Abstract
Environmental toxicants such as bisphenol-A (BPA) and polychlorinated biphenyls (PCBs) are prevalent in our water supply, soil, and many food products and can profoundly affect the central nervous system. Both BPA and PCBs can disrupt endocrine signaling, which is important for auditory development and function, but the effect of these toxicants on the auditory periphery is not understood. In this study we investigated the effect of PCB-95 and BPA on lateral line development, function, and regeneration in larval zebrafish. The lateral line is a system of mechanosensory hair cells on the exterior of the fish that are homologous to the hair cells located in the mammalian inner ear. We found that PCB-95 had no effect on lateral line development or hair cell survival. BPA also did not affect lateral line development, but instead had a significant effect on both hair cell survival and regeneration. BPA-induced hair cell loss is both dose-and time-dependent, with concentrations of 1 μM or higher killing lateral line hair cells during a 24 hr exposure period. Pharmacologic manipulation experiments suggest that BPA kills hair cells via activation of oxidative stress pathways, similar to prior reports of BPA toxicity in other tissues. We also observed that hair cells killed with neomycin, a known ototoxin, failed to regenerate normally when BPA was present, suggesting that BPA in aquatic environments could impede innate regenerative responses in fishes. Collectively, these data demonstrate that BPA can have detrimental effects on sensory systems, both in aquatic life and perhaps in terrestrial organisms, including humans.
Keywords: bisphenol-A, polychlorinated biphenyl, lateral line, hair cell, hearing, auditory
1. Introduction
Released into the environment through run-off and effluent, environmental contaminants such as polychlorinated biphenyls (PCBs) and bisphenol-A (BPA) are prevalent in our water supply, soil and food (reviewed in Halling-Sørensen, 1998). For example, a survey of over 100 streams across the US identified contaminants ranging from flame retardants to plasticizers, including BPA, in U.S. watersheds (Kolpin, 2002). BPA is also known to leach into canned foods through the breakdown of polycarbonate container linings (reviewed in Halling-Sørensen, 1998; Geens, 2012). Despite their widespread prevalence, we still do not fully understand how environmental contaminants impact human and animal health.
Banned since 1979, PCBs are the most pervasive industrial pollutants found in U.S. waterways (Stahl et al., 2009). In rats, developmental exposure in vivo is correlated with changes in synaptic development in the central nervous system and with inner ear dysfunction, while acute exposure promotes dendritic spine formation in vitro and can alter synaptic transmission in vivo (Gilbert and Liang, 1998; Crofton et al., 2000; Carpenter et al., 2002; Lein et al., 2007; Powers et al., 2009; Yang et al., 2009; Wayman et al., 2012a, 2012b; Lesiak et al., 2014). PCBs can also disrupt thyroid hormone signaling in rats and some toxic effects of PCB exposure may be attributed to altered thyroid hormone responses, particularly during development (Collins et al., 1977; Ness et al., 1993; reviewed in Kodavanti, 2006). Developmental exposure in fishes leads to muscle dysfunction and corresponding swimming defects, while acute treatment causes changes in liver metabolism and reproductive fitness (Wiseman and Vijayan, 2011; Farwell et al., 2012; Fritsch et al., 2013).
BPA is a ubiquitous environmental toxicant used in polycarbonate plastics and epoxy resins. A known endocrine disruptor, it is linked to a myriad of problems ranging from adiponectin inhibition and diabetes in humans to decreased reproductive viability and motor coordination in fishes (Sohoni et al., 2001; Lahnsteiner et al., 2005; Hugo et al., 2008; Lang et al., 2008; Wang et al., 2013). Acute BPA exposure also appears to impact hippocampal synapses in both adult and developing rodents in vivo, leading to learning and memory impairment (Eilam-Stock et al., 2012; Inagaki et al., 2012; Viberg and Lee, 2012; Kuwahara et al., 2013).
Despite demonstrated effects on the central nervous system, little is known about the influence of either PCBs or BPA on the peripheral nervous system, specifically on sensory systems. As sensory reception and processing is critical to organismal survival, environmental contaminant-induced sensory dysfunction could have profound consequences. In this study, we asked if either PCB-95 or BPA are toxic to sensory hair cells in the zebrafish lateral line.
Mechanosensory hair cells in the vertebrate inner ear transduce vibrational stimuli received by the auditory periphery into action potentials that are transmitted to the central nervous system (reviewed in Hudspeth, 2005). The lateral line is an external sensory system containing hair cells that are structurally and functionally similar to those in the mammalian ear and respond similarly to many toxins (Harris et al., 2003; Ou et al., 2007; reviewed in Coffin et al., 2010, 2014). In humans, hair cell damage results in permanent hearing loss; contaminants that damage hair cells may therefore have serious consequences for human auditory function. Unlike mammals, fish naturally regenerate hair cells (Lombarte et al., 1993; Ma et al., 2008; reviewed in Brignull et al., 2009). Therefore, hair cell damage does not have the same repercussions in fish, unless regenerative capacity is also affected, as seen after copper exposure (Hernández et al., 2006; Linbo et al., 2006). In this study we show that BPA is significantly toxic to mature hair cells in the lateral line and that exposure reduces hair cell regeneration in an innately regenerative system. PCB-95 had no detectable effect on hair cell development, survival, or regeneration. Our findings fit within the broader context of recent evidence demonstrating the prevalence of environmental contaminants and the dangers these compounds pose to the nervous system (Kolpin et al., 2002; Wayman et al., 2012a, 2012b; Elsworth et al., 2013).
2. Methods
2.1 Animals
We used 5-6 days post-fertilization (dpf) fish for all acute toxicity experiments because their hair cells exhibit mature sensitivity to known hair cell toxins (ototoxins) (Murakami et al., 2003; Santos et al., 2006). Developmental studies used animals beginning at 1 dpf, while regeneration studies were initiated in 5-6 dpf animals and concluded by 7 dpf. *AB wildtype or Brn3C:mGFP transgenic fish were used for all experiments. Hair cells of Brn3c:mGFP animals express membrane-bound GFP in all hair cells and the transgene is turned on early in hair cell development (Xiao et al., 2005), making this fish line ideal for developmental and regeneration studies. All procedures were approved by the Institutional Animal Care and Use Committee at Washington State University.
2.2 Environmental toxicant exposure
We performed three distinct series of experiments using BPA or PCB-95 to determine if either compound was toxic in the context of 1) lateral line development, 2) acute exposure of mature hair cells, or 3) regenerating hair cells. BPA concentrations were based on reported effective values in prior zebrafish studies (Lam et al., 2011; Wang et al., 2013) and on our own preliminary data that determined the range that yielded minimal mortality. We accomplished this by examining concentrations spanning several orders of magnitude across multiple time points. As PCB-95 has not, to our knowledge, been previously used in zebrafish studies, we selected our PCB-95 concentrations based on published literature for other fishes and in vitro studies in mammalian neurons (Wayman et al., 2012a; Fritsch and Pessah, 2013). Again, we corroborated these concentration ranges with empirical testing of several orders of magnitude across multiple time points. As both BPA and PCB-95 were dissolved in DMSO, control animals received the same volume of DMSO only for the same treatment duration (≤ 0.2%). Unless specified below, all animals were assessed immediately following treatment.
All experiments were performed in defined E2 embryo medium (EM) containing 994 μM MgSO4, 150 μM KH2PO4, 42 μM Na2HPO4, 986 μM CaCl2, 503 μM KCl, 14.9 mM NaCl, and 714 μM NaHCO3, with the pH adjusted to 7.2 (Westerfield, 2000). Fish were divided into treatment groups of 8-12 fish per well in a 6-well plate, with each treatment group housed in a custom transfer device constructed of PVC pipe and mesh netting that fit inside the well. Experiments were conducted in a VWR benchtop incubator set at 28 °C.
2.2.1 Development
1 dpf Brn3c:mGFP transgenic zebrafish were treated for 48 or 96 hrs with BPA (2, 10, 20, or 40 μM) or PCB-95 (0.1, 0.25, 0.50, or 2 μM). Controls were treated with DMSO (0-0.2%, matched to the volume of toxicant in DMSO), the solvent used to dissolve PCB or BPA. Counts of GFP+ hair cells were performed on fish fixed in 4% paraformaldehyde and rinsed in PBS. Hair cells of both anterior (IO1, IO2, IO3) and posterior (P1, P2) neuromasts were counted in order to assess neuromasts that develop at different time points (Raible and Kruse, 2000; Van Trump and McHenry, 2008). Hair cell counts were summed to arrive at one value per fish.
2.2.2 Acute toxicity
5-6 dpf *AB zebrafish were treated for 4-24 hrs with BPA (0.1-80 μM), 24 hrs with PCB-95 (0.5-20 μM), or with DMSO only for control animals. Fish were rinsed in EM, anesthetized with MS-222, and hair cells were assessed using DASPEI as described in section 2.2.1. A subset of BPA-treated fish were euthanized and hair cells were labeled with anti-parvalbumin and quantified as described in section 2.2.2.
2.2.3 Regeneration
To assess how an environmental contaminant affects hair cell regeneration, we used neomycin to kill hair cells in 5 dpf zebrafish (Harris et al., 2003; Ma et al., 2008), then allowed the fish to recover for 24 or 48 hrs in either PCB-95 or BPA. Fish were treated with 300 μM neomycin for 30 min, then rinsed four times in embryo medium and incubated in fresh EM for 1 hr to allow for complete loss of hair cells (Coffin et al., 2009; Owens et al., 2009). Fish were then incubated in PCB-95 (0.25-2 μM) or BPA (1-20 μM) and assessed with DASPEI labeling in live fish or by counts of anti-parvalbumin-labeled hair cells.
Regeneration proceeds more slowly after hair cell death due to toxins such as copper or cisplatin (Hernández et al., 2006; Linbo et al., 2006; MacKenzie and Raible, 2012). In order to look for a similar phenomenon following BPA damage, we next examined hair cell regeneration after BPA-induced hair cell death. 5 dpf zebrafish were exposed to 1-20 μM BPA for 24 hrs, then rinsed four times in embryo medium and allowed to recover for 24 or 48 hrs. Hair cell regeneration was assessed as described above.
2.3 Hair cell assessment
2.3.1 Vital dye labeling
Neuromasts in live, anesthetized fish were assessed using the mitochondrial dye 2-(4-(dimethylamino)styryl)-N-Ethylpyridinium iodide (DASPEI, Life Technologies), which specifically labels lateral line hair cells and olfactory receptors (Harris et al., 2003; Coffin et al., 2009; Owens et al., 2009). Fish were incubated in EM containing 0.005% DASPEI for 15 min, rinsed twice with fresh EM, then anesthetized by immersion in 0.001% MS-222 in fresh EM (3-aminobenzoic acid ethyl ester methanesulfonate, Argent Labs, Redmond, WA). We assessed the same 10 anterior neuromasts for each fish (SO1, SO2, IO1-IO4, M2, MI1, MI2, O2), with each neuromast scored as 0 (no labeling), 1 (moderate labeling), or 2 (bright labeling). Scores for each neuromast were summed resulting in a total of 0-20 per fish (Harris et al., 2003; Coffin et al., 2009, 2013a). We selected these neuromasts because they are all visible in a single field of view at 50× total magnification (our chosen magnification for DASPEI assessment) and for comparison between the present study and past work on known ototoxins, which also examined these anterior neuromasts (Coffin et al. 2009, 2013a, 2013b; Owens et al. 2009). As neuromast locations are stereotyped in zebrafish larvae, absence of a neuromast is likely a treatment effect rather than developmental variation (Raible and Kruse, 2000). While hair cell assessment was not performed blind (i.e., researcher unaware of treatment group), we have previously performed side-by-side comparisons of blinded vs. unblinded DASPEI assessment and found no differences in scoring (Coffin, unpublished data).
