Abstract
Gastrointestinal diseases are becoming increasingly prevalent in developed countries. Immortalized cells and animal models have delivered important but limited insight into the mechanisms that initiate and propagate these diseases. Human-specific models of intestinal development and disease are desperately needed that can recapitulate structure and function of the gut in vitro. Advances in pluripotent stem cells and primary tissue culture techniques have made it possible to culture intestinal epithelial cells in three dimensions that self-assemble to form ‘intestinal organoids'. These organoids allow for new, human-specific models that can be used to gain insight into gastrointestinal disease and potentially deliver new therapies to treat them. Here we review current in vitro models of intestinal development and disease, considering where improvements could be made and potential future applications in the fields of developmental modelling, drug/toxicity testing and therapeutic uses.
This article is part of the theme issue ‘Designer human tissue: coming to a lab near you'.
Keywords: disease modelling, organoids, stem cells
1. Intestinal structure and function
The intestines are a vital organ derived from definitive endoderm and can be subdivided into the small and large intestines [1]. Collectively, the small and large intestines perform the vital function of digestion, nutrient absorption and waste elimination [2]. The small intestine contains several functionally distinct areas including the duodenum, jejunum and ilium, and its surface comprises a highly folded epithelium of villi, microvilli and intestinal crypts [3]. This intricate folding of villi and microvilli serves to dramatically increase the total surface area of the small intestine, thereby facilitating greater nutrient absorption. The intestinal crypt functions as the niche in which the LGR5+ stem cells reside [4]. LGR5+ cells are relatively slow cycling cells and give rise to a highly proliferative and multipotent progenitor population known as the transit-amplifying (TA) cells, which differentiate as they migrate from the crypt towards the villi. By the time TA cells have moved out of the crypt, they are fully differentiated into one of the many cell types required for normal functionality of the epithelium [5].
The large intestine comprises four main regions: the ascending, transverse, descending and sigmoid colon [6]. While most digestion occurs in the small intestine, the large intestine functions to absorb water and ions, in addition to vitamins and short chain fatty acids (SCFA) synthesized by commensal bacteria. Production of SCFA by commensal bacteria in the large intestine is recognized as an important feature of maintaining normal gut health and has been shown to have several protective effects including creating an environment hostile to pathogenic microbes, providing an energy source for intestinal epithelial cells and enhancing mucus production [7].
2. Cells of the small and large intestine
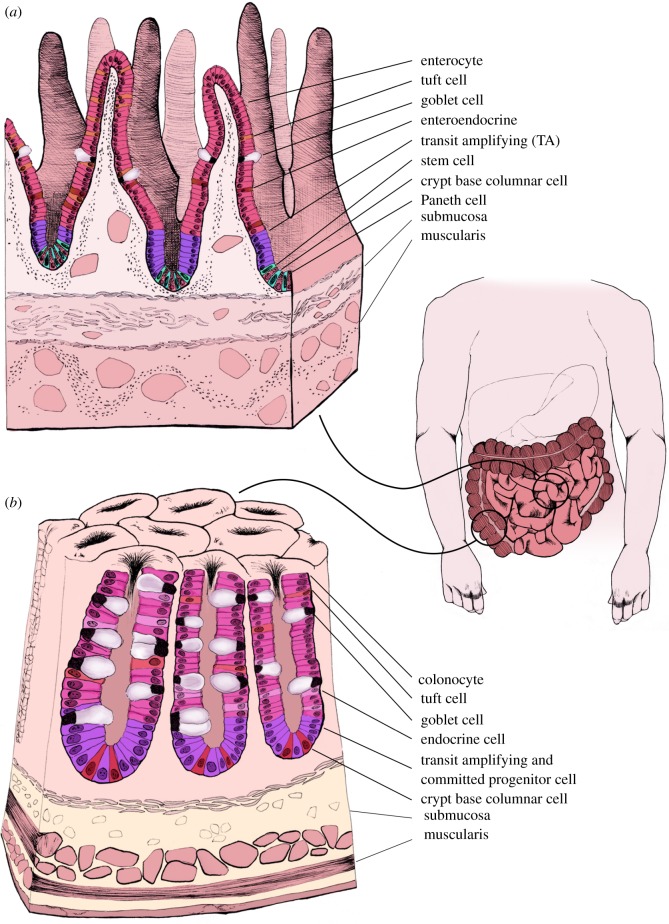
The intestinal epithelium comprises several different cell types including enterocytes, goblet cells, Paneth cells and enteroendocrine cells, all of which are derived from the resident LGR5+ stem cells found at the base of intestinal crypts [8] (figure 1). Epithelial cells are critical in maintaining immune homeostasis within intestinal tissue, closely regulating the interplay between the intestinal mesenchyme, and commensal and pathogenic bacteria. They maintain a barrier formed with tight junctions, essential to the control of macromolecular transport and immune homeostasis. Enterocytes are the most common cell type found in the epithelium, and they mainly function in nutrient absorption. Secretory epithelial cells consist of Paneth and goblet cells and multiple enteroendocrine cell types, with functions ranging from cytokine to hormone production [9].
