ABSTRACT
A primary question in dengue virus (DENV) biology is the molecular strategy for recruitment of host cell protein synthesis machinery. Here, we combined cell fractionation, ribosome profiling, and transcriptome sequencing (RNA-seq) to investigate the subcellular organization of viral genome translation and replication as well as host cell translation and its response to DENV infection. We report that throughout the viral life cycle, DENV plus- and minus-strand RNAs were highly partitioned to the endoplasmic reticulum (ER), identifying the ER as the primary site of DENV translation. DENV infection was accompanied by an ER compartment-specific remodeling of translation, where ER translation capacity was subverted from host transcripts to DENV plus-strand RNA, particularly at late stages of infection. Remarkably, translation levels and patterns in the cytosol compartment were only modestly affected throughout the experimental time course of infection. Comparisons of ribosome footprinting densities of the DENV plus-strand RNA and host mRNAs indicated that DENV plus-strand RNA was only sparsely loaded with ribosomes. Combined, these observations suggest a mechanism where ER-localized translation and translational control mechanisms, likely cis encoded, are used to repurpose the ER for DENV virion production. Consistent with this view, we found ER-linked cellular stress response pathways commonly associated with viral infection, namely, the interferon response and unfolded protein response, to be only modestly activated during DENV infection. These data support a model where DENV reprograms the ER protein synthesis and processing environment to promote viral survival and replication while minimizing the activation of antiviral and proteostatic stress response pathways.
IMPORTANCE DENV, a prominent human health threat with no broadly effective or specific treatment, depends on host cell translation machinery for viral replication, immune evasion, and virion biogenesis. The molecular mechanism by which DENV commandeers the host cell protein synthesis machinery and the subcellular organization of DENV replication and viral protein synthesis is poorly understood. Here, we report that DENV has an almost exclusively ER-localized life cycle, with viral replication and translation largely restricted to the ER. Surprisingly, DENV infection largely affects only ER-associated translation, with relatively modest effects on host cell translation in the cytosol. DENV RNA translation is very inefficient, likely representing a strategy to minimize disruption of ER proteostasis. Overall these findings demonstrate that DENV has evolved an ER-compartmentalized life cycle; thus, targeting the molecular signatures and regulation of the DENV-ER interaction landscape may reveal strategies for therapeutic intervention.
KEYWORDS: RNA virus, flavivirus, organelle protein import, translational control
INTRODUCTION
The synthesis of viral proteins, which function in viral replication, evasion of immune defenses, and virion biogenesis, is wholly dependent on host cell translation machinery. Reflecting this need, viruses have evolved diverse strategies to outcompete cellular mRNAs and co-opt host translation capacity. Some viruses have evolved mRNAs that are translated by alternative mechanisms (e.g., internal ribosomal entry site-mediated, cap-independent translation initiation) and genes that modify or inactivate host cell factors required for cap-dependent host translation, thereby providing mechanisms for viral RNAs to efficiently recruit ribosomes (1–6). Other viruses encode nucleases that specifically degrade host mRNAs, thereby significantly decreasing the competition of cellular translation activity (7). Yet others produce mRNAs that can, by nature of their extraordinary translation efficiency and/or high levels, outcompete most cellular mRNAs (8–10). As viral replication and viral protein synthesis are strictly dependent on the host cell translation machinery, understanding the mechanisms by which viruses promote translation of their RNAs within cells provides not only understanding of viral pathogenic mechanisms but also insights into host cell regulation of protein synthesis (11).
The mechanism by which dengue virus (DENV), a member of the Flavivirus genus of RNA viruses and a prominent human pathogen, usurps host cell protein synthesis is largely unknown. Like all members of the genus Flavivirus, DENV contains an enveloped 5′ m7GpppA-capped (+)-sense RNA genome with a nonpolyadenylated 3′ untranslated region (UTR). The DENV 10.7-kb genome encodes a single polyprotein, which is posttranslationally cleaved into three structural (capsid [C], premembrane/membrane [prM/M], and envelope [E]) and seven nonstructural (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) proteins required for viral replication and inactivation of antiviral cellular pathways (12–14). Neither the structural nor nonstructural proteins are known to modify or compete with the cellular translation machinery. Indeed, earlier studies report little to no effect of DENV infection on total host cell protein synthesis (15, 16). Translation initiation of the DENV plus-strand RNA is thought to occur primarily through a canonical cap-dependent mechanism (17), although alternative strategies have been described when cap-dependent translation is inhibited (15).
DENV enters cells through receptor-mediated endocytosis (18) and gains access to the cytosol compartment following fusion of the viral envelope with the endosomal membrane. Having gained access to the cytosol, the viral genome then undergoes cycles of translation and replication that can produce upwards of 10,000 infectious particles per cell within 48 h (19). Prior to the onset of viral replication, synthesis of the RNA-dependent RNA polymerase NS5, the RNA helicase NS3, and other NS proteins must occur, as these are required for assembly of the viral replication complex (20). Because minus-strand RNA synthesis and plus-strand translation compete for the same plus-strand template (21), the interplay between these two processes and their associated RNA structures is critical for optimal viral replication. Different long-range RNA-RNA interactions appear to partition the genome between a linear form devoted to protein synthesis and a circular form focused on RNA transcription (22, 23), allowing for separation of these two processes in space and time.
DENV polyprotein and genome replication occurs in association with the endoplasmic reticulum (ER) (24). This intracellular membrane affiliation reflects both the nature of the polyprotein, which contains ca. 20 transmembrane domains and is dependent on the ER protein translocation machinery for its biogenesis, and that many of the nonstructural proteins, e.g., NS4A, behave as ER-resident membrane proteins and are principal components of ER-associated replication factories (24, 25). Correspondingly, DENV replication is highly sensitive to silencing or knockout of host factors functioning in protein translocation and/or processing in the ER (26–28). Once the components of a viral particle have been synthesized, virions assemble and bud into the ER lumen, utilizing the secretory pathway to exit the cell (12).
Understanding how an ER-localized DENV plus-strand RNA serves as a template for temporally coordinated synthesis of both DENV minus-strand RNA and DENV proteins is important for understanding the DENV life cycle, yet our knowledge of these processes is limited. We considered this incomplete understanding in the context of our recent studies that point to distinct regulatory control of mRNA translation in the cytosol and ER (29–32) as well as transcriptome-wide functions for the ER-associated translation machinery in gene expression (30, 33). We thus paired ribosome profiling (Ribo-seq), which measures the position and abundance of ribosomes on mRNAs transcriptome-wide (34, 35), and transcriptome-wide quantification of mRNA abundance (RNA-seq) with biochemical cell fractionation (31, 36) to examine the subcellular organization of DENV translation through the viral life cycle. The overarching theme in the data is a DENV dependence on and selective modification of the ER-associated protein synthesis machinery. Three primary findings were revealed in this study. First, viral RNA, including the minus-strand replication template, and viral protein synthesis are wholly ER compartmentalized. Second, DENV plus-strand RNA translation is highly inefficient relative to that of host cell mRNAs, suggesting a competition/selective capture mechanism for annexing host cell ER-associated ribosomes. Third, the host translational response to DENV infection is highly compartmentalized to the ER, as most host ER-associated mRNAs are translationally suppressed, yet cytosolic host protein synthesis is relatively unchanged. Comparisons of transcriptome-wide changes in translation of host genes during DENV infection to the translational response evoked by the unfolded protein response (UPR) or treatment with beta interferon (IFN-β) demonstrated that the host translation response to DENV included both UPR and IFN response pathways and revealed a subset of genes whose translation is upregulated during DENV infection. Interestingly, we report that previously identified essential host factors for DENV infection are not translationally upregulated during infection but rather are generally repressed. These findings demonstrate that DENV specifically annexes ER-associated ribosomes, sacrificing synthesis of specific host proteins to maximize viral replication.
RESULTS
Tracking the subcellular compartmentalization of DENV genome replication and translation.
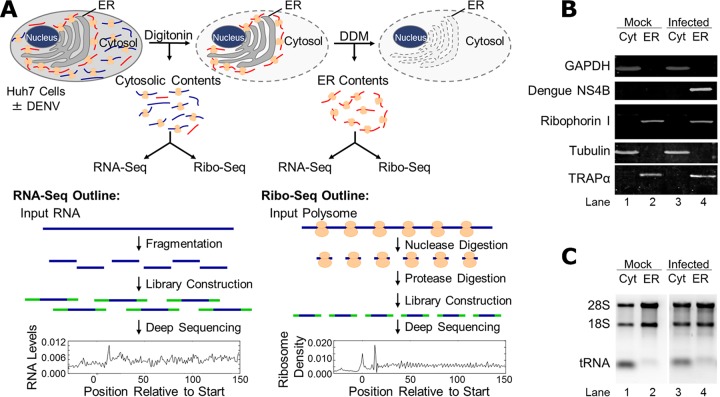
The DENV 10.7-kb plus-strand RNA, which encodes both cytosolic and integral membrane proteins, accesses the cytosol early in infection and is subsequently localized to the endoplasmic reticulum (ER), where its translation products assemble replication and virion biogenesis centers (37, 38). As a first step toward understanding the molecular strategies used by DENV to commandeer host cell translation, we examined subcellular RNA distributions and the translational status of both host cell mRNAs and DENV minus- and plus-strand RNAs through the viral life cycle (36, 39). In combining the cell fractionation protocol illustrated in Fig. 1A, which efficiently separates the two primary protein synthesis compartments of the cell, the ER, and the cytosol, with RNA-seq and ribosome profiling, we sought to determine how DENV infection impacts the subcellular distribution and translation of host cell mRNAs, as the virus captures mRNA translation capacity and secretory pathway function. In these experiments, Huh-7 hepatocarcinoma cells were infected with DENV (serotype 2; strain New Guinea-C) at a multiplicity of infection (MOI) of 10. After 1 h, the viral inoculum was removed and the cells cultured for 6, 12, 24, or 40 h postinfection. At each time point, cells were fractionated using a sequential detergent-based fractionation method (Fig. 1A) (32, 36, 40). As illustrated, cells are first treated with a digitonin-supplemented physiological salts buffer, which selectively permeabilizes the plasma membrane and releases the cytosolic contents. The digitonin-extracted cells then are treated with n-dodecyl-β-d-maltoside (DDM)-supplemented buffers to release ER-associated cellular components. Similar to data reported in prior studies (31, 32, 36, 39, 41–43), the immunoblot data shown in Fig. 1B demonstrate that the fractionation protocol yields the efficient separation and recovery of cytosolic (e.g., glyceraldehyde-3-phosphate dehydrogenase [GAPDH] and tubulin) and ER-resident (e.g., ribophorin I and TRAPα) proteins in both mock- and DENV-infected cells. Note that DENV NS4B, an integral membrane protein, was wholly ER associated in DENV-infected cells and absent from mock-infected cells (Fig. 1B). As expected, rRNAs (ribosomes) were recovered in both fractions, showing a modest ER enrichment in mock-infected cells and an approximately equal subcellular distribution at 40 h postinfection (Fig. 1C). tRNAs, in contrast, were largely recovered in the cytosol fraction (Fig. 1C). The RNA component of the two subcellular fractions was analyzed by ribosome profiling (35) to assess mRNA translation status and RNA-seq to profile mRNA transcriptome composition (see Table S1 in the supplemental material).
