Abstract
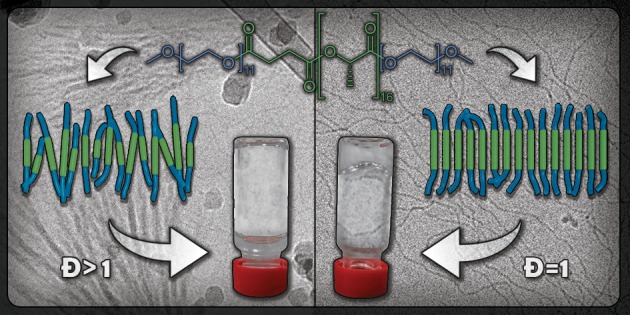
Intriguingly, little is known about the impact of dispersity on the crystallization driven self-assembly (CDSA) of amphiphilic block copolymers in aqueous media. Here, we investigate the influence of dispersity on the CDSA of ABA-type amphiphilic block co-oligomers (ABCOs). Two pairs of ABCOs are synthesized comprising discrete (Đ = 1.00) or disperse (Đ = 1.20) isotactic l-lactic acid 16-mers as the semicrystalline hydrophobic block and either oligo(ethylene glycol) methyl ether (MeOoEG) or oligo(tetraethylene glycol succinate) (oTEGSuc) as the discrete hydrophilic block. Self-assembly studies in water with 10% THF reveal uniform nanofibers/2D sheets for the discrete oligomers, but such structural regularity is largely compromised in the disperse oligomers. The results are corroborated by sharp melting transitions in both solution and bulk for the discrete ABCOs, unlike their disperse analogues that show a lack of crystallization. Interestingly, the discrete MeOoEG-LLA oligomer reveals crystallization driven gelation, illustrating the contrasting differences between the discrete oligomers and their disperse counterparts.
Self-assembly of block copolymers is a topic of considerable interest in polymer science due to the tremendous potential for applications in both biomedical engineering and nanolithography.1,2 Although abiotic polymers synthesized via controlled polymerization techniques show great diversity in their structures and functions, they still suffer from significant molar mass distribution and cannot match up with the architectural purity, precision, and complexity displayed by biomacromolecules.3,4 In contrast, most biopolymers such as DNA, RNA, and polypeptides are monodisperse and sequence-specific, which is critical to their overall three-dimensional organization and thus their properties and functions. In the recent past, polymer research started focusing on both discrete (Đ < 1.000002)4−10 and sequence-specific polymers11−15 in an attempt to mimic these aspects of biomacromolecules.
Amphiphilic block copolymers (ABCPs) have been a topic of long-standing interest in biomedical research for their ability to form nanocarriers such as micelles, vesicles, nanorods, and other tailored shapes for drug delivery.2,16,17 Depending on the hydrophilic/hydrophobic block ratio, molecular weight of the polymer, and crystallinity of the hydrophobic core, the morphology and properties of these nanoparticles can be engineered. Surprisingly, the impact of the molar mass distribution (Đ) on nanoparticle formation, shape, structural uniformity, and efficacy for uptake and release of guest molecules has hardly been investigated in amphiphilic block copolymers. Comparative self-assembly studies between ultradefined discrete ABCPs (Đ = 1.000) and their disperse counterparts could be extremely important in the fundamental understanding of the influence of dispersity on their properties after self-assembly in the aqueous phase. Contrasting differences between the discrete and disperse BCPs have been recently observed in the bulk phase. Our group has reported on the self-assembly of discrete diblock co-oligomers (BCOs) composed of oligolactic acid (oLA) and oligodimethylsiloxane (oDMS) obtained by iterative coupling-deprotection based synthetic strategies.18,19 Whereas the discrete polymer formed well-organized lamellar structures, its disperse counterpart revealed a lower extent of ordering with an increase of the domain spacing and greater stability of the phase-separated structures.19 In a complementary study, the group of Hawker observed similar differences in bulk between semidiscrete and disperse BCOs composed of oligomethyl methacrylate (oMMA) and oDMS.20
Intrigued by these results, we here aim to investigate the effect of dispersity in the aqueous phase, where the high mobility of the flexible polymers chains in solution presents an additional challenge. With this objective, we synthesized two pairs of ABA-type amphiphilic block co-oligomers (ABCOs) composed of discrete (Đ = 1.000) or disperse (Đ = 1.2) isotactic oligo(L-lactic acid) (LLA) as the hydrophobic block and either oligoethylene glycol methyl ether (MeOoEG) or oligo(tetraethylene glycol succinate) (oTEGSuc) as the discrete hydrophilic block (Figure 1A). The rationale behind choosing these blocks is as follows: the self-assembly of polylactic acid-b-polyethylene glycol (PLA-b-PEG) has been explicitly studied21−23 in the context of drug delivery and regenerative medicine because these polymers are known to be biocompatible.24,25 oTEGSuc block was chosen as a biodegradable substitute to PEG, to generate fully biodegradable ABCOs.26 Discrete chains of oTEGSuc can be synthesized following the iterative synthetic approach presented in Scheme 1. The l-lactic acid 16-mer was selected as the hydrophobic core either as a discrete 16-mer of exact molecular weight (LLA16) or as a disperse one (LLA∼16) with Đ = 1.20. Discrete LLA16 is semicrystalline and forms ordered lamellae in the bulk.27 In the context of drug delivery, the crystallization driven self-assembly (CDSA) of amphiphilic block copolymers with a crystallizable hydrophobic core has been applied to fabricate nonspherical nanostructures in solution.28,29 However, as of yet there is no study on understanding the consequence of dispersity on block crystallinity in solution, which is investigated in the present work.
