Abstract
Background
Aiming to contribute to prevention of cardiovascular disease (CVD), the National Health Service (NHS) Health Check programme has been implemented across England since 2009. The programme involves cardiovascular risk stratification—at 5-year intervals—of all adults between the ages of 40 and 74 years, excluding any with preexisting vascular conditions (including CVD, diabetes mellitus, and hypertension, among others), and offers treatment to those at high risk. However, the cost-effectiveness and equity of population CVD screening is contested. This study aimed to determine whether the NHS Health Check programme is cost-effective and equitable in a city with high levels of deprivation and CVD.
Methods and findings
IMPACTNCD is a dynamic stochastic microsimulation policy model, calibrated to Liverpool demographics, risk factor exposure, and CVD epidemiology. Using local and national data, as well as drawing on health and social care disease costs and health-state utilities, we modelled 5 scenarios from 2017 to 2040:
Scenario (A): continuing current implementation of NHS Health Check;
Scenario (B): implementation ‘targeted’ toward areas in the most deprived quintile with increased coverage and uptake;
Scenario (C): ‘optimal’ implementation assuming optimal coverage, uptake, treatment, and lifestyle change;
Scenario (D): scenario A combined with structural population-wide interventions targeting unhealthy diet and smoking;
Scenario (E): scenario B combined with the structural interventions as above.
We compared all scenarios with a counterfactual of no-NHS Health Check.
Compared with no-NHS Health Check, the model estimated cumulative incremental cost-effectiveness ratio (ICER) (discounted £/quality-adjusted life year [QALY]) to be 11,000 (95% uncertainty interval [UI] −270,000 to 320,000) for scenario A, 1,500 (−91,000 to 100,000) for scenario B, −2,400 (−6,500 to 5,700) for scenario C, −5,100 (−7,400 to −3,200) for scenario D, and −5,000 (−7,400 to −3,100) for scenario E. Overall, scenario A is unlikely to become cost-effective or equitable, and scenario B is likely to become cost-effective by 2040 and equitable by 2039. Scenario C is likely to become cost-effective by 2030 and cost-saving by 2040. Scenarios D and E are likely to be cost-saving by 2021 and 2023, respectively, and equitable by 2025. The main limitation of the analysis is that we explicitly modelled CVD and diabetes mellitus only.
Conclusions
According to our analysis of the situation in Liverpool, current NHS Health Check implementation appears neither equitable nor cost-effective. Optimal implementation is likely to be cost-saving but not equitable, while targeted implementation is likely to be both. Adding structural policies targeting cardiovascular risk factors could substantially improve equity and generate cost savings.
In a modeling study, Chris Kypridemos and colleagues assess the NHS Health Checks program for cardiovascular risk screening in Liverpool, UK.
Author summary
Why was this study done?
Previous evidence for population cardiovascular screening (e.g., National Health Service [NHS] Health Check in England) has failed to conclusively provide answers around effectiveness, cost-effectiveness, and equity.
A recent systematic review found some evidence of cost-effectiveness, but some of the studies included were methodologically flawed.
What did the researchers do and find?
The researchers simulated the life courses of synthetic individuals under different scenarios, within a close-to-reality synthetic population of Liverpool.
This study provides detailed evidence on effectiveness, cost-effectiveness, and equity of health checks while also uniquely providing estimations of the speed at which these programmes will reach these thresholds.
The results show that, while different implementation structures for health checks may improve cost-effectiveness and equity, these approaches are dominated by the addition of population-wide, structural interventions to improve diet and reduce smoking (e.g., mandatory salt reformulation of processed foods or increased taxation on tobacco products).
Liverpool is a city with a high concentration of cardiovascular risk factors and high burden of cardiovascular disease (CVD), and therefore the likelihood of programme effectiveness increases.
What do these findings mean?
The findings highlight the importance of a carefully considered approach to the implementation of health checks while also considering other approaches for CVD prevention.
The addition of structural interventions will lead to primary CVD prevention becoming equitable and cost-effective earlier.
Introduction
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality globally [1]. In 2015, almost 500,000 deaths across the United Kingdom were attributed to CVD, more than any other cause of death [2]. As part of the prevention of CVD, the National Health Service (NHS) Health Check programme was implemented across England in 2009. Local-authority commissioning of NHS Health Check has been a statutory requirement since 2013 as part of the Health and Social Care Act [3]. The programme involves CVD risk stratification—at 5-year intervals—of all adults between the ages of 40 and 74 years, excluding any with known preexisting vascular conditions (including CVD, diabetes mellitus, and hypertension, among others) [4]. Those identified as high-risk are offered appropriate treatment, including pharmacological and behavioural interventions. There are limited data available on the national programme. There is little consistency in commissioning arrangements; however, most areas use local General Practices (GPs).
There is conflicting evidence regarding the effectiveness—and cost-effectiveness—of health checks. In the UK, cost-effectiveness for public health interventions is often compared to a threshold of £20,000 per quality-adjusted life year (QALY) gained [5]. The programme was modelled by the UK Department of Health in 2008 [6], suggesting that health checks were cost-effective. However, concern was raised that assumptions about the effectiveness of lifestyle interventions might have been overestimated. A more recent study looking only at the weight-loss impact of NHS Health Check found them cost-effective, through attributing the weight-loss effect exclusively to health checks [7]. A Cochrane review found that, while health check programmes increased diagnosis, they had no significant effect on survival; however, the majority of studies in the review were from before 1980, prior to the introduction of many pharmacological interventions (such as statins for hypercholesterolaemia) [8]. A systematic review of economic evaluations for health checks reported that some modelling and observational studies found programmes to be cost-effective. However, some of these were methodologically flawed (i.e., pre–post designs without a control group) and were based on assumptions regarding uptake and treatment effectiveness that are not supported by empirical evidence [9]. A recent study has suggested that targeting health checks to those most at risk might increase cost-effectiveness [10].
There are also concerns about the potential effect of health checks on health inequalities. An analysis of the first 4 years of the NHS Health Check programme suggested low, but improving, overall uptake and higher uptake in the most deprived communities. However, this did not incorporate a complete 5-year cycle; therefore, it may reflect that, in some areas, the invitation strategy prioritised people with lower socioeconomic circumstances [11]. A rapid systematic review for the Expert Scientific and Clinical Advisory Panel identified that there was wide variability in uptake across England, and the evidence regarding differential uptake by sex, ethnicity, or deprivation was inconclusive [12]. Public Health England (PHE) recently suggested that the programme could potentially be successful in reducing inequalities, but this would require high and equitable uptake in high-risk groups [13]. Local authorities have the flexibility to focus more resources on high-risk communities, making a targeted approach on top of universal coverage possible through an approach of proportionate universalism [14]. A microsimulation study suggests that this could increase the equity of the intervention [15]. Yet there is continuing concern that interventions that target high-risk individuals such as the Health Check programme might exacerbate health inequalities given that they have the potential to favour populations with higher levels of resources [16,17].
On the other hand, population-wide policy approaches to reducing CVD can be effective, cost-effective, and improve health inequalities [16]. These approaches typically focus on tobacco and alcohol control as well as dietary interventions, such as mandatory salt reformulation of processed food or a sugar-sweetened beverage (SSB) levy [18–23]. Therefore, the optimal combination of individual and population-level strategies to reduce the unequal burden of CVD is still not well-defined.
The current study focusses on Liverpool, a city in northwest England with high levels of socioeconomic deprivation. Liverpool is ranked the fourth most deprived local authority in England when considering the proportion of neighbourhoods in the most deprived quintile [24]. Furthermore, there are high levels of inequality within the city, with notable clustering of CVD risk factors that might favour targeted, high-risk approaches to prevention. Health checks were implemented in Liverpool in 2010, but this remains suboptimal; an audit of the programme in 2015 and 2016 found large variations in practice [25]. Barely two-thirds of eligible residents were invited, of which less than one-third completed a health check. These figures are below the national average of about 86% and 49%, respectively [26]. Patients from more deprived areas were significantly less likely to attend, and men less so than women.
The aim of this study was therefore to quantify the cost-effectiveness and equity impact of the NHS Health Check programme in Liverpool, comparing current implementation, targeted implementation, or their combination with structural interventions.
