Abstract
The density of corn pollen on leaves of milkweed plants inside and outside of cornfields was measured in several studies from different localities. The purpose was to obtain a representative picture of naturally occurring pollen densities to provide a perspective for laboratory and field studies of monarch larvae feeding on milkweed leaves with Bt corn pollen. Pollen density was highest (average 170.6 grains per cm2) inside the cornfield and was progressively lower from the field edge outward, falling to 14.2 grains per cm2 at 2 m. Inside the cornfield, and for each distance from the field edge, a frequency distribution is presented showing the proportion of leaf samples with different pollen densities. Inside cornfields, 95% of leaf samples had pollen densities below 600 grains per cm2 and the highest pollen density observed was 1400 grains per cm2, which occurred in a study with a rainless anthesis period. All other studies had rainfall events during the anthesis period. A single rain event can remove 54–86% of the pollen on leaves. Leaves on the upper portion of milkweed plants, where young monarch larvae tend to feed, had only 30–50% of the pollen density levels of middle leaves.
In order to accurately interpret results of studies that examine the effects of Bt corn pollen on monarch butterfly larvae it is necessary to know the range and distribution of naturally occurring pollen densities on milkweed leaves. This provides a perspective on both laboratory and field studies in which monarch larvae feed on milkweed leaves with Bt corn pollen (1, 2). It lets us determine how frequently the pollen densities observed in these studies would occur in nature. The studies reported here contribute to the exposure characterization necessary for assessing the risk of Bt corn pollen to monarch butterflies. In particular, this paper describes the densities of corn pollen on milkweed leaves during corn anthesis for a number of geographic locations and under a variety of environmental conditions. We describe the pollen densities (pollen grains per cm2) that were found on leaves of milkweed plants within cornfields as well as near cornfields because corn pollen is wind-dispersed at least 60 m (3) and possibly more than 200 m (4). These data are used in a companion paper (5) on the results of laboratory studies on the responses of monarch larvae fed milkweed leaves with different densities of artificially applied Bt corn pollen. These data are also used in a second companion paper (6) to provide a frame of reference for the Bt pollen densities found in field trials of larvae feeding on milkweed leaves. Finally, these data are used in a summary companion paper (7) that provides a full risk assessment of monarchs and Bt corn pollen. In addition to characterizing naturally occurring pollen densities, we examined several factors that affect pollen deposition on milkweed leaves, including position of a leaf on the plant and rainfall.
Materials and Methods
This article includes the results of several studies conducted at different locations. The study locations, Iowa, Minnesota, Wisconsin, Ontario (Canada), and Maryland, were chosen to cover representative portions of the range of the Eastern population of monarch butterflies. In general, the methods involved measurements of pollen from field corn (mostly non-Bt) that had accumulated on leaves of naturally occurring milkweed plants (Asclepias syriaca), or surrogates for natural plants such as transplanted plants, potted plants, or cut plant stems. Studies either measured pollen deposition on leaves of milkweed plants inside a cornfield, outside a cornfield, or both. For milkweeds along transects into and away from the field edge, the field edge was defined as 0 m, negative numbers refer to the number of meters within a field, and positive numbers refer to the number of meters away from the field edge. Leaf samples were taken either during or at the end of pollen anthesis, or both. The state of anthesis was determined by examining a sample of tassels throughout the field and calculating the proportion that was shedding pollen. Some studies measured the ambient levels of pollen in the air at sampling locations by using pollen deposition on sticky slides or sticky plates.
Studies of In-Field and Off-Field Pollen Deposition.
Maryland, 1999.
In two eastern shore and four central Maryland counties, 1317 leaves were removed from 572 naturally occurring milkweeds located within and at various distances from 81 cornfields. Sampling was conducted during 14–24 July when the fields had reached full anthesis, during which time no rainfall had occurred in the sampled areas. For larger plants leaves from the upper, middle, and lower portions of the plant were sampled, otherwise an upper leaf was taken. Leaves were placed in Ziploc bags and transported to the laboratory. Pollen was removed by rinsing each leaf with water containing surfactant while brushing the surface with a small soft-bristled brush. The Ziploc bags were also rinsed. In some cases the rinse solution was filtered to trap pollen and the filter paper dried and folded. Filters or wash sample were sent to the U.S. Department of Agriculture, Agricultural Research Service Pollen Laboratory at College Station, TX, where the samples were processed and counted for corn pollen content according to the methods described by Erdtman (8) and Jones and Coppedge (9). After pollen removal the leaf area of each sample was measured by a Li-Cor leaf area meter (Li-Cor, Lincoln, NE). For each leaf, pollen density was calculated as the pollen count divided by the leaf area.
Maryland, 2000.
