Abstract
Epileptic activity is frequently associated with Alzheimer’s disease; this association has therapeutic implications, because epileptic activity can occur at early disease stages and might contribute to pathogenesis. In clinical practice, seizures in patients with Alzheimer’s disease can easily go unrecognised because they usually present as non-motor seizures, and can overlap with other symptoms of the disease. In patients with Alzheimer’s disease, seizures can hasten cognitive decline, highlighting the clinical relevance of early recognition and treatment. Some evidence indicates that subclinical epileptiform activity in patients with Alzheimer’s disease, detected by extended neurophysiological monitoring, can also lead to accelerated cognitive decline. Treatment of clinical seizures in patients with Alzheimer’s disease with select antiepileptic drugs (AEDs), in low doses, is usually well tolerated and efficacious. Moreover, studies in mouse models of Alzheimer’s disease suggest that certain classes of AEDs that reduce network hyperexcitability have disease-modifying properties. These AEDs target mechanisms of epileptogenesis involving amyloid β and tau. Clinical trials targeting network hyperexcitability in patients with Alzheimer’s disease will identify whether AEDs or related strategies could improve their cognitive symptoms or slow decline.
Introduction
The transient worsening of cognitive symptoms is frequent in patients with Alzheimer’s disease, sometimes raising the possibility of underlying epilepsy. Caregivers might report that the patient seems to “check out” for periods of time or appears more confused than usual on some days than on others. These changes warrant attention, since new evidence indicates that seizures and network hyperexcitability can occur in the early stages of Alzheimer’s disease and contribute to cognitive decline.1–5 Treatment options with antiepileptic drugs (AEDs) are available, but the decisions on whether to treat seizures and the choice of AED for an individual with Alzheimer’s disease can be difficult. Even more challenging is the decision on whether to treat subclinical epileptiform activity (ie, the occurrence of epileptiform activity in the absence of clinical seizures). This Review provides an update on the mechanisms and therapeutic implications of epilepsy associated with Alzheimer’s disease. We will also provide guidance on assessing and treating patients with Alzheimer’s disease and suspected seizures.
Risk of seizures in Alzheimer’s disease
The increased prevalence of seizures in patients with Alzheimer’s disease, in relation to older populations without dementia, has been noted since Alois Alzheimer’s earliest patient descriptions. Initial studies focused on seizures in patients with advanced Alzheimer’s disease, many of whom were living in institutions. However, in transgenic mouse models of Alzheimer’s disease,6 seizures and epileptiform activity can occur before amyloid β plaque deposition. This new evidence has made researchers and clinicians pay closer attention to seizures occurring in the early stages of dementia.2,3
Epidemiological studies7 have shown that unprovoked seizures occur in 10–22% of patients with Alzheimer’s disease, but reports vary, and rates as high as 64% have been observed in cohorts that were closely monitored through all stages of disease. Overall, seizures occur more frequently in patients with Alzheimer’s disease than in those with non-Alzheimer’s disease dementias, but studies have not looked beyond this crude level of distinction.8 Myoclonus, which is generally considered to be due to cortical hyperexcitability, is also common in patients with Alzheimer’s, with a prevalence of 7–10% and a cumulative risk as high as 80% by late stages of disease.9,10 In atypical presentations of Alzheimer’s disease with more neocortical involvement, myoclonus rates might be higher. For instance, in two clinicopathological studies, myoclonus was reported in five (33%) of 15 patients with corticobasal syndrome who had Alzheimer’s disease pathology at autopsy.11,12
For unclear reasons, seizures and myoclonus seem to occur more frequently in younger patients with Alzheimer’s disease.2,8 In a study of 198 patients with early-onset Alzheimer’s disease,13 defined as age at onset before 65 years, seizures were reported in 45% and myoclonus in 36% of patients; presence of myoclonus increased seizure risk by 7·7 times. The inverse association between seizure risk and age in Alzheimer’s disease contrasts with the age-associated increase in seizures in the general population, which is likely to be secondary to vascular disease and other morbidities. An explanation for the higher seizure risk in younger patients with Alzheimer’s disease might be provided by their unique risk factors, such as altered expression of genes that regulate neural network activity, when compared with older patients with Alzheimer’s disease. Alternatively, seizures might hasten onset of cognitive decline and thereby associate with earlier Alzheimer’s disease presentation.
Genes linked to Alzheimer’s disease have been implicated in aberrant network activity. Individuals with Down’s syndrome, because of an additional copy of chromosome 21 that contains the APP gene, often develop Alzheimer’s disease between the ages of 40 and 60 years. In an 8 year prospective assessment,14 41 (84%) of 49 patients with both Down’s syndrome and Alzheimer’s disease developed seizures. In people who carry APP, PSEN1, or PSEN2 mutations linked to autosomal dominant Alzheimer’s disease, risk for seizures and myoclonus is also high, with estimated seizure rates of 28% (range 15–67, highest in carriers of APP duplications),15 and myoclonus rates of 27% (range 9–31, highest in PSEN1 mutation carriers).15
Importantly, seizures can hasten cognitive decline in patients with Alzheimer’s disease.16,17 In a case-control study of ten patients with advanced Alzheimer’s disease living in institutions, those with new-onset seizures had faster declines in language assessment scores over 12 months compared with a severity-matched group of patients without seizures.16 Moreover, seizures and myoclonus have been associated with reduced survival in patients with Alzheimer’s disease.13,18
The common comorbidity of seizures and Alzheimer’s disease raises questions on its cause, versus its consequences. Does long-standing epilepsy increase the risk of developing Alzheimer’s disease or do pathological changes in the brain of patients with Alzheimer’s disease set the stage for seizure initiation? Observational studies2,3,19,20 would suggest that both mechanisms are possible, although the second scenario appears more likely. In findings from epilepsy studies,19 people with longstanding seizure disorders had a slightly increased risk of developing dementia, but this result did not reach significance unless seizures began within 10 years of Alzheimer’s disease diagnosis.19 In dementia studies that followed up participants before a dementia diagnosis, findings also suggested that, in some cases, seizures can be a harbinger of Alzheimer’s disease or begin concurrently with onset of cognitive decline.2,3 Seizures that precede onset of cognitive decline might reflect the epileptogenic potential of amyloid β, which begins to accumulate more than 10 years before clinical signs of dementia.20
Seizure semiology in Alzheimer’s disease
The predominant seizure subtype in patients with Alzheimer’s disease is non-motor complex partial seizures (table 1).2–4,21 Some symptoms of these seizures can overlap with cognitive features of Alzheimer’s disease, but they can often be distinguished as epileptic events by their recurrent and stereotyped nature, and supported by epileptiform activity on EEG. These episodes can include amnestic spells, déjà vu or jamais vu, speech arrest, staring spells, and unexplained emotions (eg, fear or euphoria) or sensory phenomena (eg, metallic taste or rising epigastric sensation). Many of these presentations are typical of focal hippocampal seizures. Seizures can induce tachycardia, bradycardia, or even asystole requiring pacemaker implantation, possibly due to involvement of insular cortical regions that influence sympathetic and parasympathetic output to the heart via the amygdala and hypothalamus.2
Table 1.
Observational studies of patients with mild cognitive impairment or dementia (predominantly Alzheimer’s disease) and comorbid epilepsy
| Diagnoses (n) | Mean age at first seizure | Mean age at onset of cognitive decline | Mean age at diagnosis of neurodegenerative disease | Seizure types | Epileptiform EEG (protocol) | Epileptic foci | |
|---|---|---|---|---|---|---|---|
| Cretin et al (2016)3 | MCI due to Alzheimer’s disease (13) | 63 | 66 | 70 | CPS (62%), SPS (23%), and GTC (15%) | 23% (routine 20-min EEGs) | Temporal (left hemisphere> right hemisphere) |
| Rao et al (2009)*21 | MCI (10), Alzheimer’s disease (9), vascular dementia (6), and dementia with Lewy bodies (5) | 62† | 71 | Not provided | CPS (72%), GTC (39%), SPS (13%), atypical absence (3%), myoclonic (8%), and unknown (3%) | 38% (unspecified EEG protocols) | Unilateral or bilateral temporal |
| Sarkis et al (2016)4 | Alzheimer’s disease‡ (64), mixed dementia (4), dementia with Lewy bodies (4), frontotemporal dementia (3), vascular dementia (1), and primary progressive aphasia (1) | 74 | 68 | 72 | CPS (53%), GTC (40%), and SPS (7%) | 36% (routine, serial, and extended EEGs) | Frontal or temporal |
| Vossel et al (2013)2 | Amnestic MCI (12) and Alzheimer’s disease (35) | 67 | 65 | 68 | CPS (47%), generalised (36%), and SPS (17%) | 62% (routine, serial, and extended EEGs) | Frontal or temporal (left hemisphere> right hemisphere) |
EEG=electrencephalogram. MCI=mild cognitive impairment. CPS=complex partial seizures. GTC=generalised tonic-clonic seizures. SPS=simple partial seizures.