Figure 1.
Anatomy of the small and large intestine. The small intestine is composed of repetitive villus and crypt structures. LGR5+ stem cells and Paneth cells are located at the base of the crypts, followed by the TA cells, and then mature epithelium composed of goblet cells, enteroendocrine cells and enterocytes (a). The large intestine lacks the villi structures of the small intestine and instead is composed of colonic crypts. At the base of colonic crypts are LGR5+ stem cells and Reg4+ cells, followed by TA cells and mature epithelium that contains a high proportion of goblet cells (b).
Goblet cells play a critical role in the functioning and protection of the intestinal tract. There are nearly twice as many goblet cells in the colon as in the small intestine, which is in proportion to the increase in bacteria [10]. Goblet cells function by producing glycoproteins known as mucins, which are vital constituents of the mucus that lines the epithelium. This mucosal layer acts as the first line of defence, preventing the bacteria present in the gut from being in direct contact with the intestinal epithelium [11]. Mucus production is increased in response to inflammatory cytokines including interferon-γ and interleukins-9, -10 and -13 [12]. The glycoproteins that comprise this mucus are toxic to many strains of bacteria. Additionally, this layer acts as a matrix to which defensins and antibodies adhere that target specific pathogenic bacteria adhere. When mucus production is hindered, this can cause commensal bacteria in the gut microbiota to penetrate the epithelial barrier, triggering an immune response [13]. Goblet cells also produce other constituents of the mucus layer, including trefoil factors that stabilize the mucus layer by forming cross-links between different components of the mucus [14]. This cross-linking creates a unique property in that the innermost layer is thicker and more viscous while the outer layer is more aqueous, allowing commensal bacteria to reside there.
The small and large intestine is a high turnover organ, with complete epithelial renewal approximately every 7 days. This process is driven by LGR5+ intestinal stem cells (figure 1a,b). The pioneering work of Hans Clevers demonstrated that LGR5+ stem cells reside at the base of the intestinal crypts that form the intestinal stem-cell niche [8,15]. LGR5+ cells are crucial for the continual renewal of the intestinal epithelium and undergo asymmetric division approximately every 24 h, giving rise to a daughter stem cell and a TA cell. These rapidly proliferating TA cells begin in the TA zone and migrate up the intestinal crypt, while dividing four to five times along the way, before terminally differentiating into any of the intestinal cell types [16]. Once differentiated, these cells continue to migrate upwards towards the top of the crypts and have a lifespan of approximately 7 days. Once these cells have reached the top of the villi, they typically undergo anoikis and are shed into the intestinal lumen.
Paneth are also generated from the asymmetrical division of the LGR5+ stem cell [16]. However, instead of migrating upwards, Paneth cells migrate downwards into the base of the crypt. Paneth cells are a longer-lived cell type, surviving for approximately 30 days at the base of intestinal crypts where they play an integral role in producing and maintaining the stem-cell niche [17,18]. In addition to maintaining the niche, Paneth cells secrete a substantial amount of antimicrobrial peptides such as defensins that help regulate intestinal microbiota and protect against invading pathogens.
Paneth cells are absent in the majority of colonic crypts. However, secretory cells expressing typical Paneth cell markers, including CD24 are present. Reg4 has been found to be a reliable marker of these cells, residing adjacent to LGR5+ stem cells in all colonic crypts [19]. These cells have been shown to secrete epidermal growth factor (EGF) and the Notch ligands DII1 and DII4, while LGR5+ stem cells were found to express the corresponding receptors [19]. These secretory Reg4+ cells were found to more closely resemble Paneth cells, rather than goblet cells found in either the small or large intestine. Reg4+ cells are integral to controlling stem-cell positioning in the crypt and for maintaining stemness of LGR5+ colonic stem cells. These cells have many distinct similarities with Paneth cells of the small intestine, with the exception that Reg4+ cells do not produce Wnt ligands, while colonic LGR5+ stem cells do express Wnt receptors [19]. Therefore, mesenchymal tissue surrounding the colonic crypts, known to produce Wnt ligands, may provide the necessary Wnt required for stem-cell maintenance in the intestinal crypt in vivo [20].
