Abstract
In biomedical imaging, nanoparticles combined with radionuclides that generate Cerenkov luminescence are used in diagnostic imaging, photon-induced therapies, and as activatable probes. In these applications, the nanoparticle is often viewed as a carrier inert to ionizing radiation from the radionuclide. However, certain phenomena such as enhanced nanoparticle luminescence and generation of reactive oxygen species cannot be explained by only Cerenkov luminescence interactions with nanoparticles. Herein, we report methods to examine the mechanisms of nanoparticle excitation by radionuclides, including interactions with Cerenkov luminescence, β particles, and γ radiation. We demonstrate that β scintillation contributes appreciably to excitation and reactivity in certain nanoparticle systems and that excitation of nanoparticles composed of large atomic number atoms by radionuclides generates X-rays, enabling multiplexed imaging through single photon emission computed tomography. These findings demonstrate practical optical imaging and therapy using radionuclides with emission energies below the Cerenkov threshold, thereby expanding the list of applicable radionuclides.
Keywords: Cerenkov, radionuclide, nanoparticle, imaging, enhancement, optical, ionise, SPECT, scintillation
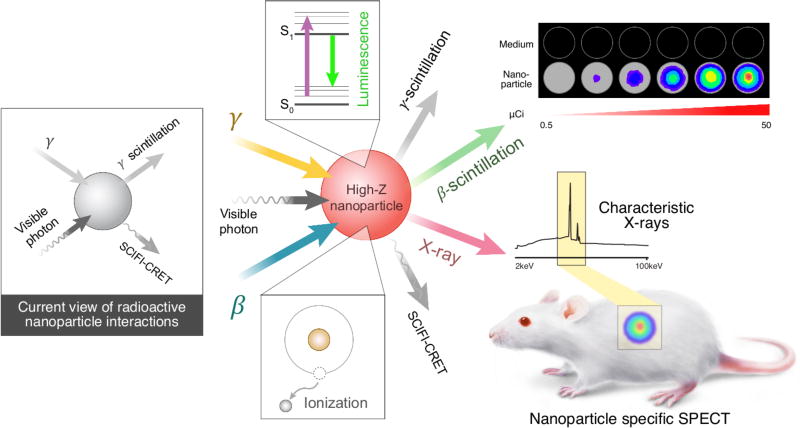
Ionizing radiation sources such as linear accelerators and more commonly radionuclides are often combined with nanoparticles (NPs) for medical applications1–5. Radiolabelled NPs have found use in clinical6, 7 and preclinical sentinel lymph node imaging4, 8, long-term NP integrity studies9, and tumour imaging through active targeting and the enhanced permeability and retention mechanisms10, 11. While radiolabelled NPs have been typically detected using single photon emission computed tomography (SPECT) or positron emission tomography (PET)12 there is increasing interest in utilizing Cerenkov luminescence (CL)13, 14. CL is the visible blue-weighted light that emanates when a charged particle, such as a β particle, travels faster than the phase velocity of light in a particular medium. Since first shown in preclinical in vivo systems in 200915, Cerenkov luminescence imaging (CLI) is being rapidly translated to the clinic16–21.
Imaging and therapeutic applications using ionizing radiation combined with NPs have generally neglected direct interactions between the ionizing radiation and the NP. Research has focused on CL generated in the medium interacting with fluorescent NPs such as quantum dots for secondary Cerenkov-induced fluorescence imaging (SCIFI)22. CL research has extended into TiO2 NP-based photodynamic therapy (PDT) with both radionuclides3 and external beams23. These studies explained the mechanism of PDT as CL from the ionizing radiation source exciting the NP-based PDT agent, generating reactive oxygen species. However, the very low CL photon fluence predicted from radionuclides (nJ cm−2) compared to external beam radiation (mJ cm−2)24 strongly suggests that additional NP excitation mechanisms are necessary for therapy.
Ionizing radiation results in the excitation and ionization of the surrounding medium, in this case the NP, as energy is dissipated to reach thermal equilibrium25. One mechanism by which NPs can relax to the ground state is through radiative processes, such as scintillation. γ-scintillation (photon production from γ-emitters) has been studied in quantum dots26 and rare-earth NPs27–31 (Fig. 1a). The contribution of β energy on NP systems has been neglected as β+ radionuclides in biomedical imaging (such as 18F and 68Ga) also ultimately emit γ photons from positron annihilation in addition to CL. Common β− (i.e. electron) emitting radionuclides such as 90Y and 32P also emit CL, thereby obscuring each mechanism contribution. Previous studies relied on physical shielding to block specific types of interactions (CL, γ, β) between a source and target. This approach led to the conclusion that Eu2O3 NPs were not excited by β interactions from the 18F positron27. However, due to the short positron range of 18F in water32 (βavg =0.25 mm, βmax = 2.0 mm) most positrons annihilated prior to interacting with the NPs, limiting the photons observed.
Figure 1.
Interactions between ionizing radiation and nanoparticles. a, gamma and visible photon sources are generally considered the excitation mechanisms of NPs by ionizing radiation that produces visible light through gamma scintillation or by Cerenkov radiation energy transfer, respectively. b, A more complete view of mechanisms resulting in excitation and ionization of NP from both β and γ radionuclides c, the fuller understanding of the consequences of NP excitation by ionizing radiation enables both optical and SPECT imaging with high atomic number (Z) NPs.
Here, we report new assays using radionuclides that do not emit CL to determine β and γ interactions with NPs. We demonstrate that β interactions significantly contribute to excitation of widely used nanoparticles such as Eu2O3 and TiO2, investigated for imaging27 and therapy3, respectively. We furthermore demonstrate that ionizing radiation in the presence of NPs containing high atomic number (Z) elements results in emission of characteristic X-rays in the 1–100 keV range. These X-rays allow for a new strategy in biomedical imaging using a SPECT scanner for multiplexed, NP-specific in vivo imaging. Our investigation into the various interactions between ionizing radiation with NPs better explains previous imaging and photodynamic therapy results in addition to a new imaging paradigm.
Visible photon output from radionuclide-NP pairings
Since clinical radionuclides decay through multiple high-energy emissions (Table 1), we first aimed to delineate NP excitation mechanisms that result in output of visible light. Radionuclides that emit only β− or γ were selected as excitation sources and added to a library of NPs that are of interest for biomedical imaging (Table 2), including amorphous silica NP (SNP), TiO2, HfO2, Eu2O3, Gd2O3, and YAG:Ce (cerium-doped yttrium aluminium garnet). The pure excitation sources were combined directly with either the NP suspension or an H2O control solution. The pure β− emitters 35S and 3H were used since the maximum energies (βmax) are below the CL threshold in water. The γ emitter 99mTc was used to assess contributions from γ excitation. Although 99mTc and 35S do not result in CL, ionization and excitation of H2O33 can produce photons but are several orders weaker than CL.
Table 1.
Radionuclides used with corresponding decay mechanisms and energies. Information populated from the Nuclear Science References database49. γ± denotes positron 511 keV photons upon annihilation.
| Radionuclide | β decay | β energy (average keV) |
γ emission |
γ energy (average) |
In vivo imaging modality |
|---|---|---|---|---|---|
| 3H | β− | 5.68 | - | - | |
| 35S | β− | 48.76 | - | - | |
| 177Lu | β− | 149.35 | γ | 208 | SPECT, CL |
| 32P | β− | 695.03 | - | - | |
| 18F | ε+β+ | 249.8 | γ± | - | PET, CL |
| 89Zr | ε+β+ | 395.5 | γ, γ± | 909 | PET, CL |
| 68Ga | ε+β+ | 836.02 | γ, γ± | 1077 | PET, CL |
| 90Y | β− | 933.7 | γ | 1761 | CL |
| 99mTc | - | - | γ | 140 | SPECT |
Table 2.