Included 14 patients with structural lesions on MRI.
Included participants with long-standing seizure disorders.
Included participants with possible Alzheimer’s disease (13%), probable Alzheimer’s disease (65%), and autopsy-proven Alzheimer’s disease (5%).
Pathological association between epilepsy and Alzheimer’s disease
Temporal lobe epilepsy and Alzheimer’s disease share several pathological and neuroimaging features. In a series of 101 patients (aged 30–61 years) with temporal lobe epilepsy and without dementia who underwent temporal lobectomy, brain samples from ten patients had amyloid β plaques. Pathological findings from this cohort were compared with temporal lobe tissue obtained from autopsies on 406 control patients without history of dementia or epilepsy (aged 30–92 years).22 Although the presence of plaques was positively correlated with age, the age-related incidence of plaques was greater in patients with temporal lobe epilepsy than in autopsy control patients without epilepsy. In a post-mortem study, chronic epilepsy was associated with increased tau neurofibrillary tangles at mid-Braak stages in patients aged 40–65 years.23 A clinicopathological study24 on tissue resected from 33 patients with refractory temporal lobe epilepsy, revealed hyperphospohorylated tau in 94% of excised temporal lobe samples, and more extensive tau pathology was associated with a decline in verbal learning, recall, and language scores over 1 year after temporal lobe resection.24 Other pathological features common to Alzheimer’s disease and epilepsy include hippocampal sclerosis25 and depletion of the calcium-binding protein calbindin-D28K in the dentate gyrus, indicating chronic overexcitation of this region.5,26 Finally, during functional MRI (fMRI), patients with either temporal lobe epilepsy, absence epilepsy, or Alzheimer’s disease had reduced resting-state activity and functional connectivity within the default mode network, a cortical network that is most active during conscious rest, suggesting shared regions of network dysfunction.27
Seizures in animal models of Alzheimer’s disease
Animal models of Alzheimer’s disease typically feature overexpression of human amyloid precursor protein (APP), presenilin 1 (PS1), or both, and express genetic mutations that are linked to familial Alzheimer’s disease. Similar to human beings, transgenic mouse models of Alzheimer’s disease can exhibit a variety of seizure types.5,26,28 Most electrographic seizures in these mice have little to no motor manifestation, with the exception of mice overexpressing APP and PS1, which present frequent motor seizures.5,26,29 Evidence from these models suggests that epileptogenesis in Alzheimer’s disease occurs through mechanisms that, in many ways, are distinct from mechanisms derived from epilepsy models (panel). The trigger for many of these mechanisms seems to be oligomeric species of amyloid β. This hypothesis is supported by the high prevalence of seizures in patients with familial Alzheimer’s disease;15 however, the relative contributions of amyloid β, APP, and other APP metabolites to network hyperexcitability is not entirely clear.26,28,50 The tau protein appears to have an enabling role for epileptogenesis in Alzheimer’s disease. Endogenous tau concentrations modulate seizure susceptibility. Amounts of wild-type tau, which can vary naturally or be changed experimentally, correlate with neuronal and network excitability in a dose-dependent manner in models of epilepsy or Alzheimer’s disease.40,41,51,52 Additionally, transgenic mice overexpressing human wildtype tau or tau with an A152T mutation, which confers a risk of Alzheimer’s disease and other tauopathies, have epileptiform activity and higher seizure susceptibility than wild-type littermates.53 The A152T mutation in tau induces higher amounts of network hyperexcitability than does wild-type tau, possibly because the A152T substitution increases production or decreases clearance of the tau protein.53 Excess tau might, in turn, stimulate presynaptic glutamate release.32 Notably, low amounts of amyloid β can also enhance synaptic transmission, but amyloid β can suppress synaptic activity at higher concentrations.54 Under scenarios of amyloid β induced synaptic suppression, epileptogenesis might be initiated by inhibition of selectively susceptible neurons within circuits. For instance, impaired cortical inputs to the reticular thalamic nucleus might lead to aberrant corticothalamic activity, which is associated with non-motor seizures.48 Emerging evidence55,56 indicates that increased neuronal activity increases both amyloid β and tau secretion; thus recurrent epileptic activity in Alzheimer’s disease could establish a vicious cycle augmenting the aberrant aggregation and spread of these disease proteins. Apolipoprotein E4 also contributes to hippocampal overexcitation in mouse models of Alzheimer’s disease by causing tau-dependent decrease in GABAergic interneurons in the hilus of the dentate gyrus.43,57
Panel. Proposed mechanisms of epileptogenesis in Alzheimer’s disease.
Extrasynaptic glutamate spillover due to impaired glial or neuronal glutamate transporters30,31
Tau-induced enhancement of presynaptic glutamate release32
Reduced axonal and dendritic transport of cargoes (eg, mitochondria) that regulate neuronal excitability33–35
Altered trafficking and surface expression of postsynaptic AMPA and NMDA receptors29,36
Altered amounts of voltage-gated ion channels in the brain37–39
Selective impairment of GABAergic interneurons in the hippocampus and parietal cortex31,37,43–46
Shortened dendrites, lowering threshold for action potential generation47
Impaired cortical input to the reticular thalamic nucleus and subsequent disinhibition of thalamic relay nuclei and their cortical and limbic targets48
Increases in cholinergic tone before the degeneration of cholinergic pathways49
Subclinical and interictal epileptiform activity
Epileptiform activity is defined as paroxysmal sharp waveforms (spikes and sharp waves) on EEG, lasting 20 to 200 ms, that disrupt background activity and are associated with a subsequent slow wave. Such activity is named interictal epileptiform activity when it is detected in patients with seizures, and subclinical epileptiform activity when detected in patients without known seizures. The sensitivity of scalp EEG recordings to detect epileptiform activity depends on the type of EEG protocol that is used. Estimates of epileptiform activity in Alzheimer’s disease are limited by the use of non-invasive scalp recordings, a limitation that does not occur in animal models, in which subdural or depth electrodes are commonly used. Consistent with studies of epilepsy in older adults, routine EEG detects interictal epileptiform activity in about a third of patients with mild cognitive impairment or dementia and comorbid seizures.2–4 The sensitivity of the EEG increases with extended recordings that capture sleep states and with serial EEGs.2
In patients with Alzheimer’s disease, epileptiform activity is commonly detected in electrodes that surround frontotemporal and temporal brain regions,2–4 and such activity is often multifocal, reflecting the diffuse networks that are affected.4,58 Since the temporal lobes are the most common regions of epileptiform activity in older adults with seizures, the location of epileptiform activity does not usually help distinguish whether seizures are secondary to Alzheimer’s disease or other causes, or if they are idiopathic.
Although EEG can guide diagnosis in the assessment of patients with Alzheimer’s disease with suspected seizures, limited information is available about the usefulness of EEG in patients without suspected seizures. Estimates of subclinical epileptiform activity in Alzheimer’s disease vary. In a study that used 30-min EEGs and kept patients awake with eyes closed during monitoring, only 31 (2%) of 1674 patients with Alzheimer’s disease had subclinical epileptiform activity or were newly diagnosed with epilepsy.59 Patients with Alzheimer’s disease and epileptiform activity were significantly younger than those without such activity (mean age, 63 years [SD 10] vs 71 years [SD 9]),59 consistent with their increased risk for seizures as reported in studies of patients with early-onset Alzheimer’s disease. In a study that used various EEG protocols and included patients with amnestic mild cognitive impairment or Alzheimer’s disease,2 subclinical epileptiform activity was detected in seven (6%) of 113 patients. In these investigations, limited longitudinal information on effects of seizures or epileptiform activity on clinical progression was provided. Motivated by findings from animal studies of Alzheimer’s disease, a prospective study1 reported on the incidence of subclinical epileptiform activity in Alzheimer’s disease and its potential effect on cognitive decline. This investigation assessed a cohort of patients with Alzheimer’s disease without a history of seizures (mean age 62 years) and age-matched healthy controls with a combination of 24-h long-term monitoring with video EEG (LTM-EEG) and a 1-h magneto-encephalography (MEG) with simultaneous EEG.1 Subclinical epileptiform activity was detected in 14 (42%) of 33 patients with Alzheimer’s disease—more than four times that in age-matched controls. 90% of epileptiform discharges in patients with Alzheimer’s disease occurred during sleep. Patients with Alzheimer’s disease and epileptiform activity did not differ clinically from those without such activity at the time of monitoring. However, over time, patients with subclinical epileptiform activity showed faster decline on the Mini-Mental State Examination (MMSE) and in executive function. These results suggest that subclinical epileptiform activity might be more common in Alzheimer’s disease than previously recognised, and raise the possibility that epileptiform activity might accelerate cognitive decline, either directly or by association with unrecognised silent seizures.
