Abstract
Muscle morphogenesis is tightly coupled with that of motor neurons (MNs). Both MNs and muscle progenitors simultaneously explore the surrounding tissues while exchanging reciprocal signals to tune their behaviors. We previously identified the Fat1 cadherin as a regulator of muscle morphogenesis and showed that it is required in the myogenic lineage to control the polarity of progenitor migration. To expand our knowledge on how Fat1 exerts its tissue-morphogenesis regulator activity, we dissected its functions by tissue-specific genetic ablation. An emblematic example of muscle under such morphogenetic control is the cutaneous maximus (CM) muscle, a flat subcutaneous muscle in which progenitor migration is physically separated from the process of myogenic differentiation but tightly associated with elongating axons of its partner MNs. Here, we show that constitutive Fat1 disruption interferes with expansion and differentiation of the CM muscle, with its motor innervation and with specification of its associated MN pool. Fat1 is expressed in muscle progenitors, in associated mesenchymal cells, and in MN subsets, including the CM-innervating pool. We identify mesenchyme-derived connective tissue (CT) as a cell type in which Fat1 activity is required for the non–cell-autonomous control of CM muscle progenitor spreading, myogenic differentiation, motor innervation, and for motor pool specification. In parallel, Fat1 is required in MNs to promote their axonal growth and specification, indirectly influencing muscle progenitor progression. These results illustrate how Fat1 coordinates the coupling of muscular and neuronal morphogenesis by playing distinct but complementary actions in several cell types.
Author summary
Fat cadherins are evolutionarily conserved cell adhesion molecules, which play key roles in modulating tissue morphogenesis through the control of collective cell behavior and polarity. We previously identified the mouse Fat1 gene as a regulator of muscle morphogenesis and reported a role for this gene in muscle progenitors to modulate their migration polarity. Recent findings have revealed a potential link between muscle patterning and non-connective tissues. Here we have analyzed the mechanisms that coordinate the behavior of two cell types, mesenchymal cells and brachial spinal motor neurons, during mouse neuromuscular morphogenesis. We show that Fat1 disruption in connective tissue robustly alters muscle morphogenesis of the cutaneous maximus muscle, affecting not only migration of progenitors and expansion of myofibers but also subsequently impairing axon growth and specification of cognate motor neurons. We observe that Fat1 acts in motor neurons in parallel to modulate axonal growth and neuronal specification, modestly influencing muscle morphology. Together, these results show that Fat1 coordinates the coupling between muscle and neuronal development by playing complementary functions in mesenchyme, muscles, and motor neurons. These findings could guide research on muscle pathologies associated with FAT1 alterations in humans.
Introduction
Neuromuscular morphogenesis involves complex tissue interactions simultaneously governing the generation of skeletal muscles and the production of the somatic motor neurons (MNs) that innervate them. Both processes independently rely on the execution of a generic regulatory program sequentially leading to cell fate determination, differentiation, and functional diversification [1–3]. These regulatory events are coupled with dynamic morphogenetic events leading to the definition of multiple muscle shapes, and the simultaneous topographical wiring of motor axonal projections. During muscle morphogenesis, myogenic progenitors migrate collectively from their origin in either somites or cephalic mesoderm to their final position in the limb, trunk, or face [4]. Trunk and limb connective tissues (CTs), which derive from lateral plate mesoderm, provide instructive signals for incoming somite-derived myogenic cells [5]. Reciprocal signals exchanged by muscle progenitors and the mesenchymal environment pattern muscle shapes by allowing the definition of fiber orientation, muscle attachment sites, and tendon development [5, 6]. Muscle progenitors subsequently engage in a complex regulatory process, through which they give rise to differentiating cells called myocytes, in charge of producing the contractile apparatus [2, 3, 7]. Myocytes then fuse with each other to form multinucleated muscle fibers. The process of muscle growth is determined by a tightly regulated balance between progenitor expansion and production of myocytes and differentiating muscle fibers [2, 3]. In parallel with muscle morphogenesis, MNs emit axons, which grow in peripheral tissues, selecting a trajectory by responding to multiple guidance cues, allowing them to find their target muscles, within which they ultimately establish a selective pattern of intramuscular arborization [8]. During these processes, axons of MNs and migratory myogenic progenitors follow converging trajectories, along which they simultaneously probe the environment and respond to instructive cues, as evidenced by classical embryological studies [5, 9–11]. Multiple signals emitted by peripheral tissues to instruct MNs’ specification and axonal pathfinding have been identified [12]. Likewise, some recent discoveries have started shedding light on how non-myogenic CTs exert their influence on muscle patterning [13]. In spite of such advances, what controls the coordinated behavior of the two cell types to orchestrate neuromuscular morphogenesis has not been studied.
In the present study, we have examined the possibility that the Fat1 cadherin, a planar cell polarity (PCP) molecule involved in tissue morphogenesis, could contribute to coordinate muscular and neuronal morphogenesis. We recently identified Fat1 as a new player in muscle morphogenesis that influences the shape of subsets of face and shoulder muscles, in part by polarizing the direction of collectively migrating myoblasts [14]. Fat1 belongs to the family of Fat-like atypical cadherins [15]. Together with their partner cadherins Dachsous, Fat-like cadherins are involved in regulating coordinated cell behaviors, such as planar cell polarity (PCP) in epithelia [15–17], collective/polarized cell migration [18–20], and oriented cell divisions [21, 22]. Through these actions, Fat/Dachsous signaling modulates cell orientation, junctional tension [23, 24], and microtubule dynamics [25], thereby influencing the mechanical coupling between cell behavior and tissue shapes [17]. Aside from their canonical role in regulating the PCP pathway [16], Fat-like cadherins also control tissue growth via the Hippo pathway [26, 27] and were recently found to contribute to mitochondria function and metabolic state by interacting with the electron transport chain [28, 29]. In vertebrates, the most studied Fat homologue, Fat4, plays multiple functions in development to coordinate kidney [22, 30–32], skeletal [21], heart [33], or neural morphogenesis [19, 34, 35]. The other family member, Fat1, is known for playing complementary functions during kidney [36, 37], muscle [14], and neural [38] morphogenesis.
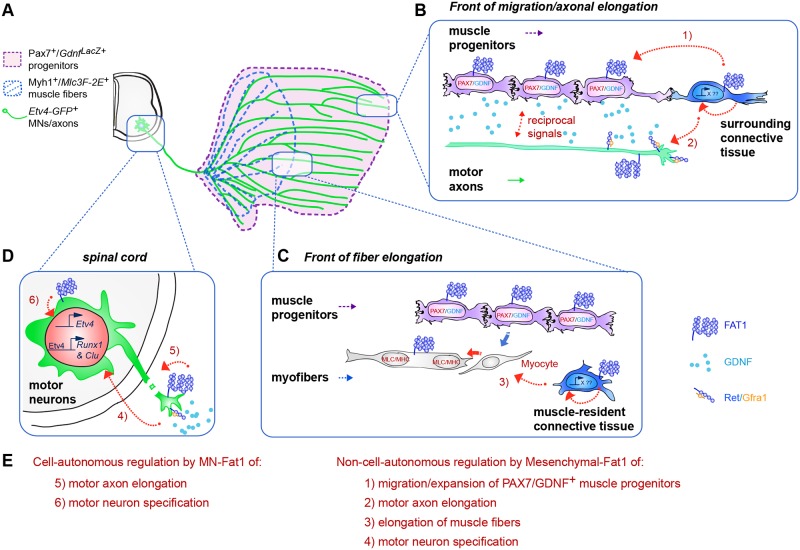
Here, to explore the mechanisms underlying the coupling of neural and muscular morphogenesis and to assess how Fat1 contributes to this process, we focused on a large flat subcutaneous muscle, the cutaneous maximus (CM), which expands under the skin by planar polarized myoblast migration. This muscle is linked to its cognate spinal MN pool through the selective production by the CM muscle of glial cell line-derived neurotrophic factor (GDNF), a secreted growth factor required to control specification of the corresponding MNs [39]. Unlike limb-innervating MNs, which are born with an intrinsic molecular program specifying their anatomical characteristics [9, 11], CM-innervating MNs are incompletely specified at the time they first send their axons and are dependent on extrinsic signals from peripheral tissues [39–41]. GDNF, produced first by the plexus mesenchyme and subsequently by the CM and latissimus dorsi (LD) muscles, is perceived by axons of a competent population of MNs when they reach the plexus and as they continue growing along the expanding muscle [39, 42]. GDNF acts through the Ret tyrosine kinase receptor in a complex with a GPI-anchored co-receptor Gfra1 [43–45] by inducing expression of the transcription factor Etv4 (Ets variant gene 4, also known as Pea3) in the MN pools innervating the CM and LD muscles [39], in synergy with another mesenchyme-derived factor, hepatocyte growth factor (HGF) [46, 47]. Etv4 in turn influences MN cell body positioning, dendrite patterning, intramuscular axonal arborization, and monosynaptic reflex circuit formation [40, 41]. Whereas MN cell body positioning is thought to involve the regulation of the Cadherin code by Etv4 [41, 48], patterning of sensory-motor connections is accounted for by the Etv4-regulated Sema3E, acting as repellent cue for sensory neurons expressing its receptor PlexinD1 [49, 50]. GDNF can also directly influence axon pathfinding, as demonstrated in the context of dorsal motor axon guidance [51–54] or of midline-crossing by commissural axons [55], and is subsequently required for survival of subsets of MNs [56, 57].
We found that inactivation of the Fat1 gene has a profound impact on the assembly of the CM neuromuscular circuit, affecting not only the rate of subcutaneous expansion of the CM muscle by progenitor migration and the subsequent rate of differentiation but also the acquisition of identity and projection patterns of their cognate MNs. Intriguingly, in addition to its function in myogenic cells [14], Fat1 is also expressed in muscle-associated mesenchymal cells and in the MN subset corresponding to the Fat1-dependent CM muscle. Through a series of genetic experiments in mice, we have selectively ablated Fat1 functions in the distinct tissue types in which it is expressed along this neuromuscular circuit and assessed the impact on muscular and neuronal morphogenesis. We uncovered two novel Fat1 functions in the mesenchymal lineage and in MNs, which synergize to coordinate the development of the CM neuromuscular circuit. Fat1 ablation in the mesenchymal lineage causes severe non–cell-autonomous alterations of CM morphogenesis, disrupting expansion of the GDNF-expressing CM progenitors and the subsequent processes of muscle fiber elongation, CM motor innervation, and the acquisition of MN pool identity. This identifies the mesenchymal lineage as a source of Fat1-dependent muscle- and MN-patterning cues. The neural consequences of mesenchyme-specific Fat1 ablation partially mimic the effects of Gdnf or Etv4 mutants and can be aggravated by further reducing Gdnf levels genetically. In parallel, we find that MN-Fat1 is required cell-autonomously for motor axon growth and MN specification. Unexpectedly, MN-specific Fat1 ablation also influences myogenic progenitor spreading in a non–cell-autonomous manner, demonstrating a reverse influence of MNs on muscle morphogenesis. Collectively, these data show that Fat1 exerts complementary functions in several tissue types along the circuit, each of which contributes to neuromuscular morphogenetic coupling through distinct mechanisms, coordinating the adaptation of MN phenotype to muscle shape.
Results
Loss of Fat1 alters posterior expansion and differentiation of the CM muscle
In this study, we have used two phenotypically equivalent constitutive knockout alleles of Fat1 (summarized in S1 Table): the first allele (Fat1- allele, also known as Fat1ΔTM) derives from the conditional Fat1Flox allele by Cre recombinase (CRE)-mediated excision of the floxed exons encoding the transmembrane domain, thus abrogating the ability of the Fat1 protein to transduce signals [14]. The second allele (Fat1LacZ allele) is a genetrap allele of Fat1, in which the inserted transgene results in expression of a Fat1-β-galactosidase chimeric protein, in the endogenous domain of Fat1 expression [14]. Both alleles cause comparable phenotypes [14].
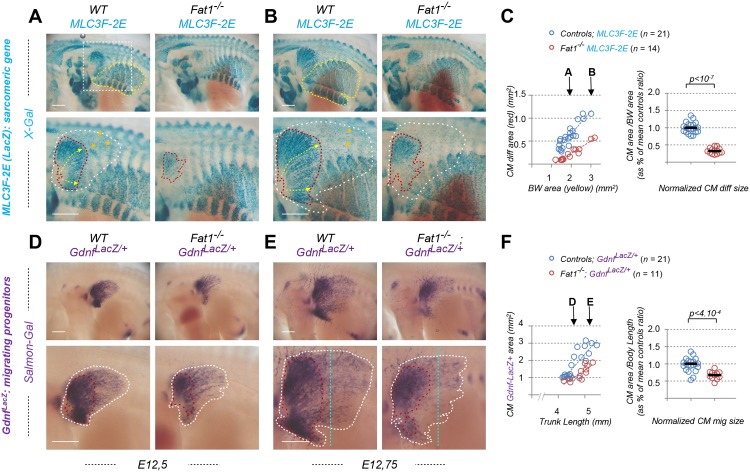
We focused on one of the muscles affected by constitutive loss of Fat1 functions, the CM muscle (Fig 1A and 1B), a flat muscle emerging from the forelimb plexus (also called brachial plexus). Completing our previous analysis [14], we first followed the establishment of the CM muscle and its evolution during development by using a transgenic line (the Mlc3f-nLacZ-2E line, later referred to as MLC3F-2E, S1 Table) expressing a nuclear LacZ reporter in differentiating muscle cells, thus behaving as a reporter of sarcomeric gene expression [58, 59]. This line was combined to the constitutive Fat1- allele, and wild-type and mutant embryos carrying the transgene were stained with X-gal. We previously reported that this approach reveals in Fat1-/- embryos (1) myocytes dispersed in the forelimb region and (2) a supernumerary muscle in ectopic position in the upper part of the forelimb (see Fig 6 in ref [14], S1A and S1B Fig). Both phenotypes can be quantified (S1B Fig) and can also be observed on histological sections using additional markers of muscle development, such as Pax7, to label myogenic progenitors, and the sarcomeric protein myosin heavy chain 1 (Myh1), to visualize muscle fibers (S1C and S1D Fig). The evolution of the CM muscle follows a very specific growth pattern. Muscle fibers can be viewed as “chains” of MLC3F-2E positive nuclei. In the CM, these fibers appear to originate from the brachial plexus, just posterior to the forelimb bud, and to extend posteriorly from this point. Individual fibers spread in a radial manner under the skin to form a fan-shaped structure, ranging from dorsally directed fibers to ventrally directed fibers, the median direction being approximately horizontal. As development proceeds, the length of such fibers (yellow arrows, Fig 1A and 1B) increases posteriorly, and the overall area covered by β-galactosidase-positive fibers (red dotted area, Fig 1A and 1B) expands. The rate of CM expansion can be measured by following the area containing differentiated fibers, plotted relative to the area of body wall (BW) muscles (yellow dotted area on upper pictures in Fig 1A and 1B), used as a proxy for the embryo stage, as these muscles are not affected by the mutation. In wild-type MLC3F-2E+ embryos, the CM area follows a positive linear evolution, strongly correlated with expansion of the BW muscle area (Fig 1C, left plot). In Fat1-/-; MLC3F-2E+ embryos, CM expansion appears severely delayed, although not abolished, with a growth rate reduced by more than 2-fold compared to control embryos (Fig 1C, left plot). As a result, at comparable stages, the differentiated CM area is systematically smaller in absence of functional Fat1. Given the highly dynamic nature of CM expansion over just one day, in order to pool data from all embryos examined, the ratio between the CM and BW areas was calculated and normalized to the median ratio of control littermate embryos, corresponding to 100% (Fig 1C, right plot). Overall, loss of Fat1 functions causes the area of differentiated CM to be reduced to a median value of about 32% compared to control embryos. Interestingly, observation of older embryos (Fig 1B) reveals that fiber length and LacZ-positive nuclei density appear more drastically reduced in the ventral part of the CM than in the dorsal part. We previously documented that at later stages, in this ventral area, occurrence of misoriented fibers crossing each other can frequently be observed [14].
Fig 1. Fat1 knockout alters expansion of the subcutaneous muscle, CM.
Whole-mount β-galactosidase staining was performed using X-gal as substrate on embryos carrying the MLC3F-2E transgene (S1 Table) (A, B) or using Salmon-Gal as substrate on embryos carrying the GdnfLacZ/+ allele (S1 Table) (D, E). In each case, two successive stages are shown, E12.5 (A,D) and E12.75 (B, E), respectively with Fat1+/+ (left) and Fat1-/- (right) embryos, with the lower panels showing a higher magnification of the flank in which the CM muscle spreads. On upper panels in (A, B), the yellow dotted line highlights the BW muscles, the area of which is being measured. The white square highlights the area shown in lower panels. Lower panels: the red dotted line highlights the area covered by differentiating MLC3F-2E+ muscle fibers constituting the CM muscle in (A, B), also matching an area of higher GdnfLacZ intensity in (D, E); the white dotted lines highlight the area corresponding to the full shape of the GdnfLacZ+ area in (D, E), in which a low density of blue (MLC3F-2E+) nuclei can also be observed in (A, B). (C) Quantifications of the relative expansion of the MLC3F-2E+ CM differentiated area. Left plot: for each embryo side, the area of differentiated CM is plotted relative to the BW area. Arrows represent the stages shown in (A) and (B), respectively. Right plot: for each embryo, the CM area/BW area was normalized to the median ratio of control embryos. Blue dots: Fat1+/+; MLC3F-2E (n = 21); red dots: Fat1-/-; MLC3F-2E (n = 14). Underlying data are provided in S1 Data. (F) Quantifications of the relative expansion of the GdnfLacZ+ area. Left plot: for each embryo side, the GdnfLacZ+ area is plotted relative to the length of the trunk (measured between two fixed points). Arrows represent the stages shown in (D) and (E), respectively. Right plot: for each embryo, the CM area/trunk length was normalized to the median ratio of control embryos. Blue dots: Fat1+/+; GdnfLacZ/+ (n = 21); red dots: Fat1-/-; GdnfLacZ/+ (n = 11). Underlying data are provided in S1 Data. Scale bars: 500 μm. BW, body wall; CM, cutaneous maximus; Gdnf, glial cell line-derived neurotrophic factor; MLC3F-2E, Mlc3f-nLacZ-2E line (S1 Table); Salmon-Gal, 6-Chloro-3-indolyl-β-D-galactopyranoside, substrate for β-galactosidase activity; WT, wild-type; X-gal, 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside, substrate for β-galactosidase activity.
Fat1 controls spreading of the GDNF-expressing CM muscle
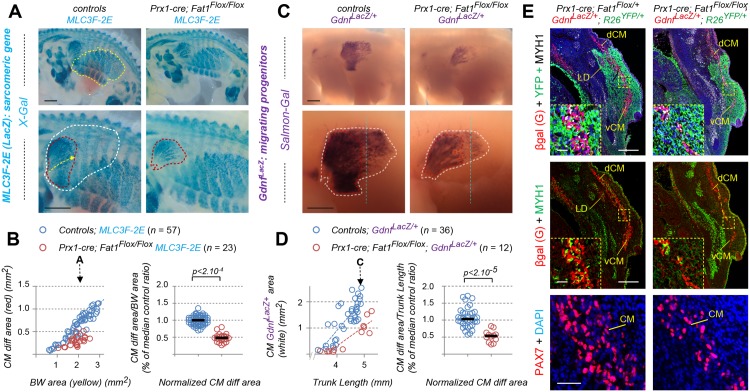
We next took advantage of the fact that the CM is a selective source of GDNF, thus offering an excellent marker to follow development of this muscle [39, 42]. Alterations of the CM muscle shape resulting from disrupted Fat1 functions can be visualized by following β-galactosidase activity in embryos carrying a GdnfLacZ allele (Fig 1D and 1E, S1 Table). We therefore produced embryos carrying one copy of the GdnfLacZ allele in wild-type or Fat1-/- contexts and performed staining with Salmon-Gal, a substrate more sensitive than X-gal, adapted to the low level of Gdnf expression (see Materials and methods). GdnfLacZ expression can be detected as early as E11.5, prior to the emergence of the CM, at the level of the plexus mesenchyme (at fore- and hind limb levels), where it serves to guide motor axons and instruct them of their identity [39, 42, 54]. Gdnf expression is then detected in the CM and in the underlying LD muscles as they emerge (around E12.0) from the brachial plexus [14, 39]. The LD muscle is not visible on our pictures because it is hidden by the CM, but it can be recognized on embryo sections. From that stage onward, these muscles progress by migrating under the skin in a posterior direction, radiating from their point of origin. As development proceeds, the area occupied by CM progenitors expands (and can be viewed through the skin by transparency in whole embryos). We focused on the time window when most of the subcutaneous progression is occurring (E12.0–E12.75). To analyze the rate of expansion, the GdnfLacZ-positive area (white dotted area in Fig 1D and 1E) was plotted relative to the trunk length, which is used as a value that increases regularly as the embryo grows, thus reflecting the stage of development. At any stage examined, the area covered by GdnfLacZ-positive cells is smaller than in control embryos (Fig 1D–1F). The rate of CM expansion is significantly reduced in Fat1-/- embryos, with a median area reduced to 66% of controls. As seen with MLC3F-2E, this effect also appears more pronounced in the ventral part of the CM. Furthermore, staining intensity in the GdnfLacZ-positive zone behind the progression front appears reduced in Fat1 mutants (compare intensity along the vertical blue dotted line in Fig 1E). Rather than reflecting a reduction in the level of GdnfLacZ expression per progenitors, this effect appears to result from a reduction in the density of LacZ-expressing cells in this front of migration.
This effect on Gdnf expression can also be observed in the context of the other null allele of Fat1 (Fat1LacZ) by following Gdnf expression by in situ hybridization on embryo sections (S2A Fig). In this context, reduced thickness of the Gdnf-expressing cell layer is observed in posterior CM sections of Fat1LacZ/LacZ embryos, reflecting a reduced number of Gdnf-expressing cells. The reduced density in muscle progenitors in constituting the CM is also visualized by following markers of myogenic cells or subsets of migratory muscle populations such as MyoD, Six1, and Lbx1 on sections at posterior levels (S2B Fig). A reduced Gdnf expression level was also detected at the level of the plexus mesenchyme in Fat1LacZ/LacZ embryos (S2A Fig). Overall, we confirm using two independent alleles that Fat1 is required for the development of the CM muscle.
Fat1 ablation disrupts innervation of the CM muscle
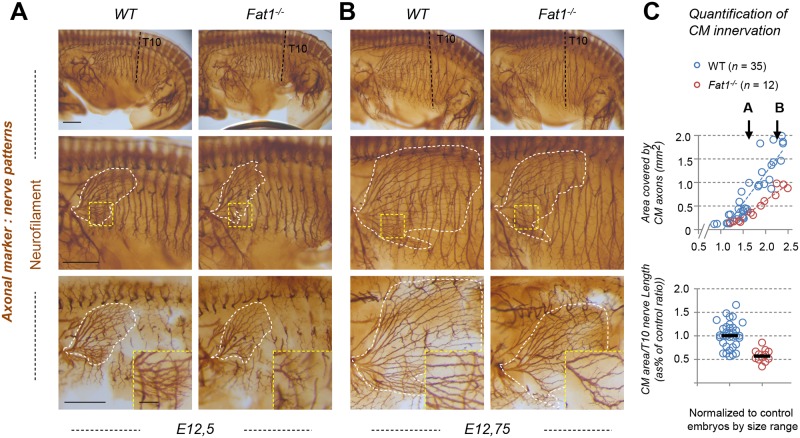
The CM muscle represents an excellent example in which to study the coupling between muscle morphogenesis and neuronal specification. Given the strong effect of Fat1 loss-of-function on expansion and differentiation of the CM muscle, we next wondered if changes could also be observed in the pattern of innervation. E12.5 control and Fat1 mutant embryos were therefore stained by whole-mount immunohistochemistry (IHC) with an anti-neurofilament antibody and visualized after clearing in benzyl-benzoate/benzyl-alcohol (BB-BA) (Fig 2). Embryos were cut in half and flat-mounted for imaging. Motor axons innervating the CM muscle can be recognized on the embryo flank by their horizontal progression, as they intersect the vertically oriented thoracic nerves. After initial imaging of flat-mounted embryos halves (Fig 2A and 2B, top and middle images), all inner structures, including thoracic nerves, were manually removed to better distinguish CM axons (Fig 2A and 2B; bottom pictures). CM motor axons cover an area with a shape very similar to that occupied by GdnfLacZ-expressing cells. This shape was affected by Fat1 loss-of-function in a similar way as was the GdnfLacZ-expressing region. At comparable stages, the area covered by CM axons was smaller in Fat1-/- embryos, which exhibited shorter CM axons than wild types (Fig 2A and 2B). As seen with muscle markers, the dorsal part of the muscle appears less affected. In the ventral muscle, mutant motor axons were shorter, with an apparent lower density of axon bundles than controls (inserts in Fig 2A, bottom images). Throughout the period considered, the subcutaneous expansion of CM-innervating axons is fast and dynamic. It is therefore best represented by showing two consecutive stages, and quantified by measuring the area covered by CM axons, plotted relative to a reference structure (such as the length of the 10th thoracic nerve [T10], black dotted line) used as an indicator of developmental age (Fig 2C). In wild-type embryos, the area covered by CM axons expanded steadily, covering the embryo flank in little more than half a day. In contrast, the rate of progression of CM innervation is reduced by approximately 2-fold in Fat1-/- embryos compared to controls. Similar observations can be made with the other null allele (Fat1LacZ, S3 Fig). In both mutants, in contrast to the abnormal behavior of CM-innervating axons, most other limb-innervating nerves are preserved and appear unaffected in Fat1-/- embryos (Fig 2). There was no obvious ectopic nerve corresponding to the supernumerary muscle in the scapulohumeral region. Thus, loss of Fat1 functions appears to predominantly affect the development of axons innervating the CM, matching the pronounced effect on muscle spreading and differentiation.
Fig 2. Fat1 knockout alters motor innervation of the CM muscle.
(A, B) The nerve pattern was analyzed by IHC with antibodies against neurofilament in E12.5 (A) to E12.75 (B) wild-type and Fat1-/- embryos. Embryos were cut in half, cleared in BB-BA, and flat-mounted. Upper panels are low-magnification images of the left flank, showing the whole trunk. Lower panels show high-magnification views of the area containing the CM muscle. The area covered by CM-innervated axons is highlighted in white (middle panels). Axons of vertically oriented thoracic spinal nerves have been manually removed by dissection in the lower panels to improve visibility of CM axons. Inserts in the lower panels represent higher magnification of the area in the yellow squares. (C) Quantifications of the relative expansion of the area covered by CM-innervating axons. Upper plot: for each embryo side, the area covered by CM-innervating axons is plotted relative to the length of a thoracic nerve (T10, from dorsal root origin to ventral tip). Arrows point the stages of representative examples shown in (A) and (B). Bottom plot: for each embryo, the CM-innervated area/T10 length was normalized to the median ratio of control embryos, by size range. Blue dots: Fat1+/+ (n = 35, same sample set as in controls of S3 Fig); red dots: Fat1-/- (n = 12). Underlying data are provided in S1 Data. Scale bars: 500 μm (large images); 100 μm (inserts in lower panels). BB-BA, benzyl-benzoate/benzyl-alcohol mix; CM, cutaneous maximus; IHC, immunohistochemistry; T10, 10th thoracic nerve; WT, wild-type.
Topographical organization of the CM muscle
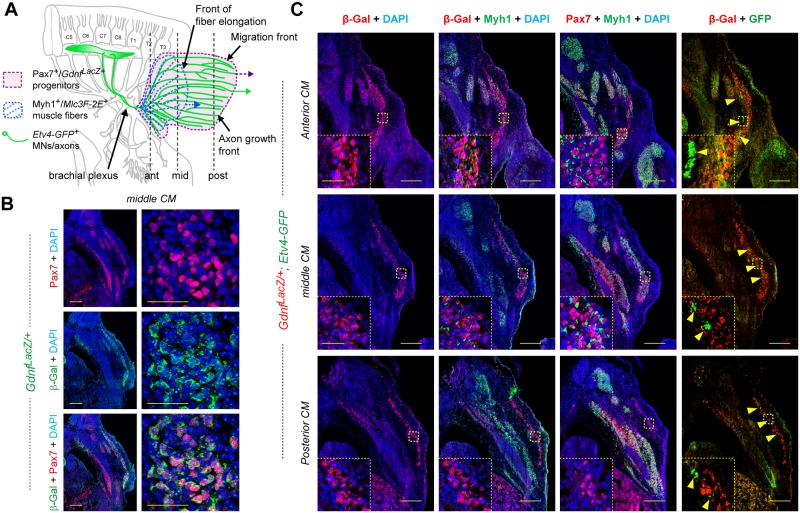
A number of important features emerge when carefully comparing MLC3F-2E+ embryos and GdnfLacZ embryos during the developmental progression of the CM muscle in wild-type embryos. For clarity in the following description, it is necessary to recall a few notions of orientation. Because the muscle progresses from anterior to posterior (see Figs 1 and 3A), the front of muscle progression is located posteriorly, whereas the rear corresponds to the point of origin of the muscle, at the brachial plexus, located anteriorly. When comparing MLC3F-2E+ embryos and GdnfLacZ embryos at similar stages (compare Fig 1A with 1D and 1B with 1E), the area occupied by GdnfLacZ cells (marked with a white dotted line) appears larger than the area occupied by MLC3F-2E+ muscle fibers (marked with the red line). This MLC3F-2E+ CM differentiation has a specific fan-like shape, in which multinucleated muscle fibers extend from a narrow zone at the anterior origin to a posterior side distributed along a wider dorsoventral extent (Fig 1A and 1B). Because progression occurs at this posterior front (called front of fiber elongation), this indicates that multinucleated fibers elongate by adding new nuclei at the posterior side. This posterior front of fiber elongation appears to be situated approximately in the middle of the muscle, at a regular distance from the even more posterior front of progression of the GdnfLacZ+ area, likely composed of migrating muscle progenitors (Fig 3A). Nevertheless, when carefully observing X-gal–stained MLC3F-2E+ embryos, one can also distinguish, beyond the front of multinucleated fiber elongation, some LacZ+ nuclei expressing β-galactosidase at lower levels, within an area matching in size and shape the GdnfLacZ+ area (white dotted line, Fig 1). This suggests that the posterior half of the CM muscle (between the two fronts) is essentially occupied by GdnfLacZ-positive migrating myogenic progenitors and a few scattered mononucleated MLC3F-2E+ myocytes. In contrast, the anterior half of the muscle contains elongating MLC3F-2E+ fibers and GdnfLacZ-positive cells. This was confirmed by analyzing serial sections of GdnfLacZ/+ embryos by IHC following β-galactosidase, the sarcomeric protein Myh1 in muscle fibers, and the muscle progenitor marker Pax7 (Fig 3B and 3C). Throughout the extent of the CM muscle (not considering the plexus mesenchyme region), we found that GdnfLacZ was co-expressed with Pax7, confirming that it labels progenitors (Fig 3B), whereas it is not co-expressed with the sarcomeric protein Myh1 (Fig 3C inserts). Thus, GdnfLacZ can be used as a specific marker of CM (and LD) progenitors. When following the CM muscle on serial sections (Fig 3C), we confirmed that only the anterior and middle sections contained a mixture of Pax7+/GdnfLacZ+ progenitors and Myh1+ fibers, whereas in the posterior sections, the CM only contained Pax7+/GdnfLacZ+ progenitors, but no fibers. Thus, there is a physical separation between the front of progenitor migration and the front of differentiation, where new differentiating myocytes are being added to growing muscle fibers on their posterior side.