This study was conducted in an 8-ha cornfield at the University of Maryland Research and Education Center at Beltsville, MD. Adjoining areas on all sides of the field consisted of low-canopy grass habitats. Before corn anthesis (10 days) milkweed plants that had been established in pots from locally collected rhizomes were transplanted both inside and outside the cornfield along two transects on each of the four sides of the field. A single plant was transplanted at each of the transect locations at −10, −5, −3, −1, 1, 3, 5, and 10 m from the field edge. Plants within the field were placed between rows and an additional plant was placed in the row directly under corn plants at the −5- and −10-m positions within 2 m of the corresponding plant situated between rows. Corn rows were the standard 0.76 m wide.
At 3, 6, and 9 days after the onset of anthesis one leaf was removed from the upper third of each milkweed. After 14 days one leaf was removed from the upper, middle, and lower thirds of each plant. Each leaf was placed upright in a 15-cm Petri dish with moist filter paper and leaves were immediately brought to the laboratory. On each leaf a slight indentation was made with a #3 cork cutter to delineate a 0.34-cm2 viewing area between the midrib and leaf margin (two such areas for day-14 leaves). The number of pollen grains was counted within the viewing area under a stereo microscope.
Ontario, 2000.
Pollen densities on leaves and sticky plates were compared in three Bt cornfields and three non-Bt cornfields in Wellington County in central southern Ontario, Canada. The fields ranged from 4.8 to 17.2 ha, and all fields chosen were greater than 150 m from adjacent cornfields to minimize pollen contamination. Four transects were established perpendicular to the field edge along one or two sides of each field; parallel transects on one side of a field were between 40 and 180 m apart. Along each transect, a potted milkweed plant and a sticky plate were placed at each of four distances: −1.5, 0, 1, and 5 m from the field edge. The sticky plates were made from Petri dishes coated with sticky material (Sticky Stuff, Olson Products, Medina, OH). Plates were placed horizontally on top of a 1-m-high wooden stake. The plants and plates were placed in the field several days before the onset of anthesis. Plates were changed daily for 16 days after the commencement of anthesis and leaves were collected from each field 6 and 11 days after the onset of anthesis. A leaf was cut from the upper, middle, and lower third of each plant. Each leaf was immediately sandwiched between two strips of contact paper (ConTact7 Brand, Decora Manufacturing, North Ridgeville, OH) to avoid the loss of pollen from its surface. Plates and leaves were brought back to the laboratory and frozen at −20 and −5°C, respectively, until pollen could be counted.
To determine pollen density, the sticky plates were stained with acid fuchsin and computer images of the stained pollen in five 1-cm2 areas were created by AIMS Lab (Fremont, CA) GRABIT IIJ version 1.10 software and a Panasonic (Secaucus, NJ) WV-D5100 system digital camera mounted on a dissecting microscope. Computer images were analyzed with Scion Image (Frederick, MD) BETA 4.0.2 software to determine the pollen density. Pollen density on milkweed leaves was evaluated by pulling the contact paper strips away from the leaf, staining them with acid fuchsin, and counting the pollen in five randomly chosen 1-cm2 areas on the top and bottom of the strips between the leaf midrib and the leaf margin. Any pollen remaining on the leaves was also counted in five 1-cm2 areas and added to the counts made on the contact paper.
Off-Field Deposition.
Iowa, 1999.
Pollen densities on leaves and sticky slides were measured at several points along transects perpendicular to the four sides of seven cornfields. The fields chosen for sampling were sufficiently isolated beyond the pollen drift zone of other cornfields. Fields were ≈2.3 ha and were surrounded by either soybeans or grass.
To measure ambient pollen levels, microscope slides were coated with glycerol-jelly (10) and each slide was attached horizontally by a clip to a bamboo pole stuck into the ground so that the slide was 1 m above ground level. To measure pollen deposition on milkweed leaves, a stem with a pair of leaves was cut from the midsection of a naturally occurring milkweed plant; this was referred to as a boutonniere. The bottom of the stem was pushed through a hole in a plastic snap cap placed on a 40-dram prescription vial containing water and Floralife (Floralife, Waterboro, SC) to increase leaf longevity. The vial was taped to a bamboo pole and stuck into the ground at the sampling location so that the boutonniere leaves were 1 m above the ground.
Boutonnieres and slides were placed along transects at 0, 1, 2, 4, 8, 20, and 100 m from the edge of the field. Both potted milkweed plants and boutonnieres were used at some sampling locations to determine whether the boutonnieres intercepted pollen in the same way as leaves on whole milkweed plants. Slides and boutonnieres were placed in the late afternoon on a day just before peak corn anthesis and remained in the field for 4 days, which covered the peak anthesis period. Slides were then collected and placed in a 50-ml centrifuge tube and capped. Leaves were removed from the boutonnieres and each leaf was placed in a Ziploc bag. Slides and leaves were placed in a refrigerator until they could be examined, usually within 2 weeks. Pollen density on leaves and slides was measured by using a dissecting microscope with an ocular grid covering 0.25 cm2. Five random locations were examined on each leaf and slide. These same leaf or slide collection and pollen density measurement procedures were followed in all of the Iowa studies described below.
