Abstract
Background
Excess body weight in adulthood is associated with risk for asthma admission (AA). Our aim was to investigate if this association also applies to the relation between body mass index (BMI) in childhood and AAs in early adulthood (age 20–45 years).
Methods
This was a prospective study of 310,211 schoolchildren (born 1930–1989) from the Copenhagen School Health Records Register. Height and weight were measured annually, and generated BMI z-scores were categorized as low (lower quartile), normal (interquartile) and high (upper quartile). Associations between BMI at ages 7–13 and AA were estimated by Cox regressions, and presented as hazard ratios (HRs) and 95% confidence intervals (CI). Main outcome was incident hospital AAs (extracted from the Danish National Patient Register) in early adulthood.
Results
During 4,708,607 person-years of follow-up, 1,813 incident AAs were observed. Nonlinear associations were detected between childhood BMI and AAs. The risk of AA increased for females in the highest BMI category in childhood, with the highest HR of 1.3 (95% CI 1.16–1.55) at the age of 13 years. By contrast, males in the low BMI category had a higher risk of AA in early adulthood, with the highest HR of 1.24 (95% CI 1.03–1.51) at the age of 12 years. Females with an increase in BMI between ages 7 and 13 years had an increased risk of AA compared with females with stable BMI (HR 1.28, 95% CI 1.10–1.50).
Conclusion
The association between childhood BMI and AA in early adulthood is non-linear. High BMI increases the risk of AA in females, whereas low BMI increases the risk in males.
Keywords: BMI, childhood, asthma, admissions, sex, adiposity
Introduction
The association between obesity and incident asthma in adults was first described by Camargo et al in 1999,1 and has more recently been reported in children.2 However, the mechanisms underlying the association are still incompletely understood.3 Furthermore, studies have also shown that obesity in both children and adults is associated with increased asthma incidence and severity,3,4 together with a less favorable response to both reliever and controller medication for asthma.5–7
Based on 18 years of follow-up of the Isle of Wight birth cohort (n=1,456), Ziyab et al8 reported that early persistent obesity is associated with a 2.2-fold increased risk of having asthma. In line with this, Egan et al9 reported from an 11-year follow-up of adolescents in the Norwegian HUNT study that individuals with general overweight and abdominal obesity had an increased risk of asthma. However, large-scale studies with longer follow-up time are likely to add important knowledge regarding the association between body mass index (BMI) in childhood and hospital admissions for asthma later in life.
Previous studies of sex differences in the impact of adiposity on the risk of asthma have yielded conflicting results.9,10 In keeping with this, Benedetti et al11 reported from a cross-sectional study of adolescents that both general overweight and abdominal adiposity in girls were associated with a high prevalence of asthma, whereas in boys it was primarily excess abdominal adiposity that was associated with a higher risk of asthma. Furthermore, based on a cross-sectional study of 4,828 adolescents, Lu et al12 reported that overweight/obesity was associated with an increased prevalence of asthma in girls but not in boys. However, by contrast, Chen et al13 reported from a cross-sectional study of approximately 7,192 boys and girls from Taiwan that the effect of asthma and high BMI on forced expiratory volume in 1 second/forced vital capacity ratio was more pronounced in boys than in girls. Our current knowledge, therefore, points to potentially important differences in the impact of childhood BMI on long-term asthma risk in males and females.
Schatz et al14 showed in a cohort of children (n=10,700) and adults (n=17,316) that a higher BMI was associated with an increased risk of asthma exacerbations and, in a further analysis of data from the same cohort, that obesity at baseline was associated with subsequent poor asthma control, including exacerbations.15 The latter observation is in accordance with a recently published study of 90 patients evaluated at a clinic specialized in severe asthma by Tay et al.16
Asthma is a heterogeneous disease. Compared with asthma with onset in childhood, adult-onset asthma is not only more often non-allergic, but also generally more difficult to control17 and has a less favorable prognosis with regard to morbidity and long-term outcomes.18–21 Furthermore, in middle-aged and older adults it can be problematic to distinguish asthma from COPD as these patients frequently have a non-reversible airflow limitation at the time of diagnosis.18 Based on these observations, we therefore decided to focus the present analysis on asthma admissions (AAs) in individuals in the age group of 20–45-years.
As far too many children are overweight or obese worldwide,22 it is of utmost importance to understand the consequences of excess body weight on the risk for asthma exacerbations of such severity that they lead to hospital admissions. In the present study of a very large cohort of schoolchildren, we therefore investigated the association between BMI in childhood and incident hospital admissions for asthma in early adulthood, focusing on potential differences between females and males.
