Abstract
BACKGROUND/OBJECTIVES
The aim of this study was to evaluate the biological and sleep-promoting effects of combined γ-aminobutyric acid (GABA) and 5-hydroxytryptophan (5-HTP) using caffeine-induced sleepless fruit flies, ICR mice, and Sprague-Dawley rats.
MATERIALS/METHODS
Video-tracking analysis was applied to investigate behavioral changes of Drosophila melanogaster. Pentobarbital-induced sleep test and electroencephalogram (EEG) patterns were used for analysis of sleep latency, duration, and quantity and quality of sleep in vertebrate models.
RESULTS
Administration of combined GABA/5-HTP could significantly reverse the caffeine induced total distance of flies (P < 0.001). Also, individually administered and combined GABA/5-HTP significantly increased the total sleeping time in the caffeine-induced sleepless ICR mice (P < 0.001). In the caffeine-induced sleepless SD-rats, combined GABA/5-HTP showed significant differences in sleep quality between individual amino acid administrations (P < 0.05).
CONCLUSIONS
Taken together, we identified inhibitory effects of combined GABA/5-HTP in locomotor activity, sleep quantity and quality in caffeine-induced sleepless models, indicating that combined GABA/5-HTP may be effective in patients with insomnia by providing sufficient sleep.
Keywords: Sleep, caffeine, GABA, 5-HTP, insomnia
INTRODUCTION
The quantity and quality of sleep are related with positive influences of life scores, are a common public health issue globally, and are closely affected by internal and external factors [1]. Studies have shown that the quality and quantity of adequate sleep (adequate sleep is defined as 6–8 hours per night regularly) are closely related to future health and well-being [2]. Sleep disorder is the most commonly reported sleep-associated problem and can be characterized in terms of duration (acute or chronic) [3]. The process of sleep and sleep-related behavior are modulated by a variety of neurochemicals operating through distributed neuronal circuits. Studies have concentrated on the pharmacological and physiological implications of specific receptor binding sites to design new drugs to treat sleep disorders during the past few decades [4]. Sleep-wake regulatory mechanisms of the central nervous system are also targets for the treatment of sleep disorder. Sleep disorder patients depend on pharmacological treatments even though these chemical agents have various side effects such as difficulty keeping balance and overdosing. For this reason, sleep disorder researches have focused on sleep-promoting properties of natural substances and biomolecule compounds to avoid adverse side effects [5,6].
Animal models have been used to understand the most common human sleep disorders such as insomnia, narcolepsy, restless legs syndrome, and sleep apnea [7]. In addition, evaluation of non-pharmacological treatments for sleep disorder will require various applied methods in animals for the development and preliminary testing of new therapeutic approaches, and for the identification and validation of sleep-inducing mechanisms. The fruit fly, Drosophila melanogaster, is a model insect with a wealth of information on circadian rhythm and sleep-related neuronal systems. The brain levels of acetylcholine, octopamine, dopamine, glutamate, and receptors for γ-aminobutyric acid (GABA) and serotonin (5-HT) are expressed in the mushroom bodies of the fly and modulate sleep/wake behavior changes [8,9]. Moreover, the fly shares an evolutionary conservation and many features with mammalian sleep, and fly sleep is controlled homeostatically by the mechanisms of sleep regulation [10]. To record sleep in flies and test sleep disorder treatment requires measuring the circadian behavior of locomotor activity of the flies from the field [11]. Moreover, preclinical studies are recommended for the reliable assessment and the validation phase of candidate treatment, with compounds generally administered to rodents, such as mice and rats. Mice and rats are the most commonly used model system for studying sleep-related behaviors, and the function of sleep and molecular processes [12]. In addition to electrophysiological measures in freely moving mice and rats under controlled laboratory conditions, sleep quantity and quality can be quantified behaviorally by measuring movement and sleep architecture. In humans and animals, the assessment of sleep architecture depends on both non-rapid eye movement (NREM) sleep (light sleep and slow-wave sleep, SWS) and rapid eye movement (REM) sleep, as well as electroencephalogram (EEG) wave patterns [13].
Although data obtained from invertebrate and vertebrate model systems have assessed the treatment properties that underlie sleep-wake cycles and neurochemical regulation, additional experimental models are needed to evaluate the interaction of behavioral changes and candidate hypnotic compounds with neuronal system. Caffeine (1,3,7-trimethylxanthine) is a psychoactive substance in many popular beverages and has been used with the purpose of maintaining physical activity and mental alertness. Even though caffeine overdose known to be associated with a variety of side effects such as tachycardia, hypertension, nausea, vomiting and gastritis, administration of caffeine has been proposed as an effective agent to model sleep-onset insomnia by antagonizing the function of all adenosine receptors (Ars: A1, A2A, A3, and A2B) [14,15]. Targeting the adenosine system with caffeine treatment is an effective tool for controlling individual weakness to the long-term effects of sleep deprivation on cognitive ability and sleep [16]. Also, previous studies have described the effects of caffeine on the cellular and behavioral levels in animal species, and chronic administration of caffeine causes a wide range of biochemical alterations in the central nervous system (CNS), including serotoergic, nicotinic, and GABAA receptors [17]. Caffeine is also known to influence the onset of sleep, total sleep time, sleep quality and efficiency, such as stage 3–4 NREM sleep [18]. In this study, caffeine was used as a stimulant to promote wakefulness and simulate the insomnia condition in invertebrate and vertebrate models to evaluate the sleep-promoting effects of two combined amino acids.
