Abstract
Background
The aim of this study was to compare the morphological changes in cerebral and cerebellar gray matter in patients with essential tremor under 60-years-of-age, with age-matched and gender-matched normal healthy volunteer control subjects, using functional magnetic resonance imaging (fMRI) and voxel-based morphometry (VBM) analysis.
Material/Methods
A retrospective, controlled, comparative clinical study included 17 patients with essential tremor, <60 years-of-age, and 17 age-matched and gender-matched healthy volunteer control subjects, recruited between June 2010–July 2012. MRI and VBM analysis were used to compare cerebral and cerebellar gray matter density between groups. The Washington Heights–Inwood Genetic Study of Essential Tremor (WHIGET) rating scale was used to assess tremor severity in the patient group. Clinical and demographic characteristics were recorded for all study participants.
Results
MRI and VBM analysis showed significant bilateral expansion of the cerebellum, occipital fusiform cortices, right inferior temporal gyrus, and precentral lobes in patients with essential tremor (P<0.005); reduction in gray matter was found in the left parietal lobe. The region of interest (ROI) analysis showed volume enlargement in the thalamus, midbrain, and the precuneus (P<0.005). No significant correlation between changes in gray matter and changes in clinical variables, including age, gender, tremor duration, the activity of daily living (ADL) scale, the mini-mental state examination (MMSE) scale, family history, and tremor severity were found.
Conclusions
Predominantly cerebellar gray matter expansion in patients less than 60 years-of-age with essential tremor might be the result of compensation for the decline in cerebellar function.
MeSH Keywords: Essential Tremor, Magnetic Resonance Imaging, Young Adult
Background
Essential tremor is one of the most common neurological movement disorders, and the key features are postural and kinetic tremor of the upper limbs. Although essential tremor is a common condition, little is known about its pathophysiology. Recently, studies on the neuropathology of essential tremor have shown loss of Purkinje cells and an increase in axonal swellings (torpedoes) in the cerebellum, indicating that the cerebellum plays an important role in the etiology of essential tremor [1,2]. However, the published literature contains controversial opinions on the role of the cerebellum in essential tremor [3,4]. These alternative views on the role of the cerebellum have been supported in a previously published study using by magnetic resonance imaging (MRI) and morphological findings using voxel-based morphometry (VBM) [5]. However, Quattrone et al. found significant atrophy of the cerebellar vermis in patients with essential tremor that included arm and head tremor, but not in individuals who presented with arm tremor alone [6]. Benito-León et al. also showed a bilateral decrease in cerebellar gray matter volume in patients with essential tremor, but with no difference between individuals with only arm tremor and those with arm and head tremor [7]. Also, other studies have shown volumetric loss of gray matter in patients with essential tremor using VBM analysis [8,9]. However, Daniels et al. reported a bilateral expansion of gray matter in the superior temporal gyrus of patients with essential tremor with intention tremor and postural tremor when compared with normal controls [10].
The contradictory findings from previous studies on essential tremor may be due to factors associated with the patient selection. There is accumulating evidence that essential tremor is a heterogeneous disorder and should be considered as a group of disorders (essential tremors) rather than a single disorder (essential tremor) [11]. As a result, a general diagnosis of essential tremor might be inadequate to select and study cohorts when only imaging analysis is involved. Therefore, it may be important to screen for more subtle differences among patients, such as the patterns of tremor manifestation, the age of onset, and the history and severity of the condition.
Some previously published studies have supported that the age of onset of essential tremor might be an important marker of disease heterogeneity. Patients with essential tremor with an age of onset at >60 years progress much more rapidly, and have greater potential for disability and cognitive decline, compared with patients with essential tremor with a younger age of onset [12,13]. Also, symptoms such as ataxia, tandem gait, and balance problems, are much more common in patients with essential tremor with an older age of onset [14]. Patients with essential tremor with a younger age of onset tend to have a more benign tremor, fewer cerebellar symptoms, and less coexisting cognitive impairment, as well as a stronger family history of tremor [14,15].
Because there appear to be differences between patients with essential tremor of older onset, it would be important to study this older group separately. Also, age bias toward elderly subjects may have resulted in important clinical clues associated with essential tremor being missed in previous studies when compared with otherwise young individuals. Therefore, the aim of this study was to compare the morphological changes in cerebral and cerebellar gray matter in patients with essential tremor under 60 years-of-age, with age-matched and gender-matched normal healthy volunteer control subjects, using functional MRI (fMRI) and VBM analysis.
