ABSTRACT
Human adenovirus 41 (HAdV-41) causes acute gastroenteritis in young children. The main characteristics of HAdV-41 infection are diarrhea and vomiting. Nevertheless, the precise mechanism of HAdV-41-induced diarrhea is unknown, as a suitable small-animal model has not been described. In this study, we used the human midgut carcinoid cell line GOT1 to investigate the effect of HAdV-41 infection and the individual HAdV-41 capsid proteins on serotonin release by enterochromaffin cells and on enteric glia cell (EGC) activation. We first determined that HAdV-41 could infect the enterochromaffin cells. Immunofluorescence staining revealed that the cells expressed HAdV-41-specific coxsackievirus and adenovirus receptor (CAR); flow cytometry analysis supported these findings. HAdV-41 infection of the enterochromaffin cells induced serotonin secretion dose dependently. In contrast, control infection with HAdV-5 did not induce serotonin secretion in the cells. Confocal microscopy studies of enterochromaffin cells infected with HAdV-41 revealed decreased serotonin immunofluorescence compared to that in uninfected cells. Incubation of the enterochromaffin cells with purified HAdV-41 short fiber knob and hexon proteins increased the serotonin levels in the harvested cell supernatant significantly. HAdV-41 infection could also activate EGCs, as shown in the significantly altered expression of glia fibrillary acidic protein (GFAP) in EGCs incubated with HAdV-41. The EGCs were also activated by serotonin alone, as shown in the significantly increased GFAP staining intensity. Likewise, EGCs were activated by the cell supernatant of HAdV-41-infected enterochromaffin cells.
IMPORTANCE The nonenveloped human adenovirus 41 causes diarrhea, vomiting, dehydration, and low-grade fever mainly in children under 2 years of age. Even though acute gastroenteritis is well described, how human adenovirus 41 causes diarrhea is unknown. In our study, we analyzed the effect of human adenovirus 41 infection on human enterochromaffin cells and found it stimulates serotonin secretion in the cells, which is involved in regulation of intestinal secretion and gut motility and can also activate enteric glia cells, which are found in close proximity to enterochromaffin cells in vivo. This disruption of gut barrier homeostasis as maintained by these cells following human adenovirus 41 infection might be a mechanism in enteric adenovirus pathogenesis in humans and could indicate a possible serotonin-dependent cross talk between human adenovirus 41, enterochromaffin cells, and enteric glia cells.
KEYWORDS: gastroenteritis, enteric adenovirus, EC cells, serotonin, enteric glia cells
INTRODUCTION
Enteric adenoviruses 40 and 41 belong to species F of human adenoviruses (HAdV) (genus Mastadenovirus of the Adenoviridae family) and are associated with acute gastroenteritis primarily in children below 2 years of age (1–3). When these viruses infect the gastrointestinal (GI) tract, watery diarrhea, vomiting, dehydration, and low-grade fever develop (4). Although the hallmarks of enteric adenovirus infection are diarrhea and vomiting, the mechanisms behind enteric adenovirus diarrhea are unresolved, primarily due to the lack of a suitable small-animal model. The mechanisms of diarrhea may include secretory diarrhea, perturbation of the intestinal barrier, and/or motility. Emerging evidence suggests perturbation of intestinal epithelial barrier function is involved in the development of different intestinal diseases (5), and that may also be applicable to enteric adenoviruses.
Several gut components participate as regulators and sentinels to maintain intestinal barrier homeostasis. One of these components is the enteric nervous system (ENS), which has been identified as a key regulator of intestinal barrier function (6–8). The ENS plays an important role in regulating fluid movement across the gut epithelium, interacting with both the endocrine and immune systems of the gut, as well as controlling gastric acid secretion (9). Enterochromaffin (EC) cells are another component associated with barrier homoeostasis. These cells represent the largest enteroendocrine cell population in the small intestine and are strategically positioned in the intestinal mucosa to release mediators from the basolateral surface, further activating afferent neuron endings mainly within the lamina propria (10, 11). EC cells are characterized by their synthesis and release of serotonin (12–14), which activates the ENS and extrinsic vagal afferents to the brain, and they may also activate enteric glia cells (EGCs) (6, 7). Moreover, the involvement of serotonin has been demonstrated in the regulation of intestinal secretion, gut motility, several GI disorders, nausea, vomiting, and acute gastroenteritis (15–21) including rotavirus disease (22). We have previously shown that rotavirus can infect human EC cells and stimulate serotonin secretion in a dose- and time-dependent manner (23).
Beneath the intestinal epithelial cells is a population of astrocyte-like cells that are known as enteric glia cells (EGCs). EGCs play an important role in maintaining intestinal barrier integrity (24–26), but they have many regulatory functions throughout the GI tract and can also be found both in the myenteric and submucosal plexuses (27). EGCs express the glia cell marker glia fibrillary acidic protein (GFAP), which is at least one downstream effector of cytokine response in enteric glia (26, 28). It has been suggested that increased GFAP expression in cells and tissue is an activation marker of illness, such as inflammatory bowel diseases (29, 30). In addition, it has been shown that vagal nerve activation of EGCs is linked to enhanced barrier function (6, 7). Several lines of evidence implicate an essential role of mucosal EGCs in regulating gut epithelium integrity (31).
Adenovirus is a nonenveloped, approximately 90-nm-diameter, double-stranded DNA-containing virus composed of three major oligomeric capsid proteins (32). The hexon proteins form the virus coat protein and are the most abundant capsid protein (33, 34). The penton base proteins are found in each corner of the 12 5-fold vertices of the icosahedral capsid and anchor trimeric fibers to the viral capsid (35, 36). Adenovirus fiber and penton base proteins of adenovirus bind to cellular receptors and coreceptors, such as coxsackievirus and adenovirus receptor (CAR) (37–39), CD46 (40), desmoglein-2 (41), sialic acid-containing glycans (42, 43), and integrins (44). Internalization of the virus is mediated by RGD (Arg-Gly-Asp) motifs in the penton base proteins that specifically recognize cellular integrins (33). Enteric HAdV-40 and HAdV-41 are distinct among HAdVs, encoding two different fibers—a longer CAR-binding fiber and a shorter fiber with unknown function—and the penton base protein lacks the otherwise conserved RGD motif (37).
In this study, we investigated whether HAdV-41 can stimulate EC cells to secrete serotonin and subsequently activate EGCs. Furthermore, we aimed to identify which capsid protein component is associated with pathogenesis, keeping in mind serotonin release from EC cells. This study aimed at gaining insight into enteric adenovirus pathogenesis, keeping in mind the lack of a small-animal model.
We found that HAdV-41 can stimulate serotonin from CAR-expressing human EC cells and that supernatant from HAdV-41-stimulated human EC cells can activate EGCs. These new observations are of interest because they propose a serotonin-dependent cross talk between HAdV-41, EC cells, and EGCs that may be relevant for the understanding of how HAdV-41 causes diarrhea.
RESULTS
HAdV-41 could infect CAR-expressing human EC cells.
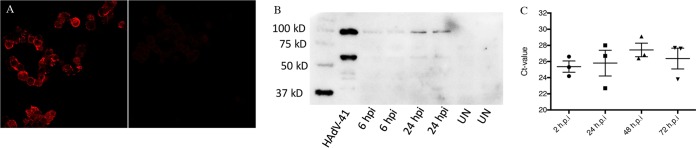
To determine if HAdV-41 can infect human EC cells, (13), the cells were grown in Lab-Tek II chamber slides and 48-well plates and infected with purified HAdV-41 (Fig. 1A and B). Immunofluorescence at 18 h postinfection (hpi) revealed that 60 to 70% of the EC cells had been infected (Fig. 1A); Western blotting at 24 hpi revealed the presence of the adenovirus-specific hexon protein (100 kDa) and the long fiber/penton base proteins (both approximately 60 kDa) (Fig. 1B).
FIG 1.
HAdV-41 infection of EC cells. (A) EC cells infected with HAdV-41 at 18 hpi. Infected (left) and uninfected (right) cells were visualized by confocal microscopy. (B) Western blot analysis of purified HAdV-41-infected cell lysate at 6 and 24 hpi and uninfected cells (UN). In the purified HAdV-41 lysate, the hexon protein is 100 kDa, the long fiber and penton base proteins are in the range 60 to 65 kDa, and the short fiber protein is in the range 40 to 45 kDa. (C) To investigate if HAdV-41 can replicate in human EC cells, a time kinetics study using qPCR was conducted, and as shown EC cells are nonpermissive for replication of HAdV-41. Ct, cycle threshold.
