Abstract
Objects
The purpose of this study was to quantitatively assess the effects of water-based Liuzijue exercise on patients with COPD and compare it with land-based Liuzijue exercise.
Materials and methods
Participants were randomly allocated to one of three groups: the water-based Liuzijue exercise group (WG), the land-based Liuzijue exercise group (LG), and the control group (CG). CG participants accepted no exercise intervention, while training groups performed Liuzijue exercise according to Health Qigong Liuzijue (People’s Republic of China) in different environments for 60-min sessions twice a week for 3 months.
Results
Of the 50 patients enrolled, 45 (90%) completed the 3-month intervention. The CG showed decreased expiratory muscle strength, extensor and flexor endurance ratio (ER) of the elbow joints and flexor peak torque (PT), total work (TW), and ER of the knee joints (p<0.05). Both training groups showed improved respiratory muscle strength, which differed from the CG (p<0.001). In addition, extensor and flexor TW of the elbow joints in the training groups were increased (p<0.01), and the WG differed from the CG in extensor TW and ER and flexor TW (p<0.01), while the LG differed from the CG in flexor TW and extensor ER (p<0.05). PT, PT/body weight (BW), and TW in the knee joint extensor in the training groups were increased as well (PT and PT/BW: p<0.05, TW: p<0.01), and the WG differed from the CG in terms of knee joints outcomes, while the LG differed from the CG in flexor TW only (p<0.05).
Conclusion
Water-based Liuzijue exercise has beneficial effects on COPD patients’ respiratory muscle strength and peripheral skeletal muscle function, and additional benefits may exist in endurance of upper limbs and strength and endurance of lower limbs when compared with land-based Liuzijue exercise.
Keywords: COPD, Liuzijue exercise, water-based exercise, respiratory muscle strength, isokinetic muscle strength, quantitative assessment
Introduction
COPD, which is characterized by persistent respiratory symptoms and limited airflow, is a preventable and treatable disease.1 As the fourth-leading cause of death in the world, COPD is primarily a lung disease, but it is associated with extrapulmonary manifestations, such as muscle weakness, pulmonary hypertension, anxiety, depression, induced exercise capacity impairment, decreased quality of life, and even death.1–3 Pulmonary rehabilitation is an effective method that can significantly improve the exercise capacity of patients and alleviate the progression of the disease.4,5
Since COPD patients mainly complain of dyspnea, previous research primarily used respiratory training, such as pursed lip breathing and inspiratory muscle training, to increase muscle strength and respiratory muscles endurance to relieve dyspnea.6–8 However, studies have shown that respiratory training does not improve exercise tolerance (6-min walking distance, 6MWD) or maximal oxygen uptake (VO2max),8 although it improves inspiratory muscle function in COPD patients. This may be attributable to the fact that respiratory training cannot improve exertion dyspnea in COPD patients.9 However, as the cornerstone of pulmonary rehabilitation, exercise training can significantly improve the exercise capacity and skeletal muscle function of COPD patients.10–12 Traditional Chinese exercises, such as Tai Chi, Liuzijue, and Yijinjing, which combine the advantages of respiratory training with skeletal muscle training, can significantly improve the function of respiratory muscles and skeletal muscle, patient exercise capacity, psychological function, and quality of life.13–16
Liuzijue exercise, which is characterized by diaphragmatic breathing and pursed lip breathing, is performed by expiration to produce six different sounds, namely, “xu”, “he”, “hu”, “si”, “chui”, and “xi”, and corresponding limb movements;13 this may be a more suitable intervention for COPD patients. In addition, using hydrotherapy as an option for exercise training appears to be popular, as evidenced by an increasing number of publications. Moreover, the safety and accessibility of water-based exercise in COPD patients has been demonstrated.17,18 Compared with land-based exercise, different intensities of water-based exercise have a similar or more significant effect on improving exercise tolerance (6MWD and endurance shuttle walk test, ESWT) and quality of life for COPD patients.19,20 This may be explained by the properties of water, such as water turbulence and resistance, which can increase muscle work when moving; buoyancy, which can support BW and reduce mechanical impact on the body; water temperature, which can promote blood circulation; water pressure on the chest and abdomen, which can activate respiratory muscles; and water flow, which has an effect on skin receptors.21,22 However, it is unclear whether water-based Liuzijue exercise can effectively improve the condition of COPD patients and whether there are additional benefits compared with land-based Liuzijue exercise.
Although there are many studies that have focused on the effects of exercise on dyspnea (Borg score), exercise capacity (6MWD, ESWT, increased shuttle walk test), quality of life, and psychological function,13,16,20,23 few studies have focused on the effects of exercise on skeletal muscle function. Hence, the current study was designed to investigate the effects of water-based Liuzijue exercise on upper and lower limb muscle function in COPD patients compared with land-based Liuzijue exercise using a CON-TREX isokinetic dynamometer.
The aim of the study was to investigate the following: 1) the effectiveness of water-based Liuzijue exercise on pulmonary function, respiratory muscle strength, and upper and lower limb muscle strength in COPD patients; and 2) whether there are additional benefits compared with land-based Liuzijue exercise. It was hypothesized that water-based Liuzijue exercise would produce better respiratory muscle function and skeletal muscle function than land- based Liuzijue exercise after 3 months of intervention.
Materials and methods
Study design
A randomized controlled trial was conducted with concealed allocation and blinded outcome assessment. Eligible patients were randomly assigned to one of three groups: the water-based Liuzijue exercise group (WG), the land-based Liuzijue exercise group (LG), and the control group (CG). Random assignment was achieved by an independent person who drew sealed numbers produced by a computer before the start of the intervention. Due to the characteristics of the interventions, it was not possible to blind the therapist or participants to their allocation, but the participants were blinded to the study hypothesis. Outcome measures were performed at baseline and at the end of the exercise program (3 months). All of the participants gave written informed consent to participate, and the study was approved by the Ethics Committee of Yue-Yang Integrative Medicine Hospital affiliated with Shanghai University of Traditional Chinese Medicine (Shanghai, People’s Republic of China).
Recruitment of patients
Participants were allocated in a rehabilitation center at Yue-Yang Integrative Medicine Hospital affiliated with Shanghai University of Traditional Chinese Medicine between March 2015 and March 2016.
The inclusion criteria were the following: diagnosed with COPD based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (forced expiratory volume in the first second [FEV1]/forced vital capacity [FVC] <0.7);24 clinically stable in the past 4 weeks; of either sex and between the age of 40 and 80 years; volunteer to participate; and have not exercised regularly for at least 6 months before the study (at least twice a week). Patients were excluded if they presented with an exacerbation of the disease; with physical comorbidities that prevented them from performing exercise; with exercise-limiting cardiovascular or neuromuscular diseases; with restricted physical activity; or they were not suitable for an assessment of muscle strength of the limbs.
Study interventions
All of the subjects continued their prescribed medical treatments. Participants in the CG did not perform any exercise intervention, while the WG and LG attended two 60-min sessions a week of supervised exercise led by the same experienced instructor for 3 months. Before formal intervention, patients were provided with three sessions weekly for 2 weeks to study the actions and the breathing methods of Liuzijue from a specialized instructor. During the study period, the pamphlet of Liuzijue Qigong25, video of Liuzijue, and exercise record manual were assigned to patients to study and record exercise. Patients were asked to record their exercise program, including metrics such as time, intensity, frequency, and exercise site. Participants were encouraged to exercise at an intensity of 4–6 on the modified Borg scale for dyspnea and perceived exertion (somewhat strong to strong/very strong).26 In addition, exercise in water and on land were matched for intensity and duration. Training intensity was measured 3 times during each exercise session, and if the intensity was below 3, the patients were encouraged to increase their intensity through adjustment of their range of motion and posture. All of the patients performed exercise while being monitored by a respiratory physician in order to avoid adverse effects related to exercise training.