MATERIALS AND METHODS
Animals
D. melanogaster Canton-S strain was obtained from the Bloomington Drosophila Stock Center (Bloomington, IN, USA). The flies were reared on Jazz-Mix Drosophila food (Fisher Scientific, Waltham, MA, USA) at 25 ± 1℃ on a 12:12 h light:dark cycle at 60% relative humidity (RH). Prior to treatment, each sample of flies (3-5 day-old male) were anesthetized for transfer by using CO2. ICR mice (4-weekold male) and SD rats (8-week old male) were obtained from Orient Bio Inc. (Gyeonggi-do, Korea). All rodents were maintained in acrylic cages with food and water at 24 ± 2℃ in 50-60% atmospheric humidity with a 12:12 h light: dark cycle. Following the 1week adaptation and recovery period vertebrate models were used for pentobarbital-induced sleep test and measurements of EEG wave patterns. The Korea University Animal Care Committee (Seoul, Korea) approved the use of animals in this protocol (KUIACUC-2015-102).
Behavioral assays
Caffeine, GABA, and 5-HTP (Sigma Aldrich, St. Louis, MO, USA) were mixed in sucrose-agar media (Normal diet: 5% sucrose and 1% agar) for the behavioral assay. For a video-tracking system, flies (10/group) were treated with GABA (10 mg/mL), 5-HTP (1 mg/mL), or combined GABA/5-HTP (10 mg/mL and 1 mg/mL each) with caffeine (1 mg/mL). A 1% caffeine concentration was used as a stimulant in the awake condition [19]. The flies acclimated to the chamber for 1 min, followed by video recording for 5 min 1 h before subjective nighttime. The chamber consisted of a circular arena (8 mm in diameter and 0.1 mm in height) which was laid down a white background. Analysis of fly activity was performed by the Noldus EthoVision-XT system (Noldus Information Technology, Netherlands).
Pentobarbital-induced sleep test
The experiments were analyzed between 13:00 and 17:00, and all mice were fasted for 24 h prior to testing. During oral administration, the individual treatments were not revealed to the observers. GABA (14/group, 60 mg/kg), 5-HTP (14/group, 6 mg/kg), and combined GABA/5-HTP (15/group, 60 mg/kg and 6 mg/kg each) were dissolved in 0.5% physiological saline. All samples were administered (post-oral injection, p.o.) with caffeine (10 mg/kg) and pentobarbital (sub-hypnotic dose: 30 mg/kg, intraperitoneal injection [i.p.]; hypnotic dose: 42 mg/kg, i.p.) was injected 1 h later to induce sleep. Following the pentobarbital injection, mice were laid down on their back in individual cages and sleep latency was recorded from this time-point. In addition, sleeping duration was analyzed, which were defined by loss of righting reflex, to recovery and beginning to move. After injection, mice that did not sleep within 15 min were excluded from the experiment.
Surgical procedures and electrophysiological analysis
Under isoflurane (Troikaa Pharmaceuticals Ltd, Gujarat, India) anesthesia in the mixture of oxygen and nitrous oxide, rats were placed to a stereotaxic instrument frame (Stoelting Inc., Wood Dale, IL, USA). The body hair of the head was removed using a shaver and cleaned to ensure disinfection with 70% ethanol before a dorsal midline incision was made in the scalp to expose the skull. Hemostasis was achieved with sterile cotton-tip applicators and bregma was marked and four holes bored through the skull +1 mm lateral to midline/+1 mm to bregma, and −1 mm lateral to midline/−3 mm to bregma for the hippocampus, frontal cortex, and striatum, respectively [20]. The EEG electrodes with a mounting screw and socket were fixed to the skull surface of rat with dental cement (AgnThós AB, Lidingö, Sweden). Seven-day post-surgery (recovery period), rats were divided into normal and control and treatment groups. Total experiments periods were recorded 1 h than with later administration of caffeine (8/group, 10 mg/kg) and the two amino acids (8/group, GABA: 60 mg/kg, 5-HTP: 6 mg/kg) and combined GABA/5-HTP (8/group, 66 mg/kg) and performed between 10:00 and 18:00. The EEG activity was acquired by Iox2 data acquisition software (version 2.8.0.13, emka Technologies, Paris, France) and EEG spectra were analyzed in 1 Hz bins. Setting for standard bands were the following: δ wave, 0.5–4; θ wave, 4–9; α wave, 9–12; β wave, 12–30; and gamma: 30–60 Hz. Analysis of EEG was calculated wave patterns, wake time, and sleep time on the recording data at 2 sec intervals by fast Fourier transform of ecgAUTO3 software (version. 3.3.0.20, emka Technologies).
