Summary
Diabetic kidney disease (DKD) is the leading cause of morbidity and mortality in diabetic patients. Defining risk factors for DKD using a reductionist approach has proven challenging. Integrative omics-based systems biology tools have shed new insights in our understanding of DKD and have provided several key breakthroughs for identifying novel predictive and diagnostic biomarkers. In this review, we highlight the role of the Warburg effect in DKD and potential regulating factors such as sphingomyelin, fumarate, and pyruvate kinase muscle isozyme M2 in shifting glucose flux from complete oxidation in mitochondria to the glycolytic pathway and its principal branches. With the development of highly sensitive instruments and more advanced automatic bioinformatics tools, we believe that omics analyses and imaging techniques will focus more on singular-cell-level studies, which will allow in-depth understanding of DKD and pave the path for personalized kidney precision medicine.
Keywords: Diabetic kidney disease, the Warburg effect, aerobic glycolysis, metabolomics, mitochondrion
Diabetic kidney disease (DKD) develops in approximately 40% of patients with diabetes and is the leading cause of chronic kidney disease worldwide.1 Metabolic alterations associated with diabetes lead to renal pathologic changes including tubulointerstitial inflammation and fibrosis, glomerular hypertrophy, and glomerulosclerosis.1 However, because only less than 10% of patients with diabetes ultimately reach end-stage renal disease, there must be something missing in our understanding of the pathophysiology of DKD. Genetic studies have not identified a major genetic contribution,2,3 although it is likely that there are important genetic determinants.4,5 As part of the International Society of Nephrology Forefronts Symposium of Systems Biology, the application of systems biology tools to understanding DKD was a major topic.
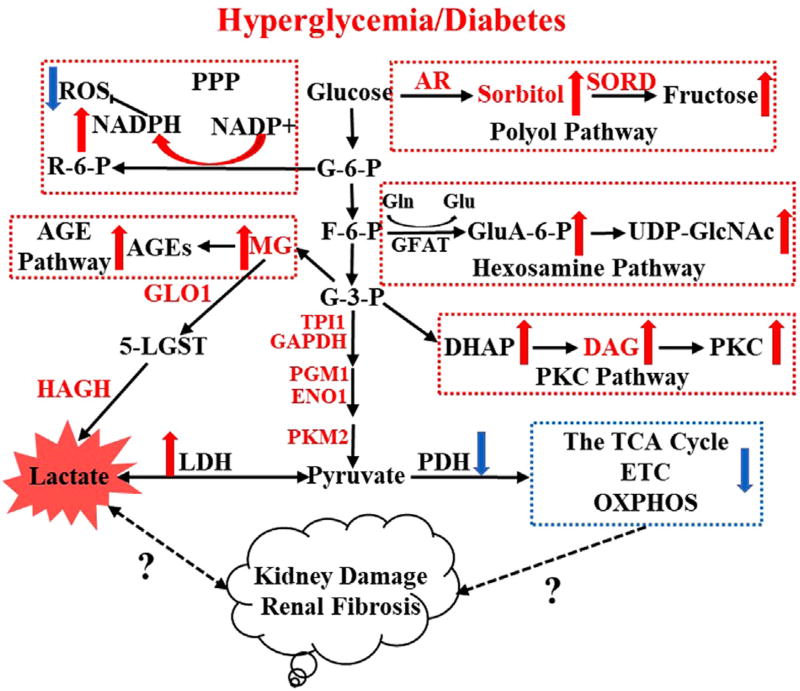
Detailed roles of mitochondria in DKD has been reviewed previously.5–7 In the presence of hyperglycemia, the metabolic flux of glucose can be shifted from complete oxidation in mitochondria to the glycolytic pathway, and the deleterious effects of hyperglycemia are considered to be owing mainly to increased activity of five different pathways: pentose phosphate pathway,8 sorbitol/polyol pathway,9 advanced glycation end-products pathway,10,11 protein kinase C pathway,12 and hexosamine pathway (Fig. 1).13,14 Activation of the earlier-mentioned pathways was reported to be associated with diabetic complications, either by causing oxidative stress, inflammation, fibrosis, DNA damage, and vascular changes, or by resulting in pathologic gene expression.5 Overproduction of superoxide in mitochondria might play a unifying role for the activation of the aforementioned metabolic pathways.15 New evidence indicates that alternative pathways such as enhanced fatty acid oxidation in mitochondria are involved in diabetic complications.15,16 However, it still is unclear whether mitochondrial dysfunction in DKD results in altered glycolysis flux, or if glycolysis flux/accumulation of glycolytic intermediates in DKD drives the metabolic flux into the five pathways discussed earlier, leading to impaired mitochondrial function, or if a bidirectional causality exists. The current review high-lights the diabetes-induced metabolic switch from oxidative phosphorylation to glycolysis and the potential link to downstream effects on kidney function and disease progression.
Figure 1.
Hyperglycemia enriches metabolic flux to glycolysis and five principal branches including the polyol pathway, pentose phosphate pathway (PPP), hexosamine pathway, protein kinase C (PKC) pathway, and advanced glycation end-products (AGE) pathway. Accumulation of four toxic glucose metabolites such as lactate, sorbitol, diacylglycerol (DAG), and methylglyoxal (MG) might contribute to the development of diabetic kidney disease. Abbreviations: 5-LGST, 5-lactoylglutathione; AR, aldose reductase; DHAP, dihydroxyacetone phosphate; ENO1, alpha-enolase; ETC, electron transport chain; F-6-P, fructose 6-phosphate; G-6-P, glucose 6-phosphate; G-3-P, glyceraldehyde 3-phosphate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFAT, glutamine:fructose-6-phosphate amidotransferase; Gln, glutamine; Glu, glutamate; GluA-6-P, glucosamine-6-phosphate; GLO1, glyoxalase 1; HAGH, hydroxyacyl glutathione hydrolase; LDH, lactate dehydrogenase; OXPHOS, oxidative phosphorylation; PGM1, phosphoglucomutase-1; R-6-P, ribulose-5-phosphate; SORD, sorbitol dehydrogenase; TPI1, triosephosphate isomerase 1; UDP-GlcNAc, uridine diphosphate N-acetylglucosamine.
WARBURG EFFECT/AEROBIC GLYCOLYSIS AND ITS POTENTIAL ROLE IN THE DEVELOPMENT OF DKD
The Warburg effect or aerobic glycolysis, first observed in 1924 by Otto Warburg, has had a profound influence on cancer metabolism.17 Warburg proposed that, independent of cellular oxygen, tumor cells synthesize adenosine triphosphate (ATP) through glycolysis and a metabolic state involving enhanced glucose uptake leads to local acidification through enhanced lactate production. Although Warburg’s18 studies suggested that mitochondrial dysfunction is the root of aerobic glycolysis, subsequent studies and technical advances have since shown that mitochondria in cancer are indeed functional and that genetic or environmental cues may contribute to this metabolic shift to the glycolysis in tumor cells.19 Although enhanced glucose entry and glycolysis has been observed in diabetic tissues, the Warburg effect has not been proposed as a major feature in diabetic complications.