Land-based Liuzijue exercise program
The LG participants performed exercise from 9:00 to 10:00 a.m. in the Rehabilitation Center of Yue-Yang Integrative Medicine Hospital. The exercise program consisted of three parts: 1) warm-up, when the trunks and limbs were stretched for 10 min; 2) Liuzijue exercise, when an intact Liuzijue exercise usually took 12–15 min to complete at the usual pace, 40 min Liuzijue exercise was performed by patients; and 3) cool down, when exercise was performed to adjust breathing and relax the muscles for 10 min.
Water-based Liuzijue exercise program
WG participants performed at 1:00–2:00 p.m. in the thermostatic swimming pool of Shanghai University of Sports from June to September 2017. The intervention program was similar to the land-based Liuzijue exercise program, but it was performed in a pool heated to 32°C±2°C. The level of water immersion of standing exercise was self-selected and always between the xiphisternum and clavicles of each participant. Patients were asked to enter the swimming pool 1 h after meals and maintain a quiet and conscious condition. After entering the pool, participants performed adaptive training taught by an instructor to adapt to the water temperature and relax their breathing. In addition, a lifeguard was in attendance during all of the exercise sessions to aid in any unforeseen medical emergencies. The exercise program was similar to that followed by the LG.
Outcome measures
All of the outcomes, including pulmonary function, respiratory muscle strength, and upper and lower limb muscle strength, were measured at baseline and study completion (3 months). The primary outcome measures were respiratory muscle strength and skeletal muscle function. The secondary outcome measure was pulmonary function. All of these data were measured or recorded at baseline and within 7 days of the end of the intervention.
Pulmonary function tests
Pulmonary function was measured using a spirometer (Masterscreen-PFT, Jaeger, Germany) by trained personnel in the pulmonary function room of the Shanghai Pulmonary Hospital affiliated with Tongji University according to the guidelines of the American Thoracic Society.27 The best of the three readings was documented, with values that varied no more than 5% of the highest value.
Respiratory muscle strength
Respiratory muscle strength was determined according to maximal inspiratory pressure (PImax) and maximal expiratory pressure (PEmax) using a spirometer (Masterscreen-PFT, Jaeger, Germany) by trained personnel in the pulmonary function room of the Shanghai Pulmonary Hospital affiliated with Tongji University according to standard protocols.28 PImax measurements required patients to inhale to the maximum extent. At the beginning of the test, the patients took several calm breaths, and when the patient exhaled to the level of functional residual volume, the instrument immediately blocked the airway and the patient was asked to complete the inhale duration of 3 s. The stable inhalation pressure readings were recorded, and the measurement was conducted at least 3 times, with the highest result being used for analysis; the interval between two consecutive measurements was at least 1 min. The PEmax measurement process was similar to that of the PImax process to a certain degree. PEmax measurements required patients to exhale to the maximum extent. At the beginning of the test, the patients took several calm breaths, and when the patient inhaled to the level of total lung volume, the instrument immediately blocked the airway, and the patient was asked to complete the exhale duration of 3 s. The stable exhalation pressure readings were recorded, and the measurement was conducted at least 3 times, with the highest result used for analysis; the interval between two consecutive measurements was at least 1 min. The variation in test values was required to be <20%.
CON-TREX isokinetic muscle function test
Isokinetic assessments of muscle function were performed on the CON-TREX isokinetic dynamometer (CON-TREX, Physiomed, Germany) by the same experimenter at the Sport and Health Science Exhibition Hall of Shanghai University of Sports according to the manufacturer’s recommendations. The isokinetic muscle strength test evaluates dynamic strength, and reliability was established, with the data output objectively reflecting the functional condition of certain muscle groups.29 This test evaluates four sets of muscles consisting of upper and lower limb muscle groups (elbow extension, elbow flexion, knee extension, and knee flexion).
Elbow joints: participants sat on the chair of the machine in an upright position, and the advanced upper limb was assessed. To minimize extraneous body movements during muscle contractions, straps were applied across the chest and pelvis. The alignment between the dynamometer rotational axis and the elbow joint rotational axis (humeral lateral condyle) was checked at the beginning of the trial. In addition, the end resistance pad was placed 2–3 cm above the wrist. Then, the patients were asked to perform flexion and extension 2–3 times to determine the range of motion and to become familiar with the test procedure.
Knee joints: participants sat on the chair of the machine in an upright position, and the advanced lower limb was assessed. To minimize extraneous body movements during muscle contractions, straps were applied across the chest, pelvis, and mid-thigh. The alignment between the dynamometer rotational axis and the knee joint rotational axis (knee joint fibula capitulum) was checked at the beginning of the trial. The end resistance pad was placed 2–3 cm above the ankle. Then, the patients were asked to perform flexion and extension 2–3 times to determine the range of motion and to become familiar with the test procedure.
Isokinetic concentric contraction mode was used, and performed at two angular velocities: 60°/s and 180°/s. At the angular velocity of 60°/s, patients were asked to perform maximal flexion and extension 5 times, and at the angular velocity of 180°/s, patients were asked to perform maximal flexion and extension for 30 consecutive repetitions. The participants were given verbal encouragement by the investigator. Parameters were recorded at 60°/s, including peak torque (PT, N m) generated from the highest of five maximal contractions as the maximal muscular strength, and relative PT was obtained by the ratio of peak torque to body weight (PT/BW, N m/kg) and can be used for comparison between individuals. Parameters were recorded at 180°/s, including total work (TW, J) measured with 30 consecutive repetitions, which was presented as peripheral muscular endurance. The muscle endurance ratio (ER) was obtained by the ratio of the TW of post 15 repetitions to the TW of pre 15 repetitions and used for muscular consecutive repetition contraction capacity.
Statistical analyses
The data were analyzed using SPSS (version 23.0; IBM Corporation, Armonk, NY, USA). The normality of the data and homogeneity of variance were tested before statistical analysis. Continuous variables were presented as mean ± SD or mean ± standard error of the mean (SEM), while categorical variables were described as frequencies (%). For continuous variables to satisfy normality and variance homogeneity, paired t-tests were used to perform analyses of within-group differences, and one-way analysis of variance and multiple comparisons with a Bonferroni test were used to analyze intergroup differences. Otherwise, a nonparametric Kruskal–Wallis test was used to analyze the intergroup differences. For categorical variables, the chi-square test was employed. A p-value of <0.05 was considered to be significant.
Results
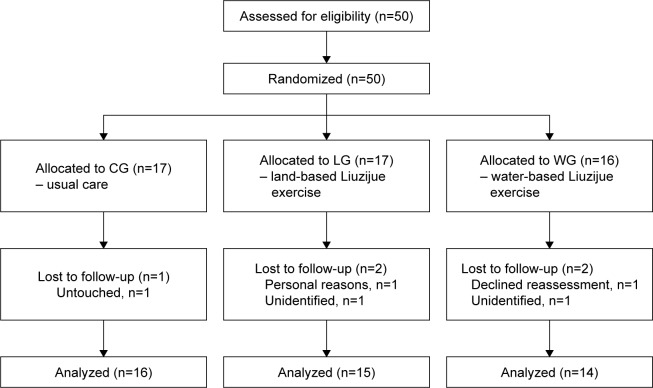
The 50 enrolled COPD patients were randomly assigned to three groups: 16 in the WG, 17 in the LG, and 17 in the CG. After a 3-month intervention, a total of 45 (90%) COPD patients completed the program and were included in the analyses (Figure 1). Groups were similar at baseline (Table 1).
Figure 1.
Study flow diagram.
