Abstract
Recent advances in the isolation of tissue-resident adult stem cells and the identification of inductive factors that efficiently direct differentiation of human pluripotent stem cells along specific lineages have facilitated the development of high-fidelity modelling of several tissues in vitro. Many of the novel approaches have employed self-organizing three-dimensional (3D) culturing of organoids, which offer several advantages over conventional two-dimensional platforms. Organoid technologies hold great promise for modelling diseases and predicting the outcome of drug responses in vitro. Here, we outline the historical background and some of the recent advances in the field of three-dimensional organoids. We also highlight some of the current limitations of these systems and discuss potential avenues to further benefit biological research using three-dimensional modelling technologies.
This article is part of the theme issue ‘Designer human tissue: coming to a lab near you’.
Keywords: pluripotent stem cells, organoids, three-dimensional culture
1. Introduction
The isolation and maintenance of mammalian cells have significantly advanced scientific research into cellular processes and mechanisms of disease, including stem cell development and differentiation, the production of monoclonal antibodies, and therapeutic proteins and for modelling cancer in vitro [1]. Although culturing tissues dates back to the late nineteenth century, present cell culture systems draw from studies on the action of serum on fibroblast cells [2] and the development of novel synthetic cell culture media [3,4]. A classic example of this was the isolation and expansion of HeLa cells from a cervical tumour on a two-dimensional monolayer culture [5].
Since then, culturing cells in two-dimensional format has remained the predominant methodology of in vitro cell growth and expansion. However, the two-dimensional platforms do not effectively recapitulate the spatial requirements that are essential for the organization and cellular interactions that occur in vivo. In addition, it is suspected that limited cell–cell contact and altered in vitro cell signalling networks can result in major discrepancies between the data acquired from two-dimensional in vitro versus in vivo research.
2. Historical background
To overcome two-dimensional platform limitations, efforts have led to the development of novel approaches to recreate a more physiologically relevant environment in the form of three-dimensional cell culture [1]. To successfully construct and maintain a three-dimensional structure, much research has been devoted to the development of synthetic or natural polymeric three-dimensional scaffolds to facilitate cell growth. These efforts have resulted in the fabrication and characterization of several non-degradable or biodegradable synthetic polymers, such as poly-lactic acid, poly-glycolic acid, poly-lactic-co-glycolic acid and poly-caprolactone [6]. Initially, a ‘top-down’ approach was adopted where cells were seeded on a prefabricated scaffold; however, difficulties in recreating the intricate microstructural characteristics of tissues have remained the major limitation of this approach [7]. Later, ‘bottom-up’ assembly of small cellularized blocks and layer-by-layer assembly (also known as three-dimensional printing) have been developed [8]. In this review, we focus on scaffold-free methods to culture cells in three-dimensions and the generation of organoids by embedding cells in semi-solidified extracellular matrices (ECM) in contrast to the use of polymeric scaffolds and three-dimensional printing, which have been reviewed extensively elsewhere [6,9,10].
3. Techniques to generate scaffold-free three-dimensional cellular aggregates
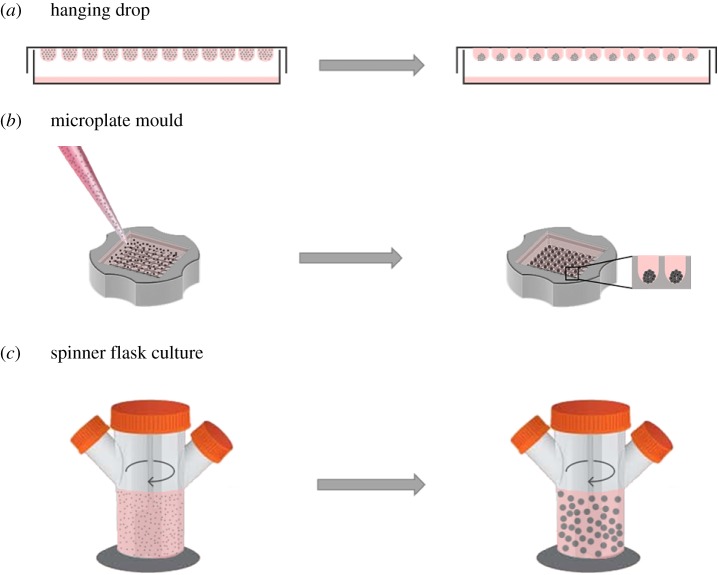
In general, scaffold-free approaches rely on cell–cell interaction and can be categorized into passive or active methodologies. The passive methodologies solely rely on cell adhesion properties, in which cells require time to form solid aggregates [11]. Various passive methods have been developed to generate scaffold-free three-dimensional aggregates robustly and consistently (figure 1).
Figure 1.
Methods for generation of three-dimensional microtissues. (a) Hanging-drop was the first method of generating and maintaining three-dimensional structures in culture. (b) Three-dimensional micromoulds have been introduced to the field to overcome both culture media restriction of the hanging drop method and size heterogeneity of spheroids formed in liquid overlay methodology. (c) The use of a spinner flask is one of the more advanced methodologies, developed for large-scale production and maintenance of three-dimensional microtissues. (Online version in colour.)
Hanging-drop was the first technique to generate three-dimensional structures, by culturing suspended droplets of the desired cell line(s) to force aggregation (figure 1a). In fact, Robert Koch et al. invented the hanging-drop methodology in the 1880s to grow anthrax bacilli in a suspended drop of fluid taken from oxen eyes in a special concave microscope slide [12]. Later, this method was adopted by Harrison to monitor nerve outgrowth [13]. Harrison's pioneering work led to the development of various techniques for short-term culture of dissected tissues during the early twentieth century. Although three-dimensional spheroids can be generated efficiently using this technique, the lack of scalability promoted the development of high-throughput culture methods that use 384-hanging-drop arrays, which are amenable to automation [14,15].
Later, the liquid overlay method was developed to generate three-dimensional microtissues on non-adherent surfaces. Using this method, random interactions of cells resulted in the formation of large numbers of spheroids, which were usually heterogeneous in size [16,17]. As the nutrients and oxygen exchange is based on passive diffusion in static culture, the formation of necrotic centres in large spheroids is a major drawback of this methodology [18]. To improve consistency and control the size of formed microtissues, micromoulds (figure 1b) and patterned microplates have more recently been used [19].
More advanced methodologies have also been developed for large-scale production of three-dimensional microtissues, which include spinner flasks, rotating wall vessel bioreactors and microfluidic systems (figure 1c).
As it is difficult to robustly generate three-dimensional microtissues from more than one cell type, several active techniques have been developed to overcome this problem. Active methodologies use additional physical stimuli such as ultrasound traps, electric fields, magnetic forces or the strong affinity between avidin and biotin to generate multicellular heterospheroids [20–23].