To investigate if HAdV-41 can replicate in human EC cells, a time kinetics study using quantitative PCR (qPCR) was conducted. As shown in Fig. 1C, EC cells are nonpermissive for replication of HAdV-41. Thus, similar to several other nonpermissive cell lines (45, 46), the fastidious HAVd-41 viruses have impaired replication yet protein expression in EC cells.
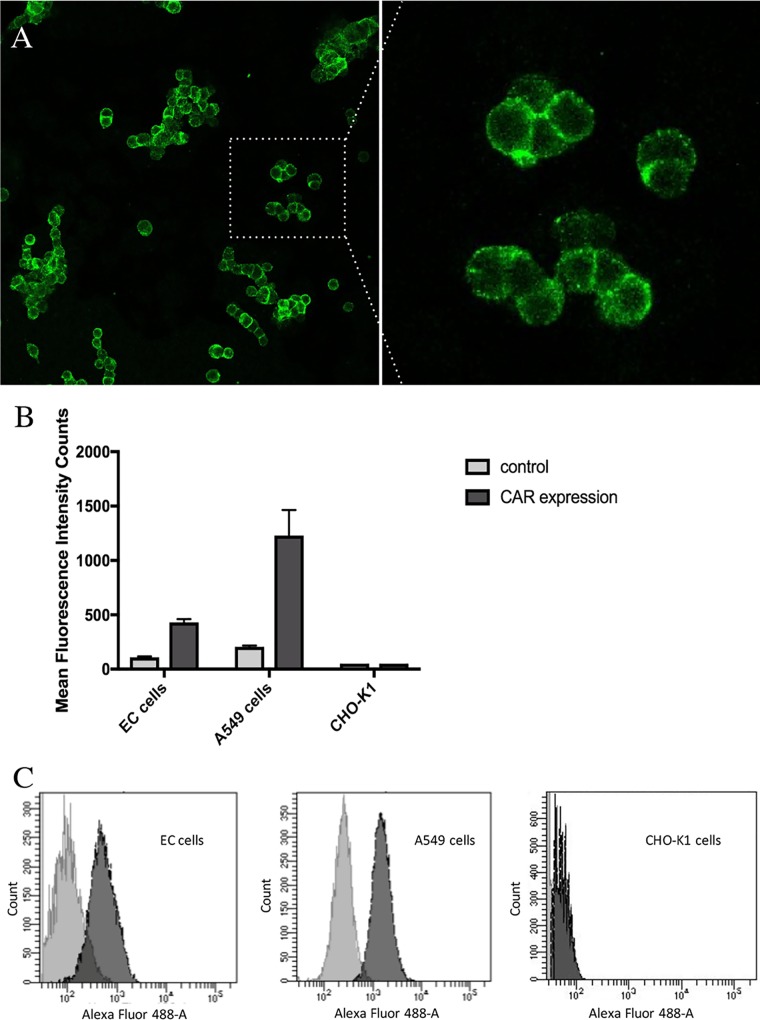
Next, we determined whether EC cells express HAdV-41-specific CAR (37). The cells were stained with CAR-specific antibody, and immunofluorescence revealed punctate, cell-membrane-associated CAR (Fig. 2A). The EC cells expressed almost three times lower levels of CAR compared to the positive-control A549 cells (Fig. 2B). Flow cytometry analysis supported the immunofluorescence findings that EC cells express CAR (Fig. 2C).
FIG 2.
Human EC cells express CAR. (A) Confocal microscopy detection of CAR (Alexa Fluor 488; green) on EC cells. Note the punctate cell membrane–associated staining of CAR on the cells. (B and C) Flow cytometry analyses of CAR expression on EC cells, A549 cells, and CHO-K1 cells. (C) Dark gray histograms show CAR expression on EC cells (left), A549 cells (middle), and CHO-K1 cells (right) compared to cells reacted with only secondary antibody (light gray histograms). Fluorescence intensity is displayed on the x axis, and counts are displayed on the y axis. Error bars represent mean ± SEM (n = 3).
HAdV-41-stimulated serotonin release from human EC cells.
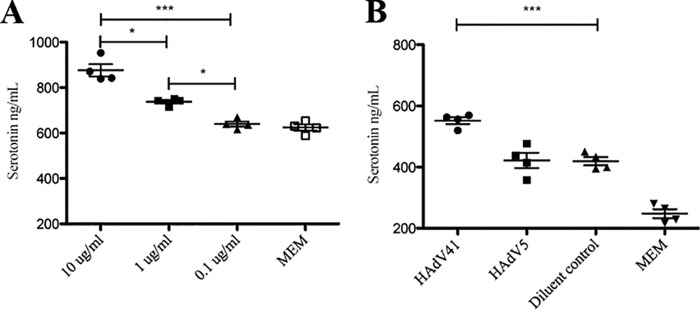
Upon stimulation, EC cells typically release serotonin, which is an important mediator in signaling and which also influences gut physiology (47). We investigated whether HAdV-41 can stimulate serotonin release from EC cells. We found that EC cells infected with purified HAdV-41 for 24 h released serotonin in a dose-dependent manner (10 to 0.1 μg/ml) (P < 0.001) (Fig. 3A). To determine if infection prior to viral replication (≤6 h) is sufficient for stimulation to induce serotonin release, the cells were infected for 6 h with purified virus, and we found that 6 h of infection is sufficient for robust stimulation of serotonin release (Fig. 3B). To determine if the serotonin-stimulating property is unique to HAdV-41, the same concentration of purified HAdV-5 (10 μg/ml) was used to infect the cells. HAdV-41, but not HAdV-5, stimulated (P < 0.001) significant serotonin release within 6 h compared to the control (Fig. 3B).
FIG 3.
HAdV-41 stimulates serotonin release from human EC cells. (A) Dose-response release of serotonin. Purified HAdV-41 was diluted in MEM and incubated (150 μl) on EC cells for 24 h, followed by collection of cell medium and serotonin determination. (B) Purified HAdV-41 but not HAdV-5 (10 μg/ml, 150 μl) stimulates serotonin release from EC cells within 6 h. The diluent control was PBS. ***, P < 0.001, and *, P < 0.05, by Student's t test. Data are means ± SEM (n = 4).
HAdV-41 short fiber knob and hexon proteins stimulated serotonin release.
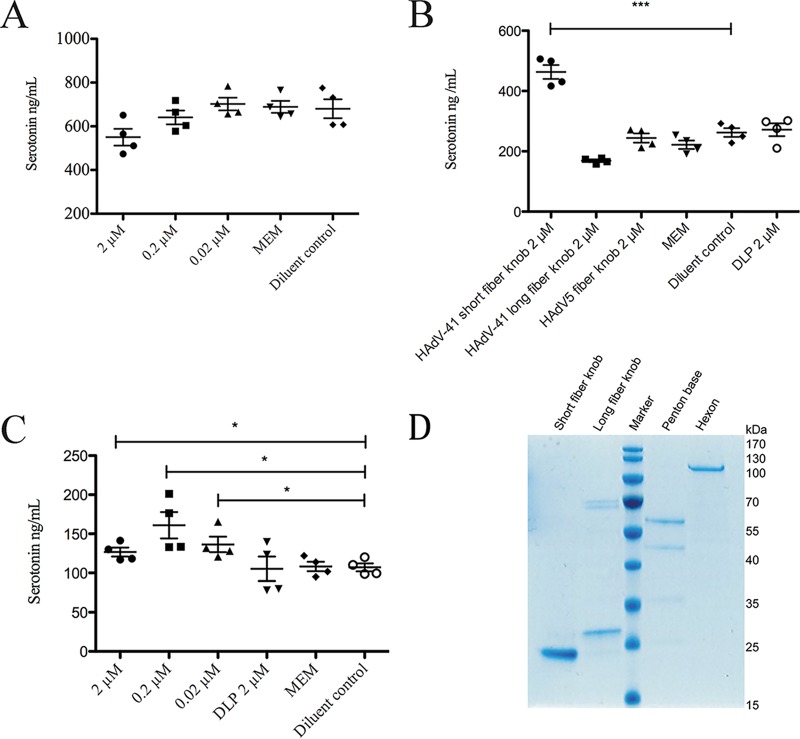
As purified HAdV-41 virus stimulated serotonin release within 6 h, the next question was whether individual capsid proteins confers serotonin-stimulating capacity. To address this question, we produced and purified full-length HAdV-41 hexon and penton base proteins as well as the knob domains of the long and short fibers (34, 48–51). In the first set of experiments, we stimulated EC cells with 2 to 0.02 μM purified HAdV-41 penton base. After 6 h of stimulation, the cell medium was collected and the released serotonin levels determined. We found that serotonin release was unaffected, suggesting that the HAdV-41 penton base does not have serotonin-stimulating properties (Fig. 4A).