Recent omic studies in diabetes and diabetes kidney disease have provided unbiased evidence that both mitochondrial dysfunction and the Warburg effect play pivotal roles in the development of DKD.16,20,21 Several groups now have found that reduced mitochondrial function plays a key role in DKD both in mouse models and in patients with DKD.20,22,23 By using transcriptomic, metabolomics, and flux approaches, Sas et al16 reported significantly increased glycolytic intermediates and enzymes in kidney cortex, along with a significant reduction in mitochondrial function in a type 2 diabetic mouse model. Interestingly, in a very recent study, Qi et al21 reported that enhanced pyruvate kinase II (PKM2) activity may preserve mitochondrial function by increasing glucose flux through glycolysis in podocytes and alleviate the progression of DKD in patients with diabetes.
To understand how the Warburg effect may play a role in DKD, here we review the major metabolic pathways and associated enzymes and intermediates that are involved in DKD pathometabolism. Compared with mitochondrial respiration, aerobic glycolysis is an inefficient pathway of generating ATP per unit of glucose.24 Although complete oxidation of one molecule of glucose via pyruvate within the tricarboxylic acid (TCA) cycle in the presence of oxygen generates 38 molecules of ATP, the glycolysis, the first step of glucose oxidation process that occurs in cytosol, generates only two molecules of ATP, leading to pyruvate as a final product. However, the rate of glucose metabolism through aerobic glycolysis is much higher (10–100 times faster) than the complete oxidation of glucose through the TCA cycle and oxidative phosphorylation in the mitochondria. Therefore, in diabetic patients, activation of aerobic glycolysis might help metabolize glucose rapidly from systemic circulation. In addition, with the evidence of mitochondrial dysfunction including low peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) levels, abnormalities in electron transport chain complex assembly/activity, alterations in pyruvate dehydrogenase complex (PDH) phosphorylation, and altered TCA cycle intermediates in DKD,20,25 it is not surprising that the glucose oxidation through glycolysis is a more favorable process toward ATP synthesis.
METABOLOMICS IDENTIFY MITOCHONDRIAL DYSFUNCTION AND WARBURG MECHANISMS IN DKD
Recent omics studies in diabetes and DKD have provided unbiased evidence that both mitochondrial dysfunction and the Warburg effect play pivotal roles in the development of DKD.16,20,21 By using targeted metabolomics and systems biology tools we reported that human DKD is associated with mitochondrial dysfunction. Gas chromatography-mass spectrometry–based targeted metabolomics were performed and 94 urinary metabolites were quantified in patients with established DKD and compared with healthy controls.20 Results indicated a marked reduction in organic anions, the TCA cycle, and amino acid metabolites. We established a 13–urinary metabolite biomarker signature of DKD, all of which were decreased significantly. Correlation analysis between each of 13 metabolites and common biomarkers of DKD showed that five of the metabolites (ie, 3-hydroxy isovalerate, aconitic acid, citric acid, glycolic acid, and uracil) were correlated significantly with estimated glomerular filtration rate, and another three metabolites (ie, 2-methyl acetoacetate, 3-methyl crotonyl glycine, and 3-methyl adipic acid) were associated with albuminuria.20 In particular, 12 of the 13 metabolites are associated with mitochondrial metabolism, the metabolites are either generated within the TCA cycle or regulated by the mitochondrial enzymes (Table 1), suggesting suppressed mitochondrial activity in DKD.20 Further analysis of kidney biopsy samples and urinary exosomes showed a significant reduction in PGC-1α, mitochondrial proteins, and mitochondrial DNA levels in diabetic nephropathy patients as compared with healthy controls. These findings suggest an overall reduction in mitochondrial biogenesis in the kidney of DKD patients, contributing to a reduction in the TCA cycle metabolites and a potential shift to glycolysis.
Table 1.
Thirteen Urinary Metabolite Biomarkers for DKD and Their Associated Enzymes and Pathways
| Metabolite Decreased in DKD |
HMDB ID of Metabolite |
Function in Intermediary Metabolism |
Enzyme(s) Producing the Metabolite | Subcellular location of Enzymes |
|---|---|---|---|---|
| 3-Hydroxyisovaleric acid | HMDB00754 | Leu metabolite | 3-methylglutaconyl CoA hydratase | Mitochondria |
| Glycolic acid | HMDB00115 | Gly (peroxisomes) and 4-hydroxyproline (mitochondria) | NADPH-glyoxylate reductase | Peroxisomes, mitochondria |
| Citric acid | HMDB00094 | The TCA cycle and lipid synthesis | Citrate synthase | Mitochondria |
| 2-Ethylhydracrylic acid | HMDB00396 | Ile metabolite | From R-pathway of Ile metabolism (with 2MBDH deficiency) | Mitochondria |
| Uracil | HMDB00300 | Pyrimidine synthesis | Coenzyme Q10: dihydroorotate dehydrogenase, UMPS | Mitochondria |
| 3-Hydroxyisobutyric acid | HMDB00023 | Val metabolite | 3HIBCH | Mitochondria |
| Aconitic acid | HMDB00072 | The TCA cycle | Aconitase | Mitochondria |
| 3-Methyladipic acid | HMDB00555 | Indicates incomplete BCFA oxidation | From decreased intake of phytanic acid or increased α-oxidation of BAFA | Mitochondria |
| Tiglylglycine | HMDB00959 | Ile metabolite | FAD and 2MBDH | Mitochondria |
| 3-Methylcrotonylglycine | HMDB00459 | Leu metabolite | FAD and IVD | Mitochondria |
| 2-Methylacetoacetic acid | HMDB03771 | Ile metabolite | NAD+ and MHBD | Mitochondria |
| Homovanillic acid | HMDB00118 | Dopamine metabolite | COMT and MAO | Cytosol, Mitochondria |
| Hydroxypropionic acid | HMDB00700 | Ile, Val, Thr, and Met metabolite | 2MAACT (Ile), NAD+ and MMSDH (Val), NAD+ and 2KBDH (Thr and Met) | Mitochondria |
Abbreviations: 2MAACT, 2-methylacetoacetyl Coa thiolase; 2MBDH, 2-methylbutyryl-CoA dehydrogenase; 2KBDH, 2-ketobutyrate dehydrogenase; 3HIBCH, 3-hydroxyisobutryl-CoA hydrolase; BAFA, branched-chain fatty acids; COMT, catechol-O-methyl transferase; FAD, flavin adenine dinucleotide; Gly, glycine; Ile, isoleucine; IVD, isovaleryl-CoA dehydrogenase; Leu, leucine; MAO, monoamine oxidase; Met, methionine; MHBD, 2-methyl-3-hydroxybutyryl CoA dehydrogenase; MMSDH, methylmalonate semialdehyde dehydrogenase; NAD+, nicotinamide adenine dinucleotide; Thr, threonine; UMPS, uridine monophosphate synthetase; Val, valine.
