Abstract
Artificial lung devices comprised of hollow fiber membranes (HFMs) coated with the enzyme carbonic anhydrase (CA), accelerate removal of carbon dioxide (CO2) from blood for the treatment of acute respiratory failure. While previous work demonstrated CA coatings increase HFM CO2 removal by 115 % in phosphate buffered saline (PBS), testing in blood revealed a 36 % increase compared to unmodified HFMs. In this work, we sought to characterize the CO2 mass transport processes within these biocatalytic devices which impede CA coating efficacy and develop approaches towards improving bioactive HFM efficiency. Aminated HFMs were sequentially reacted with glutaraldehyde (GA), chitosan, GA and afterwards incubated with a CA solution, covalently linking CA to the surface. Bioactive CA-HFMs were potted in model gas exchange devices (0.0119 m2) and tested for esterase activity and CO2 removal under various flow rates with PBS, whole blood, and solutions containing individual blood components (plasma albumin, red blood cells or free carbonic anhydrase). Results demonstrated that increasing the immobilized enzyme activity did not significantly impact CO2 removal rate, as the diffusional resistance from the liquid boundary layer is the primary impediment to CO2 transport by both unmodified and bioactive HFMs under clinically relevant conditions. Furthermore, endogenous CA within red blood cells competes with HFM immobilized CA to increase CO2 removal. Based on our findings, we propose a bicarbonate/CO2 disequilibrium hypothesis to describe performance of CA-modified devices in both buffer and blood. Improvement in CO2 removal rates using CA-modified devices in blood may be realized by maximizing bicarbonate/CO2 disequilibrium at the fiber surface via strategies such as blood acidification and active mixing within the device.
1 Introduction
Removal of CO2 from circulating blood using extracorporeal gas exchange devices is a promising treatment for acute respiratory failure [1–7]. Current respiratory assist devices used for extracorporeal carbon dioxide removal (ECCO2R) can require blood flow rates in excess of 1 L/min to extract a clinically therapeutic 50 % of metabolic CO2 production (~100 mL/min), necessitating larger cannula inserted by surgeons, rather than the intensivists typically treating these patients. This has been one reason ECCO2R has seen limited widespread use in ICUs [1, 7]. New technologies that facilitate accelerated CO2 removal may lead to the development of smaller, more efficient respiratory assist devices that can be implemented by intensivists and other nonsurgical clinicians. We recently developed strategies to covalently immobilize the enzyme carbonic anhydrase (CA) to the surface of hollow fiber membranes (HFMs), as a means to increase CO2 removal efficiency in respiratory assist devices. CA plays a critical role in blood carbon dioxide transport by catalyzing the reaction . Most of blood CO2 (>90 %) is carried as bicarbonate (HCO3−), which is dehydrated during respiration to gaseous CO2 by CA within red blood cells (RBCs). Immobilizing CA on the surface of HFMs locally catalyzes dehydration of HCO3− to CO2, increasing the CO2 partial pressure (PCO2) at the fiber surface and increasing CO2 flux through the membrane. Previous studies demonstrated bioactive CA-HFMs increase CO2 removal by 115 % in phosphate buffered saline (PBS) and 37 % in blood, compared to unmodified fibers [8, 9].
The difference in CA-HFM catalyzed CO2 removal between PBS and blood is not well understood. Compared to our current 30–40 % increase in blood CO2 removal, we anticipate at least 100 % increase will be necessary for the development of next-generation minimally invasive devices. Since both fluid systems operate under the same PCO2 of 50 mmHg, other predominant blood factors, such as RBCs or plasma proteins, must limit the CA coating performance in whole blood. The presence of endogenous CA within RBCs may compete with immobilized CA, reducing the coating efficacy [10]. Additionally, adsorption of plasma proteins and platelets are well known contributors to thrombus formation on the surfaces of polymeric materials, decreasing transport across the fibers and performance of respiratory assist devices [11]. Our use of immobilized CA on the surface of blood contacting gas exchange membranes is a new development in the field. Examining the kinetics of CO2 transport within CA-modified gas exchange devices in both PBS and blood is an important step towards elucidating strategies for improved CO2 removal efficiency.
In this study we examined the mass transport phenomena governing CO2 removal using bioactive CA-HFMs in gas exchange modules. The roles of immobilized CA activity, liquid flow rate and blood cells and proteins were clarified. Using either high activity human or low activity bovine enzyme sources, CA II was immobilized onto HFMs. The CA modified HFMs were fabricated into model gas exchange devices to assess CO2 removal performance from fluids containing individual blood components, PBS, PBS + 5 % Albumin, PBS + CA, PBS + RBC and whole blood under a mass transfer environment similar to those of commercially available oxygenators. The results of this study will be used to develop respiratory assist devices in which bioactive CA-HFMs can be employed and more substantially increase CO2 exchange in blood.
