Abstract
Treatments for metabolic diseases, such as diet and therapeutics, often provide short-term therapy for metabolic stressors, but relapse is common. Repeated bouts of exposure to – and relief from – metabolic stimuli results in a phenomenon we call “metabolic cycling”. Recent human and rodent data suggests metabolic cycling promotes an exaggerated response and ultimately worsened metabolic health. This is particularly evident with cycling of body weight and hypertension. The innate and adaptive immune systems have a profound impact on development of metabolic disease and current data suggests that immunologic memory may partially explain this association – especially in the context of metabolic cycling. In this Brief Review, we highlight recent work in this field and discuss potential immunologic mechanisms for worsened disease prognosis in individuals who experience metabolic cycling.
Introduction
In the US, over 1/3rd of adults are obese and in 2014 the World Health Organization estimated that more than 1.9 billion adults worldwide were obese. Despite increased awareness and interventions, obesity remains a health concern due to its associated comorbidities. These include metabolic diseases such as insulin resistance and diabetes; cardiovascular diseases such as dyslipidemia, atherosclerosis and stroke; and at least 13 types of cancer including esophageal, liver, pancreatic, uterine, breast, and colorectal. Concomitant with our increased knowledge regarding obesity-accelerated disease is the realization that the immune system plays a major role in the pathogenesis of all of the above-mentioned co-morbidities (1). This association is striking, and in fact, emerging therapies for these diseases now target the immune system (2–4). In addition to the health consequences of progressive weight gain, weight cycling – and its relevance to human health – has come to the forefront of recent lay and scientific literature. Similarly, an immune component to hypertension is evident in humans and rodent models, and recent studies suggest that hypertension cycling also promotes worsened disease. In this Brief Review, we will discuss what is known about immune responses to weight gain and weight loss as well as the latest studies on the role of the immune system in diseases associated with metabolic cycling, with particular focus on repeated bouts of weight gain and hypertension. As the world’s population becomes increasingly obese, metabolic cycling may be an important determinant of our health now more than ever before.
Immunometabolism
In the mid-1990’s a potential role for the immune system in metabolic disease was beginning to be identified by investigators who showed that inflammatory cytokines such as TNF-α, IL-6, CCL2, and iNOS were elevated in obese compared to lean white adipose tissue and could induce insulin resistance in adipocytes, myocytes and hepatocytes. Thus began a foray of investigation into immune regulation of metabolic processes. Scientific interest in this field was significantly piqued when, in 2003, two groups published their observation that inflammatory macrophage content was significantly increased in obese compared to lean adipose tissue [Figure 1A & B; (5, 6)]. In the past 15 years, many investigators around the world have contributed to advancements in this new field of “Immunometabolism”. It has been shown that not only do cells of innate immunity such as macrophages, eosinophils, neutrophils and mast cells exist in adipose tissue, so also do cells of adaptive immunity such as B cells and T cells. These innate and adaptive immune cells produce a concert of signaling molecules, such as the anti-inflammatory cytokines IL-4, IL-13, and IL-10 in lean adipose, as well as inflammatory cytokines such as INF-γ, IL-12, IL-8, TNF-α, and IL-1β in obese adipose. Importantly, the numbers and phenotypes of these immune cells are vastly different between lean and obese adipose tissue, such that obese adipose tissue is more inflammatory than lean adipose tissue. This extensive literature (a PubMed search using key words “adipose tissue” and “inflammation” yields over 9500 results) has been thoroughly reviewed by many groups, a few of which are referenced here (7–9). In addition to the immune cells themselves, adipokines such as leptin, adiponectin, retinol binding protein 4, fibroblast growth factors, resistin, and omentin; not to mention newly identified myokines such as irisin and myostatin; and hepatokines such as fetuin-A and fetuin-B, have also been shown to impact systemic metabolic processes. Information on these “-kines” can be found in the following recent review articles (10–12). In addition to white adipose tissue; brown adipose tissue, beige adipose tissue, muscle, liver, and the central nervous system (CNS) also plays a role in obesity-associated metabolic disease. In recent years, our understanding regarding the role of inflammation in metabolic tissues has become more nuanced. It is now understood that the first inflammatory response, possible induced by hypoxia, adipocyte death, mechanical stress or gut-derived antigens, is acute and protective. Only after long-term maladaptation does the inflammation become chronic and detrimental [reviewed in (13)]. Despite this extensive literature on immune-mediated instigation of metabolic disease, very little is known about whether and how the immune system contributes to the worsened metabolic disease and health outcomes associated with metabolic cycling – the focus of this Brief Review. In addition to the obese context, hypertension is also now known to be associated with immune activation – this phenomenon is described below.
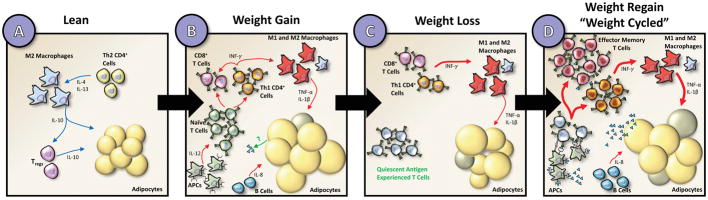
Figure 1. Inflammatory innate and adaptive immune cells in adipose tissue in lean, weight gain, weight loss, and weight-cycled mice.
Compared to lean adipose tissue (A), obese adipose tissue (B) has characteristically engorged adipocytes and infiltration of both M1-like pro-inflammatory macrophages and CD4+ and CD8+ T cells. For the sake of simplicity, other innate immune cells such as mast cells, eosinophils, and neutrophils are not shown. Adipocyte size decreases following weight loss (C), however inflammatory macrophages and memory T cells remain. Upon weight regain or weight cycling (D), effector T cell populations may interact with APCs and dramatically expand in the adipose to promote adipocyte insulin resistance through cytokine release and direct macrophage stimulation. Cell types are labeled in the figure and the grey-colored adipocytes represent dead cells. Blue arrows indicate anti-inflammatory adipose health promoting pathways and red arrows indicate pro-inflammatory metabolically unhealthy pathways.