Based on these observations, we suggest that patients with Alzheimer’s disease should be assessed carefully for epileptiform activity and silent seizures if they present with fluctuations in cognition, rapidly progressive cognitive decline, early-onset Alzheimer’s disease (eg, onset around age 50 years), and myoclonus, given the co-occurrence of myoclonus and seizures.13 A major determinant of sensitivity of an EEG to detect epileptiform activity in Alzheimer’s disease is that patients sleep during the exam.1 Therefore, extended EEGs or MEGs of 1–2 h during a time when patients can sleep are most informative.
Treatment approaches
Several factors must be considered when deciding to treat seizures in patients with Alzheimer’s disease, including age, comorbidities, drug interactions, cognitive and non-cognitive side-effects, and optimal dose. In patients with epileptiform activity but no witnessed seizures, the decision of whether to treat with AEDs is controversial, and should be based on the clinician’s judgment on whether so-called silent seizures might be contributing to cognitive symptoms (eg, cognitive fluctuations or confusion on waking). Empirical treatment with AEDs is not recommended for patients without clinical or electrographical signs of network hyperexcitability. Such decisions must be guided by future clinical trials targeting network hyperexcitability in Alzheimer’s disease. The exact mechanism of action of most AEDs is unknown, and mechanisms might differ depending on dose. Because of the absence of randomised clinical trials addressing Alzheimer’s disease-associated seizures, therapeutic choices have to be based on evidence from other studies of AEDs in older adults with or without dementia (table 2).
Table 2.
Commonly prescribed antiepileptic drugs for older adults with cognitive impairment
| Dose (mg per day) | Tolerability | Efficacy | Cognitive side-effects? | Other potential adverse effects* | Other uses | |
|---|---|---|---|---|---|---|
| Levetiracetam | 250–2000 | Excellent | Excellent | No | Aggression, asthenia, dizziness, fatigue, headache, irritability, and nausea | Treatment of myoclonus |
| Lamotrigine | 25–500 | Excellent | Excellent | No | Asthenia, ataxia, blurred vision, diarrhea, diplopia, dizziness, hypersensitivity reaction, incoordination, insomnia, nausea, rash, somnolence, Stevens-Johnson syndrome, and tremor | Mood stabilisation |
| Gabapentin | 300–1500 | Good | Good | Possible | Ataxia, dizziness, fatigue, nystagmus, nausea, peripheral oedema, somnolence, and weight gain | Treatment of insomnia, peripheral neuropathy, postherpetic neuralgia, and migraine prophylaxis |
| Carbamazepine | 600 | Fair | Good | Yes | Agranulocytosis, asthenia, ataxia, blurred vision, cardiac dysrhythmia, constipation, decreased bone density, dizziness, hepatotoxicity, hypersensitivity reaction, hyponatraemia, nausea, rash, somnolence, and xerostomia | Mood stabilisation, and treatment of trigeminal neuralgia |
| Valproic acid | 250–1000 | Fair | Good | Yes | Alopecia, asthenia, ataxia, constipation, diarrhoea, diplopia, dizziness, gait disturbance, headache, hepatotoxicity, indigestion, nausea, nervousness, nystagmus, peripheral oedema, rash, somnolence, tinnitus, tremor, weakness, and weight gain | Mood stabilisation, migraine prophylaxis, and treatment of myoclonus |
| Phenytoin | 200–300 | Poor | Good | Yes | Ataxia, constipation, decreased bone density, dizziness, dysarthria, gingival hyperplasia, hepatotoxicity, hypersensitivity reaction, incoordination, lethargy, muscle hypotonia, nausea, nervousness, nystagmus, and sedation or drowsiness | None |
| Phenobarbital | 50–100 | Poor | Excellent | Yes | Asthenia, barbiturate withdrawal, decreased bone density, hypersensitivity reaction, somnolence, and syncope | Long-term sedation |
The use of levetiracetam and lamotrigine to treat seizures associated with Alzheimer’s disease is supported by the strongest evidence. Notably, both AEDs can reduce excessive glutamate release from excitatory neurons, which might be relevant to epileptogenesis in Alzheimer’s disease (panel).65,66 Belcastro and colleagues60 did an open-label, observational study of levetiracetam (1000–1500 mg per day) treatment for 1 year or longer in 25 patients with advanced Alzheimer’s disease and epilepsy.60 16% of participants discontinued the medication because of intolerable side-effects, and 72% were seizure-free for at least 1 year. Cumbo and colleagues58 did a randomised, three-group parallel case-control study of levetiracetam (500–2000 mg per day), lamotrigine (25–100 mg per day), and phenobarbital (50–100 mg per day) in 95 patients with Alzheimer’s disease and epilepsy (mean age 72 years) and 68 age-matched control patients with Alzheimer’s disease but without epilepsy, who did not receive AEDs.58 Treatment consisted of a 4-week dose adjustment period followed by a 12-month dose evaluation period. Levetiracetam and lamotrigine caused fewer adverse events than phenobarbital, which caused somnolence in 30% of patients. 17% of patients on phenobarbital withdrew because of adverse effects. The three AEDs had equivalent effects on seizure reduction (response rates: levetiracetam 71%, lamotrigine 59%, and phenobarbital 64%). Levetiracetam and lamotrigine treatment resulted in better cognitive outcomes than phenobarbital, and lamotrigine improved mood. Patients with both Alzheimer’s disease and epilepsy who took levetiracetam had improved performance on MMSE and Alzheimer’s disease Assessment Scale-cognitive (ADAS-cog), similar to patients with Alzheimer’s disease and without epilepsy. A small, double-blind, crossover trial of lamotrigine (150 or 300 mg per day) for patients with Alzheimer’s disease without epilepsy showed that treatment with 300 mg of lamotrigine was associated with improved performance on recognition and naming tasks as well as depressed mood on the ADAS behaviour subscale after 8 weeks of treatment.67
In the Veterans Administration Cooperative Study, Rowan and colleagues61 did a randomised, double-blind, parallel trial comparing the tolerability and efficacy of gabapentin (1500 mg per day), lamotrigine (150 mg per day), and carbamazepine (600 mg per day) for 12 months in a cohort of 593 adults (mean age 72 years) with new-onset epilepsy.61 Notably, 66% of participants had vascular disease based on the prevalence of stroke, cardiac disease, and hypertension, while 35% of participants had mild cognitive impairment. Overall, patients tolerated lamotrigine and gabapentin better than carbamazepine. Seizure control was similar with all three AEDs, and more than half of participants remained seizure free at 12 months.
A multicentre, randomised, double-blind, placebo-controlled trial assessed the efficacy of valproic acid (10–12 mg/kg per day), given over 2 years, to treat agitation in 313 patients with moderate Alzheimer’s disease without epilepsy.62,63 Valproic acid treatment did not reduce incidence of agitation or psychosis, and was associated with higher rates of somnolence, gait disturbance, tremor, diarrhoea, and muscle weakness. Patients who were taking valproic acid also showed greater brain volume loss and faster decline in MMSE at 1 year than patients in the placebo group. These results should dissuade use of valproic acid, at least at the doses used in this trial, as a first-line therapy for patients with Alzheimer’s disease with or without epilepsy.
Evidence for using phenytoin in patients with Alzheimer’s disease is mostly limited to observational studies that have reported variability in its efficacy in seizure control and neurological side-effects, including ataxia, delirium, sedation, and accelerated cognitive decline.2,21,64 Phenytoin paradoxically exacerbates seizures in the APP–J20 mouse model,37 but not in mice overexpressing APP and PS1.68 The deleterious effects of phenytoin on seizures and cognition in hAPP–J20 mice might be related to the depletion of Nav1.1 in parvalbumin-positive interneurons in the parietal cortex, a change also observed in brain samples from patients with Alzheimer’s disease.37 Blocking Nav1.1 activity with phenytoin might exacerbate cortical excitability by reducing activity of Nav1.1-containing interneurons and disinhibiting neighbouring pyramidal neurons. Lamotrigine also inhibits sodium channels, but has a higher potency for inhibiting release of glutamate than of GABA, which distinguishes it from phenytoin.66 Individuals with Down’s syndrome who develop epilepsy early in life usually tolerate phenytoin well, but those who develop Alzheimer’s disease and epilepsy later in life can have deleterious cognitive side-effects when taking phenytoin.64
Benzodiazepines should be used as a last resort to treat seizures associated with Alzheimer’s disease. Although these drugs are highly effective at suppressing seizures and myoclonus, they can induce delirium and their sudden discontinuation (eg, when forgetting to take the medication) can induce withdrawal seizures, even in patients without epilepsy. Furthermore, chronic benzodiazepine use in an older population has been associated with increased risk of developing Alzheimer’s disease.69 Topiramate reduces behavioural deficits in mouse models of Alzheimer’s disease;70 however, its cognitive side-effects, such as word-finding difficulty and reduced concentration span, make it less appealing as a treatment for patients with Alzheimer’s disease. Other AEDs, such as oxcarbazepine and lacosamide, are being used successfully as monotherapy to treat seizures in older adults, but limited data are available for their use in patients with Alzheimer’s disease. Patients on carbamazepine or oxcarbazepine should have sodium levels monitored regularly, as these AEDs can cause hyponatraemia, particularly with advanced age.