Abbreviations: CG, control group; LG, land-based Liuzijue exercise group; WG, water-based Liuzijue exercise group.
Table 1.
Baseline characteristics of participants
| Characteristics | CG (n=16) | LG (n=15) | WG (n=14) | p-value |
|---|---|---|---|---|
| Age (years) | 66±8 | 65±8 | 65±11 | 0.96 |
| Sex (male/female) | 12/4 | 12/3 | 9/5 | 0.62 |
| BMI (kg/m2) | 24±3 | 23±4 | 23±3 | 0.73 |
| FEV1%pred | 59±17 | 55±17 | 59±22 | 0.77 |
| FEV1/FVC (%) | 60±8 | 60±18 | 62±14 | 0.86 |
| Years of COPD | 12±4 | 13±4 | 12±4 | 0.79 |
| COPD stage | 0.63 | |||
| Grade I | 2 (12.50) | 2 (13.33) | 3 (21.43) | |
| Grade II | 9 (56.25) | 10 (66.67) | 9 (64.29) | |
| Grade III | 3 (18.75) | 3 (20.00) | 2 (14.28) | |
| Grade IV | 2 (12.50) | 0 | 0 |
Notes: Data are expressed as mean ± SD or n (%). One-way analysis of variance was used to compare the age, BMI, FEV1%pred, and FEV1/FVC among groups, and the chi-square test was used to compare sex and COPD stage among groups. The significance level was set at p<0.05.
Abbreviations: %pred, percentage of predicted values; BMI, body mass index; CG, control group; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; LG, land-based Liuzijue exercise group; WG, water-based Liuzijue exercise group.
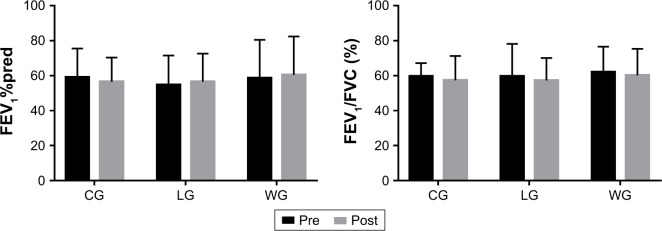
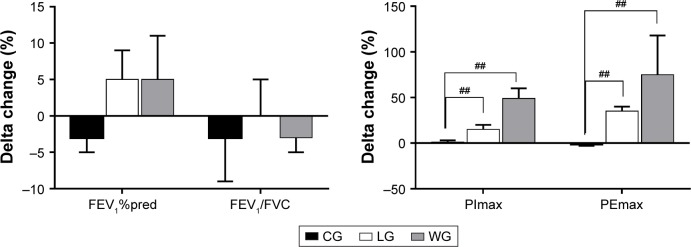
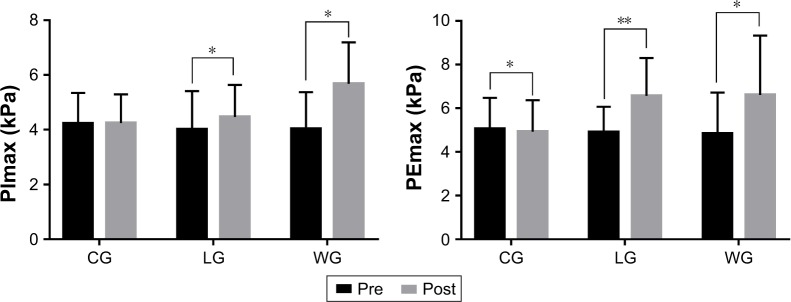
In relation to pulmonary function, none of the three groups showed changes in FEV1%pred and FEV1/FVC postintervention (Figure 2), and no significant difference was observed among the three groups after training (Table 2; Figure 3). While training groups exhibited a statistical intra-group difference in respiratory muscle strength after the exercise program (Figure 4), that is, PImax in the LG (4.01±1.4 vs 4.46±1.18 kPa) and WG (4.03±1.34 vs 5.68±1.51 kPa) and PEmax in the LG (4.9±1.17 vs 6.57±1.73 kPa) and WG (4.84±1.88 vs 6.61±2.72 kPa), CG showed a significant decrease in PEmax (p=0.022). The intergroup comparison showed that LG and WG had significantly increased PImax and PEmax compared to CG (p<0.01).
Figure 2.
Pulmonary function before and after the 3-month intervention.
Notes: Data are expressed as mean ± SD. No differences were observed between the groups at baseline and after the intervention. Within-groups comparison was made by the paired-sample t-test.
Abbreviations: %pred, percentage of predicted values; CG, control group; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; LG, land-based Liuzijue exercise group; post, postintervention; pre, preintervention; WG, water-based Liuzijue exercise group.
Table 2.
Comparisons of group changes (%) in pulmonary function and respiratory muscle strength
| Variables | CG (n=16)
|
LG (n=15)
|
WG (n=14)
|
p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Δchanges (%) | Pre | Post | Δchanges (%) | Pre | Post | Δchanges (%) | ||
| FEV1%pred | 59±17 | 57±14 | −0.03±0.02 | 55±17 | 57±16 | 0.05±0.04 | 59±22 | 60±22 | 0.05±0.06 | 0.177 |
| FEV1/FVC (%) | 60±7.57 | 57±14 | −0.03±0.06 | 60±18 | 57±13 | 0±0.05 | 62±14 | 60±15 | −0.03±0.02 | 0.911 |
| PImax (kPa) | 4.23±1.11 | 4.24±1.05 | 0.01±0.02 | 4.01±1.4 | 4.46±1.18* | 0.15±0.05## | 4.03±1.34 | 5.68±1.51* | 0.49±0.11## | <0.001 |
| PEmax (kPa) | 5.05±1.43 | 4.93±1.44* | −0.02±0.01 | 4.9±1.17 | 6.57±1.73** | 0.35±0.05## | 4.84±1.88 | 6.61±2.72* | 0.75±0.43## | <0.001 |
Notes: Pre and post data are expressed as mean ± SD, Δchanges (%) = ([post − pre]/pre) ×100, and expressed as mean ±SEM. Within groups was compared by the paired-sample t-test, one-way analysis of variance was used to compare the changes of FEV1/FVC between groups, and the nonparametric Kruskal-Wallis test was used to compare the changes of respiratory muscle strength and FEV1%pred between groups.
p<0.05, comparisons are significant within groups.
p<0.01, comparisons are significant within groups.
p<0.01, group changes (%) comparison are significant between the control group and the intervention group.
Abbreviations: %pred, percentage of predicted values; CG, control group; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; LG, land-based Liuzijue exercise group; PEmax, maximal expiratory pressure; PImax, maximal inspiratory pressure; post, postintervention; pre, preintervention; WG, water-based Liuzijue exercise group.
Figure 3.
Changes of pulmonary function and respiratory muscle strength after the 3-month intervention.
Notes: Delta changes (%) = ([post − pre]/pre) ×100, and they are expressed as mean ± SEM. Between-group comparisons used the one-way analysis of variance or the nonparametric Kruskal–Wallis test and are expressed as a p-value. The level of significance was set at p<0.05, and ##p<0.01 means it was significant when compared to the control group.
Abbreviations: %pred, percentage of predicted values; CG, control group; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; LG, land-based Liuzijue exercise group; PEmax, maximal expiratory pressure; PImax, maximal inspiratory pressure; WG, water-based Liuzijue exercise group.
Figure 4.
Respiratory muscle strength before and after the 3-month intervention.
Notes: Data are expressed as mean ± SD. No differences were observed between the groups at baseline. Within-groups comparison was made by the paired-sample t-test.
*p<0.05, comparisons were significant within groups. **p<0.01, comparisons were significant within groups.