3. Cell signalling in the intestinal epithelium
Self-renewal and differentiation of LGR5+ stem cells are achieved via tight regulation of several signalling pathways within the intestinal crypts. Wnt signalling is considered the central signalling pathway maintaining LGR5+ stem-cell proliferation, controlling cell positioning within the crypt and activating terminal differentiation of Paneth cells. Dysregulation of this pathway has been shown to be important in the development of some colon cancers [21].
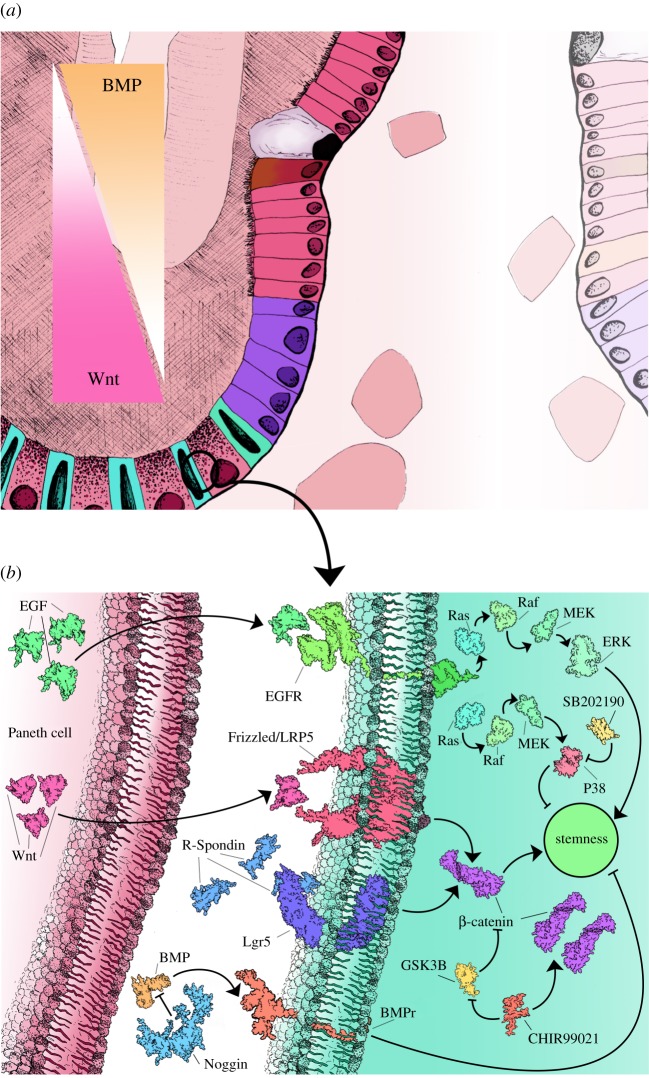
Higher concentrations of Wnt3a and EGF are present at the base of the crypts and are required for stem-cell maintenance and proliferation, respectively [22] (figure 2a). A concentration gradient of Wnt3a is established in the intestinal crypt, with highest levels found in the base of the crypt that then decrease towards the top of the crypt [23]. This concentration gradient along with a decrease in Notch signalling and increased BMP signalling at the top of the crypts facilitates the initial differentiation of the LGR5+ stem cells along with the proliferation and terminal differentiation of the TA cells [24]. Paneth cells are integral in producing the crypt environment via secretion of several factors, including Wnt3a, EGF, transforming growth factor α (TGFα) and the Notch ligand D114 [18].
Figure 2.
Regulation of stemness in the intestinal stem-cell niche. During normal maintenance of the stem-cell niche several signalling gradients are established that either promote stemness (Wnt) or differentiation (BMP) (a). Multiple signal pathway agonists and antagonists are active in the intestinal crypts that are also used during in vitro culture to simulate the crypt niche environment (b).
4. Intestinal organoids
The identification of LGR5+ stem cells and the characterization of the intestinal stem-cell niche has led to the development of three-dimensional (3D) organoid cultures and the ability to amplify intestinal epithelium in vitro. These primary tissue cultures can be maintained long term without any substantial changes to genetic integrity or tissue physiology.