NP characterization information. Dynamic light scattering of NPs in 60% glucose (Zavg), absorbance of NPs at 633 nm at 1E10 particles per mL. Kedge values reproduced from NIST X-ray transition Database38.
| Size, Absorbance, NP Characterization Table | |||||
|---|---|---|---|---|---|
| Nanoparticle | DLS (nm) | PDI | Absorbance (633 nm) |
Kedge (keV) | Vendor |
| SNP (SiO2) | 163.7±4.8 | 0.039 | 0.0725 | 1.85 | Synthesised |
| TiO2 (Anatase) | 12.8±1.5 | 0.082 | 0.192 | 4.98 | American Elements |
| HfO2 | 34.9±0.7 | 0.192 | 0.189 | 65.35 | American Elements |
| Eu2O3 | 90.7±3.6 | 0.432 | 0.443 | 48.52 | Sigma-Aldrich |
| Gd2O3 | 75.7±6.8 | 0.503 | 0.357 | 50.25 | Sigma-Aldrich |
| YAG:Ce | 36.6±4.0 | 0.401 | 0.149 | 40.45 | Nanostructured & Amorphous Materials Inc. |
| Bi2O3 | 201.2±4.4 | 0.315 | 0.242 | 90.54 | Sigma-Aldrich |
| AuNP | 85.8±0.009 | 0.092 | 0.200 | 80.72 | Synthesised |
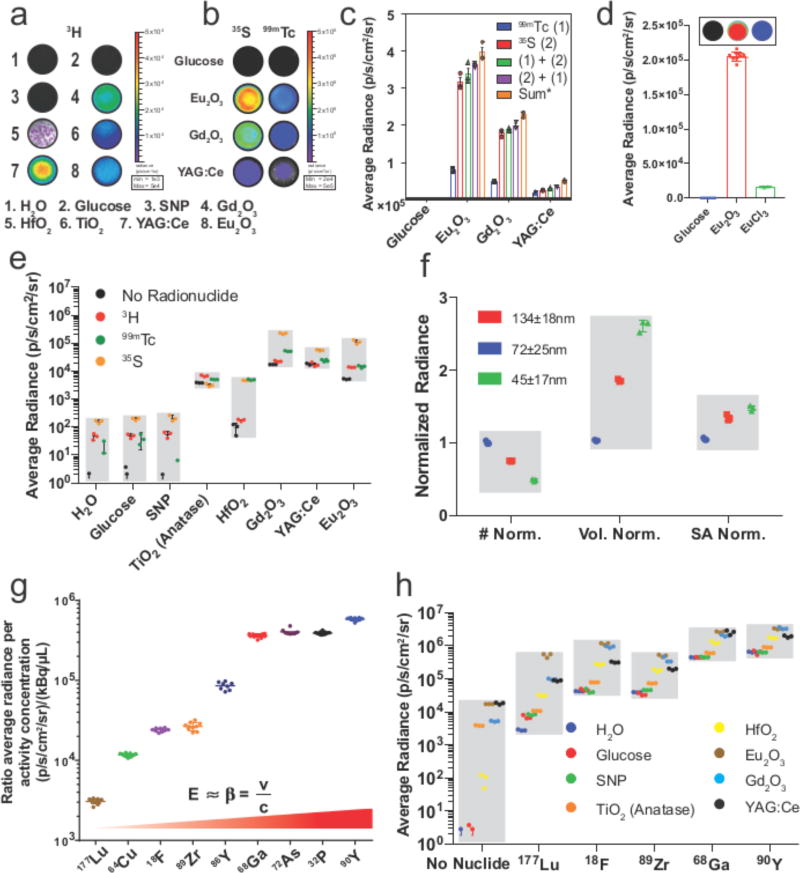
When 30 µCi of 3H, the lowest energy pure β− emitter, were combined with the NP panel, radiance enhancements over 3H in H2O were observed: SNP (1.2-fold), TiO2 (55-fold), HfO2 (4-fold), Eu2O3 (179-fold), Gd2O3 (301-fold), and YAG:Ce (376-fold) (Fig. 2a). Since the short trajectory of the low energy β emitted for 3H may only interact with a few NPs, we next tested the radiance enhancements with more energetic radionuclides. 35S yielded a greater photon flux enhancements for NP combinations: SNP (2-fold), anatase TiO2 (39-fold), HfO2 (58-fold), Eu2O3 (2750-fold), Gd2O3 (1390-fold), and YAG:Ce (690-fold). The addition of 99mTc, a pure γ-emitter, to NPs in the panel yielded similar enhancements: anatase TiO2 (268-fold), HfO2 (251-fold), Eu2O3 (2760-fold), Gd2O3 (366-fold), and YAG:Ce (775-fold). Thus, both β and γ emitting radionuclides combined with NPs led to scintillation and appreciable increases in radiance. Importantly, the radiances from Eu2O3, Gd2O3, and YAG:Ce were ca. 4-fold greater with 35S (β) compared to 99mTc (γ) demonstrating that, contrary to previous reports27, β scintillation is a crucial mechanism in NP excitation (Fig. 2b). Furthermore, the enhancement of NP radiances is dependent on the amount of radioactivity present, (Fig. S1). Both activity titrations and tissue attenuation studies were conducted to confirm linearity (Fig. S2). While both β and γ radionuclides were shown to excite NPs without involving CL, it remained to be determined if one type of excitation would dominate or if they are additive. Therefore, 35S and 99mTc were added sequentially to each NP well in both orders, (i.e. β then γ radionuclide added and vice versa). The addition of the second radionuclide yielded additive radiances thus the order of addition did not significantly matter (Fig. 2c). To assess the radiance dependence on the NP structure rather than the metal ion, an equimolar solution of europium cations was tested with 100 µCi 35S and found to yield only 13% of the radiance of Eu2O3 NP containing the same amount of Eu (Fig. 2d). Radiances observed from pure β− emitting and γ emitting radionuclides with NPs (Figs. 2a and 2b) are summarised and plotted in Fig. 2e (standard deviation shown alone in Fig. S3). The effect of NP size on enhancement was also determined by using three sizes of Eu2O3 NPs normalised to particle number, volume or surface area. When mixed with 18F-FDG, the enhancement for the different sized Eu2O3 NPs varied less than threefold (Fig. 2f) while each is nearly 100-fold brighter than 18F-FDG alone, suggesting the enhancement is more dependent on the molar amount of europium within NPs and less dependent on particle size in the range tested. While certain NPs are more luminescent than others, the addition of sub-CL energy radionuclides results in increased luminescence of up to nearly 3000 times that of the radionuclide alone (i.e. 35S with Eu2O3 NPs) and distinct from chemiluminescence (Fig. S4).
Figure 2.
Radiative output of nanoparticles with radionuclides. a, Well plate images of NPs with and without addition of low energy pure β− emitter 3H show increased radiances in wells containing HfO2, Eu2O3, and Gd2O3 (representative images, n=3 per NP). b, Head to head comparison of γ and β scintillation. Addition of 30 µCi (1.11 MBq) 99mTc (right) or 35S (left) to glucose, Eu2O3, Gd2O3 and YAG:Ce shows 35S to be brighter while both radionuclides do not appreciably produce light in the suspending medium(top row) (representative well images, n=3 per NP and radionuclide). c, Computed radiance of wells from Fig 2b including subsequent addition of the other radionuclide from Fig. 2b to the same plate to assess order of addition to radiance shows minimal difference when compared to the summation of the individual radiances by 99mTc and 35S (n=3 independent samples, column centre=mean, error bars=SD) d, Radiance with Eu2O3 NPs and radionuclides is dependent on the nanostructure of the particle and not the ion content when compared with a solution containing 35S and an equal molar solution of EuCl3 in glucose (n=8 independent samples, representative images above figure, column centre=mean, error bars=SD). e, summary of radiance enhancements seen with addition of either pure gamma emitter 99mTc or pure β emitting 3H and 35S with sub CL energy (in H2O) also result in enhanced radiance (n=3 independent samples, grey bar=min/max per NP, bar=SD). Grey bars represent min/max radiance for each particle for visual grouping. f, Three sizes of Eu2O3 NP were tested and normalised by number, volume, and surface area (n=3, independent samples grey bar=min/max per NP normalisation for visual grouping, error bars=SD). g, CL radiance of select nuclides in water per unit of activity and volume (n=8, independent samples line=mean, error bars=SD). Radionuclides that do not meet the CL threshold in water were omitted. Grey bars represent min/max radiance for each radionuclide for visual grouping. h, Radiance of NPs tested with positron-emitters 18F, 89Zr, and 68Ga and β− emitters 177Lu and 90Y at 30 µCi (1.11 MBq) per well. Particles without activity were included as a reference (n=3 independent samples, grey bar=min/max per clinical radionuclide, error bars=SD).
