Abstract
背景与目的
表皮生长因子受体-酪氨酸激酶抑制剂(epidermal growth factor receptor-tyrosine kinase inhibitors, EGFR-TKIs)是EGFR基因突变晚期非小细胞癌(non-small cell lung cancer, NSCLC)患者一线标准治疗方案, 但是临床实践中, 疗效差异较大。本项实验拟研究治疗前血清细胞角蛋白19片段(cytokeratin-19 fragments, CYFRA21-1)和癌胚抗原(carcinoembryonic antigen, CEA)的水平是否与EGFR-TKIs疗效有关。
方法
回顾性分析194例EGFR基因突变阳性且接受EGFR-TKIs治疗的NSCLC患者的治疗前的血清CYFRA21-1和CEA水平与EGFR-TKIs疗效及生存时间的关系。
结果
血清水平CYFRA21-1增高和正常的无进展生存时间(progression-free survival, PFS)分别为7.0个月和11.9个月(P < 0.001);总生存(overall survival, OS)分别为12.6个月和28.0个月(P < 0.001)。腺癌中, 血清水平增高和正常的PFS分别为7.0个月和12.0个月(P < 0.001), OS分别为13.1个月和28.1个月(P < 0.001)。鳞癌中, 血清CYFRA21-1水平高低与生存时间无关。治疗前血清CEA水平高低与生存时间无关。
结论
在EGFR突变肺腺癌患者, 治疗前血清水平CYFRA21-1增高组EGFR-TKIs治疗的PFS和OS均较正常组短。EGFR突变肺腺癌患者, 治疗前血清CYFRA21-1水平可以作为预测EGFR-TKIs治疗的疗效指标。
Keywords: 肺肿瘤, 表皮生长因子受体抑制剂, CYFRA21-1, 癌胚抗原
Abstract
Background and objective
Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) are the standard first-line treatment regimen for EGFR mutated non-small cell lung cancer (NSCLC) patients.However, the efficacy of EGFR-TKIs widely varies.The aim of this study is to determine whether the pretreatment serum cytokeratin-19 fragments (CYFRA21-1) and carcinoembryonic antigen (CEA) are associated with the efficacy of EGFR-TKIs in EGFR-mutated NSCLC patients.
Methods
We retrospectively enrolled 194 NSCLC patients harboring EGFR mutations who received EGFR-TKIs.Clinical characteristics were collected, and the relation between the efficacy of EGFR-TKIs and pretreatment serum CYFRA21-1 and CEA was analyzed.
Results
In all cases, progression-free survival (PFS) in patients with high CYFRA21-1 level was significantly shorter than PFS in patients with normal CYFRA21-1 (7.0 vs 11.9 months, P < 0.001).Overall survival (OS) in patients with high CYFRA21-1 was significantly shorter than in the normal-CYFRA21-1 group (12.6 vs 28.0 months, P < 0.001).In adenocarcinoma patients, PFS in the high-CYFRA21-1 level group was significantly shorter than in patients with normal CYFRA21-1 (7.0 vs 12.0 months, P < 0.001).OS in patients with high CYFRA21-1 was significantly shorter than that in the normal-CYFRA21-1 group (13.1 vs 28.1 months, P < 0.001).Among squamous carcinoma patients, CYFRA21-1 level did not affect survival.No significant difference in PFS and OS was observed between patients with high CEA and patients with normal CEA.
Conclusion
EGFR-mutated patients with high CYFRA21-1 had significantly shorter PFS and OS than patients with normal CYFRA21-1 after receiving EGFR-TKIs.Pretreatment serum CYFR21-1 level was a predictive marker of EGFR-TKI treatment in EGFR-mutated NSCLC patients.
Keywords: Lung neoplasms, Epidermal growth factor receptor-tyrosine kinase inhibitor, Cytokeratin 19 fragment, Carcinoembryonic antigen
肺癌是全世界发病率和死亡率最高的恶性肿瘤, 5年生存率仅为16.8%[1]。非小细胞癌(non-small cell lung cancer, NSCLC)约占所有肺癌病理类型85%, 而且 > 70%的患者确诊都是晚期[2]。全身化疗一直是这部分人群主要治疗方法。21世纪初随着分子生物学研究不断深入, 开启了NSCLC以表皮生长因子受体(epidermal growth factor receptor, EGFR)基因突变为指导的靶向治疗时代, 而IPASS、NEJGSG、WJTOG3405、OPTIMAL、EURTAC、LUX-Lun3及ICOGEN等研究, 进一步确立表皮生长因子受体-酪氨酸激酶抑制剂(epidermal growth factor receptor-tyrosine kinase inhibitors, EGFR-TKIs)在EGFR基因突变的NSCLC晚期患者治疗地位[3-9]。
血清癌胚抗原(carcinoembryonic antigen, CEA)和细胞角蛋白19片段(cytokeratin-19 fragments, CYFRA21-1)是最常见肿瘤标志物, 应用于肺癌的诊断、预后和监测。目前认为CEA是肺腺癌较好的肿瘤标志物, 而CYFRA21-1则是肺鳞癌诊断的最好肿瘤标志物。本文回顾性研究伴有EGFR基因的突变NSCLC晚期患者治疗前血清CEA和CYFRA21-1的水平和EGFR-TKIs疗效及预后的关系, 希望给临床EGFR-TKIs靶向治疗的个体化提供参考依据。
1. 资料与方法
1.1. 对象
本研究收集首都医科大学附属北京胸科医院于2010年10月-2015年3月EGFR基因突变且应用EGFR-TKIs治疗的患者194例, 所有患者均通过病理及免疫组化诊断为NSCLC, 并且为Ⅲb期-Ⅳ期或者手术后复发的晚期患者。患者年龄30岁-85岁(中位年龄58岁), 男性86例(44.3%), 女性108例(55.7%)。PS评分0分-1分166例(85.6%), 2分-4分28例(14.4%)。吸烟67例(34.5%), 非吸烟127例(65.5%)。IIIb期患者6例(3.1%), Ⅳ期188例(96.9%), 中心型26例(13.4%), 周围型168例(86.6%)。腺癌179例(92.3%), 鳞癌9例(4.6%), 非小细胞癌3例(1.5%), 腺鳞癌2例(1%), 大细胞癌1例(0.5%)。患者的临床特征见表 1。
1.
