Abstract
The purpose of this formative qualitatively driven mixed-methods study was to refine a measurement tool for use in interventions to improve colorectal cancer (CRC) surveillance care. We employed key informant interviews to explore the attitudes, practices, and preferences of four physician specialties. A national survey, literature review, and expert consultation also informed survey development. Cognitive pretesting obtained participant feedback to improve the survey’s face and content validity and reliability. Results showed that additional domains were needed to reflect contemporary interdisciplinary trends in survivorship care, evolving practice changes and current health policy. Observed dissonance in specialists’ perspectives poses challenges for the development of interventions and psychometrically sound measurement. Implications for future research include need for a flexible care model with enhanced communication and role definitions among clinical specialists, improvements in surveillance at multilevels (patients, providers, and systems), and measurement tools that focus on multispecialty involvement and the changing practice and policy environment.
Keywords: research, mixed methods; instrument development; cancer; qualitative
Colorectal cancer (CRC) is one of the most commonly diagnosed cancers in the United States and is a leading cause of cancer death (Siegel, Naishadham, & Jemal, 2013). Due to improved detection, diagnosis, and treatment over the past few decades, a majority of persons diagnosed with CRC now survive the disease. This dramatic rise in the long-term survival rate has focused national attention on the long-term care and support needs of CRC survivors (Jemal, Center, DeSantis, & Ward, 2010). Posttreatment surveillance among survivors is critical to detecting recurrence and promoting lifestyle changes to reduce risk of recurrence. Many CRC survivors, however, do not receive guideline-recommended surveillance care (i.e., office visits, carcinoembryonic antigen [CEA] tests, colonoscopy) or adhere to lifestyle-related surveillance guidelines for weight management, physical activity, and alcohol and tobacco use (National Comprehensive Cancer Network Committee, 2013; Salloum et al., 2012).
Enhancing the quality of surveillance care is critically important for the growing population of CRC survivors (de Moor et al., 2013). Quality of survivor care is influenced by the interface between primary care physicians (PCPs) and other specialists. When patients are first diagnosed with CRC, they are frequently seen by one or more specialists, including PCPs, medical oncologists, surgeons, and gastroenterologists. The role of each physician specialty in CRC surveillance care, however, has not been clarified in clinical practice guidelines, and these roles are being increasingly debated (Augestad et al., 2008) given the heightened emphasis on the patient-centered medical home (Hudson et al., 2012) and accountable care organizations (ACOs; Forster et al., 2012). To promote optimal models of care for CRC survivors, it is important to better understand specialty-specific perspectives and experiences related to survivorship care, particularly within the context of the changing health care landscape (Gage et al., 2011). This knowledge will inform the design of multilevel intervention strategies (potentially targeted at individuals, providers, and systems and policy change) to improve the quality of care and patient outcomes (Taplin et al., 2012). Efficacy testing of potential subsequent intervention strategies will also require valid and reliable assessment of potential moderating, mediating, impact, and outcome measures.
There is a growing consensus about the viability and importance of combining qualitative and quantitative methods in research, including calls to use such methods to revise previously tested quantitative surveys (Klabunde, Willis, & Casalino, 2013). Such work is essential to assuring that instruments used in large trials reflect the multilevel factors of intervention strategies and measure them reliably and accurately (Zapka, Taplin, Ganz, Grunfeld, & Sterba, 2012). This is particularly important in an era of major health care delivery and reimbursement policy change.
The overall purpose of this mixed-methods formative study was to refine a survey measurement tool to enhance progress on the design and testing of multilevel intervention strategies (National Cancer Institute [NCI], 2013) to improve the quality of surveillance care for CRC survivors. To achieve this purpose, using key informant interviews (KIIs), we explored physicians’ (primary care, gastroenterology, surgery, and oncology) attitudes, practices, and preferences in providing quality surveillance care to CRC survivors. We also pretested an instrument and used cognitive pretesting to refine the draft survey. We generated quantitative summaries of the pretest data and synthesized the findings with the findings from the KIIs. Thus, qualitative methods were essential to our work in developing rigorous survey instruments.
Method
Guiding Model
This study was guided by a broad conceptual framework considering potential factors relevant to survivorship outcomes, including those at the levels of individual patients, families, health service practitioners and organizations, community resources, and public policy (Zapka, Taplin, et al., 2012). The content of the open-ended KII guide (i.e., the broad questions and prompts) and the quantitative pilot survey draft queried physicians’ views on multilevel domains and factors related to quality surveillance care behaviors recommended by professional guidelines (National Comprehensive Cancer Network Committee, 2013; see Table 1). Broadly, we considered the predisposing, enabling, and reinforcing factors, which potentially influence physicians’ surveillance care behaviors (Green & Kreuter, 2005). Predisposing factors refer to characteristics such as knowledge, attitudes, and perceptions. Enabling factors refer to organizational factors, such as supporting systems in practices, and organizational culture with a focus on the key outcomes of communication and efficient use of resources. Finally, reinforcing factors include influences such as guideline consensus and feedback on norms as well as patient adherence.
Table 1.
Surveillance Care Behaviors and Examples of Related Factors and Survey Domains.
| Surveillance-Related Behaviorsa | Example: Social-Ecological Factors | Example: Interview/Survey Domains |
|---|---|---|
| Provide patient-centered counseling and decision making | Predisposing factors | |
| Physician/practice characteristics | Patient demographics, patient volume | |
| Apply guidelines | Knowledge, attitudes, skills | Knowledge about surveillance guidelines, posttreatment late/long-term effects |
| Make screening recommendations | Perceived barriers | |
| Assure necessary follow-up, testing | Preferences about specialty roles/responsibilities | |
| Make lifestyle recommendations | ||
| Communicate with and refer to other clinicians | Enabling factors | |
| Organizational resources | Practice location, medical record system | |
| Provide patient with oral and written plans | Coordination structures | Clinician continuing education in survivorship |
| Assess patient needs (physical, psychosocial) | Interspecialty communication | |
| Benchmarks | Care coordination resources | |
| Reinforcing factors | ||
| Patient adherence | Patient barriers, concerns, and interactions | |
| Guideline consensus | Professional association standards | |
| Professional norms | Quality improvement indicators | |
| Feedback | ||
Preliminary Work
Our survey was informed by the rigorously developed and widely cited National Cancer Institute (NCI) and American Cancer Society (ACS) Survey of Physician Attitudes Regarding the Care of Cancer Survivors (SPARCCS; Cheung et al., 2013; Forsythe et al., 2013; NCI & American Cancer Society, 2010; Potosky et al., 2011; Zapka, Klabunde, et al., 2012). Inquiry domains included care recommendations, guideline adherence, perceived survivor needs and barriers, role of specialties, preferred models of care, and demographic and practice factors. (The SPARCCS item inventory organized according to our guiding model is available from the authors.)
The SPARCCS was designed to obtain data on PCPs’ and oncologists’ care experiences with breast and CRC surveillance. We reviewed the literature for other CRC research conducted since SPARCCS implementation. Our preliminary work suggested a significant change needed to be made to the structure of the SPARCCS to encompass the multiple physician specialties that provide care for CRC survivors. Our survey was therefore framed to investigate reports and ratings among four medical specialties (primary care, medical oncologists, surgeons, and gastroenterologists) rather than only among PCPs and oncologists as in the SPARCCS (Bober et al., 2009). Therefore, to obtain data on the perspectives of these four physician specialists, we ultimately drafted one survey version for PCPs and a parallel version for the other three specialties. We also drafted items pertaining to patient–provider communication (Baravelli et al., 2009; Gage et al., 2011); attitudes related to barriers as suggested by other investigators (Hewitt, Bamundo, Day, & Harvey, 2007); and other services of current interest such as genetic counseling (Stricker et al., 2011), practice characteristics, and the political environment (public policy, litigation) as suggested by survey experts and health services researchers (Charns et al., 2012). These preliminary drafts underwent in-depth, in-person cognitive pretesting (Willis, 2005) with four physicians representing the four specialties. We then revised items for content and clarity.