2 Methods
2.1 Materials
Carbonic anhydrase II from bovine erythrocytes, glutaraldehyde (GA), chitosan (MW = 50–190 kD, based on viscosity) and glacial acetic acid were purchased from Sigma-Aldrich (St. Louis, MO). Purified recombinant human carbonic anhydrase II was provided by Dr. Silverman and Dr. McKenna from University of Florida (Gainesville, FL) [12]. Commercial microporous polypropylene HFMs (CelgardTM; OD 300 μm, ID 240 μm, Membrana GmbH, Wuppertal, Germany) were obtained from ALung Technologies, Inc. (Pittsburgh, PA) with an aminated-siloxane coating. Bovine blood with Naheparin anticoagulation (1:100 dilution of 1000 U/mL) was purchased from Lampire Biological Laboratories (Pipersville, PA). All other reagents were purchased from Sigma-Aldrich and were of analytical grade or purer.
2.2 In vitro CO2 exchange in scaled down modules of CA-HFM
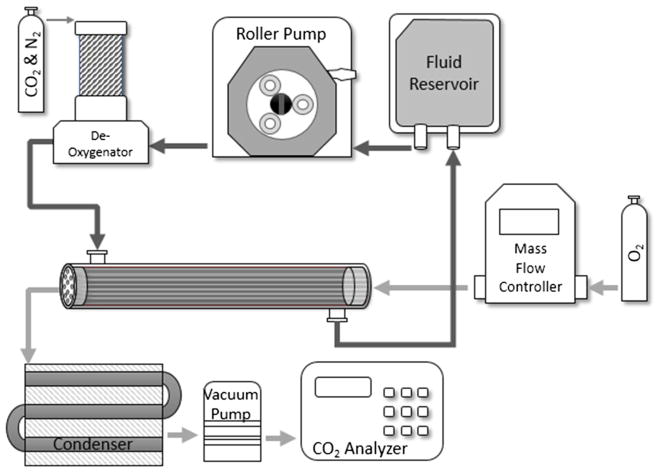
CA immobilization onto HFMs was performed as previously detailed [9]. A scaled-down gas exchange module was fabricated and an in vitro recirculating test loop was used to assess CO2 gas exchange rates using unmodified HFMs and CA-immobilized HFMs (Fig. 1) [9]. CO2 removal was assessed with PBS, PBS + 5 % Albumin, PBS + CA, PBS + RBC and whole blood. For all testing fluids, the system was maintained at 37 °C, ~50 mmHg PCO2, and liquid flow rate was 45, 90, or 200 mL/min. Under these conditions the HFM CO2 removal efficiency (mL/min/m2) matches commercially available devices. The rate of CO2 removal (V̇CO2) for each model oxygenator device was calculated using the sweep gas flow rate ( ) and CO2 fraction (FCO2) exiting the scaled-down gas exchange module and then normalized to 50 mmHg to correct for small deviations in the inlet . For each device the fluid inlet/outlet PCO2 and resulting FCO2 were measured in triplicate. The V̇CO2 for each model gas exchange device is reported as an average of these measurements. The difference in PCO2 from the device inlet to the outlet (PCO2 inlet–PCO2 outlet) is reported as PCO2 drop.
Fig. 1.
Diagram of a model respiratory device and experimental setup for measuring CO2 removal rates of unmodified and modified HFMs. Bovine blood or PBS solutions were perfused over the outside of the fibers while oxygen sweep gas was passed through the fiber lumens in the opposite direction. De-oxygenator employed the use of a heat exchanger to maintain blood temperature at 37 °C
The testing fluids were created as described. PBS: A PBS solution was prepared with a final pH 7.4 and PCO2 of 50 mmHg by diluting 780 mL PBS to 1 L with deionized water and adding 30 mMol sodium hydroxide. The basic PBS solution was introduced to the gas exchange loop and upon solvation of CO2 gas, carbonic acid is formed, decreasing PBS pH to physiologic 7.4. PBS + 5 % albumin: PBS was prepared as described, and once PCO2 equilibrated to 50 mmHg, lyophilized bovine serum albumin was dissolved to 5 % (w/v). PBS + carbonic anhydrase: PBS was prepared as described, and once PCO2 equilibrated to 50 mmHg lyophilized bovine carbonic anhydrase II was added to 16 μM. This concentration was selected to mimic physiologic RBC CAII concentration [13]. PBS + RBC: RBCs were isolated from heparinized whole bovine blood and suspended in PBS. Whole bovine blood was centrifuged at 2500 RCFs for 10 min and the buffy coat discarded. Packed RBCs were rinsed in PBS under gentle shaking for 10 min, centrifuged again and repeated, for a total of three wash steps. Packed RBCs were suspended in PBS to 35 % hematocrit, glucose was adjusted to 100–300 mg/dL prior to use by the addition of 5 % dextrose solution and allowed to incubate for 1 h [14]. Bovine Blood: Heparinized bovine blood was purchased from Lampire Biological Labs (Pipersville, PA), glucose was adjusted to 100–300 mg/dL by the addition of 5 % dextrose solution and hematocrit to 35 %, and allowed to incubate for 1 h [14].