Figure 1.
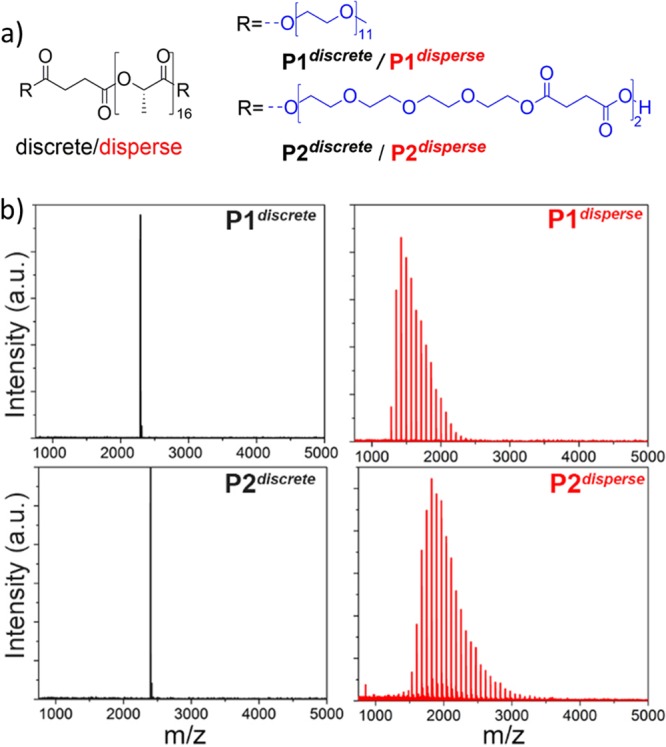
(a) Structures of discrete and disperse amphiphilic block co-oligomers and (b) their MALDI-ToF spectra.
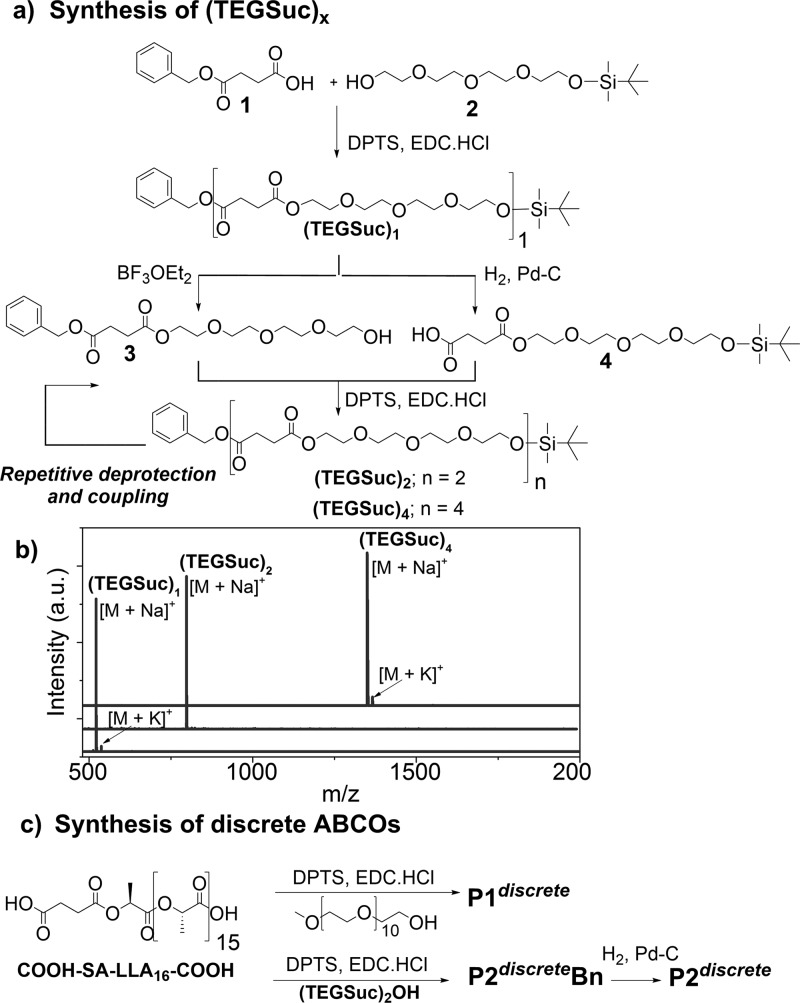
Scheme 1. Synthesis of (a) Discrete Tetraethylene Glycol Succinate (TEGSuc)x Oligomers, (b) Their MALDI-ToF Spectra, and (c) Synthesis of Discrete ABCOs.
