Abstract
Obesity is a vast public health problem, and both a major risk factor and a disease modifier for asthma in children and adults. Obese subjects have increased risk of asthma, and obese asthmatics have more symptoms, more frequent and severe exacerbations, reduced response to several asthma medications, and decreased quality of life. Obese asthma is a complex syndrome, including different phenotypes of disease, phenotypes which are just beginning to be understood. We examine the epidemiology and characteristics of this syndrome in children and adults, as well as the changes in lung function seen in each age group. We then discuss the better-recognized factors and mechanisms involved in disease pathogenesis, focusing particularly on diet and nutrients, the microbiome, inflammatory and metabolic dysregulation, and the genetics/genomics of obese asthma. Finally, we describe current evidence on the impact of weight loss, and mention some important future directions for research in the field.
Keywords: asthma, obesity, obese asthma, metabolic syndrome, microbiome
Introduction
Obesity is both a major risk factor and a disease modifier of asthma in children and adults. While obesity is defined according to a threshold BMI, recent studies suggest that BMI z-scores may be unreliable, particularly among children and adolescents with severe obesity.1–3 In adults, obesity is defined as a body mass index (BMI) of 30 kg/m2 or more, yet a given BMI may reflect vastly differing physiology and metabolic health. This distinction is likely important for asthma: while serum interleukin (IL-)6 (produced by macrophages in adipose tissue, and a marker of metabolic health) is a marker of asthma severity, some individuals with BMI’s in the non-obese range have elevated IL-6;4 Sideleva et al found that adipose tissue inflammation is increased in obese individuals with asthma, compared with obese controls.5 Metabolic dysfunction is more important than fat mass for asthma in obesity. However, most asthma studies have used BMI and metabolic dysfunction related to obesity synonymously; in this article, we will report data on metabolic dysfunction where available, but will otherwise use obesity as a marker of both fat mass and metabolic dysfunction.
Epidemiology of Obesity and Childhood Asthma
Asthma affects approximately 6.5 million children (~9% prevalence) in the U.S.6 Likewise, 17% of children in this country are obese, and another 15% are overweight.7 Obesity is now recognized as a major risk factor for asthma: several longitudinal epidemiological studies show that obesity or increased adiposity often precedes incident asthma.8–16 Many studies have reported differing obesity-asthma associations by sex,10,17–20 although results on which gender is more affected have been conflicting. Obesity is also associated with increased asthma severity.
Obesity-induced increases in asthma risk may start in utero. In a meta-analysis of over 108,000 participants, we found that maternal obesity and weight gain during pregnancy are independently associated with ~15–30% increased risk of asthma in the offspring, and others have reported very similar findings.21,22 This risk is not merely mediated by the child’s own obesity.23 Mechanisms involved may include inflammatory or other changes during pregnancy or early post-natal life,24–26 and these mechanisms may explain why excessive weight gain in infancy has also been linked to recurrent wheezing and asthma.27,28
Epidemiology of Obesity and Asthma in Adults
A meta-analysis of several prospective studies involving over 300,000 adults found a dose-response relationship between obesity and asthma: the odds ratio of incident asthma was 1.5 in the overweight, and 1.9 in the obese compared with the lean group – in effect 250,000 new asthma cases per year in the US are related to obesity.29 This relationship has radically changed the demographics of asthma in the US: the prevalence of asthma in lean adults is 7.1%, and in obese adults 11.1%. The relationship is more striking in women – the prevalence of asthma in lean versus obese women is 7.9 and 14.6%, respectively.30
Clinical Characteristics of Obese Asthma in Children
Asthma may on occasion predispose to obesity,31 obesity may confound its diagnosis,32,33 or both may simply co-occur. However, the majority of observational and experimental evidence points towards an “obese asthma” phenotype, in which obesity modifies asthma.34,35
Obese children tend to have increased asthma severity,36–38 poorer disease control,39 and lower quality of life.40 Many obese children with asthma tend to have Th1-skewed responses, particularly in response to inflammatory stimuli, with at least part of these responses mediated by systemic inflammation, insulin resistance, and/or alterations in lipid metabolism.41–43 These children and adolescents also tend to have a decreased response to asthma medications. Using data from the Childhood Asthma Management Program (CAMP), we described that overweight and obese children with asthma had a reduced response to inhaled corticosteroids (ICS), leading to increased prednisone courses and moderate-to-severe exacerbations.44 More recently, McGarry et al reported that obese black and Latino adolescents were 24% more likely to be bronchodilator unresponsive than their non-obese peers.45 Moreover, among children hospitalized for asthma, obesity is associated with longer length of stay and with higher risk of mechanical ventilation.37 Obese children with asthma may also be more susceptible to having increased symptoms with exposure to indoor pollutants.46
Clinical Characteristics of Obese Asthma in Adults
Much like children, obese adults tend to have more severe asthma than lean adults, with a 4- to 6-fold higher risk of being hospitalized compared with lean adults with asthma.47 Nearly 60% of adults with severe asthma in the US are obese.48 Obese patients also have worse asthma control and lower quality of life.49 Obese asthmatics do not respond as well to standard controller medications such as ICS and combination ICS-long acting beta agonists (LABA).50 The mechanisms behind the impaired ICS response are likely related to increased production of inflammatory cytokines in obesity, which reduce induction of mitogen-activated kinase phosphatase-1 by glucocorticoid, a signalling protein that plays an important role in steroid responses.51 Impaired response to asthma therapy in obesity is also due to the altered pathogenesis of disease, which does not respond well to medications developed to treat conventional allergic asthma.
There are likely several phenotypes within the obese asthma syndrome (Figure 1). Holguin et al reported that obese asthmatics with earlier-onset disease (who tended to have higher markers of Th2 inflammation) had the most severe disease among obese asthmatics.47 There is also a group with later-onset disease, most often female, with little in the way of airway inflammation, but significant inflammation in adipose tissue and increased airway oxidative stress.5 Some have described a phenotype with neutrophilic airway inflammation,52 which improves with weight loss in women.53 Obese individuals appear to have increased susceptibility to air-pollutants,54,55 which has been elegantly modeled in animals.56 Whether this contributes to a distinct phenotype or complicates other phenotypes is not yet clear.
Figure 1. Obese asthma syndrome.
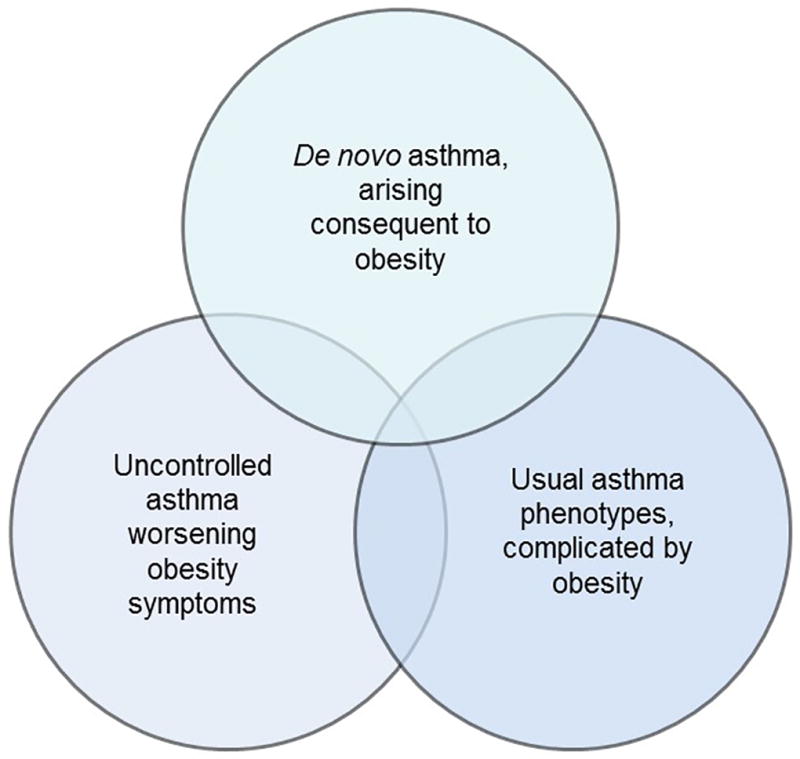
The syndrome of obese asthma likely includes many phenotypes: those typically seen in lean individuals, now complicated by obesity; disease newly arising in obese individuals; and perhaps a separate phenotype characterized by increased response to environmental pollutants. Much work remains to be done to understand whether these are unique phenotypes that require individualized therapeutic approaches.
Obesity and Lung Function in Pediatrics
Childhood obesity has a significant effect on lung function. While our initial understanding of this phenomenon derived from studies in adults (described in the following section), as early as 1997 Lazarus et al reported that height-adjusted forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) were greater in children with higher weight.57 Tantisira et al described that BMI was associated with higher FEV1 and FVC but lower FEV1/FVC among participants in the Childhood Asthma Management Program (CAMP).58 In a recent meta-analysis including dozens of studies we reported that childhood obesity is associated with normal or higher FEV1 and FVC, but a lower FEV1/FVC.59 We recently described that childhood obesity is associated with airway dysanapsis, an incongruence between the growth of the lung parenchyma and that of the caliber of the airways that is reflected in normal or supra-normal FEV1 and FVC, but with larger effects on FVC that lead to a low FEV1/FVC ratio.60 Obese children with asthma and dysanapsis had increased symptoms, medication use, and asthma exacerbations.