2.3 Carbonic anhydrase esterase activity assay
Immobilized CA enzyme activity was measured spec-trophotometrically by monitoring the hydrolysis of p-nitrophenyl acetate (p-NPA) to p-nitrophenol (p-NP) at 412 nm. CA-modified fibers were fabricated into model oxygenators as described in Sect. 2.2 and a recirculating loop of 15 mL, 100 mM phosphate buffer pH 7.5, 80 μM p-NPA was setup to measure the esterase activity under 45, 90 and 200 mL/min flow rate. Absorbance measurements at 412 nm were recorded every minute over a 5 min period, and plotted as a function of time. One activity unit was defined as the amount of enzyme required to generate 1 μmol pNP per minute.
2.4 Statistical analyses
All data are presented as a mean with standard deviation. Statistical significance between sample groups was determined using ANOVA followed by post hoc Tukey testing of specific differences. All other data was compared using a Student’s t test assuming equal sample variance. Differences were considered statistically significant for P < 0.05.
3 Results
3.1 Immobilized CA-HFM activity: bovine versus human
Human and bovine CAII HFMs were characterized for both immobilized esterase activity (μmol/min) and CO2 removal rate (mL/min/m2) in PBS buffer. Human CA-HFMs demonstrated a fivefold increase in immobilized esterase activity compared to bovine CA-HFMs, 8.7 and 1.7 U respectively (Fig. 2). Flow induced shear under clinically relevant mass transport conditions did not affect immobilized CA esterase activity, as increasing fluid flow rate between 45 and 200 mL/min had negligible effects on immobilized CA activity. In contrast to the esterase activity assay, bovine and human CA devices demonstrated similar CO2 removal rates. At 45 mL/min PBS flow rate, unmodified HFMs removed 85 mL/min/m2 while bovine and human CA-HFMs removed 135 and 143 mL/min/m2 respectively (Fig. 3). Human CA devices attained marginally higher (<10 %) removal rates over bovine CA devices with increasing PBS flow rate. This result indicates that the current level of immobilized bovine CA activity is not a limiting factor in the CO2 removal rate of bioactive CA-HFMs. Increasing p-NPA activity from 1.8 (bovine) to 8.8 U (human) did not dramatically increase gas exchange.
Fig. 2.
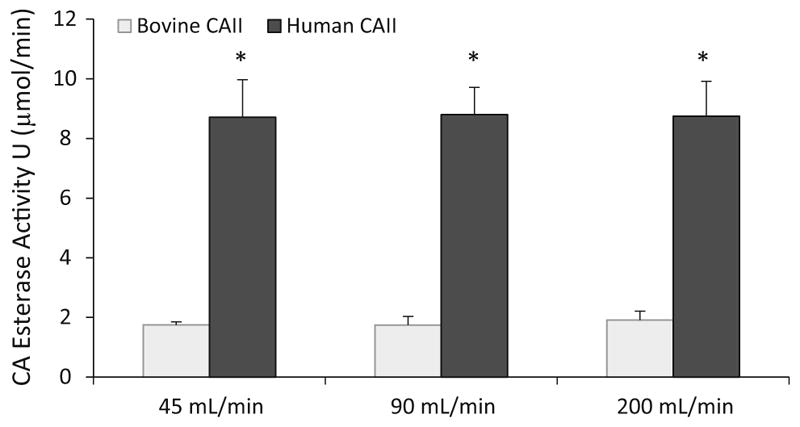
Immobilized enzyme p-NPA activity is independent of flowrate at 45, 90 and 200 mL/min (*P > 0.90)
Fig. 3.