To determine overall changes in the photon flux due to NP interactions, clinically used radionuclides from Table 1 were next explored with NPs in Table 2. Clinical β-emitting isotopes emit CL in water according to the Frank-Tamm equation (1) showing that CL increases with particle energy (Fig. 2g) and the refractive index of the medium. The clinical isotopes 18F, 68Ga, 177Lu and 90Y isotopes with high-energy emission were used to observe total radiance enhancements by NPs. Radiance enhancement for high-energy electron and γ emitting 90Y and 177Lu, and 511 keV γ emitting 18F, 68Ga and 89Zr were measured (Fig. 2h). Similar results to 90Y were observed with the addition of the pure high-energy β emitter 32P (Fig. S5). The 511 keV γ from positron annihilation was also tested with the NPs from Table 2, and were not found to produce radiances as large as direct addition of 99mTc (Figs. S6 and S21), in agreement with previous results27.
Equation 1: Frank-Tamm formula defining photon output over a distance (dN/dx), where α is the fine structure constant, β is the velocity of the particle relative to the speed of light in a vacuum, n is the refractive index of the medium, and λ is the wavelength of interest in the ultraviolet-visible region.
| (1) |
Of the NPs tested, Eu2O3 and Gd2O3 exhibited the largest enhancement in radiance. Enhancements using 177Lu, 18F, 68Ga and 90Y with Eu2O3 were found to be 180, 27, 5.7 and 5.2 times that of H2O respectively. Radiance enhancement ratios were greatest for low energy radionuclides since the control H2O radiances are the weakest. NP radiance enhancements did not correlate with NP refractive index (Figs. S7–S8), demonstrating the effect resulted from mechanisms other than CL. Overall, the increased radiance from clinical isotopes must arise from multiple mechanisms, where photon contributions are based on the type and energy distribution of radiation emitted as well as the NP composition (Fig. S9)34.
Characteristics of NP photon generation
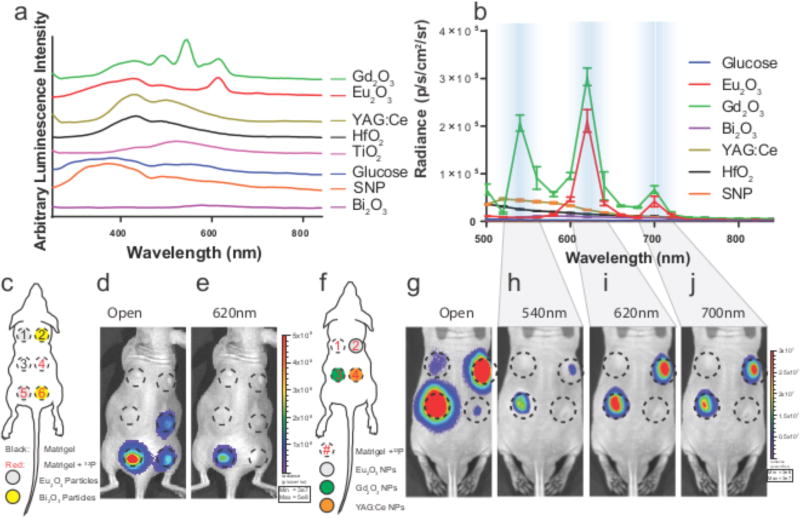
To further understand the optical output of the NP radionuclide combinations, spectra for each of the NPs with β and γ emitting radionuclides were measured from 250–840 nm in a plate reader (Fig. 3a) and in smaller but discrete step values from 500–840 nm on an IVIS optical imaging system (Fig. 3b). Disregarding material specific absorption, pure CL has the characteristic 1/λ2 wavelength intensity profile (Fig. S10), whereas discrete ionizations and excitations would exhibit known atomic transitions for scintillation or NP photoluminescence. The combination of Gd2O3 and Eu2O3 with 68Ga and 18F, respectively, produced distinct visible emission peaks (Figs. 3a, b) using a 540 nm narrow band emission filter35 for Gd3+, and characteristic f-f transitions for Eu3+ using 620 and 700 nm filters36. The less luminescent NPs such as HfO2, TiO2, Bi2O3, and YAG:Ce produced weaker and broader emission peaks in the 400–840 nm window (Figs. 3a, S10). The emission peak for HfO2 at 495 nm could not be resolved with the factory installed filters37 but is visible on the well plate scan (Fig. 3a). YAG:Ce has a photoluminescence peak at 540–560 nm38 but is weak compared to Gd2O3 and Eu2O3. No differences were observed between traditional laser excitation and emission spectra of these particles compared to spectra collected with radionuclides (Fig. S11). Spectral peaks were identical on the IVIS when excited by α emitters 225Ac or 223Ra (Fig. S12). The f-f transitions appear with both β and γ radionuclides, suggesting both as sources for scintillation with Eu2O3, Gd2O3 and YAG:Ce.
Figure 3.
The visible spectrum of radionuclides with nanoparticles between 250–840 nm on well plate and IVIS imaging systems. a, Normalised spectra of NPs and suspending medium using 6 mCi (222 MBq) 68Ga per well from 250 nm to 840 nm measured with a well plate reader. b, NPs with specific spectral emissions such as Eu2O3 and Gd2O3 with 30 µCi (1.11 MBq) 18F per well (n=3 independent samples, error bars=SD) on the IVIS imaging system. c, Diagram of in vivo phantom setup using pure β-emitting 32P. d, Open filter and e, 620 nm filter only. n=6 Matrigel phantoms per mouse (n=3) TL: Matrigel only, TR: Matrigel + 32P, ML:Eu2O3, MR: Bi2O3, BL: Eu2O3 + 32P, BR: Bi2O3 + 32P. Fig. S14, radiance spectrum for 32P phantoms showing characteristic emission peak of Eu3+ identical to in vitro emission peaks (n=3, error bars=SD). f, Diagram of in vivo phantom setup for mouse containing Matrigel phantoms with 750 µCi (27.75 MBq) 32P (clockwise from top left) Matrigel only, Eu2O3, YAG:Ce, and Gd2O3 (3×106 – 3×107 p/s/cm2/sr) (n=3) g, open filter. h, Emission specific window for Gd2O3 at 540 nm followed by similar emission peaks at 620 nm and 700 nm windows in i–j, to that seen in b. p/s/cm2/sr = photons/second/cm2/steradian
The signature emission peaks were retained in vivo (Fig. S14), where matrigel phantoms (Fig. 3c–e) showed nearly 5-fold enhanced radiance with Eu2O3 compared to 32P alone using the open filter (Fig. 3d). Using a 620 nm filter, only the emission from Eu2O3 phantom in the presence of activity was visible (Fig. 3e). A significant advantage is that the red photons from the europium emission spectrum are much less attenuated in vivo than the blue weighted light from CL13. β nuclides such as 32P can be used to produce selective optical emission both in vitro (Fig. 3b) and in vivo (Fig. 3e–j). Similarly, a comparison of Eu2O3 and Gd2O3 matrigel plugs with 32P in vivo (Fig. 3f–j) show the emission for Gd3+ using the 540 nm filter (Fig. 3h), and for both particles at longer wavelengths using 620 nm and 700 nm filters, respectively (Fig. 3i–j).
