Abstract
Ancient DNA studies have established that Neolithic European populations were descended from Anatolian migrants1–8 who received a limited amount of admixture from resident hunter-gatherers3–5,9. Many open questions remain, however, about the spatial and temporal dynamics of population interactions and admixture during the Neolithic period. Using the highest-resolution genome-wide ancient DNA data set assembled to date—a total of 180 samples, 130 newly reported here, from the Neolithic and Chalcolithic of Hungary (6000–2900 BCE, n = 100), Germany (5500–3000 BCE, n = 42), and Spain (5500–2200 BCE, n = 38)—we investigate the population dynamics of Neolithization across Europe. We find that genetic diversity was shaped predominantly by local processes, with varied sources and proportions of hunter-gatherer ancestry among the three regions and through time. Admixture between groups with different ancestry profiles was pervasive and resulted in observable population transformation across almost all cultural transitions. Our results shed new light on the ways that gene flow reshaped European populations throughout the Neolithic period and demonstrate the potential of time-series-based sampling and modeling approaches to elucidate multiple dimensions of historical population interactions.
The population dynamics of the Neolithization process are of great importance for understanding European prehistory10–13. The first quantitative model of the Neolithic transition to integrate archaeological and genetic data was the demic diffusion hypothesis10, which posited that growing population densities among Near Eastern farmers led to a range expansion that spread agriculture to Europe. Ancient DNA analysis has validated major migrations from populations related to Neolithic Anatolians as driving the introduction of farming in Europe1–8, but the demic diffusion model does not account for the complexities of the interactions between farmers and hunter-gatherers in Europe throughout the Neolithic11–16. For example, ancient DNA has shown that farmers traversed large portions of Europe with limited initial admixture from hunter-gatherers3,5,7,8, and furthermore that farmers and hunter-gatherers lived in close proximity in some locations long after the arrival of agriculture15,16. However, genetic data have yet to be used systematically to model the population interactions and transformations during the course of the Neolithic period. Key open questions include whether migrating farmers mixed with hunter-gatherers at each stage of the expansion (and if so how soon after arriving) and whether the previously observed increase in hunter-gatherer ancestry among farmers in several parts of Europe by the Middle Neolithic5–9 represented a continuous versus discrete process and a continent-wide phenomenon versus a collection of parallel, local events.
We compiled a high-resolution data set of 180 Neolithic and Chalcolithic European genomes (pre-dating the arrival of steppe ancestry in the third millennium BCE [ref 5]) from what are now Hungary, Germany, and Spain, of which 130 individuals are newly reported here, 45 with new direct radiocarbon dates (Table 1; Fig. 1A, B; Extended Data Tables 1, 2; Supplementary Tables 1, 2; Supplementary Information sections 1–3). We enriched for DNA fragments covering a set of ~1.23 million single nucleotide polymorphism (SNP) targets7 and called one allele at random per site, obtaining largely high-quality data, with at least 100,000 SNPs hit at least once (average coverage ~0.1 or higher) for 90 of the 130 samples (Methods). The majority (90) of our new samples comprise an approximately 3000-year transect of the prehistory of the Carpathian Basin (Supplementary Information section 1), from both the eastern (Great Hungarian Plain, or Alföld) and western (Transdanubia) portions of present-day Hungary. For our primary analyses, we retained 104 samples from 15 population groupings (Methods; Table 1), which we merged with 50 Neolithic individuals from the literature4,5,7,17,18. We co-analyzed these samples with 25 Neolithic individuals (~6500–6000 BCE) from northwestern Anatolia7 to represent the ancestors of the first European farmers (FEF; Supplementary Information section 4) and four primary European hunter-gatherer individuals4,7,17,19,20 (“WHG,” western hunter-gatherers; Table 1).
Table 1.
Neolithic population groups and western hunter-gatherer individuals in the study
| Population | Country | Samples* | Appx. dates (BCE) |
|---|---|---|---|
| Körös EN | HungaryE | 6/5/3† | 6000–5500 |
| Starčevo EN | HungaryW | 5/4/4 | 6000–5500 |
| ALPc MN | HungaryE | 25/20/22 | 5500–5000 |
| LBKT MN | HungaryW | 8/7/7 | 5500–5000 |
| Vinča MN | HungaryW | 6/6/0 | 5500–5000 |
| Tisza LN | HungaryE | 6/6/5 | 5000–4500 |
| TDLN | HungaryW | 15/14/14 | 5000–4500 |
| Tiszapolgár CA | HungaryE | 5/5/0 | 4500–4000 |
| Lasinja CA | HungaryW | 7/7/6 | 4300–3900 |
| Protoboleráz CA | HungaryE | 4/4/4 | 3800–3600 |
| Baden CA | Hungary | 13/12/10 | 3600–2850 |
| LBK EN | Germany | 30/15/29 | 5500–4850 |
| Germany MN | Germany | 8/4/7 | 4600–3000 |
| Blätterhöhle MN | Germany | 4/4/4† | 4100–3000 |
| Iberia EN | Spain | 7/2/7 | 5500–4500 |
| Iberia MN | Spain | 4/0/4 | 3900–3600 |
| Iberia CA | Spain | 27/15/27 | 3000–2200 |
|
| |||
| KO1 HG | HungaryE | 1/0/1 | 5700 |
| LB1 HG | Spain | 1/0/1 | 5900 |
| LOS HG | Luxembourg | 1/0/1 | 6100 |
| VIL HG | ItalyE | 1/0/1 | 12,000 |
Total number/new in this study/used in final analyses
Includes one hunter-gatherer individual treated separately
Eastern
Western
EN/MN/LN, Early/Middle/Late Neolithic; CA, Chalcolithic; HG, hunter-gatherer (LB1, La Braña 1; LOS, Loschbour; VIL, Villabruna)
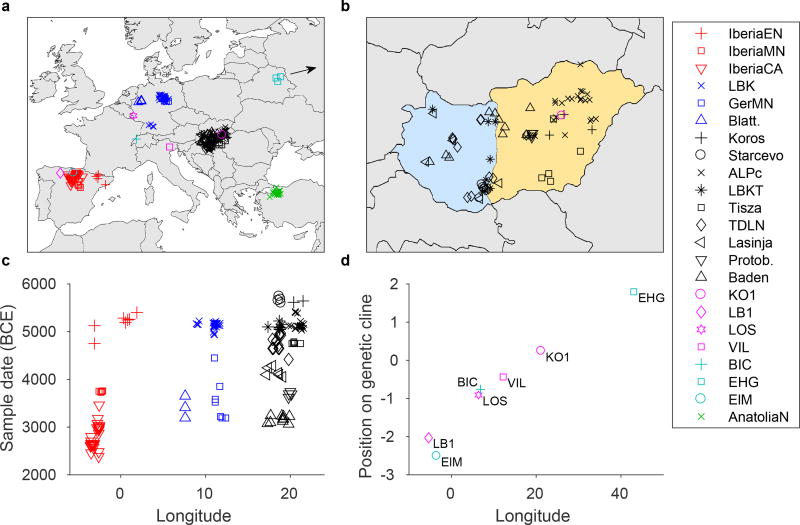
Figure 1. Spatial and temporal contexts of European Neolithic samples.
a, b, Locations of samples used for analyses, with close-up of Hungary (orange shading for Alföld and light blue for Transdanubia). c, Sample dates arranged by longitude. d, Hunter-gatherer genetic cline (derived from MDS analysis; Supplementary Information section 5) as a function of longitude. The four primary WHG individuals are shown together with “BIC” (Bichon, ~11,700 BCE from Switzerland21), “EHG” (eastern hunter-gatherers, ~7000–5000 BCE from Russia5,7), and “ElM” (El Mirón, ~17,000 BCE from Spain20). Random jitter is added to separate overlapping positions in a–c. GerMN, Germany MN; Blatt., Blätterhöhle; Protob., Protoboleráz.
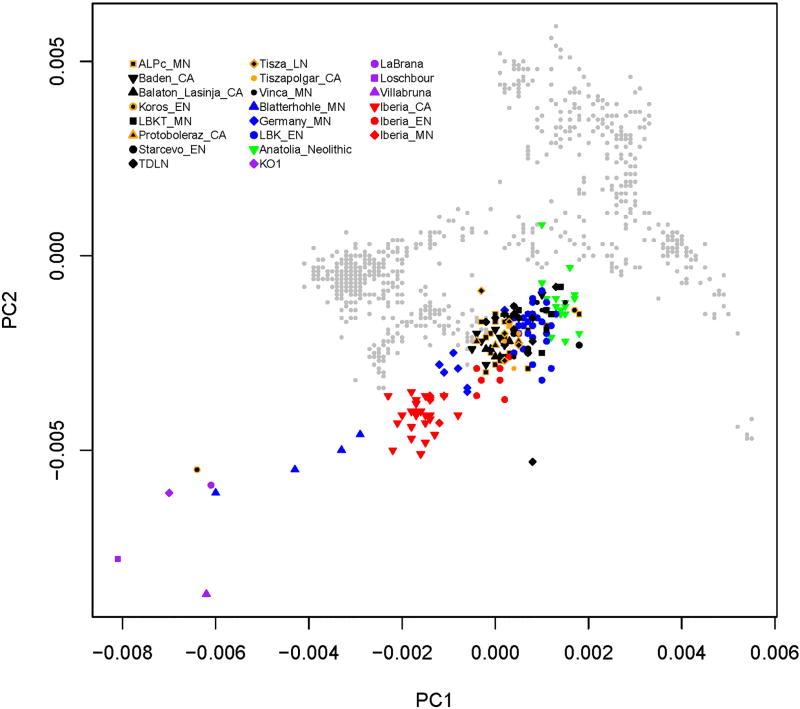
A principal component analysis (PCA) of our samples shows that, as expected, all of the Neolithic individuals fall along a cline of admixture between FEF and WHG (Extended Data Fig. 1). Y-chromosome diversity also indicates contributions from ancestral Anatolian farmer and local hunter-gatherer populations, dominated by haplogroups G and I (the latter especially common in Iberia; Supplementary Information section 3). The European populations are consistent with a common origin in Anatolia (Supplementary Information section 4), reflected in the low differentiation among EN groups in the PCA. Over the course of the Neolithic, we observe a trend of increasing hunter-gatherer ancestry in each region, although at a slower rate in Hungary than in Germany and Spain, and with limited intra-population heterogeneity (Fig. 2A; Supplementary Information section 6). We also find that this hunter-gatherer ancestry is more similar to the eastern WHG individuals (KO1 and VIL) farther east and more similar to the western WHG individuals (LB1 and LOS) farther west (Fig. 2B). While this pattern does not demonstrate directly where mixture between hunter-gatherers and farmers took place, it suggests, given that European hunter-gatherers display a strong correlation between genetic and geographic structure (Fig. 1D), that hunter-gatherer ancestry in farmers was to a substantial extent derived from populations in relatively close proximity.