Participant Key Informant and Cognitive Pretesting
The study was conducted in South Carolina where 20 physicians of varied gender and race/ethnicity were recruited using purposive sampling to represent several counties in the state, with the goal of including urban and rural geographic regions. To explore perspectives on surveillance care by physician specialty, 5 physicians from each of four specialties who treat and provide follow-up care for CRC patients (PCPs, medical oncologists, surgeons, and gastroenterologists) were identified, recruited, and interviewed. Each was offered a US$50 gift card honorarium for participation. This research received human subjects protection board approval by the sponsoring institution’s review board.
Data Collection
Three investigators experienced in qualitative research conducted KIIs with the physician participants. An open-ended question format was employed. Investigators used appropriate probes to tailor the general questions for each specialty group. The KIIs were held in the physicians’ office or preferred clinical location. One KII was held via the telephone due to problems with weather and scheduling. Interviews lasted about 45 minutes and focused on the role each physician played in caring for CRC patients: How many and what types of CRC patients they saw per year, patients’ reported physical and psychosocial concerns at the end of treatment, practices for posttreatment surveillance, communication with patients and each other about surveillance care, perceptions of roles of other physicians in treatment and surveillance, perceived barriers to surveillance care adherence, and suggested improvements in the quality of surveillance care. (Protocol is available from the authors.)
At the end of each interview, participants completed the self-administered pilot survey described above. After finishing the survey, cognitive testing of the instrument was conducted by the investigators. Any items that were unclear or that could be worded better were discussed, as well as suggestions for other questions that could be asked instead. We also inquired about preferred distribution modes (i.e., via mailed hardcopy, emailed pdf, or online survey accessed by an emailed link). The open-ended KII was conducted prior to the cognitive testing of the survey, so that the survey content would not influence the discussion points initiated by the participants.
Data Management and Analyses
Digital audio recordings of physician interviews were transcribed and analyzed using rigorous content analysis methods for systematic theme identification (Fonteyn, Vettese, Lancaster, & Bauer-Wu, 2008; LaPelle, 2004; Saldana, 2013). Codebooks were developed by reading and rereading all transcripts, outlining and organizing the key themes addressed by participants as they related to the study purpose and the ecological model components.
The codebook and related coding schemata were developed by one investigator and confirmed by two investigators. The evolving codebook and schemata (comparison table) became templates for the formal analysis of the transcripts. A template style of analysis was used at the macro level of analyses (LaPelle, 2004). This analytic approach uses a priori categories for categorization (i.e., deductive or theory driven) formulated by the analyst and may derive from the researcher’s scholarly expertise in the field (Crabtree & Miller, 1999). At the micro level, categories were more emergent relating to physician specialties and office practices. A detailed comparison table of findings was developed creating a mechanism for comparing themes voiced across participants. In each cell, the essence of a participant’s response to a particular theme was summarized or quoted. The comparison table had columns representing physician specialty (PCP, gastroenterologist, surgeon, and oncologist) and rows representing data collected from each of the five physicians of each specialty for each theme.
Survey data were entered into the Research Electronic Data Capture (REDCap) data management system, which is a web-based application that provides a mechanism for a secure system of data entry and management (Harris et al., 2009). Descriptive statistics were used to summarize responses to survey items. Frequencies of surveillance care behaviors and attitudes were explored by physician specialty type, and suggestions for survey improvement were summarized.
Findings
Table 2 reports the characteristics of the KII participants. Among the four physician specialty types, a broad array of organizational types (although most were in private practices), rural and urban geographic areas, and years since medical school graduation were represented.
Table 2.
Key Informant Participant Characteristics, N = 20.
| Participant Characteristics | n |
|---|---|
| Gender | |
| Female | 7 |
| Male | 13 |
| Medical specialty | |
| Gastroenterologist | 5 |
| Medical oncology | 5 |
| Primary care physician | 5 |
| Surgeon | 5 |
| Race/ethnicity | |
| Asian | 2 |
| African American | 4 |
| Hispanic | 2 |
| White | 12 |
| Site type | |
| Academic medical center | 1 |
| Cancer center | 3 |
| Federally qualified community health center | 2 |
| Private practice | 14 |
| Area | |
| Rural | 11 |
| Urban | 9 |
| Years since medical school graduation | |
| ≤10 | 7 |
| 11–29 | 8 |
| ≥30 | 5 |
Findings From Key Informant Open-Ended Discussions
Table 3 illustrates the themes identified according to the major SPARCCS domains and physician surveillance behaviors as well as physicians’ observations related to predisposing, enabling, and reinforcing factors. Selected specialty-specific views are also reported in the table and some general findings are noted below.
Table 3.
Summary of Themes From Key Informant Major Interview Questions.
| Themes | Summary of Comments |
|---|---|
| Perceived surveillance roles and behaviors with colorectal survivors by physician specialty | PCPs generally saw no more than 1 to 6 new CRC cases annually. They reported following recommendations of the other specialists, perceiving them as responsible for surveillance. One PCP did colonoscopies, with abnormal findings referred to the cancer center. |
| GIs saw themselves as technical consultants, as recipients of oncologists’ and surgeons’ orders. They reported doing thousands of colonoscopies, had from 1–2 to 4–5 positive cases a month and hundreds of adenomas. | |
| Surgeons reported a wide variability in CRC patients seen, from 2 to 7 per week to 1 per month to 1–10 per year. They varied in their surveillance roles depending on whether an oncologist was also involved. Some surgeons performed colonoscopies. | |
| Oncologists reported playing a major role in surveillance care with variability by level of surgeon involvement and cancer stage. | |
| Perceived surveillance care roles of other physicians | PCPs considered other specialists responsible for course of surveillance care with particular specialists for respective tests. |
| One oncologist reported that the PCP should be the gatekeeper, while another felt that the oncologist is in charge of surveillance. Several described specific timelines for the involvement of other specialists in surveillance care (e.g., PCP, GIs). | |
| GIs tended to rely on the oncologists for direction. One noted being “unsure” about what each one should be doing; “I think there are a lot of unnecessary visits.” | |
| Surgeons commented on the importance of oncologists and PCPs depending on severity (e.g., stage and treatment type). | |
| Common patient concerns and barriers at the end of treatment | All physicians commented on patient fear of recurrence. |
| Several PCPs and surgeons noted concern regarding ostomy care, side effects (e.g., diarrhea); one PCP noted “relationship” issues. | |