Methods
The IMPACTNCD model, a discrete-time stochastic microsimulation, was used to counterfactually simulate individual life courses in the scenarios. The model structure and validation is described in detail elsewhere [20,27,28]. We present, in detail, model inputs, model outputs, scenario specification, and additional results and validation in S1 Text. In brief, we set the simulation horizon to 2040 to enable this preventative intervention to have time to become effective, and we present the results for ages 30 to 84.
Model inputs
We inputted data on demographics and projections (by age, sex, and English Index of Multiple Deprivation quintile group [QIMD]) for Liverpool into the IMPACTNCD model to create a synthetic dynamic population of 200 million adults aged 30 to 84 at baseline. QIMD is a measure of relative area deprivation based on the Index of Multiple Deprivation [29]. According to this system, all Lower Super Output Areas in England (average population of 1,500) are ranked in order of increasing deprivation, based on 7 domains of deprivation: income; employment; health deprivation and disability; education, skills, and training; barriers to housing and services; crime and disorder; and living environment. Then, the QIMD is formed from the quintiles of the above index. Population exposure to 7 known CVD risks was extracted from the Health Survey for England (HSE) using a subsample of northwest England residents. The 7 risks were inadequate fruit and vegetable consumption, physical inactivity, smoking, high body mass index, hypertension, hypercholesterolaemia, and diabetes mellitus. Trends in these risks between 2002 and 2014 were projected to 2040, stratified by demographics, to estimate future exposure. We used the published relative risks from high-quality meta-analyses and cohort studies to link risk factor exposure with disease outcomes. Table 1 summarises the input sources for IMPACTNCD, and Table 2 presents the key modelling assumptions.
Table 1. IMPACTNCD data sources.
| Parameter | Outcome | Details | Comments | Source |
|---|---|---|---|---|
| Mortality rates | Deaths from nonmodelled causes | Mortality and midyear population estimates for England | Stratified by age, sex, QIMD, and cause of death. Years 2002–2013. | Data requested and obtained by the Office for National Statistics [30] |
| Population projections for Liverpool | Population size | Midyear population figures for Liverpool | Stratified by age and sex. Years 2014–2039. QIMD distribution was assumed to remain stable as in 2011. Population size for year 2040 was assumed the same as 2039. | Subnational population projections [31] |
| Exposure to risk factors | Exposure of individuals | HSE (northwest subsample) | Anonymised, individual-level datasets. Years 2001–2012. | HSE 2002–2014 [32–44] |
| RR for active smoking | CHD and stroke (ICD10: I20–I25 and I60–I69) | Re-analysis of American Cancer Society’s Cancer Prevention Study II. Prospective cohort study, 6 years of follow-up | Stratified by age and sex. Adjusted for age, race, education, marital status, ‘blue collar’ employment in most recent or current job, weekly consumption of vegetables and citrus fruit, vitamin (A, C, and E) use, alcohol use, aspirin use, body mass index, exercise, dietary fat consumption, hypertension, and diabetes at baseline. | Table 1 (Model B) in Ezzati and colleagues [45] |
| Other mortality (except CHD and stroke) | Male British doctors prospective cohort study | Age-standardised | Table 1 in Doll and colleagues [46] | |
| RR for ex-smoking | CHD (ICD10: I20–I25) | Meta-analysis. Multiple-adjusted pooled estimates from 19 prospective studies | Multiply-adjusted | Web Figure 8 in Huxley RR and colleagues [47] |
| Stroke (ICD10 I60–I69) | The Framingham study. Prospective cohort study | Stroke risk decreased significantly by 2 years and was at the level of nonsmokers by 5 years after cessation of cigarette smoking. Therefore, we considered no risk for ex-smokers. | Wolf and colleagues [48] | |
| RR for environmental tobacco smoking | CHD (ICD10: I20–I25) | Meta-analysis of 10 cohort and case-control studies | Adjusted for important CHD risk factors. | Table 3 (adjusted RR) in He and colleagues [49] |
| Stroke (ICD10 I60–I69) | Meta-analysis of 20 prospective, case-control, and cross-sectional studies | Thirteen studies adjusted for important CHD risk factors. The overall effect of all 20 studies was used. | Figure 1 in Oono and colleagues [50] | |
| RR for systolic blood pressure | CHD and stroke (ICD10: I20–I25 and I60–I69) | Meta-analysis of individual data from 61 prospective studies | Stratified by age and sex. Adjusted for regression dilution and total blood cholesterol and, where available, lipid fractions (HDL and non-HDL cholesterol), diabetes, weight, alcohol consumption, and smoking at baseline. | Figures 3 and 5 in Prospective Studies Collaboration [51] |
| RR for total cholesterol | CHD and stroke (ICD10: I20–I25 and I60–I69) | Meta-analysis of individual data from 61 prospective studies | Stratified by age and sex. Adjusted for regression dilution and age, sex, study, systolic blood pressure, and smoking. | Web Table 6 (fully adjusted) and Figure 3 in Prospective Studies Collaboration [52] |
| RR for body mass index | CHD and stroke (ICD10: I20–I25 and I60–I69) | Meta-analysis of 58 prospective studies | Stratified by age. Adjusted for age, sex, smoking status, systolic blood pressure, history of diabetes, and total and HDL cholesterol. We used the age gradient from the adjusted only for age, sex, and smoking status reported estimates. | Table 1 and Figure 2 in The Emerging Risk Factors Collaboration [53] |
| RR for diabetes mellitus | CHD and stroke (ICD10: I20–I25 and I60–I69) | Meta-analysis of 102 prospective studies | Stratified by age. Adjusted for age, smoking status, body mass index, and systolic blood pressure. | Figure 2 in The Emerging Risk Factors Collaboration [54] |
| Other mortality (except CHD and stroke) | DECODE. A collaborative prospective study of 22 cohorts in Europe | Adjusted for body mass index, blood pressure, smoking, and serum cholesterol. | The DECODE Study Group [55] | |
| RR for physical activity | CHD and stroke (ICD10: I20–I25 and I60–I69) | Meta-analysis of 18 cohort studies for CHD and 8 cohort studies for ischaemic stroke | Stratified by age and sex. Adjusted for measurement error, age, sex, smoking, blood pressure, and cholesterol. | Tables 10.19 and 10.20 in Bull and colleagues [56] |
| RR for fruit and vegetable consumption | CHD (ICD10: I20–I25) | Meta-analysis of 9 cohort studies | RR per portion of F&V. Multiply-adjusted. | Dauchet and colleagues [57] |
| Stroke (ICD10: I60–I69) | Meta-analysis of 7 cohort studies | RR per portion of F&V. Multiply-adjusted. | Dauchet and colleagues [58] | |
| Persistence with medication | Persistence to statins for primary prevention | Danish cohort study | No clear socioeconomic gradient was observed. | Wallach-Kildemoes and colleagues [59] |
| Adherence to medication | Persistence to statins for primary prevention | Danish cohort study | No clear socioeconomic gradient was observed. | Wallach-Kildemoes and colleagues [59] |
Abbreviations: CHD, coronary heart disease; F&V, fruit and vegetable; HDL, high-density lipoprotein; HSE, Health Survey for England; RR, relative risk; QIMD, Index of Multiple Deprivation quintile group.
Table 2. IMPACTNCD key assumptions and limitations.
| Assumptions and limitations |
|---|
| Migration flows and social mobility were not considered in our estimates. |
| We assumed that the data sources that we used are genuinely representative of the Liverpool population. |
| We did not explicitly model alcohol consumption. |
| We assumed multiplicative risk effects for all risk factors and log-linear exposure–response relationship for the continuous ones. |
| We explicitly modelled hypertension, diabetes, CHD, and stroke. We defined CVD as the sum of CHD and stroke cases (deaths). We did not model other noncommunicable diseases that could potentially be affected by the modelled interventions. |
| We assumed that the observed trends in exposures and CVD mortality will continue in the future. |
| We assumed that trends in CHD and stroke incidence are attributable only to the modelled risk factor exposure trends. |
Abbreviations: CHD, coronary heart disease; CVD, cardiovascular disease.
Model outputs
The following list summarises model outputs:
CVD cases and deaths prevented or postponed by a modelled intervention, cumulatively over the simulated years.
Non-CVD deaths prevented or postponed by a modelled intervention, cumulatively over the simulated years. The model only considers smoking- and diabetes-related prevented or postponed non-CVD deaths.
