Abstract
Background
As healthcare costs rise and reimbursements decrease, healthcare organization leadership and clinical providers must collaborate to provide high-value healthcare. Medications are a key driver of the increasing cost of healthcare, largely as a result of the proliferation of expensive specialty drugs, including biologic agents. Such medications contribute significantly to the inpatient diagnosis-related group payment system, often with minimal or unproved benefit over less-expensive therapies.
Objective
To describe a systematic review process to reduce non–evidence-based inpatient use of high-cost medications across a large multihospital academic health system.
Methods
We created a Pharmacy & Therapeutics subcommittee consisting of clinicians, pharmacists, and an ethics representative. This committee developed a standardized process for a timely review (<48 hours) and approval of high-cost medications based on their clinical effectiveness, safety, and appropriateness. The engagement of clinical experts in the development of the consensus-based guidelines for the use of specific medications facilitated the clinicians' acceptance of the review process.
Results
Over a 2-year period, a total of 85 patient-specific requests underwent formal review. All reviews were conducted within 48 hours. This review process has reduced the non–evidence-based use of specialty medications and has resulted in a pharmacy savings of $491,000 in fiscal year 2016, with almost 80% of the savings occurring in the last 2 quarters, because our process has matured.
Conclusion
The creation of a collaborative review process to ensure consistent, evidence-based utilization of high-cost medications provides value-based care, while minimizing unnecessary practice variation and reducing the cost of inpatient care.
Keywords: cost-containment, medication utilization, pharmacy costs, practice transformation, process improvement, specialty medications, standardized review process, stewardship, value-based care
Healthcare expenditures in the United States continue to grow at an unprecedented and unsustainable rate. With the shift to value-based care, healthcare organizations are expected to provide consistent high-quality, safe care while reducing healthcare costs.1 As reimbursements shrink, healthcare organization leadership and clinical providers must identify opportunities to minimize unnecessary practice variation while providing high-value healthcare.2
In the inpatient setting, pharmacy expenditures account for up to 20% of the operating budget, and are frequently identified as a priority area for potential savings.3 Medication cost management is often seen as an opportunity for financial savings; however, this must not come at the expense of compromising high-quality care for patients. Common pharmacy strategies include formulary restrictions, purchasing and inventory management, therapeutic interchange, and medication utilization review.4
KEY POINTS
-
▸
As we move toward value-based healthcare, standardized processes are needed to ensure safe, timely, reliable, high-quality care at an affordable cost.
-
▸
This article describes a systematic, rapid review and approval process developed by a Pharmacy & Therapeutics Committee for high-cost drugs to ensure appropriate drug utilization.
-
▸
The review criteria included clinical efficacy, safety, and appropriate inpatient use of high-cost, mostly specialty, medications.
-
▸
During 2 years, 85 requests underwent a formal review within 48 hours, resulting in pharmacy cost-savings of $79,000 in the first year and $491,000 in the second year.
-
▸
This program shows that engaging hospital leadership and clinical providers and pharmacists aligns stakeholders and promotes responsible resource utilization.
-
▸
Such a collaborative review process ensures the appropriate use of high-cost medications and leads to lower practice variation and reduced inpatient care costs.
In recent years, the therapeutic landscape has changed with the proliferation of specialty drugs, which are used in the management of an array of medical conditions, including cancers, chronic infections, autoimmune disorders, transplantation, and bleeding disorders. Loosely defined based on their high costs, the need for special handling protocols, and close patient monitoring,5 specialty drugs are projected to account for 50% of the total medical expenditure by 2019.6
Biologic agents, which are produced or derived from a living organism, are the most rapidly growing class of specialty drugs, and hold promise to revolutionize the management of a range of chronic medical conditions.7,8 The challenge, however, is reconciling the potential therapeutic benefit with the high cost of these agents. Specialty drugs contribute significantly to the inpatient diagnosis-related group payment system, often with unproved benefits over less-expensive therapies. These medications often may be more appropriate for initiation in the ambulatory setting, after the mechanism of payment for continued therapy has been established.
We describe the development and implementation of a systematic review process to reduce non–evidence-based inpatient use of high-cost medications across a large multihospital academic health system.