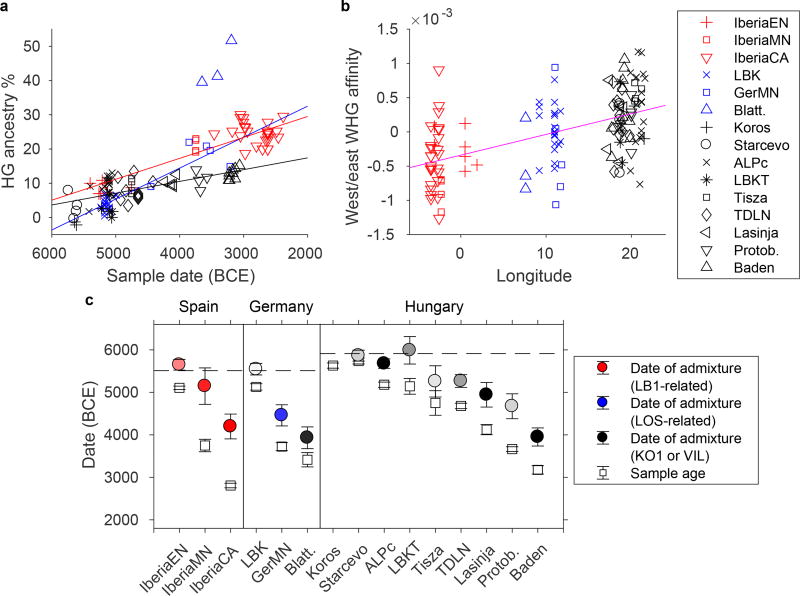
Figure 2. Admixture parameters for test individuals and populations.
a, Estimated individual hunter-gatherer ancestry versus sample date, with best-fitting regression lines for each region (excluding Blätterhöhle). Standard errors are around 2% for hunter-gatherer ancestry and 100 years for dates (Methods; Extended Data Tables 1, 2). b, Relative affinity of hunter-gatherer ancestry in Neolithic individuals, measured as f4(LB1+LOS, KO1+VIL; Anatolia, X) (positive, more similar to eastern WHG; negative, more similar to western WHG; standard errors ~5×10−4), with best-fitting regression line (|Z| > 3 for aggregate differences among the three regions). c, Population-level average sample ages and dates of admixture, plus or minus two standard errors. Colored fill indicates the inferred primary hunter-gatherer ancestry component, with darker shades corresponding to higher confidence (all admixed populations except LBK and Tisza significant at p < 0.05; see Extended Data Table 3 and Supplementary Information section 6). Dashed lines denote the approximate date of arrival of farming in each region.
To analyze admixed hunter-gatherer ancestry more formally, we modeled Neolithic farmers in an admixture graph framework. We started with a “scaffold” model (Extended Data Fig. 2) consisting of Neolithic Anatolians, the four reference WHG individuals, and two outgroups (Mbuti and Kostenki 14 [refs 20, 22]), with significant signals of admixture in LB1 and KO1 (Supplementary Information sections 5–6). We then added each Neolithic population to this model in turn, fitting them as a mixture of FEF and either one or two hunter-gatherer ancestry components. To check for robustness, we repeated our analyses using transversions or outgroup-ascertained SNPs only, with in-solution capture data for LOS, and with additional or alternative hunter-gatherers in the model (Extended Data Table 3; Supplementary Information section 6), and in all cases the results were qualitatively consistent. We find that almost all ancient groups from Hungary have ancestry significantly closest to one of the more eastern WHG individuals (KO1 or VIL); the samples from present-day Germany have the greatest affinity to LOS; and all three Iberian groups contain LB1-related ancestry (Fig. 2C; Extended Data Table 3). This pattern implies that admixture into European farmers occurred multiple times from local hunter-gatherer populations. Moreover, combining the proportions and sources of hunter-gatherer ancestry, populations from the three regions are distinguishable at all stages of the Neolithic. Thus, any further long-range migrations that may have occurred after the initial spread of agriculture in the studied regions (and before large incursions from the steppe) were not substantial enough to homogenize the ancestry of farming populations.
Additional insights about population interactions can be gained by studying the dates of admixture events. We used ALDER (ref. 23) to estimate dates of admixture for Neolithic individuals based on the recombination-induced breakdown of contiguous blocks of FEF and WHG ancestry over time (Extended Data Tables 1, 2, 4; Extended Data Fig. 3). The ALDER algorithm is not able to accommodate large amounts of missing data, so we developed a strategy for running it with the relatively low coverage of ancient DNA (Supplementary Information section 7). The dates we obtain (Fig. 2D) are based on a model of a single wave of admixture, which means that if the true history for a population includes multiples waves or continuous admixture, we will obtain an intermediate value. In particular, for later populations, this history could include mixture with previously admixed groups (either farmers with substantially different hunter-gatherer ancestry proportions or hunter-gatherers with farmer ancestry).
For our most complete time series, from Hungary, we infer admixture dates throughout the Neolithic that are on average mostly 18–30 generations old (500–840 years), indicating ongoing population transformation and admixture (Fig. 2D; Extended Data Table 4). This pattern is accompanied by a gradual increase in hunter-gatherer ancestry over time, although never reaching the levels observed in MN Germany or Iberia (Fig. 2A). While the majority of the EN samples from Hungary do not have significantly more hunter-gatherer ancestry than Neolithic Anatolians (Fig. 2A; Extended Data Tables 1, 2), one Starčevo individual, BAM17b, is inferred to have 7.8 ± 1.7% hunter-gatherer ancestry and a very recent ALDER date of 4.5 ± 1.9 generations (5865 ± 65 BCE; 1.9 ± 0.9 generations using a group-level estimate; Extended Data Table 4), consistent with having one or two hunter-gatherer ancestors in the past few generations. Additionally, one newly sampled Körös individual, TIDO2a, is similar to KO1 in having ~80% WHG and ~20% FEF ancestry and an ALDER date of 16.1 ± 3.8 generations, reinforcing the distinctive heterogeneity of the Tiszaszőlős site, the source for both TIDO2a and KO1. We also infer an average admixture date of 5675 ± 55 BCE for the ALPc MN, again suggesting that in Hungary, interaction between Anatolian migrants and local hunter-gatherers began in the Early Neolithic (cf. refs 14, 24–26). The greatest differences between Alföld and Transdanubia are observed in the MN, with substantially more hunter-gatherer ancestry in ALPc than LBKT (Fig. 2; Extended Data Table 3), and overall, we observe slight trends toward more hunter-gatherer ancestry to the north and east (Extended Data Fig. 4), as expected based on the greater archaeological evidence of hunter-gatherer settlement and interactions24. By the LN and CA, however, and especially in the Baden period (when the region became culturally unified27), our results are broadly similar over the two halves of present-day Hungary.
From Germany, we analyzed a large sample of the EN LBK culture and 11 individuals from the MN period, four of them from the Blätterhöhle site, which has been shown to have featured a combination of farmer and hunter-gatherer occupation to a relatively late date15. The average date of admixture for LBK (5545 ± 65 BCE) is more recent than the dates for EN/MN populations from Hungary, and the total hunter-gatherer ancestry proportion in LBK (~4–5%) is intermediate between LBKT and ALPc. This ancestry is most closely related to a combination of KO1 and LOS, although the assignment of the hunter-gatherer source(s) is not statistically significant (Fig. 2B; Extended Data Table 3). These results are consistent with genetic and archaeological evidence for LBK origins from the early LBKT (ref. 26), followed by additional, Central European WHG admixture after about 5500 BCE. Our “Germany MN” grouping shows increased hunter-gatherer ancestry (~17%, most closely related to LOS) and a more recent average date of admixture, reflecting gene flow from hunter-gatherers after the LBK period. We successfully sequenced a total of 17 Blätterhöhle MN samples, many of them with distinct individual labels from ref. 15, although surprisingly, the genome-wide data indicated that these corresponded to only four unique individuals (Supplementary Information section 8), for which we merged libraries to increase coverage. In accordance with previous results15, we find that the three farmer individuals (classified based on stable isotopes) harbored 40–50% hunter-gatherer ancestry, while Bla8, who showed signatures associated with a hunter-gatherer-fisher lifestyle, was closer genetically to hunter-gatherers but was also admixed, with ~27% ancestry from farmers. Our results thus provide evidence of asymmetric gene flow between farmers and hunter-gatherers at Blätterhöhle centered around the relatively late date of ~4000 BCE (ALDER dates of 10–25 generations).
In Iberia, we again see widespread evidence of local hunter-gatherer admixture, with confidently inferred LB1-related ancestry in all three population groups (EN, MN, and CA). For Iberia EN, we infer an average admixture date of 5650 ± 65 BCE, which rises to 5860 ± 110 BCE when considering only the five oldest samples (of which the earliest, CB13 [ref. 18] has an individual estimate of 5890 ± 105 BCE). Given that farming is thought to have begun in Spain around 5500 BCE (ref. 28), these dates suggest the presence of at least a small proportion of hunter-gatherer ancestry in earlier Cardial Neolithic populations acquired along their migration route (although our admixture graph analysis only confidently detected an LB1-related component). The later Iberians have large proportions of hunter-gatherer ancestry, approximately 23% for MN (from the site of La Mina, in north-central Iberia) and 27% for CA, and also relatively old ALDER dates (approximately 50 generations, or 1400 years), indicating that most of the admixture occurred well before their respective sample dates. Both populations have evidence of ancestry related to a different WHG individual in addition to LB1 (Fig. 2C; Extended Data Table 3), suggesting a non-local source for at least some of the hunter-gatherer ancestry gained between the EN and MN.
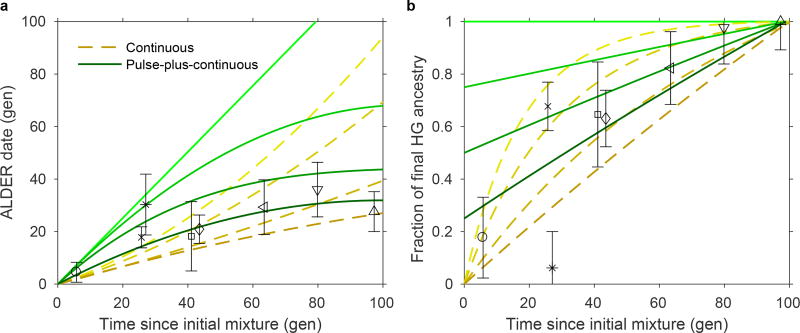
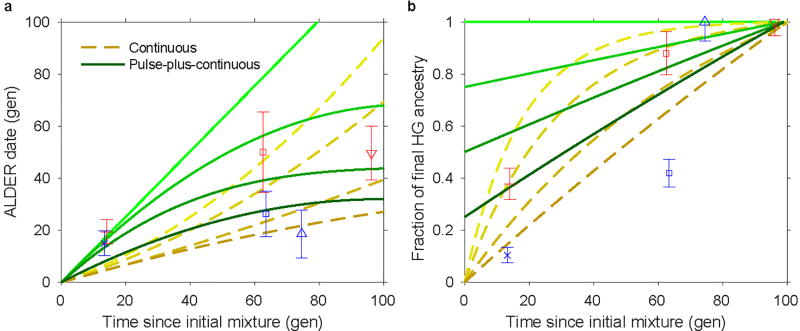
Synthesizing our time series data, we compared the observed ALDER dates and hunter-gatherer ancestry proportions of Neolithic populations to those estimated for simulated data under different temporal admixture scenarios (Fig. 3; Extended Data Fig. 5; Supplementary Information section 9). We assumed dates of 5900 BCE (Hungary) or 5500 BCE (Germany and Spain) for the onset of mixture. While none of the scenarios match the data perfectly, a good fit for Hungary is provided by a model (bottom solid green curve in both panels of Fig. 3) of an initial admixture pulse (approximately 1/4 of the total hunter-gatherer ancestry observed by the end of the time series) followed by continuous gene flow. By contrast, scenarios such as a single admixture pulse or continuous mixture decreasing by 5% or more per generation provide too much hunter-gatherer ancestry at early dates. Alföld and Transdanubia should be considered as separate series, but their parameters follow mostly similar trajectories, with the exception of the MN, where LBKT has a relatively old admixture date (albeit with large uncertainty) and ALPc a relatively high hunter-gatherer ancestry proportion (possibly influenced by the bias of sampling in favor of the middle and northern parts of the Alföld). Considering the other regions, even after normalizing for the different total hunter-gatherer ancestry proportions, we observe a high degree of local distinctiveness, for example in the older ALDER dates for Iberia MN/CA and the markedly higher hunter-gatherer ancestry in Blätterhöhle (Extended Data Fig. 5). We note that while the simulated data are generated under a model of gene flow from an unadmixed hunter-gatherer source population into a series of farmer populations in a single line of descent, observed admixture could also be influenced by flow in the other direction (from farmers to hunter-gatherers) or could reflect immigration of new farmer populations (either via their own previous hunter-gatherer admixture or new admixture between farming populations with different proportions of hunter-gatherer ancestry). Based on archaeological evidence, such a scenario is possible, for example, for the introduction of hunter-gatherer ancestry into TDLN from Southeastern European farmers via the dispersal of the northern Balkan Vinča or Sopot cultures to Transdanubia14,29,30.