QALYs gained because of a modelled intervention, cumulatively over the simulated years.
The net cost of a modelled intervention, cumulatively over the simulated years.
The incremental cost-effectiveness ratio (ICER) of a modelled intervention, as the ratio of cumulative net cost by cumulative QALYs gained (cost–utility analysis).
The net monetary benefit (NMB) assuming £20,000 willingness to pay.
The impact of an intervention on absolute and relative socioeconomic inequalities in health. We used the ‘absolute equity slope index’ and ‘relative equity slope index’—2 regression-based metrics—to measure the impact of the modelled interventions on absolute and relative socioeconomic health inequalities [27].
Uncertainty and sensitivity analysis
Second-order Monte Carlo simulation was used to estimate 95% uncertainty intervals (UIs), propagating estimated uncertainty of inputs to the outputs. Many sources of uncertainty are shared between scenarios; therefore, between-scenario results are not statistically independent and covary to an extent. Therefore, a crossover between UIs for scenarios should not be seen as evidence against statistical significance (please refer to S1 Text for between-scenario comparisons). We present the probability estimates of each scenario being cost-effective (net cost of less than £20,000 per QALY gained), cost-saving (negative net cost), and equitable (reduces both absolute and relative socioeconomic inequalities in health). We defined a probability threshold of 80% to determine when—or whether—a scenario becomes cost-effective (or cost-saving, or equitable).
Costs
We used local audit data to determine costs [25], while anonymised aggregated data from the local Clinical Commissioning Group were used to estimate prescription rates. The modelled costs were based on the payment that Liverpool City Council makes to GPs to provide the NHS Health Check programme [25]. This is £5.11 for an invitation and £13 to £19 per participant who undergoes a Health Check. The range of participation cost reflects that Liverpool City Council incentivises GP practices that achieve high uptake. Disease costs were drawn from NICE economic modelling and were separated by the first year of diagnosis, subsequent years, and fatal CVD events [60–63]. Deprivation weighting was used to match the deprivation profile of Liverpool, as there is evidence that there is a social gradient of costs [64]. Estimates assume that costs are equal for all ages and sexes, while myocardial infarction is used as a proxy for coronary heart disease (CHD). Non-CVD complications of diabetes are not included in cost estimates. Costs are inflated using the UK Treasury GDP deflator, November 2016 [65].
Health-related quality of life
We searched for disease utility index score multipliers that used the Euroqol 5-dimension scale (EQ-5D), which is seen as the ‘gold standard’ for health technology assessment in the UK and is recommended by NICE [66]. The evidence base was used to determine baseline utility scores by age [67]. A multiplier of 0.778 was used for CHD [68], 0.629 for stroke [69], and 0.901 for diabetes [70]. Hypertension was not given a multiplier because there was no consistent evidence, especially considering the link between hypertension and other morbidities.
Productivity losses
Productivity losses were estimated separately for CHD and stroke, accounting for both working years lost due to early mortality and sickness absence [71,72]. Prices were inflated using ONS data, then weighted based on Liverpool median earnings (95% of the median earnings for the UK). Indirect costs (such as informal care) were not included, and productivity losses were not included in the main cost estimations, which were from a health and social care perspective.
Discounting
An annual discount rate of 3.5% was applied from 2016 to net costs and QALYs. Results from prior to 2016 were inversely discounted. This rate was selected based on guidance from the UK Treasury [73].
Scenarios
In total, 20 scenarios were progressively developed through an iterative process with public health practitioners in Liverpool. Those scenarios included isolated improvements in coverage, uptake, prescription, referrals to lifestyle services, and some of their combinations. None were shown to be substantially better than the current implementation except the 5 scenarios we present in this study (for a detailed description and justification, please refer to S1 Text).
(A) Current implementation. In this scenario, we modelled the impact and costs assuming that the NHS Health Check programme continued unchanged in Liverpool throughout the modelled period to 2040. This assumed that annual coverage would remain at 13.8%, annual uptake would be 32.3%, and prescription rates would be 9.1% for low-risk, 25.8% for medium-risk, and 41.7% for high-risk participants. The costs were estimated at £5.11 per invitation and £13.28 per successful participation in NHS Health Check.
(B) Current plus targeted. In this scenario depicting a proportionate universalism approach, we modelled the impact and costs if the NHS Health Check programme was targeted toward individuals in the most deprived quintile, with increased coverage and uptake in this population while coverage and uptake in all other groups remain as the current implementation scenario. The coverage in the most deprived quintile is assumed to be 20%, with uptake at 66%. Crucially, in this scenario, we assume that the hypothetical recruitment strategy in the most deprived areas manages to attract participants with a higher cardiovascular risk profile than in scenario A. Prescription rates are unchanged from the current implementation scenario. The costs were estimated at £5.11 per invitation and £15.00 per successful participation in NHS Health Check. The increase in participation cost is in line with current Liverpool City Council practice to monetarily incentivise GP practices that achieve high uptake.
(C) Optimal uptake and prescription rates. In this scenario, we modelled the impact and costs if the NHS Health Check programme met the PHE requirements to invite 20% of the eligible population, and uptake was 66% [74]. Prescription rates in this scenario were set to 9.1% for low-risk, 80% for medium-risk, and 80% for high-risk participants to better reflect current prescription guidelines. In addition, we assumed highly effective lifestyle services. The costs were estimated at £5.11 per invitation and £15.00 per participant.
(D) Current plus structural interventions. This scenario modelled the impact and costs of the current implementation of NHS Health Check (scenario A) with the addition of structural interventions. The structural interventions were stricter tobacco control, an increase of fruit and vegetable consumption by a portion per day in 50% of individuals in the population, mandatory salt reformulation of processed foods, and an SSB tax of 20%. Their effectiveness was informed by published, mostly modelling studies [20,21,75–77].
(E) Current plus targeted plus structural interventions. This scenario modelled the impact and costs of the targeted implementation of NHS Health Check (scenario B) with the addition of structural interventions. The structural interventions are as in the previous scenario.
All results were reported following CHEERS guidelines (S1 CHEERS Checklist).
Results
The model estimated that about 94% (95% UI: 93% to 96%) of CHD incidence can be attributed to the modelled risk factors in the least-deprived quintile group. This gradually increased with deprivation to reach 96% (95% UI: 94% to 97%) for the most-deprived quintile group. Similarly, for stroke, the proportions were 86% (95% UI: 81% to 90%) and 92% (95% UI: 89% to 94%) for the least- and most-deprived quintile groups, respectively.
Effectiveness
Table 3 compares scenarios considering the number of CVD cases prevented and number of QALYs gained. The ‘current implementation’ was the worst-performing scenario for both outcomes and by both years (2030 and 2040). The ‘optimal implementation’ of Health Check performed better (estimated to prevent or postpone approximately 750 and 2,000 CVD cases by 2030 and 2040, respectively). However, that performance was dwarfed when structural interventions were added. The ‘targeted Health Check plus structural interventions’ was the best-performing scenario, estimated to prevent or postpone approximately 1,800 and 3,800 CVD cases by 2030 and 2040, respectively. Notably, even ‘current implementation plus structural interventions’ outperforms all programme-only scenarios.
Table 3. Comparison table of the effectiveness of the modelled scenarios.
Ages 30 to 84. Parentheses contain 95% UIs. Results are rounded to the first 2 significant digits.
| Model output | Scenario | By the year 2030 | By the year 2040 |
|---|---|---|---|
| Cumulative CVD cases prevented or postponed | Current (A) | 290 (150 to 500) | 570 (320 to 890) |
| Current plus targeted (B) | 530 (270 to 930) | 1,200 (730 to 1,900) | |
| Optimal (C) | 750 (400 to 1,300) | 2,000 (1,400 to 2,900) | |
| Current plus structural (D) | 1,600 (1,000 to 2,300) | 3,300 (2,400 to 4,200) | |
| Current plus targeted plus structural (E) | 1,800 (1,100 to 2,700) | 3,800 (2,900 to 5,000) | |
| Cumulative net QALYs gained (discounted) | Current (A) | 57 (−130 to 310) | 220 (−110 to 660) |
| Current plus targeted (B) | 85 (−200 to 490) | 500 (−82 to 1,300) | |
| Optimal (C) | 310 (−110 to 960) | 1,700 (700 to 3,100) | |
| Current plus structural (D) | 2,400 (1,100 to 4,300) | 7,000 (4,600 to 10,000) | |
| Current plus targeted plus structural (E) | 2,400 (1,000 to 4,500) | 7,200 (4,700 to 10,000) |
Abbreviations: CVD, cardiovascular disease; QALY, quality-adjusted life year; UI, uncertainty interval.