For the synthesis of discrete oTEGSuc, we followed a modified iterative coupling-deprotection route (Scheme 1a) recently reported by our group for monodisperse lactic acid oligomers.18 Succinic acid monobenzyl ester (1) was coupled with mono tert-butyl dimethylsilyl (TBDMS) ether protected tetraethylene glycol (TEG; 2) to obtain double-protected monomer (TEGSuc)1. Orthogonal deprotection of the TBDMS ether and the benzyl ester resulted in free hydroxyl and carboxylic acid containing 3 and 4, respectively. Carbodiimide-promoted coupling between the two afforded double-protected dimer (TEGSuc)2. By repetition of the deprotection and coupling steps, tetramer (TEGSuc)4 was obtained. A stack plot of the MALDI-ToF spectra of the double-protected (TEGSuc)x oligomers from monomer to tetramer is shown in Scheme 1b. Single peaks corresponding to the mass of the desired species complexed with sodium ion and potassium ion indicate that precisely defined block lengths were obtained.
The synthesis of discrete telechelic LLA16 with free carboxylic acid moieties (HOOC-SA-LLA16-COOH, Scheme 1c) is based on the synthetic strategy previously reported by Hawker and co-workers.30 Disperse telechelic LLA∼16 was synthesized by ring-opening polymerization. The dispersity of LLA∼16 (Đ = 1.2) was determined using size exclusion chromatography. Full synthetic details on the preparation of the hydrophobic blocks can be found in the Supporting Information. Subsequent ligation of the acid functionalized LLA block with two equivalents of hydroxyl functionalized discrete (TEGSuc)2-OH, or commercially available discrete MeOoEG11–OH resulted in the target ABA-type ABCOs P1 and P2 (Scheme 1c). The discrete ABCOs are designated as Pxdiscrete and their disperse analogues are referred to as Pxdisperse. All the compounds were purified by automated column chromatography and fully analyzed by 1H NMR, 13C NMR, and matrix-assisted laser desorption/ionization time-of-flight (MALDI-ToF) mass spectrometry (Figures S1–S9). Despite similar degrees of polymerization based on 1H NMR, the MALDI-ToF spectra of the discrete and the disperse oligomers reveal a wide distribution in the chain length for disperse samples compared to a single peak for the discrete ones (Figure 1).
The thermal behavior and degree of ordering in the bulk of the discrete and disperse ABCOs was investigated using differential scanning calorimetry (DSC; Figures S10 and S11) and small and wide angle X-ray scattering (SAXS and WAXS; Figure S12). The introduction of dispersity into the LLA block has a clear effect on the position and intensity of the DSC transitions for both the P1 and P2 systems (see SI for a detailed discussion). Notably, the SAXS data show a primary scattering peak only for the discrete samples, corresponding to d = 6.5 nm for P1discrete and d = 10.5 nm for P2discreteBn (Figure S12). A higher order scattering peak at d = 5.2 nm in case of P2discreteBn suggests a lamellar packing of the ABCO in the bulk. Such clear scattering peaks at low q values are absent for both disperse P1disperse and P2disperseBn, indicating reduced phase segregation between the two blocks and a less defined morphology. All in all, the differences in bulk properties of discrete and disperse ABCOs are in good correspondence to our previous results published on oLLA-oDMS block-co-oligomers,19 where we observed a significant loss in long-range order when dispersity was introduced into the oLLA block.