Abbreviations: CG, control group; LG, land-based Liuzijue exercise group; PEmax, maximal expiratory pressure; PImax, maximal inspiratory pressure; post, postintervention; pre, preintervention; WG, water-based Liuzijue exercise group.
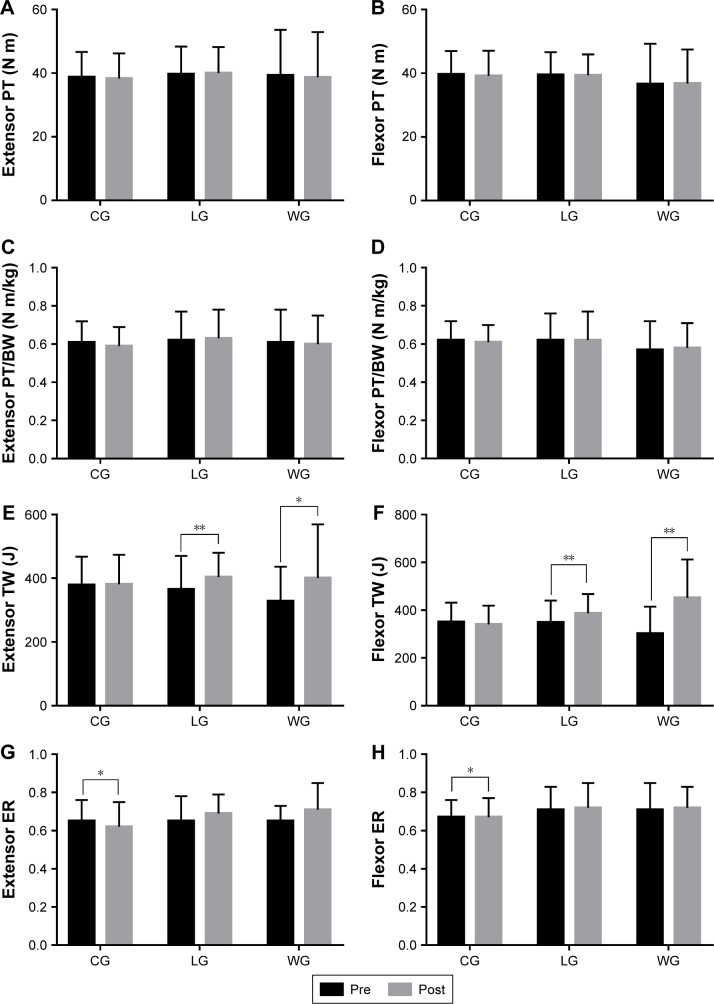
The parameters, which evaluated the muscle function of elbow joints, are shown in Table 3. There was no difference in extensor and flexor PT and PT/BW after 3 months in all of the groups (Figure 5). Extensor and flexor TW were increased significantly in the training groups (LG: p=0.008 and p=0.001, respectively; WG: p=0.031 and p<0.001, respectively), while flexor TW in the CG showed a decreasing trend. In addition, the LG had significantly increased flexor TW compared to the CG (p=0.016), and the WG had significantly improved extensor and flexor TW compared to the CG (p=0.004 and p<0.001, respectively). Extensor and flexor ER exhibited no difference in the training groups after a 3-month intervention, but they decreased statistically in the CG (p=0.035 and p=0.048, respectively). In addition, the training groups compared to the CG had a significant difference in extensor ER (p=0.012 and p=0.006, respectively).
Table 3.
Comparisons of group changes (%) in muscle function of elbow joints
| Variables | CG (n=16)
|
LG (n=15)
|
WG (n=14)
|
p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Δchanges (%) | Pre | Post | Δchanges (%) | Pre | Post | Δchanges (%) | ||
| Extensor PT (N m) | 38.75±7.93 | 38.31±7.89 | −0.01±0.01 | 39.73±8.64 | 40.07±8.17 | 0.01±0.01 | 39.3±14.33 | 38.7±14.23 | 0.02±0.06 | 0.109 |
| Flexor PT (N m) | 39.69±7.28 | 39.19±7.89 | −0.01±0.02 | 39.53±7.07 | 39.4±6.54 | 0±0.01 | 36.6±12.65 | 36.8±10.67 | 0.04±0.05 | 0.438 |
| Extensor PT/BW (N m/kg) | 0.61±0.11 | 0.59±0.1 | −0.01±0.01 | 0.62±0.15 | 0.63±0.15 | 0.01±0.01 | 0.61±0.17 | 0.6±0.15 | 0.02±0.06 | 0.109 |
| Flexor PT/BW (N m/kg) | 0.62±0.1 | 0.61±0.09 | −0.01±0.02 | 0.62±0.14 | 0.62±0.15 | 0±0.01 | 0.57±0.15 | 0.58±0.13 | 0.04±0.05 | 0.438 |
| Extensor TW (J) | 378.55±88.98 | 381.13±92.59 | 0.01±0.01 | 365.47±104.57 | 403.64±76.42** | 0.15±0.05 | 328.04±108.16 | 400.5±168.76* | 0.22±0.09## | 0.004 |
| Flexor TW (J) | 350.55±81.39 | 340.36±78.92 | −0.03±0.02 | 348.64±91.36 | 387.14±80.21** | 0.14±0.04# | 301.54±113.75 | 451.71±160.72** | 0.62±0.16## | <0.001 |
| Extensor ER | 0.65±0.11 | 0.62±0.13* | −0.06±0.02 | 0.65±0.13 | 0.69±0.1 | 0.09±0.05# | 0.65±0.08 | 0.71±0.14 | 0.1±0.05## | 0.002 |
| Flexor ER | 0.67±0.09 | 0.67±0.1* | −0.01±0.01 | 0.71±0.12 | 0.72±0.13 | 0.03±0.02 | 0.71±0.14 | 0.72±0.11 | 0.04±0.05 | 0.11 |
Notes: Pre and post data are expressed as mean ± SD, Δchanges (%) = ([post − pre]/pre) ×100, and they are expressed as mean ±SEM. Within-groups comparison was made by the paired-sample t-test and the nonparametric Kruskal–Wallis test was used to compare the changes of elbow joints muscle function between groups.
p<0.05, comparisons were significant within groups.
p<0.01, comparisons were significant within groups.
p<0.05, group changes (%) comparison was significant between the control group and the intervention group.
p<0.01, group changes (%) comparison was significant between the control group and the intervention group.
Abbreviations: BW, body weight; CG, control group; ER, endurance ratio; LG, land-based Liuzijue exercise group; post, postintervention; pre, preintervention; PT, peak torque; TW, total work; WG, water-based Liuzijue exercise group.
Figure 5.
Muscle function of elbow joints before and after the 3-month intervention.
Notes: (A–D) Within-groups comparisons in CG, LG, and WG of the elbow extensor and flexor PT and PT/BW to reflect the changes of muscle strength after intervention; (E–H) Within-groups comparisons in CG, LG, and WG of the elbow extensor and flexor TW and ER to reflect the changes of muscle endurance after intervention. Data are expressed as mean ± SD. No differences were observed between the groups at baseline. Within-groups comparisons were made with the paired-sample t-test. *p<0.05, comparisons were significant within groups, **p<0.01, comparisons were significant within groups.
Abbreviations: BW, body weight; CG, control group; ER, endurance ratio; LG, land-based Liuzijue exercise group; post, postintervention; pre, preintervention; PT, peak torque; TW, total work; WG, water-based Liuzijue exercise group.
