Abstract
In the past decade, drug delivery systems that can respond to the tumor microenvironment or external stimuli have emerged as promising platforms for treating malignancies due to their improved antitumor efficacy and reduced side effects. In particular, biodegradable polymeric micelles have attracted increasing attention and been rapidly developed as a distinct therapeutic to overcome limitations of conventional chemotherapeutic anticancer drugs. Because of their advantages with respect to biocompatibility, degradability, circulation time, and tumor accumulation, considerable effort has been dedicated to the developing and optimizing micellar systems during the past few years. This review highlights recent advances concerning stimuli-responsive micelles made of biodegradable polypeptide and polyester as nanocarries for drug delivery, and especially limits the content to pH sensitive, redox sensitive, and photo-sensitive micellar systems for safe and efficient cancer chemotherapy.
Keywords: Drug delivery, polymeric micelles, polypeptide and polyester, stimuli-responsive
INTRODUCTION
Malignancy is one of the leading causes of death around the world [1]. Due to its high mortality and morbidity, treating this devastating disease has received increasing attention over the past few decades. Among various treatment modalities, chemotherapy, surgery and radiotherapy are currently the most important treatments in clinic [2]. However, surgical treatment carries the risks of causing structural and functional abnormalities of normal tissues and organs plus the need for surveillance to monitor recurrence. Radiotherapy may cause serious side effects, such as liver and kidney dysfunction, bone marrow suppression, and immunosuppression. As for chemotherapy, although there has been a rapid development of new small-molecule chemotherapeutic drugs in recent years along with improved chemotherapeutic protocols, challenges remain relating to their implementation [3]: (i) many drugs already in clinical use (for example, doxorubicin, paclitaxel, camptothecin and cisplatin) are easily degraded under physiological conditions and thus have short half-lives in vivo; (ii) low drug bioavailability and serious systemic toxicities to normal tissue result in highly restricted in vivo applications; and (iii) it is necessary to increase drug dose in some cases since the drug-resistant cells may arise during the course of chemotherapy [4, 5].
Therefore, improving chemotherapeutic effects and reducing side effects have been a long sought-after goal for researchers. Over the past few years, the emergence of nanocarrier systems for drug delivery has offered a practical and innovative means for overcoming the challenges associated with chemotherapy [6]. Compared to small-molecule chemotherapeutic drugs, nanomedicines can more effectively and selectively accumulate and retain in tumor tissues through the abnormally leaky vasculature and relative lack of lymphatic drainage in tumor sites. This enhanced permeability and retention (EPR) effect [7] is a major driving force for passive targeting. Furthermore, nanomedicines can offer additional benefits to overcome the limitations of conventional formulations [6–9], including (i) improved solubility and stability through encapsulation of poorly soluble drugs; (ii) controlled drug release and prolonged circulation time; (iii) ability to change biological membrane transport properties and increase the trans-membrane permeability; (iv) potential for targeted drug delivery; and (v) ability to incorporate multiple drugs for combined therapy. Therefore, nanomedicines can realize some specific clinical objectives which cannot be achieved by means of traditional drug administration.
However, implementation of these functions greatly depends on the nanocarriers. Current drug delivery systems for treatment of malignancies, whether already approved in clinical trials or still undergoing research, mainly include liposomes, polymeric micelles/vesicles, dendrimers, inorganic nanoparticles (such as gold nanoparticles, magnetic nanoparticles, carbon nanotubes and mesoporoussilica, etc.) and macro/nanogels [10]. Of these nanocarriers, polymeric micelles composed of biocompatible amphiphilic block/graft copolymers have been the subject of particular interest. These micelles consist of a hydrophilic shell (e.g. poly(ethylene glycol)) surrounding a hydrophobic core, and can serve as reservoirs of bioactive molecules for sustained release. In particular, polypeptide [11–13] and polyester [14] have become the focus of current research for their unique and excellent biodegradable and biocompatible properties. Through rational molecular design, a series of functional polypeptide and polyester nanomicelles have been developed for cancer therapy. It is promising that several biodegradable polymeric micellar anticancer nanomedicines are already in early- to late-phase clinical trials [15–20]. A successful example of such a formulation that has been approved for clinical use is Genexol-PM, a biodegradable monomethoxy poly (ethylene glycol)-block-poly(D,L-lactide) (mPEG-PDLLA) copolymer micelle loaded with the anticancer drug paclitaxel (PTX). This formulation was approved in South Korea in 2007 to treat breast, lung, and ovarian cancers [21].
Although considerable progress has been made regarding polymeric drug delivery systems, there have been very few revolutionary breakthroughs in oncology chemotherapy during the last ten years. Polymeric drug delivery systems still face a series of key and basic scientific issues which are yet to be overcome. The first critical challenge is the limited penetration depth of nanomedicines into tumor tissues, leading to therapeutic failure. The second challenge comes from multidrug resistance, which is related to P-glycoprotein. P-glycoprotein is an ATP-binding multidrug transporter overexpressed in cancer cells. It prevents drug accumulation within cancer cells by actively pumping drug out of the cells, conferring resistance to a wide range of chemotherapeutic agents. Another major hurdle is intracellular drug release, a last but critical factor that contributes to the inefficacy for drug delivery. For non-intelligent nanocarriers, drug release typically relies primarily on the drug itself leaking slowly from the nanovectors. After internalization and degradation by the lysosomal enzymes, only a small fraction of free drugs remain in cytoplasm. This incomplete drug release and low drug concentration inside cancer cells may lead to relatively low inhibition of tumor cell proliferation. Even worse, this could also potentially contribute to drug resistance.
With this in mind, an ideal drug delivery system should be able to selectively deliver drugs to tumor sites and specifically and completely release drugs within target cells to maximize therapeutic efficacy and reduce systemic toxicity. At the same time, the drug should also be released in a controlled manner in response to the tumor microenvironment or slight physical or chemical changes in the surroundings. Fortunately, stimuli-responsive nanomedicines can meet all of these requirements. These kinds of nanocarriers take advantage of specific biological microenvironments and respond in a dynamic fashion to moderate drug release profiles based on parameters such as temperature, pH, the redox potential inside cells, or specific exogenous physical stimuli. As a result, not only can stimuli-responsive nanomedicines specifically release drug, but drug resistance can also be overcome to some extent by the combination of highly efficient cellular endocytosis and responsive intracellular release.
Herein, we will highlight the most significant recent progress made in the area of stimuli-responsive polymeric micelles and particularly limit the content on polypeptide and polyester micelles for cancer therapy.
STIMULI-RESPONSIVE POLYMERIC MICELLAR DRUG DELIVERY SYSTEMS
In order to design stimuli-responsive polymeric micelles for cancer therapy, it is critical to understand the physiological mechanisms of cancer pathogenesis and in particular the characteristics of the tumor microenvironment. In addition to uncontrolled cell growth, there are several features that differentiate malignant tumors from normal tissues, including [22]: (i) low extracellular pH and high lactate levels; (ii) relatively low oxygen levels (hypoxia and anoxia); (iii) specifically overexpressed proteins and enzymes; and (iv) low glucose, etc. These characteristics provide possibilities and opportunities for developing smart nanocarriers. By taking advantage of endogenous or exogenous stimuli, different stimuli-responsive drug delivery systems can be exploited.
pH-SENSITIVE POLYMERIC MICELLAR DRUG DELIVERY SYSTEM
Stimuli-responsive drug delivery systems have attracted increasing attention in the past few years and are currently center of active research due to their potential biomedical applications. The stimuli studied so far have included temperature, pH, oxidation or reduction, sound and light, etc. Each of these potential stimuli has sparked significant interest in the design, synthesis and exploitation of new smart materials. Of the various stimuli-responsive nanocarriers, pH-sensitive drug delivery system is most commonly used due to the acidic tumor microenvironment.
