Abstract
Developing non-invasive brain stimulation interventions to improve attentional control is extremely relevant to a variety of neurologic and psychiatric populations, yet few studies have identified reliable biomarkers that can be readily modified to improve attentional control. One potential biomarker of attention is functional connectivity in the core cortical network supporting attention - the dorsal attention network (DAN). We used a network-targeted cerebellar transcranial magnetic stimulation (TMS) procedure, intended to enhance cortical functional connectivity in the DAN. Specifically, in healthy young adults we administered intermittent theta burst TMS (iTBS) to the midline cerebellar node of the DAN and, as a control, the right cerebellar node of the default mode network (DMN). These cerebellar targets were localized using individual resting-state fMRI scans. Participants completed assessments of both sustained (gradual onset continuous performance task, gradCPT) and transient attentional control (attentional blink) immediately before and after stimulation, in two sessions (cerebellar DAN and DMN). Following cerebellar DAN stimulation, participants had significantly fewer attentional lapses (lower commission error rates) on the gradCPT. In contrast, stimulation to the cerebellar DMN did not affect gradCPT performance. Further, in the DAN condition, individuals with worse baseline gradCPT performance showed the greatest enhancement in gradCPT performance. These results suggest that temporarily increasing functional connectivity in the DAN via network-targeted cerebellar stimulation can enhance sustained attention, particularly in those with poor baseline performance. With regard to transient attention, TMS stimulation improved attentional blink performance across both stimulation sites, suggesting increasing functional connectivity in both networks can enhance this aspect of attention. These findings have important implications for intervention applications of TMS and theoretical models of functional connectivity.
Introduction
Attentional control, or the ability to select and maintain task-relevant information processing, is critically important for many essential activities of daily life, from effectively accomplishing work/school activities (Kalechstein, Newton, & van Gorp, 2003; Lam & Beale, 1991) to driver safety (Ball, Owsley, & Sloane, 1991; Edkins & Pollock, 1997; Schmidt et al., 2009), and is impaired in a wide range of neurological and psychiatric populations. As attention underlies many other higher-level cognitive functions, attentional impairments can result in deficits of diverse cognitive functions (Fortenbaugh et al., in press). Thus, improvement of attentional control has widespread clinical appeal.
Converging evidence directly implicates a set of frontal and parietal regions, termed the dorsal attention network (DAN), in attentional control (i.e., preparing, initiating, and maintaining goal-directed attentional control; e.g., Corbetta et al., 2000; Kastner, Pinsk, De Weerd, Desimone, & Ungerleider, 1999; Langner & Eickhoff, 2013; Fortenbaugh et al. in press). Recently, the DAN has been shown to include both cortical and cerebellar nodes (Brissenden, Levin, Osher, Halko, & Somers, 2016). In contrast, during active engagement of the DAN, the medial prefrontal cortex, the precuneus and lateral inferior parietal regions, known as the default mode network (DMN), are commonly found to be deactivated (Buckner, Andrews-Hanna, & Schacter, 2008; Raichle et al., 2001; Ralchle & Snyder, 2007). Thus, there is evidence that successful completion of attentional tasks requires some form of upregulation of the DAN and concomitant responses within the DMN.
Functional connectivity (FC) in the DAN is also related to attentional control. Specifically, increased FC within the DAN at rest corresponds with improved task performance (Hampson, Driesen, Skudlarski, Gore, & Constable, 2006). Further, when attention is engaged, it dynamically modifies FC in the DAN. For example, studies have found greater within-DAN FC during the performance of a sustained attention task compared to rest (Bray, Arnold, Levy, & Iaria, 2015). Critically, increased connectivity within the DAN, especially between cerebellar and cortical network nodes, corresponds to increased activation during multiple attentional tasks, suggesting a clear link between connectivity and cognitive performance (Brissenden, et al 2016). In addition, greater resting connectivity between DAN and DMN is associated with poor attentional control and greater distractibility (e.g., Poole et al., 2016). In contrast, within-DMN FC has been less consistently linked with attention (Barber et al., 2015; Bonnelle et al., 2011). Together, FC data suggests that internal communication within the DAN, as well as distinctiveness from task-negative DMN, are reliable markers associated with better performance during tasks requiring voluntary attentional control.
Transcranial magnetic stimulation (TMS), a non-invasive intervention, might have the potential to enhance attention or ameliorate attention deficits due to its unique ability to modulate both brain activity and connectivity (Walsh & Pascual-Leone, 2003; Eldaief, Halko, Buckner, & Pascual-Leone, 2011; Halko, Farzan, Eldaief, Schmahmann, & Pascual-Leone, 2014; Wang et al., 2014; Wang & Voss, 2015). Specifically, a key feature of TMS is that the effects are not limited to the stimulated region, but are further distributed to anatomically-connected sites (Ilmoniemi et al., 1997; Bohnin et al., 1999; Morishima et al., 2008). These distributed effects of TMS, achieved through trans-synaptic activation (Amassian et al., 1990; Paus et al., 1997), can induce changes in functional connectivity (FC) between regions that include both the stimulated region (Eldaief et al., 2011; Halko et al., 2010; Wang et al., 2014; Wang & Voss, 2015), and its connected networks (Grosbras & Paus, 2002; Taylor et al., 2006; Silvanto et al., 2006; Ruff et al., 2006; Halko et al., 2014). Thus, effective therapeutic TMS interventions must rely upon an understanding of the targeted network, distributed effects (Durbin et al., 2017; Drysdale et al., 2017), as well as the state of the stimulated neuronal population (Silvanto & Muggelton, 2008; Miniussi et al., 2010).
TMS applied to regions of the DAN, such as the frontal eye field (FEF) and other frontal-parietal regions, has shown to decrease performance of orienting, distractor suppression, visual detection, and sustained attention (Chambers & Mattingley, 2005; Capotosto et al., 2012; Esterman et al., 2015). Stimulation of the FEF has also been found to increase BOLD responses in the distal, but connected visual cortex, improving visual detection and an enhanced excitability of visual areas (Grosbras & Paus, 2002; Taylor et al., 2006; Silvanto et al., 2006; Ruff et al., 2006). In contrast to the DAN, no work to our knowledge has considered the effects of DMN stimulation on attentional performance; this may partially be due to the inaccessibility of the core cortical regions of this network. Notably, an increase in the resting FC within the DAN and the DMN occurred after stimulation of distinctly localizable regions of the cerebellum (Halko et al., 2014), although this study did not measure cognition. Specifically, intermittent theta burst TMS (iTBS) increased resting FC within the DAN or within the DMN (Halko et al., 2014) after stimulation that targeted midline and lateral regions of the cerebellum respectively, regions exhibiting functional connectivity with cortical DAN and DMN regions. This stimulation of the cerebellum could induce these network-specific modulations by virtue of an indirect connection to brain networks via the thalamus (Schmahmann & Pandya, 1997; Strick, Dum, & Fiez, 2009) which can be observed with resting state FC (Buckner, Krienen, Castellanos, Diaz, & Yeo, 2011).