(a). Three-dimensional organoid formation
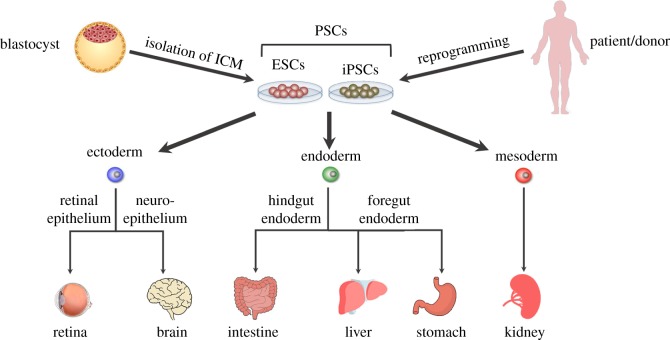
Derivation of reconstituted collagen from rat tail [24], the discovery of fibronectin [25,26], isolation of a matrix from chondrosarcoma murine cells [27] and characterization of laminin [28] have set the building blocks for subsequent progress in the field of three-dimensional cell culture. In 1989, Barcellos-Hoff and colleagues reported the functional differentiation and alveolar morphogenesis of primary mammary cultures on a reconstituted basement membrane matrix derived from Engelbreth–Holm–Swarm murine tumour, today known as Matrigel™ [29]. However, it took nearly two decades to widely use the self-organizing capacity of cells cultured in this laminin-rich ECM to form three-dimensional organ-like structures known as organoids [30]. The generation of organoids has made significant impact and led to the establishment of organoid culture from various tissues (figure 2), which will be discussed in more detail here.
Figure 2.
Schematic of some of the organoids generated from pluripotent stem cells (PSCs). Embryonic stem cells (ESCs) are generated following the expansion of cells isolated from the inner cell mass of an embryo at the blastocyst stage while iPSCs can be generated from somatic cells following reprogramming by key master regulators known as Yamanaka factors. Organoids of various tissues have been generated following treatment of MatrigelTM-embedded PSCs with cocktails of various growth factors. They can also be generated following isolation and culture of specific populations of progenitor cells, which maintain homeostasis of tissues during adulthood, such as cells expressing leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5). (Online version in colour.)
4. An insight into three-dimensional organoid cultures
The production of three-dimensional organoid-based culture systems from multiple organs has received considerable attention over the last 10 years [31]. The term ‘organoid’ is defined as self-organizing three-dimensional structures that are cultured in vitro while embedded in an ECM. These three-dimensional structures closely resemble their organ of origin [32]. Organoids can be derived from various cell sources such as primary tissue, cell lines, adult stem cells (ACSs) and pluripotent stem cells (PSCs) [33]. Organoids from human PSCs (hPSCs) are great tools to enhance our knowledge of human embryonic development while ASC-derived organoids can closely mimic the in vivo stem cell niche and can be considered as useful tools to enhance our understanding of the underlying mechanisms involved in tissue regeneration following injury.
(a). Organoids derived from the intestine and colon
The epithelium of intestine is derived from the definitive endoderm (DE) during embryonic development [34]. In a pioneering work, Ootani et al. developed an air–liquid interface model by culturing fragments of intestine that contained mesenchymal and epithelial cells from neonatal mice. In this model, cyst-like structures were formed in a medium supplemented with fetal bovine serum. Interestingly, these cyst-like structures were composed of all major cell types of the adult mouse intestine and could be maintained for over 1 year in culture [35].
Later on, Hans Clevers' research group proposed an alternative technique that aided the formation of epithelial organoids (mini-guts) from single Lgr5+ stem cells. The LGR5 protein is produced by a small population of stem cells residing in a variety of adult organs including intestine, stomach, kidney and skin [36]. By using a specialized cell culture medium and the support of Matrigel™ as an ECM, the stem cell niche of the crypt was mimicked and enabled long-term survival of LGR5+ cells [37]. These ‘organoids’ were composed of a central lumen surrounded by outgrowths or ‘buds’, which resemble the intestinal crypts and make them distinctive from the cystic structures previously described by Ootani and co-workers [35]. In this model, self-renewal of the stem cell population relied on LGR5+ stem cells, which terminally differentiated into enterocytes, and enteroendocrine or goblet cells. This methodological advancement played a key role in mimicking near-physiological conditions of in vivo mouse models while having an easy-to-maintain in vitro culture system [37]. Owing to the low level of Lgr5 expression, other research groups have investigated other stem cells markers such as CD24 [38], EphB2 [39] and CD166+/GRP78 [40] to generate intestinal organoids. In addition, a step-wise protocol was developed to generate intestinal organoids from hPSCs using activin A to induce initial transition into DE. Then WNT3A and BMP4 were used to promote hindgut and intestinal specification [41].
(b). Liver organoids
During embryonic development and early hepatogenesis, progenitor cells migrate from the foregut endoderm to form very dense and vascularized ‘liver buds’. The key cross-signalling pathways between mesenchymal, endodermal epithelial and endothelial progenitors have been studied extensively using these to better understand human liver development. In an attempt to recapitulate liver development, three-dimensional aggregates were formed by culturing human PSC-derived hepatocytes with mesenchymal stem cells and endothelial cells on a Matrigel™-coated plate. It was reported that these liver aggregates contained blood vessels and following transplantation into mice become connected to the host vessels within 48 h. The functional activity of the liver as determined by protein production and drug metabolism activity was significantly increased over time. Furthermore, the recipient mice were recovered from drug-induced liver failure following liver bud transplantation [42].
Hepatocytes and bile duct cells are the two major cell types in the liver, which have extremely slow turn-over in comparison with the small intestine and colon. In the healthy adult mouse liver, Lgr5 is not expressed at high levels. However, upon tissue damage or injury, small Lgr5+ cells located near bile ducts with high-level wingless (Wnt) signalling. It has been reported that following tissue injury, hepatocytes and bile duct cells are generated in vivo. With slight alteration, the single Lgr5+ cells could be clonally expanded as organoids by inhibiting the notch signalling pathway and differentiation into functional hepatocytes [43]. In a similar study, culture conditions were optimized for the long-term expansion of human liver progenitor cells. Similarly, long-term expanded liver organoids remained genetically stable and were transplanted into recipient mice to provide liver support [44].
(c). Pancreatic organoids
The adult pancreas is composed of several different cell types such as exocrine/acinar and endocrine cells with a very slow turn-over. Similar to the liver, under normal physiological conditions, the Wnt signalling pathway is not active and the Lgr5 gene is not highly expressed in the pancreas. Upon tissue injury, Wnt signalling is activated while pancreatic ducts regenerate through proliferation of Lgr5+ cells. In a similar setting to the mini-gut culture condition, clonal pancreas organoids were differentiated and successfully transplanted in vivo [45]. In an elegant study, Boj and colleagues established organoid models from both normal and neoplastic murine and human pancreatic tissues. Interestingly, these organoids exhibited ductal- and disease stage-specific characteristics and recapitulated tumour progression following in vivo transplantation [46].