Some symptomatic treatments for Alzheimer’s disease have been associated with an increased risk of seizures. In particular, the antidepressant bupropion and typical neuroleptics can decrease the seizure threshold in these patients. Acetylcholinesterase inhibitors, the mainstay of current symptomatic treatments, are likely to have neutral effects on seizure risk. A randomised, double-blind, placebo-controlled study of donepezil in patients with epilepsy did not reveal an increased frequency of seizures.71 Memantine, a non-competitive NMDA receptor antagonist, has been reported to have both pro-seizure and anti-seizure effects in rat models of kindling.72
Overall, most seizures associated with Alzheimer’s disease are responsive to AED monotherapy, often at low doses, which are preferred in an elderly population.2–4,58 Whether low-dose levetiracetam (125 mg twice a day), which seemed to improve hippocampal-based memory performance in patients with mild cognitive impair ment,73 is also effective at suppressing Alzheimer’s disease-associated seizures and epileptiform activity is unclear, but treatment with low-dose levetiracetam deserves consideration from a cognitive standpoint, particularly in patients with Alzheimer’s disease in whom seizures are mild or infrequent, and who have no history of status epilepticus. Levetiracetam should be used with caution in patients with advanced dementia and behavioural disturbances, because it can exacerbate agitation.
Treatment with many AEDs, particularly enzyme-inducing AEDs such as carbamazepine, phenytoin, and phenobarbital, is associated with decreased bone density. Therefore, long-term use of AEDs in elderly patients should be accompanied by routine examination of bone mineral density and supplementation with calcium, vitamin D, or other therapies.
Cognition and epileptiform activity
In patients with Alzheimer’s disease in whom epileptiform activity is detected, potential acute and chronic effects of such activity on cognitive functions should be considered. In rodent models and in patients with interictal epileptiform discharges in the hippocampus, epileptiform events disrupt short-term memory retrieval.74 These findings have been observed in human beings by use of depth electrodes in patients with refractory seizures. However, such invasive testing is rarely done in patients with Alzheimer’s disease, and the exact extent and acute cognitive effects of hippocampal epileptiform discharges in the Alzheimer’s disease population are unknown.
In addition to acute effects on cognition, epileptiform activity over time results in inhibitory compensatory changes in brain networks to prevent propagation of seizures. Although such changes can limit overall network hyperexcitability, they can also have deleterious consequences for neural plasticity, particularly in the hippocampus.5,75 In mouse models6,29,40,70,76,77 of Alzheimer’s disease, interventions that suppress epileptiform activity, including genetic ablation of tau and cellular prion protein (in mice overexpressing APP and PS1), and treatment with certain AEDs, reduce synaptic and cognitive deficits. Suppression of epileptiform activity acutely with AEDs does not improve cognitive function in such mouse models, whereas prolonged treatment over 3 weeks or longer improves both synaptic function and cognition.6,70,76,77 These data support the hypothesis that deleterious effects of epileptiform activity on cognition in Alzheimer’s disease occur more frequently through chronic remodelling of neuronal circuits than through abrupt disruptions of network functions.5,75 In patients with Alzheimer’s disease and epilepsy, epileptiform activity is often located in brain regions that are most impaired clinically.2 Such activity might either be interpreted as a surrogate marker of a more aggressive form of the disease, or it might represent network hyperexcitability that contributes causally to cognitive decline. Clinical trials with AEDs will address this essential question (eg, NCT02002819).
Transient epileptic amnesia versus Alzheimer’s disease-associated epilepsy
Alzheimer’s disease is not the only possible cause of new-onset seizures in older adults that is associated with interictal memory impairment. If underlying brain lesions and other risk factors for seizures are ruled out, many patients fall into one of two categories: either transient epileptic amnesia (or epileptic amnesic syndrome) or Alzheimer’s disease-associated epilepsy. At presentation, these conditions might overlap in some symptoms, but they also have distinguishing characteristics, as listed in table 3. Transient amnesia refers to discrete episodes of mixed anterograde and retrograde memory loss followed by return to baseline. Patients presenting with non-degenerative transient epileptic amnesia are typically middle-aged men who report recurrent amnestic attacks, lasting 30–60 min, often occurring on awakening.78 These patients might have other, more common, seizure manifestations, especially olfactory hallucinations that occur in about 42% of patients with transient epileptic amnesia.78 The epileptic amnesic syndrome, a term coined by Galassi,81 overlaps substantially with transient epileptic amnesia, but also includes patients with subtle, non-amnestic, temporal lobe seizures giving rise, for example, to episodes of déjà vu or brief complex partial seizures. Between seizures, patients with transient epileptic amnesia function well cognitively, although they often report a distinctive loss of memory for personal events (autobiographical memory), such as holidays or the birth of a child, which can stretch back decades before the onset of seizures. By contrast, these patients have preserved semantic, or factual, memory for events that occurred over the same time period (eg, where one went to school and knowledge about famous people). They also often report forgetting newly learned information over days to weeks (accelerated long-term forgetting), and difficulties navigating previously familiar routes. Patients with transient epileptic amnesia perform normally or near-normally on standardised neuropsychological tests, but show accelerated long-term forgetting, often associated with a patchy autobiographical amnesia for salient remote events.79 The amnesic seizures usually cease promptly with AED treatment, while the interictal memory problems either stabilise or improve. The brief duration of amnesia, recurrence, and interictal cognitive changes distinguish transient epileptic amnesia from transient global amnesia, a syndrome of profound anterograde amnesia lasting 4–10 h, often occurring after stressful events or exercise, and rarely recurring.78
Table 3.
Main features of transient epileptic amnesia and Alzheimer’s disease-associated epilepsy
| Transient epileptic amnesia | Alzheimer’s disease-associated epilepsy | |
|---|---|---|
| Mean age at onset of seizures | 62–69 years | 62–74 years |
| Sex | More common in men | Equally common in men and women |
| Seizure semiology | Amnestic spells on waking (100%), olfactory hallucinations (about 42%), automatisms (about 36%), and generalised tonic-clonic seizures (about 4%) | Automatisms (about 31%), sensory phenomena or unexplained emotions (about 22%), speech or behavioural arrest (13–39%), déjà vu or jamais vu (5–8%), amnestic spells (4–54%), staring spells (about 3%), and generalised tonic-clonic seizures (15–40%) |
| Interictal cognitive complaints | Autobiographical memory loss, accelerated forgetting over days to weeks, and topographical memory loss | Short-term memory loss, word-finding difficulty, difficulty with multitasking and calculations, and visuospatial impairments |
| Neuropsychological features | Normal or near-normal performance on standardised neuropsychological tests; impairments in recall of verbal and visual information at extended delays (>30 min); loss of autobiographical memories for events extending decades before onset of seizures. | Impairments in multiple cognitive domains on standardised neuropsychological tests including learning and recall of verbal and visual information, executive function, language abilities, and visuospatial function. |
| EEG epileptiform foci | Temporal or frontotemporal | Temporal, frontotemporal, or frontal |
| Brain MRI | Normal (group studies suggest subtle atrophy in limbic structures) | Grey matter atrophy in medial temporal lobes or posterior cortical regions |
| Alzheimer’s disease biomarkers* | Probably negative (not systematically studied) | Positive |
| Response of seizures to antiepileptic drugs | Good | Good |
PET measures of brain amyloid β deposition and cerebrospinal fluid measures of amyloid β, tau, and phosphorylated tau. These biomarkers are less accurate in assessing the likelihood of Alzheimer’s disease in people over 80 years old.80
Patients who develop seizures in association with Alzheimer’s disease might also report amnestic attacks. However, a broader variety of seizure types than those in transient epileptic amnesia, occur in patients with Alzheimer’s disease, reflecting the diffuse brain regions involved. Between seizures, patients with Alzheimer’s disease have a progressive decline in multiple cognitive domains, and these deficits are reflected in their decreased performance on neuropsychological tests. Long-term memories for personal and factual events are relatively preserved. EEG can reveal frontotemporal or temporal epileptiform activity in both transient epileptic amnesia and Alzheimer’s disease-associated epilepsy, but Alzheimer’s disease biomarkers (MRI; amyloid-PET; and CSF amyloid β, tau, and phospho-tau concentrations) can help distinguish the two syndromes, particularly in early-onset patients, in whom positive biomarkers are more likely to be due to Alzheimer’s disease than age-related changes.82
Network hyperexcitability in Alzheimer’s disease
In patients with mild cognitive impairment due to Alzheimer’s disease, task-based fMRI studies have shown increased hippocampal activation during memory encoding.83 Although this activation can be interpreted as the compensatory recruitment of neural reserves to meet cognitive demands within compromised brain regions, some evidence indicates that hyper activation of the hippocampus and multiple other cortical and subcortical brain regions during encoding is inefficient and might contribute to Alzheimer’s disease pathogenesis.83,84 The amount of hippocampal hyper activation in patients with mild cognitive impairment due to Alzheimer’s disease might predict future cognitive decline.83 Furthermore, in patients with amnestic mild cognitive impairment, suppressing hippocampal dentate gyrus and CA3 hyperactivation with levetiracetam improves their performance on a hippocampal-based pattern-separation task.73 Interestingly, 125 mg twice a day of levetiracetam was more effective than 250 mg twice a day at normalising fMRI changes and improving memory function in these patients.73 Since fMRI is an indirect measure of neuronal activity, the association between hippocampal hyperactivation detected by fMRI and epileptiform activity (detected by EEG) is poorly understood.