Methods
Setting
University of Washington Medicine consists of 2 academic medical centers, 2 community hospitals, and a network of ambulatory clinics. Serving the Northwestern United States, Harborview Medical Center (HMC) is a Level 1 pediatric and adult trauma and burn center. The University of Washington Medical Center (UWMC) provides complex quaternary care, including solid-organ transplant and, in collaboration with the Seattle Cancer Care Alliance (SCCA), serves an oncologic and bone marrow transplant population. Together, HMC and UWMC have a case-mix index that ranks among the highest in the country.
As the largest hospitals within University of Washington Medicine, with a combined inpatient capacity in excess of 900 beds, HMC and UWMC handle more than 35,000 admissions annually. Although inpatient pharmacy expenditures totaled $38 million at these 2 institutions in fiscal year 2012, this figure jumped to $58 million in fiscal year 2016, representing more than a 50% increase during the 5-year span. Given their similarities in size, medical acuity, service structure (with shared clinical faculty and a shared UWMC, HMC, and SCCA Pharmacy & Therapeutics [P&T] Committee), and a common electronic medical record (EMR) system, HMC and UWMC were deemed the most appropriate locations for piloting a review process for high-cost medications.
Committee Structure
The High-Cost Medication Review Committee, a subcommittee of the P&T Committee, was established to promote consistent evidence-based use of an increasing number of expensive therapies in a fiscally responsible manner. A multidisciplinary team across both hospitals was assembled, and included hospital leadership, physician representatives, clinical pharmacists, administrative leaders from pharmacy, and representation from the Department of Bioethics. The committee first met in the fall of 2014, at which time the scope of work was defined and a project charter was developed.
The objective of this effort was to develop a standardized process for a rapid, patient-specific review, and for the approval of inpatient requests for high-cost medication use, based on clinical effectiveness and appropriateness. In addition, evidence-based guidelines were developed for the use of common high-cost medications.
The committee members agreed to the following set of 4 guiding principles that would inform the review process:
Adjudication of individual requests will be fair and consistent
Recommendations must be evidence-based
Decisions must be made in a timely fashion
Review and adjudication process will be performed without knowledge of the patient's ability to pay for the medication.
Review Workflow
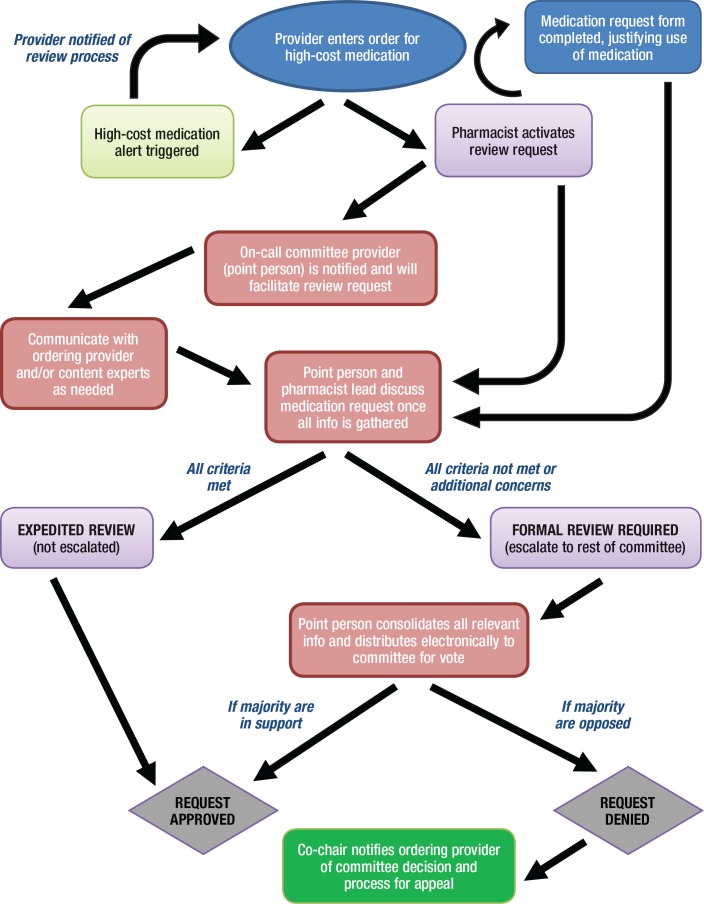
A standardized workflow was developed for reviewing all patient-specific high-cost medication orders, as depicted in Figure 1.
