Abstract
Thrombospondin-2 (TSP2) is a potent inhibitor of angiogenesis whose expression is dynamically regulated following injury. In the present study, it is shown that HIF-1α represses TSP2 transcription. Specifically, in vitro studies demonstrate that the prolyl hydroxylase inhibitor DMOG or hypoxia decrease TSP2 expression in fibroblasts. This effect is shown to be via a transcriptional mechanism as hypoxia does not alter TSP2 mRNA stability and this effect requires the TSP2 promoter. In addition, the documented repressive effect of nitric oxide (NO) on TSP2 is shown to be non-canonical and involves stabilization of hypoxia inducible factor-1a (HIF-1α). The regulation of TSP2 by hypoxia is supported by the in vivo observation that TSP2 has spatiotemporal expression distinct from regions of hypoxia in gastrocnemius muscle following murine hindlimb ischemia (HLI). A role for TSP2 regulation by HIF-1α is supported by the dysregulation of TSP2 expression in SM22α-cre HIF-1α KO mice following HLI. Indeed, there is a reduction in blood flow recovery in the SM22a-cre HIF-1α KO mice compared to littermate controls following HLI surgery, associated with impaired recovery and increased TSP2 levels. Moreover, SM22α-cre HIF-1α KO smooth muscle cells mice have increased TSP2 mRNA levels that persist in hypoxia. These findings identify a novel, ischemia-induced pro-angiogenic mechanism involving the transcriptional repression of TSP2 by HIF-1α.
Keywords: Thrombospondin, Hypoxia, Hypoxia-inducible factor (HIF), Angiogenesis, Transcription
Introduction
Pathologic hypoxia occurring in wound healing, tissue ischemia, and cancer is a strong inducer of angiogenesis [1]. Oxygen homeostasis is regulated, in part, by Hypoxia inducible factor-1α (HIF-1α) [1,2]. Under normal oxygen tension, HIF-1α is marked for degradation through hydroxylation by prolyl hydroxylases (PHDs) and ubiquitination by the E3 ligase von Hippel Lindau (VHL). As oxygen levels decrease, and oxygen becomes a limiting metabolite, hydroxylation is inhibited, allowing for HIF-1α stabilization and nuclear translocation. In the nucleus, HIF-1α binds to hypoxia response elements (HREs) in the promoter region of numerous genes. HIF-1α target genes result in broad adaptations for survival in hypoxic conditions, inducing glycolytic pathway mediators, vasodilation, erythropoiesis, and angiogenesis [1].
Germline deletion of HIF-1α in mice results in early embryonic lethality due to severe cardiac and vascular malformations [3–6]. Detailed analysis of HIF-1α KO embryos suggested that HIF-1α is dispensable for embryonic vasculogenesis and patterning, but local oxygen sensing is necessary for proper vascular network maturation and stability. In hindlimb ischemia models, blood flow recovery to the ischemic leg is delayed in HIF-1α heterozygous mice, leading to decrease in function, necrosis, and loss of limb [7]. Further, adenoviral delivery of constitutively active HIF-1α improved the response to hindlimb ischemia, with increased blood flow recovery [7]. More recently, tissue- and cell-specific deletions of HIF-1α have improved our understanding of oxygen sensing in post-natal biology. Tie2-cre/HIF-1α (flox/flox) mice (HIF-1α EC KO) survive to maturity but display delayed wound healing characterized by incomplete wound re-epithelialization and less revascularization at the wound margins [8]. Moreover, HIF-1α heterozygous mice and HIF-1α EC KO mice fail to induce neovascularization in a burn wound model [9]. HIF-1α mediates blood pressure homeostasis, as vascular smooth muscle cell (VSMC)-specific HIF-1α KO mice display increased mean arterial pressure due to increased angiotensin II type receptor I (ATR1) expression [10]. Collectively, these studies demonstrate the necessity of HIF-1α signaling in restoring vascular homeostasis following a hypoxic event.
Extensive research has been dedicated to identification of induced HIF-1α-responsive genes, many of which are pro-angiogenic targets. Less well understood is the mechanism by which genes are down regulated in hypoxia in a HIF-1α-dependent manner [11]. Furthermore, it is not well established that HIF-1α may actively suppress an anti-angiogenic gene.
Endogenous angiogenesis inhibitors come from several sources including ECM cleavage products, ECM-associated proteins, growth factors, and other soluble proteins [12]. Thrombospondin (TSP)-1 was the first identified endogenous inhibitor of angiogenesis and belongs to the TSP family of matricellular proteins [13–15]. TSP2 is a homologue of TSP1 and is itself a potent inhibitor of angiogenesis which is temporally induced following injury [13,16]. Consistent with its anti-angiogenic role, mice that lack TSP2 display enhanced angiogenesis following wound healing [17–19], hindlimb ischemia [20], and during the foreign body response [21–23]. TSP2 inhibits angiogenesis, in part, by enhancing uptake and degradation of matrix metalloproteinases (MMP) -2 and -9 [24,25] and by interacting with the surface receptors αvβ3, VLDLR, CD36, and CD47 [13]. TSP2 KO wounds have increased MMP2/9, which is associated with higher levels of soluble vascular endothelial growth factor (VEGF) in the granulation tissue [19]. Furthermore, TSP2 KO mice have altered collagen ultrastructure, characterized by irregularly assembled collagen fibers that contribute to greater skin and tendon laxity [21,26]. Decellularized ECM produced in vitro by primary TSP2 KO dermal fibroblasts is more permissive to EC migration and favorable EC spreading [20,27].
In several injury models, TSP2 expression occurs following the inflammatory phase and is associated with matrix remodeling and regulation of angiogenesis and arteriogenesis [18,20]. Interestingly, this pattern is distinct from the temporal course of hypoxia and presumably HIF-1α expression, an observation that prompted us to hypothesize that oxygen levels, and specifically HIF-1α stabilization, might regulate TSP2. Despite the plethora of reported HIF-1α effects on gene transcription, it has not been widely appreciated as a regulator of anti-angiogenic molecules.
In contrast to the abundant information about the anti-angiogenic mechanism of TSP2 and its well-established upregulation in settings of injury, relatively little is known about its regulation. There is evidence to support TSP2 is redox sensitive [28–30] and a target of the microRNA lef-7fm [30]. Recently, we described that eNOS KO mice have increased TSP2 levels at baseline and after injury, and demonstrated that TSP2 is transcriptionally repressed by NO [31]. Similarly, in Akt1 KO mice, TSP2 expression is elevated, leading to delayed tissue repair after full-thickness excisional wounding. This phenotype is rescued in Akt1/TSP2 DKO mice, highlighting that repression of TSP2, in this case by Akt1 (a critical eNOS kinase), is necessary in curtailing the anti-angiogenic properties of TSP2 [32].
In the present study, the hypothesis that HIF-1α’s pro-angiogenic activity involves the negative regulation of TSP2 was investigated. We demonstrate that in ischemic tissues, the deposition of TSP2 is excluded from hypoxic regions. Additionally, we show that DMOG and hypoxia decrease TSP2 transcription. More importantly, we demonstrate that TSP2 expression is higher in VSMC-specific HIF-1α KO mice following hindlimb ischemia, and that VSMCs isolated from these mice produce more TSP2 mRNA. This data demonstrates that TSP2 transcription is suppressed by hypoxia through a mechanism that requires HIF-1α.