FIG 4.
HAdV-41 short fiber knob and hexon proteins stimulate serotonin release. (A) EC cells were stimulated with purified HAdV-41 penton base protein. The serotonin content in supernatant was analyzed after 6 h of incubation. (B) EC cells were stimulated with 2 μM purified HAdV-41 fiber knob proteins, Serotonin content in supernatant was analyzed by ELISA after 6 h of incubation. (C) EC cells were stimulated with purified HAdV-41 hexon protein. Serotonin content in supernatant was analyzed by ELISA after 6 h of incubation. (D) SDS-PAGE of purified short and long fiber knob, penton base, and hexon. The diluent control was PBS. DLP, purified rotavirus double-layer particle. ***, P < 0.001, and *, P < 0.05, by Student's t test. Data are means ± SEM (n = 4).
Next, we investigated the serotonin-stimulating properties of the HAdV-41 short and long fiber knob proteins. EC cells were stimulated with 2 μM each fiber knob protein (Fig. 4B). Following 6 h of stimulation, the cell medium was collected and the serotonin content analyzed and compared with that of cells treated with 2 μM HAdV-5 fiber knob protein, cell medium, diluent control (phosphate-buffered saline [PBS]), and purified rotavirus double-layer particles. There was no significant increase of serotonin release from cells stimulated with HAdV-41 long fiber knob protein or HAdV-5 fiber knob protein (Fig. 4B). However, cells stimulated with HAdV-41 short fiber knob protein responded with increased serotonin release compared to the diluent control (P < 0.001) (Fig. 4B). These findings suggest that HAdV-41 short fiber knob protein has serotonin-stimulating properties. The HAdV-41 hexon protein forms the most abundant capsid protein (33), and it was therefore important to evaluate the effect this coat protein would have on EC cells. EC cells were stimulated with the purified hexon protein in different concentrations for 6 h, followed by collection of the cell supernatant and serotonin determination. The hexon protein had a significant (P < 0.05) stimulatory effect on serotonin release after 6 h of stimulation (Fig. 4C).
HAdV-41 affected serotonin-containing granules in human EC cells.
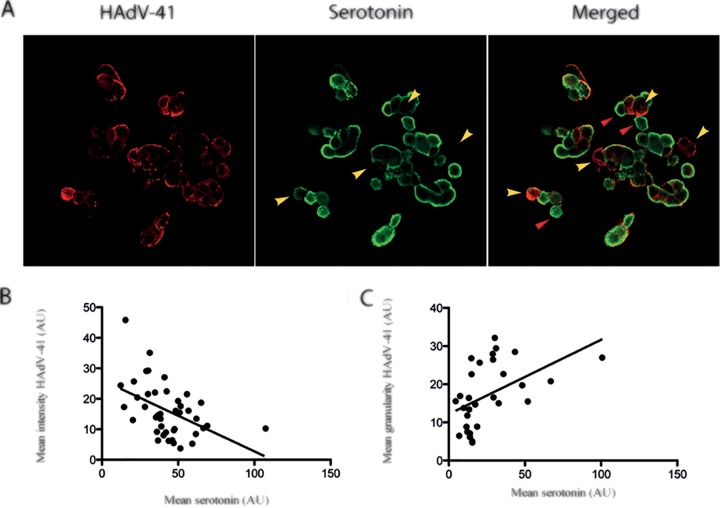
EC cells produce, store, and release serotonin (11, 13), and serotonin granules are translocated to the cell membrane upon specific stimulation (52). The fact that HAdV-41 infection of EC cells resulted in serotonin secretion raised the question of whether infection is associated with changes in the content of serotonin-containing granules. To address this question, EC cells were infected with HAdV-41 and serotonin secretion was investigated at 18 hpi by confocal microscopy. The fluorescence intensity of serotonin was altered in HAdV-41-infected (n = 30 cells) EC cells compared to uninfected cells (n = 30 cells) (Fig. 5A). The serotonin intensity showed a significant (P < 0.01) linear negative correlation between uninfected and infected cells (Fig. 5B), where HAdV-41-infected cells showing weaker serotonin immunofluorescence intensity. Hence, HAdV-41-infected EC cells had weaker serotonin immunofluorescence intensity. Next, we analyzed the granularity of serotonin-labeled immunofluorescence, delineating higher intensity with a more granular appearance in comparison to the uninfected EC cells. We found a significant (P < 0.01) positive linear correlation between the serotonin granularity and HAdV-41-infected EC cells compared to uninfected cells (n = 30 cells) (Fig. 5C). Trypan blue staining indicated cell viability (89% live in infected cell cultures versus 82% in mock-treated cultures) was unaffected in infected cell cultures, nor was any distinct cytopathic effect (CPE) observed, supporting the observation that HAdV-41 undergoes an abortive infection in EC cells.
FIG 5.
HAdV-41 affects serotonin distribution in human EC cells. (A) EC cells were infected with purified HAdV-41 (MOI of 1). At 18 hpi, the cells were fixed and double stained for serotonin (green) and HAdV-41 (red). Images were acquired by confocal microscopy. (B) Confocal analysis showed significant (P < 0.01) negative correlation (R2 = 0.22) between mean serotonin intensity and mean HAdV-41 intensity. (C) Confocal image analysis for granularity showed significant (P < 0.01) positive correlation (R2 = 0.23) between mean HAdV-41 intensity and mean serotonin intensity. Cells were identified by granular or nongranular serotonin distribution (green fluorescence) and analyzed group-wise for the intensity of adenovirus (red fluorescence). Yellow arrowheads indicate lower serotonin immunofluorescence intensity in infected cells. Red arrowheads indicate noninfected cells. Student's t test was used for statistical analysis (n = 30).
HAdV-41-activated EGCs.
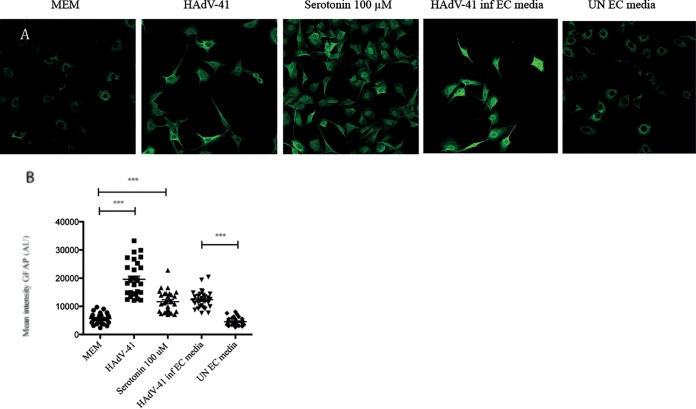
EC cells are epithelial sensor cells, and it has been proposed that EC cells communicate with the ENS and EGCs via neurotransmitters, including serotonin (53). EGCs can be found both in both the myenteric and submucosal plexuses (i.e., underneath the epithelial layer in close proximity to EC cells [27]), which led us to investigate a plausible cross talk between HAdV-41, EC cells, and EGCs. First, we investigated whether HAdV-41 can activate EGCs, as demonstrated by the altered expression of the glia cell activation marker GFAP (26), and found that EGCs were activated by purified HAdV-41 (10 μg/ml) (P < 0.001) after 6 h of stimulation (Fig. 6A and B). Considering that EC cells can release serotonin, we next investigated whether serotonin (100 μM) could activate the EGCs. Indeed, serotonin activated the glia cells (P < 0.001), as demonstrated by the significantly increased GFAP intensity (Fig. 6A and B). However, most novel was the observation that cell supernatant from HAdV-41-infected EC cells could stimulate EGCs (P < 0.001) (Fig. 6A and B). This observation, together with the fact that serotonin alone could activate EGCs, but not cell medium from uninfected EC cells, is of interest as it proposes a serotonin-dependent cross talk between HAdV-41, EC cells, and EGCs (Fig. 7).
FIG 6.
HAd4V-41 stimulates GFAP expression by EGCs. (A) Confocal microscopy images of GFAP immunofluorescence staining of EGCs stimulated with purified HAdV-41, serotonin, cell supernatant from HAdV-41-infected EC cells, and cell supernatant from uninfected EC cells. (B) GFAP immunofluorescence intensity of cells stimulated with purified HAdV-41, serotonin, cell supernatant from HAdV-41-infected EC cells, and cell supernatant from uninfected EC cells. MEM, minimum essential medium; UN, cell medium (MEM) from unstimulated cells. ***, P < 0.001 by Student's t test. Data are means ± SEM (n = 30).
