Abstract
Bell Beaker pottery spread across western and central Europe beginning around 2750 BCE before disappearing between 2200–1800 BCE. The forces propelling its expansion are a matter of long-standing debate, with support for both cultural diffusion and migration. We present new genome-wide data from 400 Neolithic, Copper Age and Bronze Age Europeans, including 226 Beaker-associated individuals. We detected limited genetic affinity between Iberian and central European Beaker-associated individuals, and thus exclude migration as a significant mechanism of spread between these two regions. However, migration played a key role in the further dissemination of the Beaker Complex, a phenomenon we document most clearly in Britain, where the spread of the Beaker Complex introduced high levels of Steppe-related ancestry and was associated with a replacement of ~90% of Britain’s gene pool within a few hundred years, continuing the east-to-west expansion that had brought Steppe-related ancestry into central and northern Europe 400 years earlier.
During the third millennium Before the Common Era (BCE), two new archaeological pottery styles expanded across Europe, replacing many of the more localized styles that preceded them1. The ‘Corded Ware Complex’ in north-central and northeastern Europe was associated with people who derived most of their ancestry from populations related to Early Bronze Age Yamnaya pastoralists from the Eurasian steppe2–4 (henceforth referred to as Steppe). In western Europe there was the equally expansive ‘Bell Beaker Complex’, defined by assemblages of grave goods that included stylised bell-shaped pots, copper daggers, arrowheads, stone wristguards and V-perforated buttons5 (Extended Data Fig. 1). The oldest radiocarbon dates associated with Beaker pottery are around 2750 BCE in Atlantic Iberia6, which has been interpreted as evidence that the Beaker Complex originated there. However, the geographic origin is still debated7 and other scenarios including an origin in the Lower Rhine area or even multiple independent origins are possible (Supplementary Information section 1). Regardless of the geographic origin, by 2500 BCE the Beaker Complex had spread throughout western Europe (and northwest Africa), and reached southern and Atlantic France, Italy and central Europe5, where it overlapped geographically with the Corded Ware Complex. Within another hundred years, it had expanded to Britain and Ireland8. A major debate in archaeology has revolved around the question of whether the spread of the Beaker Complex was mediated by the movement of people, culture, or a combination of both9. Genome-wide data have revealed high proportions of Steppe-related ancestry in Beaker Complex-associated individuals from Germany and the Czech Republic2–4, showing that they derived from mixtures of populations from the Steppe and the preceding Neolithic farmers of Europe. However, a deeper understanding of the ancestry of people associated with the Beaker Complex requires genomic characterization of individuals across the geographic range and temporal duration of this archaeological phenomenon.
Ancient DNA data
To understand the genetic structure of ancient people associated with the Beaker Complex and their relationship to preceding, subsequent and contemporary peoples, we used hybridization DNA capture4,10 to enrich ancient DNA libraries for sequences overlapping 1,233,013 single nucleotide polymorphisms (SNPs), and generated new sequence data from 400 ancient Europeans dated to ~4700–800 BCE and excavated from 136 different sites (Extended Data Table 1–2; Supplementary Table 1; Supplementary Information, section 2). This dataset includes 226 Beaker Complex-associated individuals from Iberia (n=37), southern France (n=4), northern Italy (n=3), Sicily (n=3), central Europe (n=133), The Netherlands (n=9) and Britain (n=37), and 174 individuals from other ancient populations, including 118 individuals from Britain who lived both before (n=51) and after (n=67) the arrival of the Beaker Complex (Fig. 1a–b). For genome-wide analyses, we filtered out first-degree relatives and individuals with low coverage (<10,000 SNPs) or evidence of DNA contamination (Methods) and combined our data with previously published ancient DNA data (Extended Data Fig. 2) to form a dataset of 683 ancient samples (Supplementary Table 1). We further merged these data with 2,572 present-day individuals genotyped on the Affymetrix Human Origins array11,12 and 300 high coverage genomes13. To facilitate the interpretation of our genetic results, we also generated 111 new direct radiocarbon dates (Extended Data Table 3; Supplementary Information, section 3).
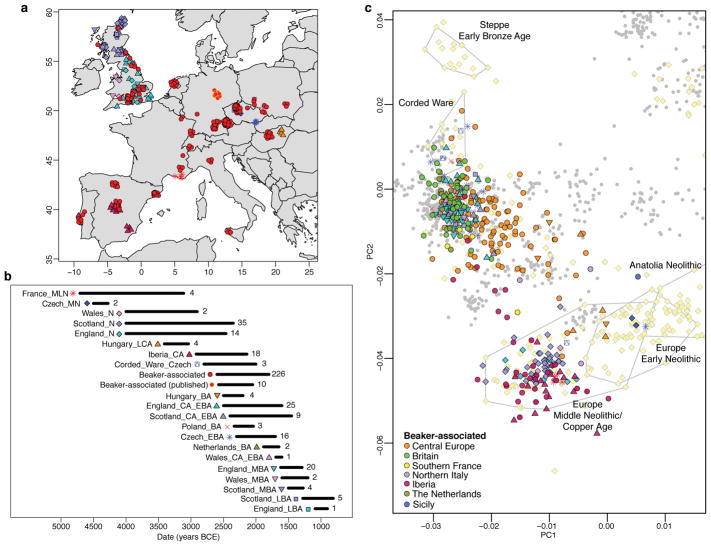
Figure 1. Spatial, temporal, and genetic structure of individuals in this study.
a, Geographic distribution of samples with new genome-wide data. Random jitter was added for sites with multiple individuals. Map data from the R package maps. b, Approximate time ranges for samples with new genome-wide data. Sample sizes are given next to each bar. c, Principal component analysis of 990 present-day West Eurasian individuals (grey dots), with previously published (pale yellow) and new ancient samples projected onto the first two principal components. This figure is a zoom of Extended Data Fig. 3a. See Methods for abbreviations of population names.
Y-chromosome analysis
The Y-chromosome composition of Beaker-associated males was dominated by R1b-M269 (Supplementary Table 4), a lineage associated with the arrival of Steppe migrants in central Europe after 3000 BCE2,3. Outside Iberia, this lineage was present in 84 out of 90 analysed males. For individuals in whom we could determine the R1b-M269 subtype (n=60), we found that all but two had the derived allele for the R1b-S116/P312 polymorphism, which defines the dominant subtype in western Europe today14. In contrast, Beaker-associated individuals from the Iberian Peninsula carried a higher proportion of Y haplogroups known to be common across Europe during the earlier Neolithic period2,4,15,16, such as I (n=5) and G2 (n=1), while R1b-M269 was found in four individuals with a genome-wide signal of Steppe-related ancestry (the two with higher coverage could be further classified as R1b-S116/P312). Finding this widespread presence of the R1b-S116/P312 polymorphism in ancient individuals from central and western Europe suggests that people associated with the Beaker Complex may have had an important role in the dissemination of this lineage throughout most of its present-day distribution.
Genomic insights into the spread of people associated with the Beaker Complex
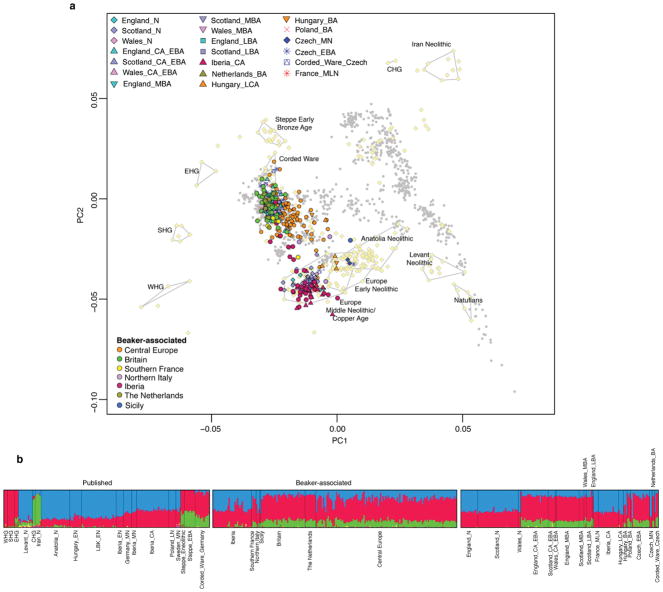
We performed Principal Component Analysis (PCA) by projecting the ancient samples onto a set of west Eurasian present-day populations. We replicated previous findings11 of two parallel clines, with present-day Europeans on one side and present-day Near Easterners on the other (Extended Data Fig. 3a). Individuals associated with the Beaker Complex are strikingly heterogeneous within the European cline—splayed out along the axis of variation defined by Early Bronze Age Yamnaya individuals from the Steppe at one extreme and Middle Neolithic/Copper Age Europeans at the other extreme (Fig. 1c; Extended Data Fig. 3a)—suggesting that the genetic differentiation may be related to variable amounts of Steppe-related ancestry. We obtained qualitatively consistent inferences using ADMIXTURE model-based clustering17. Beaker Complex-associated individuals harboured three main genetic components: one characteristic of European Mesolithic hunter-gatherers, one maximized in Neolithic individuals from the Levant and Anatolia, and one maximized in Neolithic individuals of Iran and present in admixed form in Steppe populations (Extended Data Fig. 3b).
Both PCA and ADMIXTURE are powerful tools for visualizing genetic structure but they do not provide formal tests of admixture between populations. We grouped Beaker Complex individuals based on geographic proximity and genetic similarity (Supplementary Information, section 6), and used qpAdm2 to directly test admixture models and estimate mixture proportions. We modelled their ancestry as a mixture of Mesolithic western European hunter-gatherers (WHG), northwestern Anatolian Neolithic farmers, and Early Bronze Age Steppe populations (the first two of which contributed to earlier Neolithic Europeans). We find that the great majority of sampled Beaker Complex individuals in areas outside of Iberia (with the exception of Sicily) derive a large portion of their ancestry from Steppe populations (Fig. 2a), whereas in Iberia, such ancestry is present in only eight of the 32 analysed individuals, who represent the earliest detection of Steppe-related genomic affinities in this region. We observe differences in ancestry not only at a pan-European scale, but also within regions and even within sites. For instance, at Szigetszentmiklós in Hungary we find roughly contemporary Beaker-associated individuals with very different proportions (from 0% to 75%) of Steppe-related ancestry. This genetic heterogeneity is consistent with early stages of mixture between previously established European Neolithic populations and migrants with Steppe-related ancestry. An implication is that, even at a local scale, the Beaker Complex was associated with people of diverse ancestries.