In-Field Deposition.
Iowa, 2000a.
Because rainfall may remove pollen from milkweed leaves, pollen deposition studies conducted during rainy periods may not indicate the maximum pollen densities to which a larva may be exposed. To estimate the maximum amount of pollen that could be deposited on milkweed leaves, cumulative pollen deposition over an entire rain-free anthesis period (10 days) was measured. Two boutonnieres and one slide were placed at four sampling locations within a cornfield, 6 m in from the field edge. The slide and a leaf from one of the boutonnieres were collected every 2 days from each sampling location. Slides were replaced every 2 days, whereas boutonnieres were replaced with fresh ones only as needed (the leaves remained fresh for ≈4 days). For slides the densities of pollen measured every 2 days were added together to estimate the total density of pollen available during anthesis. For leaves the densities measured in separate sampling periods were added together.
Iowa, 2000b.
Leaves were collected from naturally occurring milkweeds within two fields; plants were located more than 100 m in from the field edge. Collection was made on 18 July at the end of the anthesis period. An upper and a middle leaf were taken from three plants from two or three patches of milkweed growing in different parts of each field.
Iowa, 2000c.
Four to five weeks before anthesis, milkweeds that had been propagated from locally collected rhizomes were transplanted into 12 cornfields ≈8 m in from the field edge, 32 plants per field. These plants were examined for pollen deposition at three different times. On 14 July three of the fields that exhibited 75% anthesis were examined. On 24 July all four fields were examined, three of which had reached 100% anthesis and one that was at 75% anthesis. On 3 August all four fields were again examined; three were 10 days postanthesis and the other was at 100% anthesis. For purposes of the analysis, data from fields were combined to produce three categories: 75% anthesis, 100% anthesis, and postanthesis (10 days). At each sampling time a leaf was removed from the upper third of three randomly chosen plants from each of the 12 fields.
Iowa, 2000d.
Before anthesis potted plants were placed along a single transect within a cornfield. A cluster of six plants was placed at 0, 3, and 25 m in from the field edge. One leaf was taken from the top, middle, and bottom third of each plant on 14 August (5% anthesis), 18 August (30% anthesis), 21 August (50% anthesis), 24 August (75% anthesis), and 28 and 30 August (100% anthesis).
Data Analysis.
Laboratory and field feeding trials to determine the effects of Bt corn pollen typically use first instar larvae (5, 6). During a feeding bout these larvae carve out a leaf circle of ≈0.25 cm2, and during a 4-day feeding trial consume ≈1 cm2 of leaf tissue. Therefore, we used each subsample of pollen density on a leaf rather than a whole leaf average as representative of the dose a first instar larva might receive during a feeding bout. Subsample size varied from 0.25 cm2 (Iowa studies) to 0.34 cm2 (Maryland 2000) to 1 cm2 (Ontario 2000). For Maryland 1999 whole-leaf averages were used because the pollen counting method did not use leaf subsampling. Pollen densities were expressed on a per 1 cm2 basis. In most of the studies pollen density on the leaf was measured from locations flanking the leaf midrib. In two studies (Maryland 2000 and Iowa 2000d) deposition along the midrib itself was also measured. Only pollen on the upper surface of leaves was counted in most studies. The Ontario 2000 study measured pollen on the underside of the leaf and found that levels of pollen on the underside were 4% of those on the upper surface. Therefore, ignoring the underside will only slightly underestimate pollen densities.
For purposes of exposure characterization for risk assessment, we wish to know the probability of a larva encountering a pollen density above a toxicity threshold identified in laboratory and field bioassays. Therefore, it is more meaningful to examine a frequency distribution of observed pollen densities and determine the proportion of pollen densities that are above the threshold rather than compare the threshold to the mean pollen density. For the purpose of making generalizations, we combined the results of all studies to generate a single frequency distribution. Because young larvae (instars 1–3) do not feed on the leaf midrib (K. Oberhauser, personal communication) we used only data on samples from areas flanking the leaf midrib; midrib samples were considered separately. This frequency distribution is an average based on the frequency distribution for each study. For each study we only used data for sampling periods that had 50–100% anthesis. Each study was given equal weight in determining the average frequency distribution. The off-field results were also combined in a similar way to create an average frequency distribution for each distance away from the cornfield edge.
