Abstract
Adolescence is accompanied by the maturation of several stress-responsive areas of the brain including the amygdala, a key region for the acquisition and expression of conditioned fear. These changes may contribute to the development of stress-related disorders in adolescence, such as anxiety and depression, and increase the susceptibility to these psychopathologies later in life. Here, we assessed the effects of acute restraint stress on fear learning and amygdala activation in pre-adolescent and adult male rats. Pre-adolescents exposed to stress prior to fear conditioning showed greater resistance to the extinction of fear memories than adults. At the cellular level, the combination of stress and fear conditioning resulted in a greater number of FOS-positive cells in the basolateral nucleus of the amygdala (BLA) than fear conditioning alone, and this increase was greater in pre-adolescents than in adults. Despite age-dependent differences, we found no changes in glucocorticoid receptor (GR) levels in the amygdala of either preadolescent or adult males. Overall, our data indicate that stress prior to fear conditioning leads to extinction-resistant fear responses in pre-adolescent animals, and that the BLA may be one neural locus mediating these age-dependent effects of stress on fear learning.
Keywords: amygdala, fear conditioning, restraint stress, FOS, glucocorticoid receptors
INTRODUCTION
Adolescence is a transitional period in development characterized by major physiological and psychological changes (Sisk and Foster, 2004; Rogol et al., 2002; Spear, 2000). It is accompanied by the maturation of several stress-responsive regions of the brain including the hypothalamus, hippocampus, prefrontal cortex, and amygdala (Giedd et al., 2009; Giedd and Rapoport, 2010; Somerville and Casey, 2010). Adolescent development is also associated with a substantial shift in stress reactivity (Romeo et al., 2016; McCormick et al., 2010). Exposing animals to high levels of stress early in life increases their susceptibility to subsequent stressors (de Kloet et al., 2005; Cohen et al., 2007; Bazak et al., 2009). These changes in turn may contribute to the onset of many of the stress-related psychopathologies in humans which often occur during adolescence including both anxiety and depression (Keshavan et al., 2014; Giedd et al., 1999).
Many of the brain regions that are vulnerable to the effects of stress are involved in emotional learning including the amygdala, hippocampus and prefrontal cortex (Izquierdo et al., 2016). There has thus been considerable interest in the effects of acute and chronic stress on the neurobiological mechanisms that mediate this type of associative learning, with a particular focus on Pavlovian fear conditioning. Indeed, many psychiatric disorders including post-traumatic stress disorder are characterized by abnormalities in fear conditioning and extinction (Rothbaum and Davis, 2003). While there has been tremendous progress in understanding the cellular and molecular underpinnings of associative fear learning in adults (Pape and Pare, 2010; Izquierdo et al., 2016), it remains unclear how stress affects these neurobehavioral processes, particularly in adolescent animals.
Pavlovian fear conditioning consists of pairing a conditioned stimulus (CS) such as a tone with an aversive stimulus, such as a brief electric footshock (unconditioned stimulus; US). Learned fear responses include behavioral reactions such as the cessation of movement (freezing) as well as autonomic changes and activation of the HPA axis (Campeau and Davis, 1995; LeDoux et al., 1988). After fear learning has occurred, repeated presentations of the CS will typically lead to a reduction in the ability of the CS to elicit conditioned fear responses, a phenomenon termed fear extinction (Quirk and Mueller, 2008). This gradual reduction in fear responses which develops as the CS is repeatedly presented is termed within-session extinction. To test for long-term fear extinction memories, or between-session extinction, the CS can be presented at a later time point, typically 24 h after fear extinction learning has occurred. Fear extinction memories compete with and inhibit the original fear memory (Bouton et al., 2006; Ji and Maren, 2007). The basolateral nucleus of the amygdala (BLA) and central nucleus of the amygdala (CeA) have been identified as critical structures in the acquisition and expression of conditioned fear memories (Quirk et al., 1995; Haubensak et al., 2010).
Converging lines of evidence in adult animals suggests that acute stress enhances fear learning and memory consolidation and produces deficits in fear extinction. Stress exposure prior to fear learning enhances fear memory consolidation and increases neuronal excitability and synaptic plasticity in the BLA (Shors, 2001; Cordero et al., 2003; Rodriguez Manzanares et al., 2005; Kavushansky and Richter- Levin, 2006; Hui et al., 2006; Chauveau et al., 2012). Furthermore, a single injection of the glucocorticoid hormone corticosterone administered post-training also enhances fear memory consolidation (Zorawski and Killcross, 2002; Hui et al., 2004; Roozendaal et al., 2006). Many stress paradigms produce deficits in the recall of extinction memories including repeated restraint stress and exposure to the odor of a predator (Zhang and Rosenkranz, 2013; Miracle et al., 2006; Goswami et al., 2010). These previously stressed animals exhibit sustained levels of freezing to the CS even after extinction learning takes place. However, it has also been reported that a single 20-min session of restraint stress does not produce deficits in the recall of fear memory (Zhang and Rosenkranz, 2013).
Exposing animals to stress prior to puberty affects learning during later developmental periods in that stressed animals exhibit greater levels of fear conditioning (Toledo-Rodriguez and Sandi, 2007). This finding mirrors studies in humans revealing that stress during adolescence contributes to increased susceptibilities to psychopathologies later in life (Turner and Lloyd, 2004; Dahl and Gunnar, 2009). However, there have been relatively few studies on the effects of stress on fear conditioning and extinction in pre-adolescent animals. In response to acute stressors such as restraint stress or intermittent foot shock, pre-adolescent animals have a significantly prolonged hormonal stress response compared with adults (reviewed in Romeo et al., 2016). Moreover, there is greater FOS expression in the paraventricular nucleus of the hypothalamus (PVN) in juveniles after exposure to a single session of restraint stress (Romeo et al., 2006; Lui et al., 2012), suggesting greater neural activation in stress sensitive brain areas prior to adolescent development.