Cost-effectiveness
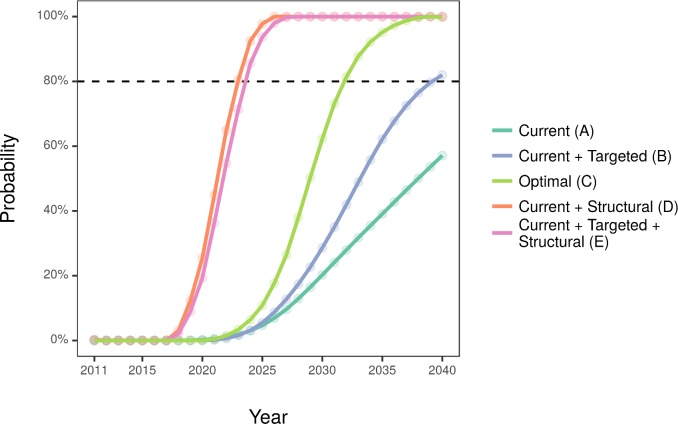
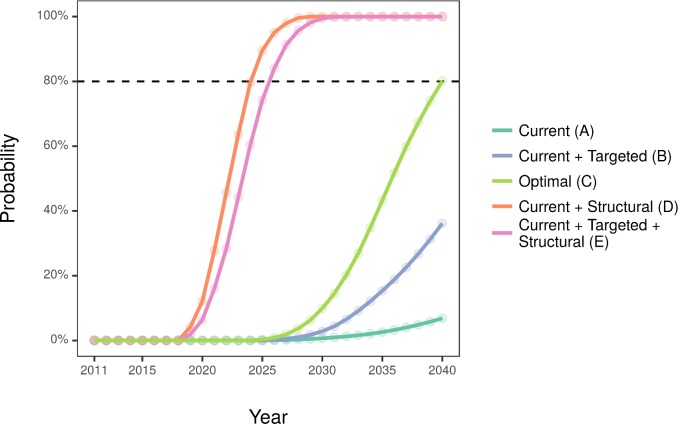
Table 4 and Fig 1 show cost-effectiveness analysis. The current implementation was unlikely to become cost-effective before 2040, while the targeted implementation would only pass the 80% threshold by 2040. The optimal implementation would pass the threshold by 2032. Comparatively, the addition of structural interventions means that scenarios D and E might become cost-effective by 2023, almost a decade earlier. Fig 2 shows that current and targeted implementations are unlikely to be cost-saving by 2040, while optimal implementation might reach the threshold by 2040. The current implementation with structural interventions could reach cost-saving fastest—by 2024—while targeted and structural approaches are likely to achieve cost-saving by 2026.
Table 4. Comparison table of the cost-effectiveness of the modelled scenarios.
Negative net costs are essentially savings. Ages 30 to 84. Parentheses contain 95% UIs. Results are rounded to the first 2 significant digits.
| Model output | Scenario | By the year 2030 | By the year 2040 |
|---|---|---|---|
| Cumulative net cost (discounted £million) | Current (A) | 4.0 (1.1 to 6.2) | 3.4 (−1.5 to 6.9) |
| Current plus targeted (B) | 4.7 (−0.1 to 7.9) | 1.3 (−8.6 to 7.5) | |
| Optimal (C) | 3.9 (−2.8 to 8.2) | −4.2 (−18.0 to 4.3) | |
| Current plus structural (D) | −13.0 (−28.0 to −3.7) | −35.0 (−60.0 to −19.0) | |
| Current plus targeted plus structural (E) | −11.0 (−27.0 to −1.7) | −35.0 (−63.0 to −18.0) | |
| Cumulative ICER (discounted £/QALY) | Current (A) | 21,000 (−650,000 to 730,000) | 11,000 (−270,000 to 320,000) |
| Current plus targeted (B) | 14,000 (−450,000 to 540,000) | 1,500 (−91,000 to 100,000) | |
| Optimal (C) | 9,700 (−170,000 to 190,000) | −2,400 (−6,500 to 5,700) | |
| Current plus structural (D) | −5,200 (−8,400 to −2,600) | −5,100 (−7,400 to −3,200) | |
| Current plus targeted plus structural (E) | −4,600 (−7,700 to −1,400) | −5,000 (−7,400 to −3,100) | |
| Cumulative NMB (discounted £million) | Current (A) | −3.0 (−8.3 to 5.0) | 0.9 (−8.6 to 14.0) |
| Current plus targeted (B) | −3.0 (−11.0 to 10.0) | 8.8 (−8.6 to 34.0) | |
| Optimal (C) | 2.5 (−10.0 to 22.0) | 38.0 (10.0 to 79.0) | |
| Current plus structural (D) | 62.0 (27.0 to 110.0) | 180.0 (120.0 to 250.0) | |
| Current plus targeted plus structural (E) | 60.0 (23.0 to 110.0) | 180.0 (120.0 to 270.0) |
Abbreviation: ICER, incremental cost-effectiveness ratio; NMB, net monetary benefit; QALY, quality-adjusted life year; UI, uncertainty interval.
Fig 1. Annual probability of the modelled scenarios to be cost-effective.
Willingness to pay £20,000 per QALY. QALY, quality-adjusted life year.
Fig 2. Annual probability of the modelled scenarios to be cost-saving.
Equity
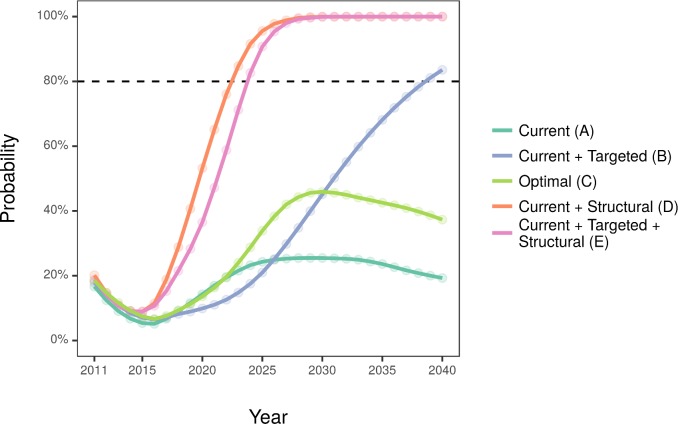
Table 5 and Fig 3 display findings concerning health inequalities. All 3 programme-only scenarios showed a decrease in absolute inequality—modest for the current implementation and substantial for the optimal implementation. When considering relative inequalities, only the targeted implementation was associated with an improvement in equity, crossing the 80% threshold by 2039. By contrast, the structural intervention scenarios both resulted in much larger improvements in both absolute and relative inequalities. Both would cross the 80% threshold before 2025.
Table 5. Comparison table of the equity of the modelled scenarios.
Positive values represent a reduction in inequalities and vice versa. Ages 30 to 84. Parentheses contain 95% UIs. Results are rounded to the first 2 significant digits.
| Model output | Scenario | By the year 2030 | By the year 2040 |
|---|---|---|---|
| Reduction in absolute socioeconomic health inequalities | Current (A) | 150 (−570 to 1,100) | 600 (−660 to 2,300) |
| Current plus targeted (B) | 410 (−1,000 to 2,600) | 2,900 (−360 to 7,700) | |
| Optimal (C) | 1,300 (−340 to 3,900) | 7,200 (3,100 to 13,000) | |
| Current plus structural (D) | 13,000 (5,800 to 22,000) | 37,000 (24,000 to 52,000) | |
| Current plus targeted plus structural (E) | 13,000 (5,300 to 23,000) | 38,000 (25,000 to 55,000) | |
| Reduction in relative socioeconomic health inequalities | Current (A) | −24 (−230 to 130) | −76 (−330 to 140) |
| Current plus targeted (B) | 11 (−150 to 200) | 120 (−110 to 400) | |
| Optimal (C) | −2.1 (−270 to 210) | −50 (−440 to 270) | |
| Current plus structural (D) | 550 (160 to 1,100) | 1,200 (630 to 1,900) | |
| Current plus targeted plus structural (E) | 550 (130 to 1,200) | 1,300 (670 to 2,000) |
Abbreviation: UI, uncertainty interval.
