Abstract
Laboratory tests were conducted to establish the relative toxicity of Bacillus thuringiensis (Bt) toxins and pollen from Bt corn to monarch larvae. Toxins tested included Cry1Ab, Cry1Ac, Cry9C, and Cry1F. Three methods were used: (i) purified toxins incorporated into artificial diet, (ii) pollen collected from Bt corn hybrids applied directly to milkweed leaf discs, and (iii) Bt pollen contaminated with corn tassel material applied directly to milkweed leaf discs. Bioassays of purified Bt toxins indicate that Cry9C and Cry1F proteins are relatively nontoxic to monarch first instars, whereas first instars are sensitive to Cry1Ab and Cry1Ac proteins. Older instars were 12 to 23 times less susceptible to Cry1Ab toxin compared with first instars. Pollen bioassays suggest that pollen contaminants, an artifact of pollen processing, can dramatically influence larval survival and weight gains and produce spurious results. The only transgenic corn pollen that consistently affected monarch larvae was from Cry1Ab event 176 hybrids, currently <2% corn planted and for which re-registration has not been applied. Results from the other types of Bt corn suggest that pollen from the Cry1Ab (events Bt11 and Mon810) and Cry1F, and experimental Cry9C hybrids, will have no acute effects on monarch butterfly larvae in field settings.
The potential for adverse effects of Bacillus thuringiensis (Bt) Berliner corn, Zea mays L., on nontarget organisms has received much attention since a correspondence to Nature suggested that pollen from Bt corn could be hazardous to the larvae of the monarch butterfly, Danaus plexippus (L.) (1). In that study, young monarch larvae were given no choice but to feed on milkweed (Asclepias curassavica L.) leaves dusted with pollen from a Bt corn hybrid. They ate less, grew more slowly, and had a significantly higher mortality rate than larvae feeding on leaves dusted with nontransgenic pollen.
B. thuringiensis subspecies are differentiated by their insecticidal activity. Generally, only insect species within an order are susceptible to a given insecticidal Bt δ endotoxin, also referred to as a Cry (crystal) protein. The toxicity of Bt proteins expressed by transgenic corn to larval stages of butterflies and moths is well known (2, 3). Many studies, particularly those addressing the extensive use of Bt sprays for gypsy moth control in forests, have shown that Bt Cry proteins can adversely affect nontarget Lepidoptera (4, 5). Field data from these studies indicated a temporary reduction in lepidopteran populations during periods of prolonged Bt use, although widespread irreversible harm has not been reported (6). Based on such information, the U.S. Environmental Protection Agency (EPA) made the assumption that lepidopteran-active B. thuringiensis insecticides are likely to be hazardous to all Lepidoptera, although exposure from agricultural uses was not expected to be as high as in forest spraying (http://www.epa.gov/pesticides/reregistration/status.htm#B). In the initial assessments of transgenic corn, the EPA predicted that the impact of Bt corn pollen on nontarget butterflies and moths would be minimal because of low exposure (7). The possible impact of Bt corn pollen on D. plexippus larvae, however, was not known.
Ecological risk is a function of exposure (environmental dose) and effect (toxicological response). The amount of pollen dusted onto the milkweed leaves was not quantified in the initial study (1); as a result, it is not possible to establish a relationship between pollen exposure and effect from these data. Additionally, only one transgenic event was examined in that study. Based on known differences that exist among transgenic corn events, it is likely that levels of risk associated with each event vary. Transgenic corn hybrids that are currently or have been commercially available contain cry1Ab (events Bt11, Mon810, and 176), cry9C (event Cbh351), or cry1Ac (event Dbt418) Bt genes. Additionally, EPA registration was recently granted for hybrids that express a cry1F gene (event Tc1507). Event 176 hybrids contain a corn pollen-specific promoter and a corn phosphoenolpyruvate carboxylase promoter; toxin is expressed in pollen and photosynthetic tissues (8). Event Tc1507 hybrids contain the ubiquitin promoter, and the other commercial Bt hybrids contain the cauliflower mosaic virus 35S promoter and express toxin in all plant tissues (8, 9). Another factor not considered was the relative susceptibility of different developmental stages of monarchs (1). Toxicity of Bt proteins has been shown to vary considerably throughout larval development of European corn borer, Ostrinia nubilalis (Hübner) (10). A similar response by monarch larvae could have important implications for risk assessment depending on synchrony of monarch larval development and corn anthesis.
A potential hazard for monarch butterfly larvae consuming milkweed leaves (Asclepias spp.) containing surface-deposited pollen from Bt corn has been suggested by two studies (1, 11). Such hazard identification, however, should not be equated with ecological consequence until an accurate assessment of ecological risk can be formulated. In this investigation, a series of laboratory assays were conducted to establish the relative toxicity of Bt toxins that have been or are likely to be present in transgenic Bt corn hybrids. Three methods were used to examine the potential toxicity of Bt pollen: (i) purified toxins incorporated into an artificial diet, (ii) Bt pollen collected from commercially available and experimental Bt hybrids applied directly to milkweed leaf discs, and (iii) Bt pollen contaminated with corn tassel material applied directly to milkweed leaf discs. The latter method was examined because it appears that contaminated samples could skew results. Information generated from these experiments provides the toxicological response data for monarch larvae that is an essential component of a detailed ecological risk assessment.
Materials and Methods
Protein Bioassay.
Insects.
Monarch eggs were purchased from Monarch Butterfly Farm (St. Petersburg, FL) or from Monarch Watch (Lawrence, KS). Additional eggs were obtained from field-collected adult females during August 2000 on the campus of the University of Nebraska (Lincoln). Eggs were held in environmental chambers at 20°C until hatching, and first instars were used in bioassays within 24 h of hatching. For bioassay of later instars, the larvae were reared on artificial diet (see below) and then transferred to treated diet for bioassay.
Bioassays.
