Abstract
Background
Death domain-associated protein (DAXX), originally identified as a pro-apoptotic protein, is now understood to be either a pro-apoptotic or an anti-apoptotic factor with a chromatin remodeler, depending on the cell type and context. This study evaluated DAXX expression and its clinical implications in squamous cell carcinoma of the esophagus.
Methods
Paraffin-embedded tissues from 60 cases of esophageal squamous carcinoma were analyzed immunohistochemically. An immune reaction with more than 10% of tumor cells was interpreted as positive. Positive reactions were sorted into 2 groups: reactions in 11%–50% of tumor cells and reactions in more than 51% of tumor cells, and the correlations between expression and survival and clinical prognosticators were analyzed.
Results
Forty-three of the 60 cases (71.7%) showed strong nuclear DAXX expression, among which 19 cases showed a positive reaction (31.7%) in 11%–50% of tumor cells, and 24 cases (40.0%) showed a positive reaction in more than 51% of tumor cells. A negative reaction was found in 17 cases (28.3%). These patterns of immunostaining were significantly associated with the N stage (p=0.005) and American Joint Committee on Cancer stage (p=0.001), but overall survival showed no significant difference. There were no correlations of DAXX expression with age, gender, or T stage. However, in stage IIB (p=0.046) and stage IV (p=0.014) disease, DAXX expression was significantly correlated with survival.
Conclusion
This investigation found upregulation of DAXX in esophageal cancer, with a 71.7% expression rate. DAXX immunostaining could be used in clinical practice to predict aggressive tumors with lymph node metastasis in advanced-stage disease, especially in stages IIB and IV.
Keywords: Esophageal squamous carcinoma, Death domain-associated protein (DAXX)
Introduction
Esophageal cancer is uncommon; however, its prognosis is poor [1]. The 5-year overall survival rate for esophageal squamous cell carcinoma ranges from 20% to 30% [1]. Early metastasis and late diagnosis are responsible for the poor prognosis of esophageal cancer [1,2]. Surgical resection has traditionally been the mainstay treatment for localized esophageal cancers [2]. However, survival after surgery alone for advanced esophageal cancer is not satisfactory. Therefore, the identification of biomarkers that enable early diagnosis, identify disease aggressiveness, and predict the prognosis of patients undergoing treatment is important.
In some tumors, several candidates for biological markers of aggressiveness have been suggested, including p53 [3,4]. However, the results are inconclusive, and the clinical impact of these candidate markers remains low. Gene-expression patterns associated with various clinicopathological parameters during tumor development and progression have been proposed [3,4].
To enable these kinds of studies, clinics routinely preserve and archive tissue samples, mainly through formalin fixation and paraffin embedding. To retrieve information at the protein level from these invaluable materials, which are associated with long and extensive patient follow-up data, immunohistochemistry is an ideal approach [5].
Death domain-associated protein (DAXX), a predominantly nuclear protein, has been reported to play important roles in carcinogenesis, transcriptional regulation, resistance to viral infection, and apoptosis [6–9]. DAXX can shuttle between the nucleus and cytoplasm or other cellular substructures, suggesting that it might have different functions in different cellular compartments and stages of the cell cycle [6–9]. The aim of this study was to evaluate DAXX expression and its clinical implications as a biological regulator of aggressiveness in esophageal carcinoma.
Methods
1) Patient characteristics
This study considered 60 cases of esophageal squamous cell carcinoma. Formalin-fixed and paraffin-embedded tissues from 60 patients who underwent esophagectomy from 2003 to 2006 were obtained from the Pathology Services Department of Kosin University Gospel Hospital (Table 1). Enrolled patients were followed up at 3-month intervals for 60 months, starting at 3 months after surgery. The mean follow-up duration was 32 months, and 2 cases were lost to follow-up for unknown reasons.
Table 1.