Figure 1. Workflow for Review of High-Cost Medication Requests.
Once an order for a high-cost agent is entered in the EMR system, an electronic alert is sent, notifying the ordering provider of the need for approval by the High-Cost Medication Review Committee. The provider is then directed to contact the inpatient pharmacy and complete a 1-page form that provides the rationale for administering the requested agent. This justification form consists of 5 questions, based on the 5 criteria outlined in Table 1.
Table 1.
High-Cost Medication Review: Criteria and Rationale
| Review criteria | Rationale |
|---|---|
| Clinical effectiveness | What is the strength of evidence? Results of well-constructed randomized controlled trials outweigh findings of observational case series, individual case reports, or personal anecdotal experience |
| Alternative therapies | Is there evidence of superiority to other less-expensive agents? If 2 medications are comparable in their likelihood to produce the same desired clinical outcome with a similar risk for side effects, then the less-expensive alternative should be selected |
| Safety concerns | Is inpatient administration necessary? Certain infused agents (particularly biologics) have a potential risk for significant side effects or toxicities that warrant closer observation or monitoring |
| Timing of treatment | Can administration of the agent be safely deferred to the outpatient setting, or does delaying treatment carry a risk for harm? |
| Duration of treatment | If ongoing treatment is indicated, is there a mechanism for continuing therapy after discharge? If not, then initiation in the acute care setting may not be prudent |
In addition, the ordering provider is asked to list any relevant references and/or provide additional supporting evidence as warranted. If a consultant service is making the recommendation to start a high-cost medication, the justification form may be completed by the specialist involved in the care of the patient.
The completed justification form is then sent to the physician and the pharmacist who are on call for the High-Cost Medication Review Committee. If needed, the on-call dyad may seek additional information by reviewing the clinical notes in the EMR, clarifying with the ordering team, and/or discussing with clinical content experts. Once all relevant information has been gathered, the on-call committee provider and pharmacist discuss the merit of the request. If both are satisfied that all the criteria have been met, the request is approved through an expedited review process without the need to involve other committee members. However, if the 2 on-call representatives think that not all the criteria have been met, or if they should have other reservations or questions, the request is denied.
At this stage, a discussion typically takes place with the ordering team, explaining why the request is not being approved. If the ordering team agrees with the rationale provided, it may withdraw the request. If the ordering team believes that treatment with the medication is justified, the request is escalated to the full committee for further discussion and a subsequent vote. If a majority of committee members are in support, the request is approved at this point. If a majority is opposed, the request is denied, with direct communication to the ordering provider notifying him or her of the committee's decision.
The provider is also informed of the mechanism for an appeal process through the medical director's office. The expectation is that all requests should be vetted and a decision be rendered within 48 hours.
Guideline Development
Our evidence-based guidelines for use of common high-cost medications are developed collaboratively with medical and pharmacy experts. These guidelines promote consistent use by the clinical services, as well as serve as a repository to support and streamline the patient-specific review process.
Results
Our patient-specific review of inpatient requests to administer select high-cost medications commenced in 2015. Initially, a limited number of drugs were selected to undergo a formal review process, based strictly on their volume of use. As the process matured, and the committee workflow was established, the scope of the formal review process was expanded to include any medications with a cost threshold of $5000 per a single dose, or $10,000 for a course of therapy.
With these parameters in place, a formal review is now required for approximately 50 medications from various types of drugs, including biologics and immunomodulators, hematologic drugs, antineoplastic drugs, metabolic drugs, and antimicrobials. A list of all medication requests that have undergone a formal review at our health system through the end of calendar year 2016 is provided in Table 2.
Table 2.
Medications That Have Undergone Formal Reviewa
| Alemtuzumab | Nelarabine |
| Alglucosidase alfa | Obinutuzumab |
| Belimumab | Obizur |
| Bendamustine (3) | Octreotide LAR |
| Bevacizumab (4) | Palifermin |
| Blinatumomab | Panhematin (3) |
| Bortezomib | Pegfilgrastim |
| Daratumumab | Pertuzumab |
| Defibrotide | Plerixafor (4) |
| Eculizumab (7) | Rituximab (10) |
| Factor VIIa | Romiplostim |
| Feiba | Ruxolitinib |
| Glucarpidase (2) | Siltuximab (7) |
| Hemin | Teduglutide |
| Infliximab (8) | Tocilizumab (10) |
| IVIG (3) | Trastuzumab |
| Liposomal doxorubicin | Vedolizumab |
| Natalizumab |
All medications had at least 1 review through the end of 2016. The number in parentheses indicates the number of requests the medication had for a formal review and approval.
