Abstract
Plant phenolics are being increasingly consumed globally with limited scientific and clinical evidence pertaining to safety and efficacy. The oil palm fruit contains a cocktail of phenolics, and palm oil production results in high volumes of aqueous by-products enriched in phenolics and bioactives. Several lines of evidence from in vitro and in vivo animal studies confirmed that the aqueous extract enriched in phenolics and other bioactives collectively known as oil palm phenolics (OPP) is safe and has potent bioactivity. A phase one clinical trial was conducted to evaluate the safety and effects of OPP in healthy volunteers. In this single-blind trial, 25 healthy human volunteers were supplemented with 450 mg gallic acid equivalent (GAE)/day of OPP or control treatments for a 60-day period. Fasting blood and urine samples were collected at days 1, 30 and 60. Medical examination was performed during these trial interventions. All clinical biochemistry profiles observed throughout the control and OPP treatment period were in the normal range with no major adverse effect (AE) or serious adverse effect (SAE) observed. Additionally, OPP supplementation resulted in improvement of total cholesterol and LDL-C levels, compared to the control treatment. The outcomes support our previous observations that OPP is safe and may have a protective role in reducing cholesterol levels.
Introduction
Phenolics are believed to be major contributors to the disease-protective effects of fruit and vegetables. They are important naturally occurring antioxidants and broadly characterized as aromatic metabolites that possess one or more ‘acidic’ phenolic hydroxyl groups1. Phenolics are mainly categorized into two classes; namely flavonoids and phenolic acids. Several types of flavonoids have been identified comprising flavones, flavonols, flavanols, flavanones, isoflavones, proanthocyanidins and anthocyanins. Several phenolic acid compounds such as caffeic, chlorogenic and ferulic acids are commonly found in daily food and beverages.
The oil palm fruit contains numerous phenolic compounds. During the extraction of palm oil from the oil palm fruit bunch, a large volume of water-soluble by-products rich in phenolic compounds is generated and discarded in the aqueous waste stream. Several phenolic compounds have been identified in the aqueous phase such as protocatechuic acid, p-hydroxybenzoic acid and three isomers of caffeoyl-shikimic acid2. Previously we successfully developed and patented a novel process to recover an extract enriched in phenolics and other bioactive compounds from the aqueous by-product3–5.
Condensed from the literature, a number of physiological and therapeutic effects of phenolics have been demonstrated. Phenolics may reduce cholesterol absorption due to the interaction of these compounds with cholesterol carriers and transporters present across the brush border membrane6. Several in vitro studies suggested that phenolics inhibit LDL-oxidation7,8 and aggregation of platelets9. OPP exhibits free-radical scavenging activity, by acting as a hydrogen donor and has been demonstrated to scavenge DPPH (2,2-diphenyl-picryl-hydrazyl) free radicals2,10. In mouse cell culture studies, OPP inhibited the progression of various cancer cell lines2. Potent anti-cancer activity of OPP was indicated in BALB/c mice injected with myeloma cells. The tumor numbers in the mice supplemented with OPP as a drink were significantly lower compared to controls11. Microarray profiling of BALB/c mice supplemented with OPP showed that several genes related to hepatic lipid catabolism were up-regulated, indicating suppression of liver fat and visceral fat accumulation in the body. Additionally, genes involved in cholesterol biosynthesis were down-regulated, suggesting a possible role in preventing hypercholesterolemia12. OPP also attenuated atherosclerosis13,14 as well as responded positively towards the distal colonic contractility and motility15 in animal models.
Long-term intake of OPP protected healthy, young Nile rats against diabetes onset, as measured by glucose, blood lipids, and weights of livers and kidneys11. These outcomes were probably due partly at least to the phenolic compounds in OPP and their protection of ß-cells in the pancreatic islets against oxidative stress, thereby maintaining the integrity and ability of ß-cells to produce insulin16. Recently, the anti-diabetic potential by OPP supplementation has been postulated to be a result of enhanced insulin sensitivity and reduced glucose absorption/output and not an increase in insulin secretion, as indicated by down-regulation of insulin signaling genes17.
The wide spectrum of bioactivities demonstrated by OPP in in vitro and in vivo systems suggests its potential application for a range of chronic diseases. Currently however, there is still no clinical data on the effects of OPP in humans in terms of safety and efficacy. Therefore, we conducted a phase one clinical trial to evaluate the physiological effects of OPP in healthy human subjects. The primary objective of this trial was to evaluate if there were any Adverse Effects (AE), and/or Serious Adverse Effects (SAE) following OPP administration (up to 60 days supplementation). Secondary objectives were to evaluate physiological effects which resulted from OPP supplementation. Data from this trial would be important for regulatory requirements and developing a safety profile of OPP besides understanding the physiological roles of OPP in humans under normal conditions.
Methods
Volunteers
Twenty-five healthy volunteers, consisting of 11 males and 14 females were recruited from the Malaysian Palm Oil Board (MPOB). Volunteers were thoroughly briefed on the objectives, design, and trial protocol before they signed the informed consent forms. All volunteers were normolipaemic, nonsmokers, and did not show any clinical symptoms associated with cardiovascular disease. They were examined for their health status and medical history by a medical officer before participation. Through the administration of a questionnaire and dietary interview, we established that none of the volunteers consumed any vitamin or herbal supplements, or were taking any prescribed medication. Female volunteers were not pregnant, lactating, or taking contraceptives at the time of recruitment. The recruitment was completed with the following baseline characteristics: Means ± SDs: Age, 29.24 ± 4.31 y; body mass index, 22.41 ± 3.99 kg/m2 (Table 1). The trial was approved by the Medical Research Ethics Committee (MREC), Malaysian Ministry of Health (NMRR-08-1616-3108) and registered with the Australian New Zealand Clinical Trial Registry (ANZCTR) database: www.anzctr.org.au/ (Trial Reference No: ACTRN 12611001122943, registration date: 27 Oct 2011). This registry is recognised by the World Health Organization International Clinical Trial Registry Platform (WHO ICTRP) as a Primary Registry. All methods and protocols were performed in accordance with the Declaration of Helsinki, The International Conference of Harmonisation (ICH) of Technical Requirements for Human Use and Good Clinical Practice (GCP).
Table 1.