Adapted with permission from Sharma et al.20
The mechanisms of mitochondrial dysfunction and reduction in TCA metabolites in DKD are not fully understood. It is unclear if low TCA cycle metabolite flux is the causal or consequence of the mitochondrial dysfunction. Recent studies by Sas et al16 using a systems-based approach combining transcriptomic, metabolomic, and metabolomic flux in a mouse model of type 2 diabetic (db/db) kidney cortex and in type 2 diabetic human patients identified enhanced metabolic flux into glycolysis, fatty acid oxidation, and TCA cycle pathways. Several of the glycolytic enzyme transcripts including hexokinase, phosphofructokinase, and pyruvate kinase were increased significantly in glomeruli-depleted kidney cortex of the db/db mice as compared with the control db/m mice. No significant changes were observed in the expression of TCA cycle pathway-related genes, suggesting a diabetes-induced metabolic shift to glycolysis. This was confirmed further by metabolomic analysis of kidney cortex, urine, plasma, and isolated mitochondria. Several glycolytic metabolites were up-regulated in the diabetic mouse kidney and urine at both 12 and 24 weeks. Likewise, several of the TCA cycle metabolites and acylcarnitines were increased in kidney cortex, isolated mitochondria, and urine samples of the diabetic mice, suggesting that mitochondrial metabolic alterations in the kidney cortex are reflective of whole-body metabolism. The TCA cycle metabolites and acylcarnitines were increased significantly in the mitochondria from kidney cortex of 24-week-old compared with 12-week-old mice, indicating a progressive increase in metabolism in the mitochondria with disease progression in DKD. Metabolomic flux analysis using [U-13C6]glucose, [2,3-13C2]pyruvate, and [13C16]palmitate were performed to assess flux through glycolysis, the TCA cycle, and fatty acid oxidation, respectively. Results from these studies showed that db/db kidney tissues had significantly increased flux of all three metabolic pathways, although there were no changes in corresponding ATP. Analysis of oxygen consumption of isolated mitochondria from kidney cortex further identified that the db/db kidney mitochondria have reduced state 3 respiration (ADP-stimulated respiration) and enhanced proton leaking, suggesting uncoupling of electron transport chain from ATP synthesisoccurs. Therefore, a compensatory increase in metabolic flux and a switch to alternate pathways including glycolysis for ATP synthesis occurs. Interestingly, the increased metabolism also was associated with enhanced protein acetylation. Sirtuin 1 (SIRT1) dependent deacetylation of PGC-1α is one of the major regulatory mechanisms that PGC-1α activity and PGC-1α levels and activity have been shown to be reduced in several animal models and human studies of DKD.26–28
SPHINGOMYELIN: A NOVEL MEDIATOR FOR ENHANCED ATP PRODUCTION VIA GLYCOLYSIS IN DIABETES
Adenosine monophosphate (AMP)-activated protein kinase (AMPK) is a major cellular energy sensor and is activated in the state of caloric depletion (eg, low ATP/high AMP).29 Reduced glucose oxidation in diabetes is expected to decrease ATP and increase AMP and AMPK activity. However, a reduction in renal AMPK activity was reported in human DKD and mouse models of DKD and in high-fat-diet–fed mice.23,30 PGC-1α is a key regulator of mitochondrial biogenesis.31 Suppressed AMPK and PGC-1α path-ways have been recognized in diabetic kidneys from both human and animal models, however, the regulating mechanisms were not fully illustrated.23,32
One of the hypotheses for energy regulation was that high levels of circulating glucose may increase the glycolytic flux and increase glycolytic ATP and ATP/AMP, which suppresses AMPK activity and PGC-1α expression. The hypothesis of an enhanced glycolytic pathway in diabetes was supported by recent studies from our laboratory using mass spectrometry imaging (MSI)-based spatial metabolomics in DKD animal models and human kidney tissue.33 We applied matrix-assisted laser desorption/ionization (MALDI)-MSI to localize and quantify renal nucleotides.33 MALDI-MSI at 70-µmol/L resolution identified ATP, adenosine diphosphate, and AMP widely distributed throughout the kidney and showed a significant increase in ATP and decrease in AMP, leading to significantly increased ATP/AMP in glomeruli of diabetic mice when compared with nondiabetic ones. Furthermore, biochemical analysis of freshly extracted nucleotides from total kidney cortex showed a similar increase in total ATP levels in db/db diabetic mice compared with the db/m controls.
To identify analytes that may regulate ATP production in the diabetic kidney, we used untargeted high spatial resolution (25-µmol/L resolution) MALDI-MSI to analyze both human and mouse kidney sections. Particularly, three peaks (ie, m/z values) of analytes were distributed mainly in mouse and human glomeruli compared with the nearby regions. Based on high accuracy measurements of parent m/z’s using high resolving power Fourier transform ion cyclotron resonance mass spectrometry and their subsequent tandem mass spectrometry analysis, three m/z values, 703.578 (+H+), 725.558 (+Na+), and 741.534 (+K+), were annotated as a specific sphingomyelin (ie, SM [d18:1/16:0]) in METLIN and LIPID MAPS databases.34,35 High spatial resolution MALDI-MSI in kidney tissue samples from type 1 diabetic and high-fat diet–fed mice tissue samples identified a significant accumulation of glomerular SM(d18:1/16:0) compared with the controls.