Statistical Analyses
The Statistical Package for Social Sciences version 12.0 (SPSS Inc., Chicago, IL, USA) was used to analyze the data. Data from the caffeine-induced sleepless model systems are represented as mean ± standard error (SE). Differences between groups were evaluated by one-way analysis of variance (ANOVA) with Tukey's and Dunnett's multiple comparison test.
RESULTS
Effects of two amino acids and combined GABA/5-HTP on the distance moved, turn angle, and mobility
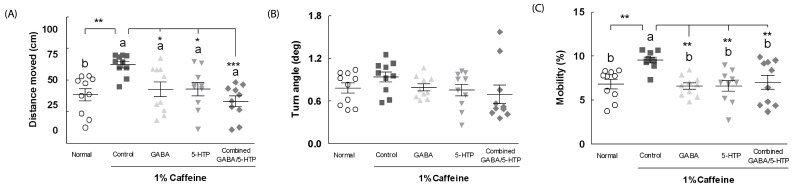
The effects of two amino acids and combined GABA/5-HTP on the distance moved, turn angle, and mobility in the caffeine-induced sleepless fly are presented in Fig. 1. The control group (flies treated with the caffeine) traveled a significantly greater distance (1.61-fold, F(4,85) = 5.57, P < 0.01) compared to the normal (untreated) group (Fig. 1A). Administration of GABA (0.69-fold, P < 0.05) and 5-HTP (0.69-fold, P < 0.05) significantly decreased the distance moved compared to the control group. Furthermore, the distance moved was significantly decreased by combined GABA/5-HTP (0.52-fold, P < 0.001) compared to control group. As shown in Fig. 1B, the turn angle provides the angle formed by the change in the direction of the individual fly movement. However, there was no significant difference in the turn angle between groups. In Fig. 1C, mobility is defined on the basis of changes in the pixels caused by the spatial displacement of individual flies. The control group showed significant increased mobility (1.40-fold, F(4,85) = 5.34, P < 0.01) compared to the normal group. Two amino acids and combined GABA/5-HTP groups showed significantly reduced mobility compared to the control group (P < 0.01).
Fig. 1. Effects of two amino acids and combined GABA/5-HTP on (A) the distance moved, (B) turn angle, and (C) mobility of Drosophila during the 5-min observation period in the open field assay.
Values are mean ± SE (n = 90). Different letters indicate significant differences at P < 0.05 by Tukey's test and different symbols indicate significant differences at *P < 0.05, **P < 0.01, ***P < 0.001 by Dunnett's test. GABA, γ-aminobutyric acid; 5-HTP, 5-hydroxytryptophan.
Effects of two amino acids and combined GABA/5-HTP on meander and velocity
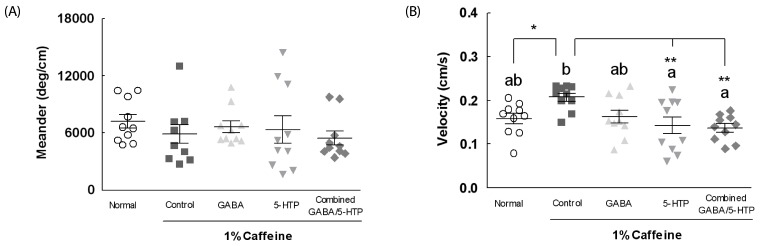
The meander measure represents the change in the direction of the individual fly movement of a subject relative to the distance moved by the subject (Fig. 2A). Administration of caffeine, and caffeine with two amino acids and combined GABA/5-HTP did not significantly change the meander of individual flies compared to the normal group. Fig. 2B shows the walking speed (velocity) during the 5-min observation period; this is a measure of the activity of the individual flies. Caffeine-treated flies showed significantly higher walking speeds compared to normal flies (1.32-fold, F(4,85) = 4.27, P < 0.05). Although GABA treatment did not affect velocity, administration of 5-HTP and combined GABA/5-HTP caused a significantly lower velocity compared to the control group (P < 0.01).
Fig. 2. Effects of two amino acids and combined GABA/5-HTP on (A) meander and (B) velocity of Drosophila during the 5-min observation period in the open field assay.
Values are means ± SE (n = 90). Different letters indicate significant differences at P < 0.05 by Tukey's test and different symbols indicate significant differences at *P < 0.05, **P < 0.01 by Dunnett's test. GABA, γ-aminobutyric acid; 5-HTP, 5-hydroxytryptophan.