Volunteers demographic characteristics. Data is tabulated as mean ± SD, or n (%).
| OPP treatment (n = 25) | Control treatment (n = 25) | |
|---|---|---|
| Age (years) | 29.24 ± 4.31 | |
| Sex | ||
| Male | 18 (72%) | |
| Female | 17 (68%) | |
| Body mass index (kg/m2) | 22.41 ± 3.99 | |
Study protocol
The trial was a single-blind trial, with comparison to control treatment. The study design enabled each subject to serve as his/her own control. On day one of the trial, volunteers attended a clinic for baseline measurement of plasma clinical profiles, after an overnight fast of at least 10 hours. Fasting blood (20 mL) and urine samples were taken. Volunteers were assigned into two intervention groups, where one group (treatment group) was given 150 mL of OPP (containing 1500 mg/L GAE OPP), twice per day. The total amount of phenolic compounds supplemented was 450 mg GAE/day. The composition of OPP was analyzed according to the method described previously2. Concentrations of major phenolic compounds are tabulated in Table 2. The other group (control group), was given 150 mL of control drinks (drinking water), twice per day. Both treatment and control drinks were made available in 250 mL amber glass bottles and kept refrigerated until distribution to the volunteers. In order to ensure compliance, all volunteers consumed both drinks in front of the investigator. All drinks consumption record was verified by the investigator and documented accordingly. The trial design allowed the investigators to detect early physiological outcomes from OPP intake. The trial period for OPP and control treatments was 60 days each where a four-week (28 days) wash-out period was allowed between both treatments. Volunteers were allowed to maintain their habitual diets and life styles during this period. Fasting blood (20 mL) and urine were also sampled at day 30 and 60. In every clinic visit, volunteers were thoroughly examined for their health status by the medical officer. The measurement of body weight (Table 3) and blood pressure (Table 4) was documented before each bleeding session.
Table 2.
Mean concentrations of major phenolic compounds in oil palm phenolics (OPP).
| Concentration (mg/L) | |
|---|---|
| Protocatechuic acid | 50 |
| p-Hydroxybenzoic acid | 577 |
| Caffeoylshikimic acid | 890 |
| Total major phenolics | 1517 |
| Gallic acid equivalent (GAE) | 1500 |
| Amount supplemented to volunteers per day (150 mL OPP, twice per day) | 450 mg/day |
Table 3.
Body weight changes in volunteers (mean ± SD).
| OPP treatment (n = 25) | Control treatment (n = 25) | |
|---|---|---|
| Body weight (kg) | ||
| Day 1 | 60.38 ± 14.2 | 60.4 ± 13.52 |
| Day 30 | 60.35 ± 14.14 | 60.21 ± 13.79 |
| Day 60 | 60.41 ± 14.0 | 60.2 ± 13.69 |
Table 4.
Blood pressure profiles (mean ± SD).
| OPP treatment (n=25) | Control treatment (n=25) | |
|---|---|---|
| Systolic (mm Hg) | ||
| Day 1 | 117.60 ± 13.32 | 115.20 ± 10.46 |
| Day 30 | 112.24 ± 11.35 | 112.64 ± 12.08 |
| Day 60 | 114.64 ± 10.32 | 112.64 ± 13.26 |
| Diastolic (mm Hg) | ||
| Day 1* | 79.92 ± 7.91 | 76.40 ± 7.44 |
| Day 30 | 74.48 ±7.33 | 74.24 ± 7.38 |
| Day 60 | 74.32 ± 7.02 | 76.88 ± 8.81 |
| Pulse rate (bpm) | ||
| Day 1 | 68.72 ± 3.26 | 69.12 ± 3.96 |
| Day 30 | 70.56 ± 3.81 | 71.04 ± 4.62 |
| Day 60 | 68.80 ± 4.04 | 69.04 ± 3.61 |
*Baseline diastolic level (day 1) was significantly different between treatments, p = 0.043 (Wilcoxon’s signed-rank test). Values were further used as covariate in repeated measures. The time × treatment analysis was however not significant, p = 0.118 (2-factor repeated measures ANOVA).
Blood samples were drawn from the volunteers by an experienced and well-trained phlebotomist using syringes. A 20 mL blood sample was then transferred into blood collection tubes either with or without ethylenediamine tetra acetic acid (EDTA). The EDTA treated blood samples were centrifuged at 3000 × g for 20 minutes at 7° C to obtain plasma samples. Urine samples were also taken prior to the blood sampling. Samples were immediately analyzed by Cobas 8000- Integrated chemistry and immunoassay Electrochemiluminescence–ECLIA platform (Roche, Indianapolis USA), Immulite 2000 XPi-Chemiluminescence immunoassay platform (Siemens, Erlargen, Germany), Urisys 2400-Fully automated Urine FEME analyzer (Roche, Indianapolis, USA) and Cell-Dyn Ruby–Fully automated 5-part hematology analyser (Abbott, Illinois, USA) for clinical biochemistry profiles (as tabulated in Table 5 to Table 11). A comprehensive clinical trial protocol of the study is provided in the Supplementary File.
Table 5.
Plasma liver function profiles (mean ± SD).
| OPP treatment (n = 25) | Control treatment (n = 25) | |
|---|---|---|
| Total protein (g/L) | ||
| Day 1 | 77.72 ± 3.85 | 77.88 ± 3.77 |
| Day 30 | 79.28 ± 3.37 | 78.04 ± 4.99 |
| Day 60 | 78.80 ± 4.75 | 79.84 ± 3.87 |
| Albumin (g/L) | ||
| Day 1 | 45.12 ± 1.81 | 45.08 ± 3.30 |
| Day 30 | 46.36 ± 1.87 | 45.72 ± 2.46 |
| Day 60 | 45.84 ± 2.69 | 46.24 ± 2.07 |
| Globulin (g/L) | ||
| Day 1 | 32.60 ± 4.12 | 32.80 ± 3.25 |
| Day 30 | 32.92 ± 3.58 | 32.28 ± 4.01 |
| Day 60 | 32.96 ± 3.98 | 33.60 ± 3.61 |
| ALP (U/L) | ||
| Day 1 | 62.32 ± 18.65 | 65.16 ± 15.79 |
| Day 30 | 69.40 ± 19.93 | 68.28 ± 16.05 |
| Day 60 | 63.64 ± 17.22 | 64.00 ± 16.30 |
| AST (U/L) | ||
| Day 1 | 19.92 ± 4.31 | 19.68 ± 5.43 |
| Day 30 | 21.00 ± 3.50 | 21.04 ± 3.61 |
| Day 60 | 20.00 ± 6.76 | 18.92 ± 3.76 |
| ALT (U/L) | ||
| Day 1 | 19.92 ± 11.24 | 18.76 ± 11.71 |
| Day 30 | 19.60 ± 9.53 | 18.96 ± 7.35 |
| Day 60 | 20.12 ± 13.48 | 18.16 ± 9.09 |
| GGT (U/L) | ||
| Day 1 | 21.04 ± 13.16 | 20.40 ± 11.38 |
| Day 30 | 23.60 ± 11.92 | 22.68 ± 14.47 |
| Day 60 | 22.48 ± 12.57 | 23.16 ± 13.60 |
| Total bilirubin (µmol/L) | ||
| Day 1 | 10.28 ± 4.14 | 9.28 ± 3.60 |
| Day 30 | 10.28 ± 4.42 | 12.20 ± 5.91 |
| Day 60 | 11.56 ± 5.49 | 12.36 ± 5.99 |
Table 11.