As attentional performance has been associated with FC within the DAN (Hampson, Driesen, Skudlarski, Gore, & Constable, 2006; Bray, Arnold, Levy, & Iaria, 2015; Brissenden, et al. 2016), we examined whether TMS applied to the cerebellar node of the DAN could enhance attention. As attentional performance is less associated with FC within the DMN, we contrasted cerebellar DAN stimulation to cerebellar DMN stimulation (as an active control). Specifically, we compared performance on a sustained attention task, the gradual onset continuous performance task (gradCPT), and a transient attention task, the attentional blink task pre/post iTBS. ITBS was applied at the midline cerebellar node of the DAN and the right cerebellar node of the DMN. We accessed the DAN/DMN via a network-based localization method (see localization approach in method section). Behavioral blocks preceding cerebellar-iTBS served as baseline performance measures. Given the importance of DAN activity and FC for sustained and selective aspects of attention, we hypothesized that stimulation of the DAN cerebellar node would improve attentional performance. Specifically, following cerebellar DAN stimulation, we predicted 1) reduced commission errors on the gradCPT, particularly while in the zone (Esterman et al., 2015), 2) enhanced ability to overcome the attentional blink as measured by fewer errors in reporting the second target, and 3) absence of attentional modulation following DMN cerebellar stimulation.
Method
Participants
Fifteen healthy participants (11 males, mean age = 22.27, SD = 3.69) were recruited from Northeastern University and Boston University. All participants met the screening criteria for TMS (Rossi, Hallett, Rossini, & Pascual-Leone, 2009) and reported to be free of neurologic and psychiatric conditions. Subjects gave informed consent and the study was approved by the VA Boston Healthcare System IRB.
Overall Experimental Procedure
Using a within-subjects cross-over design, participants completed two 1–2 h TMS sessions separated by a washout period of 48 hours to 2 weeks. Participants received stimulation to two different cerebellar sites (Figure 1; based on DAN or DMN connectivity, see Localization below) with order randomly counterbalanced across participants. Note the current study considered DMN an active control, which has distinct advantages to using sham (Duecker & Sack 2015).
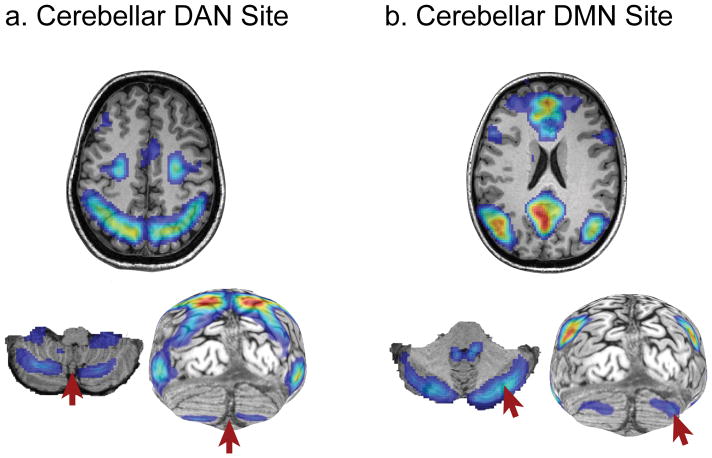
Figure 1.
TMS targets (arrows) localized using individual resting-state cortical functional connectivity (left) with a) cortical dorsal attention network (DAN) and b) default mode network (DMN).
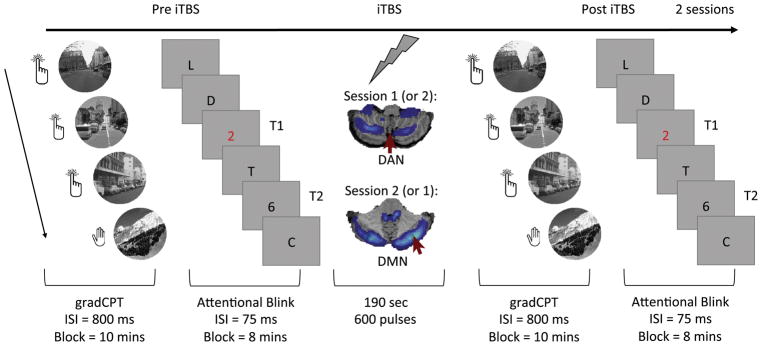
At both sessions, immediately pre- and post- TMS, participants completed 1) the gradual onset continuous performance task (gradCPT), a go/no-go sustained attention task, and 2) the attentional blink task, a measure of selective attention. GradCPT was always performed before the attentional blink task (Figure 2). The average time between gradCPT performance before and after stimulation was 35.21 min (SD = 5.03) for DAN sessions and 37.93 min (SD = 6.43) for DMN sessions, p >.34.
Figure 2.
A schematic of the testing session. Two sessions were conducted in each participant, with the order of stimulation sites counterbalanced (DAN vs. DMN stimulation).
Localization of TMS
Prior to the TMS session, each participant completed a T1-weighted structural (MPRAGE) and two resting-state fMRI scans on a 3T Siemens Trio Scanner with a 32-channel head coil at the Neuroimaging Research Center for Veterans (NeRVe) at the VA Boston Healthcare System. For 12 subjects, functional runs included 300 whole-brain volumes acquired using an echoplanar imaging sequence with the following parameters: TR = 1050 ms, TE = 34.8 ms, flip angle = 65°, acquisition matrix = 104×104, in-plane resolution = 2.0 mm2, 72 oblique slices, voxel size = 2 mm3. Two participants did not undergo multiband scans; instead functional scans were acquired with the following parameters: TR = 2000 ms, TE = 30 ms, flip angle = 90°, acquisition matrix = 64 × 64, in-plane resolution = 3.0 mm2, 33 oblique slices, voxel size = 3 mm3. MPRAGE parameters were as follows: TE = 3.32, TR = 2530 ms, flip angle = 7°, acquisition matrix = 256×256, in-plane resolution = 1.0 mm2, 176 sagittal slices, slice thickness = 1.0 mm.
We selected two targets in the cerebellum, based on functional connectivity (FC) with the cortical dorsal attention (DAN) and default mode network (DMN) for each individual subject. The cerebellar targets were functionally localized using individual resting-state MRI scans by seeding the cortical DAN and DMN respectively (using parcellation from Yeo et al., 2011; see Figure 1). For each network, TMS targets were defined as the peak activation of either the DAN or DMN cerebellar nodes as follows. The “cerebellar DAN” target was placed in the hotspot in the midline cerebellum (see Fig 1a). Modeling of the optimal location for placing a coil on the cerebellum to impact the DAN nodes have shown that this midline position represents the ideal location relative to any other position upon the posterior skull (Halko, Dannhauer and Brooks, personal communication; Brissenden et al., 2016). Although bilateral, the “cerebellar DMN” target was placed in the right lateral cerebellum (see Fig 1b), as the cerebellar connections decussate to the other hemisphere cortically and the DMN is left hemisphere dominant (Halko et al., 2014). The average error between the TMS trajectory and the fMRI-localized target was .69 mm (range: 0.37–1.40; SD = .37) for the DAN target and .73 mm (range: 0.34–2.47; SD = .61) for the DMN target; thus TMS was delivered fairly accurately to the selected target regions. Nevertheless, taking into account that TMS is mostly used to investigate regions close to the cortical surface (Barker, 1999) and that this error could be meaningful, we decided to control for stimulation error from the optimal target position as a covariate in our analyses.