Fig 3. Annual probability of the modelled scenarios to be equitable.
We defined equitable as reducing both absolute and relative socioeconomic inequalities in health.
Productivity
All 5 scenarios would produce productivity savings by 2040 (Table 6). These savings would be largest for the targeted Health Check plus structural interventions (scenario E), with savings of approximately £23 million above the current implementation. This was over 5 times greater savings than for targeted implementation (scenario B) alone and 4 times greater than the savings increase for optimal implementation.
Table 6. CHD, stroke, and CVD net productivity discounted cost by the year 2040 (30 years).
Ages 30 to 64. Parentheses contain 95% UIs. Negative values represent savings. Results are rounded to the first 2 significant digits.
| Scenario | CHD net productivity cost (£million) | Stroke net productivity cost (£million) | Total CVD net productivity cost (£million) |
|---|---|---|---|
| Current (A) | −2.0 (−4.1 to −0.84) | −0.74 (−1.7 to −0.18) | −2.7 (−5.5 to −1.2) |
| Current plus targeted (B) | −5.2 (−10.0 to 2.6) | −1.7 (−3.7 to −0.69) | −6.8 (−14.0 to −3.6) |
| Optimal (C) | −5.5 (−11.0 to −2.9) | −2.6 (−4.9 to −1.2) | −8.1 (−15.0 to −4.5) |
| Current plus structural (D) | −13.0 (−20.0 to −8.0) | −9.8 (−15.0 to −6.1) | −22.0 (−34.0 to −15.0) |
| Current plus targeted plus structural (E) | −15.0 (−25.0 to −9.7) | −10.0 (−17.0 to −6.7) | −26.0 (−41.0 to −17.0) |
Abbreviations: CHD, coronary heart disease; CVD, cardiovascular disease; UI, uncertainty interval.
Discussion
Our study suggests that simply continuing the current implementation of the Health Check programme in Liverpool is unlikely to be cost-effective or equitable, even when modelled 2 decades into the future. If implementation of the programme met optimal PHE recommendations for uptake and prescribing [74], then cost-effectiveness might be achieved by 2030 and cost-savings achieved by 2040 for the Liverpool population. However, it would not improve relative health inequalities. Conversely, a targeted approach towards the most deprived (those at the highest risk) would likely improve equity by 2039 but only reach cost-effectiveness by 2040.
Moreover, our findings may have wider implications nationally and beyond. NHS Health Check is an intervention that targets high-risk individuals. Theoretically, these interventions are more effective when the risk is concentrated in some subgroups of the population [78]. Historically, Liverpool is a city with concentrated deprivation and concentrated CVD risk that translates consistently to worse CVD burden and outcomes compared to the national average [79]. Therefore, we expect the effectiveness and cost-effectiveness of NHS Health Check—even considering optimal implementation—to be worse elsewhere than in Liverpool.
These findings add to the results of a systematic review by Lee and colleagues in 2017 [9]. Analysing the evidence for the cost-effectiveness of population-wide CVD screening, the review found that there was a lack of robust evidence to support the implementation of such screening. As a result, the authors recommended that further evidence is required to identify the cost-effectiveness of such screening, and different delivery models should be examined, such as targeted implementation or population-wide interventions. This modelling study examines these issues directly and provides the evidence recommended.
The addition of structural interventions addressing smoking, diet, and an SSB tax provides a stark contrast. These scenarios would almost certainly be cost-effective, cost-saving, and equitable and reach these 80% thresholds much more quickly. This is unsurprising when remembering Rose’s paradigm about sick individuals and sick populations [80].
The UK Department of Health estimated that the ICER for NHS Health Check would be under £3,000 per QALY gained [6]. However, our findings suggest the ICER would be substantially higher than this by 2030, regardless of implementation. Furthermore, substantial improvements to the delivery and implementation of NHS Health Check would be required to achieve an ICER under £3,000 per QALY gained by 2040. Our estimates are comparable to those from Crossan and colleagues suggesting that optimal implementation would reduce ICERs, perhaps to less than £10,000 (versus £23,000 or more for typical implementation) [10]. The different estimates of these studies are due to differences in the modelled NHS Health Check implementation—and thus effectiveness—and wider differences in the modelling assumptions overall (i.e., assumptions regarding the future burden of CVD or use of different simulation horizons).
Public health implications
These results from our IMPACTNCD model may be of particular interest to local commissioners. Public health interventions have generally been found to be cost-saving [81], thus NHS Health Check appears to represent a comparatively expensive approach to prevention [82]. However, the NHS Health Check programme is currently a statutory requirement for local governments in England. Given this and the major pressures on local-authority budgets [83], the need to improve the cost-effectiveness of prevention programmes with the addition of cost-saving interventions is becoming increasingly clear.
The UK government introduced a sweetened drinks industry levy (SSB tax) in April 2018 [84]. However, other population strategies to reduce CVD could be added. Rather than wait for further action at the national level, we would advocate that local governments take the initiative in implementing population health-improvement strategies. Liverpool did that in the past. In 2004, the local government took the initiative to make Liverpool a smoke-free city. This local initiative was instrumental in the passage of the relevant national law 2 years later [85].
A number of cities across the United States, such as Boston and Oklahoma [86,87], have engaged in collective approaches to encourage healthier diets and weight loss. New York City implemented a number of approaches to improve fruit and vegetable consumption, particularly among low-income individuals [88]. These approaches included financial support to reduce cost barriers, incentives to improve access, support to improve supply and demand for healthy food, improving food quality in different organisations (including schools and childcare centres), and individual support programmes. This combined approach was associated with small but significant improvements in fruit and vegetable consumption. The legal and political feasibility of similar local public health policies for English cities is largely unexplored. Moreover, devolution deals such as those in Liverpool and Manchester may give local governments additional power to implement more structural preventive policies.
Strengths and limitations
To our knowledge, IMPACTNCD is the first dynamic stochastic microsimulation model to estimate cost-effectiveness and equity of the NHS Health Check programme at a city level. Focusing on the city level allowed us to use real-world data on disease rates, costs, and programme success that are not easily available at the national level. In addition, the choice of Liverpool—a city in which the concentration of CVD risk theoretically would favour NHS Health Check—allows the derivation of useful analogies for other areas.
There are, however, a number of limitations. First, health-related quality of life decrements were assumed equal across all socioeconomic groups. However, when considering CVD, people from lower socioeconomic backgrounds have significantly reduced quality of life compared to higher socioeconomic backgrounds. This is likely to underestimate our cost-effectiveness and equity estimates for scenarios B and E [89]. Second, the costs for NHS Health Check only consider the prices paid by the Liverpool City Council. We have assumed that these costs are consistent and have not considered the potential effects of saturation or diminishing returns. The costs of medications for CHD, stroke, diabetes, and hypertension are included in the broad healthcare unit cost estimates. Only the costs of statins for people with no other diseases are not explicitly included, but these costs are small, at around £20 to £40 per patient per annum. Furthermore, we did not include opportunity costs, resulting in the potential for the Health Check to displace more effective health interventions [90]. Third, while most of the structural policies we modelled are incremental improvements on existing policies, their political feasibility is unclear in the current environment. Fourth, our model only considers CVD and diabetes mellitus. Both NHS Health Check and the structural interventions we modelled can potentially reduce the burden of other noncommunicable diseases, too.
Conclusions
Our results suggest that current NHS Health Check implementation appears neither equitable nor cost-effective for local authorities. Optimal administration and implementation might result in better value for money in the next few decades. However, the addition of structural interventions could substantially reduce CVD risks, improve equity, and generate cost savings within short time-scales.
Supporting information
(DOCX)
(PDF)
Acknowledgments
The authors would like to thank Chris Williamson for his support in collecting the local-level data.