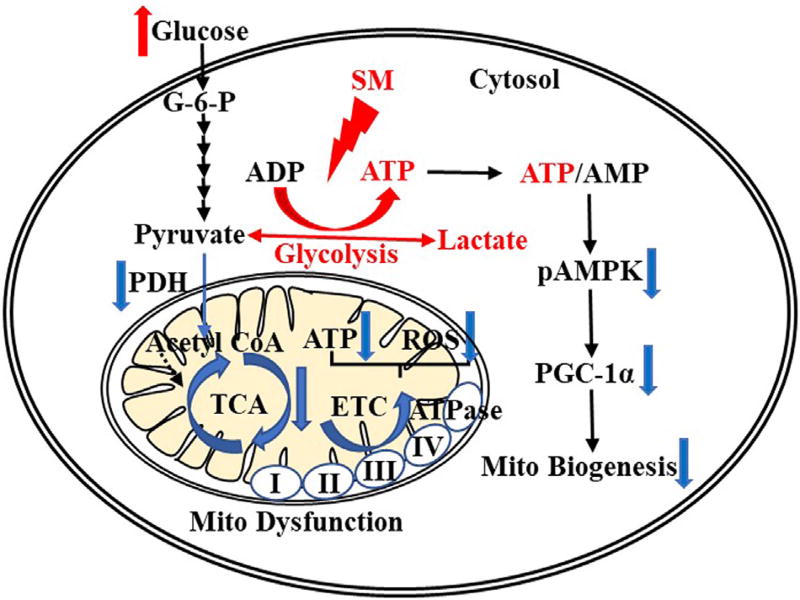
Sphingolipid metabolism is a critical cellular process and has been characterized extensively in the mammalian system.36,37 During the de novo biosynthesis ceramide is converted to SM by SM synthase (SMS), whereas SM levels are maintained by sphingomyelinase (SMase), which regulates lipid homeostasis by degrading SM to ceramide.37 Accumulation of ceramide and other sphingolipid signaling intermediates have been reported in type 1 and type 2 diabetic animal models and human studies.38,39 By using immunohistochemical staining of SMS and SMase, we found increased expression of SMS1 and SMS2, and decreased neutral SMase2 in the glomeruli of diabetic mice compared with WT mice. In addition, in murine mesangial cells, we found that SM (d18:1/16:0) suppressed AMPK activity and reduced PGC-1α protein expression.33 The liposomal SM (d18:1/16:0) treatment significantly increased lactate secretion in murine mesangial cells, suggesting that accumulation of SM and an increase in ATP in the diabetic kidney may be owing to the result of an increase in glycolysis. This was proved further by the treatment of glycolytic inhibitor 2-deoxy-D-glucose, which significantly inhibited ATP production, suggesting the potential role of SM (d18:1/16:0) in enhancing the glycolytic pathway to enhance ATP production and thus contribute to a Warburg type effect in glomeruli of the diabetic kidney (Fig. 2). These findings suggest alterations to sphing-homyelin signaling in DKD may contribute to enhanced ATP synthesis via glycolysis leading to metabolic imbalance and oxidative stress resulting in tissue damage.
Figure 2.
Accumulation of sphingomyelin (SM), a potential mediator in enhancing glycolysis-derived ATP, leads to reduced phosphorylation of AMP-activated protein kinase (pAMPK) and mitochondrial function. Abbreviations: ADP, adenosine diphosphate; ATPase, ATP synthase; ETC, electron transport chain; G-6-P, glucose 6-phosphate; Mito, mitochondrial.
PKM2 IN DKD: NOVEL MECHANISMS LINKING GLYCOLYSIS TO MITOCHONDRIAL FUNCTION
Although current understanding is that hyperglycemia enhances glycolysis and other toxic glucose-metabolite pathways as outlined in Figure 1, a recent study from the Joslin Diabetes Center has found that higher enzyme levels corresponding to glycolytic pathways can protect against diabetic nephropathy by reducing toxic glucose metabolites and increasing mitochondrial function. The study analyzed proteins from postmortem glomeruli of the kidneys of patients with long-standing diabetes with (nonprotected group) and with-out (protected) histologic signs of DKD,21 and showed that the protected group had increased levels of proteins related to glucose metabolism compared with nonprotected individuals. In particular, enzymes from the glycolytic (PKM2, PKM1, and enolase 1), methylglyoxal (eg, glyoxalase 1, and hydroxyacyl glutathione hydrolase), polyol (aldose reductase and sorbitol dehydrogenase), and mitochondrial nicotinamide adenine dinucleotide (NADH):ubiquinone oxidoreductase (complex I) and cytochrome c oxidase (complex pathways were up-regulated in the protected group. Up-regulation of glycolytic, methyl glyoxal, and polyol pathways have been considered to contribute to induce deleterious effects in diabetes. Therefore, the finding of an increase in these pathways in protective individuals was rather unexpected. Plasma metabolomics analysis further identified that an increase in glomerular enzymes may be associated with a decrease in metabolites from sorbitol and methyl glyoxal pathways in protected individuals, suggesting an increased ability to process the toxic metabolites by up-regulating the enzymes. In addition to the glycolytic enzymes, the study also found an increase in mitochondrial complex I– and complex IV–related proteins in the protected group, suggesting improved mitochondrial function. In addition, proteomic studies indicated that the protected group had significantly increased glycolytic enzyme pyruvate kinase M2 isoform. Pyruvate kinase, a rate-limiting enzyme in the glycolytic pathway, catalyzes the irreversible conversion of phosphoenolpyruvate to pyruvate.21 There are four PK isoforms in mammals including PKL (in liver), PKR (in red blood cell), PKM1 (predominantly in adult muscle, brain, bladder, and fibroblasts), and PKM2 (in most cells except for adult muscle cells).40,41 The role of PKM2 in glycolysis and cancer progression (eg, the Warburg effect) has been reviewed by several investigators.41–44 PKM2 is overexpressed in the majority of cancer cells, such as renal cell carcinoma.45–48 Increased PKM2 is associated with increased glucose uptake and lactate production, and reduced oxygen consumption, therefore PKM2 has been well studied for its role in cancer and the Warburg effect.41,46 The study by Qi et al21 using animal models as well as PKM2-specific activators in mice reported that PKM2 activation enhances glucose flux through glycolysis, reduces toxic metabolites such as sorbitol and methylglyoxal, and also enhances mitochondrial metabolism by improving PGC-1α, mitochondrial biogenesis, and mitochondrial function. Their findings suggest that in podocytes, activation of PKM2 may be beneficial in protecting against diabetic nephropathy, however, the regulation of PKM2 is complex and whether sole regulation of PKM2 will be sufficient in human disease remains to be determined. Nevertheless, the proteomic, metabolomic, and transcriptomic studies from human samples all indicate a key role for glycolysis and mitochondrial metabolism to be critical in human diabetic nephropathy.
REACTIVE OXYGEN SPECIES, NOX4, AND FUMARATE IN DKD
Oxidative Stress and NOX4 in DKD
Oxidative stress resulting from enhanced reactive oxygen species (ROS) is recognized as a key factor in the development of diabetic complications.8,49 The major sources of endogenous ROS in the diabetic kidney include mitochondria, nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, nitric oxide synthases, xanthine oxidase, and lipoxygenase.8,49,50 ROS include the free radical superoxide anion , the nonradical hydrogen peroxide (H2O2), highly reactive hydroxyl free radical (.OH), peroxynitrite (ONOO−), and singlet oxygen (1O2). Specifically, , H2O2, and ONOO− have been investigated mostly in the diabetic kidney.
