Abstract
Coral reefs face many stressors associated with global climate change, including increasing sea surface temperature and ocean acidification. Excavating sponges, such as Cliona spp., are expected to break down reef substrata more quickly as seawater becomes more acidic. However, increased bioerosion requires that Cliona spp. maintain physiological performance and health under continuing ocean warming. In this study, we exposed C. orientalis to temperature increments increasing from 23 to 32 °C. At 32 °C, or 3 °C above the maximum monthly mean (MMM) temperature, sponges bleached and the photosynthetic capacity of Symbiodinium was compromised, consistent with sympatric corals. Cliona orientalis demonstrated little capacity to recover from thermal stress, remaining bleached with reduced Symbiodinium density and energy reserves after one month at reduced temperature. In comparison, C. orientalis was not observed to bleach during the 2017 coral bleaching event on the Great Barrier Reef, when temperatures did not reach the 32 °C threshold. While C. orientalis can withstand current temperature extremes (<3 °C above MMM) under laboratory and natural conditions, this species would not survive ocean temperatures projected for 2100 without acclimatisation or adaptation (≥3 °C above MMM). Hence, as ocean temperatures increase above local thermal thresholds, C. orientalis will have a negligible impact on reef erosion.
Introduction
Increasing global temperatures are requiring organisms to acclimate to greater thermal extremes, migrate, or suffer reduced fitness and, potentially, local extirpation. The earth’s climate is already estimated to be 0.85 °C warmer than it was in 1880, which is affecting both terrestrial and marine ecosystems1. Much of the thermal energy (~60%) associated with warming has been absorbed by the oceans, resulting in melting sea ice, rising sea levels1, and record temperatures in tropical waters2,3. Ocean warming has already resulted in extensive coral mortality3,4, as evidenced in 2015/2016, when extreme temperatures led to consecutive mass coral bleaching events around the world2,3.
Corals contain photosynthetic dinoflagellates (genus Symbiodinium) that provide them with organic carbon5. However, the symbiosis is thermally sensitive and exposure to elevated temperature disrupts Symbiodinium photosynthesis6 and causes coral bleaching7,8. Some coral species and Symbiodinium ‘types’ are more thermally tolerant than others9–11, but even tolerant genotypes can be overwhelmed by severe temperature stress3. Nonetheless, in some cases, previous exposure to high temperature or association with tolerant Symbiodinium can lead to greater thermal tolerance of the coral symbiosis9,10,12,13 and accelerate recovery following bleaching9,14,15.
In comparison to reef-building corals, sponges are thought to be relatively tolerant of increasing sea surface temperatures16,17. In particular, some bioeroding sponges can tolerate temperatures that induce bleaching in sympatric corals18–22. Bioeroding sponges, principally the genus Cliona, are important members of coral reef communities as they erode the limestone substratum by reducing the pH at the sponge:substratum interface23, dissolving the substratum, and extracting microscopic ‘chips’ of calcium carbonate24,25. Like corals, many bioeroding sponge species form symbioses with photosynthetic Symbiodinium and photosynthesis enhances their growth and bioerosion26–28. However, while dependence on Symbiodinium may increase the thermal sensitivity of Cliona, little is known about how these sponges will tolerate predicted incremental temperature increases or whether they can recover from extreme thermal stress29,30.
Experimental research combining elevated temperature and reduced pH has shown that sponge bioerosion rates will likely increase under conditions of ocean acidification31–35. However, warming can have negative effects on bioeroding sponges, including bleaching or necrosis; and it is likely that these negative effects will override all other environmental factors29,30,32. For instance, under temperature and pH conditions predicted for 2100, the bioeroding sponge Cliona orientalis bleaches, and the associated reduction in photosynthetic productivity results in a negative energy budget for the sponge despite accelerated rates of erosion29,34. Warming was subsequently identified as the primary stressor inducing bleaching in a bioeroding sponge30. However, temperature tolerance appears to vary among bioeroding sponge species as bleaching or mortality was not observed in all studies31,36. Therefore, the net effect of climate change on bioeroding sponges, and on their erosion rates, appears to be species-specific.
Identifying thermal thresholds under near-future warming requires measurement of performance across a broad range of incremental temperature changes. This incremental approach enables a holistic understanding of temperature effects by allowing quantification of the optimal temperature for peak physiological performance, along with derivation of sub-lethal and lethal temperature thresholds37,38. A similar approach has been applied to corals to quantify adaptation to local thermal regimes39 and to determine how coral respiration and photosynthesis varies with temperature40. Here, we experimentally assessed the ability of C. orientalis to tolerate incrementally increasing sea surface temperatures between 23–32 °C, which represents the annual temperature range for the studied sponges (22–30 °C) and warmer temperatures predicted for 2100 (31, 32 °C)1. In addition, we monitored recovery from temperature stress to evaluate the impact of thermal exposure on sponge survival. Photosynthetic performance of Symbiodinium and energy reserves of the sponge were quantified to identify temperature optima and define thermal thresholds. To contextualize the laboratory experiment, we assessed bleaching severity for C. orientalis during the 2017 mass coral bleaching event.
Results
Response to laboratory thermal exposure
Bleaching and Symbiodinium identity
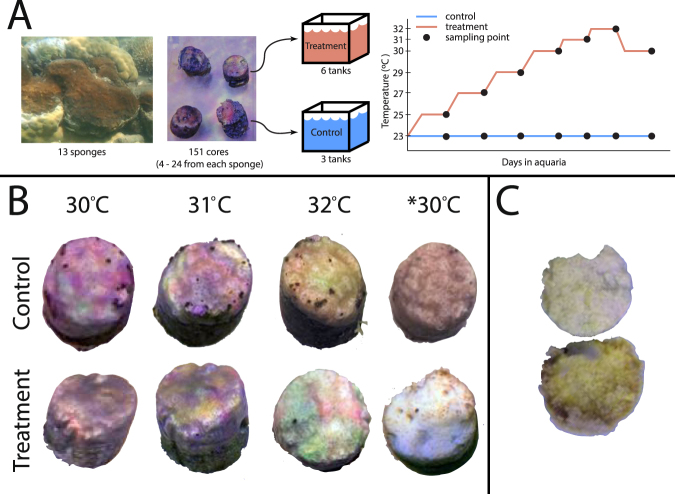
Cliona orientalis survived temperatures up to 31 °C with no visual signs of bleaching and little evidence of compromised health, such as discolouration or tissue regression (Fig. 1B). However, at 32 °C, more than 50% of the cores became visibly bleached within three to five days and this increased to 70% bleached after 8 days (Fig. 1B). The condition of some cores also visibly deteriorated in the four weeks following bleaching, including the presence of algae at the margin of the cores (Fig. 1C). The narrow bleaching threshold of 32 °C identified for C. orientalis is 2.9 °C above the maximum monthly mean at Orpheus Island. A low level of mortality occurred in both temperature treatments but was constrained to cores from a few specific sponge genotypes.
Figure 1.
(A) Sponge sampling and temperature treatments. Healthy and bleached Cliona orientalis cores. (B) A time-series of two cores from the control and heated treatments, respectively. Cores in the heated treatment visibly bleached at 32 °C and had not recovered four weeks later at *30 °C. (C) The top surface and pinacoderm of a bleached core at the end of the experiment. The pinacoderm of bleached cores (interior) remained healthy despite the absence of Symbiodinium for four weeks.
Only a single Symbiodinium ITS2 type was associated with C. orientalis, regardless of temperature treatment or bleaching state. ITS2 sequences clustered into 61 OTUs at 97% sequence similarity, but only 21 of these matched the Symbiodinium database, and only 9 were confidently matched with bitscores greater than 100. Of the nine Symbiodinium OTUs, one OTU comprised 96% of the Symbiodinium sequences and was the most abundant OTU in every sponge core. The ITS2 sequence of the dominant OTU was identical to Symbiodinium clade G previously sequenced from C. orientalis (Genbank accession JQ247051) and which was recently described as Symbiodinium endoclionum41. One other OTU occurred in 97% of the cores and was also most similar to Symbiodinium clade G from C. orientalis, differing from the dominant ITS2 sequence by one insertion of eight nucleotide substitutions. The remaining Symbiodinium OTUs were most similar to Symbiodinium clades A, B, or C, however these OTUs comprised <1% of total sequences and occurred in <20% of the samples.