Methods
Study population
We studied 310,211 children born from 1930 through 1989 who were included in the Copenhagen School Health Records Register (CSHRR). All schoolchildren underwent mandatory annual health examinations at public or private schools in Copenhagen from 1st to 7th grade through 1983 and thereafter only at school entrance and exit, unless the child had specific health requirements. As part of the examinations, performed by school doctors or nurses, height and weight were measured (with the child either naked or wearing light clothes). All children were assigned a health card on which the child’s name, date of birth, birth weight as reported by the parents, and yearly height and weight were recorded. As described previously, the register can be considered virtually complete with regard to inclusion of Copenhagen’s school children.23
BMI was calculated as weight in kilograms divided by the square of height in meters for each child at each age. Internal age- and sex-specific BMI references were calculated using data from health examinations performed for children born from 1955 to 1960, when the prevalence of obesity was low and stable. Afterwards, BMI z-scores were calculated using the Lambda Mu Sigma method.24 Positive values indicate a BMI value higher than the average and negative values indicate a BMI value lower than the average in our reference population. BMI data and corresponding z-scores (and percentiles) were available from ages 7 through 13 years.
From 1968 onwards, all Danish citizens were assigned a unique identification number.25 These numbers were recorded on the health cards for children attending school at this time or thereafter, and they were retrieved for children who left school prior to this time.23 Using the identification numbers, linkages were made to the Danish National Patient Register (DNPR), which contains information on discharge diagnoses from all somatic hospitals in Denmark since 1977 and from emergency and outpatient departments since 1995.26
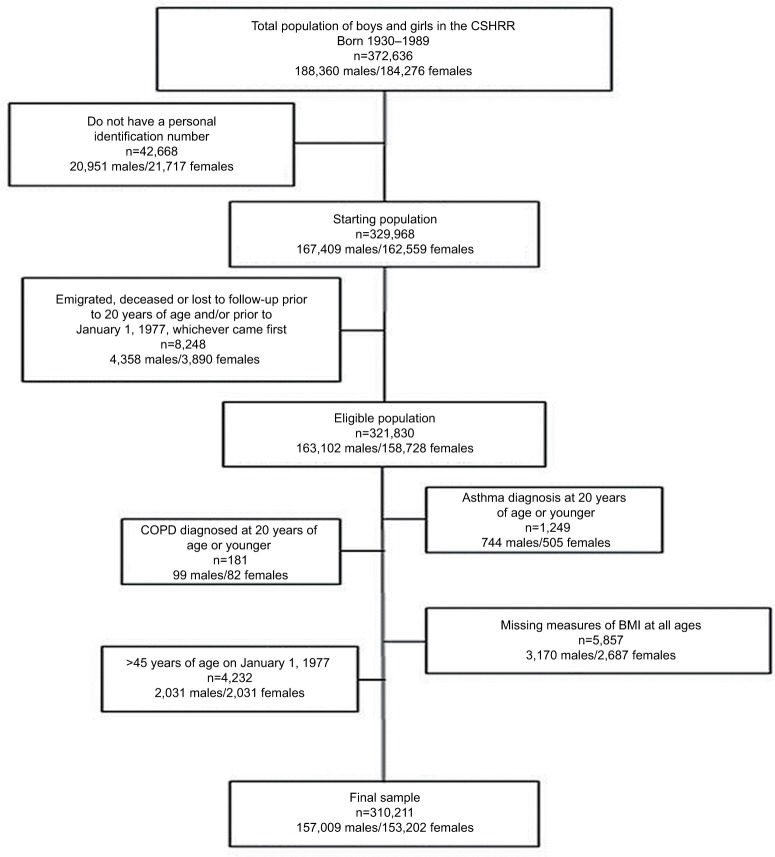
Information about hospital admissions for asthma and chronic obstructive lung disease up to age 45 years for children (n=321,830) included in the CSHRR was obtained by linking to the DNPR. All diagnoses in DNPR are classified according to the International Classification of Diseases (ICD); ICD-8 until 1994 and ICD-10 thereafter as ICD-9 codes were never implemented in Denmark. Data were extracted for first-ever diagnoses of asthma (ICD-8: 493 and ICD-10: J45-46) and COPD (ICD-8: 490-92, 494-96 and ICD-10: J40-44) as primary diagnoses. As the focus of the present analysis was incidence of first-ever AA, individuals with asthma or COPD admission before the age of 20 years (n=1,249 and n=181, respectively) were excluded; further details are given in Figure 1.
Figure 1.
Flowchart of individuals from the CSHRR included in the present analysis.