Organoids are now becoming an increasingly popular option for exploring an array of diseases currently lacking suitable treatments. Multiple organoid culture platforms have now been described including liver and pancreatic organoids [25–28], kidney organoids [29], central nervous system organoids [30] and intestinal organoids [31–33]. An organoid is often defined as ‘an in vitro 3D cellular cluster derived exclusively from primary tissue, ESCs or IPSCs, capable of self-renewal and self-organization, and exhibiting similar organ functionality as the tissue of origin' [34]. Indeed, intestinal organoids are clusters of cells that self-organize in 3D structures that recapitulate major features of their native tissue. Intestinal organoids have been derived from both human stem cells and direct biopsy of adult intestinal tissue. In each case, the resulting intestinal organoids share many features, including a highly folded epithelium structure consisting of crypts and villi similar to native intestinal epithelium. Once embedded in Matrigel™, they self-assemble so that the luminal surface of epithelium is directed towards the centre of the organoid and the basolateral side is in contact with the Matrigel™ and surrounding medium. Analysis of the different cell types present within intestinal organoids has shown that all cell types usually found in vivo are present, and are therefore useful for studying the complexities of interplay between cell types during homeostasis and disease states.
Intestinal organoids have been shown to exhibit the same functions as those that occur in vivo, including mucus production, and absorptive and secretory functions [24]. Intestinal organoids mimic in vivo epithelial regenerative capacity, with apoptotic cells being continually released into the lumen of the organoid as new cells are differentiated from the LGR5+ cells within the crypts to replenish the epithelium.
5. Isolation and culture of intestinal organoids
There are two approaches to creating intestinal organoids: either through isolation of intestinal crypts from patient donors or via in vitro differentiation of human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hIPSCs). Both methods result in organoids comprising all intestinal epithelial cell types found in vivo, in similar proportions and arrangements.
Culture conditions for both primary tissue and stem-cell-derived organoids are essentially the same, requiring a basal media comprising Advanced DMEM/F12, supplemented with N2 and B27, nicotinamide and N-acetylcysteine. To the basal medium additional growth factors are added to support the growth of the organoids including EGF, Noggin and gastrin, which are essential for regulation of gut mucosal growth and proliferation of intestinal epithelial cells. The presence of Wnt3a is crucial to regulate self-renewal, proliferation and differentiation, as expression of LGR5 in stem cells found in intestinal crypts is dependent upon the canonical Wnt signalling pathway, regulated by R-Spondin-1 and BMP4 inhibition by Noggin [35,36] (figure 2b).
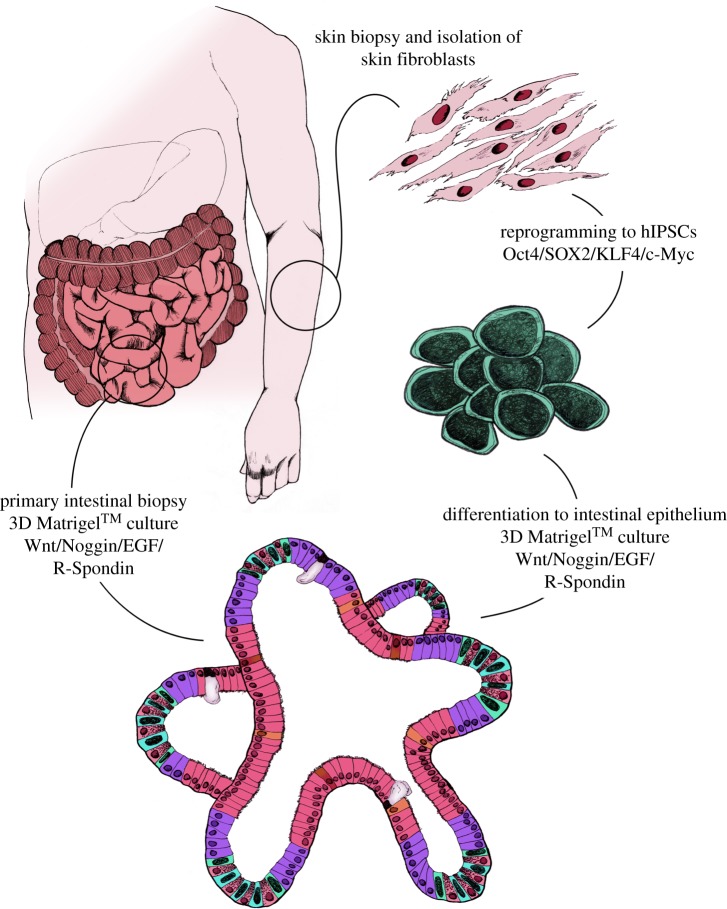
Intestinal crypts can be isolated from intestinal biopsies during endoscopy or surgical resection [18,37] (figure 3). Crypts are manually dissected from the epithelium and embedded into a Matrigel™ containing Wnt, R-Spondin, EGF and Noggin. Once Matrigel™ has solidified and encapsulated the isolated crypts, the Matrigel™ is overlaid with tissue culture medium containing all additives and growth factors. Over the course of 7–10 days intestinal crypts elongate and expand to form the first organoid structures, which can then be manually dissociated and expanded. In addition to LGR5+ stem cells, mature enteroids and colonoids comprised enterocytes, enteroendocrine cells, goblets cells and Paneth cells.