Photosynthesis and respiration
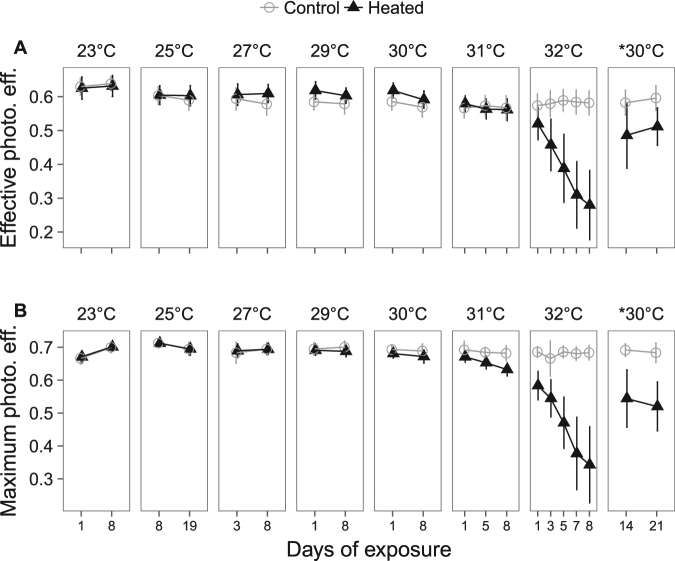
Temperature effects were interpreted as significant temperature*treatment interactions which indicate the temperatures at which heated sponge cores responded differently than control cores (Table 3). Photosynthesis was largely unaffected by temperatures up to and including 31 °C, but became inhibited at 32 °C. Sponges at 27 and 29 °C had higher effective (∆F/Fm’), but not maximum (Fv/Fm), photochemical efficiency than sponges maintained at 23 °C (Fig. 2; eff. 27 °C: z = −4.5, p < 0.01; eff. 29 °C: z = −3.2, p = 0.01). At 29–31 °C, sponges had significantly lower maximum photochemical efficiencies than control sponges (p < 0.01), but the differences were small, and C. orientalis maintained 93% of maximum photochemical efficiency of control sponges at 31 °C. However, at 32 °C, maximum and effective photochemical efficiencies were 52% and 50% of control sponges, respectively (Table 4). As with photochemical efficiency, the photosynthetic rate (gross oxygen production) did not differ from controls at temperatures up to and including 31 °C (Fig. 3A; p > 0.86) but was 43% lower than controls in sponges exposed to 32 °C (Table 4).
Table 3.
Statistical results of the laboratory experiment. Parameters tested include maximum photochemical efficiency (Fv/Fm), and effective photochemical efficiency (∆F/Fm’), photosynthetic rate (P), respiration rate (R), the ratio P:R, Symbiodinium density (Symb.), chlorophyll a and c density (chl), protein content, and ash-free dry weight (AFDW).
| Trans. | Out. rem. | Treatment (num. df = 1) | Temperature (num. df = 7) | Treatment: Temperature (num. df = 7) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | MS | D. df | F | p | SS | MS | D. df | F | p | SS | MS | D. df | F | p | |||
| Photosynthesis | |||||||||||||||||
| Fv/Fm | p/(1 − p) | N | 18.9 | 18.9 | 686.1 | 676.4 | <0.01 | 35.5 | 5.1 | 679.0 | 181.9 | <0.01 | 25.1 | 3.6 | 677.9 | 128.5 | <0.01 |
| ∆F/Fm’ | p/(1 − p) | N | 0.7 | 0.7 | 9.8 | 106.2 | <0.01 | 23.1 | 3.3 | 674.0 | 106.2 | <0.01 | 11.3 | 1.6 | 672.5 | 52.0 | <0.01 |
| Gross P | — | N | <0.1 | <0.1 | 121.4 | 4.0 | <0.01 | 0.3 | <0.1 | 24.1 | 128.3 | <0.01 | <0.1 | <0.1 | 7.6 | 124.7 | <0.01 |
| R | — | Y | <0.1 | <0.1 | 121.0 | 18.9 | <0.01 | <0.1 | <0.1 | 128.8 | 14.9 | <0.01 | <0.1 | <0.1 | 125.0 | 3.0 | 0.01 |
| Gross P:R | — | Y | 14.3 | 14.3 | 119.9 | 41.3 | <0.01 | 25.3 | 3.6 | 10.5 | 126.2 | <0.01 | 12.9 | 1.8 | 5.3 | 122.8 | <0.01 |
| Sponge condition | |||||||||||||||||
| Symb. | — | N | 18944 | 18944 | 125.2 | 8.5 | <0.01 | 173824 | 24823 | 129.7 | 11.1 | <0.01 | 89443 | 12778 | 127.4 | 5.7 | <0.01 |
| Chl a | — | N | 25624 | 25624 | 123.2 | 19.6 | <0.01 | 121098 | 17300 | 13.2 | 128.4 | <0.01 | 94888 | 13556 | 10.4 | 13556 | <0.01 |
| Chl c | — | N | 990 | 990 | 123.1 | 20.3 | <0.01 | 7072 | 1010 | 20.8 | 128.1 | <0.01 | 4372 | 624.6 | 12.8 | 125.2 | <0.01 |
| Chl a:c | Log | Y | <0.1 | <0.1 | 123.3 | 0.4 | 0.52 | 0.8 | 0.1 | 5.3 | 128.6 | <0.01 | 0.1 | <0.1 | 0.8 | 126.1 | 0.59 |
| Protein | Log | N | 0.8 | 0.8 | 120.5 | 10.4 | <0.01 | 4.0 | 0.6 | 7.9 | 130.2 | <0.01 | 2.3 | 0.3 | 4.6 | 124.8 | <0.01 |
| AFDW | Log | Y | 0.3 | 0.3 | 122.1 | 14.3 | <0.01 | 0.7 | 0.1 | 5.8 | 129.2 | <0.01 | 0.7 | 0.1 | 5.5 | 125.0 | <0.01 |
Units for each measure are provided in Figs 3–4. Data were analysed using a linear mixed model with four components: temperature treatment, temperature, treatment:temperature interaction, and a random intercept for the C. orientalis sponge. Symbiodinium density was analysed with an additional random intercept for each aquarium. Parameters that were significantly affected by the temperature exposure have a significant treatment:temperature interaction term. The table includes the transformation used (Trans.), whether any outlying observations were removed, and statistical estimates: sums of squares (SS), mean square (MS), denominator degrees of freedom (D. df), F ratio (F), and P value (p).
Figure 2.
Photochemical efficiency of Symbiodinium within C. orientalis. Effective (A) and maximum (B) photochemical efficiencies are shown for control cores measured at 23 °C (grey open circles and lines) and heated cores at elevated temperature (black triangles and lines). Panels separate the temperature of the heated treatment. Points represent means and error bars indicate one SD.
Table 4.
Results of post-hoc comparisons.
| Exposure | Recovery | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 32 °C: Heated vs Ctrl. | Heated: *30 vs 32 °C | *30 °C: Heated vs Ctrl | ||||||||
| Min. sig. temp | z | p | z | p | z | p | ||||
| Photosynthesis | ||||||||||
| Fv/Fm | 29 | C > 32 | 27.8 | <0.01 | *30 > 32 | 11.3 | <0.01 | C > H | 14.2 | <0.01 |
| ∆F/Fm’ | 32 | C > 32 | 15.8 | <0.01 | *30 > 32 | 12.2 | <0.01 | C > H | 5.4 | <0.01 |
| Gross P | 32 | C > 32 | 7.8 | <0.01 | *30 > 32 | −5.6 | <0.01 | C > H | 3.7 | <0.01 |
| R | — | — | −0.8 | 0.99 | *30 > 32 | −4.8 | <0.01 | — | 1.2 | 0.89 |
| Gross P:R | 30 | C > 32 | 6.1 | <0.01 | *30 > 32 | 5.8 | <0.01 | C > H | 3.2 | <0.01 |
| Sponge condition | ||||||||||
| Symb. | 32 | C > 32 | 4.3 | <0.01 | — | 1.9 | 0.42 | C > H | 5.3 | <0.01 |
| Chl a | 32 | C > 32 | 3.7 | <0.01 | 32 > *30 | 12.8 | 0.33 | C > H | 8.6 | <0.01 |
| Chl c | 32 | C > 32 | 3.9 | <0.01 | 32 > *30 | 3.33 | <0.01 | C > H | 9.5 | 0.01 |
| Chl a:c | — | — | — | — | — | — | — | — | — | — |
| Protein | 32 | C > 32 | 3.0 | 0.03 | 32 > *30 | 3.2 | 0.01 | C > H | 4.8 | <0.01 |
| AFDW | 32 | C > 32 | 2.8 | 0.05 | 32 > *30 | 4.3 | <0.01 | C > H | 6.6 | <0.01 |
To compare the sensitivity of different parameters, the table includes the lowest temperature increment with a significant difference between heated and control sponges. While post-hoc tests were used to test for differences after each temperature increase, detailed results are presented from 3 post-hoc tests that indicate 1) whether cores heated to 32 °C differed from control cores, 2) whether cores heated to 32 °C differed from cores returned to *30 °C (i.e., recovery), and 3) whether cores reduced to *30 °C differed from controls (i.e., recovery). For each test, the table indicates the direction of the difference between groups, the z value, and p value. p values were corrected for multiple comparisons using a single-step correction. – indicates where there was no difference between treatments. Parameter abbreviations are listed with Table 3.