Immunologic Memory in Adaptive Immune Responses
As mentioned above, both lean and obese adipose tissues contain cells of the adaptive immune system. Because the focus of this article is on immunologic memory in metabolic cycling, we will expand on general concepts of adaptive immune responses here. One of the cardinal features of adaptive immunity is the establishment of immunological memory that confers protection in response to previously encountered antigens. The concept of immunological memory has been recognized since Ancient Greek times, and is a process with several defined phases (14). The classical T cell immune response to initial antigen presentation induces priming of naïve T cells and proliferation to effector T cells, which is known as the expansion phase (15). During the subsequent contraction phase, the majority of effector T cells die, and a few remaining cells become a specialized pool of memory T cells, resulting in a memory phase, which can remain stable for the life of the individual. This state of immunological memory is acquired based on the initial encounter to a specific antigen that is independent of a recurrent encounter. These long-lived memory T cells have distinct properties from effector T cells in terms of surface receptor expression, response to stimuli, and expression of memory T cell survival genes. Transcription factors such as T-bet, EOMES, Blimp, Id3, and Bcl-6 all influence their effector and memory fate, as does regulation of the T cell metabolic state (16). In addition, some cytokines are essential for development of memory; for example, IL-7 and IL-15, whereby mice deficient in these cytokines fail to maintain memory cells upon antigen rechallenge (17, 18). Memory T cells are characterized with a unique tissue distribution and surface marker expression. For instance, some of these memory cells return to secondary lymphoid organs such as lymph nodes and the spleen and are referred to as central memory (TCM) cells. These cells are characterized by the surface markers CD44hi/CD62Lhi/CCR7+. A second recently identified subset is resident memory (TRM) cells, which are CD44hi/CD62Llo/CD103+/CD69+. Others remain in the periphery as effector memory (TEM) cells bearing the surface markers CD44hi/CD62Llo/CCR7−. A recurrent exposure to the original antigen leads to TCM and TRM activation and their conversion to effector memory (TEM) cells, which involves a rapid expansion of cell numbers, production of cytokines and a change in surface markers (19). Formation of memory cells requires the interaction of CD27 on T cells with CD70 on activated antigen presenting cells (APCs). This co-stimulatory interaction is analogous to that of T cell CD28 with the B7 ligands required for naïve T cell activation. Thus, mice lacking either CD27 or CD70 also fail to develop memory T cells upon antigen re-challenge (20). This topic has recently been elegantly reviewed by Goldrath and colleagues (14). Given the close association between immunity and metabolism, and the exagerated metabolic responses to conditions such as weight cycling and hypertension cycling, we and others have begun to consider whether metabolic cycling can lead to immunological memory.
Immunologic Memory in Weight Cycling
Adverse consequences of weight cycling on human health
The literature on weight cycling in humans, sometimes called “weight fluctuation” or “weight variability”, has spanned from the early 1980’s to today. Early studies focused primarily on propensity for increased weight regain and decreased resting metabolic rate. The evidence for adverse health consequences to weight cycling is not consistent in all reports; however, many of them point to this possibility, and the most commonly reported health outcome associated with weight cycling in humans is cardiovascular disease, although diabetes and even increased mortality have been reported (21). One thing that is important to note is that in human and rodent studies, repeated bouts of weight regain often result in metabolic adaptation that manifests as reduced resting metabolic rate (RMR). Thus, many times, the weight cycling actually results in increased body weight, which is referred to as “weight rebound.” The mechanisms for metabolic adaptation and slowing of RMR are unknown and are likely centrally mediated and relevant to the idea of body weight set point (22). This change in RMR and weight rebound may be part of the reason for worsened metabolic outcomes in weight cycling; however, many studies exist, including some detailed below, where weight rebound does not occur and yet metabolic health is impaired.
One of the most prominent recently published articles that caught the eye of the lay press was a follow-up metabolic analysis of subjects from the “Biggest Loser” television series (23). A cohort of 14 participants was observed for 6 years following the competition. These individuals had lost an average of almost 60 kg of body mass during the competition. After 6 years, most of the weight was regained, and this was attributed to a reduction in RMR of an astonishing ~500 kcal/day! At the 6-yr follow-up, the average weight and BMI of the contestants was lower than at baseline by ~12%; yet plasma glucose, insulin, and HOMA-IR were not improved (as would be expected given the modest weight loss). Plasma levels of adiponectin were higher – and those of leptin were lower – than baseline, tracking with the reduced body weight, and thus the adipokines cannot explain the worsened metabolic phenotype of the contestants. These data suggest the possibility that the weight fluctuations reduced the beneficial effects of the lower body weight seen after 6 years.
In another high profile paper, a post hoc analysis of the 2004 Treating to New Targets trial data, conducted in 2017, identified increased rates of diabetes, cardiovascular events and death in patients who had fluctuations in body weight (21). The immune phenotype and adipokine profiles were not reported. These studies highlight potential associations between weight cycling and negative metabolic health, but they lack insight into the potential underlying mechanisms. With the advent of larger clinical trials and also large data sets collected from electronic medical records, more extensive studies on the impact of weight cycling will be possible.
Rodent Models of Weight Cycling
To gain mechanistic insight into the metabolic effects of weight cycling, rodent models have been developed by various groups. Although these models are designed to robustly produce fluctuations in animal weight over time, the method by which weight changes are induced and the ultimate number of cycles vary from study to study and can have a dramatic effect on outcomes. For instance, some models focused on the effects of only one or two cycles of weight gain while others used more than ten cycles of weight gain and weight loss over long periods of time. Interestingly, numerous repeated cycles result in reduced RMR, metabolic adaptation, and ultimately weight rebound (24), while fewer cycles generally don’t result in exacerbated weight rebound, but still result in worsened metabolic profiles (25–28). Length and frequency of cycles was also variable in previous studies. Another common variable is the diet used to induce weight changes in rodents. High fat diet, containing up to 60% kcal from fat, is often used to induce weight gain. However, many different methods, such as caloric restriction, laboratory chow or low fat diet, and exercise have been used to induce weight loss in rodent models. Consideration of diet composition in these studies and future studies is critical. Using micronutrient matched low and high fat diet to induce weight cycling is preferred when observing cell populations that can be adversely impacted by variable nutrient composition, such as with chow diets.
Four models have recently been reported in which a possible immune-component to the metabolic defects was identified. Barbosa-da-Silva and colleagues modeled weight cycling using three alternating 8 week bouts of exposure to either 60% high fat diet or laboratory chow. They observed that weight-cycled mice quickly reached similar body weights to lean or high fat-fed controls upon diet switch. Weight-cycled mice were more glucose intolerant and had increased blood lipid levels relative to high fat diet fed controls. Furthermore, levels of IL-6 were at high levels during initial weight gain and remained high during subsequent weight loss and weight regain, but TNF-α, was reduced following weight loss and increased during weight regain (28). Our own lab has developed a weight cycling model in which C57BL/6J male mice were exposed to 9-week long cycles initiated by 60% high fat diet to induce obesity, a 10% low fat diet to induce weight loss, and a final exposure to 60% high fat diet to promote weight regain. Following sacrifice at 27-weeks of age, weight-cycled mice had worsened glucose tolerance and decreased insulin sensitivity compared to high fat fed controls, even though length of time on diet, body mass, and body composition were identical (25). More recently, Zou et al. published a model of weight cycling in C57BL/6J mice that utilized 60% high fat diet exposure and caloric restriction to induce a single cycle of weight cycling (27). Mice were fed high fat diet for 4 weeks and then calorie restricted until body mass was identical to chow fed controls before being fed high fat diet ad libitum to induce weight cycling. Weight-cycled mice had an increased body mass compared diet-induced obese control mice, indicating the likelihood of reduced RMR and what these authors called “obesogenic memory”. Furthermore, weight-cycled mice had worsened post-prandial blood glucose levels and decreased insulin sensitivity. A model of weight cycling that used exercise to induce weight loss has also been reported. Wainright et al. published that mice given intermittent access to an exercise wheel had normalized adiposity but worsened glucose tolerance (26). The current models of weight cycling will allow for mechanistic studies to determine changes in immune composition that may promote negative metabolic health.