Figure 3.
Schematic of human intestinal organoid creation. Intestinal organoids can be generated directly from intestinal biopsy or tissue from surgical resection. Isolated crypts can be placed directly into 3D Matrigel™ cultures along with Wnt, Noggin, EGF and R-Spondin to establish stable cell lines. An alternative approach to generating patient-specific intestinal organoids requires a skin biopsy, isolation and amplification of skin fibroblasts. Skin fibroblasts can then be reprogrammed to hIPSCs using the Yamanaka factors and differentiated into endoermal cells and finally, intestinal epithelium before long-term culture in 3D Matrigel™ conditions.
Using a stem-cell-based approach, hESCs or hIPSCs can be differentiated following normal developmental stages to generate intestinal epithelium (figure 3). This includes differentiation of cells via the major developmental milestones of definitive endoderm, hindgut endoderm and intestinal progenitor cell stages. Once differentiated into sufficiently committed intestinal progenitor cells, they can be transitioned from what is normally a two-dimensional (2D) differentiation platform into the 3D organoid platform, where cells then spontaneously rearrange themselves into intestinal organoids.
6. Functional analysis of organoids
A variety of assays have been used to assess functionality of intestinal organoids. A primary function of enterocytes is transport of water and electrolytes across the intestinal barrier. In vitro, organoids have been shown to maintain this function via derivation of organoids lacking the apical transporters SGLT-1 or PEPT1. These organoids were shown to have inhibited d-glucose, d-fructose and peptide transport across the epithelial membrane, validating their potential use as a model of nutrient and drug transport mechanisms [38].
Permeability of the intestinal epithelium is an important factor in both health and disease, affecting the diffusion of small molecules across the intestinal barrier and preventing bacterial translocation via the bloodstream. Recently, it has been demonstrated that intestinal organoids can be used to model epithelial permeability and changes can be recorded in real time [39]. Hill et al. [39] demonstrated this technique by injecting fluorescently labelled dextran into the lumen of organoids while automated microscopy was used to capture, in real time, live cells and quantify breaks in the luminal surface. Using this technique, alterations to intestinal permeability can be studied under chronic inflammatory conditions and during bacterial invasions.
Forskolin-induced organoid swelling assay has also been used to demonstrate functionality of the cystic fibrosis transmembrane conductance regulator (CFTR) ion channel. Organoids with a functional CFTR swell in response to forskolin treatment, while any changes to the function of CFTR due to inhibition or genetic mutation can be detected due to lack of swelling [40]. This assay has the potential to be adapted to a drug-screening platform for cystic fibrosis (CF).
7. Applications of organoid technology
Owing to its differing physiology and distinct functions, the gastrointestinal tract is affected by an array of disorders. The intestinal epithelium is continuously exposed to antigens from commensal bacteria and consumed food, hence it has an extensive immune system, inclusive of adjacent lymphoid tissue, heavily populated with lymphocytes. The mucosa is the main site at which the immunological functions take place; however, loss of this carefully maintained immune homeostasis can cause chronic inflammation and multiple disorders. Intestinal organoids have now become increasingly popular as a platform to model many intestinal diseases caused by chronic inflammation or physical injury.
Inflammatory bowel disease (IBD) is a chronic, progressive and relapsing disease affecting the entire gastrointestinal tract. It is a result of dysregulated innate and adaptive immune responses against antigens present in the gastrointestinal tract. IBD is categorized into two main subtypes: ulcerative colitis (UC), which is restricted to the colon, and Crohn's disease (CD), which can affect any area of the digestive tract [41]. During active IBD, the small and large intestine can become highly inflamed, leading to destruction of the epithelium and deterioration in digestive capacity. Intestinal organoids derived from IBD patients present an opportunity to understand how innate immunity is regulated in IBD patients and to identify novel therapeutics to reduce the severity of the disease.