FIG 1.
Experimental schematic and validation of cell fractionation protocol. (A) Schematic of the experimental approach. Mock- or DENV-infected Huh7 cells were fractionated by a sequential detergent extraction protocol where cell cultures are first treated with digitonin-supplemented buffers to release the cytosolic contents followed by a subsequent treatment with DDM-supplemented buffers to release the ER-associated contents. Total RNA was isolated from each fraction and analyzed by RNA-seq to assess gene expression. In parallel, polysomes in each fraction were nuclease digested, and ribosome footprints were isolated and analyzed by Ribo-seq. (B) Immunoblot analysis of the distributions of cytosolic (GAPDH and tubulin) and ER-resident membrane (ribophorin I and TRAPα) proteins in the cytosol (Cyt) and ER fractions of mock-infected cells and following 40 h of DENV infection (MOI of 10). (C) Ribosome and tRNA distributions in the two subcellular fractions were determined by isolation of total RNA, separation by agarose gel electrophoresis, and visualization with SYBR green staining. 18S, 28S, and tRNA components are indicated.
As depicted in Fig. 2A, the 40-h time course captured the major phases of the DENV life cycle. DENV plus-strand RNA levels mirrored a logistic growth curve, with an apparent lag phase extending to approximately 12 h followed by a replication phase. DENV minus-strand RNA levels, in contrast, steady increased until 24 h postinfection, followed by a decline. The relative levels of the DENV plus strand, determined from the deep sequencing data sets, were approximately an order of magnitude higher than those of the DENV minus strand throughout infection. We calculated the relative rates of minus- and plus-strand DENV RNA synthesis from the changes in RNA levels (Fig. 2B). Under the indicated experimental conditions, the peak rate of increase for the plus-strand RNA occurred between 12 and 24 h postinfection, with a doubling time of 20 min (±3.8 min). It should be noted that at a multiplicity of infection (MOI) of 10, each cell was likely exposed to ≥1,000 DENV genomes, many of which could be defective in typical infections (44), likely lowering the calculated initial plus-strand synthesis rate. The pattern of change in minus-strand RNA levels differed markedly from those of plus-strand RNA, peaking early in infection and dropping throughout the remainder of the time course. The minus-strand RNA produced early in infection presumably serves as a subsequent template for robust plus-strand synthesis. These data identify an important temporal transition in the viral life cycle, where early periods of infection are weighted to minus-strand synthesis and later time periods to plus-strand synthesis and virion production.
FIG 2.
Spatiotemporal organization of DENV replication and translation. (A) Abundance of DENV plus- and minus-strand RNA over a 40-h infection time course, as assessed by RNA-seq. (B) Rate of accumulation for DENV plus- and minus-strand RNA. Each point indicates the average rate of change of RNA abundance between the two adjacent time points, expressed as percent change per hour in an exponential growth model. (C) Percentage of DENV plus-strand RNA, minus-strand RNA, and plus-strand translation that is ER associated throughout the experimental time course. (D) Translational efficiency of DENV RNA relative to that of the host transcriptome. The translation efficiency distributions of host mRNAs encoding TMHMM-predicted ER-targeted proteins are shown in black, with the translation efficiency of DENV RNA in red. The translation efficiency distribution is calculated as an average value of all mRNAs at all time points. For all panels, error bars represent ± standard deviations (n = 2).
We next investigated the subcellular localization of minus- and plus-strand RNA, as well as plus-strand translation, over the time course of infection (Fig. 2C). Both minus- and plus-strand RNAs were highly partitioned to the ER, where the minus-strand RNA remained almost entirely ER bound throughout the time course despite not being translated. This finding may reflect localization of the minus strand to the ER-associated replication center and association with ER-associated template plus strand. While the plus strand is mostly ER bound early in the infection, at late time points a discernible increase of plus-strand RNA in the cytosol was observed. The precise subcellular disposition of this fraction of plus-strand RNA is, however, not known, as at these late time points plus-strand RNA that scored as cytosolic includes maturing viral particles packaged within secretory pathway transport vesicles. In support of this interpretation, the translation of viral proteins remained highly ER enriched at all time points, which is consistent with non-virion-complexed plus-strand RNA being largely ER associated throughout the experimental time course (Fig. 2C).
In addition to defining the subcellular locale of DENV translation, the ribosome profiling data allowed assessment of the translation status of the plus-strand RNA. Because DENV first accesses the cytosol compartment in early infection and subsequently uses the ER as a platform for virion production, we calculated the translation efficiency of the DENV plus-strand RNA in both the cytosolic and ER compartments, where translation efficiency is defined as the ribosome density within the coding sequence normalized to the level of the corresponding mRNA and is a proxy for mRNA translational status. The translation efficiency of cytosolic plus-strand RNA was low throughout the experimental time course. Intriguingly, for ER-bound DENV plus-strand RNA, translation efficiency is relatively low at the 6-h time point but increases by 12 h postinfection, where it is sustained (data not shown). This period of relatively low translation efficiency on the ER overlaps with the period of high minus-strand synthesis rates, suggesting that at early infection, plus-strand translation is suppressed in favor of RNA replication. This transition may reflect a regulated transition from a primarily circularized, replication-dedicated plus-strand structure to a linearized, translationally competent structure, as suggested previously (23, 45). Notably, even at the time points where DENV plus-strand RNA translation efficiency was highest, the relative translation efficiency was quite low relative to that of the host mRNA transcriptome, scoring in the bottom 5th percentile (Fig. 2D). As we do not know the relative fraction of DENV plus-strand RNA engaged in transcription versus translation and whether the two processes are biochemically exclusive, the precise translation efficiency score cannot be stated with certainty. Nonetheless, these data suggest that the DENV plus-strand RNA is an intrinsically weak substrate for translation. The inefficiency of DENV translation may reflect, at least in part, its highly structured 5′ UTR (46–48). There was essentially no detectable translation of the minus-strand RNA (Fig. 2D).
Ribosome footprinting analysis of DENV plus-strand RNA translation reveals intragenic variations in ribosome loading.
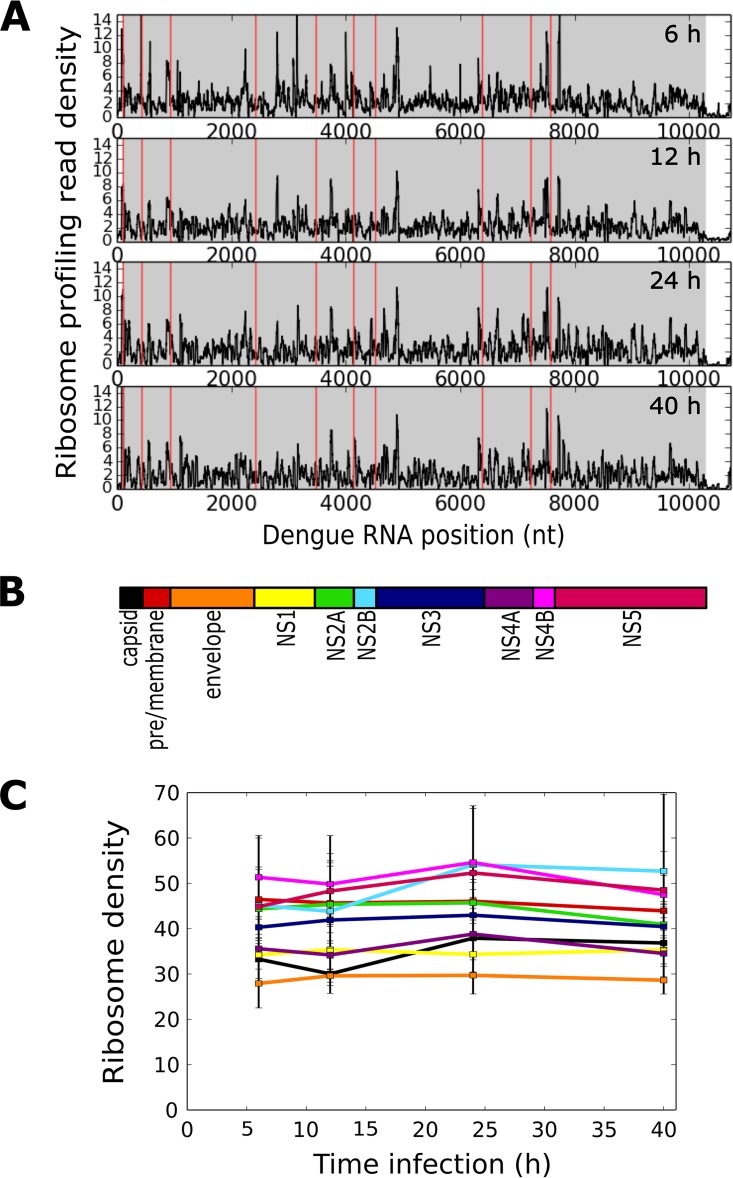
To gain insight into the translation dynamics of the DENV plus-strand RNA, we examined the positional arrangement of the ribosome profiling reads over the ∼10.3-kb coding DNA sequence (CDS) at each time point in the experimental time course of infection (Fig. 3A and C). As depicted in Fig. 3A, ribosomes were broadly distributed along the CDS, with the prominent peaks and valleys that are typical of ribosome profiling data (35). The ribosome distribution pattern was largely unchanged over the experimental time course, suggesting that synthesis of a composite balance of structural and nonstructural proteins is sustained throughout the infection cycle, which would be expected given the single open reading frame (ORF) (Fig. 3A to C). In support of this conclusion, Pearson's correlation coefficients for ribosome densities between biological replicates were indistinguishable from comparisons between time points (r = 0.85 for replicates versus 0.87 for comparisons; P value of 0.35 by two-tailed Student's t test).