Given the impact of stress on fear learning and extinction and these disparities in hormonal and neuronal responses during adolescence, the purpose of the present study was to determine whether acute stress differentially affects fear conditioning and neuronal activation in pre-adolescent versus adult rats. The precise age range that encompasses adolescent development in rats is not clearly defined. However, given that hormonal, somatic, behavioral, and neurobiological changes associated with adolescence in rats occur during a time window of approximately 30 days (between 30 and 60 days of age; Klein and Romeo, 2013; Spear, 2000), we used rats at either 30 or 70 days of age for our pre-adolescent and adult groups, respectively. Thus, using these ages, we are able to assess these neurobehavioral changes before and after adolescent maturation.
Stressors can vary in terms of type including physical, psychological, social and immunological stressors. They can also vary in duration (acute vs. chronic) and frequency (single vs. repeated). Here, we examined the effects of a single one-hour session of restraint stress, a type of physical stressor, prior to fear conditioning in both pre-adolescent and adult male rats. We then measured neuronal activation, as indexed by FOS immunohistochemistry in the BLA, CeA and PVN in preadolescent and adult animals exposed to fear conditioning and stress. Finally, we investigated potential adolescent-related changes in glucocorticoid receptors (GR) levels in the amygdala, using both immunohistochemistry and western blotting in preadolescent and adult rats.
EXPERIMENTAL PROCEDURES
Subjects
Adult (70 days of age; n = 42) or pre-adolescent (30 days of age; n = 50) male Sprague–Dawley rats (Charles River Laboratories) were housed two per cage. They were maintained on a 12-hour light/dark cycle and allowed ad libitum access to food and water. Animals were weaned at 21 days of age and were acclimated to the lab for seven days prior to the beginning of the experiments. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Columbia University’s Animal Care and Use Committee.
Restraint stress and fear conditioning
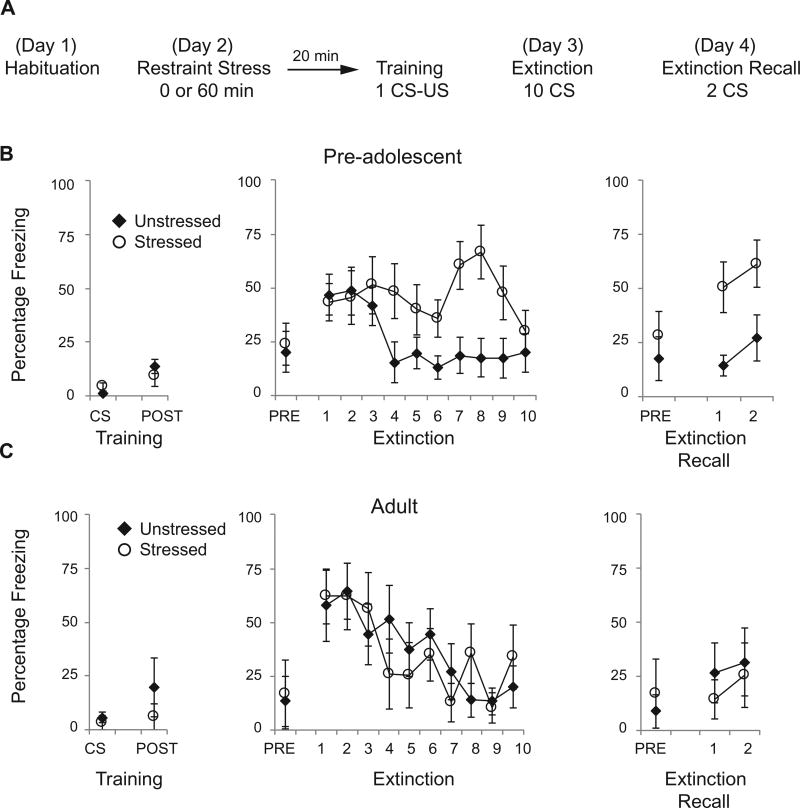
Experiment 1 examined the behavioral effects of one session of restraint stress administered prior to fear conditioning. Pre-adolescent (n = 20) and adult (n = 12) animals were exposed to one of two conditions: 1 hour of restraint stress followed by 20 min of rest followed by fear conditioning, or fear conditioning alone (Fig. 1A). Restraint stress consisted of placing animals in the prone position in wire mesh restrainers of the appropriate size. Cued fear conditioning consisted of placing rats in rodent-conditioning chambers with a metal grid floor (Coulbourn Instruments) as described previously (Davis and Bauer, 2012). Animals were habituated to this training context A 24 h prior to training. During training, rats received 1 tone-shock pairing: a 5-kHz tone, 80 dB, 30-sec duration paired with a 0.5-mA shock, 1-sec duration, with the tone co-terminating with the shock. Twenty-four hours later rats were placed in a different context (context B) with a black Plexiglas floor washed with peppermint soap. During extinction training, rats received 10 CS tones (30-sec duration; 60- to 120-sec inter-tone intervals). Extinction memory was assessed by placing the rats 24 h later in context B and delivering two CS tones. Behavior was recorded by a video camera and analyzed off-line. Time spent freezing to each CS was manually scored by an observer blind to group assignment. The fear-conditioning apparatus failed to deliver a footshock to one pre-adolescent animal, so that animal was excluded from behavioral analysis. Experiment 2 examined the effects of restraint stress and fear conditioning on the number of FOS-positive cells in the BLA, CeA and PVN. Pre-adolescent and adult animals were exposed to one of three conditions (n = 8 per age and condition): 1 hour of restraint stress followed by 20 minutes of rest, followed by fear conditioning with 1 CSUS pairing followed by 15 minutes of rest (stress + training); fear conditioning alone followed by 15 minutes of rest (training); or no behavioral manipulation (control). The 20-minute delay between restraint stress and fear conditioning was chosen because of the maximal differential stress-induced hormonal response in pre-adolescent and adult animals at this time point (Romeo et al., 2006). With a longer delay, corticosterone levels are similar in pre-adolescents and adults. In addition, there is a significant and sustained increase in FOS-positive cells in the pre-adolescent and adult rat brain 60–90 minutes after the onset of restraint stress (Romeo et al., 2006; Lui et al., 2012). Experiment 3 examined the number of GR-positive cells in the BLA and CeA, as well as total GR protein levels in the amygdala via western blots in pre-adolescent and adult male rats under control conditions (n = 6 per age). For tissues processed with immunohistochemistry in Experiments 2 and 3, at the appropriate time point animals were deeply anesthetized with sodium pentobarbital (100 mg/kg) and perfused transcardially with 0.9% saline and 4% paraformaldehyde in 0.1 M phosphate buffer (PB). The stress exposures and tissue collections were conducted between 1000 and 1300 h. Brains were removed and post-fixed for 4 h and then transferred to a 20% sucrose solution and processed for immunohistochemistry.