Innate immune contribution to immunologic effects of weight cycling
Obese adipose tissue is characterized by low-grade inflammation, thought to originate from recruitment and proliferation of M1-like pro-inflammatory macrophages (Figure 1B). Surprisingly, macrophage populations remain elevated in previously obese models after weight loss, even when metabolic parameters return to normal [Figure 1C; (29, 30)]. These findings indicate a persistent adaptation to obesity that may explain the propensity for weight regain and worsened metabolic health. However, upon weight regain in models of weight cycling, macrophage numbers are comparable to mice that have maintained high body weight, i.e. they don’t rebound to higher levels because of the weight cycling [Figure 1D; (25)]. In an intermittent exercise model of weight cycling, the mice that regularly exercised had reduced numbers of adipose tissue macrophages (26). Thus, whether the weight cycling is induced by diet or by exercise may be an important determinant in innate immune responses in the adipose tissue. None-the-less, data regarding immune-mediated mechanisms for worsened outcomes in weight cycling point away from traditional innate immune activation as the culprit.
Adaptive immune contribution to immunologic effects of weight cycling
Obesogenic memory via adaptive immunity is a potential mechanism for worsened metabolic outcomes of weight cycling. During weight gain, components of the adaptive immune system in adipose are known to increase in obese relative to lean controls [Figure 1A & B; (31–35). Recent studies have begun to characterize the secondary immune populations in the adipose during weight regain. Kyung et al. conducted global transcriptome analysis to identify genes that were differentially expressed in the adipose of weight gain and weight regain animals (36). Specifically, they observed weight regain to be associated with genes related to T cell activation, proliferation, and differentiation. Additionally, they observed increased expression of genes that regulate major histocompatibility complex II (MHC Class II). These changes correlated with increases in gonadal adipose T cell subsets by flow cytometry. Our own lab has observed both worsened metabolic parameters and increased CD4+, CD8+, and TEM in the adipose during weight cycling relative to age-matched high fat diet fed controls [Figure 1D; (25)]. Furthermore, in studies by Zou et al. CD4+ T cells were shown to be sufficient for obesogenic memory (27). Failure of obesogenic memory occurred in immunodeficient mice, but was restored following adoptive transfer of either splenocytes or CD4+ T cells from previously obese C57BL/6J mice. The activation and memory state of T cell populations in weight cycling has yet to be fully described. CD4+ TEM have been observed to increase prior to weight regain in weight cycling models (27). We have additionally observed a specific increase in CD8+ TEM cells in weight cycled mice compared to high fat fed controls [Figure 1D; (25)]. These data suggest T cell populations activity change throughout the duration of weight cycling; however, more careful characterization of these adaptive immune populations at each stage of weight loss and weight regain will be critical to understand their role in metabolic disease. Little is known, however, regarding whether TRM cells remain dormant following weight loss or whether adaptive lymphocytes can be reactivated following weight regain. Cumulatively, these data suggest a potential immunologic memory to weight cycling that is activated upon weight loss or the re-exposure to weight gain observed during weight cycling and likely contributes to metabolic dysfunction (Figure 1D).
The presence of CD4+ and CD8+ TEM cells in adipose tissue suggest that antigen presentation occurs (32, 35, 37). Furthermore, in adipose tissue, CD4 and CD8 T cells have been shown to have restricted T cell receptor repertoires (32, 35, 38), again suggesting antigenic stimulation and memory – even in the setting of simple obesity without weight cycling. Macrophages, dendritic cells, B cells and adipocytes are all capable of presenting antigen to T cells in the adipose tissue (39–44). However, which APC is most relevant and what the antigen(s) are is not known. Their identification could provide greater insight into further metabolic dysregulation that is observed in models of weight cycling, and conversely, models of weight cycling could help with the identification of the antigen(s).
Other potential mechanisms for metabolic dysfunction with weight cycling
To date, the few studies on mechanisms for weight cycling-mediated metabolic dysfunction have focused on adaptive immunity and the potential for memory and secondary immune responses. However, given our expanded understanding of how weight gain alone can impact metabolism, there are other mechanisms that should be considered. These include trained innate immunity; the contribution of other organs such as liver, muscle and the CNS; changes to the microbiome; and genetic predictors of weight regain. Future studies should illuminate the contribution of these alternate mechanisms for cycling mediated immune-driven metabolic disease. Interestingly, all of these mechanisms, including the concept of secondary immune responses we propose, have the signature of dormancy during weight loss, and reactivation during the weight regain.
Trained innate immunity is a relatively new discovery in the field of virology and bacteriology that has also been recognized in settings of Western Diet feeding (45), autoimmunity (46), and atherosclerosis (47). The concept is that innate immune cells, such as macrophages, have long-term memory to priming by certain stimuli such that they have an exaggerated response to future stimuli. Interestingly, in contrast to adaptive immunity, this second stimulus doesn’t have to be the same as the first stimulus (48, 49). Also, the reprogramming is completed via persistent epigenetic changes. There are at least 2 reasons this could be relevant to weight cycling: 1) In weight loss studies, adipose tissue macrophages maintained their inflammatory phenotype even after weight loss and normalization of metabolic parameters [Figure 1C; (29, 30)]. This same phenomenon was reported as trained innate immunity in studies using Western Diet feeding in LDLR−/− mice (45). 2) It is likely that there are many different antigens that can be recognized and responded to in obese adipose. Trained innate immunity could be particularly important if the metabolic stimuli are not the same; for example, if obesity is the first stimulus and hypertension is the second stimulus.
