Abstract
Squamous cell carcinomas of the head and neck are appearing with increased frequency in both marrow transplanted and non-transplanted Fanconi anemia (FA) patients. FA patients commonly display radiosensitivity of epithelial tissues, complicating effective radiotherapy. Fancd2−/− mice (C57BL/6J and 129/Sv background) demonstrate epithelial tissue sensitivity to single-fraction or fractionated irradiation to the head and neck and distant marrow suppression (abscopal effect), both ameliorated by intraoral administration of the mitochondrial-targeted antioxidant, GS-nitroxide, JP4-039. We now report that mice of two other FA genotypes, Fancg−/− (B6) and the most prevalent human genotype Fanca−/− (129/Sv), also demonstrate: 1. reduced longevity of hematopoiesis in long-term bone marrow cultures; 2. radiosensitivity of bone marrow stromal cell lines; and 3. head and neck radiation-induced severe mucositis and abscopal suppression of distant marrow hematopoiesis. Intraoral administration of JP4-039/F15, but not non-mitochondrial-targeted 4-amino-Tempo/F15 or F15 alone, prior to each radiation treatment ameliorated both local and abscopal radiation effects. Head and neck irradiated TGF-β-resistant SMAD3−/− (129/Sv) mice and double-knockout SMAD3−/− Fancd2−/− (129/Sv) mice treated daily with TGF-β receptor antagonist, LY364947, still displayed abscopal bone marrow suppression, implicating a non-TGF-β mechanism. Thus, amelioration of both local normal tissue radiosensitivity and distant marrow suppression by intraoral administration of JP4-039 in Fancg−/− and Fanca−/− mice supports a clinical trial of this locally administered normal tissue radioprotector and mitigator during head and neck irradiation in FA patients.
INTRODUCTION
Fanconi anemia (FA) patients often demonstrate significant toxicity from ionizing radiation, attributable to the inactivation of one or more of the 22 genes in the FA pathway, which serve as a scaffold for repair of DNA double-strand breaks (1–5). The success of bone marrow transplantation in FA patients reverses the anemia and improves survival in many patients; however, both marrow transplanted and non-transplanted FA patients are increasingly presenting with epithelial cancers, most prominently squamous cell carcinomas of the head and neck region (6–10).
FA patients with head and neck squamous cell cancers often require postoperative radiotherapy due to positive resection margins and/or the presence of regional lymph nodes (11); however, early-onset and often severe radiation-induced mucositis limits effective treatment (8, 11). Amelioration of radiation-induced mucositis in Fancd2−/− mice has been demonstrated by intraoral administration of mitochondrial-targeted GS-nitroxide, JP4-039, delivered in a formulation that localizes drug to the oral cavity and oropharynx (12–15). Furthermore, distant bone marrow suppression (abscopal effect) by head and neck irradiation in Fancd2−/− mice was also ameliorated by intraoral JP4-039 administration (14, 15).
In the current studies, we evaluated two other genotypes of FA [Fancg−/− (C57BL/6) (16)] and Fanca−/− (129/Sv) (17) for marrow cell culture radiobiology, head and neck radiation sensitivity and the therapeutic effect of intraoral JP4-039, delivered in a novel F15 emulsion. We measured duration of in vitro hematopoiesis in long-term bone marrow cultures (LTBMC), and the mitomycin-C and ionizing radiation sensitivity of clonogenic hematopoietic and bone marrow stromal cell progenitors. Each FA, heterozygote and control mouse strain genotype received either a single fraction (30 Gy), or fractionated doses (10 Gy daily × 4) to the head and neck region using a protocol that reproduces the human oral cavity toxicity of standard postoperative fractionated radiotherapy (14, 15). Subgroups of mice received JP4-039/F15, non-mitochondrial-targeted 4-amino/Tempo/F15 or the F15 formulation alone prior to each fraction. Oral cavity radiation-induced toxicity was quantitated by the severity of the histopathologic mucositis, elevation of TGF-β RNA transcripts and protein, and abscopal suppression of distant femur marrow hematopoietic colony-forming progenitor cells. We measured the possible reduction of abscopal marrow suppression by abrogation of TGF-β signaling in SMAD3−/− mice and in other mice treated with a TGF-β receptor antagonist that blocks TGF-β signaling in bone marrow stromal and hematopoietic cells. The results demonstrate that (similar to Fancd2−/− mice) both Fancg−/− and Fanca−/− mice are sensitive to head and neck radiation exposure and induced abscopal marrow suppression. Furthermore, intraoral administration of JP4-039 reduces both of these local and distant radiation toxicities.
MATERIALS AND METHODS
Mice and Animal Care
Fancg−/− mice were derived by breeding heterozygote pairs of Fancg+/− mice (16) (C57BL/6J background). Fanca−/− mice, derived by breeding homologous deletion recombinant-negative (knockout) mice directly as described elsewhere (17), were generously provided by Dr. Markus Grompe (Oregon Health Sciences Center, Portland, OR). SMAD3−/− (129/Sv background) mice (18) were obtained from Jackson Laboratory (Bar Harbor, ME). Animals were housed 4 per cage according to IACUC protocols and fed standard laboratory chow and deionized water. Double-knockout [SMAD3−/− (129/Sv)/Fancd2−/−(129/Sv)] mice were derived by breeding double heterozygotes from the crossing of each single-knockout mouse strain and each pup was genotyped by a polymerase chain reaction (PCR). To obtain the 20 double-knockout mice required for the experiments, 655 pups from double heterozygote matings were genotyped.
Long-Term Bone Marrow Cultures
The contents of femur and tibia from four of each knockout mouse strain (eight cultures), as well as each heterozygote and positive control wild-type littermate mouse were flushed into a 40-cm square plastic flask (Corning® Inc., Corning, NY) in media containing 25% fetal calf serum, 10−5M hydrocortisone hemi-succinate, and antibiotics according to previously published methods (13). Cultures were maintained in a high CO2 atmosphere and media was changed weekly with removal of nonadherent cells according to methods described elsewhere (13).
Hematopoietic Colony-Forming Assays
Nonadherent cells from long-term bone marrow cultures or single cell suspensions of fresh bone marrow were assayed for multilineage colony-forming unit-granulocyte, erythroid, megakaryocyte, macrophage (CFU-GEMM) in Iscove’s medium supplemented with hematopoietic growth factors (including IL-7, G-CSF, GM-CSF, IL-6, IL-11 and erythropoietin; Terry Fox Laboratories, Vancouver, Canada) in 0.8% methylcellulose containing Iscove’s medium. Colonies ≥50 cells were scored on days 7 and 14, as described elsewhere (13). Results are presented as mean and standard error of at least six colony plates of 104 cells/plate (13). Colonies of committed granulocyte-macrophage cells, CFU-GM, were smaller in size at days 7 and 14 and were scored separately (13).
Derivation of Clonal Bone Marrow Stromal Cell Lines and Interleukin-3 (IL-3)-Dependent Hematopoietic Progenitor Cell Lines
Nonadherent cells from 4-week-old LTBMCs were subcultured in suspension in Iscove’s medium containing 15% fetal bovine serum, antibiotics and 100 μg/ml recombinant murine IL-3. Cultures were split weekly, and clonal cell lines derived and maintained in IL-3 according to methods described elsewhere (13).
For deriving adherent stromal cell lines, 4-week-old LTBMCs of each genotype were trypsinized, and the adherent layer was subcultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum. Adherent stromal cell lines were subcultured weekly, and after eight weeks, clonal sublines were derived by subculture in 96-well plates using a single cell depositor and confirmation was obtained by observation of a single cell per well in each plating. Single cell-derived clonal lines were passaged according to methods described elsewhere (13).
Clonogenic Radiation Survival Curves In Vitro
Fresh bone marrow, IL-3-dependent cell lines or bone marrow stromal cell lines were irradiated, using a JL Shepherd IV irradiator (San Fernando, CA), at 70 cGy per min receiving doses ranging from 0–800 cGy. Cells were plated in colony assay (semisolid media for fresh bone marrow or IL-3-dependent cell lines) supplemented with hematopoietic growth factors as described above, and bone marrow stromal cell lines were plated in media supplemented with 10% fetal calf serum on nonadherent surfaces. Colonies of ≥50 cells were scored at day 7–10 according to published methods (19). Every experiment was repeated at least three times with six cultures scored per radiation dose per experiment. Results are presented as the mean of at least three experiments using single-hit/multi-target and linear-quadratic models according to previously published methods (19). D0 is defined as dose required to reduce the surviving fraction to 37%. ñ is defined as the back extrapolation to the y axis of the final linear portion of the survival curve using a log- (y axis) to-linear [horizontal axis for radiation dose (Gy)] plot.
Mouse Head and Neck Irradiation
The head and neck region (full-body shielded) of mice from each genotype were irradiated using a Varian 6 MV linear accelerator, at a dose rate of 200 cGy/min, according to previously published methods (15). Groups of mice received a single 30 Gy fraction, and other groups received 10 Gy daily fractions for four consecutive days. Mice were immobilized and irradiated while under Nembutal anesthesia as described elsewhere (14, 15). Lower single- or fractionated radiation doses did not induce mucositis (14, 15).
Oral Administration of Radiation Protector/Mitigator Drugs
Immediately prior to receiving each head and neck fraction, mice received intraoral administration of 100 μl of one of the tested drugs. Subgroups of mice (n = 20, for each of two replicate experiments) were comprised of the following: nonirradiated control, irradiation only and subgroups receiving each of three drug formulations immediately prior to each radiation fraction (JP4-039/F15 at 20 mg/ kg, 4-amino-Tempo/F15 at 20 mg/kg or F15 alone), all standardized in a 100-μl volume (15). The components and preparation of F15 liposome-emulsion and dispersion of drugs within the emulsion by sonication have been described in detail elsewhere (14, 15, 20, 21). Drug administration was performed on non-anesthetized mice using a 1-ml insulin syringe with no needle.
Real-Time Polymerase Chain Reaction (RT-PCR) Analysis of RNA Transcripts
Tongue tissue was removed from mice of each strain and prepared for RT-PCR as described elsewhere (14, 15). Tongue tissue was obtained from the nonirradiated controls, irradiation-only group and the irradiated group pretreated with JP4-039/F15, 4-amino-Tempo/F15 or F15. Tissue was harvested at day 5 after the final fraction. Tissue from each mouse was individually homogenized and RNA extracted using TRIzol® reagent (Invitrogen™, Carlsbad, CA). A total of 2 μg of RNA was used to synthesize cDNA in a 20-μl reaction system, according to the instructions for the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems®, Carlsbad, CA) with the reaction conditions of 40 cycles of 95°C (denaturation) for 15 s and 60°C (annealing and elongation) for 1 min using the Mastercycler® RealPlex2 (Eppendorf Inc., Westbury, NY) (15).
The RT-PCR analysis was performed to measure radiation-inducible transcripts for TGF-β (15). Results were standardized by comparison to control protein (glyceraldehyde phosphate dehydrogenase; GAPDH). The results are presented as fold increase in gene transcript expression above baseline level, which was adjusted to that of nonirradiated wild-type mouse tongue tissue from control mice of each genotype.
