference between rHuN4 and rHuN4-T544A or between rCH-1a and rCH-1a-A544T (*, P < 0.05; **, P < 0.01); #, significant difference between rHuN4 and rHuN4-S519T-T544A or between rCH-1a and rCH-1a-T519S-A544T (#, P < 0.05; ##, P < 0.01).
Viral plaque assays.
Plaque assay analysis consistently showed that rHuN4-S519T-T544A-, rHuN4-S519T-, and rHuN4-T544A-infected cells developed smaller plaques than the parental rHuN4 strain. In particular, rHuN4-T544A and rHuN4-S519T-T544A plaques were approximately 2-fold smaller than those observed for rHuN4. The sizes of the rHuN4-S519T plaques were slightly smaller than those of rHuN4 (Fig. 6). Conversely, rCH-1a-T519S-A544T-, rCH-1a-T519S-, and rCH-1a-A544T-infected cells developed plaques approximately 3-fold larger than those of the parent virus, rCH-1a (Fig. 6).
FIG 6.
Plaque morphology in Marc-145 cells of the parental strains rHuN4 and rCH-1a and the mutant viruses containing single or double amino acid mutations.
Detection of negative-strand gRNA by reverse transcription (RT)-PCR.
The level of negative-strand genomic RNA (gRNA) synthesis was approximately 2-fold higher for rHuN4 than for rHuN4-S519T-T544A, and that for rCH-1a-T519S-A544T was approximately 4-fold higher than that for rCH-1a (Fig. 7). Thus, a significant difference in negative-strand gRNA synthesis was observed between mutant viruses and their corresponding parental viruses, which indicated that the mutations in NSP9 changed viral genomic replication in the startup phase of replication.
FIG 7.
Comparison of the abilities of mutant viruses and their parent viruses to synthesize negative-strand RNA. (A) The amounts of PCR products of negative-strand gRNA [(−)gRNA] and β-actin were determined by electrophoresis. β-Actin was used as an internal control. (B) The ratio of gray values for PCR products (negative-strand gRNA/β-actin) was calculated using Image J software. *, P < 0.05; **, P < 0.01.
The identified amino acid mutations affected the pathogenicity of PRRSV in piglets.
Next, we assessed the pathogenicity of rHuN4-S519T-T544A, rCH-1a-T519S-A544T, and their parent viruses in vivo in piglets. The rHuN4-infected piglets presented a higher temperature that rose faster and reached a peak of 41.1°C at 4 days postinoculation (dpi) for some individuals. From 2 to 14 dpi, the mean temperature of the HuN4 group was approximately 0.5°C higher than that of the group infected with rHuN4-S519T-T544A. Piglets infected with rHuN4-S519T-T544A showed a trend similar to that of piglets infected with rHuN4 within the first 2 days of infection (Fig. 8A). However, the temperature of piglets inoculated with rHuN4-S519T-T544A decreased at 3 dpi, with some animals reaching a normal temperature at 4 dpi. Four days postinoculation, the temperature of the group infected with rHuN4-S519T-T544A rose moderately and then decreased again to normal levels 14 dpi. There was no obvious temperature difference between rCH-1a- and rCH-1a-T519S-A544T-infected piglets, and no body temperature changes were observed in the control group during the experiment period. The clinical symptoms of the infected piglets were also monitored and scored. Compared with the rHuN4-infected group, rHuN4-S519T-T544A-infected piglets showed a moderate and slower disease progression (Fig. 8B), exhibiting only a transient fever and milder respiratory symptoms with lower clinical symptom scores than the rHuN4-infected group. No obvious clinical signs were observed in the rCH-1a- and rCH-1a-T519S-A544T-infected groups during the entire experiment, and their scores were similar to those of the control group.
FIG 8.
Measures of pathology in piglets inoculated with mutant and parental virus strains. (A) Body temperatures of piglets inoculated with rHuN4-S519T-T544A and rCH-T519S-A544T and the parental viruses presented as means and standard deviations. (B) Total scores of clinical signs. (C) WGR between the beginning and end of the experiment. (D) Thymic atrophy of piglets infected with mutant and parental viruses. *, significant differences in weight gain rate and thymic atrophy between mutant and parental viruses (*, P < 0.05; **, P < 0.01).
The body weights of all the piglets were measured at 0 dpi and 21 dpi to calculate the weight gain rate (WGR) (weight gain/primary weight). All of the groups, except for the rCH-1a-infected group, had a significantly lower WGR than the uninfected control group (P < 0.01 and P < 0.05) (Fig. 8C). However, the rCH-1a-infected group had a significantly higher WGR than the rCH-1a-T519S-A544T-infected group (P < 0.05). Piglets in the rHuN4-infected group showed a trend similar to that shown in the rHuN4-S519T-T544A-infected group.
We also examined the thymuses of infected animals, demonstrating that the thymuses of rHuN4-infected piglets were reduced in size compared to thymuses from piglets infected with rHuN4-S519T-T544A. For the rHuN4- and rHuN4-S519T-T544A-infected piglets, the average thymus weight/body weight ratios were 0.8 and 0.94, respectively. In contrast, the average thymus weight/body weight ratios of piglets infected with rCH-1a-T519S-A544T and rCH-1a were 1.24 and 1.31, respectively. The thymus weight/body weight ratios of rHuN4 and rHuN4-S519T-T544A were significantly lower than those of the infected group (Fig. 8D) (P < 0.01 and P < 0.05).