Regions outside the hippocampus also show excess network activity and synchrony in early stages of Alzheimer’s disease. For instance, patients with mild cognitive impairment or mild Alzheimer’s disease, and cognitively healthy people with PET evidence for amyloid β deposition have aberrant activity in the default network, including the precuneus and posterior cingulate cortex, indicated by failure to deactivate this network during memory encoding.85 Additionally, resting-state MEG has shown network hypersynchronisation across multiple frequency bands in frontoparietal and interhemispheric networks in patients with amnestic mild cognitive impairment.86
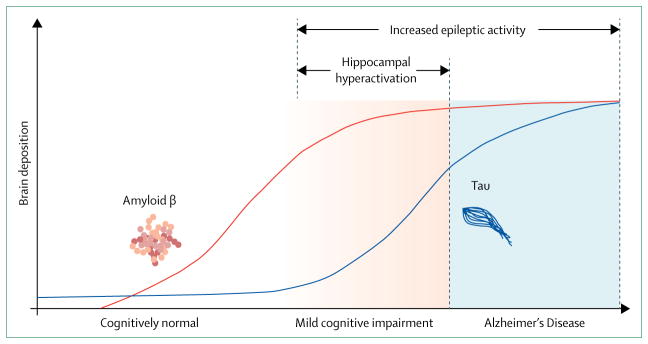
A hypothetical model for the temporal association of network alterations with amyloid β and tau deposition in the brain is shown in the figure.20,87 We propose that a crucial period during mild cognitive impairment stages exists, in which hippocampal hyperactivation is associated with an increase in deposition of neurofibrillary tangles while amyloid β deposition begins to plateau.83 Brain deposits of amyloid β and tau are not as tightly linked with cognitive dysfunction in human beings as soluble forms of these proteins are in animal models of Alzheimer’s disease.88 Since non-invasive means of measuring soluble protein in the brain are not available, functional imaging and neurophysiological recordings might become crucial biomarkers of the functional effects of these proteins, both for protein-lowering approaches and other network-stabilising therapeutic strategies.
Figure. Hypothetical model of the correlation between amyloid β and tau deposition and network alterations in Alzheimer’s disease.
Proposed temporal associations between total brain amyloid β (plaques) and tau (neurofibrillary tangles) deposition, hippocampal hyperactivation, and epileptic activity. Although hippocampal hyperactivation is restricted to the stage of mild cognitive impairment, epileptiform activity and seizures can occur throughout the course of mild cognitive impairment and Alzheimer’s disease..2,20,73,83,87
Conclusions and future directions
Patients with mild cognitive impairment or Alzheimer’s disease are prone to seizures and clinically silent forms of aberrant network activity, which can be associated with cognitive decline. Evidence for detrimental network hyperexcitability in the early stages of Alzheimer’s disease raises new therapeutic opportunities for antiepileptic strategies that might complement or enhance existing approaches, and potentially modify disease progression. No guidelines are available for treating clinically silent forms of aberrant network activity in Alzheimer’s disease. As we have learned from other medical conditions, such as hypertension and diabetes, recognising presymptomatic and subclinical forms of disease seems imperative for protecting against future organ damage. Clinical trials are required to establish the benefits and risks of treating network hyperexcitability in patients with Alzheimer’s disease. The optimal degree to which excessive brain activity and network synchrony can be suppressed in these patients, without compromising the network activity that elicits normal cognitive functions, is unknown. Therefore, early phase clinical trials will benefit from adaptive designs, such as those that include multiple treatment dose groups initially and eventually include participants into the most efficacious treatment group.
Several trials are ongoing or soon to be initiated (table 4). A phase 2 study of levetiracetam for Alzheimer’s disease-associated network hyperexcitability (LEV-AD; NCT02002819) is underway, and a multicentre phase 3 clinical trial of levetiracetam to treat amnestic mild cognitive impairment due to Alzheimer’s disease is scheduled to begin in 2017. In the LEV-AD trial, participants are prescreened for seizures or epileptiform activity with overnight video LTM-EEG and 1-h MEG–EEG exams, to identify those with or without epileptiform activity before trial entry. Given the association of epileptiform activity with cognitive decline, neurophysiological measures at trial screening might be important to avoid biases in future studies, regardless of the therapeutic target.
Table 4.
Treatments that might suppress Alzheimer’s disease-associated network hyperexcitability
| Proposed mechanism | Phase of investigation in Alzheimer’s disease | |
|---|---|---|
| Antiepileptic drug | ||
|
| ||
| Levetiracetam | Binds SV2A and prevents synaptic vesicle release,65 and increases glutamate transporter expression89 | Animal model;6,70 case-control;58 phase 2 (NCT02002819);73 and phase 3 (registering in 2017) |
| Brivaracetam | Binds SV2A with higher affinity than levetiracetam90 | Animal model76 |
| Lamotrigine | Inhibits Na+ channel activity; more potent at inhibiting glutamate release than other antiepileptic drugs in this class66 | Animal model;77 case-control58 |
| Topiramate | Inhibits GSK-3β activation and histone deacetylase activity,70 inhibits Na+ and Ca2+ channels, enhances GABAA receptor function, and blocks AMPA and kainate receptors | Animal model70 |
|
| ||
| Antineoplastic drug | ||
|
| ||
| Bexarotene | Retinoid X receptor agonist, alters gene expression | Animal model91 |
|
| ||
| Cell-replacement therapies | ||
|
| ||
| GABAergic interneurons derived from stem cells | Increase local GABAergic interneuron populations (eg, somatostatin-positive interneurons in hilus of dentate gyrus) | Animal model57 |
|
| ||
| Dietary therapies | ||
|
| ||
| Ketogenic diet | Increases mitochondrial biogenesis and oxidative phosphorylation, and enhances GABA levels92 | Phase 2 (NCT02551419, NCT02709356, and NCT00142805);93 interventional placebo-controlled, single blind (NCT02521818); phase 4 (NCT00777010) |
| Taurine | Activates GABA receptors94 | Animal model95 |
|
| ||
| Gene therapy | ||
|
| ||
| Enhancing Nav1.1 expression | Improves function of parvalbumin-positive interneurons | Animal model37 |
|
| ||
| Kinase inhibitors | ||
|
| ||
| Src family kinase inhibitor | Suppresses Fyn kinase phosphorylation of Tyr-1472 in NR2B subunit of NMDA receptors42 | Phase 1 (AZD0530 [saracatinib], completed, NCT01864655);96 phase 2 (saracatinib, NCT02167256) |
| Tyrosine kinase inhibitor | Reduction in mast cell-mediated inflammation through c-Kit inhibition; Src family kinase inhibition, including Fyn kinase97 | Phase 2 (masitinib, completed, NCT00976118);97 and phase 3 (masitinib, NCT01872598) |
|
| ||
| Tau reduction therapies | ||
|
| ||
| Salsalate | Inhibits tau acetylation, which enhances tau turnover98 | Animal model; phase 1 for progressive supranuclear palsy (NCT02422485) |
| Antisense oligonucleotides | Reduces tau mRNA levels52 | Animal model52 |
| Methylene blue | Inhibits Hsp70 enzymatic activity99 | Phase 2 (NCT02380573) |
GSK-3β=glycogen synthase kinase 3β. SV2A=synaptic vesicle protein 2A.