IVIG indicates intravenous immunoglobulin therapy; LA, long-acting.
Volume of High-Cost Medications Reviewed
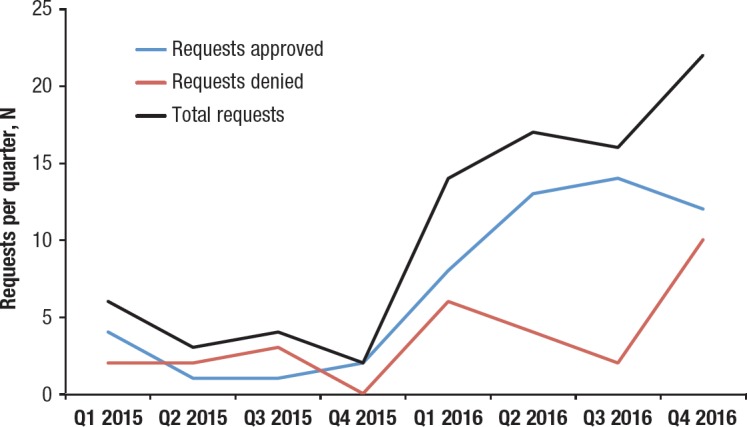
Figure 2 shows the volume of requests over a 2-year period. Through 2015, the number of requests averaged less than 2 monthly. As the number of medications that require a formal review increased, based on cost thresholds, the volume of requests increased in 2016 to an average of 4 to 5 monthly. Over the 2-year period, 70% of all requests were approved; 30% of requests were withdrawn by the ordering provider or were denied after a final vote by the committee.
Figure 2. Trend of Quarterly Volume of Requests for High-Cost Medications.
Financial Impact
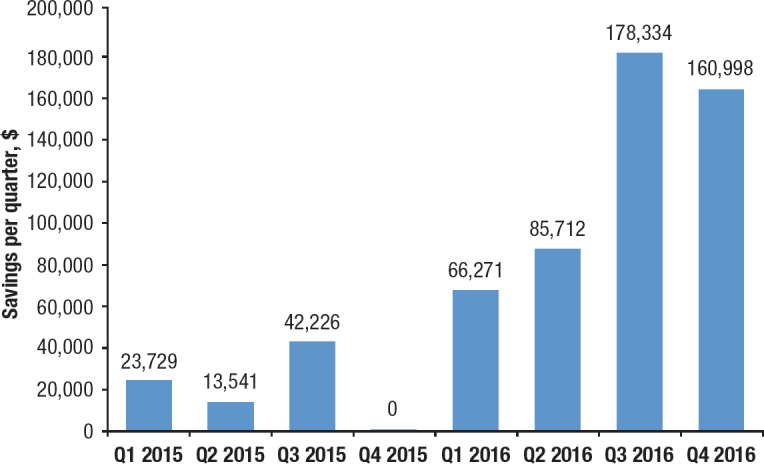
The financial impact of the high-cost medication review process has been monitored, based on the cost avoidance that resulted from requests that were denied or withdrawn by the ordering team. As shown in Figure 3, the implementation of a formal review process resulted in an estimated saving of $79,000 in 2015. As the process matured, the annual savings increased to $491,000 in 2016.
Figure 3. Quarterly Cost-Savings in 2015 and 2016 Resulting from High-Cost Medication Review Process.
Operational Efficiency and Provider Satisfaction
It was imperative that the review process for high-cost medication requests did not inadvertently lead to barriers in care delivery. Although it is the High-Cost Medication Review Committee expectation for all reviews to be vetted, and a decision rendered, within 48 hours, most requests have been adjudicated within 24 hours. Almost all the cases were able to undergo expedited review, and only 2 cases required escalation to the whole committee for discussion and vote. In situations when the request was denied, the ordering provider generally accepted the committee's decision, with only 1 case undergoing formal appeal.