Through examination of macroscopic lesions, we found that the lungs of rHuN4-infected piglets were more severely damaged than those of piglets infected with rHuN4-S519T-T544A. These animals were all characterized by consolidation and some edema, but the lungs infected with rHuN4-S519T-T544A had fewer lesions than those infected with rHuN4 (Fig. 9A, B, and C). The lungs of rHuN4-infected piglets also exhibited more severe histopathological changes that were characterized by an obvious thickening of the interlobular septum, necrotic debris infiltration within the alveolar spaces, and a large number of inflammatory cells (Fig. 9D). Conversely, the lungs of rHuN4-S519T-T544A-infected piglets displayed alleviated histopathological changes, with some slight microscopic lesions of the lungs, including slight thickening of the interlobular septum and small regions containing inflammatory cells in the alveolar spaces (Fig. 9E). The lungs of rCH-1a- and rCH-1a-T519S-T544A-infected piglets were similar to those of the uninfected group (data not shown). The average histopathological scores of the rHuN4-S519T-T544A and rHuN4 groups showed significant differences (P < 0.05) (Fig. 9G).
FIG 9.
Macroscopic lung lesions and microscopic lesion scores. (A to C) Macroscopic lesions in lungs of piglets infected with rHuN4 and rHuN4-S519T-T544A compared to uninfected controls. (D to F) Microscopic analysis of lungs infected with rHuN4 and rHuN4-S519T-T544A versus uninfected controls. (G) Mean scores for the abundance of microscopic lung lesions. *, significant difference in the scores of microscopic lung lesions in piglets infected with mutant and parental viruses (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
The identified amino acid mutations affected the in vivo replication efficiency of PRRSV.
We also determined the viral loads in the sera of infected pigs using real-time quantitative RT-PCR (qRT-PCR) (Fig. 10A). This analysis showed no significant differences in the viral loads of the rHuN4-S519T-T544A-infected group during the early time points. At 7 dpi, the viral RNA copy numbers in the sera of the pigs infected with rHuN4 were higher than those in pigs infected with rHuN4-S519T-T544A. Additionally, there was a marked reduction in viral loads in the rHuN4-S519T-T544A-infected group relative to the rHuN4-infected group at 14 and 21 dpi (P < 0.05 and P < 0.01). In contrast, the viral loads in the sera of piglets infected with rCH-1a-T519S-A544T and rCH-1a were low at most time points during the experiment. However, the rCH-1a-T519S-A544T viral loads were significantly higher than those of the rCH-1a parental strain at 21 dpi (P < 0.05), and the viral loads at 7 and 14 dpi were also higher than those for rCH-1a. Because qRT-PCR does not distinguish between viable and nonviable forms of the virus, the infectious-virus titer was measured by a microtitration assay using Marc-145 cells to further quantify infectious virus particles in serum samples. At 7 and 14 dpi, infectious-virus titers in groups of pigs infected with rHuN4-S519T-T544A were ∼1 log unit lower than those in pigs infected with the parent virus, and the difference was significant (P < 0.05) (Fig. 10B). Additionally, at 7 dpi, infectious-virus titers in groups of pigs infected with rCH-1A-T519S-A544T were higher than those in pigs infected with rCH-1a, and the difference was significant (P < 0.05) (Fig. 10B). Regardless of whether the virus copy number or virus titer was measured in the sera of infected pigs, the replication ability of rHuN4-S519T-T544A was lower than that of rHuN4 and the replication ability of rCH-1a-T519S-A554T was higher than that of rCH-1a. These data further demonstrate that the two amino acid mutations in PRRSV NSP9 are involved in in vivo virus replication efficiency. In addition, to explore the genetic stability of mutant viruses in pigs, the sera of PRRSV-infected piglets at 3, 7, 14, and 21 dpi was applied to Marc-145 cells, followed by plaque purification, where 20 individual plaques for each serum were randomly selected and the region harboring mutations in NSP9 was amplified by RT-PCR. The PCR products were purified and cloned into the pMD18-T vector and then sent for Sanger sequencing. The alignment report showed that no reversion occurred at positions 519 and 544 in the viruses isolated from sera from rHuN4-S519T-T544A- and rCH-1a-T519S-A544T-infected piglets.
FIG 10.
Comparison of viral loads in serum samples. (A) Viral loads in serum samples were quantified by qRT-PCR and calculated as viral RNA copies per milliliter. (B) Infectious-virus titers in serum samples were determined by a microtitration assay and calculated as log TCID50 per milliliter. The statistical significance of differences between wild-type-virus-infected groups and mutant-virus-infected groups was determined using Student's t test (*, P < 0.05; **, P < 0.01).
DISCUSSION
PRRSV is an important global disease that causes significant economic loss to the world pig production industry. In China, a more pathogenic form of the disease, HP-PRRS, has resulted in several expensive outbreaks in the Chinese pig industry since its emergence in 2006 (13). Recent evidence has revealed that HP-PRRSV originated from a Chinese CH-1a-like PRRSV strain (14) and has also shown that the replicative capacity of HP-PRRSV is higher than that of classical PRRSV isolates (19). However, the exact mechanisms that contribute to the increased pathogenicity and replicative capacity of HP-PRRSV are controversial and remain unclear. Additionally, most studies investigating HP-PRRSV have focused on large fragment substitutions, and until the present study, there has been no analysis of amino acid mutations that are associated with the increased replication and pathogenicity of HP-PRRSV.