The extracellular pH in tumor tissue is slightly lower than normal tissues (i.e. 7.4). This intrinsic feature mainly results from the well-known Warburg effect [23]. Due to the rapid growth and metastasis of cancerous cells, the blood vessels in tumors are unable to provide adequate nutrients and oxygen to the tumor cells for proliferation. As a result, lactic acid is produced via anaerobic glycolysis. In addition, the relative lack of internal tumor vasculature makes complete clearance of lactic acid difficult, resulting in an acidic tumor microenvironment. The average extracellular pH of tumors has been shown to be approximately 7.06 with a range of 5.7–7.8 [24]. Although this value varies with tumor histology, tumor volume, and the location inside the tumor, acidic pH can serve as an impetus for drug release. Based on this feature, the methyl maleate bond has been increasingly studied given that it can be broken under weakly acidic conditions (pH ~6.8) [25]. Wang et al. designed and synthesized charge-conversion micelles to deliver doxorubicin by attaching pH-sensitive methyl maleate groups to the hydrophilic polyphosphate block of an amphiphilic block copolymer poly(ε-caprolactone) (PCL) and poly(allyl ethylene phosphate) (PCL-b-PAEP) (see Fig. 1) [26]. The prepared micelles were neutrally charged under physiological conditions, but were shown to be activated by the acidic extracellular environment of the tumor. In the tumor microenvironment, the micelles became positively charged due to the generation of free amino groups by cleaving off methyl maleate, resulting in efficient cellular uptake and enhanced therapeutic effects. A similar concept was also recently applied by Chen’s group and Zhang’s group to make polypeptide and PEG-b-PCL copolymer micelles with pH-triggered charge reversal for promoting cell uptake and enhanced intracellular delivery of cisplatin and doxorubicin, respectively [27, 28].
Fig. 1.
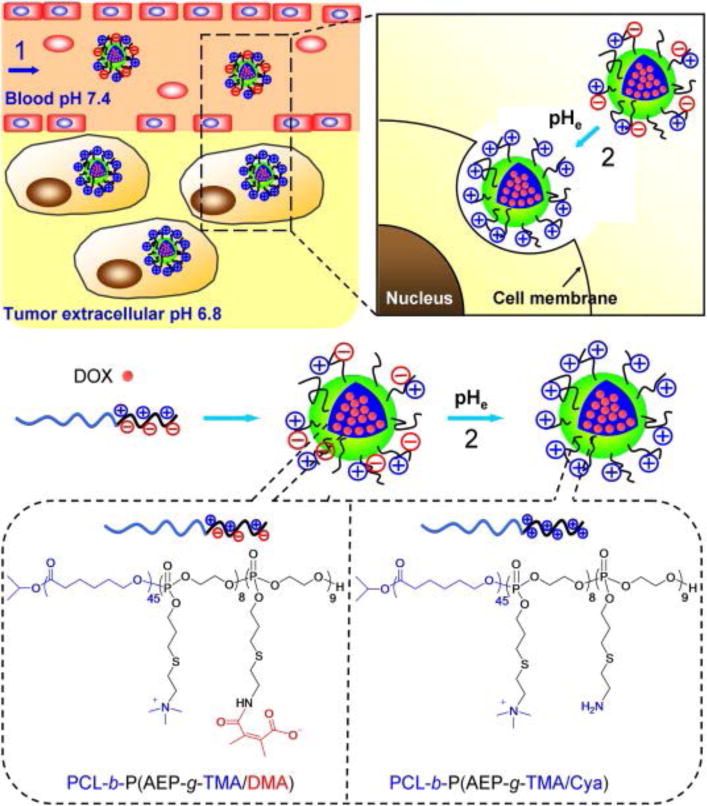
DOX-loaded zwitterionic polymer-based micelles change their surface properties in response to tumor extracellular pH. (1) PCL-b-P(AEP-g-TMA/DMA), an amphiphilic zwitterionic block copolymer, assembles into micelles and encapsulates DOX at pH 7.4. The micelles are of appropriate size to take advantage of tumors’ enhanced permeability and retention effect in vivo. (2) When the extracellular pH decreases to 6.8, as in the tumor extracellular environment, DMA is cleaved from the anionic component of the polymer, forming PCL-b-P(AEP-g-TMA/Cya). The resulting micelles are positively charged and are more readily taken up by tumor cells. Reproduced with permission [26]. Copyright 2012, Wiley-VCH.
Although the intracellular pH of tumor cells is similar to normal cells, intracellular pH gradients can still be used to design stimuli-responsive nanocarriers to moderate the drug release behavior. In most cases, the nanocarriers are uptaken via endocytosis and then entrapped in the endosomes. Early endosomes have a pH of around 5–6 [29], while late lysosomes, the most acidic organelles, have a pH of around 4–5 [30, 31]. These dramatic variations in the pH value provide opportunities for designing specific intracellular drug delivery systems.
Generally, pH responsive polymeric nanomicelles responsive to the tumor intracellular microenvironment can be divided into two categories. One category contains proton donor group with a specific pKa value in the polymer chain, such as L-histidine [30], pyridine [32] and tertiary amino groups [33], etc. When the environmental pH is higher than the pKa, the neutrally charged amphiphilic copolymer can self-assemble into nanoparticles. When the environmental pH is lower than pKa, the segment with the proton donor groups will be protonated and thus becomes positively charged. As a result, the conformation or hydrophobic/hydrophilic balance of the micelles is disrupted, leading to micelle swelling or disintegration and thus the rapid release of loaded drugs. Among these types of nanomicelles, poly(L-histidine) based copolymers have drawn increasing interest and are being widely investigated for pH-sensitive drug delivery systems due to their biodegradability, low toxicity, and combined pH triggered release and endolysosomal escape properties [34]. Bae’s group has pioneered a series of poly(L-histidine) based copolymer micelles that destabilize at tumor pH [35–38]. For example, they have developed a mixed micellar platform composed of polyHis-b-poly(ethylene glycol) (PEG) and poly(L-lactic acid) (PLLA)-b-PEG [39]. The as-prepared mixed micelles showed good stability at physiological pH, but when the pH dropped to pH 7.0 or lower, they destabilized as the polyHis block in the micelle core was ionized. Additionally, by varying the ratio of the two components, controlled pH-triggered drug release could be achieved within a certain pH range. Most importantly, in vivo studies showed significantly enhanced antitumor efficacy with intravenous administration of DOX-loaded micelles to MCF-7 xenograft nude mice, in comparison with free drug administration. Likewise, Gu et al. fabricated pH-sensitive micelles composed of poly(ethylene glycol)-poly(l-histidine)-poly(l-lactide) triblock copolymers [40]. Their in vitro study also showed much better inhibition of tumor cell proliferation by the DOX-loaded micelles compared to free drug.
The other type of pH-sensitive drug delivery system contains pH-sensitive chemical bonds that are susceptible to hydrolysis in the acidic intracellular microenvironment. When the environmental pH drops, the acid-labile bonds can be cleaved, resulting in micelle dissolution or degradation. To date, the typical acid-labile bonds include hydrazone [41, 42], oxime [43], imide [44, 45], orthoesters [46, 47], vinyl ethers [48], and β-thiopropionate [49]. Among these, hydrazone bonds have been the most widely studied. A typical example of pH responsive nanomicelles bearing hydrazone bonds was reported by Kataoka’s group. They successfully prepared the prodrug micelle by conjugating doxorubicin to poly(ethylene glycol)-block-poly(aspartate) (PEG-b-PAsp) via a pH-labile hydrazone linkage [50, 51]. As expected, the anti-tumor efficacy, pharmacokinetics, and biodistribution of this pH sensitive prodrug micelle were much better than free DOX. Recently, Jing et al. reported polycarbonate/Pirarubicin prodrug micelles with different linkage groups including hydrazone, ester and amide bonds [52]. Micelles with hydrazone linkages released faster than those with ester or amide linkages. Furthermore, studies in Balb/c mice models showed that implanted EMT6 tumors were most susceptible to those micelles with hydrazone linkages in vivo. PEG-b-PLLA micelles conjugated with cisplatin by the same linkage by Zhang’s group also showed controlled pH-dependent drug release [53].