Subsequently,
the self-assembly of the ABCOs was studied in aqueous
media. Due to the more hydrophobic nature of TEGSuc as compared to
MeOoEG, P2 could not be dissolved in pure water. As a
result, all the studies were performed in water/THF mixtures with
10% THF.31 To prepare the solutions, each
compound was dissolved in THF, and water was added dropwise to reach
a 1:9 THF/water binary mixture at 1–5 mg ABCO per mL. The formation
of the nanoparticles was studied with light and X-ray scattering (LS
and SAXS), micro-DSC, and microscopy (cryoTEM and total internal reflection
fluorescence (TIRF) microscopy). Diffusion coefficients were obtained
from multiangle light scattering by fitting the decay rate (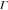 ) versus the scattering vector (q) plot (Figure S13). Using the
Stoke–Einstein equation, the hydrodynamic radius (Rh) of the particles was calculated. After self-assembly
in water, the Rh was found to be larger
for the discrete variants (Rh = 74 nm
for P1discrete and 125 nm for P2discrete) than for the disperse analogues (Rh = 42 nm for P1disperse and 90 nm for P2disperse). When comparing the LS data of P1 with P2, it appears that larger particles are for the P2 pairs. However, fitting the decay rate versus the scattering vector
reveals some anisotropy in the structures, indicating that the particles
are not spherical, and thus, the Stoke–Einstein equation does
not apply. To get an indication of the shape of the particles formed,
the scattering intensity (I) was plotted against q (Figure S14). The slope of
−1 for both P1discrete and P1disperse indicates that this ABCO self-assembles into cylindrical
micelles, whereas P2discrete and P2disperseself-assemble into vesicles or flat
bilayers (slope of −2). The morphology of an ABCP is largely
dependent on the hydrophobic/hydrophilic block ratio.2 For an invariant ratio, MeOoEG was replaced with a TEGSuc
block of comparable molar mass. Possibly, the more hydrophobic nature
of the TEGSuc block26 changes this balance,
leading to the formation of vesicles or bilayers.
) versus the scattering vector (q) plot (Figure S13). Using the
Stoke–Einstein equation, the hydrodynamic radius (Rh) of the particles was calculated. After self-assembly
in water, the Rh was found to be larger
for the discrete variants (Rh = 74 nm
for P1discrete and 125 nm for P2discrete) than for the disperse analogues (Rh = 42 nm for P1disperse and 90 nm for P2disperse). When comparing the LS data of P1 with P2, it appears that larger particles are for the P2 pairs. However, fitting the decay rate versus the scattering vector
reveals some anisotropy in the structures, indicating that the particles
are not spherical, and thus, the Stoke–Einstein equation does
not apply. To get an indication of the shape of the particles formed,
the scattering intensity (I) was plotted against q (Figure S14). The slope of
−1 for both P1discrete and P1disperse indicates that this ABCO self-assembles into cylindrical
micelles, whereas P2discrete and P2disperseself-assemble into vesicles or flat
bilayers (slope of −2). The morphology of an ABCP is largely
dependent on the hydrophobic/hydrophilic block ratio.2 For an invariant ratio, MeOoEG was replaced with a TEGSuc
block of comparable molar mass. Possibly, the more hydrophobic nature
of the TEGSuc block26 changes this balance,
leading to the formation of vesicles or bilayers.
To substantiate the formation of cylindrical micelles by P1 in solution, SAXS measurements were performed. The SAXS profile obtained of P1discrete was best fitted with a flexible cylinder model (Figures 2a and S15a). The radius of 3.2 nm, the Kuhn length of 103 nm and the overall length of 1038 nm agrees well with the cryoTEM observations (Figure 3b), which confirms the formation of elongated thin fibers of consistent width. In contrast, the cryoTEM image (Figure 3a) of the P1disperse reveals the coexistence of two populations. Next to the elongated thin fibers, bundles of shorter but much wider fibers are present. This bundling effect might be due to the coassembly of LLA blocks of varying lengths. The SAXS profile of P1disperse could not be fit well, which is likely due to an overlay of the scattering of multiple species present in solution (Figures 2a and S15b). The results above clearly exemplify the pronounced impact of LLA block dispersity on the homogeneity of the self-assembled structures. In addition, the morphologies formed were highly stable over time. CryoTEM images of aged samples for both P1discrete and P1disperse (Figure S16) retained the same morphologies even after keeping the solutions at room temperature for around 90 days.
Figure 2.
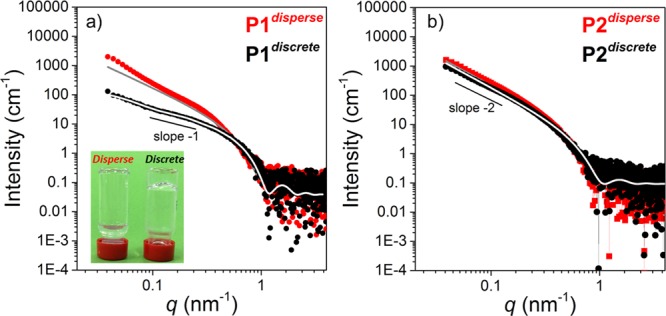
Solution SAXS traces of (a) P1discrete and P1disperse (0.5 mg mL–1) and of (b) P2discrete and P2disperse (0.6 mg mL–1) after self-assembly in water with 10% THF; (a, inset) self-assembly of P1discrete (gel) and P1disperse (sol) at 5 mg mL–1 in water with 10% THF. The lines represent the best fit to the data using either a flexible cylinder (a) or the lamellar (b) model.
Figure 3.