Oxidative stress occurs in tissues when excessive ROS or oxidant production exceeds local antioxidant capacity. Studies have shown that accumulation of ROS in diabetes induces β-cell dysfunction, increases insulin resistance, and enhances diabetes-related complications in both type 1 and type 2 diabetes.51 Almost all published studies have shown a uniform over-production of ROS in the tissues of animals and patients with diabetes.52–54 In particular, a common belief is that mitochondrial superoxide is a major contributor for diabetic complications, such as DKD.49,55,56 However, anti-oxidant–based clinical trials have proven to be largely negative.57 Based on the new evidence, the theory of "mitochondrial hormesis" has been proposed wherein optimal levels of ROS are considered a marker for functional mitochondria.85 In steptozotocin-induced and Akita type 1 diabetic mouse models, using a combination of in vivo real-time imaging and electron paramagnetic resonance approaches, we reported that diabetic kidneys have reduced overall along with a reduction in mitochondrial function, PDH activity, and mitochondrial biogenesis, which all were rescued with activation of AMPK using an AMPK activator.23 A persistent reduction in mitochondrial oxidative phosphorylation can lead to a reduction in mitochondrial superoxide and a simultaneous activation of cytosolic oxidative pathways triggering cytosolic ROS, resulting in increased oxidative stress. Indeed, the PDH complex, a major gateway for pyruvate carbons to enter the TCA cycle in mitochondria, was hyperphosphorylated and inhibited in diabetic kidneys.58 Therefore, although convincing data support increases in oxidative stress in diabetes, the source of oxidative stress and ROS needs further investigation.
NADPH oxidase (NOX) family enzymes are one of the major sources of cytosolic ROS. NOX enzymes catalyze the reduction of molecular oxygen to super-oxide and hydrogen peroxide using NADPH as an electron donor.59 Among the seven members of the NOX family, NOX1, NOX2, and NOX4 are expressed in kidneys of human beings and mice.60,61 In the NOX family, NOX4 in particular has been reported to be upregulated in DKD by different researchers, including our laboratory.61–64 Several studies have shown the beneficial effects of NOX inhibitors in reducing oxidative stress in DKD.22,65 However, the mechanisms underlying NOX4 in DKD and the key down-stream targets that mediate the progression of DKD still remain unclear. Recently, in our laboratory, by using podocyte-specific NOX4 transgenic mice, we showed that podocyte-specific induction of NOX4 alone was sufficient to induce characteristic glomerular changes associated with DKD.22 By using urinary metabolomics in type 1 Akita diabetic mice after intervention with a NOX1/4 inhibitor, we identified a reduction in the specific TCA cycle metabolite fumarate.
Fumarate in DKD
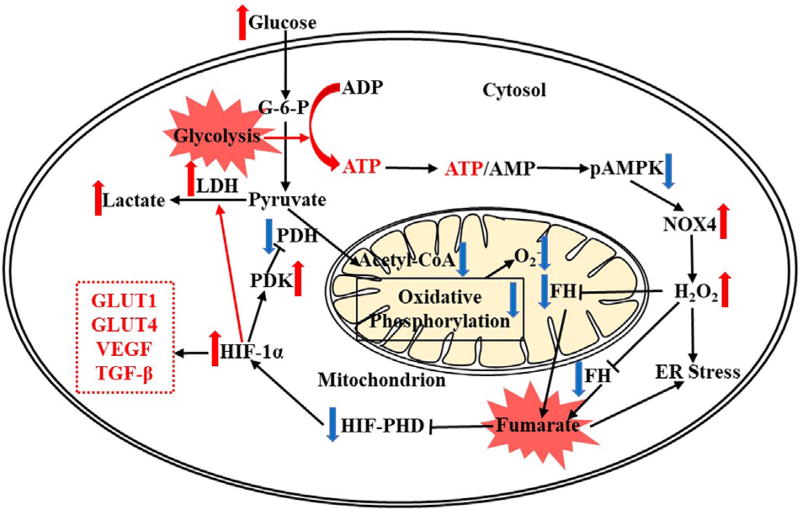
In the TCA cycle fumarate is generated from the precursor adenylsuccinate by succinate dehydrogenase and is converted to malate by fumarate hydratase (FH).22 The accumulation of fumarate can be attributed to the inhibition of FH or stimulation of succinate dehydrogenase. Our further studies showed that FH protein is reduced in the F1 Akita and in human diabetic kidney tissue. In addition, FH levels were reduced in the podocyte-specific Nox4 transgenic mice and in the cells expressing NOX4, suggesting FH is a direct target of NOX4. Administration of the NOX1/4 inhibitor increased glomerular FH levels in diabetic mice, suggesting NOX4 have a regulatory role in FH expression. In addition to NOX4, H2O2 treatment also reduced the expression of FH and increased fumarate levels in HEK293 cells, suggesting that the down-stream effects of NOX4 can be attributed to accumulation of H2O2. FH gene mutations have been reported in leiomyomatosis and renal cell cancer. Fumarate, known as an oncometabolite, accumulates in FH-deficient cells and has been shown to inhibit α-ketoglutarate–dependent dioxygenases including hypoxia inducible factor (HIF)-prolyl hydroxylases, thereby increasing HIF-1α levels. In our study, we found that both HIF-1α messenger RNA and protein levels were increased upon treatment with fumarate or with H2O2. Integrative data from three tiers including gene (FH) expression, protein (FH) analysis, and metabolomic (fumarate) analyses indicate that FH is a target of NOX4 in diabetic kidney.22 A reduction in FH and the subsequent accumulation of fumarate in diabetic kidney may result in renal pathology, potentially through the inhibition of HIF prolyl hydroxylase and increasing HIF-1α (Fig. 3).66–68
Figure 3.
Fumarate accumulation, HIF-1α regulation, and glycolysis in renal cells with increased intracellular glucose. The reduced pAMPK enhances NOX4 leading to increased H2O2, which reduces fumarate hydratase (FH) levels and then leads to fumarate accumulation. Both increased H2O2 and fumarate contribute to ER stress and thereafter apoptosis. The increased fumarate suppresses HIF-PHD activity resulting in HIF-1α accumulation, which drives the metabolic flux to the glycolytic pathway (eg, through increased LDH) while suppresses oxidative phosphorylation (eg, through reduced PDK and increased PDH) and reduces mitochondrial HIF-1α also increases levels of glucose transport proteins (eg, GLUT1 and GLUT4) and growth factors (eg, VEGF and TGF-β). Abbreviations: ADP, adenosine diphosphate; G-6-P, glucose 6-phosphate; GLUT1/GLUT4, glucose transporters; LDH, lactate dehydrogenase; PDK, pyruvate dehydrogenase kinase; PHD, prolyl hydroxylase; TGF-β, transforming growth factor β; VEGF, vascular endothelial growth factor.