194例携带EGFR突变的NSCLC患者的临床特征
Characteristics of the 194 NSCLC patients harboring EGFR mutations
| Characteristics | No.of patients | Percentage(%) |
| EGFR-TKIs:epidermal growth factor receptor-tyrosine kinase inhibitors; NSCLC:non-small cell lung cancer; PS:performance status. | ||
| Age (yr) | ||
| ≤70 | 165 | 85.1 |
| > 70 | 29 | 14.9 |
| Gender | ||
| Male | 86 | 44.3 |
| Female | 108 | 55.7 |
| Histology | ||
| Adenocarcinoma | 179 | 92.3 |
| Squamous | 9 | 4.6 |
| Others | 6 | 3.1 |
| PS | ||
| 0-1 | 166 | 85.6 |
| 2-4 | 28 | 14.4 |
| Staging | ||
| Ⅲb | 6 | 3.1 |
| Ⅳ | 188 | 96.9 |
| Smoking history | ||
| Smoking | 67 | 34.5 |
| Non-smoking | 127 | 65.5 |
| EGFR gene | ||
| Exton 19 (19del) | 112 | 57.7 |
| Exton 21 (L858R) | 72 | 37.1 |
| Others | 10 | 5.2 |
| EGFR-TKIs | ||
| Icotinib | 115 | 59.3 |
| Gefitinib | 44 | 22.7 |
| Erlotinib | 35 | 18 |
| Therapy | ||
| First-line | 117 | 60.3 |
| Second-line | 65 | 33.5 |
| Third-line | 9 | 4.6 |
| Fourth-line | 3 | 1.5 |
1.2. 治疗方法
所有患者都给予埃克替尼或者吉非替尼、厄洛替尼治疗。埃克替尼125 mg, 每日3次口服; 吉非替尼250 mg, 每日1次; 厄洛替尼150 mg每日1次。接受埃克替尼治疗115例(59.3%), 吉非替尼44例(22.7%), 厄洛替尼35例(18%)。
1.3. EGFR基因检测
采取两种方法:一种PCR-Sanger测序法, 由北京海思特临床检验所检测; 一种ARMS荧光定量PCR, 检测试剂为厦门艾德人类EGFR基因突变检测试剂盒。应用PCR-Sanger测序法123例(63.4%), ARMS法71例(36.6%)。标本来源:气管镜活检47例(24.2%), 肺穿刺活检88例(45.4%), 胸水沉淀包埋31例(16%), 手术标本13例(6.7%), 淋巴结活检14例(7.2%), 骨转移穿刺1例(0.5%)。EGFR基因检测结果:19外显子缺失突变112例(57.7%), 21外显子错义突变72例(37.1%), 少见突变3例(G719X)(1.5%), 伴有原发T790m突变1例(0.5%), EGFR基因突变2例(1.0%), 伴有Kras突变2例(1.0%), 19外显子错义突变2例(1.0%)。
1.4. 血清CEA和CYFRA21-1的检测
EGFR-TKIs治疗前清晨空腹静脉血, 分离血清后, 使用上海透景生命科技股份有限公司试剂盒及Luminex多功能流式点阵仪应用流式荧光发光法进行测定。根据我院试剂盒正常参考值进行结果判定:CEA≤6 ng/mL为正常, CEA > 6 ng/mL为表达水平增高; CYFRA21-1≤6 ng/mL为正常, CYFRA21-1 > 6 ng/mL为表达水平增高。
1.5. 疗效评定及生存指标
治疗前2周对疾病状况进行评估, 治疗后4周复查。以后每2个月-3个月随访1次。按实体瘤疗效评价标准(Response Evaluation Criteria in Solid Tumors, RECIST)评价近期疗效, 分为完全缓解(complete response, CR)、部分缓解(partial response, PR)、疾病稳定(stable disease, SD)和疾病进展(progressive disease, PD)。生存指标为无进展生存时间(progression-free survival, PFS)定义为EGFR-TKI治疗开始至疾病进展或未进展死亡的时间。总生存(overall survival, OS)定义为EGFR-TKI治疗开始到死亡或末次随访时间。
1.6. 统计学方法
使用SPSS V22.0软件, 采用Kaplan-Meier法并进行Log-rank检验生存分析, 用Cox比例风险模型进行多因素分析, 所有统计结果以P < 0.05为差异有统计学意义。所有患者均随访至2015年12月31日, 其中死亡103例, 存活90例, 1例失访。死亡病例为截尾数据, 存活病例为未截尾数据。
2. 结果
2.1. EGFR-TKIs疗效及多因素生存分析
本研究中患者的总体疗效:CR、PR、SD、DCR及PD分别为0.5%(1例)、68.6%(133例)、24.2%(47例)、93.3%(181例)和6.7%(13例)。中位PFS为9.0个月(95%CI:7.3-10.7);中位总生存OS为23.0个月(95%CI:20.2-25.8)。单因素生存分析显示:年龄 > 70和年龄≤70岁PFS分别为9.0个月和10.5个月(P=0.495);OS分别为23.0个月和24个月(P=0.441);男性和女性PFS分别为10.5个月和8.2个月(P=0.391);OS分别为24.0个月和21.8个月(P=0.717);吸烟和非吸烟PFS分别为10.5个月和8.9个月(P=0.406);OS分别为24.1个月和21.8个月(P=0.886);19外显子缺失突变和21外显子错义突变PFS分别为9.0个月和8.2个月(P=0.375);OS分别为24.1个月和17.2个月(P=0.143);以上单因素分析均无统计学差异。PS评分0分-1分和2分-4分PFS分别为10.5个月和5.3个月(P < 0.001);OS分别为24.8个月和9.7个月(P < 0.001);腺癌和鳞癌PFS分别为9.0个月和4.1个月(P=0.009);OS分别为23.1个月和8.1个月(P < 0.001);治疗前基线无脑转移和伴有脑转移PFS分别为10.9个月和7.5个月(P=0.001);OS分别为26.1个月和14.3个月(P < 0.001);无肝转移和伴有肝转移PFS分别为10.2个月和7.0个月(P=0.002);OS分别为23.7个月和12.0个月(P=0.008)。上述单因素分析有统计学差异。多因素生存分析显示PS评分状态和是否伴有脑转移PFS和OS都有统计学意义。而组织类型中腺癌和鳞癌PFS无统计学差异, 但在OS还是有统计学差异(表 2, 表 3)。
2.
