Abstract
Background
End-stage renal disease (ESRD) imposes significant economic and social burdens on patients and healthcare systems. In the United States alone, more than 600,000 Americans have ESRD, with an estimated annual cost of treatment of more than $30 billion. Peritoneal dialysis and hemodialysis are competing renal replacement therapies in ESRD; however, data comparing quality-of-life outcomes between these 2 modalities are limited.
Objectives
To compare the effectiveness of peritoneal dialysis with the more common treatment modality of hemodialysis on the health-related quality of life (HRQoL) of patients with ESRD in the general, physical, and psychological domains; and to determine whether the time of publication and the origin of each study influenced its findings regarding the effectiveness of the 2 modalities.
Methods
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines to collect the data. PubMed, MEDLINE, and PsycINFO were the primary databases searched. Only articles published in English were included in this meta-analysis. The measure of effect size was Cohen's standardized mean difference. A random-effects model was used to test the hypothesis of equality in the mean HRQoL.
Results
A total of 15 studies with a combined sample size of 4318 patients met the study criteria and were included in the analysis. The pooled effect sizes based on the random-effects model were 0.24 (95% confidence interval [CI], −0.17–0.66) in the general domain; 0.10 (95% CI, −0.09–0.29) in the physical-functioning domain; and 0.29 (95% CI, −0.13–0.71) in the psychological-functioning domain. None of the summary effect sizes was statistically significant. Subgroup analyses favored peritoneal dialysis regarding the time and country of publication.
Conclusion
The majority of the studies included in this analysis favored peritoneal dialysis over hemodialysis in all 3 domains. However, the pooled effect sizes were not significant, resulting in the inability to conclude that peritoneal dialysis is the more effective of these 2 treatment modalities.
Keywords: end-stage renal disease, health-related quality of life, hemodialysis, peritoneal dialysis, random-effects model, subgroup analysis
End-stage renal disease (ESRD), also known as stage 5 kidney disease, is characterized by permanent kidney failure. At this stage, renal replacement therapies, such as dialysis or kidney transplantation, are mandatory.1–4 Currently, more than 678,000 Americans have ESRD, and projections indicate that the population of patients with this disease may exceed 2 million by 2030.1,2 ESRD places an enormous economic and social burden on patients and the healthcare system, with annual treatment costs in excess of $32 billion.1 Hemodialysis and peritoneal dialysis are 2 competing renal replacement therapies for ESRD, with hemodialysis being the most common treatment.1 However, in recent years, a notable increase has been seen in the number of patients with ESRD who start dialysis with peritoneal dialysis.1
Technologic advances in dialysis therapy have contributed to the improved survival of patients with ESRD.1,3 Despite this, the day-to-day quality of life of patients with ESRD is still much lower than that of the overall population.1,3,4 With the projected rise in the incidence of ESRD, and the increasing healthcare costs, it is imperative that we identify robust interventions for patients with ESRD.1–5
Health-related quality of life (HRQoL) reflects the welfare of patients based on their functional status in the physical, mental, and social domains, balanced with expectations and experiences in the face of a changing health status.6 Because of its importance as a critical measurement of the overall well-being of patients with ESRD, the Centers for Medicare & Medicaid Services has mandated that the HRQoL of patients on dialysis be evaluated on an annual basis.7
Today, medical research is increasingly focusing on HRQoL as an important variable for decision-making, and many randomized clinical trials now include HRQoL measures in assessing morbidity and mortality.8 Furthermore, it has become customary for clinicians and for public health officials to use HRQoL data to measure the effects of chronic diseases and treatments.
Quality of life is measured through the use of a wide variety of instruments, including the Short Form 12 (SF-12) and Short Form 36 (SF-36) outcome questionnaires,9–12 and other internationally recognized variants of these instruments, such as the World Health Organization (WHO) Quality of Life Survey (WHOQOL-100) and its modified version, the WHOQOL-BREF.13–16 According to the Centers for Disease Control and Prevention, HRQoL data can include aspects of patients' employment, personal wealth, environment, physical health, mental health, education, and recreation and leisure time.9
Published studies regarding the effect of dialysis modalities on the HRQoL of patients with ESRD have been conflicting. This may, in part, be caused by the wide variety of measures used to evaluate HRQoL, as well as the diversity among patients and populations used in HRQoL assessments.17 In general, research studies have agreed that patients who have had a transplant enjoy a better HRQoL than patients undergoing dialysis. By contrast, studies that compare the relative effectiveness of hemodialysis and peritoneal dialysis have been polarized in their findings regarding the dominance of one treatment versus the other.1,18 However, meta-analysis can provide a common metric for analyzing HRQoL data, regardless of the assessment used for measuring quality of life.17,19
The main objective of this meta-analysis was to determine the relative effectiveness of peritoneal dialysis on the HRQoL of patients with ESRD versus hemodialysis, which is the predominant treatment modality in the United States. Our analysis also sought to determine whether 2 moderator variables—the time of publication and the origin of each study—influenced the findings of the studies on the relative effectiveness of hemodialysis and peritoneal dialysis.
Methods
This study used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines during data collection and management. Figure 1 provides a flow chart illustrating the search process and the inclusion and exclusion criteria.
Figure 1. Flow Chart Illustrating the Search Process, Reasons for Including or Excluding Studies, and Number of Studies in the Final Meta-Analysis.