Results
Hypoxia decreases TSP2 transcription
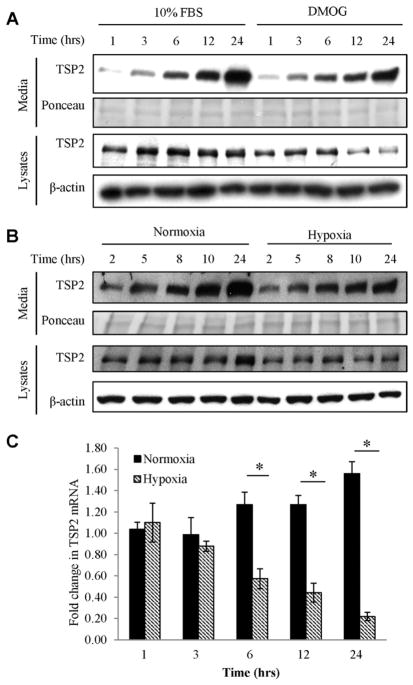
We previously described that NO decreases TSP2 mRNA levels [31] in a time frame consistent with HIF-1α stabilization by NO, implicating the latter as a putative repressor of TSP2 production. To evaluate this hypothesis, NIH3T3 cells were treated with the prolyl hydroxylase inhibitor DMOG in complete media (10% FBS). DMOG suppressed both intracellular (3 h in 10%FBS) and secreted TSP2 (6 h in 10%FBS) (Fig. 1A).
Fig. 1.
DMOG decreases TSP2 expression. (A) Western blot of NIH3T3 media and lysates treated with 1 mM DMOG in media containing 10% FBS DMOG decreased intracellular and secreted TSP2 by 6 h. (B) NIH3T3s were subjected to hypoxia for the indicated times and media and lysates samples were analyzed by Western blot for TSP2 expression. Both the secreted and intracellular pool of TSP2 was decreased following hypoxia treatment. Western blots are representative of at least three independent experiments. (C) Qualitative RT-PCR of mRNA from NIH3T3s demonstrated that hypoxia (hatched bars) resulted in decreased levels TSP2 mRNA by 6 h of treatment. * denotes p value ≤ 0.05. mRNA data are averaged from at least three independent experiments.
Furthermore, incubation of NIH3T3 cells under hypoxic conditions (0.5% O2) decreased the levels of TSP2 protein in lysates and conditioned media at 10 h (Fig. 1B). Hypoxia decreased TSP2 mRNA levels by 6 h of treatment and TSP2 levels remained significantly decreased for the remaining 24 h time course (Fig. 1C). Under these conditions, hypoxia induced the expression of the known HIF-responsive genes GLUT1 and DEC1 at 3 and 6 h of hypoxia (Supplemental Fig. 1), consistent with the time course of hypoxia-mediated suppression of TSP2 expression.
Hypoxia impacts TSP2 by a transcriptional mechanism
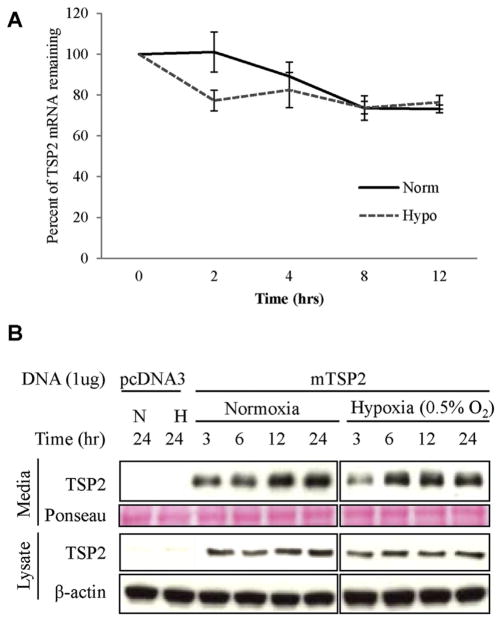
To determine the effect of hypoxia on TSP2 mRNA stability, NIH3T3s were pretreated with the transcriptional inhibitor Actinomycin D and monitored for the loss of TSP2 mRNA in both normoxia and hypoxia. As previously described [31], the half-life of TSP2 is longer than 12 h. Hypoxia did not alter the rate of decay of TSP2 mRNA (Fig. 2A). Together with the mRNA data, these findings imply that the changes in TSP2 by hypoxia are primarily mediated by a transcriptional effect. To evaluate the ability of hypoxia to modulate TSP2 mRNA or protein stability independent of the TSP2 promoter, HEK293T cells that do not express endogenous TSP2 (Fig. 2B), were transfected with plasmid mTSP2 pcDNA3, which is controlled by a constitutive promoter (CMV). Plasmid-derived TSP2 was expressed and secreted at similar levels in hypoxia and normoxia, indicating that TSP2 stability is not hypoxia-sensitive. Based on these observations, we conclude the hypoxia-mediated downregulation of TSP2 is mediated predominantly by a transcriptional mechanism. However, the existence of additional regulatory effects involving post-translational modifications cannot be entirely excluded.
Fig. 2.
Hypoxia depresses TSP2 production at the transcriptional level. (A) NIH3T3 cells were treated with Actinomycin-D and the decay of TSP2 mRNA in normoxia and hypoxia was determined by Qualitative RT-PCR and expressed as a percentage of original for the time indicated (hr). Experiments were performed three independent times. (B) HEK293T cells, which do not produce endogenous TSP2, were transfected with 1 μg of either empty vector (pcDNA3) or mouse TSP2 (mTSP2). Neither secreted nor intracellular production of TSP2 by HE293Ts was not altered by hypoxia (0.5% oxygen) as assessed by Western blot. Western blots are representative from at least three independent experiments.
Inverse relationship between hypoxia and TSP2 expression in vivo
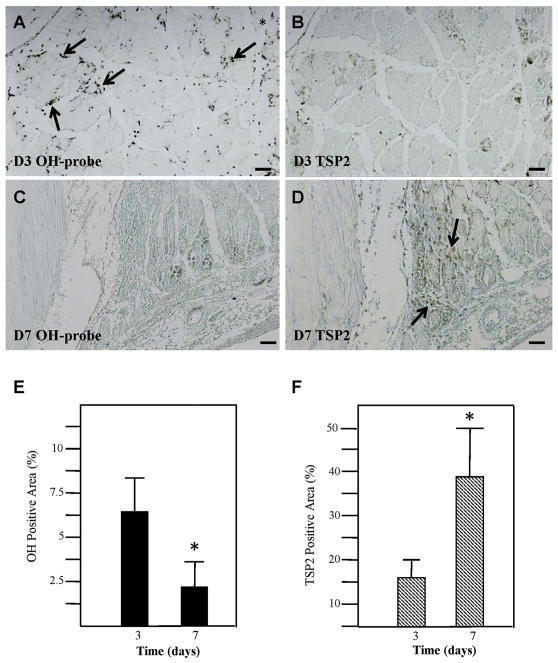
Hindlimb ischemic surgery rapidly induces hypoxia in the gastrocnemius muscle, while TSP2 expression is detectable at 7 and 28 days post-ischemia [20]. The time course and localization of TSP2 and hypoxic zones in the gastrocnemius muscle after hindlimb ischemia was evaluated in serial sections. Hydroxyp-robe staining revealed hypoxia at 3 days post-surgery, which was resolved by day 7 (Fig. 3A, C). Temporally, TSP2 expression was limited when hypoxia was observed. TSP2 was minimally detected at day 3 following injury (Fig. 3B) and was increased at day 7 (Fig. 3D). Changes in the extent of hypoxia and TSP2 expression were verified by histomorphometric analysis (Fig. 3E, F). These data support the in vitro observation that there is an inverse relationship between hypoxia and TSP2 levels.
Fig. 3.
TSP2 expression is separate from hypoxic cells in HLI. Serial sections from gastrocnemius muscle at 3D (A and B) and 7D (C and D) following hindlimb ischemia were stained for hydroxyprobe (OH-probe, A and C) and TSP2 (B and D). As indicated by the brown staining, hydroxyprobe expression is abundant at 3D and decreased at 7D. TSP2 expression demonstrates the opposite pattern, where it is limited at 3D and increased at 7D. Scale bars: 100 μm. (E–F) Percent of area exhibiting positive staining was determined for OH-probe (black bars) and TSP2 (hatched bars). * denotes p value ≤ 0.05. Experiments were performed in three mice per time point.