Bioassay of monarch larvae involved exposure to Bt toxins incorporated into artificial diet. A multispecies lepidopteran diet (Southland Products, Lake Village, AK) augmented with 2% powdered leaf tissue from air-dried common milkweed plants, Asclepias syriaca L., was used for both the bioassay diet and for rearing individual larvae to obtain later instars.
The trypsin-resistant core of four Bt toxins (Cry1Ab, Cry1Ac, Cry9C, and Cry1F), which represent toxins expressed by various commercial and noncommercial Bt corn hybrids, were uniformly incorporated into the larval diet. Depending on availability of larvae, each bioassay was replicated at least twice for each toxin with 16 larvae at each of five different Bt concentrations per replication. Bioassays were performed in 128-well trays (16 × 16 mm; CD International, Pitman, NJ). For early instars, ≈1 ml of diet was dispensed into each well and allowed to solidify. Controls consisted of artificial diet in the absence of Bt toxin. Larvae were transferred to individual wells covered with vented lids (CD International) and held at 27°C, 24-h scotophase, and 80% relative humidity. For older instars, cohorts of first instars were maintained on artificial diet for 4 days to obtain late second and early third instars and 10 days to obtain late third and early fourth instars. After maintenance on untreated diet, individual larvae were weighed to obtain a preexposure weight and then transferred to bioassay trays and exposed to various concentrations of Bt toxins as described above.
Analyses.
Both larval weight and mortality were recorded after 7 days. Mortality data were analyzed by probit analysis to determine lethal concentrations. Observed mortality was corrected for mortality in control treatments and lethal concentrations with 95% fiducial limits were calculated by probit analysis using POLO as adapted for PC use (12, 13). Larval weights were transformed to percentage of growth inhibition relative to the controls and analyzed by nonlinear regression procedures to obtain EC50 values (i.e., concentrations that produce 50% growth inhibition relative to controls) and 95% confidence intervals (14). For later instar bioassays, the difference between preexposure and postexposure weights was used to calculate growth inhibition relative to controls.
Pollen Bioassay.
Insects.
The Iowa monarch colony was established from eggs and larvae collected near Ames, IA, during the summers of 1999 and 2000. The Ontario colony was established from adult monarchs that were purchased from Swallowtail Farms (Carmichael, CA). In both cases, adult monarchs were placed in mating cages with potted tropical milkweed plants, A. currasavica, from which larvae were harvested.
Pollen.
Bioassays were conducted with field-collected pollen. Tassel bags (Lawson 404 Showerproof, Northfield, IL) were placed on shedding Bt and non-Bt corn plants for up to two pollen-shedding daily cycles. Contents of tassel bags were air dried for 48 h in darkness. Dried pollen was sieved first through a 60-mesh (250-μm) screen (Forestry Suppliers, Jackson, MS). Further sieving was necessary when it was learned that some samples had tassel contaminants. This entailed sieving with 170-mesh (90-μm) screen and then capturing the pollen with a 250-mesh (63-μm) screen. The sieved pollen was placed in glass vials and stored at −80°C until use. Unless otherwise indicated, all pollen samples were processed in this way.
In Iowa, pollen was collected from Cry1Ab event Bt11 (N3030Bt, N7070Bt, N4242Bt, N67-T4, and N58-D1, Syngenta Seeds, Golden Valley, MN) and non-Bt isoline (N3030, N7070, N4242, N67-H6, and N59-Q9, Syngenta Seeds) hybrids; Cry1Ab event Mon810 (38G17 and 34R07, Pioneer Hi-Bred International, Johnston, IA) and non-Bt isoline (3893 and 3489, Pioneer Hi-Bred International) hybrids; Cry1Ab event 176 (2657Bt, Dow AgroSciences, Indianapolis; Maximizer 21, 88, and 454, Syngenta Seeds) and non-Bt isoline (2657, Dow AgroSciences; 4394, 4273, and 4494, Syngenta Seeds) hybrids; Cry9C event Cbh351 (G8600Bt and G8539Bt, Garst Seed, Slater, IA) and non-Bt isoline (G8600, G8539 Garst Seed) hybrids; Cry1Ac event Dbt418 (DK595Bt, Monsanto, St. Louis) and non-Bt isoline (DK595, Monsanto) hybrids, and Cry1F event Tc1507 (experimental, Dow AgroSciences) and non-Bt isoline (M2722, Dow AgroSciences) hybrids. In Ontario, pollen was collected from Bt11 (N27-M3, Syngenta Seeds) and non-Bt isoline (N26-L6, Syngenta Seeds) hybrids, an event 176 (Maximizer 357, Syngenta Seeds) hybrid, and a non-Bt hybrid (Enerfeast, Syngenta Seeds).
Iowa bioassay.
Pollen bioassays involved exposing a first instar to two leaf discs from common milkweed, A. syriaca, or tropical milkweed, A. currasavica, in a small arena (60 × 10 mm Falcon culture plate; Franklin Lakes, NJ). Leaf discs were treated with different types and densities of pollen. In most cases, the effect of pollen from a Bt hybrid was compared with that of pollen from a near-isoline hybrid and a no-pollen control.
Milkweed leaves harvested the morning of the experiment were surface-sterilized by immersion in a 5% chlorine bleach solution. A #14 cork borer was used to cut 20-mm-diameter leaf discs that included ≈10 mm of the natural leaf margin. Discs from several leaves were randomly placed on trays lined with paper toweling soaked with deionized water. Three microscope slides (2.5 × 7.6 cm, clear) were interspersed on the trays and used to estimate pollen densities on the leaf discs. Measured amounts of pollen were suspended in 4 ml of deionized water in the applicator tube of a TLC sprayer (model 422550; Kimble-Kontes, Vineland, NJ) and applied to the leaf discs and slides with the sprayer within 2 min from when the water and pollen were mixed. The tray of leaf discs was held at a 45–60° angle and the sprayer was held perpendicular to the tray. Generally, four passes of the TLC sprayer were used to spray 3 ml of the suspended pollen solution onto the top surface of the leaf discs to produce a targeted pollen density. Deionized water was sprayed on leaf discs in a similar way for the no-pollen controls. Mean pollen density from each of the three slides per treatment was determined by counting pollen grains in each of six randomly selected 25-mm2 squares by using a stage micrometer on a Stereo-Zoom dissecting microscope (Nikon SMZ-1000).