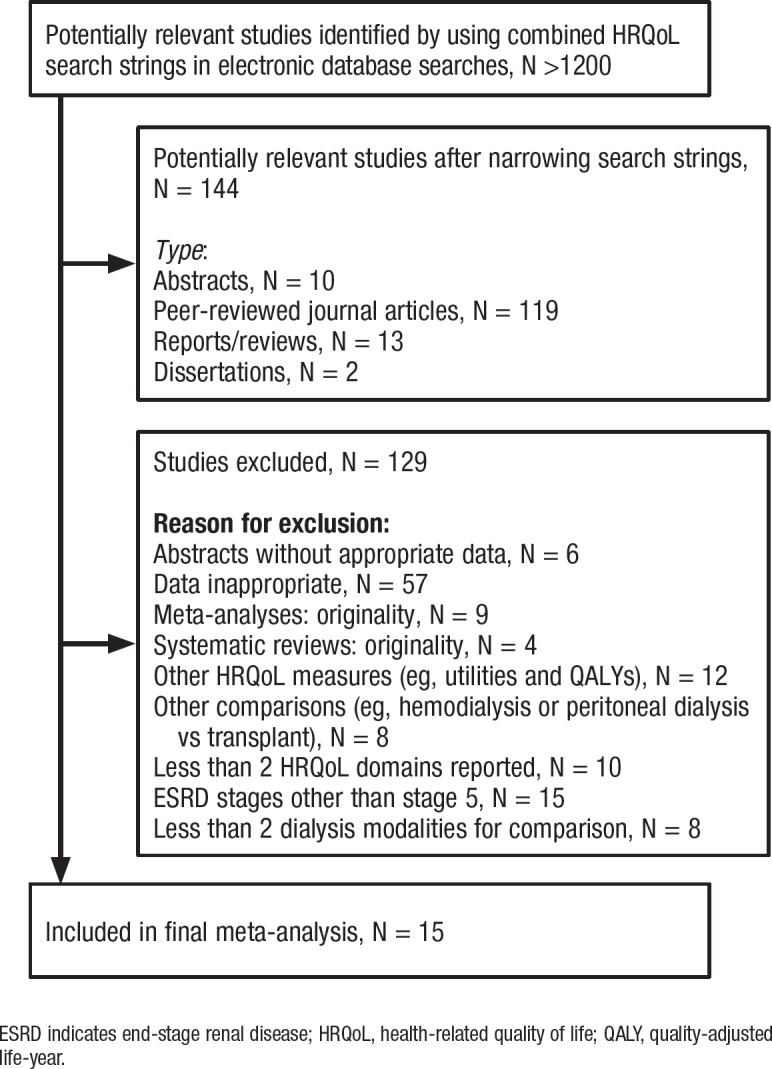
ESRD indicates end-stage renal disease; HRQoL, health-related quality of life; QALY, quality-adjusted life-year.
First, we conducted a review of the literature (studies in English only) on the effectiveness of dialysis modalities for patients with ESRD. The primary strategy used for finding published studies was a search of the PubMed, MEDLINE, and PsycINFO databases, which are among the most frequently used in healthcare research.
KEY POINTS
-
▸
ESRD places an economic and social burden on patients and healthcare, and its incidence is increasing.
-
▸
The main goal of this meta-analysis was to compare peritoneal dialysis and hemodialysis treatments in studies related to quality of life in patients with ESRD in 3 main domains.
-
▸
The second goal was to establish whether the study's year of publication and origin impacted the findings on the effectiveness of hemodialysis and peritoneal dialysis.
-
▸
Pooled effects based on the 15 studies that met the study criteria were 0.24 in the general domain, 0.10 in the physical-functioning domain, and 0.29 in the psychological-functioning domain.
-
▸
Most studies favored peritoneal dialysis over hemodialysis in all 3 domains.
-
▸
Although none of the pooled effect sizes was statistically significant, subgroup analyses favored peritoneal dialysis over hemodialysis in terms of year and country of origin.
-
▸
But the results could not confirm that peritoneal dialysis is more effective than hemodialysis.
-
▸
Future studies are needed to determine which of these 2 modalities improves the quality of life for patients with ESRD.
The next strategy was to contact a content expert to find studies that might have been missed in the primary search. All relevant studies between the years 2000 and 2017 were considered for inclusion. The key words used during the search phase included:
“ESRD/ESKD,” “end-stage renal disease,” and “end-stage kidney disease” used as interchangeable terms
“HD vs PD” and “hemodialysis vs peritoneal dialysis” used as interchangeable terms and refer to comparative studies of the 2 main treatment modalities investigated
“Dialysis modalities compared” used to extract studies that compared modalities for ESRD
“Quality of life” and “health-related quality of life” used to extract studies that covered topics on HRQoL of patients with ESRD.
We also screened meta-analyses related to HRQoL for additional citations.
Inclusion and Exclusion Criteria
To be included in the analysis, studies had to meet the following criteria: have a publication date between the years 2000 and 2017; journal articles had to report the original data (ie, sample sizes, means, and standard deviations) to enable the calculation of the effect sizes; the instruments used for measuring HRQoL had to meet WHO standards; the studies were required to compare the 2 dialysis modalities (ie, hemodialysis and peritoneal dialysis); the patients' disease progression had to be stage 5 (end-stage), and patients had to be on dialysis for more than 1 year and at the time the study was done; the study data had to be reported for at least 2 of the 3 HRQoL domains addressed in our analysis.