In addition to AEDs, diverse therapies have been shown to suppress aberrant network activity in animal models of Alzheimer’s disease (table 4). Reducing tau protein is one strategy that has shown broad antiepileptic effects, both in animal models of Alzheimer’s disease and of epilepsy.40,41,51,52 In addition to these therapies, neuromodulatory approaches, such as deep brain stimulation, transcranial magnetic stimulation, and vagus nerve stimulation, are being developed to improve cognitive function in Alzheimer’s disease and show capacity to treat seizures.100,101 Such approaches offer the advantage of precisely targeting zones of hyperexcitability and dysfunction, but also need to consider potential adverse effects of stimulating the circuits involved. For instance, deep brain stimulation of the anterior thalamus effectively treats seizures, but carries the risk of behavioural and mood changes.102
In conclusion, patients with Alzheimer’s disease and seizures can show accelerated cognitive decline and might stand to benefit from antiepileptic treatments; network hyperexcitability, more generally, offers a possible target for treatment in Alzheimer’s disease. Prolonged neurophysiological monitoring is often required to detect epileptiform activity in patients with Alzheimer’s disease, and the precise extent of pathological network hyperactivity in Alzheimer’s disease is unknown. Clinical trials using AEDs and related strategies will address the efficacy of assessing and treating aberrant network activity in Alzheimer’s disease and identify the subgroups of patients with seizures, silent neurophysiological abnormalities, or broader populations that might benefit from network stabilising strategies.
Search strategy and selection criteria.
We selected references by reviewing the authors’ personal files and by searching PubMed for manuscripts published in English before Dec 1, 2016, with the term “Alzheimer’s disease” and assorted combinations of the following terms: “epilepsy”, “seizures”, “epileptiform activity”, “network hyperexcitability”, “antiepileptic drugs”, “antiseizure drugs”, “anticonvulsants”, “Down syndrome”, “trisomy 21”, “dementia”, “neurodegenerative disease”, “early-onset”, “presenilin 1’, “presenilin 2”, and “amyloid precursor protein”. We reviewed reference lists within original research and review articles for additional references. We finalised the reference list on the basis of originality and relevance to the scope of this Review. We focused on scientific literature of the past 8 years, but also included older publications of high merit or originality. Additional information about current clinical trials was obtained from ClinicalTrials.gov.
Acknowledgments
KAV received support from the National Institutes of Health grant K23 AG038357 and a grant from the Alzheimer’s Association (PCTRB-13-288476) made possible by Part the Cloud. We thank Caroline Prioleau for her assistance with preparing the figure.
Footnotes
Contributors
KAV developed the manuscript concept and structure. All authors contributed to the literature review and preparation of the manuscript.
Declaration of interests
We declare no competing interests.
References
- 1.Vossel KA, Ranasinghe KG, Beagle AJ, et al. Incidence and impact of subclinical epileptiform activity in Alzheimer’s disease. Ann Neurol. 2016;80:858–70. doi: 10.1002/ana.24794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vossel KA, Beagle AJ, Rabinovici GD, et al. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol. 2013;70:1158–66. doi: 10.1001/jamaneurol.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cretin B, Sellal F, Philippi N, et al. Epileptic prodromal Alzheimer’s disease, a retrospective study of 13 new cases: expanding the spectrum of Alzheimer’s disease to an epileptic variant? J Alzheimers Dis. 2016;52:1125–33. doi: 10.3233/JAD-150096. [DOI] [PubMed] [Google Scholar]
- 4.Sarkis RA, Dickerson BC, Cole AJ, Chemali ZN. Clinical and neurophysiologic characteristics of unprovoked seizures in patients diagnosed with dementia. J Neuropsychiatry Clin Neurosci. 2016;28:56–61. doi: 10.1176/appi.neuropsych.15060143. [DOI] [PubMed] [Google Scholar]
- 5.Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci. 2010;13:812–18. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez PE, Zhu L, Verret L, et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc Natl Acad Sci USA. 2012;109:E2895–903. doi: 10.1073/pnas.1121081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman D, Honig LS, Scarmeas N. Seizures and epilepsy in Alzheimer’s disease. CNS Neurosci Ther. 2012;18:285–94. doi: 10.1111/j.1755-5949.2011.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherzai D, Losey T, Vega S, Sherzai A. Seizures and dementia in the elderly: nationwide inpatient sample 1999–2008. Epilepsy Behav. 2014;36:53–56. doi: 10.1016/j.yebeh.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Hauser WA, Morris ML, Heston LL, Anderson VE. Seizures and myoclonus in patients with Alzheimer’s disease. Neurology. 1986;36:1226–30. doi: 10.1212/wnl.36.9.1226. [DOI] [PubMed] [Google Scholar]
- 10.Chen JY, Stern Y, Sano M, Mayeux R. Cumulative risks of developing extrapyramidal signs, psychosis, or myoclonus in the course of Alzheimer’s disease. Arch Neurol. 1991;48:1141–43. doi: 10.1001/archneur.1991.00530230049020. [DOI] [PubMed] [Google Scholar]
- 11.Lee SE, Rabinovici GD, Mayo MC, et al. Clinicopathological correlations in corticobasal degeneration. Ann Neurol. 2011;70:327–40. doi: 10.1002/ana.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alladi S, Xuereb J, Bak T, et al. Focal cortical presentations of Alzheimer’s disease. Brain. 2007;130:2636–45. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- 13.Samson WN, van Duijn CM, Hop WC, Hofman A. Clinical features and mortality in patients with early-onset Alzheimer’s disease. Eur Neurol. 1996;36:103–06. doi: 10.1159/000117218. [DOI] [PubMed] [Google Scholar]
- 14.Lai F, Williams RS. A prospective study of Alzheimer disease in Down syndrome. Arch Neurol. 1989;46:849–53. doi: 10.1001/archneur.1989.00520440031017. [DOI] [PubMed] [Google Scholar]
- 15.Shea YF, Chu LW, Chan AO, Ha J, Li Y, Song YQ. A systematic review of familial Alzheimer’s disease: differences in presentation of clinical features among three mutated genes and potential ethnic differences. J Formos Med Assoc. 2016;115:67–75. doi: 10.1016/j.jfma.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Volicer L, Smith S, Volicer BJ. Effect of seizures on progression of dementia of the Alzheimer type. Dementia. 1995;6:258–63. doi: 10.1159/000106956. [DOI] [PubMed] [Google Scholar]
- 17.Lott IT, Doran E, Nguyen VQ, Tournay A, Movsesyan N, Gillen DL. Down syndrome and dementia: seizures and cognitive decline. J Alzheimers Dis. 2012;29:177–85. doi: 10.3233/JAD-2012-111613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prasher VP, Corbett JA. Onset of seizures as a poor indicator of longevity in people with down syndrome and dementia. Int J Geriatr Psychiatry. 1993;8:923–97. [Google Scholar]
- 19.Breteler MM, de Groot RR, van Romunde LK, Hofman A. Risk of dementia in patients with Parkinson’s disease, epilepsy, and severe head trauma: a register-based follow-up study. Am J Epidemiol. 1995;142:1300–05. doi: 10.1093/oxfordjournals.aje.a117597. [DOI] [PubMed] [Google Scholar]
- 20.Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer〉s disease: a prospective cohort study. Lancet Neurol. 2013;12:357–67. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 21.Rao SC, Dove G, Cascino GD, Petersen RC. Recurrent seizures in patients with dementia: frequency, seizure types, and treatment outcome. Epilepsy Behav. 2009;14:118–20. doi: 10.1016/j.yebeh.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackenzie IR, Miller LA. Senile plaques in temporal lobe epilepsy. Acta Neuropathol. 1994;87:504–10. doi: 10.1007/BF00294177. [DOI] [PubMed] [Google Scholar]
- 23.Thom M, Liu JY, Thompson P, et al. Neurofibrillary tangle pathology and Braak staging in chronic epilepsy in relation to traumatic brain injury and hippocampal sclerosis: a post-mortem study. Brain. 2011;134:2969–81. doi: 10.1093/brain/awr209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tai XY, Koepp M, Duncan JS, et al. Hyperphosphorylated tau in patients with refractory epilepsy correlates with cognitive decline: a study of temporal lobe resections. Brain. 2016;139:2441–55. doi: 10.1093/brain/aww187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson YS, Raby S, Foulds PG, et al. TDP-43 pathological changes in early onset familial and sporadic Alzheimer’s disease, late onset Alzheimer’s disease and Down’s syndrome: association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol. 2011;122:703–13. doi: 10.1007/s00401-011-0879-y. [DOI] [PubMed] [Google Scholar]
