Abstract
Benzodiazepines are one of the most prescribed medications as first-line treatment of anxiety, insomnia, and epilepsy around the world. Over the past two decades, advances in the neuropharmacological understanding of gamma aminobutyric acid (GABA)A receptors revealed distinct contributions from each subtype and produced effects. Recent findings have highlighted the importance of α1 containing GABAA receptors in the mechanisms of addiction and tolerance in benzodiazepine treatments. This has shown promise in the development of tranquilizers with minimal side effects such as cognitive impairment, dependence, and tolerance. A valium-like drug without its side effects, as repeatedly demonstrated in animals, is achievable.
Keywords: benzodiazepines, subtype, tolerance, dependence, anxiolytic, GABAA receptor
Introduction
Benzodiazepines are a class of tranquilizers that enhance gamma aminobutyric acid (GABA)ergic transmission. They are seen ubiquitously in the modern health care system as >5% of the total adults in USA are prescribed benzodiazepines each year.1 The major behavioral and psychoactive effects of benzodiazepines include anticonvulsive, sedative, muscle relaxant, and anxiolytic effects.2 They are readily prescribed by physicians and are regarded as a frontline treatment for many common psychiatric disorders such as anxiety, obsessive-compulsive disorder, seizures, as well as a number of sleep disorders.3
Benzodiazepines were discovered in 1955 by chemist Leo Sternbach and, when first introduced, were proposed as a promising replacement for barbiturates, another similar class of tranquilizers that also act on GABA.4,5
The medical use of barbiturates was prominent until the 1950s when serious side effects, such as high incidence of abuse, dependence, and overdose finally started to surface.6
When benzodiazepines hit the market in the 1960s, they were thought to be the successor to barbiturates due to lower toxicity and side effects. Despite having a lower abuse profile, benzodiazepines still cause dependence after repeated use, which was not widely recognized until the 1980s.7,8
Many attempts to produce dependence and tolerance-free benzodiazepine drugs have been investigated in the past. The selective agonist zolpidem was marketed, as promising data showed reduced abuse potential.9 However, these results did not translate in the clinic since zolpidem causes dependence after repeated use.10
Studies have also attempted to investigate neuropharmacological mechanisms of benzodiazepines. At first, the benzodiazepine site was categorized into benzodiazepine subtype I and benzodiazepine subtype II, where traditional benzodiazepines bind to both, but triazolopyridazines (TPZs) have high affinity for only type I.11 It was later found that TPZ was actually just a selective agonist for one of many subtypes that exist within the GABAA receptor family.12
Progress in neuropharmacology has revealed various subtypes within the GABAA receptor family, as well as anatomical and pharmacological differences between them. Investigations have also been directed toward the addiction and tolerance mechanisms of benzodiazepines; their relationships with specific subtypes within the GABAA receptor family will be discussed in further depth.
Pharmacological targets of benzodiazepine
Benzodiazepines, although referred to as a positive allosteric modulator (PAM) of the GABAA receptor, does not actually enhance GABA’s binding to the receptor, like conventional PAMs. Benzodiazepines increase the frequency of chloride channel influx which hyperpolarizes the GABA receptor, resulting in increased inhibitory postsynaptic potential.13,14
α1–6, β1–3, γ1–3, δ, ε, θ, and π make up the currently defined GABAA subunits in the human brain.15 Classic benzodiazepines such as diazepam binds to α1, α2, α3, and α5 containing GABAA receptors.16 α1 containing GABAA receptors are the most abundant subtype and can be found throughout the brain, while α2, α3, and α5 subtypes are more region specific.17
Although numerous combinations exist, most GABAA receptors structurally contain two α, two β, and a single γ subunit surrounding a chloride ion channel as shown in Figure 1.18 The benzodiazepine site is located between the α and γ subunit.
Figure 1.
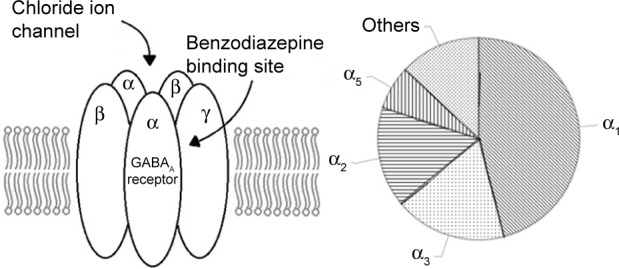
The GABAA receptor and its approximate subtype composition (adapted from Wafford’s study).
Note: Data from Wafford.19
Abbreviation: GABA, gamma aminobutyric acid.
Roles of GABAA receptor α1, α2, α3, and α5 subunits in benzodiazepine pharmacology
GABAA subtype selective compounds and rodent models of subunit point mutation have provided promising data for identifying different subunit contributions toward each clinical effect. We will discuss clinical effects of sedation first, as it impairs the cognitive performance of benzodiazepine-prescribed patients.20
We know that the α1 subtype plays a major role in sedation because α1(H101R) point mutated mice were resilient to sedative effects of benzodiazepines.21 Benzodiazepines that possess sparing efficacy at α1 subtype such as L-838,417 act as a sedation-free anxiolytic in animal models.21,22 L-838,417 has seen popular use in research and is a partial agonist of α2, α3, and α5 containing GABAA receptors.22 Other compounds with low or absent α1 subtype efficacy such as imidazenil, TPA123, and TPA023, are also shown to be sedation-free anxiolytics, revealing the importance of the α1 subtype in the mediation of sedation.23,24
Moreover, α1 subtype selective agonists such as zolpidem are unable to produce anxiolytic effects other than sedation.25 The α5 inverse agonist, α5IA, improves memory without producing anxiety, increased awakeness, or proconvulsant effects in animals.26 So far, we could conclude that anxiety is mediated by either α2 or α3 subtypes or both, while sedation is mediated by α1 containing GABAA receptors.
Because α5IA reversed alcohol-induced memory deficit when given prior to alcohol administration in humans, α5 subtype is thought to contribute toward amnesic effects.26 This was further confirmed when the α5 subtype was found to be responsible for amnestic effects of GABAA receptor PAM etomidate, which correspond with earlier studies on the role of the α5 subtype in memory and its anatomical presence in the hippocampus.27
However, it seems α5 is not the sole subtype that contributes to amnesic effects. α1 subtype full agonist zolpidem seemed to produce more memory and cognitive impairment compared to an equivalent dose of triazolam, an agonist of all benzodiazepine sites.28 Given that zolpidem produces almost no efficacy at the α5 subtype, we can conclude that α1 subtype is also involved in amnesic effects of benzodiazepines.28
In 2000, it was thought that α2 subtype solely mediated anxiolytic actions because only α2(H101R) point mutated mice were still anxious after diazepam treatment.29 On the contrary, two studies 5 years later, the first study using TP003, an α3 subtype selective agonist, demonstrated that TP003 produced strong anxiolytic effects.30 The second study used an α3 subtype inverse agonist α3IA to produce anxiogenic effects in mice, further confirming the role of α3 subtype in mediating anxiety.31
Although α3IA’s weak inverse efficacy at α2 subtype could be argued to be anxiogenic, novel non-benzodiazepine compounds such as adipiplon (NG2-73) and SB-205,384 produced anxiolytic effects without any efficacy at the α2 subtype.32
Moreover, it was shown by data that various novel benzodiazepine compounds with stronger efficacy at α3 than α2 subtype showed more anxiolytic effects. Löw et al’s study received several responses suggesting that the data from elevated plus maze in α2(H101R) mice could be more related and influenced by the motor activity mediated through α2 subtype than anxiolysis.33,34
This is important because almost all past models and reviews have categorized the anxiolytic effects of benzodiazepines solely from efficacy at the α2 subtype, some further suggesting novel benzodiazepine anxiolytics should be α2 selective.35–39 However, the most recent investigations on this matter suggest that both α2 and α3 subtypes mediate anxiety.40,41 Therefore, the design of subtype selective benzodiazepine anxiolytics should not be limited to α2 containing GABAA receptors. This paper will present a revised and comprehensive model of benzodiazepine pharmacology.