Effects of two amino acids and combined GABA/5-HTP on pentobarbital-induced sleeping behaviors
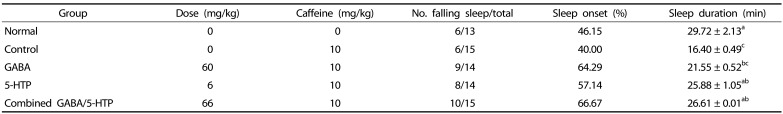
The effects of two amino acids and combined GABA/5-HTP on sleep latency and duration of caffeine-induced sleepless mice induced by a sub-hypnotic and hypnotic dosage of pentobarbital are shown in Table 1 and Fig. 3, respectively. Oral administration of caffeine significantly decreased the sleep onset ratio and duration compared to the normal group (F(4,66) = 21.96, P < 0.01). The sleep onset ratio and duration were significantly increased by Two amino acids and combined GABA/5-HTP compared to the control group (Table 1). Particularly, the administration of combined GABA/5-HTP increased the change of the sleep onset ratio and duration when compared to the control group. In mice treated with the hypnotic dose of pentobarbital (Fig. 3), caffeine administration significantly increased sleep latency (F(4,45) = 6.46, P < 0.001) and decreased sleep duration (F(4,45) = 21.54, P < 0.001). In addition, sleep latency was significantly decreased (GABA and combined GABA/5-HTTP: P < 0.001, 5-HTP: P < 0.01) and the duration of sleep was prolonged by administration of two amino acids and combined GABA/5-HTP compared to the control group (P < 0.001).
Table 1. Effects of two amino acids and combined GABA/5-HTP on sleep onset and duration in caffeine-induced sleepless mice intraperitoneal injected with a sub-hypnotic dosage of pentobarbital (30 mg/kg, i.p.).
Mice received pentobarbital 45 min after the administration of all treatments. Sleep onset (%) = no. falling asleep/total no. × 100. Sleep duration value represents mean ± SE (n = 13–15/group). Different letters indicate significant differences at P < 0.05 by Tukey's test. GABA, γ-aminobutyric acid; 5-HTP, 5-hydroxytryptophan.
Fig. 3. Effects of two amino acids and combined GABA/5-HTP on (A) sleep onset and (B) duration in caffeine-induced sleepless mice intraperitoneal injected a hypnotic dosage of pentobarbital (42 mg/kg, i.p.).
Values are mean ± SE (n = 50). Different letters indicate significant differences at P < 0.05 by Tukey's test and different symbols indicate significant differences **P < 0.01, ***P < 0.001 by Dunnett's test. GABA, γ-aminobutyric acid; 5-HTP, 5-hydroxytryptophan.
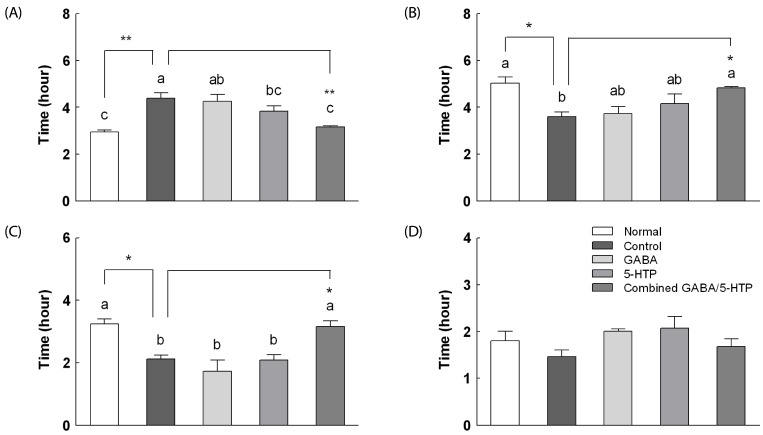
Effects two amino acids and combined GABA/5-HTP on sleep/wake architecture
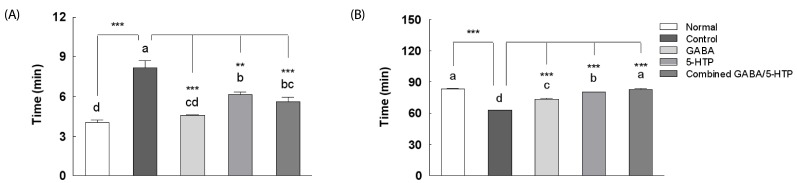
The processed recording per 8 h and sleep/wake architectures of two amino acids and the combined GABA/5-HTP administration in a caffeine-induced awake model are presented in Fig. 4. Doses for the caffeine-induced awake model were chosen based on previous literature [21]. Caffeine administration (10 mg/kg) to rats caused significant reductions in sleep time (F(4,35) = 10.68, P < 0.05) and parallel increases in wake time (F(4,35) = 11.36, P < 0.01) compared to normal rats. Caffeine combined with GABA/5-HTP administration caused a significant reduction in the wake time (P < 0.05) and a parallel increase in the sleep time compared to caffeine administration (P < 0.05). There was no significant change in REM sleep time in response to the combined GABA/5-HTP treatment. The only significant effect of the combined GABA/5-HTP administration was an increase in the NREM sleep time compared to caffeine administration (F(4,35) = 15.41, P < 0.05).
Fig. 4. Effects of two amino acids and combined GABA/5-HTP on (A) wake, (B) sleep, (C) NREM and D) REM in caffeine-induced sleepless rats.
Values are mean ± SE (n = 40). Different letters indicate significant differences at P < 0.05 by Tukey's test and different symbols indicate significant differences at * P < 0.05, ** P < 0.01 by Dunnett's test. GABA, γ-aminobutyric acid; 5-HTP, 5-hydroxytryptophan.