(d). Lung organoids
The lung is derived from Nk2 homeobox1+ (Nkx2.1+) progenitor cells, which are generated in the ventral foregut endoderm region during embryonic development. In a pioneering work, a cytokeratins 5 (KRT5)-CreERT2 transgenic mouse model was used to trace and characterize basal cells which act as progenitors to generate differentiated cells during postnatal growth and repair. Following identification of integrin subunit alpha 6 (ITGA6) and nerve growth factor receptor (NGFR) as two specific cell surface markers, an organoid culture was established to generate both mouse and human luminal cells including differentiated ciliated cells [47]. In order to model lung development in vitro, the generation of lung organoids from PSCs has also been investigated. In an early attempt, induction of PSCs towards the endodermal fate was achieved following activin A induction and TFG-β/BMP inhibition and subsequent combinatorial induction of BMP and fibroblast growth factor (FGF) signalling to generate lung progenitors, which can recapitulate the early embryonic development of the lung [48]. More recently, an efficient protocol was developed to generate most cell types of the respiratory system, including basal, goblet, Clara, ciliated, type I and type II alveolar epithelial cells capable of performing specific functions such as surfactant protein-B uptake and stimulated surfactant release [49].
(e). Stomach organoids
During embryogenesis, the stomach derives from the posterior foregut. Stomach organoids have been generated from both ASCs and PSCs. D'Amour and colleagues proposed a method for the efficient derivation of DE from hESCs. It was reported that in the presence of activin A and low serum, up to 80% of the cells were differentiated into DE cells. It was also suggested that the process of differentiation into DE requires epithelial-to-mesenchymal transition [50]. Later, it was shown that DE can be derived from PSCs with only activin A following temporal manipulation of retinoic acid, FGF, WNT, BMP and epithelial growth factor (EGF) signalling pathways to generate three-dimensional human gastric organoids [51]. In addition, gastric organoids can be generated from single Lgr5+ cells that reside at the base of pyloric glands of the adult mouse stomach. Importantly, generated organoids closely recapitulate mature pyloric epithelium and can be expanded and maintained for an extended period in culture [52]. Moreover, at the base of the gastric corpus, there are specialized chief cells called Troy cells. Upon exposure to damage, these cells undergo dedifferentiation to become multipotent epithelial stem cells in vivo. Using this knowledge, gastric organoids were generated by culturing Troy+ chief using a previously established protocol that allows long-term expansion of single Lgr5+ pyloric stem cells [53].
(f). Brain organoids
During embryonic development, neural ectoderm forms the central nervous system (CNS), initially through the formation of the neural plate, which subsequently forms the neural tube via folding and fusion. Similar to other organs, morphogenic gradients in the tube establish a dorsal–ventral and a rostral–caudal axis. Neurons are the major cell types of the CNS and they are generated from neural stem cells (NSCs), which are located near the ventricles [54,55].
In ESC culture, spontaneous neural differentiation can be achieved following inhibition of signalling pathways such as BMP, Nodal and WNT. This process is very similar to the neural-default mechanism of ESCs. Based on this knowledge, Sasai and colleagues developed SFEBq: serum-free floating culture of embryoid body (EB)-like aggregates with quick re-aggregation [56]. In this culture setting, ESCs were isolated from growth-factor-free two-dimensional cultures. The cells were then re-aggregated in 96-well non-adhesive culture plates. The cells were maintained in serum-free medium containing no or very low levels of growth factors for 7 days, after which they were transferred into adhesion plates. Following formation of the lumen, ESCs polarize and differentiate to generate polarized neuroectoderm-like epithelium. It was further concluded that under certain conditions, the embryonic spatial and temporal events can be recapitulated in vitro, which can, in turn, lead to the generation of neural structures in the brain [56].
In another study, cerebral organoids were generated that were called ‘mini-brains’ as several regions of the brain were represented in each organoid. Very similar to the previous study, the floating EBs were cultured in the absence of growth factors to derive specific brain region identity. Further, aggregates were embedded in a laminin-rich ECM. With this technique, large neuro-epithelial buds were formed representing different brain regions. Interestingly, it was reported that brain regions such as retina, ventral forebrain, midbrain–hind-brain boundary and dorsal cortex were observed in these cultures [55].
(g). Retinal organoids
Embryonic development of the retina occurs through lateral evagination of the diencephalon, which in turn forms pseudostratified neuro-epithelium known as optic vesicle (OV). Later, sensory neural retina (NR) is derived from the distal portion of the OVs, while the proximal portion gives rise to retinal pigment epithelium (RPE). Following invagination of OVs at the distal portion, a bi-layered optic cup is formed with the RPE and NR at its outer and inner walls, respectively. The NR progenitor cells give rise to photoreceptors (rods and cones), ganglion cells and all supportive cell types [57]. Pioneering work in chick embryos that demonstrated the retinal capacity to form different cell types in the distinct laminated structure of retina paved the way for the development of PSC-derived retinal organoids [58].
Following initial studies that demonstrated the successful formation of retinal epithelium from three-dimensional floating mouse ESC-derived EB-like aggregates in a low-serum medium [59], retinal organoids were generated from self-organizing human ESCs forming a multi-layered tissue containing both rod and cone photoreceptors. Remarkably, retinal organoids formed from human ESCs were much larger in size than organoids derived from mouse ESCs, potentially reflecting species-specific differences [60].
(h). Other organs
More recently, organoids from other organs such as the prostate [61], fallopian tube [62], mammary gland [63,64], taste buds [65], salivary glands [66,67] and oesophagus [68] have all been developed.
The basal and luminal cells are two major cell types that form the pseudostratified epithelium of the prostate. In 2014, a mini-gut-based culture method was developed to support the long-term expansion of primary mouse and human prostate organoids. The structure of these three-dimensional organoids consisted of mature and differentiated basal and luminal cells. It was also reported that luminal cell-derived organoids closely resembled prostate glands. Luminal cell induction depends on WNT or R-spondin activation to some extent, and subsequently this will form prostate-like pseudostratified organoid structures [61]. Furthermore, an alternative culture system was established to derive prostate organoids using Matrigel™, EGF and androgen supplementation independently [69].
The fallopian tube is an anatomically simple organ, which is composed of columnar epithelium. Secretory cells produce tubular fluid and ciliated cells support the transfer of gametes within the tube. Self-renewal capacity of the epithelium is of utmost importance owing to the monthly cyclical hormonal fluctuations. In 2015, the long-term three-dimensional organoid culture of the human fallopian tube was established following adaptation of mini-gut culture protocols. The resulting clonal organoids were composed of ciliated and secretory cells, which provide the opportunity to study human fallopian tube epithelium in more details [62].
5. Applications of three-dimensional organoids
Organoids can be exploited for various applications such as disease modelling, drug toxicity testing, organoid biobanking, personalized therapy and host–pathogen interaction studies. In addition, organoids are a useful tool to perform omics analysis (transcriptomics, proteomics, epigenomics and metabolomics) of healthy and diseased tissues to gain a better understanding of mechanisms underlying pathological conditions [70]. Some of these applications are discussed below in further detail.