The RT-PCR reaction conditions were as follows: 96-well plates were prepared with 10 μl of TaqMan® Gene Expression Master Mix, 5 μl of RNase-free water, 1 μl of the corresponding TaqMan Gene Expression probe and 4 μl of cDNA (totaling 2 μg of cDNA) using the Eppendorf epMotion® 5070 automated pipetting system. PCR amplification of the GAPDH gene was used as the housekeeping gene. Expression of TGF-β and GAPDH was determined by RT-PCR and data were presented as fold increase over baseline level for each genotype in nonirradiated control mice.
Histopathology of Oral Cavity Tissues
Tongue tissue was removed from irradiated mice at multiple time points and prepared for microscopic evaluation by fixation in 10% formalin/saline. Sections were evaluated for mucositis, as defined by thinning or loss of the epithelial layer of the tongue tissue by examination of 10 high-power microscopic sections per tongue per animal, as previously described elsewhere (15). Each section was evaluated from the dorsal, ventral and lateral side of the tongue for every animal. At least five animals for each time point for each mouse genotype were evaluated. Results were presented as percentage of tongue showing mucositis. Mucositis was quantitated as previously described elsewhere (15). Briefly, mucositis was indicated with thinning of the keratinizing epithelium and destruction of the superficial squamous layer. Microtome artifact tearing of the keratinizing layer was avoided in the scoring process.
Paraffin sections (5 μm) were stained with hematoxylin and eosin (H&E). The H&E stained slides were scored for percentage ulceration, blinded, by two observers (15). For each genotype, at least five tongue specimens with six sections per tongue (100 microscopic fields per section) were scored per condition. Mucositis was quantitated as percentage ulceration using LabWorks Image Acquisition and Analysis Software (UVP®, Upland, CA) (15). Data was presented as mean percentage mucositis ± standard deviation (15). Comparisons between groups were made using the two-sided two-sample t test. Significance was considered at P < 0.05.
TGF-β Protein Levels in Plasma
Blood was collected from the Fancg and Fanca mice at the time when the oral cavity tissue was harvested, as described above. The blood was collected in EDTA tubes (BD Microtainer® Tubes with K2E; Becton, Dickinson and Co., Franklin Lakes, NJ), placed on ice, centrifuged at 10,000 rpm for 10 min and the plasma collected and frozen at −80°C. Plasma TGF-β protein concentration was determined by analyzing 12.5 μl of plasma by Luminex® following the manufacturer’s protocol using a TGF-B Luminex Assay Kit (Milli-poreSigma, Burlington, MA) on a MAGPIX® Luminex instrument (Luminex Inc., Austin, TX).
Administration of TGF-β Receptor Antagonist LY364947
TGF-β receptor antagonist LY364947 was administered (25 mg/kg) (22) daily for 5 days, beginning 1 h prior to a single 30 Gy dose, by intraperitoneal (IP) injection of 100 μl stock solution [phosphate buffered saline (PBS), 5 mg/ml]. Previously reported studies have demonstrated that IP injection of 10 mg/kg every two days (in 100 μl) for 80 days to C57BL/6 mice blocked TGF-β signaling and effectively reversed the TGF-β-mediated progression of chronic myeloid leukemia by reducing numbers of TGF-β-dependent leukemia-initiating cells in vivo (22). In other experiments, mice were administered LY364947 daily for 5 days, then sacrificed, and bone marrow was removed from the femur and tibia and assayed in vitro for abrogation of each of the five signal transduction pathways of the TGF-β stimulated response.
TGF-β Administration to Head and Neck Irradiated Fancg−/− and Fanca−/− Mice
Mice received 2 μg TGF-β (PeproTech®, Rocky Hill, NJ) prepared as follows: 100 μg TGF-β was dissolved in 500 μl PBS, frozen in 10 μl aliquots, which were diluted 1:100 in PBS and 100 μl was IP injected to deliver 2 μg daily (36) on days 1, 2, 3, 4 and 5 after 30 Gy head and neck irradiation.
Measurement of Five TGF-β Signaling Pathways
Bone marrow stromal cell lines from Fancg−/− and Fanca−/− and control mice of each background strain (C57BL/6 and 129/Sv, respectively) were assayed for each of five TGF signaling pathways (28). Bone marrow from Fanca−/− and Fanca+/+ mice was freshly removed after 5 days of daily treatment with LY364947 (25 mg/kg in 100 μl PBS IP injection). Marrow was explanted and assayed for CFU-GEMM in colony assay. Each marrow sample was assayed for TGF-β signaling in five pathway assays, as described elsewhere (28).
Fancg−/−, Fancg+/−, Fancg+/+, Fanca−/−, Fanca+/− and Fanca+/+ marrow stromal cell lines were treated in vitro with 100 μM LY364947 for 24 h and then washed in serum-free media and treated with TGF-β 100 μM, then assayed 2 h later for signaling in each of the five TGF-β pathways as described elsewhere (28, 35).
Western blot analysis was performed for confirmation of each intact or blocked TGF-β signaling pathway, as described elsewhere (28, 35). Briefly, in the first canonical pathway, TGF-β binding to the TGF-β receptor results in phosphorylation of SMAD3; therefore, a measurement of phosphorylation-SMAD3 (P-SMAD) was performed. In the four noncanonical pathways, TGF-β binding also results in phosphor-ylation of TGF-β receptor type 1 and type 2, and along with Shc, activates the second erk pathway. This second pathway results in the phosphorylation of erk, which was measured as phospho-ERK (PERK). In the third pathway, binding of TGF-β to its receptor and the subsequent interaction with TRAF6 activates JNK and p38 kinase, which is detected by measuring phosphorylation of p38 or JNK, and we measured P-JNK (28). The fourth pathway involves TGF-β activation of the mTOR pathway through P13K, AKT, mTOR and phosphorylation of S6K measured as P-S6K. We performed Western blot analysis of P-S6K (28). TGF-β also activates a fifth RhoA kinase GTPase pathway, which requires phosphorylation of PAK and is detected by Western blot analysis using an antibody to P-PAK (28). Reagents used included antibodies to the following: phospho-Smad3 and phospho-p38 (Cell Signaling Technology®, Danvers, MA), pERK and p-JNK, as well as actin (Santa Cruz Biotechnology® Inc., Dallas, TX), phospho-S6K and phospho-PAK (Abcam, Cambridge, MA).
Statistical Evaluation
Data analysis for LTBMC was performed according to methods reported elsewhere (13). Briefly, we measured weekly cobblestone island numbers, nonadherent cell numbers, percentage confluence of adherent cells, day 7 colony-forming cells at each weekly harvest and day 14 colony-forming cell counts at each weekly harvest. Data were summarized as mean ± standard error (SEM), and P values were calculated at each week using one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison tests (46) comparing Fanca−/− with Fanca+/+ and Fanca+/−, and comparing Fancg−/− with Fancg+/+ and Fancg+/−.
The in vitro radiation survival curves were analyzed with the single-hit multitarget model, and were compared between groups by calculating D0 (final slope representing multiple-event killing), and ñ (extrapolation number measuring width of the shoulder on the radiation survival curve) for each curve (19). Results for D0 and ñ are presented as the mean ± SEM from measurements with five separate experiments. The analysis of percentage tissue damage and colony assays for hematopoietic cells was performed as previously described elsewhere (13). In all these experiments and other experiments, comparisons between each group and the control groups (i.e., Fancg+/+, Fancg+/− and irradiation only where applicable) were also performed with ANOVA followed by Dunnett’s tests.
For all cell culture experiments, as well as data analysis of oral cavity histopathology, the comparison between groups was done with ANOVA followed by Dunnett’s multiple comparison test. The P values shown are Dunnett’s adjusted and P < 0.05 is regarded as significant. All mice were studied individually.
Evaluation of the effect of JP4-039/F15 compared to irradiation alone, and each of the other treatment groups (F15 alone, F15/4-amino-Tempo compared to irradiation) was performed using Tukey’s test. In addition, each treatment group was compared with each other. As shown in the bar graphs of Figs. 4C and D, 5C and D, 6 and 7, there was a significant effect in a treatment group compared to irradiation alone. Results of three comparisons, including those that were not significant, are shown in the legend for that figure for each genotype in the same order for every figure (+/+ first, followed by +/−, then −/−) whether Fancg or Fanca.
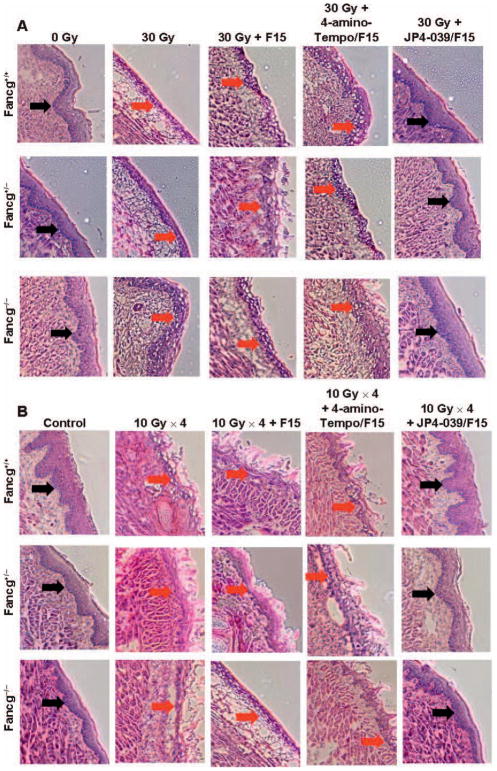
FIG. 4.
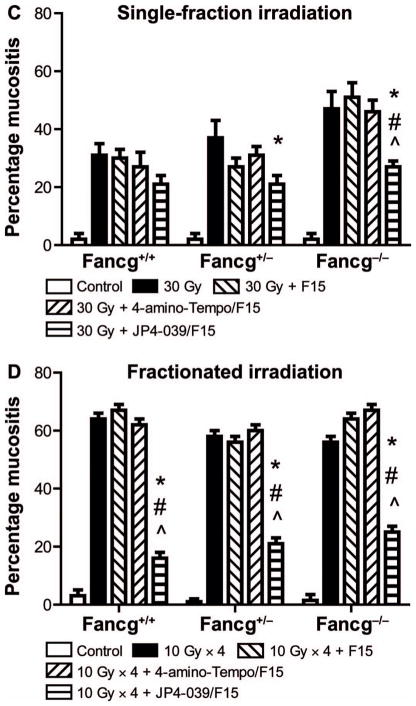
Histopathologic appearance of oral cavity tissue after single-fraction or fractionated head and neck irradiation of Fancg−/− mice and amelioration of radiation damage by JP4-039/F15 treatment. Groups of mice (Fancg−/−, Fancg+/− and Fancg+/+; n = 5 per group) were head and neck irradiated with a single 30 Gy fraction (panels A and C) or four successive days of 10 Gy (panels B and D) and all evaluated at 5 days after the final radiation fraction. Panels A and B: H&E staining (×100) for single-fraction and fractionated irradiation groups, respectively. Black ← arrows indicate normal epithelium; red arrows indicate damaged/ ulcerated epithelium. Panels C and D: Damage quantitation after single-fraction and fractionated irradiation, respectively. In panel C, for Fancg+/+, Fancg+/− and Fancg−/− values, respectively, when JP4-039 is compared to 30 Gy, *P = 0.1859, *P = 0.0230 and *P = 0.0312; when JP4-039 is compared to 30 Gy + F15, #P < 0.2571, #P < 0.5987 and #P < 0.008; and when JP4-039 is compared to 30 Gy + 4-amino-Tempo/F15, ^P < 0.7889, ^P < 0.3051 and ^P < 0.0132. For Fancg+/+, Fancg+/− and Fancg−/− values in panel D, P < 0.0001 when JP4-039 is compared to *30 Gy, #30 Gy + F15 or ^30 Gy + 4-amino-Tempo/F15.