Electrolyte profiles (mean ± SD).
| OPP treatment (n = 25) | Control treatment (n = 25) | |
|---|---|---|
| Plasma sodium (mmol/L) | ||
| Day 1 | 139.92 ± 1.71 | 140.04 ± 1.49 |
| Day 30 | 140.12 ± 1.94 | 139.56 ± 2.08 |
| Day 60 | 138.48 ± 1.56 | 138.48 ± 1.08 |
| Plasma potassium (mmol/L) | ||
| Day 1 | 4.30 ± 0.37 | 4.24 ± 0.31 |
| Day 30 | 4.36 ± 0.42 | 4.54 ± 2.00 |
| Day 60 | 4.20 ± 0.36 | 4.05 ± 0.37 |
| Plasma chloride (mmol/L) | ||
| Day 1 | 104.08 ± 1.68 | 104.52 ± 2.22 |
| Day 30 | 102.36 ± 1.89 | 102.76 ± 1.74 |
| Day 60 | 102.64 ± 1.73 | 102.40 ± 1.50 |
Statistical analysis
Wilcoxon-Signed Test was performed to compare significance of differences between parameters of interest before (baseline, day 1) and after treatment (days 30, and 60) for each treatment. Effects of treatment on parameters of interest were analyzed for their time × treatment interaction, using Two-factor repeated measures analysis of variance (ANOVA) with an interaction term to detect whether there was a significant difference of plasma and urine profiles between OPP and control treatments. If there was any significant time × treatment interaction, the value at the specific day of treatment was extensively compared using Wilcoxon-Signed Rank Test. To increase the stringency of the analysis, Bonferroni correction for multiple testing was applied. Statistical analysis was performed using Statistical Package for Social Sciences (SPSS®) for WINDOWS software (Version 10.0, SPSS Inc. Chicago, USA) and MS Excel 2003 (Microsoft Corp. California, USA). The MS Excel software was used for tabulation of statistical charts. The SPSS® software was utilized for calculation of plasma profiles and analyses of Repeated Measures ANOVA, Bonferroni and Wilcoxon-Signed Rank Test. Values were considered significant at P < 0.05.
Results
All 25 volunteers completed the trial. Table 1 shows the demographic characteristics of the volunteers. The trial was completed with the following baseline characteristics: Means ± SDs: Age, 29.24 ± 4.31 y; body mass index, 22.41 ± 3.99 kg/m2. There was no change in body weight of all volunteers (Table 3). During the trial, there was no dropout and none of the volunteers demonstrated any adverse effects (AE) or serious adverse effects (SAE).
Sample size or number of volunteers was determined based on calculation of Effect Size (ES). Data from other human studies investigating the antioxidant effects of phenolic compounds on several plasma biochemistry profiles18–21 were pooled and calculated for their ES. Estimation of sample size was conducted by introducing the ES value into the Altman Normogram. Calculations and statistical analysis for this part were carried out according to previous statistical methods22,23. This analysis indicated that the minimum number of volunteers required for this study was 23 per group. Hypothetically, this number would allow investigators to observe any difference between control and treatment groups.
All clinical biochemistry parameters including blood pressure (Table 4), liver function (Table 5), renal function (Table 6), haematology (Table 7), lipid profiles (Table 8), glucose and insulin levels (Table 9), hsCRP, whole blood HbA1C, urine albumin, ACR profile (Table 10) and electrolytes (Table 11) were in the normal range according to standard clinical safety references. There were no significant differences in any of these clinical biochemistry profiles between OPP and control treatments during the whole intervention period.
Table 6.
Plasma renal function profiles (mean ± SD).
| OPP treatment (n = 25) | Control treatment (n = 25) | |
|---|---|---|
| Urea (mmol/L) | ||
| Day 1 | 3.87 ± 0.98 | 3.99 ± 0.92 |
| Day 30 | 4.10 ± 1.11 | 3.81 ± 0.84 |
| Day 60 | 4.07 ± 0.89 | 3.85 ± 0.91 |
| Creatinine (µmol/L) | ||
| Day 1 | 68.72 ± 15.27 | 68.96 ± 15.16 |
| Day 30 | 72.72 ± 16.64 | 70.08 ± 15.62 |
| Day 60 | 72.84 ± 15.77 | 71.00 ± 17.07 |
| Uric acid (g/L) | ||
| Day 1 | 0.33 ± 0.09 | 0.31 ± 0.08 |
| Day 30 | 0.33 ± 0.10 | 0.32 ± 0.08 |
| Day 60 | 0.33 ± 0.09 | 0.33 ± 0.10 |
| Corrected calcium (mmol/L) | ||
| Day 1 | 2.21 ± 0.06 | 2.21 ± 0.07 |
| Day 30 | 2.20 ± 0.06 | 2.18 ± 0.07 |
| Day 60 | 2.20 ± 0.09 | 2.21 ± 0.10 |
| Phosphate (mmol/L) | ||
| Day 1 | 1.10 ± 0.08 | 1.10 ± 0.13 |
| Day 30 | 1.12 ± 0.14 | 1.11 ± 0.14 |
| Day 60 | 1.14 ± 0.13 | 1.17 ± 0.11 |
Table 7.