单因素生存分析
Factor associated with PFS and OS
| Factor | n | Median PFS (m) | P | Median OS (m) | P |
| PFS:progression-free survival; OS:overall survival. | |||||
| Age (yr) | 0.495 | 0.441 | |||
| ≤70 | 165 | 9.0 | 23.0 | ||
| > 70 | 29 | 10.5 | 24.0 | ||
| Gender | 0.391 | 0.717 | |||
| Male | 86 | 10.5 | 24.0 | ||
| Female | 108 | 8.2 | 21.8 | ||
| Smoking hisory | 0.406 | 0.886 | |||
| Smoking | 67 | 10.5 | 24.1 | ||
| Non-smoking | 127 | 8.9 | 21.8 | ||
| PS | < 0.001 | < 0.001 | |||
| 0-1 | 166 | 10.5 | 24.8 | ||
| 2-4 | 28 | 5.3 | 9.7 | ||
| Histology | 0.009 | < 0.001 | |||
| Adenocarcinoma | 179 | 9.0 | 23.1 | ||
| Squamous | 9 | 4.1 | 8.1 | ||
| EGFR gene | 0.375 | 0.143 | |||
| Exton 19 (19del) | 112 | 9.0 | 24.1 | ||
| Exton 21 (L858R) | 72 | 8.2 | 17.2 | ||
| CEA | 0.294 | 0.122 | |||
| ≤6 ng/mL | 69 | 10.2 | 24.0 | ||
| > 6 ng/mL | 125 | 8.9 | 21.8 | ||
| CYFRA21-1 | < 0.001 | < 0.001 | |||
| ≤6 ng/mL | 129 | 11.9 | 28.0 | ||
| > 6 ng/mL | 65 | 7.0 | 12.6 | ||
| Distant metastases | |||||
| Cerebral metastases | 58 | 7.5 | 0.001 | 14.3 | < 0.001 |
| Non-cerebral metastases | 136 | 10.9 | 26.1 | ||
| Hepatic metastases | 19 | 7.0 | 0.002 | 12.0 | 0.008 |
| Non-hepatic metastases metastases | 175 | 10.2 | 23.7 | ||
| EGFR-TKIs | 0.954 | 0.465 | |||
| Lcotinib | 115 | 8.2 | 21.1 | ||
| Gefitinib | 44 | 9.0 | 23.0 | ||
| Erlotinib | 35 | 11.0 | 24.8 | ||
| Method | 0.868 | 0.159 | |||
| PCR-Sanger | 123 | 9.0 | 21.1 | ||
| ARMS | 71 | 10.2 | 23.0 | ||
3.