Disclaimer: The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Abbreviations
- CHD
coronary heart disease
- CVD
cardiovascular disease
- EQ-5D
Euroqol 5-dimension scale
- GP
General Practice
- HDL
high-density lipoprotein
- HSE
Health Survey for England
- ICER
incremental cost-effectiveness ratio
- NHS
National Health Service
- NMB
net monetary benefit
- PHE
Public Health England
- QALY
quality-adjusted life year
- QIMD
Index of Multiple Deprivation quintile group
- SSB
sugar-sweetened beverage
- UI
uncertainty interval
Data Availability
All relevant data are within the paper, its Supporting information files, and the GitHub repository (https://github.com/ChristK/IMPACTncd_Liverpool). The source code of the model is available at https://github.com/ChristK/IMPACTncd_Liverpool. All data inputs are available as .csv files in this repository, except the Health Survey for England microdata, which are accessible through the UK Data Service (https://www.ukdataservice.ac.uk/) to registered researchers.
Funding Statement
Liverpool City Council provided modest funding to support the analysis. CK was supported by the Medical Research Council Health eResearch Centre grant MR/K006665/1, MOF and SC by the Higher Education Funding Council for England, and HB and BC were partly supported by the US National Institute of Health R01HL130735. This work was also partly funded by the NIHR health technology assessment programme, project HTA 16/165/01. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388: 1459–1544. 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.British Heart Foundation. Cardiovascular Disease Statistics 2017 [Internet]. 2017. [Cited 26 Feb 2018]. Available from: https://www.bhf.org.uk/research/heart-statistics/heart-statistics-publications/cardiovascular-disease-statistics-2017.
- 3.Parliament. Health and Social Care Act 2012 [Internet]. 2012. [Cited 26 Feb 2018]. Available from: http://www.legislation.gov.uk/ukpga/2012/7/contents/enacted.
- 4.NHS Choices. NHS Health Check [Internet]. 2018. [Cited 26 Feb 2018]. Available from: http://www.healthcheck.nhs.uk/.
- 5.National Institute for Health and Care Excellence. Judging whether public health interventions offer value for money | Guidance and guidelines | NICE [Internet]. 2013. [Cited 26 Feb 2018]. Available from: https://www.nice.org.uk/advice/lgb10/chapter/judging-the-cost-effectiveness-of-public-health-activities.
- 6.Department of Health. Economic modelling for vascular checks [Internet]. London: Department of Health; 2008; [Cited 18 March 2017]. Available from: http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_085917.pdf. [Google Scholar]
- 7.Hinde S, Bojke L, Richardson G, Retat L, Webber L. The cost-effectiveness of population Health Checks: have the NHS Health Checks been unfairly maligned? J Public Health. 2017;25: 425–431. 10.1007/s10389-017-0801-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krogsboll LT, Jørgensen KJ, Gronhoj Larsen C, Gotzsche PC. General health checks in adults for reducing morbidity and mortality from disease: Cochrane systematic review and meta-analysis. BMJ. 2012;345: e7191–e7191. 10.1136/bmj.e7191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JT, Lawson KD, Wan Y, Majeed A, Morris S, Soljak M, et al. Are cardiovascular disease risk assessment and management programmes cost effective? A systematic review of the evidence. Prev Med. 2017;99: 49–57. 10.1016/j.ypmed.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 10.Crossan C, Lord J, Ryan R, Nherera L, Marshall T. Cost effectiveness of case-finding strategies for primary prevention of cardiovascular disease: a modelling study. Br J Gen Pract J R Coll Gen Pract. 2017;67: e67–e77. doi.org/10/f9jfrz [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robson J, Dostal I, Sheikh A, Eldridge S, Madurasinghe V, Griffiths C, et al. The NHS Health Check in England: an evaluation of the first 4 years. BMJ Open. 2016;6: e008840 10.1136/bmjopen-2015-008840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Usher-Smith J, Mant J, Martin A, Harte E, MacLure C, Meads C, et al. NHS Health Check programme rapid evidence synthesis [Internet]. Cambridge: The Primary Care Unit, University of Cambridge and RAND Europe; 2017. January [Cited 18 March 2017]. Available from: http://www.healthcheck.nhs.uk/document.php?o=1251. [Google Scholar]
- 13.Public Health England. Health inequalities briefing for London NHS Health Checks and target diseases: Inequalities by protected characteristics and socioeconomic factors [Internet]. 2015. [Cited 26 February 2018]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/431384/20150520_NHS_Health_Checks_a_health_inequalities_briefing_for_London.pdf.
- 14.Association of Directors of Public Health. Guidance on the ringfenced public health grant conditions and mandated functions in England [Internet]. London: PHE; 2016. [Cited 18 March 2018]. Available from: http://www.adph.org.uk/wp-content/uploads/2016/09/Interpreting-the-ringfenced-grant-conditions-and-mandateGATEWAY.pdf. [Google Scholar]
- 15.Mytton OT, Jackson C, Steinacher A, Goodman A, Langenberg C, Griffin S, et al. The current and potential health benefits of the National Health Service Health Check cardiovascular disease prevention programme in England: A microsimulation study. PLoS Med. 2018;15(3): e1002517 10.1371/journal.pmed.1002517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capewell S, Graham H. Will cardiovascular disease prevention widen health inequalities? PLoS Med. 2010;7(8): e1000320 10.1371/journal.pmed.1000320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallach-Kildemoes H, Diderichsen F, Krasnik A, Lange T, Andersen M. Is the high-risk strategy to prevent cardiovascular disease equitable? A pharmacoepidemiological cohort study. BMC Public Health. 2012;12: 610 10.1186/1471-2458-12-610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGill R, Anwar E, Orton L, Bromley H, Lloyd-Williams F, O’Flaherty M, et al. Are interventions to promote healthy eating equally effective for all? Systematic review of socioeconomic inequalities in impact. BMC Public Health. 2015;15: 457 10.1186/s12889-015-1781-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorenc T, Petticrew M, Welch V, Tugwell P. What types of interventions generate inequalities? Evidence from systematic reviews. J Epidemiol Community Health. 2013;67: 190–193. 10.1136/jech-2012-201257 [DOI] [PubMed] [Google Scholar]
- 20.Kypridemos C, Guzman-Castillo M, Hyseni L, Hickey GL, Bandosz P, Buchan I, et al. Estimated reductions in cardiovascular and gastric cancer disease burden through salt policies in England: an IMPACTNCD microsimulation study. BMJ Open. 2017;7: e013791 10.1136/bmjopen-2016-013791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen K, Kypridemos C, Hyseni L, Gilmore AB, Diggle P, Whitehead M, et al. The effects of maximising the UK’s tobacco control score on inequalities in smoking prevalence and premature coronary heart disease mortality: a modelling study. BMC Public Health. 2016;16: 292 10.1186/s12889-016-2962-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barton P, Andronis L, Briggs A, McPherson K, Capewell S. Effectiveness and cost effectiveness of cardiovascular disease prevention in whole populations: modelling study. BMJ. 2011;343: d4044 10.1136/bmj.d4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams J, Mytton O, White M, Monsivais P. Why are some population interventions for diet and obesity more equitable and effective than others? The role of individual agency. PLoS Med. 2016;13(4): e1001990 10.1371/journal.pmed.1001990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ministry of Housing, Communities & Local Government, Office for National Statistics. English indices of deprivation 2015—GOV.UK [Internet]. 2015. [Cited 26 Feb 2018]. Available from: https://www.gov.uk/government/statistics/english-indices-of-deprivation-2015.
- 25.Jones R, Gomes C, Lloyd K. Review of the NHS Health Check programme in Liverpool—update. Liverpool: Liverpool city council; 2016. August. [Google Scholar]
- 26.NHS. Public—Interactive map—NHS Health Check [Internet]. 2018. [Cited 2 Mar 2018]. Available from: https://www.healthcheck.nhs.uk/commissioners_and_providers/data/.
- 27.Kypridemos C, Allen K, Hickey GL, Guzman-Castillo M, Bandosz P, Buchan I, et al. Cardiovascular screening to reduce the burden from cardiovascular disease: microsimulation study to quantify policy options. BMJ. 2016;353: i2793 10.1136/bmj.i2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kypridemos C. Modelling the effectiveness and equity of primary prevention policies in England: a stochastic dynamic microsimulation for the joint prevention of non communicable diseases [Internet]. PhD Thesis, University of Liverpool. 2016. [Cited 11 May 2018]. Available from: https://elements.liverpool.ac.uk/repository.html?pub=0&com=get-file&rfurl=http%3A%2F%2Flivrepository.liverpool.ac.uk%2Frt4eprints%2Ffile%2F87606%2F201001644_Oct2016.pdf.