Fig 3. Topographic organization of myogenesis and nerve pattern in the CM muscle.
(A) Scheme representing the shape of the CM muscle seen from the side of an embryo, featuring the area covered by GdnfLacZ+ muscle progenitors in purple, MLC3F-2E+ muscle fibers represented in blue, and CM-innervating axons represented in green, indicating (vertical lines) the level corresponding to sections shown in (B) and (C). (B) Cross section of an E12.5 GdnfLacZ/+ embryo, at the middle CM level, immunostained with antibodies to Pax7 (red) and β-galactosidase (green), showing that GdnfLacZ is expressed in Pax7+ progenitors. (C) Cross sections of an E12.5 GdnfLacZ/+; Etv4-GFP+ embryo at the anterior CM (top pictures), middle CM (middle row), and posterior CM (bottom row) levels and immunostained with antibodies to Pax7 (red), β-galactosidase (red), Myh1 (green), and GFP (green) and with DAPI (blue). At each level, three neighboring sections of the same embryo were used with the indicated antibody combinations. In (C), inserts show high magnifications of the area highlighted with the yellow dotted square. Scale bars: low-magnification pictures in C: 200 μm; inserts in C: 40 μm; high-magnification (right) pictures in B: 40 μm; low-magnification pictures (left) in B: 200 μm. ant, anterior CM level; β-Gal, β-galactosidase; CM, cutaneous maximus; C5 to C8, Cervical levels 5 to 8; Etv4, Ets variant gene 4; Etv4-GFP, transgenic line in which expression of an Etv4-GFP fusion protein is driven by the Etv4 locus (S1 Table); GFP, green fluorescent protein; mid, middle CM level; Myh1, myosin heavy chain 1; Pax7, paired box 7; post, posterior CM level; T1 to T3, Thoracic levels 1 to 3.
Interestingly, the shape of the area covered by CM-innervating motor axons (Fig 2) also appears more similar to the shape covered by GdnfLacZ-expressing progenitors than to the shape of the area covered by differentiated fibers (as visualized with the MLC3F-2E transgene) (Fig 1). The population of spinal MNs innervating the Gdnf-producing muscles CM (Fig 3A) and LD is characterized by expression of the transcription factor Etv4 [39, 41]. We therefore took advantage of an Etv4-GFP transgene (S1 Table, [60]), in which GFP expression reproduces that of Etv4 and enables detection of the corresponding axons, to follow CM-innervating motor axons on serial sections of GdnfLacZ; Etv4-GFP+ embryos (Fig 3A and 3C). GFP-positive axons can be seen throughout the extent of the CM, initially running as large bundles located along the interior side of the muscle in anterior sections and progressively detected as smaller bundles, posteriorly (Fig 3C). Even in the posteriormost sections, in which Myh1+ fibers are no longer detected in the GdnfLacZ-positive CM sheet, GFP-positive axon bundles are found intermingled with GdnfLacZ progenitors. Thus, Etv4-GFP+ motor axons cover the entire zone enriched in GdnfLacZ-expressing progenitors, as previously observed [39]. These observations imply that the front of migration contains GdnfLacZ progenitors and distal tips of Etv4-GFP+ motor axons, which appear to progress hand in hand, but not differentiated fibers. Thus, there is a significant topographical separation between the front of progenitor migration and axonal elongation, and the front of progression of muscle fiber elongation, where new myocytes are added to growing muscle fibers on their posterior side. These observations raise the interesting possibility that the speed and direction of CM muscle fiber elongation could be influenced by the direction/speed of progenitor migration or axon elongation, or by a combination of both. In addition, the simultaneous progression of CM progenitors and Etv4-GFP+ motor axons raises the issue of determining whether axons or muscle progenitors influence more the progression of CM expansion and the subsequent expansion of muscle fibers.
Fat1 is expressed in multiple cell types delineating the CM neuromuscular circuit morphogenesis
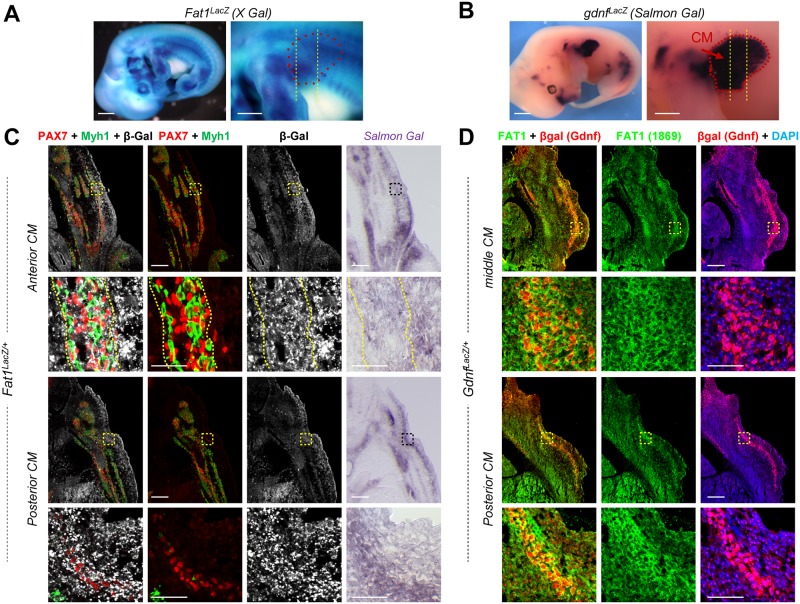
We next asked in which cell type Fat1 is required to exert the function(s) underlying this complex event of CM muscle and nerve morphogenesis. Although we previously showed that Fat1 is required in the myogenic lineage to modulate myoblast migration polarity, the consequences of Fat1 ablation driven in trunk myoblasts by Pax3cre were milder than in constitutive knockouts [14], suggesting that Fat1 might be required in other cellular components of the circuit for its muscle-patterning function. We therefore first analyzed Fat1 expression during CM development, focusing on all the cell types involved, including muscles, surrounding CTs, and MNs. Fat1 expression was followed either with anti-β-galactosidase antibodies on serial sections of Fat1LacZ/+ embryos (Fig 4A and 4C, S3 and S4 Figs), by anti-Fat1 IHC on sections of GdnfLacZ/+ embryos (Fig 4B and 4D, in which the β-galactosidase pattern reproduces Gdnf expression), or by in situ hybridization with a Fat1 RNA probe (Fig 5). As previously reported [14], in addition to its expression in migrating myogenic cells (with Fat1LacZ expression detected in both Pax7-positive progenitors and Myh1-positive muscle fibers, Fig 4C), we also found that Fat1 was expressed in mesenchymal cells surrounding the CM muscle, thus constituting a sheet of Fat1-expressing cells, through which the CM muscle expands (Fig 4C and 4D; see also [14]). Similarly, Fat1 protein is detected not only in GdnfLacZ-positive muscle precursor cells (Fig 4D) but also in cells surrounding the layer of GdnfLacZ-expressing progenitors (Fig 4D). β-galactosidase staining intensity in Fat1LacZ/+ embryos appears higher in the subcutaneous mesenchymal layer at the level of posterior CM sections than it is at anterior levels (Figs 4C and S3B). This expression was preserved in genetic contexts leading to depletion of migratory muscles, as evidenced by the robust Fat1LacZ expression detected in embryos in which myoblast migration is abrogated, such as in mutants of the HGF receptor gene Met [61, 62] or of Pax3 [63–65] (S5A and S5B Fig, S1 Table). In the latter case, following cells derived from the Pax3cre lineage using the R26-YFP reporter (in which expression of the yellow fluorescent protein, driven by the ubiquitous Rosa26 locus, is conditioned by cre-mediated removal of a stop cassette, S1 Table, [66]) (S5B Fig) reveals that most of the Fat1-β-galactosidase fusion protein detected in the dorsal forelimb remains unchanged, even in absence of the YFP+ myogenic component in Pax3cre/cre; Fat1LacZ/+ compared to Pax3cre/+; Fat1LacZ/+ embryos (the remaining YFP+ cells correspond to Schwann cells along the nerves). Together, these findings indicate that a large part of Fat1 expression surrounding or within the CM muscle corresponds to mesenchymal cells (or CT).
Fig 4. Fat1 is expressed in CM progenitors and the surrounding subcutaneous mesenchyme.
(A) Fat1 expression is visualized in an E12.5 Fat1LacZ/+ embryo by X-gal staining. Left panel: whole embryo picture; right panel: higher magnification of the forelimb and flank region in which the CM spreads. In the right panel, the approximate CM shape is highlighted by red dotted lines, and the level of sections shown in (C) is indicated by vertical lines. (B) Gdnf expression is visualized in an E12.5 GdnfLacZ/+ embryo (S1 Table) by Salmon-Gal staining. Left panel: whole embryo left side view. Right panel: higher magnification of the upper forelimb and flank region, showing that the CM exhibits a high level of GdnfLacZ+ expression (highlighted with red dotted lines). The level of sections shown in (D) is represented by vertical bars. (C) Cross sections of an E12.5 Fat1LacZ/+ embryo at anterior and posterior CM levels were immunostained with antibodies against Pax7 (red), Myh1 (green), and β-galactosidase (white). The right panels show neighboring sections of the same Fat1LacZ/+ embryo in which β-galactosidase activity was revealed by Salmon-Gal staining. (D) Comparison between expression of GdnfLacZ (visualized with an anti-β-galactosidase antibody [red]) and that of Fat1 (green, Ab FAT1-1869 Sigma) on two cross sections of an E12.5 GdnfLacZ/+ mouse embryo at middle and posterior CM levels, as indicated in (B). Fat1 protein is detected both within and around the GdnfLacZ/+ CM progenitors. Scale bars (A, B): 1 mm (left), 500 μm (right); (C, D): 200 μm (low magnification), 50 μm (high magnification). β-Gal, β-galactosidase; CM, cutaneous maximus.
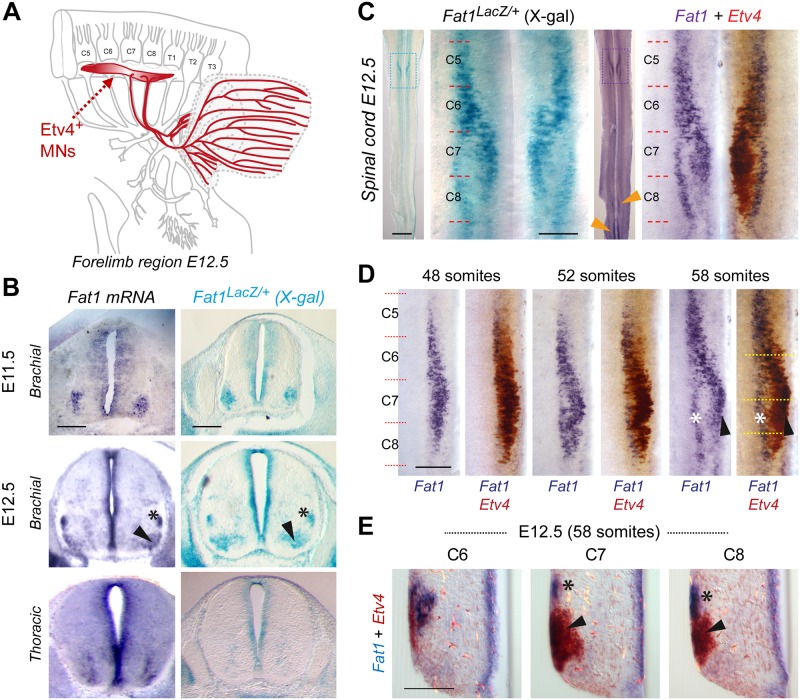
Fig 5. Fat1 is expressed in subsets of brachial MN pools, including CM-innervating Etv4+ MNs.
(A) Scheme representing the brachial region of a mouse embryo at E12.5 with the C4–T2 portion of the spinal cord, the corresponding spinal nerves and their projections to the forelimb, with Etv4+ MNs and their axons highlighted in red, whereas the target muscles CM and LD are delineated in blue (the LD being underneath the CM). (B) Fat1 expression in the mouse brachial spinal cord is shown by in situ hybridization in wild-type embryo sections (left panels) and by X-gal staining on sections of Fat1LacZ/+ spinal cords at E11.5 (top) and E12.5 (bottom), showing expression in all neural progenitors in the ventricular zone and in pools of MNs, visible as one single cluster at E11.5 and two separate pools (arrowhead and asterisk) at E12.5. (C) Fat1 expression in the mouse brachial spinal cord at E12.5 is shown through an X-gal staining of a Fat1LacZ/+ spinal cord (left) or a double in situ hybridization with Fat1 (purple) and Etv4 (brown) RNA probes on a wild-type spinal cord. Fat1 expression is detected in Etv4-expressing MN pools (arrowheads) but also expressed in a distinct dorsal column (asterisk). Spinal cords are flat-mounted, such that the ventral midline is seen as a vertical line in the middle, and motor columns are seen on both sides. For both stainings, the entire spinal cord is shown on the left, and a magnification of the brachial region is shown on the right (corresponding to the delineated zone). Left and right sides of the in situ hybridization panel show a mirror image of the same spinal cord side before and after developing the brown (Etv4) reaction. (D) Double in situ hybridizations with Fat1 (purple) and Etv4 (brown) RNA probes on wild-type spinal cords at three successive time points, 48 somites (E11.5), 52 somites (E12.0), and 58 somites (E12.5), showing the left side of the brachial spinal cord after developing Fat1 only (purple, left) or after developing the second reaction, in brown (right). (E) Cross section on the 58 somite spinal cord shown in (D, right panel) at the three levels indicated by the dotted line in (D), showing the partial overlap (arrowhead) between Fat1 and Etv4 expression and the dorsal pool of MNs expressing Fat1 only. Scale bars, (B) 200 μm; (C) low magnification: 1 mm; (C) high magnification: 200 μm; (D) 200 μm; and (E) 100 μm. CM, cutaneous maximus; C5 to C8, Cervical segments 5 to 8; Etv4, Ets variant gene 4; LD, latissimus dorsi; MN, motor neuron.
The population of spinal MNs innervating the Gdnf-producing muscles CM and LD is characterized by expression of the transcription factor Etv4 (Fig 5A) [39, 41]. Interestingly, in addition to its peripheral expression, we also detected Fat1 expression in groups of MNs at brachial levels encompassing the pools of Etv4-expressing MNs (Fig 5C–5E). Aside from expression in neural precursors in the ventricular zone all along the dorsoventral axis (Fig 5B, S6C Fig), this brachial MN column was the main site of high Fat1 expression in the spinal cord (Fig 5C). Fat1 expression was also detected in a column of ventral neurons at thoracic levels (S6A Fig) and, with later onset, in subsets of lumbar and sacral MNs (Fig 5C [orange arrowheads] and S4B Fig). The overlap between Etv4 and Fat1 expression in brachial MNs was maximal at E11.5 (Fig 5D), whereas additional groups of Fat1-positive; Etv4-negative (Fat1-only) neurons become detectable in dorsal positions at E12.5 (Fig 5D and 5E). At that stage, the CM MN pools have completed their shift in cell body position in the spinal cord [41], resulting in the dorsoventral split of Fat1 expression domain at C7–C8 levels into a dorsal Fat1-only pool (Fig 5D and 5E, asterisk) and a ventral Fat1+/Etv4+ pool (Fig 5D and 5E, arrowheads), matching position of CM MNs.
Given the Fat1/Etv4 co-expression, we asked whether Fat1 could be a transcriptional target of Etv4 or whether its expression was dependent on factors acting upstream of Etv4, such as GDNF and HGF [39, 41, 47]. Fat1 expression only appeared modestly reduced in shape in Etv4-/- and Gdnf-/- spinal cords and unchanged in Metd/d spinal cords at E11.5 (S6A and S6B Fig). These data indicate that Fat1 induction occurred independently of HGF/Met, GDNF, and Etv4, in spite of the subtle changes in shape of Fat1-expressing columns in Etv4 and Gdnf mutants. Nevertheless, at E12.5, after the dorsoventral split into two Fat1-expressing columns, the ventral Fat1-expressing pool was missing in the Etv4-/-, Gdnf-/-, and Metd/d spinal cord (S6A and S6B Fig), consistent with previously reported changes in the fate of CM MNs [39, 41, 47]. In contrast, the dorsal column appeared increased in the Etv4-/- and Gdnf-/- spinal cord (S6B Fig), also consistent with the altered positioning of some CM neurons [39, 41]. This dorsal column appeared reduced in Metd/d spinal cord, possibly resulting from the onset of enhanced MN death in the absence of the target muscle [46, 47, 60]. Altogether, these data are consistent with Fat1 being expressed in CM motor pools. The reiterated use of Fat1 expression in several components of the GDNF/Etv4 circuit and the altered shape of CM muscle and innervation pattern in Fat1 mutants raise the possibility that loss of Fat1 functions might influence development of this neuromuscular circuit, either by acting directly in MNs or as an indirect consequence of its role in muscle patterning.
Loss of Fat1 causes CM-innervating MN specification defects
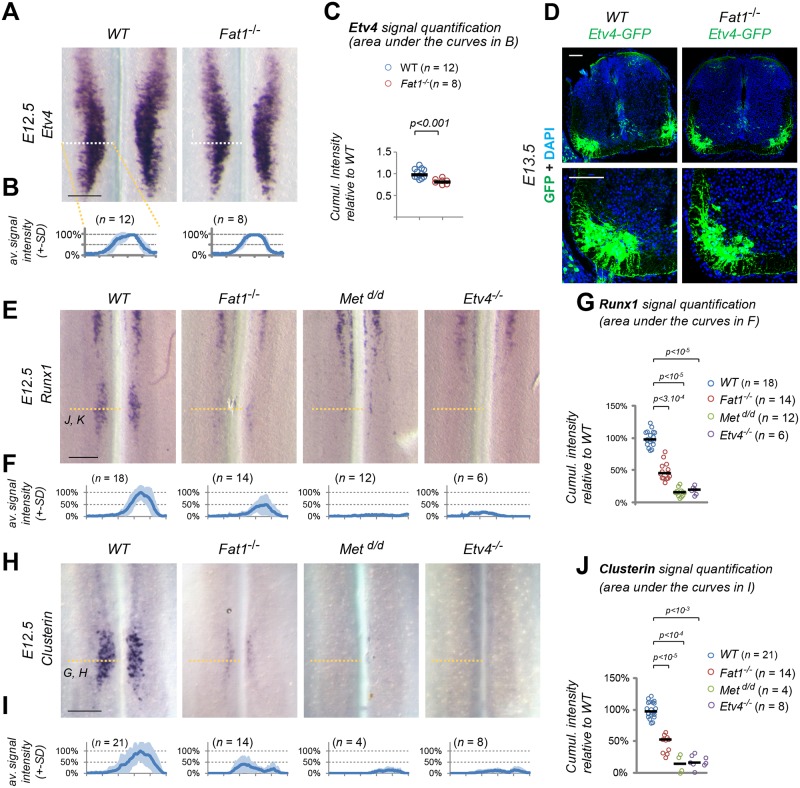
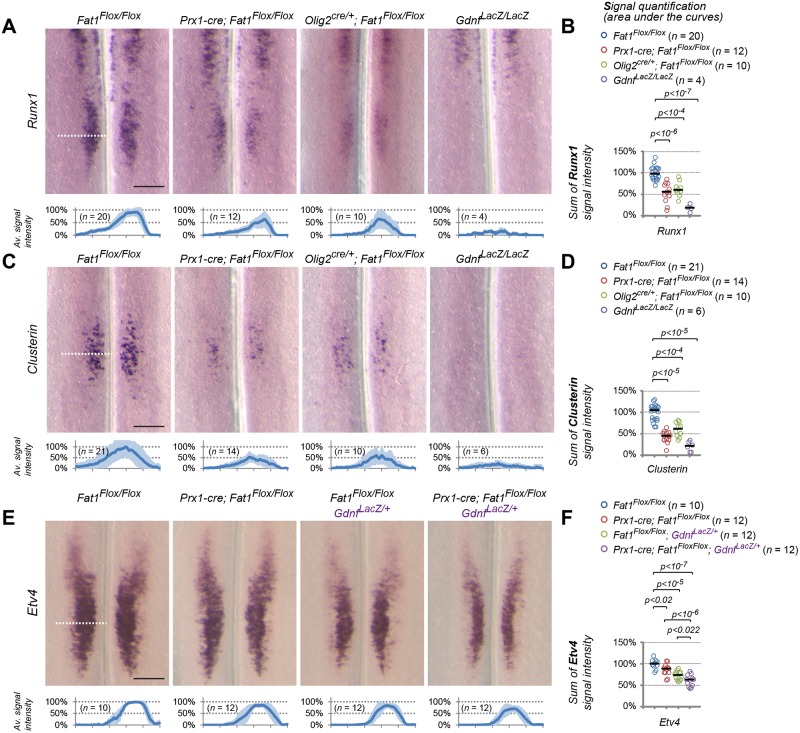
We next asked whether the CM muscle phenotype and the changes in motor axon patterns observed in Fat1 mutants were also associated with molecular defects in the corresponding spinal MN pools. The co-expression of Fat1 with Etv4 in MNs and the selective alteration in shape and nerve projections to the CM prompted us to focus on specification of the CM motor pools and to examine whether Etv4 expression was altered in Fat1 mutants. This analysis revealed that Fat1 is dispensable for the establishment of Etv4 expression domain in Fat1-/- spinal cord (Fig 6A–6C and S7A Fig). Using the Etv4-GFP transgene (S1 Table, [60]) also allowed detecting a near normal appearance of the GFP-positive motor columns in Fat1-/- spinal cords (Fig 6D). Nevertheless, analysis of signal intensity of Etv4 mRNA detected a modest but significant lowering of Etv4 signal intensity of around 20% (Fig 6B and 6C). We next asked whether such modest changes in Etv4 levels may be sufficient to impact expression of some of its transcriptional targets (for example, affecting low-affinity but not high-affinity targets).
Fig 6. Fat1 knockout alters the specification of CM motor neuron pools.
(A) Etv4 expression was analyzed by in situ hybridization in E12.5 wild-type and Fat1-/- embryos. The images represent flat-mounted spinal cords in the brachial region. (B) Quantifications of Etv4 signal: each plot represents the average signal distribution (± standard deviation in light blue) measured on the indicated number of spinal cord sides along the white dotted line in each image in (A) (Fat1+/+ [n = 12; this set of controls includes the same samples as those shown in S7 Fig]; Fat1-/- [n = 8]). (C) Quantifications and statistical analyses of the sum of signal intensity corresponding to the area under the curves in plots shown in (C): each dot represents the sum of Etv4 intensity for each spinal cord side, the number of samples being indicated (the two sides of each embryo are considered independent). (B–C) Underlying data are provided in S1 Data. (D) Sections of spinal cords from E13.5 Fat1+/+; Etv4-GFP and Fat1-/-; Etv4-GFP embryos (Etv4-GFP transgene, S1 Table) were stained with antibodies against GFP and with DAPI. (E–J) Analysis by ISH of Runx1 (E) and Clusterin (H) expression in flat-mounted brachial spinal cords from E12.5 wild-type, Fat1-/-, Metd/d, and Etv4-/- embryos: expression of Clusterin and Runx1 in the C7–C8 segments is lost in both Etv4 and Met mutants and severely reduced in Fat1-/- spinal cords, whereas the rostral domain of Runx1 expression is independent of Met, Etv4, and Fat1. Quantifications of Runx1 (F, G) and Clusterin (I, J) signal intensity: each plot in (F, I) represents the average signal distribution (± standard deviation in light blue) measured on the indicated number of spinal cord sides along the orange dotted line in each image above (with the corresponding genotype), in (F) for Clusterin and (I) for Runx1. (F, G) Clusterin probe: Fat1+/+ (n = 21); Fat1-/- (n = 14); Metd/d (n = 4); Etv4-/- (n = 8); (I, J) Runx1 probe: Fat1+/+ (n = 18); Fat1-/- (n = 14); Metd/d (n = 12); and Etv4-/- (n = 6). Underlying data are provided in S1 Data. (G, J) Quantifications and statistical analyses of the sum of signal intensity corresponding to the area under the curves in plots shown in (F) and (I), respectively: each dot represents the sum of Runx1 or Clusterin intensity for each spinal cord side, the number of samples being indicated. Underlying data are provided in S1 Data. Scale bars: 200 μm (A, E, H); 100 μm (D). CM, cutaneous maximus; ISH, in situ hybridization; WT, wild-type.
We therefore analyzed expression of several Etv4 target genes expressed in subsets of Etv4+ neurons, some of these markers being also deregulated by loss of Met, a situation that we showed partially alters Etv4 expression [41, 47]. We first studied Sema3E and Cadherin 8, two known Etv4 targets in the CM motor pool [41]. Consistent with previous reports [41], their expression is absent in Etv4-/- spinal cords, whereas it was reduced but not lost in Metd/d spinal cords (S7E Fig). Sema3E and Cadherin 8 expression appeared unaffected in Fat1-/- spinal cords (S7E Fig), with these two genes behaving as expected for robust “high-affinity” Etv4 targets. We next studied expression of Clusterin and runt related transcription factor 1 (Runx1), two genes that we selected for their expression in the CM motor pool. Runx1 is a transcription factor expressed in a rostral column of ventrally located neurons spanning C1–C6 [67] and in a separate pool at C7–C8, matching the CM subset of Etv4+ MNs (Fig 6E and S5C Fig), where its expression was shown to require Met signaling [60]. Clusterin is a glycoprotein known to accumulate in neurons after axotomy, injury, or in neurodegenerative diseases (such as amyotrophic lateral sclerosis [ALS], Alzheimer) that was proposed to modulate cell death, autophagy, and clearance of protein aggregates or cell debris [68–71]. Clusterin expression in the developing spinal cord was restricted to a subset of MNs matching the position of the Etv4+ CM pool (Fig 6H). In contrast to Sema3E and Cadherin 8, expression of both Clusterin and Runx1 was completely abolished in Etv4 and Met knockout spinal cords (Fig 6E–6J). Clusterin and Runx1 signal intensities were severely reduced in the C7–C8 MN pool in Fat1-/- spinal cords, although not as severely as in Met and Etv4 mutants (Fig 6E–6J). The severe effect on Clusterin and Runx1 expression and mild effect on Etv4 expression detected at E12.5 in Fat1 mutant spinal cords is unlikely to result from increased cell death. First, Runx1 is co-expressed with Sema3E (S6C Fig), the expression of which is unaffected by loss of Fat1 (S7E Fig), indicating that the neurons that fail to express Clusterin/Runx1 in Fat1 mutants are still present and retain Sema3E expression. Second, naturally ongoing MN death only peaks at E13.5 in mice, one day after the stage analyzed here. Absence of muscle development in mutants of genes such as Pax3 or Met was shown to have a minor effect on MN numbers prior to the establishment of the trophic dependency of MNs for muscle-derived factors [47]. Thus, at E12.5, the stage of our current analysis, cell death is unlikely to contribute to the effects of Fat1 loss-of-function on Clusterin/Runx1 expression. Thus, their sensitivity to the absence of Fat1 is consistent with Clusterin and Runx1 expression being low-affinity targets of Etv4, affected by subtle changes in Etv4 levels. Altogether, these data confirm that loss of Fat1 compromises the correct acquisition of the molecular identity of the CM-innervating MN pool, in addition to its effect on CM muscle growth and differentiation.
Fat1 ablation in mesenchymal cells, but not in MNs, causes drastic muscle shape–patterning defects
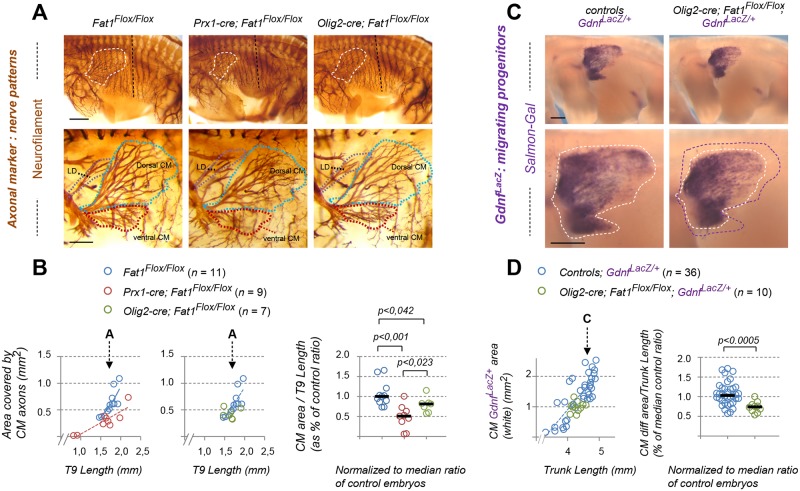
There is a strong topological connection between altered CM muscle morphology observed in Fat1 mutants and the selective defects in the corresponding MN population. This topology is even more puzzling when considering that Fat1 is expressed in several cell types, including Etv4+ MN, along the GDNF/Etv4 circuit. Neural and muscular aspects of the phenotypes could represent the consequences of one single primary phenotype resulting from Fat1 deletion in one single cell type. Given the known dependency of Etv4 induction on GDNF [39, 41, 47], the neural phenotypes could arise as a consequence of the reduced amount of GDNF-producing cells. However, the discovery of Fat1 selective expression in Etv4+ MNs raises the alternative possibility that some aspects of these phenotypes may result from its ablation in MNs. Thus, the overall knockout phenotype could represent the cumulated consequences of phenotypes resulting from ablation of independent Fat1 assignments in distinct cell types. To tackle this question, we used a conditional approach to selectively interfere with Fat1 functions in a tissue-specific manner. This allowed assessing in which cell type it was necessary and sufficient to ablate Fat1 functions to reproduce each aspect of the muscular and neural phenotypes observed in constitutive mutants. We combined our Fat1Flox conditional allele [14] with several Cre lines matching the different sites of Fat1 expression (see S1 Table for full descriptions of each mouse line): Pax3-cre knock-in for ablation in the premigratory myogenic lineage [64], Olig2-cre knock-in for MN precursors [72], Prx1-cre transgenic line for limb mesenchymal cells [73, 74], and Wnt1-cre transgenic line in neural crest derivatives [75]. We first focused on the strong alterations of CM muscle appearance observed in the constitutive knockout. We followed muscle differentiation using the MLC3F-nLacZ-2E transgene. Although we previously described significant alterations of myogenesis resulting from Fat1 ablation in the premigratory myogenic lineage (driven by Pax3-cre [14]), the appearance of the CM muscle in E14.5 Pax3-cre; Fat1Flox/Flox embryos was grossly normal, albeit with reduced density of differentiated muscle cells. This contrasts with constitutive Fat1 knockouts, in which the severe effect on CM shape persists at later stages, when the muscle has further extended to cover most of the trunk [14]. This discrepancy indicated that the effect on muscle growth caused by premigratory ablation in myogenic cells was attenuated at later stages. Thus, Fat1 function underlying proper CM development must therefore also be exerted in a distinct cell type.