Fig. 1.
Differential effects of restraint stress on cued fear conditioning in pre-adolescent and adult rats. (A) Schematic of behavioral protocol. (B) Mean ± SE percent freezing to one CS tone and to the 30-s postshock period (POST) during training, to the 30-s pre-CS period (PRE) and to ten CS tones during extinction, and to the pre-CS period and two CS tones during extinction recall in pre-adolescent rats receiving one hour of restraint stress prior to fear conditioning (n = 9) or unstressed rats (n = 10). Pre-adolescents receiving restraint stress were deficient at extinguishing conditioned fear responses. (C) Mean ± SE percent freezing to one CS tone and postshock freezing during training, the pre-CS period and ten CS tones during extinction and the pre-CS period and two CS tones during extinction recall in adult rats receiving one hour of restraint stress prior to fear
Immunohistochemistry
Coronal brain sections (40-µm) were cut using a cryostat (Jung Frigocut 2800 E) and stored in cryoprotectant at −20 °C. Tissue was processed for FOS and GR as described previously (Goble et al., 2011; Dziedzic et al., 2014). Free-floating sections containing either the amygdala or PVN were washed in 0.1 M PB, incubated for 10 min in 0.05% H2O2 in 0.1 M phosphate-buffered saline (PBS) and washed in 0.1 M PB with 0.1% Triton X-100 (PBT). After blocking in 2% normal goat serum (NGS) in PBT for 1 h, slices were incubated in one of two primary antibodies: anti-FOS (1:20,000; rabbit; Santa Cruz Biotechnology, sc-52) or anti-GR (1:10,000 anti-rabbit, Santa Cruz Biotechnology, M-20) in 2% NGS in PBT at 4 °C overnight. Sections were washed in PBS and then incubated in goat anti-rabbit secondary antibody (1:200; Vector, Burlingame, CA) in PBT for 1 h. After three washes in PBS, slices were incubated in avidin–biotin horseradish peroxidase complex (1:250; Vectastain ABC Kit, Vector) in PBT for 1 h. To visualize horseradish peroxidase, slices were developed using 3,3′diaminobenzidine (DAB; Sigma) enhanced with nickel. Sections were washed in PBS, mounted on to Fisher Brand Plus slides (Fisher Scientific, Pittsburgh, PA), dried, dehydrated in increasing concentrations of alcohol, placed in xylenes, and coverslipped with DPX Mountant (Sigma–Aldrich, St. Louis, MO).
FOS- or GR-positive cells were quantified in two anatomically equivalent sections, separated by 120 µm using a light microscope (Nikon, Eclipse E400). Ocular grid placement covered an area of either 250,000 µm2 (BLA), 90,000 µm2 (CeA) or 15,625 µm2 (PVN) and was based upon both a standard rat brain atlas and adjacent nissl-stained sections (plates 48 and 49 for PVN and plates 51–53 for BLA and CeA; Paxinos and Watson, 2005). Two bilateral counts were made for each nucleus and averaged.
Western blot
For western blot, approximately 500-µm-thick tissue punches were made using a 0.5-mm corer tool and snap frozen on dry ice. Blots were conducted as previously described (Dziedzic et al., 2014). Tissues were homogenized in lysis buffer (1% SDS with a Roche Complete, Mini, ETDA-free protease inhibitor cocktail; Roche Diagnostics, Basel, Switzerland) and exposed to boiling water for 10 min. Protein levels were determined by the BCA method (Pierce; Rockford, IL). Lysates were subjected to SDS gels, blotted on nitrocellulose membranes, and probed with anti-GR (1:5000) or anti-actin (1:1000; Sigma, St. Louis, MO). Proteins were visualized using chemiluminescence (Pierce) and Kodak XAR film. Films were scanned and ImageJ was used to measure relative optical densities (RODs). Specifically, background signal was subtracted from the film and a rectangular tool was placed over the GR- or actin-positive band and the ROD measurement was recorded. Actin bands were used as a loading control to normalize potential differences in the amount of protein loaded in each lane of the gel.
Statistical analyses
All data are presented as mean ± SEM. We tested for normal distributions using the Shapiro–Wilk test and for equal variances using the Levene test in SPSS. Data that did not satisfy these two requirements were analyzed using non-parametric tests. For Experiment 1, behavior was analyzed using repeated measures ANOVAs and Mann–Whitney’s U tests. Pre-adolescent and adult animals were analyzed separately. For Experiment 2, FOS-positive cell counts were analyzed using two-way ANOVAs (age × behavioral condition). For Experiment 3, GR-positive cell counts were analyzed using unpaired Student’s t-tests. ROD measurements were analyzed using the Mann–Whitney U test. For all experiments, differences were considered significant when P < 0.05.