Abbreviations: BMI, body mass index; CSHRR, Copenhagen School Health Records Register.
The primary outcome of interest was first-ever hospital admission for asthma until age 45 years. The age limit of 45 years was chosen, as distinguishing asthma from COPD can be problematic in older adults21 together with previous reports showing that late-onset asthma differs from early-onset asthma with regard to morbidity and outcomes.20
Follow-up of individuals began at 20 years of age or on 1st January 1977 (when the DNPR was established), whichever came later. We chose 20 years as the earliest entry age to restrict our analyses to new-onset asthma in adulthood. Individuals were followed up until date of asthma hospital admission, the date of death, emigration or December 31, 2013, whichever came last.
Statistical analysis and ethics
Descriptive statistics (mean and standard deviation [SD]) were computed for BMI from age 7 through 13 years. Furthermore, the incidence rate per 10,000 person-years of follow-up by age group, sex and AA was computed as the incidence divided by the person-years at risk.
Cox proportional hazards regressions were used to examine association between childhood BMI z-scores at each age from 7 to 13 years and the risk of hospital admission for asthma between 20 and 45 years of age with age as the underlying time scale. Linearity was assessed by testing against a restricted cubic spline with four knots. Analyses were performed with individuals stratified according to birth cohort and separately for females and males. The results of the analyses are presented as the estimated hazard ratio (HR) and the corresponding 95% confidence intervals (95% CI); furthermore, the fitted association and corresponding confidence bands were plotted. The proportional hazard assumptions were assessed by a test based on Schoenfeld residuals, and no significant deviations were detected.
Further analyses of the risk for AA were carried out by categorizing the childhood BMI z-scores into three groups by age and sex: lower quartile, interquartile range and upper quartile. The estimation of risk was analyzed in a Cox proportional hazards regression model with categorized BMI z-score as a factor and with individuals stratified according to birth cohort; the underlying time scale was age and the analyses were performed separately for males and females.
This study was approved by the Danish Data Protection Agency. According to Danish law, ethical approval is not required for secondary analyses of data derived from registers. The data used in the present study are based on a combination of data with personal identification numbers from third parties, the CSHRR and data from national health registers. Furthermore, also according to Danish law, this information cannot be publicly available, but access to the subset of data included in the present study may be obtained by submitting a project application to the senior author Jenifer L Baker and pending approval by the data steering committee.
Results
Cohort characteristics
Height and weight measurements, and therefore BMI data, were available for 310,211 individuals. As expected for children, the mean BMI values and SDs increased with age from 15.5 kg/m2 (SD 1.3) at age 7 years to 18.4 kg/m2 (SD 2.4) at age 13 years. During the 36-year study period, 1,148 females and 665 males had at least one hospital admission for asthma between age 20 and 45 years. There were 4,708,607 person-years of follow-up.
The incidence rate for first AA in adulthood was 3.85 per 10,000 person-years of follow-up and was very similar across age groups. As expected, a higher incidence rate was observed for females than for males in all age groups (Figure 2).
Figure 2.
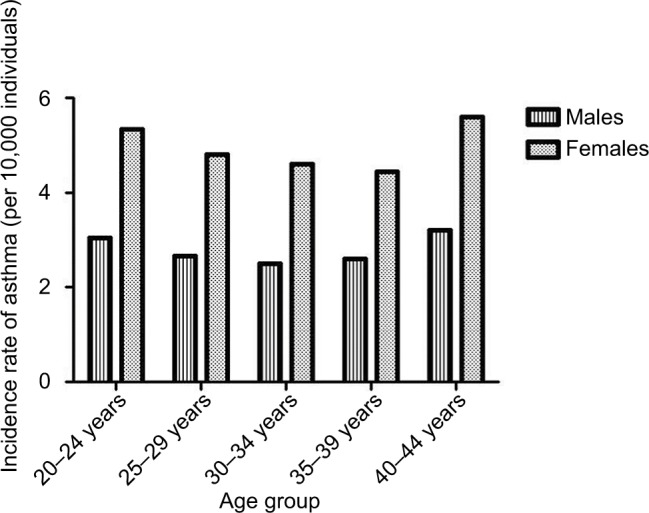
Incidence rate of asthma admissions between age 20 and 45 years in individuals from the Copenhagen School Health Records Register (n=310,211) by age group and sex.