DISCUSSION
Although questions at the behavioral and electrophysiological levels regarding the caffeine-induced sleepless model system remain unaddressed, the present study, based on behavior investigations and EEG wave patterns observations, provides a convincing explanation for the inhibitory effects of two amino acids and combined GABA/5-HTP on sleep disorder in animals. Currently, most studies of hypnotic drugs and candidate treatments focus on GABAergic inhibitory synaptic transmission, the GABAA receptor-binding site in the brain and selective 5-HT2A receptor inverse agonist remains a target of sleep disorder treatments [22,23]. In the fruit fly, downregulation of slow metabotropic GABAB receptors in the pigment dispersing factor-positive ventrolateral clock neurons using RNAi reduced sleep maintenance in the second half of the night [24]. In addition, GABAB receptor agonists are potentially useful in schizophrenia and insomnia related to schizophrenia, and are involved in inhibiting dopamine release and regulating glutamatergic systems of dopamine [25,26,27]. Arnaud et al. [28] assessed sleep-waking regulation of GABAC receptors, which have a higher sensitivity than other GABA receptors, using (1,2,5,6,-tetrahydropyridine)-methylphosphinic acid, a specific antagonist, in rats. Five-HTP is a serotonin precursor and easily crosses the blood brain barrier without a transport molecule [29]. Moreover, 5-HTP regulates sleep-wake behavior and 5-HT receptors interact with GABAergic inhibitory synaptic transmission in flies and mammals [30,31]. The polysomnography data support an increase in sleep maintenance following treatment with several selective 5-HT2A receptor antagonists [32]. In a previous study, we also evaluated the dose-dependent sleep-promoting effect of two amino acids and combined GABA/5-HTP on locomotor activity and the quantity and quality of sleep in animal models [33,34].
In the present study, we utilized the caffeine-induced sleepless Drosophila to investigate the inhibitory effect of two amino acids and combined GABA/5-HTP and regulated significant difference in locomotion such as distance moved, turn angle, mobility, meander, and velocity (Fig. 1 and 2). Following an action potential, GABA is released to terminate GABA-stimulated responses from presynaptic neurons into the synaptic cleft in both vertebrate and invertebrate nervous systems [35]. The GABAergic system is the target of pharmacological approaches and GABA transport inhibitors resulted in diminished locomotor activity [36]. D. melanogaster locomotor activity follows circadian regulation and biochemical alterations [11]. In addition, previous reports suggested that 5-HTP increases serotonin levels in the CNS of both insects and mammals, and doses of 5-HTP treatment from 1 mg/mL to 5 mg/mL and 2 mg/kg to 200 mg/kg have been found to significantly increase sleep time [9,37,38,39,40].
This study was designed to validate the inhibitory and sleep-promoting effects of combined GABA/5-HTP and demonstrate that the caffeine-induced sleepless model is necessary to evaluate sleep at both the behavioral and electrophysiological levels. Caffeine (10 mg/kg) was used to simulate the insomnia condition in pentobarbital-induced sleep test and electrophysiological analysis [41]. To understand the inhibitory effect of combined GABA/5-HTP on behavioral changes, we used the pentobarbital-induced sleep test for evaluating caffeine-induced sleepless mice (Table 1 and Fig. 3). Caffeine (control group) significantly decreased sleep onset and duration relative to the normal group at the sub-hypnotic dose pentobarbital. However, although sleep behavior depends on caffeine administration, the combined GABA/5-HTP treatment plus caffeine group showed significant differences compared to the control group. In addition, administration of caffeine alone with the hypnotic dose showed a significant arousal effect compared to the normal group, while two amino acids and combined GABA/5-HTP significantly increased total sleeping time compared to mice treated with caffeine only (Fig. 3B). Other evidence from the pentobarbital-induced sleep test has shown that caffeine produces a dose-dependent increase in sleep latency and decrease in sleep duration. Kuribara et al. [42] assessed the interaction of caffeine with ethanol, diazepam, and pentobarbital, and classified them as CNS depressants or stimulants by observing the ambulatory activity of mice. In addition, the hypnotic effect, and the interaction of caffeine with pentobarbital, was studied in 42 medical and surgical patients [43]. In male NIH Swiss strain mice, chronic ingestion of caffeine increased the densities of cortical 5 HT1 and 5 HT2 serotonergic receptors (26–30%) and benzodiazepine-binding sites associated with GABAA receptors (65%) [44]. Taken together, administration of caffeine produced arousal effects and a wide range of biochemical alterations in the CNS and the combined GABA/5-HTP treatment produced inhibitory effects by binding to upregulated GABAergic and serotonergic receptors.