A complication in combining the results from these studies is that the studies differed in which leaves were sampled on the plant. In some studies investigators examined upper leaves (Maryland 2000, Iowa 2000c); some upper and middle leaves (Iowa 2000b); some upper, middle, and lower leaves (Maryland 1999, Ontario 2000, Iowa 2000d); and some used boutonnieres, which are similar in size and orientation to middle leaves (Iowa 1999, 2000a). Average pollen density differs among leaf positions (see Results). In addition, first instar larvae are not found equally on upper, middle, and lower leaves (see Discussion). Rather than parse out this variation, which was minor compared with rainfall effects on deposition, we simply used data from whatever leaves were sampled, weighting each leaf position equally.
For testing plant or leaf position effects, all data were tested for normality and homogeneity of variances by using Spearman's Rank Correlation and Shapiro-Wilk's W test. For data not meeting the assumptions of ANOVA, variances were grouped before analysis (11). Pollen densities per square centimeter were analyzed by ANOVA [PROC MIXED (12)]. Plant or leaf position was tested as fixed effects, whereas each transect was treated as a random block effect. Means were separated by using the Tukey's Studentized range test. P values less than 0.05 were considered significant in all statistical analyses.
Results
Pollen Deposition.
In field.
Mean in-field pollen densities on milkweed leaves for the different studies are shown in Table 1. Means varied among the individual studies because of degree of anthesis and rainfall during the anthesis period as discussed below. A composite frequency distribution was developed from the results of all these studies showing the proportion of leaf subsamples that fell within different pollen density categories (Table 2). Of the leaf subsamples, 99% had pollen densities below 900 grains per cm2 and 95% of the samples had pollen densities below 600 grains per cm2.
Table 1.
Mean pollen densities on milkweed leaves inside a cornfield for different studies
| Study | Anthesis level | Mean pollen density (cm2) | No. of leaf subsamples | No. of rain events* |
|---|---|---|---|---|
| Maryland 1999 | Near peak | 65.7 | 66 | 0 |
| Iowa 2000b | 100% | 425.6 | 136 | 1 |
| Iowa 2000c | 75% | 203.8 | 180 | 2 |
| 100% | 157.1 | 180 | 2 | |
| Post anthesis (10 days) | 101.2 | 135 | 1 | |
| Iowa 2000d† | 5% | 58.0 | 36 | |
| 30% | 109.5 | 36 | 1 | |
| 50% | 122.7 | 36 | 2 | |
| 75% | 115.7 | 32 | 2 | |
| 100% | 231.4 | 38 | 0 | |
| Ontario 2000 | Day 6 | 69.7 | 350 | 1–3 |
| Day 11 | 97.7 | 305 | 0–2 | |
| Maryland 2000‡ | Day 3 | 9.7 | 48 | 2 |
| Day 6 | 67.3 | 46 | 2 | |
| Day 9 | 161.3 | 46 | 3 | |
| Day 14 | 61.8 | 86 | 2 |
Number of rain events since previous sample, since the start of anthesis, or in the previous 7 days; for Ontario the intersampling intervals for each of the six fields covered different time periods.
Only data from 25 m within the field were used.
Data from 1, 3, 5, and 10 m within the field were used; only upper leaves included.
Table 2.
Frequency distribution of pollen density levels on milkweed leaves inside a cornfield and at different distances from the cornfield edge (0 m)
| Pollen density, cm2 | Inside a cornfield | From edge of cornfield
|
|||
|---|---|---|---|---|---|
| 0 m | 1 m | 2 m | 4–5 m | ||
| 0–100 | 0.527 | 0.833 | 0.900 | 0.974 | 0.996 |
| 100–200 | 0.170 | 0.093 | 0.062 | 0.024 | 0.004 |
| 200–300 | 0.101 | 0.033 | 0.022 | 0.000 | |
| 300–400 | 0.072 | 0.017 | 0.006 | 0.002 | |
| 400–500 | 0.041 | 0.008 | 0.002 | ||
| 500–600 | 0.041 | 0.007 | 0.002 | ||
| 600–700 | 0.021 | 0.002 | 0.001 | ||
| 700–800 | 0.009 | 0.002 | 0.000 | ||
| 800–900 | 0.009 | 0.003 | 0.001 | ||
| 900–1000 | 0.002 | 0.001 | 0.000 | ||
| 1000–1100 | 0.000 | 0.001 | 0.001 | ||
| 1100–1200 | 0.003 | 0.000 | |||
| 1200–1300 | 0.001 | 0.001 | |||
| 1300–1400 | 0.000 | ||||
| 1400–1500 | 0.000 | ||||
| 1500–1600 | 0.002 | ||||
| Average | 170.6 | 63.1 | 35.4 | 14.2 | 8.1 |
| Sample size | 1456 | 1265 | 1107 | 422 | 1056 |
Based on data from sampling dates at 50–100% anthesis (days 6 and 11 for Ontario 2000 and days 6, 9, and 14 for Maryland 2000).
Off field.