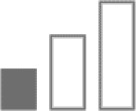
α5 subtype has also been shown to play a role in mediating anxiety and especially conditioned fear memory, as shown by α5 subtype inverse agonists, RY024, and α5(H105R) point mutated mice.42,43 However, the α5 subtype does not seem to play a major role in mediating anxiety as another α5 subtype inverse agonist, α5IA, does not produce anxiety as assessed by elevated plus maze.44 α5 subtype also appears to play a minor role in anticonvulsive actions as proconvulsive as α5IA did not increase seizure thresholds as assessed by pentylenetetrazol.44 As a result, the α5 subtype most probably mediates a narrow category of anxiety, such as conditioned fear, but could be clinically relevant.
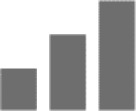
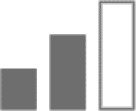
Moreover, several models of GABAA subunit contribution in benzodiazepine pharmacology suggest anticonvulsive action only at α1 subtype.36 TPA023 devoid of activity at the α1 subtype was still able to produce anticonvulsive effects, through α2 and possibly α3 subtype.24
Diazepam was anticonvulsive in both α2(H101R) and α3(H126R) mice; however, more α2(H101R) mice convulsed after treatment toward pentylenetetrazol, revealing that the α2 subtype contributes more than α3 subtype in the anticonvulsant properties of benzodiazepines.29
Most anticonvulsive actions of benzodiazepines are mediated by α1 as diazepam only mildly attenuated pentylenetetrazol-induced seizures in α1(H101R) mice. However, this data should not discourage anticonvulsant drug discovery that has no efficacy at α1 subtype. Recent investigations actually showed that the α1 subtype antagonist, α2, α3, and α5 subtype partial agonist imidazenil was actually more potent than diazepam at attenuating diisopropyl fluorophosphate-induced convulsions and neuronal damage.46
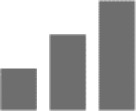
Myorelaxation has been identified to involve α2/α3 subtype using respective point mutated mice; however, the action appears to be primarily mediated through α2 subunit because high doses were needed to induce myorelaxation in the α2(H101R) mice.47 α1 and α5 subtypes might also contribute minorly toward myorelaxation because its respective selective antagonists are able to bluntly alleviate diazepam-induced myorelaxation.48,49
Not only does insufficient information support that α2 subtype exclusively mediates anxiety, other than a single study, current evidence for the anxiolytic effects of benzodiazepines also points toward a dual contribution from α2 and α3 subtypes.29–41 Therefore, in Table 1, anxiolytic effects were assigned to both α2/α3 containing GABAA receptors.
Table 1.
Model of GABAA receptor subtypes and their contribution toward benzodiazepine’s psychopharmacological effects
| Reference GABAA Receptor |
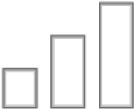
25, 28, 35–39, 49–52 α1 |
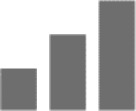
28, 35–39, 49–52, 64 α2 |
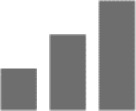
29–41,50–53, 65 α3 |
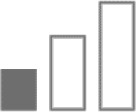
26–39, 42–49 α5 |
|---|---|---|---|---|
| Sedation |
|

|

|

|
| Addiction |
|

|

|

|
| Anxiolysis |
|
|
|
|
| Myorelaxation |

|
|

|

|
| Anticonvulsive |
|
|
|
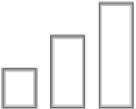
|
| Amnesia |
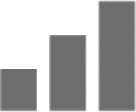
|
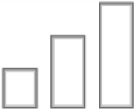
|
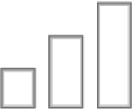
|
|
|
| ||||
| Key: contribution toward clinical effect |
 Negligible |
 Minor |
 Moderate |
 Significant |
Abbreviation: GABA, gamma aminobutyric acid.
In 2010, in a disappointing human trial on MRK-409, a GABAA receptor α2, α3, and α5 subtype partial agonist that promised to be a sedation-free anxiolytic in animals, demonstrated that its minor agonism on α1 subtype produced sedation in humans.54 However, the question of whether other subunits were involved in sedation was quickly overthrown as the clinical results of TPA023B, published shortly after, as an α1 subtype antagonist and α2/α3 subtype partial agonist, produced a sedation-free anxiety suppressing profile,50 demonstrating that any efficacy at α1 subtype could cause sedation in humans.
α5 subtype might be differentially distributed in different sexes. The selective α5 subtype allosteric modulator, SH-053-2′F-R-CH3, seemed to particularly impact female but not male mice at alleviating stress.55 In humans, long-term benzodiazepine use inducing alterations in long-term memory was only significant in women.56
Recent advances in novel clinical applications of benzodiazepines revealed densely populated α2 containing GABAA receptors within the dorsal root involved in relieving pain, in which its inhibitory currents are believed to contribute to nociception.21 Furthermore, although α1 subtype agonists such as zolpidem and diazepam are efficacious for pain and neuropathy, α2, α3 and α5 subtype partial agonists seem to produce similar results without the sedative, amnesic, and addictive properties of α1 subtype agonists.57,58
Mechanism of benzodiazepine addiction
Benzodiazepine-induced activation of mesolimbic dopamine pathway was observed for the first time in 2009. Benzodiazepine indirectly acts upon the dopaminergic neurons in the ventral tegmental area (VTA), a brain region that plays a major role in addiction and reward.59
Both the selective α1 subtype agonist zolpidem as well as diazepam were able to modulate glutamatergic transmission upon dopamine neurons in the VTA. This is groundbreaking because zolpidem has a significantly higher affinity toward α1 (Ki =20 nM) containing GABAA subtypes than α2 and α3 (Ki =400 nM) subtypes, and is almost inactive at α5.60 This suggests that α1 containing GABAA receptors unquestionably play a role in addiction.
However, in the same study, diazepam had a significantly higher effect than zolpidem upon VTA; could there be more subtypes than just α1 subtype in the role of addiction? The role of α5 subtype in addiction was ruled out because α5 subtype inverse agonists were unable to prevent self-administration of ethanol.61 Another study demonstrated that while α2, α3, and α5 point mutated mice showed clear preference toward drinking water contaminated with midazolam, α1(H101R) mice did not show any bias between water and midazolam solution,62 implying that α1 containing GABAA receptors are required for addictive behaviors associated with benzodiazepines. Furthermore, α1 subtype inactive compounds such as TPA023 show almost no abuse properties.60
Recently, opposing evidence has shown that α2 and α3 subtypes might also be implicated in the addiction of benzodiazepines. After α2 subtype within the nucleus accumbens (NAcc) are knockdown in mice, midazolam were no longer reinforcing. Implying that the α2 subtype is at least in part involved in the reinforcing effects of benzodiazepines.63 Rhesus monkeys with a history of benzodiazepine use, but not cocaine use, have been shown to self-administer α1 subtype inactive compound L-838,417 and the α3 subtype selective agonist TP003.64 These findings hint that the α3 containing GABAA receptors could be reinforcing in experienced benzodiazepine users. The experienced rhesus monkeys had been previously treated with numerous different benzodiazepines for around 6 months.64
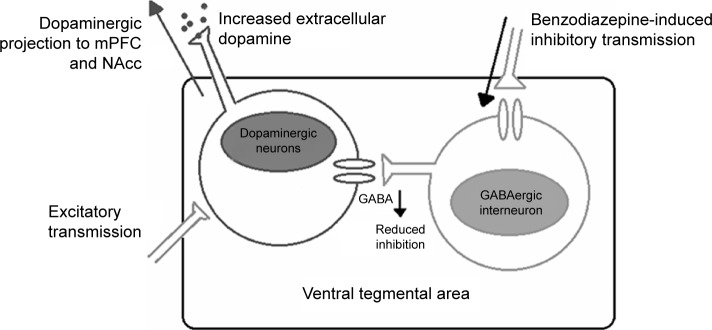
The α1 containing GABAA receptors expressed within the VTA favor its inhibitory projection toward GABA interneurons.62,65 With interneurons being responsible for the inhibitory control over dopaminergic neurons of the VTA, inhibiting the inhibition of dopaminergic neurons results in free firing of the dopaminergic neurons. This results in increased dopamine levels as shown in Figure 2.62,65 This finding is in congruence with the lack of midazolam self-administration in α1(H101R) mice.62 It seems that α1 subtype should be completely avoided since even partial agonists at this site could still produce sedation and addiction as seen in bretazenil and MRK-409.54,66
Figure 2.