(a). Disease modelling
Although several animal models have been generated to recapitulate clinical characteristics of human monogenic disorders following the introduction of single-gene mutations, the introduction of such a mutation does not guarantee the recapitulation of the clinical features of these disorders in recipient animals. However, organoids generated from patient-specific iPSC lines can recapitulate the clinical features of various monogenic disorders and can be used as in vitro models to further study these disorders.
A clear example is an early attempt to generate an in vitro model of cystic fibrosis (CF) using patient-derived tissue fragments [71]. CF is an autosomal recessive genetic disorder caused by a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene affecting multiple organs including the lung, intestine, liver, pancreas and reproductive tract [72]. Dekkers et al. initially developed an organoid-based assay, whereby forskolin promoted a rapid swelling of wild-type-derived organoids from mouse and human intestinal samples through activation of cyclic AMP. They further concluded that the drug-induced swelling was significantly reduced in mice carrying the F508del mutation in the CFTR disease model. With the development of this advanced methodology, it was suggested that this is a promising tool to study gene therapy models to correct CFTR mutations [71]. In a follow-up study, the same assay was used to assess the potential of CRISPR/CAS9 technology to correct the CFTR F508del allele. Interestingly, organoids with the correct set of alleles regained the ability to swell upon exposure to forskolin. These studies concurrently demonstrated a proof of concept for gene replacement therapy for future clinical translation [73]. More recently, in vitro organoid models of other monogenic disorders such as Alagille syndrome [74], and retinitis pigmentosa [75] have been generated, which are reviewed more extensively elsewhere [76].
(b). Cell-based therapies
Despite advances in therapeutic regimens, there are various inherited, degenerative and chronic disorders that have remained incurable through conventional approaches. Stem cell-based therapies have the potential to alleviate symptoms or possibly cure these conditions by replacing damaged or lost cells. The ability to generate organoids that contain representative cell populations of the desired organs has made organoid culture a powerful tool to obtain various progenitor cells for cell-based therapies.
In an early attempt, Yui and co-workers prepared a large batch of organoids, which originally derived from single Lgr5+ colon stem cells, to study long-term genetic stability of the organoids. These organoids were transplanted per annum into multiple mice suffering from experimental colitis. They further confirmed that organoids were readily integrated and acted as functional epithelial patches, which could not be easily distinguished from the host epithelium [77]. In another elegant study, improvement of vision impairment was successfully demonstrated following transplantation of functional rod photoreceptors in adult Gnat1–/– mice, which lack rod function as a model of congenital stationary night blindness [78]. Therefore, generation of transplantation-competent photoreceptor precursors from hPSCs has been investigated to treat blindness [79–82].
In addition, multi-lineage approaches have been developed to generate composite organoids for the liver, lung, intestine, heart, kidney and brain [42,83]. Despite promising outcomes in the preliminary studies, clinical translation of hPSC-derived organoids faces several major challenges, including reliance on current protocols on undefined and animal-derived ingredients, that need to be resolved to facilitate their clinical applications.
(c). Drug screening, organ-on-chips and personalized medicine
High attrition rate is the biggest challenge facing the pharmaceutical industry. Lack of suitable preclinical models to accurately predict efficacy and toxicity of novel lead compounds has been considered to be one of the major contributors. To improve productivity and predictability, two-dimensional cell-based screenings have been used as a convenient means to evaluate novel therapeutic candidates. However, the emerging evidence has revealed poor predictability of two-dimensional screening platforms for certain diseases, such as cancers [84]. In addition, the predictability of preclinical animal models has been a matter of debate owing to considerable interspecies differences in disease phenotypes and reactions to drugs [85–87]. Lack of predictability and growing ethical concerns regarding the use of laboratory animals have encouraged exploration of new avenues to develop novel screening platforms to mitigate the high attrition rate.
To overcome these issues, various mono- and co-culture three-dimensional systems have been developed for oncology research and drug screening. Nutrients, oxygen, metabolites and soluble factors induce the formation of a heterogeneous population of cells within three-dimensional microtissues to mimic tumour microenvironments more closely than monolayer cultures [88].
Despite various practical challenges, three-dimensional drug screening platforms have grown in popularity and both tumour and healthy organoids of various tissues have been generated from patients' biopsies and ASCs or PSCs. In a pioneering work, Wong et al. demonstrated the usefulness of in vitro organoid models for the screening of lead compounds following treatment of patient-derived organoids with a novel small molecule to correct for a common CF-processing mutation that resulted in enhanced membrane localization of mature CFTR protein [89].
Considering heterogeneity of tumour pathophysiology, patient-derived organoids have proven to be a useful tool for cancer drug discovery. The heterogeneous response of neoplastic tumours to anti-cancer treatment was demonstrated following screening of 83 authorized and experimental anti-cancer agents on tumour organoids derived from resected colorectal tissues obtained from 20 patients [90]. Similarly, organoids from three major subtypes of liver cancers were propagated and used for drug screening. Interestingly, liver cancer-derived organoids preserved gene expression, genomic landscape and metastatic properties of the original tumours even after long-term in vitro expansion. In addition, SCH772984 (an extracellular signal-regulated kinase inhibitor) was identified as a potential therapeutic compound for primary liver cancer [91]. More recently, organoids were derived from a large number of patients enrolled in four prospective phases 1 and 2 clinical trials. While notable morphological similarities were observed between patient-derived organoids and the patient biopsies from which they were originally derived, the data from a screening of anti-cancer agents suggested that patient-derived organoids can recapitulate patient responses in the clinic [92]. The above-mentioned studies reiterate the importance of patient-specific organoids to identify an appropriate anti-tumour regimen for the efficient treatment of neoplastic disorders. To this end, organoid biobanks have been established from patient tumours as a valuable tool for drug screening and personalized medicine [90,93,94].
Three-dimensional organoids have also been used in conjunction with microfluidic devices, known as organ-on-chips, as a powerful tool for drug screening. Although organ-on-chips are designed to represent the functional complexity of a particular organ such as the intestine [95] or liver [96], recent efforts have been focused on the development of more sophisticated platforms by interconnecting several organ-on-chips [97]. Development of such platforms can substitute for mandatory preclinical studies in animal models to increase the success rate and improve the productivity of drug screening while addressing growing ethical concerns regarding the use of animal models for drug screening.