Investigating linearity of the association between childhood BMI and AA
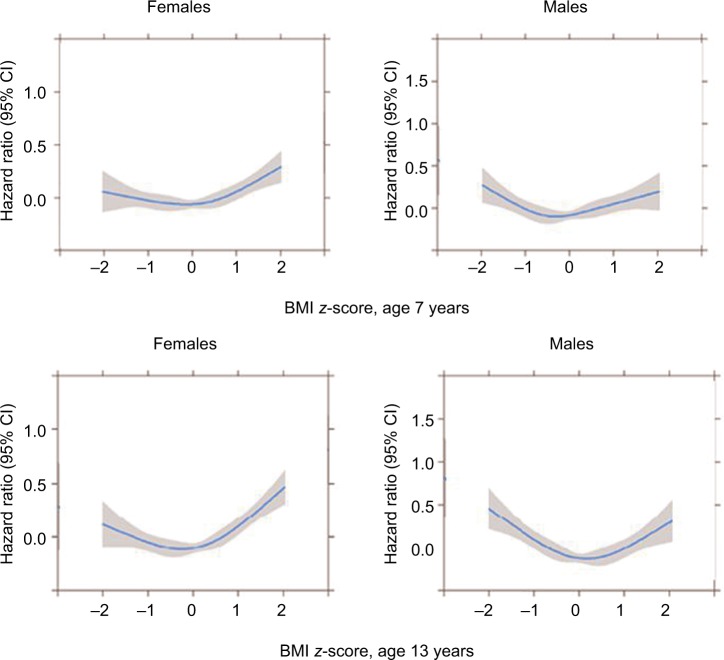
The fitted functional form and corresponding confidence intervals for associations between childhood BMI z-score for females and males at ages 7 and 13 years, respectively, and risk of AA in early adulthood (age 20–45 years) are given in Figure 3. Significant deviations from linearity were observed at all ages for females and males (all p-values ≤0.009).
Figure 3.
Association between childhood BMI z-scores at ages 7 and 13 years for females and males, respectively, and first-ever hospital admissions for asthma in early adulthood (between age 20 and 45 years) among individuals in the Copenhagen School Health Records Register (n=310,211).
Abbreviation: BMI, body mass index.
In females, higher BMI z-scores at age 7 years increased the risk of hospital admission for asthma in early adulthood (Figure 3). The risks started to significantly increase around values of 0.5 z-scores, which is equivalent to the 70th percentile of the BMI distribution. BMI z-scores below 0.5 were not significantly associated with AAs as the confidence intervals included 1. At age 13 years, the pattern was similar except that BMI z-scores just smaller and greater than average (z-score=0) were significantly associated with a reduced risk of AAs. In contrast with the females, among males low BMI z-scores at age 7 years increased the risk of AAs (Figure 3). At age 13 years, the estimates were stronger, and the risks began to increase for boys with a BMI z-score of approximately −1, which is equivalent to the 16th percentile of the BMI distribution. Similar to the females, boys with z-scores just around the average (z-score=0) had significantly reduced risks for AAs.
Childhood BMI and risk for AA in early adulthood
The risk of AA was significantly higher for females with a BMI in the highest quartile in childhood than in females with a normal BMI (value within the interquartile range), with the highest HR of 1.34 (95% CI 1.16–1.55) at the age of 13 years (Table 1). The risk of AA for females who had a BMI in the lowest quartile in childhood was not significantly different from the risk in females with a normal BMI (HR 1.05, 95% CI 0.90–1.23, at age 13 years). By contrast, among males, and similar to what is illustrated in Figure 3, there was a consistent pattern that males with a childhood BMI in the lowest quartile in childhood had a higher risk of AA in early adulthood than males with normal BMI, although these differences did not always reach statistical significance. The highest HR was observed at the age of 12 years, with a value of 1.24 (95% CI 1.03–1.51) (Table 1). The risk of incident AAs for males with high BMI values in childhood was not significantly higher than for males with normal BMI at ages from 7 to 13 years.
Table 1.