All relevant journal articles that compared the effectiveness of peritoneal dialysis and hemodialysis were considered. These included abstracts, conference proceedings, case reports, dissertations, randomized controlled trials, and observational studies. By including small and large studies in the analysis, we enhanced our opportunities for reducing publication bias. Furthermore, we increased the potential for capturing important effects that otherwise might not have been detected.20,21
For example, researchers often omit small studies because of their perceived insignificance, which is not always a good practice. In many cases, small representative studies, particularly those representative of patients' comorbid conditions and health interventions being compared, can capture treatment effects that would have been lost if these studies were omitted. In addition, randomized controlled trials are the standard for making evidence-based decisions about the effectiveness of health interventions. A small clinical trial that is externally valid (ie, representative) can yield important findings about the interventions being compared. Therefore, evidence-based research should focus not only on large, but also on small studies, based on the assumption that they are representative studies.
The enlarged sample size afforded by including all relevant journal articles also facilitated subgroup analyses that could potentially reveal patterns of effectiveness related to the time and place of study. Finally, combining data from studies of different sizes, time frames, and country of origin can improve external validity.20,21 For studies with the same health outcome indicator, the interventions are comparable.22
Therefore, all eligible studies reporting measures of HRQoL in the physical, psychological, and general domains in patients with ESRD who undergo peritoneal dialysis or hemodialysis were selected. Studies reporting the clinical and epidemiologic aspects of ESRD were not considered. Systematic reviews and meta-analyses with secondary data were not considered for inclusion.
Assessment of Study Quality
The primary function of coding assigned to each study was used to establish the criteria for assessing the quality of selected studies, and to identify the potential moderator variables. Two independent coders were used during the coding process. Whenever discrepancies were found, they were reconciled through discussion. Coders were not blinded to authors, affiliations, or journal names, because previous studies have shown this to be unnecessary.23
Data Analysis
Test for homogeneity. The between-studies test of homogeneity for the outcome variable was achieved through the calculation of Q-statistics. These statistics provide descriptive information of within- and between-study variations. Tests for homogeneity have low power, and failure to reject the null hypothesis does not provide conclusive evidence of the absence of between-study variation.23 Because of the wide variability in extracted studies (ie, sample sizes, time, and country of publication), a random-effects model was used to calculate the overall (ie, average) effect sizes and 95% confidence intervals (CIs) for the outcome variable within each HRQoL domain.
To assess the effectiveness of interventions for ESRD, we evaluated the effectiveness of peritoneal dialysis using hemodialysis as the control. The decision to use hemodialysis as the control was a result of its popularity as the most frequently used dialysis modality.19 We used the comprehensive meta-analysis software to fit random-effects models to the data to generate pooled effect sizes, their corresponding 95% CIs, standard errors, and P values. These estimates were used to identify significant peritoneal dialysis effects.
The statistical analysis system Base SAS version 9.3 (SAS Institute, Inc; Cary, NC) was used to generate forest plots using the effect sizes and 95% CIs obtained from each study.19 The direction of the effect size is indicative of which intervention was dominant, so negative effect sizes indicate hemodialysis as the dominant intervention.
Calculation of effect sizes. The effect sizes were computed based on a 1988 formula developed by Cohen to generate standardized mean differences.24 The formula is:
In this formula, d is the standardized mean difference from the treatment control comparison, is the average of the treatment or intervention group, is the average of the control group, and is the pooled sample standard deviation.
According to Cohen, an effect size of 0.20 is considered a small effect; 0.50, a medium-sized effect; and 0.80, a large effect.24
Assessment of moderator variables. The objectives of this meta-analysis included the determination of whether the year of publication and the country where the studies were done influenced the study findings. Two categorical variables representing the data collection year and the countries where the studies were conducted were identified during the coding process. The variable representing the year category was coded “new” for the newer studies (ie, after 2006) and “old” for the older studies (ie, 2000–2006). The other variable representing the country where the study was done was treated likewise, with “U.S.” representing studies conducted in the United States and “non-U.S.” for studies conducted in other countries.
We sought to determine whether these 2 variables were predictors of the reported effectiveness of peritoneal dialysis in the selected studies. The inclusion of these moderator variables was contingent on the belief that more recent studies (ie, after 2006) would find more significant intervention effects, because they were conducted when more advanced technology was available. With the United States being a developed country with high living standards, studies done in the United States may report better quality-of-life estimates than most studies done primarily in developing countries. However, the type of healthcare system adopted by the respective countries was not taken into consideration.
The subgroup analysis was confined to the effectiveness of peritoneal dialysis compared with hemodialysis, and, for convenience, we combined all 3 domains rather than performing 3 individual tests, which would be required for the pairwise comparisons across 3 domains.
Publication bias. As a precaution, we evaluated the studies to determine whether they were subjected to publication bias. The test for publication bias was achieved through the use of the Comprehensive Meta-Analysis version 2 software (Biostat; Englewood, NJ) by plotting the effect sizes against their standard errors to produce a funnel plot. The null hypothesis test of no publication bias was based on the classic fail-safe N test. A modified SAS code was used to assess the scores obtained from 2 independent coders across 15 items relating to study quality.
Results
The initial search strings returned more than 1200 related articles combined. However, narrower search strings requesting modality comparisons reduced the number to 144 potential studies that were retrieved for further review. After further screening, only 15 studies met the study criteria and were used in the comparison of the effectiveness of hemodialysis and peritoneal dialysis.10–13,15,25–34 An outline of the screening process and reasons for exclusion are illustrated in Figure 1.