多因素生存分析
Multivariate analysis of PFS and OS
| Factor | Median PFS | Median OS | |||
| HR (95%CI) | P | HR (95%CI) | P | ||
| PS (0-1 vs 2-4) | 0.53 (0.34-0.82) | 0.005 | 0.33 (0.19-0.54) | < 0.001 | |
| Histology (adenocarcinoma vs squamous) | 0.69 (0.33-1.40) | 0.301 | 0.46 (0.22-0.97) | 0.042 | |
| CEA (≤6 ng/mL vs > 6 ng/mL) | 0.86 (0.61-1.22) | 0.394 | 0.72 (0.47-1.11) | 0.138 | |
| CYFRA21-1 (≤6 ng/mL vs > 6 ng/mL) | 0.62 (0.44-0.87) | 0.006 | 0.30 (0.19-0.47) | < 0.001 | |
| Non vs Cerebral metastases | 0.63 (0.45-0.89) | 0.009 | 0.45 (0.29-0.68) | < 0.001 | |
| Non vs Hepatic metastases | 0.64 (0.37-1.09) | 0.100 | 0.82 (0.44-1.53) | 0.533 | |
2.2. 治疗前血清CYFRA21-1水平与PFS、OS的相关性分析
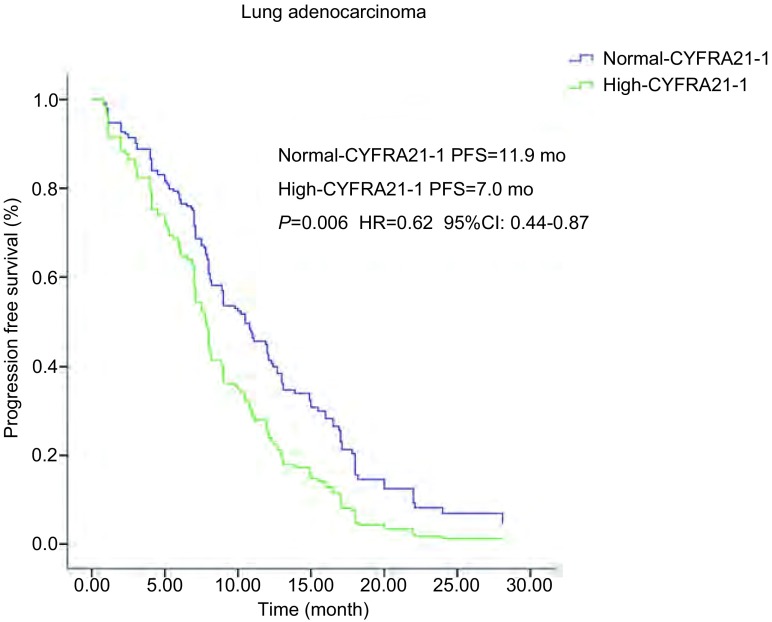
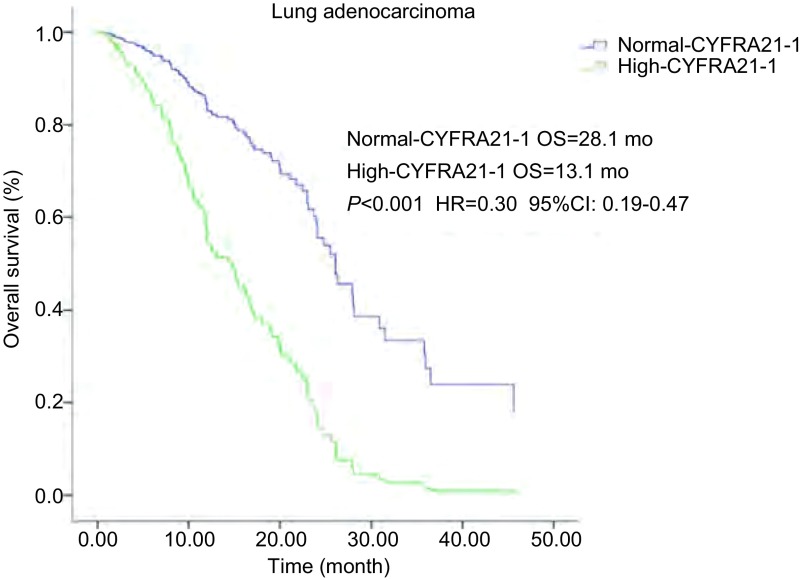
194例患者中129例血清水平CYFRA21-1正常, 65例血清CYFRA21-1水平增高, 血清水平CYFRA21-1正常和增高的PFS分别为11.9个月和7.0个月(P < 0.001), OS分别为28.0个月和12.6个月(P < 0.001), 均有统计学差异(表 2)。亚组分析中, 腺癌组120例血清CYFRA21-1水平正常, 59例血清水平增高, 它们的PFS分别为12.0个月和7.0个月(P < 0.001), OS分别为28.1个月和13.1个月(P < 0.001)均有统计学差异(表 4)。多因素分析中(表 3), PFS(P=0.006, HR=0.62, 95%CI:0.44-0.87)(图 1), OS(P < 0.001, HR=0.30, 95%CI:0.19-0.47)(图 2), 也均有统计学差异。鳞癌组4例血清水平CYFRA21-1正常, 5例血清水平增高, 它们的PFS分别为4.1个月和3.1个月(P=0.529), OS分别为8.1个月和7.0个月(P=0.359), 均无统计学差异。
4.
血清CEA、CYFRA21-1水平亚组生存分析
Subset analysis of PFS and OS
| Factor | n | Median PFS(mo) | P | Median OS(mo) | P | |
| Adenocarcinoma | CEA | 0.436 | 0.104 | |||
| ≤6 ng/mL | 64 | 9.0 | 24.8 | |||
| > 6 ng/mL | 115 | 9.0 | 23.0 | |||
| CYFRA21-1 | < 0.001 | < 0.001 | ||||
| ≤6 ng/mL | 120 | 12.0 | 28.1 | |||
| > 6 ng/mL | 59 | 7.0 | 13.1 | |||
| Squamous | CEA | 0.103 | 0.381 | |||
| ≤6 ng/mL | 2 | 7.8 | 9.1 | |||
| > 6 ng/mL | 7 | 3.1 | 7.0 | |||
| CYFRA21-1 | 0.529 | 0.359 | ||||
| ≤6 ng/mL | 4 | 4.1 | 8.1 | |||
| > 6 ng/mL | 5 | 3.1 | 7.0 |
1.
肺腺癌患者血清CYFRA21-1水平和PFS生存曲线关系。血清CYFRA21-1水平正常和增高PFS分别为12.0个月和7.0个月(P=0.006, HR=0.62, 95%CI:0.44-0.87)。
Kaplan-Meier survival curves of progression-free survival (PFS) according to serum CYFRA21-1 level in lung adenocarcinoma patients.PFS in normal-and high serum CYFRA21-1 level was 12.0 months and 7.0 months, respectively (P=0.006, HR=0.62, 95%CI:0.44-0.87).
2.