Associations (HR and 95% CI) between childhood BMI z-score and incident hospital admissions for asthma (n=1,813) in early adulthood (between age 20 and 45 years) among individuals in the Copenhagen School Health Records Register (n=310,211)
| Age (years) | Childhood BMI category
|
|||
|---|---|---|---|---|
| Low (quartile 1) | Normal (interquartile range) | High (quartile 4) | ||
| Females | Reference | |||
| 7 | 1.07 (0.92–1.24) | 1.18 (1.02–1.36) | ||
| 8 | 1.08 (0.93–1.24) | 1.12 (0.97–1.29) | ||
| 9 | 1.00 (0.86–1.16) | 1.12 (0.97–1.29) | ||
| 10 | 1.05 (0.90–1.22) | 1.26 (1.09–1.46) | ||
| 11 | 1.01 (0.86–1.18) | 1.27 (1.09–1.46) | ||
| 12 | 1.05 (0.90–1.22) | 1.24 (1.07–1.44) | ||
| 13 | 1.05 (0.90–1.23) | 1.34 (1.16–1.55) | ||
| Males | Reference | |||
| 7 | 1.10 (0.91–1.34) | 1.23 (0.98–1.41) | ||
| 8 | 1.14 (0.95–1.38) | 1.06 (0.87–1.28) | ||
| 9 | 1.18 (0.98–1.42) | 1.05 (0.87–1.28) | ||
| 10 | 1.19 (0.98–1.44) | 1.03 (0.85–1.26) | ||
| 11 | 1.19 (0.98–1.44) | 1.10 (0.90–1.35) | ||
| 12 | 1.24 (1.02–1.51) | 1.09 (0.89–1.33) | ||
| 13 | 1.19 (0.98–1.45) | 1.03 (0.84–1.26) | ||
Note: Adjusted for age (underlying time scale) stratified by birth cohort.
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio.
Change in BMI during school age and risk for AA in early adulthood
The risk of incident AA was higher for girls who gained weight from 7 to 13 years of age, i.e., those with an increase in their BMI z-score from interquartile range to the upper quartile (HR 1.28, 95% CI 1.10–1.50), than for girls with a stable weight (HR 1.06, 95% CI 0.90–1.24). The risk of AA until age 45 years was similar in boys whose body weight increased during these years (HR 1.09, 95% CI 0.90–1.34) and those who had a stable weight (HR 1.10, 95% CI 0.90–1.34).
Discussion
In this very large, population-based cohort study of 310,211 children, we found non-linear associations between childhood BMI and AA in early adulthood. Higher childhood BMI increases the risk for hospital admission for asthma in early adulthood for females, whereas for males low BMI values in childhood were associated with a higher risk of AA in early adulthood. Our study has therefore revealed important differences between males and females with regard to the association between childhood BMI and risk for more severe asthma in adulthood.
The very large cohort studied provided us with the statistical power to investigate the effects of childhood BMI separately by sex. We observed an association between high BMI in childhood and subsequent risk of more severe asthma in females, which is in line with previous, albeit much smaller cohorts with shorter observation periods, studies of children and adolescents.11,12,27–29 Furthermore, Ho et al30 reported from a cross-sectional study of approximately 4,000 individuals aged 13–15 years that overweight girls had higher odds ratio for physician-diagnosed asthma compared with normal weight participants. Among males we observed that low BMI increased the risk of hospital admissions for asthma. This contrasts with the findings of Ho et al in which they did not find any significant associations between BMI and asthma in boys at ages 13–15 years. These results are consistent with those from a Norwegian study of 2,300 adolescents that also did not detect associations.29 Therefore, in contrast with previously published studies, our study revealed a novel finding of an association between having a low BMI in childhood and risk for AA in early adulthood among males. Furthermore, Chen et al31 published a systematic review and meta-analysis addressing sex differences in childhood overweight/obesity in predicting the risk of incident asthma. They reported from their review of six studies fulfilling the inclusion criteria that obese boys had a higher risk of asthma compared with obese girls; however, they included only pediatric studies of individuals from 5 to 18 years of age that examined incident asthma in childhood. After puberty, incident asthma is more common in females than in males,32 which is in line with our findings (Figure 2), whereas in childhood the incidence of allergic asthma is much higher in boys than in girls.32
Additionally, we investigated how change in body size from the ages of 7 to 13 years is associated with AAs. For females, we found that girls with a normal BMI at the age of 7 years that increased to a high one at the age of 13 years had a significantly increased risk of AAs. We defined “high” as the 75th percentile or more as we chose to focus on the effects at the upper end of the distribution rather than on extremely heavy girls. In boys, we did not detect significant effects of increasing BMI from ages 7 to 13 years. Our findings are consistent with those from the Tucson Children’s Respiratory Study in which Castro-Rodríquez et al28 reported that girls who became overweight or obese between 6 and 11 years of age were more likely to develop asthma symptoms than those with stable BMI, but this relationship was not seen in boys.