Mechanism of benzodiazepines at VTA.
Note: Data adapted from Rudolph et al37 and Heikkinen et al.59
Abbreviations: VTA, ventral tegmental area; GABA, gamma aminobutyric acid; NAcc, nucleus accumbens; mPFC, medial prefrontal cortex.
The increased AMPA/NMDA ratio in VTA has been shown in another GABAA PAM gaboxadol; however, it does not produce reinforcing effects.67 Whether gaboxadol has another mechanism to avert addiction is unclear, but more regions than VTA could be involved. Selective α1 subtype antagonist βCCt and 3-PBC were shown to be able to prevent the reinforcing effects of alcohol.68,69 α1 subtype antagonists effectively blocking addictive behaviors in mice indicate that α1 containing GABAA receptors are highly involved and most likely contribute most or all of the reinforcing effects of GABAergic and benzodiazepine site acting substances. Furthermore, infusions of α1 subtype antagonist into the ventral pallidum and extended amygdala, which accommodates a large amount of α1 containing GABAA receptors, resulted in a reduction in ethanol-induced addictive behaviors.68,70
Opioid mechanisms are associated with reward pathways and a study has shown the competitive opioid receptor antagonist naloxone attenuating the addictive properties of benzodiazepines.71 However, more studies opposing the above study have been published. Interestingly, naloxone is able to block anxiolytic and sedative properties of benzodiazepines.72 More recent studies showed that opioid peptides are involved in benzodiazepine’s anxiolytic rather than addictive effects.73
The role of nicotinic acetylcholine receptor (nAChR), especially the α4β2 subtype, has been highlighted in drug addiction.74 Since nAChR is a major modulator of GABA release in regions such as the thalamus, hippocampus, and VTA,68,75 there is surprisingly less research in this direction. Is there really no link between acetylcholine and benzodiazepine addiction?
nAChRs, especially the α4β2 subtype, are upregulated after chronic exposure to drugs such as nicotine.76 This phenomenon is also seen in naloxone-induced morphine withdrawal, alcohol withdrawal, and what we’re interested in: flumazenil-induced benzodiazepine withdrawal.77,78
Most interestingly, this acetylcholine release was not seen in the partial agonists imidazenil and abecarnil, which has no efficacy at the α1 subtype.79 The fact that benzodiazepine withdrawal is marked by an acetylcholine increase in the nucleus accumbens, which is also seen in other drug withdrawals, further begs clarification of the benzodiazepine–acetylcholine affiliation, and possibly of subunit-specific involvement, which has been overlooked.78,79
Although a recent primate study showed that α3 subtype might be reinforcing in those who are already dependent, α1 containing GABAA receptors contribute to most if not all of the reinforcing effects of benzodiazepines.
Mechanisms of benzodiazepine tolerance
Efficacy of benzodiazepine progressively reduces after long-term exposure; not only is a higher dosage of the drug required to experience the same therapeutic effects, but also discontinuation of prolonged treatment induces withdrawal.80
A simple way to explain tolerance to any drug is down-regulation of the receptor as the aftermath of neuroplasticity. However, it is demonstrated that even after chronic administration of benzodiazepines, the number of benzodiazepine sites is not reduced, neither is the sensitivity of the benzodiazepine site.81,82
Benzodiazepine site downregulation does not seem to happen unless astronomical doses are given, in many studies over 100 mg/kg in rats, which translated into human doses that are far above the therapeutic range.83,84 Not to mention, only inconclusive and inconsistent results have been presented regarding possible changes in subtype composition and their mRNA expression.85,86 So, if its not downregulation, what is the underlying mechanism?
While tolerance to sedative and anticonvulsant effects seems to build quickly in both humans and animal models, a lack of tolerance regarding the anxiolytic and amnesic effects of long-term benzodiazepine use has been consistently demonstrated in clinical trials.87–89
Diazepam and alprazolam for the treatment of panic attacks, social phobia, and other anxiety-related disorders are effective even after chronic use.90,91 Could this mean α2 and α3 containing GABAA receptors, which mediate anxiety, have less significance in the tolerance building mechanism of benzodiazepines?
Other than putting the blame of addiction on α1 subtype, α5 subtype might be required for tolerance toward sedative effects of benzodiazepines. With the guidance of α1, α2, α3, and α5 subtype point mutated mice, repetitive dosing of diazepam showed that α5 containing GABAA receptors of the dentate gyrus lead the adaptive changes associated with sedative tolerance to benzodiazepines.92 After chronic benzodiazepine administration, mice of the α5 point mutated species retained most of the motor-depressant effects of benzodiazepines, whereas α1, α2, and α3 point mutated mice started habituating from the sedative effects of benzodiazepines.92
Now one might ask, how would you then explain tolerance of zolpidem since it has no α5 subtype efficacy? Well, in the same study, α1(H101R) mice showed downregulation of α5 containing GABAA receptors in the dentate gyrus of the hippocampus, but not in α2 and α3 point mutated mice.92 Therefore, a hypothesis that the involvement of α5 as well as α1 subtypes is required to induce tolerance in benzodiazepine use was put forward.
Although long-term benzodiazepine treatment did not reduce the number of benzodiazepine sites or alter the binding affinity as assessed by 3H-flumazenil,93 it did downregulate adenosine receptors in the striatum by almost half in mice of the same investigation. It has been consistently shown that benzodiazepine use is associated with downregulation of adenosine A1 and A2 receptors in animals.94,95 Although the mechanism of how benzodiazepines can influence adenosine receptors is unclear, a possible explanation for the downregulation of adenosine is for the attenuation of benzodiazepine-induced sedation.
It has been proven that benzodiazepines can indirectly increase adenosine after acute administration, through inhibition of adenosine reuptake. The adenosine reuptake inhibitor dipyridamole and the adenosine deaminase EHNA were able to reverse the sedative effects of benzodiazepines, as measured by excitatory currents within the hippocampus.96 This mechanism was recently further validated when the sedative effects of benzodiazepines, barbiturates, and propofol all appeared to be mediated by the adenosine system.97 Downregulation of adenosine receptors as discussed could in part explain the tolerance to the sedative effects of benzodiazepines.
It came forth that not only adenosine is involved in the tolerance toward benzodiazepines. NDMA and AMPA receptor upregulation was observed in the cerebral cortex of mice after abrupt ending of chronic diazepam administration.98,99 More recent inspections of this mechanism showed comparable results especially in the rat hippocampus.100,101
It appears that NMDA upregulation happens acutely after benzodiazepine administration, because NMDA antagonists dizocilpine (MK-801) and CPP were able to prevent tolerance to sedative and withdrawal effects after benzodiazepine administration.102,103 While the NMDA antagonist can suppress withdrawal symptoms in acute benzodiazepine abstinence, the AMPA antagonist prevented withdrawal during the long-term phase in mice.104,105 The upregulation and changes of glutamatergic system are most likely a compensatory mechanism.
Numerous investigations have observed uncoupling between allosteric linkage of GABA and the benzodiazepine site. Uncoupling is a mechanism wherein the benzodiazepine site loses its allosteric modulatory effects over GABAergic activities.18,89 It explains the reduced efficacy of benzodiazepines after chronic use and is further verified in numerous in vivo demonstrations.89 Benzodiazepine site uncoupling is associated with negative modulation of GABAergic transmission and is likely a result of compensation to suppress repeated benzodiazepine-induced GABAergic enhancement.106
In rats continuously exposed to either full agonist, partial agonist, or the antagonist flumazenil, the benzodiazepine efficacy correlates to the degree of uncoupling. Full agonists resulted in the highest percentage of benzodiazepine site uncoupling, especially in benzodiazepine sites that regulated anticonvulsant actions.107 This not only explains the rapid tolerance building toward anticonvulsant effects but also indicates that partial agonists may be able to produce less tolerance.