Based on direct feedback from front-line providers and review through our incident-reporting system, to date, no safety concerns have been identified that resulted from perceived delays in treatment. Furthermore, providers have appreciated the transparency of cost information.
Discussion
Given the demands of healthcare reform to improve quality of care delivery while simultaneously lowering costs, hospitals must identify strategic interventions to reduce expenses.9 Accounting for up to 20% of the operating budget, the pharmacy department is often identified by the leadership as an opportune area for stewardship.10 In contrast to other hospital departments where personnel costs are the greatest expense, drug acquisition costs are the biggest source of expenditure in the pharmacy department, and account for 80% of the total budget.3
A recent survey of more than 1400 hospitals nationwide revealed that the total inpatient drug expenditure increased by approximately 23% annually from fiscal year 2013 to fiscal year 2015, with an approximate 39% increase on a per-admission basis.11 Furthermore, more than 90% of hospitals reported that pharmaceutical expenditures are having a moderate-to-severe effect on their ability to manage costs.11
Specialty drugs, especially biologics, are a major driver of drug costs in hospitals; although their volume of the total prescriptions written is less than 1%, specialty drugs accounted for 31.8% of the total drug expenditures in 2014, a significant increase from the 27.7% in 2013.12 Specialty drug expenditure has greatly outpaced the annual drug price inflation over the past decade, and is projected to account for 50% of all drug spending by 2019.6 Therefore, it is imperative that hospitals manage the use of these expensive medications with a fair, standardized evidence-based process.
To ensure consistent, evidence-based use of expensive biologics and specialty medications in a fiscally responsible manner, University of Washington Medicine has created a High-Cost Medication Review Committee. In this article we describe a standardized process to review the appropriateness of high-cost medication requests in the inpatient setting. A standard set of criteria are applied in adjudicating all requests; these criteria include the strength of evidence supporting clinical effectiveness, superiority to less-expensive agents, safety concerns warranting inpatient administration, and the risk for significant harm in delaying treatment to the outpatient setting.
Over a 2-year period, a total of 85 patient-specific requests underwent formal review by the committee. In all, approximately 30% of these requests were deemed inappropriate for inpatient administration and, therefore, were denied or withdrawn by the ordering provider. The financial impact of the patient-specific review process for high-cost medications has been significant, with an estimated savings of $491,000 in fiscal year 2016, based on cost-avoidance.
Value in healthcare is conventionally defined as providing high-quality care while reducing healthcare costs. As shown in this article, the implementation of a standardized review process for high-cost agents based on available evidence fosters high-quality care through the appropriate and consistent use of specialty drugs when they provide a clinical benefit, while preventing their use when chosen arbitrarily by the clinician without supporting evidence. Under a diagnosis-related group payment structure, high-cost medications contribute significantly to the total hospital expenditures.
Through better resource stewardship and cost-containment as a result of the medication review process, we are able to promote value-based care delivery in the hospital setting. One may counter, however, that costs are not truly being reduced but are rather shifted from the inpatient to the ambulatory setting. We are confident that our process has resulted in an absolute reduction in costs, because in a number of instances, the ordering provider withdrew the request for a high-cost agent in favor of a less-expensive alternative. In situations where drug administration was delayed to the outpatient setting, prior authorization was obtained and the facility received reimbursement for the high-cost agent. Furthermore, the cost for the patient is often much lower in the ambulatory setting, because of pricing structures.
Ultimately, however, we need to work toward reducing medication expenditures across the care continuum. In the future, our hope is to adopt a similar review process for expensive medications in the ambulatory setting, because this will provide the best opportunity to truly reduce costs and promote value-in-care delivery at a systems-based level.