Material and Methods
Patients with essential tremor and healthy controls
Initially, 18 patients with essential tremor, who were <60 years-of-age, and 20 age-matched and gender-matched healthy volunteer control subjects, were recruited from June 2010–July 2012 in Shanxi Province, China. All patients met the diagnosis criteria of essential tremor, as defined by the Consensus Statement of the Movement Disorder Society on Tremor [16]. Two neurologists interviewed the patients independently and carefully recorded the following data: age, gender, number of years of education, the age of onset of tremor, duration of tremor (in years), on-medication or off-medication state, family history, relieving or aggravating factors, and sensitivity to alcohol.
The effect of the tremor on the ability to perform routine activities, and mental abilities in each subject were assessed using an activity of daily living (ADL) scale and the mini-mental state examination (MMSE) scale, respectively. All patients with arm tremor were examined using the Washington Heights-Inwood Genetic Study of Essential Tremor (WHIGET) rating scale, rather than the Fahn-Tolosa-Marin Tremor Rating Scale, to assess the severity of tremor (range, 0–3 for each test), and the total tremor score ranged from 0–36 [17]. Before recruitment into this study, thyroid function and ceruloplasmin levels were tested to ensure that the tremor was primary and not secondary. Patients with a history of dementia, stroke, epilepsy, head injury, neuropsychiatric diseases, or other movement disorders, except essential tremor, were excluded from the study.
This study included patients with essential tremor and healthy volunteer control subjects who were matched in age, gender, and education years. None of the healthy control subjects had a history of neurological disorders, for example, Parkinson’s disease, dementia, or a neurological diagnosis from a physician, and control subjects had no first-degree or second-degree relatives with essential tremor. All patients with essential tremor, and healthy volunteer control subjects were right-handed. All study participants signed written informed consents before participating in the study, which was approved by the local ethics committee.
Magnetic resonance imaging (MRI)
Magnetic resonance imaging (MRI) data were acquired using a GE Signa HDxt 3.0T scanner (GE Healthcare, Chicago, Il, USA) with an eight-channel head coil. An axial T1-weighted three-dimensional (3-D) fast spoiled gradient echo-sequence included: TR/TE=6.6/2.8 ms; TI=380 ms; field-of-view (FOV)=256×256 mm2; acquisition matrix=256×256; slice thickness=1 mm; spacing=0 mm; and flip angle=15°. T1-weighted MRI resulted in an isometric resolution of 1×1×1 mm3. A total of 176 contiguous slices were acquired and the transverse T2 fluid attenuation and inversion recovery (FLAIR) sequence was: TR/TE=9,100/165 ms; TI=2,250 ms; FOV=240×240 mm2; acquisition matrix=288×224; slice thickness=5 mm; and spacing=1.5 mm. Imaging acquisition was also acquired to exclude subjects with organic brain disease. Patients with structural brain abnormalities that affected the grey or white matter were excluded, prior to the voxel-based morphometry (VBM) analysis. MRI image interpretation, and image post-processing (see below), were performed by a radiologist who was blinded to the clinical diagnosis of each study participant.
Voxel-based morphometry (VBM) analysis
Structural data were analyzed using FSL-VBM 1.1 (Oxford University, Oxford, U.K), and VBM style analysis was carried out using FSL tools [18]. Firstly, Digital Imaging and Communications in Medicine (DICOM) files were completely converted to nii.gz format using the MRI-convert command. Structural images were brain-extracted using the BET tool in FSL software to remove skin and skull imaging data. Then, gray matter, white matter, and cerebrospinal fluid (CSF) were segmented using the FAST4 segmentation algorithm package. The resulting grey-matter partial volume images were aligned with MNI152 standard-space T1-weighted average structural template using the FMRIB’s Linear Image Registration Tool (FLIRT), followed optionally by nonlinear registration using FMRIB’s Non-Linear Image Registration Tool (FNIRT), which used B-splines representation of the registration warp fields.
The resulting images were averaged to create a study-specific template in which the native gray matter images were non-linearly re-registered. The registered partial-volume images were modulated by dividing them by the Jacobian of the warp field. The modulated segmented images were smoothed with an isotropic Gaussian kernel for threshold-free cluster enhancement (TFCE)-based analysis. Finally, the differences in cerebral gray matter density between the patient group and control group were assessed by a voxel-wise general linear model (GLM) using permutation-based non-parametric testing (5,000 permutations).