It is worth noting that dual pH-sensitive micelles have already been engineered to simultaneously achieve long half-lives in blood with efficient tumor cell uptake while maintaining their ability to rapidly release drug once inside the cell. For example, Wang et al. have reported a dual pH-sensitive polymer-DOX micelle that can reverse its surface charge to encourage cell uptake (Fig. 2) [54]. At tumor extracellular pH (~6.8), the negatively charged portion of the polymer is cleaved to reveal a positively charged amine group. At endosomal pH (~5.5), the hydrazone bond is cleaved to release DOX. In vitro studies have shown that these dual pH-sensitive micelles are more cytotoxic to drug-resistance SK-3rd cancer stem cells than conventional micellar systems.
Fig. 2.
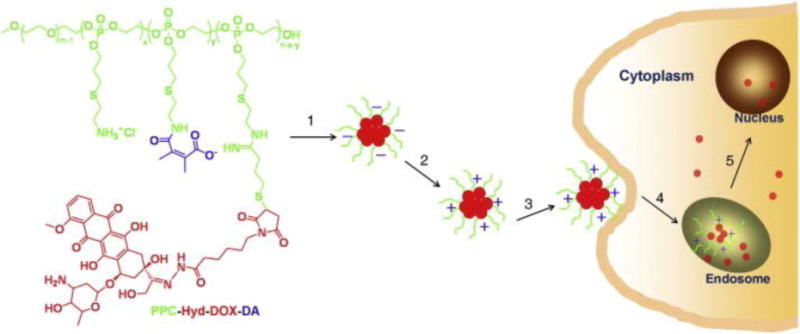
Dual pH-responsive polymer-Dox conjugates (mPEG-b-PAEP-Cya-Hyd-DOX-DA) self-assemble into negatively charged micelles in water (1). When the micelle is exposed to the acidic tumor microenvironment (pH ~6.8), the negatively-charged portion of the polymer is cleaved off, revealing an amine group and rendering the particles positively charged (2). The positively charged micelles are more readily endocytosed by tumor cells (3, 4) and accumulate in endolysosomal compartments within the cell. At the even more acidic pH of endolysosomes (~5.5), the hydrazone bond is cleaved to release DOX, which diffuses into the nucleus of the cell (5). Reproduced with permission from Reference [54]. Copyright 2012, American Chemical Society.
REDOX-SENSITIVE POLYMERIC MICELLAR DRUG DELIVERY SYSTEM
Because the extracellular and intracellular compartments are at different redox potentials, disulfide bonds have been extensively exploited to create redox-sensitive nanocarriers for intracellular drug delivery. The disulfide bond, a dynamic covalent bond existing in numerous biological systems, can be rapidly cleaved by glutathione (GSH). The concentration of GSH found in intracellular compartments (~2–10 mM) is significantly higher than that in extracellular compartments (∼2–10 μM) [55]. Furthermore, due to the reductive environment caused by severe hypoxia within some tumor tissues, a several-fold increase in GSH concentration has been found in tumor cells compared to healthy cells [56]. These uniquely high GSH concentrations in tumor cells provide the opportunity to implement specific drug delivery systems that respond to intracellular conditions. Therefore, such smart nanovehicles containing reductively degradable disulfide bonds show promise as a means for efficient intracellular release of anticancer drugs.
One way to achieve redox sensitive polymeric micelles is to self-assemble amphiphilic copolymers comprising hydrophobic and hydrophilic blocks linked by a single disulfide bond into shell-shedding nanocarriers. Cleavage of the disulfide bond leads to detachment of the shell and instability of the nanocarriers, resulting in rapid release of the trapped contents. Zhong et al. have used this principle to prepare shell detachable block polymer micelles from poly(ε-caprolactone) and poly(ethylene glycol) (PEG) (PCL-SS-PEG) containing a disulfide bridge to deliver DOX [57]. In in vitro studies, intracellular accumulation of DOX from these redox-responsive micelles was significantly improved compared to non-reducible PEG-PCL micelles. A similar idea was reported by Wang et al. using the block polymer poly(ε-caprolactone) and poly(ethyl ethylene phosphate) (PCL-SS-PEEP) [58]. Notably, Wang et al. further revealed that accelerated release of free drug from these intracellular shell detachable micelles significantly enhanced their ability to overcome multidrug resistance in MCF-7/ADR breast cancer cells [59]. Currently, redox-responsive shell-shedding nanocarriers have also been synthesized with other polymers, including the linear PEG-SS-PLA [60, 61], the star-shaped PEG-PCL [62], PEG-SS-poly(γ-benzyl-L-glutamate) [63], and PEG-SS-poly(ε-benzyloxycarbonyl-L-lysine) [64], etc.
In addition to redox-responsive shell-shedding nanocarriers, redox-sensitive micelles with degradable main- or side-chains have also been harnessed for intracellular drug release. For instance, Huang et al. prepared biodegradable pseudo-polypeptide micelles containing disulfide bonds in the polymer backbone [65]. Their in vitro studies revealed these DOX loaded main-chain degradable micelles had a higher anticancer efficiency towards Hela cells than controls with non-reductive carbon bonds.
Furthermore, redox-sensitive reversible disulfide bonds can also be introduced either in the shell or the core to fabricate shell or core cross-linked micelles, respectively [66–68]. This cross-linking approach is a powerful strategy to stabilize micelles. By cross-linking the shell or core with redox-sensitive bonds, the micelles can maintain their high stability even after dilution in blood. Upon reaching the target site, the micelles are taken up by tumor cells and the cross-linker is cleaved under the reductive conditions within the cells. Once the cross-linker is cleaved, the micelles rapidly disintegrate to specifically and intracellularly release anticancer drugs. For example, Jing et al. have designed interfacially reversible cross-linked biodegradable micelles based on the amphiphilic block copolymer mPEG-b-PLG-b-(PLA)2 (PLG: poly(glutamic acid)) [69]. The micelles are formed by cross-linking mPEG-b-PLG-b(PLA)2 poylmers via the carboxyl group on their PLG segments using cystamine, a disulfide-containing cross-linker. In vitro studies with DOX-loaded mPEG-b-PLG-b(PLA)2 micelles showed both responsiveness to reducing conditions and markedly enhanced stability. Furthermore, Jing et al. have developed reduction-sensitive core-cross-linked micelles based on mPEG-b-P(LA-co-MTCSH) block copolymer (MTCSH: thiol-functionalized polycarbonate). These core-cross-linked micelles exhibit enhanced stability even under extremely disruptive conditions (Fig. 3) [70]. In vitro studies of DOX release suggested that the core-cross-linked micelles released drug slowly in the absence of glutathione (GSH), but rapidly when exposed to 10 mM GSH, the approximate intracellular concentration of GSH in tumor cells. Moreover, in vitro MTT assays showed greater inhibition of cellular proliferation as the intracellular concentration of GSH was increased from 0 to 20 mM in cells exposed to the cross-linked micelles. Recently, a robust core cross-linked PEG-polypeptide micellar system based on the disulfide containing cross-linker cysteine N-carboxyanhydride (cysteine-NCA) has also been independently reported by Chen’s and Yan’s groups [71, 72].
Fig. 3.
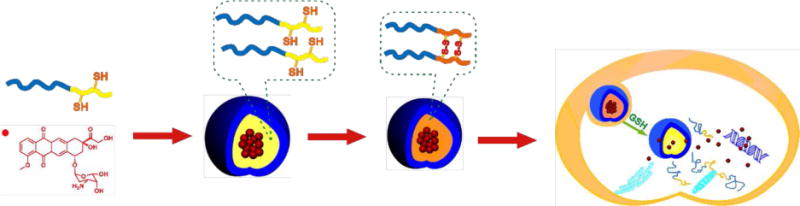
Schematic showing the formation of and drug release from cross-linked mPEG-b-P(LA-co-MTCSH) micelles. Reproduced with permission from Reference [70]. Copyright 2011, Royal Society of Chemistry.