肺腺癌患者血清CYFRA21-1水平和OS生存曲线关系。血清CYFRA21-1水平正常和增高OS分别为28.1个月和13.1个月(P < 0.001, HR=0.30, 95%CI:0.19-0.47)。
Kaplan-Meier survival curves of overall survival (OS) according to serum CYFRA21-1 level in lung adenocarcinoma patients.OS in normal-and high serum CYFRA21-1 level was 28.1 months and 13.1 months, respectively (P < 0.001, HR=0.30, 95%CI:0.19-0.47).
2.3. 治疗前血清CEA水平与PFS和OS的相关性分析
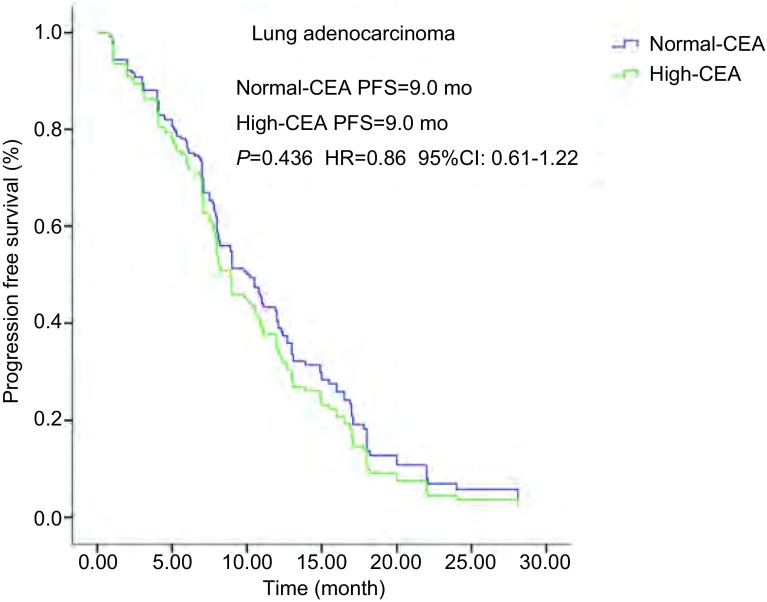
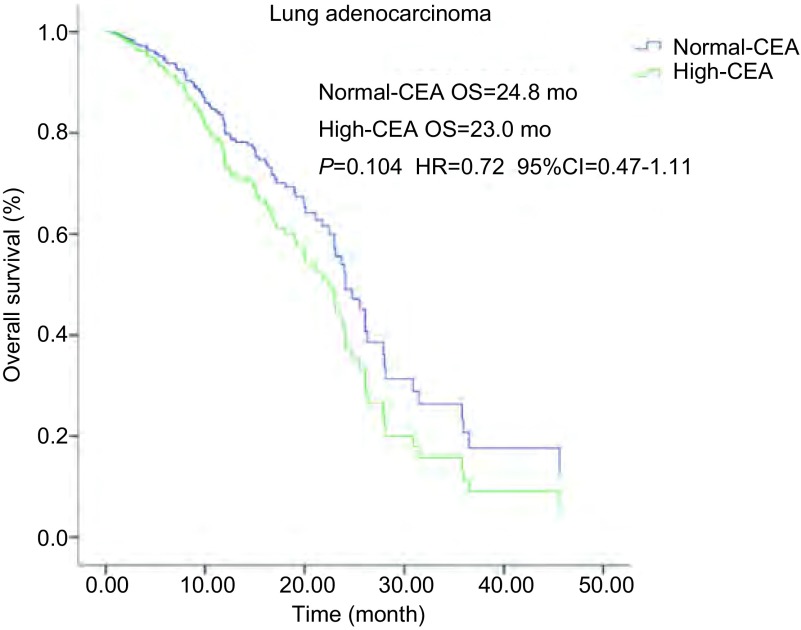
血清CEA水平正常69例, 血清CEA水平增高125例。它们的PFS分别为10.2个月和8.9个月(P=0.294), OS分别为24.0个月和21.8个月(P=0.122)均无统计学差异。腺癌亚组血清CEA水平正常及增高的PFS均为9.0个月(P=0.436), 无统计学差异(图 3); OS分别为24.8个月和23.0个月(P=0.104), 无统计学差异(图 4)。鳞癌亚组血清CEA水平正常2例, 血清水平增高7例; 它们的PFS分别为7.8个月和3.1个月(P=0.103), 无统计学差异; OS分别为9.1个月和7.0个月(P=0.381), 无统计学差异(表 2-表 4)。
3.
肺腺癌患者血清CEA水平和PFS生存曲线关系。血清CEA水平正常和增高PFS均为9.0个月(P=0.436, HR=0.86, 95%CI:0.61-1.22)。
Kaplan-Meier survival curves of progression-free survival (PFS) according to serum CEA level in lung adenocarcinoma patients.PFS in normal-and high serum CEA level both were 9.0 months (P=0.436, HR=0.86, 95%CI:0.61-1.22).
4.
肺腺癌患者, 血清CEA水平和OS生存曲线关系。血清CEA水平正常和增高OS分别为24.8个月和23.0个月(P=0.104, HR=0.72, 95%CI:0.47-1.11)。
Kaplan-Meier survival curves of overall survival (OS) according to serum CEA level in lung adenocarcinoma patients.OS in normal-and high serum CEA level was 24.8 months and 23.0 months, respectively (P=0.104, HR=0.72, 95%CI:0.47-1.11).