It is well known that late-onset asthma is generally more severe and difficult to control than early-onset asthma, especially compared to allergic asthma.33 The low BMI-AA association observed in males, in contrast with our observations in females, in the present study might be due to differences in adipokine levels between males and females, including serum leptin, as an adipokine imbalance is associated with both pro-inflammatory status and asthma.34 Differences in leptin levels are possibly triggered by different performances of the leptin gene caused by differences in diet and exercise habits between males and females.3,35,36 On the other hand, apart from differences in levels of obesity-related inflammatory markers between males and females, the observed association between high childhood BMI and AA in adulthood observed in girls might be explained by higher estrogen levels in obese compared with normal weight females.3,37,38 However, there might also be a more simple explanation for the observed differences between males and females caused by differences in smoking rates, as the smoking rate is higher for females than for males in Denmark, especially in the younger age groups. It is well known that adiposity tracks from childhood into adulthood, so young adult females may take up or continue smoking because they believe in a weight reduction effect, and by that increase their risk of episodes of uncontrolled asthma leading to hospital admission, as tobacco exposure has substantial negative impact on asthma control.39 On the contrary, the risk of poor asthma control in thin young males may also be related to smoking as it may be seen as having positive impact on self-confidence.
Strengths and limitations
Our study takes advantage of being based on a very large cohort. The CSHR register includes virtually all schoolchildren in Copenhagen from 1930 to 1989, where mandatory health examinations were performed at all public and private schools, and thus selection bias due to socioeconomic status is eliminated from the present study. Furthermore, due to the unique identification number for each individual and the effectiveness of the DNPR, complete follow-up was available for eligible individuals. In the present study, we have only analyzed hospital admissions for asthma, and by that more severe asthma. It may, therefore, be argued that our findings do not necessarily apply to the asthmatic population in general. However, it has previously been shown in a Danish study that episodes of poor asthma control, including hospital admissions, are seen in as many as 25% of patients classified as having mild to moderate asthma, defined according to level of treatment;40 furthermore, in a country with free access to healthcare, many admissions for asthma are caused by low adherence with controller therapy,41 primarily inhaled corticosteroids, and lack of recognition of need for controller therapy by both patients and doctors. Furthermore, the remission rate of asthma is very low in individuals aged 20 years and older, in contrast to asthma earlier in life; so by using this cutoff point in the present study, we have most likely primarily identified individuals with life-long asthma.42,43
Perspectives
As the prevalence and severity of asthma in young adults are increasing, the associations that we identified with childhood body size are of great importance. Our findings are based upon children born during different phases of the obesity epidemic. In all years, the prevalence of childhood overweight and obesity is much lower than what is observed in contemporary populations where approximately 30% of children in economically developed countries are classified as overweight or obese. Noteworthy is our finding among females that the risk of AAs begins to increase at BMI levels that are far lower than international definitions of childhood overweight and obesity. Our results suggest that the current obesity epidemic will make a significant contribution to AAs in young females. By contrast, we found that low BMI values increased the risk of admissions in males. We speculate that low BMI may be a marker of illness or other disease processes, and it highlights the importance of screening for underweight individuals in this era where there is large focus on excess weight.
Conclusion
The present long-term study of 310,211 schoolchildren showed that the association between childhood BMI and incident AA in early adulthood is non-linear for both males and females; importantly, our study also revealed that having a high BMI in childhood is associated with a higher risk of incident hospital admission for asthma in early adulthood in females, whereas having a low BMI in childhood increases this risk in males. Our findings may, therefore, offer guidance for measures aiming at reducing the future burden of severe asthma.
Acknowledgments