Characteristics of extracted studies. Of the 15 studies extracted, 14 included all 3 quality-of-life domains (Table 1).10–13,15,25–34 The studies were characterized by considerable variation in sample sizes, the types of measurement instruments used, and the countries where they were done. The majority of studies were non-US (N = 13), were published after 2005 (N = 11), and used cross-sectional data obtained through questionnaires (N = 14) to measure HRQoL. The most common quality-of-life measurement instruments were the Kidney Disease Quality of Life (KDQOL) SF-36 survey, along with some of its adapted versions, and the WHOQOL-100 with its variants.13–16 The sample size included in the meta-analysis was 4318 patients.
Table 1.
Characteristics of Studies Included for Data Synthesis and Analysis
| Study | Study title | Location | Sample size: hemodialysis vs peritoneal dialysis, N | Assessment method(s) | HRQoL domain(s) covered |
|---|---|---|---|---|---|
| Atapour et al, 201628 | A comparison of the quality of life of the patients undergoing hemodialysis versus peritoneal dialysis and its correlation to the quality of dialysis | Iran | 46:46 | Questionnaire (KDQOL SF-36) |
General HRQoL Physical functioning Psychological functioning |
| Brown et al, 201010 | Broadening Options for Long-term Dialysis in the Elderly (BOLDE): differences in quality of life on peritoneal dialysis compared to haemodialysis for older patients | United Kingdom | 70:70 | Questionnaire (HRQOL SF-12) version 2 |
Physical functioning Psychological functioning |
| Zhang et al, 200730 | Comparison of quality of life and causes of hospitalization between hemodialysis and peritoneal dialysis patients in China | China | 408:654 | Questionnaire (KDQOL SF-36) |
General HRQoL Physical functioning Psychological functioning |
| Theofilou, 201113 | Quality of life in patients undergoing hemodialysis or peritoneal dialysis treatment | Greece | 84:60 | Questionnaire (WHOQOL-BREF)a |
General HRQoL Physical functioning Psychological functioning |
| Ginieri-Coccossis et al, 200815 | Quality of life, mental health and health beliefs in haemodialysis and peritoneal dialysis patients: investigating differences in early and later years of current treatment | Greece | 38:17 | Questionnaire (WHOQOL-BREF)a |
General HRQoL Physical functioning Psychological functioning |
| Al Wakeel et al, 201231 | Quality of life in hemodialysis and peritoneal dialysis patients in Saudi Arabia | Saudi Arabia | 151:41 | Questionnaire (KDQOL-SF) |
General HRQoL Physical functioning Psychological functioning |
| Ogutmen et al, 200626 | Health-related quality of life after kidney transplantation in comparison intermittent hemodialysis, peritoneal dialysis, and normal controls | Turkey | 302:207 | Questionnaire (KDQOL SF-36) |
General HRQoL Physical functioning Psychological functioning |
| Wu et al, 200411 | Changes in quality of life during hemodialysis and peritoneal dialysis treatment: generic and disease specific measures | United States | 432:133 | Questionnaire (KDQOL SF-36) |
General HRQoL Physical functioning Psychological functioning |
| Kutner et al, 200512 | Health status and quality of life reported by incident patients after 1 year on haemodialysis or peritoneal dialysis | United States | 455:413 | Questionnaire (KDQOL SF-36) |
General HRQoL Physical functioning Psychological functioning |
| Niu and Li, 200532 | Quality of life of patients having renal replacement therapy | Taiwan | 80:80 | Questionnaire (WHOQOL-BREF TAIWAN)a |
Physical functioning Psychological functioning |
| Sayin et al, 200725 | Quality of life in hemodialysis, peritoneal dialysis, and transplantation patients | Turkey | 75:20 | Questionnaire (KDQOL SF-36) | General HRQoL Physical functioning Psychological functioning |
| Mau et al, 200833 | Health-related quality of life in Taiwanese dialysis patients: effects of dialysis modality | Taiwan | 182:51 | Questionnaire (KDQOL SF-36) Taiwanese version |
General HRQoL Physical functioning Psychological functioning |
| Czyz.ewski et al, 201427 | Assessment of health-related quality of life of patients after kidney transplantation in comparison with hemodialysis and peritoneal dialysis | Poland | 40:30 | Questionnaire (KDQOL-SF v.1.3) |
General HRQoL Physical functioning Psychological functioning |
| Okpechi et al, 201329 | Health-related quality of life in patients on hemodialysis and peritoneal dialysis | South Africa | 56:26 | Questionnaire (KDQOL SF-36) |
General HRQoL Physical functioning Psychological functioning |
| Rodrigues Fructuoso et al, 201134 | Quality of life in chronic kidney disease | Portugal | 37:14 | Questionnaire (KDQOL SF-36 v.1.3) |
General HRQoL Physical functioning Psychological functioning |
WHOQOL-BREF is a 28-item modified version of the WHOQOL-100 + questionnaire soliciting patient subjective assessment of HRQoL. HRQoL indicates health-related quality of life.
Statistical Analyses
The effectiveness of the interventions across the 3 quality-of-life domains, summarizing the effect sizes, as well as the effect sizes for individual studies, are illustrated in the respective forest plots in Figure 2, Figure 3, and Figure 4. Higher scores on quality-of-life scales indicate better quality of life; therefore, a negative effect size favors the control (ie, hemodialysis).