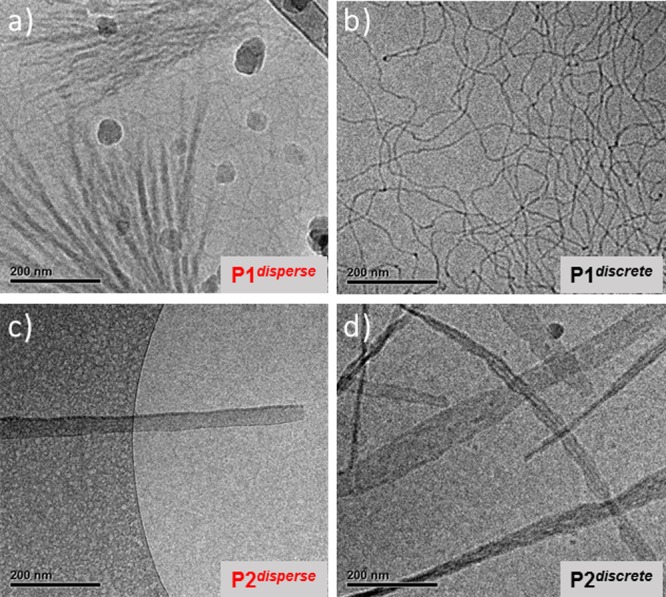
CryoTEM images at 25000 magnification of (a) P1disperse and (b) P1discrete, both at 5 mg mL–1 in water with 10% THF, and (c) P2disperse and (d) P2discrete both at 2 mg mL–1 in water with 10% THF. Large dark round particles in “a” are crystalline ice particles and not part of the sample. The images were recorded at 10 μm (a, b, d) and 5 μm defocus (c). For corresponding low-magnification images see Figure S17.
Interestingly, the discrete oligomer P1discrete formed a transparent gel at 5 mg mL–1 when the solution was heated and cooled back to room temperature (Figure 2a, inset). The gelation process was found to be reversible and repeatable. The gel–sol transition (Tgel), as determined visually for multiple cycles, varied between 42 and 48 °C upon heating the sample. This transition is very close to the melting temperature (Tm = 41 °C) of discrete LLA16 block measured in the bulk (Figure S10a), as well as the transition temperature of 43 °C measured in solution by micro-DSC (Figure 4, vide infra). Notably, no gelation was observed for P1disperse under identical conditions, although gelation in disperse PEG-PLLA-PEG based triblock copolymer is well reported via interdigitation of micelles through PEG chains.32 Such discrepancy in the gelation behavior of P1discrete and P1disperse nicely corroborates with their distinctly different morphologies as observed from cryoTEM.
Figure 4.
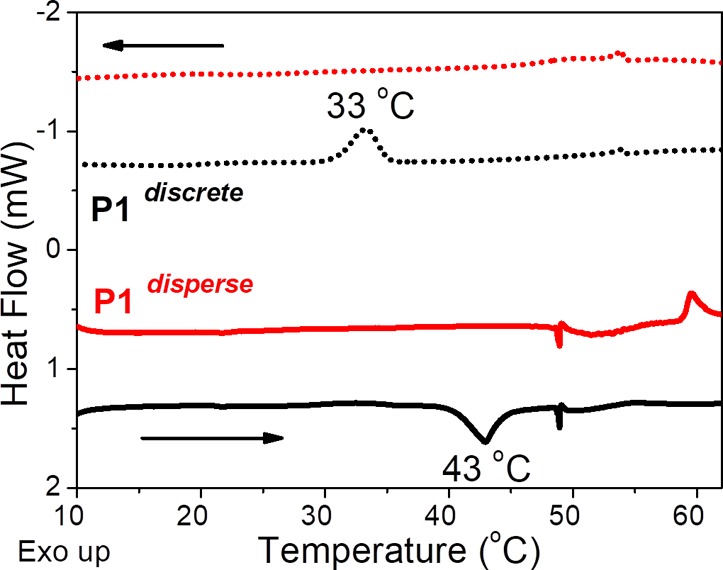
Micro-DSC traces of P1discrete and P1disperse at 5 mg mL–1 after self-assembly in water with 10% THF.
The self-assembly of the P2 pair was further investigated using SAXS in solution. The SAXS profile for both P2discrete and P2disperse shows a slope of −2, indicating the formation of vesicles or flat bilayers (Figures 2b and S15c,d). In contrast to the P1 pair, both profiles for P1discrete and P1disperse are very similar. The SAXS profiles were fitted best using the lamellae model, indicating a lamellae thickness of 7 nm (P2discrete) or 8 nm (P2disperse). Since the P2disperse sample was not transparent, indicative of the formation of large aggregates, cryoTEM imaging of P2disperse was first performed of a filtered sample, revealing the presence of uniform sheets rolled into U-shape (Figures 3c and S18). Flat sheets were observed in a sample that was not filtered prior to imaging (Figure S18). To visualize the aggregates of P2disperse at the μm length scale, TIRF microscopy was performed after the addition of the dye Nile Red. This hydrophobic dye is weakly fluorescent in water but becomes highly fluorescent after incorporation into hydrophobic domains.33 Besides the sheets rolled into U-shape, other spherical morphologies and large aggregates were present (Figure S18). In contrast, for P2discrete only the formation of long sheets was observed by cryoTEM imaging (Figure 3d), as well as by TIRF microscopy (Figure S19). Also for the P2 set, the results clearly exemplify that the dispersity of the LLA∼16 block inhibits the uniformity of the self-assembled structures in solution.