3. 讨论
2004年Lynch等[10]研究发现EGFR基因突变状态与EGFR-TKIs的疗效相关, 并且近几年多项国际多中心的临床研究已显示EGFR-TKIs在EGFR基因突变的NSCLC晚期患者有着非常好的疗效及低毒性[3-9]。2012年在美国国立综合癌症网络(National Comprehensive Cancer Network, NCCN)NSCLC临床实践指南推荐对于EGFR基因突变的晚期, 复发或转移的NSCLC患者EGFR-TKIs作为一线治疗。随着对EGFR-TKIs进一步研究, 发现同样有EGFR基因突变, 但不同的临床特征, EGFR-TKIs疗效是有差异的。Lee等[11]meta分析中认为女性, 非吸烟患者, 腺癌及19外显子缺失突变, 相对疗效更好一些。我们的研究显示, 性别、年龄、吸烟状态及EGFR基因敏感突变类型之间无统计学差异。但是PS评分0分-1分较2分-4分疗效更好, 有统计学差异; 腺癌较鳞癌患者EGFR-TKIs疗效更好, 有统计学差异。在合并有远处转移, 有脑转移和肝转移的患者PFS和OS均有缩短。多因素生存分析显示:PS状态差, 鳞癌和伴有脑转移的患者预后差, OS有统计学意义。
CYFRA21-1是我们临床实践中肺癌诊断常用肿瘤标志物。当肿瘤细胞发生溶解时, 其中的细胞角质蛋白释放入血, 而使血中CYFRA21-1升高。其对肿瘤的诊断敏感性约为50%, 且以鳞癌敏感性最高。有报道其对鳞癌的敏感性和特异性分别为66.5%和95%[12], 因此众多学者一致认为CYFR21-1是鳞癌鉴别诊断的最好肿瘤标志物, 其水平高低和敏感度与病情呈正相关。近年Tanaka等[13]提出伴有EGFR突变的NSCLC, 治疗前血清CYFRA21-1水平是预测EGFR-TKIs疗效的指标, 但相关报道国内外还是比较少。我们的研究显示治疗前血清水平CYFRA21-1正常和增高的PFS分别为11.9个月和7.0个月(P < 0.001), OS分别为28.0个月和12.6个月(P < 0.001), 均有差异。亚组分析中, 腺癌组血清CYFRA21-1水平正常和增高的PFS分别为12.0个月和7.0个月(P < 0.001), OS分别为28.1个月和13.1个月(P < 0.001)均有差异。多因素分析中, PFS(P=0.006, HR=0.62, 95%CI:0.44-0.87), OS(P < 0.001, HR=0.30, 95%CI:0.19-0.47)也均有统计学差异。鳞癌组血清水平CYFRA21-1正常和增高的PFS分别为4.1个月和3.1个月(P=0.529), OS分别为8.1个月和7.0个月(P=0.359), 均无差异。我们研究显示伴有EGFR基因突变的肺腺癌患者, 治疗前血清CYFRA21-1水平增高较正常水平相比较, PFS和OS都有缩短; 治疗前血清CYFRA21-1水平可以预测EGFR-TKIs疗效及预后。而肺鳞癌在我们研究中由于病例数较少, 并未显示治疗前血清CYFRA21-1水平与EGFR-TKIs的疗效和预后的关系。
EGFR基因突变, 在肺腺癌患者比较常见, PIONEER临床试验显示东亚肺腺癌患者50.2%的EGFR基因的突变率[14]。而肺鳞癌患者EGFR基因的突变比较少见, Dearden等[15]meta分析中, 东亚肺鳞癌患者EGFR基因的突变率仅为4.6%, 而中国报道肺鳞癌患者EGFR基因的突变则在14%-25%[16-18]。并且在既往的报道EGFR基因突变肺鳞癌患者采用EGFR-TKIs治疗的PFS和OS明显低于肺腺癌, PFS在3个月-7个月, OS在9.4个月-14.7个月[19-21]。本研究中EGFR基因的突变患者鳞癌患者9例, 为4.6%。与肺腺癌相比PFS分别为4.1个月和9.0个月(P=0.009), OS分别为8.1个月和23.1个月(P < 0.001)。肺鳞癌EGFR突变患者临床受益程度明显低于EGFR突变肺腺癌患者, 似乎提示肺鳞癌中具有EGFR突变基因也许并不是靶向治疗的“驱动基因”, 可能存在其他肿瘤驱动机制。肺癌的组织类型较多, 且以腺癌的组织分型最复杂, 异质性最明显。Travis等[22]认为由于肿瘤组织的异质性, 基于小标本的病理组织诊断是有限, 有可能会带来相反的组织类型诊断。并且由于肿瘤组织的异质性, 小标本病理活检有可能未体现出整体的病理组织类型状态。我们的研究患者均为Ⅲb期-Ⅳ期晚期患者, 标本来源:气管镜活检47例(24.2%), 肺穿刺活检88例(45.4%), 胸水沉淀包埋31例(16%), 手术标本13例(6.7%), 淋巴结活检14例(7.2%), 骨转移穿刺1例(0.5%), 绝大部分是小标本的病理活检。肺腺癌患者血清水平CYFRA21-1异常增高, 我们考虑由于肿瘤异质性, 可能混有鳞癌的成份, 而肺鳞癌较肺腺癌相比较, EGFR-TKIs疗效不佳, 那么治疗前血清CYFRA21-1水平异常增高可能带来EGFR-TKIs治疗的不佳疗效。而我们研究也显示伴有EGFR基因突变的肺腺癌者, 治疗前血清CYFRA21-1水平增高, PFS和OS都短, 血清CYFRA21-1水平可能是预测EGFR-TKIs治疗的疗效及预后的指标。
CEA是最早发现, 目前临床应用最广的一种肿瘤抗原, 在成人肺、乳腺和胃肠等腺癌组织有表达。肺癌细胞可合成和释放CEA, 目前认为CEA是肺癌尤其是腺癌的进展、疗效和预后评估较好的一个肿瘤标志物[23]。与血清CYFRA21-1不同, 近年的相关报道显示治疗前血清CEA水平并不能预测EGFR-TKIs治疗的疗效。我们的研究显示治疗前血清CEA水平正常和增高的PFS分别为10.2个月和8.9个月(P=0.294), OS分别为24.0个月和21.8个月(P=0.122)均无统计学差异。腺癌亚组血清CEA水平正常及增高的PFS均为9.0个月(P=0.436), 无统计学差异; OS分别为24.8个月和23.0个月(P=0.104), 无统计学差异。鳞癌亚组血清CEA水平正常和增高的PFS分别为7.8个月和3.1个月(P=0.103), OS分别为9.1个月和7.0个月(P=0.381), 均无统计学差异。这些表明治疗前血清CEA水平与伴有EGFR突变的NSCLC的EGFR-TKIs治疗的疗效无关, 并不能预测EGFR-TKIs治疗的疗效。
我们的研究显示, 肺腺癌中治疗前血清水平CYFRA21-1增高与正常相比较EGFR-TKIs治疗PFS和OS都有缩短, 而国外相关研究报道治疗前血清水平CYFRA21-1增高的患者EGFR-TKIs治疗仅有PFS缩短, 因为进展后的相关治疗, 两组总的OS是无统计学差异的。那么在我们的研究中出现的OS的显著性缩短, 分析其原因可能是, 我们研究中194例患者中有159例出现进展, 而42例(26.4%)患者随后未再进行化疗、放疗或者其他的靶向药物治疗, 而仅仅给予姑息维持治疗, 从而导致OS进一步缩短。说明在我们临床实践中初次的EGFR-TKIs治疗明显影响OS。NCCN指南中, 对于伴有EGFR突变的NSCLC的推荐单药EGFR-TKIs一线治疗, 但关于如何延长EGFR-TKIs治疗PFS的探索性研究近年不断涌现。FASTACTII、NEJ005及JO2557等[24-26]多中心的临床研究显示, EGFR-TKIs和化疗交替应用, 或者联合化疗药物, 抗血管药物可以延长PFS至15个月-16个月。但由于临床研究设计的局限性或两药联合应用增加的副反应, 给这些研究带来许多争议。那么对于肺腺癌EGFR基因突变患者, 治疗前血清CYFRA21-1水平异常增高且预测单药EGFR-TKIs可能疗效不佳, 给予EGFR-TKIs和化疗交替应用或者联合化疗药物, 是否能延长这部分人群PFS, 从而提高OS, 这还需要将来临床实验进一步探索研究。
我们的研究存在许多局限性和不足, 首先这是个回顾研究, 随访时间2个月-3个月随访一次; 并且EGFR基因突变的检测方法没有统一, 而是采取两种方法, PCR-Sanger测序法和ARMS荧光定量PCR法, 但两种方法PFS和OS都没有统计学差异; 我们的研究应用EGFR-TKIs药物也不是统一, 包括有埃克替尼、吉非替尼和厄洛替尼, 但是在我们的研究里, 这三个EGFR-TKI药物无论PFS和OS也都没有统计学差异。虽然有许多局限性和不足, 但是从我们的研究中可以看到伴有EGFR突变的NSCLC的EGFR-TKIs治疗疗效还是有许多差异, 提示我们可以针对特定人群给予个体化治疗。