NP-multiplexed SPECT imaging
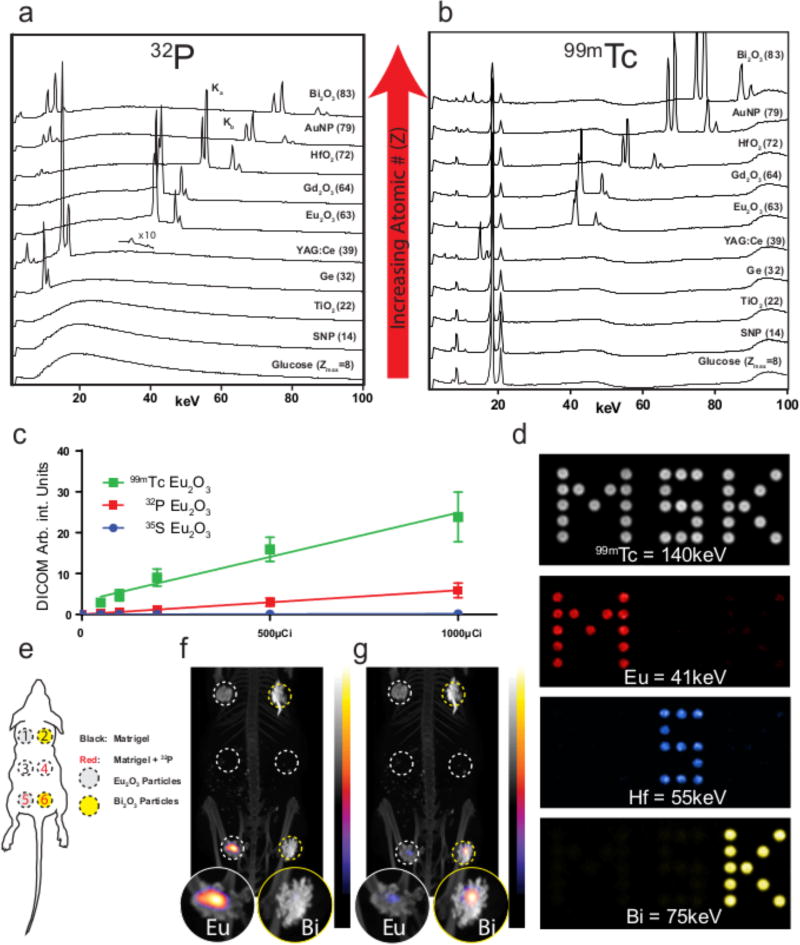
β particles can interact with matter via mechanisms that include CL, electron excitation, ionization, bremsstrahlung, and annihilation (β+ only) while γ particles can interact with matter through the photoelectric or Compton effects, coherent scattering, or pair production (Fig. S9). During ionization, characteristic X-rays are generated through an L shell electron occupying the vacancy of a previously ejected K shell electron. As the ejected X-ray represents a specific energy loss from an element, these X-rays are commonly used to determine elemental composition of materials based upon X-ray fluorescence (XRF)39, 40. Therefore, the NP panel was combined with β- or γ-emitting radionuclides, and evaluated for X-rays originating from the various NPs below 100 keV. Each of the β− radionuclides tested (except 3H, which has energy emissions too low to eject K shell electrons) showed a broad bremsstrahlung emission profile as expected in addition to characteristic X-rays, denoted Kα and Kβ, that were dependent on the NP composition. Fig. 4a presents the typical X-ray spectra for each of the NPs from the pure β− emitter 32P on top of a background of bremsstrahlung. Characteristic X-ray peaks were observed for Ge, YAG:Ce, Eu2O3, Gd2O3, HfO2, Au, and Bi2O3, while no X-ray peaks were observed for glucose, silica, and anatase TiO2. Characteristic X-ray production rates increase with both energy of the β radionuclide and atomic number41, explaining the lack of observed characteristic X-ray peaks for lower Z materials like silica, TiO2, and glucose. Here, we observe that in addition to scintillation occurring to produce visible light (Figs. 2 and 3), NPs are ionised producing characteristic X-rays in addition to and bremsstrahlung by β− emitting nuclides. The ionization peaks were identical using 99mTc (Fig. 4b). The efficiency of X-ray peak generation by the radionuclides was not calculated; however, an overlay of α, β+, γ and β− emitting nuclides tested with Eu2O3 all show the formation of characteristic X-rays (Fig. S15).
Figure 4.
High energy spectra and imaging of NP ionization from radionuclides. a, Characteristic X-ray spectra with the pure β− emitter 32P showing qualitative abundance and increasing Kα and Kβ energy with increasing atomic number (Z) from 1–100 keV. ×10 magnification above YAG:Ce trace represents characteristic X-rays of the cerium dopant upon longer measurement (representative spectra shown). b. Identical characteristic X-rays generated using 99mTc from 1–100 keV. For Eu2O3, characteristic X-ray peaks were produced using clinical radionuclides 90Y, 68Ga, 18F, and 177Lu (Fig. S15). c, X-ray generation curves from 99mTc, 32P and 35S in Eu2O3 NPs by SPECT based upon region of interest drawn over phantom cylinder (n=1 phantom per activity level per radionuclide, centre value=median intensity reported from 1cc volumetric cylinder, error bars=SD). d, Proof of concept in vitro using Eu2O3, HfO2, and Bi2O3 NPs with 99mTc to produce a multicolour image based upon characteristic X-rays captured by SPECT. e, Diagram of mouse phantom used for dual spectrum imaging of either europium or bismuth particles with 32P. f, Characteristic X-rays imaging on SPECT produced from Eu2O3 with enlarged phantom regions to highlight signal presence and absence g, Bi2O3 NPs (1×1011NPs) with 32P show signal localization to the Bi2O3 NP by SPECT and do not show signal from either NP alone nor activity alone.
While rare earth NPs have been excited in vivo using exogenous X-ray beams to produce photons30, 42, X-rays emitted from the NP by ionization from endogenous radionuclides can be used for multicolour NP specific imaging. With an adjustable keV photon energy window, a SPECT instrument represents an ideal imaging platform for this modality. For NP composed of higher Z atoms like europium, gadolinium, gold, and bismuth, the characteristic keV photons generated from ionization enables direct detection by X-ray imaging, and the different X-ray energy emitted (Table 2) enables multimodal detection. A titration curve was produced for Eu2O3 using 35S, 32P, and 99mTc, where 99mTc showed the greatest signal of europium X-rays per unit of activity (Fig. 4c) and demonstrated that imaging on a preclinical platform was both feasible and quantitative (Fig. S16). To test the multicolour capability of this system, a well plate was prepared containing either Eu2O3, HfO2, or Bi2O3 with 99mTc and X-ray emissions were detected at the energies corresponding to the predominant metal in each particle (Fig. 3d). The top panel in Fig. 3d shows the 140 keV photopeak from 99mTc activity, while the subsequent panels show X-ray emission for europium, hafnium, and bismuth. A corresponding study was also done using mixtures of these NP (Fig. S17) demonstrating that X-rays from mixtures can be resolved with an X-ray signal dependent on the composition of the mixture. To test the multiplexing in vivo with a pure β− emitter, matrigel plugs containing Eu2O3 or Bi2O3 NPs were implanted into a nude mouse with or without 32P (Fig. 4e–g). The matrigel phantom containing both 32P and the Eu2O3 show a distinct signal for europium that is not seen in any other phantom (Fig. 4f). Similarly, the phantom containing Bi2O3 and 32P shows the highest intensity for bismuth characteristic X-rays (Fig. 4g) and is specific to the detection channel used (Fig. S18) provided the detector can resolve the X-ray energy difference.