Arena preparation involved four steps: (i) two layers of solidified agar (2.5% wt/vol, 1.5 and 2.5 mm thickness) were prepared in separate culture plates, (ii) the 1.5-mm layer was removed from its plate and put over the 2.5-mm layer, (iii) a #12 cork borer was used to produce two 16-mm holes (10 mm apart in the middle of the arena) through both layers of agar, and (iv) the top agar layer was pulled back and a treated leaf disc was put over each of the holes of the bottom layer, after which the top layer was repositioned to seal the discs between the agar layers. This setup delayed leaf disc dehydration. The natural leaf edge was positioned in a manner that allowed larvae to move freely between the top and bottom surfaces of the leaf. One D. plexippus larva was transferred with a camelhair brush into each culture plate on top of the upper agar surface between the two leaf discs. Larvae were matched for maturity throughout the experiment and especially within blocks. Bioassay arenas were sorted in a randomized complete block design with 9–24 blocks. Arenas were incubated at 25°C, 8-h scotophase, and 60% relative humidity.
Ontario bioassay.
The first bioassay included six densities of Bt11 and non-Bt pollen at ≈150, 300, 750, 1100, 1500, and 4000 grains/cm2, applied to whole tropical milkweed leaves. Pollen was deposited evenly on the leaf surface through a glass Venturi-shaped tube (35 × 7.5 cm diameter). The pollen sample was placed on mesh at the top of the tube and forced through the column with air pressure. Pollen density in two 1-cm2 areas on either side of the leaf was counted to verify proximity to the desired density. A cohort of ten first instars was weighed and then placed on the top of each leaf with a camelhair brush. Each leaf was placed in a ventilated plastic arena (15 × 15 × 5.5 cm, Ziploc container) and the arenas were incubated at 20°C, 16-h scotophase, and 60% relative humidity. Larvae were exposed to pollen for 48 h, after which the milkweed leaf with pollen was removed and replaced with an untreated milkweed leaf. Each bioassay was replicated four times. There were 12 control replicates that consisted of whole leaves without pollen. A bioassay was conducted in which larvae were exposed to mean densities of about 1500 grains Bt11 pollen/cm2, 1500 grains non-Bt pollen/cm2, or pollen-free leaves for a period of 5 days, after which they received fresh milkweed leaves with no pollen for an additional 5-day period. Conditions for this 10-day bioassay were the same as those described above.
Contamination study.
Preliminary studies showed that some of the pollen samples that were collected and processed contained corn tassel material, particularly fractured anthers. The nonpollen contamination made the samples appear lighter yellow than pure pollen. The 96-hour leaf disc bioassay was used to determine whether nonpollen material in samples influences the survival and development of monarch larvae when it is applied to milkweed leaf discs. Pollen from Cbh351 (event Cry9C) hybrid 8539Bt was sifted with a 250-μm screen (initial sample) or through a 90-μm screen capturing the pollen on a 63-μm screen (finely sifted). Material remaining after sifting (siftings, >90 and <250 μm) also was tested. The initial pollen, finely sifted pollen, and sifting treatments were applied to milkweed leaf discs as described above. The pollen densities were about 600 pollen grains/cm2. Arenas were sorted in a randomized complete block design with 10 blocks. A similar experiment was conducted with pollen from Bt11 (Cry1Ab) hybrid N7070Bt and its near isoline N7070 with 12 blocks. Pollen densities were about 900 grains/cm2.
Analyses.
For the Iowa bioassay, larval survival and leaf consumption were assessed after 48 and 96 h. Larval weights were measured at 96 h. During the 10-day Ontario bioassay, survival, weight, and leaf consumption were recorded on days 5 and 10. In Iowa, a Nikon Stereo-Zoom dissecting microscope with an eyepiece reticle grid was used to assess leaf consumption. In Ontario, leaf consumption was measured by creating a digital image of the leaf with a XC-75CE black-and-white video camera module with a Cosmicar/Pentax 16-mm TV lens (Lyndhurst, NJ).
Normality and homogeneity of variance were assessed by examination of normal probability plots and residual plots, respectively. Based on these examinations, log transformations were applied as required. For all survival proportions, arcsine transformations were applied. Differences among treatment means were analyzed by ANOVA (PROC MIXED, ref. 15). Paired contrasts were used to detect differences between Bt and non-Bt treatments at the same pollen levels and Bt and no-pollen treatments for survival, leaf consumption, and larval weight. A Tukey Studentized range test was used to separate treatment means (P < 0.05) when conducting all pairwise comparisons, and Dunnett's test (P < 0.05) was used to separate treatment means when comparing all treatments to a control (15). Because a simple dose-response relationship was not found, the data were divided into five sets: 100–300, 301–600, 601-1000, 1001–1600, and >1600 mean grains/cm2. The higher value in each the first four ranges represents the 80th, 95th, 99th, and 99.9th percentile mean corn pollen density, respectively, as observed in field monitoring studies (16). Pooled effects of Bt pollen, non-Bt pollen, and no pollen were computed by fitting ANOVA models (PROC MIXED) adjusting for trial and block and allowing for variances to differ by trial.
Results
Bioassay of Purified Bt Toxins.