Figure 2. Effect Sizes and 95% CIs for Peritoneal Dialysis versus Hemodialysis in the General HRQoL Domain.
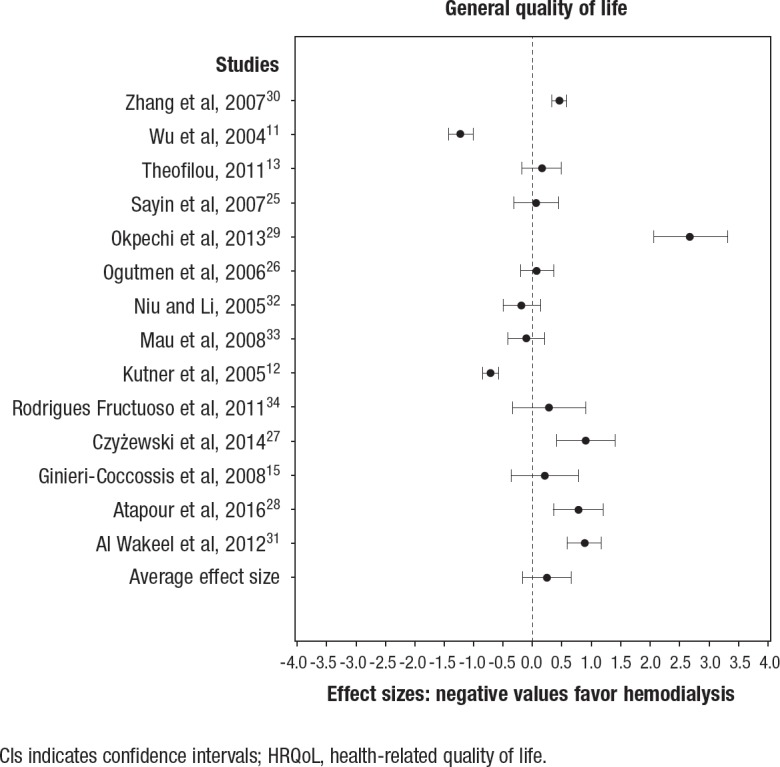
CIs indicates confidence intervals; HRQoL, health-related quality of life.
Figure 3. Effect Sizes and 95% CIs for Peritoneal Dialysis versus Hemodialysis in the Physical HRQoL Domain.
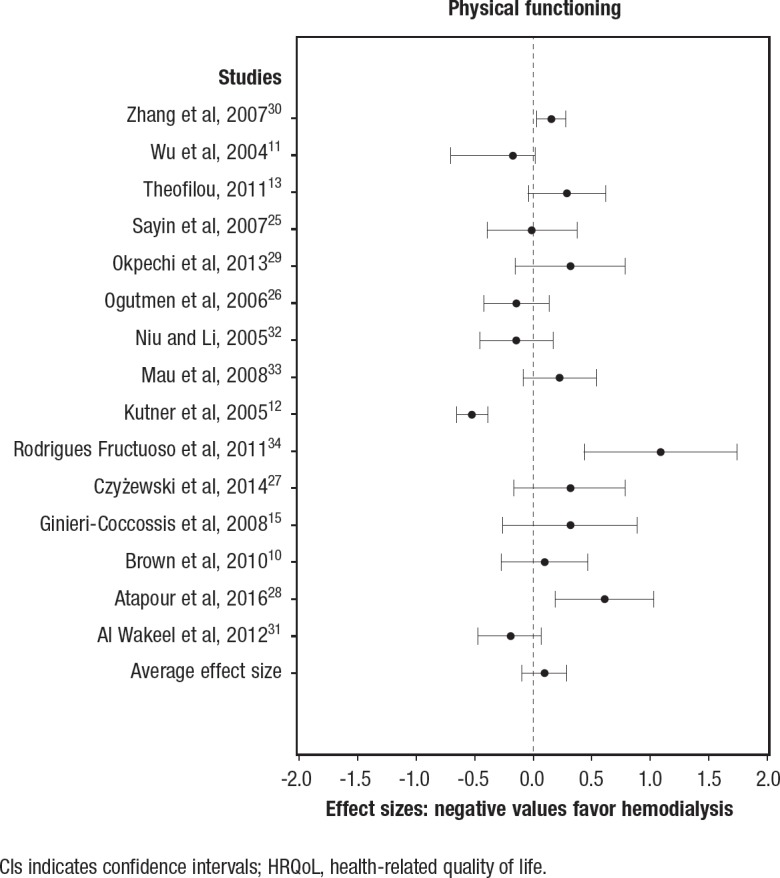
CIs indicates confidence intervals; HRQoL, health-related quality of life.
Figure 4. Effect Sizes and 95% CIs for Peritoneal Dialysis versus Hemodialysis in the Psychological HRQoL Domain.