Many studies of spirometric lung function have found differential associations based on sex. In the study by Tantisira, the decrease in FEV1/FVC with BMI was more pronounced in boys than in girls.58 Accordingly, we found that the risk of dysanapsis from obesity was higher in boys. However, others have found stronger obesity-lung function associations in girls. Similarly, age is a critical factor. In a large meta-analysis of 24 birth cohorts, Den Dekker et al showed that greater birthweight and faster infant weight gain was associated with higher FEV1 and FVC, but lower FEV1/FVC, in school-age children.61 Conversely, Strunk et al reported that CAMP participants who were not obese during the trial but became obese later on had significant decreases in FEV1 and FEV1/FVC (compared to participants who were never obese) at ~26–30 years of age, with no significant associations with FVC.62 This is consistent with data in adults: obese patients with early-onset asthma have more severe airflow limitation than obese adults with late-onset asthma.47
The effect of childhood obesity on lung volumes has been less studied, with only a handful of reports that have described conflicting findings. Davidson et al reported that obese non-asthmatic children had lower functional residual capacity (FRC), residual volume (RV), and expiratory reserve volume (ERV) than their non-obese counterparts.63 Rastogi et al recently reported similar findings, further describing associations with insulin resistance and reduced high-density lipoprotein (HDL).64 However, others have reported higher total lung capacity (TLC) in obese adolescents, and yet others have reported no significant associations.65,66 Similarly, it is not clear whether obesity leads to changes in airway hyperresponsiveness (AHR) in children, with some studies reporting higher67 and others lower AHR.68
Lung Function and Airway Hyperresponsiveness in Obese Adults
Obesity causes significant changes to normal lung physiology in adults (Figure 2). Excessive accumulation of fat in the thoracic and abdominal cavities lead to lung compression and an attendant reduction in lung volume.69 The most notable changes include a reduction in FRC and ERV.70–72 Radial traction of lung parenchymal attachments around the airways is attenuated at low lung volumes,73 which may contribute to airway collapse. Indeed, we have shown that obesity increases the collapsibility of the peripheral airways and parenchyma, especially among asthmatics with late-onset disease.74
Figure 2. Effect of obesity on biomarkers and clinical outcomes of asthma in adults.
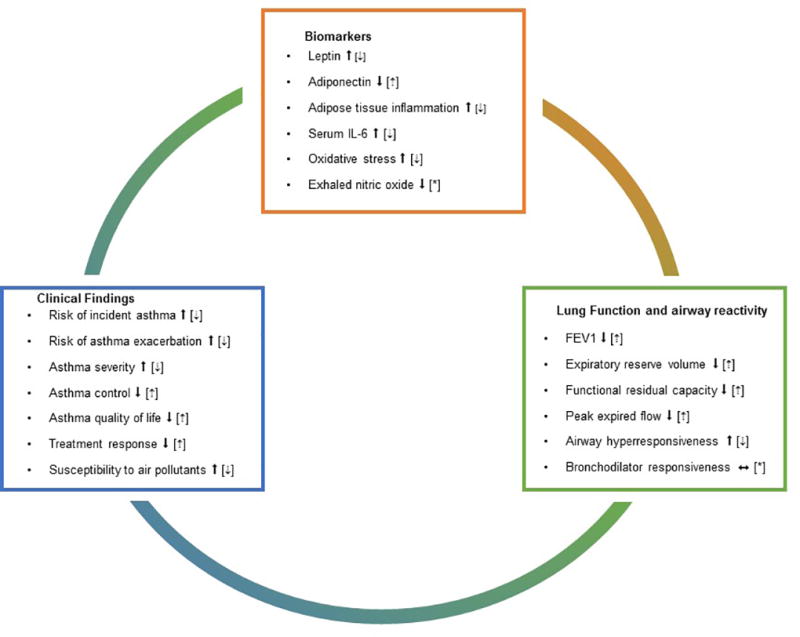
Proposed pathways that have been found altered in obesity and asthma in adults. Solid arrows indicate the effect of obesity. Dashed arrows inside brackets indicate changes that have been seen after weight loss. *Pathway has not been studied or no significant associations have been reported.
AHR is a distinguishing feature of asthma. The only prospective longitudinal cohort study that investigated the relationship between obesity and AHR –in more than 7,000 adults– reported that the risk for AHR increases with BMI, and weight gain was a risk factor for developing AHR.75 Some smaller studies failed to find a consistent relationship between AHR and obesity;76,77 this might be related to small sample sizes or differences between patient populations.
Misdiagnosis of asthma is common, but no more common in obese than non-obese patients. Aaron et al conducted a prospective study of 540 individuals with physician-diagnosed asthma and after rigorous assessment with bronchial reversibility and methacholine challenge, and withdrawal of asthma medication, they concluded that 31.8% of obese and 28.7% of non-obese patients diagnosed with asthma were actually misclassified as having asthma.78 Diagnosis of asthma should be confirmed by physiological testing in both lean and obese patients with respiratory symptoms.
The Role of Diet
Specific micronutrients may be involved in the association between obesity and asthma. Obesity is associated with low circulating vitamin D.79 Vitamin D deficiency may be a risk factor for the development of both obesity and asthma: prenatal vitamin D insufficiency has been associated with obesity in the offspring,80 and prenatal vitamin D supplementation led to a small decrease in the risk of wheezing illness at age 3 (though this did not reach formal statistical significance).81,82 This could be highly significant for asthma in obesity; there is a growing literature on the association between vitamin D deficiency and the risk of respiratory infections, asthma exacerbations83 and corticosteroid resistance,84 and so vitamin D deficiency may predispose to the development of obesity, and then to a phenotype of increased asthma severity and corticosteroid resistance. The efficacy of supplementation of vitamin D specifically on asthma in obesity is not known.
Diets that promote obesity, such as the Western diet pattern, tend to be high in saturated fatty acids, low in fiber and anti-oxidants, and high in sugars like fructose. There is a growing literature on the harmful effects of these specific dietary components on asthma. Ingestion of a single meal high in saturated fatty acids increases neutrophilic airway inflammation and decreases bronchodilator responsiveness.85 Animal studies suggest that a high fat diet increases the number of innate lymphoid cells in the lung, and can induce both innate AHR and allergic airway inflammation through an IL1β pathway.86,87 Supplementation with a high versus low anti-oxidant diet for 14 days (but not an anti-oxidant supplement) improved spirometric lung function and increased subsequent time to asthma exacerbation, though this has not been studied specifically in obese asthma.88 Studies in a mouse model of asthma suggest that a diet high in fructose promotes systemic metabolic dysfunction, and increases AHR and airway oxidative stress.89 There are few studies of dietary interventions promoting healthy dietary patterns in obese asthmatics, though a recent pilot study suggests that this approach may constitute an alternative to simply promoting weight loss.90
Specific dietary and nutritional risk factors may affect children. Breastfeeding has been associated with lower risks of both obesity and asthma.91,92 High-sugar containing beverages are a risk factor for asthma,93 as is a diet poor in vegetables and grains but rich in sweets and dairy products.94 Omega-3 has been associated with lower incidence of asthma, whereas omega-6 fatty acids are associated with higher risk of asthma in the pediatric age.95,96
The studies described above implicate dietary factors as potentially having direct effects on the airways, but it is also possible that diet could have indirect effects on the airway through effects on the gut microbiome.
The Microbiome and the Pathophysiology of Obese Asthma
Dietary changes lead to alterations in gut microbiome, and the changes characteristic of a Western dietary pattern which promote obesity might also affect the development of allergic airway disease. Bacterial colonization of the gut plays a key role in the fermentation of dietary fiber and the generation of short chain fatty acids (SCFA). Obesogenic diets are typically high in fat and low in soluble fiber; low fiber is associated with changes in gut microbiome and circulating levels of SCFA.97 Bacteroidetes bacteria –a major producer of SCFA– is reduced in the gut in obesity,98 and in the lungs in asthma.99 A low fiber diet decreases levels of the SCFA propionate; Trompette et al showed that a low fiber diet with low circulating propionate was associated with exaggerated allergic airway inflammation in a mouse model, and that increasing propionate decreased the ability of dendritic cells to promote Th2 responses and so attenuated allergic airway inflammation.100 Conversely, Thorburn et al showed that a high fiber diet increases levels of acetate, which inhibits the development of allergic airway inflammation in a mouse model through effects on histone deacetylase 9 and Treg function; this effect could also be seen in offspring of mice fed a high fiber diet, likely through epigenetic modification of the FoxP3 promoter.101 The authors also reported that high serum acetate in women was associated with lower risk of allergic airway disease in children. These studies suggest that diets low in fiber lead to changes in the gut microbiome, which can promote both obesity and allergic asthma.
Another factor that could alter microbiome is antibiotic exposure. Antibiotic exposure early in life has been associated with asthma102 and with obesity.103 The leading theory is that early changes in the microbiome may alter the maturation of the immune system, as anomalous responses to these changes have been reported to precede asthma and atopy.104 Probiotic supplementation in early life (in utero and/or infancy to either the mother, the infant, or both) has been shown to reduce the risk of atopy but not asthma.105
The airway microbiome may be altered in obese asthma. A recent study in bronchial brushings from severe asthmatics showed that BMI was associated with changes in airway microbial composition and with fewer lung tissue eosinophils.106 It is unclear whether these microbiome changes cause the reduced eosinophils, or are simply an unrelated consequence of other changes seen in obesity (such as other immune derangements or increased gastroesophageal reflux).
Inflammatory and Metabolic Changes in Obese Asthma
Many studies have reported that obesity-related asthma is more often non-atopic; yet, some studies have reported that obesity in itself is associated with atopy.107,108 In adolescents participating in the National Health and Nutrition Examination Survey (NHANES), we found that obesity was associated with asthma only among participants with normal or low exhaled nitric oxide (FeNO); however, among those who already had asthma and high FeNO, obesity was associated with increased asthma severity.109
Classic Th2 inflammation is complicated by the obese state. For example, eosinophilic airway inflammation is altered in obesity, with altered trafficking of eosinophils into the airway lumen – sputum eosinophils and exhaled nitric oxide might underestimate the degree of Type 2 inflammation in obesity.110 Obesity skews CD4 cells towards Th1 polarization, which is associated with worse asthma severity and control, and abnormal lung function.42 It is worth noting that eosinophils, alternatively activated macrophages and Type 2 cytokines are thought to play an important homeostatic role in healthy adipose tissue, with infiltration of pro-inflammatory M1 macrophages and decreased eosinophils associated with the development of obesity and insulin resistance – how this relates to airway disease is not clear.111,112
Innate immune responses involving Th17 pathways and innate lymphoid cells (ILCs) have also been implicated.86,87 ILCs play an important role in adipose tissue homeostasis, and thus changes in ILC function in obese adipose tissue could contribute to both obesity and asthma.111,113 Macrophage activation by ILCs and other pathways may constitute an important link between adiposity and worse asthma outcomes.114 In patients, Zheng et al. reported increased sputum neutrophils in “non-atopic obese asthmatics”, while subjects with “atopy-obesity overlap” exhibited higher sputum macrophage counts, suggesting the importance of these innate pathawys in obesity-related asthma.115
Changes in pulmonary innate immune function likely contribute to asthma in obesity. For example, bronchoalveolar fluid levels of surfactant protein A (SPA) –which helps modulate response to infectious and other insults– are lower in obese asthmatic subjects compared to their lean counterparts, and mouse models have shown that administration of SPA reduces lung tissue eosinophilia following allergen challenge,116 suggesting that changes in surfactant protein function could alter airway eosinophilia in obese asthma.