Notably, Shuai and co-workers recently reported the first example of an interlayer-cross-linked polypeptide micelle sensitive to both reducing conditions and acidic pH [73]. The micelles were stable and show no premature drug leakage under physiological pH, but burst to completely release the loaded anticancer drug when exposed to acidic and reductive intracellular conditions. Most importantly, this DOX loaded dual sensitive nanocarrier exhibited remarkable antitumor efficacy both in vitro and in vivo in comparison with nonsensitive PEG-PCL micelles (see Fig. 4).
Fig. 4.
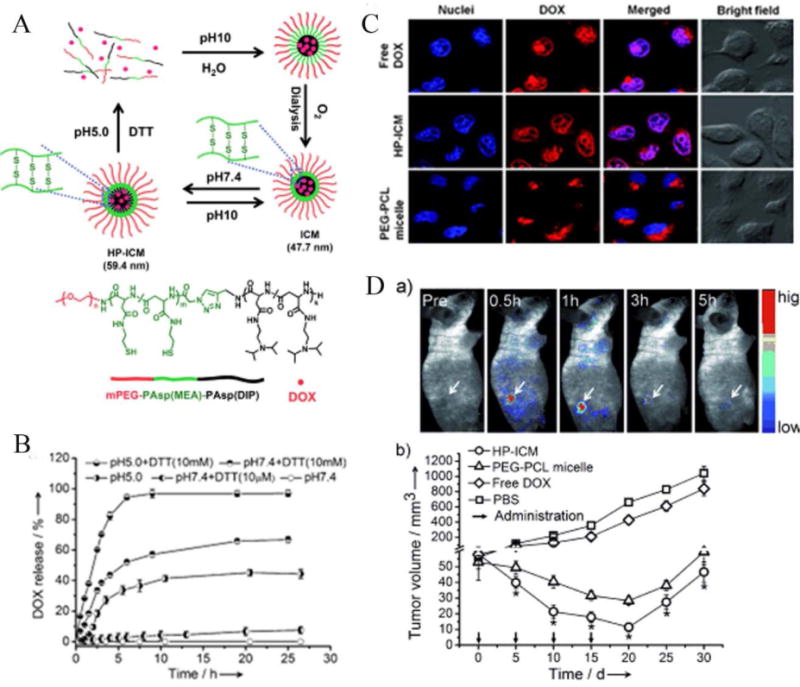
(A) Dual-sensitive interlayer-cross-linked micelles (HP-ICM) can be assembled from mPEG-PAsp(MEA)-PASM(DIP) with DOX loading at pH 7.4, and are stable up to pH 10. However, at pH 5.0 or under reducing conditions (DTT), the micelles rapidly disintegrate to release DOX. (B) Release profile of DOX from HP-ICM at various pH and reducing conditions. (C) Cellular uptake of DOX delivered as free drug, HP-ICM, and control PEG-PCL micelles. (D) Fluorescence images showing in vivo accumulation of DOX into tumor-bearing nude mice. DOX-loaded HP-ICM passively accumulate in the tumor. (a) Tumor growth inhibition is greatest when DOX-loaded HP-ICM are injected via tail vein, compared to controls (b). Reproduced with permission from Reference [73]. Copyright 2011, Wiley-VCH.
Beyond applying disulfide linkage, the diselenide bond, which can also respond to relatively low concentrations of a reducing or oxidizing agents, was recently used in redox-responsive drug delivery systems [74]. Xu and co-workers have developed a series of main-chain, side-chain and dendritic selenium-containing polymers for controlled drug release [75–78]. Meanwhile, Chen’s group has also reported a polypeptide micellar system containing a diselenide bond [79]. Doxorubicin release from this diselenide cross-linked micellar system was minimal under non-reductive conditions, while rapid release was observed at a low concentration of GSH.
PHOTO-SENSITIVE POLYMERIC MICELLAR DRUG DELIVERY SYSTEM
Light is a particularly attractive exogenous stimulus for triggering responsive nanocarriers both because it is noncontact and because it is possible to precisely control the wavelength, direction, illumination area, and intensity of a light source. As a result, it is possible to trigger on-demand drug release with specific spatial and temporal control at room temperature [80, 81]. Additionally, developments in laser and light-wave guide transmission technology provide practical possibilities for photo-chemotherapy, where light is used as a trigger to initiate the release of desired therapeutic agents [82, 83].
Among various photoresponsive groups, the o-nitrobenzyl group and its derivatives are particularly promising because of their photo-reactivity to UV-irradiation. These o-nitrobenzyl groups easily can be integrated into the backbone or side chain of a polymer. Upon exposure to UV irradiation, they undergo a Norrish type II intramolecular rearrangement and are removed [84]. Amphiphilic biodegradable copolymers containing o-nitrobenzyl groups have been used to synthesize light-sensitive micelles. For example, Jing and coworkers have used polycarbonate polymers containing o-nitrobenzyl groups in their side chains to prepare UV-induced core disruptive micelles [85]. These micelles exhibited rapid drug release behavior upon external light stimulation due to the photolysis of the o-nitrobenzyl groups. Furthermore, this approach has been extended to some other biodegradable polypeptides, such as PEG-poly(S-(2-nitrobenzyl)-l-cysteine) [86]. The release profile of loaded model drug Nile Red was observed in a few seconds upon UV-irradiation at 365 nm.
However, because light in the ultraviolet-visible region is strongly scattered and has poor penetration depth (~10 mm) in biological tissues, the application of most current light-triggered drug delivery systems requires direct illumination and is hence limited to surface regions of the body such as the skin [87]. As a result, near infrared (NIR) light-triggered drug delivery systems are promising alternatives. Unlike UV-region light, NIR light in the 700–1000 nm region has reduced optical scattering and significantly improved penetration depth (e.g. up to a few centimeters) in biological samples. Moreover, NIR light is less harmful to the cells than high-energy ultra-violet (UV) light, further justifying its use in vivo. Some recent work has already been done in this area. For instance, Zhao and co-workers have reported a biocompatible polypeptide micelle based on PEO-poly(glutamic acid) that can facilitate drug release upon irradiation by NIR light [88]. By attaching hydrophobic coumarin groups to the side chains of poly(glutamic acid), the as-prepared block copolymer can self-assemble into micelles in aqueous solution. Irradiation by 794 nm NIR light induces cleavage of the coumarin groups from the polypeptide block, leading to a shift in the hydrophilic-hydrophobic balance and disruption of the micellar structure to release the loaded antibacterial drug (Rifampicin) or anticancer drug (Paclitaxel).
Despite the promising proof of concept, NIR light-responsive biodegradable micellar drug delivery systems remain largely unexplored, and evidence of the feasibility of such a system in vivo is still needed. An important and interesting future direction will be the development of biocompatible and biodegradable photo-responsive materials that will play a critical role in the clinical translation of this system.
OTHER STIMULI-RESPONSIVE DRUG DELIVERY SYSTEMS
In addition to the major classes of stimuli-responsive drug delivery systems mentioned above, other important systems including thermo-responsive [89, 90], enzyme-responsive [91–93], magnetically-responsive [94, 95] and ultrasound-responsive [96, 97] nanocarries are also being widely explored. A typical example is the DOX loaded thermally sensitive liposome system Thermo-Dox [98], which has already progressed to clinical trials. This thermo sensitive liposome can retain the entrapped drug at physiologic body temperature (~37 °C), but will rapidly release the drug when heat treatment (~40–42°C) is applied. Recently, thermal gradients have also been used to trigger release of DOX from biodegradable amphiphilic micelles synthesized from polyester [99] and polypeptide [100, 101], which also provide the possibility for potential clinical use. In addition to thermally-responsive systems, enzyme-responsive polymeric micellar systems are also promising for intelligent drug delivery. As we know, enzymes play key roles in metabolic processes and specific types of enzymes are usually overexpressed in tumor tissues, making it possible to mediate drug delivery behavior both in the extracellular environment and intracellular compartment. For instance, matrix metalloproteinase (MMP) is highly overexpressed in tumor cells [102]. Liu and co-workers [103] used a specific peptide sequence GPLGVRG, which can be recognized and subsequently cleaved by MMP, to prepare PEG-sheddable PEG-GPLGVRG-PAsp(DET) micelles that allowed for more efficient cellular uptake and endosomal escape. Cathepsin B, another lysosomal enzyme overexpressed in several malignant tumors, has also been harnessed to cleave the peptide sequence Gly-Phe-Leu-Gly-Phe (GFLGF) from mPEG-GFLGF-PDLLA polymerosomes to accelerate drug release [104].