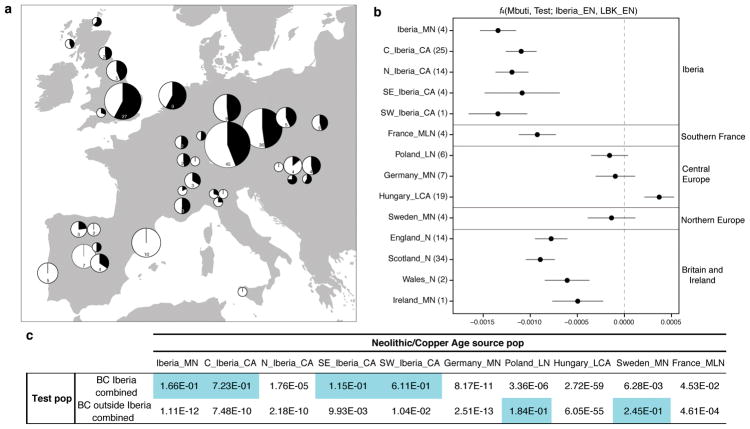
Figure 2. Investigating the genetic makeup of Beaker Complex individuals.
a, Proportion of Steppe-related ancestry (shown in black) in Beaker Complex-associated groups, computed with qpAdm under the model Steppe_EBA + Anatolia_N + WHG. The area of the pie is proportional to the number of individuals (shown inside the pie if more than one). Map data from the R package maps. b, f-statistics of the form f4(Mbuti, Test; Iberia_EN, LBK_EN) computed for European populations (number of individuals for each group is given in parentheses) before the emergence of the Beaker Complex (Supplementary Information section 7). Error bars represent ±1 standard errors. c, Testing different populations as a source for the Neolithic ancestry component in Beaker Complex individuals. The table shows the P-values (highlighted if >0.05) for the fit of the model: Steppe_EBA + Neolithic/Copper Age source population.
While the Steppe-related ancestry in Beaker-associated individuals had a recent origin in the East2,3, the other ancestry component (from previously established European populations) could potentially be derived from several parts of Europe, as genetically closely related groups were widely distributed during the Neolithic and Copper Ages2,4,11,16,18–23. To obtain insight into the origin of this ancestry component in Beaker Complex-associated individuals, we looked for regional patterns of genetic differentiation within Europe during the Neolithic and Copper Age periods. We examined whether populations predating the emergence of the Beaker Complex shared more alleles with Iberian (Iberia_EN) or central European Linearbandkeramik (LBK_EN) Early Neolithic populations. As previously described2, there is genetic affinity to Iberian Early Neolithic farmers in Iberian Middle Neolithic/Copper Age populations, but not in central and northern European Neolithic populations (Fig. 2b). These regional patterns could be partially explained by differential genetic affinities to pre-Neolithic hunter-gatherer individuals from different regions22 (Extended Data Fig. 4). Neolithic individuals from southern France and Britain are also significantly closer to Iberian Early Neolithic farmers than to central European Early Neolithic farmers (Fig. 2b), consistent with the analysis of a Neolithic genome from Ireland23. By modelling Neolithic populations and WHG in an admixture graph framework, we replicate these results and further show that they are not driven by different proportions of hunter-gatherer admixture (Extended Data Fig. 5; Supplementary Information, section 7). Our results suggest that a portion of the ancestry of the Neolithic populations of Britain was derived from migrants who spread along the Atlantic coast. Megalithic tombs document substantial interaction along the Atlantic façade of Europe, and our results are consistent with such interactions reflecting south-to-north movements of people. More data from southern Britain and Ireland and nearby regions in continental Europe will be needed to fully understand the complex interactions between Britain, Ireland, and the continent during the Neolithic24.
The distinctive genetic signatures of pre-Beaker Complex populations in Iberia compared to central Europe allow us to test formally for the origin of the Neolithic-related ancestry in Beaker Complex-associated individuals. We grouped individuals from Iberia (n=32) and from outside Iberia (n=172) to increase power, and evaluated the fit of different Neolithic/Copper Age groups with qpAdm under the model: Steppe_EBA + Neolithic/Copper Age. For Beaker Complex-associated individuals from Iberia, the best fit was obtained when Middle Neolithic and Copper Age populations from the same region were used as the source for their Neolithic-related ancestry, and we could exclude central and northern European populations (P < 0.0063) (Fig. 2c). Conversely, the Neolithic-related ancestry in Beaker Complex individuals outside Iberia was most closely related to central and northern European Neolithic populations with relatively high hunter-gatherer admixture (e.g. Poland_LN, P = 0.18; Sweden_MN, P = 0.25), and we could significantly exclude Iberian sources (P < 0.0104) (Fig. 2c). These results support largely different origins for Beaker Complex-associated individuals, with no discernible Iberia-related ancestry outside Iberia.
Nearly complete turnover of ancestry in Britain
British Beaker Complex-associated individuals (n=37) show strong similarities to central European Beaker Complex-associated individuals in their genetic profile (Extended Data Fig. 3). This observation is not restricted to British individuals associated with the ‘All-Over-Cord’ Beaker pottery style that is shared between Britain and Central Europe, as we also find this genetic signal in British individuals associated with Beaker pottery styles derived from the ‘Maritime’ forms that were the predominant early style in Iberia. The presence of large amounts of Steppe-related ancestry in British Beaker Complex-associated individuals (Fig. 2a) contrasts sharply with Neolithic individuals from Britain (n=51), who have no evidence of Steppe genetic affinities and cluster instead with Middle Neolithic and Copper Age populations from mainland Europe (Extended Data Fig. 3). A previous study showed that Steppe-related ancestry arrived in Ireland by the Bronze Age23, and here we show that – at least in Britain – it arrived earlier in the Copper Age/Beaker period.
Among the different continental Beaker Complex groups analysed in our dataset, individuals from Oostwoud (Province of Noord-Holland, The Netherlands) are the most closely related to the great majority of the Beaker Complex individuals from southern Britain (n=27). The two groups had almost identical Steppe-related ancestry proportions (Fig. 2a), the highest level of shared genetic drift (Extended Data Fig. 6b), and were symmetrically related to most ancient populations (Extended Data Fig. 6a), showing that they are likely derived from the same ancestral population with limited mixture into either group. This does not necessarily imply that the Oostwoud individuals are direct ancestors of the British individuals. However, it shows that they were genetically closely-related to the population (perhaps yet to be sampled) that moved into Britain from continental Europe.
We investigated the magnitude of population replacement in Britain with qpAdm,2 modelling the genome-wide ancestry of Neolithic, Copper and Bronze Age individuals (including Beaker Complex-associated individuals) as a mixture of continental Beaker Complex-associated samples (using the Oostwoud individuals as a surrogate) and the British Neolithic population (Supplementary Information, section 8). During the first centuries after the initial contact (between ~2450–2000 BCE), ancestry proportions were variable (Fig. 3), consistent with migrant communities that were just beginning to mix with the previously established Neolithic population of Britain. After ~2000 BCE, individuals were more homogeneous, with less variation in ancestry proportions and a modest increase in Neolithic-related ancestry (Fig. 3), which could represent admixture with persisting British populations with high levels of Neolithic-related ancestry (or alternatively incoming continental populations with higher proportions of Neolithic-related ancestry). In either case, our results imply a minimum of 90±2% local population turnover by the Middle Bronze Age (~1500–1000 BCE), with no significant decrease observed in 5 samples from the Late Bronze Age. While the exact turnover rate and its geographic pattern will be refined with more ancient samples, our results imply that for individuals from Britain during and after the Beaker period, a very high fraction of their DNA derives from ancestors who lived in continental Europe prior to 2450 BCE. An independent line of evidence for population turnover comes from uniparental markers. While Y-chromosome haplogroup R1b was completely absent in Neolithic individuals (n=33), it represents more than 90% of the Y-chromosomes during Copper and Bronze Age Britain (n=52) (Fig. 3). The introduction of new mtDNA haplogroups such as I, R1a and U4, which were present in Beaker-associated populations from continental Europe but not in Neolithic Britain (Supplementary Table 3), suggests that both men and women were involved.
Figure 3. Population transformation in Britain associated with the arrival of the Beaker Complex.
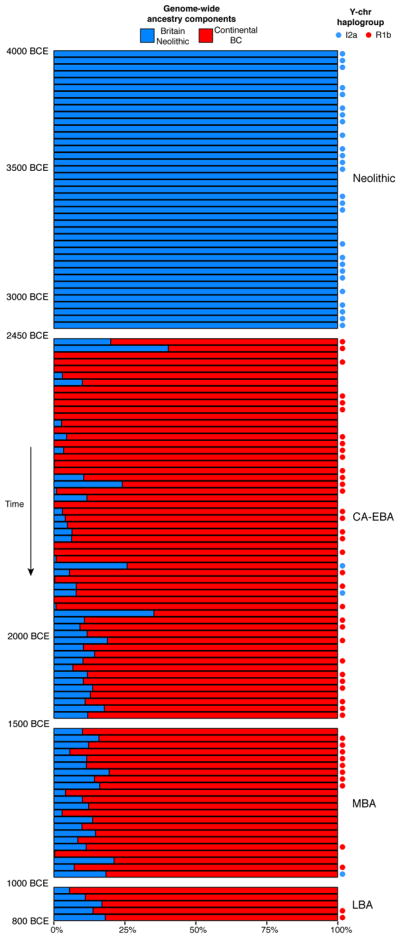
Modelling Neolithic, Copper and Bronze Age (including Beaker Complex-associated) individuals from Britain as a mixture of continental Beaker Complex-associated individuals (red) and the Neolithic population from Britain (blue). Each bar represents genome-wide mixture proportions for one individual. Individuals are ordered chronologically and included in the plot if represented by more than 100,000 SNPs. Circles indicate the Y-chromosome haplogroup for male individuals.
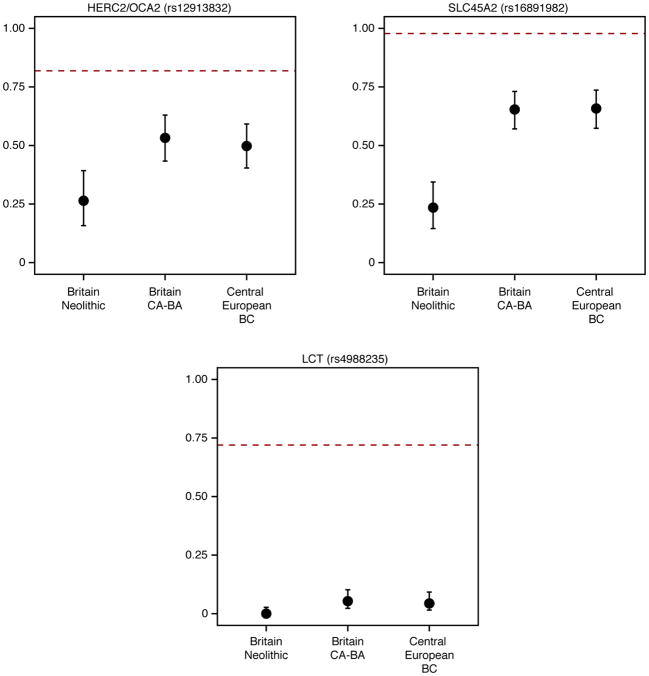
Our genetic time transect in Britain also allowed us to track the frequencies of alleles with known phenotypic effects. Derived alleles at rs16891982 (SLC45A2) and rs12913832 (HERC2/OCA2), which contribute to reduced skin and eye pigmentation in Europeans, dramatically increased in frequency between the Neolithic period and the Beaker and Bronze Age periods (Extended Data Fig. 7). Thus, the arrival of migrants associated with the Beaker Complex significantly altered the pigmentation phenotypes of British populations. However, the lactase persistence allele at SNP rs4988235 remained at very low frequencies across this transition, both in Britain and continental Europe, showing that the major increase in its frequency occurred in the last 3,500 years3,4,25.