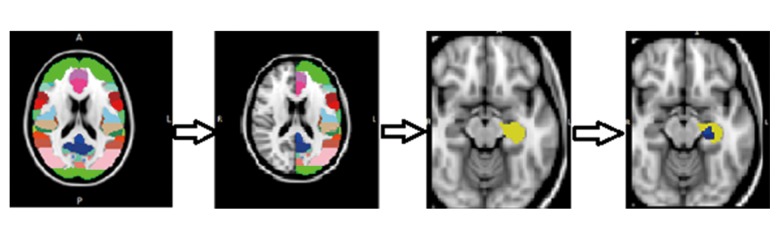
After whole-brain analysis, regions of interest (ROI) VBM analysis was performed. The ROI VBM is more sensitive than whole-brain VBM, which is used for testing a priori hypotheses [19]. According to previous studies, the thalamus, caudate, putamen, pallidum, brainstem, insula, precuneus, and middle frontal lobe were chosen as ROIs using the Harvard-Oxford Subcortical Structural Atlas [6,7,20]. Briefly, after identifying eight ROIs of different intensities, the whole brain was split into two halves to analyze the right or left side of ROIs separately. The specific ROI was then chosen as new region mask. For example, the left side of the insula was chosen as one ROI, and VBM analysis was performed. Analysis of the eight ROI VBM was then carried out sequentially, one after the other (Figure 1).
Figure 1.
The procedure of voxel-based morphometry (VBM) magnetic resonance imaging (MRI) analysis of the region of interest (ROI). First different regions of the brain are shown, according to the Harvard-Oxford subcortical structural atlas, divided into two sides. The region of interest (ROI) was chosen, and ROI voxel-based morphometry (VBM) analysis was done. As an example, the insula was chosen as one ROI, and the new mask is shown in yellow. ROI VBM analysis resulting in the area in blue, as shown, which meant that there was a reduced volume in patients with essential tremor compared with healthy volunteer control subjects.
Statistical analysis
Data were analyzed using SPSS version 19.0 (SPSS Inc, Chicago, IL, USA). The χ2 test was used to compare the sex distribution between patients with essential tremor and healthy volunteer control subjects. The t-test of two independent-samples was performed to compare differences in age, education years, ADL scores, and MMSE scores between the two groups. The primary comparative analysis maps of gray matter between patients with essential tremor and healthy volunteer control subjects were performed, on a voxel-by-voxel basis, using FSL-VBM and a two-sample t-test. The relationship between regional brain changes and clinical variables were examined, including age, gender, age at onset, tremor duration, education years, ADL scores, MMSE scores, family history, and tremor severity, using a separate analysis within the essential tremor patient group. Regions were traced manually, which indicated significant differences between groups using FSLView. The mean voxel value of each region was extracted using MATLAB 6.1. Linear regression analysis was performed between mean values and clinical variables. A P-value <0.05 was considered to be statistically significant.
Results
Clinical findings
From the initial subjects enrolled in this study, one patient with essential tremor, and three healthy volunteers were excluded from the analysis due to abnormal brain lesions detected on T2 fluid attenuation and inversion recovery (FLAIR) sequence magnetic resonance imaging (MRI). No data were discarded because of image artifacts.
The final number of eligible subjects included in the study was 17 patients with essential tremor and 17 healthy volunteer control subjects. Two patients with essential tremor had taken medication (propranolol) for a very short time and stopped taking this medication two weeks before the study. Eight patients with essential tremor did not drink alcohol, and four of the remaining nine patients did drink alcohol, none drank alcohol one week before the study.
All patients had symmetrical postural and dynamic tremor with no resting tremor. To evaluate arm tremor, the Washington Heights–Inwood Genetic Study of Essential Tremor (WHIGET) rating scale was used. The mean total tremor score was 14.35±7.43, with one patient’s score >30 (tremor score=33). There was no difference in the activity of daily living (ADL) and the mini-mental state examination (MMSE) scores between patients with essential tremor and control subjects (Table 1). There were no significant differences in age, gender, education duration, cognitive function, and daily living ability between the control group and patients with essential tremor, which were comparable between the two groups.
Table 1.