The results of studies on off-field pollen deposition on milkweed leaves were combined and the frequency distributions of pollen density values for distances of 0, 1, 2, and 4–5 m from the field edge are shown in Table 2. The 95th percentiles of pollen densities are 300, 200, 75, and 25 grains per cm2 for distances of 0, 1, 2, and 4–5 m, respectively. The mean pollen density at 0 m is 37% of that inside the field and the mean pollen density is approximately halved with each successive distance category away from the edge.
Factors Affecting Deposition.
Leaf characteristics.
Only a portion of the airborne pollen adheres to milkweed leaves. Three of the seven fields in the Iowa 1999 study of off-field deposition had sufficiently high levels of pollen to allow a comparison of the amount of pollen in the air, as measured by amounts on sticky slides, and on leaves at the same sampling position. The regressions between the pollen density on a slide and on its neighboring leaf were significant in all three of these fields (r2 = 0.37, n = 29, slope = 0.21; r2 = 0.77, n = 22, slope = 0.62; r2 = 0.78, n = 31, slope = 0.43). The average slope was 0.42. In the Iowa 2000a study of in-field deposition the slope of the regression between pollen densities on leaves and slides was 0.38 (r2 = 0.68, n =20). This indicates that in general ≈40% of the ambient pollen at any location adheres to milkweed leaves.
Pollen density along the midrib of a leaf is higher than areas flanking the midrib [Maryland 2000: midrib density 117.6, flanking density 78.2, (F(2, 540) = 7.48, P < 0.0001); Iowa 2000d: midrib density 234.4, flanking density 120.8, paired comparisons t test, t = 6.48, P < 0.0001, n = 178). The midrib density was higher by a factor of 1.5–1.9.
Leaf position on plant.
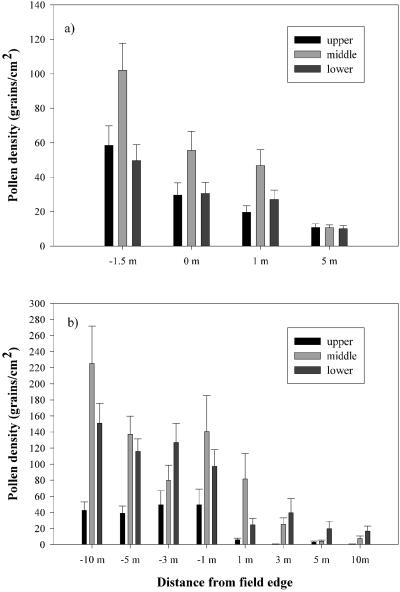
The Ontario 2000 study found significant differences in the amounts of pollen deposited on upper, middle, and lower leaves of plants [F(2, 519) = 22.7, P < 0.0001]. Middle leaves had higher levels of pollen than the upper or lower leaves within the field and up to 1 m from the field, but this pattern did not persist beyond 1 m (Fig. 1a shows the data for day 6; the pattern for day 11 is nearly identical). The mean pollen levels for all samples from −1.5 to 1 m were 35.8, 68.0, and 35.7 grains per cm2 for upper, middle, and lower leaves, respectively. The Maryland 2000 study (Fig. 1b) also found that middle leaves had significantly higher pollen levels than upper or lower leaves [F(2, 540) = 19.91, P < 0.0001], but again this pattern changed beyond 1 m. The mean pollen levels for all samples from −10 to 1 m were 37.3, 132.9, and 103.0 grains per cm2 for upper, middle, and lower leaves, respectively. The Iowa 2000c study, which only included plants inside a cornfield, also found significant leaf position effects with middle leaves having levels higher than upper leaves [mean pollen density: upper 61.4, middle 121.2, and lower 119.8 grains per cm2; F(2, 540) = 19.91, P < 0.0001].
Figure 1.
Mean (and 1 SE) pollen density on leaves from the upper, middle, and lower third of milkweed plants at different distances from the field edge. 0 m, the field edge; negative numbers, inside the field; positive numbers, outside the field. (a) Day 6 from the start of anthesis, Ontario 2000. (b) Day 14 from the start of anthesis, Maryland 2000.
Plant position in corn canopy.
In the Maryland 2000 study, pollen deposition on leaves of plants within rows was compared with levels on leaves of plants between rows (only −10 and −5 m within the field). The mean pollen density within rows (176.6 grains per cm2) was significantly higher than the pollen density between rows [118.9 grains per cm2 (F(1, 247) = 8.47, P = 0.0039)].
Wind direction.
For off-field deposition, wind direction plays a large role in the deposition level at any sampling location. For example, for one of the sites in the Iowa 1999 study the average pollen densities on leaves at 0, 1, 2, 4, and 8 m were 732.2, 312.8, 60.0, 30.2, and 1.2 grains per cm2, respectively, for the downwind sides of the field (north and east transects) and 12.6, 2.6, 3.0, 2.2, 0, and 0 grains per cm2, respectively, for the upwind sides of the field (west and south transects).