Figure 3. Hungary time series and simulated data.
a, Dates of admixture. b, Hunter-gatherer ancestry proportions, normalized by the total in the most recent (rightmost) population. Symbols are as in Figs 1 and 2, here showing population-level averages plus or minus two standard errors. Yellow dashed lines represent continuous admixture simulations: from top to bottom, diminishing 5% per generation, diminishing 3%, diminishing 1%, and uniform. Green solid lines represent pulse-plus-continuous admixture simulations: from top to bottom, all hunter-gatherer ancestry in a pulse at time zero; 3/4 of final hunter-gatherer ancestry in an initial pulse, followed by uniform continuous gene flow; half in initial pulse and half continuous; and 1/4 in initial pulse.
Our results provide greatly increased detail in understanding population interactions and admixture during the European Neolithic. In each of our three study regions, the arrival of farmers prompted admixture with local hunter-gatherers, which unfolded over many centuries: almost all sampled populations have more hunter-gatherer ancestry and more recent dates of admixture than their local predecessors, suggesting recurrent changes in genetic composition and significant hunter-gatherer gene flow beyond initial contact. These transformations left distinct signatures in each region, implying that they resulted from a complex web of local interactions rather than a uniform demographic phenomenon. Our transect of Hungary, in particular, with representative samples from many archaeological cultures across the region and throughout the Neolithic and Chalcolithic, illustrates the power of dense ancient DNA time series. Future work with continually improving data sets and statistical models promises to yield many more insights about historical population transformations in space and time.
Methods
Experimental procedures
Prehistoric teeth and petrous bone samples from Hungary were taken under sterile conditions in the Hungarian Museums and anthropological collections. Samples other than Gorzsa were documented, cleaned, and ground into powder either in the Anthropological Department of the Johannes Gutenberg University of Mainz, during the course of the German Research Foundation project AL 287-10-1, or in Budapest, in the Laboratory of Archaeogenetics of the Institute of Archaeology, Research Centre for the Humanities, Hungarian Academy of Sciences, following published protocols26. DNA was extracted in Budapest using 0.08–0.11g powder via published methods31, using High Pure Viral NA Large Volume Kit columns (Roche)32,33. DNA extractions were tested by PCR, amplifying the 16117–16233 bp fragment of the mitochondrial genome, and visualized on a 2% agarose gel. DNA libraries were prepared from clean and successful extraction batches using UDG-half and no-UDG treated methods5,34. We included milling (hydroxylapatite blanks to control for cleanness) and extraction negative controls in every batch. Bar-code adapter ligated libraries were amplified with TwistAmp Basic (Twist DX Ltd), purified with Agencourt AMPure XP (Beckman Coulter), and checked on 3% agarose gel5. Library concentration was measured on a Qubit 2.0 fluorometer. Promising libraries after initial quality control analysis were shipped to Harvard Medical School, where further processing took place. All other samples were prepared similarly in dedicated clean rooms at Harvard Medical School and the University of Adelaide in accordance with published methods5,7,33. For samples LHUE2010.11 (one library) and MIR202-037-n105, we used magnetic bead cleanups instead of MinElute column cleanups between enzymatic reactions with magnetic bead cleanups and SPRI bead cleanup instead of the final PCR cleanup35,36.
We initially screened the libraries via in-solution hybridization to a set of probes targeting mitochondrial DNA (mtDNA)37 plus roughly 3000 nuclear SNP targets, using a protocol described previously5,33 with amplified baits synthesized by CustomArray, Inc. Libraries with good screening results—limited evidence of contamination, reasonable damage profiles, and substantial coverage on targeted segments—were enriched for a genome-wide set of ~1.2 million SNPs7,33 and sequenced to greater depth. Raw sequencing data were processed by trimming bar-codes and adapters, merging read pairs with at least 15 base pairs of overlapping sequence, and mapping to the human reference genome (version hg19). Reads were filtered for mapping and base quality, duplicate molecules were removed, and two terminal bases were clipped to eliminate damage (five for UDG-minus libraries)5. All libraries had a rate of at least 4.8% C-to-T substitutions in the final base of screening sequencing reads (Supplementary Table 1), consistent with damage patterns expected for authentic ancient DNA (refs 34, 38). Pseudo-haploid genotypes at each SNP were called by choosing one allele at random from among mapped reads. Sex determinations for each individual were made by manually examining the factions of reads mapping to the X and Y chromosomes and imposing thresholds for males and females (with any indeterminate samples labeled as unknown).
Mitochondrial DNA sequences were reassembled in Geneious R10 to rCRS (ref. 39) and RSRS (ref. 40), and SNPs with at least 3× coverage and a minimum variant frequency of 0.7 were called. The assembly and the resulting list of SNPs were double-checked against phylotree.org (mtDNA tree Build 17; 18 Feb 2016). Haplotype calls are given in Extended Data Tables 1 and 2 and Supplementary Table 2. On the Y chromosome, 15,100 SNPs were targeted and sequenced, and the detected derived and ancestral alleles were compared to the ISOGG Y-tree (www.isogg.org) version 12.34, updated on 5th February 2017. Haplogroup definitions are detailed in Supplementary Information section 3.
We merged libraries from the same individual (for those with more than one) and then combined our new samples with genome-wide data from the literature (ancient individuals as described and as listed in Extended Data Tables 1 and 2 and present-day individuals from the SGDP [ref. 41]) using all autosomal SNPs (~1.15 million) from our target set. For two replications of our admixture graph analyses, we restricted either to the subset of transversions (~280K SNPs) or to the subset from panels 4 and 5 of the Affymetrix Human Origins array (ascertained as heterozygous in a San or Yoruba individual; ~260K SNPs). For PCA (Extended Data Fig. 1), we merged with a large set of present-day samples33 and used all autosomal Human Origins SNPs (~593K).
To test for possible contamination, we used contamMix (ref. 42) and ANGSD (ref. 43) to estimate rates of apparent heterozygosity in haploid genome regions (mtDNA and the X chromosome in males, respectively). Any samples with > 5% mtDNA mismatching or > 2% X contamination were excluded from further analyses, with the exception of Bla5 (Supplementary Information section 8). We also removed samples identified as clear outliers in PCA or with significant population genetic differences between all sequencing data and genotypes called only from sequences displaying ancient DNA damage signatures. A total of 19 samples were excluded based on one of these criteria. For individual-level f-statistic analyses (Fig. 2A–B), we restricted to samples with a maximum level of uncertainty, defined as a standard error of at most 7×10−4 for the statistic f4(Mbuti, WHG; Anatolia, X). This threshold (corresponding to an average coverage of approximately 0.05, or ~60K SNPs hit at least once) was met by 89 of the 112 samples passing QC (and 49 of the 50 samples from the literature). We did not impose such a threshold for ALDER analyses, but because low coverage results in a weaker signal, only one of the 23 high-uncertainty individuals in our primary data set provided an ALDER date (as compared to 89 of the 130 low-uncertainty individuals).
Population assignments
In most cases, population groupings were used that correspond to archaeological culture assignments based on chronology, geography, and material culture traits. Occasionally, we merged populations that appeared similar genetically in order to increase power: we pooled samples from all phases and groups of the eastern Hungarian MN into a single ALPc population; merged six Sopot with eight Lengyel individuals for the western Hungarian TDLN; combined one Hunyadihalom (Middle CA from the Danube-Tisza interfluve in central Hungary) with Lasinja; pooled four LBK samples from Stuttgart with the majority from farther to the northeast (primarily Halberstadt); and merged several cultures of the German MN into a single group. Other populations vary in their degrees of date and site heterogeneity, with Iberia MN the most homogeneous and Iberia EN and CA among the least (Extended Data Tables 1, 2; Supplementary Table 1). For our main analyses, we excluded the Vinča and Tiszapolgár population groups because they lacked sufficient high-quality data.
We note that the designations EN, MN, LN, and CA have different meanings in different areas. For our study regions, each term generally refers to an earlier period in Hungary than in Germany and Spain (for example, ALPc and LBKT MN in Hungary are roughly contemporaneous with LBK and Iberia EN). In order to maintain agreement with the archaeological literature, we use the established definitions, with the appropriate word of caution that they should be treated separately in each region.
Sample dates
We report 52 newly obtained accelerator mass spectrometry (AMS) radiocarbon dates for Neolithic individuals (45 direct, 7 indirect), focusing on representative high-quality samples from each site and any samples with chronological uncertainty. These are combined with 58 radiocarbon dates from the literature4,5,7,17,18,26,29,30,44,45. We report the 95.4% calibrated confidence intervals (CI) from OxCal (ref. 46) version 4.2 with the IntCal13 calibration curve47 in Extended Data Tables 1 and 2. For use in ALDER analyses (Supplementary Information section 7), we use the mean and standard deviation of the calibrated date distributions; while the distributions are non-normal, we find that on average the mean plus or minus two standard deviations contains more than 95.4% of the probability density. For samples without direct radiocarbon dates but with dates from other samples or materials at the same site, we form a conservative 95.4% CI by taking the minimum and maximum bounds of any of the calibrated CIs from the site. Finally, for the remaining samples, we use plausible date ranges based on archaeological context; we assume independence across individuals but as a result take a conservative approach and treat the assigned range as ± one standard error (e.g., an estimated range of 4800–4500 BCE becomes 4650 ± 150 BCE).
Population genetic analyses
We performed PCA by computing components for present-day populations and then projecting ancient individuals using the “lsqproject” and “shrinkmode” options in smartpca (ref. 48). Admixture graphs and f-statistics were implemented through ADMIXTOOLS (ref. 49). To obtain calendar dates of admixture, we combine the ALDER results (in generations in the past) with the ages of the Neolithic individuals, assuming an average generation time of 28 years50,51. All analytical procedures are described in full detail in Supplementary Information sections 4–9.
Data availability
The aligned sequences are available through the European Nucleotide Archive under accession number PRJEB22629. Genotype datasets used in analysis are available at https://reich.hms.harvard.edu/datasets.
Extended Data
Extended Data Figure 1. First two principal components from PCA.
We computed PCs for a set of 782 present-day western Eurasian individuals genotyped on the Affymetrix Human Origins array (background gray points) and then projected ancient individuals onto these axes. Shown is a closeup omitting the present-day Bedouin population.
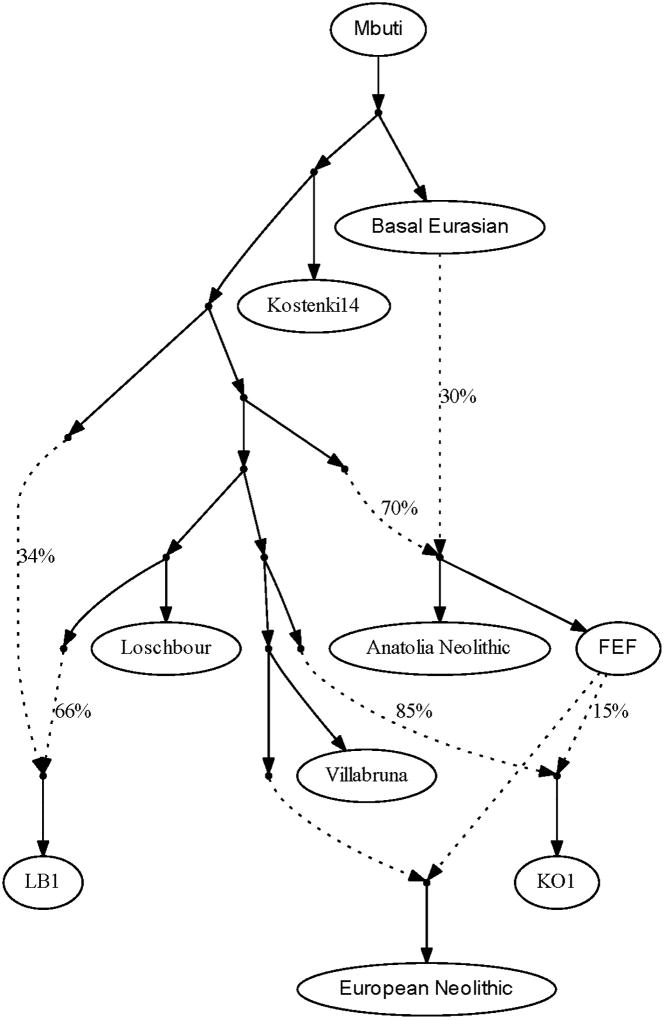
Extended Data Figure 2. Scaffold admixture graph used for modeling European Neolithic populations.