Transcranial Magnetic Stimulation
A Magstim Super Rapid Plus with a D702 figure-eight coil was used to administer the TMS pulses. Brainsight 2 system and software were used for neuronavigation (Rogue-Research Inc., Montreal, Canada).
Before the main experiment, active (AMT) and resting motor threshold (RMT) were determined by the minimum amount of stimulator output needed to observe a motor evoked potential (MEP) of at least 50 mv for 5/10 single pulses in 1 of 2 simultaneously recorded hand muscles. The TMS coil was oriented toward the frontal pole during motor thresholding. TMS pulses were delivered to the primary motor cortex in the left hemisphere, and activity was measured on the right hand.
We administered intermittent theta burst TMS (iTBS, a 2 s train of TBS [3 pulses at 50Hz, repeated at 200 ms intervals] is repeated every 10 s for a total of 190 s [600 pulses]; Huang, Edwards, Rounis, Bhatia, & Rothwell, 2005) to the midline cerebellar node (DAN) and, as a control, the right cerebellar node (DMN). TMS was administered at a lower intensity between either 80% RMT or 100% AMT (mean stimulator output = 40%; range = 30% – 45%), such that neither was exceeded based on previous literature and guidelines. We did not exceed a stimulator output of 45% to minimize participant discomfort. The TMS coil was oriented vertically with the handle directed upward for cerebellar stimulation and placed tangential to the skull.
Behavioral Tasks
gradCPT
Participants performed the gradCPT, a go/no-go continuous performance task with gradual transitions between stimuli (e.g., Fortenbaugh et al., 2015; Esterman et al., 2013). The stimuli consisted of 20 grayscale photographs of scenes cropped in a circle. Ten photographs were mountain scenes, and the other ten were city scenes. On any trial, there was a 10% chance that a mountain scene was presented, and a 90% chance that a city scene was presented. For each administration of gradCPT, the order of scenes was random. Each scene gradually transitioned into the next scene using linear pixel-by-pixel interpolation, with the complete transition occurring over ~800 ms. Participants were instructed to press the space bar on the keyboard for every city scene but withhold their response for the mountain scenes. Response accuracy was emphasized without reference to speed. However, given that the next stimulus would replace the current stimulus in ~800 ms, a response deadline was implicit in the task (see reaction time and coefficient of variation below).
The gradCPT was presented using an Apple MacBook Pro. MATLAB (MathWorks) and Psychophysics toolbox (Brainard, 1997) were used to present stimuli and collect responses. The participant sat in a chair approximately 24 in from the display. The images subtended approximately ~6° of visual angle. The participant responded by pressing the space bar on the keyboard.
Analyses
Reaction time (RT) and coefficient of variation (CV)
RTs were calculated relative to the beginning of each image transition, such that an RT of 800 ms indicates a button press at the moment image n was 100% coherent and not mixed with other images. A shorter RT indicates that the current scene was still in the process of transitioning from the previous, and a longer RT indicates that the current scene was in the process of transitioning to the subsequent scene. For example, an RT of 720 ms would be at the moment of 90% image n and 10% image n − 1. On rare trials with highly deviant RTs (before 70% coherence of image n and after 40% coherence of image n + 1) or multiple button presses, an iterative algorithm maximized correct responses as follows: the algorithm first assigned unambiguous correct responses, leaving few ambiguous button presses (presses before 70% coherence of the current scene and after 40% coherence of the following scene). Second, ambiguous presses were assigned to an adjacent trial if one of the two had no response. If both adjacent trials had no response, the press was assigned to the closest trial, unless one was a no-go target, in which case subjects were given the benefit of the doubt that they correctly omitted. Slight variations to this algorithm yielded highly similar results, as most button presses showed a 1-1 correspondence with presented images. Raw RTs on correct trials were used to calculate RT variability, or coefficient of variation (CV = standard deviation of RT/meanRT).
Accuracy
Trials in which participants correctly inhibited a button press to mountain scenes were considered correct omissions. Trials in which participants erroneously responded to mountains were considered commission errors. Commission error rate (CE) served as our primary measure of accuracy. Errors of omission, or failing to respond to city scenes, occurred very rarely (mean = .6%, SD=.001) and were thus not considered in subsequent analyses. As omission errors were so infrequent (in fact 0% in at least one block for 7 subjects), we chose not to use a signal detection approach, as the lack of false alarms would bias or invalidate these analyses (Miller, 1996). CE Rate test-re-test reliability was r = .65 in a previous study, and does not exhibit a strong practice effect (Esterman et al., 2013). Note that reaction time and accuracy are not computed within the same trials. The reaction time performance was computed based on city scene go-trials where subjects correctly responded (~90% of the 750 trials). The CE rate was computed based on no-go mountain scene trials (~10% of the 750 trials).
Variance time course analysis (VTC): defining in the zone vs. out of the zone
To assess trial-to-trial changes in RT, we conducted a within-subject analysis called the variance time course (VTC; Kucyi et al., 2016; Fortenbaugh et al., 2015; Esterman et al., 2013; Esterman, Rosenberg, & Noonan, 2014; Rosenberg, Noonan, DeGutis, & Esterman, 2013). VTCs were computed from the correct responses to cities in each run of gradCPT (following z-transformation of RTs within-subject to normalize the scale of the VTC), where the value assigned to each trial represented the absolute deviation of the trial’s RT from the mean RT of the run. Evidence shows that extremely fast RTs often indicate premature responding and inattention to the potential need for response inhibition (Cheyne, Carriere, & Smilek, 2009; Esterman et al., 2013), while extremely slow RTs might indicate reduced attention to or inefficient processing of the ongoing stream of visual stimuli, requiring more time to accurately discriminate scenes (Esterman et al., 2013; Weissman, Roberts, Visscher, & Woldorff, 2006). To emphasize attention-related fluctuations and reduce high frequency noise, based on prior literature (Di Martino et al., 2008; Esterman et al., 2013), the VTC was smoothed using a Gaussian kernel of 20 trials (~16 s) full-width at half maximum (FWHM). As in previous work, we divided performance into low- or high-variability periods (in-the-zone and out-of-the-zone periods) with a median split on the smoothed VTC for each run. This yielded 5 min each of being in the zone (lowest half of the VTC) and out of the zone (highest half of the VTC). The patterns of results were identical when considering other divisions of in the zone and out of the zone, including a tertile split and other smoothing kernels. Note that differences in performance measures between in the zone and out of the zone were highly significant (and statistically guaranteed in the case of CV), thus we do not report these main effects in the Results section.
Attentional Blink
Participants performed a visual attentional blink task (Raymond, Shapiro, & Arnell, 1992; Shapiro, Raymond, & Arnell, 1994), composed of a rapid serial visual presentation of 14 items presented in the center of the screen. The stimuli subtended 2° of visual angle vertically and 1° horizontally. Two target numbers (3, 4, 5, 6, 7, 8, 9) were embedded in 12 distractor letters (B, C, D, E, F, G, K, L, M, N, P, R, S, T) (for a full description see Van Vleet & Robertson, 2006). Each stimulus was presented on the screen for 75 ms with a 0 ms inter-stimulus interval. The first target number (T1) was red to maximize identification while the second target number (T2) was black like the distractor letters and therefore more difficult to identify. T2 appeared either two positions after T1 (75 ms after T1; lag2) or six positions after T1 (375 ms after T1; lag6). There were 80 total trials with 40 lag2 trials and 40 lag6 trials.