Figure 3.
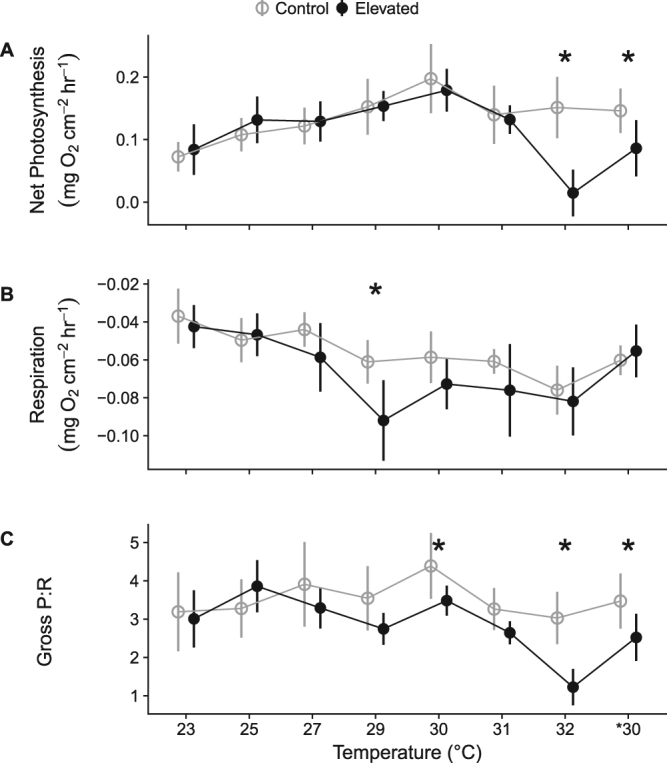
Oxygen flux rates for net photosynthesis (A), respiration (B), and the ratio of gross photosynthesis to respiration (C) for C. orientalis between 23 and 32 °C. Control cores (grey open circles and lines) were all measured at 23 °C, while heated cores (black circles and lines) were sampled at the temperature indicated in the legend. *30 indicates samples that were exposed to 32 °C and then returned to 30 °C for four weeks following bleaching. Points represent means and error bars indicate one standard error. Asterisks indicate temperature increments where sponges in control and heated treatments had significantly different responses.
In contrast to photosynthesis, the effect of thermal exposure on sponge respiration was greatest at 29 °C, where heated sponges had 47% higher respiration rates than control sponges (Fig. 3B; z = −3.4, p < 0.01). At temperatures close to 29 °C (27, 30, and 31 °C), respiration was 28–35% higher than controls, but these differences were not significant (0.09 < p < 0.29). For sponges at 32 °C, respiration rates were similar to control sponges (Table 4). The ratio of gross photosynthesis to respiration (P/R) was affected at a similar temperature as the respiration rates (Fig. 3C). Sponges at 29, 30, and 31 °C had 79% of the sponge P/R of control sponges, coinciding with faster respiration rates (Fig. 3B), but the difference was only statistically significant at 30 °C (z = 3.1, p = 0.01). Sponges at 32 °C had 37% of the P/R of control sponges (Table 4), indicating a loss of productivity of the symbiosis.
Sponge condition
Temperatures less than 32 °C did not significantly affect the condition of C. orientalis and the density of Symbiodinium, chlorophylls a and c2, protein, and organic matter were similar to control sponges (Fig. 4; p > 0.20). At 32 °C, the bleached sponges contained 25% of the Symbiodinium, 35% of chlorophyll a, and 42% of chlorophyll c2 of the control sponges (Table 4). Moreover, sponges at 32 °C contained 83% of the organic matter and 66% of the protein found in control sponges (Table 4), signifying reduced condition of the sponge.
Figure 4.
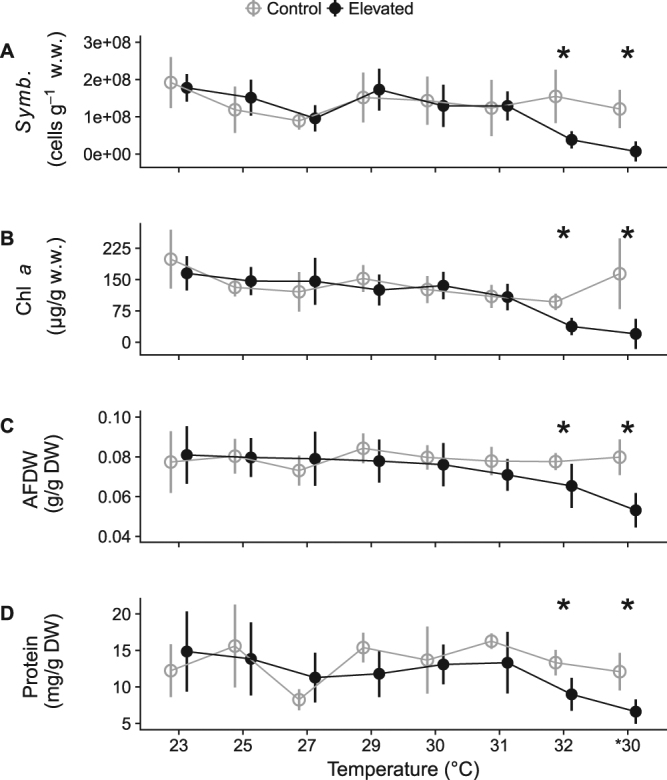
Tissue contents of Symbiodinium (A), chlorophyll a (B), ash-free dry weight (C; AFDW), and protein content (D) for C. orientalis between 23 and 32 °C. Controls (grey open circles and lines) were all sampled at 23 °C while heated cores (black circles and lines) were sampled at the temperature indicated in the legend. *30 indicates samples that were exposed to 32 °C, bleached, and were returned to 30 °C for four weeks. Points represent means and error bars indicate standard error. Asterisks indicate temperature increments where cores in control and heated treatments had significantly different responses.
While the a:c2 ratio decreased over the course of the experiment (Table 3, Temperature), it was not strongly affected by temperature, as heated sponges were 91–107% that of control sponges throughout the experiment and differences between treatments were not significant (Table 3, Treatment:Temperature).
Recovery from laboratory thermal exposure
Bleaching and Symbiodinium identity
The Symbiodinium associated with C. orientalis did not change following bleaching, with all cores dominated by Symbiodinium endoclionum. Four weeks following bleaching, C. orientalis cores appeared white, and both the Symbiodinium density and chlorophyll content revealed that the sponges had not recovered from their bleached state, indicating prolonged holobiont disruption (Fig. 4).
Photosynthesis and respiration
C. orientalis recovered some photosynthetic capacity when returned to *30 °C, with photochemical efficiencies, photosynthetic rates, and P/R being higher than when sponges were at 32 °C (Fig. 3, Table 4). However, this response was likely due to other photosynthetic colonisers as Symbiodinium densities remained low in sponges at *30 °C (Fig. 3). Regardless, recovery of photosynthesis was incomplete, as photochemical efficiencies, photosynthetic rates, and P/R remained lower than control sponges (Fig. 3, Table 4). The respiration rates of sponges returned to *30 °C were higher than the sponges at 32 °C, but not significantly different from controls (Fig. 3, Table 4).
Sponge condition
Tissue contents indicated that the condition of the 32 °C sponges continued to deteriorate after they were returned to *30 °C (Fig. 4, Table 4). Chlorophyll content, organic matter, and protein content were lower in sponges at *30 °C than at 32 °C, but Symbiodinium density was not significantly different (Table 4). In addition, all measured tissue contents remained lower in sponges returned to *30 °C than control sponges (Fig. 4, Table 4).
Field bleaching surveys
A total of 133 C. orientalis sponges, 1891 branching corals, and 1068 massive corals were counted among the six survey sites. No bleached C. orientalis sponges were observed in any of the video transects. In contrast, 83% (±6.0 SD) of branched coral colonies and 51% (±3.2 SD) of massive coral colonies were bleached (Fig. 5B).
Figure 5.