Discussion
The term ‘Bell Beaker’ was introduced by late 19th-century and early 20th-century archaeologists to refer to the distinctive pottery style found across western and central Europe at the end of the Neolithic, initially hypothesized to have been spread by a genetically homogeneous population. This idea of a ‘Beaker Folk’ became unpopular after the 1960s as scepticism grew about the role of migration in mediating change in archaeological cultures26, although J.G.D. Clark speculated that the Beaker Complex expansion into Britain was an exception27, a prediction that has now been borne out by ancient genomic data.
The expansion of the Beaker Complex cannot be described by a simple one-to-one mapping of an archaeologically defined material culture to a genetically homogeneous population. This stands in contrast to other archaeological complexes, notably the Linearbandkeramik first farmers of central Europe2, the Early Bronze Age Yamnaya of the Steppe2,3, and to some extent the Corded Ware Complex of central and eastern Europe2,3. Our results support a model in which cultural transmission and human migration both played important roles, with the relative balance of these two processes depending on the region. In Iberia, the majority of Beaker-associated individuals lacked Steppe affinities and were genetically most similar to preceding Iberian populations. In central Europe, Steppe-related ancestry was widespread and we can exclude a substantial contribution from Iberian Beaker-associated individuals. The presence of Steppe-related ancestry in some Iberian individuals demonstrates that gene-flow into Iberia was, however, not uncommon during this period. These results contradict initial suggestions of gene flow into central Europe based on analysis of mtDNA28 and dental morphology29. In particular, mtDNA haplogroups H1 and H3 were proposed as markers for an out-of-Iberia Beaker expansion28, yet H3 is absent among our Iberian Beaker-associated individuals.
In other parts of Europe, the Beaker Complex expansion was driven to a substantial extent by migration. This genomic transformation is clearest in Britain due to our densely sampled time transect. The arrival of people associated with the Beaker Complex precipitated a profound demographic transformation in Britain, exemplified by the presence of individuals with large amounts of Steppe-related ancestry after 2450 BCE. We considered the possibility that an uneven geographic distribution of samples could have caused us to miss a major population lacking Steppe-derived ancestry after 2450 BCE. However, our British Beaker and Bronze Age samples are dispersed geographically, extending from England’s southeastern peninsula to the Western Isles of Scotland, and come from a wide variety of funerary contexts (rivers, caves, pits, barrows, cists and flat graves) and diverse funerary traditions (single and multiple burials in variable states of anatomical articulation), reducing the likelihood that our sampling missed major populations. We also considered the possibility that different burial practices between local and incoming populations (cremation versus inhumation) during the early stages of interaction, could result in a sampling bias against local individuals. While it is possible that such a sampling bias makes the ancestry transition appear more sudden than it in fact was, the long-term demographic impact was clearly profound, as the pervasive Steppe-related ancestry observed during the Beaker period and absent in the Neolithic persisted during the Bronze Age, and indeed remains predominant in Britain today2. These results are notable in light of strontium and oxygen isotope analyses of British skeletons from the Beaker and Bronze Age periods30, which have provided no evidence of substantial mobility over individuals’ lifetimes from locations with cooler climates or from places with geologies atypical of Britain. However, the isotope data are only sensitive to first-generation migrants and do not rule out movements from regions such as the lower Rhine area or from other geologically similar regions for which DNA sampling is still sparse. Further sampling of regions on the European continent may reveal additional candidate sources.
By analysing DNA data from ancient individuals, we have been able to provide constraints on the interpretations of the processes underlying cultural and social changes in Europe during the third millennium BCE. Our results motivate further archaeological research to identify the changes in social organization, technology, subsistence, climate, population sizes31 or pathogen exposure32,33 that could have precipitated the demographic changes uncovered in this study.
Methods
Ancient DNA analysis
We screened skeletal samples for DNA preservation in dedicated clean rooms. We extracted DNA34–36 and prepared barcoded next generation sequencing libraries, the majority of which were treated with uracil-DNA glycosylase to greatly reduce the damage (except at the terminal nucleotide) that is characteristic of ancient DNA37,38 (Supplementary Information, section 4). We initially enriched libraries for sequences overlapping the mitochondrial genome39 and ~3000 nuclear SNPs using synthesized baits (CustomArray Inc.) that we PCR amplified. We sequenced the enriched material on an Illumina NextSeq instrument with 2x76 cycles, and 2x7 cycles to read out the two indices40. We merged read pairs with the expected barcodes that overlapped by at least 15 bases, mapped the merged sequences to hg19 and to the reconstructed mitochondrial DNA consensus sequence41 using the samse command in bwa (v0.6.1)42, and removed duplicated sequences. We evaluated DNA authenticity by estimating the rate of mismatching to the consensus mitochondrial sequence43, and also requiring that the rate of damage at the terminal nucleotide was at least 3% for UDG-treated libraries43 and 10% for non-UDG-treated libraries44.
For libraries that were promising after screening, we enriched in two consecutive rounds for sequences overlapping 1,233,013 SNPs (‘1240k SNP capture’)2,10 and sequenced 2x76 cycles and 2x7 cycles on an Illumina NextSeq500 instrument. We processed the data bioinformatically as for the mitochondrial capture data, this time mapping only to the human reference genome hg19 and merging the data from different libraries of the same individual. We further evaluated authenticity by studying the ratio of X-to-Y chromosome reads and estimating X-chromosome contamination in males based on the rate of heterozygosity45. Samples with evidence of contamination were either filtered out or restricted to sequences with terminal cytosine deamination to remove sequences that derived from modern contaminants. Finally, we filtered out from our genome-wide analysis dataset samples with fewer than 10,000 targeted SNPs covered at least once and samples that were first-degree relatives of others in the dataset (keeping the sample with the larger number of covered SNPs) (Supplementary Table 1).
Mitochondrial haplogroup determination
We used the mitochondrial capture bam files to determine the mitochondrial haplogroup of each sample with new data, restricting to sequences with MAPQ≥30 and base quality ≥30. First, we constructed a consensus sequence with samtools and bcftools46, using a majority rule and requiring a minimum coverage of 2. We called haplogroups with HaploGrep247 based on phylotree48 (mtDNA tree Build 17 (18 Feb 2016)). Mutational differences compared to the revised Cambridge Reference Sequence (rCRS) and corresponding haplogroups can be viewed in Supplementary Table 2. We computed haplogroup frequencies for relevant ancient populations (Supplementary Table 3) after removing close relatives with the same mtDNA.
Y-chromosome analysis
We determined Y-chromosome haplogroups for both new and published samples (Supplementary Information, section 5). We made use of the sequences mapping to 1240k Y-chromosome targets, restricting to sequences with mapping quality ≥30 and bases with quality ≥30. We called haplogroups by determining the most derived mutation for each sample, using the nomenclature of the International Society of Genetic Genealogy (http://www.isogg.org) version 11.110 (21 April 2016). Haplogroups and their supporting derived mutations can be viewed in Supplementary Table 4.
Merging newly generated data with published data
We assembled two datasets for genome-wide analyses:
HO includes 2,572 present-day individuals from worldwide populations genotyped on the Human Origins Array11,12,49 and 683 ancient individuals. The ancient set includes 211 Beaker Complex individuals (195 newly reported, 7 with shotgun data3 for which we generated 1240k capture data and 9 previously published3,4), 68 newly reported individuals from relevant ancient populations and 298 previously published12,18,19,21–23,50–57 individuals (Supplementary Table 1). We kept 591,642 autosomal SNPs after intersecting autosomal SNPs in the 1240k capture with the analysis set of 594,924 SNPs from Lazaridis et al.11.
HOIll includes the same set of ancient samples and 300 present-day individuals from 142 populations sequenced to high coverage as part of the Simons Genome Diversity Project13. For this dataset, we used 1,054,671 autosomal SNPs, excluding SNPs of the 1240k array located on sex chromosomes or with known functional effects.
For each individual, we represented the allele at each SNP by randomly sampling one sequence, discarding the first and the last two nucleotides of each sequence.
Abbreviations
We used the following abbreviations in population labels: E, Early; M, Middle; L, Late; N, Neolithic; CA, Copper Age; BA, Bronze Age; BC, Beaker complex; N_Iberia, Northern Iberia; C_Iberia, Central Iberia; SE_Iberia, Southeast Iberia; SW_Iberia, Southwest Iberia.
Principal component analysis
We carried out principal component analysis (PCA) on the HO dataset using the smartpca program in EIGENSOFT58. We computed principal components on 990 present-day West Eurasians and projected ancient individuals using lsqproject: YES and shrinkmode: YES.
ADMIXTURE analysis
We performed model-based clustering analysis using ADMIXTURE17 on the HO reference dataset, including 2,572 present-day individuals from worldwide populations and the ancient individuals. First, we carried out LD-pruning on the dataset using PLINK59 with the flag --indep-pairwise 200 25 0.4, leaving 306,393 SNPs. We ran ADMIXTURE with the cross validation (--cv) flag specifying from K=2 to K=20 clusters, with 20 replicates for each value of K and keeping for each value of K the replicate with highest log likelihood. In Extended Data Fig. 3b we show the cluster assignments at K=8 of newly reported individuals and other relevant ancient samples for comparison. We chose this value of K as it was the lowest one for which components of ancestry related both to Iranian Neolithic farmers and European Mesolithic hunter-gatherers were maximized.
f-statistics
We computed f-statistics on the HOIll dataset using ADMIXTOOLS49 with default parameters (Supplementary Information, section 6). We used qpDstat with f4mode:Yes for f4-statistics and qp3Pop for outgroup f3-statistics. We computed standard errors using a weighted block jackknife60 over 5 Mb blocks.
Inference of mixture proportions
We estimated ancestry proportions on the HOIll dataset using qpAdm2 and a basic set of 9 Outgroups: Mota, Ust_Ishim, MA1, Villabruna, Mbuti, Papuan, Onge, Han, Karitiana. For some analyses (Supplementary Information, section 8) we added additional outgroups to this basic set.
Admixture graph modelling
We modelled the relationships between populations in an Admixture Graph framework with the software qpGraph in ADMIXTOOLS49, using the HOIll dataset and Mbuti as an outgroup (Supplementary Information, section 7).