FIG 3.
Ribosome footprinting pattern of the DENV RNA. (A) Ribosome density across the DENV RNA at each infection time point as a 30-nucleotide moving window. Red lines separate different coding sequences, while the gray area indicates the entire polyprotein coding sequence. (B) Schematic representation of the DENV polyprotein, with each protein color coded as shown. (C) Ribosome density for each viral protein-coding region over the course of infection (colored according to panel B).
We next examined ribosome densities relative to the established N- and C-terminal boundaries of the encoded proteins of the polyprotein as a measure of intragenic translational variation (Fig. 3C). Such analyses are useful for defining alternative open reading frames, multiple ORFs, and ribosomal frame shifting, as recently reported for the coronavirus murine hepatitis virus (MHV), a plus-strand RNA virus (49). Programmed ribosomal frame shifting and/or multiple ORFs are not known to be strategies utilized by DENV (50, 51). However, intragenic variations in ribosome density are evident, where ribosome densities are lowest in the intragenic region encoding capsid and highest for the regions encoding NS2B, NS4B, and NS5. Although relatively modest (net change of <1.5-fold), the intragenic variations in ribosome densities could arise through cis-encoded translational regulation, perhaps coupled to the ordered cotranslational proteolytic processing of the DENV polyprotein into individual proteins (52, 53). The variations in ribosome density might also reflect a molecular strategy to compensate for differential stabilities of the processed proteins and so will require a detailed understanding of both intragenic ribosomal processivity and the stability/turnover rates of the individual processing-derived proteins to determine biological relevance.
A large number of RNA-seq reads mapped to the 3′ UTR. These reads increased with time of infection and thus likely reflect production of the subgenomic flaviviral RNA (sfRNA) (54) (Fig. 4). This interpretation is supported by the lack of similar changes in 5′ UTR RNA-seq map read densities as a function of time of infection. The sfRNA is a product of degradation of the viral genome by 5′ to 3′ exonucleases. The functions of the sfRNA in viral infection remain to be fully elucidated, but it is known to play a role in suppressing interferon-stimulated gene expression, thereby helping the virus evade the immune system. In contrast to the full DENV plus-strand RNA, the sfRNA was not highly enriched on the ER, consistent with previous work demonstrating functions for the sfRNA in the regulation of cytosolic antiviral immunity factors (Fig. 4B) (55). Ribosome profiling reads mapping to the UTR, particularly the 3′ UTR, were also obtained (Fig. 3A), but they were at a much lower density, and their size distribution was discernibly different from those of other genes, suggesting that they represent nuclease protection by means other than ribosomes (e.g., RNA binding proteins and highly structured/nuclease-resistant RNA domains) rather than translation (Fig. 4C).
FIG 4.
sfRNA abundance and subcellular localization. (A) RNA-seq read density in the cytosol and ER fractions along the DENV RNA sequence. The coding sequence is indicated by the gray shaded area and different coding sequences by red lines. The sfRNA is derived from the DENV 3′ UTR. (B) Subcellular localization of the sfRNA relative to the plus-strand DENV RNA. (C) Distribution of read lengths for ribosome profiling reads mapping to the transcriptome, DENV coding sequence, and DENV 3′ UTR.
DENV infection predominantly remodels translation on the ER compartment.
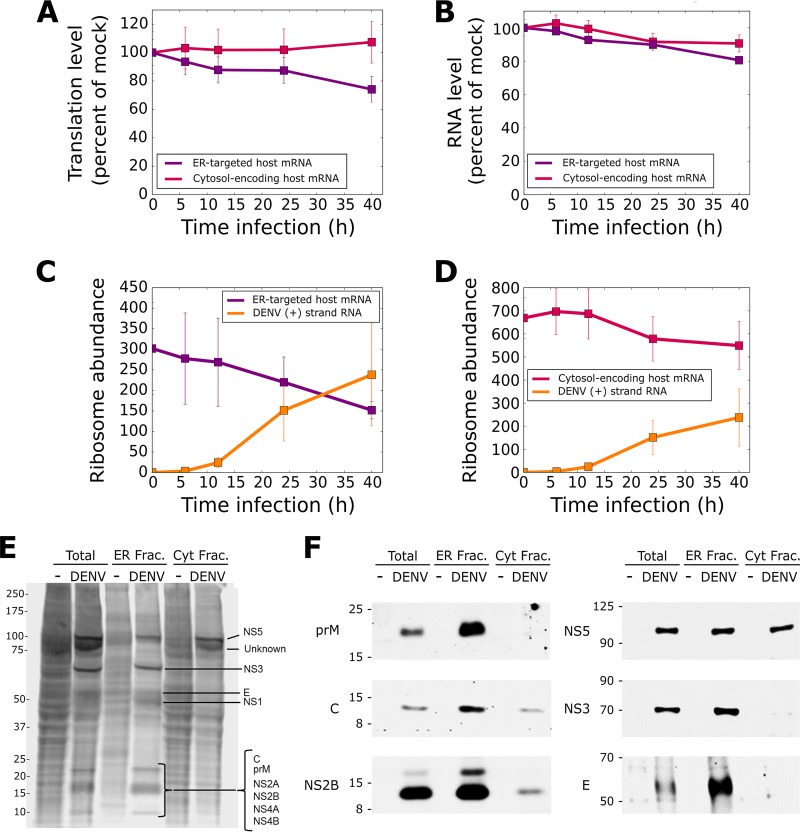
With the ribosome profiling data demonstrating that DENV plus-strand RNA translation was almost entirely localized to ER-bound ribosomes (Fig. 2C), we next examined the impact of viral RNA translation on global host cell protein synthesis via the cell fractionation methodology introduced above. We first compared the relative abundance of ribosome footprint reads on ER-targeted mRNAs (mRNAs which encode an N-terminal hydrophobic signal sequence and/or transmembrane domains and are localized to the ER for translation and translocation) and cytosolic protein-coding mRNAs (mRNAs which do not encode a signal sequence or transmembrane domain and are abundantly translated in the cytosol) (31, 33, 56, 57) (Fig. 5A). As illustrated in Fig. 5A, it was apparent that DENV infection resulted in a time-dependent decrease in the translation of host ER-targeted mRNAs, beginning early in infection and progressing throughout the experimental infection period. In contrast, the translation of cytosolic protein-encoding mRNAs was, on average, unchanged. As illustrated in Fig. 5B, analysis of the total RNA-seq data sets revealed that the levels of mRNAs coding for both ER-targeted and cytosolic proteins decreased somewhat over the course infection. The Ribo-seq and RNA-seq analyses thus indicate that reductions in both ribosome loading and overall mRNA levels contribute to a reduction of total translation on the ER.
FIG 5.
DENV selectively remodels the ER translational landscape. (A) Change in the total translation of mRNAs over the infection time course, as assessed by ribosome footprinting. Two categories of mRNAs are plotted: mRNAs encoding ER-targeted proteins, which encode a signal sequence or transmembrane domain, and mRNAs encoding cytosolic proteins, which do not. Total translation was measured as the total number of ribosome footprinting reads mapped to an mRNA cohort, normalized for library size and then expressed as a percentage of that value in mock-infected cells. DENV RNAs were excluded from these calculations. (B) As described for panel A, except mRNA levels are depicted, as measured by RNA-seq and normalized to length. (C) Time course of ribosome recruitment by ER-associated DENV plus-strand RNA. Illustrated is the fractional capture of ER-bound ribosomes translating topogenic signal-coding mRNAs (signal sequence and/or transmembrane domains, i.e., ER-targeted host mRNA) and DENV plus-strand RNA, as determined from the Ribo-seq data sets. Ribosome abundance is calculated as RPKM × CDS length × 106. (D) Fraction of ER-bound ribosomes translating nontopogenic signal-encoding mRNAs (i.e., cytosol-encoding host mRNA) and DENV plus-strand RNA, as calculated for Fig. 4C. (E) Metabolic labeling of newly synthesized total, ER-associated, and cytosolic proteins. Cells were infected with DENV for 36 h and then pulse labeled with [35S]Met/Cys. Cells were either directly detergent extracted (total) or fractionated as in illustrated in Fig. 1 to obtain ER and cytosol (Cyt) fractions and analyzed by SDS-PAGE followed by autoradiography. Bands appearing for samples from the DENV-infected samples are presumed to be DENV proteins and are labeled based on known molecular weight. (F) Immunoblot analyses of a subset of DENV proteins confirms expression and subcellular distributions indicated in panel E.
As previously reported, cytoplasmic protein-encoding mRNAs are broadly represented on the ER, although they are enriched in the cytosol compartment (31, 33, 56, 57). In the ER compartment specifically, the host ER-targeted mRNA cohort showed a 50% reduction in translation levels (Fig. 5C), whereas ER-associated cytosol-encoding mRNAs were only modestly altered (ca. 15%) (Fig. 5D), indicating that the impact of DENV on ER-associated translation was largely restricted to ER-associated secretory/membrane protein-encoding mRNAs. Because the DENV plus-strand RNA encodes ca. 20 transmembrane domains, its polyprotein translation product would be expected to compete with the translation products of host ER-targeted mRNAs for access to the protein translocation machinery. Thus, we further examined the impact of DENV infection on the translation of ER-targeted host mRNAs. To obtain a quantitative estimate of the impact of ER-localized DENV plus-strand RNA on the translation of ER-targeted host mRNAs, the reads per kilobase million (RPKM) values for ER-targeted mRNAs were multiplied by corresponding ORF length to provide a measure of gene-specific ribosome abundance (Fig. 5C and D). This transformation accounts for the fact that ribosome loading is, in general, a function of ORF length; longer ORFs tend to be more populated with ribosomes and, in this scenario, occupy a greater fraction of the protein translocation machinery than shorter ORFs (58, 59). Furthermore, with prior studies demonstrating that ribosomes engaged in the translation of secretory or transmembrane proteins are bound to the Sec61 protein translocation machinery, this metric provides a measure of the fractional utilization of the ER secretory capacity by this mRNA cohort (60–62). As depicted, by 24 h postinfection, DENV plus-strand RNA occupies levels of ER translocon-bound ribosomes similar to those of the host ER-targeted mRNAs, and by 40 h postinfection, ribosome loading onto DENV plus-strand RNA surpassed ER-targeted host mRNAs, at which point the DENV plus-strand RNA had commandeered a majority of the ER secretory capacity (Fig. 5C). That the sum of the ER-targeted host mRNA and DENV plus-strand RNA ribosome abundance values at 40 h exceeds the ribosome abundance of ER-targeted host mRNAs at the zero time point is consistent with the observation that DENV infection promotes expansion of the ER compartment, as previously reported, and thus an increase in total ER translocation activity and ribosome binding capacity (63).