Demographic and clinical features of ET patients and HVs.
| HVs (n=17) | ET Patients (n=17) | Statistic results (P, t, and P, χ2) | |
|---|---|---|---|
| Age, y | 42.24±9.47 | 39.65±8.12 | 0.40 (t=0.86) |
| Sex, F/M | 10: 7 | 9: 8 | 0.73(χ2=0.12) |
| Family history (Y/N) | – | 10: 7 | – |
| Handedness(R) | 17 | 17 | – |
| Age at onset (y) | – | 27.53±13.00 | – |
| Duration of ET (y) | – | 12.71±7.55 | – |
| Education years | 12.35±3.77 | 12.47±3.52 | 0.93 (t=−0.09) |
| WHIGET | – | 14.35±7.43 | – |
| ADL | 20±0.00 | 20.53±1.07 | 0.06 (t=−2.05) |
| MMSE | 29.24±1.03 | 28.18±2.27 | 0.09 (t=1.75) |
Voxel-based morphometry (VBM) analysis
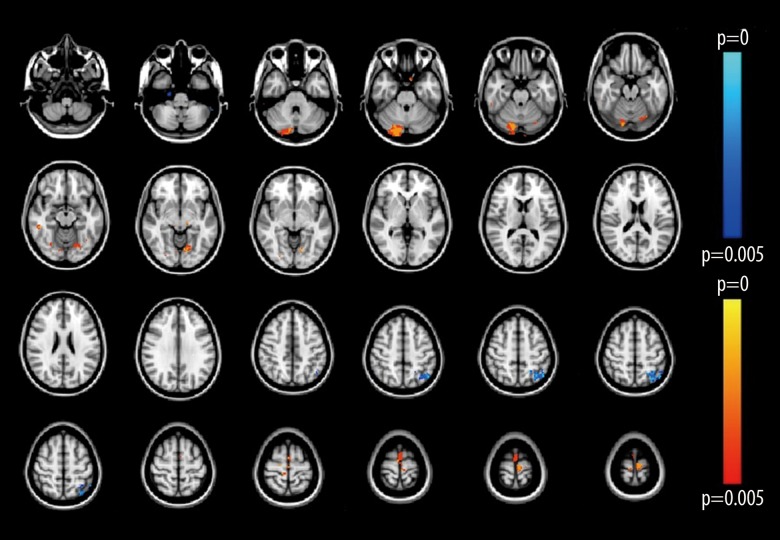
Optimized voxel-based morphometry (VBM) analysis was used to compare the differences between the patients with essential tremor and healthy volunteers and showed several significant differences in gray matter volume between the two groups. Reduced gray matter volume was detected only in the left parietal lobe; significantly increased gray matter volume was detected bilaterally in the cerebellum, precentral lobe, right inferior temporal gyrus, left temporal occipital fusiform, and the right occipital fusiform gyrus, in patients (Puncorrected<0.005) (Table 2; Figure 2). VBM analysis showed that there was a difference in gray matter structure between patients with essential tremor and the normal healthy controls, indicating that there was a structural change in essential tremor.
Table 2.
Voxel-based morphometric differences between ET patients and HVs.
| Structure name | Voxels | Z-MAX | X | Y | Z |
|---|---|---|---|---|---|
| Right CrusII* | 540 | 0.998 | 12 | −86 | −34 |
| Left Crus II* | 10 | 0.998 | −2 | −82 | −38 |
| Cerebellar left VI* | 192 | 0.998 | −28 | −72 | −24 |
| Precentral gyrus* | 257 | 0.998 | 00 | −18 | 66 |
| Right inferior temporal gyrus* | 35 | 0.998 | 54 | −36 | −16 |
| Left temporal occipital fusiform* | 19 | 0.998 | −38 | −60 | −14 |
| Right occipital fusiform gyrus* | 11 | 0.996 | 28 | −70 | −16 |
| Left parietal lobe** | 47 | 0.998 | −30 | −68 | 52 |
The areas of expansion in ET patients compared to HV subjects.
The area of reduction in ET patients compared to HV subjects.
All the areas were significant at P<0.005, uncorrected at a TFCE-based result. Local maxima were reported, including cluster size and anatomical region. Locations for statistical findings were reported in the standard Montreal Neurological Institute coordinate space (x, y, and z).
Figure 2.
A voxel-based statistical map was created that identified differences in gray matter between the two study groups. The red color shows areas of grey matter expansion, while the blue color shows areas of grey matter reduction, in patients with essential tremor compared with healthy volunteer control subjects (P<0.005).