Clinicopathological features of 60 patients with esophageal cancer who underwent esophagectomy using a gastric conduit (N=60)
| Variable | Total (%) | DAXX expression | p-value | ||
|---|---|---|---|---|---|
| Negative (%) | Positive (%) | ||||
| 11%–50% of tumor cells | >50% pf tumor cells | ||||
| 5-Year survival | 0.272 | ||||
| Alive | 20 (33.3) | 8 (13.3) | 5 (8.3) | 7 (11.7) | |
| Dead | 40 (66.6) | 9 (15.00) | 14 (23.3) | 17 (28.3) | |
| Mean survival (mo) | 45.22±40.31 | 54.52±37.82 | 37.42±42.65 | 44.79±40.42 | 0.264 |
| Distant metastasis | 0.004 | ||||
| Absence | 43 (71.7) | 16 (26.7) | 14 (23.3) | 13 (21.7) | |
| Presence | 17 (28.3) | 1 (1.7) | 5 (8.3) | 11 (18.3) | |
Values are presented as number (%) or mean±standard deviation.
DAXX, death domain-associated protein.
Radical lymphadenectomy dissection, consisting of regional lymph node resections, was performed over the middle or lower mediastinal, the superior mediastinal, the perigastric, and celiac axis areas. On average, 15–20 regional lymph node dissections were performed. Seventeen patients with stage IV disease received palliative surgery due to severe dysphagia, which was possible because they did not have unresectable or advanced cancer with comorbidities.
Before processing for this study, all tissues were fixed in 10% neutral buffered formalin for approximately 24 hours. The microscopic slides with routine hematoxylin and eosin staining were retrieved from the archives and reviewed by a specialized pathologist. The original diagnosis was confirmed in all cases.
The Institutional Review Board of Kosin University Gospel Hospital approved the present study (IRB approval no., 2014-10-143). And the requirement for informed patient consent was waived due to the retrospective nature of the study.
2) Immunohistochemistry
The paraffin blocks were cut at a 4-micron thickness, dewaxed in xylene, and rehydrated through graded percentages of ethanol. Microwave treatment for antigen retrieval was performed for 20 minutes at 98°C using 0.01 M citric acid buffer (pH 6.0). Endogenous peroxidase activity was quenched by incubating the sections in 3% hydrogen peroxide for 10 minutes at room temperature. To block the non-specific binding sites, the slides were pre-incubated with 5% normal goat serum in phosphate-buffered saline for 10 minutes at room temperature. DAXX immunostaining was performed using a polyclonal antibody (1:200; Sigma-Aldrich, St. Louis, MO, USA). Subsequently, the antigen-antibody complex was visualized using a peroxidase/DAB Envision Detection System kit (DAKO, Glustrop, Denmark) and counterstained with hematoxylin. Negative controls were used for the tested antibodies; the primary antibody was replaced by either mouse or rabbit non-immune serum, as appropriate.
An appropriate slide including the deepest invasion of the tumor was selected in each case, and immunostaining was performed on the paraffin-embedded tissue. The microscopic slide with immunostaining was interpreted by a specialized molecular and tumor pathologist using consistent criteria. All stained sections were evaluated in a blinded manner without prior knowledge of patient data.
3) Immunohistochemistry evaluation
An immune reaction from more than 10% of tumor cells was interpreted as positive, and positive reactions were classified into 2 groups: a reaction in 11%–50% of tumor cells and a reaction in more than 51% of tumor cells. The correlations between expression and clinical prognosticators and survival were analyzed. The intensity of positive staining was initially scored according to mean optical density into 4 groups: 0, no staining; 1, weak staining (light yellow); 2, moderate staining (yellow brown); and 3, strong staining (brown). Staining intensity was found not to be discriminative in a preliminary study. Therefore, positive tumor cell staining was assigned a score using a semiquantitative 3-category grading system: 0, <10% positive cells; 1, 11%–50% positive cells; 2, >51% positive cells.
