Abstract
The widespread planting of corn genetically modified to produce Bacillus thuringiensis endotoxin has led to speculation that pollen from these fields might adversely affect nearby nontarget lepidopterans. A previous study of Bt corn engineered with Monsanto event 810 failed to detect an effect of pollen exposure on the black swallowtail, Papilio polyxenes, in either the field or the laboratory. Here, we report results of a field study investigating the impact of exposure to pollen from a Bt corn hybrid containing Novartis event 176 on two species of Lepidoptera, black swallowtails and monarch butterflies, Danaus plexippus. Nearly half of the 600 monarch larvae died within the first 24 h; this and subsequent mortality was not associated with proximity to Bt corn and may have been due in part to predation. Survivorship of black swallowtails was much higher than that of the monarchs and was also independent of proximity to the transgenic corn. However, despite five rainfall events that removed much of the pollen from the leaves of their host plants during the experiment, we observed a significant reduction in growth rates of black swallowtail larvae that was likely caused by pollen exposure. These results suggest that Bt corn incorporating event 176 can have adverse sublethal effects on black swallowtails in the field and underscore the importance of event selection in reducing environmental impacts of transgenic plants.
With the exception of herbicide-tolerant soybeans, Bt corn (Zea mays engineered to express genes from the soil bacterium Bacillus thuringiensis that encode the insecticidal protein toxins Cry1Ac, Cry1Ab, or Cry9C) is the most widely grown transgenic crop plant in the United States. In 1999, Bt corn was planted on 9.6 million hectares (1). The principal target species for Bt corn is the European corn borer (ECB), Ostrinia nubilalis, one of the most damaging pests of corn in North America (http://www.extensionumn.edu/Documents/D/C/DC7055.html). Losses to ECB damage and costs of control range upward of $1 billion annually in the United States. In addition to direct damage, ECB damage leaves corn vulnerable to infection by Fusarium fungi; these pathogens can produce highly toxic fumonisins, which pose a risk to human health if ingested.
Although Bt corn has been touted as an environmentally friendly alternative to the synthetic organic insecticides traditionally used for ECB control in sweet corn (including permethrin, bifenthrin, lambda-cyhalothrin, and methyl parathion) (2), concerns have been raised that there may be adverse effects of Bt corn use on nontarget lepidopterans and their consumers (3). In a laboratory feeding study, Losey et al. (4) demonstrated that exposure to Bt corn pollen can cause mortality in neonate monarch caterpillars (Danaus plexippus). Despite the fact that the authors cautioned that “it would be inappropriate to draw any conclusion about the risk to monarch populations in the field based solely on these initial results,” the study created a widespread perception of risk, particularly among nonscientists (5). In a second study, Hansen-Jesse and Obrycki (6) fed milkweed foliage, which was “naturally dusted” under field conditions with pollen from Bt corn, to monarch caterpillars in laboratory feeding trials; they reported significantly greater mortality of larvae that consumed foliage contaminated with Bt pollen, although no dose-dependent effect of pollen concentration was observed.
To date, the only published study done to examine the consequences of exposure to Bt corn pollen on nontarget lepidopterans in the field is Wraight et al. (7). In this study, which dealt not with D. plexippus but rather with Papilio polyxenes, the black swallowtail, no mortality could be directly attributable to exposure to MON810 corn pollen under field conditions. How representative P. polyxenes is of nontarget lepidopterans that live alongside cornfields is an open question. In a companion laboratory experiment, however, these authors demonstrated that P. polyxenes is sensitive to pollen from Novartis event 176, which contained 40-fold higher concentrations of endotoxin than does MON810.
Environmentalists as well as government regulators are calling for more detailed studies on possible nontarget impacts of Bt corn. In the Federal Insecticide, Fungicide and Rodenticide Act Scientific Advisory Panel Report No. 99-06, released February 4, 2000 (http://www.epa.gov/scipoly/sap/1999/december/report.pdf), “Characterization and non-target organism data requirements for protein plant-pesticides,” the Panel concluded that current nontarget testing requirements were inadequate, in that they were limited in terms of species numbers, and called for “additional research . . . on the various possible effects of plant pesticidal proteins on non-target insects.” Here, we report the results of a study comparing responses of two different nontarget species with larval ecologies that place them at risk of exposure; both the black swallowtail P. polyxenes and the monarch caterpillar D. plexippus feed on weedy forbs that are frequently found in or around cornfields throughout the Midwest. Moreover, for the first time, we document sublethal effects of Bt corn pollen on growth and development of P. polyxenes in the field.