Adipokines and other cytokines produced or induced by adipose tissue may also affect the lungs and the airways (Figure 2).117,118 Higher leptin levels in obese adolescents correlate inversely with FEV1, FVC, and FEV1/FVC,41,119 and visceral fat leptin expression correlates with airway reactivity in adults.5 Leptin and adiponectin have also been associated with exercise-induced changes in lung function.120 Most existing literature is based on cross-sectional studies, and it is not clear whether adipokine levels may serve as biomarkers to identify at risk patients, to follow response to management, or are pathogenic mediators of disease.
Metabolic dysregulation plays an important role in many complications of obesity, including asthma.121,122 Hyperglycemia and hyperinsulinemia may contribute to AHR and remodeling via epithelial damage and airway smooth muscle proliferation.123–125 The metabolic syndrome has been associated with asthma and with lower lung function in adults.126–128 Similarly, we previously reported that insulin resistance and the metabolic syndrome are associated with lower lung function in adolescents with and without asthma (Figure 3).129 Obesity-related pro-inflammatory cytokines like IL-6 may play a crucial role in the relationship between the metabolic syndrome, lung function, and asthma severity.4 Innovative approaches such as breath condensate analytics using nuclear magnetic resonance have recently identified distinct metabolic signatures in obese asthma, suggesting unique pathogenic pathways compared to obesity or asthma alone.130 Research is needed to determine whether pharmacological management of the metabolic syndrome may lead to improvements in asthma outcomes. A recent retrospective study reported that metformin use was associated with improved asthma outcomes among subjects with both diabetes and asthma,131 although a prospective study of pioglitazone did not show efficacy in obese asthma.132
Figure 3. FEV1/FVC in adolescents with metabolic syndrome.
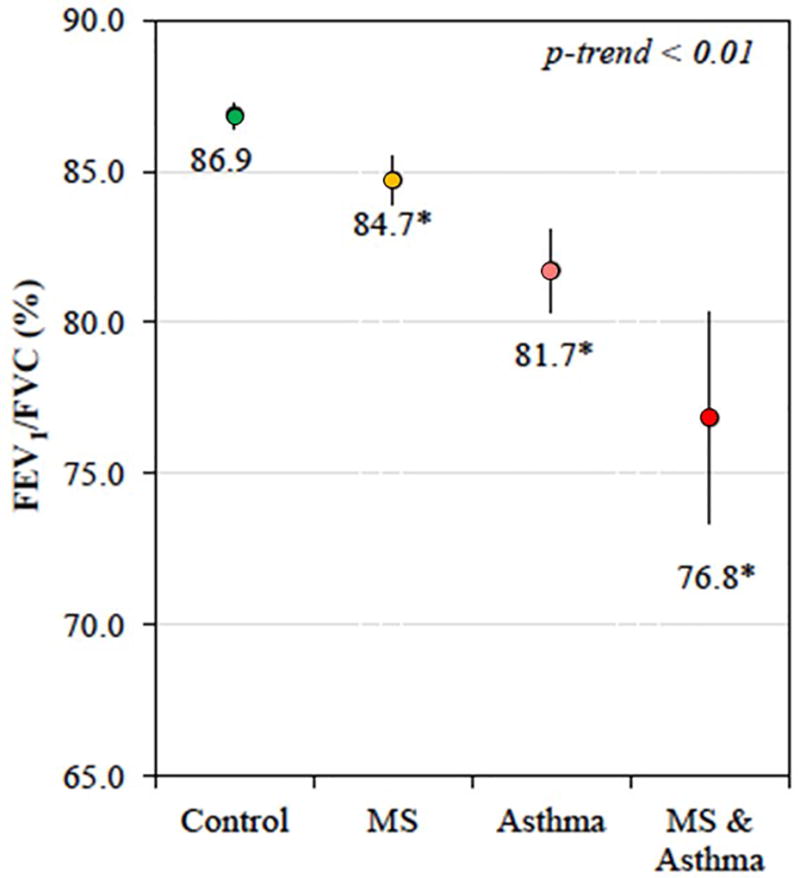
Metabolic syndrome (MS) and asthma synergistically reduce FEV1/FVC in adolescents. Data from NHANES. Models adjusted for age, sex, race/ethnicity, health insurance, family history of asthma, tobacco smoke exposure, C-reactive protein, and BMI z-score. Reproduced with permission from JACI 2015 (PMID 25748066).
Increased oxidative stress occurs in obesity, and increased airway oxidative stress has been found particularly in obese adults with late onset asthma.133 This is thought to be related to reduction in the biovailability of arginine, a substrate for the production of nitric oxide (NO): NO is an endogenous bronchodilator, and so reduced NO bioavailability may contribute to airway disease in obesity.53 Altered adaptive and innate immune responses, adipose related inflammation and increased oxidative stress all likely contribute to asthma in obesity.
Genetics, Epigenetics, and Genomics
Both asthma and obesity have a considerable hereditary component, and thus investigators have studied whether there are genetic variants that may represent a link. Yet to date these studies have been somewhat inauspicious. Candidate gene studies have reported a few genes associated with asthma and BMI, such as PRKCA, LEP, and ADRB3.134–136 The largest pediatric genome-wide association study (GWAS) to date, which included over 23,000 children and adults, reported gene DENND1B, although the main single nucleotide polymorphism (SNP) did not replicate in the independent cohorts.137
Other approaches have yielded more encouraging results. In a gene-by-environment analysis, Wang et al found seven SNPs in 17q21 (a well-known asthma-associated locus) were associated with BMI only among subjects with asthma in two independent cohorts.138 In a small pilot study in 32 children, Rastogi et al described differential epigenome-wide DNA methylation patterns in children according to their obesity and asthma status.139 In a CD4+ T-cell transcriptome analysis, they also reported Th1-related pathways that are differentially expressed in obese vs non-obese asthmatic children.140 And a study in mice and humans by Ahangari and colleagues found that expression of gene CHI3L1 can be induced by a high-fat diet, and that its product (chitinase 3-like 1) may contribute to both truncal obesity and asthma symptoms.141 Thus, while obese asthma may not be directly determined by genetic polymorphisms, it may be influenced by epigenetic or transcriptomic regulation.
Genetics have also allowed investigators to dissect the many potential factors that may confound the association between obesity and asthma, including socioeconomic status, lifestyle factors, and environmental exposures. Using a Mendelian randomization approach, Granell et al recently constructed a weighted allele score using 32 SNPs known to be associated with BMI, and demonstrated that the score was also strongly associated with asthma in school-aged children.142 Similarly, in adults, Skaaby and colleagues used a score of 26 BMI-associated SNPs, and found that BMI is related to higher risk of asthma and lower lung function.143
Lifestyle Weight Loss Interventions
Studies published on the effects of various life-style weight loss on asthma control find significant improvements in asthma control and spirometric lung function with sufficient weight loss (Table 1).53,144–152 Interventions vary from liquid diet replacement, to a more graduated dietary education approach. In adults, it appears that weight loss of at least 5% is required to produce a significant improvement in asthma control.150 This is typically associated with improvements in peak flow, spirometric lung function, and ERV. Studies which have produced the most weight loss appear to be associated with the most significant improvements in asthma control. Effects on AHR have been variable, with some studies151 –but not all145– reporting significant improvements in airway reactivity; we speculate the reasons for these contrasting findings may be related to the different phenotypes of obese asthmatics. Few studies have reported the effects on markers of airway inflammation. One recent study compared dietary intervention versus exercise plus dietary intervention and found improvements in exhaled nitric oxide with exercise plus dietary intervention;152 but it is not clear if it this was related to exercise (exercise may reduce allergic airway inflammation through effects on Treg cell function153) or the weight loss. Scott et al reported a decrease in airway neutrophilic inflammation in proportion to gynoid fat loss in women (and reduction in saturated fatty acid intake in men).53
Table 1.