Dotted lines denote admixture events. Neolithic Anatolians, LB1, and KO1 are modeled as admixed, with Basal Eurasian ancestry, deeper European hunter-gatherer ancestry, and FEF ancestry, respectively. European test populations are fit as a mixture of FEF and ancestry related to one or two of the four WHG individuals (here VIL-related as an example). See Supplementary Information section 6 for full details.
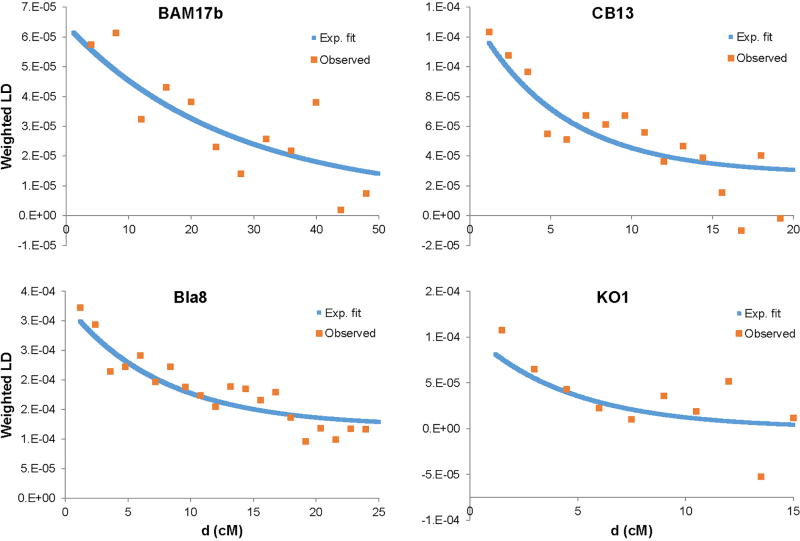
Extended Data Figure 3. Examples of ALDER weighted LD decay curves.
Weighted LD is shown as a function of genetic distance d, using Neolithic Anatolians and WHG as references, for four individuals: BAM17b (Starčevo EN), CB13 (Iberia EN), Bla8 (Blätterhöhle hunter-gatherer), and KO1. The results shown here use helper individuals M11–363 (Neolithic Anatolian), L11–322 (Neolithic Anatolian), BIC, and LB1, respectively, and have fitted dates (blue curves) of 3.8±1.2, 18.3±6.0, 13.1±2.7, and 21.6±8.8 generations (compared to final individual-level dates of 4.5±1.9, 17.5±3.5, 12.1±2.9, and 21.0±7.0 generations; see Supplementary Information section 7). Note different x-axis scales for the four individuals.
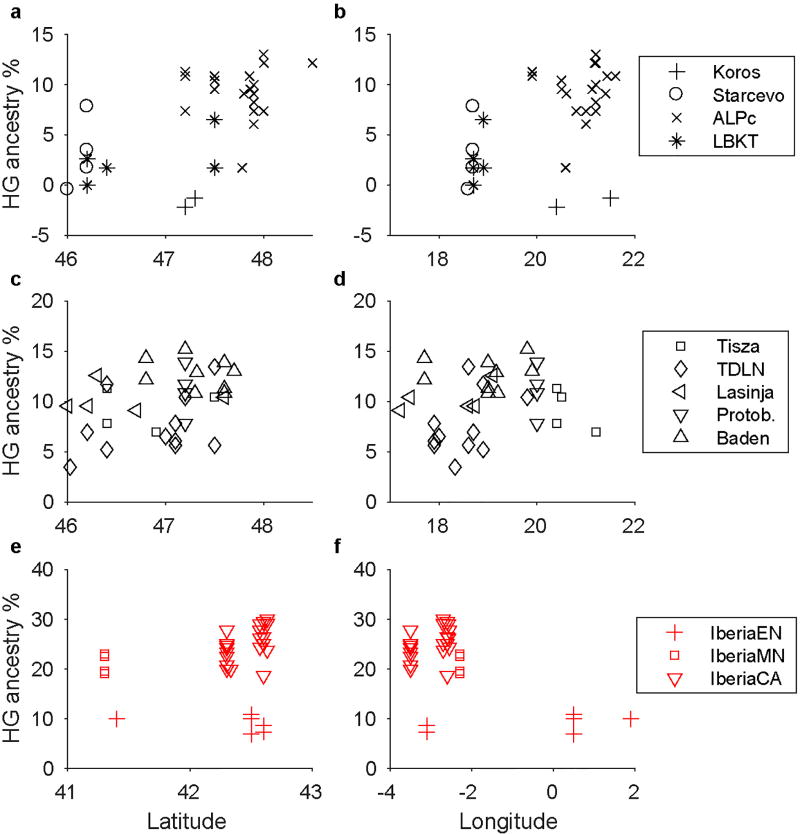
Extended Data Figure 4. Hunter-gatherer ancestry as a function of latitude and longitude for Neolithic individuals.
a, b, EN/MN Hungary. c, d, LN/CA Hungary. e, f, Iberia. Protob., Protoboleráz.
Extended Data Figure 5. Germany and Iberia time series and simulated data.
a, Dates of admixture. b, Hunter-gatherer ancestry proportions, normalized by the total in the most recent (rightmost) population. Symbols are as in Figs 1 and 2, here showing population-level averages plus or minus two standard errors. Yellow dashed lines represent continuous admixture simulations: from top to bottom, diminishing 5% per generation, diminishing 3%, diminishing 1%, and uniform. Green solid lines represent pulse-plus-continuous admixture simulations: from top to bottom, all hunter-gatherer ancestry in a pulse at time zero; 3/4 of final hunter-gatherer ancestry in an initial pulse, followed by uniform continuous gene flow; half in initial pulse and half continuous; and 1/4 in initial pulse.
Extended Data Table 1.
Information for Neolithic individuals from Hungary.
| ID | Population | Site | Lat. | Long. | Date | Sex | Mt Hap | γ Hap | Cov. | HG% | ALD | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GEN68 | Körös | Törökszentmiklós road 4 site 3 | 47.2 | 20.4 | 5706–5541 | F | k1a | ‥ | 6.16 | −2.16±1.5 | 0±0.0 | |
| HUNG276, KO2 | Körös | Berettyóújfalu-Morotva-liget | 47.3 | 21.5 | 5713–5566 | F | K1a | ‥ | 0.91 | −1.49±1.6 | 0±0.0 | [7, 17] |
| TIDO2a | Körös | Tiszaszőlős-Domaháza | 47.6 | 20.7 | 5736–5547 | M | K1 | I2a2 | 0.45 | 79.3±2.1 | 16±3.8 | |
| BAM17b | Starčevo | Alsónyék-Bátaszék, Mérnöki telep | 46.2 | 18.7 | 5832–5667 | M | T1a2 | H2 | 1.47 | 7.76±1.7 | 4.5±1.9 | |
| BAM25 | Starčevo | Alsónyék-Bátaszék, Mérnöki telep | 46.2 | 18.7 | 5702–5536 | M | N1a1a1 | H2 | 0.22 | 1.62±1.9 | 0±0.0 | [5, 7] |
| BAM4a | Starčevo | Alsónyék-Bátaszék, Mérnöki telep | 46.2 | 18.7 | 5641–5547 | M | K1a4 | G2a2a1 | 0.20 | 3.39±2.0 | 0±0.0 | |
| LGCS1a | Starčevo | Lánycsók | 46.0 | 18.6 | 5800–5500 | M | W5 | G2a2b2b1a | 0.77 | −0.63±1.6 | 0±0.0 | |
| BAL25b | LBKT | Bátaszék-Lajvér | 46.2 | 18.7 | 5208–4948 | M | K1b1a | G2a2a1 | 2.77 | 0.06±1.5 | 0±0.0 | |
| BOVO1b | LBKT | Bölcske-Gyűrűsvölgy | 46.7 | 19.0 | 5300–4900 | F | H | ‥ | 0.01 | 10.9±6.3 | 0±0.0 | |
| BUD4a | LBKT | Budakeszi-Szőlőskert | 47.5 | 18.9 | 5300–4900 | M | T1a | G2a2b2a | 0.17 | 6.72±2.3 | 36±6.1 | |
| BUD9a | LBKT | Budakeszi-Szőlőskert | 47.5 | 18.9 | 5300–4900 | F | U2 | ‥ | 1.10 | 1.87±1.6 | 13±5.3 | |
| GEN18 | LBKT | Alsónyék, site 11 | 46.2 | 18.7 | 5309–5074 | M | T2c1 | G2a2b2b1 | 1.48 | 2.66±1.5 | 35±12 | |
| KON3 | LBKT | Enese elkerülő, Kóny, Proletár-dülö, M85, site 2 | 47.6 | 17.4 | 5300–4900 | F | T2b | ‥ | 0.03 | 2.79±4.0 | 0±0.0 | |
| SZEH4 | LBKT | Szemely-Hegyes | 46.4 | 18.7 | 5207–4944 | F | N1a1a1a3 | ‥ | 0.07 | 1.88±3.0 | 0±0.0 | [5, 7] |
| CEG07B | ALPc | Cegléd, site 4/1 | 47.2 | 19.9 | 5300–4900 | M | J2b1 | G2a2b2a | 0.30 | 11.4±1.9 | 0±0.0 | |
| CEG08b | ALPc | Cegléd, site 4/1 | 47.2 | 19.9 | 5300–4900 | F | J1c1 | ‥ | 0.19 | 11.0±2.2 | 23±3.0 | |
| EBSA2a | ALPc | Ebes-Sajtgyár | 47.5 | 21.5 | 5300–4900 | F | K1a | ‥ | 0.05 | 16.2±3.1 | 0±0.0 | |
| EBVO5a | ALPc | Ebes-Zsongvölgy | 47.5 | 21.5 | 5300–4900 | M | V1a | CT | 0.04 | 9.25±3.3 | 0±0.0 | |
| HAJE10a | ALPc | Hajdúnánás-Eszlári út | 47.9 | 21.4 | 5221–5000 | M | J2b1 | I | 0.29 | 10.8±1.8 | 0±0.0 | |
| HAJE7a | ALPc | Hajdúnánás-Eszlári út | 47.9 | 21.4 | 5302–5057 | M | K1a | I2 | 1.57 | 9.15±1.7 | 6.2±5.7 | |
| HELI11a | ALPc | Hejőkürt-Lidl | 47.9 | 21.0 | 5209–4912 | M | N1a1a1 | I2a2a1b | 0.99 | 6.01±1.8 | 14±2.0 | |
| HELI2a | ALPc | Hejőkürt-Lidl | 47.9 | 21.0 | 5300–4900 | M | U8b1b | I | 0.09 | 7.39±2.6 | 4.4±1.7 | |
| HUNG302, NE2 | ALPc | Debrecen Tocopart Erdoalja | 47.5 | 21.6 | 5291–5056 | F | H | ‥ | 4.88 | 11.0±1.7 | 0±0.0 | [7, 17] |
| HUNG372, NE5 | ALPc | Kompolt-Kígyósér | 47.2 | 20.8 | 5295–4950 | M | J1c1 | C1a2 | 4.25 | 7.48±1.6 | 0±0.0 | [7, 17] |
| HUNG86, NE3 | ALPc | Garadna-Elkerülő út site 2 | 48.5 | 21.2 | 5281–5026 | F | X2b-T226C | ‥ | 3.32 | 12.1±1.7 | 18±3.2 | [7, 17] |
| MEMO24b | ALPc | Mezőkövesd-Mocsolyás | 47.8 | 20.6 | 5500–5300 | M | U8b1b | CT | 0.04 | 11.7±3.3 | 26±12 | |
| MEMO2b | ALPc | Mezőkövesd-Mocsolyás | 47.8 | 20.6 | 5500–5300 | F | K1a1 | ‥ | 2.28 | 8.99±1.7 | 24±5.2 | |
| MEMO7a | ALPc | Mezőkövesd-Mocsolyás | 47.8 | 20.6 | 5481–5361 | F | HV | ‥ | 0.26 | 1.64±1.9 | 13±6.1 | |
| PF325, NE1 | ALPc | Polgár-Ferenci-hát | 47.9 | 21.2 | 5306–5071 | F | U5b2c | ‥ | 1.52 | 8.12±1.8 | 11±3.9 | [7, 17] |
| PF839/1198, NE4 | ALPc | Polgár-Ferenci-hát | 47.9 | 21.2 | 5211–5011 | F | J1c5 | ‥ | 3.49 | 9.95±1.7 | 25±10 | [7, 17] |
| POPI5a | ALPc | Polgár-Piócás | 47.9 | 21.1 | 5300–4900 | M | K1a1 | I2a2a | 0.31 | 9.75±2.0 | 11±3.7 | |
| PULE1.18a | ALPc | Pusztataskony-Ledence | 47.5 | 20.5 | 5300–4900 | F | T2c1d1 | ‥ | 0.29 | 10.6±1.8 | 0±0.0 | |
| PULE1.23a | ALPc | Pusztataskony-Ledence | 47.5 | 20.5 | 5300–4900 | F | H1e | ‥ | 0.17 | 9.52±2.2 | 11±3.4 | |