Allele frequency estimation from read counts
We used allele counts at each SNP to perform maximum likelihood estimation of allele frequencies in ancient populations as in ref.4. In Extended Data Fig. 7, we show derived allele frequency estimates at three SNPs of functional importance for different ancient populations.
Data availability
All 1240k and mitochondrial capture sequencing data are available from the European Nucleotide Archive, accession number PRJEB23635.
The genotype dataset is available from the Reich Lab website at https://reich.hms.harvard.edu/datasets.
Extended Data
Extended Data Figure 1. Beaker complex artefacts.
a, ‘All-Over-Cord’ Beaker from Bathgate, West Lothian, Scotland. Photo: ãNational Museums Scotland. b, Beaker Complex grave goods from La Sima III barrow, Soria, Spain61. The set includes Beaker pots of the so-called ‘Maritime style’. Photo: Junta de Castilla y León, Archivo Museo Numantino, Alejandro Plaza.
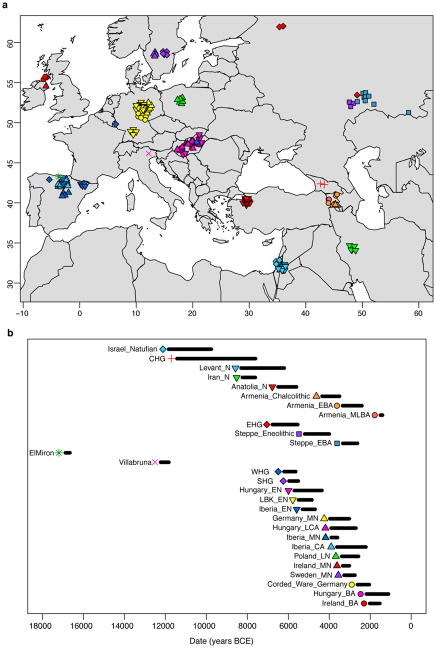
Extended Data Figure 2. Ancient individuals with previously published genome-wide data used in this study.
a, Sampling locations. b, Time ranges. W/E/S/CHG, Western/Eastern/Scandinavian/Caucasus hunter-gatherers; E, Early; M, Middle; L, Late; N, Neolithic; CA, Copper Age; BA, Bronze Age. Map data from the R package maps.
Extended Data Figure 3. Population structure.
a, Principal component analysis of 990 present-day West Eurasian individuals (grey dots), with previously published (pale yellow) and new ancient samples projected onto the first two principal components. b, ADMIXTURE clustering analysis with k=8 showing ancient individuals. W/E/S/CHG, Western/Eastern/Scandinavian/Caucasus hunter-gatherers; E, Early; M, Middle; L, Late; N, Neolithic; CA, Copper Age; BA, Bronze Age.
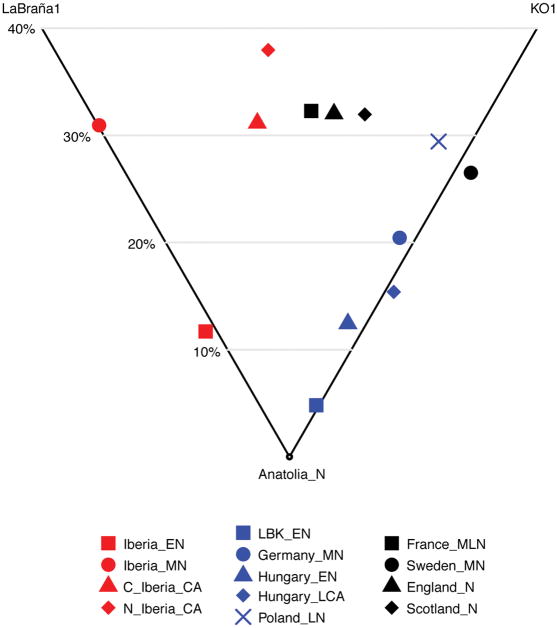
Extended Data Figure 4. Hunter-gatherer affinities in Neolithic/Copper Age Europe.
Differential affinity to hunter-gatherer individuals (LaBraña156 from Spain and KO162 from Hungary) in European populations before the emergence of the Beaker Complex. See Supplementary Information, section 8 for mixture proportions and standard errors computed with qpAdm. E, Early; M, Middle; L, Late; N, Neolithic; CA, Copper Age; BA, Bronze Age; N_Iberia, Northern Iberia; C_Iberia, Central Iberia.
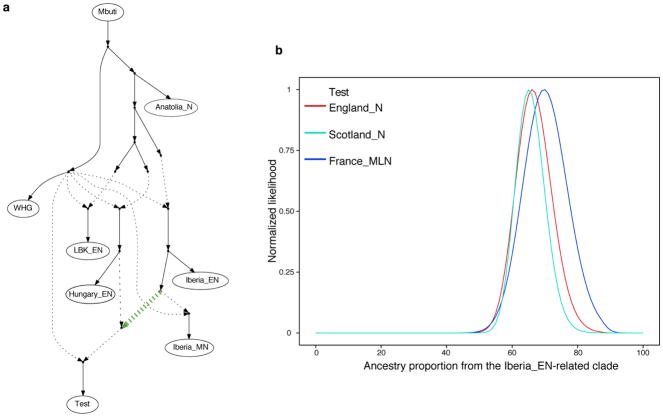
Extended Data Figure 5. Modelling the relationships between Neolithic populations.
a, Admixture graph fitting a Test population as a mixture of sources related to both Iberia_EN and Hungary_EN. b, Likelihood distribution for models with different proportions of the source related to Iberia_EN (green admixture edge in (a)) when Test is England_N, Scotland_N or France_MLN. E, Early; M, Middle; L, Late; N, Neolithic.
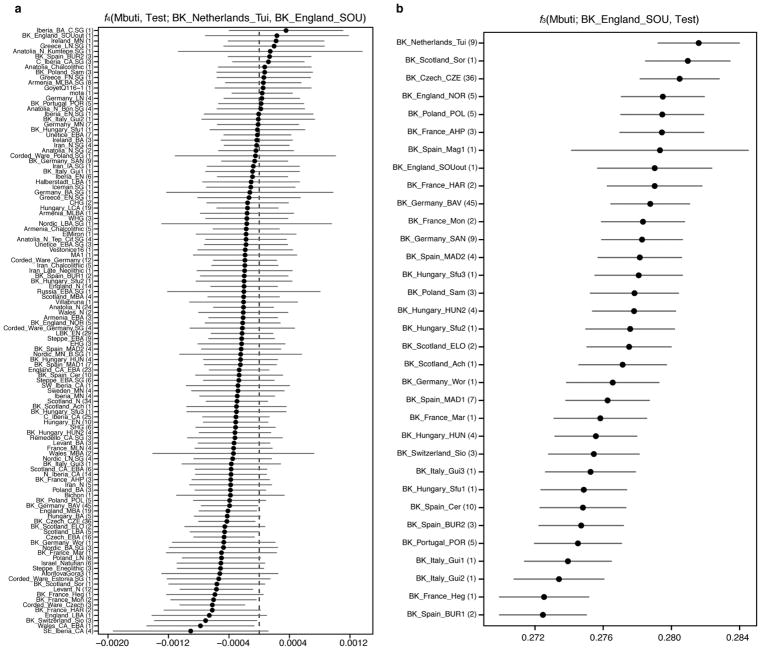
Extended Data Figure 6. Genetic affinity between Beaker Complex-associated individuals from southern England and the Netherlands.
a, f-statistics of the form f4(Mbuti, Test; BK_Netherlands_Tui, BK_England_SOU). Negative values indicate that Test is closer to BK_Netherlands_Tui than to BK_England_SOU, and the opposite for positive values. Error bars represent ±3 standard errors. b, Outgroup-f3 statistics of the form f3(Mbuti; BK_England_SOU, Test) measuring shared genetic drift between BK_England_SOU and other Beaker Complex-associated groups. Error bars represent ±1 standard errors. Number of individuals for each group is given in parentheses. BK_Netherlands_Tui, Beaker-associated individuals from De Tuithoorn, Oostwoud, the Netherlands; BK_England_SOU, Beaker-associated individuals from southern England. See Supplementary Table 1 for individuals associated to each population label.
Extended Data Figure 7. Derived allele frequencies at three SNPs of functional importance.
Error bars represent 1.9-log-likelihood support interval. The red dashed lines show allele frequencies in the 1000 Genomes GBR population (present-day people from Great Britain). Sample sizes are 50, 98, and 117 for Britain Neolithic, Britain CA-BA and Central European BC, respectively. BC, Beaker Complex; CA, Copper Age; BA, Bronze Age.
Extended Data Table 1.
Sites from outside Britain with new genome-wide data reported in this study.