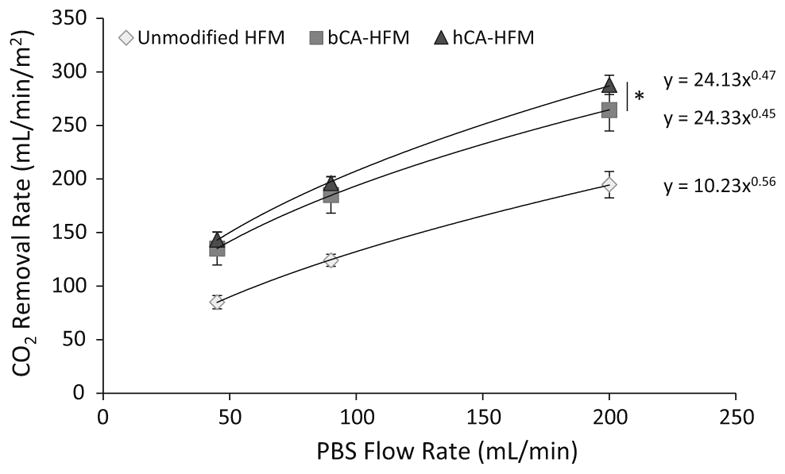
CO2 removal rates for gas exchanges devices in PBS modified with human or bovine CA, compared to unmodified HFMs. Human CA modified HFMs with 4× activity of bovine CA does not significantly increase CO2 removal (*P > 0.10)
3.2 Blood component effects on CA-HFM CO2 removal rates
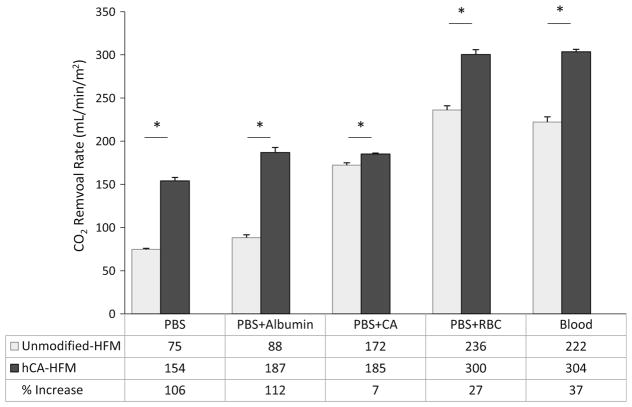
CO2 removal rates were assessed on unmodified and human CA-HFMs in PBS, PBS + 5 % Albumin, PBS + CA, PBS + RBC, and whole blood under a mass transfer environment similar to those of commercially available oxygenators. Albumin solution had no negative effects on CO2 removal performance by unmodified or CA-HFMs. In PBS and PBS + 5 % albumin, unmodified HFMs removed 75 and 88 mL/min/m2 respectively, while CA-HFMs removed 154 and 187 mL/min/m2 respectively, for a 106 % (PBS) or 112 % (PBS + 5 % albumin) increase in CO2 removal rate (Fig. 4). Albumin adsorbing onto HFMs did not impede CA coating performance. Conversely, solutions containing CA significantly decreased the enhancement of CA coatings over unmodified HFMs. Unmodified HFMs CO2 removal increased from 75 in PBS to 172 mL/min/m2 with PBS + CA, 236 mL/min/m2 with PBS + RBC and 222 mL/min/m2 with whole blood. Free CA increased unmodified HFM CO2 removal rate, which limited immobilized CA contribution to CO2 removal. CA-HFMs increased CO2 removal over unmodified fibers by 7 % (PBS + CA), 27 % (PBS + RBC) and 37 % (whole blood). This substantial decrease in CA coating performance as compared to 106 % in PBS is attributed to competition between immobilized and free solution CA to catalyze bicarbonate dehydration to CO2.
Fig. 4.
CO2 removal by unmodified and human CA immobilized HFMs in PBS, PBS + albumin, PBS + CA, PBS + RBC and bovine blood in a model respiratory assist device. In PBS and PBS + albumin, the modified fibers demonstrated a 106 and 112 % increase in CO2 removal rate. In solutions containing carbonic anhydrase, PBS + CA, PBS + RBC and bovine blood, percentage increase in CO2 removal rate decreases to 7, 27 and 37 % (*P <0.05)
3.3 Mass transport limitations by CA-HFMs in blood CO2 removal
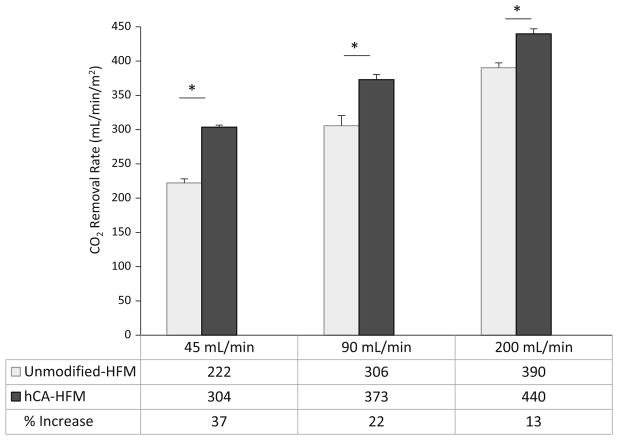
Bioactive CA-HFM CO2 removal rate and fluid PCO2 were assessed in whole bovine blood with varying flow rates. Increasing fluid flow rate adversely affects CA-HFM performance, with a 37, 22 and 13 % increase in CO2 removal at 45, 90 and 200 mL/min, respectively (Fig. 5). The difference in PCO2 from device inlet to outlet (PCO2 drop) decreased from 13.9 mmHg at 45 mL/min to 11.0 mmHg at 90 mL/min and 5.0 mmHg at 200 mL/min (Fig. 6) (P <0.01). This PCO2 drop behavior was similar between unmodified and CA-HFMs within each flow rate. Increasing fluid flow decreased CO2 removal per unit volume passing through the scaled-down gas exchange module, and adversely affected CA coating contribution to total CO2 removal rate. Increasing PCO2 drop improves bioactive CA-HFMs performance.