4) Statistical analysis
For analyses of esophageal cancer-specific mortality, death as a result of cancer was the primary end point, and deaths from other causes were censored. To adjust for potential confounding, age and year of diagnosis were used as continuous variables, and all other covariates were used as categorical variables. The correlation between each variable and expression was determined using the Kaplan-Meier method and the log-rank test. The chi-square test was used to examine associations between categorical variables. The t-test assuming unequal variance was performed to compare mean ages. All analyses used SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA), and all p-values were 2-sided. Significance was assigned at p<0.05.
Results
1) Clinical characteristics
The mean age of patients was 62.5±12.3 years. Six patients (10.0%) were female, and 54 patients (90.0%) were male. By tumor invasion depth, 9 cases (15.5%) were T1a, 6 cases (10.0%) were T1b, 16 cases (26.7%) were T2, and 29 cases (48.3%) were T3. By pN stage, 30 cases (50.0%) were N0, 25 cases (41.7%) were N1, and 5 cases (8.3%) were N2. Seventeen of the 60 cases (28.3%) showed distant metastasis. Using the seventh edition of the American Joint Committee on Cancer (AJCC) staging system, 11 cases (18.3%) were stage IA, 3 cases (5.0%) were stage IB, 12 cases (20.0%) were stage IIA, 7 cases (11.7%) were stage IIB, 10 cases (16.7%) were stage III, and 17 cases (26.3%) were stage IV. The 5-year survival rate was 33.3% (20 of 40). Forty-three patients without distant metastasis received adjuvant chemotherapy, whereas 17 patients with stage IV disease with distant metastasis received only palliative surgery and postoperative care. Patients diagnosed with pathologic stage I, II, or III disease were treated with adjuvant chemotherapy, which was administered following assessment by independent clinicians. These patients received adjuvant chemotherapy within 2 months after surgery. Patients received vinorelbine ditartrate (25 mg/m2/day, administered for only 1 day per month)+cisplatin (25 mg/m2/day, administered for only 1 day per month). Patients with stage I or II disease received 3 cycles of chemotherapy and a follow-up study was performed. We decided to add 3 cycles of chemotherapy if there was no evidence of metastatic lesions. Patients with stage III disease received chemotherapy with the same regimen for 9 cycles. No patients received neoadjuvant chemotherapy or radiation therapy, and no patients received postoperative radiation therapy. The survival period ranged from 2 months to 128 months, with a mean of 45.2±5.2 months.
2) Results from immunostaining and correlations with clinicopathological prognostic factors
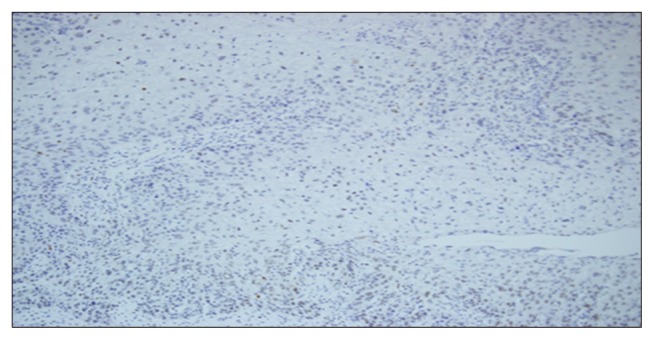
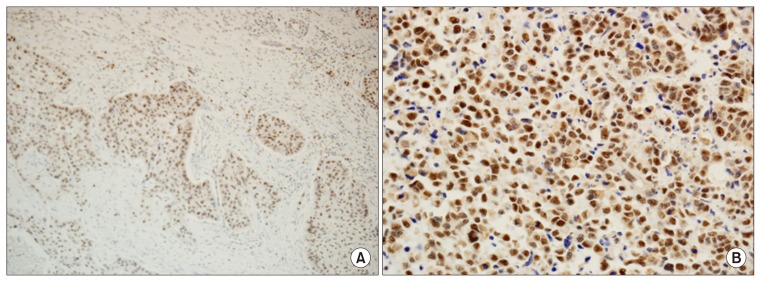
Non-neoplastic squamous epithelium adjacent to the cancer showed negative or weak staining. A negative reaction to DAXX (score of 0) was found in 17 (28.3%) of the 60 cases of esophageal squamous cell carcinoma (Table 2, Fig. 1). In this study, strong nuclear staining was predominant (Table 2, Fig. 2).