Given the selective co-expression of Fat1 and Etv4 in the CM MN pool and the fact that the front of CM expansion is led by both muscle progenitors and axons, we next asked if Fat1 deletion in the corresponding MNs had an impact on CM muscle development. However, MN-specific Fat1 deletion in Olig2-cre; Fat1Flox/Flox; MLC3F-2E embryos did not cause any detectable change in the appearance (S8A Fig) and expansion rate of the CM, as assessed by measuring the CM area in X-gal–stained embryos carrying the MLC3F-2E-LacZ transgene (S8B Fig). MN-specific mutants also lacked all the other muscle phenotypes observed in constitutive knockouts, with no ectopic muscle in the scapulohumeral region (S8A Fig), no myocyte dispersed in the forelimb (S8A and S8B Fig), and an overall normal appearance at E14.5 (S9 Fig). Together, these observations establish that the progression of myogenic differentiation in the CM is not significantly influenced by Fat1 activity in the pool of MNs innervating this muscle.
In contrast, we found that Fat1 ablation driven in the limb and trunk mesenchyme by Prx1-cre led to a severe and robust change in the appearance of the CM muscle (Fig 7A and 7B) and of upper forearm muscles (S8A and S8B Fig). This phenotype was already visible at E12.5 during the phase of CM posterior extension, with a significant reduction of the rate of CM expansion assessed by X-gal staining in Prx1-cre; Fat1Flox/Flox; MLC3F-2E embryos (Fig 7A and 7B). This leads to a phenotype as severe as that observed in constitutive knockouts, as measured when comparing the growth rate of the CM differentiation area to that of BW muscles (Fig 7B). Again, the ventral half of the CM was severely shortened, whereas spreading in the dorsal part appeared less affected (Fig 7A). Moreover, Prx1-cre; Fat1Flox/Flox; MLC3F-2E embryos consistently exhibited changes in forearm muscles, such as the appearance of ectopic muscles in the scapulohumeral area (S8A Fig) or a mild but significant increase in the number of dispersed myocytes in the forelimb (S8B Fig). At E14.5, the stage at which the CM is fully extended in the trunk, up to the point of emergence of the hind limbs, Prx1-cre; Fat1Flox/Flox embryos exhibited a pronounced hypoplasia of the ventral cutaneous maximus (vCM), with only very few MLC3F-2E-positive fibers having successfully extended in random orientations (S9A and S9C Fig), thus strongly recapitulating the changes seen in the germ line deletion. Again, the dorsal cutaneous maximus (dCM) appeared less affected in E14.5 Prx1-cre; Fat1Flox/Flox; MLC3F-2E embryos, although the density of LacZ+ nuclei nevertheless appeared reduced compared to Fat1Flox/Flox; MLC3F-2E embryos (S9A Fig).
Fig 7. Mesenchyme-specific Fat1 deletion non–cell-autonomously alters CM expansion.
(A, C) Whole-mount β-galactosidase staining was performed using X-gal as a substrate on embryos carrying the MLC3F-2E transgene (A), or using Salmon-Gal as substrate on embryos carrying the GdnfLacZ/+ allele (C), in the context of mesenchyme-specific deletion of Fat1, driven by Prx1-cre (S1 Table) at E12.5. Top images show a side view of the whole flank of an embryo. Yellow dotted lines highlight the area occupied by body wall muscles. Lower images are higher magnification of the area in which the CM spreads. Red and white dotted lines correspond (as in Fig 1) to the areas covered by MLC3F-2E+ CM fibers (red, A) and to the area covered by GdnfLacZ+ progenitors (white, C), respectively. (B) Quantification of the expansion rate of differentiated CM fibers. Left graph: For each embryo side, the area covered by differentiated CM fibers was plotted relative to the area occupied by body wall muscles. Right plot: for each embryo, the CM area/body wall area was normalized to the median ratio of control embryos. Blue dots: Fat1Flox/Flox; MLC3F-2E (n = 57, includes the same set of controls as S8C Fig); red dots: Prx1-cre; Fat1Flox/Flox-; MLC3F-2E (n = 23). Underlying data are provided in S1 Data. (D) Quantification of the expansion rate of the area occupied by GdnfLacZ+ progenitors. Left plot: for each embryo side, the area covered by GdnfLacZ+ progenitors was plotted relative to the trunk length. Right plot: for each embryo, the GdnfLacZ+ CM area/trunk length was normalized to the median ratio of control embryos. Blue dots: Fat1Flox/Flox; GdnfLacZ/+ (n = 36, pooling respective littermates); red dots: Prx1-cre; Fat1Flox/Flox-; GdnfLacZ/+ (n = 12). Underlying data are provided in S1 Data. (E) Cross sections of E12.5 Prx1-cre; Fat1Flox/+; GdnfLacZ/+; R26YFP/+ and Prx1-cre; Fat1Flox/Flox; GdnfLacZ/+; R26YFP/+ embryos at equivalent rostro-caudal positions (caudal CM level) were immunostained with antibodies against GFP/YFP (green) to reveal the domain of Prx1-cre activity (green) against β-galactosidase (red), against Myh1 (white on top panels and inserts, green on middle panels), against Pax7 (bottom panels), and with DAPI (blue). The yellow dotted boxes indicate the areas magnified in inserts and in the bottom panels, in equivalent positions of the CM. Images show that lowered Gdnf levels represent a non–cell-autonomous consequence of lack of Fat1 signaling in the mesenchyme of Prx1-cre; Fat1Flox/Flox embryos and result from a reduced number of Pax7-GDNF-expressing progenitors cells rather than from a lower level of GdnfLacZ expression per cell. Scale bars: (A, C) 500 μm; (E) low magnification: 200 μm; inserts: 20 μm; lower panels: 40 μm. CM, cutaneous maximus; cre, cre recombinase; dCM, dorsal cutaneous maximus; GFP, green fluorescent protein; Prx1-cre, mesenchymal cre expression driven by a regulatory enhancer of the paired related homeobox 1 gene; R26, Rosa26 locus; vCM, ventral cutaneous maximus; X-gal, substrate for β-galactosidase; YFP, yellow fluorescent protein.
Constitutive Fat1 knockouts also exhibit abnormal morphology of subcutaneous muscles in the face, mostly visible at E14.5 [14]. Unlike the CM muscle, this group of facial subcutaneous muscles appeared unaffected in E14.5 Prx1-cre; Fat1Flox/Flox; MLC3F-2E embryos (S9 Fig), consistent with the lack of Prx1-cre activity in craniofacial mesenchyme [73, 74]. Ablation of Fat1 functions in the craniofacial mesenchyme was achieved using Wnt1-cre (S1 Table, [75]), which drives cre expression in all neural crest cells, including the cephalic neural crest, from which most craniofacial mesenchyme derives [76]. This led to profound morphological alterations in the appearance of facial subcutaneous muscles in Wnt1-cre; Fat1Flox/Flox; MLC3F-2E embryos, with a severely reduced fiber density and drastic changes in fiber orientation and position of fiber origins (S9 Fig). The same severe effect on morphology of facial subcutaneous muscles was also observed in E14.5 Pax3cre/+; Fat1Flox/Flox embryos (S9 Fig). This reflects the known activity of Pax3-cre in the neural crest as well. By contrast, the observation of intact morphology of scapulohumeral muscles, normal rate of progression of the CM muscle, and lack of myocyte dispersion in the forelimb in Wnt1-cre; Fat1Flox/Flox; MLC3F-2E embryos (S8 and S9 Figs) indicates that Fat1 activity in the trunk neural crest is dispensable for its influence on trunk muscle development. Altogether, these data identify mesenchyme as a cell type in which Fat1 signaling is required for muscle morphogenesis, with the trunk mesenchyme deriving from Prx1-cre lineage and the craniofacial mesenchyme deriving from the Wnt1-cre/neural crest lineage. In contrast, these results establish that Fat1 activity in MNs and the trunk neural crest is dispensable for myogenic differentiation.
Fat1 ablation in mesenchymal cells non–cell-autonomously disrupts posterior spreading of GDNF-expressing CM progenitors
So far, we have established that Fat1 is required in the mesenchyme for the progression of differentiation and fiber elongation in the CM. Because the delay in CM expansion observed in constitutive knockouts was associated with a reduced rate of expansion of the sheet of GdnfLacZ-expressing progenitors, we next asked if this progenitor progression was also affected, by bringing the GdnfLacZ allele in the mesenchyme-specific mutant context and performing Salmon-Gal staining on whole-mount embryos. As in knockouts, we observed an important reduction in the rate of progression of the area occupied by GdnfLacZ-expressing progenitors (related to the evolution of trunk length) in Prx1-cre; Fat1Flox/Flox; GdnfLacZ/+ embryos compared to Fat1Flox/Flox; GdnfLacZ/+ embryos (Fig 7C and 7D). When comparing embryos of similar stage, the GdnfLacZ sheet appeared truncated in the vCM and shorter in the dCM, with an apparent reduction in staining density, visible by comparing staining intensity along a dorsoventral line positioned similarly (blue dotted line in Fig 7C). This approach does not distinguish a reduction in expression level from a reduced number/density of cells expressing GdnfLacZ. Therefore, to discriminate between the two options, we next analyzed the level of β-galactosidase protein (visualized by IHC) driven by the GdnfLacZ allele on control and mutant embryo sections. At middle and posterior CM levels, there was a clear reduction in thickness of the sheet of GdnfLacZ+ cells detected in a Prx1-cre; Fat1Flox/Flox; GdnfLacZ/+ embryo compared to a control embryo, resulting from a lowered number of stained cells rather than a reduced expression level per cells (Fig 7E, inserts). GdnfLacZ-expressing cells, however, exhibited comparable β-galactosidase staining intensity, ruling out an effect on Gdnf expression levels. Staining with antibodies against Pax7 to mark progenitors confirmed a reduction in the number of Pax7+ progenitors at comparable anteroposterior (AP) levels (Fig 7E). Thus, the reduced CM thickness in Prx1-cre; Fat1Flox/Flox; GdnfLacZ/+ embryos results in large part from a reduced amount of Pax7+ progenitors, and from a consequent reduction in the amount of differentiated fibers (highlighted with anti-Myh1 antibodies), compared to Prx1cre; Fat1Flox/+; GdnfLacZ/+ controls.
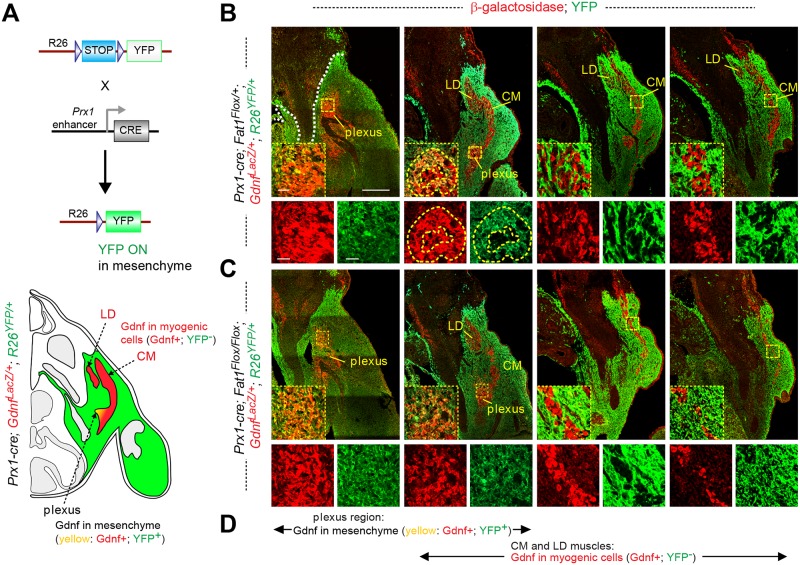
Our histological analysis of serial sections of mesenchyme-specific mutants and controls was done in a genetic context, allowing lineage tracing of Prx1-cre activity with an R26-YFP reporter (S1 Table, [66]) to highlight cells in which cre-mediated recombination is occurring (Fig 8A). This context (Prx1-cre; R26YFP/+; GdnfLacZ/+) allowed visualizing both reporters (β-galactosidase and YFP) simultaneously by IHC on transverse sections spanning from the brachial plexus to the CM muscle, comparing Fat1Flox/+ (Fig 8B) with Fat1Flox/Flox (Fig 8C) mutant settings (see also Fig 7E). This analysis confirmed that the Gdnf expression domain is subdivided into two compartments: in posterior sections, GdnfLacZ-expressing CM muscle progenitors detected throughout the length of the muscle do not derive from mesenchymal progenitors (Figs 7E and 8). These myogenic cells appear to slide along and be surrounded by a territory of YFP-expressing CT mesenchymal lineage composed of Prx1-cre; R26-YFP+ cells, up to a dorsoventral boundary corresponding to the limits of the Prx1-cre lineage [73] (Figs 7E and 8). In contrast, in anterior sections, at the level of the brachial plexus, GdnfLacZ-expressing cells co-express β-galactosidase and YFP, indicating that the plexus component of GdnfLacZ domain is constituted of mesenchyme-derived cells (Fig 8). Thus, Gdnf expression domain is constituted of two subdomains of distinct developmental origins (myogenic and mesenchymal, respectively), which are anatomically connected at the position of origin of migration of the CM (and LD) progenitors.
Fig 8. Mesenchymal Fat1 is required for expansion of the myogenic component of Gdnf expression domain but dispensable for Gdnf expression in plexus mesenchyme.
(A) Top: principle of the genetic paradigm used to follow the Prx1-cre lineage, using the R26Lox-STOP-Lox-YFP reporter line combined with Prx1-cre. In tissues in which cre is not expressed, YFP expression is prevented by the STOP cassette. In CRE-expressing mesenchymal cells, STOP cassette excision allows YFP expression. Bottom: scheme of a cross section of a Prx1-cre; R26YFP/+; GdnfLacZ/+ embryo, highlighting in green the cells in which YFP expression is activated, in red, the cells expressing GdnfLacZ, and in white or gray, the other non-recombined tissues. (B, C) Cross sections of E12.5 Prx1-cre; Fat1Flox/+; GdnfLacZ/+; R26YFP/+ (B) and Prx1-cre; Fat1Flox/Flox; GdnfLacZ/+; R26YFP/+ (C) embryos stained with antibodies against GFP (YFP; to reveal the domain of Prx1-cre activity, in green), with an anti-β-galactosidase antibody (for GdnfLacZ, red), visualized at four successive rostro-caudal positions spanning from the brachial plexus to the caudal half of the CM muscle. For each level, the inserts below represent a high-magnification view of the area indicated in the yellow dotted boxes, showing red only, green only, and overlay. (D) Visual summary of the two components of GdnfLacZ expression domain, spanning the sections shown in (B) and (C): at the plexus level, GdnfLacZ is expressed in YFP+ cells derived from Prx1-cre mesenchyme, whereas in the CM and LD muscles (emerging from the plexus and extending dorsally and caudally), GdnfLacZ-positive cells do not express YFP, as they are from the myogenic rather than the mesenchymal lineage. At the point where the first myogenic patches emerge from the plexus, such myogenic patches (red only, yellow dotted line in [B], second section) can be surrounded by mesenchymal-Gdnf cells (red + green = yellow, white dotted lines). The overall analysis shows that Prx1-cre-mediated Fat1 ablation does not affect Gdnf expression in the plexus mesenchyme but causes non–cell-autonomous reduction in the myogenic component of Gdnf expression domain through a reduction of the number of GdnfLacZ-expressing myogenic progenitors. Scale bars: (B, C) low magnification: 200 μm; inserts: 20 μm. CM, cutaneous maximus; cre, cre recombinase; LD, latissimus dorsi; Lox, recombination sites for the CRE recombinate; Lox-STOP-Lox, cassette in which STOP signal for transcription/translation is flanked by Lox sites; Prx1-cre, transgene driving cre expression in the mesenchyme; R26, Rosa26 locus; YFP, yellow fluorescent protein.
Among the two subdomains of GdnfLacZ expression, only the myogenic component was affected in Prx1-cre; Fat1Flox/Flox; GdnfLacZ/+; R26YFP/+ embryos (Fig 8C), whereas the mesenchymal subdomain of GdnfLacZ expression appeared unaffected, both anatomically and in β-galactosidase intensity. In this mutant context, Fat1 activity is disrupted in the YFP-positive cells, and not in the GdnfLacZ/Pax7+-expressing progenitors. Thus, mesenchymal Fat1 depletion has no effect on mesenchymal Gdnf expression at plexus level, in contrast to the constitutive knockout (S2 Fig). This indicates that this part of the knockout phenotype did not result from depletion of mesenchymal Fat1 and most likely reflects Fat1 activity in another cell type. In contrast, Fat1 ablation in the Prx1-cre lineage has a drastic impact on the myogenic component of the GdnfLacZ domain not derived from the Prx1-cre lineage. This demonstrates that Fat1 acts in a non–cell-autonomous manner. It is required in YFP-positive cells of the mesenchymal lineage to promote expansion of the sheet of migrating GdnfLacZ/Pax7+ progenitors, possibly by modulating the production by mesenchymal cells of signals controlling progenitor pool expansion and/or migration. Interestingly, at E12.5, the CM and LD muscles are almost entirely surrounded by Prx1-cre-derived mesenchymal cells, with the exception of the dorsalmost tip of the CM, which lies beyond the dorsoventral limit of lateral-plate mesoderm–derived mesenchyme [73] (Figs 7E and 8). Interestingly, dorsal to this dorsoventral limit, thickness of the CM appears reinforced, suggesting that once the myoblasts reach the non-recombined mesenchymal zone, the unaltered Fat1 activity available in this dorsal environment allows them to resume their normal growth behavior, providing a possible explanation for the apparent sparing of dCM at E14.5 (Fig 5C). Altogether, these data support a model in which muscle-associated mesenchymal cells exert a Fat1-dependent positive influence on CM muscle growth/extension.
Fat1 activity in the Pdgfrα-expressing CT lineage
Thus far, we have identified the Prx1-cre lineage as the cell type in which Fat1 is required to control CM expansion and scapulohumeral muscle patterning. However, the Prx1-cre-derived lineage is broad (Fig 8) and includes several distinct subtypes of CT. These comprise specialized CT, such as bones and cartilage, dense regular CT, such as tendons, and dense irregular CT (here referred to as loose CT), such as muscle-resident mesenchymal derivatives or the perimuscular environment [13]. All of these subtypes express Fat1 at relatively high levels (Fig 4, S3 and S4 Figs). The CM grows towards a subcutaneous layer of CT, in which we observed increasing levels of Fat1 expression. Furthermore, CM extension appears to be affected on the side of its growth towards the skin interface. Altogether, this suggests that this subcutaneous interface with the CM muscle might be where this Fat1 function is taking place. Whole-mount in situ hybridization with the tenocyte/tendon marker Scleraxis highlights sites of intense expression in the limb tendons or at the interface between intercostal muscles and ribs (visible by transparency, beneath the CM), but only shows background Scleraxis levels in the region in which CM is migrating (S5A Fig). When analyzing the expression of other markers of CT subtypes by IHC on embryo sections, we found that this subcutaneous CT expresses high levels of platelet-derived growth factor receptor alpha (Pdgfrα) and Tenascin C but not transcription factor 7 like 2, T cell specific 4 (Tcf4/Tcf7L2) (S10 Fig), which was otherwise detected at other muscle extremities and in subsets of limb muscle progenitors (S10 Fig), as previously reported [77, 78].
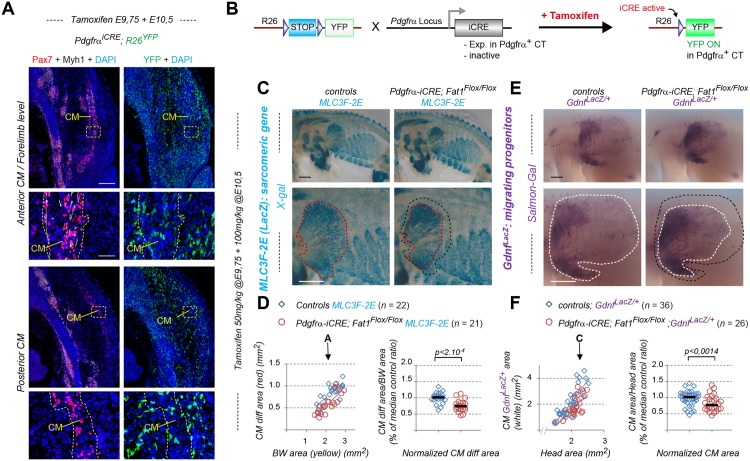
We next asked whether restricting Fat1 ablation to this subtype of mesenchyme could be sufficient to interfere with CM spreading and differentiation. Our characterization prompted us to consider inactivating Fat1 in the Pdgfrα-expressing lineage. However, as Pdgfrα is expressed at early stages in cells from which a large lineage will derive, we chose to use an inducible Pdgfrα-cre/ERT2 transgenic line (S1 Table, here referred to as Pdgfrα-iCre), in which a CRE-ERT2 fusion protein (iCRE, CRE fused with the estrogen receptor tamoxifen-binding domain) is expressed in the Pdgfrα domain but remains catalytically inactive unless tamoxifen is provided [79]. This strategy is expected to highlight only a subset of mesenchymal cells expressing Pdgfrα at the time of tamoxifen administration, including the loose CT at the skin–muscle interface (Fig 9A and 9B). To establish the conditions to obtain a reliable excision rate, we first combined the Pdgfrα-iCre line to the R26-YFP reporter [66] and exposed pregnant females to tamoxifen treatment. Optimal excision efficacy was obtained when injecting a first dose of 50 mg/kg at E9.75 (earlier injections frequently lead to developmental arrest), followed by a second injection of 100 mg/kg at E10.5 (Fig 9A and 9B). This allowed consistent detection of the R26-YFP signal when screening whole embryos just after dissection (S10B Fig). Analysis of YFP expression by IHC on sections of Pdgfrα-iCre; R26YFP/+ embryos confirmed that recombined YFP-positive cells (“Pdgfrα-iYFP” cells) were mostly localized in the loose CT surrounding the CM, whereas none of the Myh1-positive fibers exhibited any detectable recombination (Fig 9A). Although our experimental conditions do not allow simultaneous detection of YFP and Pax7 (the heat-induced-epitope-recovery treatment required to detect Pax7 protein abrogates detection of YFP), analysis of neighboring sections was consistent with the Pax7-containing area not exhibiting any YFP activity (Fig 9A). Thus, these experimental conditions allow an approximate excision rate of 30% in the loose subcutaneous CT surrounding the CM, whereas no activity in the myogenic lineage was detected.
Fig 9. Inducible Fat1 deletion in the pdgfrα connective tissue lineage alters progression of CM migration and differentiation.
(A) Cross sections of a Pdgfrα-iCre; R26YFP/+ embryo collected at E12.5, after in utero administration of tamoxifen at E9.75 (50 mg/kg) + E10.5 (100 mg/kg). Alternate sections at anterior and posterior CM levels, respectively, were immunostained with antibodies against: left panels: Pax7 (red) and myh1 (white), plus DAPI (blue); right panels: GFP (green), plus DAPI (blue), to reveal the outcome of Pdgfrα-iCre-mediated R26-YFP recombination (right panels). The yellow dotted boxes indicate the areas magnified in the bottom panels. (B) Principle of the genetic paradigm used to follow the Pdgfrα-iCre lineage, using the R26Lox-STOP-Lox-YFP reporter line combined with Pdgfrα-iCre. iCRE (CRE/ERT2) is expressed in the domain of Pdgfrα expression but remains catalytically inactive. iCRE activity is permitted by in utero treatment with tamoxifen. Catalytic activity is triggered in the cells expressing iCRE at the time of tamoxifen treatment, thus allowing the stop cassette to be deleted and YFP to be permanently expressed. (C, E) Whole-mount β-galactosidase staining was performed using X-gal as substrate on embryos carrying the MLC3F-2E transgene (C) or using Salmon-Gal as substrate on embryos carrying the GdnfLacZ/+ allele (E) in the context of tamoxifen-induced Fat1 deletion in the Pdgfrα lineage driven by Pdgfrα-iCre at E12.5. Top images show a side view of the whole flank of an embryo. Lower images are higher magnification of the area in which the CM spreads. Red and white dotted lines correspond to the areas covered in control embryos by MLC3F-2E+ CM fibers (C) and by GdnfLacZ+ progenitors (E), respectively. In comparison, the corresponding areas observed in mutants are indicated as black dotted lines in both cases. (D) Quantification of the expansion rate of differentiated CM fibers. Left graph: For each embryo side, the area covered by differentiated CM fibers was plotted relative to the area occupied by body wall muscles. Right plot: for each embryo, the CM area/body wall area was normalized to the median ratio of control embryos. Blue dots: Fat1Flox/Flox; MLC3F-2E (n = 22, control embryos from tamoxifen-treated litters); red dots: Pdgfrα-iCre; Fat1Flox/Flox- ; MLC3F-2E (n = 21). Underlying data are provided in S1 Data. (F) Quantification of the expansion rate of the area occupied by GdnfLacZ+ progenitors. Left plot: for each embryo side, the area covered by GdnfLacZ+ progenitors was plotted relative to the trunk length. Right plot: for each embryo, the GdnfLacZ+ CM area/trunk length was normalized to the median ratio of control embryos. Blue dots: Fat1Flox/Flox; GdnfLacZ/+ (n = 36; control embryos from tamoxifen-treated litters); red dots: Pdgfrα-iCre; Fat1Flox/Flox-; GdnfLacZ/+ (n = 26). Underlying data are provided in S1 Data. Scale bars: (A) low magnification: 200 μm; high magnification: 50 μm; (C, E) 500 μm. CM, cutaneous maximus; CRE/ERT2, CRE fused with the estrogen receptor Tamoxifen-binding domain; iCre, short form of CRE/ERT2; Pdgfrα, platelet-derived growth factor receptor alpha.
We next asked whether Fat1 ablation in around 30% of the loose CT surrounding the CM was sufficient to interfere with its expansion and with progression of differentiation. The Pdgfrα-iCre line was combined with the Fat1Flox allele and with either the MLC3F-2E transgenic line, to follow muscle differentiation, or with GdnfLacZ, to follow the progression of progenitor migration (Fig 9C and 9E). Pregnant females were treated with tamoxifen as defined above and embryos collected at E12.5. In all cases, mutants were compared to control embryos from tamoxifen-treated litters. Analysis was performed as previously, by measuring the area occupied by the MLC3F-2E+ fibers in the CM as compared to the area occupied by BW muscles, or by assessing the area occupied by the GdnfLacZ-expressing progenitors as compared to the head area and/or trunk length. Fat1 ablation driven by such restricted recombination paradigm leads to a significant delay in the progression of both CM muscle fiber elongation (Fig 9C and 9D) and CM progenitor migration (Fig 9E and 9F). The median differentiated CM area measured in tamoxifen-treated Pdgfrα-iCre; Fat1Flox/Flox; MLC3F-2E embryos (ratio of CM differentiated area/BW area, normalized to median control ratio) was reduced by approximately 30% compared to tamoxifen-treated control embryos (Fig 9C and 9D). Similarly, we observed a 30% reduction of the median area covered by CM progenitors observed in tamoxifen-treated Pdgfrα-iCre; Fat1Flox/Flox; GdnfLacZ/+ embryos (Fig 9E and 9F). In contrast, there was no apparent effect on the shape of scapulohumeral muscles, and we did not detect any significant enhancement of myocyte dispersion in the forelimb (S10C and S10D Fig). In the conditions of tamoxifen treatment we used for the Pdgfrα-iCre line, the percentage of cells in which the reporter R26-YFP expression indicates cre-mediated recombination (Fig 9A) is much more restricted compared to the Prx1-cre lineage (Figs 7E and 8). This indicates that Fat1 activity is required in a significant proportion of cells among these recombined “Pdgfrα-iYFP” cells for CM muscle spreading. Overall, these data are consistent with Fat1 being required in the loose CT for its non–cell-autonomous influence on CM progenitor spreading and muscle fiber extension. In conclusion, we have identified the mesenchymal lineage as the place where Fat1 activity is required to promote CM muscle expansion. We have refined our knowledge on this lineage by uncovering that in large part, this function occurs in the Pdgfrα-dependent loose CT, such as the subcutaneous layer in which CM expansion occurs. Finally, we find that this lineage represents a subset of the Pdgfrα-iCre lineage and corresponds to the subset of cells expressing Pdgfrα between E9.5 and E10.5.