Fig. 5.
CO2 removal by unmodified and human CA immobilized HFMs in bovine blood at 45, 90 and 200 mL/ min. Increasing blood flow rate adversely affects the percentage increase by carbonic anhydrase coatings on HFMs (*P < 0.05)
Fig. 6.
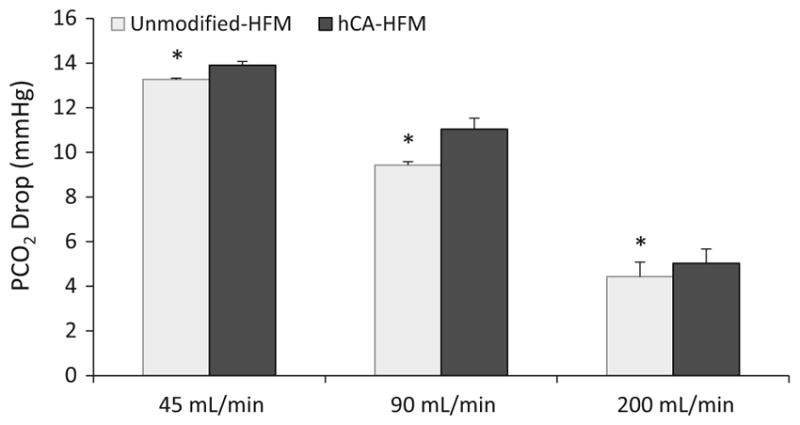
Drop in blood PCO2 across testing module oxygenator, calculated from difference between inlet and outlet, decreases with increasing blood flow rate (*P < 0.05)
4 Discussion
The goal of this study was to examine the kinetics of CO2 transport within CA-modified gas exchange devices in PBS and blood, as a means to elucidate strategies for improved blood CO2 removal using CA-HFM devices. We employed two forms of CA to empirically evaluate the kinetics and mass transport processes limiting HFM CO2 removal in prototype gas exchange devices. The CO2 removal performance by CA-HFMs is not influenced by immobilized CA activity or adsorption of blood plasma proteins (albumin). In blood, endogenous CA within RBCs participate along with surface immobilized CA to catalyze bicarbonate/CO2 equilibrium, in effect throttling immobilized CA.
The mass transport processes for CO2 removal by CA-modified gas exchange devices is comprised of four distinct physicochemical processes. Overall mass transport in the system is governed by the individual resistances of each process in series. The first resistance in HFM CO2 removal is interconversion between bicarbonate and CO2 within the bulk fluid, catalyzed by endogenous CA in blood or uncatalyzed in PBS. Second is diffusion of bicarbonate and CO2 from the bulk fluid into the liquid diffusional boundary layer adjacent to the HFM surface. Third is the catalyzed dehydration of bicarbonate to CO2 by immobilized CA on the HFM surface. Last is the diffusion of gaseous CO2 through the membrane and into the HFM sweep gas stream. Previous work demonstrated the rate limiting step in CO2 removal for unmodified HFMs is diffusion of CO2 through the liquid-side diffusional boundary layer, not diffusion through the HFM itself [15, 16]. In this application we must additionally consider the impact of immobilized CA catalytic rates, and equilibrium between bicarbonate/CO2 on system-wide CO2 transport.
The importance of immobilized CA activity on CO2 removal was studied through human and bovine CA. Immobilized human CA has fivefold increase in p-NPA activity over bovine CA, likely due to greater purity of the recombinant human CA solution as compared to the lyophilized bovine CA containing contaminants and denatured proteins. In previous studies, increasing immobilized CA activity through more efficient conjugation strategies increased CO2 removal rates [9]. We hypothesized higher activity human CA-HFMs would increase CO2 removal over bovine CA-HFMs. In contrast to the p-NPA assay, CO2 removal rates were comparable between human and bovine CA-HFMs (Fig. 3). Gas exchange was performed in PBS to avoid effects from protein adsorption on the fiber surface or endogenous CA. Chitosan immobilized CA resulted in sufficient activity such that increasing immobilized CA activity with human CA no longer increased CO2 removal rate. This suggests diffusive resistance within the liquid boundary layer, as with unmodified HFMs, is the rate limiting step for CO2 transport rather than immobilized enzyme activity. CA catalyzes bicarbonate/CO2 and p-NPA/p-NP using the same active site, however the rate constant (k) is 1 million times greater for CO2, 1 × 106 s−1 for CO2 versus 1 s−1 for p-NPA [17, 18]. CA catalyzed conversion of bicarbonate/CO2 is fast enough such that diffusion through the boundary layer dominates transport resistance [19, 20]. Decreasing boundary layer resistance by increasing fluid flow rate, increases delivery of p-NPA to the fiber surface, but does not increase p-NPA catalysis by immobilized CA (Fig. 2). Differences in immobilized CA activity are measurable with the p-NPA assay because CA p-NPA catalysis is the rate limiting resistance compared to the fluid boundary layer diffusional resistance. Conversely for bicarbonate or CO2 as the enzyme substrate, the boundary layer resistance dominates as the rate limiting step over the CA CO2 catalytic rate. Divergence in the human and bovine CA curves with increasing flow rate in Fig. 3 is interpreted as reduction in boundary layer thickness (resistance) with increasing flow rate. This hypothesis is supported by regression fits to the data resulting in exponents approaching 0.5, which is the theoretical relationship between boundary layer thickness and liquid flow rate [15]. Due to the extremely fast catalytic rate of CA (among the highest known) [17], strategies to improve CO2 removal efficacy through increased CA loading or activity on the fiber surface are not productive avenues unless a corresponding decrease in diffusional boundary layer resistance can be achieved.