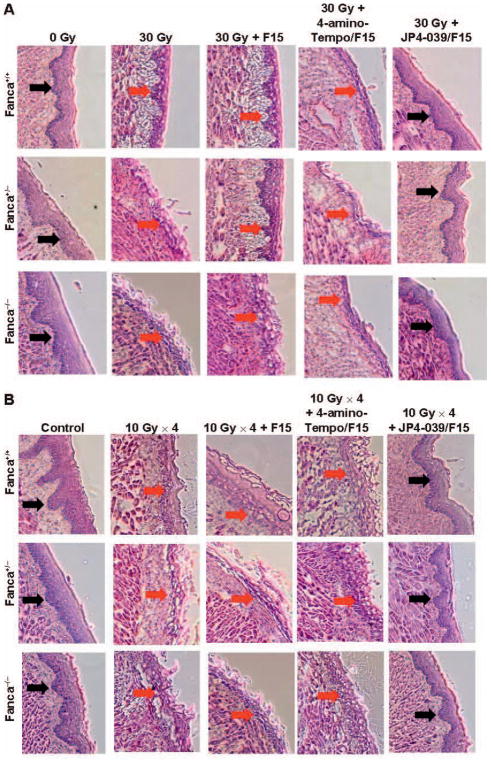
FIG. 5.
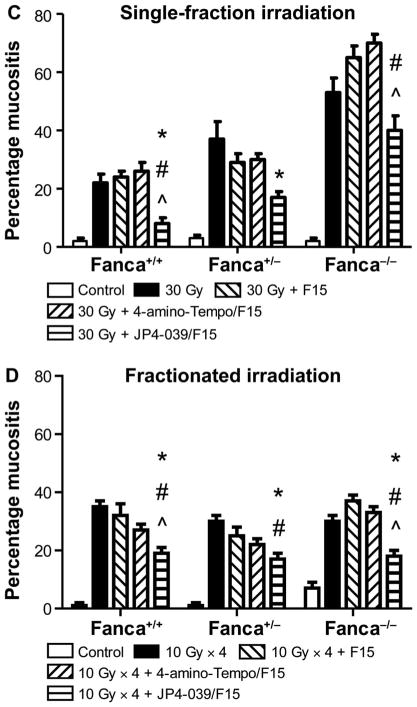
Histopathologic appearance of oral cavity tissue after single-fraction or fractionated head and neck irradiation of Fanca−/− mice and amelioration by JP4-039/F15 of radiation damage. Groups of mice (Fanca−/−, Fanca+/− and Fanca+/+; n =5 per group) were head and neck irradiated with a single 30 Gy fraction (panels A and C) or four successive days of 10 Gy (panels B and D) and all evaluated at 5 days after the final fraction. Panels A and B: H&E staining for single-fraction and fractionated irradiation groups, respectively. Black arrows indicate normal epithelium; red ← arrows indicate damaged/ulcerated epithelium. Panels C and D: Damage quantitation after single-fraction and fractionated irradiation, respectively. In panel C, for Fanca+/+, Fanca+/− and Fanca−/− values, respectively, when JP4-039 is compared to 30 Gy, *P < 0.0054, *P < 0.0075 and *P < 0.05042; when JP4-039 is compared to 30 Gy + F15, #P < 0.0005, #P < 0.2188 and #P < 0.0203; and when JP4-039 is compared to 30 Gy + 4-amino-Tempo/F15, ^P < 0.0002, ^P < 0.1435 and ^P < 0.0045. For Fanca+/+, Fanca+/− and Fanca−/− values in panel D, when JP4-039 is compared to 30 Gy, *P < 0.0001, *P < 0.0001 and *P =0.0002; when JP4-039 is compared to 30 Gy + F15, #P = 0.0006, #P = 0.0082 and #P < 0.0001; and when JP4-039 is compared to 30 Gy + 4-amino-Tempo/F15, ^P =0.0065, ^P =0.1015 and ^P < 0.0001.
FIG. 6.
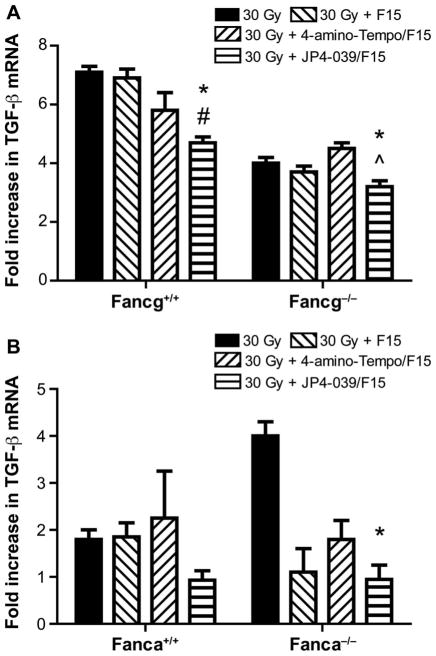
Amelioration by JP4-039/F15 treatment of head and neck radiation-induced elevated RNA transcripts for TGF-β in the oral cavity. Tongue tissue was removed for each group of mice (n = 5/ group) at day 1 or day 5 after the final dose in the single-fraction or fractionated irradiation protocols. Tongue tissue was prepared for rt-PCR and standardized to GAPDH level, as described in Materials and Methods, for Fancg−/− (panel A) and Fanca−/− (panel B) mice. In panel A, for JP4-039 compared to 30 Gy, *P < 0.0054, *P < 0.0255; for JP4-039 compared to 30 Gy + F15, #P < 0.0085, #P < 0.1761; and for JP4-039 compared to 30 Gy + 4-amino-Tempo/F15, ^P < 0.2537, ^P < 0.0015. In panel B, for JP4-039 compared to 30 Gy, *P = 0.6113, *P = 0.0001; for JP4-093 compared to 30 Gy + F15, #P = 0.5928, #P =0.9727; and for JP4-039 compared to 30 Gy + 4-amino-Tempo/F15, ^P = 0.3097, ^P = 0.1535.
FIG. 7.
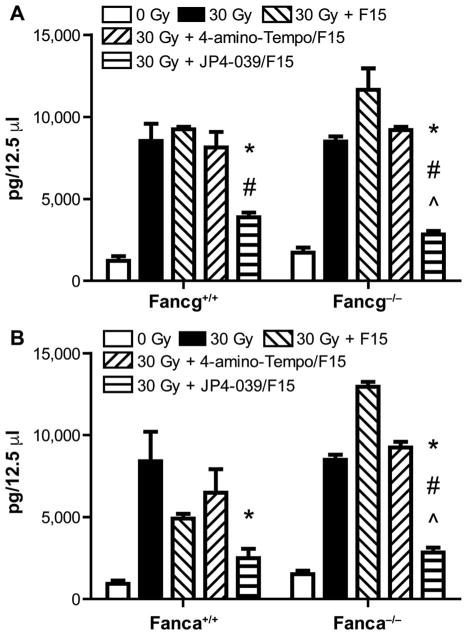
Amelioration of radiation-induced elevation of TGF-β in plasma of Fancg−/− and Fanca−/− mice (panels A and B, respectively) after single-fraction head and neck irradiation. Plasma was removed at day 5 after head and neck irradiation of each group of mice (n = 5). Plasma was prepared for protein evaluation by Luminex assays as described in Materials and Methods. Results are expressed as picogram protein/12.5 μl plasma and compare levels of protein in each treatment group with that of the irradiated groups (n = 5 mice/ group). Five marrow specimens were assayed individually for 5 mice and the value shown is the mean ± SEM for each five specimens from 5 mice/group. In panel A, for JP4-039 compared to 30 Gy, *P = 0.0411, *P = 0.0196; for JP4-039 compared to 30 Gy + F15, #P = 0.0340, #P =0.0013; and for JP4-039 compared to 30 Gy + 4-amino-Tempo/F15, ^P = 0.0788, ^P = 0.0073. In panel B, for *JP4-039 compared to 30 Gy, *P =0.0293, *P =0.0002; for #JP4-039 compared to 30 Gy + F15, #P =0.3853, #P < 0.0001; and for JP4-039 compared to 30 Gy + 4-amino-Tempo/F15, ^P = 0.0943, ^P = 0.0001.
For evaluating the effect of JP4-039/F15 on ameliorating radiation-induced distant marrow suppression by marrow hematopoietic colony-forming units, ANOVA followed by Tukey’s test was used. Here, each treatment group was compared to the nonirradiated control group. Significant differences can be seen in each of the bar graphs in Fig. 8. Results for three genotypes required 12 comparisons (irradiation alone, JP4-039/F15, 4-amino-Tempo/F15 or F15 alone) including those that were not significant and are shown in the table below each panel in the same order of genotypes as described above.
FIG. 8.
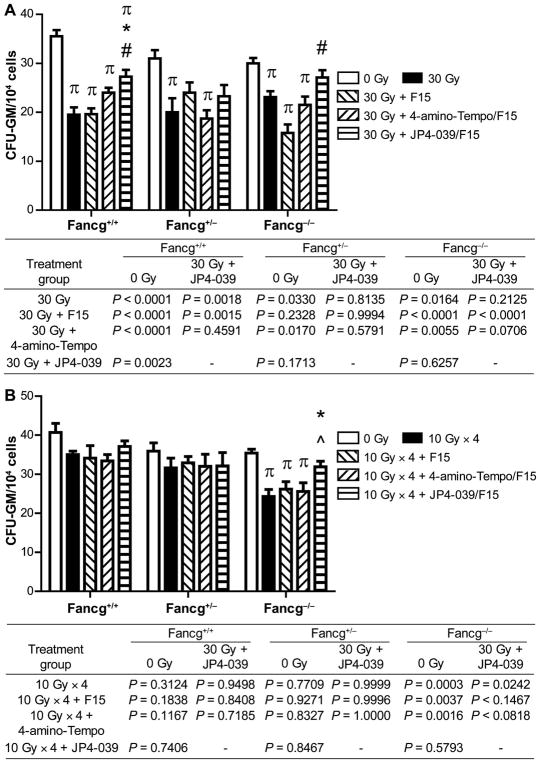
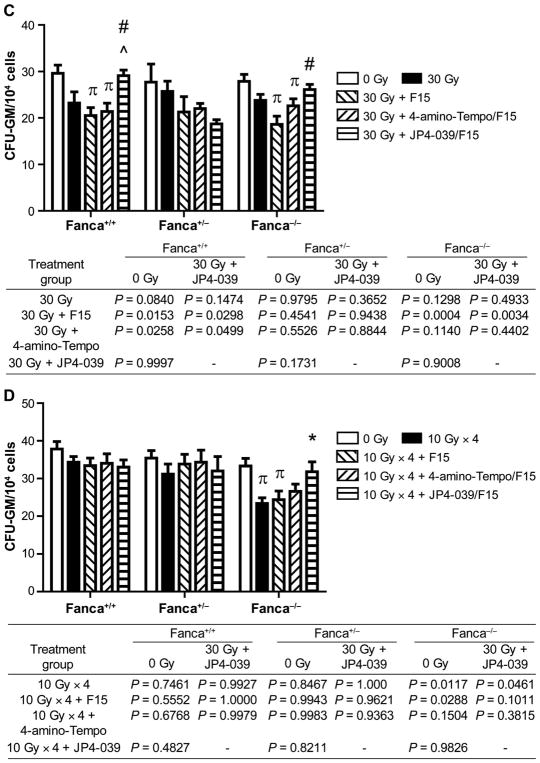
Suppression of distant marrow hematopoietic colony-forming CFU-GM by head and neck irradiation of Fancg−/− or Fanca−/− mice. Bone marrow was removed from mice in each group (n = 5/group) in single-fraction (30 Gy) or fractionated irradiation (10 Gy × 4) neck irradiation protocols, at 5 days after the final radiation fraction. CFU-GM assay was performed, as described Materials and Methods. Panel A: Fancg−/− and control mice in single-fraction irradiation protocol; panel B: Fancg−/− and control mice in fractionated irradiation protocol; panel C: Fanca−/− and control mice in single-fraction irradiation protocol; panel D: Fanca−/− and control mice in fractionated irradiation protocol. The results are presented as a mean ± SEM of four CFU-GM cultures per mouse, 5 mice/group. P value compares each group to irradiation alone, as described in Materials and Methods. In panel A, πcompared to 0 Gy; *JP4-039 compared to 30 Gy; and #JP4-039 compared to 30 Gy + F15. In panel B, πcompared to 0 Gy; *JP4-039 compared to 10 Gy × 4; and ^JP4-039 compared to 10 Gy × 4 + 4-amino-Tempo/F15. In panel C, πcompared to 0 Gy; #JP4-039 compared to 30 Gy + F15; and ^JP4 compared to 30 Gy + 4-amino-Tempo/F15. In panel D, πcompared to 0 Gy, P < 0.05; *JP4-039 compared to 4 × 10 Gy.