Haematology profiles (mean ± SD).
| OPP treatment (n = 25) | Control treatment (n = 25) | |
|---|---|---|
| Haemoglobin (g/L) | ||
| Day 1 | 137.36 ± 20.47 | 137.48 ± 20.26 |
| Day 30 | 141.04 ± 20.25 | 137.64 ± 19.98 |
| Day 60 | 138.56 ± 18.51 | 137.44 ± 19.94 |
| RBC (×1012/L) | ||
| Day 1 | 5.08 ± 0.48 | 5.04 ± 0.47 |
| Day 30 | 5.13 ± 0.48 | 5.02 ± 0.46 |
| Day 60 | 5.01 ± 0.42 | 5.05 ± 0.47 |
| PCV (L/L) | ||
| Day 1 | 0.43 ± 0.05 | 0.42 ± 0.05 |
| Day 30 | 0.43 ± 0.05 | 0.42 ± 0.05 |
| Day 60 | 0.43 ± 0.05 | 0.43 ± 0.05 |
| MCV (f/L) | ||
| Day 1 | 84.12 ± 7.95 | 84.44 ± 7.45 |
| Day 30 | 84.68 ± 7.40 | 84.20 ± 7.59 |
| Day 60 | 85.40 ± 6.92 | 84.44 ± 8.09 |
| MCH (pg) | ||
| Day 1 | 27.04 ± 3.19 | 27.20 ± 3.00 |
| Day 30 | 27.52 ± 3.04 | 27.44 ± 3.15 |
| Day 60 | 27.56 ± 2.79 | 27.363 ± 3.12 |
| MCHC (g/L) | ||
| Day 1 | 321.12 ± 12.85 | 322.40 ± 11.84 |
| Day 30 | 324.28 ± 12.91 | 325.24 ± 13.32 |
| Day 60 | 323.12 ± 12.87 | 321.72 ± 13.81 |
| RDW (%) | ||
| Day 1 | 13.87 ± 1.93 | 13.62 ± 1.47 |
| Day 30 | 14.02 ± 2.01 | 13.65 ± 1.40 |
| Day 60 | 13.83 ± 1.89 | 13.60 ± 1.50 |
| White cell count (×109 L) | ||
| Day 1 | 7.18 ± 1.77 | 7.16 ± 1.45 |
| Day 30 | 6.97 ± 1.58 | 7.85 ± 2.15 |
| Day 60 | 6.89 ± 1.80 | 6.85 ± 1.28 |
| Neutrophils (×109 L) | 3.88 ± 1.68 | 3.80 ± 1.24 |
| Day 1 | 3.84 ± 1.29 | 4.67 ± 2.01 |
| Day 30 | 3.79 ± 1.32 | 3.70 ± 1.31 |
| Day 60 | ||
| Lymphocytes (×109 L) | ||
| Day 1 | 2.53 ± 0.53 | 2.58 ± 0.71 |
| Day 30 | 2.40 ± 0.56 | 2.44 ± 0.61 |
| Day 60 | 2.38 ± 0.63 | 2.35 ± 0.52 |
| Monocytes (×109 L) | ||
| Day 1 | 0.51 ± 0.18 | 0.52 ± 0.13 |
| Day 30 | 0.49 ± 0.19 | 0.50 ± 0.17 |
| Day 60 | 0.52 ± 0.20 | 0.48 ± 0.11 |
| Eosinophils (×109 L) | ||
| Day 1 | 0.24 ± 0.14 | 0.24 ± 0.17 |
| Day 30 | 0.20 ± 0.12 | 0.21 ± 0.13 |
| Day 60 | 0.19 ± 0.10 | 0.21 ± 0.11 |
| Basophils (×109 L) | ||
| Day 1 | 0.09 ± 0.03 | 0.08 ± 0.04 |
| Day 30 | 0.07 ± 0.05 | 0.09 ± 0.04 |
| Day 60 | 0.08 ± 0.04 | 0.09 ± 0.03 |
| Platelets (×109 L) | ||
| Day 1 | 307.80 ± 69.97 | 310.68 ± 78.80 |
| Day 30 | 296.28 ± 71.44 | 296.28 ± 71.44 |
| Day 60 | 302.88 ± 65.15 | 302.88 ± 65.15 |
| ESR (mm/h) | ||
| Day 1 | 10.28 ± 7.19 | 10.72 ± 6.23 |
| Day 30 | 12.96 ± 12.17 | 15.92 ± 18.69 |
| Day 60 | 12.40 ± 10.07 | 10.64 ± 9.95 |
Table 8.
Plasma lipid profiles (mean ± SD).
| OPP treatment (n = 25) | Control treatment (n = 25) | |
|---|---|---|
| Total cholesterol, TC (mmol/L) | ||
| Day 1 | 4.99 ± 0.71 | 4.83 ± 0.74 |
| Day 30 | 5.17 ± 0.75 | 4.97 ± 0.72 |
| Day 60 | 4.94 ± 0.701 | 5.16 ± 0.82 |
| Triacylglycerol, TAG (mmol/L) | ||
| Day 1 | 1.09 ± 0.69 | 1.15 ± 0.61 |
| Day 30 | 1.14 ± 0.61 | 1.06 ± 0.54 |
| Day 60 | 0.94 ± 0.40 | 0.97 ± 0.47 |
| HDL-C (mmol/L) | ||
| Day 12 | 1.36 ± 0.32 | 1.29 ± 0.30 |
| Day 30 | 1.36 ± 0.30 | 1.31 ± 0.29 |
| Day 60 | 1.26 ± 0.26 | 1.32 ± 0.29 |
| LDL-C (mmol/L) | ||
| Day 1 | 3.13 ± 0.66 | 3.01 ± 0.66 |
| Day 30 | 3.29 ± 0.77 | 3.17 ± 0.70 |
| Day 60 | 3.25 ± 0.733 | 3.40 ± 0.79 |
| Total cholesterol/HDL ratio | ||
| Day 1 | 3.89 ± 1.13 | 3.93 ± 1.11 |
| Day 30 | 4.03 ± 1.22 | 4.00 ± 1.18 |
| Day 60 | 4.10 ± 1.21 | 4.12 ± 1.24 |
1Although time × treatment interaction was significant, p = 0.001 (2-factor repeated measures ANOVA) and TC was significantly lower for OPP compared to control treatment (at day 60), based on Wilcoxon signed rank test (p = 0.025), no significant difference of total cholesterol level was found between OPP and control treatments at day 1, 30 and 60 following Bonferroni correction.
2Baseline HDL-C level was significantly different between treatments, p = 0.038 (Wilcoxon signed rank test). The value was further used as covariate in repeated measures. Although the time × treatment analysis was significant, p = 0.011 (2-factor repeated measures ANOVA), no significant difference of HDL-C level was found between treatments at day 30 and 60 based on Wilcoxon signed rank test. Similarly, no significant difference of HDL-C level was found between treatments at day 30 and 60 after Bonferroni correction.
3Although the time × treatment interaction was significant, p = 0.018 (2-factor repeated measures ANOVA) and LDL-C was significantly lower for OPP compared to control treatment (at day 60) based on Wilcoxon signed rank test (p = 0.04), no significant difference of LDL-C level was found between OPP and control treatments at day 1, 30 and 60 following Bonferroni correction.
Table 9.