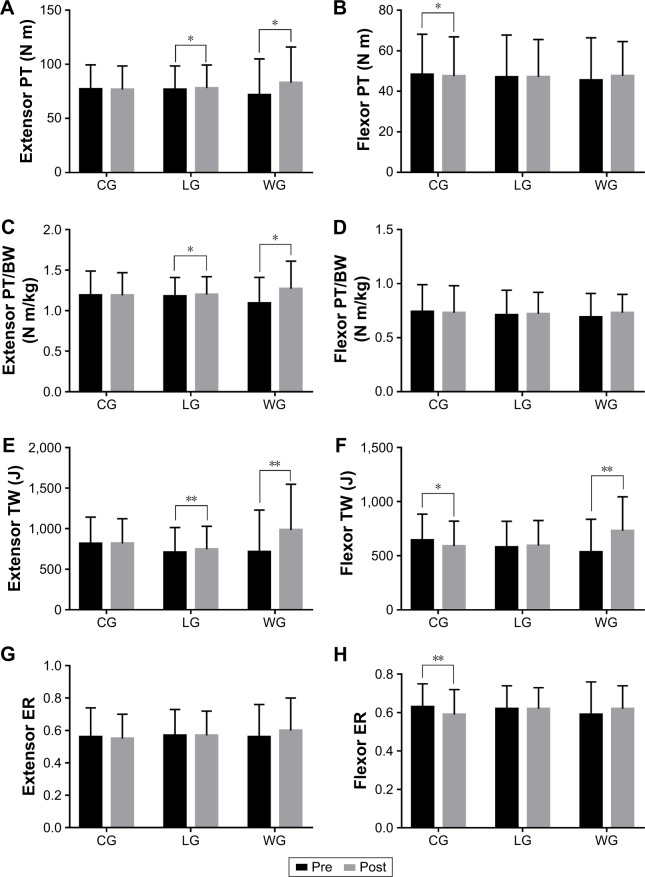
The parameters used to evaluate the muscle function of the knee joints are shown in Table 4. After 3 months of intervention, extensor PT and PT/BW in the training groups were increased significantly (extensor PT in the LG [76.62±21.86 vs 77.98±21.33] and WG [71.55±33.42 vs 82.92±33.00] and extensor PT/BW in the LG [1.18±0.23 vs 1.20±0.22] and WG [1.09±0.32 vs 1.27±0.34]), while the flexor PT was significantly decreased in the CG (p=0.036; Figure 6). An intergroup comparison showed a significant difference in the extensor and flexor PT and PT/BW between the WG and CG (p<0.05; Figure 7). In addition, the extensor TW in the training groups was increased significantly, and the flexor TW was increased in the WG (p=0.009), while the flexor TW in the CG was decreased statistically significantly (p=0.031). An intergroup comparison showed that the extensor and flexor TW and ER had significantly improved in the WG when compared to the CG (p<0.05), and only flexor TW in the LG compared to the CG showed a statistically significant difference (p=0.032).
Table 4.
Comparisons of group changes (%) in muscle function of knee joints
| Variables | CG (n=16)
|
LG (n=15)
|
WG (n=14)
|
p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Δchanges (%) | Pre | Post | Δchanges (%) | Pre | Post | Δchanges (%) | ||
| Extensor PT (N m) | 77.01±22.46 | 76.74±21.73 | 0±0.01 | 76.62±21.86 | 77.98±21.33* | 0.02±0.01 | 71.55±33.42 | 82.92±33.00* | 0.19±0.07# | 0.041 |
| Flexor PT (N m) | 48.31±19.84 | 47.50±19.39* | −0.02±0.01 | 47.03±20.76 | 47.08±18.49 | 0.01±0.01 | 45.47±20.99 | 47.66±16.89 | 0.08±0.04# | 0.024 |
| Extensor PT/BW (N m/kg) | 1.19±0.30 | 1.19±0.28 | 0±0.01 | 1.18±0.23 | 1.20±0.22* | 0.02±0.01 | 1.09±0.32 | 1.27±0.34* | 0.19±0.04# | 0.041 |
| Flexor PT/BW (N m/kg) | 0.74±0.25 | 0.73±0.25 | −0.02±0.01 | 0.71±0.23 | 0.72±0.20 | 0.01±0.01 | 0.69±0.22 | 0.73±0.17 | 0.09±0.04# | 0.028 |
| Extensor TW (J) | 816.46±326.02 | 817.64±305.62 | 0.02±0.02 | 705.89±308.43 | 745.98±283.58** | 0.09±0.03 | 712.14±516.47 | 983.58±563.44** | 0.56±0.18## | 0.002 |
| Flexor TW (J) | 643.64±240.87 | 589.78±230* | −0.08±0.03 | 579.53±238.67 | 593.47±232.71 | 0.03±0.02# | 532.63±304.65 | 731.09±312.8** | 0.51±0.15## | <0.001 |
| Extensor ER | 0.56±0.18 | 0.55±0.15 | 0.01±0.04 | 0.57±0.16 | 0.57±0.15 | 0.01±0.03 | 0.56±0.20 | 0.60±0.20 | 0.16±0.11# | 0.027 |
| Flexor ER | 0.63±0.12 | 0.59±0.13** | −0.06±0.02 | 0.62±0.12 | 0.62±0.11 | 0±0.01 | 0.59±0.17 | 0.62±0.12 | 0.09±0.06# | 0.02 |
Notes: Pre and post data are expressed as mean ± SD, Δchanges (%) = ([post − pre]/pre) ×100, and they are expressed as mean ±SEM. Within-group comparisons were made by the paired-sample t-test, one-way analysis of variance was used to compare the changes of extensor ER between groups and post hoc multiple comparisons were performed using Bonferroni test, and the nonparametric Kruskal–Wallis test was used to compare others between groups.
p<0.05, comparisons are significant within groups.
p<0.01, comparisons are significant within groups.
p<0.05, group changes (%) comparison was significant between the control group and the intervention group.
p<0.01, group changes (%) comparison was significant between the control group and the intervention group.
Abbreviations: BW, body weight; CG, control group; ER, endurance ratio; LG, land-based Liuzijue exercise group; post, postintervention; pre, preintervention; PT, peak torque; TW, total work; WG, water-based Liuzijue exercise group.
Figure 6.
Muscle function of knee joints before and after the 3-month intervention.
Notes: (A–D) Within-groups comparisons in CG, LG, and WG of the knee extensor and flexor PT and PT/BW to reflect the changes of muscle strength after intervention; (E–H) Within-groups comparisons in CG, LG, and WG of the knee extensor and flexor TW and ER to reflect the changes of muscle endurance after intervention. Data are expressed as mean ± SD. No differences were observed between the groups at baseline. Within-groups comparisons were made with the paired-sample t-test. *p<0.05, comparisons were significant within groups. **p<0.01, comparisons were significant within groups.
Abbreviations: BW, body weight; CG, control group; ER, endurance ratio; LG, land-based Liuzijue exercise group; post, postintervention; pre, preintervention; PT, peak torque; TW, total work; WG, water-based Liuzijue exercise group.
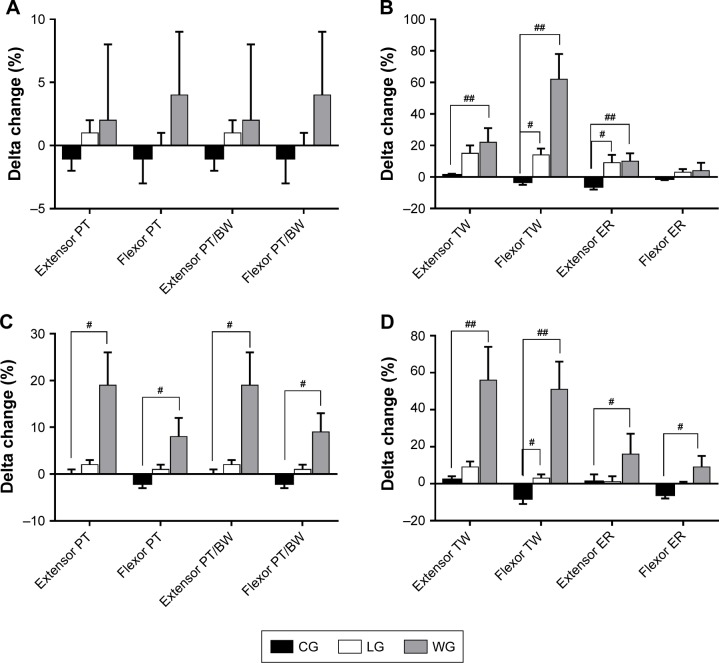
Figure 7.