| Site | N | Approx. date range (BCE) | Country |
|---|---|---|---|
| Brandysek | 12 | 2900–2200 | Czech Republic |
| Kněževes | 2 | 2500–1900 | Czech Republic |
| Lochenice | 1 | 2500–1900 | Czech Republic |
| Lovosice II | 1 | 2500–1900 | Czech Republic |
| Moravská Nová Ves | 4 | 2300–1900 | Czech Republic |
| Prague 5 - Malá Ohrada | 1 | 2500–2200 | Czech Republic |
| Prague 5, Jinonice | 14 | 2200–1700 | Czech Republic |
| Prague 8, Kobylisy, Ke Stírce Street | 12 | 2500–1900 | Czech Republic |
| Radovesice | 13 | 2500–2200 | Czech Republic |
| Veiké Přílepy | 3 | 2500–1900 | Czech Republic |
| Clos de Roque, Saint Maximin-la-Sainte-Baume | 3 | 4700–4500 | France |
| Collet Redon, La Couronne-Martigues | 1 | 3500–3100 | France |
| Hégenheim Necropole, Haut-Rhin | 1 | 2800–2500 | France |
| La Fare, Forcalquier | 1 | 2500–2200 | France |
| Marlens, Sur les Barmes, Haute-Savoie | 1 | 2500–2100 | France |
| Mondelange, PAC de la Sente, Moselle | 2 | 2400–1900 | France |
| Rouffach, Haut-Rhin | 1 | 2300–2100 | France |
| Sierentz, Les Villas d’Aurele, Haut-Rhin | 2 | 2600–2300 | France |
| Villard, Lauzet-Ubaye | 2 | 2200–1900 | France |
| Alburg-Lerchenhaid, Spedition Häring, Bavaria | 13 | 2500–2100 | Germany |
| Augsburg Sportgelände, Augsburg, Bavaria | 6 | 2500–2000 | Germany |
| Hugo-Eckener-Straße, Augsburg, Bavaria | 3 | 2500–2000 | Germany |
| Irlbach, County of Straubing-Bogen, Bavaria | 17 | 2500–2000 | Germany |
| Künzing-Bruck, Lkr. Deggendorf, Bavaria | 3 | 2500–2000 | Germany |
| Landau an der Isar, Bavaria | 5 | 2500–2000 | Germany |
| Manching-Oberstimm, Bavaria | 2 | 2500–2000 | Germany |
| Osterhofen-Altenmarkt, Bavaria | 4 | 2600–2000 | Germany |
| Unterer Talweg 58–62, Augsburg, Bavaria | 2 | 2500–2200 | Germany |
| Unterer Talweg 85, Augsburg, Bavaria | 1 | 2400–2100 | Germany |
| Weichering, Bavaria | 4 | 2500–2000 | Germany |
| Worms-Herrnsheim, Rhineland-Palatinate | 1 | 2500–2000 | Germany |
| Budakalász, Csajerszke (M0 Site 12) | 2 | 2600–2200 | Hungary |
| Budapest-Békásmegyer | 3 | 2500–2100 | Hungary |
| Mezőcsát-Hörcsögös | 4 | 3400–3000 | Hungary |
| Szigetszentmiklós-Üdülősor | 4 | 2500–2200 | Hungary |
| Szigetszentmiklós,Felső Ürge-hegyi dűlő | 6 | 2500–2200 | Hungary |
| Pergole 2, Partanna, Sicily | 3 | 2500–1900 | Italy |
| Via Guidorossi, Parma, Emilia Romagna | 3 | 2200–1900 | Italy |
| Dzielnica | 1 | 2300–2000 | Poland |
| Iwiny | 1 | 2300–2000 | Poland |
| Jordanów Œląqski | 1 | 2300–2200 | Poland |
| Kornice | 4 | 2500–2100 | Poland |
| Racibórz-Stara Wieś | 1 | 2300–2000 | Poland |
| Samborzec | 3 | 2500–2100 | Poland |
| Strachów | 1 | 2000–1800 | Poland |
| Żerniki Wielkie | 1 | 2300–2100 | Poland |
| Bolores, Estremadura | 1 | 2800–2600 | Portugal |
| Cova da Moura, Torres Vedras | 1 | 2300–2100 | Portugal |
| Galeria da Cisterna, Almonda | 2 | 2500–2200 | Portugal |
| Verdelha dos Ruivos, District of Lisbon | 3 | 2700–2300 | Portugal |
| Arroyal I, Burgos | 5 | 2600–2200 | Spain |
| Camino de las Yeseras, Madrid | 14 | 2800–1700 | Spain |
| Camino del Molino, Caravaca, Murcia | 4 | 2900–2100 | Spain |
| Humanejos, Madrid | 11 | 2900–2000 | Spain |
| La Magdalena, Madrid | 3 | 2500–2000 | Spain |
| Paris Street, Cerdanyola, Barcelona | 10 | 2900–2300 | Spain |
| Virgazal, Tablada de Rudrón, Burgos | 1 | 2300–2000 | Spain |
| Sion-Petit-Chasseur, Dolmen XI | 3 | 2500–2000 | Switzerland |
| De Tuithoorn, Oostwoud, Noord-Holland | 11 | 2600–1600 | The Netherlands |
Extended Data Table 2.
Sites from Britain with new genome-wide data reported in this study.
| Site | N | Approx. date range (BCE) | Country |
|---|---|---|---|
| Abingdon Spring Road cemetery, Oxfordshire, England | 1 | 2500–2200 | Great Britain |
| Amesbury Down, Wiltshire, England | 13 | 2500–1300 | Great Britain |
| Banbury Lane, Northamptonshire, England | 3 | 3400–3100 | Great Britain |
| Barrow Hills, Radley, Oxfordshire, England | 1 | 2300–1800 | Great Britain |
| Barton Stacey, Hampshire, England | 1 | 2200–2000 | Great Britain |
| Baston and Langtoft, South Lincolnshire, England | 2 | 1700–1600 | Great Britain |
| Biddenham Loop, Bedfordshire, England | 9 | 1600–1300 | Great Britain |
| Boscombe Airfield, Wiltshire, England | 1 | 1800–1600 | Great Britain |
| Canada Farm, Sixpenny Handley, Dorset, England | 2 | 2500–2300 | Great Britain |
| Carsington Pasture Cave, Derbyshire, England | 2 | 3700–2000 | Great Britain |
| Central Flying School, Upavon, Wiltshire, England | 1 | 2500–1800 | Great Britain |
| Cissbury Flint Mine, Worthing, West Sussex, England | 1 | 3600–3400 | Great Britain |
| Clay Farm, Cambridgeshire, England | 2 | 1400–1300 | Great Britain |
| Dairy Farm, Willington, England | 1 | 2300–1900 | Great Britain |
| Ditchling Road, Brighton, Sussex, England | 1 | 2500–1900 | Great Britain |
| Eton Rowing Course, Buckinghamshire, England | 2 | 3600–2900 | Great Britain |
| Flying School, Netheravon, Wiltshire, England | 2 | 2500–1800 | Great Britain |
| Fussell’s Lodge, Salisbury, Wiltshire, England | 2 | 3800–3600 | Great Britain |
| Lesser Kelco Cave, Giggleswick Scar, North Yorkshire, England | 1 | 3700–3500 | Great Britain |
| Hasting Hill, Sunderland, Tyne and Wear, England | 2 | 2500–1800 | Great Britain |
| Hexham Golf Course, Northumberland, England | 1 | 2000–1800 | Great Britain |
| Low Hauxley, Northumberland, England | 2 | 2100–1600 | Great Britain |
| Melton Quarry, East Riding of Yorkshire, England | 1 | 1900–1700 | Great Britain |
| Neale’s Cave, Paington, Devon, England | 1 | 2000–1600 | Great Britain |
| Nr. Ablington, Figheldean, England | 1 | 2500–1800 | Great Britain |
| Nr. Millbarrow, Wiltshire, England | 1 | 3600–3400 | Great Britain |
| Over Narrows, Needingworth Quarry, England | 5 | 2200–1300 | Great Britain |
| Porton Down, Wiltshire, England | 2 | 2500–1900 | Great Britain |
| Raven Scar Cave, Ingleton, North Yorkshire, England | 1 | 1100–900 | Great Britain |
| Reaverhill, Barrasford, Northumberland, England | 1 | 2100–2000 | Great Britain |
| River Thames, Mortlake/Syon Reach, London, England | 2 | 2500–1700 | Great Britain |
| Staxton Beacon, Staxton, England | 1 | 2400–1600 | Great Britain |
| Summerhill, Blaydon, Tyne and Wear, England | 1 | 1900–1700 | Great Britain |
| East Kent Access (Phase II), Thanet, Kent, England | 4 | 2100–1700 | Great Britain |
| Totty Pot, Cheddar, Somerset, England | 1 | 2800–2500 | Great Britain |
| Trumpington Meadows, Cambridge, England | 2 | 2200–2000 | Great Britain |
| Turners Yard, Fordham, Cambridgeshire, England | 1 | 1700–1500 | Great Britain |
| Upper Swell, Chipping Norton, Gloucestershire, England | 1 | 4000–3300 | Great Britain |
| Waterhall Farm, Chippenham, Cambridgeshire, England | 1 | 2000–1700 | Great Britain |
| West Deeping, Lincolnshire, England | 1 | 2300–2000 | Great Britain |
| Whitehawk, Brighton, Sussex, England | 1 | 3700–3400 | Great Britain |
| Wick Barrow, Stogursey, Somerset, England | 1 | 2400–2000 | Great Britain |
| Wilsford Down, Wilsford-cum-Lake, Wiltshire, England | 2 | 2400–2000 | Great Britain |
| Windmill Fields, Stockton-on-Tees, North Yorkshire, England | 4 | 2300–2000 | Great Britain |
| Yarnton, Oxfordshire, England | 4 | 2500–1900 | Great Britain |
| Aberdour Road, Dunfermline, Fife, Scotland | 1 | 2000–1800 | Great Britain |
| Achavanich, Wick, Highland, Scotland | 1 | 2500–2100 | Great Britain |
| Boatbridge Quarry, Thankerton, Scotland | 1 | 2400–2100 | Great Britain |
| Clachaig, Arran, North Ayrshire, Scotland | 1 | 3500–3400 | Great Britain |
| Covesea Cave 2, Moray, Scotland | 3 | 2100–800 | Great Britain |
| Covesea Caves, Moray, Scotland | 2 | 1000–800 | Great Britain |
| Distillery Cave, Oban, Argyll and Bute, Scotland | 3 | 3800–3400 | Great Britain |
| Doune, Perth and Kinross, Scotland | 1 | 1800–1600 | Great Britain |
| Dryburn Bridge, East Lothian, Scotland | 2 | 2300–1900 | Great Britain |
| Eweford Cottages, East Lothian, Scotland | 1 | 2100–1900 | Great Britain |
| Holm of Papa Westray North, Orkney, Scotland | 4 | 3500–3100 | Great Britain |
| Isbister, Orkney, Scotland | 10 | 3300–2300 | Great Britain |
| Leith, Merrilees Close, City of Edinburgh, Scotland | 2 | 1600–1500 | Great Britain |
| Longniddry, Evergreen House, Coast Road, East Lothian, Scotlan | 3 | 1500–1300 | Great Britain |
| Longniddry, Grainfoot, East Lothian, Scotland | 1 | 1300–1000 | Great Britain |
| Macarthur Cave, Oban, Argyll and Bute, Scotland | 1 | 4000–3800 | Great Britain |
| Pabay Mor, Lewis, Western Isles, Scotland | 1 | 1400–1300 | Great Britain |
| Point of Cott, Orkney, Scotland | 2 | 3700–3100 | Great Britain |
| Quoyness, Orkney, Scotland | 1 | 3100–2900 | Great Britain |
| Raschoille Cave, Oban, Argyll and Bute, Scotland | 9 | 4000–2900 | Great Britain |
| Sorisdale, Coll, Argyll and Bute, Scotland | 1 | 2500–2100 | Great Britain |
| Stenchme, Lop Ness, Orkney, Scotland | 1 | 2000–1500 | Great Britain |
| Thurston Mains, Innerwick, East Lothian, Scotland | 1 | 2300–2000 | Great Britain |
| Tulach an t’Sionnach, Highland, Scotland | 1 | 3700–3500 | Great Britain |
| Tulloch of Assery A, Highland, Scotland | 1 | 3700–3400 | Great Britain |
| Tulloch of Assery B, Highland, Scotland | 1 | 3800–3600 | Great Britain |
| Unstan, Orkney, Scotland | 1 | 3400–3100 | Great Britain |
| Culver Hole Cave, Port Eynon, West Glamorgan, Wales | 1 | 1600–800 | Great Britain |
| Great Orme Mines, Llandudno, North Wales | 1 | 1700–1600 | Great Britain |
| North Face Cave, Llandudno, North Wales | 1 | 1400–1200 | Great Britain |
| Rhos Ddigre, Llanarmon-yn-lâl, Denbighshire, Wales | 1 | 3100–2900 | Great Britain |
| Tinkinswood, Cardiff, Glamorgan, Wales | 1 | 3800–3600 | Great Britain |
Extended Data Table 3.