Studies of lifestyle weight loss interventions and asthma
| Author | Year | Number of Subjects | Groups/Intervention | Main Findings |
|---|---|---|---|---|
| Hakala144 | 2000 | 14 adults (all with asthma) | Single arm, very low calorie diet, 8-week intervention | Significant improvements in peak flow, spirometry and lung volumes. Mean weight loss 13.7 Kg. |
| Aaron145 | 2004 | 58 women (24 with asthma) | Single arm, meal replacement, 6-month intervention | Significant improvement in spirometry but not in AHR. Mean weight loss 20 Kg. |
| Johnson146 | 2007 | 10 adults (all with asthma) | Single arm, alternate day caloric restriction, 2-month intervention | Significant improvements in spirometry and asthma control, but not in spirometry. Mean weight loss 8% of baseline. |
| Van de Griendt154 | 2012 | 112 children (8–18 years, all without asthma) | Single arm, multidisciplinary weight loss intervention for severely obese children, 26 weeks’ duration | FEV1, TLC and ERV improved. Only ERV correlated with the reduction in BMI and in waist circumference. Mean weight loss 13.9 Kg. |
| Da Silva155 | 2012 | 76 adolescents (26 with asthma) | Single arm, multidisciplinary weight loss intervention, 1 year duration | Improvements in adipokines and lung function; improved asthma severity in the group with asthma. |
| Scott53 | 2013 | 46 adults (all with asthma) | Randomized to diet, exercise, or diet and exercise groups, 10-week intervention | Asthma control improved in diet and diet+exercise groups; 5–10% weight loss was associated with improved asthma control. |
| Jensen156 | 2013 | 28 children (8–17 years, all with asthma) | Randomized to intensive dietary intervention versus wait list control, 10-week intervention | Improvements in ERV and asthma control in the intervention group. Mean weight loss 3.4 Kg. |
| Luna-Pech148 | 2014 | 51 children (12–18 years, all with asthma) | Randomized to supervised normocaloric versus no intervention for 28 weeks | Weight loss correlated with improved asthma control and quality of life. Mean weight loss 2.5 Kg. |
| van Leeuwen149 | 2014 | 20 children (8–18 years, all with asthma) | Single arm, dietary intervention, 6 weeks’ duration | Improvement in quality of life and exercise-induced bronchoconstriction, but not in asthma control. Mean weight loss 2.6% of baseline. |
| Ma150 | 2015 | 330 adults (all with asthma) | Randomized to intensive dietary intervention versus educational intervention, 1-year study | Increased odds of improvement in asthma control in those who lost ≥ 10% weight. |
| Pakhale151 | 2015 | 22 adults (all with asthma) | Single arm, liquid meal replacement versus observation control, 3 months’ duration | Improvement in FEV1, FVC, asthma control and AHR. Mean weight loss 16.5 Kg (14.2% of baseline) |
| Willeboordse157 | 2016 | 87 children (6–16 years, all with asthma) | Randomized to multifactorial intervention vs usual care, 18-month duration | Weight, lung function, and asthma outcomes improved in both groups; intervention had greater improvement in FVC and asthma control. |
| Freitas152 | 2017 | 55 adults (all with asthma) | Randomized to dietary and exercise intervention versus diet only, 3 months’ duration | Diet and exercise lost more weight than diet alone (6.8 vs 3.1%), greater improvements in asthma control, and FeNO |
Pediatric studies of weight loss interventions for obesity and asthma are scarce. Non-controlled studies have reported improvements in FEV1, ERV and TLC that correlate with BMI,149,154 and changes in adipokine levels that correlate with improvements in FEV1 and FVC as well as exercise-induced bronchoconstriction.155 However, without control groups all of these studies are limited to small-group before/after comparisons. In a recent randomized control trial (RCT), a dietary intervention resulted in significant improvements in ERV, although the difference with the control group was not significant.156 Conversely, another RCT in children with asthma reported a greater improvement in FVC in the intervention group compared to the control group, but BMI changes were similar in both groups; there were no differences in FEV1/FVC, ERV, or TLC.157 Finally, in another RCT Luna-Pech et al showed reduced exacerbations and improved quality of life.148
Bariatric Surgery
The effects of bariatric (weight loss) surgery have been reported by a number of investigators.158–160 It is the most effective intervention for producing sustained and significant weight loss, and all studies have reported highly significant improvements in asthma control, airway reactivity and lung function (Figure 2). Bariatric surgery also has significant effects on asthma exacerbations. Hasegawa et al studied 2261 patients with asthma using a population Emergency Department and in-patient sample database; bariatric surgery led to a nearly 60% reduction in the risk of having an asthma exacerbation, with a baseline risk of asthma exacerbation in the population of approximately 22%.161 Reduced exacerbations may partly be related to effects on lung mechanics and airway reactivity, but as obesity is associated with worse outcomes related to viral and bacterial infections such as influenza and bacterial pneumonia,162,163 and impaired response to influenza vaccination,164 it is also possible that significant weight loss might reduce the risk of certain infections that precipitate asthma exacerbations.
Conclusions
Obesity is an important risk factor for asthma and for asthma morbidity, both in children and adults. While there are many common pathophysiological and clinical commonalities, certain characteristics differ between both age groups. This is a reflection of an obese asthma syndrome that is complex and multifactorial. Potential underlying mechanisms include a shared genetic component, dietary and nutritional factors, alterations in the gut microbiome, systemic inflammation, metabolic abnormalities, and changes in lung anatomy and function (Figure 4). There is growing evidence that weight loss interventions also help improve asthma outcomes. Future studies should characterize obesity beyond BMI, considering other anthropometric indices and biomarkers, much like asthma is not phenotyped merely by the presence or absence of wheezing.
Figure 4. Factors contributing to the syndrome of obesity-related asthma.
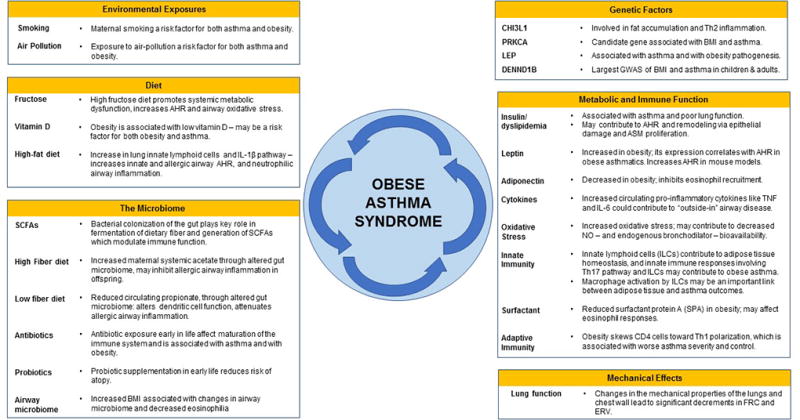
AHR: Airway hyperreactivity. ASM: Airway smoth muscle. ERV: Expiratory reserve volume. FRC: Functional residual capacity. GWAS: Genome-wide association study. NO: Nitric oxide. SCFAs: Short-chain fatty acids
Future research should aim to.
-
-
Improve our understanding of the different mechanisms and pathways that underlie obese asthma.
-
-
Identify sub-phenotypes that may have different pathophysiology and thus respond to different management strategies, as well as find biomarkers that may help identify these subgroups.
-
-
Develop new therapies and treatment approaches for these patients.
-
-
Phenotype metabolic dysfunction rather than just BMI in asthmatics.
-
-
Investigate how to effectively implement lifestyle interventions targeting obese asthmatics.
-
-
Investigate how changes in the diet and microbiome might affect outcomes in asthma.
What do we know on the subject?
Obesity is associated with increased asthma risk and morbidity.
“Obese asthma” is a complex syndrome with many phenotypes, which may differ between children and adults.
Factors and pathways implicated in obese asthma include changes in lung function, alterations in diet and nutrients, metabolic dysregulation, microbiome changes, and differences in epigenetic/genomic regulation.
What is still unknown?
How to accurately classify obese asthma phenotypes, both in children and adults.
Whether new therapies or approaches may be more successful for patients with obesity and asthma.
Whether early-life microbiome or immune alterations predispose individuals to both obesity and asthma.
Acknowledgments
Funding: Dr. Forno’s contribution was supported in part by grants HL125666 and HL119952 from the U.S. National Institutes of Health (NIH), a grant from Children’s Hospital of Pittsburgh of UPMC, and an award from the Klosterfrau Foundation. Drs. Peters and Dixon were supported by NIH grants HL133920 and HL130847.
Abbreviations/Acronyms
- AHR
Airway hyper-reactivity or hyper-responsiveness
- BMI
Body mass index
- CAMP
Childhood Asthma Management Program
- ERV
Expiratory reserve volume
- FeNO
Fractional exhaled nitric oxide
- FEV1
Forced expiratory volume in the first second
- FRC
Functional residual capacity
- FVC
Forced vital capacity
- GWAS
Genome-wide association study
- HDL
High-density lipoprotein
- ICS
Inhaled corticosteroids
- IL
Interleukin
- ILC
Innate lymphoid cells
- LABA
Long-acting beta-agonists
- NO
Nitric oxide
- RCT
Randomized controlled trial
- SCD
Stearoyl-coenzyme A reductase
- SCFA
Short-chain fatty acids
- SNP
Single nucleotide polymorphism
- SPA
Surfactant protein A
- TLC
Total lung capacity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Freedman DS, Butte NF, Taveras EM, Lundeen EA, Blanck HM, Goodman AB, et al. BMI z-Scores are a poor indicator of adiposity among 2- to 19-year-olds with very high BMIs, NHANES 1999–2000 to 2013–2014. Obesity (Silver Spring) 2017;25:739–46. doi: 10.1002/oby.21782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freedman DS, Butte NF, Taveras EM, Goodman AB, Ogden CL, Blanck HM. The Limitations of Transforming Very High Body Mass Indexes into z-Scores among 8.7 Million 2- to 4-Year-Old Children. J Pediatr. 2017;188:50–6. e1. doi: 10.1016/j.jpeds.2017.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson CM, Su H, Thomas DM, Heo M, Golnabi AH, Pietrobelli A, et al. Tri-Ponderal Mass Index vs Body Mass Index in Estimating Body Fat During Adolescence. JAMA Pediatr. 2017;171:629–36. doi: 10.1001/jamapediatrics.2017.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, et al. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med. 2016;4:574–84. doi: 10.1016/S2213-2600(16)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sideleva O, Suratt BT, Black KE, Tharp WG, Pratley RE, Forgione P, et al. Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med. 2012;186:598–605. doi: 10.1164/rccm.201203-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asthma Data, Statistics, and Surveillance. 2016 Available from http://www.cdc.gov/asthma/most_recent_data.htm.
- 7.Childhood Obesity. 2016 Available from https://www.cdc.gov/obesity/childhood/index.html.