Our bioinformatics analysis of all full-length sequences of NA-type PRRSVs revealed only three conserved amino acid differences between HP-PRRSVs and classical PRRSVs: two were located at amino acid positions 519 and 544 of NSP9, and one was located at amino acid position 408 of NSP10. The amino acids at positions 544 of NSP9 and 408 of NSP10 were also described in a previous study (21), in which some other amino acid differences were also found in NSP9 and NSP10 when aligning JXwn06, a highly pathogenic PRRS virus strain, HB-1/3.1, a low-pathogenic PRRSV strain, and about 30 representative sequences (21). In the present study, all of the available full-length NA-type PRRSV sequences (n = 204) were retrieved from GenBank in December 2014. The amino acid sequences of each of the ORFs from the 204 PRRSV strains were aligned, revealing extensive mutations in most NSPs and structural proteins; only 2 amino acids in NSP9 (positions 519 and 544) and 1 amino acid (position 408) in NSP10 were mutated under regular conditions. Some representative EU-type PRRSVs were also aligned, together with the NA-type PRRSVs. The homology between EU-type PRRSVs and NA-type PRRSVs is about 60%. The amino acid at position 519 was T in EU-type PRRSVs, which was identical to the NA-type PRRSVs with lower pathogenicity, but the amino acid at position 544 was quite different from that in NA-type PRRSVs. Considering these findings, i.e., that the NSP9 (viral RNA-dependent RNA polymerase [RdRp]) of HP-PRRSV is associated with viral replication and the fidelity of the viral genome, we focused on the mutations in NSP9. The two mutations in NSP9 proteins of different subgroups of PRRSV were hierarchical to some degree. For example, all of the HP-PRRSVs had S at position 519, whereas this amino acid was T in all of the lower-pathogenicity PRRSVs (including VR-2332-like PRRSVs and CH-1a-like PRRSVs), and the moderate-pathogenicity PRRSVs (NADC30/MN184-like PRRSVs) possessed both types of traits. The same situation was also observed at position 544. Therefore, we selected the mutations in NSP9 as the preferred research target. The effects of the mutation in NSP10 will be investigated in the future. Previous work has shown that HP-PRRSV can be attenuated by deoptimizing the codon pair bias in the RdRp of PRRSV, demonstrating that changing codon bias can alter the virulence and replication capacity of PRRSV (22). However, the mechanism by which this leads to attenuation is unclear. There is also some evidence to suggest that amino acid residues Ala283 and His421 in PRRSV NSP9 play important roles in replication by affecting RdRp fidelity (23), although these residues were not found to be involved in increasing PRRSV virulence and replication ability. In contrast, our data indicate that rHuN4-S519T-T544A, rHuN4-S519T, and rHuN4-T544A (with substitutions to make them more like classical PRRSV) all had reduced replication efficiency compared to the parental HP-PRRSV HuN4 in both Marc-145 cells and PAMs. Mutant viruses rCH-1a-T519S-A544T, rCH-1a-T519S, and rCH-1a-A544T (with substitutions to make them more like virulent HP-PRRSV) showed enhanced replication relative to the classical PRRSV strain rCH-1a in Marc-145 cells and PAMs. Based on these results, the 2 amino acids we have identified have a clear effect on viral replication efficiency in vitro. Supporting this conclusion, we found that rHuN4 was more pathogenic in vivo than rHuN4-S519T-T544A, whereas rCH-1a was less virulent than rCH-1a-T519S-A544T. Additionally, mutant viruses that included one or two swapped amino acids were able to be successfully rescued, whereas deletion of either of the two identified amino acids led to a loss of viable virus. This suggests that the 2 amino acids in NSP9 are essential for PRRSV rescue.
Our predictive-modeling analysis suggested that the effect that the 2 amino acids have on PRRSV replication efficiency may be due to alteration of the spatial structure of RdRp. Both of the amino acids are located in the C terminus of RdRp, and position T544 in particular was predicted to lie in the motif E region. In addition, the R groups of the mutant amino acids are different, resulting in alterations to the protein helices and folding. A change to this R group can also result in altered hydrogen and disulfide bonding, further affecting protein structure. Each of these small structural variations can influence the overall structure of RdRp, potentially decreasing its activity. One of several important functions of RdRp is to make minus strands of RNA virus. The synthesis of negative-strand RNA of NSP9 mutant viruses and of parent HuN4 or CH-1a viruses was detected and compared in our study. The results showed that the functions of the RdRp to make minus strands were affected after mutation, which suggests that the 2 amino acids are important for the function of the RdRp. Furthermore, in a previous study, the binding of PRRSV NSP9 and N, together with cellular DHX9 protein, was found to regulate viral RNA synthesis. It was also confirmed that the 599-to-646 amino acid fragment of the C terminus in NSP9 constituted the N protein-binding domain (24). The mutated amino acids in our study (519 and 544) were not in the area of the C terminus, and the results for the synthesis of negative-strand RNA of NSP9 mutant viruses and parent HuN4 or CH-1a viruses revealed that mutation of these amino acids may influence viral replication by influencing the synthesis of negative-strand RNA. In the present study, the changes to the replicative capacities of double mutants (rHuN4-S519T-T544A or rCH-1a-T519S-A544T) were not the sum of the changes observed for individually substituted amino acids. The reasons underlying this ambiguity will require further investigation.
In summary, our study has revealed two consistent amino acid mutations at positions 519 and 544 of NSP9 between HP-PRRSVs and classical PRRSVs. Either of these two amino acids was necessary for the rescue of PRRSV, and both were critical to the replication efficiency of HP-PRRSV and contributed to enhanced pathogenicity. These findings will help to explain the replication and pathogenicity of PRRSV and may lead to improved therapeutic and prognostic approaches to combat the disease in the global pig production industry.