RESULTS
Experiment 1 assessed the effects of a single one-hour session of restraint stress prior to fear conditioning in pre-adolescent and adult rats (Fig. 1A). In preadolescent rats, restraint stress had no effect on freezing behavior during training with 1 CS tone paired with a footshock, (Mann–Whitney’s U; p = 0.55) or on postshock freezing (Mann–Whitney’s U; 0.24). Twenty-four hours later, rats were presented with ten CS tones. There was no significant difference between groups in freezing to the 30-second pre-CS period (Mann– Whitney’s U; p = 0.84). Fear memory retrieval, based on freezing to the first CS tone, was similar for both groups (one-way ANOVA: F(1,17) = 0.07, p = 0.79). However, a repeated measures ANOVA across all ten tones revealed a significant effect of stress F(1,17) = 5.38, p < 0.05, of tones F(9,153) = 2.26, p < 0.05, and a significant interaction between the two: F(9,153) = 22.37, p < 0.05. Animals that had been previously restrained maintained high levels of freezing during extinction. This impairment persisted 24 h later when two CS tones were presented in a recall session. Again, there was no significant difference between groups in freezing to the 30-second pre-CS period (Mann–Whitney’s U; p = 0.24). However, a repeated measures ANOVA revealed a significant effect of tone F(1,17) = 25.73, p < 0.05 and group F(1,17) = 27.50, p < 0.05, but no interaction F(1,17) = 20.52; p = 0.82 (Fig. 1B).
In contrast, this single-restraint stress session had no effect on freezing behavior in adult rats at any time point (Fig. 1C). Stress did not affect freezing on the training day to either the CS (Mann–Whitney’s U, p = 0.59) or to freezing after the shock (Mann–Whitney’s U; p = 0.59). During the extinction day, there was no difference between groups in freezing to the pre-CS period (Mann–Whitney’s U; p = 0.99). There was a significant effect of tones, meaning that the animals froze less to the CS tones as extinction progressed, but there was no effect of group, or interaction (repeated measures ANOVA for tones: F(9,90) = 24.60, p < 0.001; group: F(1,10) = 20.02; p = 0.90; interaction: F(9,90) = 20.84, p = 0.58). Twenty-four hours later during the extinction recall session, there was no difference in pre-CS freezing (Mann–Whitney’s U; p = 0.82) and there were no significant effects of stress or tone (repeated measures ANOVA for tones: F(1,10) = 21.15, p = 0.31, group: F(1,10) = 20.27, p = 0.61, interaction F(1,10) = 20.17, p = 0.69). Taken together, these results suggest that previous stress results in maintained fear responses during fear extinction in pre-adolescent but not adult animals.
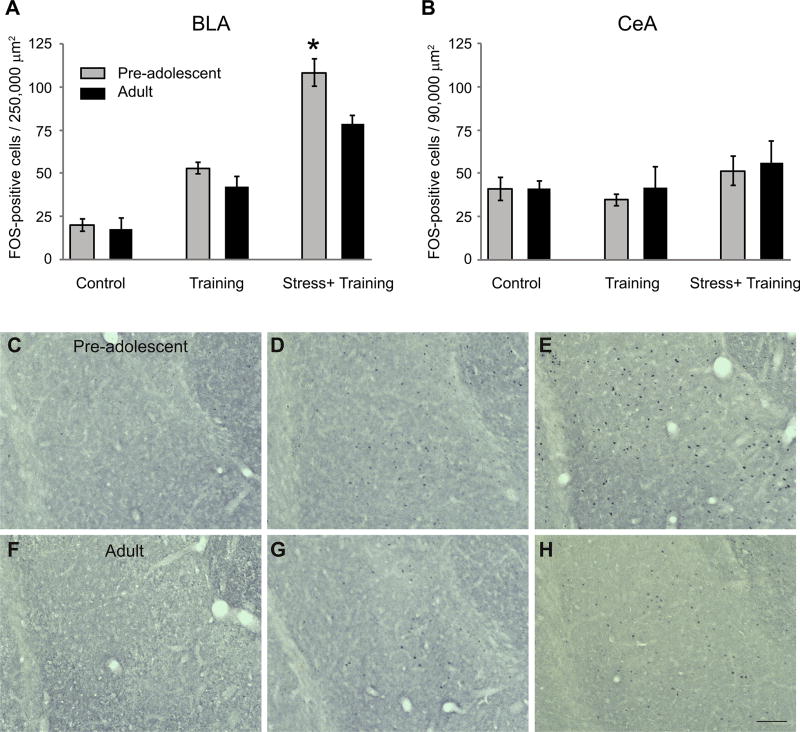
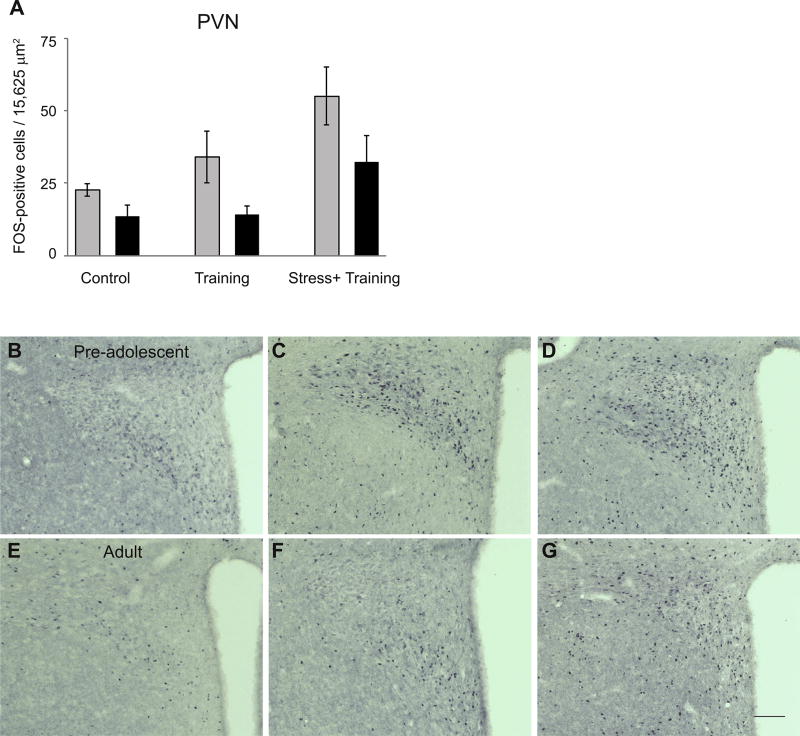
Experiment 2 examined the number of FOS-positive cells in the BLA, CeA and PVN in pre-adolescent and adult rats exposed to 1 hour of restraint stress followed by fear conditioning, fear conditioning alone, or control conditions (no behavioral manipulations). A two-way ANOVA revealed a significant interaction between age and condition (F(2,42) = 2 95.47, p < 0.05, Fig. 2A) such that training and training plus stress resulted in an increased number of FOS-positive cells in the BLA, but