Endocrinology profiles (mean ± SD).
| OPP treatment (n = 25) | Control treatment (n = 25) | |
|---|---|---|
| Serum insulin (µU/mL) | ||
| Day 1 | 9.56 ± 6.38 | 9.52 ± 6.95 |
| Day 30 | 9.24 ± 5.43 | 9.16 ± 5.47 |
| Day 60 | 9.76 ± 5.97 | 9.60 ± 4.90 |
| Plasma glucose (mmol/L) | ||
| Day 1 | 4.80 ± 0.29 | 4.75 ± 0.26 |
| Day 30 | 4.66 ± 0.35 | 4.66 ± 0.27 |
| Day 60 | 4.63 ± 0.30 | 4.56 ± 0.33 |
Table 10.
Special chemistry profile (mean ± SD).
| OPP treatment (n = 25) | Control treatment (n = 25) | |
|---|---|---|
| Serum hsCRP (mg/L) | ||
| Day 1 | 1.68 ± 2.41 | 1.58 ± 2.42 |
| Day 30 | 2.53 ± 3.97 | 1.50 ± 2.34 |
| Day 60 | 3.71 ± 6.12 | 2.66 ± 4.70 |
| Whole blood HbA1c (%) | ||
| Day 1 | 5.41 ± 0.25 | 5.43 ± 0.26 |
| Day 30 | 5.43 ± 0.24 | 5.40 ± 0.24 |
| Day 60 | 5.48 ± 0.24 | 5.47 ± 0.25 |
| Urine albumin (mg/L) | ||
| Day 1 | 19.52 ± 44.54 | 13.91 ± 25.76 |
| Day 30 | 19.56 ± 9.67 | 12.48 ± 8.00 |
| Day 60 | 16.51 ± 31.66 | 14.37 ± 10.10 |
| ACR (Alb/mmol Cr) | ||
| Day 1 | 3.13 ± 0.66 | 3.01 ± 0.66 |
| Day 30 | 3.29 ± 0.77 | 3.17 ± 0.70 |
| Day 60 | 3.25 ± 0.733 | 3.40 ± 0.79 |
The OPP dose used in the trial resulted in lower plasma TC (Fig. 1 and Table 8). After 60 days of OPP supplementation, plasma TC level in the OPP treatment group was significantly lower compared to the control treatment (p = 0.025) based on the Wilcoxon signed rank test. Plasma LDL-C was also significantly lower when volunteers were supplemented with OPP (p = 0.04), (Fig. 2 and Table 8). As several dependent and independent statistical tests were performed in this study, in order to rule out false positive results and increase the stringency of the test, Bonferroni correction was applied. Following the Bonferroni correction, the decrease in TC and LDL-C was deemed to be not significant. There was no significant difference of HDL-C (Fig. 3), TAG and TC/HDL ratio (Table 8) between OPP and control treatments based on the Wilcoxon signed rank test and the Bonferroni correction.
Figure 1.
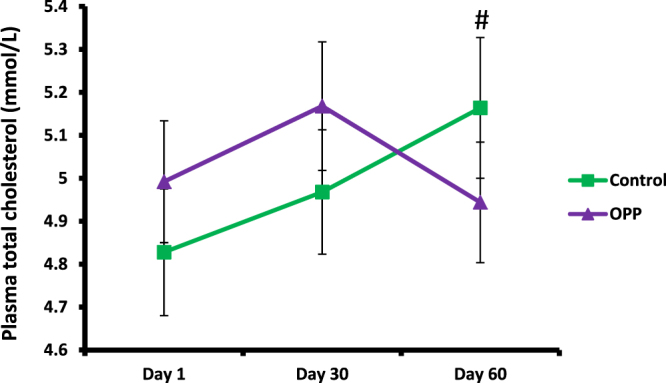
Total cholesterol level in plasma following OPP and control treatments for 60 days. #Time × treatment interaction was significant, p = 0.001 (2-factor repeated measures ANOVA). Based on Wilcoxon signed rank test, TC was significantly lower for OPP treatment compared to control treatment (at day 60), p = 0.025. However, following Bonferroni correction, no significant difference of total cholesterol level was found between OPP and control treatments at day 1, 30 and 60.
Figure 2.
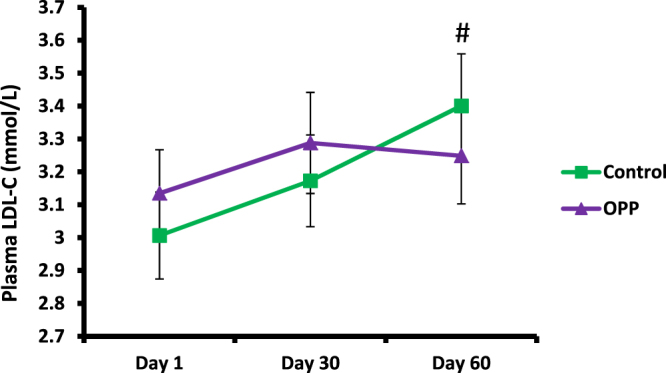
LDL-C level following OPP and control treatments for 60 days. #Time × treatment interaction was significant, p = 0.018 (2-factor repeated measures ANOVA). Based on Wilcoxon signed rank test, LDL-C was significantly lower after OPP compared to control treatment (at day 60), p = 0.04. However, following Bonferroni correction, no significant difference of LDL-C level was found between OPP and control treatments at day 1, 30 and 60.
Figure 3.
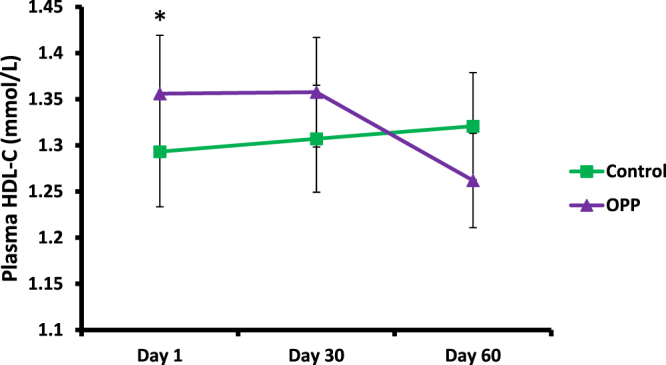
HDL-C level following OPP and control treatments for 60 days. *Baseline HDL-C level was significantly different between treatments, p = 0.038 (Wilcoxon’s signed-rank test). Value was further used as covariates in repeated measures. Although the time × treatment analysis was significant, p = 0.011 (2-factor repeated measures ANOVA), no significant difference of HDL-C level was found between treatments at day 30 and 60 (Wilcoxon signed rank test). Similarly, no significant difference of HDL-C level was found between treatments at day 30 and 60 after Bonferroni correction.