- 29.Department for Communities and Local Government. English indices of deprivation 2010—Publications—GOV.UK [Internet]. Mar 2011. [Cited 26 Aug 2014]. Available from: https://www.gov.uk/government/statistics/english-indices-of-deprivation-2010.
- 30.Office for National Statistics. Published ad hoc data: health, requests during December 2014. In: Published Ad hoc Data: Health, Requests During December 2014 [Internet]. 1 Dec 2014. [Cited 14 Oct 2015]. Available from: http://www.ons.gov.uk/ons/about-ons/business-transparency/freedom-of-information/what-can-i-request/published-ad-hoc-data/health/december-2014/number-of-registered-deaths-by-sex—cause—year—the-adjusted-index.xls.
- 31.Office for National Statistics. Subnational Population Projections for Local Authorities in England: Table 2 [Internet]. 2016. [Cited 5 Mar 2018]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/datasets/localauthoritiesinenglandtable2.
- 32.National Centre for Social Research, University College London. Department of Epidemiology and Public Health. Health Survey for England, 2002 [computer file]. 2nd Edition. Colchester, Essex: UK Data Archive [distributor]. 2010. [Cited 1 May 2014]. Available from: 10.5255/UKDA-SN-4912-1. [DOI]
- 33.National Centre for Social Research, University College London. Department of Epidemiology and Public Health. Health Survey for England, 2003 [computer file]. 2nd Edition. Colchester, Essex: UK Data Archive [distributor]. 2010. [Cited 1 May 2014]. Available from: 10.5255/UKDA-SN-5098-1. [DOI]
- 34.National Centre for Social Research, University College London. Department of Epidemiology and Public Health. Health Survey for England, 2004 [computer file]. 2nd Edition. Colchester, Essex: UK Data Archive [distributor]. 2010. [Cited 1 May 2014]. Available from: 10.5255/UKDA-SN-5439-1. [DOI]
- 35.National Centre for Social Research, University College London. Department of Epidemiology and Public Health. Health Survey for England, 2005 [computer file]. 3rd Edition. Colchester, Essex: UK Data Archive [distributor]. 2011. [Cited 1 May 2014]. Available from: 10.5255/UKDA-SN-5675-1. [DOI]
- 36.National Centre for Social Research, University College London. Department of Epidemiology and Public Health. Health Survey for England, 2006 [computer file]. 4th Edition. Colchester, Essex: UK Data Archive [distributor]. 2011. [Cited 1 May 2014]. Available from: 10.5255/UKDA-SN-5809-1. [DOI]
- 37.National Centre for Social Research, University College London. Department of Epidemiology and Public Health. Health Survey for England, 2007 [computer file]. 2nd Edition. Colchester, Essex: UK Data Archive [distributor]. 2010. [Cited 1 May 2014]. Available from: 10.5255/UKDA-SN-6112-1. [DOI]
- 38.National Centre for Social Research, University College London. Department of Epidemiology and Public Health. Health Survey for England, 2008 [computer file]. 4th Edition. Colchester, Essex: UK Data Archive [distributor]. 2013. [Cited 1 May 2014]. Available from: 10.5255/UKDA-SN-6397-2. [DOI]
- 39.National Centre for Social Research, University College London. Department of Epidemiology and Public Health. Health Survey for England, 2009 [computer file]. 2nd Edition. Colchester, Essex: UK Data Archive [distributor]. 2011. [Cited 1 May 2014]. Available from: 10.5255/UKDA-SN-6732-1. [DOI]
- 40.NatCen Social Research, Royal Free University College London. Department of Epidemiology and Public Health. Health Survey for England, 2010 [computer file]. 2nd Edition. Colchester, Essex: UK Data Archive [distributor]. 2012. [Cited 1 May 2014]. Available from: 10.5255/UKDA-SN-6986-2. [DOI]
- 41.NatCen Social Research, University College London. Department of Epidemiology and Public Health. Health Survey for England, 2011 [computer file]. Colchester, Essex: UK Data Archive [distributor]. 2013. [Cited 1 May 2014]. Available from: 10.5255/UKDA-SN-7260-1. [DOI]
- 42.NatCen Social Research, University College London. Department of Epidemiology and Public Health. Health Survey for England, 2012 [computer file]. Colchester, Essex: UK Data Archive [distributor]. 2014. [Cited 1 May 2014]. Available from: 10.5255/UKDA-SN-7480-1. [DOI]
- 43.NatCen Social Research, University College London. Department of Epidemiology and Public Health. Health Survey for England, 2013 [computer file]. Colchester, Essex: UK Data Archive [distributor]. 2015. [Cited 1 May 2016]. Available from: 10.5255/UKDA-SN-7649-1. [DOI]
- 44.NatCen Social Research, University College London. Department of Epidemiology and Public Health. Health Survey for England, 2014 [computer file]. Colchester, Essex: UK Data Archive [distributor]. 2016. [Cited 1 May 2016]. Available from: 10.5255/UKDA-SN-7919-1. [DOI]
- 45.Ezzati M, Henley SJ, Thun MJ, Lopez AD. Role of smoking in global and regional cardiovascular mortality. Circulation. 2005;112: 489–497. 10.1161/CIRCULATIONAHA.104.521708 [DOI] [PubMed] [Google Scholar]
- 46.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328: 1519 10.1136/bmj.38142.554479.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378: 1297–1305. 10.1016/S0140-6736(11)60781-2 [DOI] [PubMed] [Google Scholar]
- 48.Wolf PA, D’Agostino RB, Kannel WB, Bonita R, Belanger AJ. Cigarette smoking as a risk factor for stroke: The Framingham study. JAMA. 1988;259: 1025–1029. [PubMed] [Google Scholar]
- 49.He J, Vupputuri S, Allen K, Prerost MR, Hughes J, Whelton PK. Passive smoking and the risk of coronary heart disease—a meta-analysis of epidemiologic studies. N Engl J Med. 1999;340: 920–926. 10.1056/NEJM199903253401204 [DOI] [PubMed] [Google Scholar]
- 50.Oono IP, Mackay DF, Pell JP. Meta-analysis of the association between secondhand smoke exposure and stroke. J Public Health. 2011;33: 496–502. doi.org/10/b3vd52 [DOI] [PubMed] [Google Scholar]
- 51.Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360: 1903–1913. [DOI] [PubMed] [Google Scholar]
- 52.Prospective Studies Collaboration. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55 000 vascular deaths. Lancet. 2007;370: 1829–1839. 10.1016/S0140-6736(07)61778-4 [DOI] [PubMed] [Google Scholar]
- 53.The Emerging Risk Factors Collaboration. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377: 1085–1095. 10.1016/S0140-6736(11)60105-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375: 2215–2222. 10.1016/S0140-6736(10)60484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.The DECODE Study Group. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care. 2003;26: 688–696. doi.org/10/b4vmqv [DOI] [PubMed] [Google Scholar]
- 56.Bull FC, Armstrong TP, Dixon T, Ham S, Neiman A, Pratt M. Comparative quantification of health risks. Chapter 10: physical inactivity [Internet]. Geneva: World Health Organisation; 2004. [Cited 18 March 2018]. Available from: http://www.who.int/publications/cra/en/. [Google Scholar]
- 57.Dauchet L, Amouyel P, Hercberg S, Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. J Nutr. 2006;136: 2588–2593. 10.1093/jn/136.10.2588 [DOI] [PubMed] [Google Scholar]
- 58.Dauchet L, Amouyel P, Dallongeville J. Fruit and vegetable consumption and risk of stroke A meta-analysis of cohort studies. Neurology. 2005;65: 1193–1197. 10.1212/01.wnl.0000180600.09719.53 [DOI] [PubMed] [Google Scholar]
- 59.Wallach-Kildemoes H, Andersen M, Diderichsen F, Lange T. Adherence to preventive statin therapy according to socioeconomic position. Eur J Clin Pharmacol. 2013;69: 1553–1563. 10.1007/s00228-013-1488-6 [DOI] [PubMed] [Google Scholar]
- 60.National Institute for Health & Care Excellence. CG127; Hypertension in adults: diagnosis and management. Cost-effectiveness analysis [Internet]. London; 2011. [Cited 26 July 2018]. Available from: https://www.nice.org.uk/guidance/cg127/documents/hypertension-update-appendix-j-costeffectiveness-analysis-diagnosis2.