| One PCP mentioned emotional issues—for example, facing mortality. | |
| Two oncologists noted financial concerns, insurance, copays, and travel to treatment location. | |
| Barriers to adherence to recommended surveillance care | Patient-related barriers: Education, aversion to colonoscopy, perceptions of cancer as the “brown ribbon,” lack of insurance and ability to pay copayment charges, transportation in rural areas. One African American (AA) GI noted that AA have fears of tests and the system and worry more about stigma. One GI commented on “babysitting patients too much.” They need to take care of themselves, while admitting “we don’t empower patients with good education.” |
| Health system or provider-related barriers: Lack of EMR capability to share information, inconsistent interaction/ reporting between PCP/patient and specialists, lack of reminders, providers’ lack of guideline knowledge. | |
| One PCP emphasized that guidelines are always in flux and not evidence based; some testing may not be cost-effective. | |
| A surgeon stressed the lack of agreement on national care guidelines. | |
| Physician practices/behaviors for posttreatment surveillance | PCPs generally followed guidelines. PCPs reported addressing routine health issues and continuing CEAs once survivors were “cut loose” by the oncologist. |
| Recommendations for colonoscopy periodicity and follow-up visits varied considerably within and between the three specialist groups. CT scan ordering was consistently done on a regular basis by surgeons and oncologists. | |
| About half of the non-PCP specialists reported making lifestyle recommendations; several noted this as an important role for PCPs. | |
| One surgeon reported referring all CRC patients to oncologists, whereas others reported overseeing follow-up care themselves. | |
| Reported surveillance care discussions with survivors varied significantly; few reported providing comprehensive care plans, that is, many reported limited written materials such as descriptions of treatment options. | |
| Surveillance care resources and training | All GIs reported having reminder systems to assure patients’ adherence. |
| One oncologist noted that he had a navigator in-training. | |
| Few other specialists reported surveillance support systems, but several had reminder systems. | |
| Communication patterns among physicians | All PCPs reported receiving reports from specialists albeit variable mode and frequency. One PCP noted that she had to “track information down.” |
| Specialists reported variable communication with PCPs by phone, email/fax, or in writing. | |
| GIs noted frequent communication about needed tests and results. Four noted always sending test results to PCPs. | |
| Surgeons noted variable mode and frequency of communication with oncologist based on the case. | |
| Oncologists mentioned getting back with referring PCP, particularly surgeon, and GI regarding testing. | |
| Suggestions for improvements to surveillance care quality | Need better interspecialty communication, free colonoscopies, patient tracking systems, and more PCPs to oversee the patient-centered medical home, pay for performance regarding surveillance care, and get written plans with checklists. |
| EMR issues were emphasized by all specialties but surgeons. | |
| Surgeons emphasized the need for MD education and adherence to National Comprehensive Cancer Network guidelines. | |
| Patient education is key; patients need to become more proactive. | |
| Almost no mention was specifically made of “care plans.” | |
| Support systems were highlighted, for example, navigators, support from volunteers, community education. |
Note. PCP = primary care physician; CRC = colorectal cancer; GI = gastroenterologist; EMR = electronic medical record; CEA = carcinoembryonic antigen; CT = computed tomography.
Predisposing factors such as CRC patient volume varied greatly by specialty. In addition, substantial differences in CRC surveillance were reported by the physician specialty types with respect to perceptions, opinions, and practices, as has been noted in studies conducted within and outside the United States (Greenfield et al., 2009). Oncologists and surgeons reported feeling prepared to manage posttreatment care and were viewed as such by the gastroenterologists and PCPs. Involvement in CRC surveillance care was often described as a dynamic process with involvement of specific physician types in patient visits and tests varying according to specific timelines of various care-related activities (e.g., follow-up colonoscopies, office visits). Views on the multilevel factors that affect quality care were dissonant among the specialties. Despite general agreement about care guidelines, the majority of physicians reported tailoring care based on a variety of factors, such as clinical factors, patient expectations, litigation concerns, and interspecialty communication.
Enabling factors in the practice context such as medical records, clinic type, and access to specialty care in rural areas also influenced perceptions and care practices. For example, PCPs in more geographically remote, rural settings tended to be more likely than PCPs in urban areas to report being actively engaged in surveillance care.
Reported reinforcing factors included professional trends (e.g., focus on genetic testing/counseling and patient-centered care) and policy, notably reimbursement. Participants reported variable impact of these factors on perceptions and practices of CRC surveillance care across specialties.
Findings From Survey
This section reports selected observations from the survey frequencies relating to the broad themes identified in the KIIs according to the major survey domains.
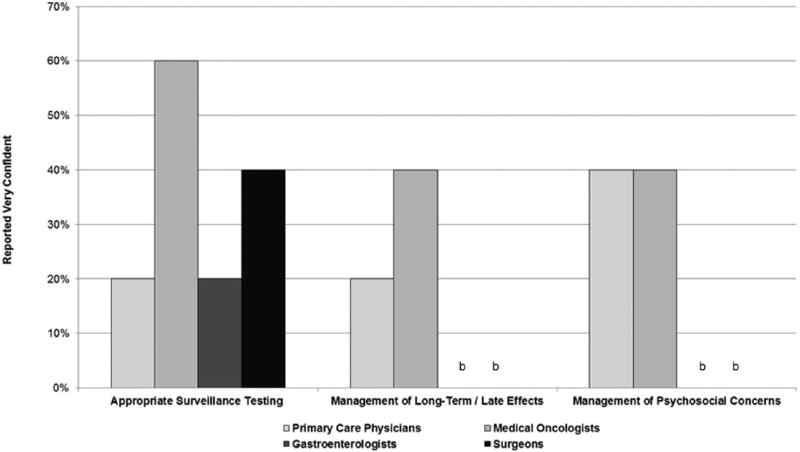
There was a considerable variability in the perceived roles and responsibilities for care between PCPs and the other three specialties. Oncologists, surgeons, and gastroenterologists reported strong feelings that PCPs should not be in charge of surveillance care, because cancer care is very specialized. Their survey responses generally highlighted their agreement that PCPs have too many competing priorities to provide surveillance care. The PCPs also agreed that they did not have time to keep up with the scientific literature on cancer surveillance guidelines and reported that cancer specialists could do a better job of promoting adherence to current CRC surveillance guidelines. An example of differences among the physician types is noted in Figure 1, which highlights differences in reported self-efficacy by specialty for three surveillance tasks.
Figure 1.
Physician reported self-efficacy for surveillance care.a
aVery confident.
bResponse is 0%.
In responding to a hypothetical CRC patient case, there was a clear variability among all four specialties in reports about the appropriate recommendations for and timing of CRC surveillance tests (e.g., colonoscopies and CEA tests). This finding perhaps reflects predisposing factors, including lack of knowledge of evidence-based guidelines, perceived need for tailoring to specific patients, and/or disagreement with guideline elements. With respect to their surveillance behavior, 46% of the non-PCP specialists reported that they often, almost always, or always gave their patients a written survivorship care plan summarizing their treatment and recommendations for follow-up. However, none of the PCPs reported that a specialist had given any patient a written care summary. While 73% of non-PCP specialists reported they frequently provided the PCP a comprehensive summary of cancer care (often, almost always, or always), only 60% of PCPs reported they received cancer care summaries for their patients (often, almost always, or always).