Voxel-based morphometry (VBM) in regions of interest (ROIs)
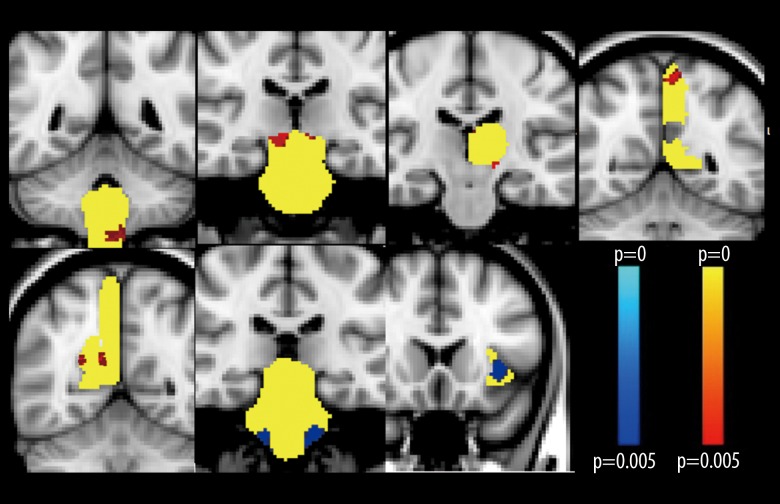
Regions of interest (ROIs) were applied for further comparison between the essential tremor group and healthy volunteers and showed no significant difference in the putamen, pallidum, and middle frontal lobe between the two groups. However, there was a significant volume increase in the midbrain, medulla, left thalamus, and bilateral precuneus lobes in the essential tremor group when compared with the healthy volunteer group. Reduced amounts of gray matter were found in the left insula and pons in the essential tremor patient group compared with the normal healthy volunteer group. The ROIs and their x, y, and z coordinates are shown in Table 3 and Figure 3. The results showed that local volume enlargement might be associated with functional or compensatory change, whereas the decreased left insula volume might be linked to cognitive decline in clinically identified patients with essential tremor.
Table 3.
Voxel-based morphometric differences between ET patients and HVs subjects in ROIs.
| Structure name | Voxels | Z-MAX | Coordinates | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Left thalamus* | 16 | 0.996 | −14 | −22 | −6 |
| Right precuneus* | 10 | 0.998 | 12 | −56 | 22 |
| 8 | 0.998 | 24 | −58 | 20 | |
| Left precuneus* | 22 | 0.998 | −8 | −54 | 62 |
| Brainstem(midbrain)* | 103 | 0.998 | 10 | −24 | −12 |
| Brainstem(medulla oblongata)* | 5 | 0.996 | −8 | −48 | −62 |
| Brainstem(pons)** | 5 | 0.996 | −12 | −28 | −44 |
| Left insula** | 43 | 0.998 | −36 | 18 | −2 |
The expanded areas in ET patients compared to HV subjects.
The reduced areas in ET patients compared to HV subjects.
All the areas were significant at P<0.005, uncorrected at a TFCE based result. Local maxima were reported, including cluster size and anatomical region. Locations for statistical findings were reported in the standard Montreal Neurological Institute coordinate space (x, y, and z).
Figure 3.
The volume of gray matter of the region of interest (ROI) was compared between patients with essential tremor and the healthy volunteer control group. Coronal images were taken for each abnormal region of interest (ROI). The corresponding ROI is yellow; the red color shows areas of grey matter expansion; the blue color shows areas of grey matter reduction, in patients with essential tremor compared with healthy volunteer control subjects (P<0.005).
Clinical variables and changes in gray matter
No significant differences or correlations were found between the clinical variables in the patients and the control group, including age, gender, the age of onset of tremor, tremor duration, education years, ADL score, MMSE score, family history, and gray matter changes. Although tremor duration was positively correlated with precentral lobe expansion, the difference was not statistically significant (r=0.47, P=0.056). There was an association between tremor duration and precentral lobe gray matter volume increase, although this was not statistically significant. It is possible to speculate that if these findings represent a trend, the volume increase in the cerebellar gray matter might be an early change associated with essential tremor, while other changes could arise secondarily.
Discussion
In this study, functional magnetic resonance imaging (fMRI) using voxel-based morphometry (VBM) analysis and the FSL-VBM method were used to detect the cerebral and cerebellar morphological changes associated with an essential tremor of the arm in patients under 65 years-of-age. The findings of this study showed the bilateral increased volume of gray matter in the cerebellum. To our knowledge, this is the first study to have reported these findings in patients with essential tremor in this age group.