RESULTS
Reduced Longevity of Hematopoiesis in Long-Term Bone Marrow Cultures of Fancg−/− and Fanca−/− Mice
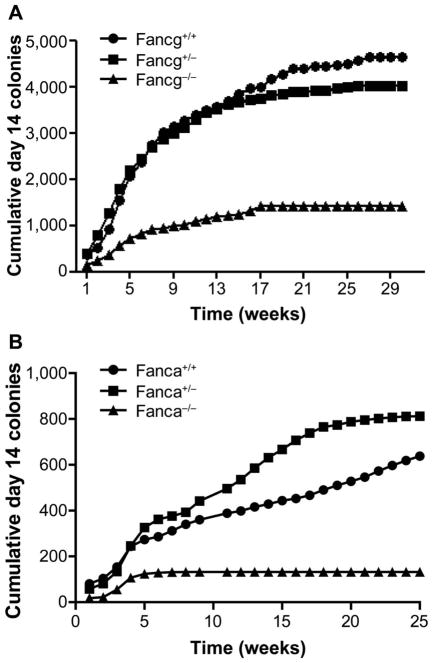
Long-term bone marrow cultures from femur and tibia marrow from groups of four mice (eight cultures) from each genotype (Fancg−/−, Fancg+/−, and Fancg+/+, Fanca−/−, Fanca+/−, and Fanca+/+) were maintained and fed as described elsewhere (13). We harvested nonadherent cells from eight cultures per genotype, and cell counts were performed for each culture separately. Then, harvested cells were pooled from each group of eight. The pooled cells from eight cultures per genotype were used in CFU-GEMM assays, as described in Materials and Methods. The data for the cumulative production of day 14 CFU-GEMM for each FA genotype and each heterozygote and control mouse strain are shown in Fig. 1. As in previously published studies with Fancd2−/− mouse LTBMCs (13, 15), both Fancg−/− and Fanca−/− marrow showed exhaustion of production of colony-forming cells earlier in vitro compared to other groups, reflecting a decreased capacity for prolonged hematopoiesis, and were consistent with the FA patient phenotype of hematopoietic failure. The other parameters of hematopoiesis in LTBMCs are shown for Fancg−/− and controls in Supplementary Fig. S1 and Tables S1–S5 (http://dx.doi.org/10.1667/RR14878.1.S1) and for Fanca−/− mouse LTBMCs and controls in Supplementary Fig. S2 and Tables S6–S10.
FIG. 1.
Reduced duration of hematopoiesis in long-term bone marrow cultures (LTBMCs) derived from Fancg−/− and Fanca−/− mice. Quantitated weekly: cumulative production of nonadherent cells forming day 14 CFU-GEMM colonies for Fancg−/− and controls (panel A) and Fanca−/− and controls (panel B). Results shown here are described in the Materials and Methods (Supplementary Figs. S1 and 2, and Tables S1–10).
Radiosensitivity of Bone Marrow Stromal Cell Lines Compared to Hematopoietic Cells in IL-3-Dependent Hematopoietic Progenitor Cell Lines and Fresh Bone Marrow from Fancg−/− and Fanca−/− Mice
Cell lines from the adherent layer of Fancg−/− and Fanca−/− and heterozygote and control wild-type long-term marrow cultures, and nonadherent IL-3-dependent hematopoietic progenitor cell lines from cells harvested at week 4 from LTBMC of each genotype group of marrow cultures were analyzed, as described in Materials and Methods.
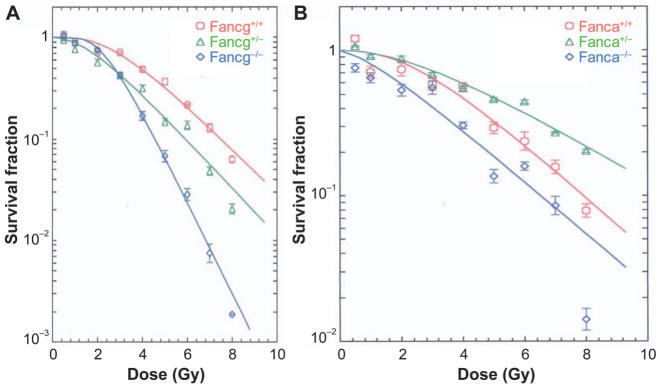
The results of radiation survival curve experiments showed radiosensitivity of bone marrow stromal cell lines derived from both Fancg−/− and Fanca−/− marrow (Fig. 2A and B, respectively; Table 1). The differences between survival curves were largely seen both in the D0, which is the dose required to reduce clonogenic survival by 37%, and in the ñ, which reflects the shoulder on the curve. The D0 for Fanca−/− marrow stromal cell lines was 2.16 ± 0.24 compared to 2.13 ± 0.22 for the Fanca+/+ marrow stromal cell lines. In contrast, the ñ showed significant radiosensitivity of the Fanca−/− cell line, ñ = 2.03 ± 0.34, compared to that for the Fanca+/+ marrow stromal cell line (ñ = 5.67 ± 1.36) P = 0.0269. The Fancg−/− mouse marrow stromal cell line also showed significant radiosensitivity, but reflected in the D0 = 1.06 ± 0.01 compared to the Fancg+/+ marrow stromal cell line D0 = 1.74 ± 0.12 (P = 0.0001). These results confirm and extend previously published studies showing radiosensitivity of bone marrow stromal cell lines derived from Fancd2−/− (C57BL/6) (13) and Fancd2−/− (129/Sv) (14) LTBMC and extend these findings to two additional FA genotypes (Table 1).
FIG. 2.
Radiosensitivity of bone marrow stromal cell lines from Fancg−/− (panel A) and Fanca−/− (panel B) mice. Clonogenic radiation survival curves were performed, as described in Materials and Methods and shown in Table 1.
TABLE 1.
Radiosensitivity of Fancg−/− and Fanca−/− Marrow Stromal Cell Lines and Radioresistance of IL-3-Dependent Hematopoietic Progenitor Cell Lines and Fresh Marrow CFU-GEMM
| Cell lines | D0 | ñ |
|---|---|---|
| Stromal | ||
| Fancg+/+ | 1.74 ± 0.12 (n = 3) | 5.97 ± 0.64 (n = 3) |
| Fancg+/− | 1.61 ± 0.12 (n = 3) | 4.07 ± 1.09 (n = 3) |
| Fancg−/− | 1.06 ± 0.02 (n = 3) (P = 0.0046a) | 8.83 ± 1.43 (n = 3) |
| Fanca+/+ | 2.13 ± 0.22 (n = 3) | 5.67 ± 1.36 (n = 4) |
| Fanca+/− | 2.34 ± 0.12 (n = 3) | 2.70 ± 0.46 (n = 3) |
| Fanca−/− | 2.16 ± 0.24 (n = 3) | 2.03 ± 0.34 (n = 3) (P = 0.0201b) |
| IL-3-dependent | ||
| Fancg+/+ | 2.45 ± 0.47 (n = 3) | 2.03 ± 0.18 (n = 3) |
| Fancg+/− | 2.44 ± 0.42 (n = 3) | 2.30 ± 0.40 (n = 3) |
| Fancg−/− | 1.98 ± 0.13 (n = 3) | 3.63 ± 0.48 (n = 3) (P = 0.0418a) |
| Fanca+/+ | 2.55 ± 0.36 (n = 3) | 2.15 ± 0.15 (n = 5) |
| Fanca+/− | 2.36 ± 0.03 (n = 4) | 3.40 ± 0.42 (n = 5) (P = 0.0304b) |
| Fanca−/− | 2.51 ± 0.22 (n = 5) | 3.78 ± 0.33 (n = 5) (P = 0.0065b) |
| Fresh bone marrow | ||
| Fancg+/+ | 1.37 ± 0.03 (n = 3) | 1.69 ± 0.23 (n = 3) |
| Fancg+/− | 1.43 ± 0.03 (n = 3) | 5.17 ± 0.59 (n = 3) (P = 0.0141a) |
| Fancg−/− | 1.32 ± 0.05 (n = 3) | 4.40 ± 0.89 (n = 3) (P = 0.0397a) |
| Fanca+/+ | 1.42 ± 0.07 (n = 3) | 3.20 ± 0.20 (n = 3) |
| Fanca+/− | 1.40 ± 0.04 (n = 3) | 5.77 ± 0.48 (n = 3) (P = 0.0019b) |
| Fanca−/− | 1.32 ± 0.01 (n = 3) | 5.20 ± 0.10 (n = 3) (P = 0.0065b) |
Notes. For each end point (D0 or ñ), and for each cell line (marrow stromal, IL-3-dependent) or fresh marrow, one-way ANOVA was performed, followed by Dunnett’s multiple comparison tests to compare Fanca+/+ with Fanca+/− and Fanca−/− each, and comparing Fancg+/+ with Fancg+/− and Fancg−/− each. Shown here are the mean ± SEM in each group and significant P values with the Dunnett’s tests (Supplementary Fig. S3; http://dx.doi.org/10.1667/RR14878.1.S1).
Significance when compared to Fancg+/+.
Significance when compared to Fanca+/+.
In contrast to the data for the clonogenic survival of bone marrow stromal cell lines, IL-3-dependent hematopoietic progenitor cell lines and fresh marrow from the same Fancg−/− mice (Supplementary Fig. S3A and C; http://dx.doi.org/10.1667/RR14878.1.S1) and Fanca−/− mice (Supplementary Figs. S3B and D) showed significant radioresistance compared to wild-type or heterozygote cells by clonogenic survival curve analysis (Table 1). These data confirm and extend the finding of radioresistance of clonogenic IL-3-dependent hematopoietic cell line and fresh marrow CFU-GEMM obtained from Fancd2−/− mice (13–15).