Clinical relevance and new imaging technology
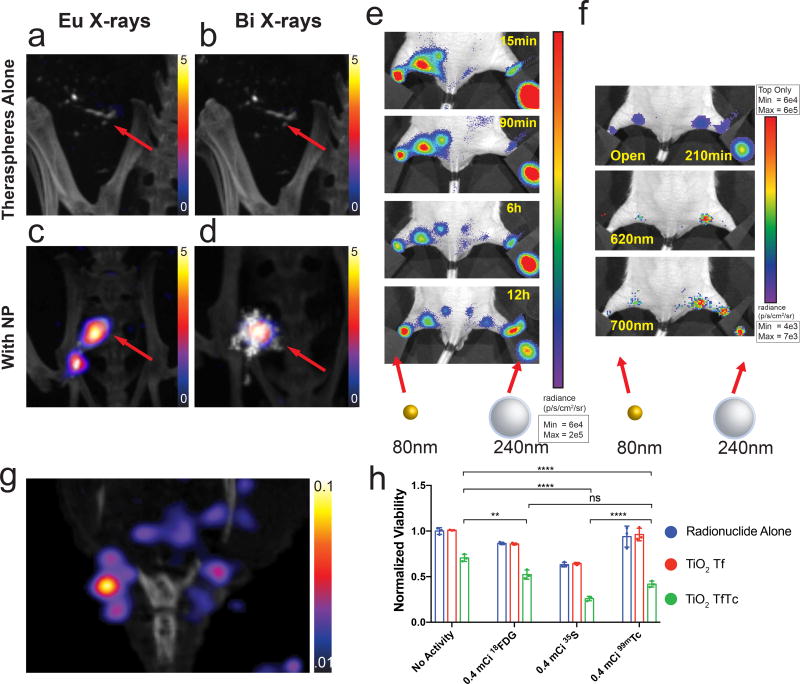
Beyond SiO2, TiO2, and AuNP, most NPs investigated face a large barrier for clinical translation. In particular, there is known toxicity for uncoated rare earth oxides in lysosomes43. Rare earth NP toxicity must be mitigated by increasing surface biocompatibility and facilitate biological clearance. However, our results highlight that NPs in proximity to clinical radionuclides provide enhanced photon radiance and generate characteristic X-rays that can be used in several modes of imaging. The high energy of 90Y makes this new SPECT mode of imaging immediately clinically relevant with Theraspheres® or other 90Y sources. Theraspheres® are glass microspheres containing 90Y and are FDA approved for radioembolization of primary liver tumours when delivered by catheter directly to the tumour via the hepatic artery branches. Imaging their location in vivo has proved challenging. Therefore, we assessed our imaging technique on the combination of Theraspheres® and bismuth or europium NPs. This is advantageous over e.g. mixing the spheres with small molecule radiotracers which could quickly dissipate from the injection side (e.g. 99mTc-MDP). Tumour bearing mice received intratumoural injections of Theraspheres® or Theraspheres® co-injected with either bismuth or europium NPs. In the europium and bismuth energy windows (~10 keV width total), Theraspheres® alone showed no appreciable signal (Fig. 5a and 5b) compared to Theraspheres® co-injected with Eu2O3 or Bi2O3 NPs (Fig. 5c and 5d). A modest CT contrast was observed with Theraspheres®, and enhanced with either europium or bismuth NPs (Fig S19) for visual location recognition. Here, select high Z NPs served as an imaging agent for Therasphere® detection by SPECT, opening up the possibility for multimodal detection with other NP agents simultaneously.
Figure 5.
Imaging of clinical radioembolization agent 90Y Theraspheres® with characteristic X-rays. a,b, Representative images of 1 mCi Theraspheres® injected intratoumorally into BT-474 orthotopic breast tumours (red arrow) using characteristic X-ray energy windows for Eu2O3 and Bi2O3 NPs, respectively, shows no appreciable signal (n=3 animals, representative image shown). c,d, Imaging of Theraspheres® co-injected with Eu2O3 or Bi2O3 NPs shows selective signal near NP injection site (n=4 animals, representative image shown). Lymph node imaging of drainage from right and left hind footpads with 90Y labelled Eu2O3 and AuNP respectively with e, IVIS open filter, and f, select 620nm and 700nm wavelengths to identify Eu2O3 selective drainage (n=3, representative single mouse sequence shown). g, SPECT-CT showing characteristic X-rays from 66.9keV ionization of gold from Au-PEG-DOTA(90Y) NPs draining into sentinel lymph nodes 48h post intradermal tail injection (n=3 animals, representative mouse image shown). Corresponding IVIS image to (Fig. 5g) can be found in Fig. S20. h, Non-CL light based PDT. Radionuclides 18F, 35S, and 99mTc were added to cells in vitro, along with TiO2 NP with transferrin attached (Tf) with and without titanocene (Tc). Therapeutic effects were demonstrated with radionuclides that do not emit CL, highlighting NP derived light can be as useful for PDT alongside CL (n=3 independent samples, centre value=mean, error bars=SD). **=P< 0.01, ****=P< 0.0001, ns=not significant.
Next, this technique was used to visualise lymphatic drainage and identify sentinel, locoregional draining lymph nodes, a procedure critical in oncologic surgery8. Eu2O3 NPs coated with silica and PEGylated AuNPs were synthesised to visualise drainage using chelator free4 and chelate methods with 90Y respectively. NPs were injected into either hind footpad to monitor lymph node drainage by IVIS (Fig. 5e). The smaller AuNPs (80 nm vs. 240 nm) drained nearly fifteen times faster (>30 µm/s) and reached the sentinel and higher echelon nodal stations faster as compared to the larger Eu2O3 NPs. This method would allow analysing lymphatic functionality preclinically. Furthermore, 620 nm and 700 nm emission windows enabled selective imaging of Eu2O3 only drainage (Fig. 5f). Sentinel lymph node drainage of AuNPs was also seen using the X-ray energy window for gold (Fig. 5g, S20). While other means of detecting sentinel nodes exist there are no clinical approved means to determine lymphatic function both non-invasively with SPECT for pre-surgical mapping and optically during surgery using the same agent and thus imaging the same biodistribution. Further miniaturisation and targeting could allow this system to move beyond lymph node drainage to image specifically tumour distribution for preclinical mapping and intraoperative imaging.
Combining TiO2 with sub CL radionuclides produced appreciable photon flux enhancements. Therefore, based on the photodynamic therapy previously described through CL3, we sought to evaluate and compare the therapeutic effect in vitro of sub-CL radionuclides together with TiO2 transferrin (Tf) and titanocene (Tc) NPs. The radionuclides 18F, 35S, and 99mTc were chosen to test if CL alone, or NP derived visible light from β or γ scintillation, are sufficient to elicit a therapeutic effect. No significant difference in viability was seen between radionuclide and the addition of TiO2Tf for all three radionuclides tested; however, the addition of TiO2TfTc produced a significant therapeutic effect (Fig. 5h). By using the non-CL radionuclides 35S and 99mTc, we demonstrate each aforementioned mechanism (not just CL) can be a contributor to a PDT effect from radiotracers and TiO2, as hypothesised previously.
Conclusions
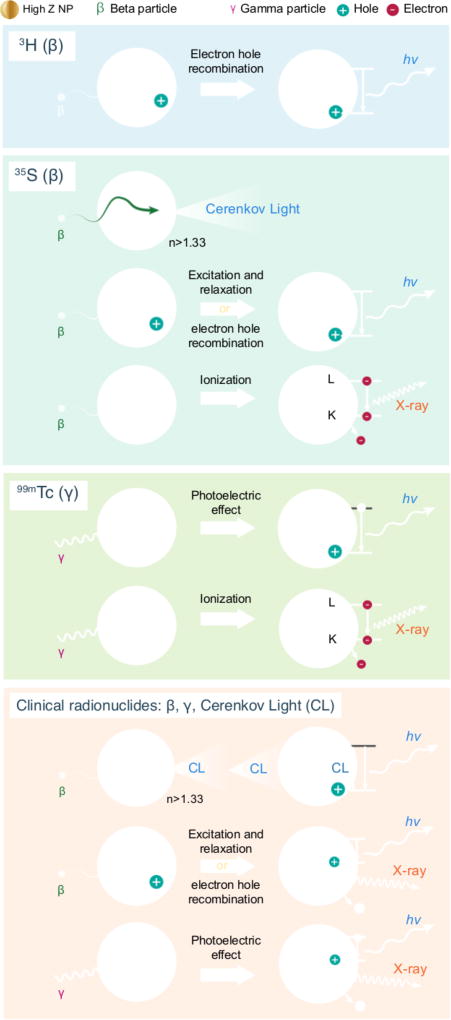
Radionuclide-NP combinations are widely used in vivo because of the high specific activity, payload, and multifunctional properties achievable with NPs. The types of interactions between ionizing radiation and NP that produce photons are dependent on both the NP’s and the radiotracer’s physical characteristics, summarised in Figure 6. Low energy radionuclides such as 3H can polarise regions on a NP while higher energy β emitters can produce CL in a NP but can also ionise, causing characteristic X-ray production. Gamma emitting radionuclides operate through the photoelectric effect where an incident gamma ray is converted into electrons which could recombine or relax to generate light. Gamma radiation can also generate characteristic X-rays if a K-shell electron is ionised. Clinical radionuclides can produce several high-energy β and γ emissions, each producing light through the aforementioned mechanisms on a given NP.