- 8.Gilliland FD, Berhane K, Islam T, McConnell R, Gauderman WJ, Gilliland SS, et al. Obesity and the risk of newly diagnosed asthma in school-age children. Am J Epidemiol. 2003;158:406–15. doi: 10.1093/aje/kwg175. [DOI] [PubMed] [Google Scholar]
- 9.Gold DR, Damokosh AI, Dockery DW, Berkey CS. Body-mass index as a predictor of incident asthma in a prospective cohort of children. Pediatr Pulmonol. 2003;36:514–21. doi: 10.1002/ppul.10376. [DOI] [PubMed] [Google Scholar]
- 10.Mannino DM, Mott J, Ferdinands JM, Camargo CA, Friedman M, Greves HM, et al. Boys with high body masses have an increased risk of developing asthma: findings from the National Longitudinal Survey of Youth (NLSY) Int J Obes (Lond) 2006;30:6–13. doi: 10.1038/sj.ijo.0803145. [DOI] [PubMed] [Google Scholar]
- 11.Mamun AA, Lawlor DA, Alati R, O'Callaghan MJ, Williams GM, Najman JM. Increasing body mass index from age 5 to 14 years predicts asthma among adolescents: evidence from a birth cohort study. Int J Obes (Lond) 2007;31:578–83. doi: 10.1038/sj.ijo.0803571. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Lai HJ, Roberg KA, Gangnon RE, Evans MD, Anderson EL, et al. Early childhood weight status in relation to asthma development in high-risk children. J Allergy Clin Immunol. 2010;126:1157–62. doi: 10.1016/j.jaci.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinmayr G, Forastiere F, Buchele G, Jaensch A, Strachan DP, Nagel G, et al. Overweight/obesity and respiratory and allergic disease in children: international study of asthma and allergies in childhood (ISAAC) phase two. PLoS One. 2014;9:e113996. doi: 10.1371/journal.pone.0113996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mebrahtu TF, Feltbower RG, Greenwood DC, Parslow RC. Childhood body mass index and wheezing disorders: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2015;26:62–72. doi: 10.1111/pai.12321. [DOI] [PubMed] [Google Scholar]
- 15.Ho WC, Lin YS, Caffrey JL, Lin MH, Hsu HT, Myers L, et al. Higher body mass index may induce asthma among adolescents with pre-asthmatic symptoms: a prospective cohort study. BMC Public Health. 2011;11:542. doi: 10.1186/1471-2458-11-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flaherman V, Rutherford GW. A meta-analysis of the effect of high weight on asthma. Arch Dis Child. 2006;91:334–9. doi: 10.1136/adc.2005.080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willeboordse M, van den Bersselaar DL, van de Kant KD, Muris JW, van Schayck OC, Dompeling E. Sex differences in the relationship between asthma and overweight in Dutch children: a survey study. PLoS One. 2013;8:e77574. doi: 10.1371/journal.pone.0077574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu KD, Billimek J, Bar-Yoseph R, Radom-Aizik S, Cooper DM, Anton-Culver H. Sex Differences in the Relationship between Fitness and Obesity on Risk for Asthma in Adolescents. J Pediatr. 2016;176:36–42. doi: 10.1016/j.jpeds.2016.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maltz L, Matz EL, Gordish-Dressman H, Pillai DK, Teach SJ, Camargo CA, Jr, et al. Sex differences in the association between neck circumference and asthma. Pediatr Pulmonol. 2016;51:893–900. doi: 10.1002/ppul.23381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castro-Rodriguez JA. A new childhood asthma phenotype: obese with early menarche. Paediatr Respir Rev. 2016;18:85–9. doi: 10.1016/j.prrv.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Forno E, Young OM, Kumar R, Simhan H, Celedon JC. Maternal obesity in pregnancy, gestational weight gain, and risk of childhood asthma. Pediatrics. 2014;134:e535–46. doi: 10.1542/peds.2014-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumas O, Varraso R, Gillman MW, Field AE, Camargo CA., Jr Longitudinal study of maternal body mass index, gestational weight gain, and offspring asthma. Allergy. 2016;71:1295–304. doi: 10.1111/all.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harskamp-van Ginkel MW, London SJ, Magnus MC, Gademan MG, Vrijkotte TG. A Study on Mediation by Offspring BMI in the Association between Maternal Obesity and Child Respiratory Outcomes in the Amsterdam Born and Their Development Study Cohort. PLoS One. 2015;10:e0140641. doi: 10.1371/journal.pone.0140641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malti N, Merzouk H, Merzouk SA, Loukidi B, Karaouzene N, Malti A, et al. Oxidative stress and maternal obesity: feto-placental unit interaction. Placenta. 2014;35:411–6. doi: 10.1016/j.placenta.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Wilson RM, Marshall NE, Jeske DR, Purnell JQ, Thornburg K, Messaoudi I. Maternal obesity alters immune cell frequencies and responses in umbilical cord blood samples. Pediatr Allergy Immunol. 2015;26:344–51. doi: 10.1111/pai.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa SM, Isganaitis E, Matthews TJ, Hughes K, Daher G, Dreyfuss JM, et al. Maternal obesity programs mitochondrial and lipid metabolism gene expression in infant umbilical vein endothelial cells. Int J Obes (Lond) 2016 doi: 10.1038/ijo.2016.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popovic M, Pizzi C, Rusconi F, Galassi C, Gagliardi L, De Marco L, et al. Infant weight trajectories and early childhood wheezing: the NINFEA birth cohort study. Thorax. 2016 doi: 10.1136/thoraxjnl-2015-208208. [DOI] [PubMed] [Google Scholar]
- 28.Casas M, den Dekker HT, Kruithof CJ, Reiss IK, Vrijheid M, de Jongste JC, et al. Early childhood growth patterns and school-age respiratory resistance, fractional exhaled nitric oxide and asthma. Pediatr Allergy Immunol. 2016 doi: 10.1111/pai.12645. [DOI] [PubMed] [Google Scholar]
- 29.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–6. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akinbami LJ, Fryar CD. NCHS data brief, no 239. Hyattsville, MD: National Center for Health Statistics; 2016. Asthma prevalence by weight status among adults: United States, 2001–2014. NCHS Data Brie. Hyattsville, MD: National Center for Health Statistics, 2016. [PubMed] [Google Scholar]
- 31.Chen Z, Salam MT, Alderete TL, Habre R, Bastain TM, Berhane K, et al. Effects of Childhood Asthma on the Development of Obesity among School-aged Children. Am J Respir Crit Care Med. 2017;195:1181–8. doi: 10.1164/rccm.201608-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang JE, Hossain J, Holbrook JT, Teague WG, Gold BD, Wise RA, et al. Gastro-oesophageal reflux and worse asthma control in obese children: a case of symptom misattribution? Thorax. 2016;71:238–46. doi: 10.1136/thoraxjnl-2015-207662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lang JE, Hossain MJ, Lima JJ. Overweight children report qualitatively distinct asthma symptoms: analysis of validated symptom measures. J Allergy Clin Immunol. 2015;135:886–93. e3. doi: 10.1016/j.jaci.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lang JE, Hossain J, Dixon AE, Shade D, Wise RA, Peters SP, et al. Does age impact the obese asthma phenotype? Longitudinal asthma control, airway function, and airflow perception among mild persistent asthmatics. Chest. 2011;140:1524–33. doi: 10.1378/chest.11-0675. [DOI] [PubMed] [Google Scholar]
- 35.Wood LG. Metabolic dysregulation. Driving the obese asthma phenotype in adolescents? Am J Respir Crit Care Med. 2015;191:121–2. doi: 10.1164/rccm.201412-2221ED. [DOI] [PubMed] [Google Scholar]
- 36.Ahmadizar F, Vijverberg SJ, Arets HG, de Boer A, Lang JE, Kattan M, et al. Childhood obesity in relation to poor asthma control and exacerbation: a meta-analysis. Eur Respir J. 2016;48:1063–73. doi: 10.1183/13993003.00766-2016. [DOI] [PubMed] [Google Scholar]
- 37.Okubo Y, Nochioka K, Hataya H, Sakakibara H, Terakawa T, Testa M. Burden of Obesity on Pediatric Inpatients with Acute Asthma Exacerbation in the United States. J Allergy Clin Immunol Pract. 2016;4:1227–31. doi: 10.1016/j.jaip.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Aragona E, El-Magbri E, Wang J, Scheckelhoff T, Scheckelhoff T, Hyacinthe A, et al. Impact of Obesity on Clinical Outcomes in Urban Children Hospitalized for Status Asthmaticus. Hosp Pediatr. 2016;6:211–8. doi: 10.1542/hpeds.2015-0094. [DOI] [PubMed] [Google Scholar]
- 39.van Gent R, van der Ent CK, Rovers MM, Kimpen JL, van Essen-Zandvliet LE, de Meer G. Excessive body weight is associated with additional loss of quality of life in children with asthma. J Allergy Clin Immunol. 2007;119:591–6. doi: 10.1016/j.jaci.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Borrell LN, Nguyen EA, Roth LA, Oh SS, Tcheurekdjian H, Sen S, et al. Childhood obesity and asthma control in the GALA II and SAGE II studies. Am J Respir Crit Care Med. 2013;187:697–702. doi: 10.1164/rccm.201211-2116OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eising JB, Uiterwaal CS, Evelein AM, Visseren FL, van der Ent CK. Relationship between leptin and lung function in young healthy children. Eur Respir J. 2014;43:1189–92. doi: 10.1183/09031936.00149613. [DOI] [PubMed] [Google Scholar]