111 newly reported radiocarbon dates.
| Sample | Date | Location | Country |
|---|---|---|---|
| I5024 | 2278–2032 calBCE (3740±35 BP, Poz-84460) | Knĕževes | Czech Republic |
| I4946 | 2296–2146 calBCE (3805±20 BP, PSUAMS-2801) | Prague 5, Jinonice, Butovická Street | Czech Republic |
| I4895 | 2273–2047 calBCE (3750±20 BP, PSUAMS-2852) | Prague 5, Jinonice, Butovická Street | Czech Republic |
| I4896 | 2288–2142 calBCE (3785±20 BP, PSUAMS-2853) | Prague 5, Jinonice, Butovická Street | Czech Republic |
| I4884 | 1882–1745 calBCE (3480±20 BP, PSUAMS-2842) | Prague 8, Kobylisy, Ke Stírce Street | Czech Republic |
| I4885 | 2289–2143 calBCE (3790±20 BP, PSUAMS-2843) | Prague 8, Kobylisy, Ke Stírce Street | Czech Republic |
| I4886 | 2205–2042 calBCE (3740±20 BP, PSUAMS-2844) | Prague 8, Kobylisy, Ke Stírce Street | Czech Republic |
| I4887 | 2201–2039 calBCE (3730±20 BP, PSUAMS-2845) | Prague 8, Kobylisy, Ke Stírce Street | Czech Republic |
| I4888 | 2190–2029 calBCE (3700±20 BP, PSUAMS-2846) | Prague 8, Kobylisy, Ke Stírce Street | Czech Republic |
| I4889 | 2281–2062 calBCE (3765±20 BP, PSUAMS-2847) | Prague 8, Kobylisy, Ke Stírce Street | Czech Republic |
| I4891 | 2281–2062 calBCE (3765±20 BP, PSUAMS-2848) | Prague 8, Kobylisy, Ke Stírce Street | Czech Republic |
| I4892 | 1881–1701 calBCE (3475±20 BP, PSUAMS-2849) | Prague 8, Kobylisy, Ke Stírce Street | Czech Republic |
| I4893 | 4449–4348 calBCE (5550±20 BP, PSUAMS-2850) | Prague 8, Kobylisy, Ke Stírce Street | Czech Republic |
| I4894 | 4488–4368 calBCE (5610±20 BP, PSUAMS-2851) | Prague 8, Kobylisy, Ke Stírce Street | Czech Republic |
| I4945 | 2291–2144 calBCE (3795±20 BP, PSUAMS-2854) | Prague 8, Kobylisy, Ke Stírce Street | Czech Republic |
| I4305 | 4825–4616 calBCE (5860±35 BP, PSUAMS-2225) | Clos de Roque, Saint Maximin-la-Sainte-Baume | France |
| I4304 | 4787–4589 calBCE (5830±35 BP, PSUAMS-2226) | Clos de Roque, Saint Maximin-la-Sainte-Baume | France |
| I4303 | 4778–4586 calBCE (5820±30 BP, PSUAMS-2260) | Clos de Roque, Saint Maximin-la-Sainte-Baume | France |
| I1392 | 2833–2475 calBCE (4047±29 BP, MAMS-25935) | Hégenheim Necropole, Haut-Rhin | France |
| I3875 | 2133–1946 calBCE (3655±25 BP, PSUAMS-1834) | Villard, Lauzet-Ubaye | France |
| I3874 | 2200–2035 calBCE (3725±25 BP, PSUAMS-1835) | Villard, Lauzet-Ubaye | France |
| I3593 | 2397–2145 calBCE (3817±26 BP, BRAMS-1215) | Alburg-Lerchenhaid, Spedition Häring, Stkr. Straubing, Bavaria | Germany |
| I3590 | 2335–2140 calBCE (3802±26 BP, BRAMS-1217) | Alburg-Lerchenhaid, Spedition Häring, Stkr. Straubing, Bavaria | Germany |
| I3592 | 2457–2203 calBCE (3844±33 BP, BRAMS-1218) | Alburg-Lerchenhaid, Spedition Häring, Stkr. Straubing, Bavaria | Germany |
| I5017 | 2460–2206 calBCE (3855±35 BP, Poz-84458) | Augsburg Sportgelände, Augsburg, Bavaria | Germany |
| I4250 | 2433–2149 calBCE (3825±26 BP, BRAMS-1219) | Irlbach, County of Straubing-Bogen, Bavaria | Germany |
| I5021 | 2571–2341 calBCE (3955±35 BP, Poz-84553) | Osterhofen-Altenmarkt, Bavaria | Germany |
| E09537_d | 2471–2298 calBCE (3909±29 BP, MAMS-29074) | Unterer Talweg 58–62, Augsburg, Bavaria | Germany |
| E09538 | 2464–2210 calBCE (3870±30 BP, MAMS-29075) | Unterer Talweg 58–62, Augsburg, Bavaria | Germany |
| I5385 | 2455–2147 calBCE (3827±33 BP, SUERC-71005) | Achavanich, Wick, Highland, Scotland | Great Britain |
| I2457 | 2199–2030 calBCE (3717±28 BP, SUERC-69975) | Amesbury Down, Wiltshire, England | Great Britain |
| I2416 | 2455–2151 calBCE (3830±30 BP, Beta-432804) | Amesbury Down, Wiltshire, England | Great Britain |
| I2596 | 2273–2034 calBCE (3739±30 BP, NZA-32484) | Amesbury Down, Wiltshire, England | Great Britain |
| I2566 | 2204–2035 calBCE (3734±25 BP, NZA-32490) | Amesbury Down, Wiltshire, England | Great Britain |
| I2598 | 2135–1953 calBCE (3664±30 BP, NZA-32494) | Amesbury Down, Wiltshire, England | Great Britain |
| I2418 | 2455–2200 calBCE (3836±25 BP, NZA-32788) | Amesbury Down, Wiltshire, England | Great Britain |
| I2565 | 2457–2147 calBCE (3829±38 BP, OxA-13562) | Amesbury Down, Wiltshire, England | Great Britain |
| I2457 | 2467–2290 calBCE (3890±30 BP, SUERC-36210) | Amesbury Down, Wiltshire, England | Great Britain |
| I2460 | 2022–1827 calBCE (3575±27 BP, SUERC-53041) | Amesbury Down, Wiltshire, England | Great Britain |
| I2459 | 2455–2150 calBCE (3829±30 BP, SUERC-54823) | Amesbury Down, Wiltshire, England | Great Britain |
| I5373 | 2194–1980 calBCE (3694±25 BP, BRAMS-1230) | Carsington Pasture Cave, Brassington, Derbyshire, England | Great Britain |
| I2988 | 3516–3361 calBCE (4645±29 BP, SUERC-68711) | Clachaig, Arran, North Ayrshire, Scotland | Great Britain |
| I2860 | 969–815 calBCE (2738±29 BP, SUERC-68715) | Covesea Cave 2, Moray, Scotland | Great Britain |
| I2861 | 976–828 calBCE (2757±29 BP, SUERC-68716) | Covesea Cave 2, Moray, Scotland | Great Britain |
| I3132 | 2118–1887 calBCE (3614±33 BP, SUERC-69070) | Covesea Cave 2, Moray, Scotland | Great Britain |
| I3130 | 977–829 calBCE (2758±29 BP, SUERC-68713) | Covesea Caves, Moray, Scotland | Great Britain |
| I2859 | 910–809 calBCE (2714±29 BP, SUERC-68714) | Covesea Caves, Moray, Scotland | Great Britain |
| I2452 | 2198–1980 calBCE (3700±30 BP, Beta-444979) | Dairy Farm, Willington, England | Great Britain |
| I2452 | 2276–2029 calBCE (3735±35 BP, Poz-83405) | Dairy Farm, Willington, England | Great Britain |
| I2659 | 3761–3643 calBCE (4914±27 BP, SUERC-68702) | Distillery Cave, Oban, Argyll and Bute, Scotland | Great Britain |
| I2660 | 3513–3352 calBCE (4631±29 BP, SUERC-68703) | Distillery Cave, Oban, Argyll and Bute, Scotland | Great Britain |
| I2691 | 3700–3639 calBCE (4881±25 BP, SUERC-68704) | Distillery Cave, Oban, Argyll and Bute, Scotland | Great Britain |
| I6774 | 2287–2044 calBCE (3760±30 BP, SUERC-74755) | Ditchling Road, Brighton, Sussex, England | Great Britain |
| I2605 | 3631–3372 calBCE (4710±35 BP, Poz-83483) | Eton Rowing Course, Buckinghamshire, England | Great Britain |
| I1775 | 1730–1532 calBCE (3344±27 BP, OxA-14308) | Great Orme, Llandudno, North Wales | Great Britain |
| I2574 | 1414–1227 calBCE (3065±36 BP, SUERC-62072) | Great Orme, Llandudno, North Wales | Great Britain |
| I2612 | 2464–2208 calBCE (3865±35 BP, Poz-83492) | Hasting Hill, Sunderland, Tyne and Wear, England | Great Britain |
| I2609 | 2022–1771 calBCE (3560±40 BP, Poz-83423) | Hexham Golf Course, Northumberland, England | Great Britain |
| I2636 | 3519–3361 calBCE (4651±33 BP, SUERC-68640) | Holm of Papa Westray North, Orkney, Scotland | Great Britain |
| I2637 | 3629–3370 calBCE (4697±33 BP, SUERC-68641) | Holm of Papa Westray North, Orkney, Scotland | Great Britain |
| I2650 | 3638–3380 calBCE (4754±36 BP, SUERC-68642) | Holm of Papa Westray North, Orkney, Scotland | Great Britain |
| I2651 | 3360–3098 calBCE (4525±36 BP, SUERC-68643) | Holm of Papa Westray North, Orkney, Scotland | Great Britain |
| I2630 | 2580–2463 calBCE (3999±32 BP, SUERC-68632) | Isbister, Orkney, Scotland | Great Britain |
| I2932 | 2570–2347 calBCE (3962±29 BP, SUERC-68721) | Isbister, Orkney, Scotland | Great Britain |
| I2933 | 3010–2885 calBCE (4309±29 BP, SUERC-68722) | Isbister, Orkney, Scotland | Great Britain |
| I2935 | 3335–3011 calBCE (4451±29 BP, SUERC-68723) | Isbister, Orkney, Scotland | Great Britain |
| I3085 | 3338–3026 calBCE (4471±29 BP, SUERC-68724) | Isbister, Orkney, Scotland | Great Britain |
| I2978 | 3335–3023 calBCE (4464±29 BP, SUERC-68725) | Isbister, Orkney, Scotland | Great Britain |