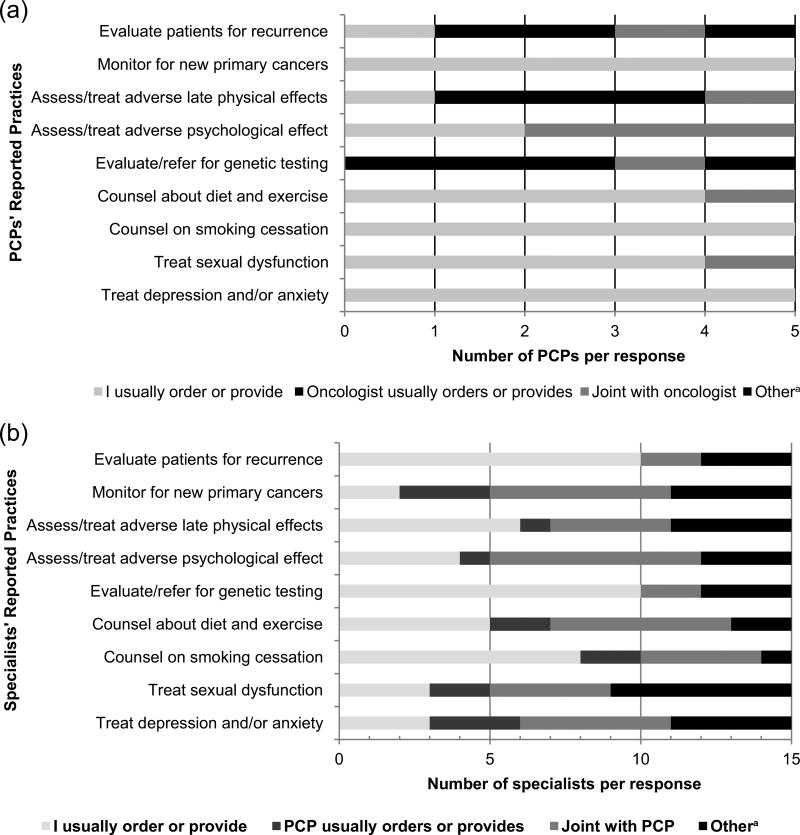
All four physician types were asked about their respective practices in providing CRC surveillance care and perceptions about shared practices for specific aspects of patient care. Figure 2(a) reports the responses of the PCPs for nine follow-up tasks related to CRC surveillance care. For several tasks (monitoring for new cancers, counseling on smoking cessation, and treating depression and anxiety), 100% of PCPs reported they usually ordered or provided them.
Figure 2.
Reported practices by physicians involved in colorectal surveillance care (N = 20): (a) Perceived roles of PCPs (n = 5) and (b) perceived roles of oncologists, surgeons, and gastroenterologists (n = 15).
Note. PCPs = primary care physicians.
aOther = “Another specialist usually orders or provides this service” or multiple responses.
Figure 2(b) illustrates the practices reported by the 15 gastroenterologists, surgeons, and oncologists for nine follow-up care tasks. Of note is the relatively small proportion who reported that the PCP usually orders or provides a certain aspect of care, although there was a great variability among and within the three other MD specialists (data not shown). For example, 100% of oncologists and 40% of surgeons said they ordered or provided counseling on diet and physical activity, whereas 40% of oncologists and no surgeons reported treatment for depression. For the smoking cessation counseling task, 53% of the other three specialists reported that they usually ordered or provided counseling on smoking. For the treatment of depression and anxiety, 20% reported that care was provided primarily by the PCP.
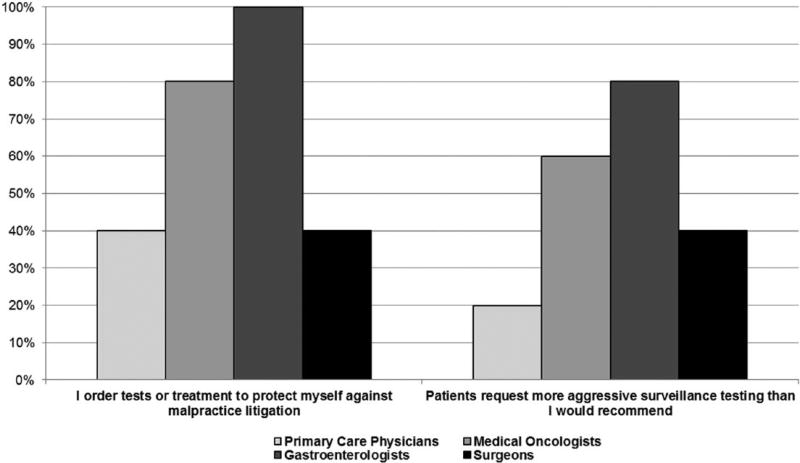
Figure 3 highlights the reports by specialty related to the frequency of ordering tests due to malpractice concerns. The question was asked given published concerns about over-ordering of tests (Emanuel & Fuchs, 2008; Han et al., 2013). Seventy-three percent of the non-PCP specialists reported sometimes to always ordering unneeded tests to protect themselves from litigation. A complementary question about patients requesting more tests than their physicians preferred to recommend is also illustrated in Figure 3 demonstrating a high frequency of such requests.
Figure 3.
Frequency of test ordering factors.
Note. Includes sometimes, often, almost always, and always responses.
Medical care delivery is frequently affected by enabling structural features in the practice setting. Very few specialists (20%) reported having designated staff to coordinate surveillance care for survivors. Two fifths of PCPs reported they often have difficulty in coordinating patient surveillance care between specialists. Increasing emphasis has been placed on the role of electronic medical records (EMRs) in improving quality of care, including interspecialty communication. In our sample, 45% of physicians reported having a full EMR system, 30% of physicians reported they were in transition to a full EMR, 20% had a partial EMR (e.g., for lab results), and 5% reported all paper records.
When hypothesizing reasons for lack of adherence to follow-up CRC care in South Carolina, all physicians noted that the following multilevel factors were moderate or major barriers: patients’ lack of insurance (75%), patients lack understanding of what care they should receive (80%), shortage of physicians in some areas (65%), patients’ nonadherence (65%), and patients had other priority chronic conditions (70%).
Findings From Cognitive Pretesting of Survey
As noted in the “Method” section, after the open-ended KII segment, physicians completed the self-administered survey, and were then asked for suggestions and reactions to improve the survey instrument. Several physicians requested clarification of the definition of “survivorship,” reflecting prior published work with patients, which highlighted the lack of common connotation of the term (Khan, Rose, & Evans, 2012). In addition, it was necessary to remind many participants to focus on surveillance testing, rather than on initial CRC screening processes and treatment, reflecting the far greater number of patients seen for screening than for surveillance care. Many respondents reported difficulty in estimating numbers and percentages, such as “estimate how many patients ever diagnosed with CRC you cared for in the last 12 months” and “approximately what percentage of your patients in your main practice location has the following insurance status.” To derive these numbers, some physicians reported that they first had to mentally review their total denominator of patients and then calculate the subset. Other respondents just quickly guessed.
This pilot survey was developed to be applicable to other specialists in addition to PCPs and oncologists. However, some respondents stated that some of the response categories listed on the survey related to specialty type were “too limited.” For example, one problematic response category was “… the oncologist/PCP and I usually share responsibility for ordering or providing this service.” Some respondents noted that they wanted to write in another specialty type with whom they shared surveillance care. One physician found the response referring to “another specialist” too vague. With respect to reporting typical behaviors concerning ordering of tests and recommendations about follow-up care visit frequency for one’s patients, participants frequently reported that “it depends” so that it was difficult to simply report recommended intervals.
These comments gleaned from the qualitative interviews represent reliability and validity challenges for construction of survey items which investigators must consider.
Discussion
In general, observations from the key informant open-ended interview and pilot survey frequencies converged, providing encouragement that the investigators’ preliminary work with literature review and KIIs was helpful in refining and adding survey items to improve reliability and content validity of our instrument. Overall, findings from this formative work highlight four important issues: (a) some similarities in observations were found between the SPARCCS national survey and our revised version; (b) results clearly confirmed the need to include other specialists when investigating care of CRC survivors; (c) results confirmed the need to consider content validity issues with new items reflecting changes in practice norms, services, and policy; and (d) findings documented the need for continued psychometric work in survey refinement for the study of surveillance care in CRC survivors.
