Synopsis
Over the last several years, anticoagulation pharmacy has been dramatically altered with U.S. FDA approval of 5 direct oral anticoagulants, one novel reversal agent and a second designated for fast track approval. Trial data surrounding current trends in anticoagulant choice for VTE, reversal and bridging are constantly redefining modern day practice. Extended therapy for unprovoked VTE has expanded to include low dose DOACs, ASA and the use of a new HERDOO2 scoring system to identify women who can stop anticoagulant therapy without increased risk of recurrent VTE. Trends in thromboprophylaxis include extended duration low dose DOACs to prevent VTE in high risk orthopedic and medical patients.
Keywords: Direct oral anticoagulants, Venous thromboembolism, Deep venous thrombosis, VTE chemoprophylaxis, VTE extended therapy, Anticoagulation, Perioperative bridging
Introduction
In the last several years, anticoagulation pharmacology has been dramatically altered in the United States with the FDA approval of five new direct oral anticoagulant (DOAC) agents. In 2012, the American College of Chest Physicians recommended treatment of acute VTE with vitamin K antagonists, while recognizing a major shift on the horizon, “Given the paucity of currently available data and that new data are rapidly emerging, we give a weak recommendation in favor of vitamin K antagonists and LMWH therapy over dabigatran and rivaroxaban…”(1) By the time the updated ACCP guidelines were released in 2016, DOACs were a routine part of the prevention and treatment of venous thromboembolism (VTE). With the advent of a new drug class, an explosion of clinical studies is underway to determine the utility of each medication for a myriad of indications.
Within this article we explore some of the most rapidly developing, exciting and important areas of change for the practicing clinician. These include new details on how to choose the best anticoagulant for VTE treatment, considerations for extended anticoagulation for prevention of recurrent VTE, developments in extended thromboprophylaxis for patients at high risk for VTE and data and trends in perioperative bridging. Within 2017 alone, two useful clinical prediction trials emerged from large studies: the SAMe-TT2R2 score for predicting the quality of anticoagulation with vitamin K antagonists (VKA) and the HERDOO2 score for identifying women at low risk of recurrent VTE. Within this same time period a fifth DOAC (Betrixaban, Bevyxxa, Portola Inc.) underwent FDA approval, adding to the array of available anticoagulants. We have designed the following sections to place newer studies in the context of historical data to allow for perspective on how this information may be practically incorporated into every day practice.
Anticoagulant Choice for VTE
Approved in 1954, VKAs (Warfarin, Coumadin) for greater than half a century, had been the mainstay of therapy for thrombotic diseases. Given the established safety profile of VKAs, as well as the efficacy in reducing the risk for fatal pulmonary embolism and recurrent thrombosis, they represent the gold standard by which every new agent now must be compared. There are several shortcomings associated with VKAs: they require monitoring, the metabolism of the drug is affected by diet and other medications, and they have a defined bleeding risk of 5–6% per year,(24) which cannot be mitigated by targeting a lower INR.(25, 26) Until recently, few alternatives existed. The other commonly prescribed anticoagulants prior to the emergence of DOACs were LMWHs. Despite the downside of subcutaneous administration, (necessitating daily or twice daily home injections), rather than oral route, the LMWHs had several advantages: dosing was weight based and predictable; routine laboratory monitoring did not need to occur; and efficacy was similar to VKAs. In a pooled analysis of LMWH and VKAs in the treatment of VTE the rate of fatal PE during treatment of DVT was 0.4% and of PE was 1.5%; the rates were similarly low following cessation of anticoagulation.(27) In certain patient populations, LMWH proved superior to VKAs: for instance, in patients with malignancy treatment with LMWH decreased the risk of recurrent VTE by about 50% at six months to one year compared to warfarin without a difference in bleeding rates.(28) In the HOMELITE trial, Tinazpine (a LMWH), was superior to warfarin for the prevention of post-thrombotic syndrome, development of leg ulcers and treatment satisfaction.(29)
While VKAs and LMWH still remain viable options for the treatment of VTE, in the last few years there has been a rapid development of a significant body of scientific evidence supporting the use of DOACs in VTE. The two categories of DOACs are the direct Xa inhibitors and direct thrombin inhibitors. Currently of the direct Xa inhibitors apixaban, rivaroxaban, and edoxaban have all been FDA approved for the treatment of VTE. Dabigatran is the sole approved direct thrombin inhibitor. The DOACs are appealing because they are all administered orally, have fixed doses that do not need to be weight based adjusted, and there is no need for monitoring. Two major initial clinical concerns regarding the DOACs were the efficacy/safety and the reversibility in the event of major bleeding. In the last 10 years all of the previously mentioned DOACs each have at least one large randomized controlled trial demonstrating non-inferiority in the treatment of VTE compared to VKAs.(30–33) Furthermore, in every study there was no increased incidence of bleeding in patients who used DOACS compared to standard VTE therapy. Of all of the DOACs, apixaban alone demonstrated a superior reduction in bleeding (major bleeding and clinically relevant non-major bleeding) compared to VKAs.
One of the appealing aspects of VKAs, (and to a lesser extent LMWH), is the ability to be quickly reversed by protamine (for heparin and to a lesser degree LMWH), fresh frozen plasma (FFP) or prothrombin complex concentrate (PCC) if indicated clinically. In addition, there is an available lab test (international normalized ratio for VKAs and anti-Xa level for heparin and LMWH) to quantify the adequacy of reversal. Historically, the same had not been true for the DOACs with reversal being limited to supportive measures, PCC or selective dialysis.(34) While the issue of reversibility has not been fully resolved, progress was achieved with the FDA approval of idarucizumab (Praxbind™) in October 2015. Idarucizumab is a monoclonal antibody that binds to dabigatran and can reverse its anticoagulant effects within minutes.(35) Two other agents currently in development include andexanet alfa and aripazine. Andexanet alfa is recombinant factor Xa protein that acts as decoy for all factor Xa inhibitors, including DOACs, LMWHs and fondaparinux.(36) Clinical trials have shown the andexanet alfa can effectively reverse DOACs anticoagulant effect by about half without any known thrombotic events in healthy patients.(37) Currently, ANNEXA-4 (NCT02329327), a Phase III open label study, is ongoing to evaluate the use of the medication in patients with ongoing major bleeding, with an estimated completion date of 2022. The final reversal agent progressing in development with FDA fast track designation for hemorrhage is Aripazine (PER-977, ciraparantag, Perosphere Inc.). It is a water soluble, catatonic molecule available in intravenous formulation that non-covalently binds to and reverses the anticoagulation of all anticoagulation agents (LMWH, UFH, FXa inhibitors, dabigatran) in both animal models and healthy volunteers.(37) In total, at least 5 phase I/II trials have been completed or are ongoing to evaluate the utility of Aripazine for anticoagulant reversal.
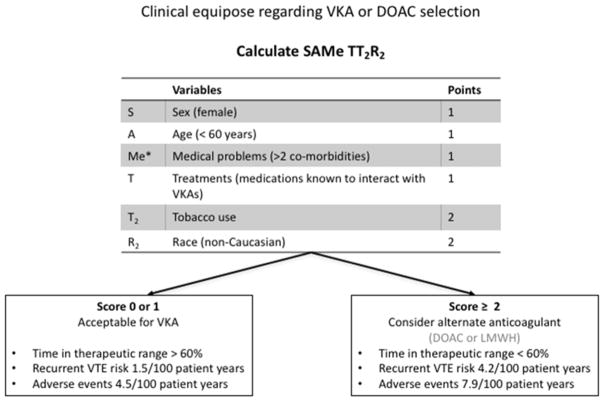
As a result of the multitude of evidence showing both the effectiveness and safety of DOACs, in 2016 the American College of Chest Physicians recommended DOACs as first line treatment of acute VTE over VKAs in patients whom do not have an associated cancer (Grade 2B).(38) In patients with cancer who develop acute VTE, LMWH remains the recommended first line treatment. Although guidelines recommend DOACs over VKA in non-malignancy related VTE there are several scenarios which would preclude the use of DOACs; for example, patients with mechanical heart valves, patients who cannot afford the cost of the medication, and patients with impaired renal function. Given that all of the comparison studies showed non inferiority, in many cases either a DOAC or a VKA is acceptable. However, recent work by Kataruka and colleagues have shown that certain patients are at much higher risk of treatment failure with VKAs than others.(39) They applied the scoring system SAMe-TT2R2 (Figure 1), which had been previously successfully validated for use in atrial fibrillation, and demonstrated that patients with a high score were significantly more likely to experience adverse events and recurrent VTE on VKA therapy than those with a low score. As the anticoagulation field continues to expand with increased agents and changing guidelines it will be important for providers to critically decide which anticoagulation treatment would be best for each individual patient (Table 1).