In obesity, even the immune-privileged CNS demonstrates an inflammatory response, with recruitment of immune cells and inflammatory cytokine secretion leading to both insulin and leptin resistance [reviewed in (50)]. In contrast to white adipose tissue, muscle, and liver, inflammatory activation in the CNS occurs before obesity develops, indicating a different inflammatory stimulus. This inflammation can be from dietary fatty acids or gut-derived hormones, and can manifest as inflammatory stimulation of neuronal cells, activation of resident microglial macrophage-like cells, or recruitment of new monocyte-derived macrophages. Although CNS inflammation has not been studied in the context of weight cycling, it is interesting to note that many studies of weight cycling demonstrate decreased RMR and increased weight gain or “weight rebound” over time. This phenomenon is seen in rodents and humans and is likely explained by maladaptive changes to synaptic plasticity, potentially mediated by heightened CNS inflammation.
Emerging evidence suggests an important role for inflammation in muscle and liver. This can be both via immune cell recruitment and activation, but also to secretion of myokines and hepatokines, respectively. As in adipose tissue, the macrophages that accumulate in liver with obesity have an M1-like pro-inflammatory phenotype. Whether the macrophages or T cells in liver are activated differently in the context of weight cycling has not been explored. Smooth muscle is an important organ to consider in the metabolic effects of weight cycling because the majority of insulin stimulated glucose uptake occurs in muscle; thus, immune mediated insulin resistance in muscle can have profound effects on whole body metabolism. Obesity results in an increase in inflammation and immune cell infiltration into muscle; however, whether weight cycling impacts this immune activation in muscle is also not known. In addition to muscle and liver, immune-mediated metabolic changes in brown adipose tissue or beige adipose tissue cannot be ruled out.
The microbiome is an area of intense investigation for its role at the intersection of immunity and metabolism. Extensive evidence links the microbiome with body weight regulation [reviewed in (51, 52)]; however, only recently has it also been associated with weight regain. Thaiss et al. report that certain microbiome signatures persist in obese mice after weight loss and are predictive of weight rebound and metabolic perturbations (53). Furthermore, this “accelerated weight regain phenotype” could be transferred to germ free mice via fecal transfer. They were also able to connect the altered microbiome with reduction in the flavonoids apigenin and naringenin. Whether this microbiome-mediated response to weight cycling intersects with any immune interactions is not yet known, but is an important area of future investigation. Furthermore, even aside from the microbiome, gut inflammation could be relevant to weight cycling-mediated metabolic disease.
Extensive GWAS analyses have been performed to identify genetic predictors that are associated with propensity for being overweight and obese; however, much less has been discovered with predictors of weight loss and weight regain. In a 2012 report on a post-hoc analysis of data from the Diabetes Prevention Program, SNPs in three genes, ENGR1, BDNF, and PPARG, were shown to be associated with weight regain (54). This work was completed with the intent of identifying individuals who might need more comprehensive support to maintain their weight loss. However, in the future, similar studies linking phenotypic, genotypic, and electronic medical record data could help identify individuals who are not only at greater risk of regaining weight, but who are also at greater risk of having worsened diseases associated with weight cycling. In addition to genetic predictors, another area of future investigation is whether any circulating factors are predictive of weight cycling in metabolic dysfunction. To date, studies have shown that hormones such as ghrelin and leptin are predictive of future weight gain (55); however, there is no evidence, either for or against, a role for adipocytokines in metabolic cycling accelerated disease.
Immunologic Memory in Hypertension
Adverse consequences of hypertension on human health
Hypertension is an enormous health care burden in Western Societies and is a major risk factor for stroke, myocardial infarction, and heart failure. One third of the population is hypertensive, while another third has “pre-hypertension” and commonly develops overt hypertension in two years (56–58). Despite the frequency of this disease, its cause in most adults remains unknown. Perturbations of the CNS, vasculature, and the kidney have all been implicated in hypertension, however the manner in which these interact remain poorly defined. Emerging evidence suggests that inflammation and immunity play an important role in the pathogenesis of hypertension [reviewed in (59)].
Adaptive immune contribution to hypertension
It has been known for over a decade that the immune system contributes to development of hypertension. In both experimental animal models and in humans with hypertension, T cells infiltrate the kidney and perivascular tissue (60–63). Injurious cytokines released by these cells seem to have major effects on vascular and renal function and injury. This has led many different groups to study the effects of specific T cell populations in the development of hypertension. In the absence of B and T cells in mice with severe combined immune deficiency (SCID) mice or RAG-1 deficiency, resistance to hypertension caused by angiotensin II (ang II), DOCA-salt, or norepinephrine is noted (63–65). Even in Dahl salt sensitive rats, elimination of T cells via deletion of the RAG-1 gene or the zeta chain of the TCR, blunts hypertension and also reduces renal damage caused by salt intake (66, 67). Conversely, adoptive transfer of T cells into RAG-1−/− mice restores hypertension and its attendant end-organ dysfunction (63). Other studies showed that T regulatory cells suppress hypertension (68–70). Thus, there is much interest in harnessing the immune system for treatment of hypertension and in fact, inhibition of T cell co-stimulation with Abatacept prevented and reversed ang II and DOCA-salt hypertension (71).
In mechanistic studies, it has been shown that stimuli such as ang II, high salt and norepinephrine promote T cell activation and accumulation in the vasculature and kidney (63). Dr. Harrison’s group recently showed that several renal sodium transporters, including the sodium hydrogen exchanger, the sodium chloride co-transporter and the sodium potassium chloride co-transporter, are regulated by the cytokines IL-17A and IFN-γ. Furthermore, mice lacking these cytokines are protected against the anti-natriuretic and anti-diuretic effects of ang II (72). Focusing on in IL-17A, Nguyen et al. showed that IL-17A causes inhibitory phosphorylation of the endothelial nitric oxide synthase, leading to endothelial dysfunction and hypertension (73) and this endothelial dysfunction is absent in mice lacking IL-17A (74). IL-17A contributes to aortic fibrosis and stiffening (75), and prevention of inflammation by scavenging isoketals prevents renal fibrosis, albuminuria and nephrinuria (76). Thus, inflammation and immune cells mediate the events that Guyton proposed in the genesis of hypertension, i.e. a shift in the renal function curve and ultimately an increase in systemic vascular resistance (77). Dr. Guyton attributed the latter to systemic autoregulation, but we now know that vascular dysfunction and remodeling contribute, and that inflammation mediates these events (78).