While the results by clonogenic survival curve analysis (D0 and ñ) (Table 1) showed radiosensitivity of bone marrow stromal cell lines, and radioresistance of both IL-3-dependent cell lines and fresh marrow CFU-GEMM, actual survival curves were in many cases indistinguishable between groups for survival above 10%. The pragmatic consequence of the apparent differences in radiation survival curves was consistent with the phenotypic differences in total-body-irradiation (TBI) sensitivity of organs and tissues in vivo of Fancg−/− mice (16) and Fanca−/− mice (17). In particular, the data with bone marrow stromal cell lines receiving 1–2 Gy in vitro correlates with observations on the in vivo sensitivity of mice receiving the same dose of TBI.
Mitomycin-C Sensitivity of Fancg−/− and Fanca−/− Bone Marrow Stromal Cells
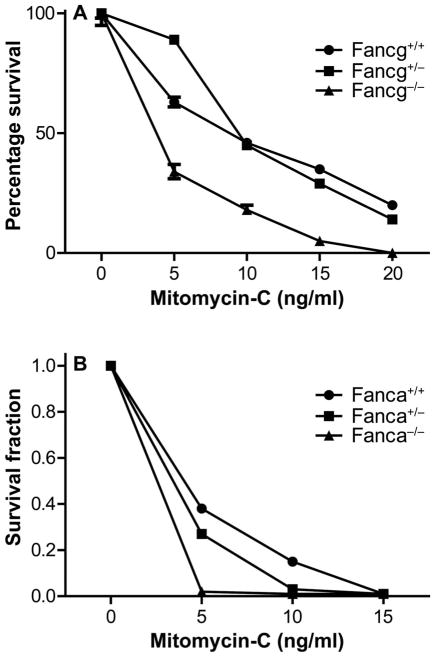
One diagnostic criterion for Fanconi anemia in both animal models and in patients is cellular sensitivity to the DNA cross-linking agent mitomycin-C (1). Bone marrow stromal cell lines from Fancg−/− and Fanca−/− mouse bone marrow cultures, as well as heterozygote and controls of each genotype, were tested for mitomycin-C sensitivity in vitro using the clonogenic survival curve assay. As shown in Fig. 3, both Fancg−/− (Fig. 3A) and Fanca−/− (Fig. 3B) marrow stromal cell lines were sensitive to mitomycin, each compared to its respective control line, Fancg+/+and Fanca+/+, respectively (P < 0.0001 and P < 0.0001). These results establish that mitomycin-C sensitivity, as well as ionizing radiation sensitivity, assayed in clonogenic survival curves, was greater in Fancg−/− and Fanca−/− marrow stromal cell lines compared to heterozygote, or wild-type control cell lines of each FA genotype.
FIG. 3.
Mitomycin-C sensitivity of bone marrow stromal colony-forming cells by cell lines derived from adherent layers of Fancg−/− and Fanca−/− mouse LTBMCs. Adherent stromal cell lines derived from each genotype were assayed, as described in Materials and Methods. Panel A: Fancg−/− and controls. Panel B: Fanca−/− and controls. Results are shown as the mean of five experiments for each cell line genotype bone marrow stromal cell line with six culture plates for each drug dose (total of 18 cultures scored for each drug dose for each cell line).
Increased Radiation-Induced Oral Cavity Mucositis in Both Fancg−/− and Fanca−/− Mice is Ameliorated by Intraoral Administration of GS-Nitroxide, JP4-039
Mice of each genotype were immobilized according to methods described elsewhere (14, 15), and received head and neck exposure to either a single 30 Gy fraction or daily 10 Gy fractions for four days. Mice were treated on a 6-MV linear accelerator and only the head and neck region was irradiated (15).
The Fancg−/− mouse oral cavity demonstrated increased radiation-induced mucositis compared to that detected in Fancg+/− or Fancg+/+ mice (Fig. 4A–D). Both single fraction (Fig. 4A and C) and fractionated irradiation (Fig. 4B and D) experiments demonstrated increased mucositis in Fancg−/− tissue compared to that in control genotypes. In each experiment, those Fancg−/− mice treated with JP4-039/F15 demonstrated a significant reduction in percentage mucositis compared to those receiving non-mitochondrial-targeted 4-amino-Tempo/F15 or F15 alone (Fig. 4).
The results for Fanca−/− mice that received either a single dose or fractionated doses to the head and neck were similar to the results for Fancg−/− mice (Fig. 5). There was significantly increased mucositis in Fanca−/− mice after a single fraction (Fig. 5A and C) and fractionated doses (Fig. 5B and D) to the head and neck. We detected a significant reduction in radiation-induced mucositis by treatment with JP4-039/F15, but not by treatment with 4-amino-Tempo/ F15 or F15 alone (Fig. 5).
We evaluated oral cavity tissue for TGF-β RNA levels, as well as plasma for TGF-β levels in head and neck irradiated Fanca−/− and control Fanca+/+ mice and in Fancg−/− and control Fancg+/+ mice. These studies were performed to determine whether the oral cavity mucositis in Fanca−/− mice was associated with an increased level of TGF-β production. We sought to determine if JP4-039/F15, but not 4-amino-Tempo/F15 or F15 treatment, ameliorated the radiation-induced levels of TGF-β RNA in tongue tissue, and TGF-β protein in plasma of Fancg−/− and Fanca−/− mice. There was a significant increase in TGF-β mRNA in tongue tissue (fold increase over baseline) (Fig. 6), and TGF-β protein in plasma was also elevated by radiation exposure (Fig. 7). The elevated levels of cellular TGF-β mRNA and plasma protein that were induced by radiation were modulated downward by intraoral JP4-039/F15 treatment prior to 30 Gy irradiation (Figs. 6 and 7). These results confirm and extend those previously reported with Fancd2−/− mice (C57BL/6 and 129/Sv) (14, 15).
The current results establish that both biochemical (RNA and protein) and histopathologic biomarkers of radiation damage to the oral cavity in Fancg−/− and Fanca−/− mice were significantly increased compared to that in control genotype mouse strains. Furthermore, treatment of mice prior to irradiation and intraoral administration of the mitochondrial-targeted antioxidant, JP4-039/F15, but not with control non-mitochondrial-targeted 4-amino-Tempo, or F15 formulation alone, significantly reduced these parameters of radiation damage. With Fancg+/+ and Fanca+/+ mice, there was some decrease (although not statistically significant) in levels of these biomarkers with F15 alone.
Radiation-Induced Distant Bone Marrow Suppression (Abscopal Effect) is Increased in Fancg−/− and Fanca−/− Head and Neck Irradiated Mice, and is Ameliorated by Intraoral JP4-039/F15
Bone marrow was explanted at day 5 after the final irradiation in both the single dose and fractionated doses experiments. Marrow was plated in the CFU-GEMM assay and colonies scored at days 7 and 14, as described in Materials and Methods.
As shown in Fig. 8, the Fanca−/− (Fig. 8A and B), and Fancg−/− (Fig. 8C and D) mice in both radiation protocols demonstrated significant suppression of distant femur marrow colony-forming progenitor cells. The level of suppression in mice of each FA genotype was greater than the level detected in the heterozygote, or control mice of each genotype (Fig. 8). The statistical significance of the improved level of CFU-GM in the group treated with JP4-039 after irradiation was compared to the irradiation alone group. Mice in each radiation protocol receiving JP4-039/ F15, but not control 4-amino-Tempo/F15 or F15 alone, showed significant restoration of distant bone marrow colony-forming progenitor cell numbers (Fig. 8). These results establish that there was an improvement in CFU-GM numbers in the marrow of both Fancg−/− and Fanca−/− mice treated with intraoral JP4-039 compared to that observed with irradiation alone. They also extend the effectiveness of JP4-039/F15 as a radiation protector and mitigator observed for a Fancd2−/− [C57BL/6J (15) and 129/Sv genetic background (14)] to the irradiated oral cavity of both Fancg−/− and Fanca−/− mice.
Abscopal Distant Bone Marrow Suppression is Observed in Head and Neck Irradiated TGF-β-Resistant SMAD3−/− (129/ Sv) and Double-Knockout SMAD3−/− (129/Sv) Fancd2−/−(129/Sv) Mice
We next evaluated the possible effect of abrogation of canonical TGF-β signaling on distant marrow suppression in SMAD3−/− (129/Sv) mice (18). SMAD3−/− mice display abrogation of the canonical TGF-β signaling pathway and absence of radiation-induced late tissue fibrosis (24, 25) and show reduced migration of bone marrow stromal cells into the irradiated lungs (26, 27).
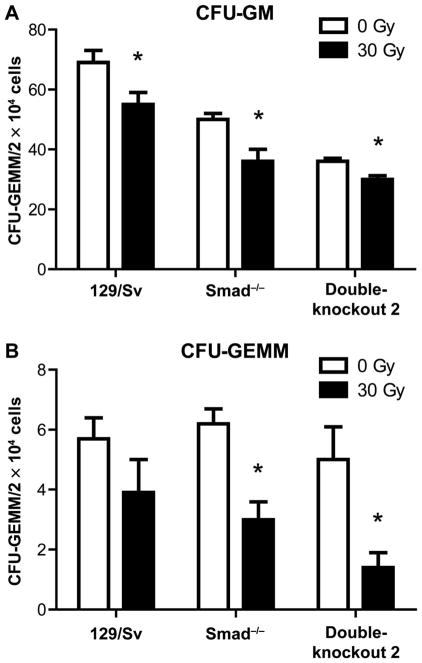
SMAD3−/− mice that received head and neck irradiation displayed persistent distant bone marrow suppression of CFU-GM (Fig. 9A), and the more primitive multilineage CFU-GEMM cells (Fig. 9B) at day 5 postirradiation. The level of suppression was of a magnitude equivalent to that seen with wild-type control mice (129/Sv background) (Fig. 9). We next tested SMAD3−/− (129/Sv) Fancd2−/− (129/Sv) double-knockout mice for head and neck radiation-induced marrow suppression at day 5 after 30 Gy irradiation. These double-knockout mice, with both absent canonical TGF-β signaling and Fancd2 protein, also demonstrated abscopal bone marrow suppression (Fig. 9). Thus, the absence of the canonical TGF-β signaling pathway through SMAD3 did not abrogate the distant abscopal bone marrow suppression by head and neck irradiation in SMAD3−/− or SMAD3−/− Fancd2−/− mice.
FIG. 9.
Bone marrow suppression in head and neck irradiated SMAD3−/−, Fancd2−/− compared to double-knockout SMAD3−/− (129/ Sv) Fancd2−/− (129/Sv) mice. Mice of each genotype (all 129/Sv genetic background) (n = 5/group) were 30 Gy irradiated, and at 5 days postirradiation, femur marrow was assayed for CFU-GEMM and CFU-GM/2 × 144 cells explanted, as described in Materials and Methods. Results are for 5 mice per mouse strain and summary of 4 plates per mouse. Panel A: CFU-GM; panel B: CFU-GEMM. In panel A, compared to 0 Gy, *P = 0.0330 (129/Sv), *P =0.0171 (Smad3−/−) or *P = 0.0438 (DKO2). In panel B, compared to 0 Gy, *P =0.0041 (Smad3−/−) or *P = 0.0159 (double-knockout 2).