Changes of elbow and knee joints of muscle function after 3-month intervention. Muscle function of elbow joints are shown in A and B, while knee joints are shown in C and D.
Notes: Delta changes (%) = ([post − pre]/pre) ×100, and they are expressed as mean ± SEM. Between-group comparisons used the one-way analysis of variance or the nonparametric Kruskal–Wallis test and are expressed as a p-value. The level of significance was set at p<0.05, and #p<0.05 and ##p<0.01 mean it was significant when compared to the CG.
Abbreviations: BW, body weight; CG, control group; ER, endurance ratio; LG, land-based Liuzijue exercise group; post, postintervention; pre, preintervention; PT, peak torque; TW, total work; WG, water-based Liuzijue exercise group.
Discussion
The present study demonstrated that water-based Liuzijue exercise can improve respiratory muscle strength, extensor and flexor endurance of the elbow joints, and maximum muscle contraction strength and endurance capacity of the knee joints in patients with COPD. Furthermore, it seems that water-based Liuzijue exercise has more significant effects on the muscle function of the knee joints in COPD patients when compared with the land-based Liuzijue exercise.
Studies have shown that even mild dyspnea in COPD patients significantly reduced physical activity (combined with a reduction in 6MWD and a more pronounced lower diffusion capacity of carbon monoxide),30 and the reduction in physical activity further exacerbated the condition of the patients, leading to worsened symptoms, muscle weakness, and decreased exercise capacity, even impacting the rate of decline in lung function.31,32 GOLD (version 2017)1 states that COPD patients should accept pulmonary rehabilitation with exercise as the primary component to improve respiratory symptoms, quality of life, physical activity, and participation in daily activities, and the safety applied to different disease grades of COPD patients has been confirmed. However, conventional exercise training requires equipment, an exercise location, and medical personnel to supervise its safety, which poses a heavy burden on patients’ families and health resources. Therefore, this study adopted traditional Chinese exercise with Liuzijue exercise, which is easy to learn and not limited by location and equipment. As a low-intensity aerobic exercise, the effectiveness and safety of Liuzijue exercise applied to COPD patients have also been confirmed.13 In addition, this study examined Liuzijue exercise in an aquatic environment as applied to the rehabilitation of patients with COPD to investigate whether the aquatic element can bring additional benefits. The safety of the water environment in the rehabilitation of COPD patients, even with physical comorbidities, has also been demonstrated and studies have shown that patients prefer to exercise in water because exercise in water reduces discomfort.17,33 Although the blood pressure and vital capacity of COPD patients decreased to some extent and the Borg score slightly increased during exercise in water, vital signs, such as heart rate, respiration rate, and oxygen saturation, did not change significantly; there was no significant discomfort or adverse events during exercise.18 Similar to previous studies, patients with COPD in this study were highly compliant and did not experience adverse events associated with intervention, further confirming the feasibility and safety of this program.
The current study corroborates the results from previous studies:34,35 neither of these interventions significantly improved FEV1%pred and FEV1/FVC% in patients with COPD. In contrast, studies have shown that both low-intensity land-based exercise and water-based exercise significantly increase FEV1 in COPD patients.19 This may be explained by the higher baseline FEV1%pred level in patients enrolled in the present study having less improvement. In addition, the present outcomes corroborate previous results of water exercise intervention19 that land-based Liuzijue exercise and water-based Liuzijue exercise can significantly improve respiratory muscle strength in patients with COPD, and a water environment did not bring additional effects. Respiratory muscle training alone can significantly improve respiratory muscle strength in COPD patients,8 but when combined with aerobic exercise, it has an additional effect on improving PImax.35 Beneficial effects obtained may be attributed to the following: the improvement in the shape of the rib cage, accessory respiratory muscle function, electromyogram fatigability of the diaphragm, and the increase in the proportion of type I fibers and size of type II fibers in the external intercostal muscles.8,36 Although there was no significant difference in PImax between the LG and the WG in this study, the data showed a trend of greater improvement in the WG. This may be explained by the characteristic of water applying pressure on the chest and abdomen of the patients, increasing the resistance to inhalation and further activating the inspiratory muscle. However, respiratory muscle strength did not improve, while improved specific airway conductance in COPD patients with Liuizjue exercise has been found.13 Larger samples and long-term intervention should be carried out in clinical studies to further confirm whether a water environment can confer additional benefits on respiratory muscle strength in COPD patients.
Studies have shown that skeletal muscle dysfunction (declined skeletal muscle strength and endurance compared with healthy sedentary controls) exists in patients with mild COPD, even before respiratory symptoms are present.37,38 Muscle atrophy appears as a major cause of decreased skeletal muscle strength,39 while the circumference of the upper and lower limbs can strongly predict COPD mortality compared to BMI.40 Similarly, skeletal muscle dysfunction as an independent risk factor can predict mortality in patients with COPD,41 and it is closely related to reduced physical activity, exercise capacity, and quality of life.42 Therefore, skeletal muscle function is an important component of the comprehensive condition of COPD patients. A variety of methods and techniques can be used to assess skeletal muscle function. Of these, one-repetition measurement (1 RM), which assesses dynamic skeletal muscle strength, and hand-grip strength, which assesses isometric muscle contractile strength, are commonly used in COPD patients and have good reliability and validity.43 These methods are simple and do not require expensive equipment, but they are vulnerable to the subjective awareness of the participants. Alternatively, computerized dynamometers with demonstrated good test–retest reliability, as well as safety and accuracy,29 can be used to assess the dynamic skeletal muscle function in patients with COPD. Therefore, this study used the CON-TREX isokinetic dynamometer to assess the skeletal muscle function of the elbow and knee joints in patients with COPD.
This study showed that no significant difference was found in the muscle strength of elbow joints (expressed by extensor and flexor PT and PT/BW) after 3 months of training, while muscle endurance improved significantly (expressed by extensor and flexor TW). These results indicated that land-based Liuzijue and water-based Liuzijue exercise can improve the muscle endurance of the elbow joints, which is consistent with a previous study.37 Generally, skeletal muscle function in the upper and lower limbs was significantly decreased in COPD patients, with the exception of upper limb endurance, which may be attributed to the continual recruitment of upper limb muscles to support ventilation and daily activities.37 In the present study, patients in the CG exhibited decreased extensor and flexor ER after the 3-month intervention, and significant differences in extensor ER and flexor TW were found when compared to the LG. A significant difference in extensor ER and extensor and flexor TW was found when compared to the WG. Additionally, the improvement in extensor TW (15%) of LG was equal to the minimal clinical importance difference value,38 while the improvement of extensor TW (22%) and flexor TW (62%) of the WG was significantly greater. The improved upper limb muscle endurance may be explained by the fact that these muscles were not being trained to the maximum extent during physical activity.