(d). Modelling infectious diseases to mimic complex interaction between the host and pathogens
The Zika virus (ZIKV) is a flavivirus that was isolated from a rhesus monkey in the Zika region of Uganda in 1947 and can be transmitted by Aedes species mosquitoes [98]. Following entry to the human body, ZIKV binds to innate immune Toll-like receptor 3 (TRL3), which leads to the activation of genes causing disregulation of neurogenesis, which is a common side effect seen following ZIKV infection. Using hESC-derived cerebral organoids, it was demonstrated that TLR3 inhibition reduced the phenotypic effects of ZIKV infection [98]. Other studies also suggested that the mechanism of action of this lethal virus is concerned with TRL3-mediated apoptosis, hence cell death of NSC and impaired development in humans [99,100]. These experiments also demonstrated that microcephaly (i.e. a low level of NSC proliferation and more cell death) can be observed as a side effect of ZIKV infection in organoids [99,101]. Based on this knowledge, another research group employed a unique miniaturized spinning bioreactor system to grow forebrain-specific organoids derived from hiPSCs to be used as a major platform for high-throughput drug screening [102].
(e). Techniques for the introduction of microorganisms into organoids
Organoids are dense three-dimensional structures that are composed of apical and basal membranes as two main compartments. The apical side of the epithelium is towards the lumen (inside) of the organoids and the basal membrane appears on the outside. Microorganisms tend to target the apical membrane in vivo. Therefore, recapitulating the exact interactions between the host and the microbes is crucial. Hence, three independent strategies have been developed to reproduce host–pathogen interactions [70].
(i). Infection of dissociated spheroids before forming three-dimensional organoids
In this technique, organoids are forced to undergo mechanical shear stress or enzymatic digestion to become single-cell suspensions to expose the apical side. Following infection of dissociated cells, the infected cells are seeded in a three-dimensional matrix to form three-dimensional organoids within a few days. This method was employed to study gene expression manipulations using a specific lentiviral system [103] and can be used to model different infectious disease models [98,104,105].
(ii). Microinjection of viruses or bacteria into the lumen side of organoids
This technique was previously developed to inject ESCs into mice to study genetics. With slight modifications, microorganisms can be injected directly into the organoid's lumen [106,107]. As the organoids remain intact and no dissociation occurs, the necessary interaction between the host and pathogens can be easily detected and monitored. Although this method seems promising, there are some limitations including the availability of a microinjector device and precise quantification of delivered pathogens can be difficult due to the size variation of organoids in culture [70].
(iii). Two-dimensional culture-derived organoids and interaction with microorganisms
Three-dimensional organoids can be dissociated and seeded onto an ECM such as Matrigel™ or collagen-coated plates. The cells will expand in two-dimensions and the apical surface will be exposed on the surface; therefore, when microorganisms are added to the dish, the host–microbe interaction proceeds. With this technique, microbes can be quantified; however, it does not resemble the in vivo three-dimensional setting [108].
6. Future directions
The ability to generate organ-specific organoids using hPSCs or tissue-specific progenitor cells alongside the development of cancer organoids has made organoid technology a powerful tool to study various biological aspects including organ development, tissue morphogenesis, modelling diseases in vitro, and testing the efficacy and toxicity of therapeutic compounds [41,43,44,51,55,71,91,109–113]. The advancement in microfabrication and microfluidic technology can set the stage for the development of new devices to enable high-throughput screening and biosensing, which subsequently would expand organoid application as a tool for drug toxicity screening of novel compounds [114].
To achieve the full potential of three-dimensional organoids, it is important to overcome limitations associated with current methodologies, particularly phenotypic immaturity of derived cells. For instance, suboptimal expression of hepocyte-specific CYP450 enzymes and low levels of albumin secretion were reported in liver organoids compared with primary hepatocytes, which restricts their downstream industrial and clinical applications [115]. In addition, Matrigel™, as an undefined animal product, has been an indispensable element of three-dimensional organoid methodologies that would undermine their therapeutic value. Therefore, it is important to develop new methodologies to establish protocols that are compatible with current good manufacturing practice for the generation of three-dimensional microtissues by using xeno-free and well-defined matrices to facilitate their potential clinical applications.
Acknowledgements
We are also immensely grateful to Dr Paresh Parmar for his comments on the text. Apologies to those scientists whose work could not be cited owing to space restrictions.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
M.T. and S.A. are supported by an NC3R SBRI CRACK-IT InMutagene award with funding from GSK and Novartis and a Brunel University London Scholarship award. H.R. is supported by an award from the UK Regenerative Medicine Platform (MRC MR/L022974/1).
References
- 1.Abbott A. 2003. Cell culture: biology's new dimension. Nature 424, 870–872. ( 10.1038/424870a) [DOI] [PubMed] [Google Scholar]
- 2.Carrel A, Ebeling AH. 1923. Action of serum on fibroblasts in vitro. J. Exp. Med. 37, 759–765. ( 10.1084/jem.37.6.759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White PR. 1946. Cultivation of animal tissues in vitro in nutrients of precisely known constitution. Growth 10, 231–289. [PubMed] [Google Scholar]
- 4.Eagle H. 1955. Nutrition needs of mammalian cells in tissue culture. Science 122, 501–504. ( 10.1126/science.122.3168.501) [DOI] [PubMed] [Google Scholar]
- 5.Sharrer T. 2006. The first immortal cell line. Scientist 20, 88. [Google Scholar]
- 6.Jafari M, Paknejad Z, Rad MR, Motamedian SR, Eghbal MJ, Nadjmi N, Khojasteh A. 2017. Polymeric scaffolds in tissue engineering: a literature review. J. Biomed Mater. Res. B 105, 431–459. ( 10.1002/jbm.b.33547) [DOI] [PubMed] [Google Scholar]
- 7.Nichol JW, Khademhosseini A. 2009. Modular tissue engineering: engineering biological tissues from the bottom up. Soft Matter 5, 1312–1319. ( 10.1039/b814285h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Y, Zhang XF, Gao GF, Yonezawa T, Cui XF. 2017. 3D bioprinting and the current applications in tissue engineering. Biotechnol. J. 12, 1600734 ( 10.1002/biot.201600734) [DOI] [PubMed] [Google Scholar]