Enhanced TSP2 production in VSMC-specific HIF-1α KO mice
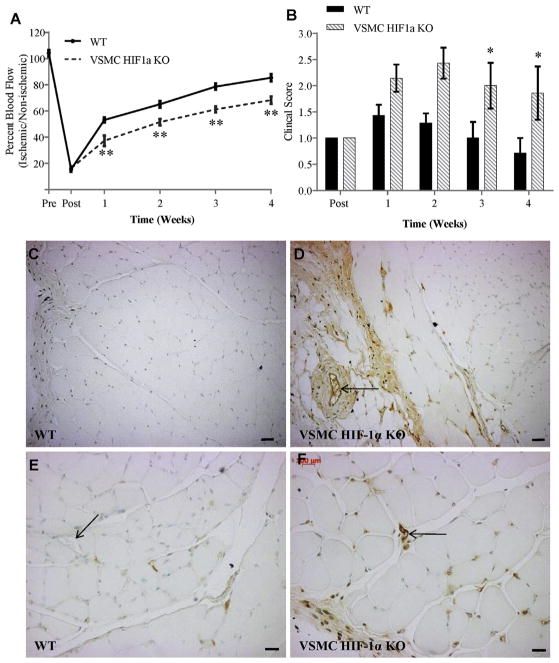
To explore the role of HIF-1α in directly regulating TSP2, SM22α-cre+ HIF-1α floxed mice, referred to here as VSMC-HIF-1α KO mice, and cre-floxed HIF-1α littermates (WT) were subjected to hindlimb ischemia. VSMC-HIF-1α KOs recovered poorly following HLI surgery as evident by reduced blood flow recovery as early as 7 days post-surgery (Fig. 4A) and compromised clinical score (Fig. 4B). Four weeks after ischemic injury, sections from the gastrocnemius were analyzed by immunohistochemistry for TSP2. VSMC-HIF-1α KO mice displayed increased levels and altered distribution and levels of TSP2. In WT mice, TSP2 levels are relatively low and restricted to the interstitial spaces between muscle fibers and weakly in the perimysium (Fig. 4C,E). In contrast, VSMC-HIF-1α KO mice had more TSP2 immunoreactivity with stronger staining in the perimysium and around large and small vessels, along with increased interstitial TSP2 (Fig. 4D,F).
Fig. 4.
Increased TSP2 associated with compromised HLI recovery in VSMC-HIF-1α KO. (A) Blood flow recovery following HLI surgery was lower in VSMC-HIF-1α KO than WT littermates. Data are from five mice per genotype. (B) VSMC-HIF-1α KO displayed lower clinical score following HLI. (C–F) Representative images of HLI tissues from WT (C, E) and VSMC-HIF-1α KO (D, F) stained for TSP2 and visualized with the peroxidase reaction (brown color) are shown. Nuclei were counterstained with methyl green. HIF-1α KO tissues displayed increased TSP2 immunoreactivity and altered distribution pattern with TSP2 stain around large vessels (arrows in D) as well as small vessels (compare Arrows in E and F). * denotes p value ≤ 0.05, ** denotes p value ≤ 0.001. Scale bars = 200 μm (C, D) and 100 μm (E, F). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
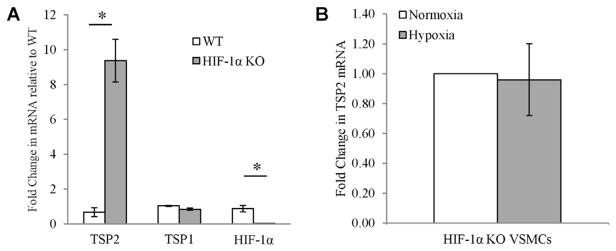
VSMCs were isolated from WT and HIF-1α KO aortas. In normoxia, HIF-1α KO VSMC had significantly more TSP2, but not TSP1 mRNA (Fig. 5A). In addition, these cells failed to repress TSP2 mRNA under hypoxic conditions (Fig. 5B). Expression of TSP2 in WT VSMCs was barely detectable under normoxic conditions, which limited further analysis in hypoxia (not shown). These data recapitulate the in vivo findings that HIF-1α KO mice have increased TSP2 and further demonstrate a basal role for HIF-1α in regulating TSP2 expression. Altogether, these changes imply that HIF-1α in VSMCs is important for the down-regulation of TSP2.
Fig. 5.
Increased TSP2 in VSMCs from HIF-1α KO mice. (A) Aortic VSMCs were isolated from WT and HIF-1α KO and RNA analyzed for the relative levels of TSP1, TSP2 and HIF-1α. (B) The level of TSP2 mRNA was not decreased in HIF-1α KO VSMCs cultured in normoxia (22% oxygen) or hypoxia (0.5% oxygen) for 6 h.
Mapping the mouse TSP2 minimal promoter
We previously described transcriptional repression of the 1kb and 2kb TSP2 promoter by DETANO [31]. In order to characterize the TSP2 promoter and identify NO-sensitive regions, a set of 5′ and 3′ deletion constructs and a set of 3′ to 5′ deletion constructs were generated (Supplemental Fig. 2A). Deletions from the 5′ end of the 2kb promoter resulted in robust activity through the 250bp length (Supplemental Fig. 2). Promoter activity declined sharply between 250bp and 150bp, and activity was lost at the 50bp length, indicating that the 250bp contains the minimal promoter. Activity of every 5′ deletion construct decreased in the presence of 1 mM DETA-NO (Supplemental Fig. 2B hatched bars). These data indicate that a critical NO-sensitive factor resides within the remaining 150bp of promoter that demonstrated luciferase activity.
To further characterize the TSP2 promoter proximal to the transcriptional initiation site, 3′ to 5′ deletions of the 1kb promoter were generated (Supplemental Fig. 2A). The deletions resulted in removing a region of Exon 1 (1kbΔ60bp) or the entire Exon 1, as well as the “TATA-like” motif presumed to be the transcriptional start site (1kbΔ150bp). Both constructs resulted in substantial diminution of baseline promoter activity, indicating that these regions are critical for transcriptional activation of TSP2. The 1kbΔ60bp promoter decreased baseline promoter activity by 42% relative to the 1kb promoter, and the 1kbΔ150bp lost promoter activity (Supplemental Fig. 2C). In the presence of 1 mM DETANO, the 1kbΔ60bp significantly decreased luciferase activity, indicating that regions of NO-sensitivity were retained in this construct. Altogether, these data suggest that there are several regions on the TSP2 promoter that confer NO-sensitivity and that at least one of these regions is located proximal to the transcriptional start site.