Consistent with a previous study of skeletal muscle dysfunction, which commonly exists in lower limb muscles in COPD patients,44 this study showed that flexor strength (PT) and endurance (TW, ER) of the knee joint were significantly decreased in the CG patients. Meanwhile, extensor muscle strength (PT, PT/BW) and endurance (TW) of the knee joint were significantly increased in the LG and WG patients. Although the exercises were different, the results were consistent with previous studies,37,38,45 indicating that Liuzijue exercise has a significant effect on improving extensor function of the knees in COPD patients. As for flexor function, TW was significantly increased in the WG only, and an intergroup comparison showed a significant difference in flexor strength and endurance between the WG and CG, while there was only a significant difference in TW between the LG and CG. Considering the clinical value, 15% of the minimal improvement in muscle strength was applied to the comparison. The present results exhibited that water-based Liuzijue exercise provides a clinical improvement in muscle strength (extensor PT and extensor PT/BW, 19%) and endurance (extensor TW, 56%; flexor TW, 51%; extensor ER, 16%) of the knee joint, while land-based Liuzijue exercise did not. The results showed that water-based Liuzijue exercise may have additional benefits to the muscle function of the knee joints than land-based Liuzijue exercise. This may be ascribed to the semiflexed position of the knees during Liuzijue exercise, which induces greater stimulation on the extensor of the knee. In addition, water-based exercise can provide greater resistance to limb movement on the premise of supporting BW, which may be one of the reasons why water-based Liuzijue exercise can further improve skeletal muscle function. Furthermore, aerobic metabolism is the energy source used to sustain physical activity in water, and water can promote peripheral blood circulation, which may explain why water-based Liuzijue exercise can further improve skeletal muscle endurance.
The main advantage of this study was the quantitative assessment of the effects of exercise on pulmonary function, respiratory muscle strength, and isokinetic muscle strength in COPD patients. Second, the study uses Liuzijue exercise, one of the traditional Chinese exercises, as an intervention in which the intensity is low and suitable to various conditions of COPD patients. Hydrotherapy, one of the emerging interventions in recent years, has become widely popular in COPD patients. Hence, the results of this study have high reliability and repeatability, and the intervention program has extensive field to be applied. However, this study did not assess the clinically related indicators of COPD patients, while previous studies have shown that the improvement of skeletal muscle function is closely related to clinical indicators, such as exercise capacity, quality of life, and even survival in patients with COPD.46 Additionally, the promotion of Liuzijue exercise may be limited by different cultural backgrounds. However, tai chi, another form of traditional Chinese exercise, has been applied in the field of fitness and rehabilitation of elderly people across the world.
Conclusion
The results of this study demonstrated that Liuzijue exercise and water-based Liuzijue exercise can significantly improve respiratory muscle strength, muscle endurance of elbow joints, and muscle strength and endurance of knee joints in COPD patients. Water-based Liuzijue exercise has an additional effect on improving extensor endurance of elbow joints and muscle function of knee joints. Therefore, long-term and regular practice of water-based Liuzijue exercise should be an alternative rehabilitation for COPD patients. Further studies should be conducted to comprehensively assess the effects of water-based Liuzijue exercise in patients with COPD.
Acknowledgments
This study was supported by Key Laboratory of Exercise and Health Sciences of Ministry of Education, Shanghai University of Sport, the national fitness project of General Administration of Sport of China (Nos 2015B077 and 2017B021), the research project of the Fitness Qigong Administrative Centre of General Administration of Sport of China (QG2017057), the “Qi Kang” young innovative talents project of School of Rehabilitation Medicine, Shanghai University of Traditional Chinese Medicine, and National Natural Science Foundation of China (No 81472163).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Global initiative for chronic obstructive lung disease (GOLD) Global strategy for the Diagnosis, Management and Prevention of chronic obstructive pulmonary disease [homepage on the Internet] 2017. [Accessed July 23, 2017]. report. Available from: http://www.goldcopd.com.
- 2.Cielen N, Maes K, Gayan-Ramirez G. Musculoskeletal disorders in chronic obstructive pulmonary disease. Biomed Res Int. 2014;2014:9657–9664. doi: 10.1155/2014/965764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379(9823):1341–1351. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnardóttir RH, Sörensen S, Ringqvist I, Larsson K. Two different training programmes for patients with COPD: a randomised study with 1-year follow-up. Respir Med. 2006;100(1):130–139. doi: 10.1016/j.rmed.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 5.Güell MR, Cejudo P, Ortega F, et al. Benefits of long-term pulmonary rehabilitation maintenance program in patients with severe chronic obstructive pulmonary disease. Three-year follow-up. Am J Respir Crit Care Med. 2017;195(5):622–629. doi: 10.1164/rccm.201603-0602OC. [DOI] [PubMed] [Google Scholar]
- 6.Spahija J, de Marchie M, Grassino A. Effects of imposed pursed-lips breathing on respiratory mechanics and dyspnea at rest and during exercise in COPD. Chest. 2005;128(2):640–650. doi: 10.1378/chest.128.2.640. [DOI] [PubMed] [Google Scholar]
- 7.de Araujo CL, Karloh M, Dos Reis CM, Palú M, Mayer AF. Pursed-lips breathing reduces dynamic hyperinflation induced by activities of daily living test in patients with chronic obstructive pulmonary disease: a randomized cross-over study. J Rehabil Med. 2015;47(10):957–962. doi: 10.2340/16501977-2008. [DOI] [PubMed] [Google Scholar]
- 8.Ramirez-Sarmiento A, Orozco-Levi M, Guell R, et al. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: structural adaptation and physiologic outcomes. Am J Respir Crit Care Med. 2002;166(11):1491–1497. doi: 10.1164/rccm.200202-075OC. [DOI] [PubMed] [Google Scholar]
- 9.Larson JL, Covey MK, Wirtz SE, et al. Cycle ergometer and inspiratory muscle training in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(2):500–507. doi: 10.1164/ajrccm.160.2.9804067. [DOI] [PubMed] [Google Scholar]
- 10.Mador MJ, Bozkanat E, Aggarwal A, Shaffer M, Kufel TJ. Endurance and strength training in patients with COPD. Chest. 2004;125(6):2036–2045. doi: 10.1378/chest.125.6.2036. [DOI] [PubMed] [Google Scholar]
- 11.Ortega F, Toral J, Cejudo P, et al. Comparison of effects of strength and endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(5):669–674. doi: 10.1164/rccm.2107081. [DOI] [PubMed] [Google Scholar]
- 12.Iepsen UW, Munch GD, Rugbjerg M, et al. Effect of endurance versus resistance training on quadriceps muscle dysfunction in COPD: a pilot study. Int J Chron Obstruct Pulmon Dis. 2016;11:2659–2669. doi: 10.2147/COPD.S114351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao CM, Zhuang YC. Efficacy of Liuzijue Qigong in individuals with chronic obstructive pulmonary disease in remission. J Am Geriatr Soc. 2015;63(7):1420–1425. doi: 10.1111/jgs.13478. [DOI] [PubMed] [Google Scholar]
- 14.Chan AW, Lee A, Suen LK, Tam WW. Tai chi Qigong improves lung functions and activity tolerance in COPD clients: a single blind, randomized controlled trial. Complement Ther Med. 2011;19(1):3–11. doi: 10.1016/j.ctim.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M, Xv G, Luo C, Meng D, Ji Y. Qigong Yi Jinjing promotes pulmonary function, physical activity, quality of life and emotion regulation self-efficacy in patients with chronic obstructive pulmonary disease: a pilot study. J Altern Complement Med. 2016;22(10):810–817. doi: 10.1089/acm.2015.0224. [DOI] [PubMed] [Google Scholar]