Structural abnormalities, including volumetric loss of gray matter in the cerebellum of patients with essential tremor, are the most prevalent findings in previously published structural neuroimaging studies [6,7,20]. These findings are supported by the findings from a post-mortem study, in which Louis et al. described the presence of cerebellar Purkinje cells loss in patients with essential tremor [1]. Several recent resting-state fMRI studies have also shown decreased functional connectivity within the cerebello-thalamo-cortical network in patients with essential tremor [21,22]. Also, the wide spectrum of clinical features of essential tremor, including intentional tremor, gait and balance abnormalities, oculomotor abnormalities, have suggested a relationship between dysfunction of the cerebellum, cerebellar blood flow, and essential tremor [23]. A previously published study that included positron emission tomography (PET) imaging showed that regional cerebral blood flow in the bilateral cerebellum was increased in patients with essential tremor during rest and tremor [5]. Clinical observations have reported that when a stroke has occurred in the cerebellum or has involved the efferent pathways of the cerebellum, essential tremor is eradicated on the affected side [24]. These observations might indicate that cerebellar overactivity, rather than dysfunction, might be the trigger for essential tremor.
The cerebellum and the cerebellar neural networks play a key role in the pathophysiology of essential tremor, and the activation of different structures along the neural pathway might lead to in different outcomes [25]. The main cerebellar efferent pathway, which is known as the dentate-thalamo-cortical tract, runs from the dentate nucleus to the ventral lateral nucleus in the thalamus and the contralateral motor cortex, and has a facilitatory effect on the thalamus and motor cortex. If the dentate nucleus is over-activated, the thalamus and the motor cortex activity will increase and could potentially induce tremor. However, Purkinje cells located in the cerebellar cortex have an inhibitory effect on the dentate nucleus. Loss of Purkinje cells reduce the inhibitory effect and will be linked to the over-activity of the cerebellum dentate nucleus, resulting in tremor [5]. Therefore, it is important to make clear whether essential tremor affects the cerebellar cortex or selectively affects the deep cerebellar nuclei.
The findings of this study showed that in patients with essential tremor compared with healthy volunteer control subjects, it was the cerebellar cortex, rather than the deep cerebellar nuclei that showed a volume increase. This finding, together with the findings from previously published studies, might make it reasonable to speculate that the increase in volume in the cerebellar cortex is a compensatory mechanism to for the effects of cerebellar dysfunction, particularly at the early stage of essential tremor. Because the patients recruited into the present study were less than 65 years, which is younger than in most studies, they would be expected to progress more slowly, and compensatory mechanisms should be more effective [15]. This finding also explains why no increase in volume was found in previous studies on patients with essential tremor when study groups were mixed as to the age of onset. Essential tremor can be regarded as a neurodegenerative disease and follows a gradual, yet progressive, clinical course [26]. At the most severe clinical stage of essential tremor, compensatory mechanisms will decline with time, and by the time that the volume of cerebellar gray matter has decreased, cerebral atrophy may be seen, which may explain the pathology and neuroimaging findings in previously published studies [1,26,7,20].
From the findings of the present study, further support for a compensation hypothesis of essential tremor was the enlargement of the thalamus seen in the ROI results. The thalamus plays an important role in the oscillating central network of essential tremor, as shown by deep brain stimulation (DBS) and its suppressive effect on the activity of the thalamus, which is an effective treatment for essential tremor [27]. According to a previously published study, afferent projections from the cerebellar dentate nucleus to thalamus are excitatory [25]. If increased cerebellar gray matter volume results in functional enhancement, the inhibitory output of the cerebellar cortex (mainly from Purkinje cells) will increase, leading to a decrease in thalamic activation, which is inconsistent with clinical findings. Therefore, it must be assumed that enlargement of the cerebellar cortex results from the reduced function. However, although it remains unclear why the enlargement was only found the left side of the cerebellar cortex, it should be noted that this finding may be due to the right-handedness of all the study participants, or to left parietal reduction.
The findings of this study also showed an increase in gray matter volume in the right inferior temporal gyrus, the left temporal occipital fusiform gyrus, and the right occipital fusiform gyrus, which are supported by previously published findings [10]. Multiple brain regions can exhibit plasticity following conditioning, and temporal and occipital lobes are mainly associated with auditory and visual function [28]. To co-ordinate the fine movements of patients with essential tremor with arm tremor, the brain needs to perform additional mechanisms of control and so the results of this study might represent adaptive reorganization and compensation through increased demands on the visuospatial control of skilled movements in patients with essential tremor. This view is supported by the findings of the study by Tuleasca et al. that included a one-year follow-up of ventral intermediate nucleus (VIM) radiosurgery for essential tremor with voxel-based morphometry before and after treatment, which showed a significant change in gray matter volume in the left temporal pole and occipital cortex of the brain before and after treatment, resulting in the conclusion that visual areas might be linked with tremor arrest and be involved in the tremor loop [29].