- 9.Langer R, Vacanti J. 2016. Advances in tissue engineering. J. Pediatr. Surg. 51, 8–12. ( 10.1016/j.jpedsurg.2015.10.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodfield T, Lim K, Morouço P, Levato R, Malda J, Melchels F. 2017. 5.14 Biofabrication in tissue engineering A2. In Comprehensive biomaterials II (ed. Ducheyne P.), pp. 236–266. Oxford, UK: Elsevier. [Google Scholar]
- 11.Lin RZ, Chou LF, Chien CCM, Chang HY. 2006. Dynamic analysis of hepatoma spheroid formation: roles of E-cadherin and β1-integrin. Cell Tissue Res. 324, 411–422. ( 10.1007/s00441-005-0148-2) [DOI] [PubMed] [Google Scholar]
- 12.Landecker H. 2009. Culturing life. Cambridge, MA: Harvard University Press. [Google Scholar]
- 13.Harrison RG. 1907. Observations on the living developing nerve fiber. Anat. Rec. 1, 116–118. ( 10.1002/ar.1090010503) [DOI] [Google Scholar]
- 14.Tung YC, Hsiao AY, Allen SG, Torisawa YS, Ho M, Takayama S. 2011. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 136, 473–478. ( 10.1039/C0AN00609B) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsiao AY, Tung YC, Qu XG, Patel LR, Pienta KJ, Takayama S. 2012. 384 hanging drop arrays give excellent Z-factors and allow versatile formation of co-culture spheroids. Biotechnol. Bioeng. 109, 1293–1304. ( 10.1002/bit.24399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlsson J, Yuhas JM. 1984. Liquid-overlay culture of cellular spheroids. Recent Results Canc. 95, 1–23. ( 10.1007/978-3-642-82340-4_1) [DOI] [PubMed] [Google Scholar]
- 17.Landry J, Bernier D, Ouellet C, Goyette R, Marceau N. 1985. Spheroidal aggregate culture of rat liver cells: histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J. Cell Biol. 101, 914–923. ( 10.1083/jcb.101.3.914) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta G, Hsiao AY, Ingram M, Luker GD, Takayama S. 2012. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control. Release 164, 192–204. ( 10.1016/j.jconrel.2012.04.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Napolitano AP, Chai P, Dean DM, Morgan JR. 2007. Dynamics of the self-assembly of complex cellular aggregates on micromolded nonadhesive hydrogels. Tissue Eng. 13, 2087–2094. ( 10.1089/ten.2006.0190) [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Kuznetsova LA, Edwards GO, Xu JS, Ma MW, Purcell WM, Jackson SK, Coakley WT. 2007. Functional three-dimensional HepG2 aggregate cultures generated from an ultrasound trap: comparison with HepG2 spheroids. J. Cell. Biochem. 102, 1180–1189. ( 10.1002/jcb.21345) [DOI] [PubMed] [Google Scholar]
- 21.Sebastian A, Buckle AM, Markx GH. 2007. Tissue engineering with electric fields: immobilization of mammalian cells in multilayer aggregates using dielectrophoresis. Biotechnol. Bioeng. 98, 694–700. ( 10.1002/bit.21416) [DOI] [PubMed] [Google Scholar]
- 22.Ino K, Ito A, Honda H. 2007. Cell patterning using magnetite nanoparticles and magnetic force. Biotechnol. Bioeng. 97, 1309–1317. ( 10.1002/bit.21322) [DOI] [PubMed] [Google Scholar]
- 23.Kojima N, Takeuchi S, Sakai Y. 2011. Establishment of self-organization system in rapidly formed multicellular heterospheroids. Biomaterials 32, 6059–6067. ( 10.1016/j.biomaterials.2011.04.081) [DOI] [PubMed] [Google Scholar]
- 24.Ehrmann RL, Gey GO. 1956. The growth of cells on a transparent gel of reconstituted rat-tail collagen. J. Natl Cancer Inst. 16, 1375–1403. [PubMed] [Google Scholar]
- 25.Gahmberg CG, Hakomori SI. 1973. Altered growth-behavior of malignant cells associated with changes in externally labeled glycoprotein and glycolipid. Proc. Natl Acad. Sci. USA 70, 3329–3333. ( 10.1073/pnas.70.12.3329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruoslahti E, Vaheri A, Kuusela P, Linder E. 1973. Fibroblast surface antigen—new serum-protein. Biochim. Biophys. Acta 322, 352–358. ( 10.1016/0005-2795(73)90310-3) [DOI] [PubMed] [Google Scholar]
- 27.Orkin RW, Gehron P, Mcgoodwin EB, Martin GR, Valentine T, Swarm R. 1977. Murine tumor producing a matrix of basement-membrane. J. Exp. Med. 145, 204–220. ( 10.1084/jem.145.1.204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. 1979. Laminin - glycoprotein from basement-membranes. J. Biol. Chem. 254, 9933–9937. [PubMed] [Google Scholar]
- 29.Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. 1989. Functional-differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement-membrane. Development 105, 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lancaster MA, Knoblich JA. 2014. Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345, 1247125 ( 10.1126/science.1247125) [DOI] [PubMed] [Google Scholar]
- 31.Simian M, Bissell MJ. 2017. Organoids: a historical perspective of thinking in three dimensions. J. Cell Biol. 216, 31–40. ( 10.1083/jcb.201610056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fatehullah A, Tan SH, Barker N. 2016. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 18, 246–254. ( 10.1038/ncb3312) [DOI] [PubMed] [Google Scholar]
- 33.Clevers H. 2016. Modeling development and disease with organoids. Cell 165, 1586–1597. ( 10.1016/j.cell.2016.05.082) [DOI] [PubMed] [Google Scholar]
- 34.Barbara PD, Van den Brink GR, Roberts DJ. 2003. Development and differentiation of the intestinal epithelium. Cell. Mol. Life Sci. 60, 1322–1332. ( 10.1007/s00018-003-2289-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ootani A, et al. 2009. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 15, 701–706. ( 10.1038/nm.1951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barker N, Clevers H. 2010. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology 138, 1681–1696. ( 10.1053/j.gastro.2010.03.002) [DOI] [PubMed] [Google Scholar]
- 37.Sato T, et al. 2009. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265. ( 10.1038/nature07935) [DOI] [PubMed] [Google Scholar]
- 38.von Furstenberg RJ, Gulati AS, Baxi A, Doherty JM, Stappenbeck TS, Gracz AD, Magness ST, Henning SJ. 2011. Sorting mouse jejunal epithelial cells with CD24 yields a population with characteristics of intestinal stem cells. Am. J. Physiol. Gastrointestin. Liver Physiol. L 300, G409–GG17. ( 10.1152/ajpgi.00453.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jung P, et al. 2011. Isolation and in vitro expansion of human colonic stem cells. Nat. Med. 17, 1225–1227. ( 10.1038/nm.2470) [DOI] [PubMed] [Google Scholar]
- 40.Wang F, et al. 2013. Isolation and characterization of intestinal stem cells based on surface marker combinations and colony-formation assay. Gastroenterology 145, 383–395.e21. ( 10.1053/j.gastro.2013.04.050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spence JR, et al. 2011. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109. ( 10.1038/nature09691) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takebe T, et al. 2013. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481–484. ( 10.1038/nature12271) [DOI] [PubMed] [Google Scholar]