The performance of CA-HFMs were examined in whole blood at 45, 90 and 200 mL/min and compared with unmodified HFMs. Increasing blood flow rate increased CO2 removal rate for unmodified and CA-HFMs, by decreasing the fluid boundary layer resistance. A decrease in CA coating enhancement from 37 to 13 % was observed with increasing blood flow rate from 45 to 200 mL/min (Fig. 5). Shear-induced enzyme activity loss as a consequence of increased liquid flow rate is a potential hypothesis. Data from p-NPA activity assay suggest fluid induced shear did not negatively impact CA under these flow rates (Fig. 2). Additionally, CO2 removal at 45 mL/min is repeatable after exposure to 200 mL/min flow rate (data not shown), indicating permanent shear-induced effects are unlikely, although transient shear effects under clinically relevant conditions still need to be explored. While increasing blood flow rate increases total CO2 removal, fluid residence time within the device decreases and consequently the CO2 removal per fluid volume declines (Fig. 6). In PBS, HFMs remove CO2 faster than bicarbonate can dehydrate to CO2 (uncatalyzed), resulting in a pool of bicarbonate as PBS is depleted of gaseous CO2. As a drop in bulk PCO2 is observed, disequilibrium between bicarbonate/CO2 is created. Approximately 103 CO2 molecules are removed per second by CA-HFMs at a liquid flow rate of 45 mL/min, while the uncatalyzed bicarbonate to CO2 conversion rate is ~1 s−1. Bioactive CA-HFMs facilitate the dehydration reaction to restore CO2 pressure in the fluid boundary layer. In blood, endogenous CA within RBCs catalyzes bicarbonate/CO2 conversion in the bulk fluid, but cannot maintain equilibrium within the fluid boundary layer, evidenced by the impact of CA coatings on HFM CO2 removal. The fluid PCO2 drop can be considered a metric of disequilibrium between bicarbonate and CO2 where the higher the fluid flow rate, the smaller the PCO2 drop, and the smaller the impact of bioactive CA coatings on HFM CO2 removal.
We consistently observed a significant reduction in CA coating efficacy in blood compared to PBS. CO2 removal by bioactive CA-HFMs was assessed in solutions containing individual blood components: plasma protein albumin, free CA and RBCs. Protein adsorption onto CA-HFMs did not decrease CO2 removal by unmodified or CA-HFMs. In contrast, endogenous CA within circulating RBCs reduced enhancement of the immobilized CA coating on CO2 removal. Blood maintains bicarbonate/CO2 equilibrium more effectively than PBS and directly limits the CA-HFM CO2 removal enhancement, evident by the 106 % increase in CO2 removal (PBS) versus 37 % increase (blood). RBC CA solutions increased CO2 removal rates for both unmodified and CA-immobilized devices compared to PBS solution (Fig. 4). Free CA spiked in PBS decreased CA coating efficacy to only 7 % improvement over non CA-HFM devices. Since CA-HFM performance increases when CA is localized to RBCs (7 % free CA vs. 27 % RBC CA), diffusion of bicarbonate/CO2 across the RBC membrane potentially contributes to the resistance limiting bulk fluid equilibrium from restoring gaseous CO2 as it is depleted by HFMs. Free CA spiked in PBS may localize closer to the HFM surface similar to immobilized CA, whereas RBCs tend to migrate away from stationary surfaces due to hydrodynamic forces, a phenomenon known as the Fahraeus effect for blood flow in small tubes [21].