Interestingly, a single flumazenil dose can reverse the anticonvulsant tolerance after chronic benzodiazepine exposure.108,109 The mechanisms of how flumazenil completely reverses the allosteric uncoupling of benzodiazepine and GABA are unclear. Flumazenil appears to be capable of yielding opposite downstream mechanisms of benzodiazepine agonists, and actually upregulate benzodiazepine binding and GABAergic chloride uptake.110 Similar results have been observed in clinics, where flumazenil was able to reverse tolerance of daily clonazepam in users with partial seizures, who have been taking the medication for over a year.111,112
Benzodiazepine uncoupling recovers after 2 days of abstinence in rats, and only happens after repeated administration.113 This correlates with previously mentioned human clinical data in terms of the quick adaptations to benzodiazepine’s anticonvulsant tolerance. Because about one third of all postsynaptic GABAA receptors are continuously activated, uncoupling might impact continual GABA transmission, which could also contribute to withdrawal.114
In chick cortical neurons, benzodiazepine treatment leads to mild reductions of GABAA receptors on membrane surface; these missing receptors are discovered intracellularly and contribute around 7% of all GABAA receptors.115 Tehrani et al also demonstrated that these intracellular GABAA receptors located on clathrin-bound vesicles are uncoupled as benzodiazepine sites with reduced affinity and allosteric modulatory control over the receptor.116–118
Long-term benzodiazepine treatment caused a significant 83% increase in the number of GABAA receptors on clathrin-coated vesicles versus control. Although benzodiazepines are still able to bind at these sites, they produce no modulatory effect. The internalization also meant a 12% reduction of GABAA receptors located on synaptic membranes, as assessed by 3H-flunitrazepam after a 7-day benzodiazepine treatment.118
This internalization of GABAA receptors at the synaptic membrane possibly contributes to the tolerance of benzodiazepines. Although benzodiazepine agonists are able to bind to intracellular GABA receptors, they produce little or no allosteric modulation. A recent examination of benzodiazepine allosteric uncoupling has shown that benzodiazepine site internalization is part of the uncoupling mechanism.114 This finding is likely the observation of benzodiazepine sites located on clathrin vesicles.
Considerable evidence has been compiled recently underlying a complete and complex molecular mechanism of phosphorylation and posttranslational modifications of GABAA as a response to benzodiazepines that possibly contribute to the observed uncoupling and tolerance building mechanism.119 These complex molecular mechanisms of posttranslational modifications of the GABAA receptor resulting from palmitoylation, ubiquitination, and especially phosphorylation, are believed to dictate the role in regulating the recycling of GABAA receptors through different protein kinases and ultimately impact inhibitory currents.18,84,119
cAMP-dependent protein kinase A (PKA) has been identified to lead the changes and alterations of GABAA receptor functioning of CA1 pyramidal cells within the hippocampus after long-term flurazepam administration.120 Further evidence accumulated over the importance of PKA in the formation of benzodiazepine tolerance showed that mutations to a single PKA phosphorylation site prevented uncoupling, even after chronic diazepam treatment.121
As compared to wild-type mice, a wide range of transcripts that are thought to contribute to the neuroplastic mechanisms of tolerance remained unchanged in α1(H101R) point mutated mice after diazepam administration.122 Transcripts changes such as brain-derived neurotrophic factor and calcium/calmodulin-dependent kinase II play important roles in synaptic plasticity; α1(H101R) mice did not produce transcript changes after diazepam treatment.122 This is direct evidence regarding the involvement of α1 containing GABAA receptors in the role of benzodiazepine tolerance. It agrees with earlier studies wherein α1 subtype inactive benzodiazepines imidazenil and TPA023 consistently failed to show anticonvulsant tolerance in chronic dosing in mice and monkeys.123–129
Interestingly, there seems to be almost no downregulation of GABAA receptors despite long-term heavy administration of imidazenil as compared to diazepam.83 Future studies could compare the uncoupling and posttranslational modification differences between traditional benzodiazepine agonists such as diazepam and α1 subtype ineffective compounds like TPA023 and imidazenil.
Conclusion
Although partial agonism at α1 containing GABAA receptors appears to reduce abuse potential, it appears insufficient. Bretazenil and etizolam which showed reduced abuse potential in animal models do not keep their promise.129,130 When the bretazenil dose increased from 2 to 4 mg in humans, users reported liking the drug. While etizolam has seen popular recreational use, it is sold online as a research chemical capable of inducing euphoria.131 As a result, if clinical effects can be achieved without the involvement of α1 subtype, it should be avoided in future drug design of benzodiazepines and similar compounds.
As already discussed, both α2 and α3 containing GABAA receptors and their involvement in anxiety suppressing effects of benzodiazepines are critical. This is because most current models have characterized α2 containing GABAA receptors as the sole mediator of anxiety in benzodiazepines.35–39 As highlighted in the model presented in this review, the search for a selective anxiolytic should not be constrained to α2 selective.29–41
Benzodiazepine tolerance is complicated and appears to result from a combination of various factors. Complex mechanisms involving uncoupling, intracellular trafficking, post-translational modifications of GABAA receptors all appear to contribute toward benzodiazepine tolerance.89,119 Various studies have also suggested the involvement of other neurotransmitters, especially adenosine and glutamate, during the tolerance and withdrawal effects of benzodiazepines.92–105
The efficacy of benzodiazepines may also play a role in tolerance as full agonists produce more tolerance than partial agonists.107
In the light of current evidence, α1 dormant, α2, α3, and α5 subtype partial agonists not only possess low abuse potential, but are also low or devoid of tolerance building. There is evidence that α1 containing GABAA receptors directly contribute to the downstream effects of tolerance, because α1(H101R) mice have been shown to maintain expressions in neuroplasticity-coding transcripts after diazepam administration.120 This perfectly agrees with data from animal studies regarding the lack of tolerance in α1 subtype inactive compounds such as TPA023B and imidazenil.121–126 Future drug discovery involving tranquilizers should look for partial agonists of α2, α3, and α5 containing GABAA receptors; Valium without its side effects is potentially achievable.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136–142. doi: 10.1001/jamapsychiatry.2014.1763. [DOI] [PubMed] [Google Scholar]
- 2.Dantzer R. Behavioral effects of benzodiazepines: a review. Neurosci Biobehav Rev. 1977;1(2):71–86. [Google Scholar]
- 3.Shader R, Greenblatt D, Balter M. Appropriate use and regulatory control of benzodiazepines. J Clin Pharmacol. 1991;31(9):781–784. doi: 10.1002/j.1552-4604.1991.tb01910.x. [DOI] [PubMed] [Google Scholar]
- 4.Sternbach L. The benzodiazepine story. J Med Chem. 1979;22(1):1–7. doi: 10.1021/jm00187a001. [DOI] [PubMed] [Google Scholar]