Short bowel syndrome (SBS) develops often due to partial resection of large intestine as a result of IBD, colorectal cancer (CRC) or ischaemic disease, or can be present at birth. Owing to a significantly reduced surface area of the intestinal epithelium, malabsorption and malnutrition are major issues. Congenital SBS has a genetic basis linked with the CLMP gene. Patient-derived small intestinal organoids offer a chance to study the genetic susceptibility in greater detail, including the molecular mechanisms involved in impaired intestinal elongation during development that are currently not well understood [42]. Furthermore, the combination of intestinal organoids with tissue-engineering techniques for the lengthening of the small intestine, offer a potential method for the restoration of normal functioning of the bowel irrespective of the cause of SBS.
Coeliac disease is an example of an autoimmune disease mainly affecting the small intestine upon consumption of gluten. The resulting inflammation causes malabsorption, leading to a range of other successive symptoms. Without exclusion of all gluten products from the diet, prolonged inflammation can lead to osteoporosis and cancer. Organoids cultured from patients suffering from coeliac disease can offer insight into genetic predispositions and mechanisms driving the disease forward. Alterations to intestinal barrier integrity, which are known to occur in coeliac patients, can be explored using organoids [43]. Similarly, patient-derived organoids could be used as a drug-screening platform for the identification of novel therapies.
Diverticular disease occurs typically in the sigmoid colon and is the result of physical damage to the intestines leading to loss of function. Diverticula are sacs that form from the herniation of the intestinal epithelium, erupting through the surrounding muscular layer. They are suspected to form due to excessive pressure throughout the large intestine, due, in part, to a low-fibre diet, however, aetiology remains largely unknown. These diverticula can become infected and inflamed, leading to the patient developing fever, pain, nausea and diarrhoea. A genetic element is suspected that predisposes individuals to developing diverticulitis. Therefore, genetic factors involved in the pathogenesis of diverticulitis can be studied using patient tissue-derived organoids from a relatively non-invasive intestinal biopsy. These can then be compared to healthy intestinal tissue to determine genetic and functional differences. Owing to the mechanical nature of the development of diverticular disease, mechanical stress could be applied to the individual organoids to examine the response of the intestinal epithelium under differing levels of stretch stress. However, a tissue-engineering approach is required in combination with organoid technology to model the development of diverticula. Intestinal organoids alone would not be suitable to model diverticula development due to the involvement of the mesenchyme and muscular layer surrounding the intestinal epithelium in vivo.
Cystic fibrosis is perhaps best known for its effect in the lung but also causes complications in other organs, including the pancreas and intestines. The most serious acute complication of the intestine in CF is obstruction of the terminal ileum or proximal large intestine, which if untreated can result in rupture and sepsis. Intestinal organoids are a suitable model of CF due to their expression of CFTR and by using a combination of forskolin-induced swelling and voltage-gate measurements, fluid and ion transport can be accurately measured. This assay has been performed on human intestinal organoids, demonstrating physiologically accurate CFTR function [44]. Drug responsiveness can, therefore, be measured using the current organoid model to identify the most effective treatment for CF or as a diagnostic tool [45].
CRCs are among the most commonly diagnosed cancers in the developed world. There are many contributing factors to development of CRC, but importantly long-term CD or UC significantly increases lifetime risk. Organoids are increasingly being used as models of CRC. Current models of CRC are unable to reproduce the progression of the disease at the early stages and are not representative of the heterogeneous nature of tumours. Genome-editing techniques, such as CRISPR/Cas9, however, can be used as a means to introduce genetic mutations into genes of interest. Following genetic manipulation organoids can then be transplanted into mice, in which the in vivo mechanisms of tumour progression and invasion can be measured [46].
8. Host–pathogen interactions
Different methods are employed to expose intestinal organoids to bacteria. Microinjection of live bacteria or bacterial proteins is a common approach to study intestinal infections, including Salmonella and Clostridium difficile infections. For example, Forbester et al. [31] used hIPSCs to generate intestinal organoids that were then microinjected with Salmonella. mRNA sequencing was used to create a global profile of changes in gene expression in response to Salmonella infection [31]. Similarly, Leslie et al. [35] used a microinjection methodology to deliver C. difficile into the lumen of hIPSC-derived intestinal organoids. They then observed that C. difficile remained in the lumen for a prolonged duration, suggesting that organoids possess suitable conditions for the survival of C. difficile and hence other obligate anaerobes. Microinjection of C. difficile toxins has also been shown to exhibit expected effects on epithelial integrity and changes to the expression of certain tight junctions [35].