MATERIALS AND METHODS
Cells, antibodies, and viruses.
Marc-145 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) (Gibco, Thermo Fisher Scientific, Waltham, MA, USA), 100 μg/ml streptomycin, and 100 IU/ml penicillin. The cells were maintained at 37°C with 5% CO2. PAMs were obtained from lung lavage samples from 4-week-old specific-pathogen-free (SPF) pigs. The PAMs were cultured in RPMI 1640 medium supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 mg/ml). An anti-PRRSV N protein monoclonal antibody (MAb) labeled with fluorescein isothiocyanate (FITC)-SR30 (RTI LLC, Brookings, SD, USA) was used to detect PRRSVs. HP-PRRSV strain HuN4 (GenBank accession no. EF635006) and the classical PRRSV CH-1a strain (GenBank accession no. AY032626) were used for all subsequent experiments.
Bioinformatics analysis of PRRSV sequences.
All of the currently available full-length NA-type PRRSV sequences (n = 204) were retrieved from GenBank in December 2014. The amino acid sequences of each of the ORFs from the 204 PRRSV strains were aligned using Lasergene software (DNAStar Inc., Madison, WI, USA), and a phylogenetic tree based on the full-length genomic sequences was generated using a distance-based neighbor-joining method with MEGA 7.2 (25). Bootstrap values were calculated using 1,000 replicates. The classifications of subgroups followed those described in previous studies (14–17, 26). To analyze the positions of the two consistently identified amino acid mutations in the spatial structure of NSP9, we predicted the three-dimensional structure of the protein using the I-TASSER online tool (http://zhanglab.ccmb.med.umich.edu/I-TASSER/), as previously described (24), as there is no direct structural information on any of the nidovirus RdRps (27). Representative NA- and EU-type PRRSV strains were also aligned, and their secondary structures and motifs were predicted using I-TASSER.
Construction of infectious cDNA clones of HP-PRRSV HuN4 and classical PRRSV CH-1a.
The viral RNA of HuN4 and CH-1a was extracted and used as a template for cDNA synthesis, according to the method of our previous study (28). In total, 12 different PCR primers were used to amplify six overlapping fragments spanning the entire genomes of HuN4 and CH-1a (Table 2). These fragments were then assembled into the pOK12-m vector (modified in our laboratory through the addition of a eukaryotic promoter from the pCAGGS vector). This led to two full-length infectious cDNA clones, pHuN4 and pCH-1a. Next, 4 μg each of pHuN4 and pCH-1a was transfected into Marc-145 cells using X-treme Gene HP DNA reagent (Roche Applied Science, Penzberg, Germany) according to the manufacturer's instructions. At 96 h posttransfection, viral infection was confirmed using immunofluorescent staining with MAb SR-30 and Western blot analysis using protocols from a previous study (29). To distinguish between wild-type virus and rescued virus, a unique restriction site for the enzyme MluI was introduced into the full-length infectious clones. This site does not exist in the genome of the wild-type virus and was used as a genetic marker by digesting PCR products with MluI.
TABLE 2.
Primers used in the study
| Primer name | Sequence (5′-3′) | Positions | Application |
|---|---|---|---|
| 5UTR-F | ATGCTCGAGTGTTAAGCGTCTGATGAGTCCGTGAGGACGAAACTATAGGAAAGGAATTCTATAGTCATGACGTATAGGTGTTG | 1–17 | CH-1a and HuN4 clone |
| 5UTR-R | CCCGCGGCCGCAAACACCCTGGCATTGG | 224–251 | CH-1a and HuN4 clone |
| C-A-NotI-F | TTATGGCGGCCGCCCAAGTCT | 240–260 | CH-1a clone |
| C-A-FseI-R | ACTGGGGCCGGCCCACTCAAA | 2890–2910 | CH-1a clone |
| C-B-FseI-F | CCTAGGCCAGTGACACCT | 2873–2890 | CH-1a clone |
| C-B-NheI-R | GCTGGCGGCTAGCAGTTT | 7693–7710 | CH-1a clone |
| C-C-NheI-F | GGAGCAGTGTTTAAACTG | 7681–7698 | CH-1a clone |
| C-C-AscI-R | CTGGTGGCAGTAGCCTTA | 11997–12014 | CH-1a clone |
| C-D-AscI-F | TTCGGGCGCGCCAGAAAG | 11969–11986 | CH-1a clone |
| C-D-MluI-R | GACGACAAACGCGTGGTT | 14763–14780 | CH-1a clone |
| H-A-NotI-F | GTGGCGGCCGCCCAGGTC | 241–258 | HuN4 clone |
| H-A-FseI-R | ACTGGGGCCGGCCCACTC | 2889–2906 | HuN4 clone |
| H-B-FseI-F | GAGTGGGCCGGCCCCAGT | 2889–2906 | HuN4 clone |