Figure 1.
In the event of clinical equipoise regarding selection of VKAs or DOACs for the treatment of VTE, the SAMe-TT2R2 score can be utilized to predict individuals likely to have adverse events, VTE recurrence an poor time in therapeutic range with VKA treatment.
*medical co-morbidities include diabetes, hypertension, renal disease, hepatic disease, pulmonary disease, congestive heart failure, coronary artery disease, peripheral vascular disease or previous stroke.
Table 1.
Dosing and considerations for various anticoagulant choices.
| Dosing | Half Life | Considerations | |
|---|---|---|---|
| Apixaban | 10mg BID x7 days Then 5mg BID (2.5mg BID for long-term therapy) |
7–11hrs |
|
| Rivaroxaban | 15mg BID x3 wk Then 20mg daily |
12 hrs |
|
| Dabigatran | LMWH for 5–10d 150mg BID |
8–15hr |
|
| Edoxaban | LMWH for 5–10 days 60mg Daily 30mg Daily ≤60kg or CrCl 15–50 |
10–14 hrs |
|
| Warfarin | Variable dosing titrate to goal INR | ~ 40 hrs |
|
Extended anticoagulation treatment to reduce the risk of recurrent VTE
The recommended duration of anticoagulation for the treatment of provoked proximal and symptomatic distal VTE is 3 months (Grade 1B). Extended therapy traditionally is considered under the following circumstances:
Previous VTE event
Thrombophilias associated with a high risk of recurrence
Unprovoked (idiopathic) VTE
Virtually all authorities agree that for a second idiopathic VTE, anticoagulant therapy should be continued due to the high recurrence risk well past 3 months in those patients with low and moderate bleeding risks (grades 1B, 2B).(38) For patients with diagnosed hereditary thrombophilias, those that are associated with a higher risk of VTE recurrence include protein C or protein S deficiency (especially with a family history), antithrombin deficiency, homozygous factor V Leiden and homozygous prothrombin 20210 gene mutation, and multiple thrombophilias in the same patient. Factor V Leiden heterozygous mutation alone does not confer an increased risk; however, when combined with prothrombin 20210 gene mutation recurrence is increased and prolonged anticoagulant therapy is recommended. Amongst acquired thrombophilias, the presence of antiphospholipid antibodies and active cancer mandate extended therapy.(40)
Idiopathic first-time VTE remains a difficult clinical scenario, as one must balance the competing risks and implications of recurrent VTE and major bleeding. D-dimer and repeat duplex testing have both been advocated as useful tests to determine risk of recurrence with cessation of anticoagulation amongst these patients. D-dimer is measured approximately 1 month after stopping oral anticoagulation. If elevated, this suggests that active thrombosis is occurring and the rate of recurrence is 15% compared to 6.2% with a normal D-dimer. This risk can be mitigated with resumption of anticoagulation which reduces the VTE rate to 2.9%.(38, 41) The use of repeat serial lower extremity ultrasound to determine the state of the thrombosed veins has been studied, with the assumption that if the veins are occluded with fibrotic scar tissue, flow will be sluggish and the risk of recurrent VTE elevated. The usefulness of this test is less certain, as this approach utilized a very difficult quantification scheme, which is difficult to reproduce in day to day clinical practice.
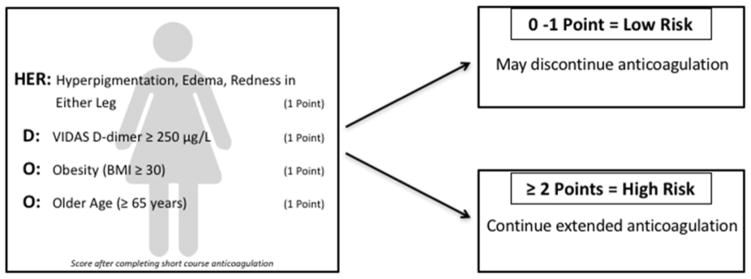
Two major new advances that have come about in recent years include the validation of the HERDOO2 score and low dose anticoagulant/antiplatelet therapies for the prevention of recurrent VTE. Recently, the HERDOO2 score was developed and prospectively validated. This identified women at low risk of recurrence following unprovoked VTE who may safely discontinue anticoagulants following short-term (standard) treatment. Women receive a point for (a) hyperpigmentation, edema, or redness in either leg, (b) VIDAS D-dimer ≥ 250 μg/L, (c) Obesity (BMI ≥ 30), and (d) Older age (≥65 years). Patients with 0 or 1 point were considered low risk for recurrent VTE and discontinued long-term anticoagulation therapy (Figure 2). Women who were defined as both low risk and discontinued therapy had a 3.0% risk of recurrent VTE per patient year, compared to an 8.1% risk in high-risk women who discontinued anticoagulants (Figure 2).(42) This clinical algorithm should be easily adopted in day to day clinical practice.
Figure 2.
In women with first time unprovoked VTE, the HERDOO2 score can identify those that are at low risk of recurrent VTE after initial standard anticoagulant therapy and can safely stop anticoagulation.
The advent of the DOACs has also re-opened the possibility of low dose anticoagulation for the prevention of recurrent thrombosis as a method of improving the risk-benefit profile by decreasing the bleeding risks associated with anticoagulant therapy. Previous studies with low dose VKAs failed to demonstrate an improvement in bleeding profile and has been largely abandoned in clinical practice.(43) Interestingly, aspirin has recently been investigated as an alternative to mitigate bleeding risk. Several studies, including the INSPIRE trial (a collaboration of the WARFASA and ASPIRE trials, Table 2) have demonstrated a reduced risk of VTE recurrence by over one-third in patients treated with aspirin compared to placebo.(44, 45) More recently, both treatment and thromboprophylactic doses of rivaroxaban and apixaban have been shown to be more effective in prevention of VTE recurrence with no increased risk of bleeding compared to placebo or ASA (Table 2).(46, 47) While evidence exists that lower dose anticoagulants may effectively reduce VTE recurrence, the most effective therapy with the safest treatment profile and lowest cost is still being debated.
Table 2.