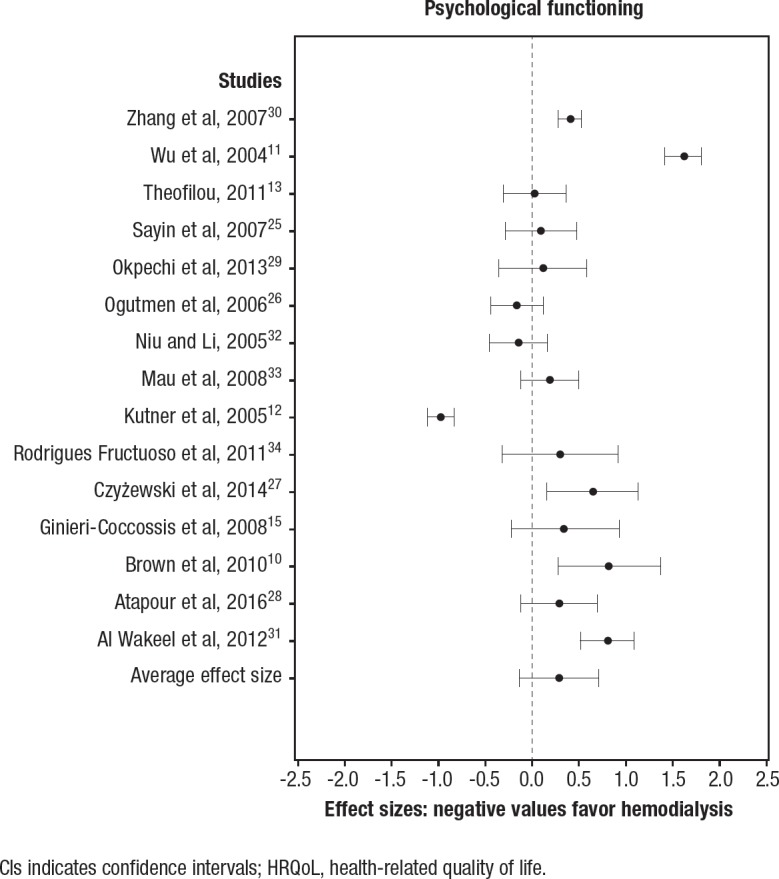
CIs indicates confidence intervals; HRQoL, health-related quality of life.
Peritoneal dialysis versus hemodialysis (general domain). In all, 14 studies were used in the comparison of the effectiveness of peritoneal dialysis relative to hemodialysis in the general HRQoL domain (Figure 2). A total of 5 studies indicated that peritoneal dialysis was more effective in improving HRQoL27–31; 2 studies indicated that hemodialysis was more effective.11,12 The summary effect size was 0.28 (95% CI, −0.14–0.69; not significant).
Peritoneal dialysis versus hemodialysis (physical domain). A total of 15 studies were used in the comparison of the effectiveness of peritoneal dialysis relative to hemodialysis in the physical HRQoL domain (Figure 3). In all, 3 studies indicated that peritoneal dialysis was more effective in improving HRQoL.25,27,29 Only 1 study indicated that hemodialysis was more effective.12 The summary effect size was 0.10 (95% CI, −0.09–0.29; not significant).
Peritoneal dialysis versus hemodialysis (psychological domain). In all, 15 studies were included in the comparison of the effectiveness of peritoneal dialysis relative to hemodialysis in the psychological HRQoL domain (Figure 4). A total of 5 studies indicated that peritoneal dialysis was more effective in improving HRQoL,10,11,25,26 whereas 1 study indicated that hemodialysis was more effective.12 The summary effect size was 0.29 (95% CI, −0.13–0.71; not significant).
Assessing the impact of moderator variables. Subgroup analyses were performed to determine whether 2 moderator variables, time of study, and country of study origin were important predictors of the effectiveness of the interventions. The results of the subgroup analyses are shown in Table 2.
Table 2.
Subgroup Analyses of Moderator Variables for the 3 HRQoL Domains Combined
| Study grouping | Studies, N | Effect size | Z value | P value | Qdf,p |
|---|---|---|---|---|---|
| United States | 2 | –.033 (95% CI, −0.75–0.08) | –1.58 | .110 | 12.85 1, 0.000 |
| Non–United States | 13 | 0.30 (95% CI, 0.12–0.47) | 3.33 | .001 | |
| Older (year 2000–2006) | 4 | –0.24 (95% CI, −0.05–0.03) | –1.64 | .102 | 7.58 1, 0.006 |
| Newer (year 2006–2017) | 11 | 0.39 (95% CI, 0.20–0.57) | 4.06 | .000 |
CI indicates confidence interval; HRQoL, health-related quality of life.
Time of study. The majority of studies (N = 11) were published after 2006, and these studies tended to report larger effects than those published before 2006 (0.39 vs −0.24, respectively). For studies published after 2006, the standardized mean difference was 0.39 (95% CI, 0.20–0.57; Z = 4.06; P <.001).
The 11 newer studies tended to indicate peritoneal dialysis as the more effective modality. For the 4 older studies, the standardized mean difference was −0.24 (95% CI, −0.05–0.03; Z = −1.64; P = .10). Although the older studies advocated hemodialysis as the more effective modality, this finding was not statistically significant. The test of homogeneity indicated significant variation in effects between the studies (Qbetween = 12.85; P <.001).
Country of study. The majority of studies (N = 13) used in this study were published outside of the United States. The standardized mean difference for the non-US studies was 0.30 (95% CI, 0.12–0.47; Z = 3.33; P = .001). The non-US studies indicated that peritoneal dialysis is the more effective modality. For US studies, the standardized mean difference was −0.33 (95% CI, −0.75–0.08; Z = −1.58; P = .11). Studies done within the United States reported numerically larger standardized mean differences in favor of hemodialysis; however, this finding was not statistically significant. The test of homogeneity indicated significant variation in effects between the US and non-US studies (Qbetween = 7.58; P = .006).