- 43.Huch M, et al. 2013. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494, 247–250. ( 10.1038/nature11826) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huch M, et al. 2015. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160, 299–312. ( 10.1016/j.cell.2014.11.050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huch M, et al. 2013. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 32, 2708–2721. ( 10.1038/emboj.2013.204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boj SF, Hwang CI, Baker LA, Engle DD, Tuveson DA, Clevers H. 2016. Model organoids provide new research opportunities for ductal pancreatic cancer. Mol. Cell Oncol. 3, e1014757 ( 10.1080/23723556.2015.1014757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BLM. 2009. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl Acad. Sci. USA 106, 12 771–12 775. ( 10.1073/pnas.0906850106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Longmire TA, et al. 2012. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell 10, 398–411. ( 10.1016/j.stem.2012.01.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang SXL, et al. 2014. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol. 32, 84–91. ( 10.1038/nbt.2754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. 2005. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 23, 1534–1541. ( 10.1038/nbt1163) [DOI] [PubMed] [Google Scholar]
- 51.McCracken KW, et al. 2014. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516, 400–404. ( 10.1038/nature13863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barker N, et al. 2010. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25–36. ( 10.1016/j.stem.2009.11.013) [DOI] [PubMed] [Google Scholar]
- 53.Stange DE, et al. 2013. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 155, 357–368. ( 10.1016/j.cell.2013.09.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eiraku M, Sasai Y. 2012. Self-formation of layered neural structures in three-dimensional culture of ES cells. Curr. Opin. Neurobiol. 22, 768–777. ( 10.1016/j.conb.2012.02.005) [DOI] [PubMed] [Google Scholar]
- 55.Lancaster MA, et al. 2013. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379. ( 10.1038/nature12517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eiraku M, et al. 2008. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532. ( 10.1016/j.stem.2008.09.002) [DOI] [PubMed] [Google Scholar]
- 57.Fuhrmann S. 2010. Eye morphogenesis and patterning of the optic vesicle. Curr. Top. Dev. Biol. 93, 61–84. ( 10.1016/B978-0-12-385044-7.00003-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rothermel A, Willbold E, Degrip WJ, Layer PG. 1997. Pigmented epithelium induces complete retinal reconstitution from dispersed embryonic chick retinae in reaggregation culture. Proc. R. Soc. Lond. B 264, 1293–1302. ( 10.1098/rspb.1997.0179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y. 2011. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56. ( 10.1038/nature09941) [DOI] [PubMed] [Google Scholar]
- 60.Nakano T, et al. 2012. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771–785. ( 10.1016/j.stem.2012.05.009) [DOI] [PubMed] [Google Scholar]
- 61.Karthaus WR, et al. 2014. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 159, 163–175. ( 10.1016/j.cell.2014.08.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kessler M, et al. 2015. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat. Commun. 6, 2487 ( 10.1038/ncomms9989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Linnemann JR, et al. 2015. Quantification of regenerative potential in primary human mammary epithelial cells. Development 142, 3239–3251. ( 10.1242/dev.123554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rios AC, Fu NY, Lindeman GJ, Visvader JE. 2014. In situ identification of bipotent stem cells in the mammary gland. Nature 506, 322–327. ( 10.1038/nature12948) [DOI] [PubMed] [Google Scholar]
- 65.Ren W, Lewandowski BC, Watson J, Aihara E, Iwatsuki K, Bachmanov AA, Margolskee RF, Jiang P. 2014. Single Lgr5- or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo. Proc. Natl Acad. Sci. USA 111, 16 401–16 406. ( 10.1073/pnas.1409064111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nanduri LS, Baanstra M, Faber H, Rocchi C, Zwart E, de Haan G, van Os R, Coppes RP. 2014. Purification and ex vivo expansion of fully functional salivary gland stem cells. Stem Cell Rep. 3, 957–964. ( 10.1016/j.stemcr.2014.09.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maimets M, et al. 2016. Long-term in vitro expansion of salivary gland stem cells driven by Wnt signals. Stem Cell Rep. 6, 150–162. ( 10.1016/j.stemcr.2015.11.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.DeWard AD, Cramer J, Lagasse E. 2014. Cellular heterogeneity in the mouse esophagus implicates the presence of a nonquiescent epithelial stem cell population. Cell Rep. 9, 701–711. ( 10.1016/j.celrep.2014.09.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chua CW, et al. 2014. Single luminal epithelial progenitors can generate prostate organoids in culture. Nat. Cell Biol. 16, 1–4. ( 10.1038/ncb3047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dutta D, Heo I, Clevers H. 2017. Disease modeling in stem cell-derived 3D organoid systems. Trends Mol. Med. 23, 393–410. ( 10.1016/j.molmed.2017.02.007) [DOI] [PubMed] [Google Scholar]
- 71.Dekkers JF, et al. 2013. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med. 19, 939–945. ( 10.1038/nm.3201) [DOI] [PubMed] [Google Scholar]
- 72.Ratjen F, Doring G. 2003. Cystic fibrosis. Lancet 361, 681–689. ( 10.1016/S0140-6736(03)12567-6) [DOI] [PubMed] [Google Scholar]
- 73.Dekkers JF, et al. 2016. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci. Transl. Med. 8, 344ra84 ( 10.1126/scitranslmed.aad8278) [DOI] [PubMed] [Google Scholar]
- 74.Guan Y, et al. 2017. Human hepatic organoids for the analysis of human genetic diseases. JCI Insight. 2, e94954 ( 10.1172/jci.insight.94954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma TP, et al. 2017. Patient-specific induced pluripotent stem cells to evaluate the pathophysiology of TRNT1-associated retinitis pigmentosa. Stem Cell Res. 21, 58–70. ( 10.1016/j.scr.2017.03.005) [DOI] [PubMed] [Google Scholar]
- 76.Perez-Lanzon M, Kroemer G, Maiuri MC. 2018. Organoids for modeling genetic diseases. In International review of cell and molecular biology (ed. Galluzzi L.), pp. 49–81. New York, NY: Academic Press. [DOI] [PubMed] [Google Scholar]
- 77.Yui SR, et al. 2012. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat. Med. 18, 618–623. ( 10.1038/nm.2695) [DOI] [PubMed] [Google Scholar]
- 78.Pearson RA, et al. 2012. Restoration of vision after transplantation of photoreceptors. Nature 485, 99–103. ( 10.1038/nature10997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gonzalez-Cordero A, et al. 2013. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat. Biotechnol. 31, 741–747. ( 10.1038/nbt.2643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lakowski J, et al. 2015. Transplantation of photoreceptor precursors isolated via a cell surface biomarker panel from embryonic stem cell-derived self-forming retina. Stem Cells 33, 2469–2482. ( 10.1002/stem.2051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gonzalez-Cordero A, et al. 2017. Recapitulation of human retinal development from human pluripotent stem cells generates transplantable populations of cone photoreceptors. Stem Cell Rep. 9, 820–837. ( 10.1016/j.stemcr.2017.07.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kruczek K, et al. 2017. Differentiation and transplantation of embryonic stem cell-derived cone photoreceptors into a mouse model of end-stage retinal degeneration. Stem Cell Rep. 8, 1659–1674. ( 10.1016/j.stemcr.2017.04.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takebe T, et al. 2015. Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell 16, 556–565. ( 10.1016/j.stem.2015.03.004) [DOI] [PubMed] [Google Scholar]