Recent trials evaluating different extended duration therapies for prevention of recurrent VTE.
| Study name | Number (n) | Study Drug | Primary efficacy endpoint | Primary safety endpoint | Summary |
|---|---|---|---|---|---|
| INSPIRE | 1224 | Aspirin 100mg once a day for two years (v. placebo) |
Recurrent VTE 7.5%/year placebo v. 5.1%/year ASA, p=0.008 |
Major bleeding 0.4%/year placebo v. 0.4%/year ASA, p=0.67 |
ASA after anticoagulant treatment reduces the overall risk of recurrent VTE without increasing risk of bleeding |
| EINSTEIN CHOICE | 3396 | Rivaroxaban 20mg once a day or 10mg once a day for 12 months (v. aspirin 100mg once a day for 12 months) |
Composite of symptomatic, recurrent fatal or nonfatal VTE or unexplained death for which PE could not be excluded Treatment Dose (20mg) 1.5% (study) v. 4.4% (aspirin) (hazard ratio 0.34; 95% CI, 0.20 to 0.59, p<0.001) Thromboprophylactic dose (10mg) 1.2% (study) v. 4.4% (aspirin) (hazard ratio 0.26, 95% CI, 0.14 to 0.47, p<0.001) |
Major bleeding Treatment Dose (20mg) 0.5% (study) v. 0.3% (aspirin) (hazard ratio 2.01; 95% CI, 0.50–8.04, p=0.32) Thromboprophylactic dose (10mg) 0.4% (study) v. 0.3% (aspirin) (hazard ratio 1.64; 95% CI, 0.39–6.84, p=0.50) |
Rivaroxaban (both treatment and prophylactic dose) was superior to aspirin in the prevention of recurrent VTE, with no difference in bleeding risk. The study was not powered to show noninferiority of prophylactic dose v. treatment dose. |
| AMPLIFY EXTEND | 2486 | Apixaban 2.5mg twice a day or 5mg twice a day for 12 months (v. placebo) |
Composite of symptomatic recurrent VTE or death from any cause Treatment Dose (5mg) 4.2% (study) v. 11.6% (placebo) (relative risk 0.36, 95% CI, 0.25 to 0.53) Thromboprophylactic dose (2.5mg) 3.8% (study) v. 11.6% (placebo) (relative risk 0.33, 95% CI 0.22 to 0.48) |
Major bleeding Treatment Dose (5mg) 0.1% (study) v. 0.5% (placebo) (relative risk 0.25, 95% CI 0.03 to 2.24) Thromboprophylactic dose (2.5mg) 0.2% (study) v. 0.5% (placebo) (relative risk 0.49, 95% CI 0.09 to 2.64) |
Extended anticoagulation with either treatment dose or prophylactic dose Apixaban is superior to placebo in the prevention of recurrent VTE, with no increase in bleeding rates compared to placebo. |
Extended VTE prophylaxis for patients at high risk
Extended DVT prophylaxis refers to chemoprophylaxis that is continued beyond the initial inhospital 5 to 14 days, for up to 35 days. This concept reflects an important fact about the epidemiology of VTE: namely, that many VTE events occur after the index hospitalization.(2) Analysis of large administrative databases indicates that for both recently hospitalized surgical patients(3) and medical patients(4) approximately 56% of VTE occur after discharge. Over the last decade considerable progress has been made in identifying high risk surgical cohorts and mitigating risk. Most recently, an effort has been made towards decreasing post discharge VTE in medical patients.
Extended thromboprophylaxis for elective cancer operations
The highest incidence of VTE after inpatient surgery is in the 2 to 3 weeks after the procedure,(5) suggesting that thromboprophylaxis in this vulnerable period following hospital discharge could be beneficial. The ENOXACAN I study identified that 15% of patients undergoing elective cancer operations with 10 days of LMWH prophylaxis suffer from VTE.(6) Subsequently, the ENOXACAN II trial demonstrated a 60% relative risk reduction in VTE following abdominal or pelvic cancer surgery, without an increased risk of bleeding.(7) Therefore, the first group in which extended VTE prophylaxis was advocated for was those undergoing major operations with the additional risk factor of malignancy. The current ACCP guidelines (Grade IB) and American Society of Clinical Oncology clinical practice guidelines suggest that for these patients undergoing abdominal or pelvic surgery for cancer, who are not otherwise at high risk for a major bleeding complications, should be treated with extended duration pharmacological prophylaxis for 4 weeks.(8, 9) Most recently, extended antithrombotic prophylaxis was shown to have a 91% relative risk reduction amongst patients undergoing laparoscopic colorectal cancer resection.(10)
Extended thromboprophylaxsis for orthopedic procedures
Similar to operations for malignancy, orthopedic procedures have been historically associated with a high post-operative VTE; up to 15–30% prior to 1980, and modern day estimates of 4.3% out to 35 days(11) use of LMWH has been associated with about 60% decrease in VTE. The median date of VTE diagnosis following major orthopedic surgery is 7 days for total knee arthroplasty (TKA) and 17 days for total hip arthroplasty (THA), which has been an impetus for testing the utility of extended VTE prophylaxis in this patient population.(12) The current ACCP guidelines suggest “extending thromboprophylaxis in the outpatient setting for up to 35 days from the day of surgery rather than only 10 to 14 day (Grade 2B).(13) This recommendation was given on the basis of compiled data from 7 studies evaluating placebo compared to extended prophylaxis with LMWH (largely in THA patients), demonstrating a decrease in 9 fewer symptomatic VTE per 1,000 patients without an increase in major bleeding.
From 2007 to 2011, a remarkable number of trials evaluating the use of DOACs for 6–10 days and extended (28–39 days) thromboprophylaxis in orthopedic surgery patients was undertaken. In the RE-NOVATE II trial, dabigatran was found to be non-inferior to LMWH in THA patients, with a similar bleeding rate.(14) In the ADVANCE-3(15) and RECORD-1(16) trials, extended duration apixaban and rivaroxaban were both found to be superior to extended duration LMWH for VTE risk reduction amongst THA patients, with a similar safety profile. Despite these trials, the American Association of Orthopedic Surgery (AAOS) leaves the decision up to the surgeon and patient regarding the use of extended thromboprophylaxis. The reasoning is that contrary to the ACCP guidelines, the emphasis is placed on “critical” endpoints (PE, major bleeding and mortality) rather than “non-critical” endpoints (symptomatic DVT, any DVT, and proximal DVT) and extended prophylaxis is more effective in these non-critical endpoints.(17) The result is a widely variable practice across developed countries with regards to the type and duration of VTE thromboprophylaxis following orthopedic operations.
Extended thromboprophylaxsis in acutely medically ill patients
Within the last several years, there have been a flurry of studies aimed at decreasing the post discharge risk of VTE amongst medical patients. These include the EXCLAIM,(18) ADOPT,(19, 20) MAGELLAN,(21) and APEX trials.(20) The summary of these trials is presented in Table 3. Taken together, these trial have evaluated the utility of prophylactic enoxaparin, apixaban, rivaroxaban, and betrixaban in acutely ill patients for an extended period of 28–35 days compared to standard therapy. With the exception of the APEX trial (betrixaban), every extended regimen has had a higher risk of major bleeding, which offsets a decreased VTE risk. A pooled meta-analysis of all four trials, encompassing 28,000 patients found a decrease in VTE and symptomatic DVT, but an increase in major bleeding.(22) The number needed to treat for VTE was 239 and number needed to harm for major bleeding was 247, suggesting that the general use of extended thromboprophylaxis in this patient population should not be undertaken. It is important to note that of all the agents, betrixaban lacked the bleeding profile and decreased the relative risk of VTE by 24%. The study utilized a smaller “enriched” cohort (patients with elevated D-dimer) as methodology to decrease heterogeneity and demonstrate efficacy. However, this failed to demonstrate statistical significance (p=0.054), despite apparent significance in the larger, unselected study population (Table 3). In June 2017, betrixaban received FDA approval for extended prophylaxis in medically ill patients. Whether it will be widely adopted into clinical practice is still uncertain. The MARINER trial (NCT02111564) is a current, ongoing trial evaluating thromboprophylaxis with rivaroxaban in selected medically ill patients based on IMPROVE VTE risk score and D-dimer.(23) This study may further define how best to treat this patient population.
Table 3.