DETANO induces HIF-1α activity in NIH3T3 fibroblasts
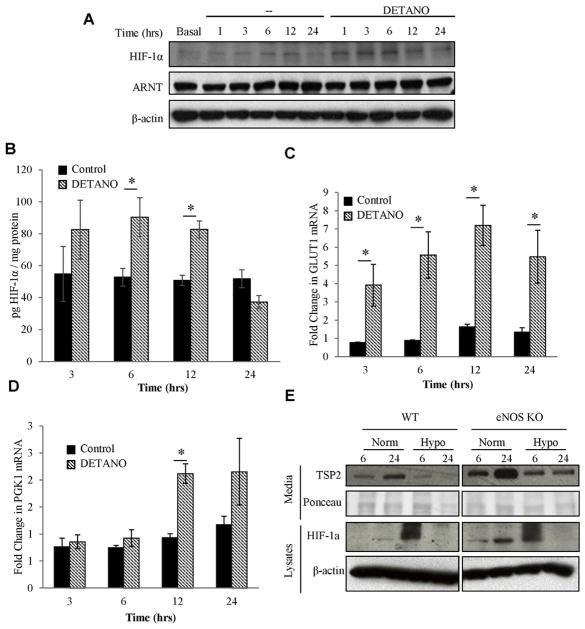
Since NO has been shown to stabilize HIF-1α in a number of cell systems, we chose to determine the HIF-1α status in NIH3T3 cells treated with DETANO. HIF-1α levels were induced rapidly and transiently by DETANO, with HIF-1α stabilization occurring between 1 and 6 h, and returning to baseline by 12 h (Fig. 6A). These observations were confirmed by HIF-1α specific ELISA, which demonstrated a significant increase in HIF-1α protein after 6 and 12 h of DETANO treatment (Fig. 6B). DETANO treatment of NIH3T3s was sufficient to increase GLUT1 and PGK1 mRNA, indicating that HIF-1α stabilization in these cells altered gene transcription (Fig. 6C,D). GLUT1 mRNA was increased between 3 and 24 h of DETANO treatment, while PGK1 mRNA increased between 12 and 24 h of DETANO treatment.
Fig. 6.
DETANO stabilizes HIF-1α in NIH3T3s. (A) Western blot of NIH3T3 lysates treated with 1 mM DETANO reveal a transient stabilization of HIF-1α between 1 and 6 h of treatment that is restored to baseline after 24 h of treatment. There is no detectable change in HIF-1β/ARNT over the same time course. (B) HIF-1α ELISA confirmed that HIF-1α was stabilized in DETANO treated NIH3T3s at 6 h and this induction was restored to baseline by 24 h. * denotes p value <0.05 (C–D) Qualitative RT-PCR of mRNA from NIH3T3s demonstrated the time-course of GLUT1 and PGK1 were induction following DETANO treatment (hatched bars). (E) MLECs from WT and eNOS KO mice were cultured in normoxia or hypoxia (0.5% oxygen). Samples from cell lysates and media were collected at 6 and 24 h post treatment and tested for TSP2. In all cells tested, hypoxic MLECs exhibited decreased secreted TSP2. All data are representative of three independent experiments. Representative Western blots are shown.
Hypoxia-mediated TSP2 downregulation is independent of the Akt1-eNOS signaling axis
We have previously documented enhanced production of TSP2 both in eNOS KO mice as well as in Akt1 KO mice, a critical eNOS kinase [31,32]. Given that NO can enhance stabilization of HIF-1α, we sought to determine whether the NO-mediated dysregulation of TSP2 is reversed by hypoxia. WT and eNOS KO ECs were subjected to 0.5% oxygen for 6 and 24 h. Similar to NIH3T3s, both WT and eNOS KO MLECs exhibited decreased TSP2 secretion after 24 h of treatment (Fig. 6E). HIF-1α stabilization was observed in both WT and eNOS KO cells under hypoxia (Fig. 6E). These data demonstrate that the hypoxia-mediated suppression of TSP2 mRNA levels in ECs does not depend on eNOS-derived NO.
Discussion
In this study, we demonstrate that oxygen tension regulates TSP2 expression. Furthermore, we make the novel observation that TSP2 is repressed in hypoxia by transcriptional activity downstream of HIF-1α. These observations are supported as fibroblasts, which are a predominant source of TSP2, lose TSP2 expression when maintained in low oxygen via a mechanism requiring the TSP2 promoter. A chemical mimic of hypoxia, DMOG, was sufficient to reduce TSP2 transcription. These observations are strengthened by in vivo observation that the temporal and spatial distribution of TSP2 in hindlimb ischemia are distinct from the hypoxic zones. In support of a direct role of HIF-1α in regulation of TSP2, we find that the levels of TSP2 are aberrantly upregulated in VSMC-specific HIF-1α-deficient mice following hindlimb ischemia and isolated VSMCs in culture. Lastly, we characterize that the minimal promoter of TSP2 is located within the 250bp proximal to the TSP2 transcriptional start site. Altogether, these findings provide novel observations to understand TSP2 transcriptional regulation by hypoxia.
Previously, it was shown that TSP2 is undetectable in uninjured tissue, but is stimulated after injuries such as burns, dermal wounding, and hindlimb ischemia [16,33]. Furthermore, TSP2 is reciprocally regulated during adipogenic and osteogenic differentiation [34]. Despite extensive characterization of the spatiotemporal expression of TSP2 in injury models, little is known about its transcriptional regulation [35]. It was recently described that TSP2 is negatively regulated by NO. Given the work that demonstrated that NO regulates HIF-1α levels through increasing HIF-1α protein synthesis [36,37], direct nitrosylation of HIF-1α [38], or by inactivation of prolyl hydroxylases (PHD) [39], we sought to determine whether hypoxia or HIF-1α regulated TSP2 expression. Indeed, either the chemical hypoxia mimic DMOG or hypoxia reduced TSP2 mRNA in fibroblasts. Furthermore, the observation that hypoxyprobe adducts and TSP2 expression maintained distinct spatiotemporal localization in hindlimb ischemia suggests an unappreciated connection between HIF-1α and repression of TSP2.
We sought to determine whether the transcriptional regulation of TSP2 by NO was associated with HIF-1α stabilization. In normoxia, HIF-1α can be stabilized by NO donors in a number of cell types including ECs, SMCs and epithelial cells [36–40]. Consistent with these reports, we observed that DETANO stabilized HIF-1α and induce HIF-1α downstream genes in fibroblasts (Fig. 5). However, both WT and eNOS KO MLECs in hypoxia reduced both intracellular and secreted TSP2 protein levels, indicating that hypoxia is able to control TSP2 expression independently of eNOS.
To explore the role of HIF-1α in regulating TSP2 expression, we utilized a HIF-1α-deficient model and found that HIF-1α VSMC KO mice have increased TSP2 protein expression after hindlimb ischemia. VSMC-specific HIF-1α deficiency was previously attributed to altered lipid uptake and monocyte recruitment within the vessel wall [41] as well as in systemic blood pressure regulation [10]. Our report is the first to demonstrate the role of VSMC-specific HIF-1α in the hindlimb ischemia model. Consistent with the reduction in angiogenic remodeling in EC-specific HIF-1α KOs [3,8,9], we demonstrate that the VSMC HIF-1α KO mice have reduced blood flow recovery after surgery (Fig. 4). Furthermore, we demonstrate that these mice exhibited increased TSP2 expression, indicating that at least part of the reduced blood flow recovery could be owing to the increased anti-angiogenic state induced by TSP2 overexpression. Previous reports have observed a reciprocal relationship between HIF-1α and TSP2, but failed to provide a direct relationship between HIF-1α and control of TSP2 expression. Salvianolic acid was found to modulate a wide variety of genes in vitro, including decreased HIF-1α and increased TSP2, but this study did not establish a mechanistic link between the two [42]. Recently, TSP2-null fractures were reported to be less hypoxic than WT, which coincided with improved healing time in the TSP2-null fracture, both alone and combined with ischemic surgery [43]. Furthermore, it was determined that the phenotype was due to increased angiogenic potential and not altered mesenchymal stem cell activation. However, this study did not evaluate the role of oxygen in influencing TSP2 levels.
Interestingly, compared to TSP2, hypoxia has a distinct effect on the modulation of its close homologue TSP1. It consistently decreased TSP1 in several immortalized cell and tumor lines, including clear-cell renal carcinoma [44], MEFs [45] and glioblastoma cells [46]. However, in pathologic settings hypoxia increases TSP1. Samples from patients with pulmonary hypertension, as well as experimental models of hypertension, demonstrate increased TSP1 [47], fitting with the observation that TSP1 KO mice are protected from pulmonary remodeling induced by chronic hypoxia [48]. Furthermore, hypoxic conditioning increases TSP1 levels in fibroblasts from healthy and systemic sclerosis patients [49], SMCs [50], and endothelial cells [51]. Other anti-angiogenic molecules have been shown to be increased by hypoxia, including endostatin, SPARC, and angiopoietin 2 [52]. It is proposed that these anti-angiogenic genes are part of a negative-feedback loop initiated downstream of VEGF signaling [52]. TSP2 is unique, then, as it supports a role for HIF-1α in promoting angiogenesis by blocking anti-angiogenic genes.