Rain effects.
The effect of rain was examined by taking advantage of a rainfall event that occurred during the Iowa 1999 study and one that occurred in an Iowa study in 2001. The pollen density on one leaf of each plant before a heavy rain was compared with the pollen density of the opposite leaf at the same node after the rain (amount of precipitation: 1.9 cm in 1999 and 1.27 cm in 2001). In 1999 and 2001 there were significant linear regression relationships between the before and after values (1999: r2 = 0.70, P < 0.0001, n = 17; 2001: r2 = 0.56, P < 0.008, n = 13). The slopes for the 1999 and 2001 data were 0.14 and 0.45, indicating that the pollen levels after the rainfall event were only 14 and 45% of those before the rainfall. Thus, a single rain event removed 86% of the pollen in one case, which involved leaves outside a cornfield, and 54% in another case, which involved leaves from within a cornfield.
The Iowa 2000a study was designed to estimate the maximum possible pollen levels on leaves if no rainfall occurred. The amounts on leaves sampled at different times during the entire anthesis period were summed to determine maximum accumulation. The four sample values were 752, 1349, 1440, and 1449 grains per cm2. Amounts as high as 1400 grains per cm2 were very rarely found in other in-field studies (Table 2) and the average pollen densities (Table 1) were much lower. This may be because all but one of the studies on in-field deposition had rainfall events during the sampling period (Table 1).
Discussion
Mean in-field levels of pollen varied among studies (Table 1). This variation may be due to several factors. First, leaves were collected at various times during anthesis. One might expect that levels of pollen would be highest on leaves sampled at the end of anthesis, because these leaves would have the cumulative pollen deposition of the entire pollination period. In some studies, leaves were sampled during anthesis as well as at the end. However, it was not always the case that leaves sampled later had more pollen (Table 1). This indicates that there are processes that remove pollen from leaves.
The most important process that removes pollen from leaves is rainfall. In a comparison of leaves before and after a single rain event, 54–86% of pollen was removed. One or more periods of rainfall occurred in all but one of the studies reported (Table 1). The highest in-field pollen levels were found in the Iowa 2000b study from samples collected at the end of anthesis during which there had been just one light rain event. These were still well below the 1400 grains per cm2 found in the Iowa 2000a study, where samples were collected at the end of a rainless anthesis period. This may indicate the upper limit of pollen density.
Factors other than rain could also remove pollen from leaves. The surface of a milkweed leaf has a low density of hairs. Thus, it is probable that pollen grains do not adhere well to the leaf surface and move around on the leaf in response to gravity, wind, or leaf movement, causing pollen to fall off.
Some of the observed in-field pollen density differences may be due to differences in the position of the leaves sampled. In the three studies in which leaf position effects were examined, pollen densities on upper leaves were 30–50% of the densities on middle leaves. The studies differed in how pollen densities on lower leaves compared with middle leaves; pollen densities on lower leaves were 50–100% of middle leaf densities. Jesse and Obrycki (2) noted a similar pattern for upper, middle, and lower leaves. The reason that upper leaves had less pollen could be the effect of rainfall. Rain is more likely to wash pollen from upper leaves than middle or lower leaves that are protected by the leaves above them. In an Iowa 2001 study the pollen density level for upper leaves was 90% of the level for middle leaves before a rain event, but only 65% afterward (J.P., unpublished data). Lower leaves may have less pollen than middle leaves because pollen deposition on lower leaves is blocked by the leaves above them. Leaf orientation also could contribute to the differences among leaf positions in pollen density. In the Ontario 2000 study upper leaves were found to have a more upright orientation (leaf angle = 32.6o, where 0o is horizontal), whereas middle leaves tended to be horizontal (leaf angle is −2.3o) and lower leaves tended to be slightly declined (leaf angle is −24.2o). Pollen is less likely to be removed from horizontal leaves by rain, wind, or shaking, and it was the middle, more horizontal leaves that had the highest pollen densities. Leaf area also may play a role. Upper leaves tend to be smaller than middle and lower leaves [top = 50.1 cm2, middle = 90.8 cm2, and bottom = 73.1 cm2 (Maryland 1999)]. In the Iowa 1999, 2000a, and 2000b studies boutonnieres were used as surrogates for natural plants. In side-by-side comparisons it was found that boutonnieres had ≈50% more pollen than upper leaves of potted plants (amounts deposited on leaves of potted plants were regressed against amounts on adjacent boutonniere leaves; slope = 1.52, r2 = 0.84, n = 17). Leaf angle and leaf area may explain these differences. Boutonniere leaves are larger and more horizontal than the upper leaves of potted plants, so they are more like middle leaves on natural plants in terms of their pollen capture.