Trials evaluating extended thromboprophylaxis in the medically ill.
| Study name | Number (n) | Study Drug | Primary efficacy endpoint | Primary safety endpoint | Summary |
|---|---|---|---|---|---|
| ADOPT | 6528 | Apixaban 2.5mg twice daily for 30 days (v. enoxaparin 6–14d) |
30-day composite of death related to VTE, PE, symptomatic DVT, or asymptomatic proximal-leg DVT 2.7% (study) v. 3.1% (placebo) (relative risk 0.87; 95% confidence interval [CI], 0.62 to 1.23; P=0.44) |
Major bleeding 0.47% (study) v. 0.17% (placebo) (relative risk, 2.58; 95% CI, 1.02 to 7.24; P=0.04) |
Extended thromboprophylaxis with apixaban was not superior to a shorter course with enoxaparin. Apixaban was associated with significantly more major bleeding events. |
| APEX | 7513 | Betrixaban 80mg once daily for 35 days (v. enoxaparin 10 days) |
Asymptomatic proximal DVT and symptomatic VTE Patients with elevated D dimer: 6.9% (study) v. 8.5% (placebo) (relative risk 0.81; 95% confidence interval [CI], 0.66 to 0.98; P=0.054) * Overall population: 5.3% (study) v. 7.0% (placebo) (relative risk 0.76; 95% confidence interval [CI], 0.63 to 0.92; P=0.0006) |
Major bleeding 0.7% (study) and 0.6% placebo (relative risk 1.19; 95% confidence interval [CI], 0.67 to 2.12; P=0.55) |
Amongst the pre- specified cohort (patients will elevated D dimer), no significant difference. In the overall population, exploratory analysis suggests a benefit in VTE reduction in the treatment group without an increased risk of bleeding complications. |
| EXCLAIM | 5963 | Enoxaparin 40mg once daily for 28 days (v. enoxaparin 10 days) |
Venous thromboembolism (symptomatic/asymptomatic DVT, symptomatic/fatal PE) 2.5% (study) v. 4.0% (placebo) (absolute risk difference −1.53%; 95% CI −2.54% to −0.52%) |
Major bleeding events 0.8% (study) v. 0.3% (placebo) (absolute risk difference 0.51%; 95% CI 0.12% to 0.89%) | Use of extended duration enoxaparin reduced VTE rates but had higher major bleeding events compared to placebo. |
| MAGELLAN | 8101 | Rivaroxaban 10mg once a day for 35 days (v. enoxaparin 10 days) |
Asymptomatic proximal or symptomatic venous thromboembolism 4.4% (study) v. 5.7% (placebo) (relative risk, 0.77; 95% CI, 0.62 to 0.96; P=0.02) |
Composite of major or clinically relevant nonmajor bleeding 4.1 (study) v. 1.7% (placebo) P<0.001 |
Rivaroxaban was noninferior to enoxaparin for standard-duration thromboprophylaxis. Extended-duration rivaroxaban reduced the risk of VTE, but was associated with an increased risk of bleeding. |
Exploratory analysis according to trial design.
Perioperative bridging
Historically, all but the lowest risk patients with an indication for anticoagulation such as VTE, atrial fibrillation, prosthetic heart valve or left ventricular assist device were managed per-operatively with an anticoagulant “bridge,” to avoid the risk of thromboembolism (TE). The VKA would be discontinued 5 days prior to the proposed procedure and either intravenous heparin or LMWH subcutaneous administered from when the INR was sub therapeutic (below 2.0 or 2.5 depending upon the indication), until 6–12 hours prior to the procedure. Anticoagulation was then immediately resumed with both a bridging agent and the VKA typically 12–24 hours post–procedure, or whenever it was felt safe to do so based on the procedure being performed. In modern times, this is still true for high risk patients under anticoagulant therapy for recent VTE, but a major paradigm shift has occurred for atrial fibrillation.
Bridging in the setting of atrial fibrillation
Nearly one in six patients treated with warfarin for atrial fibrillation undergo invasive procedures each year. Previous guidelines for bridging anticoagulation were primarily founded on observational studies, with the question of necessity of bridging therapy largely unanswered in the literature. More recently, the randomized control trial Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation (BRIDGE) addressed the question of bridging requirements in patients with atrial fibrillation on VKA therapy and found routine bridging of low and moderate risk patients to be harmful. Specifically, forgoing bridging anticoagulation was shown to be non-inferior to bridging therapy in the prevention of thromboembolism (TE); 0.4% vs 0.3%, and the incidence of major bleeding was 3.2% in bridged patients compared to 1.3% in the non-bridged group.(48) While the current body of evidence demonstrates that bridging anticoagulation of low and moderate risk patients with atrial fibrillation leads to increased rates of bleeding with no clear evidence of minimizing TE events, it must be noted that the majority of trials have underrepresentation of high risk atrial fibrillation patients (CHADS2 ≥ 5), patients with mechanical heart valves, and those with recent venous or arterial thrombosis.
Changes to anticoagulation practices should therefore be extrapolated to these populations with caution. Overall, most authorities now suggest patients with atrial fibrillation at low risk of TE who require oral anticoagulant therapy interruption temporarily discontinue anticoagulation without use of bridging therapies. In patients at high risk of TE, anticoagulation may be temporarily discontinued, but bridging therapy should be assessed individually based on bleeding and TE risk and is still strongly encouraged by some organizations including the American College of Cardiology (ACC) (grade 2C evidence).(49, 50)
Bridging in the setting of mechanical heart valves
Until recently mechanical heart valves were considered to be at very high risk of thromboembolism with recommendations for perioperative bridging in all patients requiring oral anticoagulant therapy interruption. More recent studies demonstrate a perioperative risk of TE in patients with mechanical heart valves to be approximately 1%, with previous estimates likely influenced by a larger proportion of very high-risk valves (ex cage-ball, tilting disk).(51) To date, there have been no RCTs evaluating “bridging” versus “no bridging” in patients with prosthetic heart valves, and results from trials including only atrial fibrillation patients should be generalized cautiously. However, based on the results of the BRIDGE trial, the AHA/ACC 2017 Updates on Bridging Therapy for Prosthetic Valves modified the class of recommendation supporting bridging anticoagulation from I to IIa. Noting rising concerns of bridging anticoagulation exposing patients to increased rates of bleeding with no reduction in risk of TE, the recommendations now support bridging on an individualized basis, accounting for the risk of both TE and bleeding.(52) Ongoing studies, including the PERIOP-2 trial, are evaluating the need for bridging therapy in moderate risk patients with mechanical heart valve.
Bridging in the setting of DOAC therapy
Similarly, the introduction of DOACs for the treatment of atrial fibrillation and VTE introduced the practice of avoiding bridging due to the short half-life. Initially, it was thought that dabigatran would require 3–4 days of cessation, and the direct Xa inhibitors 2–3 days of cessation based upon half-life.(2) However, since their FDA approval, additional data from the RE-LY trial and Dresden DOAC registry amongst others have better quantified the peri-operative bleeding and thrombosis risks associated with DOAC use. Additionally, there are a subset of low risk procedures for which it has been found safe to continue uninterrupted DOAC use.
In patients where anticoagulation interruption is required peri-operatively, management of DOACs aims to minimize or eliminate residual anticoagulant effect at the time of surgery. For procedures with moderate bleeding risk, dabigatran, rivaroxaban and apixaban should be held 1 day prior to surgery. This corresponds to 2–3 half-lives elapsing prior to surgery with possible 12–25% residual anticoagulant effect at the time of surgery. For high risk bleeding procedures and major operations, DOACs should be held 2 days prior to surgery, allowing 4–5 half-lives to elapse and a residual anticoagulant effect of <10% (Table 4). Importantly, the specific duration of time for these medications to be held is significantly impacted by patients’ renal function, with recommendations to discontinue DOACs for greater periods of time in patients with even moderate renal impairment.(53, 54)
Table 4.