this increase was greater in pre-adolescent compared to adults (Fig. 2C–H). On the other hand, no main effects or interaction between age and condition were detected on the number of FOS-positive cells in the CeA (Fig. 2B). Finally, a two-way ANOVA showed significant main effects of age and condition on FOS-positive cells in the PVN (age: F(1,31) = 27.81; condition: F(2,31) = 26.46, p’s < 0.05, Fig. 3), such that independent of experimental condition, pre-adolescent males had greater numbers of FOS-positive cells than adults, while independent of age, animals exposed to training plus stress had greater numbers of FOS-positive cells than animals exposed to training alone or control conditions.
Fig. 2.
Mean ± SE FOS-positive cells in the BLA (A) and CeA (B) in pre-adolescent (gray bars) and adult (black bars) male rats exposed to control conditions (Control), fear conditioning training alone (Training) or 1-hr restraint stress followed by training (Stress + Training). There was increased FOS expression in the BLA in the Training and Stress + Training groups. There was a significant interaction between age and condition in the BLA such that this increase was greater in pre-adolescents than in adults (p < 0.05). Asterisk indicates a significant difference between the ages under that condition. Representative photomicrographs of FOS-positive cells in the BLA of pre-adolescents and adults exposed to control conditions (C and F), training alone (D and G) or restraint stress followed by training (E and H). Scale bar=100 µm.
Fig. 3.
Mean ± SE FOS-positive cells in the PVN (A) in pre-adolescent (gray bars) and adult (black bars) rats exposed to control conditions (Control), fear conditioning training alone (Training) or 1 h restraint stress followed by training (Stress + Training). There was increased FOS in the Stress + Training group in both age groups (p < 0.05). Across all conditions, pre-adolescents had greater numbers of FOS-positive cells than adults (p < 0.05). Representative photomicrographs of FOS-positive cells in the PVN of pre-adolescents and adults exposed to control conditions (B and E), training alone (C and F), or restraint stress followed by training (D and G). Scale bar=100 mm. conditioning (n = 6) or unstressed rats (n = 6).
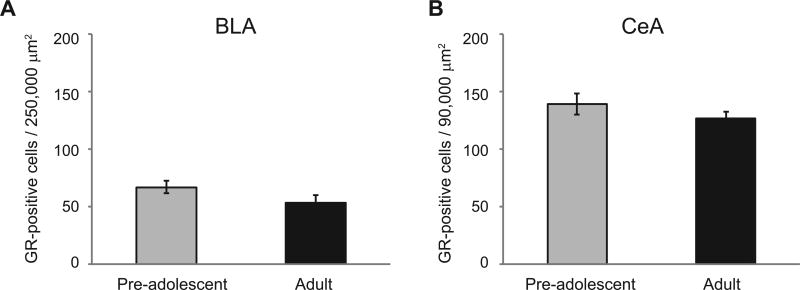
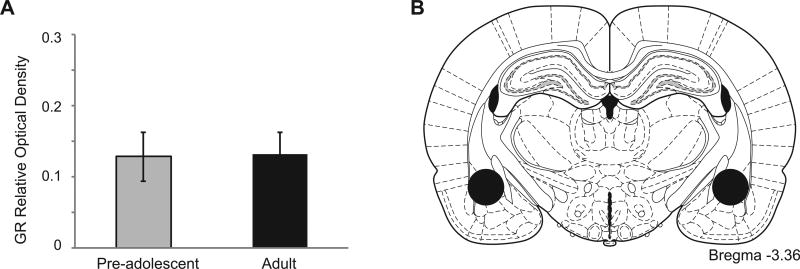
Experiment 3 measured GRs in the BLA and CeA of pre-adolescent and adult rats using both immunohistochemistry and western blotting. T-tests showed there were no significant differences in the number of GR-positive cells in the BLA or CeA in preadolescent compared to adults. Similarly, no significant differences were found between these ages in the relative optical density of GR-immunoreactivity bands from the amygdala (Figs. 4 and 5).
Fig. 4.
Mean ± SE GR-positive cell number in the BLA (A) and CeA (B) in pre-adolescent and adult male rats. There were no significant effects of age in either the BLA or CeA.
Fig. 5.
(A) Mean ± SE GR relative optical density (ROD) in the amygdala of pre-adolescent and adult male rats. There was no significant effect of age. (B) Approximate location of tissue punches in the amygdala. Adapted from Paxinos and Watson (2005).