- 5.Olsen R, Wamsley J, Lee R, Lomax P. Benzodiazepine/barbiturate/GABA receptor-chloride ionophore complex in a genetic model for generalized epilepsy. Adv Neurol. 1986;44:365–378. [PubMed] [Google Scholar]
- 6.Harris I. Addiction to barbiturates and the barbiturate abstinence syndrome. Ann Intern Med. 1950;33(1):108–121. doi: 10.7326/0003-4819-33-1-108. [DOI] [PubMed] [Google Scholar]
- 7.Wolf B, Grohmann R, Biber D, Brenne P, Rüther E. Benzodiazepine abuse and dependence in psychiatric inpatients. Pharmacopsychiatry. 1989;22(2):54–60. doi: 10.1055/s-2007-1014578. [DOI] [PubMed] [Google Scholar]
- 8.Busto U, Sellers E, Naranjo C, Cappell H, Sanchez-Craig M, Simpkins J. Patterns of benzodiazepine abuse and dependence. Addiction. 1986;81(1):87–94. doi: 10.1111/j.1360-0443.1986.tb00299.x. [DOI] [PubMed] [Google Scholar]
- 9.Perrault G, Morel E, Sanger D, Zivkovic B. Lack of tolerance and physical dependence upon repeated treatment with the novel hypnotic zolpidem. J Pharmacol Exp Ther. 1992;263(1):298–303. [PubMed] [Google Scholar]
- 10.Victorri-Vigneau C, Dailly E, Veyrac G, Jolliet P. Evidence of zolpidem abuse and dependence: results of the French Centre for Evaluation and Information on Pharmacodependence (CEIP) network survey. Br J Clin Pharmacol. 2007;64(2):198–209. doi: 10.1111/j.1365-2125.2007.02861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klepner C, Lippa A, Benson D, Sano M, Beer B. Resolution of two biochemically and pharmacologically distinct benzodiazepine receptors. Pharmacol Biochem Behav. 1979;11(4):457–462. doi: 10.1016/0091-3057(79)90125-4. [DOI] [PubMed] [Google Scholar]
- 12.Atack J. Regional differences in the inhibition of mouse in vivo [3H] Ro 15-1788 binding reflect selectivity for α1 versus α2 and α3 subunit-containing GABAA receptors. Neuropsychopharmacology. 1999;20(3):255–262. doi: 10.1016/S0893-133X(98)00052-9. [DOI] [PubMed] [Google Scholar]
- 13.Sieghart W. GABAA receptors: ligand-gated Cl− ion channels modulated by multiple drug-binding sites. Trends Pharmacol Sci. 1992;13(12):446–450. doi: 10.1016/0165-6147(92)90142-s. [DOI] [PubMed] [Google Scholar]
- 14.Study R, Barker J. Diazepam and (–)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci U S A. 1981;78(11):7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sieghart W, Sperk G. Subunit composition, distribution and function of GABA-A receptor subtypes. Curr Top Med Chem. 2002;2(8):795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- 16.Rudolph U, Crestani F, Benke D, et al. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401(6755):796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 17.Fritschy J, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359(1):154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 18.Vinkers C, Olivier B. Mechanisms underlying tolerance after long-term benzodiazepine use: a future for subtype-selective receptor modulators? Adv Pharmacol Sci. 2012;2012:1–19. doi: 10.1155/2012/416864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wafford K. GABAA receptor subtypes: any clues to the mechanism of benzodiazepine dependence? Curr Opin Pharmacol. 2005;5(1):47–52. doi: 10.1016/j.coph.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Hindmarch I, Trick L, Ridout F. A double-blind, placebo- and positive-internal-controlled (alprazolam) investigation of the cognitive and psychomotor profile of pregabalin in healthy volunteers. Psychopharmacology. 2005;183(2):133–143. doi: 10.1007/s00213-005-0172-7. [DOI] [PubMed] [Google Scholar]
- 21.McKernan R, Rosahl T, Reynolds D, et al. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nat Neurosci. 2000;3(6):587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 22.Knabl J, Witschi R, Hösl K, et al. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451(7176):330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- 23.Auta J, Kadriu B, Giusti P, Costa E, Guidotti A. Anticonvulsant, anxiolytic, and non-sedating actions of imidazenil and other imidazo-benzodiazepine carboxamide derivatives. Pharmacol Biochem Behav. 2010;95(4):383–389. doi: 10.1016/j.pbb.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Atack J. TPA023 [7-(1,1-Dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine], an agonist selective for α2- and α3-containing GABAA receptors, is a nonsedating anxiolytic in rodents and primates. J Pharmacol Exp Ther. 2005;316(1):410–422. doi: 10.1124/jpet.105.089920. [DOI] [PubMed] [Google Scholar]
- 25.Huang M, Radadia K, Macone B, Auerbach S, Datta S. Effects of eszopi-clone and zolpidem on sleep–wake behavior, anxiety-like behavior and contextual memory in rats. Behav Brain Res. 2010;210(1):54–66. doi: 10.1016/j.bbr.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atack J. GABAA receptor subtype-selective efficacy: TPA023, an α2/α3 selective non-sedating anxiolytic and α5IA, an α5 selective cognition enhancer. CNS Drug Rev. 2008;14(1):25–35. doi: 10.1111/j.1527-3458.2007.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng V, Martin J, Erin M, et al. α5GABAA receptors mediate the amnestic but not sedative-hypnotic effects of the general anesthetic etomidate. J Neurosci. 2006;26(14):3713–3720. doi: 10.1523/JNEUROSCI.5024-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roehrs T, Merlotti L, Zorick F, Roth T. Sedative, memory, and performance effects of hypnotics. Psychopharmacology. 1994;116(2):130–134. doi: 10.1007/BF02245054. [DOI] [PubMed] [Google Scholar]
- 29.Löw K, Crestani F, Keist R, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290(5489):131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 30.Dias R. Evidence for a significant role of α3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. J Neurosci. 2005;25(46):10682–10688. doi: 10.1523/JNEUROSCI.1166-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atack J, Hutson P, Collinson N, et al. Anxiogenic properties of an inverse agonist selective for α3 subunit-containing GABAA receptors. Br J Clin Pharmacol. 2005;144(3):357–366. doi: 10.1038/sj.bjp.0706056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navarro J, Burón E, Martín-López M. Anxiolytic-like activity of SB-205384 in the elevated plus-maze test in mice. Psicothema. 2006;18(1):100–104. [PubMed] [Google Scholar]
- 33.Crestani F, Möhler H, Rudolph U. Anxiolytic-like action of diazepam: mediated by GABAA receptors containing the α2-subunit. Trends Pharmacol Sci. 2001;22(8):403. [Google Scholar]
- 34.Reynolds D, McKernan R, Dawson G. Anxiolytic-like action of diazepam: which GABAA receptor subtype is involved? Trends Pharmacol Sci. 2001;22(8):402. doi: 10.1016/s0165-6147(00)01773-9. [DOI] [PubMed] [Google Scholar]
- 35.Rudolph U, Crestani F, Möhler H. GABAA receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol Sci. 2001;22(4):188–194. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- 36.Tan K, Rudolph U, Lüscher C. Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends Neurosci. 2011;34(4):188–197. doi: 10.1016/j.tins.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov. 2011;10(9):685–697. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samardzic J, Svob Strac D. New Developments in Anxiety Disorders. Croatia, Rijeka: InTech; 2016. Benzodiazepines and anxiety disorders: from laboratory to clinic. [Google Scholar]
- 39.Mirza N, Munro G. The role of GABA(A) receptor subtypes as analgesic targets. Drug News Perspect. 2010;23(6):351–360. doi: 10.1358/dnp.2010.23.6.1489909. [DOI] [PubMed] [Google Scholar]
- 40.McEown K, Treit D. α2 GABAA receptor sub-units in the ventral hippocampus and α5 GABAA receptor sub-units in the dorsal hippocampus mediate anxiety and fear memory. Neuroscience. 2013;252:169–177. doi: 10.1016/j.neuroscience.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Morris H, Dawson G, Reynolds D, Atack J, Stephens D. Both α2 and α3 GABAA receptor subtypes mediate the anxiolytic properties of benzodiazepine site ligands in the conditioned emotional response paradigm. Eur J Neurosci. 2006;23(9):2495–2504. doi: 10.1111/j.1460-9568.2006.04775.x. [DOI] [PubMed] [Google Scholar]