Bridging and reversal strategy for DOACs.
| Agent | Mechanism of Action | # days to hold for minor procedures | # days to hold for major procedures | Reversal Agent | Alternative Treatment Option | Bridging therapy required |
|---|---|---|---|---|---|---|
| Apixaban | Factor Xa inhibitor | 1 (2 doses) | 2 (4 doses) | Unavailable |
|
No |
| Rivaroxaban | Factor Xa inhibitor | 1 (1 dose) | 2 (2 doses) | Unavailable |
|
No |
| Dabigatran | Direct thrombin inhibitor | 1 (2 doses) *hold for 2 days if renal insufficiency | 2 (4 doses) *hold 4 days if renal insufficiency | Idarucizumab |
|
No |
| Edoxaban | Factor Xa inhibitor | 1 (1 dose) | 2 (2 doses) | Unavailable |
|
No |
The rapid offset and onset of action of DOACs negates the need for perioperative bridging. Additionally, in trials where patients have received bridging anticoagulation for DOAC interruption, increased rates of bleeding with no benefit in TE risk have been shown. Interruptions in anticoagulation occurred frequently in the RE-LY (Randomized Evaluation of Long Term Anticoagulation Therapy with Dabigatran Etexilate) trial. In that study, 15.4% of dabigatran treated patients whose anticoagulation was interrupted peri-procedurally were bridged, with increased rates of major bleeding in the bridged group compared to non-bridged (6.5% vs 1.8%, OR 3.68) and no significant difference in rates of TE (0.5% vs 0.3%).(48) While most experts agree that given DOACs’ pharmokinetics no pre-operative bridging is required, given the rapid onset and lack of reversal agents, in rare cases patients may require postoperative bridging therapy for high risk patients who have delayed re-initiation of DOACs.(50)
Procedures in which continuing anticoagulation may be lower risk than bridging therapy
Procedures with moderate to high bleeding risk often require interruption of oral anticoagulant therapy even in high-risk patients. However, for certain low bleeding risk procedures, uninterrupted VKA therapy was found to have a lower risk of bleeding than therapy interruption with bridging.(55) Prospective data regarding uninterrupted anticoagulation with DOACs is limited; however, many suggest continued therapy for procedures with low bleeding risk based on the experience with VKA therapy. (Table 5).(50, 53, 56) Alternatively, given the lack of prospective data with uninterrupted therapy and lack of widely accessible reversal agents, some recommend continuing medications until the day of surgery and performing the procedure at time of the drug’s trough level to achieve an overall decreased anticoagulant effect without significant disruption of therapy.(54)
Table 5.
Low bleeding risk procedures not requiring therapy interruption.
| Category | Procedure |
|---|---|
| Dental |
|
| Opthamology |
|
| Cardiology |
|
| Dermatology |
|
| Gastroenterology |
|
| Other Selected Procedures |
|
Summary
After a half a decade of incremental gains, and in some areas, stagnation of forward progress, anticoagulation pharmacology has abruptly become a rapidly developing, expansive and progressive field. The standard of care is quickly evolving, and even for an experienced clinician, the onslaught of newly approved medications and studies is difficult to interpret and implement in the context of day to day practice. We have identified the most recent trends in anticoagulation in the context of current standard of care (Table 6). It is our hope that this may serve as a practical guide.
Table 6.
New trends in anticoagulation.
| Current (or Historical) Standard of Care |
New Trend | |
|---|---|---|
| Anticoagulant choice | LMWH/VKA 1st line | DOACs 1st line SAMeT2R2 to identify patients at high risk of VKA failure. |
| Extended duration therapy for unprovoked VTE | Prolonged anticoagulation with no testing, or d dimer or duplex to determine length of anticoagulation. | HERDOO score to identify low risk women who can avoid long term anticoagulation. |
| Long term full dose anticoagulation with VKAs | Long term prophylactic dosing with rivaroxaban or apixaban or ASA. | |
| Extended thromboprophylaxis | Extended duration prophylaxis for open abdominal/pelvic cancer operations only. | Include laparoscopic cancer resection. Betrixaban for extended thromboprophylaxis in the medically ill. |
| Bridging | Routine bridging for atrial fibrillation and prosthetic valves. | No bridging for CHADS2 ≤ 2; selective bridging for prosthetic valves. |
| Always hold anticoagulation for invasive procedures. | Select procedures safer to continue anticoagulation. | |
| Hold DOACs 2–4 days | Hold DOACs 1–2 days |
Anticoagulant choice
Whether these new trends become widely adopted remains uncertain. Certainly, the prescription of DOACs for the treatment of VTE has become widespread, but whether daily use of the SAMe-TT2R2 score is as readily adopted, as say CHADS2 score for atrial fibrillation, remains uncertain. In fact, in most cases, there will be overriding co-morbidities or patient preference that will drive the selection of anticoagulant. In our own practice, the SAMe-TT2R2 score serves to identify patients at high risk of low time in therapeutic range, and thereby allow for shorter term and more frequent follow up.
Extended duration therapy for unprovoked VTE
Similarly, the validation of the HER2DOO score for identification of low risk women who may safely discontinue anticoagulation after a standard course following unprovoked VTE represents a new clinical scoring system, with an uncertain fate in terms of clinical implementation. A similar scoring system incorporating men and elderly patients would represent a much needed major advancement in the management of first time unprovoked VTE. While apixaban and rivaroxaban have shown net clinical benefit for prevention of recurrent VTE, the issue of cost remains. Even with insurance coverage, it becomes an ethical question of social responsibility – is the risk reduction so great as to offset the cost to society? Aspirin, while not as effective, does offer risk reduction and is inexpensive. In practice, the patients likely to benefit from ASA are those at high bleeding risk who have a contraindication (such as end stage renal disease) to DOACs (or who cannot afford them).
Extended thromboprophylaxis
While much money and effort was expended in the early DOAC trials (RECORD, ADVANCE) proving extended duration thromboprophylaxis was efficacious in reducing VTE, these have yet to be adopted into clinical practice. This is likely because of the priority placed by orthopedic surgeons on avoiding bleeding complications, and that the trials used LMWH (rather than placebo) as comparison group. Similarly, APEX trial resulted in approval of betrixaban for thromboprophylaxis in the medically ill, but due to study design limitation, resulting in failure to meet the primary efficacy endpoint, it is uncertain as to whether this strategy will be widely adopted. With so many available FDA approved DOACs, it remains certain that any other newcomers to the field will similarly have to expand into indications outside of primary non-valvular atrial fibrillation and VTE treatment to gain approval, such as laparoscopic cancer resection. This may prove to be beneficial as there are still gains to be made in thromboprophylaxis.
Bridging
While thromboembolism is always a feared risk in patients stopping anticoagulation, the BRIDGE trial was the first rigorous trial to bring to light the very tangible risks associated with bridging therapy. While it is accepted that the recurrent thromboembolism risk decreases over time, there is no predictive algorithm or similar trial with VTE patients to clearly delineate the risk-benefit profile of bridging and is very much needed. Other advancements in this area include the exploration of procedures in which it is safe to continue anticoagulation and the discovery that safety of shorter interruptions of DOACs prior to invasive procedures.
Future directions
The fact remains that despite an incredible number of trials and drug development over the last decade, there remains a multitude of unanswered questions and studies to be done. For instance, there is as of yet, no compelling data regarding the use of DOACs in cancer patients, representing a large group of individuals relegated to daily or twice daily LMWH injections. As the APEX study inadvertently highlighted, we still have much to learn about how to pick the highest risk medical patients who would benefit from extended thromboprophylaxis. Furthermore, even with perfect in-hospital compliance with thromboprophylaxis, development of VTE is in some cases inevitable – a fact that should prompt us all not become complacent with the current anticoagulation options. As hematologist and thrombosis researcher Dr. Robert Flaumenhaft has stated “inhibiting thrombosis without inducing bleeding is the holy grail of anticoagulant therapy… there are no commercially available anticoagulants that achieve that goal.” Much of decision making regarding anticoagulation therapy centers around risk and benefit; such an agent would absolve much of the imperfect and complicated decision making that occurs daily. Until such an ideal agent is discovered, we can envision that a male version of the HERDOO2 scoring system and a similarly designed BRIDGE trial for VTE patients would offer useful clinical information. Finally, as newer expensive anticoagulants paired with even more expensive reversal agents enter the market, the issue of cost-effectiveness has begun to permeate the medical literature; suggesting that the modern day doctor cannot function in isolation as a steward for his or her patient’s wellbeing, but must take into consideration the social and economic constraints surrounding such choices.