FIG 7.
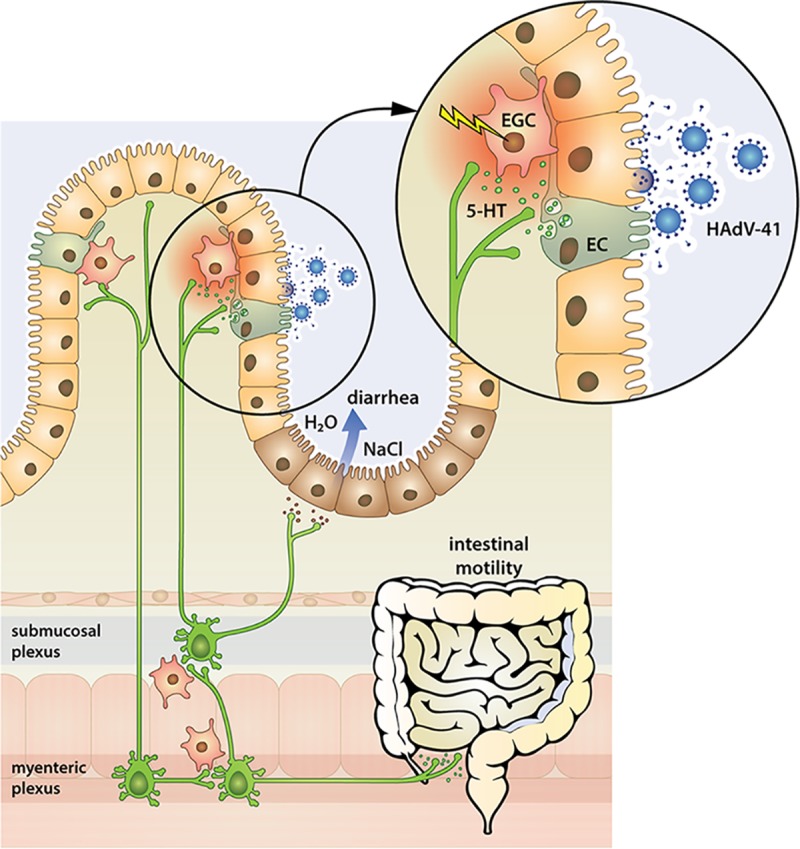
Proposed model for how human enteric adenovirus (HAdV) causes secretory diarrhea. Adenovirus infects enterocytes in the small intestine. Released virus, the knob domain of the short fiber, and hexon protein stimulate enterochromaffin (EC) cells to release serotonin (5-HT). Released 5-HT activates enteric nerves and enteric glia cells (EGC), the latter located near EC cells, and is associated with regulation of gut barrier and intestinal motility functions. Nerves within the submucosal plexus activate crypt cells to stimulate NaCl and water secretion, resulting in diarrhea. Stimulation of the myenteric plexus results in increased motility. The proposed model is based on a human rotavirus disease model (5, 22, 23, 72).
DISCUSSION
Our knowledge on how HAdV-40 and -41 cause acute diarrhea and vomiting is limited, partly due to the lack of suitable small-animal models and lack of previous access to relevant gut target cells normally associated with intestinal motility, permeability, and electrolyte and water secretion. Using a human EC cell line and EGCs, we show that HAdV-41 can stimulate serotonin release from EC cells and that the cell supernatant from HAdV-41-stimulated EC cells can activate EGCs. These new observations are interesting because they suggest a serotonin-dependent cross talk between HAdV-41, EC cells, and EGCs that might be relevant to the understanding how HAdV-41 causes diarrhea (Fig. 7). Our observations are in accordance with previous observations showing that serotonin is involved in several GI disorders (15, 16, 18), including rotavirus gastroenteritis (22, 23, 54).
EC cells, a subtype of neuroendocrine cells (55) residing in the intestinal epithelium, are considered the principal sensory cells that respond to chemical/mechanical stimulation to secrete serotonin and activate mucosal afferents. The luminal EC cells ‘‘taste'’ and ‘‘sense'’ the luminal contents and respond by releasing mediators such as serotonin to activate ENS and EGCs, as well as extrinsic vagal afferents to the brain. They are strategically positioned in the intestinal mucosa to release these mediators mainly from the basolateral surface. Using a human midgut carcinoid tumor cell line, previously characterized for specific EC cell markers (23, 56), we found that purified HAdV-41 stimulated serotonin release as early as 6 hpi.
To identify which viral protein or proteins carry the serotonin-stimulating property, we produced and purified the four main capsid proteins of HAdV-41. While no effect on serotonin release was observed from cells stimulated with the HAdV-41 penton base or long fiber knob protein or with the HAdV-5 fiber knob protein, even up to 2 μM, cells stimulated with the HAdV-41 hexon protein and in particular with the HAdV-41 short fiber knob protein responded with robust serotonin release (P < 0.001). These findings suggest that the HAdV-41 short fiber and hexon proteins have serotonin-stimulating properties. While the HAdV-41 long fiber protein binds to CAR on target cells, the target receptor for the short fiber protein remains to be determined. Our findings suggest that the short fiber knob domain interacts with distinct receptors on human EC cells. Similar to the CAR-binding fiber of multiple HAdVs (57), the short fiber may also be produced in excess and secreted from infected cells. Accordingly, whereas the secreted long fiber facilitates the transmission of progeny virions from infected cells (58), a putative function of secreted short fiber may be to trigger serotonin release from EC cells. In the present study, immunofluorescence and flow cytometry showed that EC cells express CAR, which previously has been shown to be a candidate cell receptor for HAdV-41 (37). While HAdV-41 was expressed in EC cells, as demonstrated by immunofluorescence and Western blotting, virus did not undergo productive replication in those cells. This is similar to several other nonpermissive cell lines (45, 46). The replication block is supposed to be within the early phase of the infectious cycle (59). Furthermore, block of release of progeny virus and a high particle/infectious unit ratio also contribute to poor growth of HAdV-41 in nonpermissive cell cultures (60).
As the adenovirus hexon protein is the most abundant capsid protein (33), it was of interest to investigate its serotonin-stimulating capacity. Similar to the short fiber protein, the hexon protein demonstrated a robust (P < 0.05) stimulatory effect on serotonin release.
Newly synthesized serotonin is normally transported into and stored in secretory granules by the vesicular monoamine transporter (VMAT) (61) or the serotonin reuptake transporter (SERT) (62, 63) and/or degraded to maintain extracellular serotonin homeostasis. Upon specific stimulation, these secretory granules are transported to the cell membrane and their contents, including serotonin, are released by exocytosis. In this way, extracellular serotonin reaches the ENS, where it can stimulate nerve terminals and EGCs. We found that adenovirus infection/stimulation of EC cells resulted in a robust (P < 0.01) linear negative correlation between the fluorescence intensity of serotonin in uninfected cells versus infected cells. Hence, HAdV-41 stimulation of EC cells resulted in decreased serotonin immunofluorescence intensity compared to uninfected cells, presumably due to the depletion of the serotonin content following HAdV-41 stimulation and thus weaker serotonin-specific fluorescence. This proposal is supported by the fact that the EC cells secreted premade serotonin within 6 h of stimulation. We also found a significant (P < 0.01) positive linear correlation between the serotonin granularity and HAdV-41 infection, suggesting that the infection induces serotonin accumulation in granules in parallel. A similar observation was reported previously for rotavirus-infected EC cells (22).
EGCs are the predominant cell type in the ENS and are similar in structure and function to astrocytes of the central nervous system (CNS). Enteric glia cells regulate intestinal motility, a well-characterized reflex controlled by enteric neurons, but also interact with several nonneuronal cell types in the gut, such as enterocytes, enteroendocrine cells, and immune cells, and are therefore emerging as important local regulators of diverse gut functions (64). Furthermore, EGCs play an important role in modulating gut inflammation and maintaining intestinal barrier integrity and repair following injury. The fact that EGCs can be found in close proximity to EC cells (27) led us to investigate a plausible cross talk between adenovirus-infected EC cells and EGCs.
The first observation was that HAdV-41 could stimulate EGCs, as demonstrated by its effect on the appearance of GFAP, a commonly used EGC activation marker for responses to various stimuli (28, 30, 65).