Table 2.
The correlation of DAXX expression with clinicopathological prognostic factors (N=60)
| Variable | Total (%) | DAXX expression | p-value | ||
|---|---|---|---|---|---|
| Negative (%) | Positive (%) | ||||
| 11%–50% of tumor cells | >50% pf tumor cells | ||||
| DAXX expression | 60 | 17 (28.3) | 19 (31.7) | 24 (40.0) | |
| Mean age (yr) | 62.45 | 62.65±6.736 | 60.53±10.33 | 63.83±7.118 | 0.718 |
| Gender | 0.162 | ||||
| Male | 54 (90.0) | 14 (23.3) | 17 (28.3) | 23 (38.3) | |
| Female | 6 (10.0) | 3 (5.0) | 2 (3.3) | 1 (1.7) | |
| T stage | 0.128 | ||||
| T1a | 9 (15.5) | 5 (8.3) | 2 (3.3) | 2 (3.3) | |
| T1b | 6 (10.0) | 2 (3.3) | 1 (1.7) | 3 (5.0) | |
| T2 | 16 (26.7) | 4 (6.7) | 6 (10.0) | 6 (10.0) | |
| T3 | 29 (48.3) | 6 (10.0) | 10 (16.7) | 13 (21.7) | |
| T4 | 0 | 0 | 0 | 0 | |
| pN stage | 0.007 | ||||
| N0 | 30 (50.0) | 12 (20.0) | 10 (16.7) | 8 (13.3) | |
| N1 | 25 (41.7) | 5 (8.3) | 8 (13.3) | 12 (20.0) | |
| N2 | 5 (8.3) | 0 | 1 (1.7) | 4 (6.7) | |
| AJCC staging | |||||
| IA | 11 (18.3) | 6 (10.0) | 2 (3.3) | 3 (5.0) | 0.001 |
| IB | 3 (5.0) | 1 (1.7) | 1 (1.7) | 1 (1.7) | |
| IIA | 12 (20.0) | 5 (8.3) | 6 (10.0) | 1 (1.7) | |
| IIB | 7 (11.7) | 3 (5.0) | 1 (1.7) | 3 (5.0) | |
| III | 10 (16.7) | 1 (1.7) | 4 (6.7) | 5 (8.3) | |
| IV | 17 (28.3) | 1 (1.7) | 5 (8.3) | 11 (18.3) | |
Values are presented as number (%) or mean±standard deviation.
DAXX, death domain-associated protein; AJCC, American Joint Committee on Cancer.
Fig. 1.
Negative DAXX expression in esophageal squamous cell carcinoma. DAXX, death domain-associated protein.
Fig. 2.
Positive nuclear reaction in esophageal squamous cell carcinoma. (A) Strong positive reaction in 11%–50% of tumor cells (DAXX, ×100). (B) Strong positive reaction in more than 50% of tumor cells. DAXX, death domain-associated protein.
Forty-three of the 60 cases (71.7%) showed strong DAXX expression. Of those, 19 cases (31.7%) showed a positive reaction in 11%–50% of tumor cells (score of 1), and 24 cases (40.0%) showed a positive reaction in more than 51% of tumor cells (score of 2). Twelve of the 30 cases (40.0%) without lymph node metastasis showed a negative reaction, whereas cases with lymph node metastasis were negative in only 5 of the 30 cases (16.7%).
Of the 17 cases with distant metastasis, 16 (94.1%) showed significant DAXX expression, including 11 cases classified with a score of 2 (positive reaction in more than 51% of tumor cells). By AJCC stage, DAXX expression was found in 5 of the 11 stage IA cases (45.5%), 2 of the 3 stage IB cases (66.7%), 7 of the 13 stage IIA cases (61.5%), 4 of the 7 stage IIB cases (57.1%), 9 of the 10 stage III cases (90.0%), and 16 of the 17 stage IV cases (94.1%).