2.3.2 Immunocytochemistry
We validated DASPEI scores with counts of anti-parvalbumin-labeled hair cells in fixed tissue (Heller et al., 2002; Coffin et al., 2013a, 2013b). All chemicals are from Sigma-Aldrich unless indicated. Fish were euthanized with MS-222 and fixed in 4% paraformaldehyde either overnight at 4 °C or for 1 hr at room temperature. Fish were then rinsed in phosphate-buffered saline (PBS, Life Technologies), followed by a rinse in distilled H2O, and blocked in PBS with 0.1% Triton-X (PBST) and 5% normal goat serum. Fish were then incubated overnight at 4 °C in primary antibody to parvalbumin (mouse monoclonal antibody, EMD Millipore) diluted 1:500 in PBST with 1% goat serum, rinsed 4 times in PBST and incubated in secondary antibody for 4 hrs with Alexa Fluor 488 or 568 goat anti-mouse (Life Technologies) diluted 1:500 in PBST. Fish were rinsed again in PBS, then mounted on bridged coverslips using 1:1 PBS:glycerol and viewed with a Leica DMRB or DMI 4000 B compound fluorescent microscope or a Leica SP8 confocal microscope. Hair cells from five neuromasts (IO1, IO2, IO3, M2 and OP1; Raible and Kruse, 2000) were counted and summed to arrive at one value for each fish.
2.4 Cell death pathway analysis
To give insight as to how BPA causes hair cell death, we performed a cell death inhibitor screen using a library of 50 compounds that each inhibit different cell death pathways (Coffin et al., 2013a). 5-6 dpf larvae were used for all experiments (5-8 fish per treatment). Fish were pretreated in 1 of 50 cell death inhibitors for 1 hr, then co-treated with inhibitor and 20 μM BPA for 24 hrs. Following treatment, fish were rinsed four times in EM and hair cells were assessed with DASPEI. Unlike previous experiments, the entire fish head was holistically assessed where a score of 1 (neuromasts generally dim), 3 (moderate neuromast fluorescence), or 5 (bright neuromast fluorescence) was given to each fish to expedite the screening process.
Inhibitors that resulted in an average score >4.5 were retested twice using the same scoring method as in the initial screen. For compounds that again scored high (conferred significant protection), we conducted dose-response analyses using variable concentrations of protectant concurrently with 24 hr BPA administration. For these experiments hair cells were assessed with our 0-20 DASPEI scoring system described in section 2.2.1.
2.5 BPA uptake assays
Hair cell toxins such as aminoglycoside antibiotics and cisplatin enter hair cells through the mechanotransduction channel at the apical tip of the hair bundle (Gale et al., 2001; Steyger et al., 2003; Marcotti et al., 2005; Thomas et al., 2013; Vu et al., 2013). We therefore asked if BPA toxicity was attenuated by transduction channel blockade, which would implicate a similar entry route. Previous studies have shown that high calcium can block entry of aminoglycosides into hair cells (Marcotti et al., 2005; Coffin et al., 2009). Fish were treated for 4 hrs with 40 μM BPA in EM containing either 900 μM (normal EM) or 2.1 mM (high Ca2+ EM) calcium. As a second test of BPA entry via the transduction channel, hair cell tip links were broken using Ethylenediaminetetraacetic acid (EDTA), which abolishes mechanotransduction current (Berg and Watson, 2002; Suli et al., 2012). Fish were pretreated for 20 min in 1 mM EDTA (Sigma-Aldrich), then rinsed in EM and treated with 40 μM BPA for 4 hrs. Hair cell survival was assessed with DASPEI labeling for both experiments. For these experiments we used a 4 hr treatment with higher BPA concentration because high calcium proved toxic to fish when combined with BPA for long exposure times. In addition, tip links in lateral line hair cells begin to regenerate after 4 hrs (Suli et al., 2012). In both cases, the aminoglycoside neomycin was used as a positive control, where fish were pretreated as described, then exposed to 200 μM neomycin for 30 min, followed by a 1 hr recovery period and assessment of hair cell survival (Coffin et al., 2009).
2.6 Statistical analysis
For all experiments hair cell presence was analyzed by t-test, one- or two-way ANOVA, as appropriate, with GraphPad Prism (v. 6.0), followed by posthoc testing for multiple comparisons (see figure legends for details). Data are presented as mean ± 1 s.e.m.
3. Results
3.1 Hair Cell Development
Neither BPA or PCB-95 treatment affected hair cell number in the developing lateral line of Brn3c:mGFP transgenic zebrafish (Figs. 1 and 2). In the BPA experiment, control fish at 3 dpf had 48 ± 2 hair cells (mean ± 1 s.e.m., summed over 5 neuromasts), while 5 dpf control fish had 55 ± 3 hair cells, consistent with prior studies demonstrating hair cell addition during this time period (Harris et al., 2003; Wada et al., 2013). Hair cell number in BPA-treated fish was not significantly different from controls (Fig. 1). For example, at the highest BPA concentration tested (40 μM), 3 dpf fish had 42 ± 3 hair cells and 5 dpf fish had 51 ± 2 hair cells. Similar results were seen in PCB-95-treated fish, where fish treated with PCB-95, even at the maximum concentration of 2 μM, were not significantly different from age-matched controls (Fig. 2). We also examined neuromast deposition and noted no difference in neuromast number between control and BPA- or PCB-95-treated animals, nor did we see qualitative differences in neuromast spacing (data not shown). Collectively, these data suggest that neither BPA nor PCB-95 affect developing lateral line hair cells.
Fig. 1.
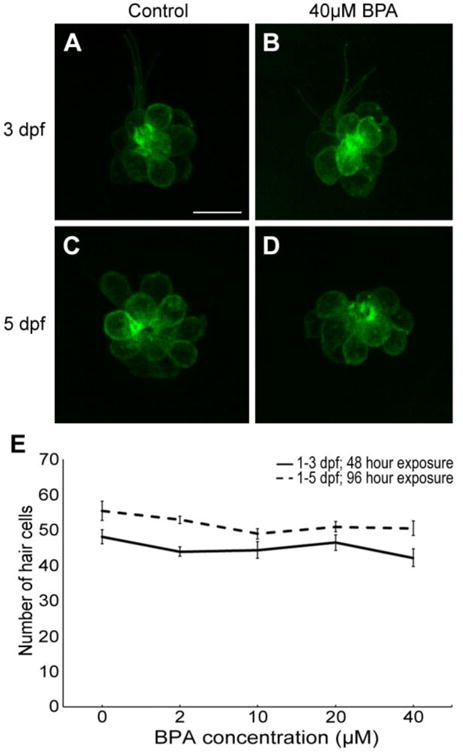
BPA does not affect lateral line development in zebrafish. (A-D) Confocal images (maximum projections) of the IO3 neuromast from Brn3c:mGFP transgenic zebrafish at 3 dpf (A-B) or 5 dpf (C-D). Incubation in BPA for 48 hrs (from 1-3 dpf, panel B) or 96 hrs (from 1-5 dpf, panel D) did not alter hair cell numbers as compared to age-matched controls (A, C). Scale bar in A is 10 μm and applies to all panels. (E) Hair cell quantification from 5 neuromasts (IO1, IO2, IO3, P1, P2), summed to arrive at one value per fish. There was no significant effect of BPA treatment on hair cell number (One-way ANOVA; 1-3 dpf: F4,45 =1.20, p=0.32; 1-5 dpf: F4,42 =1.94, p=0.12). N= 8-11 per treatment. Data are presented as mean ± 1 s.e.m.
Fig. 2.
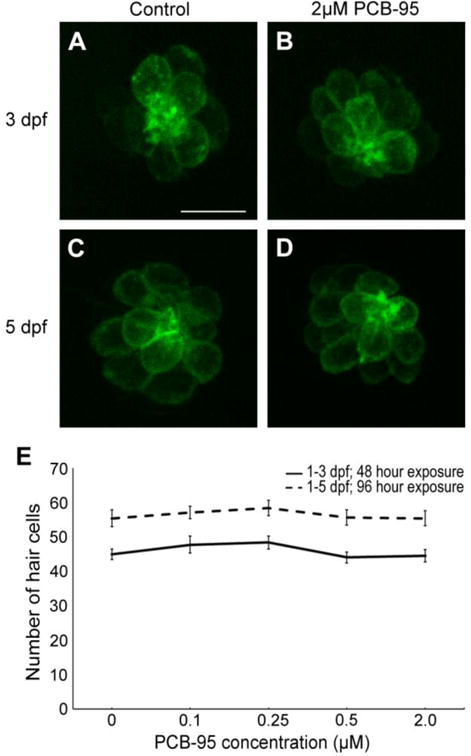
Zebrafish lateral line development is unaffected by PCB-95. (A-D) Confocal microscopy maximum projection images of the IO3 neuromast from Brn3c:mGFP transgenic zebrafish. 48 hrs (1-3 dpf) of exposure to 2 μM PCB-95 (B) was not visibly different from the age-matched control (A). Similarly, hair cells after 96 hrs (1-5 dpf) of exposure to 2 μM PCB-95 (D) were also not different from the age-matched control (C). The 10 μm scale bar in A applies to all panels. (E) Hair cells from 5 neuromasts were summed for each fish, and then averaged for each treatment group. There was no significant effect of PCB-95 on hair cell number. (One-way ANOVA; 1-3 dpf: F4,44 =1.12, p=0.36; 1-5 dpf: F4,46 =0.36, p=0.84). N=9-11 per treatment. Data are shown as ± 1 s.e.m.
3.2 Acute hair cell toxicity
Because immature hair cells are relatively resistant to known hair cell toxins such as aminoglycoside antibiotics (Murakami et al., 2003; Santos et al., 2006), we asked if PCB-95 or BPA were toxic to mature hair cells. Lateral line hair cells in 5 dpf larvae are considered mature, showing adult-like responses to known ototoxins (Murakami et al., 2003; Santos et al., 2006). We found no hair cell damage in 5 dpf larvae acutely exposed to PCB-95, while substantial, yet incomplete, hair cell loss was visible in acutely BPA-exposed fish, as illustrated in Figure 3. As shown in Figure 4, hair cell survival in 5 dpf larvae exposed to varying concentrations of PCB-95 for 24 hrs was not significantly different from controls, even at 20 μM, the highest concentration tested.
Fig. 3.
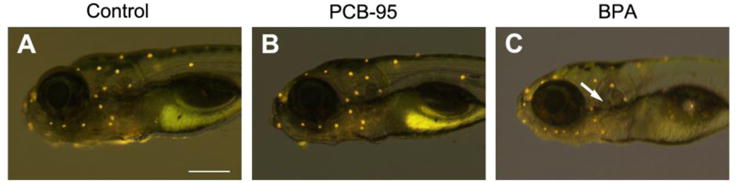
BPA is toxic to lateral line hair cells. Neuromasts were labeled with the vital dye DASPEI. (A) Control fish, (B) fish treated for 24 hrs with 2 μM PCB-95, and (C) fish treated for 24 hrs with 20 μM BPA. DASPEI labeling is less intense in the BPA-treated fish than in the other images, and the arrow in C points to the location of a missing neuromast. Scale bar in A is 250 μm and applies to all panels.