Figure 6.
Interactions between ionizing radiation and NP. Ionizing radiation interactions with NP may result in a number of emissions. Low-energy β emissions from radionuclides like 3H with TiO2 can cause electron hole recombination and resultant emission of photons. β emissions in the tens to hundreds of keV can result in numerous interactions with nanoparticles, such as Cerenkov emission within the NP, excitation or electron hole formation, or atom ionization in the NP of interest. High-energy photons, such as the 140 keV gamma from 99mTc, can result in the photoelectric effect or ionization within the NP. Clinical radionuclides such as PET tracers and radiotherapeutics, can result in the all of the aforementioned interactions along with CL interactions from the NP.
The visible light emanating from β-emitting radiotracers has allowed preclinical and clinical luminescence imaging through CLI16, but the photon flux is quite low. NPs in proximity to either β or γ emitting radionuclides were shown to exhibit enhanced total photon flux and emit at discrete wavelengths in systems with luminescent metal ions. Exploring the mechanisms of NP radiance with radionuclides that emit only a single type of radiation revealed unforeseen contributions of NP luminescence from the β particle. Our study sets the stage for imaging with all β-emitting radionuclides, exploiting the advantages of an enhanced signal emanating from the NP compared to CL alone. Using radionuclides with radiosensitizing agents for photodynamic therapy is increasingly investigated,44–46 and a greater understanding of how NPs can increase photon density for PDT should aid progress. Additionally, the heretofore unappreciated characteristic X-rays resulting from these interactions yields a new imaging modality in SPECT. These findings have broad implications for nuclear detection, optical imaging, and design of high Z NPs for imaging and therapy. Beyond the biosciences, these results can be exploited in environmental and material analytics.
Methods
Nanoparticle preparation
All NPs except SiO2 and AuNP were purchased from either American Elements or Sigma Aldrich. SiO2 NPs were prepared via a modified Stöber method4. Silicated Eu2O3 NPs were prepared based upon a modified gold NP method described previously47. Nanopowders were suspended in an aqueous solution of 60% by weight glucose with the aid of a 500 W tip sonicator to create a NP suspension. Au-PEG-DOTA NPs were prepared using an established AuNP protocol followed by thiol-PEG-NHS coupling (Nanocs Inc. HS-PEG10k-NHS) and immediate conjugation with S-2-(4-Aminobenzyl)-1,4,7,10-tetraazacyclododecane tetraacetic acid (macrocyclics) under slightly basic conditions. Particles were purified and concentrated by tangential flow filtration using a Spectrum hollow fiber microkros 50kD mPES membrane and stored at 4°C until chelation with 90Y as described previously.
Nanoparticle characterization
NP morphology and diameter were determined using both a Jeol 200 kV TEM and a Malvern Zetasizer Nano ZS dynamic light scattering. A Malvern Nanosight NS500 instrument was used to determine particle concentration. The absorbance and photo-luminescence was determined on a SpectraMax M5 plate reader via quartz cuvette using 1E10 particles per mL or lower and extrapolated when exceeding 1 OD. Excitation and emission spectra were acquired on a Cary Eclipse Fluorescence Spectrometer and the SpectraMax M5 plate reader at 1 mg per mL.
Radiotracer production (SI)
Hydrogen-3, phosphorus-32, sulfur-35, lutetium-177, and yttrium-90 were purchased from PerkinElmer as liquid solutions. When appropriate, solutions were brought to neutral pH prior to addition to NP solutions. Zirconium-89 was produced on the MSK cyclotron and purified as zirconium oxalate. This was neutralised with sodium carbonate prior to use. Gallium-68 was produced on a germanium generator and eluted in 0.3N HCl. This was neutralised with 28% ammonium hydroxide prior to use. Fluorine-18 was obtained from the MSK nuclear pharmacy in the form of 18F-fluoro-2-deoxyglucose ([18F]-FDG). Technecium-99 metastable was obtained from the MSK nuclear pharmacy in the form of 99mTc pertechnetate. 90Y Theraspheres® were obtained through a material trade agreement and generosity of BTG interventional medicine. 225Ac was generously gifted from the Lewis Lab at MSKCC and 223Ra was obtained through a material trade agreement with the Thorek lab at Johns Hopkins University.
Beta, Gamma and X-ray interactions for visible light modulation
24 well black walled plates from Greiner were used for IVIS optical imaging. NP solutions were prepared as previously stated at 1E10, 1E11 and 1E12 particles per mL. 1 mL of nanosuspension was diluted with 5 µL of the radionuclide of interest, targeting 30 µCi (1.11MBq) per well at time of measurement. Imaging was performed on an IVIS Spectrum at f=1 for durations of 60 s to 300 s with an open filter. Initially the NPs in the panel (Table 2) were measured without any radioactivity to determine background radiative output. Spectrum scans were acquired using 20 nm increment filters between 500 nm and 840 nm on the IVIS Spectrum or between 250–840nm in 10nm increments on the SpectraMax M5. For the IVIS Spectrum ROIs were drawn over each well and radiance values were reported in p/s/cm2/sr after background subtraction averaging n=3 wells and decay correction. Enhancement was normalised to the radiance of an identical activity of radionuclide in H2O. Well plate IVIS experiments were done in triplicate with spectral measurements done over the course of three hours per plate. Replicate plates were done on subsequent weeks with at least n=3 replicate experiments for a nanoparticle radionuclide combination. Radionuclide radiances alone were determined on the IVIS using a minimum of 4 replicates per activity level per nuclide with at least 5 different activity amounts. Radiances were then normalised to the intensity per unit of activity per volume.
High-energy photon luminescence measurements
The radionuclide of interest at identical concentrations to above was placed in 1 mL H2O in a 24 well black walled plate upon which a poly(methyl methacrylate) plate and black paper was then placed. On top of the black paper another 24 well plate containing in triplicate wells of H2O, 60% glucose, or NP solutions was then placed. IVIS imaging was conducted with an open filter at F1 for 300s per image. ROIs were drawn over the wells and background subtracted. See Fig. S22 for setup diagram.
X-ray and gamma ray spectroscopy
Measurement of the X-ray and gamma spectrum between 1 and 1700 keV was performed on a Canberra broad energy Ge detector. 1×1012 particles in an Eppendorf tube were placed at a fixed height in the detector, and activity was added such that crystal dead time was between 1–5% for gamma and pure β emitters. Clinical Isotopes were added at similar activities, resulting in ~60% dead time due to additional counts from emitted γ’s. Spectra were qualitatively compared to blank tubes containing identical amounts of activity in 60% glucose only. Channel peaks were calibrated to energies with the use of an 241Am 1.0 µCi source found in a Family Gard® FG200 Smoke Detector. Spectra peaks were compared to atomic transitions of the NP elements using the NIST X-ray Transitions Database48.