To study whether the self-assembly in solution is driven by the crystallization of the LLA block, microdifferential scanning calorimetry (microDSC) in 1:9 THF/water was performed. The solutions were heated from 5 to 70 °C at a rate of 0.5 K min–1 (Figure 4). P1discrete showed clear endothermic and exothermic transitions in the heating and the cooling runs corresponding to melting (Tm = 43 °C) and crystallization (Tc = 33 °C), respectively. The Tm in solution (Figure 4) corresponds well with the melting (Tm = 41 °C) in bulk (Figure S10a) for P1discrete indicating that this transition is indeed connected to the LLA16 crystallization. Further, close matching of the Tm with the Tgel–sol of P1discrete substantiates crystallization driven gelation. Interestingly, no phase transitions were observed for the discrete P2discrete (Figure S20), suggesting that the change in the hydrophilic block from MeOoEG to TEGSuc might influence the crystallization of the LLA16 core. This corroborates well with the amorphous nature of TEGSuc block as observed from its DSC profile (Figure S11a) in contrast to the semicrystalline PEG chain (Figure S11b). The very slow rate of crystallization of P2discreteBn in the bulk (Figure S10b,c) further supports our interpretation that the PEG chains aid the ordering of the LLA16 block unlike TEGSuc chains for discrete pairs.
No clear transitions were observed for P1disperse (Figure 4) or P2disperse (Figure S20). Such distinct differences between P1discrete and P1disperse reveal the negative impact of dispersity on the core crystallinity of the amphiphiles in the solution phase, leading to their varying self-assembly behavior. Possibly, the dispersity in the hydrophobic block does not allow effective packing of the LLA chains of varying length within the core of the nanoparticles in P1disperse, unlike in P1discrete. Although there are multiple reports on crystallization driven self-assembly of block copolymer amphiphiles, this is the first demonstration of the consequence of dispersity on the crystallization mediated self-assembly of oligomeric amphiphiles in aqueous solution.
In summary, we have methodically manifested the effect of dispersity on the assembly behavior of two sets of discrete amphiphilic block co-oligomers by comparing their solution self-assembly behaviors with their disperse counterparts. The finding of this work reveals remarkable differences between the discrete and the disperse ABCOs not just in the bulk but also in the solution phase in terms of crystallinity, gelation, morphology, and homogeneity of the self-assembled structures. We anticipate that further fundamental studies on pharmaceutically relevant PEG–PLLA based block co-oligomers will pave the way for synthesis of tailor-made nanocarriers with more control over their structures, dynamics, and functions as delivery vehicles.
Acknowledgments
The authors acknowledge financial support from the Dutch Ministry of Education, Culture and Science (Gravity Program 024.001.035). The BM29 beamline at the European Synchrotron Radiation Facilities (Grenoble, France) is acknowledged for access to the synchrotron facilities and for help with acquiring SAXS data. We gratefully thank G.M. ter Huurne for his help of acquiring the SAXS data and S. P. W. Wijnands for his help with TIRF microscopy.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmacrolett.8b00168.
Experimental procedures, synthesis, and characterization data for all compounds, bulk characterization, and Figures S1–S20 (PDF).