Fig. 4.
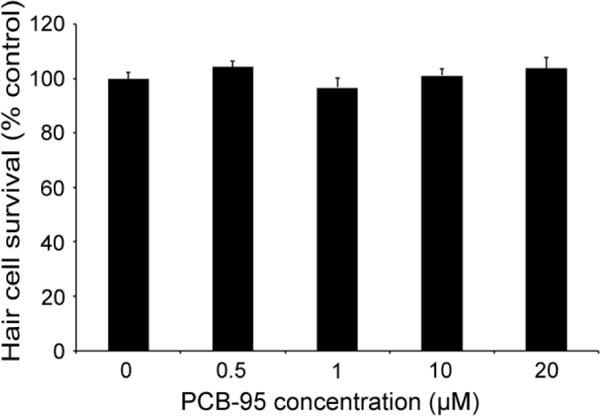
PCB-95 is not acutely toxic to lateral line hair cells. 24 hr incubation in 0.5 – 20 μM PCB-95 did not kill lateral line hair cells of wild-type zebrafish (One-way ANOVA, F4,50 =1.182, p=0.33). Hair cells were quantified by DASPEI fluorescence. Data are shown as the normalized mean + 1 s.e.m. N=11 fish/treatment.
In contrast, BPA significantly damaged mature lateral line hair cells in 5-6 dpf larvae (Fig. 5). BPA-induced hair cell death is both time- and dose-dependent, where 20 μM BPA significantly damaged lateral line hair cells after a minimum of 4 hrs exposure, with increased hair cell loss evident after 24 hrs. Treatment with higher BPA concentrations (60 or 80 μM) for 24 hrs was toxic to 5-6 dpf larvae, causing substantial mortality. We also saw modest but significant hair cell toxicity after 24 hr exposure to more environmentally relevant BPA concentrations (1 μM, Fig. 5D). We have elected to use the higher 20-40 μM BPA concentrations for the majority of our additional experiments, as the greater magnitude of hair cell loss better facilitates mechanistic analysis.
Fig. 5.
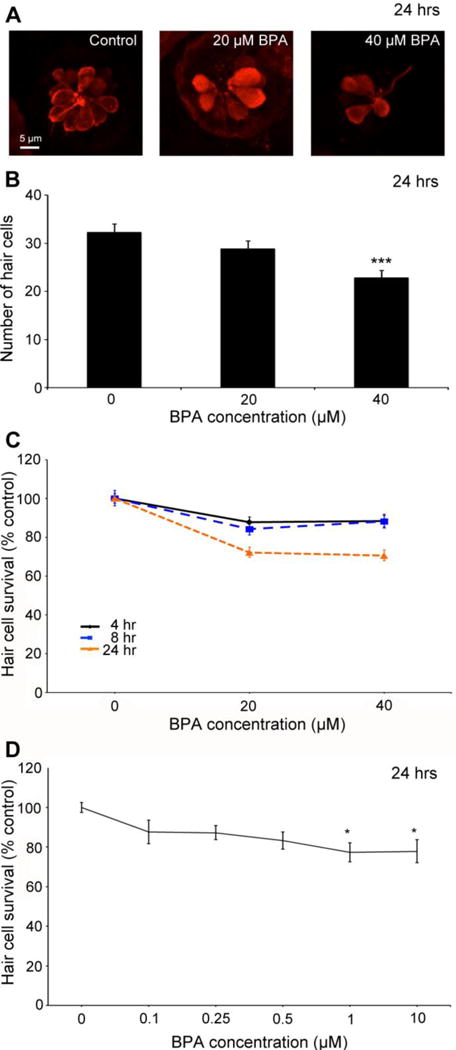
BPA is toxic to lateral line hair cells. (A) Confocal images of representative anti-parvalbumin-labeled hair cells, showing progressive cell loss with increasing BPA concentration. (B) Direct counts of labeled hair cells demonstrate significant hair cell loss with 24 hr BPA exposure (One-way ANOVA, F2,23 =8.80, p=0.001). N=8-10 fish/treatment. (C) BPA kills lateral line hair cells in a dose-dependent and time-dependent manner. Hair cells were assessed with DASPEI labeling. There is a significant effect of BPA concentration but not time, on hair cell survival (Two-way ANOVA, F2,85=40.28, p<0.001; F2,85=0.59, p=0.55 for concentration and time, respectively). However, the interaction term is also significant, demonstrating that dose-dependent hair cell loss differs based on exposure time (F4,85=4.41, p=0.003). As shown by Holm-Sidak’s multiple comparisons test, both 20 and 40 μM BPA significantly reduced hair cell numbers, as compared to the control for each time point. (4 hr p<0.05 for 20 and 40 μM; 8 hr p<0.01 for 20 and 40 μM; 24 hr p<0.001 for 20 and 40 μM). N=10-12 fish. (D) 24 hr exposure to lower BPA concentrations also causes significant hair cell loss (1-way ANOVA, F5,68 =2.75, p=0.025, n=11-14 fish/treatment). Data are presented as mean ± 1 s.e.m. *p<0.05, ***p<0.001.
It’s important to note that there was some experiment to experiment variability in BPA-induced hair cell loss, possibly due to slight differences in BPA handling or age. The data shown in Figure 5 are representative of the trends we see across experiments. In addition, the experiment in Figure 5B used cell counts to assess hair cell loss, while data in Figure 5C-D were collected with DASPEI assessment of fluorescence. These two measures are tightly correlated (Coffin et al., 2013b), but DASPEI is more sensitive to hair cell damage, as a loss of mitochondrial membrane potential precedes complete loss of the cell soma (Owens et al., 2007). Therefore, hair cell survival appears slightly lower when assessed with DASPEI, as compared to direct cell counts.
To understand the underlying mechanisms responsible for BPA-induced hair cell loss, we screened a custom cell death inhibitor library for compounds that confer protection against BPA ototoxicity (Coffin et al., 2013). Several inhibitors attenuated BPA-induced hair cell damage: Bongkrekic acid, an inhibitor of cathepsin G, and three mediators of oxidative stress (glutathione, an nNOS inhibitor, and resveratrol). In the present study we focused on two oxidative stress inhibitors that conferred significant hair cell protection: glutathione, and an inhibitor of nNOS (neuronal nitric oxide synthase). nNOS inhibition blocks nitric oxide production, reducing nitrogen species and subsequent oxidative stress (Hah et al., 2001). Glutathione mitigates cell death by preventing reactive oxygen species (ROS) accumulation, which attenuates noise- and cisplatin-induced hair cell loss in rodent models (Kopke et al., 1997; Yamasoba et al., 1998). Either of these compounds significantly protects hair cells from BPA toxicity (Fig. 6), with robust protection seen at moderate nNOS inhibitor concentrations (Fig. 6B). The highest nNOS inhibitor concentration was toxic to hair cells. Antioxidant concentration ranges were selected based on our prior drug screens and on published literature in zebrafish, including a study demonstrating that glutathione attenuates cisplatin-induced hair cells damage in zebrafish larvae (Ton and Parng, 2005; Coffin et al., 2013; Cui et al., 2013). We observed minimal hair cell toxicity when fish were treated with antioxidant alone (i.e., without BPA, data not shown).
Fig. 6.
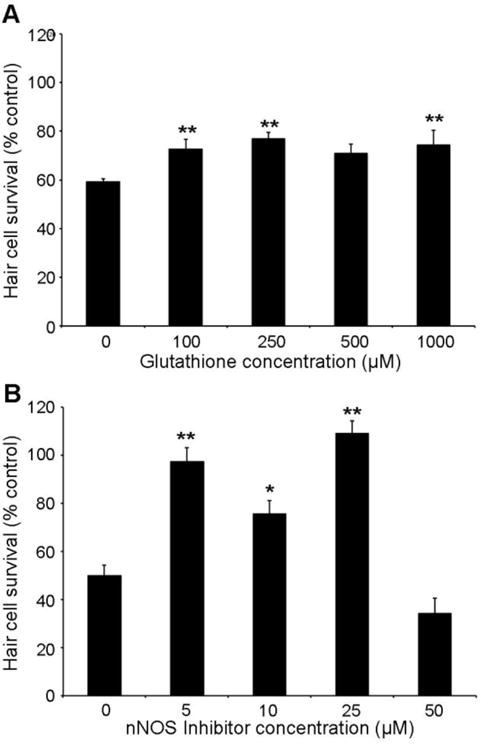
Reducing oxidative stress attenuates BPA-induced hair cell loss. Fish were treated with 20 μM BPA for 24 hrs in the presence of (A) the antioxidant glutathione or (B) an inhibitor of nNOS. Either compound confers significant protection from BPA damage (Glutathione: F4,43 = 3.57, p=0.01; nNOS inhibitor: F4,46 = 34.71, p<0.001). Holm-Sidak’s multiple comparison test shows significant differences at all glutathione or nNOS inhibitor concentrations tested, as compared to BPA-only controls (*p<0.05, **p<0.01). Hair cell survival was assessed with DASPEI scoring. N=8-12 fish/treatment, data are presented as mean + 1 s.e.m.
Aminoglycoside antibiotics and the chemotherapy drug cisplatin, all well-established ototoxins, enter hair cells via the mechanotransduction channel (Gale et al., 2001; Steyger et al., 2003; Thomas et al., 2013; Vu et al., 2013). Here, we asked if BPA enters hair cells through the same route. High extracellular calcium reduces the open probability of the channel, thereby reducing aminoglycoside uptake (Richardson and Russell, 1991; Marcotti et al., 2005; Coffin et al., 2009). Similarly, breaking tip links, extracellular filaments associated with transduction channel gating, reduces channel opening and therefore aminoglycoside entry (Assad et al., 1991; Zhao et al., 1996; Gale et al., 2001). We found that bath application of high calcium EM did not protect hair cells from BPA damage, nor did tip link breakage using the calcium chelator EDTA (Fig. 7). In contrast, either high calcium or EDTA treatment significantly protected hair cells from neomycin damage, consistent with published work on aminoglycoside uptake (Gale et al., 2001; Coffin et al., 2009). These data suggest that BPA does not enter hair cells through the transduction channel, indicating an alternate entry route such as endocytosis, or perhaps an external mechanism of action.
Fig. 7.