As EC cells and EGCs appear to be located in close proximity in vivo and EGCs can respond to serotonin by increasing GFAP expression (66), it was of great interest to investigate if cell supernatant from HAdV-41-stimulated EC cells could activate EGCs. We confirmed the previous observation (66) that serotonin alone can activate EGCs, but the most interesting finding was the observation that cell medium from HAdV-41-infected/stimulated EC cells, but not that from noninfected EC cells, could activate GFAP. This observation may explain how enteric adenovirus may cause diarrhea. However, the finding that supernatant from infected EC cells can activate GFAP in EGCs only proves indirectly, and not per se, that it was an effect of the released serotonin, as the medium contained serotonin, and serotonin alone can activate GFAP in EGCs. Moreover, the GFAP levels remained unchanged when exposed to supernatant from infected and uninfected (A549) cells, which neither secrete nor synthesize serotonin.
EGCs regulates intestinal barrier function via glia-derived S-nitrosoglutathione (GSNO) (31), and Flamant and coworkers (67) reported that EGCs significantly reduced barrier lesions induced by Shigella flexneri. It was suggested that the effect is associated with EGCs and GSNO, and it was proposed that GSNO is a major glia mediator involved in intestinal epithelial protection (67). As GSNO is released from activated EGCs and helps maintain the intestinal barrier, the observations presented allow us to speculate that this may also hold true for HAdV-41 infections in vivo. However, the current lack of an animal model for HAdV infection prevents confirmation of this hypothesis in vivo.
In summary, we show that HAdV-41 stimulates serotonin release from human EC cells and that supernatant from HAdV-41-stimulated/infected EC cells activates EGCs and presumably the ENS and GNSO release. These unexpected and novel observations are interesting because they propose a serotonin-dependent cross talk between HAdV-41 and human EC cells and EGCs and provide a new context on how on enteric adenovirus causes diarrhea (Fig. 7).
MATERIALS AND METHODS
Cells, viruses, and antibodies.
Human EC cells obtained from the GOT1 midgut carcinoid tumor cell line (56) were cultivated in RPMI 1640 medium (R0883; Sigma-Aldrich, USA) supplemented with 10% inactivated fetal bovine serum (FBS), 1× minimal essential medium (MEM) with nonessential amino acids, 0.02 mg/ml gentamicin, and 5 mM l-glutamine. The A549 human lung epithelial cell line was cultivated in 1× high-glucose Dulbecco's modified Eagle′s medium (DMEM) (21013-024; Thermo Scientific), supplemented with 10% FBS, 0.02 mg/ml gentamicin, and 5 mM l-glutamine. Rat enteric glia cells (EGCs) (ATCC CRL-2690) were cultured as with the A549 cells.
The cells were tested as free from Mycoplasma using a MycoAlert Mycoplasma detection kit (LT07-418; Lonza, USA). HAdV-41 (strain Tak) and HAdV-5 were used in the experiments, produced as described previously (68). These viruses were stored and diluted in PBS with 10% glycerol. In addition, purified rotavirus double-layer particles were used (69).
Rabbit anti-HAdV-41 serum (KS 1133) (70) was used for Western blotting and immunofluorescence analysis. Anti-CAR monoclonal antibody (MAb) was purchased from Millipore (anti-CAR, clone RmcB, 05-644; Millipore, USA). The monoclonal anti-serotonin antibody (M758) and the rabbit anti-GFAP antibody (Z0334) were both purchased from DakoCytomation (Glostrup, Denmark). The secondary antibodies for immunofluorescence analysis were rhodamine-labeled goat anti-rabbit IgG (diluted 1/400) (111-025-045; Jackson ImmunoResearch, USA) for HAdV-41 detection and fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG (1:200 [115-095-003; Jackson ImmunoResearch]) for serotonin detection; Alexa Fluor 488-conjugated AffiniPure goat anti-mouse IgG (1:200 [115-545-003; Jackson ImmunoResearch]) was used for CAR detection, and Alexa Fluor 488-conjugated AffiniPure goat anti-rabbit IgG (1:200 [111-545-144, Jackson ImmunoResearch]) was used for GFAP detection. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit (170-6515; Bio-Rad, USA) and goat anti-mouse (170-1011; Bio-Rad) antibodies were used as secondary antibodies in the Western blotting.
Adenovirus infection.
Adenovirus titer was determined via fluorescent focus assay to be 5 × 105 fluorescent focus units/ml. EC tumor cells (2.5 × 105 cells/well) cultured in 48-well plates (Nunclon Delta Si, Roskilde, Denmark) were infected with HAdV-41 at a multiplicity of infection (MOI) of 1 and incubated at 37°C and 5% CO2 until the cell supernatants or cell lysates were collected.
Fiber knob protein production.
DNA isolation from HAdV-41 virions was performed using a Blood & Cell Culture DNA minikit (Qiagen Nordic). DNA fragments encoding HAdV-40 short fiber knob protein (40SFK, nucleotides 29292 to 29902; GenBank accession no. AMQ95247.1) and HAdV-41 short fiber knob protein (41SFK, nucleotides 29378 to 29899; GenBank accession no. ADN06466.1) were amplified by PCR. Fragments were then cloned into a pQE30Xa expression vector encoding an N-terminal His tag (Qiagen) using restriction sites for BamHI and HindIII (Fermentas, ThermoFisher Scientific). All constructs were confirmed by sequencing (Eurofins MWG Operon). Proteins were expressed in Escherichia coli strain M15 and purified with nickel-nitrilotriacetic acid (NTA) agarose beads according to the manufacturer's protocol (Qiagen). Proteins were analyzed by denaturing gel (NuPAGE Bis-Tris; Invitrogen, Life Technologies) and Western blotting using monoclonal antibodies (MAbs) against the His tag (Qiagen).
Penton base production.
Full-length HAdV-41 penton base DNA was cloned in a pFastBac HT A vector. The vector was transformed into E. coli DH10Bac and analyzed by PCR according to a Bac-to-Bac baculovirus expression system (Invitrogen). Spodoptera frugiperda Sf9 cells were transfected with the bacmid DNA to first create a passage 1 (P1) baculovirus stock, from which a high-titer P2 viral stock was generated. The Sf9 cells were infected at an MOI of 5 with the P2 viral stock, and the cells were incubated at 28°C for 4 days under shaking conditions. After incubation, the cells were lysed and briefly centrifuged, and the expressed proteins were purified with a HiTrap Q-Sepharose column (GE Healthcare) by a liquid chromatography system (GE Healthcare). The soluble recombinant proteins were then stored in PBS with 10% glycerol at −20°C.
Hexon production.
Ten T-75 flasks of A549 cells were infected with HAdV-41 and 6 days after infection harvested by scraping. Harvested cells where subjected to multiple cycles of freeze/thawing to disrupt intact cells, followed by removal of cellular debris through centrifugation. The cleared supernatant was separated on a cesium chloride (CsCl) gradient as described previously (68). After ultracentrifugation, the top phase was recovered and the hexons purified by antibody capture using the HAdV-hexon MAb 8052 covalently attached to magnetic beads (Pierce) by dimethyl pimelimidate (DMP; Sigma). Briefly, 100 μl of magnetic beads was washed with PBS prior to the addition of 75 μg MAb 8052. After 60 min of binding, unbound antibody was removed by washing twice with PBS. To cross-link the antibody, DMP was diluted to 1 mg/ml in 0.2 M triethanolamine in PBS and added in a 1:1 ratio to the magnetic beads. After 30 min of slow agitation, the beads were washed with 0.2 M triethanolamine in PBS for 30 min. This step was repeated three times before the solution was quenched using 50 mM ethanolamine in PBS. Finally, excess antibody was removed by washing with 1 M glycine (pH 3). To capture HAdV-41 hexons, first 300 μl cleared supernatant was mixed in a 1:1 ratio with PBS and added to the MAb 8052-connected beads. The mixture was incubated at room temperature (RT) on a shaker for 60 min to allow antibody capture of the hexons. Subsequently, unbound protein was washed away with PBS and eluted using 200 mM glycine (pH 2.5). Immediately after elution, the low pH was neutralized by adding a small volume of 1 M Tris-HCl (pH 10.2). The capture was repeated several times in order to obtain sufficient amounts of hexons. Hexon purity was assessed by SDS-polyacrylamide gel electrophoresis (PAGE).
Detection of HAdV-41-infected cells.
The number of infected cells was determined by immunofluorescence staining. Briefly, HAdV-41 at an MOI of 1 was added to EC cells, and at 18 hpi, the cells were fixed with 4% paraformaldehyde (PFA) in PBS at RT for 2 h. The fixed cells were washed with PBS and then treated with 1% Triton X-100 in PBS for 15 min at RT, washed with PBS, and blocked with 5% bovine serum albumin (BSA) in PBS for 60 min at RT and then incubated with a rabbit anti-HAdV-41 antisera (1:400; KS 1133) for 1 h at RT. After washing three times with PBS, secondary rhodamine-labeled goat anti-rabbit IgG (1:400) was added and incubated for 1 h at RT. Following three more washes with PBS, the specimens were mounted with fluorescence mounting medium (S3023; Dako Cytomation) and examined under confocal microscopy.