- 26.Minkeviciene R, Rheims S, Dobszay MB, et al. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci. 2009;29:3453–62. doi: 10.1523/JNEUROSCI.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo C, Li Q, Lai Y, et al. Altered functional connectivity in default mode network in absence epilepsy: a resting-state fMRI study. Hum Brain Mapp. 2011;32:438–49. doi: 10.1002/hbm.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogt DL, Thomas D, Galvan V, Bredesen DE, Lamb BT, Pimplikar SW. Abnormal neuronal networks and seizure susceptibility in mice overexpressing the APP intracellular domain. Neurobiol Aging. 2011;32:1725–29. doi: 10.1016/j.neurobiolaging.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Um JW, Nygaard HB, Heiss JK, et al. Alzheimer amyloid-β oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat Neurosci. 2012;15:1227–35. doi: 10.1038/nn.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lei M, Xu H, Li Z, et al. Soluble Aβ oligomers impair hippocampal LTP by disrupting glutamatergic/GABAergic balance. Neurobiol Dis. 2016;85:111–21. doi: 10.1016/j.nbd.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Decker JM, Krüger L, Sydow A, et al. The Tau/A152T mutation, a risk factor for frontotemporal-spectrum disorders, leads to NR2B receptor-mediated excitotoxicity. EMBO Rep. 2016;17:552–69. doi: 10.15252/embr.201541439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vossel KA, Zhang K, Brodbeck J, et al. Tau reduction prevents Aβ-induced defects in axonal transport. Science. 2010;330:198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vossel KA, Xu JC, Fomenko V, et al. Tau reduction prevents Aβ-induced axonal transport deficits by blocking activation of GSK3beta. J Cell Biol. 2015;209:419–33. doi: 10.1083/jcb.201407065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zempel H, Luedtke J, Kumar Y, et al. Amyloid-β oligomers induce synaptic damage via Tau-dependent microtubule severing by TTLL6 and spastin. EMBO J. 2013;32:2920–37. doi: 10.1038/emboj.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cissé M, Halabisky B, Harris J, et al. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature. 2011;469:47–52. doi: 10.1038/nature09635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verret L, Mann EO, Hang GB, et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149:708–21. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall AM, Throesch BT, Buckingham SC, et al. Tau-dependent Kv4. 2 depletion and dendritic hyperexcitability in a mouse model of Alzheimer’s disease. J Neurosci. 2015;35:6221–30. doi: 10.1523/JNEUROSCI.2552-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corbett BF, Leiser SC, Ling HP, et al. Sodium channel cleavage is associated with aberrant neuronal activity and cognitive deficits in a mouse model of Alzheimer’s disease. J Neurosci. 2013;33:7020–26. doi: 10.1523/JNEUROSCI.2325-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberson ED, Halabisky B, Yoo JW, et al. Amyloid-β/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J Neurosci. 2011;31:700–11. doi: 10.1523/JNEUROSCI.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ittner LM, Ke YD, Delerue F, et al. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer’s disease mouse models. Cell. 2010;142:387–97. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 42.Kaufman AC, Salazar SV, Haas LT, et al. Fyn inhibition rescues established memory and synapse loss in Alzheimer mice. Ann Neurol. 2015;77:953–71. doi: 10.1002/ana.24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andrews-Zwilling Y, Bien-Ly N, Xu Q, et al. Apolipoprotein E4 causes age- and tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J Neurosci. 2010;30:13707–17. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krantic S, Isorce N, Mechawar N, et al. Hippocampal GABAergic neurons are susceptible to amyloid-β toxicity in vitro and are decreased in number in the Alzheimer’s disease TgCRND8 mouse model. J Alzheimers Dis. 2012;29:293–308. doi: 10.3233/JAD-2011-110830. [DOI] [PubMed] [Google Scholar]
- 45.Baglietto-Vargas D, Moreno-Gonzalez I, Sanchez-Varo R, et al. Calretinin interneurons are early targets of extracellular amyloid-beta pathology in PS1/AbetaPP Alzheimer mice hippocampus. J Alzheimers Dis. 2010;21:119–32. doi: 10.3233/JAD-2010-100066. [DOI] [PubMed] [Google Scholar]
- 46.Hazra A, Gu F, Aulakh A, Berridge C, Eriksen JL, Ziburkus J. Inhibitory neuron and hippocampal circuit dysfunction in an aged mouse model of Alzheimer’s disease. PLoS One. 2013;8:e64318. doi: 10.1371/journal.pone.0064318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Šisková Z, Justus D, Kaneko H, et al. Dendritic structural degeneration is functionally linked to cellular hyperexcitability in a mouse model of Alzheimer’s disease. Neuron. 2014;84:1023–33. doi: 10.1016/j.neuron.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 48.Hazra A, Corbett BF, You JC, et al. Corticothalamic network dysfunction and behavioral deficits in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2016;44:96–107. doi: 10.1016/j.neurobiolaging.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kam K, Duffy ÁM, Moretto J, LaFrancois JJ, Scharfman HE. Interictal spikes during sleep are an early defect in the Tg2576 mouse model of β-amyloid neuropathology. Sci Rep. 2016;6:20119. doi: 10.1038/srep20119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Born HA, Kim JY, Savjani RR, et al. Genetic suppression of transgenic APP rescues hypersynchronous network activity in a mouse model of Alzeimer’s disease. J Neurosci. 2014;34:3826–40. doi: 10.1523/JNEUROSCI.5171-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holth JK, Bomben VC, Reed JG, et al. Tau loss attenuates neuronal network hyperexcitability in mouse and Drosophila genetic models of epilepsy. J Neurosci. 2013;33:1651–59. doi: 10.1523/JNEUROSCI.3191-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeVos SL, Goncharoff DK, Chen G, et al. Antisense reduction of tau in adult mice protects against seizures. J Neurosci. 2013;33:12887–97. doi: 10.1523/JNEUROSCI.2107-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maeda S, Djukic B, Taneja P, et al. Expression of A152T human tau causes age-dependent neuronal dysfunction and loss in transgenic mice. EMBO Rep. 2016;17:530–51. doi: 10.15252/embr.201541438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puzzo D, Privitera L, Leznik E, et al. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28:14537–45. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamamoto K, Tanei Z, Hashimoto T, et al. Chronic optogenetic activation augments Aβ pathology in a mouse model of Alzheimer disease. Cell Rep. 2015;11:859–65. doi: 10.1016/j.celrep.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 56.Wu JW, Hussaini SA, Bastille IM, et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci. 2016;19:1085–92. doi: 10.1038/nn.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tong LM, Djukic B, Arnold C, et al. Inhibitory interneuron progenitor transplantation restores normal learning and memory in ApoE4 knock-in mice without or with Aβ accumulation. J Neurosci. 2014;34:9506–15. doi: 10.1523/JNEUROSCI.0693-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cumbo E, Ligori LD. Levetiracetam, lamotrigine, and phenobarbital in patients with epileptic seizures and Alzheimer’s disease. Epilepsy Behav. 2010;17:461–66. doi: 10.1016/j.yebeh.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 59.Liedorp M, Stam CJ, van der Flier WM, Pijnenburg YA, Scheltens P. Prevalence and clinical significance of epileptiform EEG discharges in a large memory clinic cohort. Dement Geriatr Cogn Disord. 2010;29:432–37. doi: 10.1159/000278620. [DOI] [PubMed] [Google Scholar]
- 60.Belcastro V, Costa C, Galletti F, Pisani F, Calabresi P, Parnetti L. Levetiracetam monotherapy in Alzheimer patients with late-onset seizures: a prospective observational study. Eur J Neurol. 2007;14:1176–78. doi: 10.1111/j.1468-1331.2007.01907.x. [DOI] [PubMed] [Google Scholar]
- 61.Rowan AJ, Ramsay RE, Collins JF, et al. New onset geriatric epilepsy: a randomized study of gabapentin, lamotrigine, and carbamazepine. Neurology. 2005;64:1868–73. doi: 10.1212/01.WNL.0000167384.68207.3E. [DOI] [PubMed] [Google Scholar]
- 62.Tariot PN, Schneider LS, Cummings J, et al. Chronic divalproex sodium to attenuate agitation and clinical progression of Alzheimer disease. Arch Gen Psychiatry. 2011;68:853–61. doi: 10.1001/archgenpsychiatry.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fleisher AS, Truran D, Mai JT, et al. Chronic divalproex sodium use and brain atrophy in Alzheimer disease. Neurology. 2011;77:1263–71. doi: 10.1212/WNL.0b013e318230a16c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsiouris JA, Patti PJ, Tipu O, Raguthu S. Adverse effects of phenytoin given for late-onset seizures in adults with Down syndrome. Neurology. 2002;59:779–80. doi: 10.1212/wnl.59.5.779. [DOI] [PubMed] [Google Scholar]