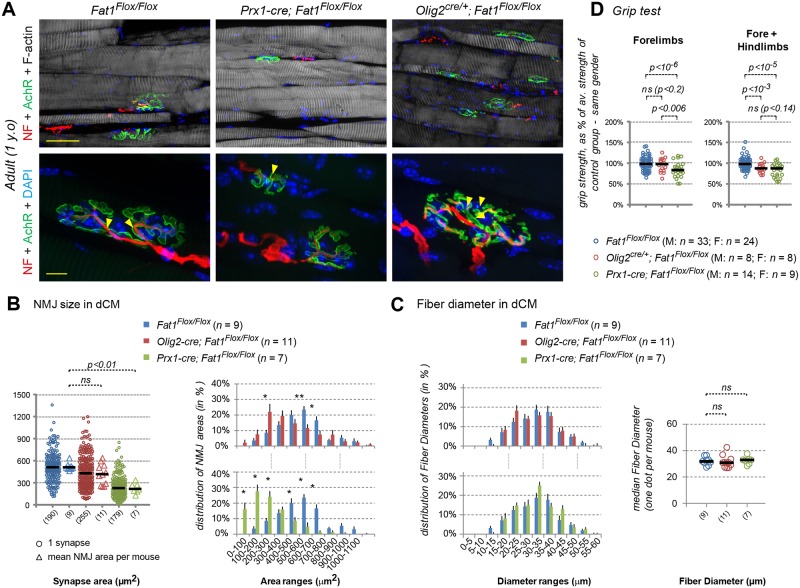
Fat1 ablation in the mesenchyme alters the pattern of axons innervating the CM
Having refined the knowledge on which CT type is required for the regulation of CM muscle expansion by Fat1, we next asked whether this activity of Fat1 in the mesenchyme-derived CT was sufficient to explain alterations of CM innervation and changes in MN pool specification, or whether Fat1 is also additionally required in MNs. To determine which of the two sites of Fat1 expression was accountable for the altered pattern of axonal arborization of the target muscle CM seen in constitutive Fat1 knockouts, we therefore performed whole-mount anti-neurofilament IHC on embryos lacking Fat1 either in MNs (Olig2cre/+; Fat1Flox/Flox) or in mesenchyme (Prx1-cre; Fat1Flox/Flox) and examined the pattern of CM motor innervation. After imaging of BB-BA cleared embryo flanks (Fig 10A, upper images), the thoracic cage and associated thoracic nerves were removed to allow easier visualization of CM-innervating axons (Fig 10A, lower panels).
Fig 10. Dual control of CM innervation by Fat1 activity in MNs and mesenchyme.
(A) Anti-neurofilament IHC was performed on E12.5 embryos in the context of Prx1-cre-mediated mesenchyme deletion of Fat1 or of Olig2-cre-mediated Fat1 deletion in MNs (S1 Table). After BB-BA clearing, the embryos were cut in half and the internal organs and skin removed to visualize the CM and brachial plexus. Upper panels show the entire flank of Fat1Flox/Flox (left), Prx1-cre; Fat1Flox/Flox (middle), or Olig2-cre; Fat1Flox/Flox (right) at comparable stages. The area covered by CM-innervating axons is outlined with white dotted lines. Lower panels represent a higher magnification of the flank area containing the CM and corresponding motor axons, after manual removal by dissection of a large part of the thoracic cage and nerves. The shape of the control area corresponding to the dCM and vCM is outlined in green and red dotted lines, respectively. (B) Quantifications of the progression of CM innervation. Left and middle plots: for each embryo side, the area covered by CM-innervating axons was plotted relative to the length of the T9 thoracic nerve, comparing Prx1-cre; Fat1Flox/Flox embryos (red dots) to controls (blue dots) on the left plot, and Olig2-cre; Fat1Flox/Flox embryos (green dots) to the same set of controls (blue dots) on the middle plot. Arrows point to the stage of representative examples shown in (A). Right plot: for each embryo, the CM-innervated area/T9 length was normalized to the median ratio of control embryos, by size range. Blue dots: Controls (n = 11, same sample set in left and middle plot, pooling respective littermates); red dots: Prx1-cre; Fat1Flox/Flox (n = 9); green dots: Olig2-cre; Fat1Flox/Flox (n = 7). Underlying data are provided in S1 Data. (C) Whole-mount β-galactosidase staining was performed using Salmon-Gal as substrate on embryos carrying the GdnfLacZ/+ allele, in the context of MN-specific deletion of Fat1, driven by Olig2-cre at E12.5. Top images: side view of the whole flank. Lower images: higher magnification of the area in which the CM spreads. White dotted lines correspond to the area covered by GdnfLacZ+ progenitors, whereas the purple line on the Olig2-cre; Fat1Flox/Flox image indicates the shape of the control area, to highlight the difference. (D) Quantification of the expansion rate of the area occupied by GdnfLacZ+ progenitors. Left plot: for each embryo side, the area covered by GdnfLacZ+ progenitors was plotted relative to the trunk length. Right plot: for each embryo, the GdnfLacZ+ CM area/trunk length was normalized to the median ratio of control embryos. Blue dots: Fat1Flox/Flox; GdnfLacZ/+ (n = 36; same control set as in Fig 7D); red dots: Olig2-cre; Fat1Flox/Flox-; GdnfLacZ/+ (n = 10). Underlying data are provided in S1 Data. Scale bars: (A) top: 500 μm; (A) bottom: 200 μm; (B) 500 μm. BB-BA, benzyl-benzoate/benzyl-alcohol mix; CM, cutaneous maximus; cre, cre recombinase; dCM, dorsal cutaneous maximus; IHC, immunohistochemistry; MN, motor neuron; Olig2, Oligodendrocyte transcription factor 2; Olig2-cre, cre expression in MN progenitors; Prx1-cre, cre expression in the mesenchyme; vCM, ventral cutaneous maximus.
We found that mesenchyme-specific mutants (Prx1-cre; Fat1Flox/Flox embryos) exhibited an overall shortening of the area covered by CM-innervating axons compared to stage-matched controls. This effect was very severe ventrally, with a complete absence of motor axon extending in the vCM (Fig 10A), whereas axons extending in the dCM were present but shorter than in control embryos. To quantify this effect, the area covered by CM-innervating axons was plotted relative to a landmark indicating developmental age, using the length of the T9 nerve (Fig 10B). The net effect quantified by following the ratio area of CM axons/T9 length was almost as severe as the effect measured in constitutive knockouts (Fig 2). This effect was strikingly reminiscent of the profound shortening and shape change of the area occupied by GdnfLacZ+ CM progenitors seen earlier (Fig 7). Thus, Fat1 ablation in the mesenchyme non–cell-autonomously influences not only expansion of the muscle progenitor area but also extension of motor axons projecting in this muscle. This indicates that even in this context of severe perturbation of CM expansion, there remains a strong coupling between motor axons and muscle progenitors, and the topography of muscle fiber extension. To determine whether growth of the CM-innervating axon was dependent on muscle, genetic depletion of the myogenic lineage was achieved through Myf5-cre-driven [80] conditional expression of diphtheria toxin A (DTA) regulated by the R26 locus (R26Lox-LacZ-STOP-Lox-DTA locus [81]). This paradigm resulted in a severe loss of muscle mass, as assessed with the MLC3F-2E-LacZ reporter. In the trunk, X-gal staining shows the loss of myogenic cells corresponding to the CM muscle, to intercostal muscles, and to the trapezius muscle, whereas epaxial muscles, although irregular, were less efficiently deleted (S11B Fig). As previously shown [46, 82], limb-innervating nerves were present and had extended in spite of the absence of muscles (S11B Fig). In contrast, the thoracic intercostal nerves, the CM-innervating motor nerve, and the spinal accessory nerve (or nerve XI), which innervates the trapezius muscle, were severely affected by muscle depletion (S11A and S11C Fig) compared to stage-matched control embryos. These results indicate that genetic suppression of myogenic cells was sufficient to cause a drastic shortening of CM-innervating axons, in contrast to the lack of effect on the trajectory of limb-innervating nerves (S11 Fig). This supports the idea that the coupling between motor and muscle phenotypes observed in Prx1-cre; Fat1Flox/Flox embryos could be mediated by muscle-derived factors.
Although the effect of MN-specific Fat1 ablation appeared less obvious, our method of quantification nevertheless uncovered a mild but significant reduction (around 20%) in the rate of expansion of the area covered by CM-innervating axons in Olig2cre/+; Fat1Flox/Flox embryos compared to age-matched controls (Fig 10A and 10B). Overall, the shape of the innervated area does not change qualitatively, but axons are shorter in Olig2cre/+; Fat1Flox/Flox embryos than in age-matched controls. This indicates that in absence of Fat1, CM motor axons extend at a slower rate. Even though the net effect was small compared to the phenotype of constitutive knockouts, this result supports the notion that Fat1 is required cell-autonomously for CM motor axon growth. Because CM motor axons progress hand in hand with Pax7+/GdnfLacZ+ progenitors, ahead of muscle fiber extension (Fig 3), we next asked if this mild but significant shortening of axons had any impact on the expansion of the CM progenitor area. We therefore brought the GdnfLacZ allele in the context of MN-specific Fat1 mutants and followed the area covered by GdnfLacZ progenitors (Fig 10C and 10D). Unexpectedly, whereas the shape of the GdnfLacZ+ area appeared qualitatively unchanged, area measurements uncovered a significant lowering (of about 25%) of the ratio GdnfLacZ area/trunk length in Olig2-cre; Fat1Flox/Flox; GdnfLacZ/+ embryos compared to Fat1Flox/Flox; GdnfLacZ/+ controls. This result uncovers that Fat1 ablation in MNs non–cell-autonomously interferes with expansion of CM progenitors. This effect may either reflect a direct control by Fat1 in MNs of the process of muscle progenitor spreading, or a Fat1-independent consequence of the slower progression of motor axons. This finding is counterintuitive, as we had previously excluded that MN-specific Fat1 loss had any impact on muscle fiber extension (S8 and S9 Figs). Thus, the shortening of the area covered by GdnfLacZ progenitors is not associated with a reduced progression of myogenic differentiation in the CM. This indicates that the slower migration seen in Olig2-cre; Fat1Flox/Flox; GdnfLacZ/+ embryos does not significantly influence the efficiency of muscle fiber extension.
Thus, the alterations in the CM innervation pattern observed in constitutive Fat1 knockouts appear to result in part from removal of Fat1 axon growth-promoting activity in MNs, and to a large extent from the non–cell-autonomous consequences of Fat1 ablation in the mesenchyme, which simultaneously affects expansion of the CM progenitor area and axonal growth. Although these two non–cell-autonomous consequences of mesenchymal Fat1 ablation could represent two independent phenotypes, the fact that axonal extension of CM-innervating axons requires the presence of myogenic cells supports the possibility that the two phenotypes might be causally linked. Given its known axonal growth promoting activity, GDNF is a likely candidate to explain how impaired myogenic progenitor expansion could lead to inhibited axon elongation in Prx1-cre; Fat1Flox/Flox embryos. In contrast, the observation of a non–cell-autonomous impact of reduced axonal elongation on progenitor expansion in Olig2-cre; Fat1Flox/Flox; GdnfLacZ/+ embryos indicate that motor axons also play a role in promoting expansion of the CM progenitor domain.
Mesenchyme and MN activities of Fat1 are required for MN fate acquisition
We next asked which tissue-specific Fat1 mutant was most closely reproducing the effects on expression of Runx1 and Clusterin in CM motor pools observed in constitutive knockouts (comparing Prx1-cre and Olig2-cre). We found that expression of both genes in the CM motor pools was significantly reduced not only in mesenchyme-specific Prx1-cre; Fat1Flox/Flox mutants but also in MN-specific Fat1 mutants (Olig2cre/+; Fat1Flox/Flox) (Fig 11A–11D). These observations demonstrate that Fat1 simultaneously exerts two complementary functions in MNs and in the mesenchyme, each of which cooperatively contributes to the acquisition of complete CM motor pool identity. Thus, the constitutive knockout phenotype results from the cumulated consequences of abrogating Fat1 functions in both MNs and mesenchyme. Interestingly, the reduction of Runx1/Clusterin expression in Fat1 conditionals is partially reminiscent of the phenotypes of Etv4 (Fig 6E–6J) and of Gdnf mutants (Fig 11A–11D). Furthermore, the degree of reduction in Runx1 and Clusterin expression correlates with the degree of reduction of the area covered by Gdnf expressing myogenic progenitors observed in Prx1-cre; Fat1Flox/Flox and in Olig2cre/+; Fat1Flox/Flox embryos, respectively (Figs 7 and 10). This suggests that the lowering of Runx1 and Clusterin expression could be a consequence of lowering Gdnf levels and of the subsequent changes in Etv4 expression, even though subtle (Fig 6A–6C). However, although comparable, the MN pool specification phenotype of either conditionals or constitutive Fat1 mutants is less severe than the effect of complete Gdnf elimination. In spite of the reduced expression of the myogenic component of Gdnf expression domain in Prx1-cre; Fat1Flox/Flox mutants, the overall GDNF level is maintained, owing to the remaining mesenchymal-Gdnf expression at plexus levels (Fig 8). This minimizes the impact on induction of Etv4, the transcription factor acting upstream of Runx1 and Clusterin (Fig 6). As a result, Etv4 expression is less severely affected in Prx1-cre; Fat1Flox/Flox embryos than in embryos with only one functional copy of Gdnf (compare Fat1Flox/Flox; GdnfLacZ/+ and Fat1Flox/Flox [Fig 11E and 11F]). As a consequence, the residual expression of Etv4 is sufficient to ensure correct mediolateral positioning of MNs in the spinal cord of Prx1-cre; Fat1Flox/Flox embryos (S12A Fig), contrasting with what occurs in Gdnf-/- or Etv4-/- spinal cords (S12A Fig, [39, 41]). However, genetic lowering of Gdnf exacerbates the effect of mesenchyme-specific Fat1 ablation on Etv4 expression when both conditions are combined (Fig 11E and 11F). Consequently, Etv4 expression is significantly lower in Prx1-cre; Fat1Flox/Flox; GdnfLacZ/+ embryos than in either Fat1Flox/Flox; GdnfLacZ/+ or Prx1cre/+; Fat1Flox/Flox embryos (Fig 11E and 11F). The possibility that Gdnf could itself be involved in regulating the subcutaneous progression and number of CM progenitors (thereby indirectly influencing the number of other factors secreted by CM progenitors) is ruled out by the observation of normal expansion of the area covered by GdnfLacZ expressing CM progenitors in GdnfLacZ/LacZ embryos, compared to GdnfLacZ/+ (S12B Fig). Altogether, these data identify Gdnf as an essential mediator of the effect of mesenchyme-specific Fat1 ablation on acquisition of CM motor pool fate, part of which involves the dose-dependent fine-tuning of Etv4 expression.
Fig 11. Fat1 is required in both peripheral mesenchyme and motor neurons for proper specification of CM motor pool identity.
(A–B) Analysis of Runx1 (A, B) and Clusterin (C, D) mRNA expression in brachial spinal cords from E12.5 Fat1Flox/Flox, Prx1-cre; Fat1Flox/Flox, Olig2cre/+; Fat1Flox/Flox, and GdnfLacZ/LacZ embryos. Spinal cords are presented as open book and flat-mounted so that the line in the middle of each picture corresponds to the ventral midline, and the motor columns are visible on each side. Fat1 ablation in both the mesenchyme and MNs leads to significant lowering of Clusterin and Runx1 expression levels, whereas expression is absent in the GdnfLacZ/LacZ embryos. Quantifications in (A–D): each plot in (A) and (C) represents, respectively, the average Runx1 and Clusterin signal distribution (± standard deviation in light blue) measured on the indicated number of spinal cord sides along the white dotted line in each image above. Underlying data are provided in S1 Data. (B) The cumulated Runx1 signal intensity (area under the curves in [A]) was plotted for each spinal cord side. Fat1Flox/Flox (n = 20) Prx1-cre; Fat1Flox/Flox (n = 12), Olig2cre/+; Fat1Flox/Flox (n = 10), and GdnfLacZ/LacZ (n = 4). Underlying data are provided in S1 Data. (D) The cumulated Clusterin signal intensity (area under the curves in (C) was plotted for each spinal cord side. Fat1Flox/Flox (n = 21) Prx1-cre; Fat1Flox/Flox (n = 14), Olig2cre/+; Fat1Flox/Flox (n = 10), and GdnfLacZ/LacZ (n = 6). Underlying data are provided in S1 Data. (E) In situ hybridization analysis of the Etv4 expression domain in brachial spinal cord of flat-mounted E12.5 Fat1Flox/Flox, Prx1-cre; Fat1Flox/Flox, Fat1Flox/Flox; GdnfLacZ/+, and Prx1-cre; Fat1Flox/Flox; GdnfLacZ/+ embryos at E12.5. Whereas Fat1 ablation in mesenchyme mildly affects Etv4 expression, genetic lowering of Gdnf levels further prevents Etv4 induction in Prx1-cre; Fat1Flox/Flox; GdnfLacZ/+ embryos, when compared to either Prx1-cre; Fat1Flox/Flox or Fat1Flox/Flox; GdnfLacZ/+. (E, F) Quantifications and statistical analyses of the sum of signal intensity corresponding to the area under the curves in plots shown in (E): each dot represents the sum of intensity for one spinal cord side. Fat1Flox/Flox (n = 10) Prx1-cre; Fat1Flox/Flox (n = 12), Fat1Flox/Flox;GdnfLacZ/+ (n = 12), and Prx1-cre; Fat1Flox/Flox; GdnfLacZ/+ (n = 12). Underlying data are provided in S1 Data. Scale bars: (A, C, E): 200 μm. CM, cutaneous maximus; cre, cre recombinase; Etv4, Ets variant gene 4; MN, motor neuron; Olig2-cre, cre expression in MN progenitors; Prx1-cre, cre expression in the mesenchyme.
Impact of MN-specific and mesenchyme-specific Fat1 depletion on adult muscle phenotypes
Finally, we sought to evaluate the respective functional relevance of the two sites of Fat1 expression for neuromuscular junction (NMJ) integrity in the adult CM muscle in both MN-specific and mesenchyme-specific mutants. We focused on the dCM, where in spite of delayed myogenesis in mesenchyme-specific mutants, muscle fibers and motor axon have extended. In this region, muscle fibers, visualized by F-actin staining with fluorescent phalloidin, have formed in both mutants (Fig 12A) and have been innervated by motor axons, leading to a normal intramuscular pattern of NMJ distribution in both MN-specific and mesenchyme-specific Fat1 mutants. We analyzed synapse morphology in NMJ-enriched regions of the dCM by labeling the postsynaptic site with alpha-bungarotoxin (α-BTX), which binds acetylcholine receptors (AchRs) and the nerve endings with anti-neurofilament antibodies. As a measurable feature of NMJ integrity, we analyzed the surface area of AchR-rich zones for an average of 23 synapses per mouse, comparing Olig2cre/+; Fat1Flox/Flox and Prx1cre/+; Fat1Flox/Flox mice with Fat1Flox/Flox mice, aged 9–13 months. This analysis uncovered that synapses are reduced in size in both genotypes compared to controls, this effect being more pronounced in Prx1cre/+; Fat1Flox/Flox mice than in Olig2cre/+; Fat1Flox/Flox, with a median synapse area of 420 μm2 (81%) and 220 μm2 (42%), respectively, compared to 512 μm2 (100%) in controls (Fig 12B). A careful analysis of the distribution of synapse areas in each genotype confirmed a significant lowering in the percentage of large synapses and an increase in the percentage of smaller synapses, with up to 68% of synapses in Prx1cre/+; Fat1Flox/Flox mice, compared to 32% in Olig2cre/+; Fat1Flox/Flox and 12% only in Fat1Flox/Flox mice, being smaller than 300 μm2 (Fig 12B). Analysis on the same sections/samples of the distribution of fiber width revealed no significant change in fiber diameter, arguing that the reduced size does not simply reflect adaptation to a change of the muscle fibers themselves (Fig 12C). Instead, this smaller synapse area is indicative of altered NMJ integrity and was accompanied by spots of local denervation of the synapses or by fragmentation of the AchR-rich synaptic area (Fig 12A).
Fig 12. Impact of mesenchyme-specific and motor neuron–specific Fat1 ablation on adult CM muscle anatomy and grip strength.
(A–C) Analysis of NMJ morphology (A) was performed by immunostaining with α-Bungarotoxin (green, detecting AchR), phalloidin (white, detecting F-actin), and anti-neurofilament antibodies on cryosections of the dCM in Fat1Flox/Flox, in Olig2cre/+; Fat1Flox/Flox, and in Prx1-cre; Fat1Flox/Flox adult mice (about 1 year old). (A) Top pictures show low 20× magnifications, whereas bottom pictures show high magnification of individual synapses. (B, C) Histograms showing quantification of NMJ area (B) and fiber diameter (C) distributions in the dCM muscle of Fat1Flox/Flox (n = 9), Olig2cre/+; Fat1Flox/Flox (n = 11), and Prx1-cre; Fat1Flox/Flox (n = 7) mice. Underlying data are provided in S1 Data. (B) Left plot shows the synapse area, with all synapses plotted (circles) for each genotype, as well as the median NMJ area per mouse (with each triangle representing one mouse). An average of 20 synapses per mouse were analyzed in the indicated number of mice. Total numbers of synapses and mice are indicated below the graph. Right plots: average distribution of synapse areas in Fat1Flox/Flox (blue bars) and Olig2-cre; Fat1Flox/Flox (red bars) mice (top) and Fat1Flox/Flox (blue bars, same data as above) and Prx1-cre; Fat1Flox/Flox (green bars) mice (bottom). (C) Left plots: average distribution of fiber diameters in the same samples. Right plots show the median fiber diameter in each mouse (one dot representing one mouse). Statistical significance: * indicates p < 0.01, ** indicates p < 0.001, Mann Whitney test. Underlying data are provided in S1 Data. (D) Measurements of grip strength in mice with the indicated genotypes. In the plots, each dot represents the average value for one mouse, assaying forelimb grip strength (left plot) or cumulative strength of forelimbs plus hind limbs (right). For each mouse, the measured strength was normalized to the mean strength of the control group of the same gender, so that males and females could be expressed as percentages and pooled on the same graph. Underlying data are provided in S1 Data. Statistical significance: p-values are indicated for the relevant comparisons, using unpaired student t test for values with normal distribution and equal variance, or Mann Whitney test, otherwise. Significance threshold: p < 0.01. Scale bars: (A) upper images: 50 μm; (A) lower images: 10 μm. AchR, acetylcholine receptor; CM, cutaneous maximus; cre, cre recombinase; dCM, dorsal cutaneous maximus; NMJ, neuromuscular junction; Olig2-cre, cre expression in MN progenitors; Prx1-cre, cre expression in the mesenchyme.
We next thought to determine if there were consequences in terms of the force-generating abilities of affected muscles. A typical assay is the grip test, which measures the grabbing force (also called prehension force) permitted by the coordinated activity of limb muscles (forelimbs and/or hind limbs). Although phenotypes in the subcutaneous CM muscle are unlikely to contribute to forelimb movements, this assay allows detecting loss of strength in other limb muscles. The subtle defect in NMJ integrity observed in Olig2cre/+; Fat1Flox/Flox mice is expected to be restricted to target muscles of Fat1-expressing MNs. These include the CM muscle and a second pool of Etv4-negative neurons for which we did not identify the target muscle. Overall, this did not result in any significant loss of forelimb grip strength in Olig2cre/+; Fat1Flox/Flox mice compared to control mice (Fig 12C). Nevertheless, Fat1 RNA, Fat1LacZ activity, and Fat1 protein were detected in subsets of lumbar MNs at E12.5 (Fig 1E) and E13.5 (S3B Fig). We therefore asked if Fat1 ablation in these lumbar MNs had any impact in the force-generation capacity of hind limb muscles. A reliable way of approaching this force is to measure forelimb plus hind limb strength (and inferring hind limb strength by subtracting forelimb strength). Consistently, we observed a significant lowering of forelimb plus hind limb grip strength in Olig2cre/+; Fat1Flox/Flox mice compared to Fat1Flox/Flox controls. This indicates that MN-Fat1 is required for the functional integrity of subsets of lumbar MN pools innervating selected hind limb muscles.
In contrast, mesenchyme-specific Fat1 ablation in Prx1cre/+; Fat1Flox/Flox mice leads to significant lowering of both forelimb and the cumulated forelimb + hind limb grip strength, with an effect more pronounced than in Olig2cre/+; Fat1Flox/Flox mice. This is consistent with the possibility that these mesenchyme-specific mice might exhibit myogenesis defects in muscles other than the CM and LD, including muscles in the scapulohumeral and lumbar regions, such defects being likely to affect the amount of muscle-derived factors supporting NMJ maintenance (Fig 7). Altogether, these results show that Fat1, by acting both in MNs and non–cell-autonomously in the mesenchyme, is required to shape CM and scapulohumeral neuromuscular circuits, thus ensuring their anatomical and functional integrity.
Discussion
While the processes of muscle morphogenesis and motor development occur simultaneously, what coordinates these two parallel processes is poorly understood. Here, we have dissected how the Fat1 cadherin controls neuromuscular morphogenesis. We studied this question in the context of morphogenesis of a subcutaneous muscle, the CM, and the associated MN population. This system is an interesting model, in which the process of myogenic differentiation is physically uncoupled from the progression of two pioneer populations—muscle progenitors and motor axons—which appear to lead the way, hand in hand, both of them exposed to cues from the surrounding CT (Fig 13). We show that loss-of-function of the Fat1 cadherin simultaneously disrupts morphogenesis of this muscle, axonal wiring along this muscle, and fate specification by the corresponding MN population. Whereas we previously analyzed what happens when removing Fat1 in myogenic progenitors, in the current study, we address the relative contribution of two other cell types expressing Fat1; these include (a) the CM-innervating MN population and (b) the subcutaneous mesenchymal CT layer, through which CM progression occurs. The present data establish that Fat1 acts in both cell types to modulate distinct aspects of a tissue cross talk coupling muscle shape to MN pool specification (Fig 13): Fat1 is required in mesenchymal cells to control muscle growth and differentiation in a non–cell-autonomous manner. This modulates the amount of a major CM-derived signal, GDNF, and consequently influences CM motor innervation and MN specification. In parallel, we show that Fat1 is required in MNs to modulate axon growth and fate specification.
Fig 13. Fat1 coordinates neuromuscular morphogenesis by playing distinct roles in mesenchyme, muscles, and motor neurons.
(A) Scheme representing the CM neuronal circuit, with Etv4+ MNs in the spinal cord and the CM muscle featuring the area covered by GdnfLacZ+ muscle progenitors in purple, MLC3F-2E+ muscle fibers represented in blue, and CM-innervating axons represented in green. (B) Scheme summarizing event occurring at the migration front, where muscle progenitors and axons of CM motor neurons encounter the surrounding connective tissue and the non–cell-autonomous functions exerted by Fat1 by acting in the mesenchyme. (C) Scheme summarizing event occurring at the front of fiber elongation, where new myocytes produced by the neighboring myogenic progenitors are added to elongating myofibers and the non–cell-autonomous function exerted by Fat1, by acting in the resident mesenchymal cells. (D) Scheme of event occurring at the level of CM-innervating MNs, in which Fat1 cell-autonomously promotes axonal growth and fine-tunes cell fate specification by modulating expression of the Etv4 target genes Clusterin and Runx1 in the CM MN pools. (E) Summary of the roles played by Fat1 in MNs and in mesenchyme/connective tissue. Right: in muscle-associated connective tissue (both at the migration front and at the front of fiber elongation), Fat1 acts by controlling the production of a signal (Fat1 protein itself or a gene activated by a signaling cascade downstream of Fat1), which influences (1) expansion of muscle progenitors, (2) motor axon elongation, (3) myofiber elongation, and (4) MN specification. As a result of mesenchyme-specific Fat1 ablation, the reduced CM muscle produces less GDNF, the depletion of which mildly affects Etv4 expression but severely impacts expression of the two Etv4-target genes Clusterin and Runx1. Left: in CM MNs, Fat1 cell-autonomously modulates axon growth and controls Clusterin and Runx1 expression, either directly or as a consequence of the non–cell-autonomous impact of MNs on myogenic progenitor expansion. Clu, Clusterin; CM, cutaneous maximus; Etv4, Ets variant gene 4; Gdnf, glial cell line-derived neurotrophic factor; Gfra1, glial cell line-derived neurotrophic factor family receptor alpha 1; MHC, myosin heavy chain; MLC, myosin light chain; MN, motor neuron; Myh1, myosin heavy chain 1; PAX7, Paired box 7; Runx1, runt related transcription factor 1.
Mesenchymal Fat1 controls CM muscle growth and impacts MN development
Ablating Fat1 in the mesenchyme profoundly disrupts the progression of progenitor migration and subsequently interferes not only with myogenic differentiation but also with axonal growth and complete specification of the cognate MN pool. This suggests that Fat1 controls the capacity of mesenchymal cells to influence (1) the subcutaneous migration of Gdnf+/Pax7+ CM progenitors, (2) the subsequent elongation of CM muscle fibers, (3) the parallel progression of CM-innervating axons of the Etv4-expressing MNs, and (4) final molecular specialization of this MN neuron pool. The fact that MN-specific Fat1 ablation only alters progenitor migration but not the rate of myogenic differentiation, whereas mesenchyme-specific ablation affects both, suggests that the alteration of fiber extension observed in Prx1-cre; Fat1Flox/Flox embryos is not simply the consequence of an impaired progression of migration but rather reflects a distinct independent function of Fat1 in the mesenchyme, which regulates fiber elongation.
The mechanism by which Fat1 acts in the mesenchyme and whether this involves bidirectional Fat-Dachsous signaling remain to be clarified. An interesting analogous situation occurs during kidney development, in which a Fat4-Dachsous1/2 cross talk controls mouse kidney morphogenesis [30–32]. In this context, Fat4 acts in the kidney stroma (analogous to the CT), whereas Dachsous1 and 2 are required in the Gdnf-expressing cap mesenchyme progenitors of renal tubules for their expansion (analogous to progenitors of the CM muscle) [30–32]. The mechanism identified in our study is also comparable to the feed-forward mechanism occurring in the drosophila wing imaginal disc, allowing wingless-dependent propagation of expression of the selector gene vestigial, to which mechanism Fat-Dachsous signaling contributes several aspects, thereby coupling cell fate and tissue growth [83].