To further explore the impact of DENV infection on host translation, [35S]Met/Cys pulse-labeling experiments were performed, again using the cell fractionation assay system depicted in Fig. 1. As a direct measure of de novo protein synthesis, [35S]Met/Cys pulse labeling provides an orthogonal test of the ribosome footprinting data and distinguishes between translating and translationally suppressed polyribosomes, which cannot be distinguished by ribosome footprinting alone. When combined with cell fractionation, this approach also reveals differences in the translational status of the cytosol and ER compartments (32, 42). In these experiments, Huh-7 cells were infected with DENV at an MOI of 10 and pulse labeled with [35S]Met/Cys 36 h postinfection. Mock- and DENV-infected cells then were fractionated, and protein synthesis activity of the two compartments was assessed by phosphorimaging analysis of SDS-PAGE-separated protein fractions (Fig. 5E). Total (unfractionated) cell extracts were obtained in parallel. As is evident in the total cell extracts, the impact of DENV infection on total proteome expression at the 36-h time point was substantial, with prominent DENV infection-dependent translation products present in the infected cells (Fig. 5E). The de novo translation patterns of the two subcellular fractions revealed distinct compartmental responses to DENV infection. Of particular interest, the overall translation pattern of the cytosol fraction at 36 h postinfection was very similar in the mock- and DENV-infected cells, with a modest suppression of overall translational activity (Fig. 5E, Cyt Frac) (16). Clearly evident in the cytosol fraction of the infected cells was a radiolabeled band of ca. 100 kDa, which is the predicted mobility of NS5, the methyltransferase polymerase (64). Lacking transmembrane domains and/or a signal peptide, NS5 would be expected to be highly enriched in the cytosol fraction; however, a portion was recovered in the ER fraction as well, which may represent NS5 polymerase associated with ER-bound minus-strand DENV RNA. The identity of the DENV infection-specific radiolabeled band migrating slightly faster than the 100-kDa band remains to be determined. Contrasting with the cytosol fraction, DENV infection elicited a dramatic remodeling of the ER-associated proteome. Previously abundant ER proteins were scarcely detectable, and DENV proteins instead dominated the output of ER protein biosynthesis (Fig. 5E, ER Frac). Of particular interest is the radiolabeled protein of ca. 68 kDa, present in the ER fraction and absent from the cytosol fraction of DENV-infected cells. The mobility of this protein in SDS-PAGE is consistent with the processing protease NS3. As NS3 lacks a signal sequence or transmembrane domain (65), it would be predicted to reside in the cytosol. Prior studies have established that NS3 associates with NS2B to form the active processing protease; with NS2B being an integral membrane protein localized to the ER, this protein-protein interaction would be expected to confer ER localization to soluble NS3 (64, 66, 67). To further explore these findings, immunoblot analyses of DENV capsid, envelope, prM, NS2B, NS3, and NS5 expression and subcellular localization were performed (Fig. 5F). As shown, the immunoblot studies were consistent with the data depicted in Fig. 5E and directly demonstrate both viral protein expression and subcellular localization.
Combined with the ribosome footprinting data, the [35S]Met/Cys pulse-labeling and DENV protein immunoblot data illustrate that DENV primarily commandeers ER translocon-associated ribosomes and suppresses translation of ER-targeted host mRNAs. Furthermore, analyses of the relative distribution of ER-bound ribosomes engaged in the translation of DENV plus-strand RNA and ER-targeted mRNAs reveal a slow process of ribosome capture by the DENV plus-strand RNA, occurring approximately in parallel with the synthesis of plus-strand DENV RNA (Fig. 2A and 5C).
Global translation response to DENV infection.
The changes in mRNA translation patterns reported above were apparent on a transcriptomic scale. Heat map analysis of the ribosome footprinting data sets indicates a broad spectrum of altered translation by 40 h of infection, although translation was largely unaffected at earlier time points when DENV plus-strand RNA levels are relatively low (Fig. 6A). Using a cutoff of a 2-fold change in total translation at the 40-h point, 948 mRNAs had enhanced translation and 880 mRNAs had suppressed translation. Importantly, the changes in translation status seen at early infection time points largely reflected lower-magnitude variants of the late infection time points. While there are specific genes that are expressed early in response to DENV infection, the majority of changes in host mRNA translation in response to DENV represent a conserved, progressive response that increases in magnitude over the time course of infection (Fig. 6A).
FIG 6.
Host gene expression response to DENV infection. (A) Heat map of changes in total translation of host genes over the DENV infection time course. Genes are sorted by their mean response over the time course of infection. The translational response to IFN-β1A treatment is also indicated. (B) Changes during DENV infection in the interferon-induced-only gene set (defined as those genes increased at least 50% after treatment with IFN-β1A) and the UPR-induced gene set (genes increased at least 50% after 4 h of UPR induction) (UPR gene set from reference 41). (C) Venn diagram specifying the overlaps between the interferon and UPR gene sets described above and the genes increased at least 100% in total translation after 40 h of DENV infection. (D) Five most significant gene ontology terms for the DENV-only gene set, determined for biological processes using GOrilla with the full data set as the background list. (E) The contributions of changes in mRNA levels and translational efficiency to changes in total translational activity after 40 h of DENV infection. These values were calculated as described in Materials and Methods for all genes and for each set of genes that is exclusively identified as DENV, UPR, or IFN.
To assess the mechanisms driving these changes in gene expression, we first queried the roles of two gene expression programs known to be active during DENV infection: the IFN pathway and the unfolded protein response (UPR) (68). We defined a set of IFN-stimulated genes by treatment of Huh-7 cells with IFN-β for 12 h. An orthologous UPR-responsive gene set was derived from a previous ribosome profiling study that used thapsigargin treatment of mouse embryonic fibroblasts to elicit UPR activation (41). In comparing the changes in these gene sets over the course of DENV infection, each was significantly increased, indicating that the two pathways were, as expected, upregulated (Fig. 6B). With regard to UPR-responsive genes, induction was quite slow and modest, more consistent with a supportive role for the UPR, e.g., expansion of ER secretory capacity, rather than an acute, proteostatic stress response (16, 69, 70). A Venn diagram of these data sets revealed a significant overlap (P < 0.005 for all by hypergeometric test) between genes with enhanced expression in DENV infection and both IFN-induced and UPR pathways (Fig. 6C). However, there remained a substantial cohort of mRNAs (433) whose translation was enhanced during DENV infection but not by IFN or UPR, which we term the DENV-only gene set (Table S2). These genes may represent specific host cell responses to infection or changes in gene expression driven by DENV itself. Gene ontology analysis of the 433 DENV-only genes revealed the most significant biological processes are linked to the Gene Ontology (GO) categories autophagy, regulation of cell cycle, signal transduction, and cellular metabolism (Fig. 6D and Table S3).
DENV-only and IFN-induced genes differed from the rest of the transcriptome in their means of activation (Fig. 6E). While most transcriptome-wide changes in total translation were driven by changes in mRNA levels, changes in DENV-only genes and IFN-induced genes were primarily driven by changes in translational efficiency. The activation of the UPR was primarily transcriptional, likely through the activation of the UPR-linked transcription factors XBP-1, ATF4, and CHOP (71–73).