We also investigated the inhibitory effect of the combined GABA/5-HTP on sleep/wake architecture in the caffeine-induced sleepless rats (Fig. 4). Oral administration of combined GABA/5-HTP increased total sleep time and NREM sleep compared to the control group. Paterson et al. [21] assessed the dose-dependent effect of caffeine on all sleep parameters using EEG analysis in SD rats and discussed the merits of using caffeine as an insomnia model. In addition, according to the report by Lazarus et al. [45] caffeine acts to produce motivational and motor responses identified using focal RNA interference to silence the expression of adenosine A2A receptors in the rat brain. Caffeine administration led to increased wakefulness and reduced EEG slow wave activity during subsequent sleep periods [46,47]. In contrast, the GABAA receptor agonist THIP (4,5,6,7,-tetrahydroisoxazole [5,4-c] pyridine-3-ol), a muscimol derivative, enhanced spectral power in the low-frequency range of EEG and affected EEG slow wave activity patterns in both waking and NREM sleep periods in rodents and humans [48]. In our previous study, GABA showed ‘temporary but unstable’ sedation and 5-HTP had a ‘delayed but stabilized’ effect on flies. The combination of GABA and 5-HTP can support stabilization and delayed effects [33,34]. The GABA re-uptake inhibitor tiagabine dose-dependently elevated the lower frequency waves during NREM sleep [49]. In addition, studies have shown that the serotonergic system and 5-HT level are linked to slow wave activity generation and that 5-HT receptors are involved in GABAergic interneuron and motoneuron modulation [50,51,52].
In conclusion, using video tracking to measure behavior, as well as electrophysiological approaches, we identify that two neuronal substances and their mixture enhance the quantity and quality of sleep in the caffeine-induced sleepless model system. The combined evidence suggests that the caffeine-induced sleepless model is a valid system for investigating the inhibitory and sleep-promoting effects of non-pharmacological remedies, and the combined GABA and 5-HT precursors can be helpful in patients with sleep disorder who have mild or moderate insomnia. The combined GABA/5-HTP could be a potential medication that acts at low doses with fewer side effects on behavioral and neurochemical responses.
Footnotes
This research was supported by the Ministry of Trade, Industry and Energy (MOTIE) and the Korea Institute for Advancement of Technology (KIAT) through the Encouragement Program for The Industries of Economic Cooperation Region.
CONFLICT OF INTEREST: The authors declare no potential conflicts of interests.
References
- 1.Léger D, Poursain B, Neubauer D, Uchiyama M. An international survey of sleeping problems in the general population. Curr Med Res Opin. 2008;24:307–317. doi: 10.1185/030079907x253771. [DOI] [PubMed] [Google Scholar]
- 2.Chen MY, Wang EK, Jeng YJ. Adequate sleep among adolescents is positively associated with health status and health-related behaviors. BMC Public Health. 2006;6:59. doi: 10.1186/1471-2458-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L, Zhao ZX. Objective and subjective measures for sleep disorders. Neurosci Bull. 2007;23:236–240. doi: 10.1007/s12264-007-0035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wafford KA, Ebert B. Emerging anti-insomnia drugs: tackling sleeplessness and the quality of wake time. Nat Rev Drug Discov. 2008;7:530–540. doi: 10.1038/nrd2464. [DOI] [PubMed] [Google Scholar]
- 5.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- 6.Longo LP, Johnson B. Addiction: part I. Benzodiazepines--side effects, abuse risk and alternatives. Am Fam Physician. 2000;61:2121–2128. [PubMed] [Google Scholar]
- 7.Toth LA, Bhargava P. Animal models of sleep disorders. Comp Med. 2013;63:91–104. [PMC free article] [PubMed] [Google Scholar]
- 8.Haynes PR, Christmann BL, Griffith LC. A single pair of neurons links sleep to memory consolidation in Drosophila melanogaster. Elife. 2015;4:e03868. doi: 10.7554/eLife.03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol. 2006;16:1051–1062. doi: 10.1016/j.cub.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 10.Bushey D, Tononi G, Cirelli C. Sleep- and wake-dependent changes in neuronal activity and reactivity demonstrated in fly neurons using in vivo calcium imaging. Proc Natl Acad Sci U S A. 2015;112:4785–4790. doi: 10.1073/pnas.1419603112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilestro GF. Video tracking and analysis of sleep in Drosophila melanogaster. Nat Protoc. 2012;7:995–1007. doi: 10.1038/nprot.2012.041. [DOI] [PubMed] [Google Scholar]