First instars were not equally sensitive to all toxins tested (Table 1). Purified Bt toxins Cry1Ab and Cry1Ac were toxic both in terms of mortality and growth inhibition, whereas Cry9C and Cry1F were relatively nontoxic. Significant concentration–mortality regressions were obtained for all toxins except Cry1F, which did not produce mortality at any of the concentrations tested. However, growth inhibition was observed at higher concentrations, allowing calculation of an EC50 based on growth inhibition. For all four toxins tested, growth of monarch larvae was significantly inhibited at concentrations that did not cause mortality, indicating a sublethal effect of the toxins during early development as evidenced by the consistently lower EC50 values relative to LC50 values. Similar results have been observed for target pest species, including O. nubilalis and corn earworm, Helicoverpa zea (Boddie) (14, 17). However, it is unknown whether delayed development of early instars negatively affected long-term growth, development, or reproductive fitness.
Table 1.
Comparative toxicity of the four Bt endotoxins tested against D. plexippus larvae on artificial diet
| Toxin | Instar | n | LC50 (95% FL)* | EC50 (95% CI)† |
|---|---|---|---|---|
| Cry1Ab | 1st | 318 | 3.3 (2.2–4.8) | 0.8 (0.6–0.9) |
| 2nd–3rd | 141 | 35.1 (30–100) | 9.6 (6.0–15) | |
| 3rd–4th | 125 | >100‡ | 18.3 (9.4–40) | |
| Cry1Ac | 1st | 192 | 13.8 (3.0–26) | 0.9 (0.9–1.0) |
| Cry9C | 1st | 164 | 316 (203–428) | 34.9 (22–55) |
| Cry1F | 1st | 62 | >30,000‡ | 5,220 (2930–8520) |
Nanograms of Cry1Ab per milliliter treated artificial diet. FL, fiducial limit.
Concentration of Cry1Ab that produces 50% growth inhibition relative to untreated controls. Calculated by nonlinear regression fitted to a probit model. CI, confidence interval.
Highest concentration tested.
Third and fourth instars were less susceptible to Cry1Ab toxin compared with first instars (Table 1). Significant mortality of older instars was not observed over the range of concentrations tested. However, based on growth inhibition, late second to early third instars were nearly 12-fold more tolerant and late third to early fourth instars were about 23-fold more tolerant than first instars.
Bt Pollen Bioassays.
Cry1Ab events Bt11 and Mon810.
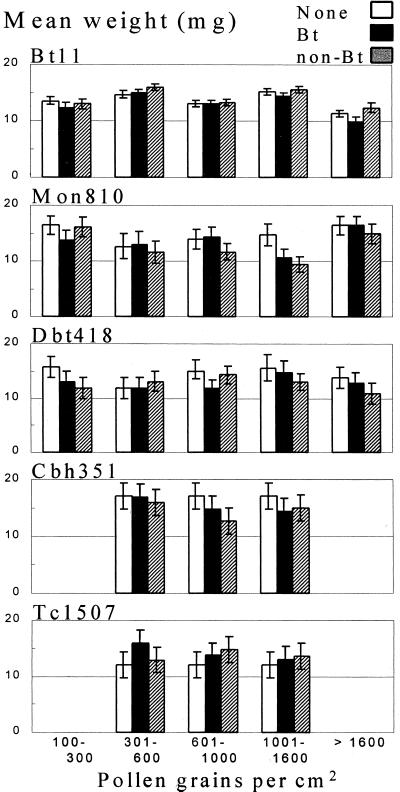
In the laboratory bioassays, pollen from Bt11 hybrids did not significantly influence the weights of monarch first instars for any of the pollen levels tested (Fig. 1; see Table 3, which is published as supporting information on the PNAS web site, www.pnas.org). The overall treatment effects, which included the no-pollen control, were not significantly different, except for the N67-H6/N67-T4 hybrid (Bt11, Trial 3, F = 3.53; df = 10,104; P = 0.0005). Mean larval weights at the highest non-Bt and Bt pollen treatments (>1600 mean pollen grain/cm2) were significantly lower than the mean larval weights in the no-pollen treatment (mean mg weight ± SE; non-Bt, 6.4 ± 2.7; Bt, 8.6 ± 2.7; no pollen, 22.9 ± 2.7; P < 0.05, Dunnett's test). Larvae feeding on milkweed leaves with pollen from Bt11 hybrid (N27-M3) at 4000 mean grains/cm2 (many times higher than average environmental exposure) weighed less than larvae feeding on a similar amount of non-Bt pollen. This difference was nearly statistically significant (non-Bt, 12.2 ± 1.4; Bt, 9.2 ± 1.4; F = 3.96; no pollen, 10.0 ± 1.4; df = 1,36; P = 0.054). The same larvae were similar in weight to larvae feeding on milkweed leaves with no pollen (F = 0.15; df = 1,36; P = 0.705). The 10-day Ontario leaf bioassay indicated monarch larvae were unaffected by Bt11 pollen after a 5-day exposure. Weights of larvae that consumed milkweed leaves dusted with 1500 mean grains of Bt11 pollen/cm2 were similar to larvae from the no-pollen and non-Bt pollen control treatments after 5 days (non-Bt, 13.0 ± 0.87; Bt, 13.3 ± 0.87; no pollen, 13.5 ± 1.0; F = 0.07; df = 2,8; P = 0.94) and 10 days (non-Bt, 113.8 ± 33.2; Bt, 112.9 ± 17.5; no pollen, 97.7 ± 11.8; F = 0.12; df = 2,8; P = 0.89).
Figure 1.
Mean ± SE 96-h weight (mg) of monarch larvae after exposure to milkweed leaves with no pollen and leaves with various levels of pollen from Bt11 and Mon810 events (Cry1Ab), Dbt418 event (Cry1Ac), Cbh351 event (Cry9C), and Tc1507 event (Cry1F) hybrids, and near-isoline hybrids. Number of trials combined for each event were (Bt11: 7), (Mon810: 3), (Dbt418: 2), (Cbh351: 2), and (Tc1507: 1).