Contrary to previous studies [6,7], the only decrease in gray matter volume that was detected in the present study, by whole brain VBM analysis, was in the left parietal lobe. This finding may be due to the enrollment of younger patients in this study, many of whom were in the early stages of the disease. It is now clear that cerebellar output is connected to more widespread regions of the cerebral cortex than previously thought, including regions of higher functional relevance, such as the prefrontal and posterior parietal cortex [30]. The decrease in gray matter in the posterior parietal lobe may be the result of cerebellum dysfunction and may represent the diminished capacity of spatial orientation and proprioceptive sensation in patients with an essential tremor that includes arm tremor [31].
This study had several limitations. To minimize the heterogeneity bias of essential tremor, recruitment involved only middle-aged patients with isolated arm tremor, and so the results do not reflect all phenotypes of essential tremor. Also, this study was a cross-sectional study, and it was not possible to conclude whether the cerebellum or other sites of brain morphological changes were primary or secondary changes. Because the study was of short duration, the patients should undergo further follow-up to observe the long-term changes in the brain gray matter. Finally, multiple functional imaging techniques should be used to confirm the findings of this study.
Conclusions
Patients with an essential tremor occurring at a younger age, of less than 65 years, with tremor involving only the arm, showed unique brain morphological patterns with characteristic changes in the gray matter, including expanded and reduced areas. The study findings further support the view that the cerebello-thalamo-cortical network is involved in essential tremor, and that the cerebellum is a critical hub for the activity of this network.
Acknowledgments
The authors appreciate Dr. Haubenberger, Dr. Dongying Ma, Dr. Xiao Song, and Dr. Devee for providing comments and revision of the manuscript.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by the Natural Science Foundation of Shanxi Province (No. 2013JM4014)
References
- 1.Louis ED. Essential tremor: Evolving clinicopathological concepts in an era of intensive post-mortem enquiry. Lancet Neurology. 2010;9:613–22. doi: 10.1016/S1474-4422(10)70090-9. [DOI] [PubMed] [Google Scholar]
- 2.Shill HA, Adler CH, Beach TG. Pathology in essential tremor. Parkinsonism Relat Disord. 2012;18(S1):S135–37. doi: 10.1016/S1353-8020(11)70042-6. [DOI] [PubMed] [Google Scholar]
- 3.Rajput AH, Robinson CA, Rajput ML, Rajput A. Cerebellar Purkinje cell loss is not pathognomonic of essential tremor. Parkinsonism Relat Disord. 2011;17:16–21. doi: 10.1016/j.parkreldis.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Rajput AH, Robinson CA, Rajput ML, et al. Essential tremor is not dependent upon cerebellar Purkinje cell loss. Parkinsonism Relat Disord. 2012;18:626–28. doi: 10.1016/j.parkreldis.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Cerasa A, Quattrone A. Linking essential tremor to the cerebellum – neuroimaging evidence. Cerebellum. 2016;15:263–75. doi: 10.1007/s12311-015-0739-8. [DOI] [PubMed] [Google Scholar]
- 6.Quattrone A, Cerasa A, Messina D, et al. Essential head tremor is associated with cerebellar vermis atrophy: A volumetric and voxel-based morphometry MR imaging study. Am J Neuroradiol. 2008;29:1692–97. doi: 10.3174/ajnr.A1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benito-Leon J, Alvarez-Linera J, Hernandez-Tamames JA, et al. Brain structural changes in essential tremor: voxel-based morphometry at 3-Tesla. J Neurolog Sci. 2009;287:138–42. doi: 10.1016/j.jns.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 8.Klein JC, Lorenz B, Kang JS, et al. Diffusion tensor imaging of white matter involvement in essential tremor. Hum Brain Mapp. 2011;32:896–904. doi: 10.1002/hbm.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang W, Lv F, Luo T, et al. Abnormal regional homogeneity in patients with essential tremor revealed by resting-state functional MRI. PLoS One. 2013;8:e69199. doi: 10.1371/journal.pone.0069199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniels C, Peller M, Wolff S, et al. Voxel-based morphometry shows no decreases in cerebellar gray matter volume in essential tremor. Neurology. 2006;67:1452–56. doi: 10.1212/01.wnl.0000240130.94408.99. [DOI] [PubMed] [Google Scholar]