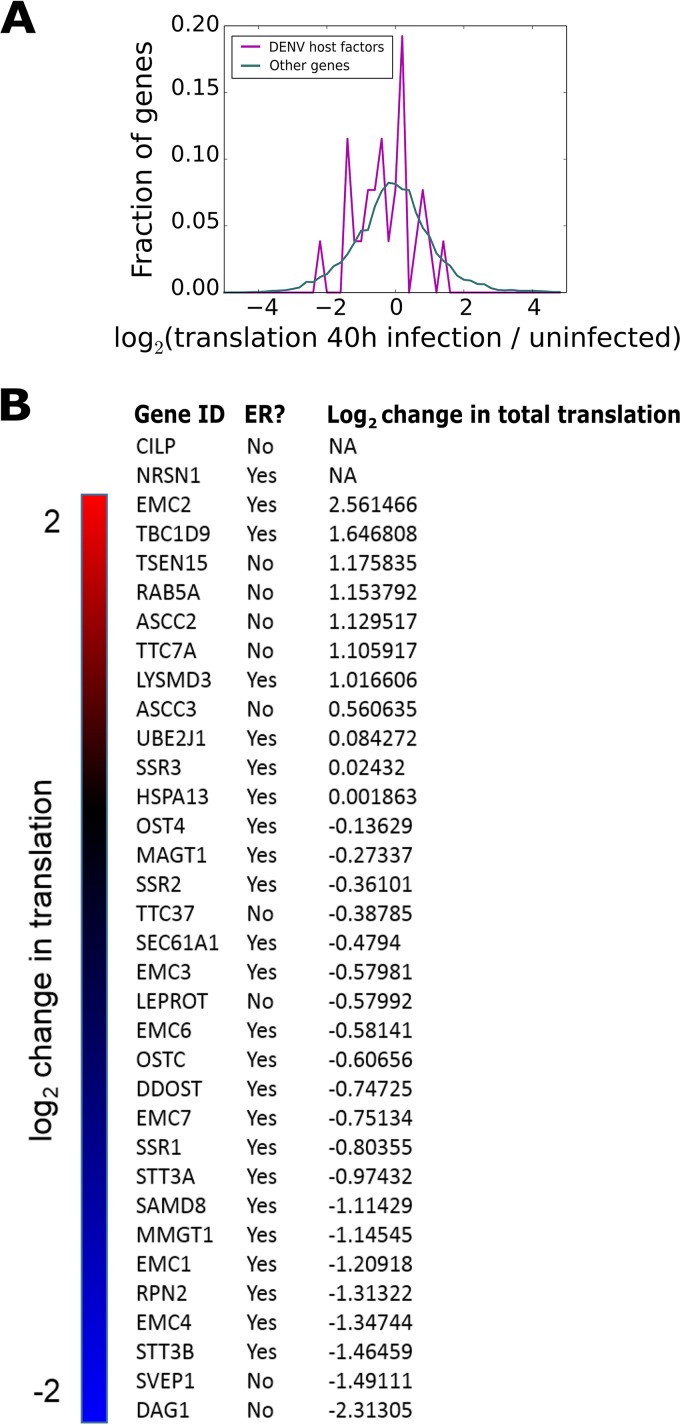
Given the ER-centric translational response to DENV described thus far and recent CRISPR screens for flaviviral host factors identifying primarily ER-resident proteins (27, 28), we examined how DENV infection affects the expression of high-confidence DENV host factors. We focused our analysis on the Marceau et al. (27) screen, as it utilized DENV serotype 2 and Huh-7 cells, as in the current study (Table S4) (27). This analysis revealed many of the CRISPR-identified essential host factors to be translationally downregulated, whereas host genes were, on average, unchanged (Fig. 7A). Specifically, of the 23 ER-resident CRISPR-identified host factor genes also present in our ribosome footprinting data set, 17 genes were translationally downregulated at 40 h postinfection [log2(Ribo-seq RPKM at 40 h/Ribo-seq RPKM in uninfected) < 0] and 6 were translationally upregulated [log2(Ribo-seq RPKM at 40 h/Ribo-seq RPKM in uninfected) > 0], although this host factor gene set is not substantially up- or downregulated (Fig. 7B). Non-ER-resident CRISPR-identified host factors did not have a particular bias for up- or downregulation (5 genes and 4 genes, respectively). The same trends in changes to translation for these CRISPR-identified host factors were seen at earlier time points, though to a lesser magnitude, as was observed with global translational changes (Fig. 6A). It is also of note that the changes in translation of CRISPR-identified host factors during DENV infection do not correlate with changes in their RNA levels, suggesting transcript-specific regulation of translation (Table S1).
FIG 7.
Translational changes of CRISPR-identified host factors during DENV infection. (A) Histogram showing changes in total translation for CRISPR-identified essential genes for DENV2 as determined in Marceau et al. (27). Genes that were essential for DENV replication and with a RIGER score of >1 were operationally scored as essential, while all other genes were scored as nonessential. (B) List of log2 change in translation for CRISPR-identified essential genes for DENV2 as determined in Marceau et al. (27), with a RIGER score of >1, after 40 h of infection. These values were calculated as described in Materials and Methods. The ER localization status of each gene product is also indicated.
DISCUSSION
Whereas the general trajectory and biochemical machinery of DENV replication are increasingly well understood (12, 74), major gaps in our understanding of how DENV coordinately regulates the synthesis of its RNAs and proteins remain. In addition, the fundamental question of how DENV plus-strand RNA competes for host cell translation capacity is largely unknown. Here, we mapped the landscape of transcriptional and translational responses to DENV infection in the host and mapped the succession and subcellular organization of the RNA replication and protein synthesis events that define the DENV life cycle. DENV executes a major annexation of translation on the ER, substantially reducing the translation of most host ER-targeted mRNAs. In addition to sequestering ER-associated ribosomes, the very low translation efficiency of DENV plus-strand RNA identified here may represent a strategy for minimizing the proteostatic stress on the ER protein-folding machinery, thereby limiting activation of the unfolded protein response, with its attendant PERK-mediated suppression of cap-dependent translation and general protein synthesis (72).
Combining the findings obtained in RNA-seq and Ribo-seq analysis of RNA abundance and translational status in the cytosol and ER compartments of DENV-infected human cells, a temporal order of molecular events was documented. Following viral RNA entry into the cytosol, the primary activity of DENV is minus-strand RNA synthesis. This activity, however, must be preceded by plus-strand translation for synthesis of the NS5 RNA polymerase. Once a critical concentration of DENV proteins is accumulated, the early commitment to minus-strand RNA synthesis serves as an investment that supports plus-strand replication and virion biogenesis. As infection progresses, minus-strand RNA synthesis drops and is replaced by two primary functions: robust translation of plus-strand RNA and rapid synthesis of additional plus-strand RNA from the now-abundant minus-strand template. Following the continued buildup of DENV proteins and RNA, a population of untranslated (ribosome-free) plus-strand RNA begins to populate the cytosol, likely representing virions in the process of secretion. These data therefore reinforce the concept that the two functions of the plus-strand DENV RNA, a template for minus-strand synthesis and an mRNA for translation, are in direct competition and are temporally skewed; synthesis of minus-strand RNA from the plus-strand template is prioritized through the 6-h time point, whereas translation of the plus strand dominates thereafter.
The critical processes of DENV protein and RNA synthesis are contingent upon the virus's ability to co-opt the structure and activity of the ER. Data included here demonstrate the localization of the vast majority of the viral RNA to the ER, including the minus-strand RNA, which is untranslated and not captured in nascent viral particles. Strikingly, and as further evidence of the importance of an ER-restricted life cycle, DENV RNAs were enriched on the ER to a greater degree than host ER-targeted mRNAs (31), suggesting that there exist DENV-specific mechanisms for ensuring the highly efficient partitioning and anchoring of the plus-strand RNA to the ER. Nonstructural DENV proteins, many of which are themselves integral membrane proteins, may serve important functions in this RNA anchoring process. It is also possible that DENV co-opts previously identified host cell factors that function in mRNA anchoring to the ER (40, 75, 76).
Given the intricate nature of DENV transmembrane domain synthesis and the complex polytopic topology of the polyprotein, the low translational efficiency of DENV RNA identified here may be adaptive, as it could serve as a “kinetic trap” and thereby divert ribosomes from host mRNAs to the DENV plus-strand RNA translation. Such inefficient translation may also be adaptive from the viewpoint of ER proteostasis. For example, if plus-strand RNA translation were to be highly efficient, the increased protein-folding load on the ER would be expected to trigger activation of the UPR, leading to suppression of protein synthesis. In contrast, inefficient translation as an adaptive feature would allow for abundant plus-strand RNA for virion production while avoiding deleterious levels of UPR activation. Here, we do not observe an acute or pronounced activation of the UPR but rather a slow increase in the transcription and translation of select UPR-associated genes (Fig. 6B and C). Such a model would be consistent with earlier findings that DENV infection intersects with the UPR pathway in a complex and temporally selective manner (16). The inefficiency of DENV translation likely reflects, at least in part, its highly structured 5′ UTR and a low rate of translation initiation (47, 48). These characteristics distinguish DENV plus-strand RNA from other plus-strand RNA viruses, such as the coronavirus MHV, whose single-stranded RNA genome is translated at an efficiency similar to that of host transcripts (49).
The means by which DENV controls host gene expression also reveals a highly ER-centric strategy. Over the course of DENV infection, non-DENV membrane protein synthesis is reduced at multiple levels. Thus, on the ER there is a significant reduction in the translation of these host mRNAs relative to mRNAs encoding cytosolic proteins that are also translated on ER-bound ribosomes (30, 31, 39, 77). Although there is a modest impact on host translation generally, the impact that DENV has specifically on host ER translation is large and broadly inhibitory, including a set of previously identified essential host factors (discussed below). While these findings bear similarity to those recently reported by Roth and coworkers (78), the two studies differ in conclusions regarding the overall magnitude of the translational inhibition observed in response to DENV infection. These differences likely reflect different assay systems used to asses translation, and in that regard we note that the magnitude of translational suppression reported by Roth and coworkers via ribopuromycylation assay is similar to that reported here by [35S]Met/Cys incorporation and compartmental analysis of translation via Ribo-seq.
The exceptions to the trend of suppressed translation hint at an important role for translational regulation of host mRNAs by DENV itself, e.g., the enhanced translation of mRNAs encoding components of the secretory pathway likely increases the cellular capacity for secreting DENV virions. How this is accomplished awaits further study and speaks to the emerging view of the ER as a central hub participating in the translation of the mRNA transcriptome, with mRNAs localized and anchored by diverse mechanisms, and the capacity for selective regulation of the translation of mRNA subsets (30).
The view that DENV-directed translational changes contribute to the remodeling of host cell gene expression is supported by comparison of the ribosome footprinting data of cells infected with DENV versus cells treated with IFN-β or thapsigargin, which activate interferon response pathways or UPR, respectively. These two cellular response pathways are associated with flavivirus infection and could be the driving factors for the translational responses observed during DENV infection (Fig. 6). In this comparison of transcriptionally activated genes, however, only subsets of IFN-activated and UPR-associated genes are translationally upregulated during DENV infection. The DENV-only subset of genes is generally related to regulation of catabolic processes (see Table S3 in the supplemental material). These biological processes ultimately could favor viral replication and virion production by dedicating cellular anabolic activities to the viral life cycle, replication of viral RNA, and folding and packaging of viral proteins. It should be considered that the specific genes found in the DENV-only category (Table S2) may be used most directly by the virus during its life cycle and could comprise therapeutic targets.
The high-confidence links between ER physiology and the DENV viral life cycle discussed above was also observed in recent genome-wide CRISPR screens for essential flavivirus host factors (27, 28). Interestingly, many of the identified DENV2 host factors in these past studies were found to be translationally repressed in our data sets. Though somewhat counterintuitive, this pattern suggests a novel way of evaluating how pathogens utilize host factors. In a genetic deletion screen, as referenced here, cells experience a complete loss of gene function before they encounter a pathogen. During infection of nongenetically modified cells, however, cells are fully equipped with essential host factors at the start of infection. After the initial infection, two response branches are likely to occur: (i) cells may respond by downregulating specific factors as a strategy to combat the infection, and (ii) the virus may evoke strategies to upregulate host factors that are beneficial to its survival. As the virus has already gained access to the cell and replication and translation have begun before the cell is able to detect and respond to the infection, the evolutionary pressure to develop a mechanism that prevents host translational repression is likely low for most genes. In this way, the virus likely allows for the translational downregulation of host factors required early in infection. It is also likely the virus has developed strategies to upregulate specific factors that are required throughout the viral life cycle. By this logic, host factors identified by loss of function that are translationally repressed during infection may be therapeutically relevant targets to minimize or block initial infection, whereas host factors that are translationally activated during infection may impact viral success at later stages of infection (i.e., when an individual is already infected). This proposed bimodal evaluation of host factors, which considers not only the outcome of the virus but how the protein is regulated during infection, will require experimental validation but may provide an opportunity for insight into the questions of how and when a host factor contributes to the viral life cycle.