Materials and Methods
Host Plants.
The host plant selected for testing P. polyxenes susceptibility to Bt corn pollen was wild parsnip, Pastinaca sativa. P. sativa grows extensively along field edges throughout central Illinois and is available as a food plant during the period when corn sheds pollen. Plants were grown in the greenhouse from seed collected in the field in Champaign County, IL and sown in plastic pots measuring 17 cm in diameter and 21 cm in height and containing a mixture of one part soil, two parts peat, and two parts perlite. At the time of the experiment, these plants were rosettes with several large compound leaves.
The host plant selected for testing D. plexippus susceptibility to Bt corn was Asclepias syriaca, like parsnip, a conspicuous element of the flora found at field edges in central Illinois (8). All but five of the milkweed plants used in the experiment were grown in the greenhouse from locally collected seeds. Seeds were sown in pots and soil identical to those used for the parsnips. At the time of the experiment, these plants were between 60 and 90 cm tall. Several of the milkweeds became diseased, as evidenced by a darkening of the leaves, and were discarded. As a result, there were five fewer seed-grown plants than the 25 plants required for the experiment. Consequently, additional milkweeds were obtained by transplanting A. syriaca in the vicinity of the experimental plot to pots containing field soil.
Insects.
Black swallowtail eggs were obtained from 23 females caught in central Illinois shortly before the start of the experiment. The females were caged together with wild parsnips to elicit oviposition. Monarch eggs deposited on milkweed foliage were provided by Dr. Patrick Hughes from a colony maintained at the Boyce Thompson Institute (Ithaca, NY). Although the use of insects from a laboratory colony may introduce artifacts due to unusual behaviors or physiology resulting from continuous rearing, the requirement for large numbers of eggs on a particular date precluded use of local wild-caught females, which were not sufficiently abundant at the time of the experiment to provide a large single cohort of neonates.
Field Experiment.
Field plot design.
A 30 × 30-m plot of Max 454 Bt corn, which contains Novartis event 176, was planted in late May with rows oriented north–south at the University of Illinois Phillips Tract research area, located 1.5 km northeast of Urbana. To ensure that we had an edge consisting of plants of uniform and typical size, an 8-m swath of the corn was mown along the north side of the plot two weeks before initiation of the experiment.
The corn first began to shed pollen on July 24, 2000. The following day, we placed 20 potted parsnips and 25 potted milkweeds, numbers sufficient to accommodate the larvae available, in an array on the north side of the plot. The first row of plants, consisting of four parsnips and five milkweeds, was situated 0.5 m from the edge of the corn. Additional rows were positioned 1, 2, 4, and 7 m from the corn. Except for the extra milkweed plant, within a row, parsnips and milkweeds were paired. Within a row, the spacing between pairs and between a pair and the lone milkweed was 1 m.
Rate of pollen deposition.
Commencing on July 26 and on each subsequent day between 9:00 am and noon for the duration of the experiment, we sampled pollen from two randomly selected parsnips and milkweeds at each distance from the cornfield. The pollen was sampled by applying 2–3 drops of a mixture of 1 part Duco cement (Devcon Consumer Products, Rivera Beach, FL) and 3 parts acetone onto a leaf. After the glue dried, it was carefully peeled off with forceps, removing all of the pollen grains while maintaining their spatial pattern. This method of pollen sampling is superior to other methods of pollen monitoring in that it allows accurate determination of pollen densities on the foliage without removing foliage, which may alter plant chemistry. Moreover, the method also prevents loss of pollen through disturbance and permits grains to be stained in their original dispersion pattern.
After the peel was removed, the location on the leaf that was sampled was marked with a small “x” in black indelible ink to ensure that it would not be subsequently resampled. These “pollen peels” were brought to the laboratory and stained with a dye consisting of 5 ml of glycerol, 10 ml of ethanol, 15 ml of water, and enough crystals of basic fuchsin to render the staining solution a deep red. The peels were rinsed in distilled water, and the pollen grains were counted at 40× magnification. Counts were converted to number of grains per cm2 of leaf area and averaged for the two samples per host plant at each distance from the cornfield.
Caterpillar performance.