- 42.Rastogi D, Fraser S, Oh J, Huber AM, Schulman Y, Bhagtani RH, et al. Inflammation, metabolic dysregulation, and pulmonary function among obese urban adolescents with asthma. Am J Respir Crit Care Med. 2015;191:149–60. doi: 10.1164/rccm.201409-1587OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vinding RK, Stokholm J, Chawes BL, Bisgaard H. Blood lipid levels associate with childhood asthma, airway obstruction, bronchial hyperresponsiveness, and aeroallergen sensitization. J Allergy Clin Immunol. 2016;137:68–74. e4. doi: 10.1016/j.jaci.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 44.Forno E, Lescher R, Strunk R, Weiss S, Fuhlbrigge A, Celedon JC, et al. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011;127:741–9. doi: 10.1016/j.jaci.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGarry ME, Castellanos E, Thakur N, Oh SS, Eng C, Davis A, et al. Obesity and bronchodilator response in black and Hispanic children and adolescents with asthma. Chest. 2015;147:1591–8. doi: 10.1378/chest.14-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu KD, Breysse PN, Diette GB, Curtin-Brosnan J, Aloe C, Williams DL, et al. Being overweight increases susceptibility to indoor pollutants among urban children with asthma. J Allergy Clin Immunol. 2013;131:1017–23. 23 e1–3. doi: 10.1016/j.jaci.2012.12.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, et al. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol. 2011;127:1486–93. e2. doi: 10.1016/j.jaci.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schatz M, Hsu JW, Zeiger RS, Chen W, Dorenbaum A, Chipps BE, et al. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2014;133:1549–56. doi: 10.1016/j.jaci.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Vortmann M, Eisner MD. BMI and health status among adults with asthma. Obesity (Silver Spring) 2008;16:146–52. doi: 10.1038/oby.2007.7. [DOI] [PubMed] [Google Scholar]
- 50.Boulet LP, Franssen E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respiratory medicine. 2007;101:2240–7. doi: 10.1016/j.rmed.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 51.Sutherland ER, Goleva E, Strand M, Beuther DA, Leung DY. Body mass and glucocorticoid response in asthma. American journal of respiratory and critical care medicine. 2008;178:682–7. doi: 10.1164/rccm.200801-076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott HA, Gibson PG, Garg ML, Wood LG. Airway inflammation is augmented by obesity and fatty acids in asthma. Eur Respir J. 2011;38:594–602. doi: 10.1183/09031936.00139810. [DOI] [PubMed] [Google Scholar]
- 53.Scott HA, Gibson PG, Garg ML, Pretto JJ, Morgan PJ, Callister R, et al. Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: a randomized trial. Clin Exp Allergy. 2013;43:36–49. doi: 10.1111/cea.12004. [DOI] [PubMed] [Google Scholar]
- 54.Dong GH, Qian Z, Liu MM, Wang D, Ren WH, Fu Q, et al. Obesity enhanced respiratory health effects of ambient air pollution in Chinese children: the Seven Northeastern Cities study. Int J Obes (Lond) 2013;37:94–100. doi: 10.1038/ijo.2012.125. [DOI] [PubMed] [Google Scholar]
- 55.Bennett WD, Ivins S, Alexis NE, Wu J, Bromberg PA, Brar SS, et al. Effect of Obesity on Acute Ozone-Induced Changes in Airway Function, Reactivity, and Inflammation in Adult Females. PLoS One. 2016;11:e0160030. doi: 10.1371/journal.pone.0160030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnston RA, Theman TA, Lu FL, Terry RD, Williams ES, Shore SA. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J Appl Physiol (1985) 2008;104:1727–35. doi: 10.1152/japplphysiol.00075.2008. [DOI] [PubMed] [Google Scholar]
- 57.Lazarus R, Colditz G, Berkey CS, Speizer FE. Effects of body fat on ventilatory function in children and adolescents: cross-sectional findings from a random population sample of school children. Pediatr Pulmonol. 1997;24:187–94. doi: 10.1002/(sici)1099-0496(199709)24:3<187::aid-ppul4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 58.Tantisira KG, Litonjua AA, Weiss ST, Fuhlbrigge AL Childhood Asthma Management Program Research G. Association of body mass with pulmonary function in the Childhood Asthma Management Program (CAMP) Thorax. 2003;58:1036–41. doi: 10.1136/thorax.58.12.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Forno E, Han YY, Mullen J, Celedon JC. Overweight, obesity, and lung function in children and adults – a meta-analysis. 2017 doi: 10.1016/j.jaip.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Forno E, Weiner DJ, Mullen J, Sawicki G, Kurland G, Han YY, et al. Obesity and Airway Dysanapsis in Children with and without Asthma. Am J Respir Crit Care Med. 2017;195:314–23. doi: 10.1164/rccm.201605-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.den Dekker HT, Sonnenschein-van der Voort AM, de Jongste JC, Anessi-Maesano I, Arshad SH, Barros H, et al. Early growth characteristics and the risk of reduced lung function and asthma: A meta-analysis of 25,000 children. J Allergy Clin Immunol. 2016;137:1026–35. doi: 10.1016/j.jaci.2015.08.050. [DOI] [PubMed] [Google Scholar]
- 62.Strunk RC, Colvin R, Bacharier LB, Fuhlbrigge A, Forno E, Arbelaez AM, et al. Airway Obstruction Worsens in Young Adults with Asthma Who Become Obese. J Allergy Clin Immunol Pract. 2015;3:765–71. e2. doi: 10.1016/j.jaip.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davidson WJ, Mackenzie-Rife KA, Witmans MB, Montgomery MD, Ball GD, Egbogah S, et al. Obesity negatively impacts lung function in children and adolescents. Pediatr Pulmonol. 2014;49:1003–10. doi: 10.1002/ppul.22915. [DOI] [PubMed] [Google Scholar]
- 64.Rastogi D, Bhalani K, Hall CB, Isasi CR. Association of pulmonary function with adiposity and metabolic abnormalities in urban minority adolescents. Ann Am Thorac Soc. 2014;11:744–52. doi: 10.1513/AnnalsATS.201311-403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mendelson M, Michallet AS, Perrin C, Levy P, Wuyam B, Flore P. Exercise training improves breathing strategy and performance during the six-minute walk test in obese adolescents. Respir Physiol Neurobiol. 2014;200:18–24. doi: 10.1016/j.resp.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 66.Mansell AL, Walders N, Wamboldt MZ, Carter R, Steele DW, Devin JA, et al. Effect of body mass index on response to methacholine bronchial provocation in healthy and asthmatic adolescents. Pediatr Pulmonol. 2006;41:434–40. doi: 10.1002/ppul.20368. [DOI] [PubMed] [Google Scholar]
- 67.Karampatakis N, Karampatakis T, Galli-Tsinopoulou A, Kotanidou EP, Tsergouli K, Eboriadou-Petikopoulou M, et al. Impaired glucose metabolism and bronchial hyperresponsiveness in obese prepubertal asthmatic children. Pediatr Pulmonol. 2016 doi: 10.1002/ppul.23516. [DOI] [PubMed] [Google Scholar]
- 68.Sposato B, Scalese M, Migliorini MG, Riccardi MP, Tosti Balducci M, Petruzzelli L, et al. Obesity can influence children's and adolescents' airway hyperresponsiveness differently. Multidiscip Respir Med. 2013;8:60. doi: 10.1186/2049-6958-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Watson RA, Pride NB, Thomas EL, Fitzpatrick J, Durighel G, McCarthy J, et al. Reduction of total lung capacity in obese men: comparison of total intrathoracic and gas volumes. J Appl Physiol (1985) 2010;108:1605–12. doi: 10.1152/japplphysiol.01267.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mehari A, Afreen S, Ngwa J, Setse R, Thomas AN, Poddar V, et al. Obesity and Pulmonary Function in African Americans. PLoS One. 2015;10:e0140610. doi: 10.1371/journal.pone.0140610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130:827–33. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 72.Pelosi P, Croci M, Ravagnan I, Tredici S, Pedoto A, Lissoni A, et al. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg. 1998;87:654–60. doi: 10.1097/00000539-199809000-00031. [DOI] [PubMed] [Google Scholar]
- 73.Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol. 1970;28:596–608. doi: 10.1152/jappl.1970.28.5.596. [DOI] [PubMed] [Google Scholar]
- 74.Al-Alwan A, Bates JH, Chapman DG, Kaminsky DA, DeSarno MJ, Irvin CG, et al. The nonallergic asthma of obesity. A matter of distal lung compliance. Am J Respir Crit Care Med. 2014;189:1494–502. doi: 10.1164/rccm.201401-0178OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Litonjua AA, Sparrow D, Celedon JC, DeMolles D, Weiss ST. Association of body mass index with the development of methacholine airway hyperresponsiveness in men: the Normative Aging Study. Thorax. 2002;57:581–5. doi: 10.1136/thorax.57.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schachter LM, Salome CM, Peat JK, Woolcock AJ. Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax. 2001;56:4–8. doi: 10.1136/thorax.56.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bustos P, Amigo H, Oyarzun M, Rona RJ. Is there a causal relation between obesity and asthma? Evidence from Chile. Int J Obes (Lond) 2005;29:804–9. doi: 10.1038/sj.ijo.0802958. [DOI] [PubMed] [Google Scholar]
- 78.Aaron SD, Vandemheen KL, Boulet LP, McIvor RA, Fitzgerald JM, Hernandez P, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121–31. doi: 10.1503/cmaj.081332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Earthman CP, Beckman LM, Masodkar K, Sibley SD. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes (Lond) 2012;36:387–96. doi: 10.1038/ijo.2011.119. [DOI] [PubMed] [Google Scholar]
- 80.Boyle VT, Thorstensen EB, Thompson JMD, McCowan LME, Mitchell EA, Godfrey KM, et al. The relationship between maternal 25-hydroxyvitamin D status in pregnancy and childhood adiposity and allergy: An observational study. Int J Obes (Lond) 2017 doi: 10.1038/ijo.2017.182. [DOI] [PubMed] [Google Scholar]
- 81.Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O'Connor GT, et al. Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. JAMA. 2016;315:362–70. doi: 10.1001/jama.2015.18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chawes BL, Bonnelykke K, Stokholm J, Vissing NH, Bjarnadottir E, Schoos AM, et al. Effect of Vitamin D3 Supplementation During Pregnancy on Risk of Persistent Wheeze in the Offspring: A Randomized Clinical Trial. JAMA. 2016;315:353–61. doi: 10.1001/jama.2015.18318. [DOI] [PubMed] [Google Scholar]