In the Iowa leaf-disc bioassay of Bt corn pollen from Mon810 hybrids 38G17 and 34R07, with one exception, mean weights of larvae feeding on leaf discs with Bt pollen, non-Bt pollen and no pollen in the three bioassays were not significantly different (see Table 3). At the third pollen level (601–1000 grains/cm2) larvae feeding on leaf discs with Bt pollen from 34R07 hybrids (Trial 3) weighed significantly more than the larvae from the non-Bt pollen treatment (non-Bt, 11.3 ± 1.7; Bt, 17.2 ± 1.7; no pollen, 16.4 ± 1.7; F = 5.74; df = 1,110; P = 0.018), but were similar to larvae from the no-pollen treatment (F = 1.38; df = 1,110; P = 0.242). For the Bt11 and Mon810 trials, the degree of leaf consumption by larvae essentially mirrored weight gain results, and no differences in larval survival were found.
Cry1Ac, Cry9C, and Cry1F events.
Larvae whose diet included pollen from Dbt418 (Cry1Ac), Cbh351 (Cry9C), or Tc1507 (Cry1F) hybrids after 4 days had weights that were not significantly different from larvae that consumed no pollen or pollen from near isoline hybrids for any of the pollen levels tested (Fig. 1; see Table 3).
Combined analyses.
There was a tendency for weights of larvae exposed to mean Bt11 pollen densities above 1000 grains/cm2 to be lower than those of larvae exposed to non-Bt pollen. (This tendency may reflect the fact that more trials were performed with Bt11 pollen than with pollen from the other events.) Thus, for each event, data from the various trials were pooled to increase the power to detect differences. There were no significant interactions between trial and protein expression (Bt, non-Bt, and no pollen) when the data were pooled. The protein (Bt and non-Bt) by pollen density interaction was not significant (F = 1.58; df = 1,569; P = 0.21). Pollen density was not a significant covariate (F = 0.76; df = 1,570; P = 0.38), indicating no significant linear dose-response relationship between pollen density and larval weight gain. When the Bt11 data were grouped as in Table 3 (Fig. 1), there were no significant differences between Bt, non-Bt, and no-pollen treatments when mean pollen treatments were between 100 and 300 (F = 0.71; df = 2,62; P = 0.496), 301 and 600 (F = 1.47; df = 2,163; P = 0.233), 601 and 1000 (F = 0.05; df = 2,138; P = 0.951), 1001 and 1600 (F = 2.22; df = 2,196; P = 0.112), and >1600 grains/cm2 (F = 1.97; df = 2,75; P = 0.147). No differences in survival among larvae exposed to Bt, non-Bt, and no-pollen treatments were detected when data from mean pollen densities >1000 grains/cm2 were pooled (Bt, 0.97 ± 0.01; non-Bt, 0.98 ± 0.01; no pollen, 0.99 ± 0.01; F = 1.24; df = 2,45; P = 0.299).
Pooled data from the Mon810 and Dbt418 trials, except for one case, revealed no significant differences in mean larval weight (mg): Mon810, 301–600 (F = 0.18; df = 2,14; P = 0.839), 601-1000 (F = 0.99; df = 2,29; P = 0.383), and >1,000 mean grains/cm2 (non-Bt, 11.2 ± 1.3; Bt, 10.4 ± 1.4; no pollen, 16.4 ± 1.7; F = 3.98; df = 2,52; P = 0.025); and Dbt418, 601-1000 (F = 1.26; df = 2,22; P = 0.305) and >1000 grains/cm2 (non-Bt, 13.1 ± 1.4; Bt, 13.6 ± 1.3; no pollen, 15.6 ± 2.2; F = 0.47; df = 2,44; P = 0.626). Protein was a significant factor when pollen on the leaf discs from the Mon810 trials was greater than 1000 grains/cm2. Bt and non-Bt treatments were not different from each other, but both were significantly lower than the no-pollen treatment (P < 0.03, Tukey's test). Pollen densities higher than 300 were pooled for each of the events Cbh351 and Tc1507 and no significant differences were found: Cbh351 (non-Bt, 14.4 ± 1.8; Bt, 15.1 ± 1.8; no pollen, 21.4 ± 3.8; F = 1.39; df = 2,40; P = 0.262); Tc1507 (non-Bt, 14.0 ± 1.2; Bt, 14.1 ± 1.2; no pollen, 12.0 ± 2.3; F = 0.34; df = 2,105; P = 0.710).
Cry1Ab event 176.
Larvae feeding on leaf discs treated with more than 100 mean grains of event 176 pollen/cm2 were significantly smaller than those fed leaves with a comparable amount of non-Bt pollen in both the Iowa bioassay (mean mg ± SE: non-Bt pollen, 31.6 ± 5.6, Bt pollen, 0.8 ± 0.1; F = 129.3; df = 1,54; P < 0.0001) and the Ontario bioassay (non-Bt pollen, 14.9 ± 2.3, Bt pollen, 3.9 ± 0.6; F = 42.4; df = 1,17; P < 0.0001). Subsequent tests focused on lower densities of pollen where statistically significant growth inhibition relative to no-pollen controls was observed at mean pollen levels between 11 and 20 grains/cm2, and in some cases at levels between 5 and 10 grains/cm2 (Table 2). Results for consumption paralleled those observed for weight gain. Proportion of survivors decreased as Bt pollen levels (grains/cm2) increased (no pollen, 0.99 ± 0.1; 5–10, 0.89 ± 0.03; 11–20, 0.76 ± 0.3; 21–40, 0.67 ± 0.04; 41–80, 0.55 ± 0.04; 81–130, 0.28 ± 0.05). Survival of larvae from the no-pollen control was significantly higher than all Bt pollen categories except the 5–10 grains/cm2 category (P < 0.05, Dunnett's test).
Table 2.