DISCUSSION
These data indicate that acute restraint stress prior to fear conditioning has differential effects on pre-adolescent and adult rats, in that fear memories of pre-adolescents exposed to stress were more resistant to extinction. In addition, the combination of restraint stress followed by fear conditioning in pre-adolescents was associated with greater activation of the BLA. However, the stress-related sensitivity exhibited by the BLA in preadolescents was independent of any gross alterations in GR protein levels in the amygdala.
In pre-adolescent rats, restraint stress administered prior to fear conditioning did not enhance freezing to the CS tone during fear acquisition nor did it enhance postshock freezing. Differences in postshock freezing can be interpreted as altered footshock sensitivity, sensory processing of the US or short-term fear memory in response to the shock (Fanselow, 1980). Our lack of an effect on freezing to the tone CS during training as well as postshock freezing suggests that the elevated freezing during extinction was a result of altered memory consolidation, rather than stress-enhanced conditioning.
Indeed, prior stress did not enhance freezing to the first CS tone on testing day. Rather, as extinction progressed, previously stressed animals exhibited sustained freezing responses to CS presentations, while unstressed animals showed extinction. This suggests that the initial consolidated fear memory in stressed animals was significantly resistant to fear extinction processes. These findings are in agreement with reports that restraint stress and other forms of physical stressors produce deficits in fear extinction (Chauveau et al., 2012; Baratta et al., 2007). It has been previously reported that stress prior to puberty affects learning during later developmental periods (Toledo-Rodriguez and Sandi, 2007). Our data extend these findings to reveal that acute stress can also affect fear learning in preadolescent animals, particularly in the context of extinction.
To determine whether stress led to generalized fear between Context A and Context B, we analyzed freezing during the 30 seconds before the first CS tone on extinction day in Context B. In both pre-adolescents and adults, stress did not contribute to increased freezing in the new context. Further, maintained fear responses in stressed pre-adolescent animals during the extinction session did not lead to second-order context fear conditioning to Context B, as the amount of freezing to the pre-CS period during extinction retention was similar between groups.
In contrast to our present results, previous studies in adult rats have shown that acute stress results in potentiated fear memories that are resistant to extinction (Rau et al., 2005; Takahashi et al., 2006). Here however, a one-hour session of restraint stress had no effect on fear conditioning or extinction in adult animals. One possibility is that restraint stress is qualitatively different from other types of acute stressors which affect fear conditioning in adults, such as single prolonged stress (Yamamoto et al., 2009). Alternatively, a one-hour session might be too mild a stressor, as the effects of stress on fear learning have been reported to be dependent on the severity of the stressor (Rau and Fanselow, 2009). In contrast, a one-hour session of restraint stress was sufficient to alter fear memories in pre-adolescents, suggesting that this type of acute stress might be a useful method to uncover differential effects of stress in pre-adolescents and adults.
Stress might affect fear conditioning in preadolescents differently because the mechanisms of fear learning and extinction in the amygdala might themselves be different in pre-adolescents and adults (Baker and Richardson, 2015). A common finding in rodents and humans is that retention of fear extinction is impaired in adolescents relative to both younger and older age groups (McCallum et al., 2010; Pattwell et al., 2012). In humans, fear extinction learning is also impaired in adolescents (Johnson and Casey, 2015). In rodents, however, there is conflicting data on whether fear extinction learning itself is impaired (Kim et al., 2011; Pattwell et al., 2012). The neural circuits involved in fear extinction in adolescence are also different from those recruited at other ages (Baker et al., 2016). Adolescent rodents do not show increases in phospho-MAP kinase (pMAPK), or upregulation of c-Fos in the medial prefrontal cortex (mPFC) compared to both juveniles and adults following fear extinction (Baker and Richardson, 2015; Pattwell et al., 2012). Further, pMAPK is down-regulated in the BLA but upregulated in the CeA after fear extinction in adolescents; an opposite pattern of activity compared to adults (Baker and Richardson, 2015).
Using FOS immunoreactivity as a marker for neuronal activation, we found that the combination of acute stress and fear conditioning led to greater activity in the BLA of pre-adolescents compared to adults. One possibility is that the enhanced consolidation of fear memories in pre-adolescents observed here is a result of greater activation of glucocorticoid-sensitive cells in the BLA. Indeed, converging lines of evidence indicate that acute stress enhances consolidation of fear memories by activating GRs in the BLA and by stimulating local release of norepinephrine in the BLA (Hui et al., 2004; Roozendaal et al., 2006; Quirarte et al., 1997). However, our present data showing similar levels of GR in the amygdala before and after adolescence would indicate any change in GR sensitivity would likely be mediated by changes in the function of these receptors (i.e., translocation to the nucleus, transcriptional activity) and not due to changes in the overall levels of these receptors. Interestingly, a recent report has shown shifts in stress-induced GR nuclear translocation during adolescence (Green et al., 2016). In contrast to our results in the BLA, there were no significant effects of pre-conditioning stress or age on FOS activation in the CeA. These data support the previous studies implicating the BLA as a key structure mediating the effects of stress on fear learning (Rodrigues and Sapolsky, 2009) and indicate adolescent changes in stress-induced activation in the amygdala demonstrate some anatomical specificity.
An alternate interpretation of our data stems from the observation that acute stressors can modulate behavior for several days. Indeed, predator stress, underwater stress and single-prolonged stress can all affect anxiety-like behavior in the elevated plus maze several days after termination of the stressor (reviewed in Armario et al., 2008). Of particular relevance is the finding that acute restraint stress causes a gradual increase in spine density in the BLA over 10 days paralleled by the development of anxiety-like behavior (Mitra et al., 2005). Thus, it is possible that restraint stress exerted some modulatory effect on extinction in adolescents, as extinction learning occurred only 24 h after training. However, we did find that the combination of stress and conditioning increased neuronal activity in the BLA after conditioning, suggesting that at least some of the effects of stress on fear extinction are the result of processes occurring immediately after training.
