Abstract
Cannibalism is a commonly observed phenomenon in arthropod species having relevant consequences for population dynamics and individual fitness. It is a context-dependent behaviour and an understanding of the factors affecting cannibalism rate is crucial to highlight its ecological relevance. In mosquitoes, cannibalism between larval stages has been widely documented, and the role of density, food availability and length of contact between individuals also ascertained. However, although mosquitoes can develop in temporary water habitats with very heterogeneous topologies, the role of the site shape where cannibals and victims co-occur has been instead overlooked. In this paper, we investigated this issue by using a simulation approach and laboratory cannibalism experiments between old (third- and fourth-instars) and young (first-instar) larvae of the tiger mosquito Aedes albopictus. Three virtual spaces with different shapes were simulated and the number of larval encounters was estimated in each one to assess whether the spatial shape affected the number of encounters between cannibal and victims. Then, experimental trials in containers with similar shapes to those used in the simulations were performed, and the cannibalism rate was estimated at 24 and 48h. Our results showed that the spatial shape plays a role on cannibalism interactions, affecting the number of encounters between individuals. Indeed, in the experimental trials performed, we observed the highest cannibalism rate in the container with the highest number of encounters predicted by the simulations. Interestingly, we found also that spatial shape can affect cannibalism not only by affecting the number of encounters, but also the number of encounters “favorable” for cannibalistic events. Temporary waters are inhabited by several species other than mosquitoes. Our results, showing an influence of the spatial shape on cannibalism in Ae. albopictus larvae, add a new critical factor to those affecting ecological interactions in these habitats.
Introduction
Cannibalism consists of killing and eating an entire or a part of a conspecific individual [1]. This form of predator-prey interaction that occurs at intraspecific level is taxonomically spread, being observed in both vertebrate and invertebrate species [1–3].
In insects, a considerable body of work has addressed the ecological consequences of cannibalism and the factors affecting it [1–5]. Cannibalism was observed to drastically reduce population size, thus contributing to the self-regulation of populations, increasing their stability and decreasing the risk of extinction. Furthermore, cannibalism was found to increase the resilience of populations to environmental stressors, as cannibalistic individuals and those who survive to their attacks are likely to be the more vigorous individuals [5–8]. Finally, cannibalism has been shown to affect the ability of population propagules to colonize and persist in new stressful environments by affecting the dispersal, nutritional ability and development time of individuals [2,9]. Likewise, cannibalism can have some consequences also at the individual level by affecting fitness benefits and costs. Benefits include the acquisition of nutritional advantages for the cannibal that increase its development rate, survival and fertility [4,10,11], and the reduction in intraspecific competition for food, mates and other resources [4,5]. Fitness costs of cannibalistic behavior include disease transmission from victims to cannibals, the consuming of potential partners and the risk of injury by the prey [4,5]. Cannibalism is therefore recognized to be a context-dependent behavior, inducible by factors affecting the benefit-cost balance, such as the relative sizes of cannibals and victims, the quality and quantity of diet, the population density and the length of contact between cannibal and victim [1,2,4,5].
Mosquitoes include more than 3.500 species, and some of them are important vectors of human and animal diseases. The immature stages develop in temporary water habitats, where different inter- and intra-specific interactions occur, and the dynamics and the rapidly changing conditions can lead to an extreme competition between individuals, favoring cannibalistic behavior [5,12–17]. Size-dependent cannibalism has been observed in larvae of Toxorhynchites, Armigeres and Anopheles genus, where young first-instar larvae were cannibalized by old fourth-instar larvae [18–23]. Density and the length of contact between larvae have been also shown to affect cannibalism in several mosquito species [18,22]. Food availability has been shown to play a role as well, such as in the mosquito Armigeres subalbatus, where the cannibalism rate between late instars (third- and fourth-instars) was higher when food was restricted than when it was given ad libitum [20].
On the other hand, in some cases cannibalism has not been explained by any of the above factors, but primarily by the probability of encountering a vulnerable individual. For example, in the mosquitoes Anopheles gambiae s.s. and An. arabiensis, cannibalism between old fourth-instar and young first-instar larvae was increased not by reduced food quantity, but by reduced space, which suggested that larval cannibalism in these species was enhanced by more frequent encounters within smaller environments [22,23]. Likewise, the frequency of intra-instar cannibalism between larvae of the mosquito Toxorynchites amboinensis was smaller in short and large than in tall and thin containers [19]. From the examples above, the spatial shape of the sites where cannibals and victims co-occur seems to significantly affect the cannibalism rate by affecting the number of encounters between individuals. Spatial shape can also affect the cannibalism rate by affecting the success of cannibalistic attacks, as the escape chances of victims can differ according to location. If an encounter between the cannibal and the potential victim occurs in open water, the victim has more chance of escaping than if it occurs, for example, near the water surface or in a corner of the container [24]. Despite the fact that the spatial shape of mosquito breeding sites, where larval cannibalism occurs in nature, can be highly heterogeneous regarding dimensions and origin (e.g. small water pools, tree holes, cattle hoof prints, man-made containers), its role in the cannibalism rate between mosquito larvae remains poorly investigated. In this paper, we aimed to investigate this issue using as study system the tiger mosquito Aedes albopictus.