Cumulatively, these findings highlight the ER as not only the site of viral replication but also as an organelle that DENV dramatically remodels to fulfill the need for both biogenesis and an exit strategy from the cell. This viral habitat provides not only entry into the secretory pathway but also a distinct environment for translational regulation that DENV controls to optimize conditions for replication (30, 79, 80). Targeting any of these points where DENV interacts with or controls the ER may be a promising area to explore antiviral pharmaceuticals.
MATERIALS AND METHODS
Cells and viruses.
Huh-7 cells (human hepatocarcinoma cells; ATCC) were grown in 4.5 g/liter glucose Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% fetal bovine serum, nonessential amino acids (Gibco), 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco). Cells were cultured at 37°C in a humidified 5% CO2 incubator. DENV strain DENV2-NGC (GenBank accession number M29095.1) was used for experiments. Viruses were grown in C6/36 cells, and titers were determined by standard Vero focus-forming assay.
Viral infection protocol.
Huh-7 cells were plated at a density of 2 × 106 cells per 10-cm2 dish. Cells were infected with the DENV2-NGC strain at an MOI of 10 for 1 h, the virus inocula were removed, and cells were washed once with phosphate-buffered saline (PBS). PBS was then removed and replaced with prewarmed complete media. MOIs were calculated using Vero cell-based titers as noted above. Interferon treatment was performed using recombinant IFN-β1A (Millipore) for 12 h at 500 U/ml.
Cell fractionation.
Cells were treated with 180 μM cycloheximide for 30 s and then washed with cold PBS. Cells then were separated into their cytosolic and ER compartments as previously described (31, 39, 40, 43, 77, 81). Briefly, the cytosol fraction was extracted by addition of a buffer containing 0.03% digitonin, 110 mM potassium acetate (KOAc), 25 mM K-HEPES, pH 7.2, 15 mM MgCl2, and 4 mM CaCl2 to the dish and incubated on ice for 5 min. The buffer was collected, and cells were washed with the same buffer containing 0.0015% digitonin. The first lysis and the wash were combined and represent the cytosolic contents of the cell. The ER fraction then was collected by lysis of the digitonin-extracted cells with an ER lysis buffer containing 2% DDM, 200 mM KOAc, 25 mM K-HEPES, pH 7.2, 15 mM MgCl2, and 4 mM CaCl2.
Ribo-seq and RNA-seq.
Cell lysates were diluted to 100 mM KOAc and treated with 10 μg/ml micrococcal nuclease for 30 min at 37°C. Ribosomes were pelleted by ultracentrifugation through a 0.5 M sucrose cushion in a Beckman TL100 ultracentrifuge using the TLA100.2 rotor (24 min, 90,000 rpm). Ribosomal pellets were subjected to phenol-chloroform extraction, and the RNA was isolated and subsequently treated with polynucleotide kinase (New England BioLabs). Ribosome-protected mRNA fragments then were size selected by acrylamide gel electrophoresis, extracted, and assembled into cDNA libraries as described in previous publications from this laboratory and summarized below (41, 82).
For mRNA-seq, total RNA was isolated from lysates by phenol-chloroform extraction. rRNA was depleted using RiboZero (Illumina). Eluted mRNA was fragmented by resuspending in 100 μl 40 mM Tris-OAc, pH 8.3, 100 mM KOAc, 30 mM MgOAc and heating to 95°C for 10 min. Fragmented RNA was precipitated by addition of NAOAc to 300 mM and 300 μl ethanol, the solution chilled on ice, and RNA collected by centrifugation. The RNA pellet was resuspended in a 10-μl solution containing 10 mM ATP, 10 U polynucleotide kinase (New England BioLabs), and 1× PNK buffer. This solution was incubated at 37°C for 30 min and then heat inactivated at 95°C for 10 min.
Each of the RNA fragment pools was converted into a cDNA library using the NEBNext small RNA library preparation set for Illumina (New England BioLabs) as described by the manufacturer, except using half the recommended volume of all components. cDNA libraries were amplified using 16 cycles of PCR and then pooled and sequenced using a HiSeq 2500 (Illumina).
Analysis of protein and RNA compositions of subcellular fractions.
Huh-7 cells were mock infected or DENV infected (MOI of 10) and fractionated into cytosol and ER fractions as described above. Fractions were either subjected to trichloroacetic acid (TCA) precipitation to recover the protein fraction or extracted with TRIzol to obtain the total RNA fraction. To analyze protein distributions in the two subfractions, samples were resuspended in SDS-PAGE sample buffer, separated on 12.5% SDS-PAGE gels, and transferred to nitrocellulose membranes, and protein distributions were analyzed by immunoblotting using the following monoclonal antibodies: DSHB-hGAPDH-2G7 against GAPDH, 6G7 against tubulin, 6G7, and rabbit polyclonal antisera recognizing ribophorin I and TRAPα. Monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, Iowa. Rabbit antisera were generated by immunization with keyhole limpet hemocyanin-synthetic peptide conjugates and were characterized in prior reports from the Nicchitta laboratory (36, 39, 40). For analysis of viral protein expression, Huh7 cells were plated at 3 × 105 cells in a six-well dish and infected the following day with DENV2-NGC at an MOI of 10, as described above. Infection was allowed to carry on for 36 h, and then cells were fractionated into cytoplasmic and ER fractions as described above. Proteins were TCA precipitated and resuspended in 1× LDS loading buffer (Novex). Proteins were heated at 95°C for 5 min, and the same volume of lysate for each compartment was separated on a 4 to 12% SDS-PAGE gel (Novex). The proteins were transferred to nitrocellulose membranes, and expressed proteins were detected using antibodies against C, prM, E, NS1, NS2B, NS4B, NS3, or NS5 (Genetex) and fluorescence-based detection (LI-COR).
To assess RNA compositions, samples were separated on agarose gels, stained with SYBR green II, and imaged on a GE Healthcare Amersham Imager 600.
Metabolic labeling of tissue culture cells.
Huh-7 cells were plated at 3 × 106 cells per well in a six-well dish and infected as described above. At the end of infections, cells were incubated in methionine- and cysteine-free medium for 30 min to deplete internal pools of these amino acids. Cells then were labeled by addition of 0.2 mCi/ml [35S]Met/Cys medium for 30 min, washed with PBS three times, and lysed with a buffer consisting of 400 mM KOAc, 15 mM Mg(OAc)2, 25 mM HEPES, pH 7.6, 1% NP-40, and 1 mM dithiothreitol. Proteins were TCA precipitated and resuspended in 1× LDS loading buffer (Novex). Proteins were separated on a 4 to 12% acrylamide gel (Novex) and dried, and the gels were phosphorimaged using a GE Typhoon Trio.
Data analysis.
Reads were first trimmed of their 3′ adapters using Cutadapt (83). A reference transcriptome was generated with TopHat and Cufflinks (84), using combined RNA-seq data to generate a consensus transcriptome from Refseq release 68. The most abundant isoform of each gene was selected and compiled into a reference transcriptome. All reads then were mapped using Bowtie (85), allowing no mismatches. Reads within the coding sequence were counted and normalized by coding sequence length and library size to give total translation and mRNA counts. Genes where fewer than 4 reads were mapped were discarded for that sample. sfRNA levels were determined via the equation (3′ UTR read density/CDS read density) × RPKM (DENV CDS).
To calculate the rates of change in DENV RNA levels, changes in RNA levels were fitted to the exponential growth model yt+1 = yt × ekΔt, where y is the RNA level time, t, and k is the growth rate. This equation was solved for k and converted to a percentage: k = 100 × ln(yt+1/yt)/Δt.
Relative contributions of mRNA levels and ribosome loading to overall changes in ribosome footprinting data were performed as described in reference 86, where the percentage of change driven by mRNA levels is calculated by the geometric mean of correlations between RNA-seq fold changes and ribosome footprinting fold changes, divided by the correlations between ribosome footprinting replicates. Changes in ribosome loading are inferred to contribute the remainder of the fold change.
Accession number(s).
All sequencing data are available under Gene Expression Omnibus (GEO) accession number GSE69602.
Supplementary Material
ACKNOWLEDGMENTS
We thank the members of the Nicchitta, Garcia-Blanco, and Vasudevan laboratories, as well as Shirish Shenolikar at Duke-NUS Medical School, for providing an energizing intellectual environment and critical feedback on the manuscript.
This work was supported through Duke/Duke-NUS Research Collaboration Awards 2014/0013 and 2016/0025 (C.V.N. and S.G.V.), NMRC/MOHIAFCat1/0018/2014 (S.G.V.), NIH RO1AI089526 and RO1AI101431 (M.A.G.-B.), and NIH GM101533-05A1 and GM118630-01A1 (C.V.N.). We thank Shirish Shenolikar, Duke-NUS, for partial funding of salary support for D.R. and the National Science Foundation Graduate Research Fellowship Program Grant (no. DGS-1644868) for funding tuition and stipend for J.C. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JVI.01766-17.