This study supported some findings of the national SPARCCS instrument, which focused on the need to understand primary care and oncologists’ views and practices related to survivorship care (Cheung et al., 2013; Klabunde, Han, et al., 2013; Potosky et al., 2011). In both the original SPARCCS study and the current study testing the modified instrument, oncologists were found to have limited confidence in PCPs’ ability to provide ongoing cancer care, PCPs lacked confidence in some of their own surveillance skills, and both groups reported significant departures from national surveillance care guidelines (Potosky et al., 2011). Also, both studies highlighted divergent views on perceptions about who the main providers of psychosocial care are (Forsythe et al., 2012), further highlighting the concern about where and how survivors will receive this critical care. Our findings about the use of and communication about survivorship care plans were congruent with SPARCCS findings (Forsythe et al., 2013). Both studies demonstrated different opinions about strategies to improve the quality of survivorship care (Cheung et al., 2013).
However, our study highlighted the importance of including other specialists in studies of CRC surveillance care, in this case gastroenterologists and surgeons, who sometimes play a major role in providing follow-up care. In both KIIs and survey reports, we found differences among the four specialty groups on several predisposing, enabling, and reinforcing factors as well as practice behaviors. A clear example was respondents’ perceived roles in survivorship care. PCPs raised issues and concerns prevalent among the PCP community, including perceptions that cancer care is now so diversified and patient cases are so individualized that it is difficult to keep abreast of current evidence-based guidelines given the small number of patients they see with various cancer types. This finding has been reported by others (Merport, Lemon, Nyambose, & Prout, 2012; Nissen et al., 2007) but does differ from some studies done outside the United States. For example, Del Giudice, Grunfeld, Harvey, Piliotis, and Verma (2009) found that Canadian PCPs were willing to assume exclusive responsibility for cancer surveillance care, provided there was appropriate information and support in place. This may be attributable in part to the national EMR system and primarily single-payer system in Canada, which together may enable and reinforce adherence to national CRC surveillance guidelines. This is a reminder that researchers must consider policy and practice systems structural features when comparing study findings.
Our formative work suggests that the interactions between specialists in surveillance care for CRC survivors are even more complex than discussed in other studies, notably, among various types and stages of cancer. As noted, much of the recent work has focused on a shared care model between medical oncology and primary care. However, participants in our study reported a variety of shared care experiences reflecting clinical factors and settings. For example, in both KIIs and survey responses, surgeons reported a major role in CRC surveillance in many settings, and gastroenterologists and surgeons were sometimes scarce in rural areas leading to their assumption of greater surveillance care responsibilities than are borne by their urban counterparts The results of this study employing mixed methods for survey improvement show that it may be important to consider new mediating and moderating factors reflecting the current practice environment when developing intervention strategies. Our adapted survey incorporated additional items related to these potential new predisposing, enabling, and reinforcing factors. For example, there is a concern about overuse of CRC surveillance testing (Goodwin, Singh, Reddy, Riall, & Kuo, 2011). The current study showed providers’ concerns about patients expecting unnecessary care which led to providers and ordering these tests and procedures because of fear of litigation. These are concerns that have not been previously addressed in other studies. Regardless of whether the overuse stems from physicians’ or patients’ requests, it reflects a significant departure from evidence-based CRC surveillance guidelines.
The importance of patient–provider communication is well documented (Thorne & Stajduhar, 2012), and patient–clinician engagement improves adherence to CRC surveillance (Tan, Bourgoin, Gray, Armstrong, & Hornik, 2011). However, patients and physicians may have discordant expectations with respect to the roles of PCPs and specialists, just as different physician specialists may have discordant expectations about these roles (Cheung, Neville, Cameron, Cook, & Earle, 2009). Therefore, our survey added detailed questions about communication between providers and patients as well as communication between providers (Foy et al., 2010). In addition, our findings underscore the need for coordination of care related to psychosocial and lifestyle guidelines, which others have found to be underemphasized with CRC survivors (Anderson, Steele, & Coyle, 2013; Daudt, Cosby, Dennis, Payeur, & Nurullah, 2012), as well as the need to better assign roles concerning which providers will be responsible for this care.
Clearly, the push for coordinated care is gaining strength in the United States and should be considered when designing interventions and measurement tools. There is a growing call for professional societies’ and individual physician commitment to quality indicators (Institute of Medicine, 2013b; Neuss et al., 2005). The American College of Surgeons has put forth “Cancer Program Standards of Care—Ensuring Patient Centered Care” (American College of Surgeons Commission on Cancer, 2012), which includes standards for clinical services, and the continuum of care and patient outcomes. The National Comprehensive Cancer Network Committee (2013) has published “National Comprehensive Cancer Network Practice Guidelines in Oncology: Survivorship.” These standards will become the accepted model for all accredited cancer centers. Included in the standards is emphasis on development of survivorship care plans (Grunfeld & Earle, 2010; Howell et al., 2012; Salz, Oeffinger, McCabe, Layne, & Bach, 2012). Much work needs to be initiated and tested at multiple levels (the organization, providers, and patients and their families) to assure that these expectations are accomplished. As we have shown, there is a lack of clarity among physician specialties about their roles and responsibilities in providing CRC surveillance care, and much less is known about their expected interactions within their organizations and with families and patients.
To some extent, the ACO Act calls for mechanisms to incentivize the discussions among organizations that address some of the issues of relationships, communication, and referrals (Patient Protection and Affordable Care Act, 2010). The Act presents challenges and opportunities related to CRC surveillance care. For example, the ACO Act incents the adoption of EMRs, so that organizations could establish software that tracks patient care and summarizes surveillance plans, thus facilitating communication among all providers involved (Bates & Bitton, 2010; Hesse, Hanna, Massett, & Hesse, 2010). As receiving care from different systems without appropriate communication between systems may negatively affect patient outcomes (Tarlov et al., 2012), coordinated cancer care will require considerable organizational attention and funding for information technology upgrades and enhancements. Even with a commitment to adopting EMRs, the establishment and maintenance of platforms and processes to facilitate interorganizational communication are far from guaranteed and may require resources that are beyond the scope of individual health care settings (Kern et al., 2013; Singh, Spitzmueller, Petersen, Sawhney, & Sittig, 2013). This has implications for medically underserved populations who receive care in resource-poor environments and who are therefore vulnerable to receiving fragmented, guideline-discordant care.
We initiated this study with the concern that important factors relating to planning interventions needed to be identified and that standardized measurement tools may require modification to adequately improve content and construct validity in the context of health policy changes (Dillman, Smyth, & Christian, 2009; Luyt, 2012). The changing landscape of the policy environment described above and its reinforcing impact on provider behavior are a case in point. Furthermore, cancer survivorship guidelines will continue to evolve and affect instrument content (Koo et al., 2013). Given this scenario, the application of qualitative methods to refine quantitative measures was essential.
With respect to item generation, findings from our pilot survey testing demonstrate the need for attention to rigorous cognitive pretesting. For example, “survivorship” and “surveillance testing” did not reflect a common connotation of the terms among physician specialists (Khan et al., 2012). In addition, because many providers reported difficulty in estimating numbers and percentages, this represents a reliability issue, and it may be most efficient to have a companion survey for practice administrators to more accurately capture this information.
Other feedback about difficulties with survey completion also highlighted needed changes to response categories. Because our survey, unlike the SPARCCS survey, was not only applied to PCPs and oncologists but was also administered to other specialists as well, the available responses could be expanded to better fit the typical models of care from multiple specialists.