Discussion
Dosage of the OPP was determined based on the safety and functionality of a similar intake of GAE from pomegranate juice. Previous investigation demonstrated that pomegranate dietary supplement is safe when ingested by healthy human volunteers in amounts up to 1420 mg/day, providing a total of 870 mg GAE/day21. No adverse effects or changes in haematology, serum chemistry, or urinalyses related to the pomegranate dietary supplement consumption were observed during the trial period. In the current trial, volunteers were given a total of 300 mL of OPP with a concentration of 1500 mg/L GAE (150 mL of OPP in the morning session and another 150 mL of OPP in the afternoon session). This provided a total of 450 mg GAE/day to each volunteer for 60 days.
The dose was selected based on translation of the effective dose used in our previous pre-clinical trials on animal models supplemented with OPP where positive effects on clinical biochemistry profiles were demonstrated2,11,12,14,16. Most clinical trials have attempted to deliver large quantities of individual polyphenols to overcome the issues of rapid polyphenol clearance in the human circulation24. Supplementation of phenolics in high doses appears to be the only reliable method to achieve measurable concentrations approaching those seen in in vitro and in vivo animal studies, thus enabling investigators to observe any beneficial effects that may occur due to the supplementation.
In most clinical trials investigating the physiological effects of phenolic substances, safety aspects are rarely documented. It is further suggested that detailed safety profiles should be included when findings are reported and published24. Following OPP supplementation in our trial, no AE/SAE or changes in haematology, serum and plasma chemistry, as well as urinalyses were observed. Moreover, none of the volunteers showed any allergic reactions towards OPP supplementation. All clinical biochemistry liver profiles were in the normal range according to the standard clinical safety references, indicating no toxic effects from OPP supplementation. In our previous investigations on animals, no toxicity effects from OPP supplementations (up to 2400 mg GAE/L dose) were found, as far as the histology, haematology and clinical biochemistry profiles were concerned2,11,12,14,16. Therefore, it can be concluded that OPP at the dose tested in the current trial is generally safe for human consumption.
Introduction of the control treatment arm into the trial was necessary as previously recommended25,26. Furthermore, a much longer wash out period (four-weeks) was implemented in the current trial, to ensure the duration was sufficient to avoid any possible effects of the repeated ingestions25. Although it has been reported that a one-week wash out period is adequate27,28 given that most polyphenols are rapidly metabolized and reach maximum concentration within hours20, this does not take into account the fact that phenolic extracts including pomegranate supplement and OPP contain other compounds such as fibre, sugars, organic acids and vitamins besides phenolics. A longer washout period is thus advantageous.
Most of the human trials investigating the effects of phenolics and polyphenols were focused on evaluating beneficial effects. Safety issues such as side effects, acute or chronic/long-term effects have rarely been reported in the literature24. A primary aim of this trial however, was to ascertain the safety of consuming OPP.
Among all results, we carefully evaluated the response of plasma lipid profiles (TC, LDL-C and HDL-C), glucose and insulin levels towards OPP supplementation, given that OPP had demonstrated positive effects in lowering lipid and diabetic profiles in our previous studies using animal models11. The OPP dose utilized in the current trial reduced plasma TC and LDL-C compared to the control treatment. Although the reduction was significant based on the Wilcoxon signed rank test, the application of the Bonferroni correction suggested that the results were not significant. Previous pre-clinical studies demonstrated the ability of OPP in lowering plasma TC11,16 in diabetic rats. Additionally, previous studies showed that genes involved in cholesterol biosynthesis in BALB/c mice were down-regulated by OPP, hence eliciting a hypocholesterolaemic effect12,14. Further clinical tests to specifically confirm the effect of OPP on lipid profiles in humans with raised TC may be necessary.
Blood lipid profiles (TAG, TC, LDL-C, HDL-C) have been routinely used as indicators in evaluating the physiological potential of phenolic supplementations in human trials. In most cases, no improvement has been clearly demonstrated in healthy subjects when phenolic substances were supplemented29. A previous human intervention trial also found no significant differences in lipid profiles namely TAG, TC, LDL-C, and HDL-C between treatment and control groups following red wine consumption for two weeks30. However, the phenolic content used in that trial was much lower (62 mg epicatechin equivalent/day) compared to our trial. Even larger doses such as 852 mg GAE/day of total phenolic intake of cranberry juice for the same period did not significantly alter plasma lipid profiles in healthy population31.
Our findings are in agreement with a previous trial where 49 males and 11 females were supplemented with 544 mg/day polyphenol extract powder (dissolved as drinks) for two months32. There were no significant differences in serum TAG, TC, HDL-C and LDL-C after consumption of the polyphenols in two different intervention groups. In another human trial, supplementation of 710 mg/day or 1420 mg/day of pomegranate polyphenol extract in the form of capsules for four weeks in 64 healthy subjects also did not significantly improve plasma TAG, TC, HDL-C and LDL-C levels21. Supplementation with 120 mg/day of isoflavones through consumption of soy preparation powder for 12 weeks significantly reduced TC, LDL-C and cholesterol/HDL ratio but not HDL-C levels. These effects were however demonstrated in diabetic volunteers27. In a later study which applied the same recruitment period and the same type of isoflavone preparation but at a slightly higher dose (132 mg/day), TAG, TC, LDL-C and HDL-C levels were not significantly changed as well33. Theoretically as an antioxidant, OPP may provide positive effects in improving the lipid profiles in volunteers with raised TC levels. However, in the current trial, no significant differences in TAG and HDL-C levels between OPP and control treatments were observed. It is important to note that the volunteers were healthy and their lipid profiles were already in the normal range and hence it is not surprising that no significant changes were observed following OPP supplementation.
The effect of phenolic supplementations on lipid profiles in humans is rather unique. Several contradictory findings from the literature suggest that recruitment periods, doses and forms of phenolic supplementations, as well as health condition of recruited volunteers affect the results of the supplementations. A short supplementation period of 150 mg/day of polyphenols rich pine bark extract in capsule form for six weeks significantly improved LDL-C and HDL-C profiles in 25 healthy subjects34. In comparison to the placebo treatment, supplementation with the same type of polyphenols34 but at slightly lower dose for a much longer period (125 mg/day) in diabetics with mild hypertension resulted in significantly lower LDL-C levels after 8 and 12 weeks35. A significant LDL-C lowering effect was also demonstrated in diabetic volunteers even after four weeks of supplementation with 321 mg/day of flavanol-rich drinks36. However, a two-week supplementation period of 800 mg/day polyphenol capsules did not significantly alter blood lipid profiles in 35 healthy male subjects28. Even high dose of phenolic substance at longer period did not improve lipid profiles, as previously demonstrated in obese subjects who received 800 mg/day of catechin supplement capsules for eight weeks37.