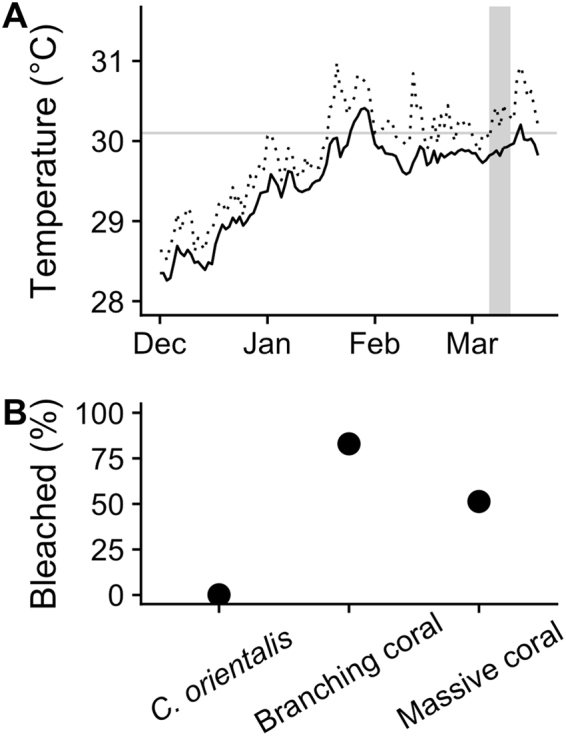
The temperature (A) and bleaching severity (B) during a natural bleaching event in the Palm Islands, Great Barrier Reef, Australia in February-March 2017. The daily mean (solid black line) and maximum (dotted line) temperature for the twelve weeks preceding the surveys are shown. The horizontal grey line indicated the local coral bleaching threshold (30.1 °C) and the vertical grey bar denotes the survey period.
Temperatures did not reach 32 °C during the 2017 bleaching event at Orpheus Island (Fig. 5A). During the two weeks preceding the surveys, daily mean temperatures averaged 29.8 °C at 5.8 m depth (Source: Australian Institute of Marine Science; http://data.aims.gov.au) and, during the 12 weeks preceding the surveys, the cumulative thermal exposure summed to 1 degree heating week (weeks above 30.1 °C). In comparison, the laboratory experiment indicated that C. orientalis bleached at 32 °C after accumulating 2.5 degree heating weeks (3 days at 32 °C; Table 1).
Table 1.
Target and actual temperatures during the laboratory experiment.
| Time point |
Target temperature |
Heated (n = 6) |
Control (n = 3) |
°C above MMM + 1 |
Accumulated DHW |
|---|---|---|---|---|---|
| 17 | 23.0 | 23.5 ± 0.1 | 23.4 ± 0.2 | 0 | 0 |
| 43 | 25.0 | 25.2 ± 0.1 | 23.1 ± 0.1# | 0 | 0 |
| 56 | 27.0 | 27.0 ± 0.1 | 23.3 ± 0.1 | 0 | 0 |
| 71 | 29.0 | 29.0 ± 0.2 | 23.0 ± 0.2 | 0 | 0 |
| 84 | 30.0 | 30.0 ± 0.1 | 23.1 ± 0.1 | 0 | 0 |
| 96 | 31.0 | 30.9 ± 0.1# | 23.2 ± 0.1 | 0.8 | 1.3 |
| 108 | 32.0 | 32.0 ± 0.1 | 23.1 ± 0.2 | 1.9 | 4.4 |
| 144 | *30.0 | 30.1 ± 0.1 | 23.1 ± 0.3 | 0 | 4.4 |
For each target temperature, tank temperatures were recorded every 5 minutes over 8 days (mean ± SD°C). #Indicates where the SD was less than 0.05 °C. °C above the maximum monthly mean (MMM) at Orpheus island (29.1 + 1.0 °C) indicates the thermal anomaly above long-term summer temperatures which was used to calculate the accumulated degree heating weeks (DHW) as the product of the thermal exposure and the thermal duration (°C-weeks).
Discussion
Bioeroding sponges are generally thought to be tolerant of environmental stressors, including elevated temperature, ocean acidification, and eutrophication, raising concerns about increased reef erosion under future projected climate scenarios42,43. Here, we show that incremental increases in ocean temperature up to 30 °C have negligible effects on C. orientalis, but C. orientalis bleaches when exposed to 32 °C, and exhibits little potential for recovery. At the collection site, 32 °C represents an increase of 3 °C above the maximum monthly mean temperature and corresponds to the increase expected under very high greenhouse gas emissions by 2100, but could represent the mean temperature as soon as 2078 (RCP 8.5)1, suggesting that C. orientalis could bleach regularly by the end of this century. The results of this study do not support the hypothesis that bioeroding sponges (particularly those species with photosynthetic symbionts) will play a larger role in structuring future reefs.
Temperature exposure in the laboratory revealed a narrow thermal threshold for C. orientalis, with sponges appearing visibly healthy after 10 days at 31 °C, but bleaching after only 3 days at 32 °C. This narrow threshold is similar to several sympatric coral species that bleached following 1 °C temperature increases between 31 and 33 °C44. In thermally sensitive corals, bleaching coincides with reduced condition and growth7 and C. orientalis exhibited similar negative responses, including a 75% reduction in Symbiodinium density, 17% reduction in organic matter and 44% reduction in protein content of C. orientalis. Few bleached cores exhibited necrosis which had been previously reported in C. orientalis from Orpheus Island after exposure to only 2 °C above MMM. The discrepancy between studies likely results from the faster temperature increases or acute exposures (3–72 h) used in previous research32,45. The thermal threshold identified here is consistent with findings for C. orientalis in the southern GBR, which tolerates exposure to +2.0 °C above MMM (27.3 °C)MMM = 27.3 °C34, but bleaches at +2.7 °C30, and dies at +3.5 °C34. Taken together, these experimental and field results suggest that C. orientalis can tolerate current ocean temperatures, but will have little capacity to cope with the warmer oceans projected for 2100.
The primary cause of coral bleaching is exposure to extreme ocean temperature, although longer exposure to moderate increases in temperature can also induce bleaching46,47. Cumulative thermal exposure is a product of the amount and the duration of stress, which is incorporated into the degree heating weeks (DHW) index, which can be used to accurately predict bleaching3. In the laboratory, C. orientalis bleached after 2.5 DHW, similar to corals that bleach after 2 DHW under natural conditions3. Consistent with our field observations at Orpheus Island, there are few reports of C. orientalis bleaching under natural conditions. In most cases, other Cliona species (C. aprica, C. caribbaea, C. varians, and C. vermifera) have tolerated periods of elevated temperature better than neighbouring corals18–20, including exposures exceeding 31 °C18 and even 33 °C20. In our surveys, C. orientalis did not bleach although temperatures did not exceed 31 °C, which is below the 32 °C thermal threshold identified during our experiment. A 32 °C threshold is consistent with other Cliona-Symbiodinium symbioses, as C. varians was recently reported to bleach when mean temperatures exceeded 31 °C for 10 days48. The combination of a 32 °C laboratory bleaching threshold with the lack of bleaching during the 2017 coral bleaching event suggests that current summer temperatures could lead to faster local erosion rates in the near future.
Coral bleaching is often preceded by disruption of Symbiodinium photosynthesis6,49 which leads to the production of toxic oxygen radicals, which must be neutralized to prevent damage to lipids, proteins and DNA50. The mechanisms of bleaching in sponges may be similar, however, if damage to the photosystems was responsible for triggering bleaching in C. orientalis, the response must have been very rapid: when C. orientalis bleached, Symbiodinium still retained ~66% of Fv/Fm which had only declined for 3 days. After eight days of exposure to 32 °C, the photosynthetic capacity of the symbiosis was diminished, coinciding with a loss of Symbiodinium and chlorophyll. Similar effects have previously been observed in bleached C. orientalis30,34, and scleractinian corals, where a loss of Symbiodinium coincides with a loss of lipids, proteins, and organic matter51,52. In addition, bleaching can disrupt the bacterial symbioses in C. orientalis53 and scleractinian corals54.
Prior to C. orientalis bleaching, there was some evidence that respiration rates increased (29–31 °C) and that energy reserves were reduced (31 °C), suggesting that the sponges expend resources to maintain their symbiosis at sub-bleaching temperatures. Respiration in C. orientalis was fastest at intermediate temperatures, likely contributing to the significant decline in the productivity of the symbiosis. Nevertheless, bleached C. orientalis had similar respiration rates to control sponges despite their reduced condition30. The absence of an effect of bleaching (i.e., absence of Symbiodinium) on respiration rates highlights the need to separate measurement of host and Symbiodinium respiration55. Based on their low biomass relative to the biomass of the sponge tissue, it is likely that Symbiodinium makes a minor contribution to overall respiration, and other factors such as pumping or feeding rates may dictate energetic demand and respiration in thermally stressed sponges56.
The ability to persist in warming oceans will depend upon recovery of symbionts and energy reserves, before exposure to any subsequent bleaching-inducing temperatures52. After C. orientalis bleached at 32 °C, the sponges did not recover during four weeks at *30 °C, with no recovery of the symbiosis or sponge condition. The only parameter that changed during recovery was photosynthesis, where the rates of oxygen production and photochemical efficiency were higher in sponges returned to *30 °C than in sponges at 32 °C. However, based on visual observations and the lack of recovery of Symbiodinium, relatively high photochemical efficiency was likely due to fouling by photosynthetic epibionts rather than a re-establishment of the Symbiodinium population. In corals, recovery can take between 1.5 and 10 months and some species do not recover within 12 months9,51,52,57,58. Our experiment indicated that C. orientalis did not recover Symbiodinium under aquarium conditions, but the availability of Symbiodinium may have limited recovery. In other laboratory studies, C. orientalis have recovered Symbiodinium following irradiance-induced bleaching59,60, but further study is necessary to determine whether C. orientalis can regain symbionts following thermal bleaching under natural conditions. Observations in the Florida Keys, USA indicate that C. varians can recover from thermal bleaching (M. Hill pers. comm.), although some Symbiodinium likely remained within the sponge48.