DAXX was expressed in 31 (77.5%) of the 40 patients who died within 5 years of disease occurrence. Among them, 17 cases showed a positive reaction in more than 51% of tumor cells.
These immunostaining patterns were significantly associated with N stage (p=0.005), AJCC stage (p=0.001), and distant metastasis (p=0.004), but the overall survival analysis showed no significant correlations. There were no correlations of DAXX expression with age, gender, or T stage (Table 2).
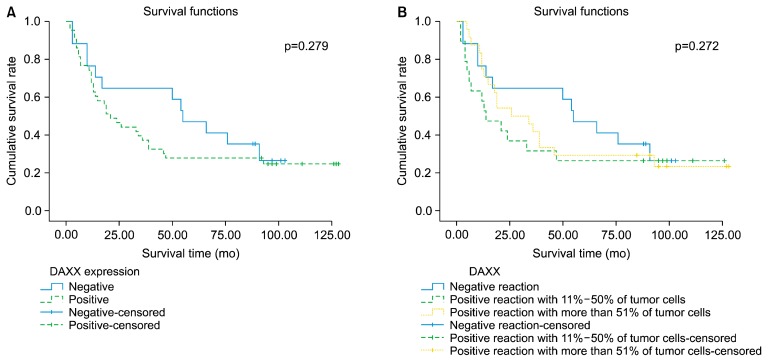
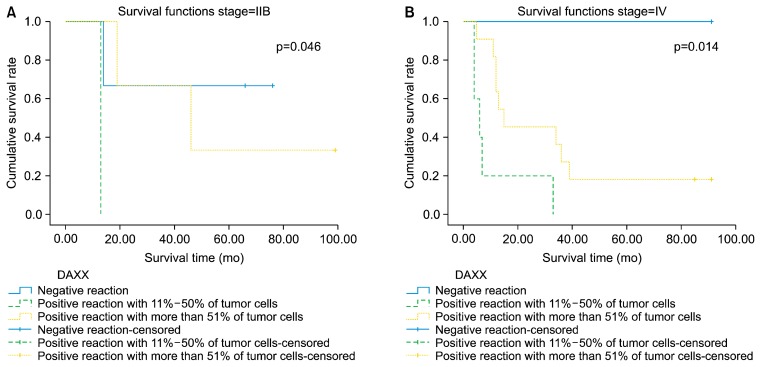
The Kaplan-Meier survival analysis found no significant difference in survival according to DAXX expression (Fig. 3). However, a significant correlation of DAXX expression with survival was demonstrated in patients with stage IIB (p=0.046) or IV (p=0.014) disease using the log-rank Mantel-Cox test (Fig. 4).
Fig. 3.
Kaplan-Meier survival curves according to DAXX expression. (A) DAXX-positive versus DAXX-negative. (B) DAXX expression pattern. DAXX, death domain-associated protein.
Fig. 4.
Significant survival curves according to DAXX expression in stage IIB (A) and IV (B) disease. By log-rank Mantel-Cox test. DAXX, death domain-associated protein.
Discussion
DAXX, an enigmatic protein, was originally suggested to be a proapoptotic protein, and is now known to be a multifunctional protein that regulates a wide range of cellular signaling pathways involved in both cell survival and apoptosis [6–9]. Because of these characteristics, DAXX has been considered to play important roles in the onset and worsening of cancer. Several authors have investigated potential relationships between DAXX expression and various cancers, such as bladder cancer, ovarian cancer, and pancreatic neuroendocrine tumors [4,10,11].
Very few studies have been conducted on the relationship between DAXX and esophageal cancer or the role of DAXX in esophageal cancer. Therefore, we planned a study to analyze the role of DAXX as a biomarker using the paraffin-embedded tissue of 60 esophageal cancer patients.
In this study, DAXX was expressed in 71.7% of esophageal squamous cell carcinoma patients and was significantly correlated with lymph node metastasis and stage. Its expression increased as stage advanced.