Our data establish that Fat1 activity controls mesenchyme-derived cues acting on myogenesis. HGF and the chemokine cxcl12 are two ideal candidates for mediating such mesenchyme-derived activity. Both factors are known to induce myoblasts’ motility and promote their migration towards the limb bud [62, 84, 85] and to act simultaneously on subsets of MNs to modulate their specification and axon guidance [46, 47, 86]. We recently showed that mesenchyme-specific overexpression of the Met receptor tyrosine kinase, without interfering with HGF expression and secretion, nevertheless prevented the release of biologically active HGF from mesenchymal cells, thereby leading to the absence of migration in the limb [84]. This phenotype was, however, much more severe than the alterations of CM shape observed in mesenchyme-specific Fat1 mutants, making it unlikely for significant changes in the amount of biologically active HGF to account for the muscle-patterning defects observed in mesenchyme-specific Fat1 mutants. The ultimate identification of mesenchyme-derived factors, the production of which is altered by deficient Fat1 signaling, will necessitate unbiased approaches such as analyses of the secretome or transcriptome of wild-type and Fat1-deficient mesenchymal cells. In addition to seeking secreted factors, future lines of research will also evaluate whether the mesenchymal Fat1 activity modulates the mechanical properties of the CT or of the extracellular matrix it produces. Indeed, recent data showed that tissue stiffening on its own is a driver for the collective migration of neural crest cells [87].
Besides acting on myogenesis, we have established that Fat1 activity in mesenchymal cells influences motor axon growth and MN fate specification. These non–cell-autonomous consequences of mesenchymal Fat1 ablation on MNs and muscles could either represent independent phenotypes or could be causally linked to one another. Limb-innervating MNs are known to be prespecified to find their peripheral targets [10, 11] and can correctly execute pathfinding decisions in the surrounding limb mesenchyme in a context in which muscles have been experimentally or genetically ablated [46, 82]. In contrast, we provide experimental evidence supporting the idea that growth of MN axons innervating the CM, the trapezius, or BW muscles is severely impacted by muscle ablation and the subsequent depletion in muscle-derived factors (S11 Fig). This is consistent with our previous work on the parallel activities of HGF/Met on muscle and MN development. Null mutation of the Met receptor abrogates muscle migration and the CM is part of the missing muscles [47, 61]. This phenotype is associated with a complete absence of CM-innervating motor axonal arborization [60]. The null allele cannot distinguish whether this axonal defect is a consequence of the absence of CM muscle or of ablation of Met in MNs. Comparing changes in axonal patterns in null mutants and in neuronal-specific mutants indicated that Met is specifically required for motor innervation of another muscle (the pectoralis minor) but dispensable for the guidance of CM motor axons to and within the CM muscles [60]. Thus, the complete lack of axons matching a CM pattern in Met null mutant embryos is exclusively a consequence of the impaired migration of myogenic progenitors, also implying that CM motor axons along and within the CM are dependent on signals from myogenic cells. Together, these past and present elements support the possibility that altered extension of CM-innervating axons in mesenchyme-specific Fat1 mutants could likewise be a consequence of the impaired CM muscle progression.
We show that in large part, MN phenotypes observed in mesenchyme-specific Fat1 mutants mimic the phenotypes resulting from depletion of one essential muscle-derived factor, GDNF, the production of which by CM myogenic progenitors must be quantitatively affected by the reduced subcutaneous spreading. While mesenchymal Gdnf expression by plexus cells is unaffected, Fat1 ablation in the mesenchyme drastically impairs the subcutaneous progression of the sheet of Gdnf+/Pax7+ progenitors (Fig 8). Our analysis of consecutive cross sections supports the idea of a reduced number of Gdnf+/Pax7+ progenitors, raising the possibility that this effect could result not only from impaired migration but also from impaired proliferation of the progenitor pool. As a result, MN specification defects exhibited by mesenchyme-specific Fat1 mutants partially mimic the effect of Gdnf loss-of-function. Depletion of myogenic-Gdnf is sufficient to impair Runx1 and Clusterin expression, with limited effect on Etv4 induction. Finally, the additive effect of genetic lowering of Gdnf levels and mesenchyme-specific Fat1 depletion indicates that leftover Gdnf is a rate-limiting factor accounting for the residual induction of Etv4 in CM motor pools in Fat1 mutants.
These results do not exclude an alternative scenario, according to which other mesenchyme-derived factors regulated by Fat1 activity would directly act on CM-innervating MNs to control their fate and axonal growth. The fact that we did not detect quantitative changes in the mesenchymal component of GDNF expression argues against the possibility of cell-autonomous regulation of Gdnf promoter by mesenchymal Fat1. As discussed earlier, the likelihood for the HGF amount to be drastically reduced is low, as this would have resulted in much more severe muscle defects. Thus, Fat1-dependent mesenchyme-derived factors influencing MN fate remain to be identified. A recent study described how regionalized mesenchyme-derived signals contribute to the acquisition of muscle-specific adaptation by proprioceptive afferents [88]. In this study, proprioceptive neuron specialization was neither affected by the MNs nor by muscle genetic ablation, pointing instead towards the contribution of mesenchymal cells. The identity of the regionalized mesenchyme-derived signals regulated by patterning activity of the Lmx1b transcription factor also remains unknown [88].
Which subtype of mesenchymal cells mediates such neuromuscular-patterning Fat1 activity? The importance of lateral plate–derived CT for muscle patterning has been evidenced long ago through classical embryological studies [5] and is currently the subject of renewed interest [13]. The CT subtypes likely to influence muscle development include tendon progenitors at the interface between bones and muscles, but also a number of muscle-associated subtypes. Genetic data supporting a contribution of the non-myogenic CT to muscle development have previously been reported [13, 77, 89–91]. Mesenchyme-specific deletions of TCF4, β-catenin, or Tbx4/5 lead to drastic alterations of muscle patterning reminiscent of the phenotypes of Fat1 mutants [77, 91]. Another example of a transcription factor acting in the limb mesenchyme to pattern both muscle shapes and neuronal patterning is Shox2 [92]. These studies converge to identify CT subtypes by the combination of transcription factors they express. Among these transcription factors, Tcf4 expression highlights a subset of CT at muscle extremities distinct from tenocytes, in addition to subsets of myogenic progenitors. Tcf4-expressing CT cells were shown to influence muscle growth and patterning during development, but also adult skeletal muscle regeneration [77, 78]. Cocultures of Tcf4-expressing CT cells with myogenic cells indicated that these cells are capable of producing factors promoting muscle growth and differentiation in vitro [77]. However, we find that the Fat1-expressing subcutaneous layer of CT that delineates the path of CM progenitor migration/extension does not express Tcf4, but instead expresses Pdgfrα and TenascinC. Taking advantage of an inducible Pdgfrα-cre/ERT2 line, we identify the Pdgfrα lineage as critical for Fat1 to influence CM growth and differentiation, because a rate of ablation of 30% of the cells in this lineage is sufficient to significantly delay progression of both progenitor expansion and myogenic differentiation. The severity of this phenotype is lower when driven by Pdgfrα-iCre than in the context of the broad deletion mediated by Prx1-cre, supporting the idea of a dosage-dependent effect affecting the concentration of secreted factors. It remains possible that some of the muscle-patterning Fat1 activity might be executed in other CT subtypes, as Fat1 ablation in the Pdgfrα-dependent lineage did not reproduce the phenotypes observed in scapulohumeral muscles. It will be interesting in the future to define the muscle-associated CT subtype that most accurately overlaps with the Fat1 expression domain and defines its domain of activity. CT at the interface between the CM muscle and the skin must be distinct from classical tendons, which connect skeletal muscles to bones for force transmission. Candidate transcription factors that could highlight a signature for the Fat1-dependent CT subtype include Gata4, Tbx3, Osr1, and Osr2 [93, 94], which were recently described as markers and essential players in defining distinct CT subtypes, respectively controlling morphogenesis of the diaphragm and of subsets of forelimb muscles [89, 90, 95]. The fact that Fat1 deficiency did not alter diaphragm development or lead to diaphragmatic hernias such as those occurring in the absence of Gata4 make it unlikely for Fat1 to be required in the Gata4+/diaphragm CT subtype. In contrast, although CM development was not directly assessed in these studies, the changes in limb muscle patterning observed upon deletion of Tbx3 or Osr1 in the lateral plate–derived lineage (Prx1-cre) include selective alterations of muscle attachment sites or fiber orientation reminiscent of those occurring in Fat1 mutants [89, 95].
The role played by the Pdgfrα-dependent lineage in Fat1-driven developmental myogenesis is interesting in light of the emerging role of muscle-resident CT lineages for adult skeletal muscle. Pdgfrα expression distinguishes a population of muscle-resident cells called fibroadipogenic progenitors (FAPs) [96, 97]. These cells do not directly contribute to the myogenic lineage but are bipotential progenitors capable of giving rise to adipocytes and to CT fibroblasts [96, 97]. Whereas FAPs are quiescent under homeostatic conditions, muscle lesions activate their proliferation and differentiation [97], in addition to activating muscle stem cells’ proliferation and triggering the production of new muscle fibers [2]. This damage-induced FAP recruitment was shown to contribute to fibrosis and fat infiltrations in the context of either acute lesions or chronic degeneration/regeneration cycles occurring in a mouse model of Duchenne muscular dystrophy (mdx mice) [98, 99]. In cocultures of FAPs and muscle stem cells, FAPs promote myogenic differentiation, indicating that the recruitment and activation of FAPs in degenerating muscles could contribute to the efficiency of muscle repair [97]. Future work will establish whether Fat1 activity is also necessary for the adult FAP lineage to exert its myogenesis-promoting activity during muscle homeostasis and repair.
Motor neuronal Fat1—Dissecting direct and indirect contributions to axonal growth, MN specification, and muscle spreading
We find that in addition to its mesenchymal contribution, Fat1 plays additional cell-autonomous functions in the MN pool innervating the CM muscle. Fat1 protein is selectively expressed in the CM MN pool, likely distributed along their axons. MN-specific Fat1 deletion negatively influences axonal growth of CM-innervating axons and alters acquisition of MN fate characteristics. Unexpectedly, although MN-specific Fat1 ablation does not significantly influence the rate of myogenic differentiation, our quantitative analysis uncovers a significant delay in the subcutaneous progression of muscle progenitor migration. This uncovers an unsuspected role of motor axons in the rate of progression of muscle progenitor migration.
The MN specification and axonal growth defects occurring in absence of MN-Fat1 may reflect a cell-autonomous role of Fat1 signaling in MNs. Future lines of investigation will determine whether such a Fat1 signaling cascade involves interactions with Dachsous cadherins and how it influences axon growth and modulates Runx1 and Clusterin expression. It will be relevant to discriminate whether MN-Fat1 (1) modulates the response to—or reception of—GDNF by controlling receptor expression, or efficiency of the GDNF/Ret signal transduction; (2) impinges on Etv4 transcriptional activity by regulating its phosphorylation; or (3) acts directly on target gene expression via other cascades known to be downstream of FAT cadherins, such as Hippo/YAP or the transcriptional repressor atrophin. As a protocadherin, Fat1 may also refine neural circuit assembly by mediating axonal self-recognition similar to functions of clustered protocadherins [100, 101]. Alternatively, the two phenotypes could simply reflect the consequence of the reduction in GDNF levels resulting from the altered progenitor progression also resulting from MN-specific Fat1 ablation. Both phenotypes are reminiscent of the consequences of Gdnf ablation (as illustrated here and in [39]). Gdnf is also known for exerting an axon growth promoting role [102], and in MN-specific Fat1 mutants, the length of CM motor axons remains correlated with the size of the Gdnf-expressing progenitor sheet.
We have uncovered that CM motor axons progress hand in hand with Gdnf+/Pax7+ muscle progenitors and shown that disrupting Fat1 activity in MNs interferes with the rate of subcutaneous progression of CM progenitors. This effect is, however, less predominant than the dependency of CM progenitor progression on mesenchymal Fat1 activity. Such an influence of MNs on myogenic progenitors is counterintuitive, because previous studies supported the idea that muscle development can occur normally in absence of motor innervation [103, 104]. These studies were examining NMJ maturation in the diaphragm muscle at fetal stages after genetic depletion of MNs. They showed that whereas a pre-pattern of AChRs prefiguring future synapses does occur in the diaphragm in absence of motor axons, the latter are required for subsequent NMJ maturation. The maturation-inducing activity elicited by MNs on muscles is mediated by signals such as Agrin, inducing AChR clustering [105]. These studies do not exclude, however, that the dynamics of muscle morphogenesis could have been altered at the primary myogenesis stage in some vulnerable muscles and compensated at secondary myogenesis stages. Such an influence of MNs on the development of another tissue type is also reminiscent of the role exerted by MNs, by way of VEGF secretion, on vascular development [106]. Finally, because axonal elongation is concomitant with spreading of the CM myogenic progenitor, motor axons and myogenic progenitors collectively expose themselves to the mesenchymal landscape and to mechanical properties of the extracellular matrix (ECM), such as its stiffness. Interfering with the progression of one of the two cell types thus exposes the other on its own to the mesenchyme, rendering it less permissive and slowing the collective progression.
In spite of this delayed progenitor progression, myogenic differentiation is not impacted in the absence of MN-Fat1, and the resulting anatomy of the adult CM is normal. MN-specific Fat1 ablation leads to significant functional impact on NMJ integrity in absence of anatomical abnormalities of the muscle and of changes in muscle fiber diameter. This highlights the importance of MN-Fat1 in ensuring NMJ integrity. This effect could be the direct or indirect consequence of (1) Clusterin or Runx1 lowering in MNs, (2) reduced GDNF levels in muscle progenitors, (3) a reduced number of muscle progenitors persisting in the adult muscle, or (4) the deregulation of other target genes influencing NMJ integrity that remain to be identified.
Relevance for facioscapulohumeral dystrophy
Muscle phenotypes caused by disruption of Fat1 functions are highly regionalized, and the topography is reminiscent of the map of muscles undergoing degeneration in facioscapulohumeral dystrophy (FSHD) [14]. FSHD is the second most frequent muscular dystrophy, characterized by the selective map of affected muscles involving facial expression and shoulder muscles [107]. In most cases, FSHD is caused by genomic alterations on chromosome 4q35, leading to excess production of the double homeodomain protein DUX4, a transcription factor encoded in a repeated macrosatellite, D4Z4, normally active only during zygotic gene activation [107, 108]. This overexpression is permitted by a genetic context simultaneously involving (1) a polymorphism stabilizing DUX4 RNA by creating a poly adenylation signal [109] and (2) the loss of epigenetic DUX4 repression [110]. Such epigenetic de-repression results (a) in the most frequent form of the disease (FSHD1), from deletions that shorten the D4Z4 repeat array [110], and (b) in less frequent cases (FSHD2), from mutations in genes involved in modulating the chromatin architecture, SMCHD1 [111], or in DNA methylation (DNMT3A [112]). DUX4 exerts a toxic effect on muscles by deregulating the transcription of multiple targets [113–115]. Individuals carrying a pathogenic context exhibit a high variability in symptom severity [116, 117], implying that other factors behave as disease modifiers.
The similarity between muscular and nonmuscular phenotypes of Fat1 mutants and FSHD symptoms indicates that Fat1 deficiency phenocopies some FSHD symptoms [14], raising the possibility that FAT1 dysfunction in FSHD-relevant tissue types could contribute to some of these phenotypes. This possibility was supported by the identification of pathogenic FAT1 variants in human patients, in which FSHD-like symptoms were not caused by the traditional genetic causes of FSHD [14, 118]. Furthermore, FAT1 is located in the vicinity of the FSHD-associated D4Z4 array on 4q35, and its expression is down-regulated in fetal and adult muscles of FSHD1 and FSHD2 patients [14, 119], suggesting that traditional FSHD-causing contexts may not only enhance DUX4 expression but also lead to lowered FAT1 expression in affected muscles. Although exogenous DUX4 can down-regulate FAT1 expression when exogenously expressed in human myoblasts, short hairpin RNA (shRNA)-mediated DUX4 silencing in FSHD1 myoblasts did not restore FAT1 expression levels [119]. Thus, FAT1 deregulation by a pathogenic 4q35 context is not attributable to DUX4 in FSHD1 myoblasts and might be caused by DUX4-independent mechanisms. First, the loss of epigenetic repression of DUX4 might be due to changes in the chromatin architecture resulting from deletions of anchoring sites for chromatin folding proteins such as CTCF or SMCHD1 [111, 120]. Given the proximity of FAT1 with the D4Z4 array, the same changes in chromatin architecture could simultaneously lead to lowered FAT1 levels. Second, among genetic variants in FAT1, a copy number variant (CNV) deleting a putative regulatory element in the FAT1 locus, which has the potential to deplete FAT1 expression in a tissue-specific manner, was enriched not only among FSHD-like patients but also among classical FSHD1 and FSHD2 [14, 119]. Furthermore, given the chromosomal proximity, genetic alterations in FAT1 have a high probability to co-segregate with a pathogenic FSHD context occurring on the same 4q chromosome. Support for this came from the recent identification of pathogenic FAT1 variants associated with a classical FSHD1 context [121]. Thus, the combination of a pathogenic 4q35 context with pathogenic FAT1 variants, whether functional or regulatory, has the potential to synergize to reduce FAT1 expression and/or activity below a functional threshold, potentially contributing to worsening FSHD symptoms. Other genetic modifiers affecting chromatin methylation [111, 112] and/or architecture of the 4q35 arm [122], hence affecting gene regulation, may simultaneously impact DUX4 and FAT1 expression.
Therefore, elucidating Fat1 functions during development could provide instructive information to guide research on FSHD pathogenesis or on pathologies associated with FAT1 alterations in humans. Our findings indicate that myoblasts may not be the only relevant cell type in which FAT1 down-regulation must occur to alter muscle development, and identify mesenchyme in developing embryos as another cell type in which Fat1 disruption is sufficient to cause muscle shape phenotypes with an FSHD-like topography. Whether FAT1 is indeed deregulated in CT of FSHD patients, and whether such regulation change is accounted for by DUX4 or by 4q35 chromatin architecture changes, remains to be established. Several FSHD mouse models exist, some of them engineered for inducible and tissue-specific DUX4 overexpression, and will be instrumental to assess to what extent and through which mechanism loss of Fat1 function could synergize with enhanced DUX4 levels [123–126]. Furthermore, our results also identify GDNF as a muscle-derived signal, the lowering of which is associated with muscular phenotypes of Fat1 mutants and has consequences at least on neuronal development and possibly on NMJ integrity in adult muscle. Interestingly, GDNF appeared significantly down-regulated in FSHD biopsies in at least two reports [127, 128]. Another study suggests instead that FSHD involves enhanced Ret signaling in myoblasts and proposes Ret inhibitors as a possible therapeutic strategy [129]. In view of these reports and together with our present findings, it may be relevant to determine whether and to what extent modulation of GDNF/Ret signaling, or of other mechanisms influenced by Fat1 signaling, can have any benefit to alleviate adult muscle symptoms and improve quality of life of FSHD patients. Finally, developmental alterations such as those occurring in the Etv4/GDNF circuit might constitute a topographic frame in which muscles have deficient functional output. It will therefore be necessary to understand whether and how this renders the muscles more susceptible to degeneration in adults in an FSHD context and whether FAT1 dysfunction at adult stages further destabilizes muscle homeostasis and contributes to degeneration.
Materials and methods
Ethics statement
All procedures involving the use of animals were performed in accordance with the European Community Council Directive of 22 September 2010 on the protection of animals used for experimental purposes (2010/63/UE). The experimental protocols were carried out in compliance with institutional Ethics Committee guidelines for animal Research (comité d’éthique pour l’expérimentation animale–Comité d’éthique de Marseille) and in compliance with French law, under an agreement (Number D13-055-21) delivered by the “Préfecture de la Région Provence-Alpes-Côte-d’Azur et des Bouches-du-Rhône.” When collecting embryos, adult mice were euthanized by cervical dislocation. For collection of adult muscle samples, mice were first anesthetized with lethal doses of Ketamin and Xylasine, and euthanized by transcardiac perfusion with 4% PFA.
Mouse lines
All the mouse lines and genetic tools used here are described in S1 Table, providing for each of them a link to the mouse genome informatics (MGI) information page, a reference describing their production and some brief explanations on what they are, how they were made, and how they are used in the present study. The alleles of the Fat1 gene used in this study included a genetrap knockout/knock-in allele Fat1KST249 (referred to as Fat1LacZ) [130], as well as a constitutive knockout Fat1ΔTM allele (here referred to as Fat1-) and the conditional-ready Fat1Fln allele from which it derived, that we previously described [14]. As the original conditional-ready Fat1Fln allele still carried a neo selection cassette flanked by FRT sites [14], we have crossed Fat1Fln/+ mice with mice carrying the Flp recombinase (ActFlpe mice, official name Tg(ACTFLPe)9205Dym [131]), thereby producing a new conditional-ready allele referred to as Fat1Flox, used throughout this study. The Cre lines used for tissue-specific ablation were either transgenic lines (official MGI nomenclature Tg(gene-cre)author), or knock-in/knockout alleles (official MGI nomenclature genetm(cre)author). These include Olig2-cre (knock-in/knockout Olig2tm1(cre)Tmj [72]); Wnt1-cre (transgenic Line Tg(Wnt1-cre)11Rth [75]); Prx1-cre (transgenic Line Tg(Prrx1-cre)1Cjt [73, 74]), Pax3-cre (knock-in/knockout line Pax3tm1(cre)Joe [64]), and the tamoxifen-inducible Pdgfrα-iCre line (BAC transgenic line Tg(Pdgfrα-cre/ERT2)1Wdr [79]). To produce embryos or mice Cre+; Fat1Flox/Flox, all cre alleles were transmitted by males only to avoid female germ line recombination by crossing Cre+; Fat1Flox/+ males or Cre+; Fat1FloxFlox males with Fat1FloxFlox females, the latter carrying one of the reporter genes described below (MLC3F-2E-LacZ, GdnfLacZ, R26-YFP). Knock-in/knockout Pea3-lacZ mice (here referred to as Etv4-) were used with the permission of S. Arber and T. Jessell and genotyped as previously described [41]. Transgenic mice carrying the BAC transgene Etv4GFP have been described previously [60]; knock-in/knockout GdnfLacZ mice were used with permission of Genentech, [39, 132]; knock-in/knockout mice carrying the Metd allele produce a signaling dead version of the Met receptor [61]. Additional lines used in this study are: Rosa26-YFP mice (Gt(ROSA)26Sortm1(EYFP)Cos line; Jackson laboratory mouse strain 006148 [66]); MLC3F-2E-LacZ mice (transgenic line Tg(Myl1-lacZ)1Ibdml [58, 59]); HB9-GFP (transgenic line Tg(Hlxb9-GFP)1Tmj allele [133]); Myf5-cre mice (Myf5tm3(cre)Sor line; Jackson laboratory mouse strain 007893 [80]); R26Lox-LacZ-STOP-Lox-DTA mice (also called R26DTA or Gt(ROSA)26Sortm2(DTA)Riet line [81]). All lines were maintained through backcrosses in B6D2 F1-hybrid background.
X-gal and Salmon-Gal staining for β-galactosidase activity
Staining for β-galactosidase activity was performed using two alternative methods. To detect LacZ expression in embryos with the MLC3F-2E-LacZ transgene or Fat1LacZ allele, we performed classical X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) staining with standard protocols using potassium ferricyanide and potassium ferrocyanide. Embryos were then postfixed in PFA, partially clarified in 1 volume glycerol/1 volume PFA 4%, and mounted in 100% glycerol on glass slides with depression, under coverslips with glass spacers for imaging. When detecting LacZ expression in embryos carrying the GdnfLacZ allele or Fat1LacZ allele on cryosections, we selected the more sensitive method using Salmon-Gal (6-chloro-3-indolyl-beta-D-galactopyranoside, Appolo Scientific UK, stock solution 40 mg/mL in DMSO) in combination with tetrazolium salt (nitro blue tetrazolium [NBT], N6876 Sigma), stock solution 75 mg/mL in 70% DMF), using the method we previously described [46]. Briefly, after initial fixation (for GdnfLacZ: 15 minutes in 1% PFA; 0.2% glutaraldehyde in PBS), embryos were rinsed in PBS and incubated overnight, in order to avoid background staining, at 37 °C in a solution containing 4 mM potassium ferricyanide, 4 mM potassium ferrocyanide, 4 mM MgCl2; 0.04% NP40, but without substrate. Embryos were then rinsed 3 times in PBS and incubated in a solution containing Salmon-Gal (1 mg/mL), NBT (330 μg/mL), 2 mM MgCl2; 0.04% NB40, in PBS. After staining completion, embryos/sections are rinsed in PBS and postfixed in 4% PFA. For GdnfLacZ, the staining reaction is completed in 30–40 minutes. For staining of cryosections of Fat1LacZ embryos, the staining reaction is completed in 1–2 hours.
Simple and double in situ hybridizations
Embryos were collected in cold PBS, and when necessary, spinal cords were dissected at that stage. Dissected spinal cords and the remaining embryo trunks or whole embryos were then fixed overnight in 4% paraformaldehyde (in PBS), dehydrated in methanol series, and stored in methanol at −20 °C. In some cases, embryos were embedded in gelatin/Albumin and sectioned on a vibratome (80 um sections). These floating sections were then dehydrated in methanol series and treated like whole-mount embryos. In situ hybridizations were done as previously described [47]. Briefly, after methanol dehydration, tissues (embryos, spinal cords, or vibratome sections) were rehydrated in inverse methanol series, treated with proteinase K, hybridized with Digoxigenin-labeled probes, washed with increasing stringency, incubated with anti-Digoxigenin antibodies conjugated with alkaline phosphatase (Roche), and developed with NBT/BCIP substrates. For double in situ hybridizations, hybridization was done by mixing a Digoxigenin-labeled and a Fluorescein-labeled probe. Revelation of the two probes was done subsequently. We first incubated the samples with the AP-conjugated anti-digoxigenin antibody (1/2,000, Roche), washed, and stained using NBT/BCIP (blue color). Samples were postfixed, cleared with 50% glycerol, and flat-mounted to be photographed with the first color only (blue). To start the second staining, the samples were then transferred back in PBT (PBS, 0.1% Tween-20). The remaining alkaline phosphatase was then inactivated for 10 minutes with 0.2 M glycine pH 2.2; 0.1% Tween-20. Samples were further rinsed 4 times in PBT, after which they were incubated with AP-conjugated anti-fluorescein antibody (Roche, 1 in 2000 dilution). After washing, this second staining was revealed using INT/BCIP substrate (Roche, red color). Samples were postfixed again, cleared in 50% glycerol, and flat-mounted for visualization.
Immunohistochemistry
Embryos were collected in cold PBS and fixed 2–4 hours in 4% PFA on ice. Adult mouse tissues were collected after perfusing anesthetized mice with 4% PFA and were postfixed for 2–4 hours in 4% PFA. The samples were rinsed in PBS, cryoprotected in 25% sucrose-PBS solution, embedded in PBS, 15% sucrose/7.5% gelatin, and cryostat sectioned (10 μm for embryos, 30 μm for NMJ analysis in adult muscle longitudinal sections). For fluorescent IHC, slides were thawed in PBS, then incubated in PBS, 0.3% triton-X-100, bleached in 6% H2O2 for 30 minutes, and rinsed again in PBS, 0.3% triton-X-100. For some antibodies (see S2 Table), heat-induced epitope retrieval was performed in 0.1 M citrate buffer pH 6 at 95 °C for 5–30 minutes (specific time depending on the antibody used). Slides were rinsed again 3× in PBS, 0.3% triton-X-100 and then incubated overnight at 4 °C with primary antibodies in 20% newborn calf serum, PBS, 0.3% triton-X-100 in a humidified chamber. Primary antibodies or detection tools used are described in S2 Table. After extensive washing in PBS, 0.3% triton-X-100, slides were incubated with secondary antibodies in blocking solution as above, for 1 hour and 30 minutes at room temperature in a humidified chamber. Secondary antibodies used were conjugated with Alexa-555, Alexa-488, or Alexa-647 (see S2 Table). After extensive washing in PBS, 0.3% triton-X-100, slides were quickly rinsed in distilled water and mounted in mounting medium with ProlongGold antifade reagent and DAPI. Slides were kept at 4 °C and imaged with a Zeiss Z1 Microscope equipped with Apotome for structured illumination, using Zen software. Whole-mount anti-neurofilament staining of E12.5 embryos was performed as previously [46], using the mouse monoclonal anti-neurofilament (2H3) antibody (bioreactor version, dilution 1/300) and anti-mouse-HRP secondary antibody (Sigma). Briefly, embryos were fixed 2–4 hours in 4% PFA (when relevant, an X-gal staining was also carried out), rinsed in PBS, then transferred in Dent’s fixative (80% methanol; 20% DMSO) and kept at 4 °C until use. A bleaching step with hydrogen peroxide was carried out by incubating embryos overnight in a solution with 1 volume 30% H2O2; 4 volumes Dent’s fixative. All washes were made in PBS, 1% triton X-100. Antibody incubations were done by incubating at least 3 days (at least one at room temperature) in a blocking solution containing 79% newborn calf serum, 20% DMSO, 1% triton X-100, and thimerosal. The final HRP color reaction was performed with DAB tablets (SIGMA) and H2O2, after rinsing in 0.1 M Tris pH 7.4. After staining completion, embryos were dehydrated in methanol, cleared in 2 volumes benzyl benzoate/1 volume Benzy-Alcohol mix (BB-BA mix; once embryos are in 100% methanol, they are transferred in a mix 50% volume methanol; 50% volume BB-BA, for 30 minutes, then twice in 100% BB-BA mix), cut in half, and mounted on glass slides with depression, under coverslips with glass spacers for imaging.
Tamoxifen induction
In cases of crosses with the Pdgfrα-iCre line, tamoxifen induction was performed by intraperitoneal (IP) injection in pregnant females. Tamoxifen (T5648, Sigma) was dissolved first in ethanol (100 mg/mL) under agitation (in a thermomixer) for 2 hours at 42 °C, then diluted in sterile corn oil (C8267, Sigma) at 10 mg/mL, kept under a chemical hood at room temperature to let the ethanol evaporate, and aliquoted (2 mL aliquots) for storage at −20 °C. One working aliquot was kept at 4 °C for 1–2 weeks. To achieve recombination in mesenchyme-derived loose CT, two successive injections were performed in pregnant females, first at E9.75 at a dose of 50 μg/g (per gram of tissue weight), and second the following day at a dose of 100 μg/g. Administration was done by IP injection using a 26G syringe. Efficiency of recombination was assessed using the reporter line R26-YFP, first by following overall intensity YFP fluorescence on freshly dissected embryos, and later by performing anti-YFP IHC on cryosections, combined with markers of tissue types.