In recent decades, Ae. albopictus has spread from its native range in East Asia to Australia, Africa, Europe, and the Americas [25–30]. This species is vector of relevant animal and human diseases [29] and is common in both suburban and rural areas, where it develops in tree holes and man-made containers of any material and form, including tires and cemetery flower pots [25]. As far as we know, no studies have focused on cannibalistic interaction between larval stages of this species. Here, we aimed i) to assess if cannibalism occurs between old and young larval instars and ii) to investigate the role of spatial shape on cannibalism rate. Firstly, we used a simulation approach to estimate the number of encounters between cannibal and victims and the number of encounters weighted for the likelihood of successful cannibalism within containers of three different shapes. Then, we performed cannibalism experiments using third-, fourth- and first-instar larvae in containers with similar shapes to those used in the simulations and estimated the cannibalism rate at two time points (24 and 48 hours).
Materials and methods
Simulations
Three grids were created to simulate three virtual spaces represented by three parallelepipeds with volumes of about 400,000 cells. The grids simulated tall and thin, intermediate and low and wide container shapes with a fixed volume. Twenty potential victims and one cannibal (i.e. 20 first-instar and one third-/fourth-instar larvae) were assumed to be in each container. The larvae were assumed to be in a homogeneous space, initially stationaries and randomly distributed and then moving of one step/time unit. An encounter between two larvae was reported when the distance between them was below a threshold number of steps (hereafter viewrange). Simulations using 4, 5 and 6 threshold values were run.
Mosquito larvae belonging to the Aedes genus must go to the water surface to breathe through the siphon. Then, they tend to remain at the water surface and move across it to filter the bacteria and organic particles contained in the water. Furthermore, mosquito larvae tend to move toward the bottom of a container and graze on the sediments [12,31]. Therefore, on the basis of the larval behavior, we simulated the larval movement within each container as follows: each larva mostly moved toward the top and the bottom of the container; when a larva arrived at the top (or at the bottom), it moved across the surface (or the bottom), then moved toward the bottom (or the top) following a random path. The time spent in top/bottom and in the other parts of the space was set at 60 and 40%, respectively for each container on the basis of our observations of larval movements (see S1 Appendix). One million time units (i.e. 10 simulations of 100,000 steps) were simulated using the Phython programming language to compute the number of encounters between cannibals and victims (N) and their 95% confidence intervals according to the Poisson distribution.
We weighted the number of encounters for the likelihood of successful cannibalism within each container, by multiplying the estimated number of encounters between cannibal and victims (N) with the cannibalism likelihood (p). In each container p was estimated as p = 1 –f, where f is the space available for the victim to escape when encounters a cannibal and its value ranged from 1 to 0.125 (e.g. it is 1 when the encounter occurs in the middle of the container; 0.5 when it occurs in the top or the bottom; 0.25 in the edges and 0.125 in a corner).
Cannibalism experiments
Mosquitoes
The Aedes albopictus mosquitoes used in this study were F2 generation derived from eggs collected by means of ovitraps during May 2016 in the urban area of Parma city (44.802 lat., 10.330 long.) and raised to adults in laboratory (no specific permissions were required for these locations/activities, and the field studies did not involve endangered or protected species). Larvae were placed into plastic trays (height = 5 cm, width = 30 cm and length = 19 cm) filled with 800 ml of distilled water, maintained in the laboratory at 27 ± 2°C, 75 ± 10% relative humidity (RH) and an L:D 16:8 h photoperiod and fed with a powder obtained by crushing dry cat food (Friskies ® Adult) (0.33g/800 ml of water) [32]. Eclosed adults were identified using the morphological keys of [33], kept in 40×40×40 cm cages and daily fed with 10% sucrose solution. Females were blood fed with fresh mechanically defibrinated bovine blood using a thermostatic apparatus [32]. Eggs were laid on paper towels in cups containing water. The paper towels were than dried and stored at 27°C until cannibalism experiments were performed. Eggs were hatched by force-hatching technique [34] to ensure uniform larval age.
Experimental setup
All experiments were performed under the same temperature, humidity and light/dark conditions of the reared colonies. We set up the experimental conditions at one larva/ml and no food shortage, providing food to the larvae during the experiments according to the rearing conditions described above (0.85mg/larva of fish food was supplied at the beginning of the experiment and after 24 h).