Under multiple influences, the subcellular localization of DAXX can be changed by modification or interaction with other proteins [7–9,12,13]. Tang et al. [9] studied the distribution and location of DAXX in cervical epithelial cells that were positive for high-risk human papillomavirus. They reported that DAXX was distributed in the nuclei of normal cervical epithelial cells and intensively distributed in the cytoplasm and cell membranes in cervical intraepithelial neoplasia (CIN) II, CIN III, and cervical cancer cells [9]. However, in this study, DAXX was expressed predominantly in the nuclei and barely heterogeneously expressed in the hyperplastic dysplastic squamous epithelium. Changing the location of DAXX can affect the cell cycle, including antiviral, pro-apoptotic, and anti-apoptotic activities, and might play a role in transcriptional regulation [7–9]. A recent study of brain tissue infected by reoviridae found that upregulation of DAXX through the interferon type I mechanism might depend on DAXX positioning in the cytoplasm or nucleus and could play a role in cell apoptosis [14]. It also showed that DAXX orientation was related to apoptosis of the host cell after viral infection. Key evidence of a possible anti-apoptotic function of DAXX is derived from DAXX knockout mouse embryos, which displayed increased global apoptosis [15]. In contrast, evidence for the pro-apoptotic functions of DAXX has been obtained from tumor cells or transformed cells treated with various stimuli, including ultraviolet light, transforming growth factor beta, hydrogen peroxide, interferon gamma, and arsenic trioxide [13]. Thus, it has been suggested that DAXX exerts an anti-apoptotic influence in unstressed primary cells, whereas it is a pro-apoptotic factor in tumor cells or transformed cells exposed to various types of stress. Pan et al. [10] provided new evidence that, in both normal ovarian cells and highly transformed ovarian cancer cells, DAXX promotes cell proliferation and represses DNA damage responses during X-ray irradiation and chemotherapy, meaning that it might function as an anti-apoptotic factor [12,13]. In this study, the expression of DAXX in esophageal squamous cell carcinoma cells was 71.7%, and it was significantly correlated with lymph node metastasis and stage, with expression increasing at advanced stages.
Therefore, the esophageal carcinomas used in this study might not have been associated with viral infection. This result suggests that DAXX plays a role as an oncoprotein or anti-apoptotic factor and does not act as a pro-apoptotic factor in esophageal squamous carcinoma. In this study, the DAXX expression level was relatively low in normal cells, while DAXX overexpression resulted in tumorigenic transformation in this tumor cell type. This result is supported by the report of Hollenbach et al. [8] that DAXX might promote chromosomal instability during cancer development.
Tsourlakis et al. [16] reported that 80.6% of prostate cancers showed DAXX expression, with predominant nuclear staining, and that stronger DAXX staining was associated with a higher Gleason grade and advanced T stage. They suggested DAXX as a novel independent prognosticator. The results of this study are consistent with those of Tsourlakis et al. [16], but no evidence of DAXX as an independent prognostic factor was found. A limitation of this study is the small number of cases. Because of the small sample size, the results may have been statistically significant only in certain stages of the disease, and different findings may be obtained if a larger patient group is analyzed. Therefore, further larger-scale studies are needed to verify DAXX as an independent prognostic factor.
In conclusion, these results for DAXX expression in esophageal carcinoma have clinical and therapeutic implications. DAXX was expressed in 71.7% of these esophageal cancer tissues and was associated with lymph node metastasis and advanced stages. Therefore, DAXX immunostaining could be used in clinical practice as a biologic marker indicating tumor aggressiveness, especially in stages IIB and IV. Inhibiting DAXX activity or DAXX accumulation could be a promising therapeutic strategy to enhance the effects of radiotherapy and chemotherapy in esophageal cancer patients.
Acknowledgments
This study was supported by a Grant of the Samsung Vein Clinic Network (Daejeon, Anyang, Cheongju, Cheonan; Fund no. KTCS04-103).