The attentional blink task was also presented using an Apple MacBook Pro. The participant sat in a chair approximately 24 in from the display. The participant reported the identity of the targets by pressing the number keys on the keyboard. T2 accuracy was calculated only for trials in which T1 was correctly identified. Chance performance for T2 accuracy is 16.67%. The attentional blink does not exhibit strong practice effects and has shown to be sensitive to intervention effects (DeGutis & Van Vleet, 2010).
Analysis Strategy
In the present study, we aimed to evaluate the interaction between stimulation site (cerebellar DAN vs. cerebellar DMN) and pre/post-stimulation attentional performance. Thus, an ANOVA was conducted for each task with these two factors for each task. Based on our previous study using 1 Hz rTMS to target the frontal eye fields of the DAN (Esterman et al., 2015), we also expected the strongest effects on the gradCPT to be during in-the-zone performance, thus for the gradCPT, we included in/out of the zone as an additional factor in the gradCPT. While our primary measure of interest for sustained attention was CE rate for the gradCPT task we examined mean reaction time and variability of reaction time. Our primary measure for the attentional blink was the % errors for the second target (T2) after a correct first target (T1). We focused our analyses on lag2 (i.e., when T2 was presented 2 positions after T1), as this is when the attention blink is maximized (see Raymond et al., 1992).
Outlier analyses
Similar to other TMS studies (Allen, Dunkley, et al., 2014; Allen, Sumner, & Chambers, 2014), we applied Chauvenet’s criterion to identify outliers (Taylor & Cohen, 1998). According to Chauvenet’s criterion, first the probability that a suspect value is drawn from a normal population is calculated. If this value multiplied by the number of participants is less than 0.5 (probability of extreme value is less than ½*n), the data point should be excluded.
Results
Participants
Our final sample consisted of 14 subjects (10 males, mean age = 22.29, SD = 3.83) after excluding one outlier (22-year-old male) based on Chauvenet’s criterion (see Methods for a description). The outlier’s pre/post change in gradCPT commission error rate (averaged across both stimulation sessions) was 2.8 SD from the mean.
TMS Effects of Cerebellar DAN and DMN Stimulation: gradCPT
Accuracy
We first sought to test whether there was an effect of cerebellar DAN vs. cerebellar DMN stimulation on commission error (CE) rate, with a three-way ANOVA with site (DAN/DMN), time (pre/post) and attentional state (in the zone/out of the zone as factors). In this model (and all subsequent models) we controlled for stimulation location precision. Specifically, we included error (in millimeters) from our ideal target as a covariate by computing, for each session, the average distance from this target across the session. As predicted, there was a significant interaction between site × time (F(1,12)=9.576, p=.009; Figure 3a). There was no further interaction with attentional state (p>.7), thus the effect was statistically equivalent for both in-the-zone and out-of-the-zone periods. Follow-up ANOVAs for each session showed the interaction was driven by a reduction in CE rate following DAN stimulation (pre-DAN CE rate: 21.5% [standard error of the mean=4.3]; post-DAN CE rate: 15.4% [SEM=2.7]; F(1,12)=7.86, p=0.016).
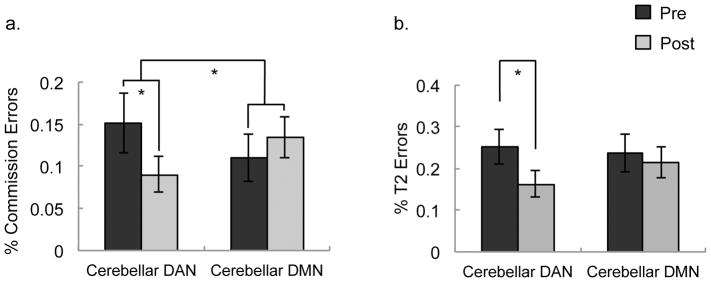
Figure 3.
Performance pre-/post- network-targeted cerebellar DMN and DAN stimulation. (a) overall gradCPT commission error (CE) (b) overall gradCPT commission error rate matched for baseline performance (see Results). Participants made fewer CEs after receiving cerebellar DAN compared to DMN stimulation. There were a significant 2-way interaction between stimulation site (DAN, DMN) and time (pre, post), *p<0.05, **p<.01.
It is noted that the CE rate interaction (Fig. 3A) could also be partially due to idiosyncratic differences at baseline between the sessions. Despite baseline differences not being significantly different between sessions (F(1,12)=2.74, p=0.12), numerically, performance was worse at baseline in the DAN session, rather than performance after stimulation. This effect was due to two participants who had poor baseline DAN performance (these participants had different session orders). To address this concern, we recomputed the overall CE ANOVA without these participants (n=12) and this created baseline scores there were numerically equivalent between the two sessions (Fig. 3B). Importantly, the site × time interaction remained significant (F(1,10)=6.339, p=.031; Fig. 3b) and is now driven by post-TMS differences. These analyses further indicate that CE rate was reduced after DAN stimulation, and no difference was observed after DMN stimulation.
Reaction time measures
Equivalent ANOVAs were conducted with mean RT and RT variability. For mean RT and for RT variability, there were no significant main effects or interactions between site and time (mean RT: 2-way interaction, F(1,12)= 1.175, p=.300; RT variability: 2-way interaction, F(1,12)=2.389, p=.148).
TMS Effects of Cerebellar DAN and DMN Stimulation: attentional blink
We next sought to test whether there was an effect of cerebellar DAN vs. cerebellar DMN stimulation on T2 error rate during the blink task, with a two-way ANOVA with site (DAN/DMN) and time (pre/post), again controlling for stimulation location precision. This interaction of site and time for selective attention was not significant (F(1, 13) = 0.38, p>.8). There was a main effect of time (F(1,12)=6.06, p=0.03), such that performance was better post- vs. pre-TMS across both sessions (pre-TMS: 24.5% error [SEM=3.8]; post-TMS: 19.8% error [SEM=3.2]). Post-hoc ANOVAs by session (DAN and DMN) found no significant improvement after either (DAN: F(1,12)=1.56, p=.24; DMN: F(1,12)=1.32, p=.27). Thus, overall, stimulation enhanced performance, although post-hoc test showed that neither stimulation type alone significantly improved performance.
Post Hoc Examination of Individual Subjects’ Performance
As a post-hoc analysis, we examined individual differences in response to stimulation. We focused on the gradCPT given the significant effect found on CE rate (i.e., significant interaction).
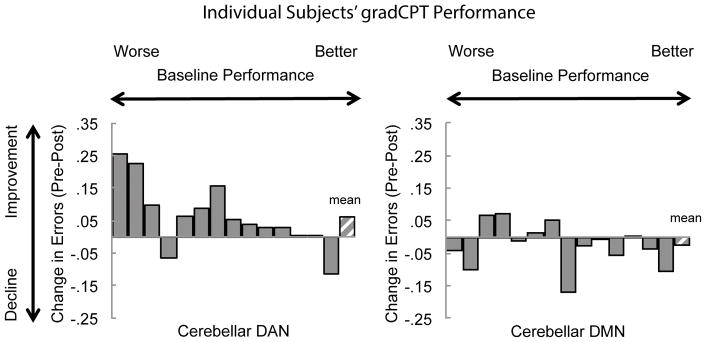
Only following DAN stimulation, participants with worse baseline functioning on the gradCPT showed greater improvements on the gradCPT task, as revealed by a correlation between improvement and average baseline accuracy (Spearman’s rho, ρ=.54, p=.048; see Figure 4-left). Critically, following DMN, this pattern was not present (ρ=−.20, p=.48; Figure 4-right), suggesting that these differences were not due to practice.