Mean 96-h larval weight (mg), standard error (SE), and treatment sample size (n) for first instar D. plexippus after exposure to milkweed leaves with no pollen and leaves with various levels of pollen from event 176 (Cry1Ab) hybrids, and near-isoline hybrids
| Trial* | n | SE | No pollen | Mean pollen levels (grains/cm2)
|
41–80 Bt | 81–130
|
|||
|---|---|---|---|---|---|---|---|---|---|
| 5–10 Bt | 11–20 Bt | 21–40 Bt | Non-Bt | Bt | |||||
| 1 | 12 | 1.1 | 11.5a | 7.3ab | 3.6bc, 2.2cd | 1.8cde | 1.1de | 11.8a | 0.7e |
| 2 | 24 | 1.1 | 15.1a | 10.3bc | 6.2cde, 5.0de | 3.0def | 2.3ef | 15.0ab | 1.7ef |
| 3 | 12 | 1.2 | 9.4ab | 5.7bc | 3.5dc | 1.8de, 1.6de | 1.3de | 14.7a | 0.7de |
| 4 | 12 | 1.2 | 17.8a | 15.1a | 6.1b, 2.4bc | 1.8c | 1.2c | — | — |
| 5 | 12 | 1.2 | 20.1a | 15.4a | 4.0b, 3.6b | 2.1b | 1.0b | — | — |
| 6 | 12 | 1.2 | 9.5ab | 5.9ab | 3.0bc | 2.2bc, 3.1abc | 1.4bc, 1.9bc | 12.7a | 1.8bc |
Means within a trial with the same letter not significantly different (Tukey's test, P < 0.05). Assigning treatments to pollen-level categories sometimes resulted in two means per trial by level combination.
Non-Bt and Bt Hybrids used in each trial include: (Trial 1, 2657, 2657Bt), (2, 4273, Maximizer 88), (3, 4494, Maximizer 454), (4, 5, and 6, 4394, Maximizer 21).
Contamination Study.
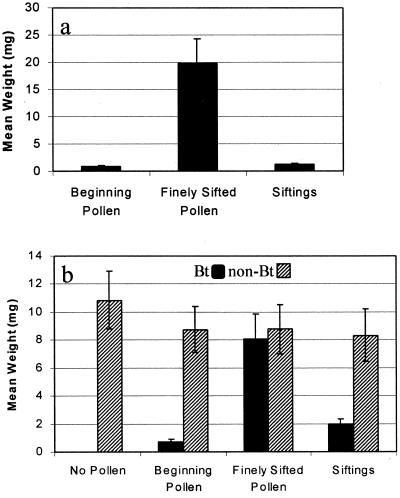
Larvae whose diet included contaminated Bt pollen, designated beginning pollen, from hybrid 8539 (Cry9C, event Cbh351) or siftings of the contaminated pollen had extremely low weights (Fig. 2a). Conversely, larvae that consumed finely sifted pollen, from which most of the contaminants had been removed, weighed 10 times more than larvae from the other two treatments (F = 43.97; df = 2,27; P < 0.0001). Similarly, larvae whose diet included contaminated pollen from Cry1Ab event Bt11 hybrid N7070Bt or siftings with contaminants had similar weights and weighed significantly less than larvae that consumed finely sifted Bt pollen, isoline (hybrid N7070) non-Bt pollen, or no pollen (Fig. 2b; F = 12.91; df = 5,66; P < 0.0001). Results from these experiments suggest that monarch larvae were affected by contaminants in the samples and not by the pollen itself.
Figure 2.
Mean ± SE weight (mg) of monarch larvae after feeding on treated milkweed discs for 96 h. Treatments included: (a) pollen from event Cbh351 (Cry9C) hybrid G8539Bt that was processed with 250-μm sieve (beginning), pollen from same hybrid processed with 90-μm sieve (finely sifted), and siftings remaining after the initial sample was finely sifted; and (b) no pollen, pollen from event Bt11 (Cry1Ab) hybrid N7070Bt, and pollen from hybrid N7070 (non-Bt) that were processed in the same manner as pollen in a. Mean pollen levels for a and b were ≈600 and ≈900 pollen grains/cm2, respectively.
Discussion
Bioassays of purified Bt toxins in artificial diet indicate that Cry9C and Cry1F proteins are relatively nontoxic to monarch first instars, whereas first instars are sensitive to Cry1Ab and Cry1Ac proteins. These assays also indicate that second-to-third and third-to-fourth instars are 12 and 23 times more tolerant, respectively, to Cry1Ab toxin than first instars. Results from pollen bioassays suggest that monarch sensitivity to Cry proteins varies depending on the amount of sample contamination with nonpollen material and the specific transgenic event.
One of the challenges of conducting a laboratory bioassay is adequately simulating field conditions. Results from pollen contamination experiments suggest that reproducing natural pollen deposition on milkweed leaves in the laboratory is difficult. Sample contaminants can dramatically influence larval weight gains and produce spurious results. Based on particle counts, the percentage of nonpollen material (predominantly fractured anthers) found in the initial pollen samples was 7 ± 0.6% for the Cry9C (Cbh351) experiment, and 8 ± 0.7% for the Cry1Ab (Bt11) experiment. Jesse and Obrycki (10) reported anther tissue contamination in their Bt11 samples at 43% ± 2%. Accordingly, their conclusion that exposure to Bt11 pollen at 135 grains/cm2 influenced larval weights should be reevaluated. This contamination issue does raise an important question. Are monarch larvae exposed to corn tissues other than pollen? A reevaluation of preserved milkweed leaves from Ontario cornfields and sticky slides and plates put in Iowa and Ontario cornfields (16) indicated that anthers were commonly found on milkweed leaves within cornfields during anthesis. None of them, however, was fractured (R.L.H., M.K.S., D.E.S.-H., and K.G.B., unpublished data). Thus, fractured anthers in pollen samples appear to be an artifact of pollen processing, but it remains unknown whether monarch larvae will consume whole anthers, which are significantly larger than pollen grains.