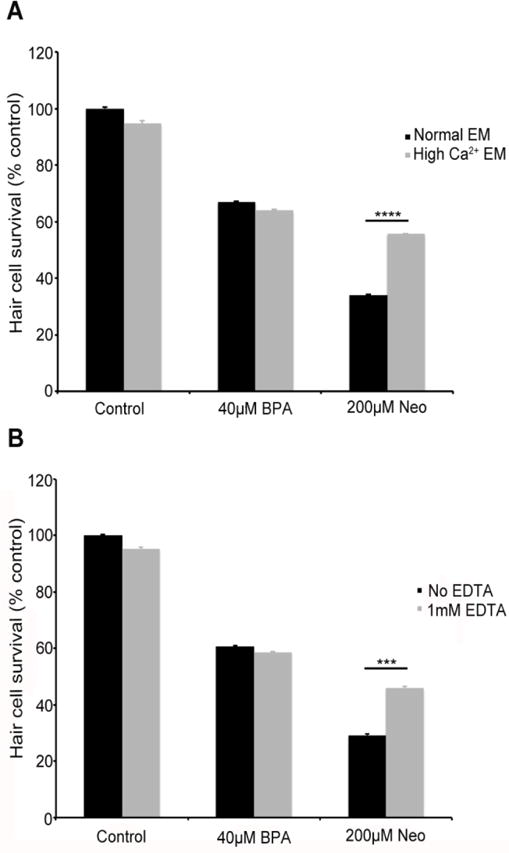
Blocking mechanotransduction does not prevent BPA-induced hair cell damage. Fish were treated for 4 hrs with 40 μM BPA in embryo medium (EM) containing either (A) 2.1 mM (high Ca2+ EM) additional calcium, which blocks the transduction channel or (B) 1mM EDTA, which inhibits transduction by breaking tip links. Neither treatment protected hair cells from BPA damage (Ca2+: t-test, p=0.42; EDTA: t-test, p=0.41). However, either treatment significantly protected hair cells from neomycin damage (***p<0.001, ****p<0.0001). Hair cell survival was assessed with DASPEI labeling. N=8-10 fish/treatment, data are presented as mean + 1 s.e.m.
Increasing public concern over BPA has led to an upsurge in analog usage. We therefore asked if BPS, a common BPA analog, was also toxic to hair cells. As shown in Figure 8A, BPS robustly damages hair cells after 4 or 24 hrs of exposure, with no difference in hair cell loss between the two exposure times. The dose-response function for 24 hr BPS exposure resembles the curve for BPA (compare Figs. 5C and 8A), except that high BPS is not overtly toxic to fish, allowing us to assess hair cell toxicity. We then exposed fish concurrently to both BPS and BPA (40 μM each) to assess potential synergistic effects on hair cells and found that co-treatment causes hair cell damage comparable to what we see for BPS or BPA alone, suggesting neither an additive nor synergistic effect of combined BPS/BPA exposure (Fig. 8B). These results suggest that BPS and BPA exert similar toxic effects on hair cells, likely via the same molecular mechanisms.
Fig. 8.
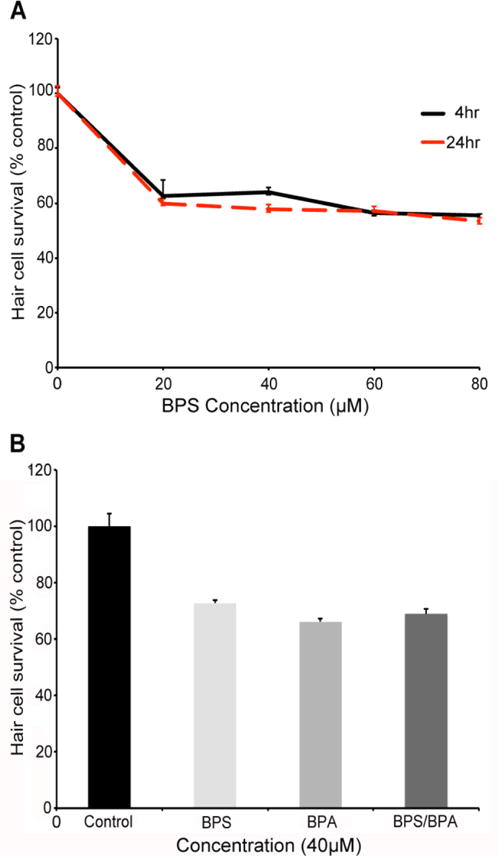
(A) BPS kills lateral line hair cells in a dose-dependent manner (Two-way ANOVA, F4,107=63.75, p<0.001). N=10-12 fish/treatment. There is a significant effect of BPA concentration at 4 hrs (One-way ANOVA, F4,54 =39.92, p<0.001) and 24 hrs (F4,53 =38.91, p<0.001). (B) The individual effects of BPA and BPS do not appear to be additive when fish are treated with 40 μM BPA and/or BPS for 4 hrs (BPA, t-test, p=0.16; BPS, t-test, p=0.09, vs. combined BPS/BPA exposure). Fish were assessed with DASPEI scoring of neuromast fluorescent intensity. N=10-12 fish/treatment, data are presented as mean ± 1 s.e.m.
3.3 Hair Cell Regeneration
Hair cell regeneration is reduced or delayed following exposure to some toxins, suggesting that they may interfere with the regenerative process (Hernández et al., 2006; Mackenzie and Raible, 2012). We therefore asked if PCB-95 or BPA affected hair cell regeneration following exposure to a known ototoxin. Fish were treated with 300 μM neomycin to ablate hair cells, then allowed to recover for 24 or 48 hrs in variable concentrations of environmental contaminant, or in EM only as a control (0 environmental contaminants groups). PCB-95 did not affect hair cell regeneration within the concentration range we tested (Fig. 9). Partial regeneration was evident 24 hrs after neomycin damage and regeneration was largely complete by 48 hrs post-neomycin, even in the presence of PCB-95. In contrast, BPA significantly attenuated regeneration following neomycin damage, with dose-dependent suppression of regeneration observed at 24 or 48 hrs post-neomycin (Fig. 10). 10 μM BPA significantly reduced regenerative responses at either time point, while 20 μM BPA eliminated regeneration entirely (Fig. 10E). These data suggest that the hair cell regeneration process is more susceptible to BPA damage than are mature hair cells.
Fig. 9.
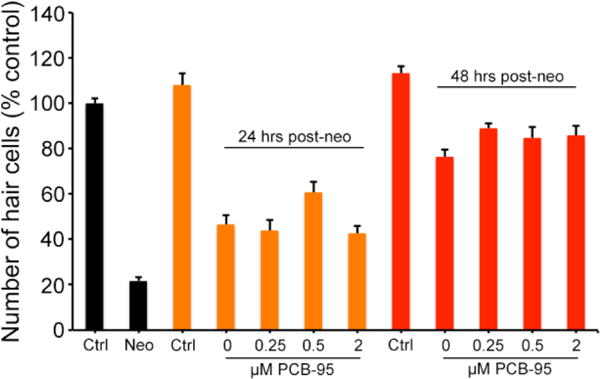
PCB-95 exposure does not affect lateral line hair cell regeneration. Neomycin treatment generated a robust hair cell lesion (black Neo bar). Hair cell addition was evident 24 hrs post-neomycin in all treatment groups (orange bars). While there was a significant effect of PCB concentration (One-way ANOVA, F3,32=4.01, p=0.016), this effect is modest and there are no significant pairwise differences when compared with the 0 PCB-95 (neo only) control. There is no effect of PCB treatment 48 hrs post-neomycin (red bars), with similar hair cell regeneration seen in all groups (One-way ANOVA, F3,39=1.84, p=0.16). Hair cells were quantified by counts of anti-parvalbumin labeled cells in five neuromasts per fish (IO1, IO2, IO3, M2, OP1) and summed to arrive at one value per animal. Data are presented as mean + 1 s.e.m., n= 9-12 fish/group.
Fig. 10.
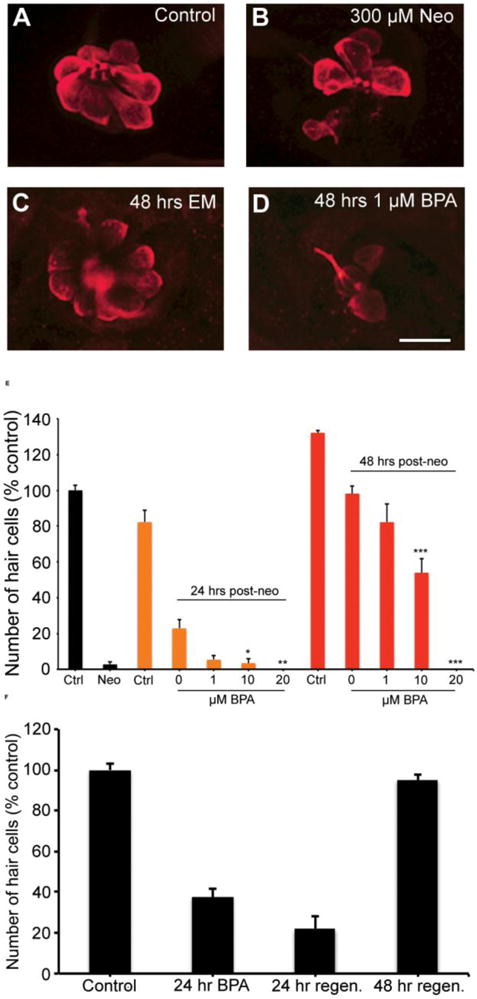
BPA attenuates hair cell regeneration. (A-D) Representative confocal images of anti-parvalbumin-labeled hair cells from (A) an untreated fish, (B) a damaged neuromast immediately after exposure to 300 μM neomycin, (C) a neomycin-treated neuromast after 48 hrs of recovery in normal EM, and (D), a neomycin-treated neuromast after 48 hrs of recovery in 1 μM BPA. (E) There is a significant effect of both BPA concentration and recovery time on hair cell survival, with more hair cells present after longer times or in lower BPA concentrations (Two-way ANOVA, BPA conc. F3,63=55.79, p<0.001; Recovery time F1,63=213.0, p<0.001). Black bars indicate fish not treated with BPA. 300 μM treatment results in almost complete hair cell loss (black “neo” bar). Orange bars represent 24 hrs of regeneration in BPA, and red bars indicate 48 hrs of regeneration in BPA. In all cases “Ctrl” are age-matched control animals treated with DMSO. Hair cell regeneration was reduced in 10 μM BPA and abolished by 20 μM BPA. (F) BPA-induced hair cell damage delays regeneration. Fish were treated for 24 hrs with 20 μM BPA and either assessed immediately, or allowed to recover in fresh EM for an additional 24 hrs. There is no difference in hair cell number in the 24-hr recovery fish as compared to fish assessed immediately after BPA exposure (t-test, p=0.13), yet there is significant recovery 48 hrs after BPA removal (t-test, p<0.001). N=9-11 fish/treatment, data are presented as mean + 1 s.e.m. *p<005, **p<0.01, ***p<0.001.
Finally, we asked if hair cells regenerate after BPA-induced damage. Fish were treated with 20 μM BPA for 24 hrs and hair cells were assessed either immediately after BPA removal or 24 hrs later (24 hr recovery period). We found that hair cells did not regenerate 24 hrs after BPA removal and that there was a trend towards increased hair cell loss (Fig. 10F). These data demonstrate that BPA can have ongoing toxic effects on mature hair cells in addition to negative effects on regeneration.
4. Discussion
4.1 BPA is toxic to hair cells
We show that BPA, a prevalent environmental contaminant, has toxic effects on mature and regenerating sensory hair cells in the zebrafish lateral line. To our knowledge, this is the first report of BPA-induced hair cell damage. Previous work has reported BPA-associated otolith formation defects in the zebrafish inner ear, but no hair cell phenotype was noted (Gibert et al., 2011). Hair cell susceptibility to chemical toxicants likely stems from multiple mechanisms, including differential toxicant uptake and high metabolic demands. Inhibiting mechanotransduction with extracellular calcium or tip link breakage did not attenuate BPA-induced damage, suggesting that BPA does not enter hair cells in the same way as aminoglycosides or cisplatin (Coffin et al., 2009; Thomas et al., 2013, Vu et al., 2013). Further studies are needed to identify BPA’s route of entry into hair cells, if in fact entry is necessary. It is possible that BPA may stimulate extracellular death receptors without being internalized.