| TISO13a | ALPc | Tiszadob-Ókenéz | 48.0 | 21.2 | 5208–4942 | M | J1c2 | I2a2a | 1.21 | 12.9±1.7 | 22±7.6 | |
| TISO1b | ALPc | Tiszadob-Ókenéz | 48.0 | 21.2 | 5300–4900 | M | H7 | I2a2a1b1 | 0.11 | 7.24±2.4 | 0±0.0 | |
| TISO3a | ALPc | Tiszadob-Ókenéz | 48.0 | 21.2 | 5300–4900 | F | U5b2b1a | ‥ | 0.27 | 12.1±2.1 | 8.4±5.2 | |
| SEKU10a | Vinča | Szederkény-Kukorica-dülö | 45.6 | 18.3 | 5320–5080 | M | K2a | G2a2b2a1a | 0.24 | 2.28±1.9 | 0±0.0 | |
| SEKU6a | Vinča | Szederkény-Kukorica-dülö | 45.6 | 18.3 | 5321–5081 | F | H26 | ‥ | 1.15 | 9.16±1.7 | 9.0±9.4 | |
| VEGI17a | Vinča | Versend-Gilencsa | 45.6 | 18.3 | 5400–5000 | F | U2 | ‥ | 0.01 | −6.14±5.6 | 0±0.0 | |
| VEGI3a | Vinča | Versend-Gilencsa | 45.6 | 18.3 | 5400–5000 | M | T2b | H2 | 0.41 | 0.53±1.8 | 0±0.0 | |
| Gorzsa18 | Tisza | Hódmezővásárhely-Gorzsa | 46.4 | 20.4 | 5000–4500 | M | U5b2c | I2a1 | 6.87 | 7.77±1.6 | 13±4.3 | |
| Gorzsa4 | Tisza | Hódmezővásárhely-Gorzsa | 46.4 | 20.4 | 5000–4500 | F | T1a | ‥ | 0.06 | 11.3±3.0 | 22±11 | |
| KOKE3a | Tisza | Hódmezővásárhely-Kökénydomb Vörös tanya | 46.4 | 20.2 | 5000–4500 | M | K1b1 | I | 0.06 | 13.7±3.2 | 0±0.0 | |
| PULE1.24 | Tisza | Pusztataskony-Ledence | 47.5 | 20.5 | 5000–4500 | F | K1a4 | ‥ | 0.40 | 10.4±1.9 | 18±7.2 | |
| VSM3a | Tisza | Vésztő-Mágor | 46.9 | 21.2 | 5000–4500 | M | H26 | G2a | 0.09 | 6.92±2.6 | 0±0.0 | |
| ALE14a | TDLN | Alsónyék-Elkerülő site 2 | 46.2 | 18.7 | 5030–4848 | M | U8b1b | G2a | 0.05 | −1.11±3.2 | 0±0.0 | |
| ALE4a | TDLN | Alsónyék-Elkerülő site 2 | 46.2 | 18.7 | 5016–4838 | M | T2c1 | F | 0.03 | 10.6±3.6 | 0±0.0 | |
| BAL3a | TDLN | Bátaszék-Lajvér | 46.2 | 18.7 | 4800–4500 | M | T2f | H1b1 | 0.91 | 6.89±1.7 | 22±9.0 | |
| CSAT19a | TDLN | Csabdi-Télizöldes | 47.5 | 18.6 | 4800–4500 | M | H | H | 0.52 | 5.82±1.8 | 34±9.6 | |
| CSAT25a | TDLN | Csabdi-Télizöldes | 47.5 | 18.6 | 4826–4602 | M | T2b | I2 | 0.43 | 13.5±1.9 | 26±8.1 | |
| FAGA1a | TDLN | Fajsz-Garadomb | 46.4 | 18.9 | 5100–4750 | M | HVOa | I | 0.09 | 5.08±2.4 | 0±0.0 | |
| FAGA2a | TDLN | Fajsz-Garadomb | 46.4 | 18.9 | 5195–4842 | F | H | ‥ | 0.49 | 11.9±1.8 | 14±4.1 | |
| FEB3a | TDLN | Felsőörs-Bárókert | 47.0 | 18.0 | 4800–4500 | M | H44 | J2a | 0.16 | 6.31±2.1 | 0±0.0 | |
| HUNG347, NE7 | TDLN | Apc-Berekalja | 47.2 | 19.8 | 4491–4357 | M | N1a1a1a | I | 4.85 | 10.6±1.6 | 19±3.1 | [7, 17] |
| SZEH5a | TDLN | Szemely-Hegyes | 46.0 | 18.3 | 4904–4709 | M | K1b1a | G | 0.01 | 10.8±6.5 | 0±0.0 | |
| SZEH7b | TDLN | Szemely-Hegyes | 46.0 | 18.3 | 4930–4715 | F | K1a | ‥ | 0.52 | 3.44±1.7 | 0±0.0 | |
| VEJ12a | TDLN | Veszprém Jutasi út | 47.1 | 17.9 | 4800–4500 | M | U8b1a2b | H | 0.10 | 6.17±2.3 | 0±0.0 | |
| VEJ2a | TDLN | Veszprém Jutasi út | 47.1 | 17.9 | 4800–4500 | M | T2b | C | 0.34 | 5.63±1.8 | 0±0.0 | |
| VEJ5a | TDLN | Veszprém Jutasi út | 47.1 | 17.9 | 4936–4742 | M | J1c2 | G2a2a1 | 0.62 | 7.78±1.8 | 15±2.9 | |
| GEN67 | Tiszapolgár | Törökszentmiklós road 4 site 3 | 47.2 | 20.4 | 4444–4257 | M | H1 | I2a2a1b | 2.28 | 13.0±1.7 | 50±15 | |
| PULE1.10a | Tiszapolgár | Pusztataskony-Ledence | 47.5 | 20.5 | 4500–4000 | M | T2c1 | I2a | 0.28 | 9.03±2.0 | 0±0.0 | |
| PULE1.13a | Tiszapolgár | Pusztataskony-Ledence | 47.5 | 20.5 | 4500–4000 | M | T2c1 | G2a2b2a1a1c1a | 0.38 | 10.3±1.9 | 0±0.0 | |
| PULE1.9a | Tiszapolgár | Pusztataskony-Ledence | 47.5 | 20.5 | 4500–4000 | M | H26 | G2a2b | 0.11 | 11.6±2.4 | 0±0.0 | |
| GEN100 | Lasinja | Alsónyék, site 11 | 46.2 | 18.7 | 4300–3900 | F | T2b | ‥ | 1.81 | 9.51±1.6 | 45±11 | |
| GEN49 | Lasinja | Nemesnádudvar-Papföld | 46.3 | 19.1 | 4228–3963 | M | T2b23 | CT | 0.97 | 12.8±1.8 | 27±6.8 | |
| KEFP2a | Lasinja | Keszthely-Fenékpuszta | 46.7 | 17.2 | 4300–3900 | F | J2b1a | ‥ | 0.74 | 9.12±1.7 | 21±5.4 | |
| KON2a | Lasinja | Enese elkerülő, Kóny, Proletár-dülö, M85, site 2 | 47.6 | 17.4 | 4333–4072 | F | K2a | ‥ | 2.13 | 10.3±1.7 | 21±6.4 | |
| M6-116.12a | Lasinja | Lánycsók, Csata-alja | 46.0 | 18.6 | 4232–4046 | F | T2f8a | ‥ | 0.64 | 9.68±1.7 | 29±11 | |
| VEJ9a | Lasinja | Veszprém Jutasi út | 47.1 | 17.9 | 4339–4237 | M | H40 | CT | 0.05 | 8.83±3.2 | 0±0.0 | |
| GEN60 | Protoboleráz | Abony, Turjányos-dűlő | 47.2 | 20.0 | 3909–3651 | M | H | G2a2b2a | 1.88 | 14.0±1.6 | 37±8.8 | |
| GEN61 | Protoboleráz | Abony, Turjányos-dűlő | 47.2 | 20.0 | 3800–3600 | M | J1c | I2c | 0.76 | 10.8±1.7 | 65±13 | |
| GEN62 | Protoboleráz | Abony, Turjányos-dűlő | 47.2 | 20.0 | 3762–3636 | F | N1a1a1a3 | ‥ | 4.81 | 8.00±1.6 | 37±9.6 | |
| GEN63 | Protoboleráz | Abony, Turjányos-dűlő | 47.2 | 20.0 | 3658–3384 | M | U5a1c1 | I2c | 1.92 | 11.9±1.7 | 34±8.1 | |
| GEN12a | Baden | Budakalász-Luppa csárda | 47.6 | 19.0 | 3340–2945 | M | H26a | G2a2b2a1a1b1 | 1.98 | 13.8±1.6 | 34±7.2 | |
| GEN13a | Baden | Budakalász-Luppa csárda | 47.6 | 19.0 | 3332–2929 | M | HV | G2a2b2a1a | 2.65 | 11.3±1.6 | 27±6.6 | |
| GEN15a | Baden | Budakalász-Luppa csárda | 47.6 | 19.0 | 3367–3103 | M | J2a1a1 | G2a2b2a1a1c1a | 1.66 | 10.8±1.7 | 22±9.3 | |
| GEN16a | Baden | Alsónémedi | 47.3 | 19.2 | 3346–2945 | F | T2b | ‥ | 4.30 | 12.9±1.6 | 38±16 | |
| GEN17a | Baden | Alsónémedi | 47.3 | 19.2 | 3359–3098 | M | U5b3f | G2a2a | 0.82 | 10.7±1.7 | 21±6.4 | |
| GEN21 | Baden | Balatonlelle-Felső-Gamász | 46.8 | 17.7 | 3600–2850 | M | K1a | I2a1 | 0.67 | 12.3±1.7 | 0±0.0 | |
| GEN22 | Baden | Balatonlelle-Felső-Gamász | 46.8 | 17.7 | 3332–2929 | M | U5a1 | I2a1a1 | 2.31 | 14.5±1.7 | 25±6.6 | |
| GEN55 | Baden | Vámosgyörk | 47.7 | 19.9 | 3600–2850 | F | T2c1d1 | ‥ | 0.81 | 13.1±1.7 | 22±6.6 | |
| HUNG353, CO1 | Baden | Apc-Berekalja | 47.2 | 19.8 | 3315–2923 | F | H | ‥ | 4.56 | 15.1±1.7 | 0±0.0 | [7, 17] |
| Vors1 | Baden | Vörs | 46.7 | 17.3 | 3300–2850 | F | T2f | ‥ | 0.03 | 4.47±4.2 | 0±0.0 |
Cov: average coverage per SNP. HG%: inferred percentage of hunter-gatherer ancestry (mean ± standard error). ALD inferred date of admixture (generations in the past; mean ± standard error; zero implies no date obtained). Ref: reference for published data; if blank, newly published sample in this study (asterisk denotes a published individual with new sequencing data added). Radiocarbon dates are in normal text, while dates estimated from archaeological context are in italics. Further information can be found in Supplementary Table 1.