- 42.Bailey D, Tetzlaff J, Cook J, He X, Helmstetter F. Effects of hippocampal injections of a novel ligand selective for the α5β2γ2 subunits of the GABA/benzodiazepine receptor on Pavlovian conditioning. Neurobiol Learn Mem. 2002;78(1):1–10. doi: 10.1006/nlme.2001.4050. [DOI] [PubMed] [Google Scholar]
- 43.Crestani F, Keist R, Fritschy J, et al. Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc Natl Acad Sci U S A. 2002;99(13):8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dawson G, Maubach KA, Collinson N, et al. An inverse agonist selective for α5 subunit-containing GABAA receptors enhances cognition. J Pharmacol Exp Ther. 2005;316(3):1335–1345. doi: 10.1124/jpet.105.092320. [DOI] [PubMed] [Google Scholar]
- 45.Rudolph U, Crestani F, Benke D, et al. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401(6755):796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 46.Kadriu B, Guidotti A, Costa E, Auta J. Imidazenil, a non-sedating anticonvulsant benzodiazepine, is more potent than diazepam in protecting against DFP-induced seizures and neuronal damage. Toxicology. 2009;256(3):164–174. doi: 10.1016/j.tox.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 47.Crestani F, Löw K, Keist R, Mandelli M, Möhler H, Rudolph U. Molecular targets for the myorelaxant action of diazepam. Mol Pharmacol. 2001;59(3):442–445. doi: 10.1124/mol.59.3.442. [DOI] [PubMed] [Google Scholar]
- 48.Milić M, Divljaković J, Rallapalli S, van Linn M, Timić T, Cook J, Savić M. The role of α1 and α5 subunit-containing GABAA receptors in motor impairment induced by benzodiazepines in rats. Behav Pharmacol. 2012;23(2):191–197. doi: 10.1097/FBP.0b013e3283512c85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Licata S, Platt D, Cook J, Van Linn M, Rowlett J. Contribution of α1 subunit-containing γ-aminobutyric acid A (GABAA) receptors to motor-impairing effects of benzodiazepines in squirrel monkeys. Psychopharmacology. 2008;203(3):539–546. doi: 10.1007/s00213-008-1401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atack J, Wafford K, Street L, et al. MRK-409 (MK-0343), a GABAA receptor subtype-selective partial agonist, is a non-sedating anxiolytic in preclinical species but causes sedation in humans. J Psychopharmacol. 2010;25(3):314–328. doi: 10.1177/0269881109354927. [DOI] [PubMed] [Google Scholar]
- 51.Ator N, Atack J, Hargreaves R, Burns H, Dawson G. Reducing abuse liability of GABAA/benzodiazepine ligands via selective partial agonist efficacy at α1 and α2/3 subtypes. J Pharmacol Exp Ther. 2009;332(1):4–16. doi: 10.1124/jpet.109.158303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rowlett J, Platt D, Lelas S, Atack J, Dawson G. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Acad Sci U S A. 2005;102(3):915–920. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fischer B, Atack J, Platt D, Reynolds D, Dawson G, Rowlett J. Contribution of GABAA receptors containing α3 subunits to the therapeutic-related and side effects of benzodiazepine-type drugs in monkeys. Psychopharmacology. 2010;215(2):311–319. doi: 10.1007/s00213-010-2142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Atack J, Hallet DJ, Tye S, et al. Preclinical and clinical pharmacology of TPA023B, a GABAA receptor α2/α3 subtype-selective partial agonist. J Psychopharmacol. 2010;25(3):329–344. doi: 10.1177/0269881109354928. [DOI] [PubMed] [Google Scholar]
- 55.Piantadosi S, French B, Poe M, et al. Sex-dependent anti-stress effect of an α5 subunit containing GABAA receptor positive allosteric modulator. Front Pharmacol. 2016;7:446. doi: 10.3389/fphar.2016.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boeuf-Cazou O, Bongue B, Ansiau D, Marquié J, Lapeyre-Mestre M. Impact of long-term benzodiazepine use on cognitive functioning in young adults: the VISAT cohort. Eur J Clin Pharmacol. 2011;67(10):1045–1052. doi: 10.1007/s00228-011-1047-y. [DOI] [PubMed] [Google Scholar]
- 57.Moldofsky H, Lue F, Mously C, Roth-Schechter B, Reynolds W. The effect of zolpidem in patients with fibromyalgia: a dose ranging, double blind, placebo controlled, modified crossover study. J Rheumatol. 1996;23(3):529–533. [PubMed] [Google Scholar]
- 58.Nickolls S, Mace H, Fish R, et al. A comparison of the α2/3/5 selective positive allosteric modulators L-838,417 and TPA023 in preclinical models of inflammatory and neuropathic pain. Adv Pharmacol Sci. 2011;2011:1–12. doi: 10.1155/2011/608912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heikkinen A, Möykkynen T, Korpi E. Long-lasting modulation of glutamatergic transmission in VTA dopamine neurons after a single dose of benzodiazepine agonists. Neuropsychopharmacology. 2009;34(2):290–298. doi: 10.1038/npp.2008.89. [DOI] [PubMed] [Google Scholar]
- 60.Crestani F, Martin J, Möhler H, Rudolph U. Mechanism of action of the hypnotic zolpidem in vivo. Br J Clin Pharmacol. 2000;131(7):1251–1254. doi: 10.1038/sj.bjp.0703717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stephens D, Pistovcakova J, Worthing L, Atack J, Dawson G. Role of GABAA α5-containing receptors in ethanol reward: the effects of targeted gene deletion, and a selective inverse agonist. Eur J Pharmacol. 2005;526(1–3):240–250. doi: 10.1016/j.ejphar.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 62.Tan K, Brown M, Labouèbe G, et al. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463(7282):769–774. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Engin E, Bakhurin K, Smith K, et al. Neural Basis of Benzodiazepine Reward: Requirement for α2 Containing GABAA Receptors in the Nucleus Accumbens. Neuropsychopharmacology. 2012;39(8):1805–1815. doi: 10.1038/npp.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shinday N, Sawyer E, Fischer B, et al. Reinforcing effects of compounds lacking intrinsic efficacy at α1 subunit-containing GABAA receptor subtypes in midazolam- but not cocaine-experienced rhesus monkeys. Neuropsychopharmacology. 2012;38(6):1006–1014. doi: 10.1038/npp.2012.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lalive A, Rudolph U, Lüscher C, Tan K. Is there a way to curb benzodiazepine addiction? Swiss Med Wkly. 2011;141:w13277. doi: 10.4414/smw.2011.13277. [DOI] [PubMed] [Google Scholar]
- 66.Busto U, Kaplan H, Zawertailo L, Sellers E. Pharmacologic effects and abuse liability of bretazenil, diazepam, and alprazolam in humans. Clin Pharmacol Ther. 1994;55(4):451–463. doi: 10.1038/clpt.1994.55. [DOI] [PubMed] [Google Scholar]
- 67.Vashchinkina E, Panhelainen A, Vekovischeva O, et al. GABA site agonist gaboxadol induces addiction-predicting persistent changes in ventral tegmental area dopamine neurons but is not rewarding in mice or baboons. J Neurosci. 2012;32(15):5310–5320. doi: 10.1523/JNEUROSCI.4697-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harvey S, Foster K, McKay P, et al. The GABAA receptor α1 subtype in the ventral pallidum regulates alcohol-seeking behaviors. J Neurosci. 2002;22(9):3765–3775. doi: 10.1523/JNEUROSCI.22-09-03765.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaminski B, Van Linn M, Cook J, Yin W, Weerts E. Effects of the benzodiazepine GABAA α1-preferring ligand, 3-propoxy-β-carboline hydrochloride (3-PBC), on alcohol seeking and self-administration in baboons. Psychopharmacology. 2012;227(1):127–136. doi: 10.1007/s00213-012-2946-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eiler W, June H. Blockade of GABAA receptors within the extended amygdala attenuates D2 regulation of alcohol-motivated behaviors in the ventral tegmental area of alcohol-preferring (P) rats. Neuropharmacology. 2007;52(8):1570–1579. doi: 10.1016/j.neuropharm.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cooper SJ. Benzodiazepine–opiate antagonist interactions and reward processes: implications for drug dependency. Neuropharmacology. 1983;22(4):535–538. doi: 10.1016/0028-3908(83)90174-0. [DOI] [PubMed] [Google Scholar]