Figure 4.
Change in gradCPT commission error (CE) rate across individual subjects. Improvement is positive and decline is negative (y-axis). Participants are sorted by baseline CE rate (average of both baseline CE rates across sessions) with worse baseline performance on the left and better baseline performance on the right (x-axis). There was a significant relationship in the DAN stimulation condition, such that worse performers showed the greatest TMS-induced enhancement (rho=.54, p<0.05).
Discussion
We applied iTBS to DAN and DMN hubs in the cerebellum in an attempt to enhance sustained and transient attentional control using a network-based localization method. Our results show that cerebellar DAN-targeted stimulation significantly reduced commission error rate during the gradCPT task post-iTBS compared to baseline pre-iTBS performance. In contrast, iTBS applied to cerebellar DMN did not modulate performance. Reaction time measures on the gradCPT were unaffected by either TMS condition. Attentional blink performance generally improved after TMS, as there was a reduction in errors post-iTBS compared to baseline pre-iTBS, indicating that the effect was not network dependent. This study is one of the first to use network-targeted TMS to enhance attention, and suggests that network-targeted cerebellar TMS could enhance other aspects of attention or cognition that were not assessed in this study.
Sustained attention improvements from midline cerebellar DAN TMS may have occurred through enhanced DAN-connectivity leading to a more efficient utilization of attentional resources. Studies have shown that during periods of optimal performance, participants show less activation in DAN regions, and have greater activation in areas of the DMN (Esterman et al., 2013; Esterman et al., 2014; Kucyi et al., 2016). These studies and others suggest that during optimal performance, attentional resources are engaged with greater efficiency and precision. Supporting this interpretation, task-irrelevant stimuli are processed with greater depth during these optimal periods, akin to lower perceptual load, or more efficient task-related processing (Esterman et al., 2014). Similarly, we found that inhibitory TMS (1 Hz) to the right frontal eye field impaired more optimal periods of performance (Esterman et al., 2015). This indicates that FEF is more critical during these periods of consistent and accurate performance. In the domain of attentional control over the motor system, Kucyi et al. (2016) found that stable and accurate periods of rhythmic finger tapping was also associated with less activation in task-positive regions, again consistent with neural efficiency. We speculate that cerebellar-DAN TMS enhanced within-DAN FC, and in turn, neural efficiency of a network critical for sustained attentional control.
Further, our data suggest that subjects with worse baseline attentional performance benefited to the greatest extent on the gradCPT from iTBS of the DAN. These results could be explained by the concept of stochastic resonance (Miniussi et al., 2010; 2013; Schwarzkopf et al., 2011). This phenomenon boosts weak signal by adding broad-spectrum noise to it. In this regard, participants who had worse baseline performance (i.e., a weaker signal) may have benefited more. It is equally important to note that subjects with better baseline attentional performance also benefited from iTBS but to a lesser extent. This effect is mainly the resultant of TMS “calling to action” the neuronal population that was less active during the baseline. Subjects with better baseline performance might have a higher ratio of volume of neuronal population activated during task, leaving iTBS to effect a smaller “inactive” volume of neuronal population. While we recruited healthy participants, this result suggests that populations with attention deficits, who have worse baseline performance, may be more sensitive to these TMS-induced improvements.
Alternatively, improvement following cerebellar DAN stimulation may be interpreted as the result of functional improvement of the cerebellum’s role in the DAN, and attention more broadly (Stoodley, 2012; Stoodley & Schmahmann, 2009; Tomasi, Chang, Caparelli, & Ernst, 2007). The dysmetria of thought hypothesis suggests that the cerebellum’s functional role in cognition is similar to its role in the motor system (Schmahmann, 1991; Andreasen, 1998). The cerebellum contains an organization that is remarkably consistent across networks. This modular organization means that TBS stimulation to different subregions will result in similar effects across distinct networks (Farzan 2016). Indeed, there has been investigation within the motor system of the effect of TBS, which is consistent with iTBS resulting in a change in distal motor cortex plasticity (Casula et al 2016; Koch et al 2008; Grimaldi et al., 2014). In the motor system, the cerebellum may contain an internal model predicting the course of future events based on past experience (Manto et al., 2012). Indeed, the physiology of the cerebellum suggests that translated to the attentional system, this same internal model system may be responsible for predictive deployment and maintenance of attention. There is evidence of the cerebellum contributing to attentional processes from imaging, transcranial magnetic stimulation and lesion studies (Brissenden et al., 2016; Arasanz et al., 2012; Baier et al., 2010). Thus, it is plausible that the iTBS-induced improvement in sustained attention may arise from improved functioning of the cerebellum’s role in attention.
Though speculative, the improvement from DAN-iTBS can also potentially be explained by considering the state of the targeted neuronal population prior to stimulation (Silvanto & Muggleton, 2008; Minuissi et al., 2013). In this study, we may have preconditioned selective neuronal populations by having participants perform the gradCPT (and attentional blink task) immediately prior to iTBS. Under certain circumstances, preconditioning has been shown to boost TMS efficacy (Romei et al., 2016). Specifically, the enhancement of gradCPT performance from cerebellar DAN iTBS could be the resultant of this prior neuronal tuning of DAN regions, know to be recruited during gradCPT as shown with fMRI (Esterman et al., 2013, 2014). This design element may have boosted TMS efficacy, and helped induced the pre/post differences in gradCPT performance.
Our results might appear to contradict a recent cerebellar TMS study (Cattaneo et al., 2014) that found that rTMS applied to the cerebellar vermis, compared to sham and no TMS blocks, reduced visual motion discrimination ability. This disparity in attentional performance outcome is likely due to the different stimulation parameters, tasks involved, and especially stimulation sites. In the current study our stimulation sites were far more inferior to the stimulation site in Cattaneo et al. (2014), which was 1 cm inferior to the inion. Additionally, in the current study we used iTBS offline whereas Cattaneo et al. (2014) used rTMS online concurrently with the task. These parameters might have led to these opposite effects, as would be predicted by our network model of the stimulation effects (Halko et al., 2014). Others have reported reaction time changes and motor physiological changes from the application of TBS to the cerebellum, typically 1cm inferior and 3cm lateral to the inion (Koch et al., 2008, 2009; Picazio et al., 2016; Casula et al 2016). These motor sites are substantially superior and lateral to the typical dorsal attention network site. Previous investigations of the present cerebellar locations demonstrated no change in the somatomotor network with identical stimulation procedures (Halko et al 2014).