HIF-1α regulation in diabetic kidney and its role in stimulating glycolysis for lactate production via enhanced lactate dehydrogenase and inhibiting PDH and oxidative phosphorylation via increased pyruvate dehydrogenase kinase has been elaborated.49 HIF-1α and HIF-2α are transcription factors that up-regulate aerobic glycolysis-associated genes (eg, glucose transporter and vascular endothelial growth factor) in cancer. Both glucose transporter and vascular endothelial growth factor enhance glucose transport and tumor vascularity.66 In both in vitro and in vivo studies, NOX4 activation sufficiently induces HIF-1α and the NOX1/NOX4 inhibitor reduces HIF-1α in DKD, therefore NOX4 might be an important mediator for HIF-1α in DKD.22 Moreover, it was reported that NOX4 is a novel target gene of HIFs, whose levels also are regulated by NOX4.69,70 Results from MSI, gene expression, protein, and metabolomic analyses suggest a potential feedback loop mechanism (ie, increased ATP/AMP, reduced AMPK, activated NOX4, increased H2O2, suppressed FH, accumulated fumarate, increased HIF-1α, and enhanced glycolysis) in the development of DKD.49 More sophisticated approaches are needed to accurately determine the sources of ATP and ROS that can provide further insights into the pathogenesis of DKD.
UNDERSTANDING DKD USING A SYSTEMS BIOLOGY APPROACH
Genomic, transcriptomic, proteomic, and metabolomic/lipidomic biomarkers of DKD have been reviewed previously.71–75 In particular, new noninvasive urinary and serum/plasma biomarkers for DKD provide valuable insights in interpreting the disease pathogenesis and show potential roles in clinical medicine. To better understand the role of DKD biomarkers in the development of disease, Saito et al76 integrated 13 previously reported DKD urinary metabolite bio-markers with publicly available protein–protein interaction networks.20 Multi-omics studies and bioinformatics tools (eg, Cytoscape [UC San Diego, La Jolla, CA] and MetBridge Generator [UC San Diego, La Jolla, CA]) have shown that mouse double minute 2 homolog in glomeruli and tubules of DKD had the highest number of protein–protein interaction connections, and its reduction was associated with two decreased metabolites biomarkers (ie, 3-methylcroto-nylglycine and uracil) in DKD.76,77
Experimental evidence supports that an altered glycolytic pathway is associated with the development of DKD.21 However, only a limited number of glycolysis-related enzymes, intermediates, or genes were studied. What is the degree of aerobic glycolysis in DKD? Whether it benefits or impairs the kidney still is not fully known. Beyond ATP production, aerobic glycolysis also support macromolecular synthesis (eg, DNA, RNA, proteins, and lipids) and connections between the glycolytic pathway and biosynthetic precursors were elaborated.78 For example, the glycolytic intermediate dihydroxyacetone phosphate is the precursor to glycerol-3-phosphate, which is vital for the biosynthesis of triacylglycerols and phospholipids that serve as primary structural lipids in cell membranes. Dihydroxyacetone phosphate is also the precursor for other lipid species such as cardiolipin, which is an important component of mitochondrial membranes and plays a pivotal role in mitochondrial function.79 Glycolytic intermediates (eg, 3-phosphoglycerate and pyruvate) also are direct precursors for the biosynthesis of amino acids (eg, alanine, cysteine, glycine, and serine).78
FUTURE PERSPECTIVES
As a complex multifactor-derived disease, the patho-mechanism of DKD is still not fully clarified. Omics-based systems biology, which incorporate different tiers of biological data, has shed new insights into our understanding of DKD and has provided several key breakthroughs for novel predictive and diagnostic biomarkers. Recent omics-based investigations coupled with systems biology tools have shown that mitochondrial dysfunction,20 oxidative stress (eg, ROS),49 alterations of multitier information (eg, genes, proteins, and metabolites), and major metabolic pathways (eg, the TCA cycle, glycolysis, fatty acid metabolism, and amino acid metabolism)16,21,22 all are altered in a manner consistent with a Warburg-type effect in diabetic nephropathy.
The end products of glycolysis are multidirectional. To understand how alterations of the glycolytic path-way contribute to the development of DKD, further comprehensive metabolic flux studies are required and integrate different tiers of information. By comparing different biological samples (eg, blood, urine, renal biopsy, and nephrostomy) obtained from nonprotected (ie, diabetes and DKD) and protected (ie, diabetes without DKD), we will know which metabolic path-ways are more activated or suppressed in DKD patients. Mouse models of DKD and in vitro studies also are warranted to confirm and validate the human data. Furthermore, with the development of high sensitive technologies such as global DNA methylations,80 singular-cell RNA sequencing,81 RNA bar-coding based on single-molecule fluorescence in situ hybridization,82 MALDI-MSI,33 laser capture micro-dissection -omics,83 and cytometry by time of flight,84 it becomes feasible to measure multiomics (ie, genomics, transcriptomics, proteomics, and metabolomics/lipidomics) data in a single-cell or cell type. In the near future, we could link pathologic changes with multi-omics in glomeruli, podocytes, and tubules of DKD, which will allow in-depth understanding of DKD and pave the path for personalized kidney precision medicine.
Acknowledgments
Financial support: Supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (5R24DK082841-08 and 1UG3DK114920-01) and the Juvenile Diabetes Research Foundation to KS.
Footnotes
Conflict of interest statement: none.