The concept of reducing pharmacy expenditures by changing clinical practice patterns is not new.13 Antibiotic stewardship programs originated in response to the inappropriate or unnecessary use of antimicrobials, which are estimated to be as high as 50% of all antibiotics prescribed in the inpatient setting.14 Through coordinated efforts to ensure the appropriate use of antimicrobial agents and optimal dosing regimens, antibiotic stewardship programs have improved the quality and safety of care delivery by reducing side effects and preventing the emergence of resistant pathogens, while also reducing costs.15–17
More recently, Trinity Regional Health System in Illinois reported an annual savings of nearly $2 million across their 4-hospital system.18 A major contributing factor to their achieved cost-savings was the effective partnership between pharmacy, revenue cycle, and physician leaders to monitor utilization practices, to benchmark against external peers, and to identify focused opportunities to substitute less-expensive clinically equivalent drugs.18 A similar approach was also reported by Lancaster General Health, a health system in central Pennsylvania, where standardization and utilization initiatives resulted in $1.5 million in annual pharmacy-related cost-savings.19
In reviewing the trend in requests for high-cost medications at our institution, it is interesting to note that the volume initially decreased from quarter 1 to quarter 4 of 2015. This was not a reflection of changes in the clinical population or demographics. Instead, it might have been a consequence of increasing awareness among front-line providers regarding cost concerns that result from the new requirement for formal review when requesting specific agents.
The built-in “pause” created by the new process might have led providers to deliberate whether treatment with a given high-cost agent was truly warranted. Simply having the process in place has raised provider awareness, such that, in certain situations, medication orders are not even being initiated. Therefore, the actual savings (through cost-avoidance) might even be greater than reported, because the review process encouraged providers to consider the indication and cost before ordering the drug.
The $5000 threshold for triggering a high-cost medication review was selected in an effort to ensure that the most expensive therapies are being prescribed in a clinically appropriate and responsible manner. We recognize that opportunities certainly remain to reduce unnecessary variation in the use of less-expensive treatments. Lowering the threshold further, and focusing on the cumulative cost for a course of therapy, particularly for high-volume utilization drugs, may provide additional savings opportunities.
Of note, the majority of high-cost medication requests were (and are) being approved. Requests that were not approved for inpatient administration should not necessarily be construed as denials. In some situations, the medication in question might have been a maintenance dose of a known therapy for a chronic disease, and its timing happened to coincide with an inpatient admission. Alternatively, it might have been a new agent that was being initiated for a nonurgent indication. A follow-up conversation between the High-Cost Medication Review Committee member and the ordering provider often led to a mutual agreement to withdraw the request and/or defer treatment to the outpatient setting, provided the treatment could be coordinated in a timely manner.
The intent of the patient-specific review process was not to create barriers or solely to reduce costs. Rather, the goal was to optimize care while using the least-expensive, safe, and effective therapies in the most appropriate setting. A byproduct of reducing the unnecessary variation in practice is cost-containment.
One of the main reasons for the success of the high-cost medication request review process has been the collaborative approach adopted from the outset. By engaging stakeholders from hospital leadership, clinical providers, and clinical pharmacists, the necessary organizational alignment was created to promote responsible resource utilization. To be successful in providing high-quality care while reducing costs, it is critical to collaborate with front-line clinicians, not disenfranchise them. We have nurtured this partnership by recruiting local clinical experts in the development of guidelines to inform the appropriate inpatient use of specific agents.
Specifically, front-line providers are being asked to review the evidence and reach a shared understanding with their peers as to when a specific treatment is clinically warranted in the inpatient setting. By taking a proactive approach to develop such guidelines, the review and evaluation of individual, patient-specific requests is streamlined and reduces variability in providers' prescribing practices.
Conclusion
Through a partnership between clinicians and pharmacists, the University of Washington has developed a standardized approach for a rapid, patient-specific review and approval of high-cost medications based on clinical effectiveness and appropriateness. The collaborative review process has resulted in more consistent, evidence-based utilization of these expensive agents. By reducing unnecessary practice variation related to drug-ordering practices, inpatient expenditures are better contained.
The high-cost medication review process serves as a useful framework that could be readily adopted by other hospital organizations. Furthermore, the principles reviewed here have important implications for promoting value-based care across the healthcare continuum.
Author Disclosure Statement
Dr Durvasula, Dr Kelly, Dr Schleyer, Dr Anawalt, Mr Somani, and Dr Dellit reported no conflicts of interest.
Contributor Information
Raghu Durvasula, Associate Professor of Medicine, Division of Nephrology, University of Washington Medical Center, Seattle.
Janet Kelly, Assistant Director of Pharmacy Services, University of Washington Medical Center.
Anneliese Schleyer, Associate Professor of Medicine, Harborview Medical Center, University of Washington.
Bradley D. Anawalt, Professor of Medicine, Division of General Internal Medicine, Department of Medicine, University of Washington.
Shabir Somani, Chief Pharmacy Officer and Assistant Dean, University of Washington School of Pharmacy.