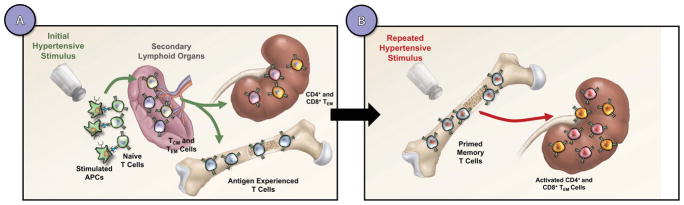
In addition to a role for TCM, TEM can be detected in the blood, vasculature, and kidneys of hypertensive mice (63, 71, 79). However, the role of these memory T cells in hypertension compared to naïve T cells is poorly understood. Itani et al. discovered a novel role of immunological memory in hypertension and showed that TEM infiltrate the kidney and bone marrow in response to repeated hypertensive challenges [Figure 2A; (80)]. These memory cells are primarily responsible for production of injurious cytokines including IFN-γ and IL-17A that lead to end-organ damage. The presence of TCM in models of hypertension suggests that there could be immunologic memory to hypertension – a theory that has been experimentally tested as described below.
Figure 2. Hypertensive stimuli prime memory formation that exacerbates responses to subsequent stimuli.
A hypertensive stimulus promotes presentation of neoantigens by dendritic cells to naïve T cell. Subsequently, this leads to T cell proliferation and memory formation in secondary lymphoid organs (A). Long-lived memory T cells reside in the bone marrow and upon repeated hypertensive stimuli (B), memory T cells migrate back to the kidney and stimulate effector T cell activity.
Rodent models of hypertension cycling
To study the concept of immunological memory in hypertension, it is crucial to introduce repeated hypertensive stimuli in mice without any surgical intervention. Itani et al. hypothesized that the presence of hypertension-specific memory T cells place the host at high risks for developing hypertension in response to mild-repeated hypertensive stimuli (80). In this study, two experimental mouse models that mimic immunological memory were used to examine the role of memory T cells in hypertension (Figure 2). The first experimental model involves an initial 2-week exposure of the nitric oxide synthase (NOS) inhibitor L-NAME (0.5mg/mL), followed by a 2-week washout, and subsequent employment of a high salt diet (4% NaCl) for three weeks (81). This model mimics immunological memory and recapitulates salt-sensitive hypertension that is common in humans. A second mouse model was also used to investigate the potential role of T-cell memory in angiotensin II–induced hypertension. In this model, C57BL/6 mice were implanted with an osmotic pump for infusion of either angiotensin II (490 ng/kg/min) or vehicle for 2 weeks, followed by a 2-week washout period. The mice were then implanted with a second osmotic minipump for infusion of low dose of angiotensin II (140 ng//kg/min), and blood pressure was monitored by radiotelemetry. TEM were formed upon these repeated challenges and contribute to both salt-sensitive and angiotensin II-induced hypertension results (80). L-NAME followed by high salt (HS) increased systolic blood pressure and caused a 2-fold increase in both renal and bone marrow CD4+ and CD8+ TEM cells. Importantly, using intracellular staining, these TEM cells are predominantly responsible for production of injurious cytokines IFN-γ and IL17A in the kidney. In keeping with this, there was a 1.8-fold increase in CD4+ TEM cells and a 3-fold increase in CD8+ TEM cells in the bone marrow following L-NAME/high salt exposure compared to non-hypertensive mice.
Development and reactivation of memory cells requires the interaction of CD27 on T cells with CD70 on activated antigen presenting cells (20, 82) and L-NAME/HS increases expression of CD70 on both macrophages and DCs in the spleen. Thus, studies of mice lacking these critical complexes have been useful. Because memory T cells are a major source of IFN-γ, the hypertensive response to the L-NAME/HS protocol in IFN-γ−/− mice was examined. While wild type mice develop severe hypertension during salt intake, the salt-sensitive hypertension in CD70−/− and IFN-γ−/− mice was markedly attenuated (80). Importantly, CD70−/− and IFN-γ−/− mice fail to develop memory T cells in the kidney in response to repeated hypertensive stimuli. Also, wild type mice develop striking proteinuria, while the CD70−/− and IFN-γ−/− mice are protected from renal injury. Together with a recent study by Kamat et al. showing that IL-17A and IFN-γ modulate sodium transport (72), these data strongly indicate a role of TEM cells in the genesis of hypertension and salt sensitivity, at least in response to this L-NAME/high salt hypertension cycling protocol (Figure 2).
Relevance to human health
Given that hypertensive stimuli could be recurrent, the concept of immunological memory in hypertension may have clinical significance. Repeated epsidodes of dietary indescretion or emotional stress have been associated with hypertension (83). Likewise, it is known that transient hypertension during pregnancy (preeclampsia), sleep apnea that involves repeated surges of sympathetic outflow, and repeated stress insults place individuals at risk for developing cardiovascular disease later in life. It is interesting to speculate that TEM contribute to these cardiovascular events. Given that the role of immunological memory in hypertension has been defined predominantly in experimental animals, Itani et al. sought to determine whether human T cells are activated in hypertension. A third humanized mouse model in which the murine immune system is replaced by the human immune system was studied (84). Angiotensin II increased systolic blood pressure in humanized mice compared to sham-treated animals. In response to angiotensin II, flow cytometric analysis of thoracic lymph nodes, aorta, and kidney induced an increase in infiltration of human leukocytes (CD45+) and T lymphocytes (CD3+ and CD4+). Angiotensin II also increased memory T cells (CD3+/CD45RO+) in the aortas and lymph nodes. Deterrence of hypertension by co-administration of hydrochlorothiazide and hydralazine abrogated the accumulation of T cells in these tissues. These human T cells were not only activated, they also invaded critical end-organ tissues in response to hypertension. To corroborate these findings in humans, 20 normotensive and 20 hypertensive humans were matched for age, sex and BMI, and assessed by staining for CD45RO+ human memory cells and performing intracellular staining for IL-17 and IFN-γ. Flow cytometric analysis revealed an increase in percent of CD4+ and CD8+/CD45RO+ circulating T cells in the hypertensive compared with normotensive humans. Also, the CD4+ T cells of humans with hypertension produced greater amounts of IL-17A than normotensive controls. Intracellular staining for IFN-γ and TNF-α revealed that both of these cytokines were increased in the CD4+ T cells and CD8+ T cells of hypertensive humans. Thus, circulating T cells of hypertensive individuals exhibit evidence of activation and increased IL-17A and IFN-γ production that seem to mimic the findings in mice.