Larvae of both species were placed onto plants in the field when sufficient numbers of eggs hatched to produce a large cohort of neonates. Neonate monarchs, 24 to a plant, were placed on a fully expanded leaf near the top of 24 of 25 plants, one of the transplanted milkweeds having died before egg hatch. All other milkweed plants remained healthy and vigorous throughout the duration of the experiment. In total, 600 larvae were put in place on July 28, 4 days after the onset of pollen release. On the following day, 15 swallowtail neonates were placed on a single leaf of each of 20 parsnip plants for a total of 300 caterpillars. The number of live larvae remaining each day was recorded for a total of 6 days for monarchs and 5 days for swallowtails. We modified our census procedure on the second day, after observing a nearly 50% decline in monarchs 24 h after placement of the larvae. Because the decline appeared to be unaffected by proximity to the corn, we suspected that predation was the cause. Accordingly, as part of the daily protocol, we continued to census caterpillars and also recorded the number and type of predaceous arthropods on each plant. After predators were identified and counted, they were destroyed.
The final census date, August 3, coincided with the cessation of pollen shed and followed by one day the last of five rainfall events, which took place on July 28, 29, 30, 31, and August 2. On the final census day, all larvae were collected and weighed to the nearest milligram in the laboratory. Because the distribution and variation in the larval mass data for black swallowtails did not conform to the requirements for analysis of variance, a Kruskal–Wallace analysis was performed to determine whether proximity to the cornfield affected larval mass (SPSS, Chicago). Survivorship curves in the field were compared as a function of distance by Kaplan–Meier analysis (survival procedure in SPSS).
Laboratory bioassay.
As reported in an earlier study (7), four pollen concentrations, 10,000, 1,000, 100, and 10 grains per cm2 of Bt pollen, together with an acetone control were bioassayed in the summer of 1999 against first instar black swallowtails in the laboratory. In that study, the results for only a single concentration of Max 454 pollen were reported. In this study, we report an LD50 for Max 454 based on all five concentrations. The LD50 of the pollen was determined by probit regression (SPSS), and survivorship curves at different doses were compared by Kaplan–Meier analysis (survival procedure in SPSS).
Results
Rates of Pollen Deposition.
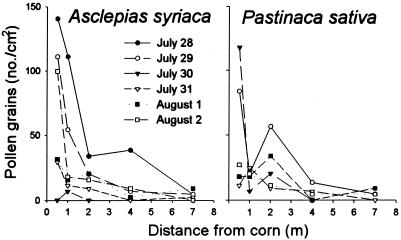
Pollen counts varied considerably, depending on the location of the sample within a leaf. Pollen was most prevalent along major veins and, on milkweed leaves, particularly along the midvein. Despite the high variation in pollen densities from sample to sample, patterns of pollen distribution observed in this study (Fig. 1) were consistent with those previously reported (6, 7, 9), in that the greatest amounts of pollen were deposited in close proximity to the cornfield.
Figure 1.
Pollen densities on foliage of milkweeds (A. syriaca) and wild parsnips (P. sativa) as a function of time and distance from a stand of event 176 Bt corn. In each case, larvae were placed on the plants on the earliest date indicated. Rainfalls of 0.20, 0.43, 0.10, and 0.10 cm occurred on July 28, 29, 30, and 31, respectively. An additional rainfall of 1.56 cm occurred on August 2 after the last pollen sampling and the day before the final larval census.
Pollen densities before placement of the larvae on the plants (July 27, data not shown in Fig. 1) were higher than those on the day the larvae were put in place. For example, on milkweed foliage, the highest average density of pollen was 260 grains/cm2 on plants 0.5 m from the corn and 170 grains/cm2 on plants located 1 m away from the corn.
On parsnip foliage, the highest average pollen density was 320 grains/cm2 0.5 m from the corn. Remarkably high densities (180 grains/cm2) were observed as far as 2 m from the corn. However, because of rain events, the test larvae did not experience these higher pollen levels. In fact, pollen densities declined dramatically after rain events (Fig. 1).
Caterpillar Performance.