It is important to note that drug delivery systems responsive to more than one stimulus are fundamentally interesting and have attracted great attention in recent years. The most common combination involves pH and redox responsiveness [105–107] or pH and thermo-responsiveness [108]. As discussed, these interlayer cross-linked polypeptide micelles are good examples for pH and redox responsiveness [73], as the use of multiple stimuli allows for further improved drug delivery and therapeutic efficacy. However, despite the superior versatility of multi-stimuli-responsive nanovectors, they often seem too complicated. To move beyond the proof-of-concept stage, further studies will be necessary both in vitro and in vivo to demonstrate the feasibility of these multi-stimuli strategies.
CONCLUSIONS AND PERSPECTIVES
Considerable progress has been made concerning biodegradable stimuli-responsive polypeptides and polyester micelles for the treatment of malignancy. The major available physiological stimuli include pH gradients, redox-potentials and overexpressed enzymes. Variations in external physical stimuli, such as light, temperature, magnetic field, and ultrasound provide additional possibility for tumor-specific, on-demand drug release. By taking advantage of the tumor microenvironment and external stimuli, biodegradable stimuli-responsive micelles have exhibited enhanced cellular internalization and rapid intracellular drug release, thus resulting in improved antitumor efficacy and reduced side effects. The concept of stimuli-responsive drug delivery by biodegradable micelles also made more attractive by their potential to mitigate multi-drug resistance.
Despite immense progress made in the past decade, most available stimuli-responsive systems remain in the proof of concept stage. More follow-up work and innovative approaches will be required to address some remaining challenges. Firstly, greater sensitivity of biodegradable micelles to either tumor microenvironment or external stimuli needs to be further improved, especially for rapid intracellular drug release to overcome drug resistance. Secondly, except for spherical micelles, the effects of different morphologies, shapes, and surface properties of stimuli-responsive micelles deserve more attention and more extensively investigation, which could benefit to improve cellular uptake efficiency, prolong circulation time, and probably enhance tumor penetration depth. Finally, since active targeting strategies already exhibit improved drug delivery to specific tissues and cells, stimuli-responsive micelles decorated with target ligands may significantly improve anticancer efficacy. With combined interdisciplinary efforts, new developments will be able to further refine the function of biodegradable stimuli-responsive micelles, making them even better drug delivery systems and leading to even further improved therapeutic outcomes.
Acknowledgments
This work was supported in part by the National Institutes of Health (NIH) under grants (R01 CA120480, R01 CA153023 and U54CA151838).
Footnotes
Send Orders for Reprints to reprints@benthamscience.ae
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Breast Cancer Chemosensitivity. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 3.Brindle K. New approaches for imaging tumour responses to treatment. Nat Rev Cancer. 2008;8(2):94–107. doi: 10.1038/nrc2289. [DOI] [PubMed] [Google Scholar]
- 4.Cho KJ, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14(5):1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 5.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5(3):219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 6.Peer D, Karp JM, Hong S, FaroKHzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 7.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J Control Release. 2000;65(1–2):271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 8.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharmaceut. 2008;5(4):505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li SD, Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol Pharmaceut. 2008;5(4):496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- 10.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12(11):991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 11.Huang J, Heise A. Stimuli responsive synthetic polypeptides derived from N-carboxyanhydride (NCA) polymerisation. Chem Soc Rev. 2013;42(17):7373–7390. doi: 10.1039/c3cs60063g. [DOI] [PubMed] [Google Scholar]
- 12.Kricheldorf HR. Polypeptides and 100 years of chemistry of alpha-amino acid N-carboxyanhydrides. Angew Chem Int Ed Engl. 2006;45(35):5752–5784. doi: 10.1002/anie.200600693. [DOI] [PubMed] [Google Scholar]
- 13.He C, Zhuang X, Tang Z, Tian H, Chen X. Stimuli-sensitive synthetic polypeptide-based materials for drug and gene delivery. Adv Healthc Mater. 2012;1(1):48–78. doi: 10.1002/adhm.201100008. [DOI] [PubMed] [Google Scholar]
- 14.Feng J, Zhuo RX, Zhang XZ. Construction of functional aliphatic polycarbonates for biomedical applications. Prog Polym Sci. 2012;37(2):211–236. [Google Scholar]
- 15.Matsumura Y, Hamaguchi T, Ura T, Muro K, Yamada Y, Shimada Y, Shirao K, Okusaka T, Ueno H, Ikeda M, Watanabe N. Phase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicin. Brit J Cancer. 2004;91(10):1775–1781. doi: 10.1038/sj.bjc.6602204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamaguchi T, Matsumura Y, Suzuki M, Shimizu K, Goda R, Nakamura I, Nakatomi I, Yokoyama M, Kataoka K, Kakizoe T. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Brit J Cancer. 2005;92(7):1240–1246. doi: 10.1038/sj.bjc.6602479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamaguchi T, Doi T, Eguchi-Nakajima T, Kato K, Yamada Y, Shimada Y, Fuse N, Ohtsu A, Matsumoto S, Takanashi M, Matsumura Y. Phase I study of Nk012, a novel sn-38-incorporating micellar nanoparticle, in adult patients with solid tumors. Clin Cancer Res. 2010;16(20):5058–5066. doi: 10.1158/1078-0432.CCR-10-0387. [DOI] [PubMed] [Google Scholar]
- 18.Nishiyama N, Okazaki S, Cabral H, Miyamoto M, Kato Y, Sugiyama Y, Nishio K, Matsumura Y, Kataoka K. Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice. Cancer Res. 2003;63(24):8977–8983. [PubMed] [Google Scholar]