Future Directions
Advanced techniques are being developed to help address questions regarding immune-mediated health and disease, but continued development of these techniques is critical. One critical question is whether certain antigens or neoantigens are particularly antigenic in the setting of metabolic cycling. Studies identifying neoantigens have primarily been in the context of cancer and have been reviewed recently (85). Future studies should consider using similar experimental approaches to identify potential antigens as mediators in memory of metabolic disease. For example, T cell receptor (TCR) sequencing techniques have been developed to look at changes in total isolated T cell repertoires. Additionally, new methods to analyze these complex TCR repertoire datasets are being developed. These experimental methods let investigators explore T cell clonality as a measurement for antigen-mediated T cell proliferation. However, T cell populations are complex and actively inflammatory cells can coexist with immunosuppressive cells. Therefore, advancement in single cell TCR sequencing have been proposed. Early experiments suggest single cell TCR sequencing can be used to elucidate T cell ancestry and activation. Furthermore, development of T cell lines with single epitopes might allow for antigen identification. In addition to these other future studies to determine immunologic mediators of worsened outcome with metabolic cycling should focus on trained innate immunity; the contribution of other organs such as liver, muscle and the CNS; changes to the microbiome; and genetic predictors of weight regain as described above.
Conclusions
The immune system is a major contributor to disease development and progression associated with metabolic cycling. Although the adaptive immune system has been well-studied in the context of metabolic disease, work completed over the past decade suggests that the adaptive immune system plays a unique role in memory of prolonged metabolic disturbances. By characterizing population changes in metabolic tissues following re-exposure to metabolic challenges, new experimental and therapeutic targets have been identified. Memory formation following early antigen stimulated effector T cell expansion underlies worsened metabolic outcomes upon exposure to subsequent metabolic stressors (Figure 3). Resident, central, and effector memory T cells have been suggested and experimentally shown to be involved in memory of metabolic disease, however these studies are early in development and more work remains to be done to better characterize these populations.
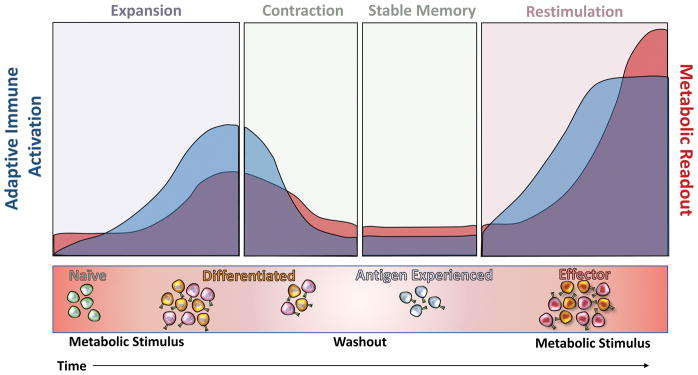
Figure 3. Proposed immunologic mechanisms for exaggerated responses to metabolic cycling.
Upon metabolic stimulus, T cell populations expand and a mild metabolic response is observed. Attenuation of stimuli results in T cell population contraction, stable memory formation, and metabolic improvement. Repeated metabolic stimulation ultimately results in an exacerbated effector T cell response that exaggerates negative metabolic outcomes.
Footnotes
Financial Support: This project was supported by a Merit Award from the Veterans Affairs (5I01BX002195) to AHH. MAC is supported by the Molecular Endocrinology Training Grant (DK07563) and HAI is supported by an American Heart Association Scientist Development Grant (17SDG33410261).
Literature Cited
- 1.Hotamisligil GS. Foundations of Immunometabolism and Implications for Metabolic Health and Disease. Immunity. 2017;47:406–420. doi: 10.1016/j.immuni.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41:36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant RW, V, Dixit D. Adipose tissue as an immunological organ. Obesity (Silver Spring) 2015;23:512–518. doi: 10.1002/oby.21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta. 2014;1842:446–462. doi: 10.1016/j.bbadis.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YS, Wollam J, Olefsky JM. An Integrated View of Immunometabolism. Cell. 2018;172:22–40. doi: 10.1016/j.cell.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi CHJ, Cohen P. Adipose crosstalk with other cell types in health and disease. Exp Cell Res. 2017;360:6–11. doi: 10.1016/j.yexcr.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen BK. Muscle as a secretory organ. Compr Physiol. 2013;3:1337–1362. doi: 10.1002/cphy.c120033. [DOI] [PubMed] [Google Scholar]
- 12.Meex RCR, Watt MJ. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol. 2017;13:509–520. doi: 10.1038/nrendo.2017.56. [DOI] [PubMed] [Google Scholar]
- 13.Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13:633–643. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 14.Chang JT, Wherry EJ, Goldrath AW. Molecular regulation of effector and memory T cell differentiation. Nature immunology. 2014;15:1104–1115. doi: 10.1038/ni.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 16.Chang JT, Wherry EJ, Goldrath AW. Molecular regulation of effector and memory T cell differentiation. Nature immunology. 2014;15:1104–1115. doi: 10.1038/ni.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nature immunology. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 18.Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. The Journal of experimental medicine. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 20.Denoeud J, Moser M. Role of CD27/CD70 pathway of activation in immunity and tolerance. Journal of leukocyte biology. 2011;89:195–203. doi: 10.1189/jlb.0610351. [DOI] [PubMed] [Google Scholar]
- 21.Bangalore S, Fayyad R, Laskey R, DeMicco DA, Messerli FH, Waters DD. Body-Weight Fluctuations and Outcomes in Coronary Disease. N Engl J Med. 2017;376:1332–1340. doi: 10.1056/NEJMoa1606148. [DOI] [PubMed] [Google Scholar]
- 22.Speakman JR, Levitsky DA, Allison DB, Bray MS, de Castro JM, Clegg DJ, Clapham JC, Dulloo AG, Gruer L, Haw S, Hebebrand J, Hetherington MM, Higgs S, Jebb SA, Loos RJ, Luckman S, Luke A, Mohammed-Ali V, O’Rahilly S, Pereira M, Perusse L, Robinson TN, Rolls B, Symonds ME, Westerterp-Plantenga MS. Set points, settling points and some alternative models: theoretical options to understand how genes and environments combine to regulate body adiposity. Dis Model Mech. 2011;4:733–745. doi: 10.1242/dmm.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, Chen KY, Skarulis MC, Walter M, Walter PJ, Hall KD. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring) 2016;24:1612–1619. doi: 10.1002/oby.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dankel SN, Degerud EM, Borkowski K, Fjaere E, Midtbo LK, Haugen C, Solsvik MH, Lavigne AM, Liaset B, Sagen JV, Kristiansen K, Mellgren G, Madsen L. Weight cycling promotes fat gain and altered clock gene expression in adipose tissue in C57BL/6J mice. Am J Physiol Endocrinol Metab. 2014;306:E210–224. doi: 10.1152/ajpendo.00188.2013. [DOI] [PubMed] [Google Scholar]