In the current study, following 60 days of OPP supplementation, plasma glucose levels declined gradually from their respective baseline values. However, this observation did not reflect any of the glucose-reducing effects as previously suggested in our animal studies11,16, since a similar trend was even demonstrated in the controls. Nevertheless, no significant changes in glucose and insulin levels were observed after 60 days of OPP supplementation. Furthermore, there was no significant difference in plasma glucose at day 30 or 60 between both OPP and control treatments. However, this is not surprising as this trial was with healthy volunteers and reduction in glucose levels was not anticipated. OPP may have elicited a different effect in diabetic or pre-diabetic volunteers.
Most of the glucose lowering effects by polyphenols in healthy populations were demonstrated in the postprandial trial, where subjects were challenged with the oral glucose tolerance test (OGTT) coupled with polyphenol treatments38,39. No significant effects were however demonstrated during long-term supplementation trials in healthy populations19,21,30,37,40 and even in pathological populations18,27,33,36,40,41. Similar to other antioxidants, the effects of polyphenol treatments are likely to be influenced by doses, durations and delivery forms of supplementations, as well as the health status of the studied populations. Higher polyphenol treatments such as ≈1500 mg/day for two weeks significantly reduced plasma glucose level in type 2 diabetic volunteers42. However, the actual effects of the polyphenol treatment in the study were not clearly established since the diabetic volunteers were also prescribed with the oral blood glucose-lowering drugs, although the comparison was made with the control treatment42. Furthermore, the relationship between blood glucose and insulin levels in diabetic volunteers has not yet been conclusive since some polyphenol treatments resulted in significantly improved insulin profiles but not glucose levels19.
Although there are no clear guidelines in determining the upper-tolerable intake of phenolic supplementations, future human trials should establish the effective minimum dose of phenolic intake that could significantly improve desired clinical profiles. Our phase one clinical trial demonstrated the safety and physiological effects of OPP, as supported by the current available data of clinical biochemistry profiles. Supplementation of 450 mg GAE/day OPP is found to be safe for human consumption. Although the results were not significant following Bonferroni correction, the observed decrease in TC and LDL-C suggests that at appropriate doses, OPP may provide several beneficial effects in improving lipid profiles in humans and the current observations justify the need to continue with dose-exploration trials of OPP in future.
Electronic supplementary material
Acknowledgements
The present trial was fully supported by the Malaysian Palm Oil Board and ScienceFund, Malaysian Ministry of Agriculture and Agro-Based Industry (0104031002).
Author Contributions
S.F. was the overall researcher in charge of the trial, having designed the trial protocol, primed the laboratory and statistical techniques, prepared the OPP supplements, and drafting the manuscript. S.S.L. conducted laboratory analysis and contributed intellectually to the final manuscript. I.N.M. examined overall medical conditions, evaluated clinical biochemistry profiles and contributed intellectually to the final manuscript. Y.A.T. participated in the preparation of OPP supplements and contributed intellectually to the final manuscript. K.S. designed the trial protocol including selection of tested clinical biochemistry profiles and contributed intellectually to the final manuscript. R.S. participated in the design of the trial and preparation of OPP supplements and contributed intellectually to the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-26384-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grace, S.C. Phenolics as antioxidants in Antioxidants and reactive oxygen species in plants (ed. Mirnoff, N.) 141–159 (Oxford: Blackwell Publishing).
- 2.Sambanthamurthi R, et al. Oil palm vegetation liquor: a new source of phenolic bioactives. Br. J. Nutr. 2011;106:1655–1663. doi: 10.1017/S0007114511002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sambanthamurthi, R., Tan, Y.A., Sundram, K. Treatment of liquors derived from oil-bearing mesocarps, for example oil palm fruit mesocarp, and products thereof. United States Patent Application No. 20030031740 (2003).
- 4.Sambanthamurthi, R., Tan, Y.A. & Sundram, K. Treatment of liquors derived from oil-bearing mesocarps, for example oil palm fruit mesocarp, and products thereof. Malaysian Patent Application No. PI 980 4378 (1998).
- 5.Sambanthamurthi, R., Tan, Y.A. & Sundram, K. Treatment of liquors derived from oil-bearing mesocarps, for example oil palm fruit mesocarp, and products thereof. Indonesia Patent Application #P-990892 (1999).
- 6.Zern TL, Fernandez ML. Cardioprotective effects of dietary polyphenols. J. Nutr. 2005;135:2291–2294. doi: 10.1093/jn/135.10.2291. [DOI] [PubMed] [Google Scholar]
- 7.Zhu QY, Huang Y, Tsang D, Chen ZY. Regeneration of α-tocopherol in human low-density lipoprotein by green tea catechin. J. Agric. Food Chem. 1999;47:2020–2025. doi: 10.1021/jf9809941. [DOI] [PubMed] [Google Scholar]
- 8.Natella F, Nardini M, Belelli F, Scaccini C. Coffee drinking induces incorporation of phenolic acids into LDL and increases the resistance of LDL to ex vivo oxidation in humans. Am. J. Clin. Nutr. 2007;86:604–609. doi: 10.1093/ajcn/86.3.604. [DOI] [PubMed] [Google Scholar]
- 9.Russo P, et al. Effects of de-alcoholated red wine and its phenolic fractions on platelet aggregation. Nutr. Metab. Cardiovascular Dis. 2001;11:25–29. [PubMed] [Google Scholar]
- 10.Balasundram N, Tan YA, Sambanthamurthi R, Sundram K, Samman S. Antioxidant properties of palm fruit extracts. Asia Pac. J. Clin. Nutr. 2005;4:319–324. [PubMed] [Google Scholar]
- 11.Sambanthamurthi R, et al. Positive outcomes of oil palm phenolics on degenerative diseases in animal models. Br. J. Nutr. 2011;106:1664–1675. doi: 10.1017/S0007114511002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leow SS, Sekaran SD, Sundram K, Tan YA, Sambanthamurthi R. Differential transcriptomic profiles effected by oil palm phenolics indicate novel health outcome. BMC Genomics. 2011;12:432. doi: 10.1186/1471-2164-12-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Idris CAC, et al. Oil palm phenolics and vitamin E reduce atherosclerosis in rabbits. J Func Foods. 2014;7:541–550. doi: 10.1016/j.jff.2014.01.002. [DOI] [Google Scholar]
- 14.Leow SS, Sekaran SD, Sundram K, Tan YA, Sambanthamurthi R. Oil palm phenolics attenuate changes caused by an atherogenic diet in mice. Eur J Nutr. 2013;52:443–446. doi: 10.1007/s00394-012-0346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patten GS, Abeywardena MY, Sundram K, Tan YA, Sambanthamurthi R. Effect of oil palm phenolics on gastrointestinal transit, contractility and motility in the rat. J Func Foods. 2016;17:928–937. doi: 10.1016/j.jff.2015.06.008. [DOI] [Google Scholar]
- 16.Bolsinger J, Pronzcuk A, Sambanthamurthi R, Hayes KC. Anti diabetic effect of palm fruit juice in the nile rat (Arvicanthis niloticus) J. Nutr. Sci. 2014;3(e5):1–11. doi: 10.1017/jns.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leow SS, Bolsinger J, Pronczuk A, Hayes KC, Sambanthamurthi R. Hepatic transcriptome implications for palm fruit juice deterrence of type 2 diabetes mellitus in young male Nile rats. Genes & Nutrition. 2016;11:29. doi: 10.1186/s12263-016-0545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenblat M, Hayek T, Aviram M. Anti-oxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages. Atherosclerosis. 2006;187:363–371. doi: 10.1016/j.atherosclerosis.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Banini AE, Boyd LC, Allen JC, Allen HG, Sauls DL. Muscadine grape products intake, diet and blood constituents of non-diabetic and type 2 diabetic subjects. Nutrition. 2006;22:1137–1145. doi: 10.1016/j.nut.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans, Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:S230–S242. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 21.Heber D, et al. Safety and antioxidant activity of a pomegranate dietary supplement in overnight individuals with increased waist size. J. Agric. Food Chem. 2007;55:10050–10054. doi: 10.1021/jf071689v. [DOI] [PubMed] [Google Scholar]
- 22.Thalheimer, W. & Cook, S. How to calculate effect sizes from published research articles: a simplified methodology. Retrieved November 31, 2002 from http://worklearning.com/effectsizes.html (2002).