- 12.Deboer T. Technologies of sleep research. Cell Mol Life Sci. 2007;64:1227–1235. doi: 10.1007/s00018-007-6533-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang ZJ, Yu B, Zhang XQ, Sheng ZF, Li SJ, Huang YL, Cao Q, Cui XY, Cui SY, Zhang YH. Correlations between depression behaviors and sleep parameters after repeated corticosterone injections in rats. Acta Pharmacol Sin. 2014;35:879–888. doi: 10.1038/aps.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribeiro JA, Sebastião AM. Caffeine and adenosine. J Alzheimers Dis. 2010;20(Suppl 1):S3–S15. doi: 10.3233/JAD-2010-1379. [DOI] [PubMed] [Google Scholar]
- 15.Jabbar SB, Hanly MG. Fatal caffeine overdose: a case report and review of literature. Am J Forensic Med Pathol. 2013;34:321–324. doi: 10.1097/PAF.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 16.Snel J, Lorist MM. Effects of caffeine on sleep and cognition. Prog Brain Res. 2011;190:105–117. doi: 10.1016/B978-0-444-53817-8.00006-2. [DOI] [PubMed] [Google Scholar]
- 17.Paterson LM, Wilson SJ, Nutt DJ, Hutson PH, Ivarsson M. A translational, caffeine-induced model of onset insomnia in rats and healthy volunteers. Psychopharmacology (Berl) 2007;191:943–950. doi: 10.1007/s00213-006-0672-0. [DOI] [PubMed] [Google Scholar]
- 18.Wright KP, Jr, Bogan RK, Wyatt JK. Shift work and the assessment and management of shift work disorder (SWD) Sleep Med Rev. 2013;17:41–54. doi: 10.1016/j.smrv.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Ko CH, Koon CM, Yu SL, Lee KY, Lau CB, Chan EH, Wing YK, Fung KP, Leung PC. Hypnotic effects of a novel anti-insomnia formula on Drosophila insomnia model. Chin J Integr Med. 2016;22:335–343. doi: 10.1007/s11655-014-1625-1. [DOI] [PubMed] [Google Scholar]
- 20.Palkovits M. The rat brain in stereotaxic coordinates. Neuropeptides. 1983;3:319. [Google Scholar]
- 21.Paterson LM, Wilson SJ, Nutt DJ, Hutson PH, Ivarsson M. Characterisation of the effects of caffeine on sleep in the rat: a potential model of sleep disruption. J Psychopharmacol. 2009;23:475–486. doi: 10.1177/0269881109104846. [DOI] [PubMed] [Google Scholar]
- 22.Revel FG, Gottowik J, Gatti S, Wettstein JG, Moreau JL. Rodent models of insomnia: a review of experimental procedures that induce sleep disturbances. Neurosci Biobehav Rev. 2009;33:874–899. doi: 10.1016/j.neubiorev.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Al-Shamma HA, Anderson C, Chuang E, Luthringer R, Grottick AJ, Hauser E, Morgan M, Shanahan W, Teegarden BR, Thomsen WJ, Behan D. Nelotanserin, a novel selective human 5-hydroxytryptamine2A inverse agonist for the treatment of insomnia. J Pharmacol Exp Ther. 2010;332:281–290. doi: 10.1124/jpet.109.160994. [DOI] [PubMed] [Google Scholar]
- 24.Gmeiner F, Kołodziejczyk A, Yoshii T, Rieger D, Nässel DR, Helfrich-Förster C. GABA(B) receptors play an essential role in maintaining sleep during the second half of the night in Drosophila melanogaster. J Exp Biol. 2013;216:3837–3843. doi: 10.1242/jeb.085563. [DOI] [PubMed] [Google Scholar]
- 25.Kantrowitz J, Citrome L, Javitt D. GABA(B) receptors, schizophrenia and sleep dysfunction: a review of the relationship and its potential clinical and therapeutic implications. CNS Drugs. 2009;23:681–691. doi: 10.2165/00023210-200923080-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Javitt DC, Hashim A, Sershen H. Modulation of striatal dopamine release by glycine transport inhibitors. Neuropsychopharmacology. 2005;30:649–656. doi: 10.1038/sj.npp.1300589. [DOI] [PubMed] [Google Scholar]
- 27.Vacher CM, Gassmann M, Desrayaud S, Challet E, Bradaia A, Hoyer D, Waldmeier P, Kaupmann K, Pévet P, Bettler B. Hyperdopaminergia and altered locomotor activity in GABAB1-deficient mice. J Neurochem. 2006;97:979–991. doi: 10.1111/j.1471-4159.2006.03806.x. [DOI] [PubMed] [Google Scholar]
- 28.Arnaud C, Gauthier P, Gottesmann C. Study of a GABAC receptor antagonist on sleep-waking behavior in rats. Psychopharmacology (Berl) 2001;154:415–419. doi: 10.1007/s002130000653. [DOI] [PubMed] [Google Scholar]
- 29.Ciranna L. Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: implications in physiological functions and in pathology. Curr Neuropharmacol. 2006;4:101–114. doi: 10.2174/157015906776359540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung BY, Kilman VL, Keath JR, Pitman JL, Allada R. The GABA(A) receptor RDL acts in peptidergic PDF neurons to promote sleep in Drosophila. Curr Biol. 2009;19:386–390. doi: 10.1016/j.cub.2009.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, Kang K, Liu X, Garrity PA, Rosbash M, Griffith LC. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanover KE, Davis RE. Role of 5-HT2A receptor antagonists in the treatment of insomnia. Nat Sci Sleep. 2010;2:139–150. doi: 10.2147/nss.s6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong KB, Park Y, Suh HJ. Sleep-promoting effects of a GABA/5-HTP mixture: Behavioral changes and neuromodulation in an invertebrate model. Life Sci. 2016;150:42–49. doi: 10.1016/j.lfs.2016.02.086. [DOI] [PubMed] [Google Scholar]