- 25.Anderson EK, Gutierrez DA, Kennedy A, Hasty AH. Weight cycling increases T-cell accumulation in adipose tissue and impairs systemic glucose tolerance. Diabetes. 2013;62:3180–3188. doi: 10.2337/db12-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wainright KS, Fleming NJ, Rowles JL, Welly RJ, Zidon TM, Park YM, Gaines TL, Scroggins RJ, Anderson-Baucum EK, Hasty AH, Vieira-Potter VJ, Padilla J. Retention of sedentary obese visceral white adipose tissue phenotype with intermittent physical activity despite reduced adiposity. American journal of physiology Regulatory, integrative and comparative physiology. 2015;309:R594–602. doi: 10.1152/ajpregu.00042.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou J, Lai B, Zheng M, Chen Q, Jiang S, Song A, Huang Z, Shi P, Tu X, Wang D, Lu L, Lin Z, Gao X. CD4+ T cells memorize obesity and promote weight regain. Cell Mol Immunol. 2017 doi: 10.1038/cmi.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbosa-da-Silva S, Fraulob-Aquino JC, Lopes JR, Mandarim-de-Lacerda CA, Aguila MB. Weight cycling enhances adipose tissue inflammatory responses in male mice. PLoS One. 2012;7:e39837. doi: 10.1371/journal.pone.0039837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zamarron BF, Mergian TA, Cho KW, Martinez-Santibanez G, Luan D, Singer K, DelProposto JL, Geletka LM, Muir LA, Lumeng CN. Macrophage Proliferation Sustains Adipose Tissue Inflammation in Formerly Obese Mice. Diabetes. 2017;66:392–406. doi: 10.2337/db16-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW., Jr Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, Fischer-Posovszky P, Barth TF, Dragun D, Skurk T, Hauner H, Bluher M, Unger T, Wolf AM, Knippschild U, Hombach V, Marx N. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1304–1310. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 33.Shirakawa K, Yan X, Shinmura K, Endo J, Kataoka M, Katsumata Y, Yamamoto T, Anzai A, Isobe S, Yoshida N, Itoh H, Manabe I, Sekai M, Hamazaki Y, Fukuda K, Minato N, Sano M. Obesity accelerates T cell senescence in murine visceral adipose tissue. J Clin Invest. 2016;126:4626–4639. doi: 10.1172/JCI88606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith CW, Ballantyne CM. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 35.Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL, Dixit VD. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185:1836–1845. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kyung DS, Sung HR, Kim YJ, Kim KD, Cho SY, Choi JH, Lee YH, Kim IY, Seong JK. Global transcriptome analysis identifies weight regain-induced activation of adaptive immune responses in white adipose tissue of mice. Int J Obes. 2017 doi: 10.1038/ijo.2017.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Tian J, Tian X, Tang X, Rui K, Tong J, Lu L, Xu H, Wang S. Adipose tissue dendritic cells enhances inflammation by prompting the generation of Th17 cells. PLoS One. 2014;9:e92450. doi: 10.1371/journal.pone.0092450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, Leong HX, Glassford A, Caimol M, Kenkel JA, Tedder TF, McLaughlin T, Miklos DB, Dosch HM, Engleman EG. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huh JY, Kim JI, Park YJ, Hwang IJ, Lee YS, Sohn JH, Lee SK, Alfadda AA, Kim SS, Choi SH, Lee DS, Park SH, Seong RH, Choi CS, Kim JB. A novel function of adipocytes in lipid antigen presentation to iNKT cells. Mol Cell Biol. 2013;33:328–339. doi: 10.1128/MCB.00552-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng T, Lyon CJ, Minze LJ, Lin J, Zou J, Liu JZ, Ren Y, Yin Z, Hamilton DJ, Reardon PR, Sherman V, Wang HY, Phillips KJ, Webb P, Wong ST, Wang RF, Hsueh WA. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 2013;17:411–422. doi: 10.1016/j.cmet.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malide D, Yewdell JW, Bennink JR, Cushman SW. The export of major histocompatibility complex class I molecules from the endoplasmic reticulum of rat brown adipose cells is acutely stimulated by insulin. Mol Biol Cell. 2001;12:101–114. doi: 10.1091/mbc.12.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng TL, Deng JY, Minze L, Xiao X, Wright V, Yu R, Li XC, Blaszczak A, Bergin S, DiSilvestro D, Judd R, Bradley D, Caligiuri M, Lyon CJ, Hsueh WA. Adipocyte adaptive immunity mediates diet-induced adipose inflammation and insulin resistance by decreasing adipose Treg cells. Nature communications. 2017:8. [Google Scholar]
- 45.Christ A, Gunther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, Scholz CJ, Oosting M, Haendler K, Bassler K, Klee K, Schulte-Schrepping J, Ulas T, Moorlag S, Kumar V, Park MH, Joosten LAB, Groh LA, Riksen NP, Espevik T, Schlitzer A, Li Y, Fitzgerald ML, Netea MG, Schultze JL, Latz E. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell. 2018;172:162–175 e114. doi: 10.1016/j.cell.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newson J, Motwani MP, Kendall AC, Nicolaou A, Muccioli GG, Alhouayek M, Bennett M, Van De Merwe R, James S, De Maeyer RPH, Gilroy DW. Inflammatory Resolution Triggers a Prolonged Phase of Immune Suppression through COX-1/mPGES-1-Derived Prostaglandin E2. Cell Rep. 2017;20:3162–3175. doi: 10.1016/j.celrep.2017.08.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leentjens J, Bekkering S, Joosten LA, Netea MG, Burgner DP, Riksen NP. Trained Innate Immunity as a Novel Mechanism Linking Infection and the Development of Atherosclerosis. Circulation research. 2018 doi: 10.1161/CIRCRESAHA.117.312465. [DOI] [PubMed] [Google Scholar]
- 48.Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, O’Neill LA, Xavier RJ. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song WM, Colonna M. Immune Training Unlocks Innate Potential. Cell. 2018;172:3–5. doi: 10.1016/j.cell.2017.12.034. [DOI] [PubMed] [Google Scholar]
- 50.Jais A, Bruning JC. Hypothalamic inflammation in obesity and metabolic disease. J Clin Invest. 2017;127:24–32. doi: 10.1172/JCI88878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boulange CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8:42. doi: 10.1186/s13073-016-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harley IT, Karp CL. Obesity and the gut microbiome: Striving for causality. Mol Metab. 2012;1:21–31. doi: 10.1016/j.molmet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thaiss CA, Itav S, Rothschild D, Meijer M, Levy M, Moresi C, Dohnalova L, Braverman S, Rozin S, Malitsky S, Dori-Bachash M, Kuperman Y, Biton I, Gertler A, Harmelin A, Shapiro H, Halpern Z, Aharoni A, Segal E, Elinav E. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature. 2016 doi: 10.1038/nature20796. [DOI] [PubMed] [Google Scholar]