The position of a milkweed plant relative to the corn canopy also can affect deposition levels. Milkweed plants within rows had more pollen than plants between rows. This could also be an effect of rain; leaves on plants within rows may have less pollen removed by rain because of sheltering by the corn canopy.
One additional factor that could account for some of the in-field pollen density differences among studies is how deep inside the field the sampling was done. Sampling distances from the field edge ranged from 1.5 m (Ontario 2000), 6 m (Iowa 2000a), 8 m (Iowa 2000c), 3 and 25 m (Iowa 2000d), and 1, 3, 5, and 10 m (Maryland 2000) to more than 100 m (Iowa 2000b). In the Iowa 2000d study there was significantly higher pollen density at 25 m inside the field than at 3 m [pollen density = 147.5 (25 m) and 55.5 (3 m); F1, 140 = 59.26, P < 0.0001]. In the Maryland 2000 study there was a trend of increasing pollen density with greater distance inside the field (Fig. 1b), but the differences were not significant. Therefore, samples that are taken close to the field edge may experience an edge effect and underestimate pollen densities deeper in the field.
Other factors that could explain differences among studies in pollen deposition are corn cultivar differences in total pollen production, environmental effects on total pollen production, and environmental influences on temporal release of pollen.
Mean off-field pollen densities on milkweed leaves were much lower than the mean in-field density (Table 2). Mean pollen density at the field edge was 37% of the density inside the field and pollen densities declined by about half with each successive distance category away from the field edge. Jesse and Obrycki (2) found a similar result with a smaller data set. The limited dispersal of corn pollen away from a cornfield is due to the fact that a corn pollen grain is 90–100 μm in diameter, one of the largest wind-dispersed pollen grains (3). Consequently, a pollen grain in the air has a greater tendency to settle out than to move upward and outward (3). Variation in pollen densities at a particular distance from the field edge can be attributed to such factors as whether it is the upwind or downwind side of the field, the rainfall history during the deposition period, and the time during anthesis when the sample was made.
Significance for Monarch Larvae.
The main purpose of these studies was to characterize the range and distribution of pollen densities, and thus potentially Bt corn pollen densities, to which monarch larvae could be exposed. Although our data were collected in somewhat different ways, the variation introduced by methodological differences is not large compared with the naturally occurring variation in deposition caused by such things as rainfall. Thus, we feel that we have produced a fairly representative picture of naturally occurring pollen densities. In general, the exposure to monarch larvae would be highest inside the cornfield; pollen density drops off very steeply away from a cornfield and only plants on the downwind side of the field receive any appreciable pollen.
Using the data in the present study, published studies on monarch larvae feeding on leaves with Bt corn pollen can be examined to determine how commonly the densities observed in those studies occur in nature. In the study by Losey et al. (1) pollen was applied to milkweed leaves, but no evaluation is possible because the pollen density was not quantified. In the field portion of the study by Jesse and Obrycki (2) their observed pollen densities within a cornfield (overall average 121.6 grains per cm2) were somewhat below the average of 170.6 grains per cm2 found in our study, but the authors note that there were three or eight rainfall events that occurred during anthesis. Their study was much more limited in scope (n = 270, one locality) than ours (n = 1450, three localities). In the laboratory portion of their study the three densities of pollen applied to leaves (14, 135, and 1300 grains per cm2) were within the natural range we found and correspond to low, medium, and very high values. A further discussion of their paper can be found in Hellmich et al. (5).
The pollen exposure period for monarch larvae will include the anthesis period, usually 7–10 days, but perhaps beyond because pollen can persist on leaves after the anthesis period, as was the case for Iowa 2000c (Table 1). The effect on monarch larvae of postanthesis Bt pollen will depend on the rate at which the Bt toxin breaks down in pollen over time, something that is currently being evaluated.
Three factors will tend to reduce the exposure risks to Bt corn pollen. The most important of these is rainfall, which removes pollen from leaves. Because of rainfall, it is unlikely that pollen levels on leaves will build up to 1400 grains per cm2, our estimate of the maximum possible pollen density. In fact, the average pollen density inside fields for the studies reported here, most of which experienced rainy periods during anthesis, was much lower at 170.6 grains per cm2. A second factor that limits exposure to first instars, the larvae most vulnerable to Bt toxins, is that they tend to feed primarily on upper leaves. The majority of monarch eggs are laid on upper leaves (13) and 55% of first instars were found on upper leaves, compared with 31% on middle leaves and 13% on lower leaves (J.P. and W. K. Lam, unpublished data; n = 159). Our data show that upper leaves have only 30–50% of the pollen density of middle leaves. Third, young larvae (instars 1–3) do not tend to feed on the leaf midrib. Pollen densities were 1.5–1.9 times higher along the leaf midrib. This would, however, mean a higher exposure for 4th and 5th instars.