| H-B-NheI-R | GCCGCTGGCGGCTAGCAG | 7605–7622 | HuN4 clone |
| H-C-NheI-F | CCAGGGCCTGACTAAGGA | 7575–7592 | HuN4 clone |
| H-C-AscI-R | CCTCATGCTGGTGGCATT | 11913–11930 | HuN4 clone |
| H-D-AscI-F | ACAATGATGCGTTTCGGG | 11866–11883 | HuN4 clone |
| H-D-MluI-R | GACGACAAACGCGTGGTT | 14628–14689 | HuN4 clone |
| N + 3UTR-F | TTTACGCGTTTGTCGTCCGGCGTC | 14673–14696 | CH-1a and HuN4 clone |
| N + 3UTR-R | CTTTAATTAACGCCCTCCCTTAGCCATCCGAGTGGACGTGCGTCCTCCTTCGGATGCGGAGGTGGAGATGCCATGCCGACCCTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTAATTACGGCCGCATGGTTCTC | 15300–15352 | CH-1a and HuN4 clone |
| MluI-F | ACGCCAGTGATGATATATGC | 14454–14473 | Enzyme digestion |
| MluI-R | GACAAGGTTTACCACTCCCTG | 14865–14885 | Enzyme digestion |
| NSP9-CH-TS-F | TGTATGCCGAGTCTCCCTCCATGCCAAAC | 9227–9255 | PCR mutagenesis |
| NSP9-CH-TS-R | GTTTGGCATGGAGGGAGACTCGGCATACA | 9227–9255 | PCR mutagenesis |
| NSP9-HuN-ST-F | TGTATGCCGAGTCTCCCACCATGCCAAAC | 9136–9164 | PCR mutagenesis |
| NSP9-HuN-ST-R | GTTTGGCATGGTGGGAGACTCGGCATACA | 9136–9164 | PCR mutagenesis |
| NSP9-CH-AT-F | GACCCAAAGAAGACAACCATAACAGACTC | 9304–9332 | PCR mutagenesis |
| NSP9-CH-AT-R | GAGTCTGTTATGGTTGTCTTCTTTGGGTC | 9304–9332 | PCR mutagenesis |
| NSP9-HuN-TA-F | GACCCAAAGAAGACAGCCATCACAGACTC | 9213–9241 | PCR mutagenesis |
| NSP9-HuN-TA-R | GAGTCTGTGATGGCTGTCTTCTTTGGGTC | 9213–9241 | PCR mutagenesis |
| NSP9-HuNΔS-F | GTATGCCGAGTCTCCCATGCCAAACTACCACTGGTGGGTTG | 9137–9180 | PCR mutagenesis |
| NSP9-HuNΔS-R | CAACCCACCAGTGGTAGTTTGGCATGGGAGACTCGGCATAC | 9137–9180 | PCR mutagenesis |
| NSP9-HuNΔT-F | GACGGACCCAAAGAAGACAATCACAGACTCACCATCATTC | 9209–9251 | PCR mutagenesis |
| NSP9-HuNΔT-R | GAATGATGGTGAGTCTGTGATTGTCTTCTTTGGGTCCGTC | 9209–9251 | PCR mutagenesis |
| NSP9-CHΔT-F | GTATGCCGAGTCTCCCATGCCAAACCACCACTGGTGGGTTG | 9228–9271 | PCR mutagenesis |
| NSP9-CHΔT-R | CAACCCACCAGTGGTGGTTTGGCATGGGAGACTCGGCATAC | 9228–9271 | PCR mutagenesis |
| NSP9-CHΔA-F | GACCCAAAGAAGACAATAACAGACTCGCCATCATTTC | 9304–9343 | PCR mutagenesis |
| NSP9-CHΔA-R | GAAATGATGGCGAGTCTGTTATTGTCTTCTTTGGGTC | 9304–9343 | PCR mutagenesis |
| PNP-F | CCCTAGTGAGCGGCAATTGT | 15002–15021 | qRT-PCR primer |
| PNP-R | ATCCTCCCTGAATCTGAC | 15074–15091 | qRT-PCR primer |
| CH-1a-SF-6 | GTATAGGTGTTGGCTCTATGC | 6–26 | Negative-strand gRNA analysis |
| CH-1a-SF-683 | GGAGCGGTAAGTTGGTTAACACA | 661–683 | Negative-strand gRNA analysis |
| CH-1a-SF-12 | GTGTTGGCTCTATGCCACGAC | 12–32 | Negative-strand gRNA analysis |
| CH-1a-SF-343 | ATAAAATAGGCCCAGCACCCC | 323–343 | Negative-strand gRNA analysis |
| HuN4-SF-6 | GTATAGGTGTTGGCTCTATGC | 6–26 | Negative-strand gRNA analysis |
| HuN4-SF-683 | GGGAGCGGTAAGTTGGTTAACAC | 661–683 | Negative-strand gRNA analysis |
| HuN4-SF-12 | GTGTTGGCTCTATGCCACGGC | 12–32 | Negative-strand gRNA analysis |
| HuN4-SF-343 | TATAAAATAGACCCAGCACCC | 323–343 | Negative-strand gRNA analysis |
| SFactin | CTTCCTGGGCATGGAGTCC | β-Actin analysis | |
| SRactin | GGCGCGATGATCTTGATCTTC | β-Actin analysis |
Construction of mutant viruses.
The amino acid residues at positions 519 and 544 of NSP9 in the HuN4 and CH-1a strains were swapped and deleted individually by site-directed mutation using a QuikChange mutagenesis kit (Agilent Technologies, Santa Clara, CA, USA). All viruses that included an amino acid mutation and deletion were generated using the C fragment from an NheI and AscI digestion. All of these mutant C fragments were then assembled into the backbones of the pHuN4 and pCH-1a constructs. Four plasmids (pHuN4-S519T, pHuN4-T544A, pHuN4-△519S, and pHuN4-△544T) were constructed based on pHuN4, and four plasmids (pCH-1a-T519S, pCH-1a-A544T, pCH-1a-△519T, and pCH-1a-△544A) were based on pCH-1a. In addition, pHuN4-S519T-T544A and pCH-1a-T519S-A544T constructs with a double mutation in HuN4 and CH-1a were also generated. These plasmids were validated by DNA sequencing and transfected into Marc-145 cells as previously described. Mutant viruses and the infectious cDNA clone virus were then recovered and examined using the previously outlined method. All viruses were passaged once in Marc-145 cells and harvested by repeated freezing and thawing. They were then titrated and stored at −80°C.