- 84.Karlsson H, Fryknas M, Larsson R, Nygren P. 2012. Loss of cancer drug activity in colon cancer HCT-116 cells during spheroid formation in a new 3-D spheroid cell culture system. Exp. Cell Res. 318, 1577–1585. ( 10.1016/j.yexcr.2012.03.026) [DOI] [PubMed] [Google Scholar]
- 85.Olson H, et al. 2000. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharm. 32, 56–67. ( 10.1006/rtph.2000.1399) [DOI] [PubMed] [Google Scholar]
- 86.Seok J, et al. 2013. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl Acad. Sci. USA 110, 3507–3512. ( 10.1073/pnas.1222878110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burkina V, Rasmussen MK, Pilipenko N, Zamaratskaia G. 2017. Comparison of xenobiotic-metabolising human, porcine, rodent, and piscine cytochrome P450. Toxicology 375, 10–27. ( 10.1016/j.tox.2016.11.014) [DOI] [PubMed] [Google Scholar]
- 88.Fang Y, Eglen RM. 2017. Three-dimensional cell cultures in drug discovery and development. SLAS Discov. 22, 456–472. ( 10.1177/1087057117696795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan LJ, Ratjen F, Ellis J, Rossant J. 2012. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat. Biotechnol. 30, 876–882. ( 10.1038/nbt.2328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van de Wetering M, et al. 2015. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945. ( 10.1016/j.cell.2015.03.053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Broutier L, et al. 2017. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 23, 1424–1435. ( 10.1038/nm.4438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vlachogiannis G, et al. 2018. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359, 920 ( 10.1126/science.aao2774) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Verissimo CS, et al. 2016. Targeting mutant RAS in patient-derived colorectal cancer organoids by combinatorial drug screening. eLife 5, 1626 ( 10.7554/eLife.18489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aboulkheyr Es H, Montazeri L, Aref AR, Vosough M, Baharvand H. 2018. Personalized cancer medicine: an organoid approach. Trends Biotechnol. 36, 358–371. ( 10.1016/j.tibtech.2017.12.005) [DOI] [PubMed] [Google Scholar]
- 95.Gao D, Liu HX, Lin JM, Wang YN, Jiang YY. 2013. Characterization of drug permeability in Caco-2 monolayers by mass spectrometry on a membrane-based microfluidic device. Lab. Chip 13, 978–985. ( 10.1039/c2lc41215b) [DOI] [PubMed] [Google Scholar]
- 96.Baudoin R, Prot JM, Nicolas G, Brocheton J, Brochot C, Legallais C, Benech H, Leclerc E. 2013. Evaluation of seven drug metabolisms and clearances by cryopreserved human primary hepatocytes cultivated in microfluidic biochips. Xenobiotica 43, 140–152. ( 10.3109/00498254.2012.706725) [DOI] [PubMed] [Google Scholar]
- 97.Ishida S. 2018. Organs-on-a-chip: current applications and consideration points for in vitro ADME-Tox studies. Drug Metab. Pharmacokinet 33, 49–54. ( 10.1016/j.dmpk.2018.01.003) [DOI] [PubMed] [Google Scholar]
- 98.Dang J, Tiwari SK, Lichinchi G, Qin Y, Patil VS, Eroshkin AM, Rana TM. 2016. Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell 19, 258–265. ( 10.1016/j.stem.2016.04.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wells MF, et al. 2016. Genetic ablation of AXL does not protect human neural progenitor cells and cerebral organoids from Zika virus infection. Cell Stem Cell 19, 703–708. ( 10.1016/j.stem.2016.11.011) [DOI] [PubMed] [Google Scholar]
- 100.Garcez PP, et al. 2016. Zika virus impairs growth in human neurospheres and brain organoids. Science 352, 816–818. ( 10.1126/science.aaf6116) [DOI] [PubMed] [Google Scholar]
- 101.Nowakowski TJ, Pollen AA, Di Lullo E, Sandoval-Espinosa C, Bershteyn M, Kriegstein AR. 2016. Expression analysis highlights AXL as a candidate Zika virus entry receptor in neural stem cells. Cell Stem Cell 18, 591–596. ( 10.1016/j.stem.2016.03.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qian X, et al. 2016. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254. ( 10.1016/j.cell.2016.04.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koo BK, Stange DE, Sato T, Karthaus W, Farin HF, Huch M, van Es JH, Clevers H. 2012. Controlled gene expression in primary Lgr5 organoid cultures. Nat. Methods 9, 81–83. ( 10.1038/nmeth.1802) [DOI] [PubMed] [Google Scholar]
- 104.Forbester JL, Goulding D, Vallier L, Hannan N, Hale C, Pickard D, Mukhopadhyay S, Dougan G. 2015. Interaction of Salmonella enterica serovar typhimurium with intestinal organoids derived from human induced pluripotent stem cells. Infect. Immun. 83, 2926–2934. ( 10.1128/IAI.00161-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang YG, Wu S, Xia Y, Sun J. 2014. Salmonella-infected crypt-derived intestinal organoid culture system for host–bacterial interactions. Physiol. Rep. 2, e12147 ( 10.14814/phy2.12147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leslie JL, Huang S, Opp JS, Nagy MS, Kobayashi M, Young VB, Spence JR, McCormick BA. 2015. Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function. Infect. Immun. 83, 138–145. ( 10.1128/IAI.02561-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bartfeld S, Clevers H. 2015. Organoids as model for infectious diseases: culture of human and murine stomach organoids and microinjection of Helicobacter pylori. J. Vis. Exp. 105, e53359 ( 10.3791/53359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ettayebi K, et al. 2016. Replication of human noroviruses in stem cell-derived human enteroids. Science 353, 1387–1393. ( 10.1126/science.aaf5211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gao D, et al. 2014. Organoid cultures derived from patients with advanced prostate cancer. Cell 159, 176–187. ( 10.1016/j.cell.2014.08.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cao L, Gibson JD, Miyamoto S, Sail V, Verma R, Rosenberg DW, Nelson CE, Giardina C. 2011. Intestinal lineage commitment of embryonic stem cells. Differentiation 81, 1–10. ( 10.1016/j.diff.2010.09.182) [DOI] [PubMed] [Google Scholar]
- 111.Sato T, et al. 2011. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141, 1762–1772. ( 10.1053/j.gastro.2011.07.050) [DOI] [PubMed] [Google Scholar]
- 112.McCracken KW, Howell JC, Wells JM, Spence JR. 2011. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat. Protoc. 6, 1920–1928. ( 10.1038/nprot.2011.410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yusa K, et al. 2011. Targeted gene correction of α1-antitrypsin deficiency in induced pluripotent stem cells. Nature 478, 391–394. ( 10.1038/nature10424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Skardal A, Shupe T, Atala A. 2016. Organoid-on-a-chip and body-on-a-chip systems for drug screening and disease modeling. Drug Discov. Today 21, 1399–1411. ( 10.1016/j.drudis.2016.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Azuma H, et al. 2007. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat. Biotechnol. 25, 903–910. ( 10.1038/nbt1326) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.