Association with tolerant Symbiodinium, especially multiple types of Symbiodinium, can aid recovery from coral bleaching14. Here, all C. orientalis cores harboured the same symbiont, S. endoclionum41, and exhibited little flexibility in their symbiotic association before or after bleaching. This may make C. orientalis more vulnerable to warming than reef taxa that can associate with multiple Symbiodinium clades10,12, as C. orientalis harbours S. endoclionum over a large geographic range41. Little is known about the genetic diversity or physiology of clade G Symbiodinium, which have only have been found in bioeroding sponges61–63, foraminifera64, and one octocoral species65. A physiological comparison of Clade G to other Symbiodinium has suggested that the Clade G from C. orientalis are more thermally tolerant than the clade C or D inhabiting scleractinian corals45. However, here we have refined the thermal threshold, showing that while the clade G symbiont in C. orientalis can tolerate current summer temperatures (<32 °C), photosynthesis is impaired at predicted future temperatures (≥32 °C).
Recent mass coral bleaching events are a clear indication that ocean warming is a primary threat to reef corals3 and accelerated bioerosion by Clionaid sponges under ocean acidification would further compound the adverse outcomes of climate change. However, here we show that while the symbiosis between C. orientalis and its associated Symbiodinium tolerates current maximum sea surface temperatures, the partnership breaks down as sea surface temperatures reach 32 °C. A relatively high tolerance of present day temperature extremes may benefit C. orientalis via coral mortality and increased substratum availability in the short term22,66, however bioeroding sponges with Symbiodinium will be severely affected by ocean temperatures expected by 2100.
Methods
Laboratory experiment
Thirteen Cliona orientalis (Thiele, 1900) sponges were collected at 2–4 m depth from Little Pioneer Bay on Orpheus Island, Queensland, Australia (18°37′40″S, 146°29′36″E) in June 2015 (Marine Parks Permit G12/35236.1). Sponges were transported by road in 60 L plastic aquaria to the National Sea Simulator (SeaSim) at the Australian Institute of Marine Science in Townsville, Queensland, where they were maintained in outdoor flow-through aquaria at ambient temperature (23.0 °C ± 0.1 SD). After seven days in aquaria, 3.5 cm-diameter cores (n = 151) were drilled from the 13 sponges. Each sponge produced between 4 and 24 cores, depending on the size of the sponge (median = 9). Each core was labelled with the identity of the original sponge to control for genotype differences. Sixteen days after drilling, the cores from each sponge were haphazardly divided amongst nine indoor aquaria (50 L), resulting in 15–18 cores per aquarium. Ten sponges were represented in every aquarium, but three sponges were not, as they were represented by fewer than nine cores (Table 2).
Table 2.
The number of cores and original sponges sampled for oxygen flux and tissue contents at each timepoint. *30 indicates the recovery period at 30.0 °C following exposure to 32 °C.
| Time point |
Target temperature |
Heated | Control | ||
|---|---|---|---|---|---|
| Sponges | Cores | Sponges | Cores | ||
| 17 | 23.0 | 9 | 9 | 6 | 6 |
| 43 | 25.0 | 10 | 12 | 6 | 6 |
| 56 | 27.0 | 9 | 12 | 6 | 6 |
| 71 | 29.0 | 9 | 12 | 6 | 6 |
| 84 | 30.0 | 8 | 12 | 6 | 6 |
| 96 | 31.0 | 7 | 12 | 5 | 6 |
| 108 | 32.0 | 8 | 16 | 4 | 5 |
| 144 | *30.0 | 8 | 16 | 4 | 6 |
Each aquarium was continuously supplied with 0.04 µm filtered seawater at 0.8 L/min. Water temperature was regulated by a SeaSim computer-controlled system to reach target temperatures; tanks were additionally buffered against temperature fluctuations using water jackets. LED lighting illuminated aquaria with 300 µmol quanta m−2 s−1 light for 11 h with an additional 1 h ramping period after dawn and before dusk. The irradiance level was less than the saturation irradiance (400 µmol quanta m−2 s−1) that was determined via rapid light curves.The irradiance level (300 µmol quanta m−2 s−1) is comparable to a cloudy day on the reef (302 µmol quanta m−2 s−1), but less than the average irradiance on a clear day (653 µmol quanta m−2 s−1) calculated from67. In terms of the total irradiance, irradiance in the aquaria was 12 mol d−1 compared to 15 or 31 mol d-1 on a cloudy or sunny day on the reef, respectively calculated from67. Cores were maintained at 23.2 ± 0.3 °C (±SD) for 17 days, after which sponge photosynthesis and respiration were measured and tissue samples were taken (detailed below).
To measure changes in C. orientalis condition and performance as a function of temperature, two temperature treatments were established: three aquaria were maintained under their initial conditions at 23 °C for the duration of the experiment (control) and six aquaria had the temperature increased every two weeks, first by 2 °C increments (23 to 29 °C) then by 1 °C increments (30 to 32 °C). Thus, the temperature targets were 23, 25, 27, 29, 30, 31, and 32 °C. These span the average range of temperatures at the collection site: 22.4 °C in July to 29.1 °C in February, with a maximum monthly mean of 30.5 °C between 2002 and 2010 (Source: Australian Institute of Marine Science; http://weather.aims.gov.au; 1.9 m depth). Each temperature increment was achieved via ramping by 0.5 °C per day up to the target temperature followed by 10 days of exposure to that temperature. The rate of temperature increase is similar to daily changes in mean temperature at the collection site calculated from67. The 25 °C temperature increment was extended from 14 to 24 days due to a logistical issue with the heating and cooling system. With this exception, temperatures were finely controlled throughout the experiment, typically within 0.1 °C of the target temperature and with ~0.1 °C SD among aquaria (Table 1). Exposure of sponges to these temperature increments occurred from July to November and coincided with the natural winter to summer temperature increase during the austral summer.
To evaluate the potential for C. orientalis to recover from bleaching, cores exposed to 32 °C were returned to 30 °C (by 0.25 °C per day) and monitored for a further 28 days. Hereafter, ‘*30 °C’ is used to distinguish the recovery period at 30 °C from the incremental increase to 30 °C. To compare the thermal exposure in the laboratory to exposure under natural conditions, degree heating weeks (DHW) of thermal exposure were calculated for each temperature target above the 29.1 + 1.0 °C bleaching threshold for Orpheus Island. DHW was calculated as the product of the °C above 30.1 °C and the duration of temperature ramping (2–4 days) and exposure to the target temperature (10 days). 30 °C was chosen as the recovery temperature as it is below the bleaching threshold and represents a typical summer temperature at the collection site.
For each temperature increment, photosynthetic measurements were taken on two days near the completion of each temperature exposure. Photochemical efficiency of all cores was measured at least twice during exposure to each increment, after one and eight days of exposure, but photochemical efficiency was measured more frequently for the 23 °C, 25 °C, 31 °C, and 32 °C temperature increments. For other photosynthetic parameters and tissue contents, 2–3 cores were selected from each tank at each temperature increment, resulting in ~6 and ~12 samples for the control and heated treatments, respectively. Oxygen flux and sponge surface area were measured after nine days at each temperature increment (due to the time required to measure oxygen flux) except for *30 °C, where measurements were taken after 28 days. After 10 days acclimation to each temperature increment (28 days at *30 °C), sponges were frozen in liquid nitrogen for DNA extraction and measurement of tissue contents.
Photosynthesis and respiration
Photochemical efficiency is an indicator of electron transport during photosynthesis68. For Symbiodinium, decreases in photochemical efficiency can precede bleaching and coincide with damage to photosystems69. The photochemical efficiency of photosystem II was measured using a mini-PAM fluorometer (Walz, Effeltrich, Germany) using standard settings (MI = 8; SI = 6; SW = 0.6; G = 2; D = 2). Clear tubing was used to maintain a constant distance between the PAM fibre optic cable and the sponge. Photochemical efficiency was measured at two timepoints: before the lights turned on (Fv/Fm; maximum efficiency) and after two hours of constant light exposure (∆F/Fm’; effective efficiency). Changes in these variables over the course of each temperature increment reflect the severity of the stress on the Symbiodinium.