Assessing publication bias. The results indicated evidence of publication bias. The Z-value for the classic fail-safe N test was 4.28 (P <.001), resulting in the rejection of the null hypothesis of no publication bias. The interrater reliability was 0.73, which is indicative of good interrater agreement and study quality.
Discussion
Despite HRQoL becoming an important rubric for determining the overall well-being of patients with ESRD in the United States, the vast majority of studies researching this topic are done in foreign countries. If healthcare officials in the United States continue to agree on the use of HRQoL as a rubric for determining the overall well-being of patients with ESRD, there is a need for more studies of this nature in the United States. The majority of studies in this analysis indicate that peritoneal dialysis is a more effective dialysis modality than hemodialysis, as was evident in all 3 HRQoL domains addressed in this study (Figures 2–4). However, this finding is a function of moderating factors, particularly the time period in which the studies were done.
The more recent studies (ie, after 2006) tend to show peritoneal dialysis as the more effective of the 2 dialysis modalities. The inconsistency in the findings between the 2 time periods is supported by the tests for homogeneity indicating significant variation within and between time periods, as well as within and between studies. It is also possible that the respective study sizes and countries of origin could have influenced the results.
The older studies, which, in general, had larger sample sizes and were conducted within the United States, might have been more representative than the majority of non-US, smaller studies. However, when the summary effect sizes were taken into consideration, peritoneal dialysis was not a more effective dialysis modality in any of the 3 HRQoL domains; therefore, this review is unable to conclude that peritoneal dialysis is the more effective of the 2 modalities.
It is important to note that recent reports by the US Renal Data System indicate that patients with ESRD are starting dialysis with peritoneal dialysis more often than in previous years.1 This trend is similar to what is already observed in non-US studies. If this trend continues in the future, studies such as our analysis may help to solidify whether patients undergoing peritoneal dialysis actually experience a better quality of life than patients undergoing hemodialysis.
Limitations
This study adhered to PRISMA protocols during the collection and management of data to compare the effectiveness of hemodialysis and peritoneal dialysis as they relate to the HRQoL of patients with ESRD. Throughout the process of identifying eligible studies, we sought to avoid publication bias by including any scholarly article that met the inclusion criteria. However, the avoidance of publication bias is often not realistically possible. Like reviews, meta-analyses are syntheses of existing data that are confined to previously selected study settings, interventions, populations, health outcomes, and designs. Thus, we are aware of the limitations of this study for not being able to fully account for variability resulting from these factors, as well as other factors, such as country-specific healthcare policies, racial, ethnic, and sex differences.
Another limitation is that our study did not address the effects of study size on the results. This is an important consideration, especially because the results showed that the larger studies tend to indicate statistically significant results. However, the inclusion of small studies is also justified, because small studies can often contain true intervention effects that would have been overlooked had they not been included.
Conclusion
Despite the noted limitations, the methodologies used in this study are conservative but efficient ways of examining the effects of dialysis modality choices, time, and country of origin on the study findings. Peritoneal dialysis and hemodialysis are 2 renal replacement therapies used for ESRD, but comparative data on quality-of-life outcomes between these modalities are limited.
Overall, the majority of studies included in this meta-analysis favored peritoneal dialysis over hemodialysis in all 3 HRQoL domains considered. Nevertheless, because the pooled effect sizes were not statistically significant, we were unable to conclude that peritoneal dialysis is more effective than hemodialysis. Additional studies are needed to determine which ESRD treatment modality is better in terms of patients' HRQoL.
Author Disclosure Statement
Dr Queeley and Dr Campbell have no conflicts of interest to report.
Contributor Information
Gilbert L. Queeley, Research Associate, Division of Economic Social and Administrative Pharmacy, College of Pharmacy and Pharmaceutical Sciences, Florida A&M University, Tallahassee..
Ellen S. Campbell, Associate Professor, Division of Economic Social and Administrative Pharmacy, College of Pharmacy and Pharmaceutical Sciences, Florida A&M University, Tallahassee..
References
- 1.United States Renal Data System. 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Volume 2: ESRD in the United States. 2016; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD.
- 2.Szczech LA, Lazar IL. Projecting the United States ESRD population: issues regarding treatment of patients with ESRD. Kidney Int. 2004;66(suppl 90):S3–S7. [DOI] [PubMed] [Google Scholar]
- 3.Kontodimopoulos N, Niakas D. An estimate of lifelong costs and QALYs in renal replacement therapy based on patients' life expectancy. Health Policy. 2008;86:85–96. [DOI] [PubMed] [Google Scholar]
- 4.National Institute of Diabetes and Digestive and Kidney Diseases. Kidney disease statistics for the United States. December 2016. www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Accessed April 16, 2018.
- 5.Menzin J, Lines LM, Weiner DE, et al. A review of the costs and cost effectiveness of interventions in chronic kidney disease: implications for policy. Pharmacoeconomics. 2011;29:839–861. [DOI] [PubMed] [Google Scholar]
- 6.Abdel-Kader K, Myaskovsky L, Karpov I, et al. Individual quality of life in chronic kidney disease: influence of age and dialysis modality. Clin J Am Soc Nephrol. 2009;4:711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen SS, Al Mawed S, Unruh M. Health-related quality of life in end-stage renal disease patients: how often should we ask and what do we do with the answer? Blood Purif. 2016;41:218–224. [DOI] [PubMed] [Google Scholar]
- 8.Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003;56:395–407. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Health related quality of life. www.cdc.gov/hrqol/concept.htm. Accessed April 16, 2018.