综上所述, 我们研究的结论是:伴有EGFR突变的NSCLC的EGFR-TKI治疗中, PS状态差、鳞癌和伴有脑转移的患者EGFR-TKIs治疗预后差。小标本病理有其局限性, 有时是不能代表整体病理组织类型。伴有EGFR突变的肺腺癌患者, 治疗前血清水平CYFRA21-1增高者与正常者相比, EGFR-TKIs治疗的PFS和OS均有缩短, 治疗前血清CYFRA21-1水平可以作为预测EGFR-TKIs疗效指标, 也可能是EGFR-TKIs治疗的预后指标; 而治疗前血清CEA水平则不能预测EGFR-TKIs疗效。
References
- 1.National Cancer Institute. Sureillance, epidemiology and end results program [S/OL]. 2014-12-10].http://seer.cancer.gov/ststfacts/html/lungb#survival
- 2.Vijayvergia N, Mehra R. Clinical challenges in targeting anaplastic lymphoma kinase in advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2014;74(3):437–446. doi: 10.1007/s00280-014-2517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mok TS, Wu YL, Thongprasert S, et al. Gefitnib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 4.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 5.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutation of the epidermal growth factor receptor (WJTOG3405):an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 6.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment of patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802):a multicenter, open-label, randomized, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 7.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC):a multicenter, open-label, randomize phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 8.Yang JC, Hirsh V, Schuler M, et al. Symptom control and quality of life in LUX-Lung 3:a phase Ⅲ study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3342–3350. doi: 10.1200/JCO.2012.46.1764. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advavced non-small-cell lung cancer (ICOGEN):a randomized, double-blind phase 3 non-inferionrity trial. Lancet Oncol. 2013;14(10):953–961. doi: 10.1016/S1470-2045(13)70355-3. [DOI] [PubMed] [Google Scholar]
- 10.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinb. N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 11.Lee CK, Wu YL, Ding PN, et al. Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR-mutant lung cancer:a meta-analysis. J Clin Oncol. 2015;33(17):1958–1965. doi: 10.1200/JCO.2014.58.1736. [DOI] [PubMed] [Google Scholar]
- 12.Kulpa J, Wójcik E, Reinfuss M, et al. Carcinoembryonic antigen, squamous cell carcinoma antigen, CYFRA21-1, and neuron-specific enolase in squamous cell lung cancer patients. http://www.clinchem.org/content/48/11/1931.short?related-urls=yesl48/11/1931. Clin Chem. 2002;48(11):1931–1937. [PubMed] [Google Scholar]
- 13.Taraka K, Hata A, Kaji R, et al. Cytokeratin 19 fragment predicts the efficacy of epidermal growth factor receptor-tyrosine kinase inhibitor in non-small-cell lung cancer harboring EGFR mutation. J Thorac Oncol. 2013;8(7):892–898. doi: 10.1097/JTO.0b013e31828c3929. [DOI] [PubMed] [Google Scholar]
- 14.Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutation in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER) J Thorac Oncol. 2014;9(2):154–162. doi: 10.1097/JTO.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dearden S, Stevenns J, Wu YL, et al. Mutation incidence and histology (mutMap) Ann Oncol. 2013;24(9):2371–2376. doi: 10.1093/annonc/mdt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai Y, Zhang Z, Li J, et al. EGFR mutations in surgically resected fresh specimens from 697 consecutive Chinese patients with non-small-cell lung cancer and their relationships with clinical features. Int J Mol Sci. 2013;14(12):24549–24559. doi: 10.3390/ijms141224549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yi SQ, Zhuang Y, Zhu WD, et al. Analysis of KRAS gene mutations in non-small cell lung cancer. Zhonghua Lin Chuan Yi Shi Za Zhi (Electronic Edition) 2013;7(20):9111–9115. doi: 10.3877/cma.j.issn.1674-0785.2013.20.025. [DOI] [Google Scholar]; 衣 素琴, 庄 园, 朱 卫东, et al. 非小细胞肺癌中KRAS基因突变分析. 中华临床医师杂志(电子版) 2013;7(20):9111–9115. doi: 10.3877/cma.j.issn.1674-0785.2013.20.025. [DOI] [Google Scholar]