- 65.Yang XF, Rothman SM. Levetiracetam has a time- and stimulation-dependent effect on synaptic transmission. Seizure. 2009;18:615–19. doi: 10.1016/j.seizure.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 66.Ahmad S, Fowler LJ, Whitton PS. Lamotrigine, carbamazepine and phenytoin differentially alter extracellular levels of 5-hydroxytryptamine, dopamine and amino acids. Epilepsy Res. 2005;63:141–49. doi: 10.1016/j.eplepsyres.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 67.Tekin S, Aykut-Bingöl C, Tanridağ T, Aktan S. Antiglutamatergic therapy in Alzheimer’s disease—effects of lamotrigine. J Neural Transm (Vienna) 1998;105:295–303. doi: 10.1007/s007020050059. [DOI] [PubMed] [Google Scholar]
- 68.Ziyatdinova S, Gurevicius K, Kutchiashvili N, et al. Spontaneous epileptiform discharges in a mouse model of Alzheimer’s disease are suppressed by antiepileptic drugs that block sodium channels. Epilepsy Res. 2011;94:75–85. doi: 10.1016/j.eplepsyres.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 69.Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ. 2014;349:g5205. doi: 10.1136/bmj.g5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi JQ, Wang BR, Tian YY, et al. Antiepileptics topiramate and levetiracetam alleviate behavioral deficits and reduce neuropathology in APPswe/PS1dE9 transgenic mice. CNS Neurosci Ther. 2013;19:871–81. doi: 10.1111/cns.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamberger MJ, Palmese CA, Scarmeas N, Weintraub D, Choi H, Hirsch LJ. A randomized, double-blind, placebo-controlled trial of donepezil to improve memory in epilepsy. Epilepsia. 2007;48:1283–91. doi: 10.1111/j.1528-1167.2007.01114.x. [DOI] [PubMed] [Google Scholar]
- 72.Löscher W, Hönack D. High doses of memantine (1-amino-3,5-dimethyladamantane) induce seizures in kindled but not in non-kindled rats. Naunyn Schmiedebergs Arch Pharmacol. 1990;341:476–81. doi: 10.1007/BF00176343. [DOI] [PubMed] [Google Scholar]
- 73.Bakker A, Albert MS, Krauss G, Speck CL, Gallagher M. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. Neuroimage Clin. 2015;7:688–98. doi: 10.1016/j.nicl.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kleen JK, Scott RC, Holmes GL, et al. Hippocampal interictal epileptiform activity disrupts cognition in humans. Neurology. 2013;81:18–24. doi: 10.1212/WNL.0b013e318297ee50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Noebels J. A perfect storm: converging paths of epilepsy and Alzheimer’s dementia intersect in the hippocampal formation. Epilepsia. 2011;52(suppl 1):39–46. doi: 10.1111/j.1528-1167.2010.02909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nygaard HB, Kaufman AC, Sekine-Konno T, et al. Brivaracetam, but not ethosuximide, reverses memory impairments in an Alzheimer’s disease mouse model. Alzheimers Res Ther. 2015;7:25. doi: 10.1186/s13195-015-0110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang MY, Zheng CY, Zou MM, et al. Lamotrigine attenuates deficits in synaptic plasticity and accumulation of amyloid plaques in APP/PS1 transgenic mice. Neurobiol Aging. 2014;35:2713–25. doi: 10.1016/j.neurobiolaging.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 78.Butler CR, Graham KS, Hodges JR, Kapur N, Wardlaw JM, Zeman AZ. The syndrome of transient epileptic amnesia. Ann Neurol. 2007;61:587–98. doi: 10.1002/ana.21111. [DOI] [PubMed] [Google Scholar]
- 79.Muhlert N, Milton F, Butler CR, Kapur N, Zeman AZ. Accelerated forgetting of real-life events in transient epileptic amnesia. Neuropsychologia. 2010;48:3235–44. doi: 10.1016/j.neuropsychologia.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 80.Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924–38. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gallassi R. Epileptic amnesic syndrome: an update and further considerations. Epilepsia. 2006;47(suppl 2):103–05. doi: 10.1111/j.1528-1167.2006.00704.x. [DOI] [PubMed] [Google Scholar]
- 82.Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA. 2015;313:1939–49. doi: 10.1001/jama.2015.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huijbers W, Mormino EC, Schultz AP, et al. Amyloid-β deposition in mild cognitive impairment is associated with increased hippocampal activity, atrophy and clinical progression. Brain. 2015;138:1023–35. doi: 10.1093/brain/awv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oh H, Jagust WJ. Frontotemporal network connectivity during memory encoding is increased with aging and disrupted by beta-amyloid. J Neurosci. 2013;33:18425–37. doi: 10.1523/JNEUROSCI.2775-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sperling RA, Laviolette PS, O’Keefe K, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–88. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maestú F, Peña JM, Garcés P, et al. A multicenter study of the early detection of synaptic dysfunction in mild cognitive impairment using magnetoencephalography-derived functional connectivity. Neuroimage Clin. 2015;9:103–09. doi: 10.1016/j.nicl.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–69. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 88.Spires-Jones TL, Hyman BT. The intersection of amyloid β and tau at synapses in Alzheimer’s disease. Neuron. 2014;82:756–71. doi: 10.1016/j.neuron.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ueda Y, Doi T, Nagatomo K, Tokumaru J, Takaki M, Willmore LJ. Effect of levetiracetam on molecular regulation of hippocampal glutamate and GABA transporters in rats with chronic seizures induced by amygdalar FeCl3 injection. Brain Res. 2007;1151:55–61. doi: 10.1016/j.brainres.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 90.Gillard M, Fuks B, Leclercq K, Matagne A. Binding characteristics of brivaracetam, a selective, high affinity SV2A ligand in rat, mouse and human brain: relationship to anti-convulsant properties. Eur J Pharmacol. 2011;664:36–44. doi: 10.1016/j.ejphar.2011.04.064. [DOI] [PubMed] [Google Scholar]
- 91.Bomben V, Holth J, Reed J, Cramer P, Landreth G, Noebels J. Bexarotene reduces network excitability in models of Alzheimer’s disease and epilepsy. Neurobiol Aging. 2014;35:2091–95. doi: 10.1016/j.neurobiolaging.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bough KJ, Wetherington J, Hassel B, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–35. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- 93.Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond) 2009;6:31. doi: 10.1186/1743-7075-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Louzada PR, Paula Lima AC, Mendonca-Silva DL, Noël F, De Mello FG, Ferreira ST. Taurine prevents the neurotoxicity of beta-amyloid and glutamate receptor agonists: activation of GABA receptors and possible implications for Alzheimer’s disease and other neurological disorders. FASEB J. 2004;18:511–18. doi: 10.1096/fj.03-0739com. [DOI] [PubMed] [Google Scholar]
- 95.Kim HY, Kim HV, Yoon JH, et al. Taurine in drinking water recovers learning and memory in the adult APP/PS1 mouse model of Alzheimer’s disease. Sci Rep. 2014;4:7467. doi: 10.1038/srep07467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nygaard HB, Wagner AF, Bowen GS, et al. A phase Ib multiple ascending dose study of the safety, tolerability, and central nervous system availability of AZD0530 (saracatinib) in Alzheimer’s disease. Alzheimers Res Ther. 2015;7:35. doi: 10.1186/s13195-015-0119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Piette F, Belmin J, Vincent H, et al. Masitinib as an adjunct therapy for mild-to-moderate Alzheimer’s disease: a randomised, placebo-controlled phase 2 trial. Alzheimers Res Ther. 2011;3:16. doi: 10.1186/alzrt75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Min SW, Chen X, Tracy TE, et al. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat Med. 2015;21:1154–62. doi: 10.1038/nm.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.O’Leary JC, 3rd, Li Q, Marinec P, et al. Phenothiazine-mediated rescue of cognition in tau transgenic mice requires neuroprotection and reduced soluble tau burden. Mol Neurodegener. 2010;5:45. doi: 10.1186/1750-1326-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cukiert A, Cukiert CM, Burattini JA, Lima AM. Seizure outcome after hippocampal deep brain stimulation in a prospective cohort of patients with refractory temporal lobe epilepsy. Seizure. 2014;23:6–9. doi: 10.1016/j.seizure.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 101.Hsu WY, Cheng CH, Lin MW, Shih YH, Liao KK, Lin YY. Antiepileptic effects of low frequency repetitive transcranial magnetic stimulation: a meta-analysis. Epilepsy Res. 2011;96:231–40. doi: 10.1016/j.eplepsyres.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 102.Hartikainen KM, Sun L, Polvivaara M, et al. Immediate effects of deep brain stimulation of anterior thalamic nuclei on executive functions and emotion-attention interaction in humans. J Clin Exp Neuropsychol. 2014;36:540–50. doi: 10.1080/13803395.2014.913554. [DOI] [PMC free article] [PubMed] [Google Scholar]