The abstract of this paper was presented at the annual meeting of the European Respiratory Society as a conference talk with interim findings. The abstract was published in the European Respiratory Journal; 2016;(suppl 60):OA 3315.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159(21):2582–2588. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 2.Byberg KK, Eide GE, Forman MR, Júlíusson PB, Øymar K. Body mass index and physical activity in early childhood are associated with atopic sensitization, atopic dermatitis and asthma in later childhood. Clin Transl Allergy. 2016;6(1):33. doi: 10.1186/s13601-016-0124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali Z, Ulrik CS. Obesity and asthma: a coincidence or a causal relationship? A systematic review. Respir Med. 2013;107(9):1287–1300. doi: 10.1016/j.rmed.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Silveira DH, Zhang L, Prietsch SO, Vecchi AA, Susin LR. Nutritional status, adiposity and asthma severity and control in children. J Paediatr Child Health. 2015;51(10):1001–1006. doi: 10.1111/jpc.12882. [DOI] [PubMed] [Google Scholar]
- 5.Juel CT, Ulrik CS. Obesity and asthma: impact on severity, asthma control, and response to therapy. Respir Care. 2013;58(5):867–873. doi: 10.4187/respcare.02202. [DOI] [PubMed] [Google Scholar]
- 6.Dixon AE, Holguin F, Sood A, et al. American Thoracic Society Ad Hoc Subcommittee on Obesity and Lung Disease An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7(5):325–335. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 7.Camargo CA, Jr, Boulet LP, Sutherland ER, et al. Body mass index and response to asthma therapy: fluticasone propionate/salmeterol versus montelukast. J Asthma. 2010;47(1):76–82. doi: 10.3109/02770900903338494. [DOI] [PubMed] [Google Scholar]
- 8.Ziyab AH, Karmaus W, Kurukulaaratchy RJ, Zhang H, Arshad SH. Developmental trajectories of Body Mass Index from infancy to 18 years of age: prenatal determinants and health consequences. J Epidemiol Community Health. 2014;68(10):934–941. doi: 10.1136/jech-2014-203808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egan KB, Ettinger AS, DeWan AT, Holford TR, Holmen TL, Bracken MB. Longitudinal associations between asthma and general and abdominal weight status among Norwegian adolescents and young adults: the HUNT study. Pediatr Obes. 2015;10(5):345–352. doi: 10.1111/ijpo.271. [DOI] [PubMed] [Google Scholar]
- 10.Maltz L, Matz EL, Gordish-Dressman H, et al. Sex differences in the association between neck circumference and asthma. Pediatr Pulmonol. 2016;51(9):893–900. doi: 10.1002/ppul.23381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benedetti FJ, Bosa VL, Giesta JM, Fischer GB. Anthropometric indicators of general and central obesity in the prediction of asthma in adolescents; central obesity in asthma. Nutr Hosp. 2015;32(6):2540–2548. doi: 10.3305/nh.2015.32.6.9851. [DOI] [PubMed] [Google Scholar]
- 12.Lu KD, Billimek J, Bar-Yoseph R, Radom-Aizik S, Cooper DM, Anton-Culver H. Sex differences in the relationship between fitness and obesity on risk for asthma in adolescents. J Pediatr. 2016;176:36–42. doi: 10.1016/j.jpeds.2016.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YC, Huang YL, Ho WC, Wang YC, Yu YH. Gender differences in effects of obesity and asthma on adolescent lung function: results from a population-based study. J Asthma. 2017;54(3):279–285. doi: 10.1080/02770903.2016.1212367. [DOI] [PubMed] [Google Scholar]
- 14.Schatz M, Zeiger RS, Zhang F, Chen W, Yang SJ, Camargo CA., Jr Overweight/obesity and risk of seasonal asthma exacerbations. J Allergy Clin Immunol Pract. 2013;1(6):618–622. doi: 10.1016/j.jaip.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Schatz M, Zeiger RS, Yang SJ, et al. Prospective study on the relationship of obesity to asthma impairment and risk. J Allergy Clin Immunol Pract. 2015;3(4):560–565.e1. doi: 10.1016/j.jaip.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Tay TR, Radhakrishna N, Hore-Lacy F, et al. Comorbidities in difficult asthma are independent risk factors for frequent exacerbations, poor control and diminished quality of life. Respirology. 2016;21(8):1384–1390. doi: 10.1111/resp.12838. [DOI] [PubMed] [Google Scholar]
- 17.Gibson PG, McDonald VM, Marks GB. Asthma in older adults. Lancet. 2010;376(9743):803–813. doi: 10.1016/S0140-6736(10)61087-2. [DOI] [PubMed] [Google Scholar]
- 18.Porsbjerg C, Lange P, Ulrik CS. Lung function impairment increases with age of diagnosis in adult onset asthma. Respir Med. 2015;109(7):821–827. doi: 10.1016/j.rmed.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Ulrik CS. Outcome of asthma: longitudinal changes in lung function. Eur Respir J. 1999;13(4):904–918. doi: 10.1034/j.1399-3003.1999.13d35.x. [DOI] [PubMed] [Google Scholar]
- 20.Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(GINA) GIfA . Asthma, COPD and the asthma-COPD overlap syndrome (ACOS) 2014. [Accessed April 22, 2018]. [Google Scholar]