Several physicians commented on the length of the survey, and clearly respondent burden and its impact on the response rate (Klabunde et al., 2012; McLeod, Klabunde, Willis, & Stark, 2013) will be a major concern in the design of instruments in this area. In a larger survey, recruitment methods also may need attention. Despite enthusiasm for the importance of research examining survivorship care quality, several physicians noted that they would not normally respond to a survey such as ours but were influenced by the incentive or by a colleague who had recommended participation. In addition, several physicians commented that if they received a similar survey via email for a future study, they would delete the email message. Indeed, careful planning of data collection continues to be a challenge, notably with researchers’ growing preference for email, the enthusiasm for which is not necessarily matched by practitioners (Dillman et al., 2009; Nicholls et al., 2011; VanGeest, Johnson, & Welch, 2007). Much more measurement work is needed to appropriately assess needs, burden, preferable survey distribution mode, and incentives (Klabunde et al., 2012; Puleo et al., 2002) to evaluate efficacy and effectiveness of interventions.
Limitations
Given the formative nature of this research, sample size is a major limitation. We also acknowledge that findings reflect differences in practice in a limited geographic area (Institute of Medicine, 2013a). Given the in-person nature of the interviews, the threat of social desirability to internal validity in responses may have been operating. While there was a general synergy between the KI unstructured interview data and survey data, with a few items the discrepancy in reports suggests the need for continued attention to construct validity and reliability. Data from the physician reports were not contrasted with data from patient reports of their interactions with and recommendations from physicians. Clearly more work with larger samples is needed to explore psychometric properties.
Implications and Conclusions
This mixed-methods study highlighted dissonance in perspectives among four physician specialties, suggesting the need for flexible models of care with enhanced communication and role definitions among CRC clinical team members. The survivorship literature, professional organizations, and the Institute of Medicine emphasize a shared care model during CRC surveillance care, promotion of primary care medical homes, and provision of written plans to patients and ACOs. Our data indicate that we have a long way to go before these goals are attained given the dissonance in perspectives among the four MD specialties. In addition, all physicians reported deviation from national guidelines to tailor surveillance care to the perceived needs of their patients. Our participants endorsed that provider and patient education and practice- level infrastructure resources are necessary and equally important factors in improving CRC surveillance care. Where and who should deliver surveillance care requires more debate (Greenfield et al., 2009). In the U.S. health care system, important barriers and facilitators will need to be addressed, including physician training, reimbursement, and compatible templates for automated data (Merport et al., 2012).
Coordinated CRC surveillance care will continue to be challenged by evolving practice certification requirements, rapidly changing science related to CRC detection, staging, and management, and the challenges of overseeing care of elders with comorbid conditions amid fragmented insurance coverage and MD reimbursement systems. Meeting these challenges will require designing interventions to improve care quality, which address MD training, systems redesign, and change and coordination of state and national health policy. Achieving recommendations for comprehensive high-quality survivorship care will require overcoming barriers at multiple levels focusing on patient-centered care (Campbell et al., 2011; McCabe et al., 2013). Improved communication and collaboration of physician specialty types may occur across health care institutions, taking into account enhanced focus on patient-centered medical homes. Indeed, a recently published Senate document states that “new patient care models will be created and disseminated, rural patients and providers will see meaningful improvements” (http://www.dpc.senate.gov/healthreformbill/healthbill52.pdf).
Clearly, the complexity of cancer care and the major multilevel changes evolving in the environment provide challenges to researchers related to measurement and intervention strategy decisions. Our work highlights the importance of qualitatively driven mixed-methods designs to improve understanding of factors which drive provider and patient behavior.
Acknowledgments
We thank Kendrea Knight for her assistance with the manuscript preparation.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the National Institutes of Health/National Cancer Institute Grant 5R21CA152865-02.
Biographies
Jane Zapka, ScD, is a research professor in the Department of Public Health Sciences, College of Medicine; an adjunct professor in the College of Nursing; and a member of the Hollings Cancer Center Cancer Control Program at the Medical University of South Carolina in Charleston, SC, USA.
Katherine R. Sterba, PhD, is an assistant professor in the Department of Public Health Sciences, College of Medicine and a member of the Hollings Cancer Center Cancer Control Program at the Medical University of South Carolina in Charleston, SC, USA.
Nancy LaPelle, PhD, is an adjunct assistant professor in the Division of Preventive and Behavioral Medicine at the University of Massachusetts Medical School in Worcester, MA, USA.
Kent Armeson, MS, is a research instructor in the Department of Public Health Sciences, College of Medicine, and a member of the biostatistics shared resource at Hollings Cancer Center at the Medical University of South Carolina in Charleston, SC, USA.
Dana R. Burshell, MPH, is a program coordinator at the South Carolina Clinical & Translational Research Institute, Community Engagement Program at the Medical University of South Carolina in Charleston, SC, USA.
Marvella E. Ford, PhD, is an associate director of cancer disparities at Hollings Cancer Center and a professor in the Department of Public Health Science, College of Medicine at the Medical University of South Carolina in Charleston, SC, USA.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- American College of Surgeons Commission on Cancer. Cancer program standards 2012: Ensuring patient-centered care. 2012 Retrieved from https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012updates.ashx.
- Anderson AS, Steele R, Coyle J. Lifestyle issues for colorectal cancer survivors-perceived needs, beliefs and opportunities. Support Care Cancer. 2013;21:35–42. doi: 10.1007/s00520-012-1487-7. [DOI] [PubMed] [Google Scholar]
- Augestad KM, Vonen B, Aspevik R, Nestvold T, Ringberg U, Johnsen R, Lindsetmo RO. Should the surgeon or the general practitioner (GP) follow up patients after surgery for colon cancer? A randomized controlled trial protocol focusing on quality of life, cost-effectiveness and serious clinical events. BMC Health Services Research. 2008;8 doi: 10.1186/1472-6963-8-137. Article 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baravelli C, Krishnasamy M, Pezaro C, Schofield P, LotfiJam K, Rogers M, Jefford M. The views of bowel cancer survivors and health care professionals regarding survivorship care plans and post treatment follow up. Journal of Cancer Survivorship. 2009;3:99–108. doi: 10.1007/s11764-009-0086-1. [DOI] [PubMed] [Google Scholar]