- 19.Plummer R, Wilson RH, Calvert H, Boddy AV, Griffin M, Sludden J, Tilby MJ, Eatock M, Pearson DG, Ottley CJ, Matsumura Y, Kataoka K, Nishiya T. A Phase I clinical study of cisplatin-incorporated polymeric micelles (NC-6004) in patients with solid tumours. Brit J Cancer. 2011;104(4):593–598. doi: 10.1038/bjc.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hrkach J, Von Hoff D, Ali MM, Andrianova E, Auer J, Campbell T, De Witt D, Figa M, Figueiredo M, Horhota A, Low S, McDonnell K, Peeke E, Retnarajan B, Sabnis A, Schnipper E, Song JJ, Song YH, Summa J, Tompsett D, Troiano G, Hoven TV, Wright J, LoRusso P, Kantoff PW, Bander NH, Sweeney C, Farokhzad OC, Langer R, Zale S. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4(128) doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 21.Gong J, Chen MW, Zheng Y, Wang SP, Wang YT. Polymeric micelles drug delivery system in oncology. J Control Release. 2012;159(3):312–323. doi: 10.1016/j.jconrel.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliver Rev. 2012;64:24–36. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 23.Heiden MGV, Cantley LC, Thompson CB. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engin K, Leeper DB, Cater JR, Thistlethwaite AJ, Tupchong L, McFarlane JD. Extracellular pH distribution in human tumours. Int J Hyperthermia. 1995;11(2):211–216. doi: 10.3109/02656739509022457. [DOI] [PubMed] [Google Scholar]
- 25.Lee Y, Fukushima S, Bae Y, Hiki S, Ishii T, Kataoka K. A protein nanocarrier from charge-conversion polymer in response to endosomal pH. J Am Chem Soc. 2007;129(17):5362–5363. doi: 10.1021/ja071090b. [DOI] [PubMed] [Google Scholar]
- 26.Yuan YY, Mao CQ, Du XJ, Du JZ, Wang F, Wang J. Surface charge switchable nanoparticles based on zwitterionic polymer for enhanced drug delivery to tumor. Adv Mater. 2012;24(40):5476–5480. doi: 10.1002/adma.201202296. [DOI] [PubMed] [Google Scholar]
- 27.Huang Y, Tang Z, Zhang X, Yu H, Sun H, Pang X, Chen X. pH-Triggered charge-reversal polypeptide nanoparticles for cisplatin delivery: preparation and in vitro evaluation. Biomacromolecules. 2013;14(6):2023–2032. doi: 10.1021/bm400358z. [DOI] [PubMed] [Google Scholar]
- 28.Deng H, Liu J, Zhao X, Zhang Y, Xu S, Deng L, Dong A, Zhang J. PEG-b-PCL copolymer micelles with the ability of pH-controlled negative-to-positive charge reversal for intracellular delivery of doxorubicin. Biomacromolecules. 2014;15(11):4281–4292. doi: 10.1021/bm501290t. [DOI] [PubMed] [Google Scholar]
- 29.Murphy RF, Powers S, Cantor CR. Endosome pH measured in single cells by dual fluorescence flow cytometry: rapid acidification of insulin to pH 6. J Cell Biol. 1984;98(5):1757–1762. doi: 10.1083/jcb.98.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee ES, Gao Z, Bae YH. Recent progress in tumor pH targeting nanotechnology. J Control Release. 132(3):164–170. doi: 10.1016/j.jconrel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin GR, Jain RK. Noninvasive measurement of interstitial pH profiles in normal and neoplastic tissue using fluorescence ratio imaging microscopy. Cancer Res. 1994;54(21):5670–5674. [PubMed] [Google Scholar]
- 32.Changez M, Kang NG, Lee CH, Lee JS. Reversible and pH-sensitive vesicles from amphiphilic homopolymer poly(2-(4-vinylphenyl)pyridine) Small. 2010;6(1):63–68. doi: 10.1002/smll.200901670. [DOI] [PubMed] [Google Scholar]
- 33.Ma X, Wang Y, Zhao T, Li Y, Su LC, Wang Z, Huang G, Sumer BD, Gao J. Ultra-pH-sensitive nanoprobe library with broad pH tunability and fluorescence emissions. J Am Chem Soc. 2014;136(31):11085–11092. doi: 10.1021/ja5053158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee ES, Shin HJ, Na K, Bae YH. Poly(L-histidine)-PEG block copolymer micelles and pH-induced destabilization. J Control Release. 2003;90(3):363–374. doi: 10.1016/s0168-3659(03)00205-0. [DOI] [PubMed] [Google Scholar]
- 35.Lee ES, Gao Z, Kim D, Park K, Kwon IC, Bae YH. Super pH-sensitive multifunctional polymeric micelle for tumor pH(e) specific TAT exposure and multidrug resistance. J Control Release. 2008;129(3):228–236. doi: 10.1016/j.jconrel.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee ES, Na K, Bae YH. Super pH-sensitive multifunctional polymeric micelle. Nano Lett. 2005;5(2):325–329. doi: 10.1021/nl0479987. [DOI] [PubMed] [Google Scholar]
- 37.Lee YJ, Kang HC, Hu J, Nichols JW, Jeon YS, Bae YH. pH-Sensitive polymeric micelle-based pH probe for detecting and imaging acidic biological environments. Biomacromolecules. 2012;13(9):2945–2951. doi: 10.1021/bm300985r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim KS, Park W, Hu J, Bae YH, Na K. A cancer-recognizable MRI contrast agents using pH-responsive polymeric micelle. Biomaterials. 2014;35(1):337–343. doi: 10.1016/j.biomaterials.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Lee ES, Na K, Bae YH. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J Control Release. 2005;103(2):405–418. doi: 10.1016/j.jconrel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 40.Liu R, Li D, He B, Xu X, Sheng M, Lai Y, Wang G, Gu Z. Anti-tumor drug delivery of pH-sensitive poly(ethylene glycol)-poly(L-histidine-)-poly(L-lactide) nanoparticles. J Control Release. 2011;152(1):49–56. doi: 10.1016/j.jconrel.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 41.Hu XL, Liu S, Huang YB, Chen XS, Jing XB. Biodegradable block copolymer-doxorubicin conjugates via different linkages: Preparation, characterization, and in vitro evaluation. Biomacromolecules. 2010;11(8):2094–2102. doi: 10.1021/bm100458n. [DOI] [PubMed] [Google Scholar]
- 42.Hu XL, Wang R, Yue J, Liu S, Xie ZG, Jing XB. Targeting and anti-tumor effect of folic acid-labeled polymer-Doxorubicin conjugates with pH-sensitive hydrazone linker. J Mater Chem. 2012;22(26):13303–13310. [Google Scholar]
- 43.Jin Y, Song L, Su Y, Zhu LJ, Pang Y, Qiu F, Tong GS, Yan DY, Zhu BS, Zhu XY. Oxime Linkage: A robust tool for the design of pH-sensitive polymeric drug carriers. Biomacromolecules. 2011;12(10):3460–3468. doi: 10.1021/bm200956u. [DOI] [PubMed] [Google Scholar]
- 44.Wang BD, Xu CJ, Xie J, Yang ZY, Sun SL. pH Controlled Release of Chromone from Chromone-Fe3O4 Nanoparticles. J Am Chem, Soc. 2008;130(44):14436–14437. doi: 10.1021/ja806519m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gu JX, Cheng WP, Liu JG, Lo SY, Smith D, Qu XZ, Yang ZZ. pH-triggered reversible “stealth” polycationic micelles. Biomacromolecules. 2008;9(1):255–262. doi: 10.1021/bm701084w. [DOI] [PubMed] [Google Scholar]
- 46.Liu R, Zhang Y, Zhao X, Agarwal A, Mueller LJ, Feng PY. pH-Responsive nanogated ensemble based on gold-capped mesoporous silica through an acid-labile acetal linker. J Am Chem Soc. 2010;132(5):1500–1501. doi: 10.1021/ja907838s. [DOI] [PubMed] [Google Scholar]
- 47.Huang XN, Du FS, Cheng J, Dong YQ, Liang DH, Ji SP, Lin SS, Li ZC. Acid-sensitive polymeric micelles based on thermoresponsive block copolymers with pendent cyclic orthoester groups. Macromolecules. 2009;42(3):783–790. [Google Scholar]
- 48.Xu ZH, Gu WW, Chen LL, Gao Y, Zhang ZW, Li YP. A Smart nanoassembly consisting of acid-labile vinyl ether PEG-DOPE and protamine for gene delivery: Preparation and in vitro transfection. Biomacromolecules. 2008;9(11):3119–3126. doi: 10.1021/bm800706f. [DOI] [PubMed] [Google Scholar]
- 49.Ali MM, Oishi M, Nagatsugi F, Mori K, Nagasaki Y, Kataoka K, Sasaki S. Intracellular inducible alkylation system that exhibits antisense effects with greater potency and selectivity than the natural oligonucleotide. Angew Chem Int Edit. 2006;45(19):3136–3140. doi: 10.1002/anie.200504441. [DOI] [PubMed] [Google Scholar]