- 61.National Institute for Health & Care Excellence. NG 28; Type 2 diabetes in adults: management. Economic modelling report [Internet]. London: NICE; 2015. [Cited 17 March 2017]. Available from: https://www.nice.org.uk/guidance/ng28/evidence/appendix-f-full-health-economics-report-2185320355.
- 62.Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of Type 1 and Type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med J Br Diabet Assoc. 2012;29: 855–862. doi.org/10/gc5p47 [DOI] [PubMed] [Google Scholar]
- 63.National Institute for Health & Care Excellence. PH25; Cardiovascular disease prevention. Costing report [Internet]. London; 2010 Jun. [Cited 26 February 2016]. Available from: https://www.nice.org.uk/guidance/ph25/resources/costing-report-67331053.
- 64.Charlton J, Rudisill C, Bhattarai N, Gulliford M. Impact of deprivation on occurrence, outcomes and health care costs of people with multiple morbidity. J Health Serv Res Policy. 2013;18: 215–223. 10.1177/1355819613493772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.HM Treasury, Office for National Statistics. GDP deflators at market prices, and money GDP: November 2016 (the Autumn Statement)—GOV.UK [Internet]. 2016. [Cited 26 Feb 2018]. Available from: https://www.gov.uk/government/statistics/gdp-deflators-at-market-prices-and-money-gdp-november-2016-the-autumn-statement.
- 66.Longworth L, Yang Y, Young T, Mulhern B, Hernández Alava M, Mukuria C, et al. Use of generic and condition-specific measures of health-related quality of life in NICE decision-making: a systematic review, statistical modelling and survey. Health Technol Assess. 2014;18 10.3310/hta18090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kind P, Hardman G, Macran S. UK population norms for EQ-5D [Internet]. Centre for Health Economics, University of York; 1999. [Cited 18 March 2017]. Report No.: 172chedp. Available from: https://ideas.repec.org/p/chy/respap/172chedp.html. [Google Scholar]
- 68.Mistry H, Morris S, Dyer M, Kotseva K, Wood D, Buxton M, et al. Cost-effectiveness of a European preventive cardiology programme in primary care: a Markov modelling approach. BMJ Open. 2012;2. doi.org/10/gb3tpb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tengs TO, Lin TH. A meta-analysis of quality-of-life estimates for stroke. PharmacoEconomics. 2003;21: 191–200. [DOI] [PubMed] [Google Scholar]
- 70.Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making. 2002;22: 340–349. 10.1177/0272989X0202200412 [DOI] [PubMed] [Google Scholar]
- 71.Liu JL, Maniadakis N, Gray A, Rayner M. The economic burden of coronary heart disease in the UK. Heart. 2002;88: 597–603. doi.org/10/b72f9w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saka O, McGuire A, Wolfe C. Cost of stroke in the United Kingdom. Age Ageing. 2009;38: 27–32. 10.1093/ageing/afn281 [DOI] [PubMed] [Google Scholar]
- 73.Lowe J. Intergenerational wealth transfers and social discounting: Supplementary Green Book guidance [Internet]. HM Treasury; 2008. [Cited 18 Mar 2017]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/193938/Green_Book_supplementary_guidance_intergenerational_wealth_transfers_and_social_discounting.pdf.
- 74.Public Health England. NHS Health Check implementation review and action plan [Internet]. London: PHE; 2013. July [Cited 18 March 2017]. Available from: https://www.gov.uk/government/publications/nhs-health-check-implementation-review-and-action-plan. [Google Scholar]
- 75.Briggs ADM, Mytton OT, Kehlbacher A, Tiffin R, Rayner M, Scarborough P. Overall and income specific effect on prevalence of overweight and obesity of 20% sugar sweetened drink tax in UK: econometric and comparative risk assessment modelling study. BMJ. 2013;347: f6189 10.1136/bmj.f6189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bartlett S, Klerman J, Olsha L, Logan C, Blocklin M, Beauregard M, et al. Evaluation of the Healthy Incentives Pilot (HIP) Final Report [Internet]. 2014. [Cited 18 March 2017]. Available from: http://www.fns.usda.gov/sites/default/files/HIP-Final.pdf.
- 77.Nnoaham KE, Sacks G, Rayner M, Mytton O, Gray A. Modelling income group differences in the health and economic impacts of targeted food taxes and subsidies. Int J Epidemiol. 2009;38: 1324–1333. 10.1093/ije/dyp214 [DOI] [PubMed] [Google Scholar]
- 78.Manuel DG, Lim J, Tanuseputro P, Anderson GM, Alter DA, Laupacis A, et al. Revisiting Rose: strategies for reducing coronary heart disease. BMJ. 2006;332: 659–662. 10.1136/bmj.332.7542.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Public Health England. Public Health Outcomes Framework [Internet]. 21 Apr 2017. [Cited 21 Apr 2017]. Available from: http://www.phoutcomes.info/public-health-outcomes-framework#page/4/gid/1000044/pat/6/par/E12000002/ati/102/are/E08000012/iid/40401/age/163/sex/4.
- 80.Rose G. Sick individuals and sick populations. Int J Epidemiol. 2001;30: 427–432. doi.org/10/dkwc5q [DOI] [PubMed] [Google Scholar]
- 81.Masters R, Anwar E, Collins B, Cookson R, Capewell S. Return on investment of public health interventions: a systematic review. J Epidemiol Community Health. 2017;71: 827–834. 10.1136/jech-2016-208141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Owen L, Morgan A, Fischer A, Ellis S, Hoy A, Kelly MP. The cost-effectiveness of public health interventions. J Public Health. 2012;34: 37–45. doi.org/10/fdvwnm [DOI] [PubMed] [Google Scholar]
- 83.Butler P. Councils “at breaking point” due to budget cuts and rising social care bills. the Guardian. London, UK; 10 Feb 2017. [Cited 26 February 2018]. Available from: http://www.theguardian.com/society/2017/feb/10/councils-budget-cuts-social-care-bills.
- 84.HM Treasury, HM Revenue & Customs, Department of Health and Social Care. Soft Drinks Industry Levy: 12 things you should know [Internet]. 2016. [Cited 26 Feb 2018]. Available from: https://www.gov.uk/government/news/soft-drinks-industry-levy-12-things-you-should-know.
- 85.World Health Organization. SmokeFree Liverpool leads way to smoke-free United Kingdom [Internet]. 2018. [Cited 28 Feb 2018]. Available from: http://www.who.int/kobe_centre/interventions/smoke_free/liverpool/en/.
- 86.Chandler R. Boston Moves (a Little) for Health. Boston Magazine. 17 Aug 2012. [Cited 26 February 2018]. Available from: https://www.bostonmagazine.com/health/2012/08/17/boston-moves-a-little-for-health/.
- 87.Healthwise Champions. Oklahoma City Million—This City is Going on a Diet [Internet]. 2014. [Cited 26 Feb 2018]. Available from: http://www.thiscityisgoingonadiet.com/.
- 88.Sacks R, Yi SS, Nonas C. Increasing Access to Fruits and Vegetables: Perspectives From the New York City Experience. Am J Public Health. 2015;105: e29–e37. doi.org/10/gc5p3t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Denvir MA, Lee AJ, Rysdale J, Walker A, Eteiba H, Starkey IR, et al. Influence of socioeconomic status on clinical outcomes and quality of life after percutaneous coronary intervention. J Epidemiol Community Health. 2006;60: 1085–1088. 10.1136/jech.2005.044255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baird B, Charles A, Honeyman M, Maguire D, Das P. Understanding pressures in general practice King’s Fund; London; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(PDF)
Data Availability Statement
All relevant data are within the paper, its Supporting information files, and the GitHub repository (https://github.com/ChristK/IMPACTncd_Liverpool). The source code of the model is available at https://github.com/ChristK/IMPACTncd_Liverpool. All data inputs are available as .csv files in this repository, except the Health Survey for England microdata, which are accessible through the UK Data Service (https://www.ukdataservice.ac.uk/) to registered researchers.