Extraction of DNA from cell lysates.
Two hundred microliters of cell lysates from HAdV-41-infected GOT1 and rat glia cells was used for DNA extraction. The extraction was done with EZ1Virus minikit v2.0 (Qiagen, GmbH, Hilden) using the EZ1 Advanced XL system (Qiagen).
Real-time qPCR for HAdV-41.
The amount of adenovirus genomic DNA after HAdV-41 infection (MOI of 1) at 2, 24, 48, and 72 hpi was quantified with the TaqMan ABI 7500 system (Applied Biosystems, Foster City, CA, USA). Samples from 2 hpi, the time for virus exposure, were used as control (background level of incoming virus). Briefly, amplification was performed with 10 μl of purified DNA samples and standard DNA templates in 25-μl reaction mixtures for 45 cycles with the QuantiTect probe PCR kit (Qiagen). Primer and probe sequences were obtained from previous studies (71). Primer concentrations of 900 nM and a probe concentration of 225 nM were used in the PCR protocol as follows: activation of the uracil N-glycosylase (2 min, 50°C) and activation of Taq polymerase for 10 min at 95°C for 45 cycles (15 s at 94°C and 1 min at 60°C). Standard curves were generated by using serial dilutions (range, 10 to 106) of known amounts of a linearized plasmid containing the entire hexon region of HAdV-41.
Cell viability.
Trypan blue staining was used to determine cell viability. A 0.4% trypan blue solution (MP Biomedicals) was mixed in a 1:1 ratio with cell suspension, and the number of blue-colored cells was calculated as a percentage of the total number of cells in a Bürker chamber.
Western blotting.
EC cell monolayers (approximately 250,000 cells per well) in 48-well plates were infected as described above. After 24 h of infection, the medium was removed, and cells were lysed with 1× radioimmunoprecipitation assay (RIPA) buffer (9806; Cell Signaling Technology) and freeze-thawed 3 to 4 times. The cell lysates were then centrifuged at 10,000 × g for 10 min, and the supernatant was collected and boiled for 10 min at 95°C in loading buffer (5% 2-mercaptoethanol [161-0710; Bio-Rad] in Laemmli sample buffer [161-0737; Bio-Rad]) before separation by 10% PAGE. The proteins were stained with Coomassie brilliant blue, and the relative protein concentration was determined. Samples were separated by PAGE and transferred (via Western blotting) to a polyvinylidene difluoride (PVDF) membrane at 375 mA for 60 min. The membrane was blocked with 3% BSA in PBS-T buffer (PBS containing 0.05% Tween 20) for 1 h. Rabbit anti-HAdV-41 antibody (1:400 in PBS-T with 1% BSA) was added and incubated for 2 h at RT. The membrane was washed four times with PBS-T. HRP-conjugated goat anti-rabbit antibody (1:10,000 [170-6515; Bio-Rad, USA]) was used as the secondary antibody, and the membrane was incubated for 90 min. After washing, the reaction was developed with Immun-Star HRP substrate (170-5041; Bio-Rad), and the bands were visualized with a Molecular Imager ChemiDoc XRS system (Bio-Rad) and Quantity One 1-D analysis software (Bio-Rad, USA).
Stimulation of EC cells with virus, penton base, hexon, and short and long fiber proteins.
EC cell monolayers (approximately 250,000 cells per well) in 48-well plates at 80% confluence were washed with serum-free medium and stimulated with purified HAdV-41 (0.1, 1.0, and 10.0 μg/ml, 150 μl), purified HAdV-5 (10.0 μg/ml, 150 μl), HAdV-41 penton base (0.02, 0.2, and 2.0 μM), HAdV-41 hexon protein (0.02, 0.2, and 2.0 μM), HAdV-41 long and short fiber knob proteins (2.0 μM), and HAdV-5 fiber knob protein (2.0 μM). Purified adenovirus was stored and diluted in PBS plus 10% glycerol, unless otherwise stated. Rotavirus was purified as previously described (69). After 6 h of stimulation at 37°C and 5% CO2, the cell supernatants was collected and stored at −80°C until enzyme-linked immunosorbent assay (ELISA) serotonin analysis. EGC stimulation was performed essentially as for EC cells.
Serotonin ELISA.
A commercial serotonin ELISA kit was used (RE59121; IBL International, Hamburg, Germany) according to the manufacturer's instruction to determine serotonin concentrations.
Immunofluorescence.
EC cells were grown to 80% confluence in Lab-Tek II chamber slides (Nunc, Thermo Fisher Scientific) and infected with HAdV-41 at an MOI of 1, as described above. At 18 hpi, cells were fixed with ice-cold acetone (A/0520/PB17; Fisher Chemical) or 4% PFA–PBS (02176; Histolab, Gothenburg, Sweden). The cells stained for serotonin and HAdV-41 were fixed with 4% PFA–PBS; cells stained for CAR and GFAP were fixed with ice-cold acetone. The PFA-fixed cells were washed with PBS, treated with 1% Triton X-100 in PBS for 15 min, and blocked with 5% BSA in PBS for 60 min at RT, and the respective primary antibodies (KS1133, 1:400; CAR, 1:100; serotonin, 1:50; GFAP, 1:200) were added to the cells and incubated for 1 h at RT. After three washes with PBS, secondary IgG was added and incubated for 1 h at RT, washed, mounted with fluorescence mounting medium (S3023; DakoCytomation), and examined by confocal microscopy.
Stimulation of EGCs.
EGCs were grown to 80% confluence on Lab-Tek II chamber slides at 37°C and 5% CO2 and stimulated for 6 h with 100 μM serotonin (H9523; Sigma-Aldrich), purified HAdV-41 (10 μg/ml), or supernatant from uninfected and HAdV-41-infected EC cells (24 hpi). Cell supernatants were centrifuged at 500 × g for 5 min and filtered through a 20-μm pore filter before use. The cell medium was removed, and the cells were fixed with ice-cold acetone for 10 min and stained for GFAP as described above.
Confocal imaging and image analysis.
An upright Zeiss Axio Imager.Z2 microscope, equipped with a LSM700 confocal module controlled by Zen2012 software (Oberkochen, Germany) was used to capture all fluorescent images. The images were acquired with a Plan-Apochromat 40×/1.3 and 20×/0.8 objective. Single-labeled samples were used to assess bleed-through; samples labeled only with secondary antibody were used to check for nonspecific binding. All control images were captured using the same confocal settings. Image J software was used to measure the mean intensities and single-cell areas. The figures presented were composed using Adobe Photoshop or Adobe Illustrator.
Flow cytometry.
EC cells and A549 cells were detached with PBS-EDTA (0.05% EDTA), counted, and then allowed to recover in growth medium at 37°C. After 1 h, the cells were transferred to a V-bottom 96-well plate (2 × 105 cells/well) and washed once with fluorescence-activated cell sorter (FACS) buffer (PBS with 2% FBS). A monoclonal antibody against CAR (clone RmcB) was diluted 1:40 in FACS buffer (PBS plus 2% FBS) and incubated with the cells for 30 min at 4°C. Before incubation with a fluorescently labeled secondary antibody, unbound primary antibody was washed away with FACS buffer. The cells were then incubated with Alexa Fluor 488-conjugated donkey anti-mouse IgG (Life Technologies, diluted 1:1,000 in FACS buffer) for 30 min at 4°C. Then the cells were washed with FACS buffer and analyzed on a BD LSRII cytometer (Becton Dickinson). The results were analyzed with FACSDiva software (Becton Dickinson).
Statistical analysis.
Statistical analysis was performed with GraphPad Prism (GraphPad Prism 5.0a or 7 Macintosh version by software MacKiev; GraphPad Software, Inc. 1994 to 2008). Data in the graphs are presented as the mean ± standard error of the mean (SEM). We used Student's t test, and P values of <0.05, <0.01, and <0.001 were considered significant.
ACKNOWLEDGMENTS
This work was supported by the Swedish Research Council 320301 (L.S.) and 2013-2753 (N.A.), MIIC, Linköping University (L.S. and K.-E.M.).
We thank Kristina Lindman and the Protein Expertise Platform at Umeå University for assistance with protein production and purification. We also thank Rada Ellegard for the iilustration.