Extended Data Table 2.
Information for Neolithic individuals from Germany and Spain.
| ID | Population | Site | Lat. | Long. | Date | Sex | Mt Hap | Y Hap | Cov. | HG% | ALD | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HAL03a | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5295–5057 | F | T2b | ‥ | 0.01 | −5.13±6.8 | 0±0.0 | |
| HAL07a | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5212–4992 | F | N1a1a1 | ‥ | 0.05 | 1.72±3.2 | 0±0.0 | |
| HAL15a | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5199–4857 | M | N1a1a1a3 | G2 | 0.02 | 5.26±5.0 | 0±0.0 | |
| HAL17b | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5500–4850 | F | V1 | ‥ | 0.02 | 9.21±4.2 | 0±0.0 | |
| HAL18a | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5500–4850 | F | K2a | ‥ | 0.02 | 0.27±4.6 | 0±0.0 | |
| HAL19 | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5500–4850 | F | K1a2 | ‥ | 0.86 | 7.10±1.7 | 16±7.6 | [7]* |
| HAL2 | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5211–4963 | M | N1a1a1a2 | G2a2a1 | 0.76 | 1.91±1.7 | 11±2.4 | [5, 7]* |
| HAL20b | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5500–4850 | M | K1a2 | G2a2a | 0.06 | 2.53±3.1 | 0±0.0 | |
| HAL21a | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5500–4850 | M | T2b | G2a2a | 0.01 | −4.41±5.8 | 0±0.0 | |
| HAL22b | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5500–4850 | F | T2b | ‥ | 0.02 | −7.71±4.7 | 0±0.0 | |
| HAL24 | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5201–4850 | M | X2d1 | G2a2a1 | 0.42 | 6.39±1.8 | 0±0.0 | [5, 7]* |
| HAL25 | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5210–5002 | M | K1a | G2a2a1 | 0.49 | 2.58±1.7 | 18±6.6 | [5, 7]* |
| HAL27a | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5500–4850 | M | N1a1a3 | G2a2a | 0.05 | 3.84±3.0 | 0±0.0 | |
| HAL31a | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5295–5057 | F | K1 | ‥ | 0.12 | 4.54±2.3 | 11±3.1 | |
| HAL32b | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5500–4850 | F | H26 | ‥ | 0.23 | 3.34±2.0 | 23±4.4 | |
| HAL34 | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5219–5021 | F | N1a1a1 | ‥ | 0.25 | 5.63±2.0 | 9.2±5.0 | [5, 7]* |
| HAL35b | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5500–4850 | F | J1c | ‥ | 0.10 | 3.93±2.4 | 0±0.0 | |
| HAL38a | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5500–4850 | F | V1 | ‥ | 0.29 | 1.10±1.9 | 0±0.0 | |
| HAL39b | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5210–5002 | M | H1e | G2a2a1 | 0.08 | 3.96±2.6 | 0±0.0 | |
| HAL4 | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5202–4852 | F | N1a1a1a | ‥ | 6.92 | 6.55±1.6 | 18±5.9 | [5, 7]* |
| HAL40a | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5500–4850 | F | T2b | ‥ | 0.17 | 2.50±2.1 | 0±0.0 | |
| HAL5 | LBK | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 5211–4991 | F | T2c1 | ‥ | 2.23 | 2.98±1.6 | 15±5.4 | [5, 7]* |
| KAR16A | LBK | Karsdorf | 51.3 | 11.7 | 5500–4850 | M | H46b | T1a | 0.09 | 0.28±2.6 | 13±5.1 | [7] |
| KAR6 | LBK | Karsdorf | 51.3 | 11.7 | 5217–5041 | M | H1/H1au1b | CT | 0.10 | 5.78±2.5 | 0±0.0 | [5, 7] |
| LBK1976 | LBK | Viesenhäuser Hof | 48.8 | 9.2 | 5500–4850 | F | T2e | ‥ | 0.44 | 3.46±1.7 | 18±4.4 | [5, 7] |
| LBK1992 | LBK | Viesenhäuser Hof | 48.8 | 9.2 | 5500–4850 | F | T2b | ‥ | 2.66 | 5.68±1.6 | 12±4.3 | [5, 7] |
| LBK2155 | LBK | Viesenhäuser Hof | 48.8 | 9.2 | 5500–4850 | F | T2b | ‥ | 3.63 | 4.84±1.5 | 13±4.4 | [5, 7] |
| Stuttgart | LBK | Viesenhäuser Hof | 48.8 | 9.2 | 5310–5076 | F | T2c1d1 | ‥ | 9.65 | 3.00±1.6 | 22±8.1 | [4]* |
| UWS4 | LBK | Unterwiederstedt | 51.7 | 11.5 | 5223–5021 | F | J1c17 | ‥ | 18.6 | 5.70±1.6 | 13±14 | [5, 7] |
| ESP30 | GermanyMN | Esperstedt | 51.4 | 11.7 | 3970–3710 | M | H1e1a | I | 0.09 | 22.0±2.7 | 0±0.0 | [5, 7] |
| HAL13a | GermanyMN | Halberstadt-Sonntagsfeld | 51.9 | 11.0 | 4600–4300 | F | V1a | ‥ | 0.11 | 9.04±2.4 | 13±4.3 | |
| QLB15D | GermanyMN | Quedlinburg | 51.8 | 11.1 | 3654–3527 | M | HV | R | 0.16 | 20.9±2.2 | 36±8.7 | [5, 7] |
| QLB18A | GermanyMN | Quedlinburg | 51.8 | 11.1 | 3640–3376 | F | T2e1 | ‥ | 0.41 | 19.6±1.8 | 23±4.9 | [5, 7] |
| SALZ3B | GermanyMN | Salzmünde-Schiebzig | 51.5 | 11.8 | 3400–3025 | M | U3a1 | G2a2a1 | 0.09 | 14.9±2.7 | 0±0.0 | [7] |
| SALZ57A | GermanyMN | Salzmünde-Schiebzig | 51.5 | 11.8 | 3345–3097 | F | H3 | ‥ | 0.02 | 25.0±4.4 | 0±0.0 | |
| SALZ77A | GermanyMN | Salzmünde-Schiebzig | 51.5 | 11.8 | 3400–3025 | M | H3 | IJK (x J) | 0.02 | 21.3±5.0 | 0±0.0 | |
| Bla16 | Blätterhöhle | Blätterhöhle Cave | 51.4 | 7.6 | 3958–3344 | M | U5b2a2 | R1b1 | 0.80 | 39.5±1.9 | 15±5.8 | |
| Bla28 | Blätterhöhle | Blätterhöhle Cave | 51.4 | 7.6 | 3337–3024 | M | J1c1b1 | R1 | 0.10 | 51.9±2.7 | 11±4.5 | |
| Bla5 | Blätterhöhle | Blätterhöhle Cave | 51.4 | 7.6 | 3704–3117 | F | H5 | ‥ | 5.07 | 41.2±1.9 | 24±4.7 | |
| Bla8 | Blätterhöhle | Blätterhöhle Cave | 51.4 | 7.6 | 4038–3532 | M | U5b2b2 | I2a1 | 4.58 | 72.6±2.0 | 12±2.9 | |
| CB13 | Iberia EN | Cova Bonica | 41.4 | 1.9 | 5469–5327 | F | K1a2a | ‥ | 0.98 | 9.97±1.7 | 17±3.5 | [18] |
| E-06-Ind1 | Iberia EN | EI Prado de Pancorbo | 42.6 | −3.1 | 4827–4692 | F | K1a4a1 | ‥ | 0.47 | 8.72±1.8 | 17±2.3 | |
| E-14-Ind2 | Iberia EN | EI Prado de Pancorbo | 42.6 | −3.1 | 5216–5031 | F | H1 | ‥ | 0.38 | 7.52±1.8 | 19±2.8 | |
| Troc1 | Iberia EN | Els Trocs | 42.5 | 0.5 | 5311–5218 | F | J1c3 | ‥ | 0.69 | 7.15±1.7 | 12±9.1 | [5, 7] |
| Troc3 | Iberia EN | Els Trocs | 42.5 | 0.5 | 5294–5066 | M | T2c1d/T2c1d2 | R1b1a | 1.31 | 9.91±1.8 | 49±22 | [5, 7] |
| Troc5 | Iberia EN | Els Trocs | 42.5 | 0.5 | 5310–5078 | M | N1a1a1 | I2a1b1 | 13.8 | 6.83±1.6 | 6.8±2.8 | [5, 7] |
| Troc7 | Iberia EN | Els Trocs | 42.5 | 0.5 | 5303–5075 | F | V | ‥ | 1.57 | 11.0±1.7 | 18±4.8 | [5, 7] |
| Mina18 | Iberia MN | La Mina | 41.3 | −2.3 | 3893–3661 | F | U5b1 | ‥ | 13.6 | 22.8±1.7 | 42±18 | [5, 7] |
| Mina3 | Iberia MN | La Mina | 41.3 | −2.3 | 3900–3600 | M | K1a1b1 | H2 | 0.38 | 19.5±1.9 | 80±20 | [5, 7] |
| Mina4 | Iberia MN | La Mina | 41.3 | −2.3 | 3900–3600 | M | H1 | I2a2a1b2 | 3.95 | 22.6±1.9 | 25±6.2 | [5, 7] |
| Mina6 | Iberia MN | La Mina | 41.3 | −2.3 | 3900–3600 | F | K1b1a1 | ‥ | 1.36 | 18.9±1.7 | 46±8.2 | [5, 7] |
| 1.-K11 | Iberia CA | La Chabola de la Hechicera | 42.6 | −2.6 | 3263–2903 | M | X2b | I2a2 | 0.18 | 27.8±2.1 | 68±28 | |
| 3.-K11 | Iberia CA | La Chabola de la Hechicera | 42.6 | −2.6 | 3627–3363 | F | J2a1a1 | ‥ | 0.12 | 24.4±2.4 | 27±11 | |
| 5.-K18 | Iberia CA | La Chabola de la Hechicera | 42.6 | −2.6 | 3090–2894 | M | J1c1 | I2a2 | 0.10 | 18.5±2.5 | 43±11 | |
| ES.1/4 | Iberia CA | EI Sotillo | 42.6 | −2.6 | 2571–2347 | M | H3 | I | 0.07 | 25.4±2.8 | 0±0.0 | |
| ES-6G-110 | Iberia CA | EI Sotillo | 42.6 | −2.6 | 2916–2714 | M | H3 | I2a2a | 0.05 | 25.4±3.2 | 0±0.0 | |
| Inventario0/4 | Iberia CA | EI Sotillo | 42.6 | −2.6 | 2481–2212 | M | X2b | I2a2a | 0.12 | 29.6±2.5 | 56±23 | |
| LHUE11J.5 | Iberia CA | Alto de la Huesera | 42.6 | −2.6 | 3092–2877 | F | U5b1 | ‥ | 1.19 | 26.7±1.9 | 40±9.7 | |