Development of improved and more efficient respiratory assist devices will enable next generation, minimally invasive extracorporeal support of failing lungs at low blood flows. Carbonic anhydrase immobilized on HFMs is a novel approach to increase CO2 removal rates, but the factors limiting the enhancement of CO2 removal by CA-HFMs were not well understood. Our results demonstrate bioactive CA-HFMs operate under Le Chatelier’s principle, catalyzing conversion of bicarbonate/CO2 to preserve chemical equilibrium within the fluid boundary layer. In blood, endogenous CA within RBCs also catalyzes bicarbonate/CO2 equilibrium, in effect throttling immobilized CA’s contribution towards CO2 removal. Mechanisms which reduce the diffusional boundary layer resistance and conditions which promote local bicarbonate/CO2 disequilibrium, such as fluid longer transit times, will result in the largest contribution from the CA coating. Development is underway on CO2 removal devices which reduce the boundary layer resistance by increasing fluid velocity past HFMs through mechanical mixing independent of fluid flow rate through the device [22–24]. This approach increases delivery of bicarbonate/CO2 to the HFM surface without decreasing blood transit time, which may take advantage of CA’s rapid catalysis and boost CO2 removal performance from increased substrate delivery. Active mixing and CA-HFMs can only restore equilibrium within the fluid boundary layer, but cannot augment fluid PCO2 above the device inlet condition (around 50 mmHg). Since bicarbonate/CO2 equilibrium is described by , increasing the H+ concentration in the fluid boundary layer will create disequilibrium, forcing CA to catalyze dehydration of bicarbonate, increasing fluid PCO2 beyond the inlet 50 mmHg, and driving further improvement in HFM CO2 removal. By using an acidic gas within the oxygen sweep gas passed through the HFM lumens, an acid product can be introduced to the fluid boundary layer, creating local disequilibrium while minimizing acidification of the whole bulk fluid. In subsequent work we demonstrate dilute sulfur dioxide in oxygen sweep gas increases CO2 removal, and when used in combination with bioactive CA-HFMs has a synergistic effect to more than double CO2 removal while maintaining physiologic pH [25].
Acknowledgments
This publication was made possible by Grant Numbers R01 HL70051 and R01 HL117637 from the National Institutes of Health, National Heart, Lung and Blood Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH. This work was also funded by research Grants from the Pennsylvania Department of Health (SAP #4100030667, #4100035341, and #4100041556). The author would like to thank Drs. Christopher D. Boone and Robert McKenna (Department of Biochemistry and Molecular Biology, College of Medicine, University of Florida, Gainesville, FL 32610) for supplying the wild-type hCA II used in these experiments, that was funded in part from the National Institutes of Health (GM25154) award. We would like to recognize the University of Pittsburgh’s McGowan Institute for Regenerative Medicine for support of this study. Primary funding for David Arazawa was provided by the NIH Training Grant (T32-HL076124) at the University of Pittsburgh: Cardiovascular Bioengineering Training Program (CBTP).
Footnotes
Conflict of interest W. J. Federspiel has an equity interest in Alung Technologies which has licensed portions of the technology described in this manuscript. For all other authors, there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- 1.Pesenti A, Patroniti N, Fumagalli R. Carbon dioxide dialysis will save the lung. Crit Care Med. 2010;38:S549–54. doi: 10.1097/CCM.0b013e3181f1fe0c. [DOI] [PubMed] [Google Scholar]
- 2.Terragni P, Maiolo G, Ranieri VM. Role and potentials of low-flow CO(2) removal system in mechanical ventilation. Curr Opin Crit Care. 2012;18(1):93–8. doi: 10.1097/MCC.0b013e32834f17ef. [DOI] [PubMed] [Google Scholar]
- 3.Kaushik M, Wojewodzka-Zelezniakowicz M, Cruz DN, Ferrer-Nadal A, Teixeira C, Iglesias E, Kim JC, Braschi A, Piccinni P, Ronco C. Extracorporeal carbon dioxide removal: the future of lung support lies in the history. Blood Purif. 2012;34(2):94–106. doi: 10.1159/000341904. [DOI] [PubMed] [Google Scholar]
- 4.Cove M, MacLaren G, Federspiel W, Kellum J. Bench to bedside review: extracorporeal carbon dioxide removal, past present and future. Crit Care. 2012;16(5):232. doi: 10.1186/cc11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batchinsky AI, Jordan BS, Regn D, Necsoiu C, Federspiel WJ, Morris MJ, Cancio LC. Respiratory dialysis: reduction in dependence on mechanical ventilation by venovenous extracorporeal CO2 removal. Crit Care Med. 2011;39(6):1382–7. doi: 10.1097/CCM.0b013e31820eda45. [DOI] [PubMed] [Google Scholar]