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Higuchi K, Koizumi W, Tanabe S, et al. Current management of esophageal squamous-cell carcinoma in Japan and other countries. Gastrointest Cancer Res. 2009;3:153–61. [PMC free article] [PubMed] [Google Scholar]
- 3.Hu Y, Correa AM, Hoque A, et al. Prognostic significance of differentially expressed miRNAs in esophageal cancer. Int J Cancer. 2011;128:132–43. doi: 10.1002/ijc.25330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segersten MU, Edlund EK, Micke P, et al. A novel strategy based on histological protein profiling in-silico for identifying potential biomarkers in urinary bladder cancer. BJU Int. 2009;104:1780–5. doi: 10.1111/j.1464-410X.2009.08674.x. [DOI] [PubMed] [Google Scholar]
- 5.Montironi R, Cheng L, Scarpelli M, Mazzucchelli R, Lopez-Beltran A. How much do you know about benign, preneoplastic, non-invasive and invasive neoplastic lesions of the urinary bladder classified according to the 2004 WHO scheme? Diagn Pathol. 2011;6:31. doi: 10.1186/1746-1596-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campos EI, Reinberg D. New chaps in the histone chaper-one arena. Genes Dev. 2010;24:1334–8. doi: 10.1101/gad.1946810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drane P, Ouararhni K, Depaux A, Shuaib M, Hamiche A. The death-associated protein DAXX is a novel histone chaper-one involved in the replication-independent deposition of H3.3. Genes Dev. 2010;24:1253–65. doi: 10.1101/gad.566910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollenbach AD, McPherson CJ, Mientjes EJ, Iyengar R, Grosveld G. Daxx and histone deacetylase II associate with chromatin through an interaction with core histones and the chromatin-associated protein Dek. J Cell Sci. 2002;115(Pt 16):3319–30. doi: 10.1242/jcs.115.16.3319. [DOI] [PubMed] [Google Scholar]
- 9.Tang J, Agrawal T, Cheng Q, et al. Phosphorylation of Daxx by ATM contributes to DNA damage-induced p53 activation. PLoS One. 2013;8:e55813. doi: 10.1371/journal.pone.0055813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan WW, Zhou JJ, Liu XM, et al. Death domain-associated protein DAXX promotes ovarian cancer development and chemoresistance. J Biol Chem. 2013;288:13620–30. doi: 10.1074/jbc.M112.446369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Wilde RF, Heaphy CM, Maitra A, et al. Loss of ATRX or DAXX expression and concomitant acquisition of the alternative lengthening of telomeres phenotype are late events in a small subset of MEN-1 syndrome pancreatic neuroendocrine tumors. Mod Pathol. 2012;25:1033–9. doi: 10.1038/modpathol.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puto LA, Reed JC. Daxx represses RelB target promoters via DNA methyltransferase recruitment and DNA hypermethylation. Genes Dev. 2008;22:998–1010. doi: 10.1101/gad.1632208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryo A, Hirai A, Nishi M, et al. A suppressive role of the prolyl isomerase Pin1 in cellular apoptosis mediated by the death-associated protein Daxx. J Biol Chem. 2007;282:36671–81. doi: 10.1074/jbc.M704145200. [DOI] [PubMed] [Google Scholar]
- 14.Dionne KR, Zhuang Y, Leser JS, Tyler KL, Clarke P. Daxx upregulation within the cytoplasm of reovirus-infected cells is mediated by interferon and contributes to apoptosis. J Virol. 2013;87:3447–60. doi: 10.1128/JVI.02324-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, He J, Li J, Tian D, Gu L, Zhou M. Methylation of RASSF1A gene promoter is regulated by p53 and DAXX. FASEB J. 2013;27:232–42. doi: 10.1096/fj.12-215491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsourlakis MC, Schoop M, Plass C, et al. Overexpression of the chromatin remodeler death-domain–associated protein in prostate cancer is an independent predictor of early prostate-specific antigen recurrence. Hum Pathol. 2013;44:1789–96. doi: 10.1016/j.humpath.2013.01.022. [DOI] [PubMed] [Google Scholar]