Modified SPECT-CT imaging using characteristic X-rays
0.5–1 mCi (18.5–37 MBq) of 32P was mixed with nanoparticles (1×1011 NPs) and matrigel and subcutaneously injected into athymic nude mice for pilot phantom studies. AuNP tail drainage experiments were done using 270 µCi (9.9 MBq) of 90Y chelated to ~10mg of AuNPs and injected intradermally into the tail of an athymic nude mouse. Energy windows for imaging were selected based upon main Kα and Kβ transitions on a Mediso nanoSPECT/CT (Fig. S23). We constructed artificial isotopes in the Mediso isotope master file and subsequently performed energy and uniformity calibrations to prepare the nanoSPECT/CT for NP characteristic X-ray imaging. SPECT images were acquired using the standard high-energy pinhole collimators, and a scan time of 1 hour, and with 35–60% smoothing for acquired SPECT images.
In Vivo Imaging with athymic nude mice
Animal experiments were done in accordance with protocols approved by the Institutional Animal Care and Use Committee of Memorial Sloan Kettering Cancer Center and followed National Institutes of Health guidelines for animal care. Athymic male nude mice were used for lymph node drainage studies while athymic female nude mice were implanted orthotopically with BT-474 cells and injected intratumourally with 1 mCi of 90Y Theraspheres® with and without 1E11 Eu2O3 or Bi2O3 NPs. Cells were authenticated but not checked for mycoplasma while in culture. Europium oxide nanoparticles were silicated and annealed in a 500°C oven for one hour before chelator free radiolabelling with 90Y. Approximately 270 µCi (9.9 MBq) of 90Y labelled NPs (10mg AuNP or 5mg Eu2O3) were injected into either hind food pad and imaged on the IVIS every 15 minutes through 3 hours and then subsequently at 6 and 12 hours.
In Vitro PDT using TiO2 NPs
Experimental conditions were based off of methods described previously for TiO2 NPs3. TiO2 NPs from Sigma were washed with Chelex grade water and dispersed in 1× DPBS at a concentration of 1 mg/mL. Apo-transferrin from Sigma was dissolved in 1× DPBS at a concentration of 10 mg/mL and diluted 1:1 with TiO2 NP dispersion. TiO2Tf dispersion was placed on a cold block and tip sonicated for 120s with a 5s on, 10s off pulse sequence. Dispersion was immediately filtered with 0.45 µm filter and measured for NP absorbance relative to a known unfiltered sample. Titanocene was dissolved in DMSO and added to the TiO2Tf NPs before incubation with HT1080 fibrosarcoma cells overnight. Cells were authenticated but not checked for mycoplasma while in culture. Radionuclides were added the following day at concentrations between 0.01–0.80 mCi/well (n=3 per condition). Plates were placed in a shielded incubator for 72h before MTS viability assay. MTS assay was read out using a M5 spectramax plate reader.
Statistical Analysis
Graphpad prism software V6.05 and V7.03 were used for data analysis and statistical calculations. A 2-way ANOVA analysis was done for the 99mTc and 35S head to head radiance analysis.
Data Availability
The data that support the plots within this paper and other finding of this study are available from the corresponding author upon reasonable request.
Code Availability
Code used to reconfigure the SPECT/CT for X-Ray imaging of select elements is available upon request. Code used requires subsequent energy and uniformity mapping for each combination before use.
Supplementary Material
Acknowledgments
We thank BTG Interventional Medicine for the supply of Theraspheres® required to conduct the in vivo imaging. The Australian Nuclear Science and Technology Organization (ANSTO) is acknowledged for their generous provision of a research-grade gallium-68 generator and purification system along with the MSKCC nuclear pharmacy for 18FDG. We thank the MSKCC Radiochemistry and Molecular Imaging Probes Core for technical assistance with the Ge detector, P. Zanzonico and V. Longo of the Small Animal Imaging Core for their direction and assistance on the nanoSPECT/CT and medical physics discussions. This work was supported by the following grants: National Institutes of Health (NIH) R01EB014944 and R01CA183953 (to J.G.), T32 CA196585 (to T.M.S.), and National Science Foundation Integrative Graduate Education and Research Traineeship grant (DGS 0965983, at Hunter College). Technical services provided by the MSKCC Small-Animal Imaging Core Facility, supported in part by NIH Centre Grant No P30 CA08748, are gratefully acknowledged. NIH Shared Instrumentation Grant No 1 S10 RR028889-01, which provided funding support for the purchase of the NanoSPECT/CT Plus, and a Shared Resources Grant from the MSKCC Metastasis Research Centre, which provided funding support for the purchase of the IVIS Spectrum, are also gratefully acknowledged.
The authors E.C.P., T.M.S., and J.G. have filed an international patent application regarding the content of the manuscript (PCT/US2016/018502).
Footnotes
Author Contributions
E.C.P., T.M.S., and Q.Z. devised and carried out the experiments. E.C.P. and T.M.S. wrote the manuscript. J.G. and C.M.D. supervised the project and edited the manuscript. All authors contributed discussions on the project.
References
- 1.Pratt EC, Shaffer TM, Grimm J. Nanoparticles and radiotracers: advances toward radionanomedicine. Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology. 2016 doi: 10.1002/wnan.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benezra M, et al. Ultrasmall integrin-targeted silica nanoparticles modulate signaling events and cellular processes in a concentration-dependent manner. Small. 2015;11:1721–1732. doi: 10.1002/smll.201402331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotagiri N, Sudlow GP, Akers WJ, Achilefu S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat Nanotechnol. 2015;10:370–379. doi: 10.1038/nnano.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaffer TM, et al. Silica nanoparticles as substrates for chelator-free labeling of oxophilic radioisotopes. Nano Lett. 2015;15:864–868. doi: 10.1021/nl503522y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaffer TM, et al. Stable Radiolabeling of Sulfur-Functionalized Silica Nanoparticles with Copper-64. Nano Lett. 2016;16:5601–5604. doi: 10.1021/acs.nanolett.6b02161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoogendam JP, et al. 99mTc-Nanocolloid SPECT/MRI Fusion for the Selective Assessment of Nonenlarged Sentinel Lymph Nodes in Patients with Early-Stage Cervical Cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016;57:551–556. doi: 10.2967/jnumed.115.164780. [DOI] [PubMed] [Google Scholar]
- 7.Phillips E, et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Science Translational Medicine. 2014;6:260ra149–260ra149. doi: 10.1126/scitranslmed.3009524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thorek DL, et al. Non-invasive mapping of deep-tissue lymph nodes in live animals using a multimodal PET/MRI nanoparticle. Nat Commun. 2014;5:3097. doi: 10.1038/ncomms4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen F, et al. In Vivo Integrity and Biological Fate of Chelator-Free Zirconium-89-Labeled Mesoporous Silica Nanoparticles. ACS nano. 2015;9:7950–7959. doi: 10.1021/acsnano.5b00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez-Medina C, et al. A modular labeling strategy for in vivo PET and near-infrared fluorescence imaging of nanoparticle tumor targeting. J Nucl Med. 2014;55:1706–1711. doi: 10.2967/jnumed.114.141861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X, et al. Self-illuminating 64Cu-doped CdSe/ZnS nanocrystals for in vivo tumor imaging. Journal of the American Chemical Society. 2014;136:1706–1709. doi: 10.1021/ja410438n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goel S, England CG, Chen F, Cai W. Positron emission tomography and nanotechnology: A dynamic duo for cancer theranostics. Advanced Drug Delivery Reviews. doi: 10.1016/j.addr.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaffer TM, Pratt EC, Grimm J. Utilizing the power of Cerenkov light with nanotechnology. Nat Nano. 2017;12:106–117. doi: 10.1038/nnano.2016.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czupryna J, et al. Cerenkov-Specific Contrast Agents for Detection of pH In Vivo. Journal of Nuclear Medicine. 2015;56:483–488. doi: 10.2967/jnumed.114.146605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson R, et al. Optical imaging of Cerenkov light generation from positron-emitting radiotracers. Phys Med Biol. 2009;54:N355–365. doi: 10.1088/0031-9155/54/16/N01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorek DL, Riedl CC, Grimm J. Clinical Cerenkov luminescence imaging of (18)F-FDG. J Nucl Med. 2014;55:95–98. doi: 10.2967/jnumed.113.127266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spinelli AE, et al. First human Cerenkography. J Biomed Opt. 2013;18:20502. doi: 10.1117/1.JBO.18.2.020502. [DOI] [PubMed] [Google Scholar]