HIF-1α stabilization is associated with broad changes in gene transcription to adapt for a low-oxygen environment. The induction of hypoxia genes, which contain the HIF-1α binding site referred to as hypoxia response elements (HREs) have received the major focus of attention [1,53]. Alternatively, microarray studies have revealed many genes that are decreased following hypoxia, but the mechanism behind this is poorly understood [11]. Many, but not all, of these genes are HIF-1α-regulated and it is not known whether these effects are due to active repression by HIF-1α or secondary to other transcription factors. One exception is melanoma-associated oncogene MITF, which is decreased in hypoxia through transcriptional repressor DEC1, a bona fide HIF-1α target gene [11]. Additionally, it has been reported that hypoxia inhibits pre-initiation complex formation by activation of the transcriptional regulator NC2, leading to target selective repression following hypoxia [54]. NC2 is believed to bind to TATA boxes and be further regulated in function by distal transcriptional enhancers.
Our studies demonstrate a role for HIF-1α in regulation of TSP2 expression via primarily a transcriptional mechanism. However, the regulation of transcriptional machinery controlling TPS2 remains largely descriptive. Studies have described TSP2 to be controlled by nitric oxide [31], reactive oxygen species [28,29], cMyc [55], and Ewing’s sarcoma fusion protein EWS/FL11 [56]. We and others [20,56], have previously tested the activity of the full-length promoter. Characterization of the minimal promoter, on the other hand, has not been described. Here, we demonstrate that the core transcriptional machinery of TSP2 is located within the 250bp range proximal to the transcriptional start site. Additionally, deletions from the 3′ to 5′ direction indicate that there are important regulatory factors for TSP2 transcription located in its first exon, as has been described for other genes including TSP1 [57]. These data underscore the importance of the sequences immediately proximal to the transcriptional start site, because deletion of 60bp halved promoter activity. Furthermore, deletion of the entire first exon and the “TATA-like” box abolished promoter activity, verifying the importance of this region for TSP2 transcription. Previously, we demonstrated that TSP2 transcription is repressed by NO in vitro and in vivo. TSP2 protein and mRNA were increased in eNOS KO mice, both at baseline and following injury models [31]. Multiple studies have demonstrated NO-mediated inactivation of transcription factors, including AP1, NFkB and Sp1 [58–61]. In the present study, we sought to address the mechanism of NO-mediated TSP2 repression. Surprisingly, every active TSP2 promoter was DETANO-sensitive. Moreover, the 150bp promoter, the shortest NO-sensitive promoter, lacked canonical AP1, NFkB, and Sp1 sites, indicating these prototypical NO-sensitive transcription factors were not mediating TSP2 repression. Even though HIF-1α is necessary for the hypoxia-induced alterations in TSP2 expression, the lack of a HRE in the TSP2 promoter region suggests that is not a direct target of HIF-1α.
In summary, our study demonstrates a difference in the spatial and temporal patterns of hypoxic cells and TSP2 following hindlimb ischemia. TSP2 protein and mRNA are decreased in hypoxia and in DMOG, the pharmacological hypoxia mimic. Further, we demonstrate that TSP2 levels are dysregulated in HIF-1α KO mice and VSMCs. Therefore, this study provides new evidence of repression of an anti-angiogenic molecule by a pro-angiogenic factor, adding a novel facet in the regulation of angiogenesis by hypoxia.
Experimental procedures
Chemicals
DMOG was purchased from Cayman Chemicals (Ann Arbor, MI) and Actinomycin-D from Sigma Aldrich (Saint Louis, MO). DETANONOate was purchased from Alexis Biochemicals (San Diego, CA).
Hindlimb ischemia, tissue isolation and immunohistochemistry
Hindlimb ischemia was performed as previously described [62,63]. Briefly, mice were anesthetized (100 mg/kg ketamine and 10 mg/kg xylazine) and ischemia was induced in the left leg by ligation of both the proximal portion of the femoral artery and the distal portion of the saphenous artery. Blood flow recovery was determined using a PeriFlux Laser Doppler System perfusion module (Perimed, North Royalton, OH) and tissue damage was assessed weekly using a previously described scoring system [63]. Statistical analysis was performed using repeated measures ANOVA with Bonferroni post-hoc test (Graphpad Prism). Hypoxic regions in C57Bl6 mice were detected following IP injection of pimonidazole 30 min prior to euthanasia and subsequent staining with the Hypoxyprobe-1 Omni Kit (Hypoxyprobe Inc., Burlington, MA). Ischemia was performed on floxed HIF-1α mice (WT) and SM22α-cre floxedHIF-1α mice (VSMC-HIF-1α KO). Generation of VSMC-HIF-1α KO mice was described previously [10]. Branches between the two sites were ligated or cauterized and arteriectomy was performed. Skin incision without femoral artery ligation was performed for control sham operations. Excised ischemic muscle from the upper and lower leg was fixed in 10% zinc-buffered formalin (Z-fix, Anatech, Battle Creek, MI) and embedded in paraffin. HLI muscle from TSP2 KO mice was used as negative control. 5 μm-thick sections were generated and stained with hematoxylin and eosin (H&E) or immunohistochemistry with an anti-TSP2 antibody was performed as described previously [19,22,26,64]). Immunoreactivity was detected by peroxidase activity and visualized with the Vector ABC Elite kit (Vector Laboratories, Burlingame, CA). Nikon Eclipse 800 microscope equipped with fluorescence optics was used for all examinations. Supplemental Fig. 3 shows analysis of sham control and TSP2 KO tissues. Metamorph software was utilized to determine the area of tissue positive for peroxidase activity. A total of at least six mice per time point per genotype were analyzed.
Cell culture
NIH3T3 fibroblasts and HEK293 cells (ATCC) were cultured in DMEM (Gibco, Grand Island, NY) with 10% FBS, penicillin, streptomycin and L-glutamine. Isolation and immortalization of MLECs was previously described [62,65]. MLECs were cultured in EBM-2 media (Lonza, Walkersville, MD) supplemented with 15% Hyclone characterized FBS, EGM-2MV Single-Quot growth factors (Cambrex, Walkersville, MD), penicillin, streptomycin, and 2× L-glutamine.
VSMCs were isolated from aortas of SMC-HIF-1α KO mice and floxed HIF-1α littermates (WT) between 3 and 4 months of age by enzymatic digestion as described previously [66]. Eight mice were used per isolation. VSMCs were maintained in DMEM supplemented with 10% FBS, penicillin, streptomycin, and L-glutamine.
Hypoxia, DMOG, and DETANO experiments
Experiments were performed with cells at 80% confluence. NIH3T3s, VSMCs, or MLECs were washed with PBS and fresh complete media was added. Cells were then returned to a room air incubator (normoxia, 18% oxygen) or exposed to hypoxia (0.5% oxygen) in a ProxOxC nitrogen-induced hypoxia system (BioSpherix, Red Field, NY). During the time-course of 24 h, cells did not demonstrate visual indications of apoptosis. DMOG was added to NIH3T3s in complete media (10% FBS). DETANO was added to NIH3T3s at a concentration of 1 mM in starvation media (DMEM supplemented with 0.5% FBS) for a 24 h period. At indicated times, media was collected, centrifuged at 1200 rpm for 5 min and the supernatant saved for analysis. Cells were lysed in RIPA buffer plus protease inhibitors (Complete Mini EDTA-free inhibitor cocktail, Roche, Indianapolis, IN) for Western blot analysis. Isolation of mRNA was performed at indicated times following hypoxia treatment using the RNeasy Kit (Qiagen) according to the manufacturer’s protocol.