- 23.Dell RB, Holleran S, Ramakrishnan R. Sample size determination. ILAR Journal. 2002;43:207–213. doi: 10.1093/ilar.43.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Bock M, Jose G, Derraik B, Cutfield WS. Polyphenols and glucose homeostasis in humans. J. Acad. Nutr. Diet. 2012;112(6):808–815. doi: 10.1016/j.jand.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Panchon MS, Villano D, Troncoso AM, Garcia-Parrilla MC. Antioxidant activity of phenolic compounds: from in vitro results to in vivo evidence. Crit. Rev. Food Sci. Nutr. 2008;48:649–671. doi: 10.1080/10408390701761845. [DOI] [PubMed] [Google Scholar]
- 26.Panossian, A. G. & Wikma, G. C. Clinical trial a signposts to efficacy (Chapter 29) in Evaluation of herbal medicinal products (eds Houghton, P. & Mukherjee, P.) 426–443 (London, Pharmaceutical Press, 2009).
- 27.Jayagopal V, et al. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care. 2002;25:1709–1714. doi: 10.2337/diacare.25.10.1709. [DOI] [PubMed] [Google Scholar]
- 28.Van Mierlo LAJ, Zock PL, van der Knaap HCM, Draijer R. Grape polyphenols do not affect vascular function in healthy men. J. Nutr. 2010;140:1769–1773. doi: 10.3945/jn.110.125518. [DOI] [PubMed] [Google Scholar]
- 29.Chong MFF, Macdonald R, Lovegrove JA. Fruit polyohenols and CVD risk: a review of human intervention studies. Br. J. Nutr. 2010;104:S28–39. doi: 10.1017/S0007114510003922. [DOI] [PubMed] [Google Scholar]
- 30.Micallef M, Lexis L, Lewandowski P. Red wine consumption increases antioxidant status and decreases oxidative stress in the circulation of both young and old humans. Nutr. J. 2007;6:27. doi: 10.1186/1475-2891-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duthie SJ, et al. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur. J. Nutr. 2006;45:113–22. doi: 10.1007/s00394-005-0572-9. [DOI] [PubMed] [Google Scholar]
- 32.Fukino Y, et al. Randomized controlled trial for an effect of green tea-extract powder supplementation on glucose abnormalities. Eur. J. Clin. Nutr. 2008;62:953–960. doi: 10.1038/sj.ejcn.1602806. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez S, Jayagopal V, Kilpatrick ES, Chapman T, Atkin SL. Effects of isoflavone dietary supplementation on cardiovascular risk factors in type 2 diabetes. Diabetes Care. 2007;30(7):1871–1873. doi: 10.2337/dc06-1814. [DOI] [PubMed] [Google Scholar]
- 34.Devaraj S, et al. Supplementation with a pine bark extract rich in polyphenols increases plasma antioxidant capacity and alters the plasma lipoprotein profile. Lipids. 2002;37(10):931–934. doi: 10.1007/s11745-006-0982-3. [DOI] [PubMed] [Google Scholar]
- 35.Zibadi S, Rohdewald PJ, Park D, Watson RR. Reduction of cardiovascular risk factors in subjects with type 2 diabetes by Pycnogenol supplementation. Nutr Res. 2008;28:315–320. doi: 10.1016/j.nutres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Balzer J, et al. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients. J. Am. Coll. Cardiol. 2008;51:2141–2149. doi: 10.1016/j.jacc.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 37.Brown AL, et al. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: randomized controlled trial. Br. J. Nutr. 2009;101:886–894. doi: 10.1017/S0007114508047727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grassi D, Lippi C, Necozione S, Desideri G, Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am. J. Clin. Nutr. 2005;81:611–614. doi: 10.1093/ajcn/81.3.611. [DOI] [PubMed] [Google Scholar]
- 39.Tsuneki H, et al. Effect of green tea on blood glucose levels and serum proteomic patterns in diabetic (db/db) mice and on glucose metabolism in healthy humans. BMC Pharmacol. 2004;4:18. doi: 10.1186/1471-2210-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukino Y, Shimbo M, Aoki N, Okubo T, Iso H. Randomized controlled trial for an effect of green tea consumption on insulin resistance and inflammation markers. J. Nutr. Sci. Vitaminol. 2006;51:335–342. doi: 10.3177/jnsv.51.335. [DOI] [PubMed] [Google Scholar]
- 41.Nagao T, et al. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity. 2008;17:310–317. doi: 10.1038/oby.2008.505. [DOI] [PubMed] [Google Scholar]
- 42.Hosoda K, et al. Antihyperglycemic effect of oolong tea in type 2 diabetes. Diabetes Care. 2003;26:1714–1718. doi: 10.2337/diacare.26.6.1714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