- 54.Delahanty LM, Pan Q, Jablonski KA, Watson KE, McCaffery JM, Shuldiner A, Kahn SE, Knowler WC, Florez JC, Franks PW G Diabetes Prevention Program Research. Genetic predictors of weight loss and weight regain after intensive lifestyle modification, metformin treatment, or standard care in the Diabetes Prevention Program. Diabetes Care. 2012;35:363–366. doi: 10.2337/dc11-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crujeiras AB, Goyenechea E, Abete I, Lage M, Carreira MC, Martinez JA, Casanueva FF. Weight regain after a diet-induced loss is predicted by higher baseline leptin and lower ghrelin plasma levels. J Clin Endocrinol Metab. 2010;95:5037–5044. doi: 10.1210/jc.2009-2566. [DOI] [PubMed] [Google Scholar]
- 56.Narayan KM, Ali MK, Koplan JP. Global noncommunicable diseases--where worlds meet. N Engl J Med. 2010;363:1196–1198. doi: 10.1056/NEJMp1002024. [DOI] [PubMed] [Google Scholar]
- 57.Lenfant C, Chobanian AV, Jones DW, Roccella EJ. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): resetting the hypertension sails. Hypertension. 2003;41:1178–1179. doi: 10.1161/01.HYP.0000075790.33892.AE. [DOI] [PubMed] [Google Scholar]
- 58.Julius S, Nesbitt SD, Egan BM, Weber MA, Michelson EL, Kaciroti N, Black HR, Grimm RH, Jr, Messerli FH, Oparil S, Schork MA. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med. 2006;354:1685–1697. doi: 10.1056/NEJMoa060838. [DOI] [PubMed] [Google Scholar]
- 59.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olsen F. Inflammatory cellular reaction in hypertensive vascular disease in man. Acta Pathol Microbiol Scand A. 1972;80:253–256. [PubMed] [Google Scholar]
- 61.Mattson DL. Effector Memory T Lymphocytes in Renal Disease. Am J Physiol Renal Physiol. 2010 doi: 10.1152/ajprenal.00452.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, Chen W, Li CI, Shyr Y, Harrison DG. Oligoclonal CD8+ T Cells Play a Critical Role in the Development of Hypertension. Hypertension. 2014;64:1108–1115. doi: 10.1161/HYPERTENSIONAHA.114.04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. The Journal of experimental medicine. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS, Ruiz P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1089–1097. doi: 10.1152/ajpregu.00373.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circulation research. 2010;107:263–270. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. American journal of physiology Regulatory, integrative and comparative physiology. 2013;304:R407–414. doi: 10.1152/ajpregu.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL PhysGen Knockout P. CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension. 2014;63:559–564. doi: 10.1161/HYPERTENSIONAHA.113.02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011;57:469–476. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 69.Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, Neves MF, Laurant P, Paradis P, Schiffrin EL. T Regulatory Lymphocytes Prevent Aldosterone-Induced Vascular Injury. Hypertension. 2011 doi: 10.1161/HYPERTENSIONAHA.111.181123. [DOI] [PubMed] [Google Scholar]
- 70.Matrougui K, Abd Elmageed Z, Kassan M, Choi S, Nair D, Gonzalez-Villalobos RA, Chentoufi AA, Kadowitz P, Belmadani S, Partyka M. Natural regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice. Am J Pathol. 2011;178:434–441. doi: 10.1016/j.ajpath.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, Weyand CM, Harrison DG, Guzik TJ. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation. 2010;122:2529–2537. doi: 10.1161/CIRCULATIONAHA.109.930446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kamat NV, Thabet SR, Xiao L, Saleh MA, Kirabo A, Madhur MS, Delpire E, Harrison DG, McDonough AA. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-gamma−/− and interleukin-17A−/− mice. Hypertension. 2015;65:569–576. doi: 10.1161/HYPERTENSIONAHA.114.04975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nguyen H, V, Chiasson L, Chatterjee P, Kopriva SE, Young KJ, Mitchell BM. Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc Res. 2013;97:696–704. doi: 10.1093/cvr/cvs422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu J, Thabet SR, Kirabo A, Trott DW, Saleh MA, Xiao L, Madhur MS, Chen W, Harrison DG. Inflammation and Mechanical Stretch Promote Aortic Stiffening in Hypertension Through Activation of p38 Mitogen-Activated Protein Kinase. Circ Res. 2014;114:616–625. doi: 10.1161/CIRCRESAHA.114.302157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J, 2nd, Harrison DG. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–4656. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guyton AC. Renal function curve--a key to understanding the pathogenesis of hypertension. Hypertension. 1987;10:1–6. doi: 10.1161/01.hyp.10.1.1. [DOI] [PubMed] [Google Scholar]
- 78.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res. 2015;116:1022–1033. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crowley SD, Rudemiller NP. Immunologic Effects of the Renin-Angiotensin System. Journal of the American Society of Nephrology: JASN. 2017;28:1350–1361. doi: 10.1681/ASN.2016101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Itani HA, Xiao L, Saleh MA, Wu J, Pilkinton MA, Dale BL, Barbaro NR, Foss JD, Kirabo A, Montaniel KR, Norlander AE, Chen W, Sato R, Navar LG, Mallal SA, Madhur MS, Bernstein KE, Harrison DG. CD70 Exacerbates Blood Pressure Elevation and Renal Damage in Response to Repeated Hypertensive Stimuli. Circulation research. 2016;118:1233–1243. doi: 10.1161/CIRCRESAHA.115.308111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, Giani JF, Nguyen MT, Riquier-Brison AD, Seth DM, Fuchs S, Eladari D, Picard N, Bachmann S, Delpire E, Peti-Peterdi J, Navar LG, Bernstein KE, McDonough AA. The absence of intrarenal ACE protects against hypertension. J Clin Invest. 2013;123:2011–2023. doi: 10.1172/JCI65460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feau S, Garcia Z, Arens R, Yagita H, Borst J, Schoenberger SP. The CD4(+) T-cell help signal is transmitted from APC to CD8(+) T-cells via CD27-CD70 interactions. Nature communications. 2012;3:948. doi: 10.1038/ncomms1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, Markovitz JH. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110:74–78. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- 84.Itani HA, McMaster WG, Jr, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, Konior A, Prejbisz A, Januszewicz A, Norlander AE, Chen W, Bonami RH, Marshall AF, Poffenberger G, Weyand CM, Madhur MS, Moore DJ, Harrison DG, Guzik TJ. Activation of Human T Cells in Hypertension: Studies of Humanized Mice and Hypertensive Humans. Hypertension. 2016;68:123–132. doi: 10.1161/HYPERTENSIONAHA.116.07237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]