4.2 BPA mechanism of damage
Despite a different entry route, BPA-induced hair cell damage shares similarities with known ototoxins. We found that immature hair cells are relatively resistant to BPA damage, consistent with aminoglycoside studies in several vertebrate taxa, including zebrafish (Marot et al., 1980; Duckert and Rubel, 1990; Murakami et al., 2003, Santos et al., 2006). The present study shows that either the antioxidant glutathione or an inhibitor of nNOS significantly protect hair cells from BPA exposure, suggesting that BPA damages hair cells through oxidative stress and the buildup of reactive oxygen and nitrogen species. Aminoglycoside antibiotics and cisplatin can also activate ROS signaling and overwhelm endogenous antioxidant mechanisms in rodent ototoxicity models (Sha and Schacht, 1999, 2000; see Porrier et al., 2010); antioxidant therapies may attenuate aminoglycoside damage in vivo (e.g., Ton and Parng, 2005; Campbell et al., 2007; Fetoni et al., 2012; LePrell et al., 2014). BPA is also known to induce ROS generation in other tissues and organisms, indicating that oxidative stress is a major mechanism underlying BPA toxicity (Kim et al., 2007; Huc et al., 2012; Jiang et al., 2014). For example, embryonic BPA exposure increases ROS generation in rat hepatocytes and oxidative DNA damage and cell death in zebrafish musculature (Wang et al., 2013; Jiang et al., 2014). Collectively, our data demonstrate that the environmental contaminant BPA can damage sensory hair cells, which may have consequences for aquatic vertebrates. Furthermore, since lateral line hair cells are structurally and functionally similar to those found in the human ear (reviewed in Coffin et al., 2010; 2014), and mammalian hair cells do not regenerate, these findings pose the disturbing possibility that BPA may cause permanent hearing loss in humans.
4.3 BPA delays regeneration of hair cells
Unlike mammals, fishes and many other vertebrates can regenerate hair cells (Corwin and Cotanche, 1988; Ryals and Rubel, 1988; reviewed in Brignull et al., 2009). In larval zebrafish, aminoglycoside-damaged hair cells fully regenerate three days after damage, with regeneration occurring primarily via proliferation of surrounding supporting cells and differentiation of their progeny into new hair cells (Harris et al., 2003, Ma et al., 2008, MacKenzie and Raible, 2012). Regeneration is mediated in part by Notch signaling, and BPA may interact with components of the Notch signaling pathway (Baba et al., 2009; Ma et al., 2008). We show that BPA attenuates regeneration post-neomycin, possibly by disrupting Notch activity. Alternatively, BPA could inhibit regeneration via a separate mechanism, perhaps by interfering with estrogen signaling.
BPA is often characterized as a xenoestrogen, and estrogenic activity of BPA has been noted in fishes, including zebrafish (Lahnsteiner et al., 2005; Kishida et al., 2001). Estrogens are known to induce glial cell proliferation and neuronal addition in the central nervous system in both fishes and mammals, but their proliferative role in the peripheral nervous system is poorly understood (reviewed in Barha and Galea, 2010, Le Page et al., 2010). Coffin et al. (2012) demonstrated a seasonal change in hair cell addition in the plainfin midshipman fish (Porichthys notatus) that is likely estrogen-dependent. An inhibitor of estrogen signaling, Fulvestrant, has also been shown to decrease hair cell regeneration in larval zebrafish (Namdaran et al., 2012). These data suggest that estrogen may be important for endogenous hair cell addition in fishes, possibly via interaction with Wnt signaling, another pathway important for hair cell regeneration in both fishes and birds (Kouzmenko et al., 2004; Gao et al., 2013; Head et al., 2013; Jacques et al., 2013). Future studies will determine which pathway(s) are targeted by BPA to reduce hair cell regeneration. It is also possible that BPA damages supporting cells, preventing their proliferation, similar to what has been suggested for high concentrations of the ototoxic agent cisplatin (Mackenzie and Raible, 2012; Slattery et al. 2014). Future experiments will quantify supporting cells during the regenerative period. Regardless of molecular mechanism, our data indicate that BPA exposure may have negative long-term consequences on fish mechanosensory systems, given that BPA both damages existing hair cells and reduces their replacement.
4.4 BPS damages hair cells
As public outcry over the use of BPA has gained traction, companies have switched to using BPA analogs. Bisphenol S (BPS) is a common substitute that unfortunately has similar estrogenic properties to BPA (Rosenmai et al., 2014). In a recent study, 81% of 315 people sampled in the U.S. had detectable concentrations of BPS in their urine (Liao et al., 2012). Like BPA, BPS also adversely affects the endocrine system and reproduction in developing and adult zebrafish, altering estrogen and testosterone levels and skewing sex ratios in favor of females (Ji et al., 2013; Naderi et al., 2014). In the present study we show that BPS damages hair cells, making its substitution as a safe alternative to BPA questionable. Similar questions have arisen for other BPA analogs, as bisphenol F also shows cytotoxic properties (Lee et al., 2013).
4.5 Developmental BPA exposure
We did not see an impact of BPA exposure on lateral line development. This contrasts with a previous study by Lam et al. (2011), which demonstrated that 5 days of exposure to 2.2 or 22 μM BPA resulted in changes in neuromast morphology. Their study used the ET20 transgenic line, which labels a subset of non-sensory mantle cells (Parinov et al., 2004), so it is possible that hair cells were not affected by their BPA treatment. Lam et al. (2011) also noted that transgenic ET20 larvae were more sensitive to overall BPA toxicity than were wildtype larvae, so differences in genetic background may account for the lack of a neuromast phenotype in our study.
In general, BPA-treated fish appeared grossly normal, although we did not assess other tissues for subtle effects. We also did not raise the fish to adulthood to examine the effect of developmental BPA exposure on adult mechanosensory function; prenatal or perinatal BPA exposure in rodents alters gene expression and neurotransmitter biochemistry in the adult CNS while affecting sex-specific behaviors, leading to BPA-dependent phenotypes long after exposure cessation (Farabollini et al., 2002; Nakamura et al., 2010; Jones and Watson, 2012; Viberg and Lee, 2012). BPA exposure is correlated with changes in DNA methylation in cultured human and mouse cells and epigenetic regulation in vivo in mice. Significant behavioral consequences of BPA exposure were reported in mouse trangenerational experiments, including changes in measures of anxiety and social behavior (Wolstenholme et al., 2012, 2013; Bastos Sales et al., 2013; Kundakovic et al., 2013). Possible epigenetic effects on sensory structure and function have yet to be explored.
4.6 Vertebrate BPA exposure
Many studies use concentrations of BPA comparable to the range where we see hair cell toxicity, and often these studies show little to no effect at lower BPA concentrations (Delclos KB et al., 2014; Sone K et al., 2004; Staples CA et al., 2011). These concentrations are generally higher than the U.S. population’s median BPA daily intake of 34 ng/kg-day (Lakind and Naiman, 2011), or the 12 μg/L (52 nM) BPA concentration reported for U.S. waters (Kolpin et al., 2002). The U.S. Environmental Protection Agency has established 50 μg/kg-day as the tolerable daily intake (TDI) for human BPA exposure (EPA website). However, BPA does not appear to exhibit a monotonic dose-response curve at concentrations in the picomolar through micromolar range (Wozniak et al., 2005; Zsarnovsky et al., 2005). Because the EPA’s TDI is based on a monotonic assumption (Myers et al., 2009), the current exposure limit may be incorrect. In addition, the majority of studies only account for oral consumption routes. Absorption and inhalation are known routes of exposure (for review see Geens et al., 2012) given that BPA is found in everything from electronic equipment to the by-product of the most commonly used flame retardant, Tetrabromobisphenol A (Banerjee et al., 2014). Indeed, high levels of BPA have been reported in cashiers that regularly handle thermal receipts, suggesting that some professions may experience dangerous levels of BPA exposure (Lu et al., 2013). Consequently, our use of higher concentrations of BPA may equate to realistic exposure levels.
4.7 Effects of PCB-95 on hair cells
We did not find a direct effect of PCB-95 on hair cells. Several studies demonstrate that PCB exposure is correlated with hearing loss in humans and animal models, including a reported association between hair cell loss and PCB treatment in rats (Crofton et al., 2000a; Poon et al., 2011; Min et al., 2014). Many of these auditory studies use a mixture of PCBs (Aroclor 1254), making it difficult to determine the precise contribution of a specific PCB to the observed auditory defect (Goldey et al., 1995; Crofton et al., 2000a, 2000b). In rats, PCB-induced auditory dysfunction is correlated with increased circulating thyroid hormone levels, consistent with PCB activity as an endocrine disrupter (McKinney and Waller, 1994; Goldey and Crofton, 1998; Crofton et al., 2000b). Constitutive thyroid hormone deficiency affects cochlear ion channel expression and hair cell function in mice (Rusch et al., 2001; Dettling et al., 2014), suggesting that PCB exposure may cause hearing loss via hair cell dysfunction and that the hair cell loss reported by Crofton et al. (2000a) is a secondary effect. Wayman et al. (2012b) reported altered synaptogenesis in hippocampal neurons cultured with PCB-95, further evidence that PCBs may exert a synaptic effect rather than overt neurotoxicity. Additional neurotransmission experiments are needed to test this hypothesis.
5. Conclusions
Our study identifies bisphenol-A as a new sensory hair cell toxicant, adding to the dangers of this environmental contaminant. In humans, BPA exposure poses a threat to sensory cells of the inner ear, which may cause deafness. BPA may also threaten the viability of fish populations by damaging their mechanosensory systems and reducing their regenerative capacity, potentially impacting prey detection, predator avoidance, and reproductive behaviors.
Acknowledgments
We thank K. Williams and P. Uribe for assistance with experiments and C. Riso for fish husbandry support. We also thank members of the Coffin Lab for feedback on early drafts of this manuscript and P. Lein for supplying the PCB-95 used for these studies.