- 22.Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376(3):254–266. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 23.Baker JL, Olsen LW, Andersen I, Pearson S, Hansen B, Sørensen T. Cohort profile: the Copenhagen School Health Records Register. Int J Epidemiol. 2009;38(3):656–662. doi: 10.1093/ije/dyn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11(10):1305–1319. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(Suppl 7):22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 26.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(Suppl 7):30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 27.Gold DR, Damokosh AI, Dockery DW, Berkey CS. Body-mass index as a predictor of incident asthma in a prospective cohort of children. Pediatr Pulmonol. 2003;36(6):514–521. doi: 10.1002/ppul.10376. [DOI] [PubMed] [Google Scholar]
- 28.Castro-Rodríguez JA, Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Increased incidence of asthmalike symptoms in girls who become overweight or obese during the school years. Am J Respir Crit Care Med. 2001;163(6):1344–1349. doi: 10.1164/ajrccm.163.6.2006140. [DOI] [PubMed] [Google Scholar]
- 29.Tollefsen E, Langhammer A, Romundstad P, Bjermer L, Johnsen R, Holmen TL. Female gender is associated with higher incidence and more stable respiratory symptoms during adolescence. Respir Med. 2007;101(5):896–902. doi: 10.1016/j.rmed.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 30.Ho WC, Lin YS, Caffrey JL, et al. Higher body mass index may induce asthma among adolescents with pre-asthmatic symptoms: a prospective cohort study. BMC Public Health. 2011;11:542. doi: 10.1186/1471-2458-11-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen YC, Dong GH, Lin KC, Lee YL. Gender difference of childhood overweight and obesity in predicting the risk of incident asthma: a systematic review and meta-analysis. Obes Rev. 2013;14(3):222–231. doi: 10.1111/j.1467-789X.2012.01055.x. [DOI] [PubMed] [Google Scholar]
- 32.Leynaert B, Sunyer J, Garcia-Esteban R, et al. Gender differences in prevalence, diagnosis and incidence of allergic and non-allergic asthma: a population-based cohort. Thorax. 2012;67(7):625–631. doi: 10.1136/thoraxjnl-2011-201249. [DOI] [PubMed] [Google Scholar]
- 33.Prevention GSfAMa Global Initiative for Asthma (GINA) 2015. [Accessed April 22, 2018]. Available from: www.ginasthma.org.
- 34.Muc M, Mota-Pinto A, Padez C. Association between obesity and asthma – epidemiology, pathophysiology and clinical profile. Nutr Res Rev. 2016;29(2):194–201. doi: 10.1017/S0954422416000111. [DOI] [PubMed] [Google Scholar]
- 35.Guler N, Kirerleri E, Ones U, Tamay Z, Salmayenli N, Darendeliler F. Leptin: does it have any role in childhood asthma? J Allergy Clin Immunol. 2004;114(2):254–259. doi: 10.1016/j.jaci.2004.03.053. [DOI] [PubMed] [Google Scholar]
- 36.Thomsen SF. Exploring the origins of asthma: lessons from twin studies. Eur Clin Respir J. 2014;1(Suppl 1):1–42. doi: 10.3402/ecrj.v1.25535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carey MA, Card JW, Voltz JW, et al. It’s all about sex: gender, lung development and lung disease. Trends Endocrinol Metab. 2007;18(8):308–313. doi: 10.1016/j.tem.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carey MA, Card JW, Voltz JW, Germolec DR, Korach KS, Zeldin DC. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Physiol Lung Cell Mol Physiol. 2007;293(2):L272–L278. doi: 10.1152/ajplung.00174.2007. [DOI] [PubMed] [Google Scholar]
- 39.Grarup PA, Janner JH, Ulrik CS. Passive smoking is associated with poor asthma control during pregnancy: a prospective study of 500 pregnancies. PLoS One. 2014;9(11):e112435. doi: 10.1371/journal.pone.0112435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Bülow A, Kriegbaum M, Backer V, Porsbjerg C. The prevalence of severe asthma and low asthma control among Danish adults. J Allergy Clin Immunol Pract. 2014;2(6):759–767. doi: 10.1016/j.jaip.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Ulrik CS, Backer V, Søes-Petersen U, Lange P, Harving H, Plaschke PP. The patient’s perspective: adherence or non-adherence to asthma controller therapy? J Asthma. 2006;43(9):701–704. doi: 10.1080/02770900600925569. [DOI] [PubMed] [Google Scholar]
- 42.De Marco R, Locatelli F, Cerveri I, Bugiani M, Marinoni A, Giammanco G, Italian Study on Asthma in Young Adults study group Incidence and remission of asthma: a retrospective study on the natural history of asthma in Italy. J Allergy Clin Immunol. 2002;110(2):228–235. doi: 10.1067/mai.2002.125600. [DOI] [PubMed] [Google Scholar]
- 43.Pesce G, Locatelli F, Cerveri I, et al. Seventy years of asthma in Italy: age, period and cohort effects on incidence and remission of self-reported asthma from 1940 to 2010. PLoS One. 2015;10(10):e0138570. doi: 10.1371/journal.pone.0138570. [DOI] [PMC free article] [PubMed] [Google Scholar]