Growth kinetics of mutant viruses in Marc-145 cells.
To investigate the replication of mutant viruses and their parental strains, Marc-145 cells were grown in 6-well plates to 80% confluence and then infected with mutant and parent viruses at a multiplicity of infection (MOI) of 0.01. After inoculation for 1 h at 37°C, the supernatants were discarded and the cells were washed with phosphate-buffered saline (PBS) three times. Fresh DMEM with 2% FBS was then added. The cells were harvested at 0, 24, 48, 72, and 96 hpi, and total RNA was extracted using an RNeasy minikit (Qiagen, Hilden, Germany) following the manufacturer's instructions. Reverse transcription reactions were performed at 2°C for 10 min and at 42°C for 1 h using the Moloney murine leukemia virus (M-MLV) reverse transcription polymerase system (TaKaRa, Dalian, China). The viral copy numbers at different time points were determined using qRT-PCR and protocols from a previous study (28).
Growth kinetics of mutant viruses in PAMs.
To further investigate the replication of mutant viruses in PAMs, cells were cultured in 6-well plates containing approximately 105 cells per well. After culturing for 12 h, the supernatant was removed and the PAMs were infected with mutant or parental viruses at an MOI of 0.01. Cells were then harvested at 0, 24, 48, 72, and 96 hpi. Total RNA was extracted, and the viral copy number was assessed as previously described.
Viral plaque assays.
Mutant and parental viruses were used to infect Marc-145 cell monolayers in 6-well plates at an MOI of 0.01. After incubation for 1 h at 37°C, the supernatants were discarded, and the cells were washed three times using PBS. The cell monolayer was then overlaid with 2× minimal essential medium (MEM) containing 4% FBS mixed with an equal volume of 2% low-melting-point agarose. The plate was then inverted and incubated for 96 h. The cells were fixed using 4% polyoxymethylene for 2 h at room temperature, and the agarose was removed. The resulting plaques were stained with crystal violet (5% [wt/vol] in 20% ethanol) (30).
Detection of negative-strand gRNA strands by RT-PCR.
PAM cells in 6-well plates were infected with the mutant viruses and their parent viruses at the same MOI (0.01) for analysis of viral negative-strand gRNA synthesis using methods and primers described previously (31). Briefly, total cellular RNA was extracted at 24 hpi and further treated with DNase I. The first-strand cDNA was synthesized, and the resultant cDNA was treated with RNase A to remove the remaining RNA. Then, nested PCR with internal primers HuN4-SF-6 and HuN4-SF-683 (CH-1a-SF-6/CH-1a-SF-683) was performed to detect the negative-strand gRNA (Table 2). As an internal control, the mRNA of β-actin was also amplified from the same RNA samples using the primers actin-F and actin-R. Finally, the gray values [the density ratio of the band (−)gRNA/β-actin] for the PCR products were measured with Image J software.
In vivo assessment of the virulence of mutant virus in piglets.
The animal experiments in this study were conducted based on the recommendations in the Chinese Regulations on Laboratory Animals, the Guidelines for the Care of Laboratory Animals (Ministry of Science and Technology of the People's Republic of China), and the Laboratory Animal Requirements of Environment and Housing Facilities (GB14925-2010; National Laboratory Animal Standardization Technical Committee). In total, 25 5-week-old SPF piglets were randomly divided into four test groups (n = 5/group) and a control group (n = 5). The five groups were separated into different rooms at the experimental facility. The piglets in the first test group (no. 441 to 445) were infected with rHuN4-T519S-A544T, those in the second test group (no. 451 to 455) were infected with rCH-1a-S519T-T544A, and those in the third test group (no. 446 to 450) and fourth test group (no. 456 to 460) were infected with rCH-1a and rHuN4, respectively. Each piglet in the test groups was inoculated intranasally with a 2 ml of solution containing 1 × 105 50% tissue culture infective doses (TCID50) and intramuscularly with 2 ml of solution containing 1 × 105 TCID50 virus. The piglets in the control group (no. 351 to 356) were sham infected with DMEM. The clinical symptoms of each piglet were monitored and scored on each test day. Sera were collected from individual pigs at 0, 3, 7, 10, 14, and 21 dpi and stored at −80°C. All the animals were euthanized 21 dpi. Tissue samples were obtained from the lungs, lymphoid nodes (submandibular, mesenteric, and superficial inguinal), heart, liver, kidneys, spleen, and tonsils for further detection and histopathological examination. Additionally, the thymus from each pig was dissected during a necropsy and weighed. The thymus atrophy level was evaluated by the changes in the weight of the thymus, as described previously (32).
Real-time RT-PCR quantification of viral RNA copies in the sera of infected piglets.
To explore the replication rates of mutant viruses and their parental strains in infected piglets, viral RNAs in the sera were collected and extracted at 0, 3, 7, 14, and 21 dpi. Subsequently, the viral RNA copy number was determined using qRT-PCR following the previously described method.
Virus titration.