Key Points.
Based on ease of dosing and large non-inferiority trials, DOACs should be considered as first line therapy for treatment of VTE.
New strategies for treatment (besides long term anticoagulation) of unprovoked (idiopathic) VTE now exist: prophylactic dose rivaroxaban or apixaban, aspirin, and use of HERDOO2 scoring system to identify women at low risk of VTE recurrence.
Betrixaban is the newest DOAC to gain FDA approval with indication for extended thromboprophylaxis in high risk medical patients.
Selective, rather than routine, bridging of anticoagulants should occur in the setting of atrial fibrillation and prosthetic heart valves.
Footnotes
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S–e96S. doi: 10.1378/chest.11-2301. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. http://www.ncbi.nlm.nih.gov/pubmed/22315268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spencer FA, Lessard D, Emery C, Reed G, Goldberg RJ. Venous thromboembolism in the outpatient setting. Arch Intern Med. 2007;167(14):1471–5. doi: 10.1001/archinte.167.14.1471. Venous thromboembolism in the outpatient setting. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17646600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost. 2003;90(3):446–55. doi: 10.1160/TH03-03-0152. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12958614. [DOI] [PubMed] [Google Scholar]
- 4.Amin AN, Varker H, Princic N, Lin J, Thompson S, Johnston S. Duration of venous thromboembolism risk across a continuum in medically ill hospitalized patients. Journal of hospital medicine. 2012;7(3):231–8. doi: 10.1002/jhm.1002. Duration of venous thromboembolism risk across a continuum in medically ill hospitalized patients. https://www.ncbi.nlm.nih.gov/pubmed/22190427. [DOI] [PubMed] [Google Scholar]
- 5.Sweetland S, Green J, Liu B, Berrington de Gonzalez A, Canonico M, Reeves G, Beral V Million Women Study c. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ. 2009;339:b4583. doi: 10.1136/bmj.b4583. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. https://www.ncbi.nlm.nih.gov/pubmed/19959589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: a double-blind randomized multicentre trial with venographic assessment. ENOXACAN Study Group. Br J Surg. 1997;84(8):1099–103. Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: a double-blind randomized multicentre trial with venographic assessment. ENOXACAN Study Group. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9278651. [PubMed] [Google Scholar]
- 7.Bergqvist D, Agnelli G, Cohen AT, Eldor A, Nilsson PE, Le Moigne-Amrani A, Dietrich-Neto F Investigators EI. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346(13):975–80. doi: 10.1056/NEJMoa012385. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. https://www.ncbi.nlm.nih.gov/pubmed/11919306. [DOI] [PubMed] [Google Scholar]
- 8.Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, Samama CM. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e227S–e77S. doi: 10.1378/chest.11-2297. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. https://www.ncbi.nlm.nih.gov/pubmed/22315263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyman GH, Bohlke K, Falanga A American Society of Clinical O. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Oncol Pract. 2015;11(3):e442–4. doi: 10.1200/JOP.2015.004473. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. https://www.ncbi.nlm.nih.gov/pubmed/25873061. [DOI] [PubMed] [Google Scholar]
- 10.Vedovati MC, Becattini C, Rondelli F, Boncompagni M, Camporese G, Balzarotti R, Mariani E, Flamini O, Pucciarelli S, Donini A, et al. A randomized study on 1-week versus 4-week prophylaxis for venous thromboembolism after laparoscopic surgery for colorectal cancer. Ann Surg. 2014;259(4):665–9. doi: 10.1097/SLA.0000000000000340. A randomized study on 1-week versus 4-week prophylaxis for venous thromboembolism after laparoscopic surgery for colorectal cancer. http://www.ncbi.nlm.nih.gov/pubmed/24253138. [DOI] [PubMed] [Google Scholar]
- 11.Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, Ortel TL, Pauker SG, Colwell CW., Jr Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e278S–e325S. doi: 10.1378/chest.11-2404. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. https://www.ncbi.nlm.nih.gov/pubmed/22315265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White RH, Romano PS, Zhou H, Rodrigo J, Bargar W. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med. 1998;158(14):1525–31. doi: 10.1001/archinte.158.14.1525. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. https://www.ncbi.nlm.nih.gov/pubmed/9679793. [DOI] [PubMed] [Google Scholar]
- 13.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ American College of Chest Physicians Antithrombotic T, Prevention of Thrombosis P. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):7S–47S. doi: 10.1378/chest.1412S3. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. https://www.ncbi.nlm.nih.gov/pubmed/22315257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eriksson BI, Dahl OE, Huo MH, Kurth AA, Hantel S, Hermansson K, Schnee JM, Friedman RJ Group R-NIS. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomised, double-blind, non-inferiority trial. Thromb Haemost. 2011;105(4):721–9. doi: 10.1160/TH10-10-0679. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomised, double-blind, non-inferiority trial. https://www.ncbi.nlm.nih.gov/pubmed/21225098. [DOI] [PubMed] [Google Scholar]
- 15.Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM Investigators A. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363(26):2487–98. doi: 10.1056/NEJMoa1006885. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. https://www.ncbi.nlm.nih.gov/pubmed/21175312. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, Bandel TJ, Beckmann H, Muehlhofer E, Misselwitz F, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358(26):2765–75. doi: 10.1056/NEJMoa0800374. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. https://www.ncbi.nlm.nih.gov/pubmed/18579811. [DOI] [PubMed] [Google Scholar]
- 17.Nikolaou VS, Desy NM, Bergeron SG, Antoniou J. Total knee replacement and chemical thromboprophylaxis: current evidence. Curr Vasc Pharmacol. 2011;9(1):33–41. doi: 10.2174/157016111793744724. Total knee replacement and chemical thromboprophylaxis: current evidence. https://www.ncbi.nlm.nih.gov/pubmed/21044024. [DOI] [PubMed] [Google Scholar]
- 18.Hull RD, Schellong SM, Tapson VF, Monreal M, Samama MM, Nicol P, Vicaut E, Turpie AG, Yusen RD study E. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153(1):8–18. doi: 10.7326/0003-4819-153-1-201007060-00004. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. https://www.ncbi.nlm.nih.gov/pubmed/20621900. [DOI] [PubMed] [Google Scholar]
- 19.Goldhaber SZ, Leizorovicz A, Kakkar AK, Haas SK, Merli G, Knabb RM, Weitz JI Investigators AT. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365(23):2167–77. doi: 10.1056/NEJMoa1110899. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. https://www.ncbi.nlm.nih.gov/pubmed/22077144. [DOI] [PubMed] [Google Scholar]