- 16.Chan AW, Lee A, Suen LK, Tam WW. Effectiveness of a Tai Chi Qigong program in promoting health-related quality of life and perceived social support in chronic obstructive pulmonary disease clients. Qual Life Res. 2010;19(8):653–664. doi: 10.1007/s11136-010-9632-6. [DOI] [PubMed] [Google Scholar]
- 17.McNamara RJ, McKeough ZJ, McKenzie DK, Alison JA. Acceptability of the aquatic environment for exercise training by people with chronic obstructive pulmonary disease with physical comorbidities: additional results from a randomised controlled trial. Physiotherapy. 2015;101(2):187–192. doi: 10.1016/j.physio.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Perk J, Perk L, Bodén C. Cardiorespiratory adaptation of COPD patients to physical training on land and in water. Eur Respir J. 1996;9(2):248–252. doi: 10.1183/09031936.96.09020248. [DOI] [PubMed] [Google Scholar]
- 19.de Souto Araujo ZT, de Miranda Silva Nogueira PA, Cabral EE, de Paula Dos Santos L, da Silva IS, Ferreira GM. Effectiveness of low-intensity aquatic exercise on COPD: a randomized clinical trial. Respir Med. 2012;106(11):1535–1543. doi: 10.1016/j.rmed.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 20.Wadell K, Sundelin G, Henriksson-Larsén K, Lundgren R. High intensity physical group training in water-an effective training modality for patients with COPD. Respir Med. 2004;98(5):428–438. doi: 10.1016/j.rmed.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 21.McNamara RJ, McKeough ZJ, McKenzie DK, Alison JA. Water-based exercise training for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;12:CD008290. doi: 10.1002/14651858.CD008290.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Lotshaw AM, Thompson M, Sadowsky HS, Hart MK, Millard MW. Quality of life and physical performance in land- and water-based pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2007;27(4):247–251. doi: 10.1097/01.HCR.0000281772.28394.30. [DOI] [PubMed] [Google Scholar]
- 23.Cortopassi F, Castro AA, Porto EF, et al. Comprehensive exercise training improves ventilatory muscle function and reduces dyspnea perception in patients with COPD. Monaldi Arch Chest Dis. 2009;71(3):106–112. doi: 10.4081/monaldi.2009.355. [DOI] [PubMed] [Google Scholar]
- 24.Global initiative for chronic obstructive lung disease (GOLD) Global Initiative for Chronic Obstructive Lung Disease: Pocket guide to COPD diagnosis, management, and prevention [homepage on the Internet] 2013. [Accessed September 23, 2014]. report. Available from: http://www.goldcopd.com.
- 25.Si QR. Health Qigong Liuzijue. Beijing, China: Peking Sport University Press; 2013. Six-Character Formula; pp. 2–71. [Chinese] [Google Scholar]
- 26.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 27.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 28.American Thoracic Society/European Respiratory Society ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166(4):518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 29.Maffiuletti NA, Bizzini M, Desbrosses K, et al. Reliability of knee extension and flexion measurements using the Con-Trex isokinetic dynamometer. Clin Physiol Funct Imaging. 2010;27(6):346–353. doi: 10.1111/j.1475-097X.2007.00758.x. [DOI] [PubMed] [Google Scholar]
- 30.Van Remoortel H, Hornikx M, Demeyer H, et al. Daily physical activity in subjects with newly diagnosed COPD. Thorax. 2013;68(10):962–963. doi: 10.1136/thoraxjnl-2013-203534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernández M, Zambom-Ferraresi F, Cebollero P, Hueto J, Cascante JA, Antón MM. The relationships between muscle power and physical activity in older men with chronic obstructive pulmonary disease. J Aging Phys Act. 2016;25(3):360–366. doi: 10.1123/japa.2016-0144. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med. 2007;175(5):458–463. doi: 10.1164/rccm.200607-896OC. [DOI] [PubMed] [Google Scholar]
- 33.McNamara RJ, McKeough ZJ, McKenzie DK, Alison JA. Water-based exercise in COPD with physical comorbidities: a randomised controlled trial. Eur Respir J. 2013;41(6):1284–1291. doi: 10.1183/09031936.00034312. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigues SL, Viegas CADA, Lima T. Efetividade da reabilitação pulmonar como tratamento coadjuvante da doença pulmonar obstrutiva crônica [The effectiveness of the pulmonary rehabilitation program as an ancillary treatment for chronic obstructive pulmonary disease] J De Pneumol. 2002;28(2):65–70. Portuguese. [Google Scholar]
- 35.Wang K, Zeng GQ, Li R, et al. Cycle ergometer and inspiratory muscle training offer modest benefit compared with cycle ergometer alone: a comprehensive assessment in stable COPD patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2655–2668. doi: 10.2147/COPD.S140093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charususin N, Dacha S, Gosselink R, et al. Respiratory muscle function and exercise limitation in patients with chronic obstructive pulmonary disease: a review. Expert Rev Respir Med. 2018;12(1):67–79. doi: 10.1080/17476348.2018.1398084. [DOI] [PubMed] [Google Scholar]
- 37.Clark CJ, Cochrane LM, Mackay E, Paton B. Skeletal muscle strength and endurance in patients with mild COPD and the effects of weight training. Eur Respir J. 2000;15(1):92–97. doi: 10.1183/09031936.00.15109200. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y, Niu M, Zhang X, Qian H, Xie A, Wang X. Effects of home-based lower limb resistance training on muscle strength and functional status in stable COPD patients. J Clin Nurs. 2018;27(5–6):e1022–e1037. doi: 10.1111/jocn.14131. [DOI] [PubMed] [Google Scholar]
- 39.Malaguti C, Napolis LM, Villaça D, Neder JA, Nery LE, Dal Corso S. Relationship between peripheral muscle structure and function in patients with chronic obstructive pulmonary disease with different nutritional status. J Strength Cond Res. 2011;25(7):1795–1803. doi: 10.1519/JSC.0b013e3181e501c1. [DOI] [PubMed] [Google Scholar]
- 40.Ho SC, Wang JY, Kuo HP, et al. Mid-arm and calf circumferences are stronger mortality predictors than body mass index for patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;11(1):2075–2080. doi: 10.2147/COPD.S107326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Passey SL, Hansen MJ, Bozinovski S, McDonald CF, Holland AE, Vlahos R. Emerging therapies for the treatment of skeletal muscle wasting in chronic obstructive pulmonary disease. Pharmacol Ther. 2016;166:56–70. doi: 10.1016/j.pharmthera.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 42.Sue DY. Peripheral muscle dysfunction in patients with COPD: comparing apples to apples? Chest. 2003;124(1):1–4. doi: 10.1378/chest.124.1.1. [DOI] [PubMed] [Google Scholar]
- 43.Robles PG, Mathur S, Janaudisfereira T, Dolmage TE, Goldstein RS, Brooks D. Measurement of peripheral muscle strength in individuals with chronic obstructive pulmonary disease: a systematic review. J Cardiopulm Rehabil Prev. 2011;31(1):11–24. doi: 10.1097/HCR.0b013e3181ebf302. [DOI] [PubMed] [Google Scholar]
- 44.Rabinovich RA, Vilaró J. Structural and functional changes of peripheral muscles in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2010;16(2):123–133. doi: 10.1097/MCP.0b013e328336438d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nyberg A, Lindstrom B, Rickenlund A, Wadell K. Low-load/high-repetition elastic band resistance training in patients with COPD: a randomized, controlled, multicenter trial. Clin Respir J. 2015;9(3):278–288. doi: 10.1111/crj.12141. [DOI] [PubMed] [Google Scholar]
- 46.Maltais F, Decramer M, Casaburi R, et al. ATS/ERS Ad Hoc Committee on Limb Muscle Dysfunction in COPD An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):e15–e62. doi: 10.1164/rccm.201402-0373ST. [DOI] [PMC free article] [PubMed] [Google Scholar]