- 6.Wearden PD, Federspiel WJ, Morley SW, Rosenberg M, Bieniek PD, Lund LW, Ochs BD. Respiratory dialysis with an active-mixing extracorporeal carbon dioxide removal system in a chronic sheep study. Intensive Care Med. 2012;38(10):1705–11. doi: 10.1007/s00134-012-2651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crotti S, Lissoni A, Tubiolo D, Azzari S, Tarsia P, Caspani L, Gattinoni L. Artificial lung as an alternative to mechanical ventilation in COPD exacerbation. Eur Respir J. 2012;39(1):212–5. doi: 10.1183/09031936.00021111. [DOI] [PubMed] [Google Scholar]
- 8.Arazawa DT, Oh H-I, Ye S-H, Johnson CA, Jr, Woolley JR, Wagner WR, Federspiel WJ. Immobilized carbonic anhydrase on hollow fiber membranes accelerates CO2 removal from blood. J Membr Sci. 2012;403–404:25–31. doi: 10.1016/j.memsci.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimmel JD, Arazawa DT, Ye S-H, Shankarraman V, Wagner WR, Federspiel WJ. Carbonic anhydrase immobilized on hollow fiber membranes using glutaraldehyde activated chitosan for artificial lung applications. J Mater Sci Mater Med. 2013;24(11):2611–21. doi: 10.1007/s10856-013-5006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geers C, Gros G. Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol Rev. 2000;80(2):681–715. doi: 10.1152/physrev.2000.80.2.681. [DOI] [PubMed] [Google Scholar]
- 11.Florchinger B, Philipp A, Klose A, Hilker M, Kobuch R, Rupprecht L, Keyser A, Puhler T, Hirt S, Wiebe K, Muller T, Langgartner J, Lehle K, Schmid C. Pumpless extracorporeal lung assist: a 10-year institutional experience. Ann Thorac Surg. 2008;86(2):410–7. doi: 10.1016/j.athoracsur.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 12.Boone CD, Habibzadegan A, Tu C, Silverman DN, McKenna R. Structural and catalytic characterization of a thermally stable and acid-stable variant of human carbonic anhydrase II containing an engineered disulfide bond. Acta Crystallogr D Biol Crystallogr. 2013;69(8):1414–22. doi: 10.1107/S0907444913008743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodgson SJ, Forster RE. Carbonic anhydrase activity of intact erythrocytes from seven mammals. J Appl Physiol. 1983;55(4):1292–8. doi: 10.1152/jappl.1983.55.4.1292. [DOI] [PubMed] [Google Scholar]
- 14.ANSI/AAMI/ISO 7199:2009—Cardiovascular implants and artificial organs—Blood-gas exchangers (oxygenators) Association for the Advancement of Medical Instrumentation (AAMI); 2009. [Google Scholar]
- 15.Federspiel WJ, Henchir KA. Lung, artificial: basic principles and current applications. Encycl Biomater Biomed Eng. 2004;9:910. [Google Scholar]
- 16.Galletti P, Colton C. Artificial lungs and blood–gas exchange devices. In: Bronzino JD, editor. The biomedical engineering handbook. Boca Raton: CRC Press; 2000. pp. 1–19. [Google Scholar]
- 17.Gilmour KM. Perspectives on carbonic anhydrase. Comp Biochem Physiol. 2010;157(3):193–7. doi: 10.1016/j.cbpa.2010.06.161. [DOI] [PubMed] [Google Scholar]
- 18.Thorslund A, Lindskog S. Studies of the esterase activity and the anion inhibition of bovine zinc and cobalt carbonic anhydrases. Eur J Biochem. 1967;3(1):117–23. doi: 10.1111/j.1432-1033.1967.tb19504.x. [DOI] [PubMed] [Google Scholar]
- 19.Khalifah RG. Carbon dioxide hydration activity of carbonic anhydrase: paradoxical consequences of the unusually rapid catalysis. Proc Natl Acad Sci. 1973;70(7):1986–9. doi: 10.1073/pnas.70.7.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasinoff BB. Kinetics of carbonic anhydrase catalysis in solvents of increased viscosity: a partially diffusion-controlled reaction. Arch Biochem Biophys. 1984;233(2):676–81. doi: 10.1016/0003-9861(84)90494-6. [DOI] [PubMed] [Google Scholar]
- 21.Truskey GA, Yuan F, Katz DF. Transport phenomena in biological systems. 2. Upper Saddle River: Prentice Hall; 2009. [Google Scholar]
- 22.Federspiel WJ, Svitek RG. Lung, artificial: current research and future directions. Encycl Biomater Biomed Eng. 2004;2:922–31. [Google Scholar]
- 23.Mihelc KM, Frankowski BJ, Lieber SC, Moore ND, Hattler BG, Federspiel WJ. Evaluation of a respiratory assist catheter that uses an impeller within a hollow fiber membrane bundle. ASAIO J. 2009;55(6):569–74. doi: 10.1097/MAT.0b013e3181bc2655. [DOI] [PubMed] [Google Scholar]
- 24.Jeffries RG, Frankowski BJ, Burgreen GW, Federspiel WJ. Effect of impeller design and spacing on gas exchange in a percutaneous respiratory assist catheter: impeller testing for a respiratory assist catheter. Artif Organs. 2014;38(12):1007–17. doi: 10.1111/aor.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arazawa DT, Kimmel JD, Finn MC, Federspiel WJ. Acidic sweep gas with carbonic anhydrase coated hollow fiber membranes synergistically accelerates CO2 removal from blood. 2015 doi: 10.1016/j.actbio.2015.07.007. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]