We tested the occurrence of cannibalism between third-instar (L3) and first-instar (L1) larvae and between fourth-instar (L4) and L1 larvae (L1 < 48 h old) and assessed the influence of the container shape on cannibalism rate. Three plastic containers with different surface/water column ratios were used, that reproduce common breeding sites of Ae. albopictus, such as flowerpots, man-made containers and flowerpot dishes: a 6×6×12 cm container (hereafter “tall and thin” container); a 12.5×12.5×4.5 cm container (hereafter “intermediate” container) and a 25×25×8 cm container (hereafter “low and wide” container). Each container was filled with 200 ml of distilled water, then twenty L1 larvae and one L3 or one L4 larva were placed into each container.
A control for each treatment was performed, placing 20 L1 larvae in each container without L3/L4 larvae. Further controls using dead larvae were also performed to test for possible larval decomposition and disappearance. Twenty L1 larvae (killed at -80°C for 5 min) were placed into the experimental containers maintained under the same experimental conditions as described before. Missing larvae were counted after 24 and 48 h that is the time span in which the larvae remain into their instar before moult to the next instar. For each experimental condition ten experimental containers of each shape were used (pseudoreplicates) and the experiment was replicated three times (biological replicates).
Data analyses
The number of L1 larvae in each container was computed after 24 and 48 h from the beginning of the experiments. Missing L1 larvae were considered cannibalized by L3 or L4 larvae [18–24]. The effect of the experimental factors and their combination on the cannibalism rate was investigated by logistic regression, using cannibalism as a binary response variable. The experimental factors used were: larval instar (L3, L4); container shape (encoded as: tall and thin = 1, low and wide = 2 and intermediate = 3); time (i.e. the length of contact between larvae, 24 and 48 hours). The fit of the model with the observed data was assessed using the Hosmer and Lemeshow goodness of fit test [35]. ANOVA analysis was performed to assess the effect of the experimental factors and their combination on the cannibalism rate. All analyses were performed using the software R version 3.0.2 [36].
Results
Simulations
The simulation results showed that the estimated number of encounters between larvae differed among the three containers. The tall and thin container showed the highest number of encounters between cannibal and victims, followed by the intermediate and the low and wide containers (Table 1). On the contrary, the space available for the victim to escape when encounters a cannibal (f) was the lowest in the low and wide container, followed by the tall and thin and intermediate containers (Table 1).
Table 1. Simulation results.
The Unweighted (N) and weighted number of encounters (Np) between cannibal and victims are shown for each container. C.I., 95% Confidence Intervals; f is the space available for the victim to escape when encounters a cannibal.
| Container | Unweighted number of encounters (N) (95% C.I.) |
f (mean ± sd) |
Weighted number of encounters (Np) (95% C.I.) | |
|---|---|---|---|---|
| Viewrange = 4 | ||||
| Tall and thin | 8,354 (8,175; 8,534) | 0.50 (0.05) | 4,154 (4,028; 4,281) | |
| Intermediate | 2,146 (2,056; 2,237) | 0.58 (0.03) | 896 (838; 955) | |
| Low and wide | 1,342 (1,271; 1,414) | 0.37 (0.01) | 843 (787; 900) | |
| Viewrange = 5 | ||||
| Tall and thin | 10,968 (10,763; 11,174) | 0.50 (0.06) | 5,472 (5,327; 5,617) | |
| Intermediate | 3,317 (3,205; 3,430) | 0.58 (0.04) | 1,378 (1,306; 1,451) | |
| Low and wide | 1,857 (1,773; 1,942) | 0.30 (0.01) | 1,306 (1,236; 1,377) | |
| Viewrange = 6 | ||||
| Tall and thin | 13,524 (13,297; 13,752) | 0.51 (0.07) | 6,740 (6,580; 6,901) | |
| Intermediate | 4,472 (4,341; 4,604) | 0.59 (0.04) | 1,855 (1,771; 1,940) | |
| Low and wide | 2,405 (2,309; 2,502) | 0.25 (0.01) | 1,810 (1,727; 1,894) |
The tall and thin container showed the highest number of encounters weighted for the likelihood of successful cannibalism (Np), while similar values were observed between the intermediate and the low and wide containers. The same pattern was observed using different values of viewrange (Table 1).
Cannibalism experiments
In the control tests no missing larvae were found after 24 and 48 hours. In the test trials instead, L1 larvae disappearance was observed under all experimental conditions, while no L3 and L4 larvae disappearance was observed.