REFERENCES
- 1.Gingras AC, Svitkin Y, Belsham GJ, Pause A, Sonenberg N. 1996. Activation of the translational suppressor 4E-BP1 following infection with encephalomyocarditis virus and poliovirus. Proc Natl Acad Sci U S A 93:5578–5583. doi: 10.1073/pnas.93.11.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borman AM, Michel YM, Kean KM. 2001. Detailed analysis of the requirements of hepatitis A virus internal ribosome entry segment for the eukaryotic initiation factor complex eIF4F. J Virol 75:7864–7871. doi: 10.1128/JVI.75.17.7864-7871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuyumcu-Martinez M, Belliot G, Sosnovtsev SV, Chang KO, Green KY, Lloyd RE. 2004. Calicivirus 3C-like proteinase inhibits cellular translation by cleavage of poly(A)-binding protein. J Virol 78:8172–8182. doi: 10.1128/JVI.78.15.8172-8182.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komarova AV, Real E, Borman AM, Brocard M, England P, Tordo N, Hershey JW, Kean KM, Jacob Y. 2007. Rabies virus matrix protein interplay with eIF3, new insights into rabies virus pathogenesis. Nucleic Acids Res 35:1522–1532. doi: 10.1093/nar/gkl1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He B, Gross M, Roizman B. 1997. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci U S A 94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aoyagi M, Gaspar M, Shenk TE. 2010. Human cytomegalovirus UL69 protein facilitates translation by associating with the mRNA cap-binding complex and excluding 4EBP1. Proc Natl Acad Sci U S A 107:2640–2645. doi: 10.1073/pnas.0914856107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abernathy E, Gilbertson S, Alla R, Glaunsinger B. 2015. Viral nucleases induce an mRNA degradation-transcription feedback loop in mammalian cells. Cell Host Microbe 18:243–253. doi: 10.1016/j.chom.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh D, Mathews MB, Mohr I. 2013. Tinkering with translation: protein synthesis in virus-infected cells. Cold Spring Harbor Perspectives Biol 5:a012351. doi: 10.1101/cshperspect.a012351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leong K, Lee W, Berk AJ. 1990. High-level transcription from the adenovirus major late promoter requires downstream binding sites for late-phase-specific factors. J Virol 64:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guarino LA, Summers MD. 1986. Interspersed homologous DNA of Autographa californica nuclear polyhedrosis virus enhances delayed-early gene expression. J Virol 60:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh D, Mohr I. 2011. Viral subversion of the host protein synthesis machinery. Nat Rev Microbiol 9:860–875. doi: 10.1038/nrmicro2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paranjape SM, Harris E. 2010. Control of dengue virus translation and replication. Curr Top Microbiol Immunol 338:15–34. [DOI] [PubMed] [Google Scholar]
- 13.Screaton G, Mongkolsapaya J, Yacoub S, Roberts C. 2015. New insights into the immunopathology and control of dengue virus infection. Nat Rev Immunol 15:745–759. doi: 10.1038/nri3916. [DOI] [PubMed] [Google Scholar]
- 14.Diamond MS, Pierson TC. 2015. Molecular insight into dengue virus pathogenesis and its implications for disease control. Cell 162:488–492. doi: 10.1016/j.cell.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edgil D, Polacek C, Harris E. 2006. Dengue virus utilizes a novel strategy for translation initiation when cap-dependent translation is inhibited. J Virol 80:2976–2986. doi: 10.1128/JVI.80.6.2976-2986.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pena J, Harris E. 2011. Dengue virus modulates the unfolded protein response in a time-dependent manner. J Biol Chem 286:14226–14236. doi: 10.1074/jbc.M111.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong H, Fink K, Zust R, Lim SP, Qin CF, Shi PY. 2014. Flavivirus RNA methylation. J Gen Virol 95:763–778. doi: 10.1099/vir.0.062208-0. [DOI] [PubMed] [Google Scholar]
- 18.Mukhopadhyay S, Kuhn RJ, Rossmann MG. 2005. A structural perspective of the flavivirus life cycle. Nat Rev Microbiol 3:13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- 19.Cologna R, Rico-Hesse R. 2003. American genotype structures decrease dengue virus output from human monocytes and dendritic cells. J Virol 77:3929–3938. doi: 10.1128/JVI.77.7.3929-3938.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klema VJ, Padmanabhan R, Choi KH. 2015. Flaviviral replication complex: coordination between RNA synthesis and 51-RNA capping. Viruses 7:4640–4656. doi: 10.3390/v7082837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gamarnik AV, Andino R. 1998. Switch from translation to RNA replication in a positive-stranded RNA virus. Genes Dev 12:2293–2304. doi: 10.1101/gad.12.15.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villordo SM, Filomatori CV, Sanchez-Vargas I, Blair CD, Gamarnik AV. 2015. Dengue virus RNA structure specialization facilitates host adaptation. PLoS Pathog 11:e1004604. doi: 10.1371/journal.ppat.1004604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villordo SM, Alvarez DE, Gamarnik AV. 2010. A balance between circular and linear forms of the dengue virus genome is crucial for viral replication. RNA 16:2325–2335. doi: 10.1261/rna.2120410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stohlman SA, Wisseman CL Jr, Eylar OR, Silverman DJ. 1975. Dengue virus-induced modifications of host cell membranes. J Virol 16:1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller S, Sparacio S, Bartenschlager R. 2006. Subcellular localization and membrane topology of the dengue virus type 2 non-structural protein 4B. J Biol Chem 281:8854–8863. doi: 10.1074/jbc.M512697200. [DOI] [PubMed] [Google Scholar]
- 26.Heaton NS, Mosca F, Fenouil R, Gardner TJ, Aguirre S, Shah PS, Zhou N, Manganaro L, Hultquist JF, Noel J, Sachs DH, Hamilton J, Leon PE, Chawdury A, Tripathi S, Melegari C, Campisi L, Hai R, Meteveli G, Gamarnik AV, Garcia-Sastre A, Greenbaum B, Simon V, Fernandez-Sesma A, Krogan NJ, Mulder LC, van Bakel H, Tortorella D, Taunton J, Palese P, Marazzi I. 2016. Targeting viral proteostasis limits influenza virus, HIV, and dengue virus infection. Immunity 44:46–58. doi: 10.1016/j.immuni.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marceau CD, Puschnik AS, Majzoub K, Ooi YS, Brewer SM, Fuchs G, Swaminathan K, Mata MA, Elias JE, Sarnow P, Carette JE. 2016. Genetic dissection of Flaviviridae host factors through genome-scale CRISPR screens. Nature 535:159–163. doi: 10.1038/nature18631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang R, Miner JJ, Gorman MJ, Rausch K, Ramage H, White JP, Zuiani A, Zhang P, Fernandez E, Zhang Q, Dowd KA, Pierson TC, Cherry S, Diamond MS. 2016. A CRISPR screen defines a signal peptide processing pathway required by flaviviruses. Nature 535:164–168. doi: 10.1038/nature18625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staudacher JJ, Naarmann-de Vries IS, Ujvari SJ, Klinger B, Kasim M, Benko E, Ostareck-Lederer A, Ostareck DH, Bondke Persson A, Lorenzen S, Meier JC, Bluthgen N, Persson PB, Henrion-Caude A, Mrowka R, Fahling M. 2015. Hypoxia-induced gene expression results from selective mRNA partitioning to the endoplasmic reticulum. Nucleic Acids Res 43:3219–3236. doi: 10.1093/nar/gkv167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reid DW, Nicchitta CV. 2015. Diversity and selectivity in mRNA translation on the endoplasmic reticulum. Nat Rev Mol Cell Biol 16:221–231. doi: 10.1038/nrm3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reid DW, Nicchitta CV. 2012. Primary role for endoplasmic reticulum-bound ribosomes in cellular translation identified by ribosome profiling. J Biol Chem 287:5518–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stephens SB, Nicchitta CV. 2008. Divergent regulation of protein synthesis in the cytosol and endoplasmic reticulum compartments of mammalian cells. Mol Biol Cell 19:623–632. doi: 10.1091/mbc.E07-07-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diehn M, Eisen MB, Botstein D, Brown PO. 2000. Large-scale identification of secreted and membrane-associated gene products using DNA microarrays. Nat Genet 25:58–62. doi: 10.1038/75603. [DOI] [PubMed] [Google Scholar]
- 34.Ingolia NT. 2014. Ribosome profiling: new views of translation, from single codons to genome scale. Nat Rev Genet 15:205–213. doi: 10.1038/nrg3645. [DOI] [PubMed] [Google Scholar]
- 35.Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. 2009. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 324:218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jagannathan S, Nwosu C, Nicchitta CV. 2011. Analyzing mRNA localization to the endoplasmic reticulum via cell fractionation. Methods Mol Biol 714:301–321. doi: 10.1007/978-1-61779-005-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inoue T, Tsai B. 2013. How viruses use the endoplasmic reticulum for entry, replication, and assembly. Cold Spring Harb Perspect Biol 5:a013250. doi: 10.1101/cshperspect.a013250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welsch S, Miller S, Romero-Brey I, Merz A, Bleck CK, Walther P, Fuller SD, Antony C, Krijnse-Locker J, Bartenschlager R. 2009. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe 5:365–375. doi: 10.1016/j.chom.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lerner RS, Seiser RM, Zheng T, Lager PJ, Reedy MC, Keene JD, Nicchitta CV. 2003. Partitioning and translation of mRNAs encoding soluble proteins on membrane-bound ribosomes. RNA 9:1123–1137. doi: 10.1261/rna.5610403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jagannathan S, Hsu JC, Reid DW, Chen Q, Thompson WJ, Moseley AM, Nicchitta CV. 2014. Multifunctional roles for the protein translocation machinery in RNA anchoring to the endoplasmic reticulum. J Biol Chem 289:25907–25924. doi: 10.1074/jbc.M114.580688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reid DW, Chen Q, Tay AS, Shenolikar S, Nicchitta CV. 2014. The unfolded protein response triggers selective mRNA release from the endoplasmic reticulum. Cell 158:1362–1374. doi: 10.1016/j.cell.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stephens SB, Dodd RD, Brewer JW, Lager PJ, Keene JD, Nicchitta CV. 2005. Stable ribosome binding to the endoplasmic reticulum enables compartment-specific regulation of mRNA translation. Mol Biol Cell 16:5819–5831. doi: 10.1091/mbc.E05-07-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stephens SB, Dodd RD, Lerner RS, Pyhtila BM, Nicchitta CV. 2008. Analysis of mRNA partitioning between the cytosol and endoplasmic reticulum compartments of mammalian cells. Methods Mol Biol 419:197–214. doi: 10.1007/978-1-59745-033-1_14. [DOI] [PubMed] [Google Scholar]