Image processing for quantification of signal intensity
Quantifications of signal intensity were performed using the Image J software (NIH) as previously described [46, 84]. Briefly, color images were first converted to gray scale and inverted to negative scale (a scale with white for highest signal intensity [250] and black for the lowest intensity [0]). An area of fixed pixel width was cropped, and signal intensity was measured with ImageJ along a horizontal band with the width of the cropped image (500 pixels, matching half a flat-mounted spinal cord, along dotted lines in quantified pictures, always ending in a fixed positional landmark, such as the spinal cord midline), and height of a few pixels. After background subtraction (background being the baseline value of the control samples in the same experiment, and baseline being the minimum value in a fixed window, systematically avoiding points such as the midline, where changes in tissue thickness influence background intensity) and threshold subtraction (to avoid negative values), the values were expressed as a percentage of the mean maximal amplitude of the control samples used in the same experiment (so that experiments developed independently can be normalized to their respective controls, and pooled). A mean distribution along the line is generated by averaging the normalized intensities between several samples of each genotype (considering left and right sides separately, the number of spinal cord sides used, for each plot being indicated in the corresponding figures) to generate an average signal distribution plot, shown ± standard deviation). The total signal intensity value was also calculated for each sample by measuring the area under the curve and plotted individually as percentage of the mean control value.
Area measurements
Area measurements were used to assess CM subcutaneous progression and NMJ size. Whole embryo images were acquired on a Leica Stereomicroscope, using Axiovision software. We used the area/distance measurement tools in the Axiovision software. For each embryo side, we measured the area occupied by the CM, detected with MLC3F-2E-LacZ by X-Gal staining, with GdnfLacZ using Salmon-Gal staining, or with anti-neurofilament IHC. As reference measurements for normalization, we measured structures that grow proportionally with the embryo age. For this, we measured, respectively, the area occupied by BW muscles (MLC3F-2E-LacZ), the length of the trunk between two fixed GdnfLacZ+ landmarks (shoulder to posterior limit of the hind limb), and the dorsoventral length of a given thoracic nerve (T10 or T9) for anti-neurofilament stainings. For all measurements, the first graphs show the raw values, whereas the second plots represent the normalized area, obtained by calculation the ratio CM area/reference value, and dividing this value by the median ratio for a set of control embryos for normalization. For LacZ-stained embryos, measurements were done on embryos irrespective of the storage time and medium (PBS/PFA or glycerol, with different degrees of tissue shrinkage); normalization was typically done by comparing embryos of the same litter and mounted/imaged at the same time. For anti-neurofilament staining, as all embryos were stored in BB-BA (where tissue shrinkage is invariable), normalization was done by ranking embryos by size range (according to the thoracic nerve length) and comparing embryos in the same size range.
For NMJ area measurements, IHC was performed on 30 μm thick longitudinal cryosections of the dCM muscle to detect the neuromuscular junctions (with α-BTX), the nerve endplate (with anti-neurofilament antibodies), and the muscle fiber F-actin (with Phalloidin) (see S2 Table for reagents). NMJ images were acquired on Zeiss Z1 Microscope equipped with Apotome for structured illumination and processed using Zen Imaging software (Zeiss) or Axiovision software. After NMJ Image acquisition, a maximal intensity projection image was generated. The latter was converted from .czi to .zvi file, and we used the area measurement tool in the Axiovision software. For each mouse, a minimum of 20 NMJs were measured in the dCM using pictures acquired at magnifications ranging from 10× to 63× (keeping the scale adapted to the magnification to measure areas in μm2), and the percentage of NMJs matching a set of area ranges was determined per mouse. As control, on the same set of images, the diameter of each muscle fiber was also measured (on longitudinal sections, the MIP shows the maximal width across the section thickness, thus accurately measuring fiber diameter). Even when no specific staining of the fiber was performed, background levels of the α-BTX staining are sufficient to accurately detect muscle fibers and measure their width. For each mouse, around 20–50 fiber diameters were measured, and the percentage of fibers matching a set of diameter ranges was determined in each mouse. The NMJ area and fiber diameter size distribution graphs in Fig 12 represent mean percentage in each area range for each genotype ± SEM, indicating the number of mice used next to the genotype indication. Control data from Fat1FloxFlox mice are shown twice (in blue) in both graphs, to make readability easier.
Grip strength measurements
Grip tests were performed on adult mice using the Grip test apparatus (Bioseb), which measures the maximum force exerted by the mouse as it pulls a sensitive device (grid) until release of the grip. We measured either forelimb strength only or the cumulated force of forelimbs + hind limbs (when a mouse uses all 4 limbs to pull the measurement grid). As raw force in the measured unit (Newton) was significantly different between males and females (controls), the data were normalized for each gender and genotype by dividing the measured value by the average value of the control group of the same gender. This allows expressing the output in percentage of the control value, set as 100% in average, hence allowing us to pool data from males and females. In the results shown in Fig 12, male and female data have been pooled, as the separate analysis of both genders gave similar results. Three to four measurements were made for each mouse, and an average value was calculated. In both the pooled and non-pooled graphs, each dot represents the average value for one mouse. Statistic tests were carried out on the average values.
Statistical analysis
StatEL (add-in to Excel, by Ad Science, Paris, France) was used for statistical analyses. For comparisons between two groups, differences were assessed either using the unpaired Student t test, when data were showing a normal distribution and equal variance, or using the nonparametric Mann Whitney test otherwise. All p-values are indicated in the figures, except for Fig 9, where * indicates p-value < 0.01, whereas ** indicates p-value < 0.001. Differences were considered significant when p < 0.05. For signal intensity distribution curves, average values ± standard deviations are shown (in light blue). For dot plots, all individual data are plotted (one per sample), and the median value is shown with a black bar.
Supporting information
(XLSX)
(XLSX)
(XLSX)
(A) Whole-mount β-galactosidase staining was performed on Fat1+/+; (left) and Fat1-/- (right) embryos carrying the MLC3F-2E transgene (S1 Table). Top images represent a side view of entire embryos and indicate the position of higher magnification areas, including that of the top pictures in Fig 1A and 1B as well as the upper forelimb region (with scapulohumeral muscles) and distal forelimb, showing a region where dispersed myocytes accumulate in Fat1-/- embryos. The second row images highlight a closer view of the upper thoracic and forelimb region. The dotted lines indicate for each genotype the levels and angle corresponding to the three consecutive sections shown in (C) and (D). (B) Quantification of the number of dispersed myocytes found in orange areas in the forelimb (left plot) and of the area occupied by the ectopic humeral muscle (ectop) appearing between the spinodeltoid (Del) and the triceps brachii (TriBra) muscles (right plot). These data reproduce and confirm our own previous results. Underlying data are provided in S1 Data. (C, D) Cross sections of control Fat1+/- and mutant Fat1-/- E12.5 embryos, featuring three consecutive sections at forelimb levels (Level 1 and Level 2) and upper thoracic level (Level 3), immunostained with antibodies against Pax7 (red), Myh1 (green), and neurofilament (white) and with DAPI (blue). Images in (D) represent high-magnification views of the area highlighted with the yellow dotted square in (C). These data confirm (1) the severe reduction in thickness of the CM muscle (Level 3, and higher magnification in [D]), (2) the presence of a robust ectopic muscle next to the triceps brachii (Levels 2 + 3, and higher magnification in [D]), and (3) the presence of dispersed myogenic progenitors and muscle fibers in the ectopic subcutaneous position in the forelimb (the image in [D] shows higher magnification of an area between the digit extensors and the skin). Lack of obvious phenotype in the diaphragm is also shown. CM, cutaneous maximus; Del, spinodeltoid; diaph, diaphragm; disp. Myo, dispersed myoblatsts; ectop, ectopic humeral muscle; ext. dig, extensor digitorum; trap, trapezius; TriBra, triceps brachii.
(TIF)
Expression of Gdnf (A) and of three markers of developing muscles (MyoD, Six1, and Lbx1 [B]) was analyzed by in situ hybridization on floating vibratome sections of E12.5 wild-type (left panels) and Fat1LacZ/LacZ embryos (right panels). (A) Gdnf expression is visualized at three successive anteroposterior levels, showing a hot spot at the brachial plexus (mesenchymal cells around passing nerves), where Gdnf expression is drastically reduced by the absence of Fat1, and two successive levels in the CM and LD muscles, where Fat1LacZ/LacZ embryos exhibit a thinner CM with less overall Gdnf signal. (B) On sections corresponding to the anterior part of the CM muscle, expression of markers of muscle differentiations (MyoD, Six1, and Lbx1) also shows that Fat1LacZ/LacZ embryos exhibit a selective loss of staining in the CM and not other neighboring muscle masses. CM, cutaneous maximus; Gdnf, glial cell line-derived neurotrophic factor; Lbx1, ladybird homeobox 1; LD, latissimus dorsi; MyoD, myogenic differentiation 1; Six1, sine oculis-related homeobox 1.
(TIF)
(A, B) The nerve pattern was analyzed by IHC with antibodies against neurofilament (2H3 antibody) (A) or by taking advantage of the Hb9-GFP transgene (S1 Table) (B), which labels motor neurons and their axons. (A) Anti-neurofilament histochemistry on whole-mount wild-type and Fat1LacZ/LacZ embryos at E12.0. (B) Hb9-GFP was visualized with antibodies against GFP (top and middle images) or by direct fluorescence imaging in Fat1+/+; Hb9-GFP+ and Fat1LacZ/LacZ; Hb9-GFP+ embryos at E12.5. For IHC, embryos were cut in half, cleared in BB-BA, and flat-mounted. Upper panels (A, B) are low-magnification images of the embryo flank. Lower panels show high-magnification views of the area containing the CM muscle. The area covered by CM-innervated axons is highlighted in white (middle panels). In the middle panels in (B), axons of vertically oriented thoracic spinal nerves have been manually removed by dissection to improve visibility of CM axons. In lower panels in (B), direct GFP imaging was done on PFA fixed embryos, after flat-mounting of the flank. (C) Quantifications of the relative expansion of the area covered by CM-innervating axons. Upper plot: for each embryo side, the area covered by CM-innervating axons is plotted relative to the length of a thoracic nerve (T10, from dorsal root origin to ventral tip). Arrows point the stages of representative examples shown in (A) and (B). Bottom plot: for each embryo, the CM-innervated area/T10 length was normalized to the median ratio of control embryos, by size range. Blue dots: Fat1+/+ (n = 35, same sample set as in controls of Fig 2); red dots: Fat1LacZ/LacZ (n = 12). Underlying data are provided in S1 Data. BB-BA, benzyl-benzoate/benzyl-alcohol mix; CM, cutaneous maximus; IHC, immunohistochemistry; PFA, paraformaldehyde.
(TIF)
(A) Scheme of the protein products of a wild-type Fat1 allele (full-length Fat1) and of a Fat1LacZ allele (producing a chimaeric protein with the first 8 cadherin domains of Fat1 extracellular domain, fused to an exogenous transmembrane domain in frame with β-galactosidase as intracellular domain). An antibody to Fat1 (Sigma 1869) directed against a portion of the common segment of Fat1 extracellular domain recognizes both proteins, whereas an antibody to β-galactosidase recognizes only the Fat1–β-gal fusion protein, most of which is sequestered in the Golgi apparatus and not localized at the cell membrane. (B) Comparison of immunohistochemical detection of Fat1 in a Fat1LacZ/+ embryo using the anti-β-galactosidase antibody (red), the Fat1-1869 antibody (green), and the pattern of β-galactosidase activity revealed by Salmon-Gal staining on cross sections of an E13.5 mouse embryo at lumbar levels where it is possible to detect both the expression in subsets of MNs and in the caudal-most part of the mesenchymal subcutaneous layer, towards which the CM extends. The Fat1–β-gal fusion protein is mainly detected by both antibodies as punctae in the Golgi, most likely because the protein is misfolded and does not reach the cell membrane. The two stainings perfectly overlap (except for some weak staining of blood vessels in the green channel, mostly due to the secondary antibody) and correspond to the signal detected by Salmon-Gal staining. β-gal, β-galactosidase; CM, cutaneous maximus; epax, epaxial; MNs, motor neurons.
(TIF)
(A) Analysis of MyoD and Scleraxis expression in Fat1LacZ/+ and Metd/d E12.5 embryos, and of Fat1LacZ expression in Fat1LacZ/+ and Metd/d; Fat1LacZ/+ E12.5 embryos was performed by whole-mount in situ hybridization and X-gal staining, respectively. Peripheral sites of Fat1LacZ and Scleraxis expression are maintained in spite of the absence of migratory appendicular muscles, indicating that expression of both genes occurs in non-myogenic/mesenchymal cells. Orange arrowheads indicate the position of the CM muscle; white arrowheads indicate the mesenchyme hot spot in the humeral region of the forelimb; and yellow arrowheads indicate positions of Scleraxis-positive tendons in the distal limb (autopod). (B) Comparison of β-gal (red) and YFP (green) IHC in Pax3cre/+; R26YFP/+; Fat1LacZ/+ (top) and Pax3cre/cre; R26YFP/+; Fat1LacZ/+ (bottom) E11.5 embryos on transverse sections at forelimb levels. The region indicated with a dotted area is shown in higher magnification on the right panels. CM, cutaneous maximus; MyoD, myogenic differentiation 1; Pax3, Paired box 3; R26, Rosa26 Locus; X-gal, substrate for β-galactosidase activity; YFP, yellow fluorescent protein.
(TIF)
(A) Double ISH analysis of Fat1 (purple) is combined with Etv4 (red) expression in WT and Metd/d E12.5 spinal cords in lower panels. (B) ISH analysis of Fat1 in WT, Etv4-/-, and Gdnf-/- spinal cords at E11.5 (upper panels) and E12.5 (lower panels). (A, B) Onset of Fat1 expression occurs independently of Met (A), of Etv4 and of Gdnf (B) functions and is preserved in Metd/d, Etv4-/-, and Gdnf-/- embryos at E11.5. In contrast, analysis of Fat1 expression at E12.5 reveals alterations consistent with (1) defective maintenance of Fat1 expression in the CM pool in Metd/d spinal cords (A), possibly due to the lack of migrating muscles and subsequent growth factor depletion, and (2) aberrant positioning of the CM pool in Pea3-/- and Gdnf-/- spinal cords (B). (C) Comparison of Fat1, Sema3E, and Runx1 expression in the brachial spinal cord at E12.5 was performed by double ISH with Fat1 (purple) and Sema3E (red) on the left, and with Runx1 (purple) and Sema3E (red) on the right. (D) Fat1 expression in the mouse thoracic spinal cord is shown X-gal staining on sections of Fat1LacZ/+ spinal cords at E12.5, showing expression in all neural progenitors in the ventricular zone, and lack of high- level expression in thoracic motor columns. CM, cutaneous maximus; Etv4, Ets variant gene 4; ISH, in situ hybridization; Runx1, runt related transcription factor 1; Sema3E, semaphoring 3E; WT, wild-type.
(TIF)
(A–C) (A) Etv4 expression was analyzed by ISH in E12.5 wild-type and Fat1LacZ/LacZ spinal cords. The images represent flat-mounted spinal cords in the brachial region. (B) Quantifications of Etv4 signal: each plot represents the average signal distribution (± standard deviation in light blue) measured on the indicated number of spinal cord sides along the orange dotted line in each image in (A) (Fat1+/+ [n = 12]; this set of controls is the same as that shown in Fig 6); Fat1LacZ/LacZ (n = 6). (C) Quantifications and statistical analyses of the sum of signal intensity corresponding to the area under the curves in plots shown in (C): each dot represents the sum of Etv4 intensity for each spinal cord side, the number of samples being indicated (the two sides of each embryo considered independent). (B–C): Underlying data are provided in S1 Data. (D) Analysis of Etv4 expression was carried out by ISH with Etv4 probe alone (top) on WT and Fat1LacZ/LacZ E13.5 spinal cords, or with Etv4 probe combined with prior X-gal staining (bottom) on Fat1LacZ/+ and Fat1LacZ/LacZ E13.5 spinal cords. (E) Analysis by ISH of Sema3E and Cadherin8 expression in flat-mounted brachial spinal cords from wild-type, Fat1LacZ/LacZ, Metd/d, and Etv4-/- embryos at E12.5: expression of Sema3E and Cadherin8 in the C7–C8 segments is lost in Etv4-/- and reduced in Metd/d mutants, whereas they are unaffected in Fat1LacZ/LacZ spinal cords. Cad8, Cadherin 8, Etv4, Ets variant gene 4; ISH, in situ hybridization; Sema3E, semaphoring 3E; WT, wild-type; X-gal, substrate for β-galactosidase activity.
(TIF)
(A) Embryonic musculature was visualized by X-gal staining on E12.5 embryos carrying the MLC3F-2E transgene in different contexts of tissue-specific deletion of Fat1, with Wnt1-cre (neural crest cells), Olig2-cre (MN precursors), and Prx1-cre (limb/lateral mesoderm-derived mesenchyme) (cre lines described in S1 Table). Top images are images of the entire flank of Fat1Flox/Flox; MLC3F-2E controls, and Wnt1-cre; Fat1Flox/Flox; MLC3F-2E, Olig-cre; Fat1Flox/Flox; MLC3F-2E, and Prx1-cre; Fat1Flox/Flox; MLC3F-2E embryos, respectively. Higher magnification pictures focus (from top to bottom) on humeral muscles, on the forelimb area, and on the flank region in which the CM spreads, respectively. (B) Quantification of the number of dispersed myocytes found in orange areas in the forelimb (left plot) and of the area occupied by the ectopic humeral muscle (ectop) appearing between the spinodeltoid (Del) and the triceps brachii (TriBra) muscles (right plot). In both graphs, data from Fat1-/- embryos (from plots in S1B Fig) have been added in the last lane, for comparison of effect size. Note that on both graphs, there is an interruption on the y axis and a change of scale. Only Prx1-cre; Fat1Flox/Flox; MLC3F-2E embryos exhibit a mild increase compared to controls. Underlying data are provided in S1 Data. (C) Quantification of the expansion rate of differentiated CM fibers in Fat1Flox/Flox; MLC3F-2E controls, Wnt1-cre; Fat1Flox/Flox; MLC3F-2E, and Olig-cre; Fat1Flox/Flox; MLC3F-2E. Quantifications of Prx1-cre; Fat1Flox/Flox; MLC3F-2E embryos are shown in Fig 7B. Left graph: for each embryo side, the area covered by differentiated CM fibers was plotted relative to the area occupied by body wall muscles. Right plot: for each embryo, the CM area/body wall area was normalized to the median ratio of control embryos. Blue dots: Fat1Flox/Flox; MLC3F-2E (n = 57, includes the same set of controls as Fig 7B); green crosses: Wnt1-cre; Fat1Flox/Flox-; MLC3F-2E (n = 8); purple triangles: Olig2-cre; Fat1Flox/Flox-; MLC3F-2E (n = 6). Underlying data are provided in S1 Data. Scale bars (A) 500 μm. BW, body wall muscles; CM, cutaneous maximus; hum, humeral; sDel, spinodeltoid; ectop, ectopic humeral muscle; TriBra, triceps brachii.
(TIF)
(A) The respective effects of depletion with Wnt1-cre, Olig2-cre, Prx1-cre, and Pax3-cre (premigratory myogenic cells + neural crest lineage) (cre lines described in S1 Table) were assessed by whole-mount X-gal staining on E14.5 Fat1Flox/Flox; MLC3F-2E controls (first column), Olig-cre; Fat1Flox/Flox; MLC3F-2E (second column), Prx1-cre; Fat1Flox/Flox; MLC3F-2E (third column), Wnt1-cre; Fat1Flox/Flox; MLC3F-2E (fourth column), and Pax3cre/+; Fat1Flox/ Flox; MLC3F-2E (fifth column) embryos. Top panels: low-magnification pictures show whole-embryo side views. Boxed areas show the position of higher magnification pictures shown in lower panels. Middle panels focus on facial subcutaneous muscles. Lower panels focus on the CM muscle. Prx1-cre; Fat1Flox/Flox embryos display normal musculature in the face but abnormal muscle shape and intramuscular orientation in the CM (higher magnification of the orange box shown in [C]), while Wnt1-cre; Fat1Flox/Flox embryos have a normal CM but severely affected subcutaneous muscles in the face, compared to controls (Fat1Flox/Flox). Thus, ablation in neural crest cells causes severe alterations in shape of the facial subcutaneous muscles in both Pax3cre/+; Fat1Flox/Flox and Wnt1-cre; Fat1Flox/Flox embryos. In contrasts, muscle shapes of Olig2-cre; Fat1Flox/Flox embryos are indistinguishable from those seen in controls. (B, C) Higher magnification views of fiber density in the CM in yellow and orange boxed areas outlined in lower panels in (A), respectively comparing control with Pax3cre/+; Fat1Flox/Flox (B) and with Prx1-cre; Fat1Flox/Flox (C) embryos. The effect of Fat1 ablation in Pax3cre/+; Fat1Flox/Flox embryos on CM appearance (B) is modest at E14.5 (high-magnification images illustrate a subtle reduction in the density of LacZ-positive nuclei). In contrast, the reduction in fiber density observed in Prx1-cre; Fat1Flox/Flox embryos (C) is very severe in the ventral CM, in contrast to more dorsal regions, coinciding with the limit above which Prx1-cre is not active. CM, cutaneous maximus; dCM, dorsal CM; fm, facial subcutaneous muscle; temp, temporalis; vCM, ventral CM; zyg, zygomatic muscle.
(TIF)
(A) Characterization of the mesenchyme subtype at the CM–skin interface. Sections of an E12.5 wild-type embryo at the level of the anterior CM (and upper forelimb) were immunostained with antibodies against Myh1 (white), TenascinC (green), Tcf4 (green), Pdgfrα (red), and DAPI (blue). The lower panels represent higher magnifications of the two bowed areas indicated in the low magnification views, focusing on the mesenchyme surrounding the CM and at the interface between CM and skin (middle panels) and the extremity of one forelimb muscle (lower panel). Whereas Tcf4 is detected in myogenic cells and connective tissue fibroblasts associated with the forelimb muscle, no expression is detected in the CM/skin connective tissue, which, in contrast, expresses TenascinC and Pdgfrα. (B) Control and Pdgfrα-iCre; R26YFP/+ embryos were collected at E12.5 from a pregnant female treated with tamoxifen at E9.5 + E10.5. Images show the direct YFP fluorescence signal acquired with the same exposure time. The vertical lines in the lower picture highlight the level of sections corresponding to Fig 9F (anterior CM and posterior CM). (C) Whole-mount β-galactosidase staining was performed on tamoxifen-treated control; MLC3F-2E (left) and Pdgfrα-iCre; Fat1Flox/Flox- ; MLC3F-2E (right) embryos at E12.5. Top images represent the upper forelimb region (with scapulohumeral muscles); bottom images represent the forelimb region, where myocyte dispersal is being quantified in (D). (D) Quantification of the number of dispersed myoblasts observed in the forelimb (counted in the areas delimited by orange lines). Blue dots: Controls; MLC3F-2E (n = 17, control embryos from tamoxifen-treated litters); red dots: Pdgfrα-iCre; Fat1Flox/Flox- ; MLC3F-2E (n = 17). Underlying data are provided in S1 Data. CM, cutaneous maximus; iCre, short form of CRE/ERT2; Myh1, myosin heavy chain I; Pdgfrα, platelet-derived growth factor receptor alpha; R26, Rosa26 locus; Tcf4, T cell factor 4; TenC, Tenascin C.
(TIF)
(A–C) Anti-neurofilament IHC was performed on E12.5 embryos, in the context of Myf5-cre-driven (S1 Table) myogenic lineage depletion, mediated by conditional DTA expression under regulatory control of the R26 locus (R26Lox-LacZ-STOP-Lox-DTA mouse line S1 Table). This nerve pattern is either visualized on its own (A, B) or combined with X-gal staining, when embryos also carry the MLC3F-2E-LacZ transgene (C). In cases with MLC3F-2E (C), X-gal staining also detects LacZ expression driven by the R26 locus in unrecombined cells (the relative expression levels in the two loci [R26 and MLC3F-2E-LacZ transgene] allows visualizing muscles in the trunk area but not in the limb). After BB-BA clearing, the embryos were cut in half and the internal organs and skin removed to visualize the CM and brachial plexus. Genotypes shown are control R26Lox-LacZ-STOP-Lox-DTA; and Myf5-cre; R26Lox-LacZ-STOP-Lox-DTA embryos (A, B); control R26Lox-LacZ-STOP-Lox-DTA; MLC3F-2E (left), Myf5-cre; R26Lox-LacZ-STOP-Lox-DTA; MLC3F-2E (middle), or Myf5-cre; R26Lox-LacZ-STOP-Lox-DTA; MLC3F-2E (right) (C). Upper panels show the entire flank of embryos at comparable stages. The area covered by CM-innervating axons is outlined with white dotted lines. The extent of the T8 thoracic nerve is outlined with a black arrow. The length of the forelimb (slightly affected by muscle depletion) is highlighted by a red arrow. Middle panels show higher magnification of the upper thoracic region containing the CM and corresponding motor axons as well as thoracic nerves, after manual removal by dissection of most cutaneous sensory nerves. Lower panels show the upper cervical region, with the trapezius muscle, innervated by the cranial nerve XI, also called spinal accessory nerve. (D, E) Quantifications of the progression of CM innervation (D) and of thoracic nerve growth (E). Left plots: for each embryo side, the area covered by CM-innervating axons (D) or the length of the T8 nerve (E) were plotted relative to the head area, comparing Myf5-cre; R26Lox-LacZ-STOP-Lox-DTA; embryos (red dots) to controls (blue dots). The T8 value represents the distance between the dorsal tip of sensory nerve roots and the ventral-most axon tips on the flank, thus including both sensory and motor components. The head area was used as reference, as it grows proportionally to the embryo, and it is not affected by muscle depletion, in contrast to other distances/areas in the trunk or limbs. Right plots: for each embryo side, we calculated the ratios CM-innervated area/head area (D) and T8 length/head area (E), and these values were normalized to the median ratio of control embryos, by size range. Blue dots: Controls (n = 8, pooling all control littermates); red dots: Myf5-cre; R26Lox-LacZ-STOP-Lox-DTA (n = 8). (D, E) Underlying data are provided in S1 Data. Arrows point to the stage of representative examples shown in (A–C). BB-BA, benzyl-benzoate/benzyl-alcohol mix; CM, cutaneous maximus; cre, cre recombinase; DTA, diphtheria toxin A; IHC, immunohistochemistry; MN, motor neuron; Myf5, myogenic factor 5; R26, Rosa26 locus; X-gal, substrate for β-galactosidase activity.
(TIF)
(A) Analysis by IHC of Clusterin, islet1, and GFP expression on spinal cord sections at the C7–C8 level from E13.5 WT; Etv4-GFP/+, from GdnfLacZ/LacZ; Etv4-GFP/+, and from Prx1-cre; Fat1Flox/Flox embryos. Clusterin and GFP staining were done on sections neighboring those analyzed with Islet1/2 IHC. Etv4-GFP could not be combined with Prx1-cre, as this combination appears to cause a phenotype (short limbs, with impaired elongation, visible from E12.5 onward) unrelated to Fat1 alleles and most likely linked to the site of integration of both transgenes. (B) X-gal staining of GdnfLacZ/+ and GdnfLacZ/LacZ embryos, showing that expansion of the area covered by GdnfLacZ-expressing CM progenitors is not affected by the lack of Gdnf. CM, cutaneous maximus; cre, cre recombinase; Etv4, Ets variant gene 4; GFP, green fluorescent protein; IHC, immunohistochemistry; MN, motor neuron; WT, wild-type; X-gal, substrate for β-galactosidase activity.
(TIF)
Acknowledgments
I thank F. Maina and R. Kelly for comments on the manuscript and suggestions throughout the work; F. Maina for Metd mutant mice; T. Jessell and S. Arber for Etv4 null and HB9-GFP mice; Genentech for GdnfLacZ mice; Irina Dudanova for sending Prx1-cre mice; Aziz Moqrich for Wnt1-cre mice; Andrea Huber-Brösamle for Olig2-cre and R26DTA mice, with permission of T. Jessell; and Pascale Durbec for providing the Pdgfrα-Cre/ERT2 mice (from William Richardson). Scleraxis RNA probe was provided by Delphine Duprez and Clusterin RNA probe by C.E. Henderson and K. Dudley; Runx1 probe was obtained from the RZPD repository and Gdnf RNA probe cloned by B. Herberth. Antibodies against Pax7 (developed by A. Kawakami), Neurofilaments (2H3) (by T. Jessell), and Myh1 (MF20) (by D.A. Fischman) were obtained from the Developmental study hybridoma bank (DSHB) created by the NIHCD of the NIH and kept under the auspices of the Iowa University. I thank Nathalie Caruso for mouse colony genotyping and technical support in early phases of this study, Dominique Fragano for mouse colony PCR genotyping, Virginia Girod-David and the IBDM animal house staff for help with mouse husbandry at the IBDM, and Angela K. Zimmermann for contribution to the histological analysis for part of Fig 12B (top graph). Imaging was performed using the PiCSL-FBI core facility (IBDM, AMU-Marseille, France).