References
- 1.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12:2032–45. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimazaki A, Kawamura Y, Kanazawa A, Sekine A, Saito S, Tsunoda T, et al. Genetic variations in the gene encoding ELMO1 are associated with susceptibility to diabetic nephropathy. Diabetes. 2005;54:1171–8. doi: 10.2337/diabetes.54.4.1171. [DOI] [PubMed] [Google Scholar]
- 3.Kottgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet. 2009;41:712–7. doi: 10.1038/ng.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas MC, Brownlee M, Susztak K, Sharma K, Jandeleit-Dahm KA, Zoungas S, et al. Diabetic kidney disease. Nat Rev Dis Primers. 2015;1:15018. doi: 10.1038/nrdp.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hallan S, Sharma K. The role of mitochondria in diabetic kidney disease. Curr Diab Rep. 2016;16:61. doi: 10.1007/s11892-016-0748-0. [DOI] [PubMed] [Google Scholar]
- 6.Sharma K. Mitochondrial dysfunction in the diabetic kidney. Adv Exp Med Biol. 2017;982:553–62. doi: 10.1007/978-3-319-55330-6_28. [DOI] [PubMed] [Google Scholar]
- 7.Bhargava P, Schnellmann RG. Mitochondrial energetics in the kidney. Nat Rev Nephrol. 2017;13:629–46. doi: 10.1038/nrneph.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–54. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 9.Lee AYW, Chung SSM. Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J. 1999;13:23–30. doi: 10.1096/fasebj.13.1.23. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen AH, Bhavsar SB, Riley EM, Caponetti GC, Agrawal DK. Association of high mobility group BOX-1 and receptor for advanced glycation endproducts with clinicopathological features of haematological malignancies: a systematic review. Contemp Oncol (Pozn) 2016;20:425–9. doi: 10.5114/wo.2016.65600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowotny K, Jung T, Hohn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5:194–222. doi: 10.3390/biom5010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47:859–66. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- 13.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 14.Naudi A, Jove M, Ayala V, Cassanye A, Serrano J, Gonzalo H, et al. Cellular dysfunction in diabetes as maladaptive response to mitochondrial oxidative stress. Exp Diabetes Res. 2012;2012:696215. doi: 10.1155/2012/696215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brownlee M. The pathobiology of diabetic complications - a unifying mechanism. Diabetes. 2005;54:1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 16.Sas KM, Kayampilly P, Byun J, Nair V, Hinder LM, Hur J, et al. Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight. 2016;1:e86976. doi: 10.1172/jci.insight.86976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warburg O, Posener K, Negelein E. On the metabolism of carcinoma cells. Biochem Z. 1924;152:309–44. [Google Scholar]
- 18.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 19.Crabtree HG. Observations on the carbohydrate metabolism of tumours. Biochem J. 1929;23:536–45. doi: 10.1042/bj0230536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R, et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol. 2013;24:1901–12. doi: 10.1681/ASN.2013020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi W, Keenan HA, Li Q, Ishikado A, Kannt A, Sadowski T, et al. Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat Med. 2017;23:753–62. doi: 10.1038/nm.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.You YH, Quach T, Saito R, Pham J, Sharma K. Metabolomics reveals a key role for fumarate in mediating the effects of NADPH oxidase 4 in diabetic kidney disease. J Am Soc Nephrol. 2016;27:466–81. doi: 10.1681/ASN.2015030302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves AE, et al. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest. 2013;123:4888–99. doi: 10.1172/JCI66218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heiden MGV, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi HY, Casalena G, Shi SL, Yu LP, Ebefors K, Sun YZ, et al. Glomerular endothelial mitochondrial dysfunction is essential and characteristic of diabetic kidney disease susceptibility. Diabetes. 2017;66:763–78. doi: 10.2337/db16-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canto C, Auwerx J. PGC-1 alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasegawa K, Wakino S, Simic P, Sakamaki Y, Minakuchi H, Fujimura K, et al. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med. 2013;19:1496–504. doi: 10.1038/nm.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higashida K, Kim SH, Jung SR, Asaka M, Holloszy JO, Han DH. Effects of resveratrol and SIRT1 on PGC-1alpha activity and mitochondrial biogenesis: a reevaluation. PLoS Biol. 2013;11:e1001603. doi: 10.1371/journal.pbio.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardie DG. AMP-activated protein kinase-an energy sensor that regulates all aspects of cell function. Gene Dev. 2011;25:1895–908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kidokoro K, Satoh M, Channon KM, Yada T, Sasaki T, Kashihara N. Maintenance of endothelial guanosine triphosphate cyclohydrolase I ameliorates diabetic nephropathy. J Am Soc Nephrol. 2013;24:1139–50. doi: 10.1681/ASN.2012080783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jornayvaz FR, Shulman GI. Regulation of mitochondrial biogenesis. Essays Biochem. 2010;47:69–84. doi: 10.1042/bse0470069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21:37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyamoto S, Hsu CC, Hamm G, Darshi M, Diamond-Stanic M, Decleves AE, et al. Mass spectrometry imaging reveals elevated glomerular ATP/AMP in diabetes/obesity and identifies sphingomyelin as a possible mediator. EBioMedicine. 2016;7:121–34. doi: 10.1016/j.ebiom.2016.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith CA, O'Maille G, Want EJ, Qin C, Trauger SA, Brandon TR, et al. METLIN - a metabolite mass spectral database. Ther Drug Monit. 2005;27:747–51. doi: 10.1097/01.ftd.0000179845.53213.39. [DOI] [PubMed] [Google Scholar]
- 35.Fahy E, Sud M, Cotter D, Subramaniam S. LIPID MAPS online tools for lipid research. Nucleic Acids Res. 2007;35:W606–W612. doi: 10.1093/nar/gkm324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segui B, Andrieu-Abadie N, Jaffrezou JP, Benoist H, Levade T. Sphingolipids as modulators of cancer cell death: potential therapeutic targets. Biochem Biophys Acta. 2006;1758:2104–20. doi: 10.1016/j.bbamem.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 37.Young SA, Mina JG, Denny PW, Smith TK. Sphingolipid and ceramide homeostasis: potential therapeutic targets. Biochem Res Int. 2012;2012:248135. doi: 10.1155/2012/248135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams JM, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25–31. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- 39.Straczkowski M, Kowalska I, Nikolajuk A, Dzienis-Straczkowska S, Kinalska I, Baranowski M, et al. Relationship between insulin sensitivity and sphingomyelin signaling pathway in human skeletal muscle. Diabetes. 2004;53:1215–21. doi: 10.2337/diabetes.53.5.1215. [DOI] [PubMed] [Google Scholar]
- 40.Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem. Cell B. 2011;43:969–80. doi: 10.1016/j.biocel.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Luo WB, Semenza GL. Emerging roles of PKM2 in cell metabolism and cancer progression. Trends Endocrinol Metab. 2012;23:560–6. doi: 10.1016/j.tem.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong GC, Mao QX, Xia WJ, Xu YT, Wang J, Xu L, et al. PKM2 and cancer: the function of PKM2 beyond glycolysis. Oncol Lett. 2016;11:1980–6. doi: 10.3892/ol.2016.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dayton TL, Jacks T, Vander Heiden MG. PKM2, cancer metabolism, and the road ahead. EMBO Rep. 2016;17:1721–30. doi: 10.15252/embr.201643300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong N, De Melo J, Tang D. PKM2, a central point of regulation in cancer metabolism. Int J Cell Biol. 2013;2013:242513. doi: 10.1155/2013/242513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bluemlein K, Gruning NM, Feichtinger RG, Lehrach H, Kofler B, Ralser M. No evidence for a shift in pyruvate kinase PKM1 to PKM2 expression during tumorigenesis. Oncotarget. 2011;2:393–400. doi: 10.18632/oncotarget.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–3. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 47.Yamada K, Noguchi T. Nutrient and hormonal regulation of pyruvate kinase gene expression. Biochem J. 1999;337:1–11. [PMC free article] [PubMed] [Google Scholar]