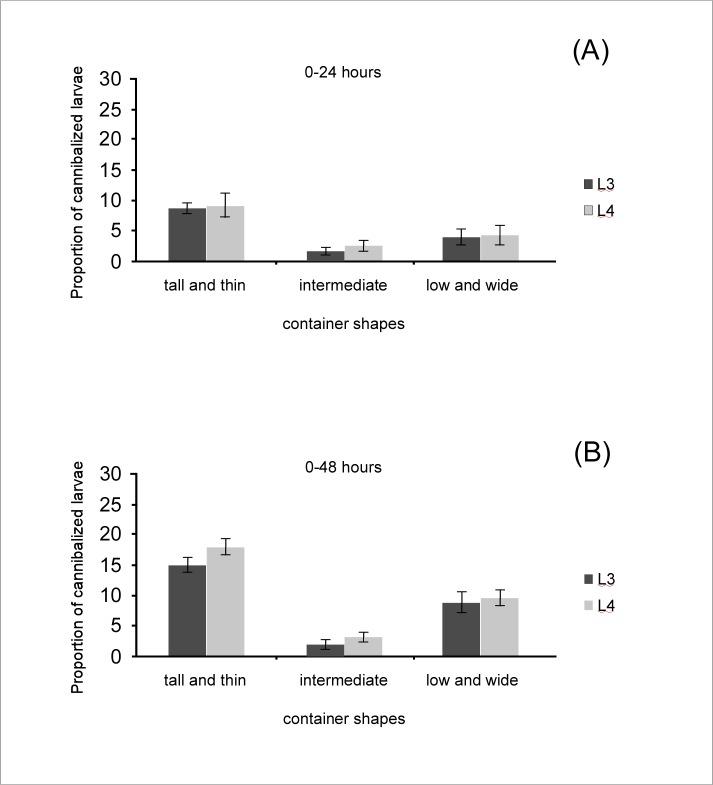
In the experimental trials between L1 and L3 larvae, a percentage equal to 8.67±0.76%, and 15.33 ±1.44% of cannibalized larvae (i.e. missing larvae out of all larvae used in the experiment) were observed in the tall and thin containers after 24 and 48 hours (mean ± standard deviation), respectively. In the intermediate containers, the percentages of missing larvae after 24 and 48 hours were 1.67±0.29%, and 2.50 ±1.53%, while in those low and wide were 4.01±1.5%, and 9.33 ±2.52% (Fig 1).
Fig 1. Cannibalism rate between Aedes albopictus larvae.
The proportion of the cannibalized first-instar larvae is shown for each container shape. The values are shown as mean ± standard deviations. L3: third-instar larvae; L4: fourth-instar larvae.
In the experimental trials between L1 and L4 larvae, a percentage equal to 9.03±2.78%, and 18.17 ±1.53% were observed in the tall and thin containers after 24 and 48 hours, respectively. In the intermediate containers the percentages of missing larvae after 24 and 48 hours were 2.67±0.76%, and 3.80 ±1.53%, while in those low and wide were 4.33±2.02%, and 9.83 ±1.61% (Fig 1).
The Hosmer and Lemeshow goodness of fit (GOF) test showed a good fit of the data with the regression logistic model (χ2 = 0.4233, df = 8, P-value = 0.99). The ANOVA analysis showed that cannibalism rate was not affected by the larval instar as no significant differences were observed by comparing cannibalism rate between (L3-L1) and (L4-L1) trials in any containers (Table 2; Fig 1). Likewise, the length of contact between old and young larvae (24 and 48 hours) affected the cannibalism rate only in the tall and thin container (Table 2; Fig 1). On the contrary, the container shape significantly affected cannibalism rate in both (L3-L1) and (L4-L1) trials, with significantly higher cannibalism rate observed in the tall and thin containers than in the other two containers (Fig 1).
Table 2. ANOVA analysis performed for the cannibalism data in Aedes albopictus.
| Df | Deviance Resid. | Df Resid. | Dev | Pr(>Chi) | |
|---|---|---|---|---|---|
| Null | 359 | 557.66 | |||
| Instar | 1 | 1.873 | 358 | 555.79 | 0.1711 |
| Shape | 2 | 130.419 | 356 | 425.37 | 2e-6*** |
| Time | 1 | 0.515 | 355 | 424.86 | 0.4729 |
| Instar ×Shape× Time | 2 | 1.077 | 348 | 414.45 | 0.5837 |
| Instar ×Shape | 2 | 1.028 | 353 | 423.83 | 0.5981 |
| Instar × Time | 1 | 0.105 | 352 | 423.72 | 0.7454 |
| Shape× Time | 2 | 8.198 | 350 | 415.52 | 0.0166 * |
*P< 0.05
***P< 0.001
Discussion
Cannibalism between old and young larvae
In this paper we found evidence that Aedes albopictus L3 and L4 larvae cannibalize L1 larvae. Under all experimental conditions, indeed, the disappearance of L1 larvae was observed, ranging from 2.5±0.5% to 18.17±1.5% after 48 h (Fig 1). We can confidently assume that the missing L1 larvae were cannibalized by older larvae as i) cannibalism between L1 larvae can be excluded because in the control tests, where no L3 and L4 larvae were introduced into the experimental chamber, no L1 disappearance was observed. Likewise, decomposition of the L1 larvae can also be excluded, because in the control tests where L1 dead larvae were used, no missing individual were observed after 48 hours; ii) the consumption of L1 larvae by L3 and L4 larvae has been described in other mosquito species and is in accordance with the expectation that cannibalism mostly occurs between older, bigger individuals and the younger [5,18–23].