- 18.Spinelli A, et al. Cerenkov and radioluminescence imaging of brain tumor specimens during neurosurgery. 2016;21 doi: 10.1117/1.JBO.21.5.050502. [DOI] [PubMed] [Google Scholar]
- 19.Hu H, et al. Feasibility study of novel endoscopic Cerenkov luminescence imaging system in detecting and quantifying gastrointestinal disease: first human results. European Radiology. 2015;25:1814–1822. doi: 10.1007/s00330-014-3574-2. [DOI] [PubMed] [Google Scholar]
- 20.Grootendorst MR, Cariati M, Kothari A, Tuch DS, Purushotham A. Cerenkov luminescence imaging (CLI) for image-guided cancer surgery. Clin Transl Imaging. 2016;4:353–366. doi: 10.1007/s40336-016-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grootendorst MR, et al. Intraoperative Assessment of Tumor Resection Margins in Breast-Conserving Surgery Using 18F-FDG Cerenkov Luminescence Imaging: A First-in-Human Feasibility Study. J Nucl Med. 2017;58:891–898. doi: 10.2967/jnumed.116.181032. [DOI] [PubMed] [Google Scholar]
- 22.Thorek DL, Ogirala A, Beattie BJ, Grimm J. Quantitative imaging of disease signatures through radioactive decay signal conversion. Nat Med. 2013;19:1345–1350. doi: 10.1038/nm.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouyang Z, Liu B, Yasmin-Karim S, Sajo E, Ngwa W. Nanoparticle-aided external beam radiotherapy leveraging the Cerenkov effect. Physica medica : PM : an international journal devoted to the applications of physics to medicine and biology : official journal of the Italian Association of Biomedical Physics (AIFB) 2016;32:944–947. doi: 10.1016/j.ejmp.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glaser AK, Zhang R, Andreozzi JM, Gladstone DJ, Pogue BW. Cherenkov radiation fluence estimates in tissue for molecular imaging and therapy applications. Physics in medicine and biology. 2015;60:6701–6718. doi: 10.1088/0031-9155/60/17/6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaffer TM, Drain CM, Grimm J. Optical Imaging of Ionizing Radiation from Clinical Sources. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016;57:1661–1666. doi: 10.2967/jnumed.116.178624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Letant SE, Wang TF. Semiconductor quantum dot scintillation under gamma-ray irradiation. Nano Lett. 2006;6:2877–2880. doi: 10.1021/nl0620942. [DOI] [PubMed] [Google Scholar]
- 27.Hu Z, et al. In vivo nanoparticle-mediated radiopharmaceutical-excited fluorescence molecular imaging. Nat Commun. 2015;6:7560. doi: 10.1038/ncomms8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao X, et al. Intensity Enhanced Cerenkov Luminescence Imaging Using Terbium-Doped Gd2O2S Microparticles. ACS Appl Mater Interfaces. 2015;7:11775–11782. doi: 10.1021/acsami.5b00432. [DOI] [PubMed] [Google Scholar]
- 29.Ma X, et al. Enhancement of Cerenkov luminescence imaging by dual excitation of ER(3+),Yb(3+)-doped rare-earth microparticles. PloS one. 2013;8:e77926. doi: 10.1371/journal.pone.0077926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karnkaew A, Chen F, Zhan YH, Majewski RL, Cai WB. Scintillating Nanoparticles as Energy Mediators for Enhanced Photodynamic Therapy. ACS nano. 2016;10:3918–3935. doi: 10.1021/acsnano.6b01401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun C, et al. Synthesis and radioluminescence of PEGylated Eu(3+) -doped nanophosphors as bioimaging probes. Adv Mater. 2011;23:H195–199. doi: 10.1002/adma.201100919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell GS, Gill RK, Boucher DL, Li C, Cherry SR. In vivo Cerenkov luminescence imaging: a new tool for molecular imaging. Philosophical transactions. Series A, Mathematical, physical, and engineering sciences. 2011;369:4605–4619. doi: 10.1098/rsta.2011.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sitharamaro DN, Duncan JF. MOLECULAR EXCITATION OF WATER BY GAMMA-IRRADIATION. J. Phys. Chem. 1963;67:2126-&. [Google Scholar]
- 34.McParland BJ, McParland BJ. Charged Particle Interactions with Matter. Springer-Verlag London Ltd; Godalming: 2010. [Google Scholar]
- 35.Carnall WT, Fields PR, Rajnak K. ELECTRONIC ENERGY LEVELS OF TRIVALENT LANTHANIDE AQUO IONS .4. EU3+ Journal of Chemical Physics. 1968;49:4450-&. [Google Scholar]
- 36.Sudheendra L, et al. NaGdF:Eu Nanoparticles for Enhanced X-ray Excited Optical Imaging. Chemistry of materials : a publication of the American Chemical Society. 2014;26:1881–1888. doi: 10.1021/cm404044n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villa I, et al. Size-dependent Luminescence in HfO2Nanocrystals: towards White Emission from Intrinsic Surface Defects. Chemistry of Materials. 2016 [Google Scholar]
- 38.Zorenko Y, et al. Luminescence properties of Y3Al5O12:Ce nanoceramics. Journal of Luminescence. 2011;131:17–21. [Google Scholar]
- 39.Geraki K, Farquharson MJ, Bradley DA. X-ray fluorescence and energy dispersive x-ray diffraction for the quantification of elemental concentrations in breast tissue. Physics in medicine and biology. 2004;49:99–110. doi: 10.1088/0031-9155/49/1/007. [DOI] [PubMed] [Google Scholar]
- 40.Margui E, Queralt I, Hidalgo M. Application of X-ray fluorescence spectrometry to determination and quantitation of metals in vegetal material. Trac-Trends in Analytical Chemistry. 2009;28:362–372. [Google Scholar]
- 41.Scofield JH. Radiative Decay Rates of Vacancies in the K and L Shells. Physical Review. 1969;179:9–16. [Google Scholar]
- 42.Wojtowicz AJ. Rare-earth-activated wide bandgap materials for scintillators. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2002;486:201–207. [Google Scholar]
- 43.Liu F, Cui Q, He J, Fei D. Preparation, Characterization and Ionizing Radiation Protection Properties of Electrospun Nanofibrous Mats Embedded with Erbium Oxide (Er2O3) Nanoparticles. Journal of Nano Research. 2014;27:121–127. [Google Scholar]
- 44.Tang R, Habimana-Griffin LM, Lane DD, Egbulefu C, Achilefu S. Nanophotosensitive drugs for light-based cancer therapy: what does the future hold? Nanomedicine. 2017;12:1101–1105. doi: 10.2217/nnm-2017-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamura Y, et al. Cerenkov Radiation-Induced Photoimmunotherapy with 18F-FDG. J Nucl Med. 2017;58:1395–1400. doi: 10.2967/jnumed.116.188789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hartl BA, Hirschberg H, Marcu L, Cherry SR. Activating Photodynamic Therapy in vitro with Cerenkov Radiation Generated from Yttrium-90. 2016;35:185–192. doi: 10.1615/JEnvironPatholToxicolOncol.2016016903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wall MA, et al. Chelator-Free Radiolabeling of SERRS Nanoparticles for Whole-Body PET and Intraoperative Raman Imaging. Theranostics. 2017;7:3068–3077. doi: 10.7150/thno.18019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deslattes RD, et al. National Institute of Standards and Technology, Gaithersburg, MD. 2005. [Google Scholar]
- 49.Pritychenko B, Běták E, Kellett MA, Singh B, Totans J. The Nuclear Science References (NSR) database and Web Retrieval System. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2011;640:213–218. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the plots within this paper and other finding of this study are available from the corresponding author upon reasonable request.