- 50.Bae Y, Fukushima S, Harada A, Kataoka K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: Polymeric micelles that are responsive to intracellular pH change. Angew Chem Int Ed Engl. 2003;42(38):4640–4643. doi: 10.1002/anie.200250653. [DOI] [PubMed] [Google Scholar]
- 51.Bae Y, Nishiyama N, Fukushima S, Koyama H, Yasuhiro M, Kataoka K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: Tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjug Chem. 2005;16(1):122–130. doi: 10.1021/bc0498166. [DOI] [PubMed] [Google Scholar]
- 52.Mo G, Hu X, Liu S, Yue J, Wang R, Huang Y, Jing X. Influence of coupling bonds on the anti-tumor activity of polymer-pirarubicin conjugates. Eur J Pharm Sci. 2012;46(5):329–335. doi: 10.1016/j.ejps.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 53.Aryal S, Hu CM, Zhang L. Polymer–cisplatin conjugate nanoparticles for acid-responsive drug delivery. ACS Nano. 2010;4(1):251–258. doi: 10.1021/nn9014032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Du JZ, Du XJ, Mao CQ, Wang J. Tailor-made dual pH-sensitive polymer-doxorubicin nanoparticles for efficient anticancer drug delivery. J Am Chem Soc. 2011;133(44):17560–17563. doi: 10.1021/ja207150n. [DOI] [PubMed] [Google Scholar]
- 55.Saito G, Swanson JA, Lee KD. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv Drug Deliver Rev. 2003;55(2):199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 56.Russo A, Degraff W, Friedman N, Mitchell JB. Selective modulation of glutathione levels in human normal versus tumor-cells and subsequent differential response to chemotherapy drugs. Cancer Res. 1986;46(6):2845–2848. [PubMed] [Google Scholar]
- 57.Sun HL, Guo BN, Cheng R, Meng FH, Liu HY, Zhong ZY. Biodegradable micelles with sheddable poly(ethylene glycol) shells for triggered intracellular release of doxorubicin. Biomaterials. 2009;30(31):6358–6366. doi: 10.1016/j.biomaterials.2009.07.051. [DOI] [PubMed] [Google Scholar]
- 58.Tang LY, Wang YC, Li Y, Du JZ, Wang J. Shell-detachable micelles based on disulfide-linked block copolymer as potential carrier for intracellular drug delivery. Bioconjug Chem. 2009;20(6):1095–1099. doi: 10.1021/bc900144m. [DOI] [PubMed] [Google Scholar]
- 59.Wang YC, Wang F, Sun TM, Wang J. Redox-responsive nanoparticles from the single disulfide bond-bridged block copolymer as drug carriers for overcoming multidrug resistance in cancer cells. Bioconjug Chem. 2011;22(10):1939–1945. doi: 10.1021/bc200139n. [DOI] [PubMed] [Google Scholar]
- 60.Li YW, Tong R, Xia HS, Zhang HJ, Xuan JA. High intensity focused ultrasound and redox dual responsive polymer micelles. Chem Commun. 2010;46(41):7739–7741. doi: 10.1039/c0cc02628j. [DOI] [PubMed] [Google Scholar]
- 61.Sourkohi BK, Cunningham A, Zhang Q, Oh JK. Biodegradable block copolymer micelles with thiol-responsive sheddable coronas. Biomacromolecules. 2011;12(10):3819–3825. doi: 10.1021/bm2011032. [DOI] [PubMed] [Google Scholar]
- 62.Ren TB, Feng Y, Zhang ZH, Li L, Li YY. Shell-sheddable micelles based on star-shaped poly(epsilon-caprolactone)-SS-poly(ethyl glycol) copolymer for intracellular drug release. Soft Matter. 2011;7(6):2329–2331. [Google Scholar]
- 63.Thambi T, Yoon HY, Kim K, Kwon IC, Yoo CK, Park JH. Bioreducible block copolymers based on poly(ethylene glycol) and poly(gamma-benzyl L-glutamate) for intracellular delivery of camptothecin. Bioconjug Chem. 2011;22(10):1924–1931. doi: 10.1021/bc2000963. [DOI] [PubMed] [Google Scholar]
- 64.Ding JX, Chen JJ, Li D, Xiao CS, Zhang JC, He CL, Zhuang XL, Chen XS. Biocompatible reduction-responsive polypeptide micelles as nanocarriers for enhanced chemotherapy efficacy in vitro. J Mater Chem B. 2013;1(1):69–81. doi: 10.1039/c2tb00063f. [DOI] [PubMed] [Google Scholar]
- 65.Wu YJ, Kuang HH, Xie ZG, Chen XS, Jing XB, Huang YB. Novel hydroxyl-containing reduction-responsive pseudo-poly(aminoacid) via click polymerization as an efficient drug carrier. Polym Chem-Uk. 2014;5(15):4488–4498. [Google Scholar]
- 66.Meng FH, Hennink WE, Zhong Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials. 2009;30(12):2180–2198. doi: 10.1016/j.biomaterials.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 67.Cheng R, Feng F, Meng FH, Deng C, Feijen J, Zhong ZY. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J Control Release. 2011;152(1):2–12. doi: 10.1016/j.jconrel.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 68.Sun HL, Meng FH, Cheng R, Deng C, Zhong ZY. Reduction-sensitive degradable micellar nanoparticles as smart and intuitive delivery systems for cancer chemotherapy. Expert Opin Drug Del. 2013;10(8):1109–1122. doi: 10.1517/17425247.2013.783009. [DOI] [PubMed] [Google Scholar]
- 69.Yue J, Wang R, Liu S, Wu SH, Xie ZG, Huang YB, Jing XB. Reduction-responsive shell-crosslinked micelles prepared from Y-shaped amphiphilic block copolymers as a drug carrier. Soft Matter. 2012;8(28):7426–7435. [Google Scholar]
- 70.Yan LS, Wu WB, Zhao W, Qi RG, Cui DM, Xie ZG, Huang YB, Tong T, Jing XB. Reduction-sensitive core-cross-linked mPEG-poly(ester-carbonate) micelles for glutathione-triggered intracellular drug release. Polym Chem-Uk. 2012;3(9):2403–2412. [Google Scholar]
- 71.Ding JX, Shi FH, Xiao CS, Lin L, Chen L, He CL, Zhuang XL, Chen XS. One-step preparation of reduction-responsive poly(ethylene glycol)-poly (amino acid)s nanogels as efficient intracellular drug delivery platforms. Polym Chem-Uk. 2011;2(12):2857–2864. [Google Scholar]
- 72.Xing T, Lai B, Ye XD, Yan LF. Disulfide core cross-linked pegylated polypeptide nanogel prepared by a one-step ring opening copolymerization of N-carboxyanhydrides for drug delivery. Macromol Biosci. 2011;11(7):962–969. doi: 10.1002/mabi.201000510. [DOI] [PubMed] [Google Scholar]
- 73.Dai J, Lin S, Cheng D, Zou S, Shuai X. Interlayer-crosslinked micelle with partially hydrated core showing reduction and pH dual sensitivity for pinpointed intracellular drug release. Angew Chem Int Ed Engl. 2011;50(40):9404–9408. doi: 10.1002/anie.201103806. [DOI] [PubMed] [Google Scholar]
- 74.Xu HP, Cao W, Zhang X. Selenium-containing polymers: Promising biomaterials for controlled release and enzyme mimics. Accounts Chem Res. 2013;46(7):1647–1658. doi: 10.1021/ar4000339. [DOI] [PubMed] [Google Scholar]
- 75.Ma N, Li Y, Xu HP, Wang ZQ, Zhang X. Dual redox responsive assemblies formed from diselenide block copolymers. J Am Chem Soc. 2010;132(2):442–443. doi: 10.1021/ja908124g. [DOI] [PubMed] [Google Scholar]
- 76.Han P, Ma N, Ren HF, Xu HP, Li ZB, Wang ZQ, Zhang X. Oxidation-responsive micelles based on a selenium-containing polymeric superamphiphile. Langmuir. 2010;26(18):14414–14418. doi: 10.1021/la102837a. [DOI] [PubMed] [Google Scholar]
- 77.Ma N, Li Y, Ren HF, Xu HP, Li ZB, Zhang X. Selenium-containing block copolymers and their oxidation-responsive aggregates. Polym Chem-Uk. 2010;1(10):1609–1614. [Google Scholar]