- 18.Zhang H, Yang XJ, Qin N, et al. Detection and analysis of EGFR and KRAS mutations in the patient with lung squamous cell carcinomas. http://www.lungca.org/index.php?journal=01&page=article&op=view&path%5B%5D=10.3779%2Fj.issn.1009-3419.2015.10.04. Zhongguo Fei Ai Za Zhi. 2015;10(18):621–625. doi: 10.3779/j.issn.1009-3419.2015.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]; 张 卉, 杨 新杰, 秦 娜, et al. 肺鳞癌EGFR与KRAS基因突变状态分析. http://www.lungca.org/index.php?journal=01&page=article&op=view&path%5B%5D=10.3779%2Fj.issn.1009-3419.2015.10.04 中国肺癌杂志. 2015;10(8):621–625. [Google Scholar]
- 19.Shukuya T, Takahashi T, Kaira R, et al. Efficacy of gefitinib for non-adenocarcinoma non-small cell lung cancer patients harboring epidermal growth factor receptor mutations:a pooled analysis of published reports. Cancer Sci. 2011;102(5):1032–1037. doi: 10.1111/cas.2011.102.issue-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hata A, Katakami N, Yoshioka H, et al. How sensitive are epidermal growth factor receptor-tyrosine kinase inhibitors for squamous cell carcinoma of the lung harboring EGFR gene-sensitive mutation? J Thorac Oncol. 2013;8(1):89–95. doi: 10.1097/JTO.0b013e31827690b5. [DOI] [PubMed] [Google Scholar]
- 21.Liu YM, Zhao Q, Tang Y, et al. Efficacy of epidermal growth factor receptor-tyrosine kinase inhibitors for advanced squamous cell lung carcinoma patients with sensitive EGFR mutations. Cancer Res Prev Treat. 2015;42(9):911–913. [Google Scholar]; 刘 咏梅, 赵 倩, 唐 源, et al. EGFR-TKIs治疗EGFR敏感突变的晚期肺鳞癌的疗效分析. 肿瘤防治研究. 2015;42(9):911–913. [Google Scholar]
- 22.Travis WD, Rekhtman N, Riley GJ, et al. Pathologic diagnosis of advanced lung cancer based on small biopsies and cytology:a paradigm shift. J Thorac Oncol. 2010;5(4):411–414. doi: 10.1097/JTO.0b013e3181d57f6e. [DOI] [PubMed] [Google Scholar]
- 23.Cedrés S, Nuñez I, Longo M, et al. Serum tumor markers CEA, CYFRA21-1 and CA-125 are associated with worse prognosis in advanced non-small-cell cancer (NSCLC) https://www.sciencedirect.com/science/article/pii/S1525730411000209. Clin Lung Cancer. 2011;22(3):172–179. doi: 10.1016/j.cllc.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Wu YL, Lee JS, Thongprasert S, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2):a randomized, double-blind trial. Lancet Oncol. 2013;14(8):777–786. doi: 10.1016/S1470-2045(13)70254-7. [DOI] [PubMed] [Google Scholar]
- 25.Sugawara S, Oizumi S, Minato K, et al. Randomized phase Ⅱ study of concurrent versus sequential alternating gefitinib and chemotherapy in previously untreated non-small cell lung cancer with sensitive EGFR mutation:NEJ005/TCOG0902. Ann Oncol. 2015;26(5):888–894. doi: 10.1093/annonc/mdv063. [DOI] [PubMed] [Google Scholar]
- 26.Seto T, Kato T, Nishio M, et al. Erlotinib along or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutation (JO25567):an open-label, randomized, multicenter, phase 2 study. Lancet Oncol. 2014;15(11):1236–1244. doi: 10.1016/S1470-2045(14)70381-X. [DOI] [PubMed] [Google Scholar]