Ae. albopictus is a vector species able to transmit several pathogenic viruses to humans, such as dengue, chikungunya and zika virus [29]. The findings of the consumption of young L1 larvae by the late instars can be particularly interesting from the epidemiological point of view. Cannibalistic behaviour, by affecting the number of individuals emerging from a breeding site could significantly affect the vector capacity of this species. On one hand, by limiting the number of larvae in the breeding sites it could maintain the population size below the carrying capacity, allowing it to grow. Furthermore, cannibalism, by offering a nutritional benefit to cannibal, can lead to the emerging of larger adult females with high fitness, high flight performance, host-seeking ability and dispersal potential, all characteristics affecting vector capacity [37–39]. On the other hand, however, cannibalism could reduce the number of emerging adults, and thus negatively impact on vector capacity [40,41]. Notably, our results, by showing that cannibalism rate is different between the experimental containers accordingly to their shape, suggest that the breeding sites can differently carry weight on vector capacity, and that the container shape plays a major role in generating this difference.
Cannibalistic behaviour between old and young larvae, could also affect the female oviposition behaviour and thus the distribution of mosquito vectors. Gravid females are highly selective in choosing oviposition sites, as immature stages are unable to move to a more suitable habitat if conditions become adverse [12]. In some mosquito species, including Ae. albopictus, the presence of conspecific larvae at low density is attractive for females, as it would indicate the site’s suitability (i.e. absence of predators and abundance of food resources). On the contrary, a high density of conspecific larvae has a repellent effect, because it would be a hallmark of intraspecific competition [42,43]. Our results showing cannibalism between late- and young-instars larvae in Ae. albopictus suggest that cannibalism can contribute to repel the oviposition of gravid females. The reduced hatchability of eggs observed in the presence of third-instar larvae in some species where cannibalism between old and young larvae occurs, such as Anopheles gambiae s.s. [44–46], would support this hypothesis. In Ae. albopictus, no studies specifically addressed if some oviposition behaviours evolved in response to cannibalism. Complex oviposition behaviours of females have been described, such as the scatter oviposition strategy, where egg batches are splitted between different breeding sites, and the asynchronous hatching of eggs [47]. Could be these strategies useful to avoid or reduce cannibalism between larvae? Or they are just an adaptation to limit larval competition? By affecting the larval density of the breeding sites, we could hypothesize that they can actually have some influence in these density-dependant interactions. Future studies specifically addressing these issues will allow to answer these questions.
Container shape and cannibalism rate
The role of spatial shape on the cannibalism rate between late (L3 and L4) and young (L1) larval instars of the tiger mosquito Ae. albopictus was investigated using both experimental and simulation approaches. Simulation results showed that a different number of encounters between cannibal and victims characterized the three containers, with the highest values observed in the tall and thin containers (Table 1). If the cannibalism rate is affected by the number of encounters between cannibals and victims, we expected that the cannibalism rate observed in our experimental trials would be higher in tall and thin containers. Our results were concordant with this prediction showing that in all trials between old and young larvae, the rate of cannibalism significantly differed among the three container shapes, and was the highest within the tall and thin container (Table 1). Therefore, empirical and simulation results jointly suggested that container shape significantly affects the cannibalism rate by affecting the probability of encounter between individuals.
Empirical results showed that after the tall and thin container, the rate of cannibalism was higher in the low and wide than in the intermediate container, contrary to what we had been led to expect by simulations results, which predicted a lower number of encounters within the low and wide than in the intermediate container. However, the two containers showed a similar weighted number of encounters, and the low and wide container had also the lowest values in the f parameter (i.e. the space available for the victim to escape when encounters a cannibal) (Table 1). Taken together these results could explain the higher cannibalism rate observed in the low and wide container by suggesting the occurrence of encounters in more “favorable” positions for the cannibal, which could balance the effect of a lower number of encounters. According to our simulation and experimental results, therefore, the container shape would affect cannibalism not only by affecting the number of encounters between cannibal and victims but also by affecting the number of “favorable” encounters for the cannibal.
Should we consider cannibalism as being affected only by the probability of encounter between larvae?