- Bates DW, Bitton A. The future of health information technology in the patient-centered medical home. Health Affairs (Millwood) 2010;29:614–621. doi: 10.1377/hlthaff.2010.0007. [DOI] [PubMed] [Google Scholar]
- Bober SL, Recklitis CJ, Campbell EG, Park ER, Kutner JS, Najita JS, Diller L. Caring for cancer survivors: A survey of primary care physicians. Cancer. 2009;115(18 Suppl):4409–4418. doi: 10.1002/cncr.24590. [DOI] [PubMed] [Google Scholar]
- Campbell MK, Tessaro I, Gellin M, Valle CG, Golden S, Kaye L, Miller K. Adult cancer survivorship care: Experiences from the LIVESTRONG centers of excellence network. Journal of Cancer Survivorship. 2011;5:271–282. doi: 10.1007/s11764-011-0180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charns MP, Foster MK, Alligood EC, Benzer JK, Burgess JF, Jr, Li D, Clauser SB. Multilevel interventions: Measurement and measures. Journal of National Cancer Institute Monograph. 2012;2012(44):67–77. doi: 10.1093/jncimonographs/lgs011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung WY, Aziz N, Noone AM, Rowland JH, Potosky AL, Ayanian JZ, Earle CC. Physician preferences and attitudes regarding different models of cancer survivorship care: A comparison of primary care providers and oncologists. Journal of Cancer Survivorship. 2013;7:343–354. doi: 10.1007/s11764-013-0281-y. [DOI] [PubMed] [Google Scholar]
- Cheung WY, Neville BA, Cameron DB, Cook EF, Earle CC. Comparisons of patient and physician expectations for cancer survivorship care. Journal of Clinical Oncology. 2009;27:2489–2495. doi: 10.1200/JCO.2008.20.3232. [DOI] [PubMed] [Google Scholar]
- Crabtree BF, Miller WL. Doing qualitative research. 2. Vol. 3. Thousand Oaks, CA: SAGE; 1999. [Google Scholar]
- Daudt HM, Cosby C, Dennis DL, Payeur N, Nurullah R. Nutritional and psychosocial status of colorectal cancer patients referred to an outpatient oncology clinic. Support Care Cancer. 2012;20:1417–1423. doi: 10.1007/s00520-011-1224-7. [DOI] [PubMed] [Google Scholar]
- Del Giudice ME, Grunfeld E, Harvey BJ, Piliotis E, Verma S. Primary care physicians’ views of routine follow-up care of cancer survivors. Journal of Clinical Oncology. 2009;27:3338–3345. doi: 10.1200/JCO.2008.20.4883. [DOI] [PubMed] [Google Scholar]
- de Moor JS, Mariotto AB, Parry C, Alfano CM, Padgett L, Kent EE, Rowland JH. Cancer survivors in the United States: Prevalence across the survivorship trajectory and implications for care. Cancer Epidemiology, Biomarkers & Prevention. 2013;22:561–570. doi: 10.1158/1055-9965.EPI-12-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillman DA, Smyth JD, Christian LM. Internet, mail, and mixed-mode surveys: The tailored design method. Hoboken, NJ: John Wiley; 2009. [Google Scholar]
- Emanuel EJ, Fuchs VR. The perfect storm of overutilization. Journal of the American Medical Association. 2008;299:2789–2791. doi: 10.1001/jama.299.23.2789. [DOI] [PubMed] [Google Scholar]
- Fonteyn ME, Vettese M, Lancaster DR, Bauer-Wu S. Developing a codebook to guide content analysis of expressive writing transcripts. Applied Nursing Research. 2008;21:165–168. doi: 10.1016/j.apnr.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Forster AJ, Childs BG, Damore JF, DeVore SD, Korch EA, Lloyd DA. Accountable care strategies: Lessons from the premier health care alliance’s accountable care collaborative. 2012 Available from http://www.commonwealthfund.org/
- Forsythe LP, Alfano CM, Leach CR, Ganz PA, Stefanek ME, Rowland JH. Who provides psychosocial follow-up care for post-treatment cancer survivors? A survey of medical oncologists and primary care physicians. Journal of Clinical Oncology. 2012;30:2897–2905. doi: 10.1200/jco.2011.39.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe LP, Parry C, Alfano CM, Kent EE, Leach CR, Haggstrom DA, Rowland JH. Use of survivorship care plans in the United States: Associations with survivorship care. Journal of the National Cancer Institute. 2013;105(20):1579–1587. doi: 10.1093/jnci/djt258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy R, Hempel S, Rubenstein L, Suttorp M, Seelig M, Shanman R, Shekelle PG. Meta-analysis: Effect of interactive communication between collaborating primary care physicians and specialists. Annals of Internal Medicine. 2010;152:247–258. doi: 10.7326/0003-4819-152-4-201002160-00010. [DOI] [PubMed] [Google Scholar]
- Gage EA, Pailler M, Zevon MA, Ch’ng J, Groman A, Kelly M, Gruber M. Structuring survivorship care: Discipline-specific clinician perspectives. Journal of Cancer Survivorship. 2011;5:217–225. doi: 10.1007/s11764-011-0174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin JS, Singh A, Reddy N, Riall TS, Kuo YF. Overuse of screening colonoscopy in the Medicare population. Archives of Internal Medicine. 2011;171:1335–1343. doi: 10.1001/archinternmed.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LW, Kreuter MW. Health program planning: An educational and ecological approach. 4. Boston, MA: McGraw-Hill; 2005. [Google Scholar]
- Greenfield DM, Absolom K, Eiser C, Walters SJ, Michel G, Hancock BW, Coleman RE. Follow-up care for cancer survivors: The views of clinicians. British Journal of Cancer. 2009;101:568–574. doi: 10.1038/sj.bjc.6605160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunfeld E, Earle CC. The interface between primary and oncology specialty care: Treatment through survivorship. Journal of National Cancer Institute Monograph. 2010;2010(40):25–30. doi: 10.1093/jncimonographs/lgq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han PK, Klabunde CN, Noone AM, Earle CC, Ayanian JZ, Ganz PA, Potosky AL. Physicians’ beliefs about breast cancer surveillance testing are consistent with test overuse. Medical Care. 2013;51:315–323. doi: 10.1097/MLR.0b013e31827da908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse BW, Hanna C, Massett HA, Hesse NK. Outside the box: Will information technology be a viable intervention to improve the quality of cancer care? Journal of National Cancer Institute Monograph. 2010;2010(40):81–89. doi: 10.1093/jncimonographs/lgq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt ME, Bamundo A, Day R, Harvey C. Perspectives on post-treatment cancer care: Qualitative research with survivors, nurses, and physicians. Journal of Clinical Oncology. 2007;25:2270–2273. doi: 10.1200/JCO.2006.10.0826. [DOI] [PubMed] [Google Scholar]
- Howell D, Hack TF, Oliver TK, Chulak T, Mayo S, Aubin M, Sinclair S. Models of care for posttreatment follow-up of adult cancer survivors: A systematic review and quality appraisal of the evidence. Journal of Cancer Survivorship. 2012;6:359–371. doi: 10.1007/s11764-012-0232-z. [DOI] [PubMed] [Google Scholar]
- Hudson SV, Miller SM, Hemler J, McClinton A, Oeffinger KC, Tallia A, Crabtree BF. Cancer survivors and the patient-centered medical home. Translational Behavioral Medicine. 2012;2:322–331. doi: 10.1007/s13142-012-0138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Interim report of the committee on geographic variation in health care spending and promotion of high-value care: Preliminary committee observations. Washington, DC: The National Academies Press; 2013a. [PubMed] [Google Scholar]
- Institute of Medicine. National cancer policy forum. 2013b Retrieved from http://www.iom.edu/Activities/Disease/NCPF.aspx.
- Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiology, Biomarkers & Prevention. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- Kern LM, Malhotra S, Barron Y, Quaresimo J, Dhopeshwarkar R, Pichardo M, Kaushal R. Accuracy of electronically reported “meaningful use” clinical quality measures: A cross-sectional study. Annals of Internal Medicine. 2013;158:77–83. doi: 10.7326/0003-4819-158-2-201301150-00001. [DOI] [PubMed] [Google Scholar]
- Khan NF, Rose PW, Evans J. Defining cancer survivorship: A more transparent approach is needed. Journal of Cancer Survivorship. 2012;6:33–36. doi: 10.1007/s11764-011-0194-6. [DOI] [PubMed] [Google Scholar]
- Klabunde CN, Han PK, Earle CC, Smith T, Ayanian JZ, Lee R, Potosky AL. Physician roles in the cancer-related follow-up care of cancer survivors. Family Medicine. 2013;45:463–474. [PMC free article] [PubMed] [Google Scholar]
- Klabunde CN, Willis GB, Casalino LP. Facilitators and barriers to survey participation by physicians: A call to action for researchers. Evaluation & the Health Professions. 2013;36:279–295. doi: 10.1177/0163278713496426. [DOI] [PubMed] [Google Scholar]
- Klabunde CN, Willis GB, McLeod CC, Dillman DA, Johnson TP, Greene SM, Brown ML. Improving the quality of surveys of physicians and medical groups: A research agenda. Evaluation & the Health Professions. 2012;35:477–506. doi: 10.1177/0163278712458283. [DOI] [PubMed] [Google Scholar]
- Koo SL, Wen JH, Hillmer A, Cheah PY, Tan P, Tan IB. Current and emerging surveillance strategies to expand the window of opportunity for curative treatment after surgery in colorectal cancer. Expert Review of Anticancer Therapy. 2013;13:439–450. doi: 10.1586/era.13.14. [DOI] [PubMed] [Google Scholar]
- LaPelle N. Simplifying qualitative data analysis with general purpose software tools. Field Methods. 2004;16:85–108. [Google Scholar]
- Luyt R. A framework for mixing methods in quantitative measurement development, validation, and revision: A case study. Journal of Mixed Methods Research. 2012;6:294–316. [Google Scholar]
- McCabe MS, Bhatia S, Oeffinger KC, Reaman GH, Tyne C, Wollins DS, Hudson MM. American society of clinical oncology statement: Achieving high-quality cancer survivorship care. Journal of Clinical Oncology. 2013;31:631–640. doi: 10.1200/jco.2012.46.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod CC, Klabunde CN, Willis GB, Stark D. Health care provider surveys in the United States, 2000–2010: A review. Evaluation & the Health Professions. 2013;36:106–126. doi: 10.1177/0163278712474001. [DOI] [PubMed] [Google Scholar]
- Merport A, Lemon SC, Nyambose J, Prout MN. The use of cancer treatment summaries and care plans among Massachusetts physicians. Support Care Cancer. 2012;20:1579–1583. doi: 10.1007/s00520-012-1458-z. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Grid enabled measures database. 2013 Retrieved from https://www.gem-beta.org/Public/Home.aspx.
- National Cancer Institute & American Cancer Society. Survey of Physician Attitudes Regarding the Care of Cancer Survivors (SPARCCS) 2010 Retrieved from http://healthservices.cancer.gov/surveys/sparccs/
- National Comprehensive Cancer Network Committee. National comprehensive cancer network practice guidelines in oncology: Survivorship. 2013 Retrieved from http://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf.
- Neuss MN, Desch CE, McNiff KK, Eisenberg PD, Gesme DH, Jacobson JO, Simone JV. A process for measuring the quality of cancer care: The Quality Oncology Practice Initiative. Journal of Clinical Oncology. 2005;23:6233–6239. doi: 10.1200/JCO.2005.05.948. [DOI] [PubMed] [Google Scholar]
- Nicholls K, Chapman K, Shaw T, Perkins A, Sullivan MM, Crutchfield S, Reed E. Enhancing response rates in physician surveys: The limited utility of electronic options. Health Services Research. 2011;46:1675–1682. doi: 10.1111/j.1475-6773.2011.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen MJ, Beran MS, Lee MW, Mehta SR, Pine DA, Swenson KK. Views of primary care providers on follow-up care of cancer patients. Family Medicine. 2007;39:477–482. [PubMed] [Google Scholar]
- The Patient Protection and Affordable Care Act, Pub. L. No. 111–148. U.S. Government Printing Office; 2010. [Google Scholar]
- Potosky AL, Han PK, Rowland J, Klabunde CN, Smith T, Aziz N, Stefanek M. Differences between primary care physicians’ and oncologists’ knowledge, attitudes and practices regarding the care of cancer survivors. Journal of General Internal Medicine. 2011;26:1403–1410. doi: 10.1007/s11606-011-1808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puleo E, Zapka J, White MJ, Mouchawar J, Somkin C, Taplin S. Caffeine, cajoling, and other strategies to maximize clinician survey response rates. Evaluation & the Health Professions. 2002;25:169–184. doi: 10.1177/016327870202500203. [DOI] [PubMed] [Google Scholar]
- Saldana J. The coding manual for qualitative researchers. 2. London: SAGE; 2013. [Google Scholar]
- Salloum RG, Hornbrook MC, Fishman PA, Ritzwoller DP, O’Keeffe Rossetti MC, Elston Lafata J. Adherence to surveillance care guidelines after breast and colorectal cancer treatment with curative intent. Cancer. 2012;118:5644–5651. doi: 10.1002/cncr.27544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salz T, Oeffinger KC, McCabe MS, Layne TM, Bach PB. Survivorship care plans in research and practice. CA: A Cancer Journal for Clinicians. 2012;62(2):101–117. doi: 10.3322/caac.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: A Cancer Journal for Clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Singh H, Spitzmueller C, Petersen NJ, Sawhney MK, Sittig DF. Information overload and missed test results in electronic health record-based settings. JAMA Internal Medicine. 2013;173:702–704. doi: 10.1001/2013.jamainternmed.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker CT, Jacobs LA, Risendal B, Jones A, Panzer S, Ganz PA, Palmer SC. Survivorship care planning after the institute of medicine recommendations: How are we faring? Journal of Cancer Survivorship. 2011;5:358–370. doi: 10.1007/s11764-011-0196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AS, Bourgoin A, Gray SW, Armstrong K, Hornik RC. How does patient-clinician information engagement influence self-reported cancer-related problems?: Findings from a longitudinal analysis. Cancer. 2011;117(11):2569–2576. doi: 10.1002/cncr.25804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taplin SH, Anhang Price R, Edwards HM, Foster MK, Breslau ES, Chollette V, Zapka J. Introduction: Understanding and influencing multilevel factors across the cancer care continuum. Journal of National Cancer Institute Monograph. 2012;2012(44):2–10. doi: 10.1093/jncimonographs/lgs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlov E, Lee TA, Weichle TW, Durazo-Arvizu R, Zhang Q, Perrin R, Hynes DM. Reduced overall and event-free survival among colon cancer patients using dual system care. Cancer Epidemiology, Biomarkers & Prevention. 2012;21:2231–2241. doi: 10.1158/1055-9965.EPI-12-0548. [DOI] [PubMed] [Google Scholar]
- Thorne SE, Stajduhar KI. Patient perceptions of communications on the threshold of cancer survivorship: Implications for provider responses. Journal of Cancer Survivorship. 2012;6:229–237. doi: 10.1007/s11764-012-0216-z. [DOI] [PubMed] [Google Scholar]
- VanGeest JB, Johnson TP, Welch VL. Methodologies for improving response rates in surveys of physicians: A systematic review. Evaluation & the Health Professions. 2007;30:303–321. doi: 10.1177/0163278707307899. [DOI] [PubMed] [Google Scholar]
- Willis G. Cognitive interviewing: A tool for improving questionnaire design. Newbury Park, CA: SAGE; 2005. [Google Scholar]
- Zapka J, Klabunde CN, Taplin S, Yuan G, Ransohoff D, Kobrin S. Screening colonoscopy in the US: A attitudes and practices of primary care physicians. Journal of General Internal Medicine. 2012;27:1150–1158. doi: 10.1007/s11606-012-2051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapka J, Taplin SH, Ganz P, Grunfeld E, Sterba K. Multilevel factors affecting quality: Examples from the cancer care continuum. Journal of National Cancer Institute Monograph. 2012;2012(44):11–19. doi: 10.1093/jncimonographs/lgs005. [DOI] [PMC free article] [PubMed] [Google Scholar]