- 72.Solhi H, Mostafazadeh B, Reza KVH, Reza GA, Shooshtarizadeh A. Benefit effect of naloxone in benzodiazepines intoxication: findings of a preliminary study. Hum Exp Toxicol. 2010;30(7):535–540. doi: 10.1177/0960327110374972. [DOI] [PubMed] [Google Scholar]
- 73.Kang W, Steven W, Marlene W. Overexpression of proenkephalin in the amygdala potentiates the anxiolytic effects of benzodiazepines. Neuropsychopharmacology. 2000;22(1):77–88. doi: 10.1016/S0893-133X(99)00090-1. [DOI] [PubMed] [Google Scholar]
- 74.Connolly J, Boulter J, Heinemann S. α4-β2 and other nicotinic acetylcholine receptor subtypes as targets of psychoactive and addictive drugs. Br J Clin Pharmacol. 1992;105(3):657–666. doi: 10.1111/j.1476-5381.1992.tb09035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sher T, Chen Y, Sharples TJ, et al. Physiological roles of neuronal nicotinic receptors subtypes: new insights on the nicotinic modulation of neurotransmitter release, synaptic transmission and plasticity. Curr Top Med Chem. 2003;4(3):283–297. doi: 10.2174/1568026043451393. [DOI] [PubMed] [Google Scholar]
- 76.Buisson B, Bertrand D. Nicotine addiction: the possible role of functional upregulation. Trends Pharmacol Sci. 2002;23(3):130–136. doi: 10.1016/S0165-6147(00)01979-9. [DOI] [PubMed] [Google Scholar]
- 77.Williams M, Adinoff B. The role of acetylcholine in cocaine addiction. Neuropsychopharmacology. 2007;33(8):1779–1797. doi: 10.1038/sj.npp.1301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rada P, Hoebel B. Acetylcholine in the accumbens is decreased by diazepam and increased by benzodiazepine withdrawal: a possible mechanism for dependency. Eur J Pharmacol. 2005;508(1–3):131–138. doi: 10.1016/j.ejphar.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 79.Dazzi L, Motzo C, Maira G, Sanna A, Serra M, Biggio G. Enhancement of acetylcholine release by flumazenil in the hippocampus of rats chronically treated with diazepam but not with imidazenil or abecarnil. Psychopharmacology. 1995;121(2):180–185. doi: 10.1007/BF02245628. [DOI] [PubMed] [Google Scholar]
- 80.Petursson H, Lader M. Benzodiazepine dependence. Addiction. 1981;76(2):133–145. doi: 10.1111/j.1360-0443.1981.tb00218.x. [DOI] [PubMed] [Google Scholar]
- 81.Tietz E, Rosenberg H, Chiu T. Autoradiographic localization of benzodiazepine receptor downregulation. J Pharmacol Exp Ther. 1986;236(1):284–292. [PubMed] [Google Scholar]
- 82.Gallager D, Lakoski J, Gonsalves S, Rauch S. Chronic benzodiazepine treatment decreases postsynaptic GABA sensitivity. Nature. 1984;308(5954):74–77. doi: 10.1038/308074a0. [DOI] [PubMed] [Google Scholar]
- 83.Zhao T, Chiu T, Rosenberg H. Reduced expression of gamma-aminobutyric acid type A/benzodiazepine receptor gamma 2 and alpha 5 subunit mRNAs in brain regions of flurazepam-treated rats. Mol Pharmacol. 1994;45(4):657–663. [PubMed] [Google Scholar]
- 84.Poisbeau P, Williams S, Mody I. Silent GABAA synapses during flurazepam withdrawal are region-specific in the hippocampal formation. J Neurosci. 1997;17(10):3467–3475. doi: 10.1523/JNEUROSCI.17-10-03467.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pesold C, Caruncho H, Impagnatiello F, et al. Tolerance to diazepam and changes in GABAA receptor subunit expression in rat neocortical areas. Neuroscience. 1997;79(2):477–487. doi: 10.1016/s0306-4522(96)00609-4. [DOI] [PubMed] [Google Scholar]
- 86.Arnot M, Davies M, Martin I, Bateson A. GABAA receptor gene expression in rat cortex: differential effects of two chronic diazepam treatment regimes. J Neurosci Res. 2001;64(6):617–625. doi: 10.1002/jnr.1115. [DOI] [PubMed] [Google Scholar]
- 87.Kales A, Scharf M, Kales J. Rebound insomnia: a new clinical syndrome. Science. 1978;201(4360):1039–1041. doi: 10.1126/science.684426. [DOI] [PubMed] [Google Scholar]
- 88.Browne T, Penry J. Benzodiazepines in the treatment of epilepsy: a review. Epilepsia. 1973;14(3):277–310. doi: 10.1111/j.1528-1157.1973.tb03965.x. [DOI] [PubMed] [Google Scholar]
- 89.Bateson A. Basic pharmacologic mechanisms involved in benzodiazepine tolerance and withdrawal. Curr Pharm Des. 2002;8(1):5–21. doi: 10.2174/1381612023396681. [DOI] [PubMed] [Google Scholar]
- 90.Schweizer E. Maintenance drug treatment of panic disorder. Arch Gen Psychiatry. 1993;50(1):51–60. doi: 10.1001/archpsyc.1993.01820130053009. [DOI] [PubMed] [Google Scholar]
- 91.Cowley D. Benzodiazepine sensitivity in panic disorder: effects of chronic alprazolam treatment. Neuropsychopharmacology. 1995;12(2):147–157. doi: 10.1016/0893-133X(94)00074-A. [DOI] [PubMed] [Google Scholar]
- 92.Rijnsoever VC. Requirement of α5-GABAA receptors for the development of tolerance to the sedative action of diazepam in mice. J Neurosci. 2004;24(30):6785–6790. doi: 10.1523/JNEUROSCI.1067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hawkins M, Pravica M, Radulovacki M. Chronic administration of diazepam downregulates adenosine receptors in the rat brain. Pharmacol Biochem Behav. 1988;30(2):303–308. doi: 10.1016/0091-3057(88)90459-5. [DOI] [PubMed] [Google Scholar]
- 94.Hawkins M, Pan W, Stefanovich P, Radulovacki M. Desensitization of adenosine A2 receptors in the striatum of the rat following chronic treatment with diazepam. Neuropharmacology. 1988;27(11):1131–1140. doi: 10.1016/0028-3908(88)90008-1. [DOI] [PubMed] [Google Scholar]
- 95.Kaplan G, Cotreau M, Greenblatt D. Effects of benzodiazepine administration on A1 adenosine receptor binding in-vivo and ex-vivo. J Pharm Pharmacol. 1992;44(8):700–703. doi: 10.1111/j.2042-7158.1992.tb05502.x. [DOI] [PubMed] [Google Scholar]
- 96.Narimatsu E, Aoki M. Involvement of the adenosine neuromodulatory system in the benzodiazepine-induced depression of excitatory synaptic transmissions in rat hippocampal neurons in vitro. Neurosci Res. 1999;33(1):57–64. doi: 10.1016/s0168-0102(98)00110-2. [DOI] [PubMed] [Google Scholar]
- 97.Narimatsu E, Niiya T, Kawamata M, Namiki A. The mechanisms of depression by benzodiazepines, barbiturates and propofol of excitatory synaptic transmissions mediated by adenosine neuromodulation. Masui. 2006;55(6):684–691. Japanese. [PubMed] [Google Scholar]
- 98.Tsuda M, Chiba Y, Suzuki T, Misawa M. Upregulation of NMDA receptor subunit proteins in the cerebral cortex during diazepam withdrawal. Eur J Pharmacol. 1998;341(2–3):R1–R2. doi: 10.1016/s0014-2999(97)01501-x. [DOI] [PubMed] [Google Scholar]
- 99.Tsuda M, Shimizu N, Yajima Y, Suzuki T, Misawa M. Hypersusceptibility to DMCM-induced seizures during diazepam withdrawal in mice: evidence for upregulation of NMDA receptors. Naunyn Schmiedebergs Arch Pharmacol. 1998;357(3):309–315. doi: 10.1007/pl00005172. [DOI] [PubMed] [Google Scholar]
- 100.Bonavita C, Ferrero A, Cereseto M, Velardez M, Rubio M, Wikinski S. Adaptive changes in the rat hippocampal glutamatergic neurotrans-mission are observed during long-term treatment with lorazepam. Psychopharmacology. 2003;166(2):163–167. doi: 10.1007/s00213-002-1373-y. [DOI] [PubMed] [Google Scholar]
- 101.Song J, Shen G, Greenfield L, Tietz E. Benzodiazepine withdrawal-induced glutamatergic plasticity involves up-regulation of GluR1-containing α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors in hippocampal CA1 neurons. J Pharmacol Exp Ther. 2007;322(2):569–581. doi: 10.1124/jpet.107.121798. [DOI] [PubMed] [Google Scholar]