In the mosquito Anopheles stephensi, it has been suggested that the cannibalism observed between fourth- and first-instar larvae could arise from circular currents created by the filtering action of mouth brushes of fourth-instar larvae sweeping up first-instar larvae into the mouth parts. The cannibalism rate has therefore been considered to be the final product of random contacts between cannibal and victims [18]. However, mosquito larvae are able to actively attack co-specifics if close to one another as well as to escape from attacks [12,48]. Further ethological studies are needed to assess whether the eating of a co-specific is a by-product of the filtering actions or the consequence of voluntary attacks. Under both hypotheses, however, the probability of cannibal and victims encountering each other is a key factor in determining cannibalism rate. According to our results, the container shape plays a role by affecting the number and the propitiousness of encounters between cannibal and victims. Future studies should be performed to assess the interactions between the spatial shape and the other known factors affecting cannibalism, such as food or density. For example, we could hypothesize that the scarcity of food can lead to an increased foraging activity promoting the encounters between larvae, and thus cannibalistic events.
Conclusions
To our best knowledge, our results represent the first evidence of larval cannibalism in the tiger mosquito Aedes albopictus. Temporary waters are inhabited by the immature stages of mosquitoes as well as many other arthropod species. Cannibalism at larval stage has been documented in several of them such as caddisflies [49], damselflies [50] and blowflies [51], and it has been shown to significantly affect both population demography and dynamics. In some cases, cannibalism has been suggested to be primarily due to the probability of encountering a vulnerable individual due to spatial topology. For example, in the pit-building antlion Myrmeleon hyalinus larvae, increased sand depth led to decreased cannibalism rate by providing a potential refuge from cannibals and reducing the encounters between cannibal and victims [52]. Our results, showing the role of spatial shape on cannibalism rates in mosquito larvae suggest that it can be a critical factor affecting the interactions occurring in this habitat, and highlight the importance of further study of its contribution in aquatic insects.
Supporting information
Observations of the time spent by the Aedes albopictus larvae at the top and the bottom in each container.
(DOCX)
Acknowledgments
We would like to thank Federica Morgante, Ambra Castigliani, Sara Maggini and Alessandra Spanò for their help and comments; Mark Eltenton for the linguistic revision; the two anonymous reviewers for their comments and suggestions.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Fox LR. Cannibalism in natural populations. Annu Rev Ecol Syst. 1975; 6:87–106. [Google Scholar]
- 2.Polis GA. The evolution and dynamics of intraspecific predation. Annu Rev Ecol Syst. 1981;12: 225–51. [Google Scholar]
- 3.Elgar MA, Crespi BJ. Cannibalism: ecology and evolution among diverse taxa. Oxford: Oxford University Press; 1992. [Google Scholar]
- 4.Richardson ML, Mitchell RF, Reagel PF, Hanks LM. Causes and consequences of cannibalism in non-carnivorous insects. Ann Rev Entomol. 2010;55: 39–53. [DOI] [PubMed] [Google Scholar]
- 5.Santana AFK, Roselino AC, Cappelari AF, Zucoloto FS. Cannibalism in insects In: Panizzi AR, Parra JLP, editors. Insect bioecology and nutrition for integrated pest management. Boca Raton: CRC Press; 2012. p. 177–194. [Google Scholar]
- 6.Via S. Cannibalism facilitates the use of a novel environment in the flour beetle, Tribolium castaneum. Heredity. 1999;82: 267–75. [DOI] [PubMed] [Google Scholar]
- 7.Tayeh A, Estroup A, Lombaert E, Guillemaud T, Kichenko N, Lawson-Handley L, et al. Cannibalism in invasive, native and biocontrol populations of the harlequin ladybird. BMC Evol Biol. 2014;14:15 doi: 10.1186/1471-2148-14-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schausberger P. Cannibalism among phytoseiid mites: a review. Exp Appl Acarol. 2003;29: 173–191. [DOI] [PubMed] [Google Scholar]
- 9.Osawa N. Population field studies of the aphidophagous ladybird beetle Harmonia axyridis (Coleoptera: Coccinellidae): life tables and key factor analysis. Res Popul Ecol. 1993;35: 335–48. [Google Scholar]
- 10.Vijendravarma RK, Narasimha S, Kawecki TJ. Predatory cannibalism in Drosophila melanogaster larvae. Nat commun. 2013;4: 1879 doi: 10.1038/ncomms2836 [DOI] [PubMed] [Google Scholar]
- 11.Bolívar-Silva DA, Guedes NMP, Guedes RNC. Larval cannibalism and fitness in the stored grain weevils Sitophilus granarius and Sitophilus zeamais. J Pest Sci. 2017; 10.1007/s10340-017-0921-5. [Google Scholar]