Administration of LY364947 Abrogates Both Canonical and Noncanonical TGF-β Signaling in Marrow Stromal Cell Lines from Fancg−/− and Fanca−/− Mice and in Marrow from Head and Neck Irradiated Fanca−/− Mice
There are five known TGF-β signaling pathways, some of which do not require signaling through SMAD3 (28). We first tested the effect of the TGF-β receptor antagonist, LY364947, for abrogation of each of the five TGF-β signaling pathways by examining the bone marrow stromal cell lines from Fancg−/−, Fanca−/− and wild-type control cell lines from each FA genotype. Fancg−/−, Fancg+/+, Fanca−/− and Fanca+/+, as well as Fanca−/−, Fanca+/− and Fanca+/+ bone marrow stromal cell lines were washed in serum-free media and incubated in serum-free media, then treated with 100 μM TGF-β alone, 100 μM LY364947 alone or LY564947 for 24 h followed by TGF-β. We assayed each of the five TGF-β signaling pathways by Western blot, as described in Materials and Methods, and the results are shown for Fancg−/− and Fanca−/− and control cell lines in Fig. 10A and B, respectively.
FIG. 10.
Administration of LY364947 blocks all five TGF-β signaling pathways in Fancg−/− and Fanca−/− bone marrow stromal cell lines in vitro and in Fanca−/− bone marrow in vivo. Western blot analysis of each of the five TGF-β signaling pathways in each of six cell lines treated with 100 μM TGF-β with or without preincubation in 100 μM LY363947. Data are shown for marrow stromal cell lines of Fancg+/+, Fancg+/− and Fancg−/− (panel A) and Fanca+/+, Fanca+/−, and Fanca−/− (panel B). Panel C: Effects on TGF-β signaling by each of five pathways in freshly explanted marrow of in vivo treatment with LY364947 daily for 5 days after 30 Gy head and neck irradiation compared to irradiation alone, as described in Materials and Methods. (Densitometry shown in Supplementary Fig. S4; http://dx.doi.org/10.1667/RR14878.1.S1.)
The data showed that all five pathways of TGF-β signaling were reduced by preincubation of cells in 100 μM LY364947 prior to adding 100 μM TGF-β in vitro to Fancg−/− and control (Fig. 10A) or Fanca−/− and control cell lines (Fig. 10B). With no added TGF-β, the baseline control levels of signaling in the P-ERK, P-S6K and P-PAK pathway for cell lines were not reduced to zero. Since these pathways are involved in other non-TGF-β signaling events, this result was expected (28). Each of the five known TGF-β signaling pathways was induced by TGF-β (28), and each was blocked by the TGF-β receptor binding inhibitor, LY364947.
We next tested the effect of LY364947 on TGF-β signaling in vivo. We administered the TGF-β receptor antagonist LY364947 intraperitoneally to Fanca−/− and control Fanca+/+ mice daily for 5 days beginning 1 h after a single 30 Gy dose to the head and neck region. Then, we quantitated the level of abscopal distant marrow suppression. Explanted marrow from mice of each genotype after LY364947 treatment in vivo was washed in serum-free media, then evaluated for level of abrogation of TGF-β signaling by each of the five pathways (Fig. 10C). As with the bone marrow stromal cell lines that were established from control 129/Sv Fanca+/+, as well as Fanca−/− mice, the data with freshly explanted marrow showed that LY364947 reduced TGF-β signaling in each of the five TGF-β pathways. Baseline control levels of P-JNK and P-PAK in freshly explanted marrow were not reduced to zero and may reflect that some non-TGF-β signaling pathways were still active. As with cell line data, fresh marrow showed increased activity in all five pathways after adding TGF-β, and the level was reduced by LY364947 (Supplementary Fig. S4; http://dx.doi.org/10.1667/RR14878.1.S1).
We next evaluated the CFU-GEMM numbers in the distant marrow of head and neck irradiated, LY364947-treated mice. There was no significant abrogation of head and neck radiation-induced distant bone marrow suppression, when LY364947 was given to either Fanca−/− or control Fanca+/+ mice (Fig. 11).
FIG. 11.
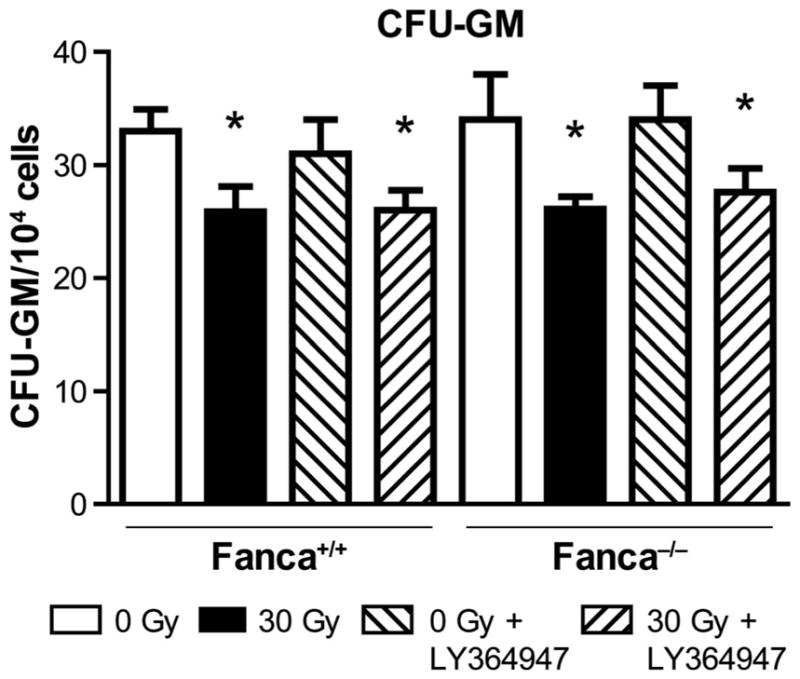
TGF-β receptor antagonist drug LY364947 does not ameliorate distant marrow suppression by head and neck irradiation of Fanca−/− mice. Blocking of each of the five TGF-β signaling pathways by daily administration of LY364947 to Fanca−/− and control wild-type mice. Mice (n = 5/group) were studied individually. Groups of mice received single-fraction head and neck irradiation (30 Gy) according to Materials and Methods. At day 5 postirradiation, femur bone marrow was removed and quantitated for the number of CFU-GM colony-forming cells scored at day 7 in secondary semisolid media culture, as described in Materials and Methods. Compared to 0 Gy, for Fanca+/+ exposed to 30 Gy and to 30 Gy + LY364947, respectively, *P =0.0245 and *P =0.0297; and for Fanca−/− exposed to 30 Gy and to Gy + LY364947, respectively, *P = 0.0015 and *P = 0.0415.
These results establish that LY364947 inhibited all five TGF-β signaling pathways in both Fancg−/− and Fanca−/− and control cell lines in vitro and in Fanca−/− and control bone marrow in vivo. Therefore, inhibition of all TGF-β signaling pathways by the TGF-β receptor antagonist, LY364947, did not abrogate distant bone marrow suppression after head and neck irradiation.
Administration of TGF-β does not Exacerbate Abscopal Marrow Suppression Induced by Head and Neck Irradiation
To confirm that TGF-β released by irradiated head and neck tissue was not solely responsible for the distant marrow suppression seen in FA mice, we treated Fanca+/+ and Fanca−/− mice, as well as Fancg−/− and Fancg+/+ mice with head and neck 30 Gy irradiation, or no irradiation. We administered 2 μg/day of TGF-β daily for 5 days to subgroups of each genotype according to published methods (36). We then quantitated numbers of CFU-GM in femur and tibia marrow at day 5. The results showed no further abscopal distant marrow suppression by administration of TGF-β to head and neck irradiated mice compared to that level of suppression seen in head and neck irradiated mice not administered TGF-β (Fig. 12). There was, in fact, a stimulation of marrow hematopoietic progenitor cell numbers in both Fancg−/− and Fanca−/− mice by administering five daily injections of TGF-β (Fig. 12). The data support the conclusion that the distant abscopal marrow suppression observed in head and neck irradiated Fanca−/− mice was mediated by a humoral factor other than TGF-β.
FIG. 12.
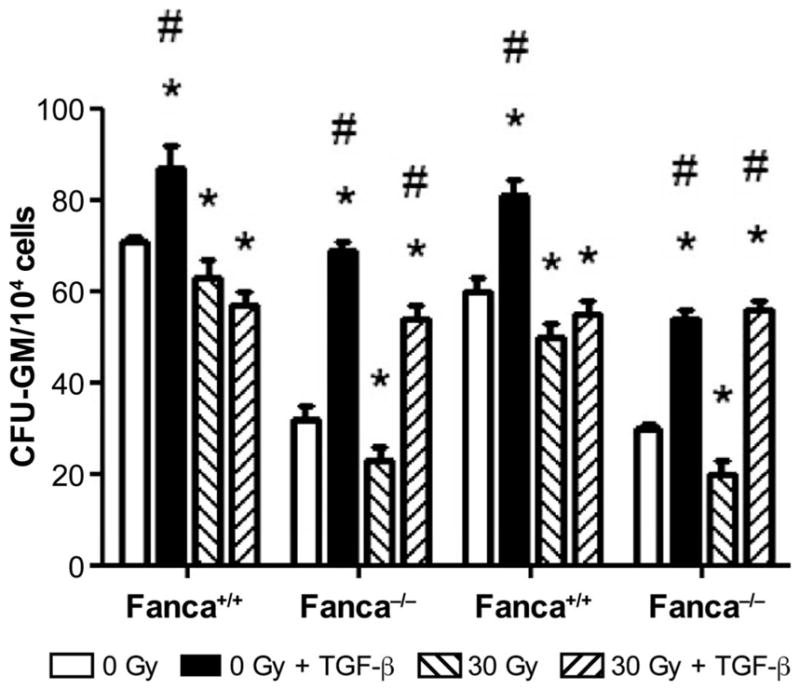
Effect on abscopal distant marrow suppression of daily IP administration of TGF-β to head and neck irradiated Fancg−/− and Fanca−/− mice. Mice (n =5/group) received: no treatment, 30 Gy head and neck irradiation or 30 Gy irradiation followed by daily 2 μg TGF-β/day IP for 5 days. Head and neck irradiated (to 30 Gy) or nonirradiated 129/Sv and Fanca−/− mice received IP administration of TGF-β daily for 5 days or control mice received saline for 5 days. On day 5 marrow was explanted for CFU-GM assay, as described in Materials and Methods. *P < 0.05 compared to 0 Gy; #P < 0.05 compared to 30 Gy.
DISCUSSION
The current results show radioprotection by intraoral JP4-039/F15 in head and neck irradiated Fancg−/− mice (C57BL/ 6 background) and Fanca−/− mice (129/Sv background) and provide further evidence that mitochondrial-targeted anti-apoptotic small molecules can ameliorate radiation-induced acute normal tissue damage in FA (12–15). The mitochondrial-targeted GS-nitroxide, JP4-039, but not the unspecific 4-amino-Tempo, or F15 formulation alone, has been previously shown to decrease radiation-induced apoptosis of the oral cavity of Fancd2−/− mice in two background mouse strains (C57BL/6 and 129/Sv) (14, 15). The current data extend the radioprotection of the oral cavity and abrogation of distant marrow suppression by JP4-039 to Fancg−/− and Fanca−/− mouse genotypes, which represent other groups of FA patients with genetic defects in components of the core-complex of FA proteins and include the most common Fanca−/− patients (1, 2, 17, 29–32).