Abbreviations
- α-BTX
alpha-bungarotoxin
- AchR
acetylcholine receptor
- ALS
amyotrophic lateral sclerosis
- AP
anteroposterior
- BB-BA
benzyl-benzoate/benzyl-alcohol mix
- BW
body wall
- CM
cutaneous maximus
- CNV
copy number variant
- CRE
Cre recombinase
- CRE/ERT2
CRE fused with the estrogen receptor tamoxifen-binding domain
- CT
connective tissue
- dCM
dorsal cutaneous maximus
- DTA
diphtheria toxin A
- ECM
extracellular matrix
- Etv4
Ets variant gene 4 (formerly called Pea3 for polyomavirus enhancer activator 3)
- FAP
fibroadipogenic progenitor
- Fat1
FAT atypical Cadherin 1
- FSHD
facioscapulohumeral dystrophy
- GDNF
glial cell line-derived neurotrophic factor
- GFP
green fluorescent protein
- Gfra1
glial cell line-derived neurotrophic factor family receptor alpha 1
- HGF
hepatocyte growth factor
- IHC
immunohistochemistry
- IP
intraperitoneal
- Lbx1
ladybird homeobox 1
- LD
latissimus dorsi
- MGI
mouse genome informatics
- MLC
myosin light chain
- MN
motor neuron
- Myh1
myosin heavy chain 1
- MyoD
myogenic differentiation 1
- NBT
nitro blue tetrazolium
- nLacZ
LacZ sequence with a nuclear localization signal
- NMJ
neuromuscular junction
- Olig2
Oligodendrocyte transcription factor 2
- Pax3
paired box 3
- Pax7
paired box 7
- PCP
planar cell polarity
- Pdgfrα
platelet-derived growth factor receptor alpha
- PFA
paraformaldehyde
- Prx1
paired related homeobox 1
- Runx1
runt related transcription factor 1
- Salmon-Gal
6-Chloro-3-indolyl-β-D-galactopyranoside
- Sema3E
Semaphorin 3E
- shRNA
short hairpin RNA
- Six1
sine oculis-related homeobox 1
- T10
10th thoracic nerve
- Tcf4/Tcf7L2
transcription factor 7 like 2, T cell specific 4
- vCM
ventral cutaneous maximus
- X-Gal
5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
FSHD Global Research foundation https://fshdglobal.org/ (grant number Grant 14). Funding to FH. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. FSH Society https://www.fshsociety.org/ (grant number FSHS-82014-05). Funding to FH. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. AFM-Telethon, Association Francaise contre les Myopathies https://www.afm-telethon.fr/ (grant number 15823; 16785; 20861). Funding to FH. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Agence Nationale pour la Recherche http://www.agence-nationale-recherche.fr/investissements-d-avenir/ (grant number France-BioImaging, ANR-10-INBS-04). Imaging was performed using the PiCSL-FBI core facility (IBDM, AMU-Marseille, France) supported by the Agence Nationale de la Recherche through the ‘Investments for the Future’ program. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Francius C, Clotman F. Generating spinal motor neuron diversity: a long quest for neuronal identity. Cell Mol Life Sci. 2014;71(5):813–29. Epub 2013/06/15. doi: 10.1007/s00018-013-1398-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Comai G, Tajbakhsh S. Molecular and cellular regulation of skeletal myogenesis. Curr Top Dev Biol. 2014;110:1–73. Epub 2014/09/25. doi: 10.1016/B978-0-12-405943-6.00001-4 . [DOI] [PubMed] [Google Scholar]
- 3.Buckingham M, Rigby PW. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev Cell. 2014;28(3):225–38. Epub 2014/02/15. doi: 10.1016/j.devcel.2013.12.020 . [DOI] [PubMed] [Google Scholar]
- 4.Vasyutina E, Birchmeier C. The development of migrating muscle precursor cells. Anat Embryol (Berl). 2006;211 Suppl 1:37–41. Epub 2006/09/16. doi: 10.1007/s00429-006-0118-9 . [DOI] [PubMed] [Google Scholar]
- 5.Chevallier A, Kieny M. On the role of the connective tissue in the patterning of the chick limb musculature. Wilehm Roux Arch Dev Biol. 1982;191(4):277–80. Epub 1982/07/01. doi: 10.1007/BF00848416 . [DOI] [PubMed] [Google Scholar]
- 6.Kardon G. Muscle and tendon morphogenesis in the avian hind limb. Development. 1998;125(20):4019–32. [DOI] [PubMed] [Google Scholar]
- 7.Braun T, Gautel M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat Rev Mol Cell Biol. 2011;12(6):349–61. Epub 2011/05/24. doi: 10.1038/nrm3118 . [DOI] [PubMed] [Google Scholar]
- 8.Catela C, Shin MM, Dasen JS. Assembly and function of spinal circuits for motor control. Annu Rev Cell Dev Biol. 2015;31:669–98. Epub 2015/09/24. doi: 10.1146/annurev-cellbio-100814-125155 . [DOI] [PubMed] [Google Scholar]
- 9.Landmesser LT. The acquisition of motoneuron subtype identity and motor circuit formation. Int J Dev Neurosci. 2001;19(2):175–82. Epub 2001/03/20. . [DOI] [PubMed] [Google Scholar]
- 10.Lance-Jones C, Dias M. The influence of presumptive limb connective tissue on motoneuron axon guidance. Dev Biol. 1991;143(1):93–110. Epub 1991/01/01. . [DOI] [PubMed] [Google Scholar]
- 11.Lance-Jones C, Landmesser L. Pathway selection by embryonic chick motoneurons in an experimentally altered environment. Proc R Soc Lond B Biol Sci. 1981;214(1194):19–52. . [DOI] [PubMed] [Google Scholar]
- 12.Stifani N. Motor neurons and the generation of spinal motor neuron diversity. Front Cell Neurosci. 2014;8:293 doi: 10.3389/fncel.2014.00293 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nassari S, Duprez D, Fournier-Thibault C. Non-myogenic Contribution to Muscle Development and Homeostasis: The Role of Connective Tissues. Front Cell Dev Biol. 2017;5:22 Epub 2017/04/08. doi: 10.3389/fcell.2017.00022 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caruso N, Herberth B, Bartoli M, Puppo F, Dumonceaux J, Zimmermann A, et al. Deregulation of the protocadherin gene FAT1 alters muscle shapes: implications for the pathogenesis of facioscapulohumeral dystrophy. PLoS Genet. 2013;9(6):e1003550 Epub 2013/06/21. doi: 10.1371/journal.pgen.1003550 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma P, McNeill H. Fat and Dachsous Cadherins. Prog Mol Biol Transl. 2013;116:215–35. doi: 10.1016/B978-0-12-394311-8.00010-8 [DOI] [PubMed] [Google Scholar]
- 16.Sharma P, McNeill H. Regulation of long-range planar cell polarity by Fat-Dachsous signaling. Development. 2013;140(18):3869–81. doi: 10.1242/dev.094730 [DOI] [PubMed] [Google Scholar]
- 17.Bosveld F, Bonnet I, Guirao B, Tlili S, Wang Z, Petitalot A, et al. Mechanical control of morphogenesis by Fat/Dachsous/Four-jointed planar cell polarity pathway. Science. 2012;336(6082):724–7. Epub 2012/04/14. doi: 10.1126/science.1221071 . [DOI] [PubMed] [Google Scholar]
- 18.Arata M, Sugimura K, Uemura T. Difference in Dachsous Levels between Migrating Cells Coordinates the Direction of Collective Cell Migration. Dev Cell. 2017;42(5):479–97 e10. Epub 2017/09/13. doi: 10.1016/j.devcel.2017.08.001 . [DOI] [PubMed] [Google Scholar]
- 19.Zakaria S, Mao Y, Kuta A, Ferreira de Sousa C, Gaufo GO, McNeill H, et al. Regulation of neuronal migration by Dchs1-Fat4 planar cell polarity. Curr Biol. 2014;24(14):1620–7. Epub 2014/07/08. doi: 10.1016/j.cub.2014.05.067 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou R, Liu L, Anees S, Hiroyasu S, Sibinga NE. The Fat1 cadherin integrates vascular smooth muscle cell growth and migration signals. J Cell Biol. 2006;173(3):417–29. Epub 2006/05/10. doi: 10.1083/jcb.200508121 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao Y, Kuta A, Crespo-Enriquez I, Whiting D, Martin T, Mulvaney J, et al. Dchs1-Fat4 regulation of polarized cell behaviours during skeletal morphogenesis. Nat Commun. 2016;7:11469 Epub 2016/05/06. doi: 10.1038/ncomms11469 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, et al. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008;40(8):1010–5. Epub 2008/07/08. doi: 10.1038/ng.179 . [DOI] [PubMed] [Google Scholar]
- 23.Bosveld F, Guirao B, Wang Z, Riviere M, Bonnet I, Graner F, et al. Modulation of junction tension by tumor suppressors and proto-oncogenes regulates cell-cell contacts. Development. 2016;143(4):623–34. Epub 2016/01/27. doi: 10.1242/dev.127993 . [DOI] [PubMed] [Google Scholar]
- 24.Marcinkevicius E, Zallen JA. Regulation of cytoskeletal organization and junctional remodeling by the atypical cadherin Fat. Development. 2013;140(2):433–43. Epub 2012/12/20. doi: 10.1242/dev.083949 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harumoto T, Ito M, Shimada Y, Kobayashi TJ, Ueda HR, Lu B, et al. Atypical cadherins Dachsous and Fat control dynamics of noncentrosomal microtubules in planar cell polarity. Dev Cell. 2010;19(3):389–401. Epub 2010/09/08. doi: 10.1016/j.devcel.2010.08.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawrence PA, Casal J. The mechanisms of planar cell polarity, growth and the Hippo pathway: some known unknowns. Dev Biol. 2013;377(1):1–8. Epub 2013/04/18. doi: 10.1016/j.ydbio.2013.01.030 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho E, Feng Y, Rauskolb C, Maitra S, Fehon R, Irvine KD. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38(10):1142–50. Epub 2006/09/19. doi: 10.1038/ng1887 . [DOI] [PubMed] [Google Scholar]
- 28.Cao LL, Riascos-Bernal DF, Chinnasamy P, Dunaway CM, Hou R, Pujato MA, et al. Control of mitochondrial function and cell growth by the atypical cadherin Fat1. Nature. 2016;539(7630):575–8. Epub 2016/11/10. doi: 10.1038/nature20170 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sing A, Tsatskis Y, Fabian L, Hester I, Rosenfeld R, Serricchio M, et al. The atypical cadherin fat directly regulates mitochondrial function and metabolic state. Cell. 2014;158(6):1293–308. Epub 2014/09/13. doi: 10.1016/j.cell.2014.07.036 . [DOI] [PubMed] [Google Scholar]
- 30.Mao Y, Francis-West P, Irvine KD. Fat4/Dchs1 signaling between stromal and cap mesenchyme cells influences nephrogenesis and ureteric bud branching. Development. 2015;142(15):2574–85. Epub 2015/06/28. doi: 10.1242/dev.122630 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bagherie-Lachidan M, Reginensi A, Pan Q, Zaveri HP, Scott DA, Blencowe BJ, et al. Stromal Fat4 acts non-autonomously with Dchs1/2 to restrict the nephron progenitor pool. Development. 2015;142(15):2564–73. Epub 2015/06/28. doi: 10.1242/dev.122648 . [DOI] [PubMed] [Google Scholar]
- 32.Das A, Tanigawa S, Karner CM, Xin M, Lum L, Chen C, et al. Stromal-epithelial crosstalk regulates kidney progenitor cell differentiation. Nat Cell Biol. 2013;15(9):1035–44. Epub 2013/08/27. doi: 10.1038/ncb2828 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ragni CV, Diguet N, Le Garrec JF, Novotova M, Resende TP, Pop S, et al. Amotl1 mediates sequestration of the Hippo effector Yap1 downstream of Fat4 to restrict heart growth. Nat Commun. 2017;8:14582 Epub 2017/02/28. doi: 10.1038/ncomms14582 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beste C, Ocklenburg S, von der Hagen M, Di Donato N. Mammalian cadherins DCHS1-FAT4 affect functional cerebral architecture. Brain Struct Funct. 2015. Epub 2015/05/02. doi: 10.1007/s00429-015-1051-6 . [DOI] [PubMed] [Google Scholar]
- 35.Cappello S, Gray MJ, Badouel C, Lange S, Einsiedler M, Srour M, et al. Mutations in genes encoding the cadherin receptor-ligand pair DCHS1 and FAT4 disrupt cerebral cortical development. Nat Genet. 2013;45(11):1300–8. Epub 2013/09/24. doi: 10.1038/ng.2765 . [DOI] [PubMed] [Google Scholar]
- 36.Gee HY, Sadowski CE, Aggarwal PK, Porath JD, Yakulov TA, Schueler M, et al. FAT1 mutations cause a glomerulotubular nephropathy. Nat Commun. 2016;7:10822 Epub 2016/02/26. doi: 10.1038/ncomms10822 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciani L, Patel A, Allen ND, ffrench-Constant C. Mice lacking the giant protocadherin mFAT1 exhibit renal slit junction abnormalities and a partially penetrant cyclopia and anophthalmia phenotype. Mol Cell Biol. 2003;23(10):3575–82. Epub 2003/05/02. doi: 10.1128/MCB.23.10.3575-3582.2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badouel C, Zander MA, Liscio N, Bagherie-Lachidan M, Sopko R, Coyaud E, et al. Fat1 interacts with Fat4 to regulate neural tube closure, neural progenitor proliferation and apical constriction during mouse brain development. Development. 2015;142(16):2781–91. Epub 2015/07/26. doi: 10.1242/dev.123539 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haase G, Dessaud E, Garces A, de Bovis B, Birling M, Filippi P, et al. GDNF acts through PEA3 to regulate cell body positioning and muscle innervation of specific motor neuron pools. Neuron. 2002;35(5):893–905. Epub 2002/10/10. . [DOI] [PubMed] [Google Scholar]
- 40.Vrieseling E, Arber S. Target-induced transcriptional control of dendritic patterning and connectivity in motor neurons by the ETS gene Pea3. Cell. 2006;127(7):1439–52. Epub 2006/12/28. doi: 10.1016/j.cell.2006.10.042 . [DOI] [PubMed] [Google Scholar]
- 41.Livet J, Sigrist M, Stroebel S, De Paola V, Price SR, Henderson CE, et al. ETS gene Pea3 controls the central position and terminal arborization of specific motor neuron pools. Neuron. 2002;35(5):877–92. Epub 2002/10/10. . [DOI] [PubMed] [Google Scholar]
- 42.Wright DE, Snider WD. Focal expression of glial cell line-derived neurotrophic factor in developing mouse limb bud. Cell Tissue Res. 1996;286(2):209–17. Epub 1996/11/01. . [DOI] [PubMed] [Google Scholar]
- 43.Garces A, Haase G, Airaksinen MS, Livet J, Filippi P, deLapeyriere O. GFRalpha 1 is required for development of distinct subpopulations of motoneuron. J Neurosci. 2000;20(13):4992–5000. Epub 2000/06/24. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Treanor JJ, Goodman L, de Sauvage F, Stone DM, Poulsen KT, Beck CD, et al. Characterization of a multicomponent receptor for GDNF. Nature. 1996;382(6586):80–3. Epub 1996/07/04. doi: 10.1038/382080a0 . [DOI] [PubMed] [Google Scholar]
- 45.Durbec P, Marcos-Gutierrez CV, Kilkenny C, Grigoriou M, Wartiowaara K, Suvanto P, et al. GDNF signalling through the Ret receptor tyrosine kinase. Nature. 1996;381(6585):789–93. Epub 1996/06/27. doi: 10.1038/381789a0 . [DOI] [PubMed] [Google Scholar]
- 46.Caruso N, Herberth B, Lamballe F, Arce-Gorvel V, Maina F, Helmbacher F. Plasticity versus specificity in RTK signalling modalities for distinct biological outcomes in motor neurons. BMC Biol. 2014;12:56 Epub 2014/08/16. doi: 10.1186/s12915-014-0056-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Helmbacher F, Dessaud E, Arber S, deLapeyriere O, Henderson CE, Klein R, et al. Met signaling is required for recruitment of motor neurons to PEA3-positive motor pools. Neuron. 2003;39(5):767–77. Epub 2003/09/02. . [DOI] [PubMed] [Google Scholar]
- 48.Price SR, De Marco Garcia NV, Ranscht B, Jessell TM. Regulation of motor neuron pool sorting by differential expression of type II cadherins. Cell. 2002;109(2):205–16. Epub 2002/05/15. . [DOI] [PubMed] [Google Scholar]
- 49.Fukuhara K, Imai F, Ladle DR, Katayama K, Leslie JR, Arber S, et al. Specificity of Monosynaptic Sensory-Motor Connections Imposed by Repellent Sema3E-PlexinD1 Signaling. Cell Rep. 2013;5(3):748–58. Epub 2013/11/12. doi: 10.1016/j.celrep.2013.10.005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pecho-Vrieseling E, Sigrist M, Yoshida Y, Jessell TM, Arber S. Specificity of sensory-motor connections encoded by Sema3e-Plxnd1 recognition. Nature. 2009;459(7248):842–6. Epub 2009/05/08. doi: 10.1038/nature08000 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dudanova I, Klein R. Integration of guidance cues: parallel signaling and crosstalk. Trends Neurosci. 2013;36(5):295–304. Epub 2013/03/15. doi: 10.1016/j.tins.2013.01.007 . [DOI] [PubMed] [Google Scholar]
- 52.Bonanomi D, Chivatakarn O, Bai G, Abdesselem H, Lettieri K, Marquardt T, et al. Ret is a multifunctional coreceptor that integrates diffusible- and contact-axon guidance signals. Cell. 2012;148(3):568–82. Epub 2012/02/07. doi: 10.1016/j.cell.2012.01.024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dudanova I, Gatto G, Klein R. GDNF acts as a chemoattractant to support ephrinA-induced repulsion of limb motor axons. Curr Biol. 2010;20(23):2150–6. Epub 2010/11/27. doi: 10.1016/j.cub.2010.11.021 . [DOI] [PubMed] [Google Scholar]
- 54.Kramer ER, Knott L, Su F, Dessaud E, Krull CE, Helmbacher F, et al. Cooperation between GDNF/Ret and ephrinA/EphA4 signals for motor-axon pathway selection in the limb. Neuron. 2006;50(1):35–47. Epub 2006/04/08. doi: 10.1016/j.neuron.2006.02.020 . [DOI] [PubMed] [Google Scholar]
- 55.Charoy C, Nawabi H, Reynaud F, Derrington E, Bozon M, Wright K, et al. gdnf activates midline repulsion by Semaphorin3B via NCAM during commissural axon guidance. Neuron. 2012;75(6):1051–66. Epub 2012/09/25. doi: 10.1016/j.neuron.2012.08.021 . [DOI] [PubMed] [Google Scholar]
- 56.Shneider NA, Brown MN, Smith CA, Pickel J, Alvarez FJ. Gamma motor neurons express distinct genetic markers at birth and require muscle spindle-derived GDNF for postnatal survival. Neural Dev. 2009;4:42 Epub 2009/12/04. doi: 10.1186/1749-8104-4-42 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, et al. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266(5187):1062–4. Epub 1994/11/11. . [DOI] [PubMed] [Google Scholar]
- 58.Kelly RG, Zammit PS, Schneider A, Alonso S, Biben C, Buckingham ME. Embryonic and fetal myogenic programs act through separate enhancers at the MLC1F/3F locus. Dev Biol. 1997;187(2):183–99. Epub 1997/07/15. doi: 10.1006/dbio.1997.8577 . [DOI] [PubMed] [Google Scholar]
- 59.Kelly R, Alonso S, Tajbakhsh S, Cossu G, Buckingham M. Myosin light chain 3F regulatory sequences confer regionalized cardiac and skeletal muscle expression in transgenic mice. J Cell Biol. 1995;129(2):383–96. Epub 1995/04/01. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lamballe F, Genestine M, Caruso N, Arce V, Richelme S, Helmbacher F, et al. Pool-specific regulation of motor neuron survival by neurotrophic support. J Neurosci. 2011;31(31):11144–58. Epub 2011/08/05. doi: 10.1523/JNEUROSCI.2198-11.2011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maina F, Casagranda F, Audero E, Simeone A, Comoglio PM, Klein R, et al. Uncoupling of Grb2 from the Met receptor in vivo reveals complex roles in muscle development. Cell. 1996;87(3):531–42. Epub 1996/11/01. . [DOI] [PubMed] [Google Scholar]
- 62.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376(6543):768–71. Epub 1995/08/31. doi: 10.1038/376768a0 . [DOI] [PubMed] [Google Scholar]
- 63.Buckingham M, Relaix F. PAX3 and PAX7 as upstream regulators of myogenesis. Semin Cell Dev Biol. 2015;44:115–25. Epub 2015/10/02. doi: 10.1016/j.semcdb.2015.09.017 . [DOI] [PubMed] [Google Scholar]
- 64.Engleka KA, Gitler AD, Zhang M, Zhou DD, High FA, Epstein JA. Insertion of Cre into the Pax3 locus creates a new allele of Splotch and identifies unexpected Pax3 derivatives. Dev Biol. 2005;280(2):396–406. Epub 2005/05/11. doi: 10.1016/j.ydbio.2005.02.002 . [DOI] [PubMed] [Google Scholar]
- 65.Tremblay P, Dietrich S, Mericskay M, Schubert FR, Li Z, Paulin D. A crucial role for Pax3 in the development of the hypaxial musculature and the long-range migration of muscle precursors. Dev Biol. 1998;203(1):49–61. Epub 1998/11/10. doi: 10.1006/dbio.1998.9041 . [DOI] [PubMed] [Google Scholar]
- 66.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4 Epub 2001/04/12. doi: 10.1186/1471-213X-1-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stifani N, Freitas AR, Liakhovitskaia A, Medvinsky A, Kania A, Stifani S. Suppression of interneuron programs and maintenance of selected spinal motor neuron fates by the transcription factor AML1/Runx1. Proc Natl Acad Sci U S A. 2008;105(17):6451–6. Epub 2008/04/23. doi: 10.1073/pnas.0711299105 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang F, Kumano M, Beraldi E, Fazli L, Du C, Moore S, et al. Clusterin facilitates stress-induced lipidation of LC3 and autophagosome biogenesis to enhance cancer cell survival. Nat Commun. 2014;5:5775 Epub 2014/12/17. doi: 10.1038/ncomms6775 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shannan B, Seifert M, Leskov K, Willis J, Boothman D, Tilgen W, et al. Challenge and promise: roles for clusterin in pathogenesis, progression and therapy of cancer. Cell Death Differ. 2006;13(1):12–9. Epub 2005/09/24. doi: 10.1038/sj.cdd.4401779 . [DOI] [PubMed] [Google Scholar]
- 70.Zhang H, Kim JK, Edwards CA, Xu Z, Taichman R, Wang CY. Clusterin inhibits apoptosis by interacting with activated Bax. Nat Cell Biol. 2005;7(9):909–15. Epub 2005/08/23. doi: 10.1038/ncb1291 . [DOI] [PubMed] [Google Scholar]
- 71.Wicher GK, Aldskogius H. Adult motor neurons show increased susceptibility to axotomy-induced death in mice lacking clusterin. Eur J Neurosci. 2005;21(7):2024–8. Epub 2005/05/05. doi: 10.1111/j.1460-9568.2005.04008.x . [DOI] [PubMed] [Google Scholar]
- 72.Dessaud E, Yang LL, Hill K, Cox B, Ulloa F, Ribeiro A, et al. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature. 2007;450(7170):717–20. Epub 2007/11/30. doi: 10.1038/nature06347 . [DOI] [PubMed] [Google Scholar]
- 73.Durland JL, Sferlazzo M, Logan M, Burke AC. Visualizing the lateral somitic frontier in the Prx1Cre transgenic mouse. J Anat. 2008;212(5):590–602. Epub 2008/04/24. doi: 10.1111/j.1469-7580.2008.00879.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33(2):77–80. Epub 2002/07/12. doi: 10.1002/gene.10092 . [DOI] [PubMed] [Google Scholar]
- 75.Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8(24):1323–6. Epub 1998/12/09. . [DOI] [PubMed] [Google Scholar]
- 76.Le Lievre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol. 1975;34(1):125–54. Epub 1975/08/01. . [PubMed] [Google Scholar]
- 77.Mathew SJ, Hansen JM, Merrell AJ, Murphy MM, Lawson JA, Hutcheson DA, et al. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development. 2011;138(2):371–84. Epub 2010/12/24. doi: 10.1242/dev.057463 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kardon G, Harfe BD, Tabin CJ. A Tcf4-positive mesodermal population provides a prepattern for vertebrate limb muscle patterning. Dev Cell. 2003;5(6):937–44. Epub 2003/12/12. . [DOI] [PubMed] [Google Scholar]
- 79.Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11(12):1392–401. Epub 2008/10/14. doi: 10.1038/nn.2220 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tallquist MD, Weismann KE, Hellstrom M, Soriano P. Early myotome specification regulates PDGFA expression and axial skeleton development. Development. 2000;127(23):5059–70. Epub 2000/11/04. . [DOI] [PubMed] [Google Scholar]
- 81.Brockschnieder D, Pechmann Y, Sonnenberg-Riethmacher E, Riethmacher D. An improved mouse line for Cre-induced cell ablation due to diphtheria toxin A, expressed from the Rosa26 locus. Genesis. 2006;44(7):322–7. doi: 10.1002/dvg.20218 . [DOI] [PubMed] [Google Scholar]
- 82.Lewis J, Chevallier A, Kieny M, Wolpert L. Muscle nerve branches do not develop in chick wings devoid of muscle. J Embryol Exp Morphol. 1981;64:211–32. Epub 1981/08/01. . [PubMed] [Google Scholar]
- 83.Zecca M, Struhl G. A feed-forward circuit linking wingless, fat-dachsous signaling, and the warts-hippo pathway to Drosophila wing growth. PLoS Biol. 2010;8(6):e1000386 Epub 2010/06/10. doi: 10.1371/journal.pbio.1000386 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fan Y, Richelme S, Avazeri E, Audebert S, Helmbacher F, Dono R, et al. Tissue-Specific Gain of RTK Signalling Uncovers Selective Cell Vulnerability during Embryogenesis. PLoS Genet. 2015;11(9):e1005533 Epub 2015/09/24. doi: 10.1371/journal.pgen.1005533 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vasyutina E, Stebler J, Brand-Saberi B, Schulz S, Raz E, Birchmeier C. CXCR4 and Gab1 cooperate to control the development of migrating muscle progenitor cells. Genes Dev. 2005;19(18):2187–98. Epub 2005/09/17. doi: 10.1101/gad.346205 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lieberam I, Agalliu D, Nagasawa T, Ericson J, Jessell TM. A Cxcl12-CXCR4 chemokine signaling pathway defines the initial trajectory of mammalian motor axons. Neuron. 2005;47(5):667–79. Epub 2005/09/01. doi: 10.1016/j.neuron.2005.08.011 . [DOI] [PubMed] [Google Scholar]
- 87.Barriga EH, Franze K, Charras G, Mayor R. Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo. Nature. 2018. Epub 2018/02/15. doi: 10.1038/nature25742 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Poliak S, Norovich AL, Yamagata M, Sanes JR, Jessell TM. Muscle-type Identity of Proprioceptors Specified by Spatially Restricted Signals from Limb Mesenchyme. Cell. 2016;164(3):512–25. Epub 2016/01/30. doi: 10.1016/j.cell.2015.12.049 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Colasanto MP, Eyal S, Mohassel P, Bamshad M, Bonnemann CG, Zelzer E, et al. Development of a subset of forelimb muscles and their attachment sites requires the ulnar-mammary syndrome gene Tbx3. Dis Model Mech. 2016;9(11):1257–69. Epub 2016/08/05. doi: 10.1242/dmm.025874 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Merrell AJ, Ellis BJ, Fox ZD, Lawson JA, Weiss JA, Kardon G. Muscle connective tissue controls development of the diaphragm and is a source of congenital diaphragmatic hernias. Nat Genet. 2015;47(5):496–504. Epub 2015/03/26. doi: 10.1038/ng.3250 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hasson P, DeLaurier A, Bennett M, Grigorieva E, Naiche LA, Papaioannou VE, et al. Tbx4 and tbx5 acting in connective tissue are required for limb muscle and tendon patterning. Dev Cell. 2010;18(1):148–56. Epub 2010/02/16. doi: 10.1016/j.devcel.2009.11.013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vickerman L, Neufeld S, Cobb J. Shox2 function couples neural, muscular and skeletal development in the proximal forelimb. Dev Biol. 2011;350(2):323–36. Epub 2010/12/16. doi: 10.1016/j.ydbio.2010.11.031 . [DOI] [PubMed] [Google Scholar]
- 93.Stricker S, Mathia S, Haupt J, Seemann P, Meier J, Mundlos S. Odd-skipped related genes regulate differentiation of embryonic limb mesenchyme and bone marrow mesenchymal stromal cells. Stem Cells Dev. 2012;21(4):623–33. Epub 2011/06/16. doi: 10.1089/scd.2011.0154 . [DOI] [PubMed] [Google Scholar]
- 94.Stricker S, Brieske N, Haupt J, Mundlos S. Comparative expression pattern of Odd-skipped related genes Osr1 and Osr2 in chick embryonic development. Gene Expr Patterns. 2006;6(8):826–34. Epub 2006/03/24. doi: 10.1016/j.modgep.2006.02.003 . [DOI] [PubMed] [Google Scholar]
- 95.Vallecillo-Garcia P, Orgeur M, Vom Hofe-Schneider S, Stumm J, Kappert V, Ibrahim DM, et al. Odd skipped-related 1 identifies a population of embryonic fibro-adipogenic progenitors regulating myogenesis during limb development. Nat Commun. 2017;8(1):1218 Epub 2017/11/01. doi: 10.1038/s41467-017-01120-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12(2):143–52. Epub 2010/01/19. doi: 10.1038/ncb2014 . [DOI] [PubMed] [Google Scholar]
- 97.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12(2):153–63. Epub 2010/01/19. doi: 10.1038/ncb2015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Contreras O, Rebolledo DL, Oyarzun JE, Olguin HC, Brandan E. Connective tissue cells expressing fibro/adipogenic progenitor markers increase under chronic damage: relevance in fibroblast-myofibroblast differentiation and skeletal muscle fibrosis. Cell Tissue Res. 2016;364(3):647–60. Epub 2016/01/09. doi: 10.1007/s00441-015-2343-0 . [DOI] [PubMed] [Google Scholar]
- 99.Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124(Pt 21):3654–64. Epub 2011/11/03. doi: 10.1242/jcs.086629 . [DOI] [PubMed] [Google Scholar]
- 100.Hayashi S, Takeichi M. Emerging roles of protocadherins: from self-avoidance to enhancement of motility. J Cell Sci. 2015;128(8):1455–64. Epub 2015/03/10. doi: 10.1242/jcs.166306 . [DOI] [PubMed] [Google Scholar]
- 101.Lefebvre JL, Kostadinov D, Chen WV, Maniatis T, Sanes JR. Protocadherins mediate dendritic self-avoidance in the mammalian nervous system. Nature. 2012;488(7412):517–21. Epub 2012/07/31. doi: 10.1038/nature11305 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Madduri S, Papaloizos M, Gander B. Synergistic effect of GDNF and NGF on axonal branching and elongation in vitro. Neurosci Res. 2009;65(1):88–97. Epub 2009/06/16. doi: 10.1016/j.neures.2009.06.003 . [DOI] [PubMed] [Google Scholar]
- 103.Yang X, Arber S, William C, Li L, Tanabe Y, Jessell TM, et al. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron. 2001;30(2):399–410. . [DOI] [PubMed] [Google Scholar]
- 104.Lin W, Burgess RW, Dominguez B, Pfaff SL, Sanes JR, Lee KF. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature. 2001;410(6832):1057–64. doi: 10.1038/35074025 . [DOI] [PubMed] [Google Scholar]
- 105.Darabid H, Perez-Gonzalez AP, Robitaille R. Neuromuscular synaptogenesis: coordinating partners with multiple functions. Nat Rev Neurosci. 2014;15(11):703–18. Epub 2014/12/11. . [PubMed] [Google Scholar]