Cell extracts were analyzed for total HIF-1α levels by the DuoSet IC ELISA kit according to the supplier’s instructions (R&D Systems).
Western blot analysis
Cell lysates (20 μg) and media (30 μL) were separated by SDS-PAGE, transferred to nitrocellulose membranes (Bio-Rad), and developed with a luminol-based chemiluminescent horseradish peroxidase substrate kit (Perkin Elmer, Waltham, MA). Western blot analysis for TSP2 (BD Bioscience, San Jose, CA) and β-actin (Sigma, St Louis, MO) was performed as described previously [20]. Analysis of ARNT (Novus Biologicals, NB100–133, Littleton, CO) and HIF-1α (Novus Biologicals, NB100–449) was done according to the supplier’s instructions.
RNA isolation and qRT-PCR
RNA samples were collected using the RNeasy Mini Kit (Qiagen, Valencia, CA). Reverse transcription (RT) was performed on 1 μg of RNA (Superscript II, Invitrogen, Carlsbad, CA) utilizing Oligo dT and Random Hexamer (Invitrogen) primers. SYBRgreen (BioRad, Hercules, CA) semi quantitative PCR was performed using a BioRad iCycler5. Primers were designed using NCBI’s primer design tool or replicated from literature references and are available upon request. Samples were run in duplicate and averaged. Experimental results reflect the average of triplicate experiments.
For the mRNA stability assay, NIH3T3 cells were pretreated with Actinomycin-D (1 μg/mL, 30 min, Sigma Aldrich, Saint Louis, MI) in starve media prior to exposure to hypoxia. RNA was isolated after onset hypoxia over a 12 hour period and compared to cells maintained in normoxia for the same time. RT and qPCR was performed as described. The experiment was performed three times and data was normalized to GAPDH and expressed as percent message remaining relative to Actinomycin-D treated cells (t = 0) plus or minus the standard error of the mean (SEM).
Generation of promoter deletion constructs and luciferase assay
Serial deletion mutants of the 2kb promoter in the 5′ to 3′ direction were generated by insertion of a second KpnI site at the desired distance from the transcriptional start site by Site-directed Mutagenesis. Plasmids were digested with KpnI, purified using the QIAquick gel extraction kit (Qiagen) and re-ligated. Deletion mutants from the 3′ to 5′ direction were generated from the 1kb plasmid, by inserting a second BglII site, a single cutter in the parent plasmid, by Site-directed Mutagenesis (Stratagene). Mutants were screened by BglII digestion (NE Biolabs), purified with Qiagen gel extraction kit and re-ligated with T4 ligase (NE Biolabs).
Luciferase assays were performed using the Dual Luciferase Reporter Assay (Promega, Madison, WI) as described previously [31]. Briefly, NIH3T3s were co-transfected with 200 ng/well of luciferase construct (pGL3 basic (Promega) or indicated mTSP2 promoter construct) and 50 ng/well renilla using Lipofectamine (Invitrogen). Transfection was performed in antibiotic-free starvation media (0.5% FBS) in cells that were either untreated or treated in the presence of 1 mM DETANO. Luciferase Assays were performed 24 h after transfection and were assayed on a Lumat luminometer (Berthold Technologies, Oak Ridge, TN). Assays were performed in duplicate and repeated four times.
Cell transfection
HEK293T cells were transfected with 1 μg/6well of either empty pcDNA3 vector (Invitrogen) or mTSP2 pcDNA3 (Addgene.org, plasmid 12,411) using Lipofectamine (Invitrogen, Carlsbad, CA) and Optimem (Gibco) as indicated by manufacturers. Twenty-four hours later, cells were washed and given fresh culture media and returned to either a room air incubator or 0.5% O2.
Statistical analysis
Experiments were performed at least in triplicate, and data are plotted as the mathematical average of the replicates plus or minus the standard error of the mean, unless otherwise noted above. Statistics were calculated with the statistical package in Prism GraphPad. Unless stated above, statistical significance was determined using unpaired Students t-tests were performed with p value less than or equal to 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Andrew Sawyer and Nina Kristofik for assistance with animal experiments. Kurt Hankenson kindly provided the original TSP2-luciferase promoter.
Funding
This work was supported by National Institutes of Health Grants HL 107205 (T.R.K, W.C.S., M.S., and M.A.S.) and (GM-072194-01 to TK). SM was funded by an AHA Predoctoral grant (09PRE2080166).
Abbreviations used
- ATR1
angiotensin II type receptor I
- ARNT
aryl hydrocarbon receptor nuclear translocator
- HIF-1α
hypoxia-inducible factor 1α
- HREs
hypoxia response elements
- KO
knockout
- MMP
matrix metalloproteinases
- MLEC
mouse lung endothelial cell
- NO
nitric oxide
- PHDs
prolyl hydroxylases
- TSP
thrombospondin
- VEGF
vascular endothelial growth factor
- VSMC
vascular smooth muscle cell
- VHL
von Hippel Lindau
- WT
wildtype
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.matbio.2017.07.002.
References
- 1.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40(2):294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rey S, Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res. 2010;86(2):236–242. doi: 10.1093/cvr/cvq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17(11):3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Compernolle V, Brusselmans K, Franco D, Moorman A, Dewerchin M, Collen D, Carmeliet P. Cardia bifida, defective heart development and abnormal neural crest migration in embryos lacking hypoxia-inducible factor-1alpha. Cardiovasc Res. 2003;60(3):569–579. doi: 10.1016/j.cardiores.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12(2):149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotch LE, Iyer NV, Laughner E, Semenza GL. Defective vascularization of HIF-1alpha-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Dev Biol. 1999;209(2):254–267. doi: 10.1006/dbio.1999.9253. [DOI] [PubMed] [Google Scholar]
- 7.Bosch-Marce M, Okuyama H, Wesley JB, Sarkar K, Kimura H, Liu YV, Zhang H, Strazza M, Rey S, Savino L, Zhou YF, McDonald KR, Na Y, Vandiver S, Rabi A, Shaked Y, Kerbel R, Lavallee T, Semenza GL. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ Res. 2007;101(12):1310–1318. doi: 10.1161/CIRCRESAHA.107.153346. [DOI] [PubMed] [Google Scholar]
- 8.Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP, Ferrara N, Johnson RS. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6(5):485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Liu L, Wei X, Tan YS, Tong L, Chang R, Ghanamah MS, Reinblatt M, Marti GP, Harmon JW, Semenza GL. Impaired angiogenesis and mobilization of circulating angiogenic cells in HIF-1alpha heterozygous-null mice after burn wounding. Wound Repair Regen. 2010;18(2):193–201. doi: 10.1111/j.1524-475X.2010.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y, Di Lorenzo A, Jiang W, Cantalupo A, Sessa WC, Giordano FJ. Hypoxia-inducible factor-1alpha in vascular smooth muscle regulates blood pressure homeostasis through a peroxisome proliferator-activated receptor-gamma-angiotensin II receptor type 1 axis. Hypertension. 2013;62(3):634–640. doi: 10.1161/HYPERTENSIONAHA.111.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greijer AE, van der Groep P, Kemming D, Shvarts A, Semenza GL, Meijer GA, van de Wiel MA, Belien JA, van Diest PJ, van der Wall E. Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1) J Pathol. 2005;206(3):291–304. doi: 10.1002/path.1778. [DOI] [PubMed] [Google Scholar]