- 78.Ren HF, Wu YT, Ma N, Xu HP, Zhang X. Side-chain selenium-containing amphiphilic block copolymers: Redox-controlled self-assembly and disassembly. Soft Matter. 2012;8(5):1460–1466. [Google Scholar]
- 79.Ding JX, Xiao CS, Yan LS, Tang ZH, Zhuang XL, Chen XS, Jing XB. pH and dual redox responsive nanogel based on poly(l-glutamic acid) as potential intracellular drug carrier. J Control Release. 2011;152:E11–E13. doi: 10.1016/j.jconrel.2011.08.091. [DOI] [PubMed] [Google Scholar]
- 80.Yan LS, Yang LX, He HY, Hu XL, Xie ZG, Huang YB, Jing XB. Photo-cross-linked mPEG-poly(gamma-cinnamyl-L-glutamate) micelles as stable drug carriers. Polym Chem-Uk. 2012;3(5):1300–1307. [Google Scholar]
- 81.Katz JS, Burdick JA. Light-responsive biomaterials: development and applications. Macromol Biosci. 2010;10(4):339–348. doi: 10.1002/mabi.200900297. [DOI] [PubMed] [Google Scholar]
- 82.Luo LL, Guo Y, Yang JC, Liu YJ, Chu S, Kong F, Wang Y, Zou ZG. An efficient visible light controlled protein delivery system. Chem Commun. 2011;47(40):11243–11245. doi: 10.1039/c1cc14100g. [DOI] [PubMed] [Google Scholar]
- 83.Fang WJ, Yang J, Gong JW, Zheng NF. Photo- and pH-Triggered release of anticancer drugs from mesoporous silica-coated pd@ag nanoparticles. Adv Funct Mater. 2012;22(4):842–848. [Google Scholar]
- 84.Bochet CG. Photolabile protecting groups and linkers. J Chem Soc Perk T1. 2002;2:125–142. [Google Scholar]
- 85.Xie ZG, Hu XL, Chen XS, Mo GJ, Sun J, Jing XB. A novel biodegradable and light-breakable diblock copolymer micelle for drug delivery. Adv Eng Mater. 2009;11(3):B7–B11. [Google Scholar]
- 86.Wu YJ, Yan LS, He HY, Li B, Xie ZG, Huang YB, Jing XB. Caged mPEG-poly(S-(2-nitrobenzyl)-L-cysteine) for photo-triggered drug release and thiol-ene functionalization. J Control Release. 2013;172(1):E83–E84. [Google Scholar]
- 87.Zhao Y. Light-responsive block copolymer micelles. Macromolecules. 2012;45(9):3647–3657. [Google Scholar]
- 88.Kumar S, Allard JF, Morris D, Dory YL, Lepage M, Zhao Y. Near-infrared light sensitive polypeptide block copolymer micelles for drug delivery. J Mater Chem. 2012;22(15):7252–7257. [Google Scholar]
- 89.Shen ZY, Ma GH, Dobashi T, Maki Y, Su ZG. Preparation and characterization of thermo-responsive albumin nanospheres. Int J Pharmaceut. 2008;346(1–2):133–142. doi: 10.1016/j.ijpharm.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 90.Abulateefeh SR, Spain SG, Thurecht KJ, Aylott JW, Chan WC, Garnett MC, Alexander C. Enhanced uptake of nanoparticle drug carriers via a thermoresponsive shell enhances cytotoxicity in a cancer cell line. Biomater Sci-Uk. 2013;1(4):434–442. doi: 10.1039/c2bm00184e. [DOI] [PubMed] [Google Scholar]
- 91.Harnoy AJ, Rosenbaum I, Tirosh E, Ebenstein Y, Shaharabani R, Beck R, Amir RJ. Enzyme-responsive amphiphilic PEG-dendron hybrids and their assembly into smart micellar nanocarriers. J Am Chem Soc. 2014;136(21):7531–7534. doi: 10.1021/ja413036q. [DOI] [PubMed] [Google Scholar]
- 92.Rao JY, Khan A. Enzyme sensitive synthetic polymer micelles based on the azobenzene motif. J Am Chem Soc. 2013;135(38):14056–14059. doi: 10.1021/ja407514z. [DOI] [PubMed] [Google Scholar]
- 93.Hu JM, Zhang GQ, Liu SY. Enzyme-responsive polymeric assemblies, nanoparticles and hydrogels. Chem Soc Rev. 2012;41(18):5933–5949. doi: 10.1039/c2cs35103j. [DOI] [PubMed] [Google Scholar]
- 94.Qin J, Asempah I, Laurent S, Fornara A, Muller RN, Muhammed M. Injectable superparamagnetic ferrogels for controlled release of hydrophobic drugs. Adv Mater. 2009;21(13):1354–1357. [Google Scholar]
- 95.Cai KY, Luo Z, Hu Y, Chen XY, Liao YJ, Yang L, Deng LH. Magnetically triggered reversible controlled drug delivery from microfabricated polymeric multireservoir devices. Adv Mater. 2009;21(40):4045–4049. [Google Scholar]
- 96.Schroeder A, Honen R, Turjeman K, Gabizon A, Kost J, Barenholz Y. Ultrasound triggered release of cisplatin from liposomes in murine tumors. J Control Release. 2009;137(1):63–68. doi: 10.1016/j.jconrel.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 97.Sirsi SR, Borden MA. State-of-the-art materials for ultrasound-triggered drug delivery. Adv Drug Deliver Rev. 2014;72:3–14. doi: 10.1016/j.addr.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Steinbach OC. Industry update: The latest developments in therapeutic delivery. Ther Deliv. 2014;5(5):505–509. doi: 10.4155/tde.14.26. [DOI] [PubMed] [Google Scholar]
- 99.Cheng YX, Hao J, Lee LA, Biewer MC, Wang Q, Stefan MC. Thermally controlled release of anticancer drug from self-assembled gamma-substituted amphiphilic poly(epsilon-caprolactone) micellar nanoparticles. Biomacromolecules. 2012;13(7):2163–2173. doi: 10.1021/bm300823y. [DOI] [PubMed] [Google Scholar]
- 100.Ding JX, Zhao L, Li D, Xiao CS, Zhuang XL, Chen XS. Thermo-responsive “hairy-rod” polypeptides for smart antitumor drug delivery. Polym Chem-Uk. 2013;4(11):3345–3356. [Google Scholar]
- 101.Cheng YL, He CL, Xiao CS, Ding JX, Zhuang XL, Chen XS. Versatile synthesis of temperature-sensitive polypeptides by click grafting of oligo(ethylene glycol) Polym Chem-Uk. 2011;2(11):2627–2634. [Google Scholar]
- 102.Zhu L, Kate P, Torchilin VP. Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting. ACS Nano. 2012;6(4):3491–3498. doi: 10.1021/nn300524f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li JJ, Ge ZS, Liu SY. PEG-sheddable polyplex micelles as smart gene carriers based on MMP-cleavable peptide-linked block copolymers. Chem Commun. 2013;49(62):6974–6976. doi: 10.1039/c3cc43576h. [DOI] [PubMed] [Google Scholar]
- 104.Lee JS, Groothuis T, Cusan C, Mink D, Feijen J. Lysosomally cleavable peptide-containing polymersomes modified with anti-EGFR antibody for systemic cancer chemotherapy. Biomaterials. 2011;32(34):9144–9153. doi: 10.1016/j.biomaterials.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 105.Chen W, Zhong P, Meng FH, Cheng R, Deng C, Feijen J, Zhong ZY. Redox and pH-responsive degradable micelles for dually activated intracellular anticancer drug release. J Control Release. 2013;169(3):171–179. doi: 10.1016/j.jconrel.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 106.Cheng R, Meng FH, Deng C, Klok HA, Zhong ZY. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials. 2013;34(14):3647–3657. doi: 10.1016/j.biomaterials.2013.01.084. [DOI] [PubMed] [Google Scholar]
- 107.Ding JX, Shi FH, Li D, Chen L, Zhuang XL, Chen XS. Enhanced endocytosis of acid-sensitive doxorubicin derivatives with intelligent nanogel for improved security and efficacy. Biomater Sci-Uk. 2013;1(6):633–646. doi: 10.1039/c3bm60024f. [DOI] [PubMed] [Google Scholar]
- 108.Singh NK, Lee DS. In situ gelling pH- and temperature-sensitive biodegradable block copolymer hydrogels for drug delivery. J Control Release. 2014;193:214–227. doi: 10.1016/j.jconrel.2014.04.056. [DOI] [PubMed] [Google Scholar]