Funding: This work is funded by a Washington State University Vancouver mini-grant and by Washington State University start-up funds, both to A. Coffin. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Assad JA, Shepherd GM, Corey DP. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron. 1991;7(6):985–94. doi: 10.1016/0896-6273(91)90343-x. [DOI] [PubMed] [Google Scholar]
- Baba K, Okada K, Kinoshita T, Imaoka S. Bisphenol A disrupts Notch signaling by inhibiting gamma-secretase activity and causes eye dysplasia of Xenopus laevis. Toxicol Sci. 2009;108(2):344–55. doi: 10.1093/toxsci/kfp025. 2009. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Periyasamy G, Pati SK. Formation mechanism and possible stereocontrol of bisphenol A derivatives: a computational study. J Phys Chem B. 2014;118(31):9258–62. doi: 10.1021/jp506822w. [DOI] [PubMed] [Google Scholar]
- Barha CK, Galea LA. Influence of different estrogens on neuroplasticity and cognition in the hippocampus. Biochim Biophys Acta. 2010;1800(10):1056–67. doi: 10.1016/j.bbagen.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Bastos Sales L, Kamstra JH, Cenijn PH, van Rijt LS, Hamers T, Legler J. Effects of endocrine disrupting chemicals on vitro global DNA methylation and adipocyte differentiation. Toxicol In Vitro. 2013;27(6):1634–43. doi: 10.1016/j.tiv.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Berg A, Watson GM. Rapid recovery of sensory function in blind cave fish treated with anemone repair proteins. Hear Res. 2002;174(1–2):296–304. doi: 10.1016/s0378-5955(02)00705-0. [DOI] [PubMed] [Google Scholar]
- Brignull HR, Raible DW, Stone JS. Feathers and fins: non-mammalian models for hair cell regeneration. Brain Res. 2009;1277:12–23. doi: 10.1016/j.brainres.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KC, Meech RP, Klemens JJ, Gerberi MT, Dyrstad SS, Larsen DL, Michell DL, El-Azizi M, Verhulst SJ, Hughes LF. Prevention of noise- and drug-induced hearing loss with D-methionine. Hear Res. 2007;226(1–2):92–103. doi: 10.1016/j.heares.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Carpenter DO, Hussain RJ, Berger DF, Lombardo JP, Park HY. Electrophysiologic and behavioral effects of perinatal and acute exposure of rats to lead and polychlorinated biphenyls. Environ Health Perspect. 2002;110(Suppl 3):377–86. doi: 10.1289/ehp.02110s3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H-L, Yuh C-H, Wu KK. Nesting is essential for zebrafish brain and eye development through control of progenitor cell apoptosis. PLoS One. 2010;5(2):e9318. doi: 10.1371/journal.pone.0009318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin AB, Brignull H, Raible DW, Rubel EW. Hearing loss, protection, and regeneration in the larval zebrafish lateral line. In: Coombset al., editors. The Lateral Line, SHAR series. 2014. [Google Scholar]
- Coffin AB, Mohr RA, Sisneros JA. Saccular-specific hair cell addition correlates with reproductive state-dependent changes In the auditory saccular sensitivity of a vocal fish. J Neurosci. 2012;32(4):1366–76. doi: 10.1523/JNEUROSCI.4928-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin AB, Ou H, Owens KN, Santos F, Simon JA, Rubel EW, Raible DW. Chemical screening for hair cell loss and protection in the zebrafish lateral line. Zebrafish. 2010;7(1):3–11. doi: 10.1089/zeb.2009.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin AB, Reinhart KE, Owens KN, Raible DW, Rubel EW. Extracellular cations modulate aminoglycoside-induced hair cell death in the zebrafish lateral line. Hear Res. 2009;253(1–2):42–51. doi: 10.1016/j.heares.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin AB, Rubel EW, Raible DW. Bax, Bcl2, and p53 diffreentially regulate neomycin- and gentamicin-induced hair cell death in the zebrafish lateral line. J Assoc Res Otolaryngol. 2013b;14(5):645–59. doi: 10.1007/s10162-013-0404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin AB, Williamson KL, Mamiya A, Raible DW, Rubel EW. Profiling drug-induced cell death pathways in the zebrafish lateral line. Apoptosis. 2013a;18(4):393–408. doi: 10.1007/s10495-013-0816-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins WT, Jr, Capen CC, Kasza L, Carter C, Dailey RE. Effect of polychlorinated biphenyl (PCB) on the thyroid glad of rats. Ultrastructural and biochemical investigations. AM J Pathol. 1997;89(1):119–36. [PMC free article] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240(4860):1772–4. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Crofton KM, Ding D, Padich R, Taylor M, Henderson D. Hearing loss following exposure during development to polychlorinated biphenyls: a cochlear site of action. Hear Res. 2000a;144(1–2):196–204. doi: 10.1016/s0378-5955(00)00062-9. [DOI] [PubMed] [Google Scholar]
- Crofton KM, Kodavanti PR, Derr Yellin EC, Casey AC, Kehn LS. PCBs, thyroid hormones, and ototoxicity in rats: cross-fostering experiments demonstrate the impact of postnatal lactation exposure. Toxicol Sci. 2000b;57(1):131–40. doi: 10.1093/toxsci/57.1.131. [DOI] [PubMed] [Google Scholar]
- Cui W, Zhang Z, Li W, Hu S, Mak S, Zhang H, Han R, Yuan S, Li S, Sa F, Xu D, Lin Z, Zuo Z, Rong J, Ma ED, Choi TC, Lee SMY, Han Y. The anti-cancer agent SU4312 unexpectedly protects against MMP+-induced neurotoxicity via selective and direct inhibition of neuronal NOS. Br J Pharmacol. 2013;168(5):1201–1214. doi: 10.1111/bph.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delclos KB, Camacho L, Lewis SM, Vanlandingham MM, Latendresse JR, Olson GR, Davis KJ, Patton RE, Gamboa da Costa G, Woodling KA, Bryant MS, Chidambaram M, Trbojevich R, Juliar BE, Felton RP, Thorn BT. Toxicity evaluation of bisphenol A administered by gavage to Sprague Dawley rats from gestation day 6 through postnatal day 90. Toxicol Sci. 2014;139(1):174–97. doi: 10.1093/toxsci/kfu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettling J, Franz C, Zimmerman U, Lee SC, Bress A, Brandt N, Feil R, Pfister M, Engel J, Flamant F, Rüttiger L, Knipper M. Autonomous functions of murine thyroid hormone receptor TRα and TRβ in cochlear hair cells. Mol Cell Endocrinol. 2014;382(1):26–37. doi: 10.1016/j.mce.2013.08.025. [DOI] [PubMed] [Google Scholar]
- Duckert LG, Rubel EW. Ultrastructural observations on regenerating hair cells in the chick basilar papilla. Hear Res. 1990;48(1–2):161–82. doi: 10.1016/0378-5955(90)90206-5. [DOI] [PubMed] [Google Scholar]
- Eilam-Stock T, Serrano P, Frankfurt M, Luine V. Bisphenol-A impairs memory and reduces dendritic spine density in adult male rats. Behav Neurosci. 2012;126(1):175–85. doi: 10.1037/a0025959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsworth JD, Jentsch JD, Vandevoort CA, Roth RH, D E, Jr, Leranth C. Prenatal exposure to bisphenol A impacts midbrain dopamine neurons and hippocampal spine synapses in non-human primates. Neurotoxicology. 2013;35:113–20. doi: 10.1016/j.neuro.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA (United States Environmental Protection Agency) Bisphenol A, Integrated Risk Information System. http://www.epa.gov/iris/subst/0356.htm [accessed on 7 698 August 20140.
- Farabollini F, Porrini S, Della Seta D, Bianchi F, Dessì-Fulgheri F. Effects of perinatal exposure to bisphenol A on sociosexual behavior of female and male rats. Environ Health Perspect. 2002;110(Suppl 3):409–14. doi: 10.1289/ehp.02110s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwell M, Drouillard KG, Heath DD, Pitcher TE. Acclimation of life-history traits to experimental changes in environmental contaminant concentrations in brown bullhead (Ameiurus nebulosus) Environ Toxicol Chem. 2012;31(4):863–9. doi: 10.1002/etc.1761. [DOI] [PubMed] [Google Scholar]
- Fetoni AR, Eramo SL, Rolesi R, Troiani D, Paludetti G. Antioxidant treatment with coenzyme Q-ter in prevention of gentamycin ototoxicity in an animal model. Acta Otorhinolaryngol Ital. 2012;32(2):103–10. [PMC free article] [PubMed] [Google Scholar]
- Fritsch EB, Pessah IN. Structure-activity relationship of non-coplanar polychlorinated biphenyls toward skeletal muscle ryanodine receptors in rainbow trout (Oncorhynchus mykiss) Aquat Toxicol. 2013;140–141:204–12. doi: 10.1016/j.aquatox.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Huang E, Zhang H, Wang J, Wu N, Chen X, Wang N, Wen S, Nan G, Deng F, Liao Z, Wu D, Zhang B, Haydon RC, Luu HH, Shi LL, He TC. Crosstalk between Wnt/β-catenin and estrogen receptor signaling synergistically promotes osteogenic differentiation of mesenchymal progenitor cells. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0082436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale JE, Marcotti W, Kennedy HJ, Kros CJ, Richardson GP. FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J Neurosci. 2001;21(18):7013–25. doi: 10.1523/JNEUROSCI.21-18-07013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geens T, Aerts D, Berthot C, Bourguignon JP, Goeyens L, Lecomte P, Maghuin-Rogister G, Pironnet Am, Pussemier L, Scippo ML, Van Loco J, Covaci A. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol. 2012;50(10):3725–40. doi: 10.1016/j.fct.2012.07.059. [DOI] [PubMed] [Google Scholar]
- Gibert Y, Sassi-Messai S, Fini JB, Bernard L, Zalko D, Cravedi JP, Balaguer P, Andersson0Lendahl M, Demeneix B, Laudet V. Bisphenol A induces otolith malformations during vertebrate embryogenesis. BMC Dev Biol. 2011;11:4. doi: 10.1186/1471-213X-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert ME, Liang D. Alterations in synaptic transmission and plasticity in hippocampus by a complex PCB mixture, Aroclor 1254. Neurotoxicol Teratol. 1998;20(4):383–9. doi: 10.1016/s0892-0362(98)00005-1. [DOI] [PubMed] [Google Scholar]
- Goldey ES, Crofton KM. Thyroxine replacement attenuates hypothyroxinemia, hearing loss, and motor deficits following developmental exposure to Aroclor 1254 in rats. Toxicol Sci. 1998;45(1):94–105. doi: 10.1006/toxs.1998.2495. [DOI] [PubMed] [Google Scholar]
- Goldey ES, Kehn LS, Lau C, Rehnberg GL, Crofton KM. Developmental exposure to polychlorinated biphenyls (Aroclor 1254) reduces circulating thyroid hormone concentrations and acuases hearing deficits in rats. Toxicol Appl Pharmacol. 1995;135(1):77–88. doi: 10.1006/taap.1995.1210. [DOI] [PubMed] [Google Scholar]
- Hah JM, Roman LJ, Martásek P, Silverman RB. Reduced amide bond peptidomimetics. (4S)-N-(4-amino-5-[aminoakyl]aminopentyl)-N′-nitroguanidines, potent and highly selective inhibitors of neuronal nitric oxide synthase. J Med Chem. 2001;44(16):2667–70. doi: 10.1021/jm0101491. [DOI] [PubMed] [Google Scholar]
- Halling-Sørensen B, Nors Nielsen S, Lanzky PF, Ingerslev F, Holten Lüzhøft HC, Jørgensen SE. Occurrence, fate and effects of pharmaceutical substances in the environment—a review. Chemosphere. 1998;36(2):357–93. doi: 10.1016/s0045-6535(97)00354-8. [DOI] [PubMed] [Google Scholar]
- Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio) J Assoc Res Otolaryngol. 2003;4:219–234. doi: 10.1007/s10162-002-3022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head JR, Gacioch L, Pennisi M, Meyers JR. Activation of canonical Wnt/β-catenin signaling stimulates proliferation in neuromasts in the zebrafish posterior lateral line. Dev Dyn. 2013;242(7):832–46. doi: 10.1002/dvdy.23973. [DOI] [PubMed] [Google Scholar]
- Heller S, Bell AM, Denis CS, Choe Y, Hudspeth AJ. Parvalbumin 3 is an abundant Ca2+ buffer in hair cells. J Assoc Res Otolaryngol. 2002;3(4):488–98. doi: 10.1007/s10162-002-2050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández PP, Moreno V, Olivari FA, Allende ML. Sub-lethal concentrations of waterborne copper are toxic to lateral line neuromasts in zebrafish (Danio rerio) Hear Res. 2006;213(1–2):1–10. doi: 10.1016/j.heares.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ. How the ear’s works work: mechanoelectrical transduction and amplification by hair cells. C R Biol. 2005;328(2):155–62. doi: 10.1016/j.crvi.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Huc L, Lemarié A, Guéraud F, Héliès-Toussaint C. Low concentrations of bisphenol A induce lipid accumulation meditated by the production of reactive oxygen species in the mitochondria of HepG2 cells. Toxicol In Vitro. 2012;26(5):709–17. doi: 10.1016/j.tiv.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adiopose tissue explants and adipocytes. Environ Health Perspect. 2008;116(12):1642–7. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Frankfurt M, Luine V. Estrogen-induced memory enhancements are blocked by acute bisphenol A in adult female rats: role of dendritic spines. Endocrinology. 2012;153(7):3357–67. doi: 10.1210/en.2012-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques BE, Montgomery WH, 4th, Uribe PM, Yatteau A, Asuncion JD, Resendiz G, Matsui JI, Dabdoub A. The role of Wnt/β-catenin signaling in proliferation and regeneration of the developing basilar papilla and lateral line. Dev Neurobiol. 2014;74(4):438–56. doi: 10.1002/dneu.22134. [DOI] [PubMed] [Google Scholar]
- Ji K, Hong S, Kho Y, Choi K. Effects of bisphenol s exposure on endocrine functions and reproduction of zebrafish. Environ Sci Technol. 2013;47(15):8793–800. doi: 10.1021/es400329t. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Xia W, Zhu Y, Li X, Wang D, Liu J, Chang H, Li G, Xu B, Chen X, Li Y, Xu S. Mitochondiral dysfuction in early life resulted from perinatal bisphenol A exposure contributes to hepatic steatosis in rat offspring. Toxicol Lett. 2014;228(2):85–92. doi: 10.1016/j.toxlet.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Jones BA, Watson NV. Perinatal BPA exposure demasculinizes males in measures of affect but has no effect on water maze learning in adulthood. Horm Behav. 2012;61(4):605–10. doi: 10.1016/j.yhbeh.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Kim K, Son TG, Kim SJ, Kim HS, Kim TS, Han SY, Lee J. Suppressive effects of bisphenol A on the proliferation of neural progenitor cells. J Toxicol Environ Health A. 2007;70(15–16):1288–95. doi: 10.1080/15287390701434216. [DOI] [PubMed] [Google Scholar]
- Kishida M, McLellan M, Miranda JA, Callard GV. Estrogen and xenoestrogens upregulate the brain aromatase isoform (P450aromB) and perturb markers of early development in zebrafish (Danio rerio) Comp Biochem Physiol B Biochem Mol Biol. 2001;129(2–3):261–8. doi: 10.1016/s1096-4959(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR. Neurotoxicity of persistent organic pollutants: possible mode(s) of action and further considerations. Dose Response. 2006;3(3):273–305. doi: 10.2203/dose-response.003.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpin DW, Furlong ET, Meyter MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999-2000: a national reconnaissance. Environ Sci Technol. 2002;36(6):1202–11. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Kopke RD, Liu W, Gabaizadeh R, Jacono A, Feghali J, Spray D, Carcia P, Steinman H, Malgrange B, Ruben RJ, Rybak L, Van de Water TR. Use of organotypic cultures of Corti’s organ to study the protective effects of antioxidant molecules on cisplatin-induced damage of auditory hair cells. Am J Otol. 1997;18(5):559–71. [PubMed] [Google Scholar]
- Kouzmenko AP, Takeyama K, Ito S, Furutani T, Sawatsubashi S, Maki A, Suzuki E, Kawasaki Y, Akiyama T, Tabata T, Kato S. Wnt/beta-catenin and estrogen signaling converge in vivo. J Biol Chem. 2004;279(39):40255–8. doi: 10.1074/jbc.C400331200. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Dugsnuk K, Franks B, Madrid J, Miller RL, Perera FP, Champagne FA. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci U S A. 2013;110(24):9956–61. doi: 10.1073/pnas.1214056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara R, Kawaguchi S, Kohara Y, Cui H, Yamashita K. J Perinatal exposure to low-dose bisphenol A impairs spatial learning and memory in male rats. Pharmacol Sci. 2013;123(2):132–9. doi: 10.1254/jphs.13093fp. [DOI] [PubMed] [Google Scholar]
- Lahnsteiner F, Berger B, Kletzl M, Weismann T. Effect of bisphenol A on maturation and quality of semen and eggs in the brown trout, Salmo trutta f. fario. Aquat Toxicol. 2005;75(3):213–24. doi: 10.1016/j.aquatox.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Lam SH, Hlaing MM, Zhang X, Yan C, Duan Z, Zhu L, Ung CY, Mathavan S, Ong CN, Gong Z. Toxicogenomic and phenotypic analyses of bisphenol-A early-life exposure toxicity in zebrafish. PLoS One. 2011;6(12):e28273. doi: 10.1371/journal.pone.0028273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;3000(11):1303–10. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- Lakind JS, Naiman DQ. Daily intake of bisphenol A and potential sources of exposure: 2005-2006 National Health and Nutrition Examination Survey, 2011. J Expo Sci Environ Epidemiol. 2011;21:272–279. doi: 10.1038/jes.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim YK, Shin TY, Kim SH. Neurotoxic effects of bisphenol AF on calcium-induced ROS and MAPKs. Nerutox Res. 2013;23(3):249–59. doi: 10.1007/s12640-012-9353-4. [DOI] [PubMed] [Google Scholar]
- Lein PJ, Yang D, Bachstetter AD, Tilson HA, Harry GJ, Mervis RF, Kodavanti PR. Ontogenetic alterations in molecular and structural correlates of dendritic growth after developmental exposure to polychlorinated biphenyls. Environ Health Perspect. 2007;115:556–563. doi: 10.1289/ehp.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Page Y, Diotel N, Vaillant C, Pellegrini E, Anglade I, Mérot Y, Kah O. Aromatase, brain sexualization and plasticity: the fish paradigm. Eur J Neurosci. 2010;32(12):2105–15. doi: 10.1111/j.1460-9568.2010.07519.x. [DOI] [PubMed] [Google Scholar]
- Le Prell CG, Ojano-Dirain C, Rudnick EW, Nelson MA, DeRemer SJ, Prieskorn DM, Miller JM. Assessment of nutrient supplement to reduce gentamicin-induced ototoxicity. J Assoc Res Otolaryngol. 2014;15(3):375–93. doi: 10.1007/s10162-014-0448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesiak A, Zhu M, Chen H, Appleyard SM, Impey S, Lein PJ, Wayman GA. The environmental neurotoxicant PCB 95 promotes synaptogenesis via ryanodine receptor-dependent miR132 upregulation. J Neurosci. 2014;34(3):717–725. doi: 10.1523/JNEUROSCI.2884-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linbo TL, Stehr CM, Incardona JP, Scholz NL. Dissolved copper triggers cell death in the peripheral mechanosensory system of larval fish. Environ Toxicol Chem. 2006;25(2):597–603. doi: 10.1897/05-241r.1. [DOI] [PubMed] [Google Scholar]
- Liao C, Liu F, Alomirah H, Loi VD, Mohd MA, Moon HB, Nakata H, Kannan K. Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures. Environ Sci Technol. 2012;46(12):6860–6. doi: 10.1021/es301334j. [DOI] [PubMed] [Google Scholar]
- Lombarte A, Yan HY, Popper An, Chang JS, Platt C. Damage and regeneration of hair cell ciliary bundles in a fish ear following treatment with gentamicin. Hear Res. 1993;64(2):166–74. doi: 10.1016/0378-5955(93)90002-i. [DOI] [PubMed] [Google Scholar]
- Lu SY, Chang WJ, Sojinu SO, Ni HG. Bisphenol A in supermarket receipts and its exposure to human in Shenzhen, China. Chemosphere. 2013;92(9):1190–4. doi: 10.1016/j.chemosphere.2013.01.096. [DOI] [PubMed] [Google Scholar]
- Ma EY, Rubel EW, Raible DW. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J Neurosci. 2008;28(9):2261–73. doi: 10.1523/JNEUROSCI.4372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie SM, Raible DW. Proliferative regeneration of zebrafish lateral line hair cells after different ototoxic insults. PLoS One. 2012;7(10):e47257. doi: 10.1371/journal.pone.0047257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, van Netten SM, Kros CJ. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J Physiol. 2005;567(Pt 2):505–21. doi: 10.1113/jphysiol.2005.085951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marot M, Uziel A, Romand R. Ototoxicty of kanamycin in developing rats: relationship with the onset of the auditory function. Hear Res. 1980;2(2):111–3. doi: 10.1016/0378-5955(80)90032-5. [DOI] [PubMed] [Google Scholar]
- McKinney JD, Waller CL. Polychlorinated biphenyls as hormonally active structural analogues. Environ Health Perspect. 1994;102(3):290–7. doi: 10.1289/ehp.94102290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JY, Kim R, Min KB. Serum polychlorinated biphenyls concentrations and hearing impairment in adults. Chemosphere. 2014;102:6–11. doi: 10.1016/j.chemosphere.2013.11.046. [DOI] [PubMed] [Google Scholar]
- Myers JP, Zoeller TR, vom Saal FS. A clash of old and new scientific concepts in toxicity, with important implications for public health. Environ Health Perspect. 2009;117(11):1652–5. doi: 10.1289/ehp.0900887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami SL, Cunningham LL, Werner LA, Bauer E, Pujol R, Raible DW, Rubel EW. Developmental differences in susceptibility to neomycin-induced hair cell death in the lateral line neuromasts of zebrafish (Danio rerio) Hear Res. 2003;186(1–2):47–56. doi: 10.1016/s0378-5955(03)00259-4. [DOI] [PubMed] [Google Scholar]
- Naderi M, Wong MYL, Gholami F. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquat Toxicol. 2014;148:195–203. doi: 10.1016/j.aquatox.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Itoh K, Yoshimoto K, Sugimoto T, Fushiki S. Prenatal and lactational exposure to low-doses of bisphenol A alters brain monoamine concentration in adult mice. Neurosci Lett. 2010;484(1):66–70. doi: 10.1016/j.neulet.2010.08.021. [DOI] [PubMed] [Google Scholar]