- 83.Brehm JM, Acosta-Perez E, Klei L, Roeder K, Barmada M, Boutaoui N, et al. Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med. 2012;186:140–6. doi: 10.1164/rccm.201203-0431OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lan N, Luo G, Yang X, Cheng Y, Zhang Y, Wang X, et al. 25-Hydroxyvitamin D3-deficiency enhances oxidative stress and corticosteroid resistance in severe asthma exacerbation. PLoS One. 2014;9:e111599. doi: 10.1371/journal.pone.0111599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wood LG, Garg ML, Gibson PG. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J Allergy Clin Immunol. 2011;127:1133–40. doi: 10.1016/j.jaci.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 86.Kim HY, Lee HJ, Chang YJ, Pichavant M, Shore SA, Fitzgerald KA, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. 2014;20:54–61. doi: 10.1038/nm.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Everaere L, Ait-Yahia S, Molendi-Coste O, Vorng H, Quemener S, LeVu P, et al. Innate lymphoid cells contribute to allergic airway disease exacerbation by obesity. J Allergy Clin Immunol. 2016;138:1309–18. e11. doi: 10.1016/j.jaci.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 88.Wood LG, Garg ML, Smart JM, Scott HA, Barker D, Gibson PG. Manipulating antioxidant intake in asthma: a randomized controlled trial. Am J Clin Nutr. 2012;96:534–43. doi: 10.3945/ajcn.111.032623. [DOI] [PubMed] [Google Scholar]
- 89.Singh VP, Aggarwal R, Singh S, Banik A, Ahmad T, Patnaik BR, et al. Metabolic Syndrome Is Associated with Increased Oxo-Nitrative Stress and Asthma-Like Changes in Lungs. PLoS One. 2015;10:e0129850. doi: 10.1371/journal.pone.0129850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blonstein AC, Lv N, Camargo CA, Wilson SR, Buist AS, Rosas LG, et al. Acceptability and feasibility of the 'DASH for Asthma' intervention in a randomized controlled trial pilot study. Public Health Nutr. 2016;19:2049–59. doi: 10.1017/S136898001500350X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dogaru CM, Nyffenegger D, Pescatore AM, Spycher BD, Kuehni CE. Breastfeeding and childhood asthma: systematic review and meta-analysis. Am J Epidemiol. 2014;179:1153–67. doi: 10.1093/aje/kwu072. [DOI] [PubMed] [Google Scholar]
- 92.Yan J, Liu L, Zhu Y, Huang G, Wang PP. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health. 2014;14:1267. doi: 10.1186/1471-2458-14-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berentzen NE, van Stokkom VL, Gehring U, Koppelman GH, Schaap LA, Smit HA, et al. Associations of sugar-containing beverages with asthma prevalence in 11-year-old children: the PIAMA birth cohort. Eur J Clin Nutr. 2015;69:303–8. doi: 10.1038/ejcn.2014.153. [DOI] [PubMed] [Google Scholar]
- 94.Han YY, Forno E, Brehm JM, Acosta-Perez E, Alvarez M, Colon-Semidey A, et al. Diet, interleukin-17, and childhood asthma in Puerto Ricans. Ann Allergy Asthma Immunol. 2015;115:288–93. e1. doi: 10.1016/j.anai.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li J, Xun P, Zamora D, Sood A, Liu K, Daviglus M, et al. Intakes of long-chain omega-3 (n-3 PUFAs and fish in relation to incidence of asthma among American young adults: the CARDIA study. Am J Clin Nutr. 2013;97:173–8. doi: 10.3945/ajcn.112.041145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.D'Vaz N, Meldrum SJ, Dunstan JA, Lee-Pullen TF, Metcalfe J, Holt BJ, et al. Fish oil supplementation in early infancy modulates developing infant immune responses. Clin Exp Allergy. 2012;42:1206–16. doi: 10.1111/j.1365-2222.2012.04031.x. [DOI] [PubMed] [Google Scholar]
- 97.Marsland BJ, Gollwitzer ES. Host-microorganism interactions in lung diseases. Nat Rev Immunol. 2014;14:827–35. doi: 10.1038/nri3769. [DOI] [PubMed] [Google Scholar]
- 98.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 99.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–66. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 101.Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ, et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun. 2015;6:7320. doi: 10.1038/ncomms8320. [DOI] [PubMed] [Google Scholar]
- 102.Pitter G, Ludvigsson JF, Romor P, Zanier L, Zanotti R, Simonato L, et al. Antibiotic exposure in the first year of life and later treated asthma, a population based birth cohort study of 143,000 children. Eur J Epidemiol. 2016;31:85–94. doi: 10.1007/s10654-015-0038-1. [DOI] [PubMed] [Google Scholar]
- 103.Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168:1063–9. doi: 10.1001/jamapediatrics.2014.1539. [DOI] [PubMed] [Google Scholar]
- 104.Dzidic M, Abrahamsson TR, Artacho A, Bjorksten B, Collado MC, Mira A, et al. Aberrant IgA responses to the gut microbiota during infancy precede asthma and allergy development. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.06.047. [DOI] [PubMed] [Google Scholar]
- 105.Elazab N, Mendy A, Gasana J, Vieira ER, Quizon A, Forno E. Probiotic administration in early life, atopy, and asthma: a meta-analysis of clinical trials. Pediatrics. 2013;132:e666–76. doi: 10.1542/peds.2013-0246. [DOI] [PubMed] [Google Scholar]
- 106.Huang YJ, Nariya S, Harris JM, Lynch SV, Choy DF, Arron JR, et al. The airway microbiome in patients with severe asthma: Associations with disease features and severity. J Allergy Clin Immunol. 2015;136:874–84. doi: 10.1016/j.jaci.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Murray CS, Canoy D, Buchan I, Woodcock A, Simpson A, Custovic A. Body mass index in young children and allergic disease: gender differences in a longitudinal study. Clin Exp Allergy. 2011;41:78–85. doi: 10.1111/j.1365-2222.2010.03598.x. [DOI] [PubMed] [Google Scholar]
- 108.Forno E, Acosta-Perez E, Brehm JM, Han YY, Alvarez M, Colon-Semidey A, et al. Obesity and adiposity indicators, asthma, and atopy in Puerto Rican children. J Allergy Clin Immunol. 2014;133:1308–14. 14 e1–5. doi: 10.1016/j.jaci.2013.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Han YY, Forno E, Celedon JC. Adiposity, fractional exhaled nitric oxide, and asthma in U.S. children. Am J Respir Crit Care Med. 2014;190:32–9. doi: 10.1164/rccm.201403-0565OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Desai D, Newby C, Symon FA, Haldar P, Shah S, Gupta S, et al. Elevated sputum interleukin-5 and submucosal eosinophilia in obese individuals with severe asthma. Am J Respir Crit Care Med. 2013;188:657–63. doi: 10.1164/rccm.201208-1470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maizels RM, Allen JE. Immunology. Eosinophils forestall obesity. Science. 2011;332:186–7. doi: 10.1126/science.1205313. [DOI] [PubMed] [Google Scholar]
- 112.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–7. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Everaere L, Ait Yahia S, Boute M, Audousset C, Chenivesse C, Tsicopoulos A. Innate lymphoid cells at the interface between obesity and asthma. Immunology. 2017 doi: 10.1111/imm.12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Periyalil HA, Wood LG, Scott HA, Jensen ME, Gibson PG. Macrophage activation, age and sex effects of immunometabolism in obese asthma. Eur Respir J. 2015;45:388–95. doi: 10.1183/09031936.00080514. [DOI] [PubMed] [Google Scholar]
- 115.Zheng J, Zhang X, Zhang L, Zhang HP, Wang L, Wang G. Interactive effects between obesity and atopy on inflammation: A pilot study for asthma phenotypic overlap. Ann Allergy Asthma Immunol. 2016;117:716–7. doi: 10.1016/j.anai.2016.09.430. [DOI] [PubMed] [Google Scholar]
- 116.Lugogo N, Francisco D, Addison KJ, Manne A, Pederson W, Ingram JL, et al. Obese asthmatic patients have decreased surfactant protein A levels: Mechanisms and implications. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kattan M, Kumar R, Bloomberg GR, Mitchell HE, Calatroni A, Gergen PJ, et al. Asthma control, adiposity, and adipokines among inner-city adolescents. J Allergy Clin Immunol. 2010;125:584–92. doi: 10.1016/j.jaci.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sood A, Shore SA. Adiponectin, Leptin, and Resistin in Asthma: Basic Mechanisms through Population Studies. J Allergy (Cairo) 2013;2013:785835. doi: 10.1155/2013/785835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang F, Del-Rio-Navarro BE, Torres-Alcantara S, Perez-Ontiveros JA, Ruiz-Bedolla E, Saucedo-Ramirez OJ, et al. Adipokines, asymmetrical dimethylarginine, and pulmonary function in adolescents with asthma and obesity. J Asthma. 2017;54:153–61. doi: 10.1080/02770903.2016.1200611. [DOI] [PubMed] [Google Scholar]
- 120.Baek HS, Kim YD, Shin JH, Kim JH, Oh JW, Lee HB. Serum leptin and adiponectin levels correlate with exercise-induced bronchoconstriction in children with asthma. Ann Allergy Asthma Immunol. 2011;107:14–21. doi: 10.1016/j.anai.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 121.Suratt BT, Ubags NDJ, Rastogi D, Tantisira KG, Marsland BJ, Petrache I, et al. An Official American Thoracic Society Workshop Report: Obesity and Metabolism. An Emerging Frontier in Lung Health and Disease. Ann Am Thorac Soc. 2017;14:1050–9. doi: 10.1513/AnnalsATS.201703-263WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vijayakanthi N, Greally JM, Rastogi D. Pediatric Obesity-Related Asthma: The Role of Metabolic Dysregulation. Pediatrics. 2016;137 doi: 10.1542/peds.2015-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cohen P, Noveral JP, Bhala A, Nunn SE, Herrick DJ, Grunstein MM. Leukotriene D4 facilitates airway smooth muscle cell proliferation via modulation of the IGF axis. Am J Physiol. 1995;269:L151–7. doi: 10.1152/ajplung.1995.269.2.L151. [DOI] [PubMed] [Google Scholar]
- 124.Dekkers BG, Schaafsma D, Tran T, Zaagsma J, Meurs H. Insulin-induced laminin expression promotes a hypercontractile airway smooth muscle phenotype. Am J Respir Cell Mol Biol. 2009;41:494–504. doi: 10.1165/rcmb.2008-0251OC. [DOI] [PubMed] [Google Scholar]
- 125.Agrawal A, Mabalirajan U, Ahmad T, Ghosh B. Emerging interface between metabolic syndrome and asthma. American journal of respiratory cell and molecular biology. 2011;44:270–5. doi: 10.1165/rcmb.2010-0141TR. [DOI] [PubMed] [Google Scholar]