Oxygen flux is an integrative measure of respiration by the sponge (and symbionts) and photosynthesis by the Symbiodinium70. For sponges, stress can manifest in altered respiration, decreased photosynthesis, or a reduced ratio of photosynthesis to respiration29,30,71. Oxygen flux was measured using an optical dissolved oxygen meter (Hach HQ30d; Hach, Colorado, USA). Sponge cores were sealed in a darkened chamber (500 mL) for one hour. Water within each chamber was mixed with a magnetic stir bar and the target temperature was maintained using an external water jacket. Respiration rates were calculated by the difference in oxygen concentration between the beginning and end of the incubations. After measurement of respiration, sponges were returned to the aquaria for one hour at 300 µmol quanta m−2 s−1 then sealed in a chamber at 400 µmol quanta m−2 s−1 to measure oxygen production. The photosynthetic rate was calculated using the change in oxygen concentration in the chamber over 40 minutes. Both respiration and photosynthetic rates were adjusted by the amount of oxygen flux in a chamber without a sponge core to account for oxygen flux by microorganisms in the water. The surface area of the selected cores was measured using the aluminium foil method72, sponge tissue was removed (top 1 cm), the cores were frozen in liquid nitrogen and stored at −80 °C.
Sponge condition (Symbiodinium, chlorophyll, protein, organic content)
Invertebrates that harbour Symbiodinium can bleach by losing symbionts or their photosynthetic pigments73. Symbiodinium cells were extracted by incubating sponge tissue in 1 M NaOH at 37 °C for 1 h74. Cells were counted using 4 replicate counts of 0.45 cm2 on a hemocytometer and standardised to the wet weight of sponge tissue. To quantify the photosynthetic pigments within C. orientalis tissues, chlorophylls were extracted in two consecutive extractions of 1 mL of ethanol (95%) to ensure complete extraction of pigments. During each extraction, the tissue was homogenized for 3 min in a bead beater, centrifuged for 5 min (10,000 g), after which the two extracts were pooled. Absorbance of the extract was measured at 630, 647, 664, and 750 nm using a Power Wave Microplate Scanning Spectrophotometer (BIO-TEK Instruments Inc., Vermont, USA). The concentrations of chlorophylls a, b, and c were estimated using the equations of Ritchie75 and standardised to sponge wet weight.
Protein content and tissue organic matter were measured as proxies for sponge condition as these parameters have been shown to decline in bleached corals51. Frozen sponges were weighed (wet weight), lyophilized, and weighed again (dry weight). Proteins were extracted from homogenized dried sponge in 2 mL of 0.125 M NaOH over 24 h at room temperature. Protein concentration was estimated using the Red660 Protein Assay with five concentrations of BSA protein standard and then standardised to the dry weight of the sponge tissue. Organic matter was measured using lyopholized sponge tissue (see Protein content). Dried sponge was combusted at 450 °C for 16 h. Organic matter was estimated as the difference between the dry weight and the ash weight and standardised to the dry weight of the sponge.
Symbiodinium identity
To determine whether the sponges switched Symbiodinium types during temperature stress, we identified the Symbiodinium associated with cores from 9 of the original sponge fragments as temperatures increased. In total, 35 cores were analysed including cores from the same genotype in each temperature increment. DNA was extracted from frozen sponge tissue (~0.2 g) using the Powerplant Pro DNA Isolation Kit (Mo Bio), including the beadbeating, RNAse, and proteinase K procedures as per the manufacturer’s instructions. DNA extracts were sent to the Australian Centre for Ecogenomics at the University of Queensland, Australia for sequencing. The ITS2 region of ribosomal rDNA was amplified using Symbiodinium-specific ITS2 primers76 and sequenced using Illumina MiSeq250 bp chemistry.
Sequences were analysed in Mothur v.1.38.077. Paired reads were combined and screened for quality and chimeric sequences were identified using Uchime in Mothur. The remaining sequences were clustered into 97% similar operational taxonomic units (OTU). The dataset was reduced to 2000 sequences per sample and the relative abundance and prevalence were calculated for each OTU. Representative sequences were defined using the sequence with the smallest distance to all other sequences within the OTU. Sequences were compared against a curated database of ITS2 sequences including all clades of Symbiodinium using the BLAST algorithm78. Blast results with bit scores less than 100 were discarded.
Bleaching surveys
Field surveys were conducted to assess the thermal tolerance of C. orientalis during a natural thermal bleaching event, and to contrast bleaching responses between C. orientalis and corals. In March 2017, video transects were filmed at six sites within the Palm Islands Group, three of which were at Orpheus Island where the experimental samples were collected. Survey sites were chosen as replicate exposed and protected locations, as well as to span the depth gradient over which Acropora cover is relatively high at Orpheus Island. At each site, two transects (50 m long and parallel to shore) were filmed at 0–4 m below the lowest astronomical tide. Cliona orientalis sponges and scleractinian coral colonies were manually counted along each video transect. Coral colonies were categorized as either branching or massive and we used a simple ‘bleached’ or ‘unbleached’ categorisation due to the absence of a reliable colour reference in the videos. White individuals, as well as individuals with fluorescent discolouration (e.g., blue or pink Acropora spp.) were considered bleached.
To compare the thermal exposure during the natural bleaching event to the laboratory experiment, daily mean and maximum temperatures at Orpheus Island (5.8 m depth) were downloaded from the Australian Institute of Marine Science (http://weather.aims.gov.au).
Statistical analysis
Photosynthesis and sponge condition data were analysed using linear mixed models with treatment, time point, and treatment * time point interaction as well as a random intercept to account for between-sponge differences using the R packages lme479, lmerTest80, and multcomp81. Only the final photochemical efficiency was analysed statistically. Symbiodinium density was analysed with an additional random intercept to account for correlations between samples from the same aquarium. Boxplots and residual plots were used to assess whether the data met the assumptions of linear models. Samples with large deviations from fitted values (>1.5*interquartile range) were removed from the analysis. Some response variables were transformed using log (respiration, chl a:c, protein, organic matter) or odds ratios (Fv/Fm, ∆F/Fm’) to meet these assumptions. Planned contrasts were used to test whether heated sponges differed from control sponges at each time point, as well as whether changes occurred after the reduction in temperature following exposure to 32 °C. Results from the planned contrasts are reported with z statistics and P values. P values were corrected using a single-step correction for multiple comparisons.
For each benthic category in the bleaching surveys, individuals were pooled between replicate transects at each site. The proportion of bleached individuals was calculated out of the total number of individuals encountered at each site. For each category of taxa, the proportion of bleached individuals was calculated using proportion of bleached individuals relative to the total number of colonies and then weighted according to the number of individuals at each site.
Data availability
Data are available from the authors upon request.
Acknowledgements
We thank C. Pascelli and M. Kupresanin for assistance collecting rocks, the AIMS Coral Health team for the respirometry chambers, and the AIMS SeaSim staff for support throughout the experiment. K. Shirur provided insightful comments on an earlier version of this manuscript.
Author Contributions
B.R., M.H., S.W. and N.W. planned the experiment. B.R. performed the experiment. B.R. and H.S. analysed the data. B.R. wrote the manuscript with significant contributions from M.H., H.S., S.W. and N.W. N.W. was funded by an Australian Research Council Future Fellowship FT120100480. B.R. was sponsored by an AIMS@JCU PhD scholarship.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.IPCC. IPCC, 2014: Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change Core Writing Team, R. K. Pachauri & L. A. Meyer. (IPCC, 2014).
- 2.Heron SF, Maynard JA, van Hooidonk R, Eakin CM. Warming Trends and Bleaching Stress of the World’s Coral Reefs 1985–2012. Sci Rep. 2016;6:38402. doi: 10.1038/srep38402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes TP, et al. Global warming and recurrent mass bleaching of corals. Nature. 2017;543:373–377. doi: 10.1038/nature21707. [DOI] [PubMed] [Google Scholar]
- 4.Doney SC, et al. Climate Change Impacts on Marine Ecosystems. Annu. Rev. Marine. Sci. 2012;4:11–37. doi: 10.1146/annurev-marine-041911-111611. [DOI] [PubMed] [Google Scholar]
- 5.Yellowlees D, Rees TAV, Leggat W. Metabolic interactions between algal symbionts and invertebrate hosts. Plant Cell Environ. 2008;31:679–694. doi: 10.1111/j.1365-3040.2008.01802.x. [DOI] [PubMed] [Google Scholar]
- 6.Warner ME, Fitt WK, Schmidt GW. Damage to photosystem II in symbiotic dinoflagellates: a determinant of coral bleaching. Proc. Natl. Acad. Sci. USA. 1999;96:8007–8012. doi: 10.1073/pnas.96.14.8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClanahan, T. R., Weil, E., Cortés, J., Baird, A. H. & Ateweberhan, M. In Coral Bleaching205, 121–138 (Springer Berlin Heidelberg, 2009).