9. Limitations of organoids
Despite increasing interest in organoid platforms to model intestinal development and disease, organoids used in today's research lack certain elements of the complete organ found in vivo (table 1). This includes a lack of mesenchymal tissue, immune and neural cells that in vivo contribute to the overall structure and functioning of the intestines. Organoids currently used in research are comprised mainly of epithelium, including the niche that enables self-renewal of intestinal stem cells.
Table 1.
Advantages and disadvantages for the use of intestinal organoids in the study of disease.
| diseases | advantages | disadvantages |
|---|---|---|
| inflammatory bowel disease (IBD) | —3D arrangement of cells functionally representative of in vivo —all intestinal cell types expressed —representative of patient's genetic background —effective drug-screening platform —mucosal healing can be closely studied —suitable model for epithelial regeneration following inflammation |
—lacks immune elements, hence interaction between immune cells cannot be observed —current models lack enteric nervous system which, in vivo, has an impact upon multiple intestinal cell responses to inflammation |
| diverticular disease | —genetic predisposition of diverticular disease can be further studied | —difficult to reproduce the conditions that would trigger formation of diverticula within organoids —lacks muscular layer and enteric nervous system, therefore, weakening of cell wall cannot be studied |
| short bowel syndrome (SBS) | —genetic predisposition of congenital SBS can be further studied —methods for increasing level of cellular functioning can be investigated, to compensate for loss of existing functional cells —combined with scaffolds, normal length and functioning of small or large intestine can be restored |
—difficult to model with isolated intestinal organoids |
| coeliac disease | —effects of gluten on genetic profile and immediate inflammatory response can be explored —suitable drug-screening platform —epithelial integrity can be measured following exposure to gluten |
—lacks immune cells —effects of gluten antibodies cannot be measured in current model |
| colorectal cancer (CRC) | —metastasis can be studied when transplanted into an in vivo model —representative of patient's genetic profile —more representative of tumour heterogeneity than other tumour cell lines —ease of genetic manipulation to introduce mutations for cancer modelling |
—mechanisms of metastasis cannot be studied in current in vitro model alone |
| bacterial infection | —able to assess the combined reaction of intestinal epithelial cells during Salmonella, Norovirus or Clostridia infection, including functional changes following exposure to different pathogenic bacterial species —epithelial permeability can be observed in response to pathogenic bacterial invasion —exposure of pathogens to basolateral and apical membrane can both be measured |
—model doesn't account for cell-based immune response present in vivo |
Organoids differentiated from induced pluripotent stem cells (IPSCs) into intestinal epithelial structures are known as induced intestinal organoids [47]. The differentiation protocols promoting the generation of such organoids also generates mesenchyme that, when transplanted under the renal capsule of mice, differentiate into smooth muscle and myofibroblasts [24]. These, therefore, are more representative of the intestines in vivo. However, they require a longer period of time to generate and are still devoid of immune cells which are necessary to address the most common intestinal disease IBD.
Other limitations of intestinal organoid include practical limitations due to the fact that cells must be embedded in Matrigel™ and grown in 3D. This creates additional complications when manipulating cells for simple procedures such as isolating mRNA, DNA and performing immuncytochemistry because organoids will require removal from Matrigel™ prior to processing. 3D culture also creates problems when attempting genetic manipulation via transfection and CRISPR/Cas9 gene editing approaches. Again, cells require removal of Matrigel™ prior to manipulation, which can create suboptimal conditions for organoid maintenance and growth.
It is clear that intestinal organoids can be used as an effective model of host–pathogen interactions that occur within the intestinal tract. However, further study is required to incorporate additional immune elements into the model to give a more complete illustration of inflammatory response. Thus far, microinjection has been used effectively to deliver the bacteria into the lumen of the organoid. However, this is a time-consuming technique. Therefore, other techniques have been trialled as more efficient alternatives. Treating a monolayer, for example, is much quicker to execute, however, this method is less indicative of how the 3D in vivo intestine would respond due to the lack of 3D architecture and the stem-cell niche. Without this native architecture it cannot be determined how intestinal stem cells would respond to such an infection.