- 34.Hong KB, Park Y, Suh HJ. Sleep-promoting effects of the GABA/5-HTP mixture in vertebrate models. Behav Brain Res. 2016;310:36–41. doi: 10.1016/j.bbr.2016.04.049. [DOI] [PubMed] [Google Scholar]
- 35.Hosie AM, Sattelle DB. Agonist pharmacology of two Drosophila GABA receptor splice variants. Br J Pharmacol. 1996;119:1577–1585. doi: 10.1111/j.1476-5381.1996.tb16075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leal SM, Neckameyer WS. Pharmacological evidence for GABAergic regulation of specific behaviors in Drosophila melanogaster. J Neurobiol. 2002;50:245–261. doi: 10.1002/neu.10030. [DOI] [PubMed] [Google Scholar]
- 37.Baier A, Wittek B, Brembs B. Drosophila as a new model organism for the neurobiology of aggression? J Exp Biol. 2002;205:1233–1240. doi: 10.1242/jeb.205.9.1233. [DOI] [PubMed] [Google Scholar]
- 38.Zhu H, Zhang L, Wang G, He Z, Zhao Y, Xu Y, Gao Y, Zhang L. Sedative and hypnotic effects of supercritical carbon dioxide fluid extraction from Schisandra chinensis in mice. J Food Drug Anal. 2016;24:831–838. doi: 10.1016/j.jfda.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrow JD, Vikraman S, Imeri L, Opp MR. Effects of serotonergic activation by 5-hydroxytryptophan on sleep and body temperature of C57BL/6J and interleukin-6-deficient mice are dose and time related. Sleep. 2008;31:21–33. doi: 10.1093/sleep/31.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao X, Cui XY, Wang LE, Zhang YH. Potentiating effect of diltiazem on pentobarbital-induced hypnosis is augmented by serotonergic system: the TMN and VLPO as key elements in the pathway. Neuropharmacology. 2009;56:937–943. doi: 10.1016/j.neuropharm.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Mabunga DF, Gpmzales EL, Kim HJ, Choung SY. Treatment of GABA from fermented rice germ ameliorates caffeine-induced sleep disturbance in mice. Biomol Ther (Seoul) 2015;23:268–274. doi: 10.4062/biomolther.2015.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuribara H, Asahi T, Tadokoro S. Ethanol enhances, but diazepam and pentobarbital reduce the ambulation-increasing effect of caffeine in mice. Arukoru Kenkyuto Yakubutsu Ison. 1992;27:528–539. [PubMed] [Google Scholar]
- 43.Forrest WH, Jr, Bellville JW, Brown BW., Jr The interaction of caffeine with pentobarbital as a nighttime hypnotic. Anesthesiology. 1972;36:37–41. doi: 10.1097/00000542-197201000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Shi D, Nikodijević O, Jacobson KA, Daly JW. Chronic caffeine alters the density of adenosine, adrenergic, cholinergic, GABA, and serotonin receptors and calcium channels in mouse brain. Cell Mol Neurobiol. 1993;13:247–261. doi: 10.1007/BF00733753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lazarus M, Shen HY, Cherasse Y, Qu WM, Huang ZL, Bass CE, Winsky-Sommerer R, Semba K, Fredholm BB, Boison D, Hayaishi O, Urade Y, Chen JF. Arousal effect of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J Neurosci. 2011;31:10067–10075. doi: 10.1523/JNEUROSCI.6730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwierin B, Borbély AA, Tobler I. Effects of N6-cyclopentyladenosine and caffeine on sleep regulation in the rat. Eur J Pharmacol. 1996;300:163–171. doi: 10.1016/0014-2999(96)00021-0. [DOI] [PubMed] [Google Scholar]
- 47.Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14:557–568. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- 48.Vyazovskiy VV, Kopp C, Bösch G, Tobler I. The GABAA receptor agonist THIP alters the EEG in waking and sleep of mice. Neuropharmacology. 2005;48:617–626. doi: 10.1016/j.neuropharm.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 49.Lancel M, Faulhaber J, Deisz RA. Effect of the GABA uptake inhibitor tiagabine on sleep and EEG power spectra in the rat. Br J Pharmacol. 1998;123:1471–1477. doi: 10.1038/sj.bjp.0701769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jouvet M. Sleep and serotonin: an unfinished story. Neuropsychopharmacology. 1999;21:24S–27S. doi: 10.1016/S0893-133X(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 51.Fukushima T, Ohtsubo T, Tsuda M, Yanagawa Y, Hori Y. Facilitatory actions of serotonin type 3 receptors on GABAergic inhibitory synaptic transmission in the spinal superficial dorsal horn. J Neurophysiol. 2009;102:1459–1471. doi: 10.1152/jn.91160.2008. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Li L, Stephens MJ, Zenner D, Murray KC, Winship IR, Vavrek R, Baker GB, Fouad K, Bennett DJ. Synthesis, transport, and metabolism of serotonin formed from exogenously applied 5-HTP after spinal cord injury in rats. J Neurophysiol. 2014;111:145–163. doi: 10.1152/jn.00508.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]