DMN stimulation did not induce any change in performance during the gradCPT task compared to baseline performance. The absence of effects could mean that the DMN-FC is not as directly involved in attentional control. In a similar line of evidence DMN FC has been less consistently linked with attention (Barber et al., 2015; Bonnelle et al., 2011). In contrast, FC between DAN and the DMN has been found to contribute to individual differences in response time variability, such that greater DAN-DMN FC is associated with greater variability and distractibility in healthy adults and ADHD patients (Kelly et al., 2008; Kucyi, et al., 2015; Poole et al., 2016). While the literature points at an existent interplay between DAN and DMN, the results of this study made a potential distinction as to their involvement in attentional control.
Reaction time analyses did not show any modulatory effects of iTBS on the gradCPT. This finding is less common in the TMS literature where most reported effects are observed at this level. However our lack of effect might be due to gradCPT task design. In many tasks, subjects typically have enough time range (usually up to seconds) to accumulate information needed before giving their response. In contrast, due to the gradual and overlapping nature of the task, as well as the rapid pace, the task has a limited range of RTs (~500ms-~1300ms), as reaction times that are too fast (which leads to errors of commission to rare mountains) and too slow (which leads to missing the implicit response deadline and thus errors of omission), will eventually be misassigned to previous/subsequent trials, are be associated with worse accuracy (Esterman et al., 2013). Other tasks for which reaction time is a primary measure of sustained attention (e.g., vigilance, X-CPTs) may reveal RT enhancements from similar stimulation protocols.
Overall, attentional blink performance did benefit from stimulation, suggesting the enhancement of functional connectivity in both DAN and DMN can improve transient aspects of attentional control. Our present findings are consistent with previous studies of attentional and visuo-motor effects of cerebellar stimulation. Most relevant, Aransaz et al. (2012) performed stimulation of the cerebellum with continuous theta burst, an inhibitory protocol (in contrast to the current study), and found reduced performance on an attentional blink paradigm. Though this is consistent with the current study, their target site in the cerebellum was more superior to our sites, so direct comparisons are not possible. Theoret et al. (2001) directly investigated lateral and midline cerebellar stimulation (similar to the current protocol), and found an aftereffect on visually paced finger tapping, such that the variability of responses was greater after midline 1 Hz stimulation, but not with lateral stimulation. This finding is consistent with an attentional influence of cerebellar stimulation (in the case of the DAN stimulation), since tapping variability has been previously associated with attentional fluctuations (Kucyi, Hove, Esterman, Hutchison, & Valera, 2016). It is important to note that post-hoc tests did not reveal significant effects for either network alone. Additionally, because the effect of time (pre/post) did not interact with stimulation site, it is possible that the effects could be non-specific to the sites tested, or due to practice. Further study is necessary to rule out these possibilities.
In conclusion, we found that network-targeted iTBS of the cerebellar DAN enhanced sustained attentional control, and cerebellar DAN and DMN stimulation enhanced transient attentional control. This is one of the few studies to show improvements in attention from network-targeted invasive brain stimulation. Our results suggest that within-DAN FC is integral for optimal sustained attention, and that cerebellar stimulation, targeted via cortical connectivity, has promise to enhance cognition. This has important theoretical implications for our understanding of models of attention, FC-MRI as a biomarker, TMS for cognitive enhancement, and the role of the cerebellum in attention. Further, while more work is needed, this study lays some of the groundwork for future clinical applications.
Acknowledgments
This work was supported by the US Department of Veteran Affairs through a Clinical Science Research & Development Career Development Award (grant number 1IK2CX000706-01A2) to M.E, Sidney Baer Jr. Foundation Awards to M.A.H and S.E.L., and the National Institutes of Health (grant number 1R01MH112706) to M.A.H.
References
- Allen CP, Dunkley BT, Muthukumaraswamy SD, Edden R, Evans CJ, Sumner P, … Chambers CD. Enhanced awareness followed reversible inhibition of human visual cortex: a combined TMS, MRS and MEG study. PLoS One. 2014;9(6):e100350. doi: 10.1371/journal.pone.0100350. PONE-D-14-05089 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CP, Sumner P, Chambers CD. The timing and neuroanatomy of conscious vision as revealed by TMS-induced blindsight. J Cogn Neurosci. 2014;26(7):1507–1518. doi: 10.1162/jocn_a_00557. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Paradiso S, O’leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophrenia Bulletin. 1998;24(2):203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Arasanz CP, Staines WR, Schweizer TA. Isolating a cerebellar contribution to rapid visual attention using transcranial magnetic stimulation. Front Behav Neurosci. 2012;6:55. doi: 10.3389/fnbeh.2012.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier B, Dieterich M, Stoeter P, Birklein F, Müller NG. Anatomical correlate of impaired covert visual attentional processes in patients with cerebellar lesions. Journal of Neuroscience. 2010;30(10):3770–3776. doi: 10.1523/JNEUROSCI.0487-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Owsley C, Sloane ME. Visual and cognitive predictors of driving problems in older adults. Experimental Aging Research. 1991;17:79–80. [PubMed] [Google Scholar]
- Barber AD, Jacobson LA, Wexler JL, Nebel MB, Caffo BS, Pekar JJ, Mostofsky SH. Connectivity supporting attention in children with attention deficit hyperactivity disorder. Neuroimage Clin. 2015;7:68–81. doi: 10.1016/j.nicl.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker AT. The history and basic principles of magnetic nerve stimulation. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:3– 21. [PubMed] [Google Scholar]
- Bonnelle V, Leech R, Kinnunen KM, Ham TE, Beckmann CF, De Boissezon X, … Sharp DJ. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J Neurosci. 2011;31(38):13442–13451. doi: 10.1523/JNEUROSCI.1163-11.2011. 31/38/13442 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Bray S, Arnold AE, Levy RM, Iaria G. Spatial and temporal functional connectivity changes between resting and attentive states. Hum Brain Mapp. 2015;36(2):549–565. doi: 10.1002/hbm.22646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissenden JA, Levin EJ, Osher DE, Halko MA, Somers DC. Functional Evidence for a Cerebellar Node of the Dorsal Attention Network. Journal of Neuroscience. 2016;36(22):6083–6096. doi: 10.1523/jneurosci.0344-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network - Anatomy, function, and relevance to disease. Year in Cognitive Neuroscience 2008. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(5):2322–2345. doi: 10.1152/jn.00339.2011. jn.00339.2011 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capotosto P, Corbetta M, Romani GL, Babiloni C. Electrophysiological correlates of stimulus-driven reorienting deficits after interference with right parietal cortex during a spatial attention task: a TMS-EEG study. Journal of cognitive neuroscience. 2012;24(12):2363–2371. doi: 10.1162/jocn_a_00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casula EP, Pellicciari MC, Ponzo V, Bassi MS, Veniero D, Caltagirone C, Koch G. Cerebellar theta burst stimulation modulates the neural activity of interconnected parietal and motor areas. Scientific Reports. 2016;6 doi: 10.1038/srep36191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo Z, Renzi C, Casali S, Silvanto J, Vecchi T, Papagno C, D’Angelo E. Cerebellar vermis plays a causal role in visual motion discrimination. Cortex. 2014;58:272–280. doi: 10.1016/j.cortex.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Mattingley JB. Neurodisruption of selective attention: insights and implications. Trends Cogn Sci. 2005;9(11):542–550. doi: 10.1016/j.tics.2005.09.010. S1364-6613(05)00272-X [pii] [DOI] [PubMed] [Google Scholar]