- 44.Junjhon J, Lausumpao M, Supasa S, Noisakran S, Songjaeng A, Saraithong P, Chaichoun K, Utaipat U, Keelapang P, Kanjanahaluethai A, Puttikhunt C, Kasinrerk W, Malasit P, Sittisombut N. 2008. Differential modulation of prM cleavage, extracellular particle distribution, and virus infectivity by conserved residues at nonfurin consensus positions of the dengue virus pr-M junction. J Virol 82:10776–10791. doi: 10.1128/JVI.01180-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu ZY, Li XF, Jiang T, Deng YQ, Ye Q, Zhao H, Yu JY, Qin CF. 2016. Viral RNA switch mediates the dynamic control of flavivirus replicase recruitment by genome cyclization. Elife 5:e17636. doi: 10.7554/eLife.17636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Araujo PR, Yoon K, Ko D, Smith AD, Qiao M, Suresh U, Burns SC, Penalva LO. 2012. Before it gets started: regulating translation at the 5′ UTR. Comp Funct Genomics 2012:475731. doi: 10.1155/2012/475731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gebhard LG, Filomatori CV, Gamarnik AV. 2011. Functional RNA elements in the dengue virus genome. Viruses 3:1739–1756. doi: 10.3390/v3091739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iglesias NG, Gamarnik AV. 2011. Dynamic RNA structures in the dengue virus genome. RNA Biol 8:249–257. doi: 10.4161/rna.8.2.14992. [DOI] [PubMed] [Google Scholar]
- 49.Irigoyen N, Firth AE, Jones JD, Chung BY, Siddell SG, Brierley I. 2016. High-resolution analysis of coronavirus gene expression by RNA sequencing and ribosome profiling. PLoS Pathog 12:e1005473. doi: 10.1371/journal.ppat.1005473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Firth AE, Blitvich BJ, Wills NM, Miller CL, Atkins JF. 2010. Evidence for ribosomal frameshifting and a novel overlapping gene in the genomes of insect-specific flaviviruses. Virology 399:153–166. doi: 10.1016/j.virol.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Firth AE, Brierley I. 2012. Non-canonical translation in RNA viruses. J Gen Virol 93:1385–1409. doi: 10.1099/vir.0.042499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Markoff L. 1989. In vitro processing of dengue virus structural proteins: cleavage of the pre-membrane protein. J Virol 63:3345–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clum S, Ebner KE, Padmanabhan R. 1997. Cotranslational membrane insertion of the serine proteinase precursor NS2B-NS3(Pro) of dengue virus type 2 is required for efficient in vitro processing and is mediated through the hydrophobic regions of NS2B. J Biol Chem 272:30715–30723. doi: 10.1074/jbc.272.49.30715. [DOI] [PubMed] [Google Scholar]
- 54.Pijlman GP, Funk A, Kondratieva N, Leung J, Torres S, van der Aa L, Liu WJ, Palmenberg AC, Shi PY, Hall RA, Khromykh AA. 2008. A highly structured, nuclease-resistant, noncoding RNA produced by flaviviruses is required for pathogenicity. Cell Host Microbe 4:579–591. doi: 10.1016/j.chom.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 55.Manokaran G, Finol E, Wang C, Gunaratne J, Bahl J, Ong EZ, Tan HC, Sessions OM, Ward AM, Gubler DJ, Harris E, Garcia-Blanco MA, Ooi EE. 2015. Dengue subgenomic RNA binds TRIM25 to inhibit interferon expression for epidemiological fitness. Science 350:217–221. doi: 10.1126/science.aab3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pyhtila B, Zheng T, Lager PJ, Keene JD, Reedy MC, Nicchitta CV. 2008. Signal sequence- and translation-independent mRNA localization to the endoplasmic reticulum. RNA 14:445–453. doi: 10.1261/rna.721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Diehn M, Bhattacharya R, Botstein D, Brown PO. 2006. Genome-scale identification of membrane-associated human mRNAs. PLoS Genet 2:e11. doi: 10.1371/journal.pgen.0020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arava Y, Boas FE, Brown PO, Herschlag D. 2005. Dissecting eukaryotic translation and its control by ribosome density mapping. Nucleic Acids Res 33:2421–2432. doi: 10.1093/nar/gki331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rogers DW, Bottcher MA, Traulsen A, Greig D. 2017. Ribosome reinitiation can explain length-dependent translation of messenger RNA. PLoS Comput Biol 13:e1005592. doi: 10.1371/journal.pcbi.1005592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beckmann R, Spahn CM, Frank J, Blobel G. 2001. The active 80S ribosome-Sec61 complex. Cold Spring Harbor Symp Quant Biol 66:543–554. doi: 10.1101/sqb.2001.66.543. [DOI] [PubMed] [Google Scholar]
- 61.Menetret JF, Hegde RS, Aguiar M, Gygi SP, Park E, Rapoport TA, Akey CW. 2008. Single copies of Sec61 and TRAP associate with a nontranslating mammalian ribosome. Structure 16:1126–1137. doi: 10.1016/j.str.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Voorhees RM, Fernandez IS, Scheres SH, Hegde RS. 2014. Structure of the mammalian ribosome-Sec61 complex to 3.4 A resolution. Cell 157:1632–1643. doi: 10.1016/j.cell.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pena J, Harris E. 2012. Early dengue virus protein synthesis induces extensive rearrangement of the endoplasmic reticulum independent of the UPR and SREBP-2 pathway. PLoS One 7:e38202. doi: 10.1371/journal.pone.0038202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perera R, Kuhn RJ. 2008. Structural proteomics of dengue virus. Curr Opin Microbiol 11:369–377. doi: 10.1016/j.mib.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo D, Xu T, Hunke C, Gruber G, Vasudevan SG, Lescar J. 2008. Crystal structure of the NS3 protease-helicase from dengue virus. J Virol 82:173–183. doi: 10.1128/JVI.01788-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chambers TJ, Nestorowicz A, Amberg SM, Rice CM. 1993. Mutagenesis of the yellow fever virus NS2B protein: effects on proteolytic processing, NS2B-NS3 complex formation, and viral replication. J Virol 67:6797–6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Falgout B, Pethel M, Zhang YM, Lai CJ. 1991. Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J Virol 65:2467–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Umareddy I, Pluquet O, Wang QY, Vasudevan SG, Chevet E, Gu F. 2007. Dengue virus serotype infection specifies the activation of the unfolded protein response. Virol J 4:91. doi: 10.1186/1743-422X-4-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walter P, Ron D. 2011. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 70.Iwakoshi NN, Lee AH, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH. 2003. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol 4:321–329. doi: 10.1038/ni907. [DOI] [PubMed] [Google Scholar]
- 71.Han J, Kaufman RJ. 2017. Physiological/pathological ramifications of transcription factors in the unfolded protein response. Genes Dev 31:1417–1438. doi: 10.1101/gad.297374.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ron D, Walter P. 2007. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 73.Schroder M, Kaufman RJ. 2005. The mammalian unfolded protein response. Annu Rev Biochem 74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 74.Bidet K, Garcia-Blanco MA. 2014. Flaviviral RNAs: weapons and targets in the war between virus and host. Biochem J 462:215–230. doi: 10.1042/BJ20140456. [DOI] [PubMed] [Google Scholar]
- 75.Chen Q, Jagannathan S, Reid DW, Zheng T, Nicchitta CV. 2011. Hierarchical regulation of mRNA partitioning between the cytoplasm and the endoplasmic reticulum of mammalian cells. Mol Biol Cell 22:2646–2658. doi: 10.1091/mbc.E11-03-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cui XA, Palazzo AF. 2014. Localization of mRNAs to the endoplasmic reticulum. Wiley Interdiscip Rev RNA 5:481–492. doi: 10.1002/wrna.1225. [DOI] [PubMed] [Google Scholar]
- 77.Lerner RS, Nicchitta CV. 2006. mRNA translation is compartmentalized to the endoplasmic reticulum following physiological inhibition of cap-dependent translation. RNA 12:775–789. doi: 10.1261/rna.2318906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roth H, Magg V, Uch F, Mutz P, Klein P, Haneke K, Lohmann V, Bartenschlager R, Fackler OT, Locker N, Stoecklin G, Ruggieri A. 2017. Flavivirus infection uncouples translation suppression from cellular stress responses. mBio 8:e02150-16. doi: 10.1128/mBio.02150-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li S, Liu L, Zhuang X, Yu Y, Liu X, Cui X, Ji L, Pan Z, Cao X, Mo B, Zhang F, Raikhel N, Jiang L, Chen X. 2013. MicroRNAs inhibit the translation of target mRNAs on the endoplasmic reticulum in Arabidopsis. Cell 153:562–574. doi: 10.1016/j.cell.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stalder L, Heusermann W, Sokol L, Trojer D, Wirz J, Hean J, Fritzsche A, Aeschimann F, Pfanzagl V, Basselet P, Weiler J, Hintersteiner M, Morrissey DV, Meisner-Kober NC. 2013. The rough endoplasmatic reticulum is a central nucleation site of siRNA-mediated RNA silencing. EMBO J 32:1115–1127. doi: 10.1038/emboj.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stephens SB, Nicchitta CV. 2007. In vitro and tissue culture methods for analysis of translation initiation on the endoplasmic reticulum. Methods Enzymol 431:47–60. doi: 10.1016/S0076-6879(07)31004-5. [DOI] [PubMed] [Google Scholar]
- 82.Reid DW, Shenolikar S, Nicchitta CV. 2015. Simple and inexpensive ribosome profiling analysis of mRNA translation. Methods 91:69–74. doi: 10.1016/j.ymeth.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martin M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17:10–12. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 84.Pollier J, Rombauts S, Goossens A. 2013. Analysis of RNA-Seq data with TopHat and Cufflinks for genome-wide expression analysis of jasmonate-treated plants and plant cultures. Methods Mol Biol 1011:305–315. doi: 10.1007/978-1-62703-414-2_24. [DOI] [PubMed] [Google Scholar]
- 85.Langmead B, Trapnell C, Pop M, Salzberg SL. 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jovanovic M, Rooney MS, Mertins P, Przybylski D, Chevrier N, Satija R, Rodriguez EH, Fields AP, Schwartz S, Raychowdhury R, Mumbach MR, Eisenhaure T, Rabani M, Gennert D, Lu D, Delorey T, Weissman JS, Carr SA, Hacohen N, Regev A. 2015. Immunogenetics. Dynamic profiling of the protein life cycle in response to pathogens. Science 347:1259038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.