- 102.File S, Fernandes C. Dizocilpine prevents the development of tolerance to the sedative effects of diazepam in rats. Pharmacol Biochem Behav. 1994;47(4):823–826. doi: 10.1016/0091-3057(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 103.Koff J, Pritchard G, Greenblatt D, Miller L. The NMDA receptor competitive antagonist CPP modulates benzodiazepine tolerance and discontinuation. Pharmacology. 1997;55(5):217–227. doi: 10.1159/000139531. [DOI] [PubMed] [Google Scholar]
- 104.Fernandes C, File S. Dizocilpine does not prevent the development of tolerance to the anxiolytic effects of diazepam in rats. Brain Res. 1999;815(2):431–434. doi: 10.1016/s0006-8993(98)01160-3. [DOI] [PubMed] [Google Scholar]
- 105.Steppuhn K, Turski L. Diazepam dependence prevented by glutamate antagonists. Proc Natl Acad Sci U S A. 1993;90(14):6889–6893. doi: 10.1073/pnas.90.14.6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jacob T, Michels G, Silayeva L, Haydon J, Succol F, Moss S. Benzodiazepine treatment induces subtype-specific changes in GABAA receptor trafficking and decreases synaptic inhibition. Proc Natl Acad Sci U S A. 2012;109(45):18595–18600. doi: 10.1073/pnas.1204994109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hernandez T, Heninger C, Wilson M, Gallager D. Relationship of agonist efficacy to changes in GABA sensitivity and anticonvulsant tolerance following chronic benzodiazepine ligand exposure. Eur J Pharmacol. 1989;170(3):145–155. doi: 10.1016/0014-2999(89)90535-9. [DOI] [PubMed] [Google Scholar]
- 108.Gonsalves S, Gallager D. Persistent reversal of tolerance to anticonvulsant effects and GABAergic subsensitivity by a single exposure to benzodiazepine antagonist during chronic benzodiazepine administration. J Pharmacol Exp Ther. 1988;244(1):79–83. [PubMed] [Google Scholar]
- 109.Klein R, Mascia M, Harkness P, Hadingham K, Whiting P, Harris R. Regulation of allosteric coupling and function of stably expressed gamma-aminobutyric acid (GABA)A receptors by chronic treatment with GABAA and benzodiazepine agonists. J Pharmacol Exp Ther. 1995;274(3):1484–1492. [PubMed] [Google Scholar]
- 110.Miller L, Heller J, Lumpkin M, Weill C, Greenblatt D, Shader R. Augmentation of GABAA receptor function by chronic exposure to GABA-neutral and GABA-negative benzodiazepine ligands in cultured cortical neurons. Biochem Pharmacol. 1990;40(6):1337–1344. doi: 10.1016/0006-2952(90)90401-6. [DOI] [PubMed] [Google Scholar]
- 111.Lugoboni F, Leone R. What is stopping us from using flumazenil? Addiction. 2012;107(7):1359. doi: 10.1111/j.1360-0443.2012.03851.x. [DOI] [PubMed] [Google Scholar]
- 112.Savic I. Feasibility of reversing benzodiazepine tolerance with flumazenil. Lancet. 1991;337(8734):133–137. doi: 10.1016/0140-6736(91)90799-u. [DOI] [PubMed] [Google Scholar]
- 113.Tietz E, Chiu T, Rosenberg H. Regional GABA/benzodiazepine receptor/chloride channel coupling after acute and chronic benzodiazepine treatment. Eur J Pharmacol. 1989;167(1):57–65. doi: 10.1016/0014-2999(89)90747-4. [DOI] [PubMed] [Google Scholar]
- 114.Gutiérrez M, Ferreri M, Gravielle M. GABA-induced uncoupling of GABA/benzodiazepine site interactions is mediated by increased GABAA receptor internalization and associated with a change in subunit composition. Neuroscience. 2014;257:119–129. doi: 10.1016/j.neuroscience.2013.10.077. [DOI] [PubMed] [Google Scholar]
- 115.Tehrani M, Barnes E. Agonist-dependent internalization of gamma-aminobutyric acid A/benzodiazepine receptors in chick cortical neurons. J Neurochem. 1991;57(4):1307–1312. doi: 10.1111/j.1471-4159.1991.tb08295.x. [DOI] [PubMed] [Google Scholar]
- 116.Tehrani M, Barnes E. Identification of GABAA/benzodiazepine receptors on clathrin-coated vesicles from rat brain. J Neurochem. 1993;60(5):1755–1761. doi: 10.1111/j.1471-4159.1993.tb13400.x. [DOI] [PubMed] [Google Scholar]
- 117.Tehrani M, Barnes E. Sequestration of γ-aminobutyric acid A receptors on clathrin-coated vesicles during chronic benzodiazepine administration in vivo. J Pharmacol Exp Ther. 1997;283(1):384–390. [PubMed] [Google Scholar]
- 118.Tehrani M, Baumgartner B, Barnes E. Clathrin-coated vesicles from bovine brain contain uncoupled GABAA receptors. Brain Res. 1997;76(1–2):195–203. doi: 10.1016/s0006-8993(97)01037-8. [DOI] [PubMed] [Google Scholar]
- 119.Gravielle M. Activation-induced regulation of GABAA receptors: is there a link with the molecular basis of benzodiazepine tolerance? Pharmacol Res. 2016;109:92–100. doi: 10.1016/j.phrs.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 120.Lilly S, Zeng X, Tietz E. Role of protein kinase A in GABAA receptor dysfunction in CA1 pyramidal cells following chronic benzodiazepine treatment. J Neurochem. 2003;85(4):988–998. doi: 10.1046/j.1471-4159.2003.01746.x. [DOI] [PubMed] [Google Scholar]
- 121.Ali N, Olsen R. Chronic benzodiazepine treatment of cells expressing recombinant GABAA receptors uncouples allosteric binding: studies on possible mechanisms. J Neurochem. 2008;79(5):1100–1108. doi: 10.1046/j.1471-4159.2001.00664.x. [DOI] [PubMed] [Google Scholar]
- 122.Huopaniemi L, Keist R, Randolph A, Certa U, Rudolph U. Diazepam-induced adaptive plasticity revealed by α1 GABAA receptor-specific expression profiling. J Neurochem. 2004;88(5):1059–1067. doi: 10.1046/j.1471-4159.2003.02216.x. [DOI] [PubMed] [Google Scholar]
- 123.Auta J, Impagnatiello F, Kadriu B, Guidotti A, Costa E. Imidazenil: a low efficacy agonist at α1- but high efficacy at α5-GABAA receptors fail to show anticonvulsant cross tolerance to diazepam or zolpidem. Neuropharmacology. 2008;55(2):148–153. doi: 10.1016/j.neuropharm.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zanotti A, Mariot R, Contarino A, Lipartiti M, Giusti P. Lack of anticonvulsant tolerance and benzodiazepine receptor down regulation with imidazenil in rats. Br J Clin Pharmacol. 1996;117(4):647–652. doi: 10.1111/j.1476-5381.1996.tb15239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ghiani C, Serra M, Motzo C, et al. Chronic administration of an anti-convulsant dose of imidazenil fails to induce tolerance of GABAA receptor function in mice. Eur J Pharmacol. 1994;254(3):299–302. doi: 10.1016/0014-2999(94)90470-7. [DOI] [PubMed] [Google Scholar]
- 126.Kadriu B, Gocel J, Larson J, et al. Absence of tolerance to the anticonvulsant and neuroprotective effects of imidazenil against DFP-induced seizure and neuronal damage. Neuropharmacology. 2011;61(8):1463–1469. doi: 10.1016/j.neuropharm.2011.08.043. [DOI] [PubMed] [Google Scholar]
- 127.Auta J, Guidotti A, Costa E. Imidazenil prevention of alprazolam-induced acquisition deficit in patas monkeys is devoid of tolerance. Proc Natl Acad Sci U S A. 2000;97(5):2314–2319. doi: 10.1073/pnas.97.5.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vinkers C, van Oorschot R, Nielsen E, et al. GABAA receptor α subunits differentially contribute to diazepam tolerance after chronic treatment. PLoS One. 2012;7(8):e43054. doi: 10.1371/journal.pone.0043054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rundfeldt C, Wlaź P, Hönack D, Löscher W. Anticonvulsant tolerance and withdrawal characteristics of benzodiazepine receptor ligands in different seizure models in mice. Comparison of diazepam, bretazenil and abecarnil. J Pharmacol Exp Ther. 1995;275(2):693–702. [PubMed] [Google Scholar]
- 130.Sanna E, Busonero F, Talani G, et al. Low tolerance and dependence liabilities of etizolam: molecular, functional, and pharmacological correlates. Eur J Pharmacol. 2005;519(1–2):31–42. doi: 10.1016/j.ejphar.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 131.O’Connell C, Sadler C, Tolia V, Ly B, Saitman A, Fitzgerald R. Overdose of etizolam: the abuse and rise of a benzodiazepine analog. Ann Emerg Med. 2015;65(4):465–466. doi: 10.1016/j.annemergmed.2014.12.019. [DOI] [PubMed] [Google Scholar]