Both Fancg−/− and Fanca−/− mouse strains showed significant normal tissue, radiosensitivity, susceptibility to radiation mucositis, reduced long-term marrow hematopoietic cell culture longevity, and both mitomycin-C, and ionizing radiation sensitivity of bone marrow stromal cell lines. Radiation mucositis and distant femur marrow suppression were ameliorated by intraoral JP4-039/F15 in both Fancg−/− and Fanca−/− mouse strains. Several experiments indicated that TGF-β elevation by radiation was not solely responsible for the abscopal effect. TGF-β resistant SMAD3−/− (129/Sv) mice that received head and neck irradiation still showed distant marrow suppression. Marrow suppression by head and neck irradiation was not reduced in Fanca−/− mice by the TGF-β receptor antagonist LY364947, which blocked all TGF-β signaling pathways. TGF-β administration to control nonirradiated mice did not suppress distant marrow colony-forming progenitor cell numbers nor did TGF-β administration to head and neck irradiated Fancg−/− and Fanca−/− mice exacerbate the level of detectable radiation-induced marrow suppression.
We applied two experimental models to determine whether elevated levels of TGF-β mediated the abscopal marrow suppression in head and neck irradiated Fanca−/−(129/Sv) mice. First, head and neck irradiation of SMAD3−/−(129/Sv) mice, and double-knockout SMAD3−/− (129/Sv) Fancd2−/− (129/Sv) mice showed significant distant bone marrow suppression. The double-knockout mouse strain we tested had absence of both SMAD3−/− and Fancd2−/− and were syngeneic on the 129/Sv background (18) common to both the previously reported Fancd2−/− (129/Sv) mice (14), and the current Fanca−/− (129/Sv) mice. Thus, the absence of an intact canonical TGF-β signaling pathway through SMAD3 (30) did not abrogate the abscopal marrow suppression from head and neck irradiation.
The results with the TGF-β receptor antagonist LY364947 were particularly interesting with respect to the potential use of TGF-β inhibitors as radiation protectors or radiation mitigators. TGF-β has been implicated as causal in both acute radiation effects ranging from the hematopoietic syndrome of total-body irradiation, to the acute radiation pneumonitis in thoracic radiotherapy patients (41–43). TGF-β has also been implicated as involved in late radiation effects, predominantly radiation fibrosis, which does not occur in SMAD3−/− mice (24, 25), and TGF-β levels have been correlated with the onset of radiation fibrosis in clinical radiotherapy patients (44). In this regard, multiple categories of TGF-β inhibitors have been described (44), including monoclonal antibodies against TGF-β, TGF-β receptor binding proteins (43), TGF-β receptor antagonists (45), and several new small molecule drugs (46) have been reported. The LY364947 (37) drug is novel in its action on multiple TGF-β signaling pathways. LY364947 has been shown to inhibit the TGF-β receptor 1 kinase and block the phosphorylation of SMAD2 and SMAD3 (38). LY364947 also inhibits the phosphorylation of ERK 1/2 (39) and AKT (40) by TGF-β stimulation, which are two of the non-canonical TGF-β pathways (28). By blocking the phosphorylation of SMAD3, ERK 1/2 and AKT, there is also an inhibition of the epithelial-mesenchymal transition (EMT) and the nuclear localization of the forkhead O transcription factors (FOXO) (38, 40). In our studies, adding five daily IP injections of TGF-β to head and neck irradiated or nonirradiated Fancg−/− or Fanca−/− mice neither induced nor exacerbated abscopal marrow suppression, and was in fact stimulatory of hematopoietic progenitor cell numbers in distant bone marrow of both Fancg−/− and Fanca−/− mice. The stimulatory effect of TGF-β on some subsets of marrow hematopoietic progenitor cells has been previously reported (47, 48).
Thus, intraoral JP4-039 amelioration of head and neck radiation-induced abscopal marrow suppression was not detectable after genetic or pharmacologic inhibition of TGF-β signaling. The molecular mechanism of radiation-induced abscopal distant bone marrow suppression remains unknown, but may include other categories of humoral factors released by irradiated tissues, including nucleic acids, oxidized lipids and small molecule inhibitors of hematopoiesis.
Previously published studies have demonstrated decreased competitive repopulation capacity of bone marrow hematopoietic progenitor cells in Fancd2−/− mice (33). The current assays did not measure true totipotential marrow stem cells, which reconstitute all cell lineages of myeloid and lymphoid hematopoiesis (33). An assay for abscopal suppression of the true primitive marrow stem cell population would require the competitive repopulation assay (35), which was not done in the current studies. The current data suggest that the radiosensitivity of cells of the bone marrow microenvironment (bone marrow stromal cells, mesenchymal stem cells) can overcome the radioresistance of hematopoietic progenitor cells (13, 14, 34). An indirect effect of radiosensitive stromal cells on the hematopoietic cells of FA patients may explain the relative ease of engraftment of donor “normal” hematopoietic progenitor cells into FA patients, since donor cells do not display the same sensitivity to TGF-β (23, 34).
Different target cell subsets and different experimental conditions may have produced different results with respect to the hyperactive TGF-β signaling pathway in FA patients (23). The hyperactive TGF-β response pathway in FA patients and mouse models may explain the anemia associated with aging as a continuous and chronic process (23). Furthermore, abscopal marrow suppression of committed hematopoietic progenitors observed in head and neck irradiated FA mice in the current and previous studies (14, 15) may involve humoral factor(s) other than TGF-β.
The current results have broad implications with respect to potential use of JP4-039 as a radiation countermeasure and also for its use to facilitate a safe radiotherapy in radiosensitive patients. With respect to radiation countermeasures, it appears that JP4-039 can be used safely in radiosensitive victims of radiation terrorism, or after unintended total-body exposure (8). In the area of clinical radiotherapy, radiosensitive patients, including those with Fanconi anemia, may be unable to receive effective radiotherapy doses because of their reduced normal tissue tolerance. While not all FA patients suffer early or severe mucositis from head and neck radiotherapy, JP4-039 administration as a radiation protector/mitigator during fractionated head and neck radiotherapy should be considered as safe and practical. The current studies support a clinical trial to investigate the potential use of JP4-039 as a clinical oral cavity radioprotector in FA patients and for the general population of head and neck radiotherapy patients, who may be treated in protocols that include radiation dose escalation for locally advanced or recurrent head and neck cancer.
Supplementary Material
Fig. S1. Hematopoiesis in Fancg−/− LTBMCs.
Fig. S2. Hematopoiesis in Fanca−/− LTBMCs.
Fig. S3. Radiation survival curves of IL-3-dependent hematopoietic progenitor cell lines and fresh marrow hematopoietic colony-forming cells from Fancg−/− and Fanca−/− mice.
Fig. S4. Densitometry for Fancg and Fanca bone marrow stromal cell Western blots shown in Fig. 10A and B, respectively, and for Fanca bone marrow shown in Fig. 10C.
Table S1. Analysis of cobblestone islands in Fancg−/− LTBMCs.
Table S2. Analysis of nonadherent cells per flask from Fancg−/− LTBMCs (×1,000,000).
Table S3. Analysis of percentage confluence in LTBMCs from Fancg−/− mice.
Table S4. Analysis of day 7 colony-forming progenitor cells from Fancg−/− LTBMCs.
Table S5. Analysis of day 14 colony-forming progenitor cells from Fancg−/− LTBMCs.
Table S6. Analysis of cobblestone islands in Fanca−/− LTBMCs.
Table S7. Analysis of nonadherent cells per flask from Fanca−/− LTBMCs (×1,000,000).
Table S8. Analysis of percentage confluence in LTBMCs from Fanca−/− mice.
Table S9. Analysis of day 7 colony-forming progenitor cells from Fanca−/− LTBMCs.
Table S10. Analysis of day 14 colony-forming progenitor cells from Fanca−/− LTBMCs.
Acknowledgments
This work was supported by the NIAID/NIH, grant no. U19-A168021 and Fanconi Anemia Research Fund. This project used the UPCI animal facility that is supported in part by award P30CA047904. Drs. Michael W. Epperly, Peter Wipf and Joel S. Greenberger are co-inventors on patents issued for the use of JP4-039 as radiation mitigators.