- Cheyne JA, Carriere JS, Smilek D. Absent minds and absent agents: attention-lapse induced alienation of agency. Conscious Cogn. 2009;18(2):481–493. doi: 10.1016/j.concog.2009.01.005. S1053-8100(09)00007-5 [pii] [DOI] [PubMed] [Google Scholar]
- Cooper AC, Humphreys GW, Hulleman J, Praamstra P, Georgeson M. Transcranial magnetic stimulation to right parietal cortex modifies the attentional blink. Exp Brain Res. 2004;155(1):24–29. doi: 10.1007/s00221-003-1697-9. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3(3):292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Degutis JM, Van Vleet TM. Tonic and phasic alertness training: a novel behavioral therapy to improve spatial and non-spatial attention in patients with hemispatial neglect. Front Hum Neurosci. 2010;4 doi: 10.3389/fnhum.2010.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Ghaffari M, Curchack J, Reiss P, Hyde C, Vannucci M, … Castellanos FX. Decomposing intra-subject variability in children with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;64(7):607–614. doi: 10.1016/j.biopsych.2008.03.008. S0006-3223(08)00300-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon ML, Andrews-Hanna JR, Spreng RN, Irving ZC, Christoff K. Anticorrelation between default and dorsal attention networks varies across default subsystems and cognitive states. bioRxiv. 2016:056424. [Google Scholar]
- Dubin MJ, Liston C, Avissar MA, Ilieva I, Gunning FM. Network-Guided Transcranial Magnetic Stimulation for Depression. Current Behavioral Neuroscience Reports. 2017:1–8. doi: 10.1007/s40473-017-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duecker F, Sack AT. Rethinking the role of sham TMS. Frontiers in psychology. 2015;6:210. doi: 10.3389/fpsyg.2015.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, … Schatzberg AF. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nature Medicine. 2016 doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edkins GD, Pollock CM. The influence of sustained attention on railway accidents. Accident Analysis and Prevention. 1997;29(4):533–539. doi: 10.1016/s0001-4575(97)00033-x. [DOI] [PubMed] [Google Scholar]
- Eldaief MC, Halko MA, Buckner RL, Pascual-Leone A. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc Natl Acad Sci U S A. 2011;108(52):21229–21234. doi: 10.1073/pnas.1113103109. 1113103109 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman M, Liu G, Okabe H, Reagan A, Thai M, DeGutis J. Frontal eye field involvement in sustaining visual attention: evidence from transcranial magnetic stimulation. Neuroimage. 2015;111:542–548. doi: 10.1016/j.neuroimage.2015.01.044. S1053-8119(15)00069-5 [pii] [DOI] [PubMed] [Google Scholar]
- Esterman M, Noonan SK, Rosenberg M, Degutis J. In the zone or zoning out? Tracking behavioral and neural fluctuations during sustained attention. Cereb Cortex. 2013;23(11):2712–2723. doi: 10.1093/cercor/bhs261. bhs261 [pii] [DOI] [PubMed] [Google Scholar]
- Esterman M, Rosenberg MD, Noonan SK. Intrinsic fluctuations in sustained attention and distractor processing. J Neurosci. 2014;34(5):1724–1730. doi: 10.1523/JNEUROSCI.2658-13.2014. 34/5/1724 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman M, Yantis S. Perceptual expectation evokes category-selective cortical activity. Cereb Cortex. 2010;20(5):1245–1253. doi: 10.1093/cercor/bhp188. bhp188 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan F, Pascual-Leone A, Schmahmann JD, Halko M. Enhancing the temporal complexity of distributed brain networks with patterned cerebellar stimulation. Scientific reports. 2016;6 doi: 10.1038/srep23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortenbaugh FC, DeGutis J, Germine L, Wilmer JB, Grosso M, Russo K, Esterman M. Sustained attention across the life span in a sample of 10,000 dissociating ability and strategy. Psychological Science. 2015;26(9):1497–1510. doi: 10.1177/0956797615594896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortenbaugh FC, DeGutis J, Esterman M. Recent theoretical, neural, and clinical advances in sustained attention research. Annals Of The New York Academy Of Sciences. doi: 10.1111/nyas.13318. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi G, Argyropoulos GP, Boehringer A, Celnik P, Edwards MJ, Ferrucci R, … Lesage E. Non-invasive cerebellar stimulation—a consensus paper. The Cerebellum. 2014;13(1):121–138. doi: 10.1007/s12311-013-0514-7. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Paus T. Transcranial magnetic stimulation of the human frontal eye field: effects on visual perception and attention. Journal of cognitive neuroscience. 2002;14(7):1109–1120. doi: 10.1162/089892902320474553. [DOI] [PubMed] [Google Scholar]
- Halko MA, Eldaief MC, Horvath JC, Pascual-Leone A. Combining transcranial magnetic stimulation and FMRI to examine the default mode network. J Vis Exp. 2010;(46) doi: 10.3791/2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halko MA, Farzan F, Eldaief MC, Schmahmann JD, Pascual-Leone A. Intermittent theta-burst stimulation of the lateral cerebellum increases functional connectivity of the default network. J Neurosci. 2014;34(36):12049–12056. doi: 10.1523/JNEUROSCI.1776-14.2014. 34/36/12049 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26(51):13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. 26/51/13338 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Newton TF, van Gorp WG. Neurocognitive functioning is associated with employment status: A quantitative review. Journal of Clinical and Experimental Neuropsychology. 2003;25(8):1186–1191. doi: 10.1076/jcen.25.8.1186.16723. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22(4):751–761. doi: 10.1016/s0896-6273(00)80734-5. S0896-6273(00)80734-5 [pii] [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39(1):527–537. doi: 10.1016/j.neuroimage.2007.08.008. S1053-8119(07)00705-7 [pii] [DOI] [PubMed] [Google Scholar]
- Kihara K, Hirose N, Mima T, Abe M, Fukuyama H, Osaka N. The role of left and right intraparietal sulcus in the attentional blink: a transcranial magnetic stimulation study. Exp Brain Res. 2007;178(1):135–140. doi: 10.1007/s00221-007-0896-1. [DOI] [PubMed] [Google Scholar]
- Kihara K, Ikeda T, Matsuyoshi D, Hirose N, Mima T, Fukuyama H, Osaka N. Differential contributions of the intraparietal sulcus and the inferior parietal lobe to attentional blink: evidence from transcranial magnetic stimulation. J Cogn Neurosci. 2011;23(1):247–256. doi: 10.1162/jocn.2010.21426. [DOI] [PubMed] [Google Scholar]
- Koch G, Mori F, Marconi B, Codecà C, Pecchioli C, Salerno S, … Caltagirone C. Changes in intracortical circuits of the human motor cortex following theta burst stimulation of the lateral cerebellum. Clinical Neurophysiology. 2008;119(11):2559–2569. doi: 10.1016/j.clinph.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Koch G, Brusa L, Carrillo F, Gerfo EL, Torriero S, Oliveri M, … Stanzione P. Cerebellar magnetic stimulation decreases levodopa-induced dyskinesias in Parkinson disease. Neurology. 2009;73(2):113–119. doi: 10.1212/WNL.0b013e3181ad5387. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Hove MJ, Biederman J, Van Dijk KR, Valera EM. Disrupted functional connectivity of cerebellar default network areas in attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2015;36(9):3373–3386. doi: 10.1002/hbm.22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Hove MJ, Esterman M, Hutchison RM, Valera EM. Dynamic Brain Network Correlates of Spontaneous Fluctuations in Attention. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw029. bhw029 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CM, Beale IL. RELATIONS AMONG SUSTAINED ATTENTION, READING PERFORMANCE, AND TEACHERS RATINGS OF BEHAVIOR PROBLEMS. Remedial and Special Education. 1991;12(2):40–47. [Google Scholar]