- 20.Cohen AT, Harrington RA, Goldhaber SZ, Hull RD, Wiens BL, Gold A, Hernandez AF, Gibson CM Investigators A. Extended Thromboprophylaxis with Betrixaban in Acutely Ill Medical Patients. N Engl J Med. 2016;375(6):534–44. doi: 10.1056/NEJMoa1601747. Extended Thromboprophylaxis with Betrixaban in Acutely Ill Medical Patients. https://www.ncbi.nlm.nih.gov/pubmed/27232649. [DOI] [PubMed] [Google Scholar]
- 21.Cohen AT, Spiro TE, Buller HR, Haskell L, Hu D, Hull R, Mebazaa A, Merli G, Schellong S, Spyropoulos A, et al. Extended-duration rivaroxaban thromboprophylaxis in acutely ill medical patients: MAGELLAN study protocol. J Thromb Thrombolysis. 2011;31(4):407–16. doi: 10.1007/s11239-011-0549-x. Extended-duration rivaroxaban thromboprophylaxis in acutely ill medical patients: MAGELLAN study protocol. https://www.ncbi.nlm.nih.gov/pubmed/21359646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dentali F, Mumoli N, Prisco D, Fontanella A, Di Minno MN. Efficacy and safety of extended thromboprophylaxis for medically ill patients. A meta-analysis of randomised controlled trials. Thromb Haemost. 2017;117(3):606–17. doi: 10.1160/TH16-08-0595. Efficacy and safety of extended thromboprophylaxis for medically ill patients. A meta-analysis of randomised controlled trials. https://www.ncbi.nlm.nih.gov/pubmed/28078350. [DOI] [PubMed] [Google Scholar]
- 23.Raskob GE, Spyropoulos AC, Zrubek J, Ageno W, Albers G, Elliott CG, Halperin J, Haskell L, Hiatt WR, Maynard GA, et al. The MARINER trial of rivaroxaban after hospital discharge for medical patients at high risk of VTE. Design, rationale, and clinical implications. Thromb Haemost. 2016;115(6):1240–8. doi: 10.1160/TH15-09-0756. The MARINER trial of rivaroxaban after hospital discharge for medical patients at high risk of VTE. Design, rationale, and clinical implications. https://www.ncbi.nlm.nih.gov/pubmed/26842902. [DOI] [PubMed] [Google Scholar]
- 24.Schulman S, Rhedin AS, Lindmarker P, Carlsson A, Larfars G, Nicol P, Loogna E, Svensson E, Ljungberg B, Walter H. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of Anticoagulation Trial Study Group. N Engl J Med. 1995;332(25):1661–5. doi: 10.1056/NEJM199506223322501. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of Anticoagulation Trial Study Group. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7760866. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Goldhaber SZ, Glynn RJ. Low-intensity versus conventional-intensity warfarin for prevention of recurrent venous thromboembolism. N Engl J Med. 2003;349(22):2164–7. author reply −7. Low-intensity versus conventional-intensity warfarin for prevention of recurrent venous thromboembolism. http://www.ncbi.nlm.nih.gov/pubmed/14658125. [PubMed] [Google Scholar]
- 26.Kearon C, Ginsberg JS, Kovacs MJ, Anderson DR, Wells P, Julian JA, MacKinnon B, Weitz JI, Crowther MA, Dolan S, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med. 2003;349(7):631–9. doi: 10.1056/NEJMoa035422. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. http://www.ncbi.nlm.nih.gov/pubmed/12917299. [DOI] [PubMed] [Google Scholar]
- 27.Douketis JD, Kearon C, Bates S, Duku EK, Ginsberg JS. Risk of fatal pulmonary embolism in patients with treated venous thromboembolism. Jama. 1998;279(6):458–62. doi: 10.1001/jama.279.6.458. Risk of fatal pulmonary embolism in patients with treated venous thromboembolism. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9466640. [DOI] [PubMed] [Google Scholar]
- 28.Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, Rickles FR, Julian JA, Haley S, Kovacs MJ, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146–53. doi: 10.1056/NEJMoa025313. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. http://www.ncbi.nlm.nih.gov/pubmed/12853587. [DOI] [PubMed] [Google Scholar]
- 29.Hull RD, Pineo GF, Brant R, Liang J, Cook R, Solymoss S, Poon MC, Raskob G Investigators LT. Home therapy of venous thrombosis with long-term LMWH versus usual care: patient satisfaction and post-thrombotic syndrome. Am J Med. 2009;122(8):762–9. e3. doi: 10.1016/j.amjmed.2008.12.023. Home therapy of venous thrombosis with long-term LMWH versus usual care: patient satisfaction and post-thrombotic syndrome. http://www.ncbi.nlm.nih.gov/pubmed/19635277. [DOI] [PubMed] [Google Scholar]
- 30.Investigators E. Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing AW, Misselwitz F, Prins MH, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–510. doi: 10.1056/NEJMoa1007903. Oral rivaroxaban for symptomatic venous thromboembolism. https://www.ncbi.nlm.nih.gov/pubmed/21128814. [DOI] [PubMed] [Google Scholar]
- 31.Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, Baanstra D, Schnee J, Goldhaber SZ, Group R-CS. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342–52. doi: 10.1056/NEJMoa0906598. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. https://www.ncbi.nlm.nih.gov/pubmed/19966341. [DOI] [PubMed] [Google Scholar]
- 32.Hokusai VTEI, Buller HR, Decousus H, Grosso MA, Mercuri M, Middeldorp S, Prins MH, Raskob GE, Schellong SM, Schwocho L, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369(15):1406–15. doi: 10.1056/NEJMoa1306638. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. https://www.ncbi.nlm.nih.gov/pubmed/23991658. [DOI] [PubMed] [Google Scholar]
- 33.Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Masiukiewicz U, Pak R, Thompson J, Raskob GE, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808. doi: 10.1056/NEJMoa1302507. Oral apixaban for the treatment of acute venous thromboembolism. https://www.ncbi.nlm.nih.gov/pubmed/23808982. [DOI] [PubMed] [Google Scholar]
- 34.Knepper J, Horne D, Obi A, Wakefield TW. A systematic update on the state of novel anticoagulants and a primer on reversal and bridging. Journal of vascular surgery Venous and lymphatic disorders. 2013;1(4):418–26. doi: 10.1016/j.jvsv.2013.04.006. A systematic update on the state of novel anticoagulants and a primer on reversal and bridging. https://www.ncbi.nlm.nih.gov/pubmed/26992768. [DOI] [PubMed] [Google Scholar]
- 35.Pollack CV, Jr, Reilly PA, Eikelboom J, Glund S, Verhamme P, Bernstein RA, Dubiel R, Huisman MV, Hylek EM, Kamphuisen PW, et al. Idarucizumab for Dabigatran Reversal. N Engl J Med. 2015;373(6):511–20. doi: 10.1056/NEJMoa1502000. Idarucizumab for Dabigatran Reversal. https://www.ncbi.nlm.nih.gov/pubmed/26095746. [DOI] [PubMed] [Google Scholar]
- 36.Siegal DM, Curnutte JT, Connolly SJ, Lu G, Conley PB, Wiens BL, Mathur VS, Castillo J, Bronson MD, Leeds JM, et al. Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. N Engl J Med. 2015;373(25):2413–24. doi: 10.1056/NEJMoa1510991. Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. https://www.ncbi.nlm.nih.gov/pubmed/26559317. [DOI] [PubMed] [Google Scholar]
- 37.Crowther M, Crowther MA. Antidotes for novel oral anticoagulants: current status and future potential. Arterioscler Thromb Vasc Biol. 2015;35(8):1736–45. doi: 10.1161/ATVBAHA.114.303402. Antidotes for novel oral anticoagulants: current status and future potential. https://www.ncbi.nlm.nih.gov/pubmed/26088576. [DOI] [PubMed] [Google Scholar]
- 38.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149(2):315–52. doi: 10.1016/j.chest.2015.11.026. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. https://www.ncbi.nlm.nih.gov/pubmed/26867832. [DOI] [PubMed] [Google Scholar]