- 8.Baird AH, Marshall PA. Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar Ecol Prog Ser. 2002;237:133–141. doi: 10.3354/meps237133. [DOI] [Google Scholar]
- 9.Grottoli AG, et al. The cumulative impact of annual coral bleaching can turn some coral species winners into losers. Global Change Biol. 2014;20:3823–3833. doi: 10.1111/gcb.12658. [DOI] [PubMed] [Google Scholar]
- 10.Abrego D, Ulstrup KE, Willis BL, van Oppen MJH. Species–specific interactions between algal endosymbionts and coral hosts define their bleaching response to heat and light stress. Proc. R. Soc. B. 2008;275:2273–2282. doi: 10.1098/rspb.2008.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Díaz-Almeyda EM, et al. Intraspecific and interspecific variation in thermotolerance and photoacclimation in Symbiodinium dinoflagellates. Proc. Biol. Sci. 2017;284:20171767. doi: 10.1098/rspb.2017.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berkelmans R, van Oppen MJH. The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc. R. Soc. B. 2006;273:2305–2312. doi: 10.1098/rspb.2006.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palumbi SR, Barshis DJ, Traylor-Knowles N, Bay RA. Mechanisms of reef coral resistance to future climate change. Science. 2014;344:895–898. doi: 10.1126/science.1251336. [DOI] [PubMed] [Google Scholar]
- 14.Bay LK, Doyle J, Logan DM, Berkelmans R. Recovery from bleaching is mediated by threshold densities of background thermo-tolerant symbiont types in a reef-building coral. R Soc Open Sci. 2016;3:160322. doi: 10.1098/rsos.160322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverstein RN, Cunning R, Baker AC. Change in algal symbiont communities after bleaching, not prior heat exposure, increases heat tolerance of reef corals. Global Change Biol. 2015;21:236–249. doi: 10.1111/gcb.12706. [DOI] [PubMed] [Google Scholar]
- 16.Bell JJ, Davy SK, Jones T, Taylor MW, Webster NS. Could some coral reefs become sponge reefs as our climate changes? Global Change Biol. 2013;19:2613–2624. doi: 10.1111/gcb.12212. [DOI] [PubMed] [Google Scholar]
- 17.Przeslawski R, Ahyong S, Byrne M, Wörheide G, Hutchings PA. Beyond corals and fish: the effects of climate change on noncoral benthic invertebrates of tropical reefs. Global Change Biol. 2008;14:2773–2795. doi: 10.1111/j.1365-2486.2008.01693.x. [DOI] [Google Scholar]
- 18.Carballo JL, Bautista E, Nava H, Cruz-Barraza JA, Chávez JA. Boring sponges, an increasing threat for coral reefs affected by bleaching events. Ecol Evol. 2013;3:872–886. doi: 10.1002/ece3.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortés J, Murillo MM, Guzman HM, Acuña J. Pérdida de zooxanthelas y muerte de corales y otros organismos arrecifales en el Caribe y Pacifico de Costa Rica. Rev. biol. trop. 1984;32:227–231. [Google Scholar]
- 20.Vicente VP. Response of sponges with autotrophic endosymbionts during the coral-bleaching episode in Puerto Rico. Coral Reefs. 1990;8:199–202. doi: 10.1007/BF00265011. [DOI] [Google Scholar]
- 21.Rützler K. Impact of Crustose Clionid Sponges on Caribbean Reef Corals. Acta Geol Hisp. 2002;37:61–72. [Google Scholar]
- 22.Schönberg, C. H. L. & Ortiz, J. C. Is sponge bioerosion increasing? Proceedings of 11th International Coral Reef Symp 7–11 (2008).
- 23.Hatch WI. The Implication of Carbonic Anhydrase in the Physiological Mechanism of Penetration of Carbonate Substrata by the Marine Burrowing Sponge Cliona celata (Demospongiae) Biol Bull. 1980;159:135–147. doi: 10.2307/1541014. [DOI] [Google Scholar]
- 24.Rützler K, Rieger G. Sponge burrowing: Fine structure of Cliona lampa penetrating calcareous substrata. Mar Biol. 1973;21:144–162. doi: 10.1007/BF00354611. [DOI] [Google Scholar]
- 25.Zundelevich A, Lazar B, Ilan M. Chemical versus mechanical bioerosion of coral reefs by boring sponges - lessons from Pione cf. vastifica. J Exp Biol. 2007;210:91–96. doi: 10.1242/jeb.02627. [DOI] [PubMed] [Google Scholar]
- 26.Hill MS. Symbiotic zooxanthellae enhance boring and growth rates of the tropical sponge Anthosigmella varians forma varians. Mar Biol. 1996;125:649–654. doi: 10.1007/BF00349246. [DOI] [Google Scholar]
- 27.Schönberg, C. H. L. Growth and erosion of the zooxanthellate Australian bioeroding sponge. Proceedings of the 10th International Coral Reef Symposium 168–174 (2006).
- 28.Weisz JB, Massaro AJ, Ramsby BD, Hill MS. Zooxanthellar symbionts shape host sponge trophic status through translocation of carbon. Biol Bull. 2010;219:189–197. doi: 10.1086/BBLv219n3p189. [DOI] [PubMed] [Google Scholar]
- 29.Fang JKH, Schönberg CHL, Mello-Athayde MA, Hoegh-Guldberg O, Dove S. Effects of ocean warming and acidification on the energy budget of an excavating sponge. Global Change Biol. 2014;20:1043–1054. doi: 10.1111/gcb.12369. [DOI] [PubMed] [Google Scholar]
- 30.Achlatis M, et al. Sponge bioerosion on changing reefs: ocean warming poses physiological constraints to the success of a photosymbiotic excavating sponge. Sci Rep. 2017;7:1–13. doi: 10.1038/s41598-017-10947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duckworth AR, Peterson BJ. Effects of seawater temperature and pH on the boring rates of the sponge Cliona celata in scallop shells. Mar Biol. 2013;160:27–35. doi: 10.1007/s00227-012-2053-z. [DOI] [Google Scholar]
- 32.Wisshak M, Schönberg CHL, Form A, Freiwald A. Effects of ocean acidification and global warming on reef bioerosion—lessons from a clionaid sponge. Aquat Biol. 2013;19:111–127. doi: 10.3354/ab00527. [DOI] [Google Scholar]
- 33.Wisshak M, Schönberg CHL, Form A, Freiwald A. Ocean Acidification Accelerates Reef Bioerosion. PLoS ONE. 2012;7:e45124. doi: 10.1371/journal.pone.0045124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang JKH, et al. Sponge biomass and bioerosion rates increase under ocean warming and acidification. Global Change Biol. 2013;19:3581–3591. doi: 10.1111/gcb.12334. [DOI] [PubMed] [Google Scholar]
- 35.Enochs IC, et al. Ocean acidification enhances the bioerosion of a common coral reef sponge: implications for the persistence of the Florida Reef Tract. Bull Mar Sci. 2015;91:271–290. doi: 10.5343/bms.2014.1045. [DOI] [Google Scholar]
- 36.Stubler AD, Furman BT, Peterson BJ. Sponge erosion under acidification and warming scenarios: differential impacts on living and dead coral. Global Change Biol. 2015;21:4006–4020. doi: 10.1111/gcb.13002. [DOI] [PubMed] [Google Scholar]
- 37.Angilletta, M. J. Thermal adaptation: a theoretical and empirical synthesis: Oxford University Press. (Oxford University Press, 2009).
- 38.Pörtner HO. Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften. 2001;88:137–146. doi: 10.1007/s001140100216. [DOI] [PubMed] [Google Scholar]
- 39.Rodolfo-Metalpa R, et al. Thermally tolerant corals have limited capacity to acclimatize to future warming. Global Change Biol. 2014;20:3036–3049. doi: 10.1111/gcb.12571. [DOI] [PubMed] [Google Scholar]
- 40.Coles SL, Jokiel PL. Effects of temperature on photosynthesis and respiration in hermatypic corals. Mar Biol. 1977;43:209–216. doi: 10.1007/BF00402313. [DOI] [Google Scholar]
- 41.Ramsby BD, et al. Sibling species of mutualistic Symbiodinium clade G from bioeroding sponges in the western Pacific and western Atlantic oceans. J Phycol. 2017;11:36–10. doi: 10.1111/jpy.12576. [DOI] [PubMed] [Google Scholar]
- 42.Wulff, J. L. Sponge Contributions to the Geology and Biology of Reefs: Past, Present, and Future. In: Coral reefs at the crossroads (eds Hubbard DK, Rogers CS, Lipps JH, Stanley GD Jr.), pp. 103–126 (2016).
- 43.Schönberg, C. H. L., Fang, J. K. H., Carreiro-Silva, M., Tribollet, A. & Wisshak, M. Bioerosion: the other ocean acidification problem. ICES J. Mar. Sci. 1–31, 10.1093/icesjms/fsw254 (2017).