- Langner R, Eickhoff SB. Sustaining attention to simple tasks: a meta-analytic review of the neural mechanisms of vigilant attention. Psychol Bull. 2013;139(4):870–900. doi: 10.1037/a0030694. 2012-30617-001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manto M, Bower JM, Conforto AB, Delgado-García JM, da Guarda SNF, Gerwig M, … Molinari M. Consensus paper: roles of the cerebellum in motor control—the diversity of ideas on cerebellar involvement in movement. The Cerebellum. 2012;11(2):457–487. doi: 10.1007/s12311-011-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. The sampling distribution of d’. Percept Psychophys. 1996;58(1):65–72. doi: 10.3758/bf03205476. [DOI] [PubMed] [Google Scholar]
- Miniussi C, Ruzzoli M, Walsh V. The mechanism of transcranial magnetic stimulation in cognition. Cortex. 2010;46(1):128–130. doi: 10.1016/j.cortex.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Miniussi Carlo, Harris Justin A, Ruzzoli Manuela. Modelling non-invasive brain stimulation in cognitive neuroscience. Neuroscience & Biobehavioral Reviews. 2013;37(8):1702–1712. doi: 10.1016/j.neubiorev.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Akaishi R, Yamada Y, Okuda J, Toma K, Sakai K. Task-specific signal transmission from prefrontal cortex in visual selective attention. Nature neuroscience. 2009;12(1):85–91. doi: 10.1038/nn.2237. [DOI] [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci. 1997;17(9):3178–3184. doi: 10.1523/JNEUROSCI.17-09-03178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picazio S, Ponzo V, Koch G. Cerebellar control on prefrontal-motor connectivity during movement inhibition. The Cerebellum. 2016;15(6):680–687. doi: 10.1007/s12311-015-0731-3. [DOI] [PubMed] [Google Scholar]
- Poole VN, Robinson ME, Singleton O, DeGutis J, Milberg WP, McGlinchey RE, … Esterman M. Intrinsic functional connectivity predicts individual differences in distractibility. Neuropsychologia. 2016;86:176–182. doi: 10.1016/j.neuropsychologia.2016.04.023. S0028-3932(16)30139-7 [pii] [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37(4):1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: an attentional blink? J Exp Psychol Hum Percept Perform. 1992;18(3):849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Rohr CS, Vinette SA, Parsons KA, Cho IY, Dimond D, Benischek A, … Bray S. Functional Connectivity of the Dorsal Attention Network Predicts Selective Attention in 4–7 year-old Girls. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw236. [DOI] [PubMed] [Google Scholar]
- Romei V, Thut G, Silvanto J. Information-based approaches of noninvasive transcranial brain stimulation. Trends in Neurosciences. 2016;39(11):782–795. doi: 10.1016/j.tins.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Rosenberg M, Noonan S, DeGutis J, Esterman M. Sustaining visual attention in the face of distraction: a novel gradual-onset continuous performance task. Atten Percept Psychophys. 2013;75(3):426–439. doi: 10.3758/s13414-012-0413-x. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. S1388-2457(09)00519-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, … Driver J. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Current Biology. 2006;16(15):1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. J Neurosci. 1997;17(1):438–458. doi: 10.1523/JNEUROSCI.17-01-00438.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD. An emerging concept: the cerebellar contribution to higher function. Archives of neurology. 1991;48(11):1178–1187. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- Schmidt EA, Schrauf M, Simon M, Fritzsche M, Buchner A, Kincses WE. Drivers’ misjudgement of vigilance state during prolonged monotonous daytime driving. Accident Analysis and Prevention. 2009;41(5):1087–1093. doi: 10.1016/j.aap.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Schwarzkopf DS, Silvanto J, Rees G. Stochastic resonance effects reveal the neural mechanisms of transcranial magnetic stimulation. Journal of Neuroscience. 2011;31(9):3143–3147. doi: 10.1523/JNEUROSCI.4863-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro KL, Raymond JE, Arnell KM. Attention to visual pattern information produces the attentional blink in rapid serial visual presentation. J Exp Psychol Hum Percept Perform. 1994;20(2):357–371. doi: 10.1037//0096-1523.20.2.357. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Lavie N, Walsh V. Stimulation of the human frontal eye fields modulates sensitivity of extrastriate visual cortex. Journal of Neurophysiology. 2006;96(2):941–945. doi: 10.1152/jn.00015.2006. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton N, Walsh V. State-dependency in brain stimulation studies of perception and cognition. Trends in cognitive sciences. 2008;12(12):447–454. doi: 10.1016/j.tics.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ. The cerebellum and cognition: evidence from functional imaging studies. The Cerebellum. 2012;11(2):352–365. doi: 10.1007/s12311-011-0260-7. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. S1053-8119(08)00972-5 [pii] [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Cohen E. An introduction to error analysis: the study of uncertainties in physical measurements. Measurement Science and Technology. 1998;9(6):1015. [Google Scholar]
- Taylor PC, Nobre AC, Rushworth MF. Combining Correlation and Interference Methods in the Human Brain. Focus on “Cortico-Cortical Interactions in Spatial Attention: A Combined ERP/TMS Study”. Journal of neurophysiology. 2006;95(5):2731–2732. doi: 10.1152/jn.00058.2006. [DOI] [PubMed] [Google Scholar]
- Theoret H, Haque J, Pascual-Leone A. Increased variability of paced finger tapping accuracy following repetitive magnetic stimulation of the cerebellum in humans. Neurosci Lett. 2001;306(1–2):29–32. doi: 10.1016/s0304-3940(01)01860-2. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Chang L, Caparelli EC, Ernst T. Different activation patterns for working memory load and visual attention load. Brain Res. 2007;1132(1):158–165. doi: 10.1016/j.brainres.2006.11.030. S0006-8993(06)03338-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vleet TM, Robertson LC. Cross-modal interactions in time and space: auditory influence on visual attention in hemispatial neglect. J Cogn Neurosci. 2006;18(8):1368–1379. doi: 10.1162/jocn.2006.18.8.1368. [DOI] [PubMed] [Google Scholar]
- Walsh V, Pascual-Leone A. Transcranial magnetic stimulation: a neurochronometrics of mind. MIT press; 2003. [Google Scholar]
- Wang JX, Rogers LM, Gross EZ, Ryals AJ, Dokucu ME, Brandstatt KL, … Voss JL. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science. 2014;345(6200):1054–1057. doi: 10.1126/science.1252900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JX, Voss JL. Long-lasting enhancements of memory and hippocampal-cortical functional connectivity following multiple-day targeted noninvasive stimulation. Hippocampus. 2015;25(8):877–883. doi: 10.1002/hipo.22416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9(7):971–978. doi: 10.1038/nn1727. nn1727 [pii] [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, … Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. jn.00338.2011 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]