Pollen is ≈90 μm in diameter when fresh and slightly smaller when dried. Multiple sieving of pollen through a 90-μm screen ending with a 63-μm screen to capture the pollen eliminated most of the nonpollen material from the samples, but could not eliminate particles that were similar in size to pollen. Researchers investigating potential environmental effects of transgenic plants should recognize that artifacts could result from the unintentional mixing of processed tissues with different Bt protein expression levels. Green tissues from Bt corn hybrids express Cry1Ab proteins at levels more than 100 times that of their pollen (ref. 18; www.epa.gov/pesticides/biopesticides/factsheets/fs006430t.htm). Because of the potential problems associated with simulating natural pollen deposition, laboratory results should be viewed as supportive information but not as a replacement for field experiments (19).
Does this information shed any light on the original monarch study? Besides the delayed development, the researchers reported that larval survival after feeding for 4 days on milkweed leaves dusted with Bt11 pollen was 56%, compared with 100% survival in the control treatments (1). In our study, survival of larvae exposed to >1000 pollen grains/cm2 for 4 days was 97% ± 1%, not significantly different from the controls. However, survival of larvae that consumed Cbh351 pollen with corn tassel contaminants was 20% ± 13% compared with 100% survival of larvae that consumed finely-sifted Cbh351 pollen; and survival of larvae that consumed Bt11 pollen with contaminants was 17% ± 11% compared with 92% ± 1% survival of larvae from the isoline control. Except for the 176 experiments, low survival was not detected unless pollen was contaminated with anther material. These results together with the pollen bioassay results suggest that the Bt11 pollen treatments used in the original monarch study may have been contaminated with nonpollen tissues, that pollen densities were well in excess of 1000 grains/cm2, or both.
Toxicity of purified Cry1Ab protein can be related to results from bioassays of event 176 pollen by comparing consumption of first instars. The estimated equivalent amount of event 176 pollen affecting monarch larvae calculated from studies with purified protein ranges from 7 to 30 pollen grains/cm2 leaf, which is in the range of pollen grains found to affect larvae in the leaf-disc bioassay.‖ However, it must be kept in mind that this estimate is based on conservative, no-choice laboratory exposures of the most sensitive larval stage. Predicting activity levels for pollen of Bt11 and Mon810 is more problematic. Cry1Ab expression in pollen from these events has been reported as <0.09 μg/g for Bt11 and Mon810 (www.epa.gov/scipoly/sap/2000/october/brad2 scienceassessment.pdf); this value corresponds to the lower limit of quantitation for the ELISA procedures. The actual levels of expression could be much lower. Using estimates derived from studies of purified Cry1Ab protein, threshold effects from Bt11 and Mon810 pollen (based on a conservative assumption that pollen contains 0.09 μg Cry1Ab/g) are predicted to occur at >366 pollen grains/cm2. Results from pollen bioassays, however, suggest that densities in excess of 1000 pollen grains/cm2, perhaps much higher, would be required to see a significant adverse effect on larval development. There are, however, factors that might influence larval susceptibility to Bt protein in pollen, including protein deterioration due to processing and storage of pollen, and larval preference or avoidance of pollen. There was some evidence that both Bt and non-Bt pollen at densities of pollen >1000 grains/cm2 may have influenced larval weight gains, which might be attributed to larvae trying to avoid the high densities of pollen.
The only transgenic corn pollen that consistently affected monarch larvae, even at low levels, was from event 176 hybrids, which use a pollen-specific promoter for expression of the cry1Ab gene. There was no evidence that pollen from Cry1Ab events Bt11 and Mon810 affected larvae at pollen densities less than 1000 pollen grains/cm2. There were some results, although not statistically significant, that suggested Bt11 pollen levels above 1000 pollen grains/cm2 could have a small effect on larval weight gain. With the combined analyses for pollen levels between 1000 and 1600 for the Bt11 study, the power (two-tailed test, alpha = 0.05) to detect a 20% differences in weight gain was excellent (0.93), but the power to detect a 10% difference in weight gain was low (0.40). Thus, with further study with larger sample sizes, small differences (presently, Bt vs. non-Bt, Bt ≈8% lower; Bt vs. no pollen, Bt ≈5% lower), if they are actually nonzero, could be detected (i.e., deemed statistically significant). However, it is sometimes difficult to determine whether small differences are real or due to experimental artifact. The laboratory bioassays used in these studies produce an extreme artificial environment where larvae are given no choice but to feed on milkweed leaves with high densities of pollen; and tassel contaminants, an artifact of pollen processing, could be an important factor. Even if the small weight gain effects were real, there should be little if any effect on monarch butterflies. Pollen densities that exceed 1000 grains/cm2 are not common, representing less than 1% of the milkweed leaves in cornfields during anthesis (16).
Larvae in these tests were exposed for up to 5 days and, with the exception of event 176 pollen, significant adverse effects in terms of weight gain or mortality were not observed. Experiments are underway to determine whether subtle effects could occur when larvae are exposed to Bt pollen for longer periods. Corn hybrids that produce event 176 pollen represent a small percentage (<2%) of the total corn planted in the U.S. (20). Additionally, seed companies producing event 176 hybrids have chosen not to seek U.S. EPA re-registration for event 176 in 2001, and will phase out the marketing of event 176 hybrids. Pollen levels measured on milkweed leaves in cornfields during anthesis do not commonly exceed 1000 grains/cm2; average values range from 10 to 425 grains/cm2 (16). These results suggest that pollen from Cry1Ab (events Bt11 and Mon810), Cry1F, and experimental Cry9C hybrids will have no acute effects on monarch butterfly larvae in field settings.