- 44.Berkelmans R, Willis BL. Seasonal and local spatial patterns in the upper thermal limits of corals on the inshore Central Great Barrier Reef. Coral Reefs. 1999;18:219–228. doi: 10.1007/s003380050186. [DOI] [Google Scholar]
- 45.Schönberg CHL, Suwa R, Hidaka M, Loh WKW. Sponge and coral zooxanthellae in heat and light: preliminary results of photochemical efficiency monitored with pulse amplitude modulated fluorometry. Mar Ecol. 2008;29:247–258. doi: 10.1111/j.1439-0485.2007.00216.x. [DOI] [Google Scholar]
- 46.Berkelmans R. Time-integrated thermal bleaching thresholds of reefs and their variation on the Great Barrier Reef. Mar Ecol Prog Ser. 2002;229:73–82. doi: 10.3354/meps229073. [DOI] [Google Scholar]
- 47.Baker AC, Glynn PW, Riegl B. Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci. 2008;80:435–471. doi: 10.1016/j.ecss.2008.09.003. [DOI] [Google Scholar]
- 48.Hill MS, Walter C, Bartels E. A mass bleaching event involving clionaid sponges. Coral Reefs. 2016;35:153–153. doi: 10.1007/s00338-016-1402-7. [DOI] [Google Scholar]
- 49.Smith DJ, Suggett DJ, Baker NR. Is photoinhibition of zooxanthellae photosynthesis the primary cause of thermal bleaching in corals? Global Change Biol. 2005;11:1–11. doi: 10.1111/j.1529-8817.2003.00895.x. [DOI] [Google Scholar]
- 50.Baird AH, Bhagooli R, Ralph PJ, Takahashi S. Coral bleaching: the role of the host. Trends in Ecology & Evolution. 2009;24:16–20. doi: 10.1016/j.tree.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Fitt WK, Spero HJ, Halas J, White MW, Porter JW. Recovery of the coral Montastrea annularis in the Florida Keys after the 1987 Caribbean bleaching event. Coral Reefs. 1993;12:57–64. doi: 10.1007/BF00302102. [DOI] [Google Scholar]
- 52.Rodrigues LJ, Grottoli AG. Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnology and Oceanography. 2007;52:1874–1882. doi: 10.4319/lo.2007.52.5.1874. [DOI] [Google Scholar]
- 53.Rodrigues LJ, Grottoli AG. Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnology and Oceanography. 2007;52:1874–1882. doi: 10.4319/lo.2007.52.5.1874. [DOI] [Google Scholar]
- 54.Bourne DG, Morrow KM, Webster NS. Insights into the Coral Microbiome: Underpinning the Health and Resilience of Reef Ecosystems. Annu. Rev. Microbiol. 2016;70:317–340. doi: 10.1146/annurev-micro-102215-095440. [DOI] [PubMed] [Google Scholar]
- 55.Hawkins, T. D., Hagemeyer, J. C. G., Hoadley, K. D., Marsh, A. G. & Warner, M. E. Partitioning of Respiration in an Animal-Algal Symbiosis: Implications for Different Aerobic Capacity between Symbiodinium spp. Front. Physiol. 7, (2016). [DOI] [PMC free article] [PubMed]
- 56.Riisgård HU, Thomassen S, Jakobsen H, Weeks JM, Larsen PS. Suspension feeding in marine sponges Halichondria panicea and Haliclona urceolus: effects of temperature on filtration rate and energy cost of pumping. Mar Ecol Prog Ser. 1993;96:177–188. doi: 10.3354/meps096177. [DOI] [Google Scholar]
- 57.Szmant AM, Gassman NJ. The effects of prolonged bleaching on the tissue biomass and reproduction of the reef coral Montastrea annularis. Coral Reefs. 1990;8:217–224. doi: 10.1007/BF00265014. [DOI] [Google Scholar]
- 58.Grottoli AG, Rodrigues LJ, Palardy JE. Heterotrophic plasticity and resilience in bleached corals. Nature. 2006;440:1186–1189. doi: 10.1038/nature04565. [DOI] [PubMed] [Google Scholar]
- 59.Riesgo A, et al. Transcriptomic analysis of differential host gene expression upon uptake of symbionts: a case study with Symbiodinium and the major bioeroding sponge Cliona varians. BMC Genomics. 2014;15:376. doi: 10.1186/1471-2164-15-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pineda M-C, et al. Effects of light attenuation on the sponge holobiont- implications for dredging management. Sci Rep. 2016;6:39038. doi: 10.1038/srep39038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hill MS, Allenby A, Ramsby B, Schönberg CHL, Hill AL. Symbiodinium diversity among host clionaid sponges from Caribbean and Pacific reefs: Evidence of heteroplasmy and putative host-specific symbiont lineages. Mol Phylogen Evol. 2011;59:81–88. doi: 10.1016/j.ympev.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 62.Schönberg CHL, Loh WK. Molecular identity of the unique symbiotic dinoflagellates found in the bioeroding demosponge Cliona orientalis. Mar Ecol Prog Ser. 2005;299:157–166. doi: 10.3354/meps299157. [DOI] [Google Scholar]
- 63.Granados C, Camargo C, Zea S, Sánchez JA. Phylogenetic relationships among zooxanthellae (Symbiodinium) associated to excavating sponges (Cliona spp.) reveal an unexpected lineage in the Caribbean. Mol Phylogen Evol. 2008;49:554–560. doi: 10.1016/j.ympev.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 64.Pochon X, Montoya-Burgos JI, Stadelmann B, Pawlowski J. Molecular phylogeny, evolutionary rates, and divergence timing of the symbiotic dinoflagellate genus. Symbiodinium. Mol Phylogen Evol. 2006;38:20–30. doi: 10.1016/j.ympev.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 65.Bo M, et al. First description of algal mutualistic endosymbiosis in a black coral (Anthozoa: Antipatharia) Mar Ecol Prog Ser. 2011;435:1–11. doi: 10.3354/meps09228. [DOI] [Google Scholar]
- 66.Chaves-Fonnegra A, et al. Bleaching events regulate shifts from corals to excavating sponges in algae dominated reefs. Global Change Biol. 2017;00:1–13. doi: 10.1111/gcb.13962. [DOI] [PubMed] [Google Scholar]
- 67.Hoogenboom MO, Connolly SR, Anthony K. Biotic and abiotic correlates of tissue quality for common scleractinian corals. Mar Ecol Prog Ser. 2011;438:119–128. doi: 10.3354/meps09271. [DOI] [Google Scholar]
- 68.Baker NR. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol. 2008;59:89–113. doi: 10.1146/annurev.arplant.59.032607.092759. [DOI] [PubMed] [Google Scholar]
- 69.Warner, M. E., Lesser, M. P. & Ralph, P. J. In Chlorophyll a fluorescence in aquatic sciences: methods and applications (eds Suggett, D. J., Prášil, O. & Borowitzka, M. A.) 209–222 (2010).
- 70.Osinga, R., Iglesias-Prieto, R. & Enrquez, S. Measuring Photosynthesis in Symbiotic Invertebrates: A Review of Methodologies, Rates and Processes. In: Applied Photosynthesis (ed Najafpour M), pp. 1–29, 10.5772/29339, InTech (2012).
- 71.Bennett HM, et al. Interactive effects of temperature and pCO2 on sponges: from the cradle to the grave. Global Change Biol. 2016;23:2031–2046. doi: 10.1111/gcb.13474. [DOI] [PubMed] [Google Scholar]
- 72.Marsh JA. Primary Productivity of Reef‐Building Calcareous Red Algae. Ecology. 1970;51:255–263. doi: 10.2307/1933661. [DOI] [Google Scholar]
- 73.Fitt W, Brown B, Warner M, Dunne R. Coral bleaching: interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs. 2001;20:51–65. doi: 10.1007/s003380100146. [DOI] [Google Scholar]
- 74.Zamoum T, Furla P. Symbiodinium isolation by NaOH treatment. J Exp Biol. 2012;215:3875–3880. doi: 10.1242/jeb.074955. [DOI] [PubMed] [Google Scholar]
- 75.Ritchie RJ. Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica. 2008;46:115–126. doi: 10.1007/s11099-008-0019-7. [DOI] [Google Scholar]
- 76.Pochon X, Pawlowski J, Zaninetti L, Rowan R. High genetic diversity and relative specificity among Symbiodinium. Mar Biol. 2001;139:1069–1078. doi: 10.1007/s002270100674. [DOI] [Google Scholar]
- 77.Schloss PD, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arif C, et al. Assessing Symbiodinium diversity in scleractinian corals via next-generation sequencing-based genotyping of the ITS2 rDNA region. Mol Ecol. 2014;23:4418–4433. doi: 10.1111/mec.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models using lme4. J Stat Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 80.Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. B. lmerTest: Tests in Linear Mixed Effects Models. (R package version 2.0–32, 2016).
- 81.Hothorn T, Bretz F, Westfall P. Simultaneous Inference in General Parametric Models. Biom J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the authors upon request.