Whether the levels of exposure inside a cornfield or within 2 m of the field edge could have any negative effects on monarch larvae depends on the expression level of endotoxin in the pollen and the pollen density threshold above which there are fitness or mortality consequences. Companion papers on laboratory and field bioassay studies (5, 6) address toxicity issues and the summary paper (7) evaluates what proportion of naturally occurring pollen densities would exceed the toxicity threshold.
Determining the potential negative impact on the monarch population of Bt corn pollen at the densities we observed requires information on the threshold pollen density above which there are fitness or mortality consequences and the probability of larvae feeding on milkweeds growing in and near Bt corn fields. Companion papers provide information on toxicity (5, 6) and exposure probabilities (14), and a summary paper (7) combines this information with pollen density data to produce a full risk assessment.
Acknowledgments
Field and laboratory assistance was provided by Pat Beaupre, Laura Timms, Bryan Muscat, Matt van Ast, and Chad Harvey (Ontario); Randy Ritland, Rachel Pleasants, and Stacy van Loon (Iowa); Jeff Miner, Greg Hess, Mike Embrey, Jessica Hopper, Annie Donnelly, Eric Olson, Mike Raupp, and Terry Patton (Maryland). Barbara Pleasants provided beneficial comments. This research was supported by a pooled grant provided by the U.S. Department of Agriculture, Agricultural Research Service, and the Agricultural Biotechnology Stewardship Technical Committee (ABSTC). Members of the ABSTC are Aventis CropScience USA LP, Dow AgroSciences LLC, E. I. du Pont de Nemours and Company, Monsanto Company, and Syngenta Seeds, Inc. Additional funding came from the Canadian Food Inspection Agency, and Environment Canada.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Losey J E, Rayor L S, Carter M E. Nature (London) 1999;399:214. doi: 10.1038/20338. [DOI] [PubMed] [Google Scholar]
- 2.Jesse L C H, Obrycki J J. Oecologia. 2000;125:241–248. doi: 10.1007/s004420000502. [DOI] [PubMed] [Google Scholar]
- 3.Raynor G S, Ogden E C, Hayes J V. Agron J. 1972;64:420–427. [Google Scholar]
- 4.Louette D. In: Gene Flow among Maize Landraces, Improved Maize Varieties and Teosinte: Implications for Transgenic Maize. Serratos J A, Willcox M C, Castillo F, editors. Mexico, D.F.: International Maize and Wheat Improvement Center; 1997. pp. 56–66. [Google Scholar]
- 5.Hellmich R L, Siegfried B D, Sears M K, Stanley-Horn D E, Mattila H R, Spencer T, Bidne K G, Daniels M J, Lewis L C. Proc. Natl. Acad. Sci. USA 98. 2001. 11925–11930. (First Published September 14, 2001; 10.1073/pnas.211297698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanley-Horn, Dively G P, Hellmich R L, Mattila H R, Sears M K, Rose R, Jesse L C H, Losey J E, Obrycki J J, Lewis L C. Proc Natl Acad Sci USA. 2001;98:11931–11936. doi: 10.1073/pnas.211277798. . (First Published September 14, 2001; 10.1073/pnas.211277798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sears M K, Hellmich R L, Siegfried B D, Pleasants J M, Stanley-Horn D E, Oberhauser K S, Dively G P. Proc Natl Acad Sci USA. 2001;98:11937–11942. doi: 10.1073/pnas.211329998. . (First Published September 14, 2001; 10.1073/pnas.211329998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erdtman G. Svensk Botanisk Tidskrift. 1960;54:561–564. [Google Scholar]
- 9.Jones G D, Coppedge J R. J Econ Entomol. 2000;93:636–643. doi: 10.1603/0022-0493-93.3.636. [DOI] [PubMed] [Google Scholar]
- 10.Dafni A. Pollination Ecology: A Practical Approach. Oxford: Oxford Univ. Press; 1992. [Google Scholar]
- 11.Russek-Cohen E, Douglas L W. Mixed Models Data Analysis: Using. 1999. PROC MIXED for biological research. Mixed model shortcourse manual (Beltsville, MD), pp. 89–103. [Google Scholar]
- 12.SAS/STAT. User's Guide. Cary, NC: SAS Inst.; 1996. [Google Scholar]
- 13.Zalucki M P, Kitching R L. J Zool. 1982;198:103–116. [Google Scholar]
- 14.Oberhauser K S, Prysby M, Mattila H R, Stanley-Horn D E, Sears M K, Dively G, Olson E, Pleasants J M, Lam W-K F, Hellmich R L. Proc Natl Acad Sci USA. 2001;98:11913–11918. doi: 10.1073/pnas.211234298. . (First Published September 14, 2001; 10.1073/pnas.211234298) [DOI] [PMC free article] [PubMed] [Google Scholar]