- 10.Brown EA, Johansson L, Farrington K, et al. Broadening Options for Long-term Dialysis in the Elderly (BOLDE): differences in quality of life on peritoneal dialysis compared to haemodialysis for older patients. Nephrol Dial Transplant. 2010;25:3755–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu AW, Fink NE, Marsh-Manzi JV, et al. Changes in quality of life during hemodialysis and peritoneal dialysis treatment: generic and disease specific measures. J Am Soc Nephrol. 2004;15:743–753. [DOI] [PubMed] [Google Scholar]
- 12.Kutner NG, Zhang R, Barnhart H, Collins AJ. Health status and quality of life reported by incident patients after 1 year on haemodialysis or peritoneal dialysis. Nephrol Dial Transplant. 2005;20:2159–2167. [DOI] [PubMed] [Google Scholar]
- 13.Theofilou P. Quality of life in patients undergoing hemodialysis or peritoneal dialysis treatment. J Clin Med Res. 2011;3:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sreejitha NS, Devi KS, Deepa M, et al. The quality of life of patients on maintenance hemodialysis and those who underwent renal transplantation. Amrita J Med. 2012;8:1–44. [Google Scholar]
- 15.Ginieri-Coccossis M, Theofilou P, Synodinou C, et al. Quality of life, mental health and health beliefs in haemodialysis and peritoneal dialysis patients: investigating differences in early and later years of current treatment. BMC Nephrol. 2008;9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wesołowski T, Szyber P. A trial of objective comparison of quality of life between chronic renal failure patients treated with hemodialysis and renal transplantation. Ann Transplant. 2003;8:47–53. [PubMed] [Google Scholar]
- 17.Tengs TO, Lin TH. A meta-analysis of quality-of-life estimates for stroke. Pharmacoeconomics. 2003;21:191–200. [DOI] [PubMed] [Google Scholar]
- 18.Wyld M, Morton RL, Hayen A, et al. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med. 2012;9:e1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landreneau K, Lee K, Landreneau MD. Quality of life in patients undergoing hemodialysis and renal transplantation—a meta-analytic review. Nephrol Nurs J. 2010;37:37–44. [PubMed] [Google Scholar]
- 20.Turner RM, Bird SM, Higgins JP. The impact of study size on meta-analyses: examination of underpowered studies in Cochrane reviews. PLoS One. 2013;8:e59202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.IntHout J, Ioannidis JP, Borm GF, Goeman JJ. Small studies are more heterogeneous than large ones: a meta-meta-analysis. J Clin Epidemiol. 2015;68:860–869. [DOI] [PubMed] [Google Scholar]
- 22.Li R, Zhang P, Barker LE, et al. Cost-effectiveness of interventions to prevent and control diabetes mellitus: a systematic review. Diabetes Care. 2010;33:1872–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liem YS, Bosch JL, Hunink MG. Preference-based quality of life of patients on renal replacement therapy: a systematic review and meta-analysis. Value Health. 2008;11:733–741. [DOI] [PubMed] [Google Scholar]
- 24.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 25.Sayin A, Mutluay R, Sindel S. Quality of life in hemodialysis, peritoneal dialysis, and transplantation patients. Transplant Proc. 2007;39:3047–3053. [DOI] [PubMed] [Google Scholar]
- 26.Ogutmen B, Yildirim A, Sever MS, et al. Health-related quality of life after kidney transplantation in comparison intermittent hemodialysis, peritoneal dialysis, and normal controls. Transplant Proc. 2006;38:419–421. [DOI] [PubMed] [Google Scholar]
- 27.Czyżewski Ł, Sańko-Resmer J, Wyzgał J, Kurowski A. Assessment of health-related quality of life of patients after kidney transplantation in comparison with hemodialysis and peritoneal dialysis. Ann Transplant. 2014;19:576–585. [DOI] [PubMed] [Google Scholar]
- 28.Atapour A, Nasr S, Boroujeni AM, et al. A comparison of the quality of life of the patients undergoing hemodialysis versus peritoneal dialysis and its correlation to the quality of dialysis. Saudi J Kidney Dis Transpl. 2016;27:270–280. [DOI] [PubMed] [Google Scholar]
- 29.Okpechi IG, Nthite T, Swanepoel CR. Health-related quality of life in patients on hemodialysis and peritoneal dialysis. Saudi J Kidney Dis Transpl. 2013;24:519–526. [DOI] [PubMed] [Google Scholar]
- 30.Zhang AH, Cheng LT, Zhu N, et al. Comparison of quality of life and causes of hospitalization between hemodialysis and peritoneal dialysis patients in China. Health Qual Life Outcomes. 2007;5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al Wakeel J, Al Harbi A, Bayoumi M, et al. Quality of life in hemodialysis and peritoneal dialysis patients in Saudi Arabia. Ann Saudi Med. 2012;32:570–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niu SF, Li IC. Quality of life of patients having renal replacement therapy. J Adv Nurs. 2005;51:15–21. [DOI] [PubMed] [Google Scholar]
- 33.Mau LW, Chiu HC, Chang PY, et al. Health-related quality of life in Taiwanese dialysis patients: effects of dialysis modality. Kaohsiung J Med Sci. 2008;24:453–460. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigues Fructuoso M, Castro R, Oliveira I, et al. Quality of life in chronic kidney disease. Nefrologia. 2011;31:91–96. [DOI] [PubMed] [Google Scholar]