References
- 1.Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res. 2009;668:4–10. doi: 10.1016/j.mrfmmm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kutler DI, Singh B, Satagopan J, Batish SD, Berwick M, Giampietro PF, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR) Blood. 2003;101:1249–56. doi: 10.1182/blood-2002-07-2170. [DOI] [PubMed] [Google Scholar]
- 3.Kim H, D’Andrea AD. Regulation of DNA cross-link repair by the Fanconi anemia/BRCA pathway. Genes Dev. 2012;26:1393–1408. doi: 10.1101/gad.195248.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kottemann MC, Smogorzewska A. Fanconi anemia and the repair of Watson and Crick DNA crosslinks. Nature. 2013;493:358–63. doi: 10.1038/nature11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimamura A, Alter BP. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev. 24:101–22. doi: 10.1016/j.blre.2010.03.002. 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houghtaling S, Timmers C, Noll M, Finegold MJ, Jones SN, Meyn MS, et al. Epithelial cancer in Fanconi anemia complementation group D2 (Fancd2) knockout mice. Genes Dev. 2003;17:2021–35. doi: 10.1101/gad.1103403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg PS, Socie G, Alter BP, Gluckman E. Risk of head and neck squamous cell cancer and death in patients with Fanconi anemia who did and did not receive transplants. Blood. 2005;105:67–73. doi: 10.1182/blood-2004-04-1652. [DOI] [PubMed] [Google Scholar]
- 8.Birkeland AC, Auerbach AD, Sanborn E, Parashar B, Kuhel WI, Chandrasekharappa SC, et al. Postoperative clinical radiosensitivity in patients with Fanconi anemia and head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2011;137:930–4. doi: 10.1001/archoto.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JW, Pitot HC, Strati K, Spardy N, Duensing S, Grompe M, et al. Deficiencies in the Fanconi anemia DNA damage response pathway increase sensitivity to HPV-associated head and neck cancer. Cancer Res. 2010;70:9959–68. doi: 10.1158/0008-5472.CAN-10-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kutler DI, Auerbach AD, Satagopan J, Giampietro PF, Batish SD, Huvos AG, et al. High incidence of head and neck squamous cell carcinomas in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg. 2003;129:106–12. doi: 10.1001/archotol.129.1.106. [DOI] [PubMed] [Google Scholar]
- 11.Greenberger JS, Epperly MW. Radiotherapy for the patient with Fanconi anemia: A challenge for the radiation oncologist. In: Greenberg JS, Epperly MW, editors. Fanconi anemia (FA): Genetic prevalence, management, and treatment outcomes. Hauppauge, NY: Nova Science Publishers; 2015. pp. 63–76. [Google Scholar]
- 12.Bernard ME, Kim H, Berhane H, Epperly MW, Franicola D, Zhang X, et al. GS-nitroxide (JP4-039) mediated radioprotection of human Fanconi anemia cell lines. Radiat Res. 2011;176:603–12. doi: 10.1667/rr2624.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berhane H, Epperly MW, Goff J, Kalash R, Cao S, Franicola D, et al. Radiobiologic differences between bone marrow stromal and hematopoietic progenitor cell lines from Fanconi anemia (Fancd2−/−) mice. Radiat Res. 2014;181:76–89. doi: 10.1667/RR13405.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berhane H, Shinde A, Kalash R, Xu K, Epperly MW, Goff J, et al. Amelioration of radiation-induced oral cavity mucositis and distant bone marrow suppression in Fancd2−/− mice by intraoral JP4-039/ F15. Radiat Res. 2014;182:35–49. doi: 10.1667/RR13633.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shinde A, Berhane H, Rhieu BH, Kalash R, Xu K, Goff J, et al. Intraoral mitochondrial-targeted GS-nitroxide, JP4-039, radioprotects normal tissue in tumor-bearing radiosensitive Fancd2−/−(C57BL/6) mice. Radiat Res. 2016;185:134–50. doi: 10.1667/RR14035.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Kuang Y, Montes De Oca R, Hays T, Moreau L, Lu N, et al. Targeted disruption of the murine Fanconi anemia gene, Fancg/ Xrcc9. Blood. 2001;98:3435–40. doi: 10.1182/blood.v98.12.3435. [DOI] [PubMed] [Google Scholar]
- 17.Cheng NC, van de Vrugt HJ, van der Valik MA, Oostra AB, Krimpenfort P, DeVries Y, et al. Mice with a targeted disruption of the Fanconi anemia homolog Fanca. Hum Mol Genet. 2000;9:1805–11. doi: 10.1093/hmg/9.12.1805. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y, Richardson JA, Parada LF, Graff JM. Smad3−/− mutant mice develop metastatic colorectal cancer. Cell. 1998;94:703–14. doi: 10.1016/s0092-8674(00)81730-4. [DOI] [PubMed] [Google Scholar]
- 19.Rwigema J-CM, Beck B, Wang W, Doemling A, Epperly MW, Shields D, et al. Two strategies for the development of mitochondrial-targeted small molecule radiation damage mitigators. Int J Radiat Oncol Biol Phys. 2011;80:860–8. doi: 10.1016/j.ijrobp.2011.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epperly MW, Goff JP, Franicola D, Wang H, Wipf P, Si S, et al. Esophageal radioprotection in thoracic irradiated mice with transgenic lung tumors by swallowed JP4-039/F15. In Vivo. 2014;28:435–40. [PMC free article] [PubMed] [Google Scholar]
- 21.Epperly MW, Rwigema J-CM, Li S, Gao X, Wipf P, Goff J, et al. Intraesophageal administration of GS-nitroxide (JP4-039) protects against ionizing irradiation-induced esophagitis. In Vivo. 2010;24:811–21. [PMC free article] [PubMed] [Google Scholar]
- 22.Naka K, Hoshii T, Muraguchi T, Tadokoro Y, Ooshio T, Kondo Y, et al. TGF-beta-FOXO signaling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature. 2010;463:676–80. doi: 10.1038/nature08734. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Kozono DE, O’Connor KW, Vidal-Cardenas S, Rousseau A, Hamilton A, et al. TGF-beta inhibition rescues hematopoietic stem cell defects and bone marrow failure in Fanconi anemia. Cell Stem Cell. 2016;18:668–81. doi: 10.1016/j.stem.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flanders KC, Sullivan CD, Fujii M, Sowers A, Anzano MA, Arabshahi A, et al. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am J Pathol. 2002;160:1057–68. doi: 10.1016/S0002-9440(10)64926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flanders KC, Major CD, Arabshahi A, Aburime EE, Okada MH, Fujji M, et al. Interference with transforming growth factor-beta/ Smad3 signaling results in accelerated healing of wounds in previously irradiated skin. Am J Pathol. 2003;163:2247–57. doi: 10.1016/s0002-9440(10)63582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epperly MW, Cao S, Goff J, Shields D, Zhou S, Glowacki J, et al. Increased longevity of hematopoiesis in continuous bone marrow cultures and adipocytogenesis in marrow stromal cells derived from SMAD3−/− mice. Exp Hematol. 2005;33:353–62. doi: 10.1016/j.exphem.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Epperly MW, Franicola D, Zhang X, Nie S, Wang H, Bahnson A, et al. Reduced irradiation pulmonary fibrosis and stromal cell migration in SMAD3−/− marrow chimeric mice. In Vivo. 2006;20:573–82. [PubMed] [Google Scholar]
- 28.Zhang YE. Non-smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–39. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Higuera I, Kuang Y, Denham J, D’Andrea AD. The Fanconi anemia proteins FANCA and FANCG stabilize each other and promote the nuclear accumulation of the Fanconi anemia complex. Blood. 2000;96:3224–30. [PubMed] [Google Scholar]
- 30.Naf D, Kupfer GM, Suliman A, Lambert K, D’Andrea AD. Functional activity of the Fanconi anemia protein FAA requires FAC binding and nuclear localization. Mol Cell Biol. 1998;18:5952–60. doi: 10.1128/mcb.18.10.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiao F, Mi J, Wilson JB, Zhi G, Bucheimer NR, Jones NJ, et al. Phosphorylation of Fanconi anemia (FA) complementation group G protein, FANCG, at serine 7 is important for function of the FA pathway. J Biol Chem. 2004;279:46035–45. doi: 10.1074/jbc.M408323200. [DOI] [PubMed] [Google Scholar]
- 32.Collins NB, Wilson JB, Bush T, Thomashevski A, Roberts KJ, Jones NJ, et al. ATR-dependent phosphorylation of FANCA on serine 1449 after DNA damage is important for FA pathway function. Blood. 2009;113:2181–90. doi: 10.1182/blood-2008-05-154294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garaycoechea JJ, Crossan GP, Langevin F, Daly M, Arends MJ, Patel KG. Genotoxic consequences of endogenous aldehydes on mouse haematopoietic stem cell function. Nature. 2012;489:571–8. doi: 10.1038/nature11368. [DOI] [PubMed] [Google Scholar]
- 34.Epperly MW, Franicola D, Dixon T, Cao S, Zhang X, Shields D, et al. Induction of TGF-beta by irradiation or chemotherapy in Fanconi anemia (FA) mouse bone marrow is modulated by small molecule radiation mitigators, JP4-039 and MMS350. In Vivo. 2017;31:159–68. doi: 10.21873/invivo.11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Epperly MW, Zhang X, Fisher R, Hou W, Franicola D, Shields D, et al. Reduced competitive repopulation capacity of multipotential hematopoietic stem cells in the bone marrow of Friend virus-infected Fv2-resistant mice. In Vivo. 2017;31:313–20. doi: 10.21873/invivo.11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zugmaier G, Paik S, Wilding G, Knabee C, Bano M, Lupu R, et al. Transforming growth factor beta1 induces cachexia and systemic fibrosis without an antitumor effect in nude mice. Cancer Res. 1991;51:3590–4. [PubMed] [Google Scholar]
- 37.Peng S-B, Yan L, Xia X, Watkins SA, Brooks HB, Beight D, et al. Kinetic characterization of novel pyrazole TGF-I kinase inhibitors and their blockade of the epithelial-mesenchymal transition. Biochem. 2005;44:2293–304. doi: 10.1021/bi048851x. [DOI] [PubMed] [Google Scholar]
- 38.Peng S-B, Yan L, Xia X, Watkins S, Brooks HB, Beight D, et al. Kinetic characterization of novel pyrazole TGF-beta receptor 1 kinase inhibitors and their blockade of the epithelial-mesenchymal transition. Biochemistry. 2005;44:2293–304. doi: 10.1021/bi048851x. [DOI] [PubMed] [Google Scholar]
- 39.Li C, Qu X, Xu W, Qu N, Mei L, Liu Y, et al. Arsenic trioxide induces cardiac fibroblast apoptosis in vitro and in vivo by upregulating TGF-beta1 expression. Toxicol Lett. 2013;219:223–30. doi: 10.1016/j.toxlet.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 40.Naka K, Hoshii T, Muraguchi T, Tadokoro Y, Ooshio T, Kondo Y, et al. TGF-beta-FOXO signaling maintains leukaemis-initiating cells in chronic myeloid leukaemia. Nature. 2010;463:676–80. doi: 10.1038/nature08734. [DOI] [PubMed] [Google Scholar]
- 41.Zhang M, Yin L, Zhang K, Sun W, Yang S, Zhang B, et al. Response patterns of cytokines/chemokines in two murine strains after irradiation. Cytokine. 2012;58:169–77. doi: 10.1016/j.cyto.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 42.Rube CE, Palm J, Erren M, Fleckenstein J, Konig J, Remberger K, et al. Cytokine plasma levels: reliable predictors for radiation pneumonitis? PLoS One. 2008;3:e2898. doi: 10.1371/journal.pone.0002898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graves PR, Siddiqui F, Anscher MS, Movsas B. Radiation pulmonary toxicity: from mechanisms to management. Semin Radiat Oncol. 2010;20:201–7. doi: 10.1016/j.semradonc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Citrin DE, Prasanna PGS, Walker AJ, Freeman ML, Eke I, Barcellos-Hoff MH, et al. Radiation-induced fibrosis: mechanisms and opportunities to mitigate. Report of an NCI Workshop, September 19, 2016. Radiat Res. 2017;188:1–20. doi: 10.1667/RR14784.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haiping Z, Takayama K, Uchino J, Harada A, Adachi Y, Kura S, et al. Prevention of radiation-induced pneumonitis by recombinant adenovirus-mediated transferring of soluble TGF-beta type II receptor gene. Cancer Gene Ther. 2006;13:864–72. doi: 10.1038/sj.cgt.7700959. [DOI] [PubMed] [Google Scholar]
- 46.Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096–121. [Google Scholar]
- 47.Blank U, Karlsson S. TGF-beta signaling in the control of hematopoietic stem cells. Blood. 2015;125:3542–50. doi: 10.1182/blood-2014-12-618090. [DOI] [PubMed] [Google Scholar]
- 48.Challen GA, Boles NC, Chambers SM, Goodell MA. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 2010;6:265–78. doi: 10.1016/j.stem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Hematopoiesis in Fancg−/− LTBMCs.
Fig. S2. Hematopoiesis in Fanca−/− LTBMCs.
Fig. S3. Radiation survival curves of IL-3-dependent hematopoietic progenitor cell lines and fresh marrow hematopoietic colony-forming cells from Fancg−/− and Fanca−/− mice.
Fig. S4. Densitometry for Fancg and Fanca bone marrow stromal cell Western blots shown in Fig. 10A and B, respectively, and for Fanca bone marrow shown in Fig. 10C.
Table S1. Analysis of cobblestone islands in Fancg−/− LTBMCs.
Table S2. Analysis of nonadherent cells per flask from Fancg−/− LTBMCs (×1,000,000).
Table S3. Analysis of percentage confluence in LTBMCs from Fancg−/− mice.
Table S4. Analysis of day 7 colony-forming progenitor cells from Fancg−/− LTBMCs.
Table S5. Analysis of day 14 colony-forming progenitor cells from Fancg−/− LTBMCs.
Table S6. Analysis of cobblestone islands in Fanca−/− LTBMCs.
Table S7. Analysis of nonadherent cells per flask from Fanca−/− LTBMCs (×1,000,000).
Table S8. Analysis of percentage confluence in LTBMCs from Fanca−/− mice.
Table S9. Analysis of day 7 colony-forming progenitor cells from Fanca−/− LTBMCs.
Table S10. Analysis of day 14 colony-forming progenitor cells from Fanca−/− LTBMCs.