The parent viruses and the mutant viruses at passage 3 were used to characterize virus growth properties in vitro. Confluent Marc-145 cells and PAMs were inoculated with the parent virus or the mutants at an MOI of 0.01. The cell culture supernatant was harvested at 0, 24, 48, 72, and 96 hpi. The virus titers were measured by a microtitration assay using Marc-145 cells in 96-well plates and calculated as TCID50 per milliliter according to the method of Reed and Muench (33). The sera from infected piglets were also probed by the same method.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism 6 (GraphPad, La Jolla, CA, USA). The measured values are expressed as means with standard deviations (SD). Significance was assessed using Student's t test. A P value of <0.05 was considered significant.
ACKNOWLEDGMENTS
We thank Hong-Ying Chen and Long Liu (Northwest A&F University, Yangling, China) for assistance in predicting the structure of PRRSV RdRp.
The study was supported by grants from the National Natural Science Foundation of China (31270045), the Foundation for Science and Technology Innovative Talents of Harbin (2016RAQXJ142), the Heilongjiang Natural Science Fund for Distinguished Young Scholars (JC2017010), and the Foundation for Basic Scientific Research of Central Public Research Institutes (Y2017JC16). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Ng KK, Arnold JJ, Cameron CE. 2008. Structure-function relationships among RNA-dependent RNA polymerases. Curr Top Microbiol Immunol 320:137–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonald SM. 2013. RNA synthetic mechanisms employed by diverse families of RNA viruses. Wiley Interdiscip Rev RNA 4:351–367. doi: 10.1002/wrna.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sexton NR, Smith EC, Blanc H, Vignuzzi M, Peersen OB, Denison MR. 2016. Homology-based identification of a mutation in the coronavirus RNA-dependent RNA polymerase that confers resistance to multiple mutagens. J Virol 90:7415–7428. doi: 10.1128/JVI.00080-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albina E. 1997. Epidemiology of porcine reproductive and respiratory syndrome (PRRS): an overview. Vet Microbiol 55:309–316. doi: 10.1016/S0378-1135(96)01322-3. [DOI] [PubMed] [Google Scholar]
- 5.Fang Y, Treffers EE, Li Y, Tas A, Sun Z, van der Meer Y, de Ru AH, van Veelen PA, Atkins JF, Snijder EJ, Firth AE. 2012. Efficient −2 frameshifting by mammalian ribosomes to synthesize an additional arterivirus protein. Proc Natl Acad Sci U S A 109:E2920–E2928. doi: 10.1073/pnas.1211145109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han M, Yoo D. 2014. Engineering the PRRS virus genome: updates and perspectives. Vet Microbiol 174:279–295. doi: 10.1016/j.vetmic.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knoops K, Bárcena M, Limpens RW, Koster AJ, Mommaas AM, Snijder EJ. 2012. Ultrastructural characterization of arterivirus replication structures: reshaping the endoplasmic reticulum to accommodate viral RNA synthesis. J Virol 86:2474–2487. doi: 10.1128/JVI.06677-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang Y, Snijder EJ. 2010. The PRRSV replicase: exploring the multifunctionality of an intriguing set of nonstructural proteins. Virus Res 154:61–76. doi: 10.1016/j.virusres.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bøtner A, Nielsen J, Bille-Hansen V. 1994. Isolation of porcine reproductive and respiratory syndrome (PRRS) virus in a Danish swine herd and experimental infection of pregnant gilts with the virus. Vet Microbiol 40:351–360. doi: 10.1016/0378-1135(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 10.Baron T, Albina E, Leforban Y, Madec F, Guilmoto H, Plana Duran J, Vannier P. 1992. Report on the first outbreaks of the porcine reproductive and respiratory syndrome (PRRS) in France. Diagnosis and viral isolation. Ann Rech Vet 23:161–166. [PubMed] [Google Scholar]
- 11.Kuwahara H, Nunoya T, Tajima M, Kato A, Samejima T. 1994. An outbreak of porcine reproductive and respiratory syndrome in Japan. J Vet Med Sci 56:901–909. doi: 10.1292/jvms.56.901. [DOI] [PubMed] [Google Scholar]
- 12.Guo BQ, Chen ZS, Liu WX, Cui YZ. 1996. Porcine reproductive and respiratory syndrome virus was isolated from abortive fetus of suspected PRRS. Chinese J Anim Poultry Infect Dis 87:1–5. (In Chinese.) [Google Scholar]
- 13.Tian K, Yu X, Zhao T, Feng Y, Cao Z, Wang C, Hu Y, Chen X, Hu D, Tian X, Liu D, Zhang S, Deng X, Ding Y, Yang L, Zhang Y, Xiao H, Qiao M, Wang B, Hou L, Wang X, Yang X, Kang L, Sun M, Jin P, Wang S, Kitamura Y, Yan J, Gao GF. 2007. Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS One 2:e526. doi: 10.1371/journal.pone.0000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.An TQ, Tian ZJ, Xiao Y, Li R, Peng JM, Wei TC, Zhang Y, Zhou YJ, Tong GZ. 2010. Origin of highly pathogenic porcine reproductive and respiratory syndrome virus, China. Emerg Infect Dis 16:365–367. doi: 10.3201/eid1602.090005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C, Zhuang J, Wang J, Han L, Sun Z, Xiao Y, Ji G, Li Y, Tan F, Li X, Tian K. 2016. Outbreak investigation of NADC30-like PRRSV in south-east China. Transbound Emerg Dis 63:474–479. doi: 10.1111/tbed.12530. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Bao H, Wang Y, Tian K. 2017. Wide spread of NADC30-like PRRSV in China: another Pandora's box for Chinese pig industry as the outbreak of highly pathogenic PRRSV in 2006? Infect Genet Evol 49:12–13. doi: 10.1016/j.meegid.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Zhao K, Ye C, Chang XB, Jiang CG, Wang SJ, Cai XH, Tong GZ, Tian ZJ, Shi M, An TQ. 2015. Importation and recombination are responsible for the latest emergence of highly pathogenic porcine reproductive and respiratory syndrome virus in China. J Virol 89:10712–10716. doi: 10.1128/JVI.01446-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou L, Wang Z, Ding Y, Ge X, Guo X, Yang H. 2015. NADC30-like strain of porcine reproductive and respiratory syndrome virus, China. Emerg Infect Dis 21:2256–2257. doi: 10.3201/eid2112.150360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han Z, Liu Y, Wang G, He Y, Hu S, Li Y, Shi W, Wu J, Wang S, Liu H, Cai X. 2015. Comparative analysis of immune responses in pigs to high and low pathogenic porcine reproductive and respiratory syndrome viruses isolated in China. Transbound Emerg Dis 62:e1–e10. doi: 10.1111/tbed.12190. [DOI] [PubMed] [Google Scholar]
- 20.Zhou L, Zhang J, Zeng J, Yin S, Li Y, Zheng L, Guo X, Ge X, Yang H. 2009. The 30-amino-acid deletion in the Nsp2 of highly pathogenic porcine reproductive and respiratory syndrome virus emerging in China is not related to its virulence. J Virol 83:5156–5167. doi: 10.1128/JVI.02678-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Zhou L, Zhang J, Ge X, Zhou R, Zheng H, Geng G, Guo X, Yang H. 2014. Nsp9 and Nsp10 contribute to the fatal virulence of highly pathogenic porcine reproductive and respiratory syndrome virus emerging in China. PLoS Pathog 10:e1004216. doi: 10.1371/journal.ppat.1004216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao L, Wang L, Huang C, Yang L, Guo XK, Yu Z, Liu Y, Yang P, Feng WH. 2015. HP-PRRSV is attenuated by de-optimization of codon pair bias in its RNA-dependent RNA polymerase nsp9 gene. Virology 485:135–144. doi: 10.1016/j.virol.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Tian D, Meng XJ. 2016. Amino acid residues Ala283 and His421 in the RNA-dependent RNA polymerase of porcine reproductive and respiratory syndrome virus play important roles in viral ribavirin sensitivity and quasispecies diversity. J Gen Virol 97:53–59. doi: 10.1099/jgv.0.000316. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Tian J, Nan H, Tian M, Li Y, Xu X, Huang B, Zhou E, Hiscox JA, Chen H. 2016. Porcine reproductive and respiratory syndrome virus nucleocapsid protein interacts with Nsp9 and cellular DHX9 to regulate viral RNA synthesis. J Virol 90:5384–5398. doi: 10.1128/JVI.03216-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q, Bai J, Hou H, Song Z, Zhao Y, Jiang P. 2017. A novel recombinant porcine reproductive and respiratory syndrome virus with significant variation in cell adaption and pathogenicity. Vet Microbiol 208:150–158. doi: 10.1016/j.vetmic.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 27.Posthuma CC, Te Velthuis AJW, Snijder EJ. 2017. Nidovirus RNA polymerases: complex enzymes handling exceptional RNA genomes. Virus Res 234:58–73. doi: 10.1016/j.virusres.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei TC, Tian ZJ, An TQ, Zhou YJ, Xiao Y, Jiang YF, Zhang SR, Peng JM, Qiu HJ, Tong GZ. 2008. Development and application of Taq Man-MGB fluorescence quantitative RT-PCR for detection of porcine reproductive and respiratory syndrome virus. Chinese J Prev Vet Med 30:12 (In Chinese.) [Google Scholar]
- 29.Gao F, Qu Z, Li L, Yu L, Jiang Y, Zhou Y, Yang S, Zheng H, Huang Q, Tong W, Tong G. 2016. Recombinant porcine reproductive and respiratory syndrome virus expressing luciferase genes provide a new indication of viral propagation in both permissive and target cells. Res Vet Sci 107:132–140. doi: 10.1016/j.rvsc.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Zheng H, Sun Z, Zhu XQ, Long J, Lu J, Lv J, Yuan S. 2010. Recombinant PRRSV expressing porcine circovirus sequence reveals novel aspect of transcriptional control of porcine arterivirus. Virus Res 148:8–16. doi: 10.1016/j.virusres.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu J, Gao F, Wei Z, Liu P, Liu C, Zheng H, Li Y, Lin T, Yuan S. 2011. A 5′-proximal stem-loop structure of 5′ untranslated region of porcine reproductive and respiratory syndrome virus genome is key for virus replication. Virol J 8:172. doi: 10.1186/1743-422X-8-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He Y, Wang G, Liu Y, Shi W, Han Z, Wu J, Jiang C, Wang S, Hu S, Wen H, Dong J, Liu H, Cai X. 2012. Characterization of thymus atrophy in piglets infected with highly pathogenic porcine reproductive and respiratory syndrome virus. Vet Microbiol 160:455–462. doi: 10.1016/j.vetmic.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Shyu D-L, Shang P, Bai J, Ouyang K, Dhakal S, Hiremath J, Binjawadagi B, Renukaradhya GJ, Fang Y. 2016. Mutations in a highly conserved motif of nsp1β protein attenuate the innate immune suppression function of porcine reproductive and respiratory syndrome virus. J Virol 90:3584–3599. doi: 10.1128/JVI.03069-15. [DOI] [PMC free article] [PubMed] [Google Scholar]