- 48.Brinck U, Eigenbrodt E, Oehmke M, Mazurek S, Fischer G. L-Pyruvate and M(2)-pyruvate kinase expression in renal-cell carcinomas and their metastases. Virchows Arch. 1994;424:177–85. doi: 10.1007/BF00193498. [DOI] [PubMed] [Google Scholar]
- 49.Sharma K. Obesity and diabetic kidney disease: role of oxidant stress and redox balance. Antioxid Redox Signal. 2016;25:208–16. doi: 10.1089/ars.2016.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coughlan MT, Sharma K. Challenging the dogma of mitochondrial reactive oxygen species overproduction in diabetic kidney disease. Kidney Int. 2016;90:272–9. doi: 10.1016/j.kint.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 51.Ha H, Hwang IA, Park JH, Lee HB. Role of reactive oxygen species in the pathogenesis of diabetic nephropathy. Diabetes Res Clin Pract. 2008;82:S42–5. doi: 10.1016/j.diabres.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 52.Hu TS, RamachandraRao SP, Siva S, Valancius C, Zhu YQ, Mahadev K, et al. Reactive oxygen species production via NADPH oxidase mediates TGF-beta induced cytoskeletal alterations in endothelial cells. Am J Physiol Renal Physiol. 2005;289:F816–25. doi: 10.1152/ajprenal.00024.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jun M, Venkataraman V, Razavian M, Cooper B, Zoungas S, Ninomiya T, et al. Antioxidants for chronic kidney disease. Cochrane Database Syst Rev. 10:CD008176. doi: 10.1002/14651858.CD008176.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pollock JS, Pollock DM. Endothelin, nitric oxide, and reactive oxygen species in diabetic kidney disease. Contrib Nephrol. 2011;172:149–59. doi: 10.1159/000329054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nishikawa T, Brownlee M, Araki E. Mitochondrial reactive oxygen species in the pathogenesis of early diabetic nephropathy. J Diabetes Invest. 2015;6:137–9. doi: 10.1111/jdi.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 57.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492–503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rardin MJ, Wiley SE, Naviaux RK, Murphy AN, Dixon JE. Monitoring phosphorylation of the pyruvate dehydrogenase complex. Anal Biochem. 2009;389:157–64. doi: 10.1016/j.ab.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 60.Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of Renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A. 2000;97:8010–4. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.You YH, Okada S, Ly S, Jandeleit-Dahm K, Barit D, Namikoshi T, et al. Role of Nox2 in diabetic kidney disease. Am J Physiol Renal Physiol. 2013;304:F840–8. doi: 10.1152/ajprenal.00511.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jha JC, Gray SP, Barit D, Okabe J, El-Osta A, Namikoshi T, et al. Genetic targeting or pharmacologic inhibition of NADPH oxidase Nox4 provides renoprotection in long-term diabetic nephropathy. J Am Soc Nephrol. 2014;25:1237–54. doi: 10.1681/ASN.2013070810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A. 2009;106:14385–90. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu YQ, Dunn SR, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118:1645–56. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gorin Y, Cavaglieri RC, Khazim K, Lee DY, Bruno F, Thakur S, et al. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am J Physiol Renal Physiol. 2015;308:F1276–87. doi: 10.1152/ajprenal.00396.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Linehan WM, Rouault TA. Molecular pathways: fumarate hydratase-deficient kidney cancer-targeting the Warburg effect in cancer. Clin Cancer Res. 2013;19:3345–52. doi: 10.1158/1078-0432.CCR-13-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–53. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 68.Tong WH, Sourbier C, Kovtunovych G, Jeong SY, Vira M, Ghosh M, et al. The glycolytic shift in fumarate-hydratase-deficient kidney cancer lowers AMPK levels, increases anabolic propensities and lowers cellular iron levels. Cancer Cell. 2011;20:315–27. doi: 10.1016/j.ccr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diebold I, Petry A, Hess J, Gorlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell. 2010;21:2087–96. doi: 10.1091/mbc.E09-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Block K, Gorin Y, Hoover P, Williams P, Chelmicki T, Clark RA, et al. NAD(P)H oxidases regulate HIF-2alpha protein expression. J Biol Chem. 2007;282:8019–26. doi: 10.1074/jbc.M611569200. [DOI] [PubMed] [Google Scholar]
- 71.Ju WJ, Smith S, Kretzler M. Genomic biomarkers for chronic kidney disease. Transl Res. 2012;159:290–302. doi: 10.1016/j.trsl.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee CH, Lam KSL. Biomarkers of progression in diabetic nephropathy: the past, present and future. J Diabetes Invest. 2015;6:247–9. doi: 10.1111/jdi.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Campion CG, Sanchez-Ferras O, Batchu SN. Potential role of serum and urinary biomarkers in diagnosis and prognosis of diabetic nephropathy. Can J Kidney Health Dis. 2017;4 doi: 10.1177/2054358117705371. 2017 2054358117705371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, Hoy WE. Biomarkers in chronic kidney disease: a review. Kidney Int. 2011;80:806–21. doi: 10.1038/ki.2011.198. [DOI] [PubMed] [Google Scholar]
- 75.Hocher B, Adamski J. Metabolomics for clinical use and research in chronic kidney disease. Nat Rev Nephrol. 2017;13:269–84. doi: 10.1038/nrneph.2017.30. [DOI] [PubMed] [Google Scholar]
- 76.Saito R, Rocanin-Arjo A, You YH, Darshi M, Van Espen B, Miyamoto S, et al. Systems biology analysis reveals role of MDM2 in diabetic nephropathy. JCI Insight. 2016;1:e87877. doi: 10.1172/jci.insight.87877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–64. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 79.Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:C33–44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- 80.Chowdhury B, Cho IH, Irudayaraj J. Technical advances in global DNA methylation analysis in human cancers. J Biol Eng. 2017;11:10. doi: 10.1186/s13036-017-0052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Der E, Ranabothu S, Suryawanshi H, Akat KM, Clancy R, Morozov P, et al. Single cell RNA sequencing to dissect the molecular heterogeneity in lupus nephritis. JCI Insight. 2017;2:9. doi: 10.1172/jci.insight.93009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kwon S. Single-molecule fluorescence in situ hybridization: quantitative imaging of single RNA molecules. Bmb Rep. 2013;46:65–72. doi: 10.5483/BMBRep.2013.46.2.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Datta S, Malhotra L, Dickerson R, Chaffee S, Sen CK, Roy S. Laser capture microdissection: big data from small samples. Histol Histopathol. 2015;30:1255–69. doi: 10.14670/HH-11-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chevrier S, Levine JH, Zanotelli VRT, Silina K, Schulz D, Bacac M, et al. An immune atlas of clear cell renal cell carcinoma. Cell. 2017;169:736–49. doi: 10.1016/j.cell.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sharma K. Mitochondrial hormesis and diabetic complications. Diabetes. 2015;64:663–72. doi: 10.2337/db14-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]