- 126.Nakajima K, Kubouchi Y, Muneyuki T, Ebata M, Eguchi S, Munakata H. A possible association between suspected restrictive pattern as assessed by ordinary pulmonary function test and the metabolic syndrome. Chest. 2008;134:712–8. doi: 10.1378/chest.07-3003. [DOI] [PubMed] [Google Scholar]
- 127.Leone N, Courbon D, Thomas F, Bean K, Jego B, Leynaert B, et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009;179:509–16. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- 128.Brumpton BM, Camargo CA, Jr, Romundstad PR, Langhammer A, Chen Y, Mai XM. Metabolic syndrome and incidence of asthma in adults: the HUNT study. Eur Respir J. 2013;42:1495–502. doi: 10.1183/09031936.00046013. [DOI] [PubMed] [Google Scholar]
- 129.Forno E, Han YY, Muzumdar RH, Celedon JC. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J Allergy Clin Immunol. 2015;136:304–11. e8. doi: 10.1016/j.jaci.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maniscalco M, Paris D, Melck DJ, D'Amato M, Zedda A, Sofia M, et al. Coexistence of obesity and asthma determines a distinct respiratory metabolic phenotype. J Allergy Clin Immunol. 2017;139:1536–47. e5. doi: 10.1016/j.jaci.2016.08.038. [DOI] [PubMed] [Google Scholar]
- 131.Li CY, Erickson SR, Wu CH. Metformin use and asthma outcomes among patients with concurrent asthma and diabetes. Respirology. 2016;21:1210–8. doi: 10.1111/resp.12818. [DOI] [PubMed] [Google Scholar]
- 132.Dixon AE, Subramanian M, DeSarno M, Black K, Lane L, Holguin F. A pilot randomized controlled trial of pioglitazone for the treatment of poorly controlled asthma in obesity. Respir Res. 2015;16:143. doi: 10.1186/s12931-015-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Holguin F, Comhair SA, Hazen SL, Powers RW, Khatri SS, Bleecker ER, et al. An association between L-arginine/asymmetric dimethyl arginine balance, obesity, and the age of asthma onset phenotype. Am J Respir Crit Care Med. 2013;187:153–9. doi: 10.1164/rccm.201207-1270OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Murphy A, Tantisira KG, Soto-Quiros ME, Avila L, Klanderman BJ, Lake S, et al. PRKCA: a positional candidate gene for body mass index and asthma. Am J Hum Genet. 2009;85:87–96. doi: 10.1016/j.ajhg.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Melen E, Himes BE, Brehm JM, Boutaoui N, Klanderman BJ, Sylvia JS, et al. Analyses of shared genetic factors between asthma and obesity in children. J Allergy Clin Immunol. 2010;126:631–7. e1–8. doi: 10.1016/j.jaci.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kuo NW, Tung KY, Tsai CH, Chen YC, Lee YL. beta3-Adrenergic receptor gene modifies the association between childhood obesity and asthma. J Allergy Clin Immunol. 2014;134:731–3. e3. doi: 10.1016/j.jaci.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 137.Melen E, Granell R, Kogevinas M, Strachan D, Gonzalez JR, Wjst M, et al. Genome-wide association study of body mass index in 23 000 individuals with and without asthma. Clin Exp Allergy. 2013;43:463–74. doi: 10.1111/cea.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang L, Murk W, DeWan AT. Genome-Wide Gene by Environment Interaction Analysis Identifies Common SNPs at 17q21.2 that Are Associated with Increased Body Mass Index Only among Asthmatics. PLoS One. 2015;10:e0144114. doi: 10.1371/journal.pone.0144114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rastogi D, Suzuki M, Greally JM. Differential epigenome-wide DNA methylation patterns in childhood obesity-associated asthma. Sci Rep. 2013;3:2164. doi: 10.1038/srep02164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rastogi D, Nico J, Johnston AD, Tobias TAM, Jorge Y, Macian F, et al. CDC42-related genes are upregulated in helper T cells from obese asthmatic children. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ahangari F, Sood A, Ma B, Takyar S, Schuyler M, Qualls C, et al. Chitinase 3-like-1 regulates both visceral fat accumulation and asthma-like Th2 inflammation. Am J Respir Crit Care Med. 2015;191:746–57. doi: 10.1164/rccm.201405-0796OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Granell R, Henderson AJ, Evans DM, Smith GD, Ness AR, Lewis S, et al. Effects of BMI, fat mass, and lean mass on asthma in childhood: a Mendelian randomization study. PLoS Med. 2014;11:e1001669. doi: 10.1371/journal.pmed.1001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Skaaby T, Taylor AE, Thuesen BH, Jacobsen RK, Friedrich N, Mollehave LT, et al. Estimating the causal effect of body mass index on hay fever, asthma and lung function using Mendelian randomization. Allergy. 2017 doi: 10.1111/all.13242. [DOI] [PubMed] [Google Scholar]
- 144.Hakala K, Stenius-Aarniala B, Sovijarvi A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest. 2000;118:1315–21. doi: 10.1378/chest.118.5.1315. [DOI] [PubMed] [Google Scholar]
- 145.Aaron SD, Fergusson D, Dent R, Chen Y, Vandemheen KL, Dales RE. Effect of weight reduction on respiratory function and airway reactivity in obese women. Chest. 2004;125:2046–52. doi: 10.1378/chest.125.6.2046. [DOI] [PubMed] [Google Scholar]
- 146.Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–74. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jensen ME, Gibson PG, Collins CE, Wood LG. Airway and systemic inflammation in obese children with asthma. Eur Respir J. 2013;42:1012–9. doi: 10.1183/09031936.00124912. [DOI] [PubMed] [Google Scholar]
- 148.Luna-Pech JA, Torres-Mendoza BM, Luna-Pech JA, Garcia-Cobas CY, Navarrete-Navarro S, Elizalde-Lozano AM. Normocaloric diet improves asthma-related quality of life in obese pubertal adolescents. Int Arch Allergy Immunol. 2014;163:252–8. doi: 10.1159/000360398. [DOI] [PubMed] [Google Scholar]
- 149.van Leeuwen JC, Hoogstrate M, Duiverman EJ, Thio BJ. Effects of dietary induced weight loss on exercise-induced bronchoconstriction in overweight and obese children. Pediatr Pulmonol. 2014;49:1155–61. doi: 10.1002/ppul.22932. [DOI] [PubMed] [Google Scholar]
- 150.Ma J, Strub P, Xiao L, Lavori PW, Camargo CA, Jr, Wilson SR, et al. Behavioral weight loss and physical activity intervention in obese adults with asthma. A randomized trial. Ann Am Thorac Soc. 2015;12:1–11. doi: 10.1513/AnnalsATS.201406-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pakhale S, Baron J, Dent R, Vandemheen K, Aaron SD. Effects of weight loss on airway responsiveness in obese adults with asthma: does weight loss lead to reversibility of asthma? Chest. 2015;147:1582–90. doi: 10.1378/chest.14-3105. [DOI] [PubMed] [Google Scholar]
- 152.Freitas PD, Ferreira PG, Silva AG, Stelmach R, Carvalho-Pinto RM, Fernandes FL, et al. The Role of Exercise in a Weight-Loss Program on Clinical Control in Obese Adults with Asthma. A Randomized Controlled Trial. Am J Respir Crit Care Med. 2017;195:32–42. doi: 10.1164/rccm.201603-0446OC. [DOI] [PubMed] [Google Scholar]
- 153.Lowder T, Dugger K, Deshane J, Estell K, Schwiebert LM. Repeated bouts of aerobic exercise enhance regulatory T cell responses in a murine asthma model. Brain Behav Immun. 2010;24:153–9. doi: 10.1016/j.bbi.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.van de Griendt EJ, van der Baan-Slootweg OH, van Essen-Zandvliet EE, van der Palen J, Tamminga-Smeulders CL, Benninga MA, et al. Gain in lung function after weight reduction in severely obese children. Arch Dis Child. 2012;97:1039–42. doi: 10.1136/archdischild-2011-301304. [DOI] [PubMed] [Google Scholar]
- 155.da Silva PL, de Mello MT, Cheik NC, Sanches PL, Correia FA, de Piano A, et al. Interdisciplinary therapy improves biomarkers profile and lung function in asthmatic obese adolescents. Pediatr Pulmonol. 2012;47:8–17. doi: 10.1002/ppul.21502. [DOI] [PubMed] [Google Scholar]
- 156.Jensen ME, Gibson PG, Collins CE, Hilton JM, Wood LG. Diet-induced weight loss in obese children with asthma: a randomized controlled trial. Clin Exp Allergy. 2013;43:775–84. doi: 10.1111/cea.12115. [DOI] [PubMed] [Google Scholar]
- 157.Willeboordse M, Kant KD, Tan FE, Mulkens S, Schellings J, Crijns Y, et al. A Multifactorial Weight Reduction Programme for Children with Overweight and Asthma: A Randomized Controlled Trial. PLoS One. 2016;11:e0157158. doi: 10.1371/journal.pone.0157158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Boulet LP, Turcotte H, Martin J, Poirier P. Effect of bariatric surgery on airway response and lung function in obese subjects with asthma. Respir Med. 2012;106:651–60. doi: 10.1016/j.rmed.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 159.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128:508–15. e1–2. doi: 10.1016/j.jaci.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.van Huisstede A, Rudolphus A, Castro Cabezas M, Biter LU, van de Geijn GJ, Taube C, et al. Effect of bariatric surgery on asthma control, lung function and bronchial and systemic inflammation in morbidly obese subjects with asthma. Thorax. 2015;70:659–67. doi: 10.1136/thoraxjnl-2014-206712. [DOI] [PubMed] [Google Scholar]
- 161.Hasegawa K, Tsugawa Y, Chang Y, Camargo CA., Jr Risk of an asthma exacerbation after bariatric surgery in adults. J Allergy Clin Immunol. 2015;136:288–94. e8. doi: 10.1016/j.jaci.2014.12.1931. [DOI] [PubMed] [Google Scholar]
- 162.Paich HA, Sheridan PA, Handy J, Karlsson EA, Schultz-Cherry S, Hudgens MG, et al. Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 influenza A virus. Obesity (Silver Spring) 2013;21:2377–86. doi: 10.1002/oby.20383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ubags ND, Stapleton RD, Vernooy JH, Burg E, Bement J, Hayes CM, et al. Hyperleptinemia is associated with impaired pulmonary host defense. JCI Insight. 2016;1 doi: 10.1172/jci.insight.82101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Neidich SD, Green WD, Rebeles J, Karlsson EA, Schultz-Cherry S, Noah TL, et al. Increased risk of influenza among vaccinated adults who are obese. Int J Obes (Lond) 2017 doi: 10.1038/ijo.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]