S.W., M.H., J.N., and S.S. performed the infection and stimulation experiments. L.S. and N.A. proposed the project, designed the study, and analyzed and interpreted the experiments. L.S., N.A., K.-E.M., S.W., S.S., and M.H. wrote the manuscript. V.L., S.W., M.H., and K.-E.M. performed the confocal microscopy and captured images. B.D.P. and A.R. cultivated, purified, and characterized the adenovirus and adenovirus structural proteins of adenovirus. A.A. performed the DNA extraction and quantitative real-time PCR analysis, and S.S. purified the rotavirus. A.R. performed the flow cytometry.
The authors declare they have no competing financial interests.
REFERENCES
- 1.Uhnoo I, Wadell G, Svensson L, Johansson ME. 1984. Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children. J Clin Microbiol 20:365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uhnoo I, Wadell G, Svensson L, Johansson M. 1983. Two new serotypes of enteric adenovirus causing infantile diarrhoea. Dev Biol Stand 53:311–318. [PubMed] [Google Scholar]
- 3.Jacobsson PA, Johansson ME, Wadell G. 1979. Identification of an enteric adenovirus by immunoelectroosmophoresis (IEOP) technique. J Med Virol 3:307–312. doi: 10.1002/jmv.1890030409. [DOI] [PubMed] [Google Scholar]
- 4.Uhnoo I, Svensson L, Wadell G. 1990. Enteric adenoviruses. Bailliere's Clin Gastroenterol 4:627–642. doi: 10.1016/0950-3528(90)90053-J. [DOI] [PubMed] [Google Scholar]
- 5.Hagbom M, Sharma S, Lundgren O, Svensson L. 2012. Towards a human rotavirus disease model. Curr Opin Virol 2:408–418. doi: 10.1016/j.coviro.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Cheadle GA, Costantini TW, Lopez N, Bansal V, Eliceiri BP, Coimbra R. 2013. Enteric glia cells attenuate cytomix-induced intestinal epithelial barrier breakdown. PLoS One 8:e69042. doi: 10.1371/journal.pone.0069042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costantini TW, Krzyzaniak M, Cheadle GA, Putnam JG, Hageny AM, Lopez N, Eliceiri BP, Bansal V, Coimbra R. 2012. Targeting alpha-7 nicotinic acetylcholine receptor in the enteric nervous system: a cholinergic agonist prevents gut barrier failure after severe burn injury. Am J Pathol 181:478–486. doi: 10.1016/j.ajpath.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Lundgren O, Peregrin AT, Persson K, Kordasti S, Uhnoo I, Svensson L. 2000. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science 287:491–495. doi: 10.1126/science.287.5452.491. [DOI] [PubMed] [Google Scholar]
- 9.Furness JB. 2012. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 10.Bertrand PP, Kunze WA, Furness JB, Bornstein JC. 2000. The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience 101:459–469. doi: 10.1016/S0306-4522(00)00363-8. [DOI] [PubMed] [Google Scholar]
- 11.Hansen MB, Witte AB. 2008. The role of serotonin in intestinal luminal sensing and secretion. Acta Physiol (Oxf) 193:311–323. doi: 10.1111/j.1748-1716.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 12.Erspamer V, Asero B. 1952. Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature 169:800–801. [DOI] [PubMed] [Google Scholar]
- 13.Cetin Y, Kuhn M, Kulaksiz H, Adermann K, Bargsten G, Grube D, Forssmann WG. 1994. Enterochromaffin cells of the digestive system: cellular source of guanylin, a guanylate cyclase-activating peptide. Proc Natl Acad Sci U S A 91:2935–2939. doi: 10.1073/pnas.91.8.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manocha M, Khan WI. 2012. Serotonin and GI disorders: an update on clinical and experimental studies. Clin Transl Gastroenterol 3:e13. doi: 10.1038/ctg.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, Moses PL. 2004. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Belai A, Boulos PB, Robson T, Burnstock G. 1997. Neurochemical coding in the small intestine of patients with Crohn's disease. Gut 40:767–774. doi: 10.1136/gut.40.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bearcroft CP, Perrett D, Farthing MJ. 1998. Postprandial plasma 5-hydroxytryptamine in diarrhoea predominant irritable bowel syndrome: a pilot study. Gut 42:42–46. doi: 10.1136/gut.42.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, Spiller RC. 2005. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol 3:349–357. doi: 10.1016/S1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 19.Gershon MD. 1999. Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther 13(Suppl 2):S15–S30. [PubMed] [Google Scholar]
- 20.Freedman SB, Steiner MJ, Chan KJ. 2010. Oral ondansetron administration in emergency departments to children with gastroenteritis: an economic analysis. PLoS Med 7:e1000350. doi: 10.1371/journal.pmed.1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine DA. 2012. Oral ondansetron decreases vomiting, as well as the need for intravenous fluids and hospital admission, in children with acute gastroenteritis. Evid Based Med 17:112–113. doi: 10.1136/ebmed.2011.100355. [DOI] [PubMed] [Google Scholar]
- 22.Bialowas S, Hagbom M, Nordgren J, Karlsson T, Sharma S, Magnusson KE, Svensson L. 2016. Rotavirus and serotonin cross-talk in diarrhoea. PLoS One 11:e0159660. doi: 10.1371/journal.pone.0159660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagbom M, Istrate C, Engblom D, Karlsson T, Rodriguez-Diaz J, Buesa J, Taylor JA, Loitto VM, Magnusson KE, Ahlman H, Lundgren O, Svensson L. 2011. Rotavirus stimulates release of serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in nausea and vomiting. PLoS Pathog 7:e1002115. doi: 10.1371/journal.ppat.1002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gershon MD, Rothman TP. 1991. Enteric glia. Glia 4:195–204. doi: 10.1002/glia.440040211. [DOI] [PubMed] [Google Scholar]
- 25.Ruhl A, Nasser Y, Sharkey KA. 2004. Enteric glia Neurogastroenterol Motil 16(Suppl 1):44–49. [DOI] [PubMed] [Google Scholar]
- 26.Jessen KR, Mirsky R. 1980. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature 286:736–737. doi: 10.1038/286736a0. [DOI] [PubMed] [Google Scholar]
- 27.Gulbransen BD, Sharkey KA. 2012. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 9:625–632. doi: 10.1038/nrgastro.2012.138. [DOI] [PubMed] [Google Scholar]
- 28.von Boyen GB, Steinkamp M, Reinshagen M, Schafer KH, Adler G, Kirsch J. 2004. Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut 53:222–228. doi: 10.1136/gut.2003.012625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cornet A, Savidge TC, Cabarrocas J, Deng WL, Colombel JF, Lassmann H, Desreumaux P, Liblau RS. 2001. Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn's disease? Proc Natl Acad Sci U S A 98:13306–13311. doi: 10.1073/pnas.231474098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Boyen GB, Schulte N, Pfluger C, Spaniol U, Hartmann C, Steinkamp M. 2011. Distribution of enteric glia and GDNF during gut inflammation. BMC Gastroenterol 11:3. doi: 10.1186/1471-230X-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, Bourreille A, Hurst R, Sofroniew MV. 2007. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology 132:1344–1358. doi: 10.1053/j.gastro.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 32.Fields BN. 2001. Adenoviruses, p 2265–2300. In Fields BN, Knipe DM, Howley PM, Griffin DE (ed), Fields virology, 4th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 33.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. 1993. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73:309–319. doi: 10.1016/0092-8674(93)90231-E. [DOI] [PubMed] [Google Scholar]
- 34.Pichla-Gollon SL, Drinker M, Zhou X, Xue F, Rux JJ, Gao GP, Wilson JM, Ertl HC, Burnett RM, Bergelson JM. 2007. Structure-based identification of a major neutralizing site in an adenovirus hexon. J Virol 81:1680–1689. doi: 10.1128/JVI.02023-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burnett RM. 1985. The structure of the adenovirus capsid. II. The packing symmetry of hexon and its implications for viral architecture. J Mol Biol 185:125–143. [DOI] [PubMed] [Google Scholar]
- 36.van Oostrum J, Burnett RM. 1985. Molecular composition of the adenovirus type 2 virion. J Virol 56:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roelvink PW, Lizonova A, Lee JG, Li Y, Bergelson JM, Finberg RW, Brough DE, Kovesdi I, Wickham TJ. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol 72:7909–7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. 1997. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 39.Tomko RP, Johansson CB, Totrov M, Abagyan R, Frisen J, Philipson L. 2000. Expression of the adenovirus receptor and its interaction with the fiber knob. Exp Cell Res 255:47–55. doi: 10.1006/excr.1999.4761. [DOI] [PubMed] [Google Scholar]