| I2979 | 3333–2941 calBCE (4447±29 BP, SUERC-68726) | Isbister, Orkney, Scotland | Great Britain |
| I2934 | 3338–3022 calBCE (4466±33 BP, SUERC-69071) | Isbister, Orkney, Scotland | Great Britain |
| I2977 | 3008–2763 calBCE (4275±33 BP, SUERC-69072) | Isbister, Orkney, Scotland | Great Britain |
| I2657 | 3951–3780 calBCE (5052±30 BP, SUERC-68701) | Macarthur Cave, Oban, Argyll and Bute, Scotland | Great Britain |
| I5441 | 1938–1744 calBCE (3512±37 BP, OxA-16522) | Neale’s Cave, Paington, Devon, England | Great Britain |
| I4949 | 3629–3376 calBCE (4715±20 BP, PSUAMS-2513) | Nr. Millbarrow, Winterbourne Monkton, Wiltshire, England | Great Britain |
| I2980 | 3360–3101 calBCE (4530±33 BP, SUERC-69073) | Point of Cott, Orkney, Scotland | Great Britain |
| I2796 | 3705–3535 calBCE (4856±33 BP, SUERC-69074) | Point of Cott, Orkney, Scotland | Great Britain |
| I2631 | 3097–2906 calBCE (4384±36 BP, SUERC-68633) | Quoyness, Orkney, Scotland | Great Britain |
| I3135 | 3640–3383 calBCE (4770±30 BP, PSUAMS-2068) | Raschoille Cave, Oban, Argyll and Bute, Scotland | Great Britain |
| I3136 | 3520–3365 calBCE (4665±30 BP, PSUAMS-2069) | Raschoille Cave, Oban, Argyll and Bute, Scotland | Great Britain |
| I3133 | 3631–3377 calBCE (4725±20 BP, PSUAMS-2154) | Raschoille Cave, Oban, Argyll and Bute, Scotland | Great Britain |
| I3134 | 3633–3377 calBCE (4730±25 BP, PSUAMS-2155) | Raschoille Cave, Oban, Argyll and Bute, Scotland | Great Britain |
| I3138 | 3263–2923 calBCE (4415±25 BP, PSUAMS-2156) | Raschoille Cave, Oban, Argyll and Bute, Scotland | Great Britain |
| I2610 | 1935–1745 calBCE (3515±35 BP, Poz-83498) | Summerhill Blaydon, Tyne and Wear, England | Great Britain |
| I2634 | 3703–3534 calBCE (4851±34 BP, SUERC-68638) | Tulach an t’Sionnach, Highland Scotland | Great Britain |
| I2635 | 3652–3389 calBCE (4796±37 BP, SUERC-68639) | Tulloch of Assery A, Highland, Scotland | Great Britain |
| I2633 | 3765–3641 calBCE (4911±32 BP, SUERC-68634) | Tulloch of Assery B, Highland, Scotland | Great Britain |
| I2453 | 2288–2040 calBCE (3760±35 BP, Poz-83404) | West Deeping, Lincolnshire, England | Great Britain |
| I2445 | 2136–1929 calBCE (3650±35 BP, PoZ-83407) | Yarnton. Oxfordshire, England | Great Britain |
| I2447 | 2115–1910 calBCE (3625±25 BP, PSUAMS-2336) | Yarnton, Oxfordshire, England | Great Britain |
| I2786 | 2458–2205 calBCE (3850±35 BP, Poz-83639) | Szigetszentmiklós-Felső-Urge hegyi dűlő | Hungary |
| I2787 | 2457–2201 calBCE (3840±35 BP, Poz-83640) | Szigetszentmiklós-Felső-Urge hegyi dűlő | Hungary |
| I2741 | 2457–2153 calBCE (3835±35 BP, Poz-83641) | Szigetszentmiklós-Felső-Urge hegyi dűlő | Hungary |
| I6531 | 2286–2038 calBCE (3755±35 BP, Poz-86947) | Dzielnica | Poland |
| I6579 | 2335–2046 calBCE (3780±35 BP, Poz-75954) | Iwiny | Poland |
| I6534 | 2456–2149 calBCE (3830±35 BP, Poz-75936) | Kornice | Poland |
| I6582 | 2343–2057 calBCE (3790±35 BP, POZ-75951) | Kornice | Poland |
| I4251 | 2431–2150 calBCE (3825±25 BP, PSUAMS-2321) | Samborzec 1 | Poland |
| I4252 | 2285–2138 calBCE (3780±20 BP, PSUAMS-2338) | Samborzec 1 | Poland |
| I4253 | 2456–2207 calBCE (3850±20 BP, PSUAMS-2339) | Samborzec 1 | Poland |
| I6538 | 2008–1765 calBCE (3545±35 BP, Poz-86950) | Strachów | Poland |
| I6583 | 2289–2050 calBCE (3770±30 BP, Poz-65207) | Zerniki Wielkie | Poland |
| I4229 | 2288–2134 calBCE (3775±25 BP, PSUAMS-1750) | Cova da Moura | Portugal |
| I0462 | 2566–2345 calBCE (3950±26 BP, MAMS-25936) | Arroyal I, Burgos | Spain |
| I4247 | 2464–2210 calBCE (3870±30 BP, PSUAMS-2120) | Camino de las Yeseras, Madrid | Spain |
| I4245 | 2460–2291 calBCE (3875±20 BP, PSUAMS-2320) | Camino de las Yeseras, Madrid | Spain |
| I0257 | 2572–2348 calBCE (3965±29 BP, MAMS-25937) | Paris Street, Cerdanyola, Barcelona | Spain |
| I0825 | 2474–2298 calBCE (3915±29 BP, MAMS-25939) | Paris Street, Cerdanyola, Barcelona | Spain |
| I0826 | 2834–2482 calBCE (4051±28 BP, MAMS-25940) | Paris Street, Cerdanyola, Barcelona | Spain |
| I4068 | 2131–1951 calBCE (3655±20 BP, PSUAMS-2318) | De Tuithoorn, Oostwoud, Noord-Holland | The Netherlands |
| I4076 | 1882–1750 calBCE (3490±20 BP, PSUAMS-2319) | De Tuithoorn, Oostwoud, Noord-Holland | The Netherlands |
| I4075 | 2118–1937 calBCE (3635±20 BP, PSUAMS-2337) | De Tuithoorn, Oostwoud, Noord-Holland | The Netherlands |
Supplementary Material
Acknowledgments
We thank D. Anthony, J. Koch, I. Mathieson and C. Renfrew for comments, and A. Cooper for support from the Australian Centre for Ancient DNA. We thank the Bristol Radiocarbon Accelerator Mass Spectrometry Facility (BRAMS), University of Bristol. We thank A. C. Sousa, A. Martín Cólliga, L. Loe, C. Roth, E. Carmona Ballesteros, M. Kunst, S.-A. Coupar, M. Giesen and G. Drinkall for assistance with samples, and E. Willerslev for supporting several co-authors at the Centre for GeoGenetics. We thank the Museo Arqueológico Regional de la Comunidad de Madrid, the Hunterian Museum, University of Glasgow, the Orkney Museum, the Museu Municipal de Torres Vedras, the Great North Museum: Hancock, the Society of Antiquaries of Newcastle upon Tyne, the Sunderland Museum and the Museum of London for facilitating sample collection. Support for this project was provided by the Czech Academy of Sciences grant RVO:67985912; by Momentum Mobility Research Group of the Hungarian Academy of Sciences; by the Wellcome Trust [100713/Z/12/Z]; by Irish Research Council grant GOIPG/2013/36 to D.F.; by the Heidelberg Academy of Sciences (WIN project ‘Times of Upheaval’) to P.W.S., J.K. and A.M.; by The Swedish Foundation for Humanities and Social Sciences grant M16-0455:1 to K.K.; by the National Science Centre, Poland grant DEC-2013/10/E/HS3/00141 to M.Fu.; by a Spanish MINECO grant BFU2015-64699-P to C.L.-F.; by a Spanish MINECO grant HAR2016-77600-P to C.L., P.R. and C.B.; by the NSF Archaeometry program BCS-1460369 to D.J.K.; by the NFS Archaeology program BCS-1725067 to D.J.K. and T. H.; and by Allen Discovery Center grant from the Paul Allen Foundation, US National Science Foundation HOMINID grant BCS-1032255, US National Institutes of Health grant GM100233, and the Howard Hughes Medical Institute to D.R.
Footnotes
Author Contributions
S.B., M.E.A., N.R., A.Sz.-N., A.M., N.B., M.F., E.H., M.M., J.O., K.S., O.C., D.K., F.C., R.P., J.K., W.H., I.B. and D.R. performed or supervised laboratory work. G.T.C. and D.J.K. undertook the radiocarbon dating of a large fraction of samples. I.A., K.K., A.B., K.W.A., A.A.F., E.B., M.B.-B., D.B., C.B., J.V.M., R.M., C.Bo., L.B., T.A., L.Bü., S.C., L.C.N., O.E.C., G.T.C., B.C., A.D., K.E.D., N.D., M.E., C.E., M.K., J.F.F., H.F., C.F., M.G., R.G.P., M.H.-U., E.Had., G.H., N.J., T.K., K.M., S.P., P.L., O.L., A.L., C.H.M., V.G.O., A.B.R., J.L.M., T.M., J.I.M, K.Mc., B.G.M., A.Mo., G.K., V.K., A.C., R.Pa., A.E., K.Kö., T.H., T.S., J.D., Z.B., M.H., P.V., M.D., F.B., R.F.F., A.H.-C., S.T., E.C., L.L., A.V., A.Z., C.W., G.D., E.G.-D., B.N., M.B., M.Lu., R.Mo., J.De., M.Be., G.B., M.Fu., A.H., M.Ma., A.R., S.L., I.S., K.T.L., J.L.C., C.L., M.P.P., P.W., T.D.P., P.P., P.-J.R., P.R., R.R., M.A.R.G., A.S., J.S., A.M.S., V.S., L.V., J.Z., D.C., T.Hi., V.H., A.Sh., K.-G.S., P.W.S., R.P., J.K., W.H., I.B., C.L.-F. and D.R. assembled archaeological material. I.O., S.M., T.B., A.M., E.A., M.L., I.L., N.P., Y.D., Z.F., D.F., D.J.K., P.d.K., T.K.H., M.G.T. and D.R. analysed data. I.O., C.L.-F. and D.R. wrote the manuscript with input from all co-authors.
Supplementary Information is available in the online version of the paper.