- 12.Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65(10):3967–3979. doi: 10.1158/0008-5472.CAN-04-2427. [DOI] [PubMed] [Google Scholar]
- 13.Kyriakides TR, Maclauchlan S. The role of thrombospondins in wound healing, ischemia, and the foreign body reaction. J Cell Commun Signal. 2009;3(3–4):215–225. doi: 10.1007/s12079-009-0077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy-Ullrich JE, Sage EH. Revisiting the matricellular concept. Matrix Biol. 2014;37:1–14. doi: 10.1016/j.matbio.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong LC, Bornstein P. Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix Biol. 2003;22(1):63–71. doi: 10.1016/s0945-053x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 16.Calabro NE, Kristofik NJ, Kyriakides TR. Thrombospondin-2 and extracellular matrix assembly. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbagen.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyriakides TR, Tam JW, Bornstein P. Accelerated wound healing in mice with a disruption of the thrombospondin 2 gene. J Invest Dermatol. 1999;113(5):782–787. doi: 10.1046/j.1523-1747.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- 18.Agah A, Kyriakides TR, Letrondo N, Bjorkblom B, Bornstein P. Thrombospondin 2 levels are increased in aged mice: consequences for cutaneous wound healing and angiogenesis. Matrix Biol. 2004;22(7):539–547. doi: 10.1016/j.matbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Maclauchlan S, Skokos E, Agah A, Zeng J, Tian W, Davidson J, Bornstein P, Kyriakides T. Enhanced angiogenesis and reduced contraction in thrombospondin-2-null wounds is associated with increased levels of matrix metalloproteinases-2 and -9, and soluble VEGF. J Histochem Cytochem. 2009;57(4):301–313. doi: 10.1369/jhc.2008.952689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krady MM, Zeng J, Yu J, MacLauchlan S, Skokos EA, Tian W, Bornstein P, Sessa WC, Kyriakides TR. Thrombospondin-2 modulates extracellular matrix remodeling during physiological angiogenesis. Am J Pathol. 2008;173(3):879–891. doi: 10.2353/ajpath.2008.080128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyriakides TR, Leach KJ, Hoffman AS, Ratner BD, Bornstein P. Mice that lack the angiogenesis inhibitor, thrombospondin 2, mount an altered foreign body reaction characterized by increased vascularity. Proc Natl Acad Sci U S A. 1999;96(8):4449–4454. doi: 10.1073/pnas.96.8.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyriakides TR, Zhu YH, Yang Z, Huynh G, Bornstein P. Altered extracellular matrix remodeling and angiogenesis in sponge granulomas of thrombospondin 2-null mice. Am J Pathol. 2001;159(4):1255–1262. doi: 10.1016/S0002-9440(10)62512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris AH, Kyriakides TR. Matricellular proteins and biomaterials. Matrix Biol. 2014;37:183–191. doi: 10.1016/j.matbio.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Z, Kyriakides TR, Bornstein P. Matricellular proteins as modulators of cell-matrix interactions: adhesive defect in thrombospondin 2-null fibroblasts is a consequence of increased levels of matrix metalloproteinase-2. Mol Biol Cell. 2000;11(10):3353–3364. doi: 10.1091/mbc.11.10.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hahn-Dantona E, Ruiz J, Bornstein P, Strickland D. The low density lipoprotein receptor-related protein modulates levels of matrix metalloproteinase 9 (MMP-9) by mediating its cellular catabolism. J Biol Chem. 2001;276(18):15498–15503. doi: 10.1074/jbc.M100121200. [DOI] [PubMed] [Google Scholar]
- 26.Kyriakides TR, Zhu YH, Smith LT, Bain SD, Yang Z, Lin MT, Danielson KG, Iozzo RV, LaMarca M, McKinney CE, Ginns EI, Bornstein P. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol. 1998;140(2):419–430. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristofik N, Calabro NE, Tian W, Meng A, MacLauchlan S, Wang Y, Breuer CK, Tellides G, Niklason LE, Kyriakides TR. Impaired von Willebrand factor adhesion and platelet response in thrombospondin-2 knockout mice. Blood. 2016;128(12):1642–1650. doi: 10.1182/blood-2016-03-702845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang Y, Scheef E, Gurel Z, Sorenson C, Jefcoate C, Sheibani N. CYP1B1 and endothelial nitric oxide synthase combine to sustain proangiogenic functions of endothelial cells under hyperoxic stress. Am J Physiol Cell Physiol. 2009 doi: 10.1152/ajpcell.00153.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Y, Scheef E, Wang S, Sorenson C, Marcus C, Jefcoate C, Sheibani N. CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression. Blood. 2009;113(3):744–754. doi: 10.1182/blood-2008-03-145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bae ON, Wang JM, Baek SH, Wang Q, Yuan H, Chen AF. Oxidative stress-mediated thrombospondin-2 upregulation impairs bone marrow-derived angiogenic cell function in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2013 doi: 10.1161/ATVBAHA.113.301609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacLauchlan S, Yu J, Parrish M, Asoulin TA, Schleicher M, Krady MM, Zeng J, Huang PL, Sessa WC, Kyriakides TR. Endothelial nitric oxide synthase controls the expression of the angiogenesis inhibitor thrombospondin 2. Proc Natl Acad Sci U S A. 2011;108(46):E1137–45. doi: 10.1073/pnas.1104357108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bancroft T, Bouaouina M, Roberts S, Lee M, Calderwood DA, Schwartz M, Simons M, Sessa WC, Kyriakides TR. Up-regulation of thrombospondin-2 in Akt1-null mice contributes to compromised tissue repair due to abnormalities in fibroblast function. J Biol Chem. 2015;290(1):409–422. doi: 10.1074/jbc.M114.618421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kyriakides TR, Bornstein P. Matricellular proteins as modulators of wound healing and the foreign body response. Thromb Haemost. 2003;90(6):986–992. doi: 10.1160/TH03-06-0399. [DOI] [PubMed] [Google Scholar]
- 34.Shitaye HS, Terkhorn SP, Combs JA, Hankenson KD. Thrombospondin-2 is an endogenous adipocyte inhibitor. Matrix Biol. 2010;29(6):549–556. doi: 10.1016/j.matbio.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stenina-Adognravi O. Invoking the power of thrombospondins: regulation of thrombospondins expression. Matrix Biol. 2014;37:69–82. doi: 10.1016/j.matbio.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasuno K, Takabuchi S, Fukuda K, Kizaka-Kondoh S, Yodoi J, Adachi T, Semenza GL, Hirota K. Nitric oxide induces hypoxia-inducible factor 1 activation that is dependent on MAPK and phosphatidylinositol 3-kinase signaling. J Biol Chem. 2004;279(4):2550–2558. doi: 10.1074/jbc.M308197200. [DOI] [PubMed] [Google Scholar]
- 37.Sandau KB, Fandrey J, Brune B. Accumulation of HIF-1alpha under the influence of nitric oxide. Blood. 2001;97(4):1009–1015. doi: 10.1182/blood.v97.4.1009. [DOI] [PubMed] [Google Scholar]