| LHUE2010.10 | Iberia CA | Alto de la Huesera | 42.6 | −2.6 | 3014–2891 | F | J1c1 | ‥ | 0.11 | 25.2±2.5 | 64±13 | |
| LHUE2010.11 | Iberia CA | Alto de la Huesera | 42.6 | −2.6 | 3092–2918 | M | V | G2a2a | 5.36 | 28.9±1.8 | 38±12 | |
| LHUE2014.11J | Iberia CA | Alto de la Huesera | 42.6 | −2.6 | 3100–2850 | F | U5b2b | ‥ | 0.06 | 26.3±3.0 | 0±0.0 | |
| LY.II.A.10.15066 | Iberia CA | Las Yurdinas II | 42.6 | −2.7 | 3350–2750 | M | U5b2b3a | I2a2a | 1.93 | 30.0±1.8 | 0±0.0 | |
| LY.II.A.10.15067 | Iberia CA | Las Yurdinas II | 42.6 | −2.7 | 3350–2750 | F | J2a1a1 | ‥ | 0.30 | 23.8±2.0 | 0±0.0 | |
| LY.II.A.10.15068 | Iberia CA | Las Yurdinas II | 42.6 | −2.7 | 3350–2750 | F | K1a4a1 | ‥ | 0.39 | 29.2±1.9 | 26±10 | |
| LY.II.A.10.15069 | Iberia CA | Las Yurdinas II | 42.6 | −2.7 | 3354–2943 | F | J1c3 | ‥ | 4.24 | 25.1±1.7 | 28±15 | |
| MIR1 | Iberia CA | EI Mirador Cave | 42.3 | −3.5 | 2900–2346 | F | K1a | ‥ | 0.24 | 24.2±2.1 | 0±0.0 | [7] |
| MIR13 | Iberia CA | EI Mirador Cave | 42.3 | −3.5 | 2900–2346 | F | H3c3 | ‥ | 0.10 | 27.8±2.4 | 0±0.0 | [7] |
| MIR14 | Iberia CA | EI Mirador Cave | 42.3 | −3.5 | 2568–2346 | M | H3 | I2a2a | 0.94 | 23.3±1.8 | 57±15 | [7] |
| MIR17 | Iberia CA | EI Mirador Cave | 42.3 | −3.5 | 2900–2346 | F | J1c1 | ‥ | 0.22 | 23.6±2.2 | 0±0.0 | [7] |
| MIR18 | Iberia CA | EI Mirador Cave | 42.3 | −3.5 | 2865–2575 | F | H1t | ‥ | 1.58 | 20.0±1.6 | 0±0.0 | [7] |
| MIR19 | Iberia CA | EI Mirador Cave | 42.3 | −3.5 | 2900–2346 | M | H3 | I | 0.06 | 21.8±3.1 | 0±0.0 | [7] |
| MIR2 | Iberia CA | EI Mirador Cave | 42.3 | −3.5 | 2857–2496 | F | K1b1a1 | ‥ | 0.98 | 22.6±1.7 | 56±8.9 | [7] |
| MIR202-037-n105 | Iberia CA | EI Mirador Cave | 42.3 | −3.5 | 2900–2346 | M | K1a | I2a2a | 5.73 | 19.9±1.7 | 0±0.0 | |
| MIR21 | Iberia CA | EI Mirador Cave | 42.3 | −3.5 | 2900–2346 | M | H3 | I | 0.11 | 24.7±2.4 | 55±17 | [7] |
| MIR22 | Iberia CA | EI Mirador Cave | 42.3 | −3.5 | 2900–2346 | F | K1a2a | ‥ | 2.79 | 22.6±1.7 | 62±10 | [7] |
| MIR24 | Iberia CA | EI Mirador Cave | 42.3 | −3.5 | 2900–2346 | M | J2b1a3 | G2a2b2b | 0.06 | 20.0±3.0 | 0±0.0 | [7] |
| MIR25 | Iberia CA | EI Mirador Cave | 42.3 | −3.5 | 2900–2346 | M | U3a1 | I2a1a1 | 0.73 | 25.3±1.7 | 34±15 | [7] |
| MIR5, MIR6 | Iberia CA | EI Mirador Cave | 42.3 | −3.5 | 2900–2679 | M | X2b | I2a2a2a | 10.4 | 20.7±1.7 | 0±0.0 | [7] |
Cov: average coverage per SNP. HG%: inferred percentage of hunter-gatherer ancestry (mean ± standard error). ALD: inferred date of admixture (generations in the past; mean ± standard error; zero implies no date obtained). Ref: reference for published data; if blank, newly published sample in this study (asterisk denotes a published individual with new sequencing data added). Radiocarbon dates are in normal text, while dates estimated from archaeological context are in italics. Further information can be found in Supplementary Table 1.
Extended Data Table 3.
Admixture graph results for Neolithic populations
| Main scaffold | Alternative scaffold | |||
|---|---|---|---|---|
|
| ||||
| Population | HG ancestry | WHG affinity | HG ancestry | WHG affinity |
| Körös EN | 0.0 ± 1.2% | 0.0 ± 1.2% | ||
| Starčevo EN | 2.3 ± 1.0% | KO1/VIL* | 2.3 ± 1.0% | VIL |
| ALPc MN | 8.8 ± 0.6% | KO1* + VIL | 9.5 ± 0.6% | KO1* + VIL |
| LBKT MN | 0.8 ± 0.9% | VIL* | 0.5 ± 0.9% | VIL |
| Tisza LN | 8.4 ± 1.3% | KO1/VIL | 9.8 ± 1.3% | KO1/VIL + EHG |
| TDLN | 8.2 ± 0.7% | KO1/VIL* | 8.4 ± 0.7% | KO1* |
| Lasinja CA | 10.7 ± 0.9% | KO1/VIL* | 10.6 ± 0.9% | KO1/VIL* |
| Protoboleráz CA | 12.7 ± 0.9% | KO1/VIL* | 12.5 ± 0.9% | KO1/VIL |
| Baden CA | 13.0 ± 0.7% | KO1/VIL* | 13.4 ± 0.7% | KO1* |
| LBK EN | 4.2 ± 0.6% | KO1 + LOS | 5.0 ± 0.6% | KO1* |
| Germany MN | 17.0 ± 1.1% | LOS* | 18.3 ± 1.1% | LOS + KO1 |
| Blätterhöhle MN | 40.6 ± 1.5% | KO1/VI L* + LOS | 42.6 ± 1.5% | KO1* + LOS |
| Iberia EN | 10.0 ± 0.8% | LB1* | 10.4 ± 0.8% | LB1* |
| Iberia MN | 23.3 ± 1.1% | LB1* + LOS | 24.8 ± 1.1% | LB1* + LOS |
| Iberia CA | 26.5 ± 0.7% | LB1* + LOS/KO1/VIL* | 27.5 ± 0.7% | LB1* + VIL* |
Hunter-gatherer ancestry in Neolithic populations as inferred from admixture graph analyses. Shown are the inferred ancestry proportions for the best-fitting FEF+WHG model, along with the WHG individual(s) inferred to be related to the hunter-gatherer sources, with * denoting statistical significance (Methods). The two sets of results are for the primary scaffold model (Extended Data Fig. 2) and an alternative admixture graph scaffold including EHG (Supplementary Information section 6). Plus signs indicate two components, while slashes indicate single components with one of two or three possibilities.
Extended Data Table 4.
Average dates of admixture for Neolithic populations
| Population | Individual-based | Group-based | Average sample date (BCE) |
|---|---|---|---|
| Körös EN | 5631 ± 31 | ||
| Starčevo EN | 4.5 ± 1.9 | 1.9 ± 0.9 | 5738 ± 35 |
| ALPc MN | 17.8 ± 2.0 | 16.4 ± 2.6 | 5180 ± 31 |
| LBKT MN | 30.3 ± 5.8 | 31.5 ± 10.9 | 5142 ± 93 |
| Tisza LN | 18.2 ± 6.6 | 12.6 ± 3.1 | 4750 ± 145 |
| TDLN | 20.9 ± 2.7 | 19.1 ± 3.8 | 4681 ± 32 |
| Lasinja CA | 29.3 ± 5.2 | 23.0 ± 4.1 | 4123 ± 59 |
| Protoboleráz CA | 44.3 ± 6.4 | 19.8 ± 5.4 | 3674 ± 35 |
| Baden CA | 27.6 ± 3.8 | 26.2 ± 6.9 | 3176 ± 49 |
| LBK EN | 14.9 ± 2.4 | 15.4 ± 3.6 | 5128 ± 38 |
| Germany MN | 26.2 ± 4.4 | 55.0 ± 41.2 | 3724 ± 46 |
| Blätterhöhle MN | 18.5 ± 4.6 | 23.1 ± 6.2 | 3414 ± 84 |
| Iberia EN | 19.4 ± 2.3 | 17.5 ± 5.9 | 5107 ± 20 |
| Iberia MN | 49.9 ± 7.7 | 40.0 ± 6.9 | 3749 ± 74 |
| Iberia CA | 49.6 ± 5.2 | 56.5 ± 7.9 | 2808 ± 27 |
Dates of admixture (in generations in the past) as inferred from ALDER through two different methods. On the left are the average individual-level dates used in our main analyses, and on the right are direct estimates for population groups. By default, for group-level estimates, we used all individuals that yielded a date in our standard ALDER procedure, but because of missing data, for some populations we used a subset of individuals (typically those with highest coverage): Starčevo (BAM17b, BAM4a, and LGCS1a; we note that in this case only BAM17b had an ALDER signal individually), ALPc (HAJE7a, HELI11a, MEMO2b, NE1, NE3, NE4, and TISO13a), Tisza (Gorzsa18 and PULE1.24), Baden (GEN12a, GEN13a, GEN15a, GEN17a, GEN22, and GEN55), LBK (HAL19, HAL2, HAL4, HAL5, LBK1992, and Stuttgart), and Iberia CA (LHUE11J.5, LHUE2010.11, LY.II.A.10.15066, LY.II.A.10.15069, MIR14, MIR2, and MIR22). For the group-level estimate for Iberia MN, we use a fitting start point of 0.8 cM instead of the program-inferred minimum of 0.6 because of a noticeably lower standard error. For our main analyses, we omit the outlier Protoboleráz individual GEN61, yielding an average date of 36.0 ± 5.2 generations, to help capture uncertainty due to the disagreement between the individual-level and group-level estimates shown here. Average sample dates (except for Körös) are based on the same weighting as the individual-level average dates of admixture for compatibility (Supplementary Information section 7).