- Namdaran P, Reinhart KE, Owens KN, Raible DW, Rubel EW. Identification of modulators of hair cell regeneration in the zebrafish lateral line. J Neurosci. 2012;32(10):3516–28. doi: 10.1523/JNEUROSCI.3905-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness DK, Schantz SL, Moshtaghian J, Hansen LG. Effects of perinatal exposure to specific PCB congeners on thyroid hormone concentrations and thyroid histology in the rat. Toxicol Lett. 1993;68(3):311–23. doi: 10.1016/0378-4274(93)90023-q. [DOI] [PubMed] [Google Scholar]
- Owens KN, Cunningham DE, MacDonald G, Rubel EW, Raible DW, Pujol R. Ultrastructural analysis of aminoglycoside-induced hair cell death in the zebrafish lateral line reveals an early mitochondrial response. J Comp Neurol. 2007;502(4):522–43. doi: 10.1002/cne.21345. [DOI] [PubMed] [Google Scholar]
- Owens Kn, Coffin AB, Hong LS, Bennett KO, Rubel EW, Raible DW. Response of mechanosensory hair cells of the zebrafish lateral line to aminoglycosides reveals distinct cell death pathways. Hear Res. 2009;253(1–2):32–1. doi: 10.1016/j.heares.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou HC, Raible DW, Rubel EW. Cisplatin-induced hair cell loss in zebrafish (Danio rerio) lateral line. Hear Res. 2007;233(1–2):46–53. doi: 10.1016/j.heares.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A, Gin KY, Lin AY, Reinhard M. Impacts of emerging organic contaminants on freshwater resources: review of recent occurrences, sources, fate and effects. Sci Total Environ. 2010;408(24):6062–9. doi: 10.1016/j.scitotenv.2010.09.026. [DOI] [PubMed] [Google Scholar]
- Parinov S, Kondrichin I, Korzh V, Emelyanov A. Tol2 transposon-mediated enhancer trap to identify developmentally regulated zebrafish genes in vivo. Dev Dyn. 2004;231(2):449–59. doi: 10.1002/dvdy.20157. [DOI] [PubMed] [Google Scholar]
- Poon E, Powers BE, McAlonan RM, Ferguson DC, Schantz SL. Effects of developmental exposure to polychlorinated biphenyls and/or polybrominated diphenyl ethers on cochlear function. Toxicol Sci. 2011;124(1):161–8. doi: 10.1093/toxsci/kfr214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers BE, Poon E, Sable HJ, Schantz SL. Developmental exposure to PCBs, MeHg, or both: long-term effects on auditory function. Environ Health Perspect. 2009;117(7):1101–7. doi: 10.1289/ehp.0800428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raible DW, Kruse GJ. Organization of the lateral line system in embryonic zebrafish. J Comp Neurol. 2000;421(2):189–98. [PubMed] [Google Scholar]
- Richardson GP, Russell IJ. Cochlear cultures as a model system for studying aminoglycoside induced ototoxicity. Hear Res. 1991;53(2):293–311. doi: 10.1016/0378-5955(91)90062-e. [DOI] [PubMed] [Google Scholar]
- Rosenmai AK, Dybdahl M, Pedersen M, Alice van Vugt-Lussenburg BM, Wedebye EB, Taxvig C, Vinggaard AM. Are structural analogues to bisphenol a safe alternatives? Roxicol Sci. 2014;139(1):35–47. doi: 10.1093/toxsci/kfu030. [DOI] [PubMed] [Google Scholar]
- Rusch A, Ng L, Goodyear R, Oliver D, Lisoukov I, Vennstrom B, Richardson G, Kelley MW, Forrest D. Retardation of cochlear maturation and impaired hair cell function caused by deletion of all known thyroid hormone receptors. J Neurosci. 2001;21(24):9792–800. doi: 10.1523/JNEUROSCI.21-24-09792.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240(4860):1774–6. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Santos F, MacDonald G, Rubel EW, Raible DW. Lateral line hair cell maturation is a determinant of aminoglycoside susceptibility in zebrafish (Danio rerio) Hear Res. 2006;213(1–2):25–33. doi: 10.1016/j.heares.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Sha SH, Schacht J. Salicylate attenuates gentamicin-induced ototoicity. Lab Investig. 1999b;79:807–813. [PubMed] [Google Scholar]
- Sha SH, Schacht J. Antioxidants attenuate gentamicin-induced free radical formation in vitro and ototoxicity in vivo: D-methionine is a potential protectant. Hear Res. 2000;142:34–40. doi: 10.1016/s0378-5955(00)00003-4. 2000. [DOI] [PubMed] [Google Scholar]
- Slattery EL, Oshima K, Heller S, Warchol ME. Cisplatin exposure damages resident stem cells of the mammalian inner ear. Dev Dyn. 2014;243(10):1328–1337. doi: 10.1002/dvdy.24150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohoni P, Tyler CR, Hurd K, Caunter J, Hetheridge M, Williams T, Woods C, Evans M, Toy R, Gargas M, Sumpter JP. Reproductive effects of long-term exposure to Bisphenol A in the fathead minnow (Pimephales promelas) Environ Sci Technol. 2001;35(14):2917–25. doi: 10.1021/es000198n. [DOI] [PubMed] [Google Scholar]
- Sone K, Hinago M, Kitayama A, Morokuma J, Ueno N, Watanabe H, Iguchi T. Effects of 17beta-estradiol, nonylphenol, and bisphenol-A on developing Xenopus laevis embryos. Gen Comp Endocrinol. 2004;138(3):228–36. doi: 10.1016/j.ygcen.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Stahl LL, Snyder BD, Olsen AR, Pitt JL. Contaminants in fish tissue from US lakes and reservoirs: a national probabilistic study. Environ Monit Assess. 2009;150(1–4):3–19. doi: 10.1007/s10661-008-0669-8. [DOI] [PubMed] [Google Scholar]
- Staples CA, Tilghman Hall A, Friederich U, Caspers N, Klecka GM. Early life-stage and multigeneration toxicity study with bisphenol A and fathead minnows (Pimephales promelas) Ecotoxicol Environ Saf. 2011;74(6):1548–57. doi: 10.1016/j.ecoenv.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Steyger PS, Peters SL, Rehling J, Hordichok A, Dai CF. Uptake of gentamicin by bullfrog saccular hair cells in vitro. J Assoc Res Otolaryngol. 2003;4(4):565–78. doi: 10.1007/s10162-003-4002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suli A, Watson GM, Rubel EW, Raible DW. Rheotaxis in larval zebrafish is mediated by lateral line mechanosensory hair cells. PLoS One. 2012;7(2):e29727. doi: 10.1371/journal.pone.0029727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AJ, Hailey DW, Stawicki TM, Wu P, Coffin AB, Rubel EW, Raible DW, Simon JA, Ou HC. Functional mechanotransduction is required for cisplatin-induced hair cell death in the zebrafish lateral line. J Neurosci. 2013;33(10):4405–14. doi: 10.1523/JNEUROSCI.3940-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton C, Parng C. The use of zebrafish for assessing ototoxic and otoprotective agents. Hear Res. 2005;208(1–2):79–88. doi: 10.1016/j.heares.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Van Trump WJ, McHenry MJ. The morphology and mechanical sensitivity of lateral line receptors in zebrafish larvae (Danio rerio) J Exp Biol. 2008;211(Pt 13):2105–15. doi: 10.1242/jeb.016204. [DOI] [PubMed] [Google Scholar]
- Viberg H, Lee I. A single exposure to bisphenol A alters the levels of important neuroproteins in adult male and female mice. Neurotoxicology. 2012;33(5):1390–5. doi: 10.1016/j.neuro.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Vu AA, Nadaraja GS, Huth ME, Luk L, Kim J, Chai R, Ricci AJ, Cheng AG. Integrity and regeneration of mechanotransduction machinery regulate aminoglycoside entry and sensory cell death. PLoS One. 2013;8(1):e54794. doi: 10.1371/journal.pone.0054794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Ghysen A, Asakawa K, Abe G, Ishitani T, Kawakami K. Wnt/Dkk negative feedback regulates sensory organ size in zebrafish. Curr Biol. 2013;23(16):1559–65. doi: 10.1016/j.cub.2013.06.035. [DOI] [PubMed] [Google Scholar]
- Wang X, Dong Q, Chen Y, Jiang H, Xiao Q, Wang Y, Li W, Bai C, Huang C, Yang D. Bisphenol A affects axonal growth, musculature and motor behavior in developing zebrafish. Aquat Toxicol. 2013;142–143:104–13. doi: 10.1016/j.aquatox.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Bose DD, Yang D, Lesiak A, Bruun D, Impey S, Ledoux V, Pessah IN, Lein PJ. PCB-95 modulates the calcium-dependent signaling pathway responsible for activity-dependent dendritic growth. Environ Health Perspect. 2012a Jul;120(7):1003–1009. doi: 10.1289/ehp.1104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Yang D, Bose DD, Lesiak A, Ledoux V, Bruun D, Pessah IN, Lein PJ. PCB-95 promotes dendritic growth via ryanodine receptor-dependent mechanisms. Envrion Health Perspect. 2012b;120(7):997–1002. doi: 10.1289/ehp.1104832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A Guide for Laboratory Use of Zebrafish (Danio Rerio) 4th. University of Oregon Press; Eugene: 2000. [Google Scholar]
- Wiseman S, Vijayan MM. Arocolor 1254 disrupts liver glycogen metabolism and enhances actue stressor-mediated glycogenolysis in rainbow trout. Comp Biochem Physiol C Toxicol Pharmacol. 2011;154(3):254–60. doi: 10.1016/j.cbpc.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Wolstenholme JT, Edwards M, Shetty SR, Gatewood JD, Taylor JA, Rissman EF, Connelly JJ. Gestsational exposure to bisphenol a produces transgenerational changes in behaviors and gene expression. Endocrinology. 2012;153(8):3828–38. doi: 10.1210/en.2012-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Goldsby JA, Rissman EF. Transgenerational effects of prenatal bisphenol A on social recognition. Horm Behav. 2013;64(5):833–9. doi: 10.1016/j.yhbeh.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak Al, Bulayeva NN, Watson CS. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-α-mediated Ca2+ fluxes and prolactin release in GH2/B6 pituitary tumor cells. Environ Health Perspect. 2005;113:431–439. doi: 10.1289/ehp.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T, Roeser T, Staub W, Baier H. A GFP-based genetic screen reveals mutations that disrupt the architecture of the zebrafish retinotectal projection. Development. 2005;132(13):2955–67. doi: 10.1242/dev.01861. [DOI] [PubMed] [Google Scholar]
- Yamasoba T, Dolan DF. The medial cochlear efferent system does not appear to contribute to the development of acquired resistance to acoustic trauma. Hear Res. 1998;120(1–2):143–51. doi: 10.1016/s0378-5955(98)00054-9. [DOI] [PubMed] [Google Scholar]
- Yang D, Kim KH, Phimister A, Bachstetter AD, Ward TR, Stackman RW, Mervis RF, Wisniewki AB, Klein SL, Kodavanti PR, Anderson KA, Wayman G, Pessah IN, Lein PJ. Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ Health Perspect. 2009;117:426–435. doi: 10.1289/ehp.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yamoah EN, Gillespie PG. Regeneration of broken tip links and restoration of mechanical transduction in hair cells. Proc Natl Acad Sci U S A. 1996;93(26):15469–74. doi: 10.1073/pnas.93.26.15469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsarnovsky A, Le HH, Wang HS, Belcher SM. Ontogeny of rapid estrogen-mediated extracellular signal-regulated kinase signaling in the rat cerebellar cortex: potent non-genomic agonist and endocrine disrupting activity of the xenoestrogen bisphenol A. Endocrinology. 2005;146(12):5388–5396. doi: 10.1210/en.2005-0565. [DOI] [PubMed] [Google Scholar]