- 40.Gaggar A, Shayakhmetov DM, Lieber A. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat Med 9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Li ZY, Liu Y, Persson J, Beyer I, Moller T, Koyuncu D, Drescher MR, Strauss R, Zhang XB, Wahl JK III, Urban N, Drescher C, Hemminki A, Fender P, Lieber A. 2011. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med 17:96–104. doi: 10.1038/nm.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnberg N, Edlund K, Kidd AH, Wadell G. 2000. Adenovirus type 37 uses sialic acid as a cellular receptor. J Virol 74:42–48. doi: 10.1128/JVI.74.1.42-48.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnberg N, Kidd AH, Edlund K, Olfat F, Wadell G. 2000. Initial interactions of subgenus D adenoviruses with A549 cellular receptors: sialic acid versus alpha(v) integrins. J Virol 74:7691–7693. doi: 10.1128/JVI.74.16.7691-7693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mathias P, Galleno M, Nemerow GR. 1998. Interactions of soluble recombinant integrin alphav beta5 with human adenoviruses. J Virol 72:8669–8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Retter M, Middleton PJ, Tam JS, Petric M. 1979. Enteric adenoviruses: detection, replication, and significance. J Clin Microbiol 10:574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gary GW Jr, Hierholzer JC, Black RE. 1979. Characteristics of noncultivable adenoviruses associated with diarrhea in infants: a new subgroup of human adenoviruses. J Clin Microbiol 10:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan WI, Ghia JE. 2010. Gut hormones: emerging role in immune activation and inflammation. Clin Exp Immunol 161:19–27. doi: 10.1111/j.1365-2249.2010.04150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez E, Romero C, Rio A, Miralles M, Raventos A, Planells L, Burgueno JF, Hamada H, Perales JC, Bosch A, Gassull MA, Fernandez E, Chillon M. 2013. Short-fiber protein of ad40 confers enteric tropism and protection against acidic gastrointestinal conditions. Hum Gene Ther Methods 24:195–204. doi: 10.1089/hgtb.2012.096. [DOI] [PubMed] [Google Scholar]
- 49.Yeh HY, Pieniazek N, Pieniazek D, Gelderblom H, Luftig RB. 1994. Human adenovirus type 41 contains two fibers. Virus Res 33:179–198. doi: 10.1016/0168-1702(94)90054-X. [DOI] [PubMed] [Google Scholar]
- 50.Roberts MM, White JL, Grutter MG, Burnett RM. 1986. Three-dimensional structure of the adenovirus major coat protein hexon. Science 232:1148–1151. doi: 10.1126/science.3704642. [DOI] [PubMed] [Google Scholar]
- 51.Arnberg N. 2012. Adenovirus receptors: implications for targeting of viral vectors. Trends Pharmacol Sci 33:442–448. doi: 10.1016/j.tips.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Racke K, Reimann A, Schworer H, Kilbinger H. 1996. Regulation of 5-HT release from enterochromaffin cells. Behav Brain Res 73:83–87. doi: 10.1016/0166-4328(96)00075-7. [DOI] [PubMed] [Google Scholar]
- 53.Gershon MD, Tack J. 2007. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Hagbom M, Novak D, Ekstrom M, Khalid Y, Andersson M, Lindh M, Nordgren J, Svensson L. 2017. Ondansetron treatment reduces rotavirus symptoms—a randomized double-blinded placebo-controlled trial. PLoS One 12:e0186824. doi: 10.1371/journal.pone.0186824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grubisic V, Verkhratsky A, Zorec R, Parpura V. 2017. Enteric glia regulate gut motility in health and disease. Brain Res Bull 136:109–117. doi: 10.1016/j.brainresbull.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kolby L, Bernhardt P, Ahlman H, Wangberg B, Johanson V, Wigander A, Forssell-Aronsson E, Karlsson S, Ahren B, Stenman G, Nilsson O. 2001. A transplantable human carcinoid as model for somatostatin receptor-mediated and amine transporter-mediated radionuclide uptake. Am J Pathol 158:745–755. doi: 10.1016/S0002-9440(10)64017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rebetz J, Na M, Su C, Holmqvist B, Edqvist A, Nyberg C, Widegren B, Salford LG, Sjogren HO, Arnberg N, Qian Q, Fan X. 2009. Fiber mediated receptor masking in non-infected bystander cells restricts adenovirus cell killing effect but promotes adenovirus host co-existence. PLoS One 4:e8484. doi: 10.1371/journal.pone.0008484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walters RW, Freimuth P, Moninger TO, Ganske I, Zabner J, Welsh MJ. 2002. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell 110:789–799. doi: 10.1016/S0092-8674(02)00912-1. [DOI] [PubMed] [Google Scholar]
- 59.Tiemessen CT, Nel MJ, Kidd AH. 1996. Adenovirus 41 replication: cell-related differences in viral gene transcription. Mol Cell Probes 10:279–287. doi: 10.1006/mcpr.1996.0037. [DOI] [PubMed] [Google Scholar]
- 60.Brown M, Wilson-Friesen HL, Doane F. 1992. A block in release of progeny virus and a high particle-to-infectious unit ratio contribute to poor growth of enteric adenovirus types 40 and 41 in cell culture. J Virol 66:3198–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schuldiner S, Shirvan A, Linial M. 1995. Vesicular neurotransmitter transporters: from bacteria to humans. Physiol Rev 75:369–392. doi: 10.1152/physrev.1995.75.2.369. [DOI] [PubMed] [Google Scholar]
- 62.Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. 1996. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci 16:2352–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen JX, Pan H, Rothman TP, Wade PR, Gershon MD. 1998. Guinea pig 5-HT transporter: cloning, expression, distribution, and function in intestinal sensory reception. Am J Physiol 275:G433–G448. [DOI] [PubMed] [Google Scholar]
- 64.Costantini TW, Bansal V, Krzyzaniak M, Putnam JG, Peterson CY, Loomis WH, Wolf P, Baird A, Eliceiri BP, Coimbra R. 2010. Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells. Am J Physiol Gastrointest Liver Physiol 299:G1308–G1318. doi: 10.1152/ajpgi.00156.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.da Cunha Franceschi R, Nardin P, Machado CV, Tortorelli LS, Martinez-Pereira MA, Zanotto C, Goncalves CA, Zancan DM. 2017. Enteric glial reactivity to systemic LPS administration: changes in GFAP and S100B protein. Neurosci Res 119:15–23. doi: 10.1016/j.neures.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 66.Boesmans W, Cirillo C, Van den Abbeel V, Van den Haute C, Depoortere I, Tack J, Vanden Berghe P. 2013. Neurotransmitters involved in fast excitatory neurotransmission directly activate enteric glial cells. Neurogastroenterol Motil 25:e151–e160. doi: 10.1111/nmo.12065. [DOI] [PubMed] [Google Scholar]
- 67.Flamant M, Aubert P, Rolli-Derkinderen M, Bourreille A, Neunlist MR, Mahe MM, Meurette G, Marteyn B, Savidge T, Galmiche JP, Sansonetti PJ, Neunlist M. 2011. Enteric glia protect against Shigella flexneri invasion in intestinal epithelial cells: a role for S-nitrosoglutathione. Gut 60:473–484. doi: 10.1136/gut.2010.229237. [DOI] [PubMed] [Google Scholar]
- 68.Johansson SM, Nilsson EC, Elofsson M, Ahlskog N, Kihlberg J, Arnberg N. 2007. Multivalent sialic acid conjugates inhibit adenovirus type 37 from binding to and infecting human corneal epithelial cells. Antiviral Res 73:92–100. doi: 10.1016/j.antiviral.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 69.Svensson L. 1984. Identification of an outer capsid glycoprotein of human rotavirus by concanavalin A. J Gen Virol 65:2183–2190. doi: 10.1099/0022-1317-65-12-2183. [DOI] [PubMed] [Google Scholar]
- 70.Murray PR, Baron EJ, Pfaller MA (ed). 1999. Manual of clinical microbiology, 7th ed ASM Press, Washington, DC. [Google Scholar]
- 71.Hernroth BE, Conden-Hansson AC, Rehnstam-Holm AS, Girones R, Allard AK. 2002. Environmental factors influencing human viral pathogens and their potential indicator organisms in the blue mussel, Mytilus edulis: the first Scandinavian report. Appl Environ Microbiol 68:4523–4533. doi: 10.1128/AEM.68.9.4523-4533.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Crawford SE, Ramani S, Tate JE, Parashar UD, Svensson L, Hagbom M, Franco MA, Greenberg HB, O'Ryan M, Kang G, Desselberger U, Estes MK. 2017. Rotavirus infection. Nat Rev Dis Primers 3:17083. doi: 10.1038/nrdp.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]