Timothy H. Dellit, Professor of Medicine, Harborview Medical Center, University of Washington School of Medicine.
References
- 1.Burwell SM. Setting value-based payment goals—HHS efforts to improve U.S. health care. N Engl J Med. 2015;372:897–899. [DOI] [PubMed] [Google Scholar]
- 2.Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds; Committee on the Learning Health Care System in America; Institute of Medicine. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2013. [PubMed] [Google Scholar]
- 3.Edwards R. In struggle to cut expenses, hospitals eye the pharmacy. Hosp Health Netw. 2011;85:28–30, 32. [PubMed] [Google Scholar]
- 4.ASHP Expert Panel on Medication Cost Management. ASHP guidelines on medication cost management strategies for hospitals and health systems. Am J Health Syst Pharm. 2008;65:1368–1384. Erratum in: Am J Health Syst Pharm 2008;65: 1491. [DOI] [PubMed] [Google Scholar]
- 5.Pew Charitable Trusts. Specialty drugs and health care costs. Fact sheet. Updated December 2016. http://pew.org/1MRtwwM. Accessed August 28, 2017.
- 6.Goozner M. New strategies to curb specialty drug costs. Mod Healthc. 2014;44:25. [PubMed] [Google Scholar]
- 7.Wu N, Lee YC, Shah N, Harrison DJ. Cost of biologics per treated patient across immune-mediated inflammatory disease indications in a pharmacy benefit management setting: a retrospective cohort study. Clin Ther. 2014;36:1231–1241, 1241.e1–1241.e3. [DOI] [PubMed] [Google Scholar]
- 8.Glover L. Why are biologic drugs so costly? U.S. News & World Report. February 6, 2015. http://health.usnews.com/health-news/health-wellness/articles/2015/02/06/why-are-biologic-drugs-so-costly. Accessed February 17, 2017.
- 9.Lee VS, Kawamoto K, Hess R, et al. Implementation of a value-driven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA. 2016;316:1061–1072. [DOI] [PubMed] [Google Scholar]
- 10.ASHP Foundation. Pharmacy Forecast 2016–2020: strategic planning advice for pharmacy departments in hospitals and health systems. December 2015. www.ashpfoundation.org/PharmacyForecast2016. Accessed December 27, 2017.
- 11.NORC at the University of Chicago. Trends in hospital inpatient drug costs: issues and challenges. Final report. October 11, 2016. www.aha.org/content/16/aha-fah-rx-report.pdf. Accessed December 27, 2017.
- 12.Express Scripts. U.S. Rx spending increased 13.1% in 2014. March 10, 2015. http://lab.express-scripts.com/lab/insights/industry-updates/us-rx-spending-increased-13-percent-in-2014. Accessed December 27, 2017.
- 13.Hoffmann RP. A strategy to reduce drug expenditures with a drug utilization review program. Hosp Pharm. 1984;19:7–8, 11–12. [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Core elements of hospital antibiotic stewardship programs. Updated February 23, 2017. www.cdc.gov/getsmart/healthcare/implementation/core-elements.html#_ENREF_17. Accessed February 24, 2017.
- 15.Schwartzberg E, Rubinovich S, Hassin D, et al. Developing and implementing a model for changing physicians' prescribing habits – the role of clinical pharmacy in leading the change. J Clin Pharm Ther. 2006;31:179–185. [DOI] [PubMed] [Google Scholar]
- 16.Roberts RR, Hota B, Ahmad I, et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis. 2009;49:1175–1184. [DOI] [PubMed] [Google Scholar]
- 17.Standiford HC, Chan S, Tripoli M, et al. Antimicrobial stewardship at a large tertiary care academic medical center: cost analysis before, during, and after a 7-year program. Infect Control Hosp Epidemiol. 2012;33:338–345. [DOI] [PubMed] [Google Scholar]
- 18.Bates C, Richards BS. Reducing pharmacy costs through improved utilization. Healthc Financ Manage. 2013;67:106–110. [PubMed] [Google Scholar]
- 19.Rebuck J. Lancaster General on reducing hospital pharmacy costs. FierceHealthcare. July 8, 2013. www.fiercehealthcare.com/finance/lancaster-general-reducing-hospital-pharmacy-costs. Accessed December 27, 2017.