- 38.Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, Vujaskovic Z, Dewhirst MW, Li CY. Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell. 2007;26(1):63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Metzen E, Zhou J, Jelkmann W, Fandrey J, Brune B. Nitric oxide impairs normoxic degradation of HIF-1alpha by inhibition of prolyl hydroxylases. Mol Biol Cell. 2003;14(8):3470–3481. doi: 10.1091/mbc.E02-12-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmer LA, Gaston B, Johns RA. Normoxic stabilization of hypoxia-inducible factor-1 expression and activity: redox-dependent effect of nitrogen oxides. Mol Pharmacol. 2000;58(6):1197–1203. doi: 10.1124/mol.58.6.1197. [DOI] [PubMed] [Google Scholar]
- 41.Liu D, Lei L, Desir M, Huang Y, Cleman J, Jiang W, Fernandez-Hernando C, Di Lorenzo A, Sessa WC, Giordano FJ. Smooth muscle hypoxia-inducible factor 1α links intravascular pressure and atherosclerosis–brief report. Arterioscler Thromb Vasc Biol. 2016;36(3):442–445. doi: 10.1161/ATVBAHA.115.306861. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, Ge PJ, Jiang L, Li FL, Zhu QY. Modulation of growth and angiogenic potential of oral squamous carcinoma cells in vitro using salvianolic acid B. BMC Complement Altern Med. 2011;11:54. doi: 10.1186/1472-6882-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burke D, Dishowitz M, Sweetwyne M, Miedel E, Hankenson KD, Kelly DJ. The role of oxygen as a regulator of stem cell fate during fracture repair in TSP2-null mice. J Orthop Res. 2013 doi: 10.1002/jor.22396. [DOI] [PubMed] [Google Scholar]
- 44.Bienes-Martinez R, Ordonez A, Feijoo-Cuaresma M, Corral-Escariz M, Mateo G, Stenina O, Jimenez B, Calzada MJ. Autocrine stimulation of clear-cell renal carcinoma cell migration in hypoxia via HIF-independent suppression of thrombospondin-1. Sci Rep. 2012;2:788. doi: 10.1038/srep00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laderoute KR, Alarcon RM, Brody MD, Calaoagan JM, Chen EY, Knapp AM, Yun Z, Denko NC, Giaccia AJ. Opposing effects of hypoxia on expression of the angiogenic inhibitor thrombospondin 1 and the angiogenic inducer vascular endothelial growth factor. Clin Cancer Res. 2000;6(7):2941–2950. [PubMed] [Google Scholar]
- 46.Tenan M, Fulci G, Albertoni M, Diserens AC, Hamou MF, El Atifi-Borel M, Feige JJ, Pepper MS, Van Meir EG. Thrombospondin-1 is downregulated by anoxia and suppresses tumorigenicity of human glioblastoma cells. J Exp Med. 2000;191(10):1789–1798. doi: 10.1084/jem.191.10.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bauer PM, Bauer EM, Rogers NM, Yao M, Feijoo-Cuaresma M, Pilewski JM, Champion HC, Zuckerbraun BS, Calzada MJ, Isenberg JS. Activated CD47 promotes pulmonary arterial hypertension through targeting caveolin-1. Cardiovasc Res. 2012;93(4):682–693. doi: 10.1093/cvr/cvr356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ochoa CD, Yu L, Al-Ansari E, Hales CA, Quinn DA. Thrombospondin-1 null mice are resistant to hypoxia-induced pulmonary hypertension. J Cardiothorac Surg. 2010;5:32. doi: 10.1186/1749-8090-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Distler JH, Jungel A, Pileckyte M, Zwerina J, Michel BA, Gay RE, Kowal-Bielecka O, Matucci-Cerinic M, Schett G, Marti HH, Gay S, Distler O. Hypoxia-induced increase in the production of extracellular matrix proteins in systemic sclerosis. Arthritis Rheum. 2007;56(12):4203–4215. doi: 10.1002/art.23074. [DOI] [PubMed] [Google Scholar]
- 50.Osada-Oka M, Ikeda T, Akiba S, Sato T. Hypoxia stimulates the autocrine regulation of migration of vascular smooth muscle cells via HIF-1alpha-dependent expression of thrombospondin-1. J Cell Biochem. 2008;104(5):1918–1926. doi: 10.1002/jcb.21759. [DOI] [PubMed] [Google Scholar]
- 51.Phelan MW, Forman LW, Perrine SP, Faller DV. Hypoxia increases thrombospondin-1 transcript and protein in cultured endothelial cells. J Lab Clin Med. 1998;132(6):519–529. doi: 10.1016/s0022-2143(98)90131-7. [DOI] [PubMed] [Google Scholar]
- 52.Messmer-Blust A, An X, Li J. Hypoxia-regulated angiogenic inhibitors. Trends Cardiovasc Med. 2009;19(8):252–256. doi: 10.1016/j.tcm.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92(12):5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Denko N, Wernke-Dollries K, Johnson AB, Hammond E, Chiang CM, Barton MC. Hypoxia actively represses transcription by inducing negative cofactor 2 (Dr1/DrAP1) and blocking preinitiation complex assembly. J Biol Chem. 2003;278(8):5744–5749. doi: 10.1074/jbc.M212534200. [DOI] [PubMed] [Google Scholar]
- 55.Bein K, Ware J, Simons M. Myb-dependent regulation of thrombospondin 2 expression. Role of mRNA stability. J Biol Chem. 1998;273(33):21423–21429. doi: 10.1074/jbc.273.33.21423. [DOI] [PubMed] [Google Scholar]
- 56.Potikyan G, Savene RO, Gaulden JM, France KA, Zhou Z, Kleinerman ES, Lessnick SL, Denny CT. EWS/ FLI1 regulates tumor angiogenesis in Ewing’s sarcoma via suppression of thrombospondins. Cancer Res. 2007;67(14):6675–6684. doi: 10.1158/0008-5472.CAN-06-4140. [DOI] [PubMed] [Google Scholar]
- 57.Janz A, Sevignani C, Kenyon K, Ngo CV, Thomas-Tikhonenko A. Activation of the myc oncoprotein leads to increased turnover of thrombospondin-1 mRNA. Nucleic Acids Res. 2000;28(11):2268–2275. doi: 10.1093/nar/28.11.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tabuchi A, Sano K, Oh E, Tsuchiya T, Tsuda M. Modulation of AP-1 activity by nitric oxide (NO) in vitro: NO-mediated modulation of AP-1. FEBS Lett. 1994;351(1):123–127. doi: 10.1016/0014-5793(94)00839-6. [DOI] [PubMed] [Google Scholar]
- 59.Nikitovic D, Holmgren A, Spyrou G. Inhibition of AP-1 DNA binding by nitric oxide involving conserved cysteine residues in Jun and Fos. Biochem Biophys Res Commun. 1998;242(1):109–112. doi: 10.1006/bbrc.1997.7930. [DOI] [PubMed] [Google Scholar]
- 60.Matthews J, Botting C, Panico M, Morris H, Hay R. Inhibition of NF-kappaB DNA binding by nitric oxide. Nucleic Acids Res. 1996;24(12):2236–2242. doi: 10.1093/nar/24.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reynaert N, Ckless K, Korn S, Vos N, Guala A, Wouters E, van der Vliet A, Janssen-Heininger Y. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc Natl Acad Sci U S A. 2004;101(24):8945–8950. doi: 10.1073/pnas.0400588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton R, Galasso G, Birnbaum M, Walsh K, Sessa W. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115(8):2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu J, de Muinck E, Zhuang Z, Drinane M, Kauser K, Rubanyi G, Qian H, Murata T, Escalante B, Sessa W. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci U S A. 2005;102(31):10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agah A, Kyriakides TR, Lawler J, Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol. 2002;161(3):831–839. doi: 10.1016/S0002-9440(10)64243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kuhlencordt P, Rosel E, Gerszten R, Morales-Ruiz M, Dombkowski D, Atkinson W, Han F, Preffer F, Rosenzweig A, Sessa W, Gimbrone MJ, Ertl G, Huang P. Role of endothelial nitric oxide synthase in endothelial activation: insights from eNOS knockout endothelial cells. Am J Physiol Cell Physiol. 2004;286(5):C1195–202. doi: 10.1152/ajpcell.00546.2002. [DOI] [PubMed] [Google Scholar]
- 66.Siow RC, Pearson JD. Vascular smooth muscle cells: isolation, culture, and characterization. Methods Mol Med. 2001;46:237–245. doi: 10.1385/1-59259-143-4:237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.