Author Contributions
All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Whitesides G. M.; Boncheva M. Beyond Molecules: Self-Assembly of Mesoscopic and Macroscopic Components. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 4769–4774. 10.1073/pnas.082065899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai Y.; Eisenberg A. Self-Assembly of Block Copolymers. Chem. Soc. Rev. 2012, 41, 5969. 10.1039/c2cs35115c. [DOI] [PubMed] [Google Scholar]
- Lutz J.-F.; Lehn J.-M.; Meijer E. W.; Matyjaszewski K. From Precision Polymers to Complex Materials and Systems. Nat. Rev. Mater. 2016, 1, 16024. 10.1038/natrevmats.2016.24. [DOI] [Google Scholar]
- Hawker C. J.; Wooley K. L. The Convergence of Synthetic Organic and Polymer Chemistries. Science 2005, 309, 1200–1205. 10.1126/science.1109778. [DOI] [PubMed] [Google Scholar]
- Lawrence J.; Lee S.-H.; Abdilla A.; Nothling M. D.; Ren J. M.; Knight A. S.; Fleischmann C.; Li Y.; Abrams A. S.; Schmidt B. V. K. J.; Hawker M. C.; Connal L. A.; McGrath A. J.; Clark P. G.; Gutekunst W. R.; Hawker C. J. A Versatile and Scalable Strategy to Discrete Oligomers. J. Am. Chem. Soc. 2016, 138, 6306–6310. 10.1021/jacs.6b03127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa K.; Tang C.; Hawker C. J. Molecularly Defined Caprolactone Oligomers and Polymers: Synthesis and Characterization. J. Am. Chem. Soc. 2008, 130, 1718–1726. 10.1021/ja077149w. [DOI] [PubMed] [Google Scholar]
- Jiang Y.; Golder M. R.; Nguyen H. V.-T.; Wang Y.; Zhong M.; Barnes J. C.; Ehrlich D. J. C.; Johnson J. A. Iterative Exponential Growth Synthesis and Assembly of Uniform Diblock Copolymers. J. Am. Chem. Soc. 2016, 138, 9369–9372. 10.1021/jacs.6b04964. [DOI] [PubMed] [Google Scholar]
- Yang J.; Gitlin I.; Krishnamurthy V. M.; Vazquez J. A.; Costello C. E.; Whitesides G. M. Synthesis of Monodisperse Polymers from Proteins. J. Am. Chem. Soc. 2003, 125, 12392–12393. 10.1021/ja035978l. [DOI] [PubMed] [Google Scholar]
- Székely G.; Schaepertoens M.; Gaffney P. R. J.; Livingston A. G. Beyond PEG2000: Synthesis and Functionalisation of Monodisperse PEGylated Homostars and Clickable Bivalent Polyethyleneglycols. Chem. - Eur. J. 2014, 20, 10038–10051. 10.1002/chem.201402186. [DOI] [PubMed] [Google Scholar]
- Williams J. B.; Chapman T. M.; Hercules D. M. Synthesis of Discrete Mass Poly(butylene glutarate) Oligomers. Macromolecules 2003, 36, 3898–3908. 10.1021/ma021056b. [DOI] [PubMed] [Google Scholar]
- Rosales A. M.; Segalman R. A.; Zuckermann R. N. Polypeptoids: A Model System to Study the Effect of Monomer Sequence on Polymer Properties and Self-Assembly. Soft Matter 2013, 9, 8400–8414. 10.1039/c3sm51421h. [DOI] [Google Scholar]
- Lutz J.-F. J.-F. Sequence-Controlled Polymerizations: The next Holy Grail in Polymer Science?. Polym. Chem. 2010, 1, 55–62. 10.1039/b9py00329k. [DOI] [Google Scholar]
- Ida S.; Ouchi M.; Sawamoto M. Template-Assisted Selective Radical Addition toward Sequence-Regulated Polymerization: Lariat Capture of Target Monomer by Template Initiator. J. Am. Chem. Soc. 2010, 132, 14748–14750. 10.1021/ja1070575. [DOI] [PubMed] [Google Scholar]
- Lutz J.-F. Defining the Field of Sequence-Controlled Polymers. Macromol. Rapid Commun. 2017, 38, 1700582. 10.1002/marc.201700582. [DOI] [PubMed] [Google Scholar]
- Robertson E. J.; Battigelli A.; Proulx C.; Mannige R. V.; Haxton T. K.; Yun L.; Whitelam S.; Zuckermann R. N. Design, Synthesis, Assembly, and Engineering of Peptoid Nanosheets. Acc. Chem. Res. 2016, 49, 379–389. 10.1021/acs.accounts.5b00439. [DOI] [PubMed] [Google Scholar]
- Pijpers I. A. B.; Abdelmohsen L. K. E. A.; Williams D. S.; van Hest J. C. M. Morphology Under Control: Engineering Biodegradable Stomatocytes. ACS Macro Lett. 2017, 6, 1217–1222. 10.1021/acsmacrolett.7b00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmohsen L. K. E. A.; Williams D. S.; Pille J.; Ozel S. G.; Rikken R. S. M.; Wilson D. A.; van Hest J. C. M. Formation of Well-Defined, Functional Nanotubes via Osmotically Induced Shape Transformation of Biodegradable Polymersomes. J. Am. Chem. Soc. 2016, 138, 9353–9356. 10.1021/jacs.6b03984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Genabeek B.; de Waal B. F. M.; Gosens M. M. J.; Pitet L. M.; Palmans A. R. A.; Meijer E. W. Synthesis and Self-Assembly of Discrete Dimethylsiloxane–Lactic Acid Diblock Co-Oligomers: The Dononacontamer and Its Shorter Homologues. J. Am. Chem. Soc. 2016, 138, 4210–4218. 10.1021/jacs.6b00629. [DOI] [PubMed] [Google Scholar]