Supplementary Material
Acknowledgments
We thank Iosif Lazaridis, Po-Ru Loh, Iain Mathieson, Iñigo Olalde, Eleftheria Palkopoulou, Nick Patterson, and Pontus Skoglund for helpful comments and suggestions; Johannes Krause for providing the Stuttgart sample for which we generated a new library in this study; Alasdair Whittle and Alex Bayliss from The Times of Their Lives project for providing the radiocarbon date for sample VEJ5a; and Bálint Havasi (Balaton Museum), György V. Székely (Katona József Museum), Csilla Farkas (Dobó István Museum), Borbála Nagy (Herman Ottó Museum), I. Pap, A. Kustár, T. Hajdu (Hungarian Natural History Museum), J. Ódor (Wosinsky Mór Museum), E. Nagy (Janus Pannonius Museum), P. Rácz (King St. Stephen Museum), L. Szathmáry (Debrecen University), N. Kalicz, V. Voicsek, O. Vajda-Kiss, V. Majerik, and I. Kővári for assistance with samples. This work was supported by the Australian Research Council (grant DP130102158; B.L. and W.H.), Hungarian National Research, Development and Innovation Office (K 119540; B.M.), German Research Foundation (AL 287-10-1; K.W.A.), FEDER and Ministry of Economy and Competitiveness of Spain (BFU2015-64699-P; C.L.-F.), National Science Foundation (HOMINID grant BCS-1032255; D.R.), National Institutes of Health (NIGMS grant GM100233; D.R.), and Howard Hughes Medical Institute (D.R.).
Footnotes
Author contributions
A.S.-N., J.B., E.B., K.W.A., C.L.-F., W.H., and D.R. designed and supervised the study. B.G.M., K.K., K.O., M.B., T.M., A.O., J.J., T.P., F.H., P.C., J.K., K.Se., A.A., P.R., J.R., J.P.B., S.F., G.S., Z.T., E.G.N., J.D., E.M., G.P., L.M., B.M., Z.B., L.D., J.F.-E., J.A.M.-A., C.A.F., J.J.E., R.B., J.O., K.Sc., H.M., A.C., J.B., E.B., K.W.A., C.L.-F., and W.H. provided samples and assembled archaeological and anthropological information. A.S.-N., A.P., B.S., V.K., N.R., K.St., M.F., M.M., J.O., N.B., E.H., S.N., and B.L. performed laboratory work. M.L., A.S.-N., S.M., and D.R. analyzed genetic data. M.L., A.S.-N., and D.R. wrote the manuscript with input from all coauthors.
The authors declare no competing financial interests.
References
- 1.Bramanti B, et al. Genetic discontinuity between local hunter-gatherers and Central Europe’s first farmers. Science. 2009;326:137–140. doi: 10.1126/science.1176869. [DOI] [PubMed] [Google Scholar]
- 2.Haak W, et al. Ancient DNA from European early Neolithic farmers reveals their Near Eastern affinities. PLoS Biol. 2010;8:e1000536. doi: 10.1371/journal.pbio.1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skoglund P, et al. Origins and genetic legacy of Neolithic farmers and hunter-gatherers in Europe. Science. 2012;336:466–469. doi: 10.1126/science.1216304. [DOI] [PubMed] [Google Scholar]
- 4.Lazaridis I, et al. Ancient human genomes suggest three ancestral populations for present-day Europeans. Nature. 2014;513:409–413. doi: 10.1038/nature13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haak W, et al. Massive migration from the steppe was a source for Indo-European languages in Europe. Nature. 2015;522:207–211. doi: 10.1038/nature14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Günther T, et al. Ancient genomes link early farmers from Atapuerca in Spain to modern-day Basques. Proc. Natl. Acad. Sci. U. S. A. 2015;112:11917–11922. doi: 10.1073/pnas.1509851112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathieson I, et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature. 2015;528:499–503. doi: 10.1038/nature16152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmanová Z, et al. Early farmers from across Europe directly descended from Neolithic Aegeans. Proc. Natl. Acad. Sci. U. S. A. 2016 doi: 10.1073/pnas.1523951113. 201523951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandt G, et al. Ancient DNA reveals key stages in the formation of Central European mitochondrial genetic diversity. Science. 2013;342:257–261. doi: 10.1126/science.1241844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ammerman AJ, Cavalli-Sforza LL. The Neolithic transition and the genetics of populations in Europe. Princeton: 1984. [Google Scholar]
- 11.Price TD. Lessons in the transition to agriculture. In: Price TD, editor. Europe’s First Farmers. Cambridge: 2000. pp. 301–18. [Google Scholar]
- 12.Zvelebil M. The agricultural transition and the origins of Neolithic society in Europe. Documenta Praehistorica. 2001;28:1–26. [Google Scholar]
- 13.Richards M. The Neolithic invasion of Europe. Ann. Rev. Anthropol. 2003:135–162. [Google Scholar]
- 14.Tringham R. Southeastern Europe in the transition to agriculture in Europe: bridge, buffer or mosaic. In: Price TD, editor. Europe’s First Farmers. Cambridge: 2000. pp. 19–56. [Google Scholar]
- 15.Bollongino R, et al. 2000 years of parallel societies in Stone Age Central Europe. Science. 2013;342:479–481. doi: 10.1126/science.1245049. [DOI] [PubMed] [Google Scholar]
- 16.Skoglund P, et al. Genomic diversity and admixture differs for Stone-Age Scandinavian foragers and farmers. Science. 2014;344:747–750. doi: 10.1126/science.1253448. [DOI] [PubMed] [Google Scholar]
- 17.Gamba C, et al. Genome flux and stasis in a five millennium transect of European prehistory. Nat. Comm. 2014;5:5257. doi: 10.1038/ncomms6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olalde I, et al. A common genetic origin for early farmers from Mediterranean Cardial and Central European LBK cultures. Mol. Biol. Evol. 2015;32:3132–3142. doi: 10.1093/molbev/msv181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olalde I, et al. Derived immune and ancestral pigmentation alleles in a 7,000-year-old Mesolithic European. Nature. 2014;507:225–228. doi: 10.1038/nature12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu Q, et al. The genetic history of Ice Age Europe. Nature. 2016;534:200–205. doi: 10.1038/nature17993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones ER, et al. Upper Palaeolithic genomes reveal deep roots of modern Eurasians. Nat. Comm. 2015;6:8912. doi: 10.1038/ncomms9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seguin-Orlando A, et al. Genomic structure in Europeans dating back at least 36,200 years. Science. 2014;346:1113–1118. doi: 10.1126/science.aaa0114. [DOI] [PubMed] [Google Scholar]
- 23.Loh P-R, et al. Inferring admixture histories of human populations using linkage disequilibrium. Genetics. 2013;193:1233–1254. doi: 10.1534/genetics.112.147330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bánffy E. Eastern, Central and Western Hungary – variations of Neolithisation models. Documenta Praehistorica. 2006;33:125–142. [Google Scholar]
- 25.Domboróczki L, Kaczanowska M, Kozńowski J. The Neolithic settlement at Tiszaszőlős-Domaháza-puszta and the question of the northern spread of the Körös Culture. Atti Soc. Preist. Protost. Friuli-VG. 2010;17:101–155. [Google Scholar]
- 26.Szécsényi-Nagy A, et al. Tracing the genetic origin of Europe’s first farmers reveals insights into their social organization. Proc. Royal Soc. B. 2015;282:20150339. doi: 10.1098/rspb.2015.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raczky P. Historical context of the Late Copper Age Cemetery at Budakalász. In: Bondár M, Raczky P, editors. The Copper Age Cemetery of Budakalász. Pytheas, Budapest: 2009. pp. 475–485. [Google Scholar]
- 28.Martins H, et al. Radiocarbon dating the beginning of the Neolithic in Iberia: New results, new problems. J. Medit. Arch. 2015;28:105–131. [Google Scholar]
- 29.Jakucs J, et al. Between the Vinča and Linearbandkeramik worlds: The diversity of practices and identities in the 54th–53rd centuries cal BC in Southwest Hungary and beyond. J. World Prehist. 2016;29:267–336. doi: 10.1007/s10963-016-9096-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oross K, et al. Midlife changes: The Sopot burial ground at Alsónyék. Bericht der Römisch-Germanischen Kommission. 2016;94:151–178. [Google Scholar]
- 31.Dabney J, et al. Complete mitochondrial genome sequence of a Middle Pleistocene cave bear reconstructed from ultrashort DNA fragments. Proc. Natl. Acad. Sci. U. S. A. 2013;110:15758–15763. doi: 10.1073/pnas.1314445110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korlević P, et al. Reducing microbial and human contamination in DNA extractions from ancient bones and teeth. BioTechniques. 2015;59:87–93. doi: 10.2144/000114320. [DOI] [PubMed] [Google Scholar]
- 33.Lazaridis I, et al. Genomic insights into the origin of farming in the ancient Near East. Nature. 2016;536:419–424. doi: 10.1038/nature19310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rohland N, Harney E, Mallick S, Nordenfelt S, Reich D. Partial uracil–DNA–glycosylase treatment for screening of ancient DNA. Phil. Trans. R. Soc. B. 2015;370 doi: 10.1098/rstb.2013.0624. 20130624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeAngelis MM, Wang DG, Hawkins TL. Solid-phase reversible immobilization for the isolation of PCR products. Nucl. Acids Res. 1995;23:4742–4743. doi: 10.1093/nar/23.22.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rohland N, Reich D. Cost-effective, high-throughput DNA sequencing libraries for multiplexed target capture. Genome Res. 2012;22:939–946. doi: 10.1101/gr.128124.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer M, et al. A mitochondrial genome sequence of a hominin from Sima de los Huesos. Nature. 2014;505:403–406. doi: 10.1038/nature12788. [DOI] [PubMed] [Google Scholar]
- 38.Sawyer S, Krause J, Guschanski K, Savolainen V, Pääbo S. Temporal patterns of nucleotide misincorporations and DNA fragmentation in ancient DNA. PloS one. 2012;7:e34131. doi: 10.1371/journal.pone.0034131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrews RM, et al. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat. Genet. 1999;23:147. doi: 10.1038/13779. [DOI] [PubMed] [Google Scholar]
- 40.Behar DM, et al. A “Copernican” reassessment of the human mitochondrial DNA tree from its root. Am. J. Hum. Genet. 2012;90:675–684. doi: 10.1016/j.ajhg.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mallick S, et al. The Simons Genome Diversity Project: 300 genomes from 142 diverse populations. Nature. 2016;538:201–206. doi: 10.1038/nature18964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu Q, et al. DNA analysis of an early modern human from Tianyuan Cave, China. Proc. Natl. Acad. Sci. U. S. A. 2013;110:2223–2227. doi: 10.1073/pnas.1221359110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korneliussen TS, Albrechtsen A, Nielsen R. ANGSD: analysis of next generation sequencing data. BMC Bioinformatics. 2014;15:356. doi: 10.1186/s12859-014-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Domboróczki L. Research at Tiszaszőlős-Domaháza-puszta in 2003. In: Anders A, Siklósi Z, editors. The First Neolithic Sites in Central/South-East European Transect. Volume III: The Körös Culture in Eastern Hungary. Oxford: 2012. pp. 107–111. [Google Scholar]
- 45.Oross K, et al. The early days of Neolithic Alsónyék: the Starčevo occupation. Bericht der Römisch-Germanischen Kommission. 2016;94:93–121. [Google Scholar]
- 46.Ramsey CB, Lee S. Recent and planned developments of the program OxCal. Radiocarbon. 2013;55:720–730. [Google Scholar]
- 47.Reimer PJ, et al. Intcal13 and marine13 radiocarbon age calibration curves 0–50,000 years cal bp. Radiocarbon. 2013;55:1869–1887. [Google Scholar]
- 48.Patterson N, Price A, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2:e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patterson N, et al. Ancient admixture in human history. Genetics. 2012;192:1065–1093. doi: 10.1534/genetics.112.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fenner J. Cross-cultural estimation of the human generation interval for use in genetics-based population divergence studies. Am. J. Phys. Anthropol. 2005;128:415–423. doi: 10.1002/ajpa.20188. [DOI] [PubMed] [Google Scholar]
- 51.Moorjani P, et al. A genetic method for dating ancient genomes provides a direct estimate of human generation interval in the last 45,000 years. Proc. Natl. Acad. Sci. U. S. A. 2016 doi: 10.1073/pnas.1514696113. 201514696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The aligned sequences are available through the European Nucleotide Archive under accession number PRJEB22629. Genotype datasets used in analysis are available at https://reich.hms.harvard.edu/datasets.