- 12.Clements AN. Behaviour and aspects of the biology of larvae In: The biology of mosquitoes: Sensory reception and behaviour. Wallingford, UK: CABI Publishing; 1999, pp. 135–205. [Google Scholar]
- 13.Juliano SA. Species interactions among larval mosquitoes: context dependence across habitat gradients. Annu Rev Entomol. 2008;54:37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andreadis TG, Wolfe BJ. Evidence for reduction of native mosquitoes with increased expansion of invasive Ochlerotatus japonicus japonicus (Diptera: Culicidae) in the Northeastern United States. J Med Entomol. 2010;47:43–52. [DOI] [PubMed] [Google Scholar]
- 15.Silberbush A, Tsurim I, Rosen R, Margalith Y, Ovadia O. Species-specific non-physical interference competition among mosquito larvae. PLOS One. 2014;9:e88650 doi: 10.1371/journal.pone.0088650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urbanelli S, Porretta D, Mastrantonio V, Bellini R, Pieraccini G, Romoli R, et al. Hybridization, natural selection and evolution of reproductive isolation: a 25-yr survey of an artificial sympatric area between two mosquito sibling species of the Aedes mariae complex. Evolution. 2014;68:3030–3018. doi: 10.1111/evo.12490 [DOI] [PubMed] [Google Scholar]
- 17.Tsurim I, Silberbush A. Detrivory, competition, and apparent predation by Culiseta longiareolata in a temporary pool ecosystem. Isr J Ecol Evol 2016;62:138–42. [Google Scholar]
- 18.Reisen WK, Emory RW. Cannibalism in Anopheles stephensi Liston. Mosquito News. 1976;36: 198–200. [Google Scholar]
- 19.Annis B, Umi TI, Sarojo B, Hamzah N, Trenggono B. Laboratory studies of larval cannibalism in Toxorhynchitesamboinensis (Diptera:Culicidae). J Med Entomol. 1990;27: 777–83. [Google Scholar]
- 20.Rajavel AR. Cannibalistic behavior in Armigeres subalbatus (Diptera: Culicidae). Southeast Asian S Trop Med Public Health. 1992; 23:453–457. [PubMed] [Google Scholar]
- 21.Lounibos LP, Escher RL, Duzak D, Martin EA. Body size, sexual receptivity and larval cannibalism in relation to protandry among Toxorhynchites mosquitoes. Oikos. 1996;77: 309–16. [Google Scholar]
- 22.Koenraadt CJM, Takken W. Cannibalism and predation among larvae of the Anopheles gambiae complex. Med Vet Entomol. 2003;17: 61–6. [DOI] [PubMed] [Google Scholar]
- 23.Koenraadt CJM, Majambere S, Hemerik L, Takken W. The effects of food and space on the occurrence of cannibalism and predation among larvae of Anopheles gambiae s.l. Entomol Exp Appl. 2004;112: 125–34. [Google Scholar]
- 24.Porretta D, Mastrantonio V, Crasta G, Bellini R, Comandatore F, Rossi P, et al. Intra-instar larval cannibalism in Anopheles gambiae (s.s.) and Anopheles stephensi (Diptera: Culicidae). Parasite Vector. 2016;9: 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawley WA. The biology of Aedes albopictus. J Am Mosq Contr Ass. 1988;1: 1–39. [PubMed] [Google Scholar]
- 26.Urbanelli S, Bellini R, Carrieri M, Sallicandro P, Celli G Population structure of Aedes albopictus (Skuse): the mosquito which is colonizing Mediterranean countries. Heredity. 2000;84: 331–337. [DOI] [PubMed] [Google Scholar]
- 27.Benedict MQ, Levine R, Hawley W, Lounibos L. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoon Dis. 2007;7:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porretta D, Mastrantonio V, Bellini R, Somboon P, Urbanelli S. Glacial history of a modern invader: phylogeography and species distribution modelling of the asian tiger mosquito Aedes albopictus. PlosOne. 2012;7(9): e44515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonizzoni M, Gasperi G, Chen X, James A. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol. 2013;29: 460–468. doi: 10.1016/j.pt.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraemer MU, Sinka ME, Duda KA, Mylne A, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and A. albopictus. Elife. 2015;4: e08347 doi: 10.7554/eLife.08347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Donnell DL, Armbruster PC. Comparison of larval foraging behavior of Aedes albopictus and Aedes japonicus (Diptera: Culicidae). J Med Entmol. 2007;44: 984–989. [DOI] [PubMed] [Google Scholar]
- 32.Bellini R, Medici A, Carrieri M, Calvitti M, Cirio U, Maini S. Applicazione della tecnica del maschio sterile nella lotta contro Aedes albopictusin Italia: ottimizzazione delle fasi di allevamento massale e messaggio. Proceedings of the XIX Italian National Congress of Entomology, Catania 2002;II: 993–998. [Google Scholar]
- 33.Schaffner F, Angel G, Geoffroy B, et al. The mosquitoes of Europe/Les moustiques d’Europe. Logiciel d’identification et d’enseignement (CD-Rom). IRD Editions & EID Méditerranée, 2001; Montpellier, France.