- 106.James JM, Mukouyama YS. Neuronal action on the developing blood vessel pattern. Semin Cell Dev Biol. 2011;22(9):1019–27. Epub 2011/10/08. doi: 10.1016/j.semcdb.2011.09.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tawil R, van der Maarel SM, Tapscott SJ. Facioscapulohumeral dystrophy: the path to consensus on pathophysiology. Skelet Muscle. 2014;4:12 Epub 2014/06/19. doi: 10.1186/2044-5040-4-12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Iaco A, Planet E, Coluccio A, Verp S, Duc J, Trono D. DUX-family transcription factors regulate zygotic genome activation in placental mammals. Nat Genet. 2017;49(6):941–5. Epub 2017/05/02. doi: 10.1038/ng.3858 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lemmers RJ, van der Vliet PJ, Klooster R, Sacconi S, Camano P, Dauwerse JG, et al. A unifying genetic model for facioscapulohumeral muscular dystrophy. Science. 2010;329(5999):1650–3. Epub 2010/08/21. doi: 10.1126/science.1189044 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van der Maarel SM, Miller DG, Tawil R, Filippova GN, Tapscott SJ. Facioscapulohumeral muscular dystrophy: consequences of chromatin relaxation. Curr Opin Neurol. 2012;25(5):614–20. Epub 2012/08/16. doi: 10.1097/WCO.0b013e328357f22d . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lemmers RJ, Tawil R, Petek LM, Balog J, Block GJ, Santen GW, et al. Digenic inheritance of an SMCHD1 mutation and an FSHD-permissive D4Z4 allele causes facioscapulohumeral muscular dystrophy type 2. Nat Genet. 2012;44(12):1370–4. doi: 10.1038/ng.2454 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van den Boogaard ML, Lemmers RJ, Balog J, Wohlgemuth M, Auranen M, Mitsuhashi S, et al. Mutations in DNMT3B Modify Epigenetic Repression of the D4Z4 Repeat and the Penetrance of Facioscapulohumeral Dystrophy. Am J Hum Genet. 2016;98(5):1020–9. Epub 2016/05/08. doi: 10.1016/j.ajhg.2016.03.013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Banerji CR, Knopp P, Moyle LA, Severini S, Orrell RW, Teschendorff AE, et al. beta-Catenin is central to DUX4-driven network rewiring in facioscapulohumeral muscular dystrophy. J R Soc Interface. 2015;12(102):20140797 Epub 2015/01/01. doi: 10.1098/rsif.2014.0797 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Geng LN, Yao Z, Snider L, Fong AP, Cech JN, Young JM, et al. DUX4 activates germline genes, retroelements, and immune mediators: implications for facioscapulohumeral dystrophy. Dev Cell. 2012;22(1):38–51. Epub 2012/01/03. doi: 10.1016/j.devcel.2011.11.013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wallace LM, Garwick SE, Mei W, Belayew A, Coppee F, Ladner KJ, et al. DUX4, a candidate gene for facioscapulohumeral muscular dystrophy, causes p53-dependent myopathy in vivo. Ann Neurol. 2011;69(3):540–52. Epub 2011/03/30. doi: 10.1002/ana.22275 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scionti I, Greco F, Ricci G, Govi M, Arashiro P, Vercelli L, et al. Large-scale population analysis challenges the current criteria for the molecular diagnosis of fascioscapulohumeral muscular dystrophy. Am J Hum Genet. 2012;90(4):628–35. Epub 2012/04/10. doi: 10.1016/j.ajhg.2012.02.019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scionti I, Fabbri G, Fiorillo C, Ricci G, Greco F, D'Amico R, et al. Facioscapulohumeral muscular dystrophy: new insights from compound heterozygotes and implication for prenatal genetic counselling. J Med Genet. 2012;49(3):171–8. Epub 2012/01/06. doi: 10.1136/jmedgenet-2011-100454 . [DOI] [PubMed] [Google Scholar]
- 118.Puppo F, Dionnet E, Gaillard MC, Gaildrat P, Castro C, Vovan C, et al. Identification of variants in the 4q35 gene FAT1 in patients with a facioscapulohumeral dystrophy-like phenotype. Hum Mutat. 2015;36(4):443–53. Epub 2015/01/24. doi: 10.1002/humu.22760 . [DOI] [PubMed] [Google Scholar]
- 119.Mariot V, Roche S, Hourde C, Portilho D, Sacconi S, Puppo F, et al. Correlation between low FAT1 expression and early affected muscle in facioscapulohumeral muscular dystrophy. Ann Neurol. 2015;78(3):387–400. Epub 2015/05/29. doi: 10.1002/ana.24446 . [DOI] [PubMed] [Google Scholar]
- 120.Ottaviani A, Rival-Gervier S, Boussouar A, Foerster AM, Rondier D, Sacconi S, et al. The D4Z4 macrosatellite repeat acts as a CTCF and A-type lamins-dependent insulator in facio-scapulo-humeral dystrophy. PLoS Genet. 2009;5(2):e1000394 Epub 2009/02/28. doi: 10.1371/journal.pgen.1000394 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Park HJ, Lee W, Kim SH, Lee JH, Shin HY, Kim SM, et al. FAT1 Gene Alteration in Facioscapulohumeral Muscular Dystrophy Type 1. Yonsei Med J. 2018;59(2):337–40. Epub 2018/02/13. doi: 10.3349/ymj.2018.59.2.337 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Robin JD, Ludlow AT, Batten K, Gaillard MC, Stadler G, Magdinier F, et al. SORBS2 transcription is activated by telomere position effect-over long distance upon telomere shortening in muscle cells from patients with facioscapulohumeral dystrophy. Genome Res. 2015;25(12):1781–90. Epub 2015/09/12. doi: 10.1101/gr.190660.115 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jones T, Jones PL. A cre-inducible DUX4 transgenic mouse model for investigating facioscapulohumeral muscular dystrophy. PLoS ONE. 2018;13(2):e0192657 Epub 2018/02/08. doi: 10.1371/journal.pone.0192657 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bosnakovski D, Chan SSK, Recht OO, Hartweck LM, Gustafson CJ, Athman LL, et al. Muscle pathology from stochastic low level DUX4 expression in an FSHD mouse model. Nat Commun. 2017;8(1):550 Epub 2017/09/17. doi: 10.1038/s41467-017-00730-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dandapat A, Bosnakovski D, Hartweck LM, Arpke RW, Baltgalvis KA, Vang D, et al. Dominant lethal pathologies in male mice engineered to contain an X-linked DUX4 transgene. Cell Rep. 2014;8(5):1484–96. Epub 2014/09/02. doi: 10.1016/j.celrep.2014.07.056 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Krom YD, Thijssen PE, Young JM, den Hamer B, Balog J, Yao Z, et al. Intrinsic epigenetic regulation of the D4Z4 macrosatellite repeat in a transgenic mouse model for FSHD. PLoS Genet. 2013;9(4):e1003415 Epub 2013/04/18. doi: 10.1371/journal.pgen.1003415 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gonzalez-Navarro FF, Belanche-Munoz LA, Silva-Colon KA. Effective classification and gene expression profiling for the Facioscapulohumeral Muscular Dystrophy. PLoS ONE. 2013;8(12):e82071 Epub 2013/12/19. doi: 10.1371/journal.pone.0082071 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rahimov F, King OD, Leung DG, Bibat GM, Emerson CP Jr., Kunkel LM, et al. Transcriptional profiling in facioscapulohumeral muscular dystrophy to identify candidate biomarkers. Proc Natl Acad Sci U S A. 2012;109(40):16234–9. Epub 2012/09/19. doi: 10.1073/pnas.1209508109 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moyle LA, Blanc E, Jaka O, Prueller J, Banerji CR, Tedesco FS, et al. Ret function in muscle stem cells points to tyrosine kinase inhibitor therapy for facioscapulohumeral muscular dystrophy. Elife. 2016;5 Epub 2016/11/15. doi: 10.7554/eLife.11405 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Leighton PA, Mitchell KJ, Goodrich LV, Lu X, Pinson K, Scherz P, et al. Defining brain wiring patterns and mechanisms through gene trapping in mice. Nature. 2001;410(6825):174–9. Epub 2001/03/10. doi: 10.1038/35065539 . [DOI] [PubMed] [Google Scholar]
- 131.Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, et al. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25(2):139–40. Epub 2000/06/03. doi: 10.1038/75973 . [DOI] [PubMed] [Google Scholar]
- 132.Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, et al. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382(6586):76–9. Epub 1996/07/04. doi: 10.1038/382076a0 . [DOI] [PubMed] [Google Scholar]
- 133.Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110(3):385–97. Epub 2002/08/15. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(A) Whole-mount β-galactosidase staining was performed on Fat1+/+; (left) and Fat1-/- (right) embryos carrying the MLC3F-2E transgene (S1 Table). Top images represent a side view of entire embryos and indicate the position of higher magnification areas, including that of the top pictures in Fig 1A and 1B as well as the upper forelimb region (with scapulohumeral muscles) and distal forelimb, showing a region where dispersed myocytes accumulate in Fat1-/- embryos. The second row images highlight a closer view of the upper thoracic and forelimb region. The dotted lines indicate for each genotype the levels and angle corresponding to the three consecutive sections shown in (C) and (D). (B) Quantification of the number of dispersed myocytes found in orange areas in the forelimb (left plot) and of the area occupied by the ectopic humeral muscle (ectop) appearing between the spinodeltoid (Del) and the triceps brachii (TriBra) muscles (right plot). These data reproduce and confirm our own previous results. Underlying data are provided in S1 Data. (C, D) Cross sections of control Fat1+/- and mutant Fat1-/- E12.5 embryos, featuring three consecutive sections at forelimb levels (Level 1 and Level 2) and upper thoracic level (Level 3), immunostained with antibodies against Pax7 (red), Myh1 (green), and neurofilament (white) and with DAPI (blue). Images in (D) represent high-magnification views of the area highlighted with the yellow dotted square in (C). These data confirm (1) the severe reduction in thickness of the CM muscle (Level 3, and higher magnification in [D]), (2) the presence of a robust ectopic muscle next to the triceps brachii (Levels 2 + 3, and higher magnification in [D]), and (3) the presence of dispersed myogenic progenitors and muscle fibers in the ectopic subcutaneous position in the forelimb (the image in [D] shows higher magnification of an area between the digit extensors and the skin). Lack of obvious phenotype in the diaphragm is also shown. CM, cutaneous maximus; Del, spinodeltoid; diaph, diaphragm; disp. Myo, dispersed myoblatsts; ectop, ectopic humeral muscle; ext. dig, extensor digitorum; trap, trapezius; TriBra, triceps brachii.
(TIF)
Expression of Gdnf (A) and of three markers of developing muscles (MyoD, Six1, and Lbx1 [B]) was analyzed by in situ hybridization on floating vibratome sections of E12.5 wild-type (left panels) and Fat1LacZ/LacZ embryos (right panels). (A) Gdnf expression is visualized at three successive anteroposterior levels, showing a hot spot at the brachial plexus (mesenchymal cells around passing nerves), where Gdnf expression is drastically reduced by the absence of Fat1, and two successive levels in the CM and LD muscles, where Fat1LacZ/LacZ embryos exhibit a thinner CM with less overall Gdnf signal. (B) On sections corresponding to the anterior part of the CM muscle, expression of markers of muscle differentiations (MyoD, Six1, and Lbx1) also shows that Fat1LacZ/LacZ embryos exhibit a selective loss of staining in the CM and not other neighboring muscle masses. CM, cutaneous maximus; Gdnf, glial cell line-derived neurotrophic factor; Lbx1, ladybird homeobox 1; LD, latissimus dorsi; MyoD, myogenic differentiation 1; Six1, sine oculis-related homeobox 1.
(TIF)
(A, B) The nerve pattern was analyzed by IHC with antibodies against neurofilament (2H3 antibody) (A) or by taking advantage of the Hb9-GFP transgene (S1 Table) (B), which labels motor neurons and their axons. (A) Anti-neurofilament histochemistry on whole-mount wild-type and Fat1LacZ/LacZ embryos at E12.0. (B) Hb9-GFP was visualized with antibodies against GFP (top and middle images) or by direct fluorescence imaging in Fat1+/+; Hb9-GFP+ and Fat1LacZ/LacZ; Hb9-GFP+ embryos at E12.5. For IHC, embryos were cut in half, cleared in BB-BA, and flat-mounted. Upper panels (A, B) are low-magnification images of the embryo flank. Lower panels show high-magnification views of the area containing the CM muscle. The area covered by CM-innervated axons is highlighted in white (middle panels). In the middle panels in (B), axons of vertically oriented thoracic spinal nerves have been manually removed by dissection to improve visibility of CM axons. In lower panels in (B), direct GFP imaging was done on PFA fixed embryos, after flat-mounting of the flank. (C) Quantifications of the relative expansion of the area covered by CM-innervating axons. Upper plot: for each embryo side, the area covered by CM-innervating axons is plotted relative to the length of a thoracic nerve (T10, from dorsal root origin to ventral tip). Arrows point the stages of representative examples shown in (A) and (B). Bottom plot: for each embryo, the CM-innervated area/T10 length was normalized to the median ratio of control embryos, by size range. Blue dots: Fat1+/+ (n = 35, same sample set as in controls of Fig 2); red dots: Fat1LacZ/LacZ (n = 12). Underlying data are provided in S1 Data. BB-BA, benzyl-benzoate/benzyl-alcohol mix; CM, cutaneous maximus; IHC, immunohistochemistry; PFA, paraformaldehyde.
(TIF)
(A) Scheme of the protein products of a wild-type Fat1 allele (full-length Fat1) and of a Fat1LacZ allele (producing a chimaeric protein with the first 8 cadherin domains of Fat1 extracellular domain, fused to an exogenous transmembrane domain in frame with β-galactosidase as intracellular domain). An antibody to Fat1 (Sigma 1869) directed against a portion of the common segment of Fat1 extracellular domain recognizes both proteins, whereas an antibody to β-galactosidase recognizes only the Fat1–β-gal fusion protein, most of which is sequestered in the Golgi apparatus and not localized at the cell membrane. (B) Comparison of immunohistochemical detection of Fat1 in a Fat1LacZ/+ embryo using the anti-β-galactosidase antibody (red), the Fat1-1869 antibody (green), and the pattern of β-galactosidase activity revealed by Salmon-Gal staining on cross sections of an E13.5 mouse embryo at lumbar levels where it is possible to detect both the expression in subsets of MNs and in the caudal-most part of the mesenchymal subcutaneous layer, towards which the CM extends. The Fat1–β-gal fusion protein is mainly detected by both antibodies as punctae in the Golgi, most likely because the protein is misfolded and does not reach the cell membrane. The two stainings perfectly overlap (except for some weak staining of blood vessels in the green channel, mostly due to the secondary antibody) and correspond to the signal detected by Salmon-Gal staining. β-gal, β-galactosidase; CM, cutaneous maximus; epax, epaxial; MNs, motor neurons.
(TIF)
(A) Analysis of MyoD and Scleraxis expression in Fat1LacZ/+ and Metd/d E12.5 embryos, and of Fat1LacZ expression in Fat1LacZ/+ and Metd/d; Fat1LacZ/+ E12.5 embryos was performed by whole-mount in situ hybridization and X-gal staining, respectively. Peripheral sites of Fat1LacZ and Scleraxis expression are maintained in spite of the absence of migratory appendicular muscles, indicating that expression of both genes occurs in non-myogenic/mesenchymal cells. Orange arrowheads indicate the position of the CM muscle; white arrowheads indicate the mesenchyme hot spot in the humeral region of the forelimb; and yellow arrowheads indicate positions of Scleraxis-positive tendons in the distal limb (autopod). (B) Comparison of β-gal (red) and YFP (green) IHC in Pax3cre/+; R26YFP/+; Fat1LacZ/+ (top) and Pax3cre/cre; R26YFP/+; Fat1LacZ/+ (bottom) E11.5 embryos on transverse sections at forelimb levels. The region indicated with a dotted area is shown in higher magnification on the right panels. CM, cutaneous maximus; MyoD, myogenic differentiation 1; Pax3, Paired box 3; R26, Rosa26 Locus; X-gal, substrate for β-galactosidase activity; YFP, yellow fluorescent protein.
(TIF)
(A) Double ISH analysis of Fat1 (purple) is combined with Etv4 (red) expression in WT and Metd/d E12.5 spinal cords in lower panels. (B) ISH analysis of Fat1 in WT, Etv4-/-, and Gdnf-/- spinal cords at E11.5 (upper panels) and E12.5 (lower panels). (A, B) Onset of Fat1 expression occurs independently of Met (A), of Etv4 and of Gdnf (B) functions and is preserved in Metd/d, Etv4-/-, and Gdnf-/- embryos at E11.5. In contrast, analysis of Fat1 expression at E12.5 reveals alterations consistent with (1) defective maintenance of Fat1 expression in the CM pool in Metd/d spinal cords (A), possibly due to the lack of migrating muscles and subsequent growth factor depletion, and (2) aberrant positioning of the CM pool in Pea3-/- and Gdnf-/- spinal cords (B). (C) Comparison of Fat1, Sema3E, and Runx1 expression in the brachial spinal cord at E12.5 was performed by double ISH with Fat1 (purple) and Sema3E (red) on the left, and with Runx1 (purple) and Sema3E (red) on the right. (D) Fat1 expression in the mouse thoracic spinal cord is shown X-gal staining on sections of Fat1LacZ/+ spinal cords at E12.5, showing expression in all neural progenitors in the ventricular zone, and lack of high- level expression in thoracic motor columns. CM, cutaneous maximus; Etv4, Ets variant gene 4; ISH, in situ hybridization; Runx1, runt related transcription factor 1; Sema3E, semaphoring 3E; WT, wild-type.
(TIF)
(A–C) (A) Etv4 expression was analyzed by ISH in E12.5 wild-type and Fat1LacZ/LacZ spinal cords. The images represent flat-mounted spinal cords in the brachial region. (B) Quantifications of Etv4 signal: each plot represents the average signal distribution (± standard deviation in light blue) measured on the indicated number of spinal cord sides along the orange dotted line in each image in (A) (Fat1+/+ [n = 12]; this set of controls is the same as that shown in Fig 6); Fat1LacZ/LacZ (n = 6). (C) Quantifications and statistical analyses of the sum of signal intensity corresponding to the area under the curves in plots shown in (C): each dot represents the sum of Etv4 intensity for each spinal cord side, the number of samples being indicated (the two sides of each embryo considered independent). (B–C): Underlying data are provided in S1 Data. (D) Analysis of Etv4 expression was carried out by ISH with Etv4 probe alone (top) on WT and Fat1LacZ/LacZ E13.5 spinal cords, or with Etv4 probe combined with prior X-gal staining (bottom) on Fat1LacZ/+ and Fat1LacZ/LacZ E13.5 spinal cords. (E) Analysis by ISH of Sema3E and Cadherin8 expression in flat-mounted brachial spinal cords from wild-type, Fat1LacZ/LacZ, Metd/d, and Etv4-/- embryos at E12.5: expression of Sema3E and Cadherin8 in the C7–C8 segments is lost in Etv4-/- and reduced in Metd/d mutants, whereas they are unaffected in Fat1LacZ/LacZ spinal cords. Cad8, Cadherin 8, Etv4, Ets variant gene 4; ISH, in situ hybridization; Sema3E, semaphoring 3E; WT, wild-type; X-gal, substrate for β-galactosidase activity.
(TIF)
(A) Embryonic musculature was visualized by X-gal staining on E12.5 embryos carrying the MLC3F-2E transgene in different contexts of tissue-specific deletion of Fat1, with Wnt1-cre (neural crest cells), Olig2-cre (MN precursors), and Prx1-cre (limb/lateral mesoderm-derived mesenchyme) (cre lines described in S1 Table). Top images are images of the entire flank of Fat1Flox/Flox; MLC3F-2E controls, and Wnt1-cre; Fat1Flox/Flox; MLC3F-2E, Olig-cre; Fat1Flox/Flox; MLC3F-2E, and Prx1-cre; Fat1Flox/Flox; MLC3F-2E embryos, respectively. Higher magnification pictures focus (from top to bottom) on humeral muscles, on the forelimb area, and on the flank region in which the CM spreads, respectively. (B) Quantification of the number of dispersed myocytes found in orange areas in the forelimb (left plot) and of the area occupied by the ectopic humeral muscle (ectop) appearing between the spinodeltoid (Del) and the triceps brachii (TriBra) muscles (right plot). In both graphs, data from Fat1-/- embryos (from plots in S1B Fig) have been added in the last lane, for comparison of effect size. Note that on both graphs, there is an interruption on the y axis and a change of scale. Only Prx1-cre; Fat1Flox/Flox; MLC3F-2E embryos exhibit a mild increase compared to controls. Underlying data are provided in S1 Data. (C) Quantification of the expansion rate of differentiated CM fibers in Fat1Flox/Flox; MLC3F-2E controls, Wnt1-cre; Fat1Flox/Flox; MLC3F-2E, and Olig-cre; Fat1Flox/Flox; MLC3F-2E. Quantifications of Prx1-cre; Fat1Flox/Flox; MLC3F-2E embryos are shown in Fig 7B. Left graph: for each embryo side, the area covered by differentiated CM fibers was plotted relative to the area occupied by body wall muscles. Right plot: for each embryo, the CM area/body wall area was normalized to the median ratio of control embryos. Blue dots: Fat1Flox/Flox; MLC3F-2E (n = 57, includes the same set of controls as Fig 7B); green crosses: Wnt1-cre; Fat1Flox/Flox-; MLC3F-2E (n = 8); purple triangles: Olig2-cre; Fat1Flox/Flox-; MLC3F-2E (n = 6). Underlying data are provided in S1 Data. Scale bars (A) 500 μm. BW, body wall muscles; CM, cutaneous maximus; hum, humeral; sDel, spinodeltoid; ectop, ectopic humeral muscle; TriBra, triceps brachii.
(TIF)
(A) The respective effects of depletion with Wnt1-cre, Olig2-cre, Prx1-cre, and Pax3-cre (premigratory myogenic cells + neural crest lineage) (cre lines described in S1 Table) were assessed by whole-mount X-gal staining on E14.5 Fat1Flox/Flox; MLC3F-2E controls (first column), Olig-cre; Fat1Flox/Flox; MLC3F-2E (second column), Prx1-cre; Fat1Flox/Flox; MLC3F-2E (third column), Wnt1-cre; Fat1Flox/Flox; MLC3F-2E (fourth column), and Pax3cre/+; Fat1Flox/ Flox; MLC3F-2E (fifth column) embryos. Top panels: low-magnification pictures show whole-embryo side views. Boxed areas show the position of higher magnification pictures shown in lower panels. Middle panels focus on facial subcutaneous muscles. Lower panels focus on the CM muscle. Prx1-cre; Fat1Flox/Flox embryos display normal musculature in the face but abnormal muscle shape and intramuscular orientation in the CM (higher magnification of the orange box shown in [C]), while Wnt1-cre; Fat1Flox/Flox embryos have a normal CM but severely affected subcutaneous muscles in the face, compared to controls (Fat1Flox/Flox). Thus, ablation in neural crest cells causes severe alterations in shape of the facial subcutaneous muscles in both Pax3cre/+; Fat1Flox/Flox and Wnt1-cre; Fat1Flox/Flox embryos. In contrasts, muscle shapes of Olig2-cre; Fat1Flox/Flox embryos are indistinguishable from those seen in controls. (B, C) Higher magnification views of fiber density in the CM in yellow and orange boxed areas outlined in lower panels in (A), respectively comparing control with Pax3cre/+; Fat1Flox/Flox (B) and with Prx1-cre; Fat1Flox/Flox (C) embryos. The effect of Fat1 ablation in Pax3cre/+; Fat1Flox/Flox embryos on CM appearance (B) is modest at E14.5 (high-magnification images illustrate a subtle reduction in the density of LacZ-positive nuclei). In contrast, the reduction in fiber density observed in Prx1-cre; Fat1Flox/Flox embryos (C) is very severe in the ventral CM, in contrast to more dorsal regions, coinciding with the limit above which Prx1-cre is not active. CM, cutaneous maximus; dCM, dorsal CM; fm, facial subcutaneous muscle; temp, temporalis; vCM, ventral CM; zyg, zygomatic muscle.
(TIF)
(A) Characterization of the mesenchyme subtype at the CM–skin interface. Sections of an E12.5 wild-type embryo at the level of the anterior CM (and upper forelimb) were immunostained with antibodies against Myh1 (white), TenascinC (green), Tcf4 (green), Pdgfrα (red), and DAPI (blue). The lower panels represent higher magnifications of the two bowed areas indicated in the low magnification views, focusing on the mesenchyme surrounding the CM and at the interface between CM and skin (middle panels) and the extremity of one forelimb muscle (lower panel). Whereas Tcf4 is detected in myogenic cells and connective tissue fibroblasts associated with the forelimb muscle, no expression is detected in the CM/skin connective tissue, which, in contrast, expresses TenascinC and Pdgfrα. (B) Control and Pdgfrα-iCre; R26YFP/+ embryos were collected at E12.5 from a pregnant female treated with tamoxifen at E9.5 + E10.5. Images show the direct YFP fluorescence signal acquired with the same exposure time. The vertical lines in the lower picture highlight the level of sections corresponding to Fig 9F (anterior CM and posterior CM). (C) Whole-mount β-galactosidase staining was performed on tamoxifen-treated control; MLC3F-2E (left) and Pdgfrα-iCre; Fat1Flox/Flox- ; MLC3F-2E (right) embryos at E12.5. Top images represent the upper forelimb region (with scapulohumeral muscles); bottom images represent the forelimb region, where myocyte dispersal is being quantified in (D). (D) Quantification of the number of dispersed myoblasts observed in the forelimb (counted in the areas delimited by orange lines). Blue dots: Controls; MLC3F-2E (n = 17, control embryos from tamoxifen-treated litters); red dots: Pdgfrα-iCre; Fat1Flox/Flox- ; MLC3F-2E (n = 17). Underlying data are provided in S1 Data. CM, cutaneous maximus; iCre, short form of CRE/ERT2; Myh1, myosin heavy chain I; Pdgfrα, platelet-derived growth factor receptor alpha; R26, Rosa26 locus; Tcf4, T cell factor 4; TenC, Tenascin C.
(TIF)
(A–C) Anti-neurofilament IHC was performed on E12.5 embryos, in the context of Myf5-cre-driven (S1 Table) myogenic lineage depletion, mediated by conditional DTA expression under regulatory control of the R26 locus (R26Lox-LacZ-STOP-Lox-DTA mouse line S1 Table). This nerve pattern is either visualized on its own (A, B) or combined with X-gal staining, when embryos also carry the MLC3F-2E-LacZ transgene (C). In cases with MLC3F-2E (C), X-gal staining also detects LacZ expression driven by the R26 locus in unrecombined cells (the relative expression levels in the two loci [R26 and MLC3F-2E-LacZ transgene] allows visualizing muscles in the trunk area but not in the limb). After BB-BA clearing, the embryos were cut in half and the internal organs and skin removed to visualize the CM and brachial plexus. Genotypes shown are control R26Lox-LacZ-STOP-Lox-DTA; and Myf5-cre; R26Lox-LacZ-STOP-Lox-DTA embryos (A, B); control R26Lox-LacZ-STOP-Lox-DTA; MLC3F-2E (left), Myf5-cre; R26Lox-LacZ-STOP-Lox-DTA; MLC3F-2E (middle), or Myf5-cre; R26Lox-LacZ-STOP-Lox-DTA; MLC3F-2E (right) (C). Upper panels show the entire flank of embryos at comparable stages. The area covered by CM-innervating axons is outlined with white dotted lines. The extent of the T8 thoracic nerve is outlined with a black arrow. The length of the forelimb (slightly affected by muscle depletion) is highlighted by a red arrow. Middle panels show higher magnification of the upper thoracic region containing the CM and corresponding motor axons as well as thoracic nerves, after manual removal by dissection of most cutaneous sensory nerves. Lower panels show the upper cervical region, with the trapezius muscle, innervated by the cranial nerve XI, also called spinal accessory nerve. (D, E) Quantifications of the progression of CM innervation (D) and of thoracic nerve growth (E). Left plots: for each embryo side, the area covered by CM-innervating axons (D) or the length of the T8 nerve (E) were plotted relative to the head area, comparing Myf5-cre; R26Lox-LacZ-STOP-Lox-DTA; embryos (red dots) to controls (blue dots). The T8 value represents the distance between the dorsal tip of sensory nerve roots and the ventral-most axon tips on the flank, thus including both sensory and motor components. The head area was used as reference, as it grows proportionally to the embryo, and it is not affected by muscle depletion, in contrast to other distances/areas in the trunk or limbs. Right plots: for each embryo side, we calculated the ratios CM-innervated area/head area (D) and T8 length/head area (E), and these values were normalized to the median ratio of control embryos, by size range. Blue dots: Controls (n = 8, pooling all control littermates); red dots: Myf5-cre; R26Lox-LacZ-STOP-Lox-DTA (n = 8). (D, E) Underlying data are provided in S1 Data. Arrows point to the stage of representative examples shown in (A–C). BB-BA, benzyl-benzoate/benzyl-alcohol mix; CM, cutaneous maximus; cre, cre recombinase; DTA, diphtheria toxin A; IHC, immunohistochemistry; MN, motor neuron; Myf5, myogenic factor 5; R26, Rosa26 locus; X-gal, substrate for β-galactosidase activity.
(TIF)
(A) Analysis by IHC of Clusterin, islet1, and GFP expression on spinal cord sections at the C7–C8 level from E13.5 WT; Etv4-GFP/+, from GdnfLacZ/LacZ; Etv4-GFP/+, and from Prx1-cre; Fat1Flox/Flox embryos. Clusterin and GFP staining were done on sections neighboring those analyzed with Islet1/2 IHC. Etv4-GFP could not be combined with Prx1-cre, as this combination appears to cause a phenotype (short limbs, with impaired elongation, visible from E12.5 onward) unrelated to Fat1 alleles and most likely linked to the site of integration of both transgenes. (B) X-gal staining of GdnfLacZ/+ and GdnfLacZ/LacZ embryos, showing that expansion of the area covered by GdnfLacZ-expressing CM progenitors is not affected by the lack of Gdnf. CM, cutaneous maximus; cre, cre recombinase; Etv4, Ets variant gene 4; GFP, green fluorescent protein; IHC, immunohistochemistry; MN, motor neuron; WT, wild-type; X-gal, substrate for β-galactosidase activity.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.