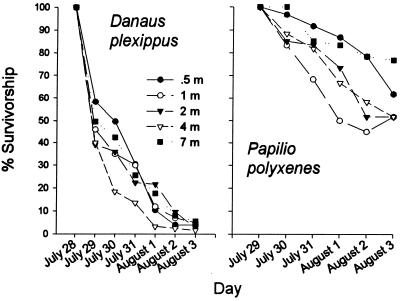
Mortality was most pronounced among the monarch larvae. After 6 days in the field, fewer than 7% of the larvae survived (Fig. 2). The decline was greatest during the first 24 h, and the rate of decline slowed after we began systematically removing predatory arthropods. Despite clear differences in exposure to pollen as function of distance (Fig. 1), survivorship curves did not differ significantly according to distance (log rank statistic = 1.22, P = 0.269). Proximity also did not affect mass of the surviving larvae (ANOVA F = 0.528, df = 4, 17, P = 0.716), although, with only 22 of the 600 monarch larvae surviving, statistical power was extremely low for this analysis.
Figure 2.
Survivorship of monarch (D. plexippus) and black swallowtail (P. polyxenes) larvae as a function of distance from a field of event 176 Bt corn.
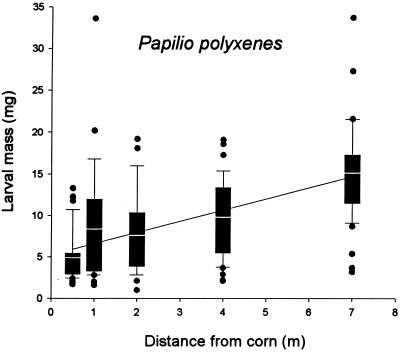
Mortality of black swallowtail larvae was noticeably less than that of monarchs (Fig. 2), and, as was the case for the monarchs, mortality was not affected by proximity to corn; there were no significant differences among survivorship curves (log rank statistic = 1.62, P = 0.203). However, larval masses were significantly negatively affected by proximity to the corn (Fig. 3). Larvae 7 m from the corn attained a biomass that on average was three times that of larvae located 0.5 m from the corn. The best-fitting regression between mean larval mass and proximity to corn was linear (F = 30.3, P = 0.012) and accounted for 91% of the variation.
Figure 3.
Larval masses of the black swallowtail (P. polyxenes) at different distances from a field of event 176 Bt corn. The white lines within the boxes are the means. The boundaries of the boxes represent the 25th and 75th percentiles, and the boundaries of the whiskers represent the 10th and 90th percentiles. ●, outliers (one outlier value of 51.8 in the 7 m position is not shown). Larval masses differed significantly among distance treatments (Kruskal–Wallace χ2 = 60.5, P < 0.001). The regression of mean larval mass against distance was also significant (P = 0.012, y = 1.36x + 5.23). Sample sizes for distances from 0.5 to 7 m were 39, 26, 25, 36, and 46, respectively.
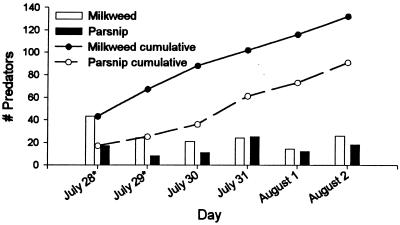
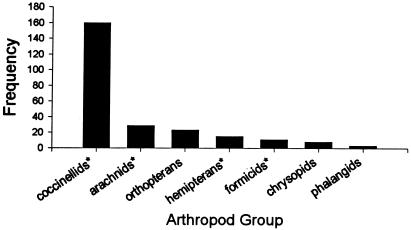
Despite daily removals, the frequency of predaceous arthropods was remarkably constant and, based on cumulative totals, remained constant because of a steady influx of individuals (Fig. 4). On two sampling dates, predator numbers were significantly greater on milkweed plants than on parsnip plants (Fig. 4); differences in predator number may have contributed to the lower survivorship of monarchs in the field. By far the most abundant predators were adult and larval coccinellids (Fig. 5). They were found on both host plants but were most numerous on milkweeds in the vicinity of the apical meristem, where aphids had become established and where many of the monarch larvae moved after being placed on a fully expanded leaf near the top of the plant. Very few acts of predation were observed during the sampling periods; on one occasion, an immature Podisus maculiventris was observed and photographed consuming a monarch larva.
Figure 4.
Daily and cumulative totals of arthropods found and removed from milkweeds and wild parsnips during the experiment. Dates with asterisks indicate significantly higher numbers of predaceous arthropods on milkweeds (χ2 values = 11.26 and 8 for July 28 and 29, respectively; both values have probabilities less than 0.05).
Figure 5.
Frequency of taxonomic groups found and removed from both milkweeds and wild parsnips during the experiment. Asterisks denote groups known to be generalist consumers of lepidopteran larvae.
Laboratory Bioassay.