- van Genabeek B.; de Waal B. F. M.; Ligt B.; Palmans A. R. A.; Meijer E. W. Dispersity under Scrutiny: Phase Behavior Differences between Disperse and Discrete Low Molecular Weight Block Co-Oligomers. ACS Macro Lett. 2017, 6, 674–678. 10.1021/acsmacrolett.7b00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oschmann B.; Lawrence J.; Schulze M. W.; Ren J. M.; Anastasaki A.; Luo Y.; Nothling M. D.; Pester C. W.; Delaney K. T.; Connal L. A.; et al. Effects of Tailored Dispersity on the Self-Assembly of Dimethylsiloxane–Methyl Methacrylate Block Co-Oligomers. ACS Macro Lett. 2017, 6, 668–673. 10.1021/acsmacrolett.7b00262. [DOI] [PubMed] [Google Scholar]
- Yang J.; Liang Y.; Luo J.; Zhao C.; Han C. C. Multilength Scale Studies of the Confined Crystallization in Poly(l-lactide)-b-Poly(ethylene glycol) Copolymer. Macromolecules 2012, 45 (10), 4254–4261. 10.1021/ma202505f. [DOI] [Google Scholar]
- Zhang J.; Wang L.-Q.; Wang H.; Tu K. Micellization Phenomena of Amphiphilic Block Copolymers Based on Methoxy Poly(ethylene glycol) and Either Crystalline or Amorphous Poly(caprolactone-b-lactide). Biomacromolecules 2006, 7, 2492–2500. 10.1021/bm0601732. [DOI] [PubMed] [Google Scholar]
- Heald C. R.; Stolnik S.; Kujawinski K. S.; De Matteis C.; Garnett M. C.; Illum L.; Davis S. S.; Purkiss S. C.; Barlow R. J.; Gellert P. R. Poly(lactic acid)–Poly(ethylene oxide) (PLA–PEG) Nanoparticles: NMR Studies of the Central Solidlike PLA Core and the Liquid PEG Corona. Langmuir 2002, 18, 3669–3675. 10.1021/la011393y. [DOI] [Google Scholar]
- Knop K.; Hoogenboom R.; Fischer D.; Schubert U. S. Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem., Int. Ed. 2010, 49, 6288–6308. 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- Nicolas J.; Mura S.; Brambilla D.; Mackiewicz N.; Couvreur P. Design, Functionalization Strategies and Biomedical Applications of Targeted Biodegradable/biocompatible Polymer-Based Nanocarriers for Drug Delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. 10.1039/C2CS35265F. [DOI] [PubMed] [Google Scholar]
- Chen S.; Wang Y.; Fan Y.; Ma J. Synthesis of Amphiphilic Poly(tetraethylene glycol succinate) and the Thermosensitivity of Its Aggregation in Water. J. Biomed. Mater. Res., Part A 2009, 88A, 769–777. 10.1002/jbm.a.31909. [DOI] [PubMed] [Google Scholar]
- van Genabeek B.; Lamers B. A. G.; de Waal B. F. M.; van Son M. H. C.; Palmans A. R. A.; Meijer E. W. Amplifying (Im)perfection: The Impact of Crystallinity in Discrete and Disperse Block Co-Oligomers. J. Am. Chem. Soc. 2017, 139, 14869–14872. 10.1021/jacs.7b08627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy J. B.; Gädt T.; Whittell G. R.; Chabanne L.; Mitchels J. M.; Richardson R. M.; Winnik M. A.; Manners I. Monodisperse Cylindrical Micelles by Crystallization-Driven Living Self-Assembly. Nat. Chem. 2010, 2, 566–570. 10.1038/nchem.664. [DOI] [PubMed] [Google Scholar]
- Pitto-Barry A.; Kirby N.; Dove A. P.; O’Reilly R. K. Expanding the Scope of the Crystallization-Driven Self-Assembly of Polylactide-Containing Polymers. Polym. Chem. 2014, 5, 1427–1436. 10.1039/C3PY01048A. [DOI] [Google Scholar]
- Takizawa K.; Nulwala H.; Hu J.; Yoshinaga K.; Hawker C. J. Molecularly Defined (L)-Lactic Acid Oligomers and Polymers: Synthesis and Characterization. J. Polym. Sci., Part A: Polym. Chem. 2008, 46, 5977–5990. 10.1002/pola.22944. [DOI] [Google Scholar]
- P1discrete/P1disperse show higher solubility (up to 5 mg mL–1 instead of only 2 mg mL–1 for P2discrete/P2disperse) and are soluble in pure water as well.
- Jeong B.; Bae Y. H.; Lee D. S.; Kim S. W. Biodegradable Block Copolymers as Injectable Drug-Delivery Systems. Nature 1997, 388, 860. 10.1038/42218. [DOI] [PubMed] [Google Scholar]
- Sackett D. L.; Wolff J. Nile Red as a Polarity-Sensitive Fluorescent Probe of Hydrophobic Protein Surfaces. Anal. Biochem. 1987, 167, 228–234. 10.1016/0003-2697(87)90157-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.