Following restraint stress, pre-adolescents display longer stress-induced hormonal responses compared to adults (Romeo, 2010). Specifically, they display ACTH and corticosterone responses that can last twice as long as adults (reviewed in Romeo et al., 2016). Here we found greater PVN activation in pre-adolescent compared to adult animals under all three behavioral conditions, in agreement with earlier studies (Romeo et al., 2006; Lui et al., 2012). Furthermore, regardless of age, all animals showed greater FOS responses in the PVN following the combination of restraint stress and fear conditioning. Together, these studies along with the current report point to a significant influence of pubertal development on stress reactivity, with restraint stress leading to greater stress responsiveness both neurobiologically and behaviorally prior to adolescent maturation.
CONCLUSION
Our data indicate that acute stress experienced prior to fear learning renders fear memories more resistance to extinction in pre-adolescent compared to adult male rats. Further, the combination of stress and fear conditioning produces enhanced activation of BLA neurons than fear conditioning alone, and this effect is greater in pre-adolescents than in adults. However, this age-dependent change in activation is independent of any gross alteration of GR protein levels within the amygdala. These findings emphasize that preadolescence is a vulnerable period in which fear memories may be particularly susceptible to stress. Given the increase in stress-related psychological vulnerabilities during adolescence (Costello et al., 2003), it will be imperative to continue to examine the interactions between stress, adolescence, and fear learning.
Acknowledgments
This work was supported in part by NIH grant MH107008-01A1 to E.P.B. and by NSF grant IOS-1456577 to R.D.R.
References
- Armario A, Escorihuela RM, Nadal R. Long-term neuroendocrine and behavioural effects of a single exposure to stress in adult animals. Neurosci Biobehav Rev. 2008;32:1121–1135. doi: 10.1016/j.neubiorev.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Baker KD, Richardson R. Forming competing fear learning and extinction memories in adolescence makes fear difficult to inhibit. Learn Mem. 2015;22:537–543. doi: 10.1101/lm.039487.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, Watkins LR, Maier SF. Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience. 2007;146:1495–1503. doi: 10.1016/j.neuroscience.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KD, Bisby MA, Richardson R. Impaired fear extinction in adolescent rodents: behavioural and neural analyses. Neurosci Biobehav Rev. 2016;70:59–73. doi: 10.1016/j.neubiorev.2016.05.019. [DOI] [PubMed] [Google Scholar]
- Bazak N, Kozlovsky N, Kaplan Z, Matar M, Golan H, Zohar J, Richter-Levin G, Cohen H. Pre-pubertal stress exposure affects adult behavioral response in association with changes in circulating corticosterone and brain-derived neurotrophic factor. Psychoneuroendocrinology. 2009;34:844–858. doi: 10.1016/j.psyneuen.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15:2312–2327. doi: 10.1523/JNEUROSCI.15-03-02312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauveau F, Lange MD, Jungling K, Lesting J, Seidenbecher T, Pape HC. Prevention of stress-impaired fear extinction through neuropeptide s action in the lateral amygdala. Neuropsychopharmacology. 2012;37:1588–1599. doi: 10.1038/npp.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Kaplan Z, Matar MA, Loewenthal U, Zohar J, Richter-Levin G. Long-lasting behavioral effects of juvenile traumain an animal model of PTSD associated with a failure of the autonomic nervous system to recover. Eur Neuropsychopharmacol. 2007;17:464–477. doi: 10.1016/j.euroneuro.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Venero C, Kruyt ND, Sandi C. Prior exposure to a single stress session facilitates subsequent contextual fear conditioning in rats. Evidence for a role of corticosterone. Horm Behav. 2003;44:338–345. doi: 10.1016/s0018-506x(03)00160-0. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Dev Psychopathol. 2009;21:1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- Davis SE, Bauer EP. L-type voltage-gated calcium channels in the basolateral amygdala are necessary for fear extinction. J Neurosci. 2012;32:13582–13586. doi: 10.1523/JNEUROSCI.0809-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Sibug RM, Helmerhorst FM, Schmidt MV. Stress, genes and the mechanism of programming the brain for later life. Neurosci Biobehav Rev. 2005;29:271–281. doi: 10.1016/j.neubiorev.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Dziedzic N, Ho A, Adabi B, Foilb AR, Romeo RD. Shifts in hormonal stress reactivity during adolescence are not associated with changes in glucocorticoid receptor levels in the brain and pituitary of male rats. Dev Neurosci. 2014;36:261–268. doi: 10.1159/000362873. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J Am Acad Child Adolesc Psychiatry. 2009;48:465–470. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goble KH, Bain ZA, Padow VA, Lui P, Klein ZA, Romeo RD. Pubertal-related changes in hypothalamic-pituitary-adrenal axis reactivity and cytokine secretion in response to an immunological stressor. J Neuroendocrinol. 2011;23:129–135. doi: 10.1111/j.1365-2826.2010.02085.x. [DOI] [PubMed] [Google Scholar]
- Goswami S, Cascardi M, Rodriguez-Sierra OE, Duvarci S, Pare D. Impact of predatory threat on fear extinction in Lewis rats. Learn Mem. 2010;17:494–501. doi: 10.1101/lm.1948910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MR, Nottrodt RE, Simone JJ, McCormick CM. Glucocorticoid receptor translocation and expression of relevant genes in the hippocampus of adolescent and adult male rats. Psychoneuroendocrinology. 2016;73:32–41. doi: 10.1016/j.psyneuen.2016.07.210. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui GK, Figueroa IR, Poytress BS, Roozendaal B, McGaugh JL, Weinberger NM. Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiol Learn Mem. 2004;81:67–74. doi: 10.1016/j.nlm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Hui IR, Hui GK, Roozendaal B, McGaugh JL, Weinberger NM. Posttraining handling facilitates memory for auditory-cue fear conditioning in rats. Neurobiol Learn Mem. 2006;86:160–163. doi: 10.1016/j.nlm.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Furini CR, Myskiw JC. Fear Memory. Physiol Rev. 2016;96:695–750. doi: 10.1152/physrev.00018.2015. [DOI] [PubMed] [Google Scholar]
- Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17:749–758. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- Johnson DC, Casey BJ. Extinction during memory reconsolidation blocks recovery of fear in adolescents. Sci Rep. 2015;5:8863. doi: 10.1038/srep08863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavushansky A, Richter-Levin G. Effects of stress and corticosterone on activity and plasticity in the amygdala. J Neurosci Res. 2006;84:1580–1587. doi: 10.1002/jnr.21058. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Giedd J, Lau JY, Lewis DA, Paus T. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry. 2014;1:549–558. doi: 10.1016/S2215-0366(14)00081-9. [DOI] [PubMed] [Google Scholar]
- Kim JH, Li S, Richardson R. Immunohistochemical analyses of long-term extinction of conditioned fear in adolescent rats. Cereb Cortex. 2011;21:530–538. doi: 10.1093/cercor/bhq116. [DOI] [PubMed] [Google Scholar]
- Klein ZA, Romeo RD. Changes in hypothalamic-pituitary-adrenal stress responsiveness before and after puberty in rats. Horm Behav. 2013;64:357–363. doi: 10.1016/j.yhbeh.2013.01.012. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui P, Padow VA, Franco D, Hall BS, Park B, Klein ZA, Romeo RD. Divergent stress-induced neuroendocrine and behavioral responses prior to puberty. Physiol Behav. 2012;107:104–111. doi: 10.1016/j.physbeh.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum J, Kim JH, Richardson R. Impaired extinction retention in adolescent rats: effects of D-cycloserine. Neuropsychopharmacology. 2010;35:2134–2142. doi: 10.1038/npp.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain Cogn. 2010;72:73–85. doi: 10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci USA. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, Ruberry EJ, Powers A, Mehta N, Yang RR, Soliman F, Glatt CE, Casey BJ, Ninan I, Lee FS. Altered fear learning across development in both mouse and human. Proc Natl Acad Sci U S A. 2012;109:16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- Quirarte GL, Roozendaal B, McGaugh JL. Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci U S A. 1997;94:14048–14053. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Rau V, Fanselow MS. Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress. 2009;12:125–133. doi: 10.1080/10253890802137320. [DOI] [PubMed] [Google Scholar]
- Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Sapolsky RM. Disruption of fear memory through dual-hormone gene therapy. Biol Psychiatry. 2009;65:441–444. doi: 10.1016/j.biopsych.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Manzanares PA, Isoardi NA, Carrer HF, Molina VA. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. J Neurosci. 2005;25:8725–8734. doi: 10.1523/JNEUROSCI.2260-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogol AD, Roemmich JN, Clark PA. Growth at puberty. J Adolesc Health. 2002;31:192–200. doi: 10.1016/s1054-139x(02)00485-8. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Front Neuroendocrinol. 2010;31:232–240. doi: 10.1016/j.yfrne.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Karatsoreos IN, McEwen BS. Pubertal maturation and time of day differentially affect behavioral and neuroendocrine responses following an acute stressor. Horm Behav. 2006;50:463–468. doi: 10.1016/j.yhbeh.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Patel R, Pham L, So VM. Adolescence and the ontogeny of the hormonal stress response in male and female rats and mice. Neurosci Biobehav Rev. 2016;70:206–216. doi: 10.1016/j.neubiorev.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Hui GK, Hui IR, Berlau DJ, McGaugh JL, Weinberger NM. Basolateral amygdala noradrenergic activity mediates corticosterone-induced enhancement of auditory fear conditioning. Neurobiol Learn Mem. 2006;86:249–255. doi: 10.1016/j.nlm.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Acute stress rapidly and persistently enhances memory formation in the male rat. Neurobiol Learn Mem. 2001;75:10–29. doi: 10.1006/nlme.1999.3956. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Curr Opin Neurobiol. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Morinobu S, Iwamoto Y, Yamawaki S. Effect of paroxetine on enhanced contextual fear induced by single prolonged stress in rats. Psychopharmacology. 2006;189:165–173. doi: 10.1007/s00213-006-0545-6. [DOI] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Sandi C. Stress before puberty exerts a sex- and age-related impact on auditory and contextual fear conditioning in the rat. Neural Plast. 2007;2007:71203. doi: 10.1155/2007/71203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RJ, Lloyd DA. Stress burden and the lifetime incidence of psychiatric disorder in young adults: racial and ethnic contrasts. Arch Gen Psychiatry. 2004;61:481–488. doi: 10.1001/archpsyc.61.5.481. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Morinobu S, Takei S, Fuchikami M, Matsuki A, Yamawaki S, Liberzon I. Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress Anxiety. 2009;26:1110–1117. doi: 10.1002/da.20629. [DOI] [PubMed] [Google Scholar]
- Zhang W, Rosenkranz JA. Repeated restraint stress enhances cue-elicited conditioned freezing and impairs acquisition of extinction in an age-dependent manner. Behav Brain Res. 2013;248:12–24. doi: 10.1016/j.bbr.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorawski M, Killcross S. Posttraining glucocorticoid receptor agonist enhances memory in appetitive and aversive Pavlovian discrete-cue conditioning paradigms. Neurobiol Learn Mem. 2002;78:458–464. doi: 10.1006/nlme.2002.4075. [DOI] [PubMed] [Google Scholar]