- 11.Louis ED. ‘Essential tremor’ or ‘the essential tremors’: Is this one disease or a family of diseases? Neuroepidemiol. 2014;42:81–89. doi: 10.1159/000356351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louis ED, Ford B, Barnes LF. Clinical subtypes of essential tremor. Arch Neurol. 2000;57:1194–98. doi: 10.1001/archneur.57.8.1194. [DOI] [PubMed] [Google Scholar]
- 13.Louis ED, Faust PL, Vonsattel JP, et al. Older onset essential tremor: More rapid progression and more degenerative pathology. Mov Disord. 2009;24:1606–12. doi: 10.1002/mds.22570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chunling W, Zheng X. Review on clinical update of essential tremor. Neurolog Sci. 2016;37:495–502. doi: 10.1007/s10072-015-2380-1. [DOI] [PubMed] [Google Scholar]
- 15.Deuschl G, Petersen I, Lorenz D, Christensen K. Tremor in the elderly: Essential and aging-related tremor. Mov Disord. 2015;30:1327–34. doi: 10.1002/mds.26265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13(Suppl 3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 17.Louis ED, Ottman R, Ford B, et al. The Washington Heights-Inwood Genetic Study of Essential Tremor: Methodologic issues in essential-tremor research. Neuroepidemiol. 1997;16:124–33. doi: 10.1159/000109681. [DOI] [PubMed] [Google Scholar]
- 18.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(S1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 19.Seidman LJ, Biederman J, Liang L, et al. Gray matter alterations in adults with attention-deficit/hyperactivity disorder identified by voxel based morphometry. Biol Psych. 2011;69:857–66. doi: 10.1016/j.biopsych.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin H, Lee DK, Lee JM, et al. Atrophy of the cerebellar vermis in essential tremor: Segmental volumetric MRI analysis. Cerebellum. 2016;15(2):174–81. doi: 10.1007/s12311-015-0682-8. [DOI] [PubMed] [Google Scholar]
- 21.Popa T, Russo M, Vidailhet M, et al. Cerebellar rTMS stimulation may induce prolonged clinical benefits in essential tremor, and subjacent changes in functional connectivity: An open label trial. Brain Stim. 2013;6:175–79. doi: 10.1016/j.brs.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Benito-Leon J, Louis ED, Romero JP, et al. Altered functional connectivity in essential tremor: A resting-state fMRI study. Medicine. 2015;94:e1936. doi: 10.1097/MD.0000000000001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benito-Leon J, Labiano-Fontcuberta A. Linking essential tremor to the cerebellum: Clinical evidence. Cerebellum. 2016;15:253–62. doi: 10.1007/s12311-015-0741-1. [DOI] [PubMed] [Google Scholar]
- 24.Dupuis MJE, Jacquerye FL, Picard PG, et al. Disappearance of essential tremor after stroke. Mov Disord. 2010;25:2884–87. doi: 10.1002/mds.23328. [DOI] [PubMed] [Google Scholar]
- 25.Lozano AM, Hallett M, editors. Brain Stimulation. Handbook of Clinical Neurology. 2013;116:11. doi: 10.1016/B978-0-444-53497-2.09986-1. [DOI] [PubMed] [Google Scholar]
- 26.Benito-Leon J. Essential tremor: A neurodegenerative disease? Tremor Other Hyperkinet Mov. 2014;4:252. doi: 10.7916/D8765CG0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flora ED, Perera CL, Cameron AL, Maddern GJ. Deep brain stimulation for essential tremor: A systematic review. Mov Disord. 2010;25:1550–59. doi: 10.1002/mds.23195. [DOI] [PubMed] [Google Scholar]
- 28.Ditye T, Kanai R, Bahrami B, et al. Rapid changes in brain structure predict improvements induced by perceptual learning. Neuroimage. 2013;81:205–12. doi: 10.1016/j.neuroimage.2013.05.058. [DOI] [PubMed] [Google Scholar]
- 29.Tuleasca C, Witjas T, Najdenovska E, et al. Assessing the clinical outcome of Vim radiosurgery with voxel-based morphometry: Visual areas are linked with tremor arrest! Acta Neurochirurgica. 2017;159(11):2139–44. doi: 10.1007/s00701-017-3317-7. [DOI] [PubMed] [Google Scholar]
- 30.Habas C, Kamdar N, Nguyen D, et al. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–94. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darling WG, Bartelt R, Rizzo M. Unilateral posterior parietal lobe lesions disrupt kinaesthetic representation of forearm orientation. Neurol Neurosurg Psychiatry. 2004;75:428–35. doi: 10.1136/jnnp.2003.026138. [DOI] [PMC free article] [PubMed] [Google Scholar]