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of the paper.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Czebreszuk J. Bell Beakers from West to East. In: Bogucki PI, Crabtree PJ, editors. Ancient Europe, 8000 B.C. to A.D. 1000: An Encyclopedia of the Barbarian World. Charles Scribner’s Sons; 2004. pp. 476–485. [Google Scholar]
- 2.Haak W, et al. Massive migration from the steppe was a source for Indo-European languages in Europe. Nature. 2015;522:207–211. doi: 10.1038/nature14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allentoft ME, et al. Population genomics of Bronze Age Eurasia. Nature. 2015;522:167–172. doi: 10.1038/nature14507. [DOI] [PubMed] [Google Scholar]
- 4.Mathieson I, et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature. 2015;528:499–503. doi: 10.1038/nature16152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czebreszuk J. Bell Beakers in Europe. Adam Mickiewicz University; 2004. Similar But Different. [Google Scholar]
- 6.Cardoso JL. Absolute chronology of the Beaker phenomenon North of the Tagus estuary: demographic and social implications. Trabajos de Prehistoria. 2014;71:56–75. [Google Scholar]
- 7.Jeunesse C. The dogma of the Iberian origin of the Bell Beaker: attempting its deconstruction. J Neolit Archaeol. 2015;16:158–166. [Google Scholar]
- 8.Fokkens H, Nicolis F. Background to Beakers. Inquiries into regional cultural backgrounds of the Bell Beaker complex. Leiden: Sidestone Press; 2012. [Google Scholar]
- 9.Vander Linden M. What linked the Bell Beakers in third millennium BC Europe? Antiquity. 2007;81:343–352. [Google Scholar]
- 10.Fu Q, et al. An early modern human from Romania with a recent Neanderthal ancestor. Nature. 2015;524:216–219. doi: 10.1038/nature14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazaridis I, et al. Ancient human genomes suggest three ancestral populations for present-day Europeans. Nature. 2014;513:409–413. doi: 10.1038/nature13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazaridis I, et al. Genomic insights into the origin of farming in the ancient Near East. Nature. 2016;536:1–22. doi: 10.1038/nature19310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mallick S, et al. The Simons Genome Diversity Project: 300 genomes from 142 diverse populations. Nature. 2016:538. doi: 10.1038/nature18964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valverde L, et al. New clues to the evolutionary history of the main European paternal lineage M269: dissection of the Y-SNP S116 in Atlantic Europe and Iberia. Eur J Hum Genet. 2015:1–5. doi: 10.1038/ejhg.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gamba C, et al. Ancient DNA from an Early Neolithic Iberian population supports a pioneer colonization by first farmers. Mol Ecol. 2012;21:45–56. doi: 10.1111/j.1365-294X.2011.05361.x. [DOI] [PubMed] [Google Scholar]
- 16.Günther T, et al. Ancient genomes link early farmers from Atapuerca in Spain to modern-day Basques. Proc Natl Acad Sci U S A. 2015;112:11917–11922. doi: 10.1073/pnas.1509851112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broushaki F, et al. Early Neolithic genomes from the eastern Fertile Crescent. Science. 2016;7943:1–16. doi: 10.1126/science.aaf7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skoglund P, et al. Genomic Diversity and Admixture Differs for Stone-Age Scandinavian Foragers and Farmers. Science. 2014;201:786–792. doi: 10.1126/science.1253448. [DOI] [PubMed] [Google Scholar]
- 20.Olalde I, et al. A Common Genetic Origin for Early Farmers from Mediterranean Cardial and Central European LBK Cultures. Mol Biol Evol. 2015;32:3132–3142. doi: 10.1093/molbev/msv181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathieson I, et al. The Genomic History of Southeastern Europe. bioRxiv. 2017 doi: 10.1038/nature25778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipson M, et al. Parallel ancient genomic transects reveal complex population history of early European farmers. Nature. 2017;551:368–372. doi: 10.1038/nature24476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cassidy LM, et al. Neolithic and Bronze Age migration to Ireland and establishment of the insular Atlantic genome. Proc Natl Acad Sci U S A. 2016;113:1–6. doi: 10.1073/pnas.1518445113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheridan JA. The Neolithisation of Britain and Ireland: the big picture. In: Finlayson B, Warren G, editors. Landscapes in transition. Oxbow; Oxford: 2010. pp. 89–105. [Google Scholar]
- 25.Burger J, Kirchner M, Bramanti B, Haak W, Thomas MG. Absence of the lactase-persistence-associated allele in early Neolithic Europeans. Proc Natl Acad Sci U S A. 2007;104:3736–41. doi: 10.1073/pnas.0607187104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke DL. The Beaker network: social and economic models. In: Lanting JN, van DerWaals JD, editors. Glockenbecher Symposion; Oberried. 18–23 März 1974; 1976. pp. 460–77. [Google Scholar]
- 27.Clark G. The Invasion Hypothesis in British Archaeology. Antiquity. 1966;40:172–189. [Google Scholar]
- 28.Brotherton P, et al. Neolithic mitochondrial haplogroup H genomes and the genetic origins of Europeans. Nat Commun. 2013;4:1764. doi: 10.1038/ncomms2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desideri J. When Beakers Met Bell Beakers: an analysis of dental remains. British archaeological Reports - International Series. 2011:2292. [Google Scholar]
- 30.Parker Pearson M, et al. Beaker people in Britain: migration, mobility and diet. Antiquity. 2016;90:620–637. [Google Scholar]
- 31.Shennan S, et al. Regional population collapse followed initial agriculture booms in mid-Holocene Europe. Nat Commun. 2013;4:2486. doi: 10.1038/ncomms3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valtueña AA, et al. The Stone Age Plague: 1000 years of Persistence in Eurasia. bioRxiv. 2016 [Google Scholar]
- 33.Rasmussen S, et al. Early Divergent Strains of Yersinia pestis in Eurasia 5,000 Years Ago. Cell. 2015;163:571–582. doi: 10.1016/j.cell.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dabney J, et al. Complete mitochondrial genome sequence of a Middle Pleistocene cave bear reconstructed from ultrashort DNA fragments. Proc Natl Acad Sci U S A. 2013;110:15758–63. doi: 10.1073/pnas.1314445110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Damgaard PB, et al. Improving access to endogenous DNA in ancient bones and teeth. Sci Rep. 2015;5:11184. doi: 10.1038/srep11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korlević P, et al. Reducing microbial and human contamination in dna extractions from ancient bones and teeth. Biotechniques. 2015;59:87–93. doi: 10.2144/000114320. [DOI] [PubMed] [Google Scholar]
- 37.Rohland N, Harney E, Mallick S, Nordenfelt S, Reich D. Partial uracil – DNA – glycosylase treatment for screening of ancient DNA. Philos Trans R Soc London B. 2015;370:20130624. doi: 10.1098/rstb.2013.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Briggs AW, et al. Removal of deaminated cytosines and detection of in vivo methylation in ancient DNA. Nucleic Acids Res. 2010;38:1–12. doi: 10.1093/nar/gkp1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maricic T, Whitten M, Pääbo S. Multiplexed DNA sequence capture of mitochondrial genomes using PCR products. PLoS One. 2010;5:e14004. doi: 10.1371/journal.pone.0014004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kircher M, Sawyer S, Meyer M. Double indexing overcomes inaccuracies in multiplex sequencing on the Illumina platform. Nucleic Acids Res. 2012;40:1–8. doi: 10.1093/nar/gkr771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Behar DM, et al. A ‘Copernican’ reassessment of the human mitochondrial DNA tree from its root. Am J Hum Genet. 2012;90:675–84. doi: 10.1016/j.ajhg.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu Q, et al. A revised timescale for human evolution based on ancient mitochondrial genomes. Curr Biol. 2013;23:553–9. doi: 10.1016/j.cub.2013.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawyer S, Krause J, Guschanski K, Savolainen V, Pääbo S. Temporal patterns of nucleotide misincorporations and DNA fragmentation in ancient DNA. PLoS One. 2012;7:e34131. doi: 10.1371/journal.pone.0034131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korneliussen TS, Albrechtsen A, Nielsen R. ANGSD: Analysis of Next Generation Sequencing Data. BMC Bioinformatics. 2014;15:1–13. doi: 10.1186/s12859-014-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–9. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weissensteiner H, et al. HaploGrep 2: mitochondrial haplogroup classification in the era of high-throughput sequencing. Nucleic Acids Res. 2016;44:W58–63. doi: 10.1093/nar/gkw233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Oven M, Kayser M. Updated comprehensive phylogenetic tree of global human mitochondrial DNA variation. Hum Mutat. 2009;30:E386–94. doi: 10.1002/humu.20921. [DOI] [PubMed] [Google Scholar]
- 49.Patterson N, et al. Ancient admixture in human history. Genetics. 2012;192:1065–93. doi: 10.1534/genetics.112.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raghavan M, et al. Upper Palaeolithic Siberian genome reveals dual ancestry of Native Americans. Nature. 2014;505:87–91. doi: 10.1038/nature12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Omrak A, et al. Genomic Evidence Establishes Anatolia as the Source of the European Neolithic Gene Pool. Curr Biol. 2016;26:270–275. doi: 10.1016/j.cub.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 52.Gallego Llorente M, et al. Ancient Ethiopian genome reveals extensive Eurasian admixture in Eastern Africa. Science. 2015;350:820–822. doi: 10.1126/science.aad2879. [DOI] [PubMed] [Google Scholar]
- 53.Fu Q, et al. The genetic history of Ice Age Europe. Nature. 2016;534:200–205. doi: 10.1038/nature17993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kilinc GM, et al. The Demographic Development of the First Farmers in Anatolia. Curr Biol. 2016;26:1–8. doi: 10.1016/j.cub.2016.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gallego-Llorente M, et al. The genetics of an early Neolithic pastoralist from the Zagros, Iran. Sci Rep. 2016;6:4–10. doi: 10.1038/srep31326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olalde I, et al. Derived immune and ancestral pigmentation alleles in a 7,000-year-old Mesolithic European. Nature. 2014;507:225–8. doi: 10.1038/nature12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hofmanová Z, et al. Early farmers from across Europe directly descended from Neolithic Aegeans. Proc Natl Acad Sci U S A. 2016;113:6886–6891. doi: 10.1073/pnas.1523951113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2:e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Purcell S, et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Busing FMTA, Meijer E, Van Der Leeden R. Delete- m Jackknife for Unequal m. Stat Comput. 1999;9:3–8. [Google Scholar]
- 61.Rojo-Guerra MÁ, Kunst M, Garrido-Pena R, García-Martínez de Lagrán I, Morán-Dauchez G. Memorias Arqueología en Castilla y León 14, Junta de Castilla y León, Valladolid. 2005. Un desafío a la eternidad. Tumbas monumentales del Valle de Ambrona. [Google Scholar]
- 62.Gamba C, et al. Genome flux and stasis in a five millennium transect of European prehistory. Nat Commun. 2014;5:5257. doi: 10.1038/ncomms6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All 1240k and mitochondrial capture sequencing data are available from the European Nucleotide Archive, accession number PRJEB23635.
The genotype dataset is available from the Reich Lab website at https://reich.hms.harvard.edu/datasets.