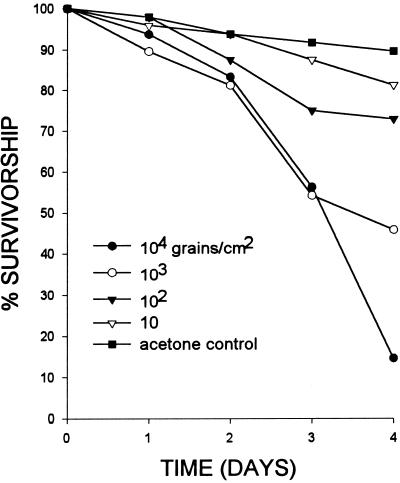
The laboratory bioassays of event 176 pollen revealed significant mortality at doses of 100 grains/cm2 and higher (log rank test P = 0.0405 for comparison of the 100 grains/cm2 dose with the control) (Fig. 6). The LD50 for Bt pollen in this bioassay was 613 grains/cm2, with a 95% confidence interval between 299 and 1151.
Figure 6.
Survivorship of first instar black swallowtails administered different doses of event 176 pollen in the laboratory. The only survivorship curve that does not differ from control is the 10 grains/cm2 (log rank test P = 0.263). Pollen was suspended in acetone and applied to leaf material. The control consisted of addition of acetone only. For details of the bioassay method, see Wraight et al. (7).
Discussion
In 1999, Wraight et al. (7) conducted a study designed to detect effects of event MON810 pollen on black swallowtails. That study failed to detect an effect of Bt pollen on either survivorship or larval mass in the field or on survivorship in the laboratory. However, a laboratory bioassay of event 176 pollen demonstrated that this pollen was toxic at high doses to black swallowtails. The bioassay reported in this present study (Fig. 6) indicates that concentrations of event 176 pollen as low as 100 grains/cm2 cause significant mortality in black swallowtails. Given the wide confidence interval, the LD50 for black swallowtails, 613 grains/cm2, is not substantially different from the LD50 of 389 grains/cm2 reported for monarchs (9).
The results of this study suggest that pollen from Bt corn varieties engineered with the 176 event may have sublethal effects on black swallowtails feeding on host plants situated outside of cornfields. The cause of the reduction in larval mass as a function of proximity to the Bt corn (Fig. 3) is most likely toxicity because of the ingestion of transgenic pollen grains. Alternative explanations not involving Bt pollen fail to account satisfactorily for the observed pattern of larval masses. Possible explanations involve modification of the thermal environment by the corn. A stand of corn may affect the thermal environment outside its boundaries via shading and mass flow of transpirationally cooled air. Because the experiment was situated on the north side of the field, where the flow of pollen outside of the field was expected to be greatest (prevailing summer winds are from the south and southwest), a shadow cast by the corn could have fallen on larvae near the corn. By virtue of their early instar black color and habit of feeding exposed on the upper sides of leaves, black swallowtail body temperatures are likely to be affected by exposure to direct sunlight. However, at the time that the experiment was performed, the sun was close to its zenith, and the broken shadow cast by the stems and leaves extended no farther than 0.7 m from the field's edge. Thus, the impact of shading could not have affected the larvae at 1 m and beyond. A reanalysis of larval masses omitting the 0.5-m data remained highly significant (Kruskal–Wallace χ2 value = 30.766, P < 0.001), ruling out the influence of shading on reduced larval mass.
Another possible explanation for reduced growth is that evaporatively cooled air from the corn caused a temperature gradient extending from the borders of the corn that in turn caused differences in growth rate. This explanation also appears unlikely to account for our results. Three days after completion of the experiment, we measured air temperatures at 0.5, 1, 2, 4, and 7 m along ten transects within the experimental plot. Temperature was measured 30 cm above the ground with a TH-65 thermocouple thermometer (Wescor, Logan, UT). We did find a significant temperature gradient increasing away from the field (y = 0.262x − 0.016x2 +25.98, F = 7.76, df = 47, P = 0.004). However, the difference in mean temperature between 0.5 m and 7 m was only 0.94°C. For a single degree difference to cause a 3-fold increase in growth, the Q10 for growth of the black swallowtail would have to have been on the order of 60,000. Growth Q10 levels based on data from Knapp and Casey (10) were on the order of 2 and 5, respectively, for two other lepidopteran species, Lymantria dispar and Malacosoma americanum. Effects on thermal environments and other potential effects of cornfields not involving pollen are further discounted by the lack of any effect on larval mass of a different and less toxic form of Bt corn (7).