- 34.Sivanathan MM. The ecology and biology of Aedes aegypti (L.) and Aedes albopictus (Skuse) (Diptera: Culicidae) and the resistance status of Aedes albopictus (field strain) against organophosphates in Penang, Malaysia. Thesis dissertation. 2006.
- 35.Hosmer DW, Lemeshow S, Rodney X. Sturdivant: Applied Logistic Regression. 3rd ed. New York: Wiley; 2013. [Google Scholar]
- 36.R Core Team. R: A Language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria: 2013. http://www.R-project.org. Accessed 15 December 2017. [Google Scholar]
- 37.Briegel H. Fecundity, metabolism, and body size in Anopheles (Diptera, Culicidae), vectors of malaria. J Med Entomol. 1990;27: 839–50. [DOI] [PubMed] [Google Scholar]
- 38.Alto BW, Reiskind MH, Lounibos LP. Size alters susceptibility of vectors to dengue virus infection and dissemination. Am J Trop Med Hyg. 2008;79:688–695. [PMC free article] [PubMed] [Google Scholar]
- 39.Shapiro LLM, Murdock CC, Jacobs GR, Thomas RJ, Thomas MB. Larval food quantity affects the capacity of adult mosquitoes to transmit human malaria. Proc R Soc B. 2016;283: 20160298 doi: 10.1098/rspb.2016.0298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith DL, Battle KE, Hay SI, Barker CM, Scott TW, McKenzie FE. Ross, macdonald and a theory for the dynamics and control of mosquito-transmitted pathogens. PLoS Pathog. 2012;8: e1002588 doi: 10.1371/journal.ppat.1002588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brady OJ, Godfray HCJ, Tatem AJ, Gething PW, Cohen JM, McKenzie FE, et al. Vectorial capacity and vector control: reconsidering sensitivity to parameters for malaria elimination. Trans. R. Soc. Trop. Med. Hyg. 2016;110(2):107–117. doi: 10.1093/trstmh/trv113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wasserberg G, Bailes N, Davis C, Yeoman K. Hump-shaped density-dependent regulation of mosquito oviposition site-selection by conspecific immature stages: theory, field test with Aedes albopictus, and a meta-Analysis. Plos One. 2014;9(3): e92658 doi: 10.1371/journal.pone.0092658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez PV, Gonzalez Audino PA, Masuh HM. Oviposition behavior in Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in response to the presence of heterospecific and conspecific larvae. J Med Entomol. 2016;53(2): 268–72. doi: 10.1093/jme/tjv189 [DOI] [PubMed] [Google Scholar]
- 44.Livdahl TP, Koenekoop RK, Futterweit SG. The complex hatching response of Aedes eggs to larval density. Ecol Entomol. 1984;9: 437–42. [Google Scholar]
- 45.Edgerly JS, Marvier MA. To hatch or not to hatch? Egg hatch response to larval density and to larval contact in a tree hole mosquito. Ecol Entomol. 1992;17: 28 32. [Google Scholar]
- 46.Gotifrid GM, Urasa MF, Katunzi G, Yarro JG, Munga S, Kweka E. Reduced hatchability of Anopheles gambiae s.s eggs in presence of third instar larvae. BMC Res Notes. 2014;7: 231–233. doi: 10.1186/1756-0500-7-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hawley WA (1988) The biology of Aedes albopictus. J Am Mosq Control Assoc Suppl 11–39. [PubMed] [Google Scholar]
- 48.Sih A. Antipredator responses and the perception of danger by mosquito larvae. Ecology. 1986;67: 434–41. [Google Scholar]
- 49.Wissinger SA, Sparks GB, Rouse GL, Brown WS, Steltzer H. Intraguild predation and cannibalism among larvae of detritivorous caddisflies in subalpine wetlands. Ecology. 1996;77: 2421–2430. [Google Scholar]
- 50.Johansson F, Rowe L. Life history and behavioral responses to time constraints in a damselfly. Ecology. 1999;80: 1242–1252. [Google Scholar]
- 51.Faria L, Trinca L, Godoy W. Cannibalistic behavior and functional response in Chrysomya albiceps (Diptera: Calliphoridae) J Ins Behav. 2004;17: 251–261. [Google Scholar]
- 52.Barkae ED, Golan O, Ovadia O. Dangerous neighbors: interactive effects of factors influencing cannibalism in pit-building antlion larvae, Behav Ecol. 2014;25: 1311–1319. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Observations of the time spent by the Aedes albopictus larvae at the top and the bottom in each container.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.