Supplementary Material
Acknowledgments
We thank Fred Gould, Bruce Tabashnik, George Kennedy, Kevin Steffey, and Anthony Shelton for their critical reviews. We thank Randy Ritland, Jim Robbins, Colothdian Tate, Patricia Anderson, Denny Bruck, Stacy Van Loon, Kate Kronback, Mary Schuster, Jaleen Brunner, Karen Doucette, Kerry Gillooly, Erin Roe, Tegwin Taylor, Christa Hoffman, Pat Beaupre, Bryan Muscat, Laura Timms and Matt van Ast for their assistance. This research was supported by a pooled grant provided by USDA-ARS and the Agricultural Biotechnology Stewardship Technical Committee (ABSTC), and funding from Canadian Food Inspection Agency (CFIA), Environment Canada, and the Ontario Ministry of Agriculture, Food and Rural Affairs. Members of ABSTC are Aventis CropScience USA LP, Dow AgroSciences LLC, E. I. du Pont de Nemours and Company, Monsanto Company, and Syngenta Seeds, Inc. This is a joint contribution from the USDA-ARS, and Journal Paper No. J-19461, the Iowa Agriculture and Home Economics Experiment Station, Ames, Iowa, Project No. 3543 (supported by Hatch Act and State of Iowa funds). This is paper No. 13415 of the Journal Series of the University of Nebraska Agricultural Research Division, and Contribution No. 1108 of the Department of Entomology, University of Nebraska-Lincoln.
Abbreviations
- Cry
crystal
- USDA-ARS
U.S. Department of Agriculture–Agricultural Research Service
- EPA
Environmental Protection Agency
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
First instars consumed 3.3 ± 0.5 mg of leaf tissue per day (daily leaf-disc area consumed 0.19 ± 3 cm2; leaf weight 17 ± 1.6 mg/cm2). Assuming artificial diet consumption is similar, protein consumption equivalent to the dietary EC50 is 0.00209 ng of protein per day (0.64 ng protein/g × 0.0033 g/day). If pollen Cry1Ab concentration is 1.1–5.0 μg/g (reported ranges of Cry1Ab concentration in event 176 pollen), the equivalent pollen concentration leading to an EC50 is 1.9–0.42 E-06 g of pollen per day. Assuming there are ≈1.5 million pollen grains per gram pollen, the equivalent number of pollen grains leading to an EC50 is 1.7–5.7. If the daily milkweed consumption by first instars is 0.19 cm2, the number of pollen grains that must be present on the leaf equivalent to the EC50 is 7–30 pollen grains/cm2 leaf.
References
- 1.Losey J E, Rayor L S, Carter M E. Nature (London) 1999;399:214. doi: 10.1038/20338. [DOI] [PubMed] [Google Scholar]
- 2.Kreig A, Langenbruch G A. In: Microbial Control of Pests and Plant Diseases. Burges H D, editor. New York: Academic; 1981. pp. 837–896. [Google Scholar]
- 3.Peacock J W, Schweitzer D F, Dale F, Carter J L, Dubois N R. Environ Entomol. 1998;27:450–457. [Google Scholar]
- 4.Miller J C. Am Entomol. 1990;36:135–139. [Google Scholar]
- 5.Johnson K S, Scriber J M, Nitao J K, Smitley D R. Environ Entomol. 1995;24:288–297. [Google Scholar]
- 6.Hall S P, Sullivan J B, Schweitzerm D F. USDA Bull. No. FHTET-98-16. 1999. [Google Scholar]
- 7.U.S. Environmental Protection Agency. Publ. No. EPA731-F-95-004. Washington, DC: U.S. Gov. Printing Office; 1995. [Google Scholar]
- 8.Koziel M G, Beland G L, Bowman C, Carozzi N B, Crenshaw R, Crossland L, Dawson J, Desai N, Hill M, Kadwell S. Bio/Technology. 1993;11:194–200. [Google Scholar]
- 9.Christensen A H, Sharrock R A, Quail P H. Plant Mol Biol. 1992;18:675–689. doi: 10.1007/BF00020010. [DOI] [PubMed] [Google Scholar]
- 10.Huang F, Buschman L L, Higgins R A. J Econ Entomol. 1999;92:547–550. [Google Scholar]
- 11.Jesse L C H, Obrycki J J. Oecologia. 2000;125:241–248. doi: 10.1007/s004420000502. [DOI] [PubMed] [Google Scholar]
- 12.Finney D J. Probit Analysis. 3rd Ed. Cambridge, U.K.: Cambridge Univ. Press; 1971. [Google Scholar]
- 13.Leora Software. POLO-PC: A User's Guide to probit and logist Analysis. Berkeley, CA: Leora Software; 1987. [Google Scholar]
- 14.Marçon P C R G, Young L J, Steffey K L, Siegfried B D. J Econ Entomol. 1999;922:799–285. [Google Scholar]
- 15.SAS Institute. SAS/STAT User's Guide. Cary, NC: SAS Institute; 1990. , Version 6.0. [Google Scholar]
- 16.Pleasants J M, Hellmich R L, Dively G P, Sears M K, Foster J E, Clark P L, Jones G D. Proc Natl Acad Sci USA. 2001;98:11919–11924. doi: 10.1073/pnas.211287498. . (First Published September 14, 2001; 10.1073/pnas.211287498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegfried B D, Spencer T, Nearman J L. J Econ Entomol. 2000;93:1265–1268. doi: 10.1603/0022-0493-93.4.1265. [DOI] [PubMed] [Google Scholar]
- 18.Fearing P L, Brown D, Vlachos D, Meghji M, Privalle L. Mol Breeding. 1997;3:169–176. [Google Scholar]
- 19.Stanley-Horn D E, Dively G P, Hellmich R L, Matilla H R, Sears M K, Rose R, Jesse L C H, Losey J E, Obrycki J J, Lewis L C. Proc Natl Acad Sci USA. 2001;98:11931–11936. doi: 10.1073/pnas.211277798. . (First Published September 14, 2001; 10.1073/pnas.211277798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hellmich R L, Siegfried B D. In: Genetically Modified Organisms in Agriculture-Economics and Politics. Nelson G C, editor. London: Academic; 2001. pp. 283–289. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.