The effect of the event 176 Bt pollen in this study is presumably less than the potential effect that would have been observed had repeated rainfalls not washed pollen off of the foliage. Only once during the swallowtail experiment did pollen levels exceed 60 grains per cm2 beyond 0.5 m (Fig. 1), whereas, before the experiment and before the rainfalls, average pollen densities were three times higher as far as 2 m from the corn (192 grains/cm2). Consequently, the effects observed in this study must be considered conservative.
Whether the observed reduction in larval mass was due to direct toxicity of ingested pollen or to antifeedant effects of pollen exposure (e.g., ref. 6) is unclear. Also unresolved are the long-term consequences of larval mass reduction over the life of the caterpillar. Hansen-Jesse and Obrycki (6) suggest that age may influence susceptibility of monarch larvae to pollen ingestion. Moreover, because the greatest amount of all food ingested over the course of caterpillar development is consumed in the ultimate instar, caterpillars experiencing developmental delays early in life may be able to compensate if they subsequently consume uncontaminated foliage.
Notwithstanding, this study documents sublethal effects of event 176 Bt corn pollen on a nontarget lepidopteran outside of a cornfield. Wraight et al. (7) suggested that nontarget impacts may be manageable by careful event selection. To some extent, this approach is already underway. During the 2000 growing season, event 176 corn comprised less than 1% of total U.S. acreage (Jeff Stein, Novartis, personal communication), and reregistration of the technology with the U.S. Environmental Protection Agency is not anticipated (http://www.nk.com/infosilo/news/release.cfm?releaseId = 113&sKeyword = newsrelease).
That there may be nontarget impacts of Bt corn is not in itself surprising; there are nontarget impacts of any pest management approach. Risks and benefits of Bt corn must be evaluated relative to alternative methods of management (3). A recent National Academy of Sciences study (NAS 2000) emphasizes the need for maintaining a “diverse toolbox” for pest management; careful use of tools, which includes use of the most appropriate tool for each situation, can help to preserve their utility as well as to maintain environmental quality.
Acknowledgments
We thank Dr. Patrick Hughes for supplying us with monarch eggs, Robert Dunker for planting and maintaining the Bt cornfield, and Steve Buck, Katrina Lustofin, Xianchun Li, and Daniel Skirvin for technical assistance. We also thank Kevin L. Steffey and Fred Gould for their careful review of the manuscript. This study was supported by funds from the University of Illinois Foundation and the University of Illinois at Urbana-Champaign Center for Advanced Study (to M.B.).
Abbreviation
- ECB
European corn borer
References
- 1.James C. Global Status of Commercialized Transgenic Crops. ISAAA Briefs #12: Preview. Ithaca, NY: International Service for the Acquisition of Agribiotech Applications; 1999. [Google Scholar]
- 2.Steffey K L, Gray M E. Illinois Agricultural Pest Management Handbook. Urbana: University of Illinois Urbana-Champaign Extension; 2001. [Google Scholar]
- 3.Wolfenbarger L L, Phifer P R. Science. 2000;290:2088–2093. doi: 10.1126/science.290.5499.2088. [DOI] [PubMed] [Google Scholar]
- 4.Losey J E, Rayor L S, Carter M E. Nature (London) 1999;399:214. doi: 10.1038/20338. [DOI] [PubMed] [Google Scholar]
- 5.Berenbaum M. Plant Physiol. 2001;125:1–4. doi: 10.1104/pp.125.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen-Jesse L C, Obrycki J J. Oecologia. 2000;125:241–248. doi: 10.1007/s004420000502. [DOI] [PubMed] [Google Scholar]
- 7.Wraight C L, Zangerl A R, Carroll M J, Berenbaum M R. Proc Natl Acad Sci USA. 2000;97:7700–7703. doi: 10.1073/pnas.130202097. . (First Published June 6, 2000; 10.1073/pnas.130202097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchholtz K P, Grigsby B H, Lee O C, Slife F W, Willard C J, Volk N J. Weeds of the North Central States. Urbana: University of Illinois Agricultural Experiment Station; 1979. [Google Scholar]
- 9.Sears M K, Stanley-Horn D. 6th International Symposium on the Biosafety of GMOs. 2000. [Google Scholar]
- 10.Knapp R, Casey T M. Ecology. 1986;67:598–608. [Google Scholar]