- 39.Kataruka A, Kong X, Haymart B, Kline-Rogers E, Almany S, Kozlowski J, Krol GD, Kaatz S, McNamara MW, Froehlich JB, et al. SAMe-TT2R2 predicts quality of anticoagulation in patients with acute venous thromboembolism: The MAQI2 experience. Vasc Med. 2017;22(3):197–203. doi: 10.1177/1358863X16682863. SAMe-TT2R2 predicts quality of anticoagulation in patients with acute venous thromboembolism: The MAQI2 experience. https://www.ncbi.nlm.nih.gov/pubmed/28145152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabriel F, Portoles O, Labios M, Rodriguez C, Cisneros E, Vela J, Nunez M Investigators R. Usefulness of thrombophilia testing in venous thromboembolic disease: findings from the RIETE registry. Clin Appl Thromb Hemost. 2013;19(1):42–7. doi: 10.1177/1076029611436193. Usefulness of thrombophilia testing in venous thromboembolic disease: findings from the RIETE registry. https://www.ncbi.nlm.nih.gov/pubmed/22327823. [DOI] [PubMed] [Google Scholar]
- 41.Palareti G, Cosmi B, Legnani C, Tosetto A, Brusi C, Iorio A, Pengo V, Ghirarduzzi A, Pattacini C, Testa S, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med. 2006;355(17):1780–9. doi: 10.1056/NEJMoa054444. D-dimer testing to determine the duration of anticoagulation therapy. https://www.ncbi.nlm.nih.gov/pubmed/17065639. [DOI] [PubMed] [Google Scholar]
- 42.Rodger MA, Le Gal G, Anderson DR, Schmidt J, Pernod G, Kahn SR, Righini M, Mismetti P, Kearon C, Meyer G, et al. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ. 2017;356:j1065. doi: 10.1136/bmj.j1065. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. https://www.ncbi.nlm.nih.gov/pubmed/28314711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kearon C, Gent M, Hirsh J, Weitz J, Kovacs MJ, Anderson DR, Turpie AG, Green D, Ginsberg JS, Wells P, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med. 1999;340(12):901–7. doi: 10.1056/NEJM199903253401201. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10089183. [DOI] [PubMed] [Google Scholar]
- 44.Simes J, Becattini C, Agnelli G, Eikelboom JW, Kirby AC, Mister R, Prandoni P, Brighton TA Investigators IS. Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation. 2014;130(13):1062–71. doi: 10.1161/CIRCULATIONAHA.114.008828. Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. https://www.ncbi.nlm.nih.gov/pubmed/25156992. [DOI] [PubMed] [Google Scholar]
- 45.Becattini C, Agnelli G, Schenone A, Eichinger S, Bucherini E, Silingardi M, Bianchi M, Moia M, Ageno W, Vandelli MR, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366(21):1959–67. doi: 10.1056/NEJMoa1114238. Aspirin for preventing the recurrence of venous thromboembolism. https://www.ncbi.nlm.nih.gov/pubmed/22621626. [DOI] [PubMed] [Google Scholar]
- 46.Weitz JI, Lensing AWA, Prins MH, Bauersachs R, Beyer-Westendorf J, Bounameaux H, Brighton TA, Cohen AT, Davidson BL, Decousus H, et al. Rivaroxaban or Aspirin for Extended Treatment of Venous Thromboembolism. N Engl J Med. 2017;376(13):1211–22. doi: 10.1056/NEJMoa1700518. Rivaroxaban or Aspirin for Extended Treatment of Venous Thromboembolism. https://www.ncbi.nlm.nih.gov/pubmed/28316279. [DOI] [PubMed] [Google Scholar]
- 47.Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Porcari A, Raskob GE, Weitz JI Investigators A-E. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368(8):699–708. doi: 10.1056/NEJMoa1207541. Apixaban for extended treatment of venous thromboembolism. https://www.ncbi.nlm.nih.gov/pubmed/23216615. [DOI] [PubMed] [Google Scholar]
- 48.Douketis JD, Healey JS, Brueckmann M, Eikelboom JW, Ezekowitz MD, Fraessdorf M, Noack H, Oldgren J, Reilly P, Spyropoulos AC, et al. Perioperative bridging anticoagulation during dabigatran or warfarin interruption among patients who had an elective surgery or procedure. Substudy of the RE-LY trial. Thromb Haemost. 2015;113(3):625–32. doi: 10.1160/TH14-04-0305. Perioperative bridging anticoagulation during dabigatran or warfarin interruption among patients who had an elective surgery or procedure. Substudy of the RE-LY trial. https://www.ncbi.nlm.nih.gov/pubmed/25472710. [DOI] [PubMed] [Google Scholar]
- 49.Rechenmacher SJ, Fang JC. Bridging Anticoagulation: Primum Non Nocere. J Am Coll Cardiol. 2015;66(12):1392–403. doi: 10.1016/j.jacc.2015.08.002. Bridging Anticoagulation: Primum Non Nocere. https://www.ncbi.nlm.nih.gov/pubmed/26383727. [DOI] [PubMed] [Google Scholar]
- 50.Doherty JU, Gluckman TJ, Hucker WJ, Januzzi JL, Jr, Ortel TL, Saxonhouse SJ, Spinler SA. 2017 ACC Expert Consensus Decision Pathway for Periprocedural Management of Anticoagulation in Patients With Nonvalvular Atrial Fibrillation: A Report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol. 2017;69(7):871–98. doi: 10.1016/j.jacc.2016.11.024. 2017 ACC Expert Consensus Decision Pathway for Periprocedural Management of Anticoagulation in Patients With Nonvalvular Atrial Fibrillation: A Report of the American College of Cardiology Clinical Expert Consensus Document Task Force. https://www.ncbi.nlm.nih.gov/pubmed/28081965. [DOI] [PubMed] [Google Scholar]
- 51.Wysokinski WE, McBane RD., 2nd Periprocedural bridging management of anticoagulation. Circulation. 2012;126(4):486–90. doi: 10.1161/CIRCULATIONAHA.112.092833. Periprocedural bridging management of anticoagulation. https://www.ncbi.nlm.nih.gov/pubmed/22825410. [DOI] [PubMed] [Google Scholar]
- 52.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(25):e1159–e95. doi: 10.1161/CIR.0000000000000503. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. https://www.ncbi.nlm.nih.gov/pubmed/28298458. [DOI] [PubMed] [Google Scholar]
- 53.Baron TH, Kamath PS, McBane RD. Management of antithrombotic therapy in patients undergoing invasive procedures. N Engl J Med. 2013;368(22):2113–24. doi: 10.1056/NEJMra1206531. Management of antithrombotic therapy in patients undergoing invasive procedures. https://www.ncbi.nlm.nih.gov/pubmed/23718166. [DOI] [PubMed] [Google Scholar]
- 54.NOACS/DOACS. Peri-Operative Management. Thrombosis Canada. 2017:1–7. Peri-Operative Management. http://thrombosiscanada.ca/wp-content/uploads/2017/09/22_NOACs-DOACs-Peri-Operative-Management-2017Jul26.pdf.
- 55.Birnie DH, Healey JS, Wells GA, Verma A, Tang AS, Krahn AD, Simpson CS, Ayala-Paredes F, Coutu B, Leiria TL, et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med. 2013;368(22):2084–93. doi: 10.1056/NEJMoa1302946. Pacemaker or defibrillator surgery without interruption of anticoagulation. https://www.ncbi.nlm.nih.gov/pubmed/23659733. [DOI] [PubMed] [Google Scholar]
- 56.Raval AN, Cigarroa JE, Chung MK, Diaz-Sandoval LJ, Diercks D, Piccini JP, Jung HS, Washam JB, Welch BG, Zazulia AR, et al. Management of Patients on Non-Vitamin K Antagonist Oral Anticoagulants in the Acute Care and Periprocedural Setting: A Scientific Statement From the American Heart Association. Circulation. 2017;135(10):e604–e33. doi: 10.1161/CIR.0000000000000477. Management of Patients on Non-Vitamin K Antagonist Oral Anticoagulants in the Acute Care and Periprocedural Setting: A Scientific Statement From the American Heart Association. https://www.ncbi.nlm.nih.gov/pubmed/28167634. [DOI] [PMC free article] [PubMed] [Google Scholar]