10. Future challenges
Intestinal organoids remain a promising, tunable model for developmental and disease modelling, drug and toxicity testing, and host–pathogen interaction studies. In the future, in conjunction with CRISPR/Cas9 technology intestinal organoids hold great potential for gene therapy and transplantation applications in humans to treat chronic inflammatory disorders, such as CD, UC and CRCs. However, there are many aspects of this technology that still require significant investment to create a model that is more representative of in vivo tissue and their translational use in humans to become a reality.
Culturing intestinal organoids in 3D creates additional layers of complexity when attempting manipulations involving gene editing, transfection or when studies require access to the apical surface of intestinal epithelium. Previously, processing organoids has required specialized and time-consuming procedures such as the use of micromanipulator and microinjection platforms. More recently, several studies have addressed this problem and have begun developing methodologies to use 2D culture of dissociated intestinal organoids as a platform for high-throughput drug testing, migration assays and host–pathogen interaction studies. By using transwells with permeable membranes, experimentation can be performed with ease of access to both the apical and basolateral sides of the newly formed epithelial membrane [24]. However, this approach also disrupts the stem-cell compartment, meaning that using current culture conditions cells cannot be propagated long term using this 2D culture approach. It is feasible, however, to maintain organoids in 3D while performing manipulations in transient 2D cultures.
Current organoid platforms require the spontaneous self-assembly of epithelial tissue following dissociation and then embedding in Matrigel™. This approach creates organoids of differing shapes and sizes that lack the gross anatomical features of the intestine. Accuracy in developmental and disease modelling will be dramatically enhanced by the use of 3D scaffolds constructed from either decellularization and then recellularization of an ex vivo extracellular matrix or a cellularized synthetic scaffold. This approach offers a base on which to culture the cells into the tubular structure with more precise patterning of epithelium and consistent numbers of cells. This technique is potentially tunable, enabling the cell composition to be altered depending on the region of intestinal tract being studied or disease process being modelled. This model, as well as being a closer approximation of the intestine, is likely to be easier to manipulate by providing easier access to apical and basolateral sides of cells. Studies carried out into the development of this model have shown that it can accumulate a mucus layer on the luminal surface, as with organoids, with a thickness of 20 µm [48]. This can, therefore, recapitulate the host–pathogen interactions, occurring in vivo, that require an intact mucosal layer. So far, these models lack neural and immune cells to form a complete organ and require many months of set-up until the platform is suitable for experimental use. A notable disadvantage to this system is the deterioration in the model after only a few weeks following the seeding of cells to the 3D scaffold, which could be due to nutrient and oxygen starvation of cells, further reinforcing the need for models that recapitulate the complete organ including vascularization. This, therefore, must be overcome before it can be used for longer-term studies, including that of chronic inflammation.
Several studies have demonstrated the potential for direct transplantation of intestinal organoids into mice and rodents [32,49]. Fordham et al. successfully transplanted fetal intestinal progenitors, which had not yet been differentiated into intestinal organoids, into a colonic injury mouse model [32]. They found that immature enterospheres have regenerative capacity when transplanted into adult damaged colonic epithelium. Additionally, they demonstrated the ability of these immature cells to mature in vivo, appropriate for the region of engraftment, expressing Mucin-2-positive goblet cells and lacking markers of small intestine. Whether the same approach could be used to treat human intestinal injury from both acute and chronic IBD is yet to be determined. When combined with decellularization/recellularization techniques, 3D printing and development of synthetic and biodegradable scaffold technologies, stem-cell-derived intestinal tissue or patient-derived intestinal tissue could be used to replace large segments of the small and large intestine.
One of the more significant drawbacks to modelling disease using intestinal organoids is the absence of an immune element that can be used to understand mechanisms driving the autoimmune destruction of the intestinal mucosa that occurs during CD and UC in vivo. Thus far, intestinal organoids have been able to model the immune response at an innate level, determining the effects of gene expression and cytokine signalling in the development of CD and in host–pathogen interaction studies. CD is known to be triggered by a combination of genetic susceptibility and environmental factors, resulting in dysregulation of immune homeostasis. Studies have shown that human intestinal organoids generated from hIPSCs are a suitable model for studying the interactions between intestinal epithelium and the enteric pathogen Salmonella typhimurium to dissect elements of the innate immune response [31]. The model, however, needs to be further developed to include elements of systemic immune regulation including cells such as macrophages, neutrophils, T cells, B lymphocytes, NK cells and dendritic cells. Addition of these cell types will create a more complete model but will be a significant challenge requiring all cell types to be from the same donor.
Intestinal organoids, whether derived from primary tissue biopsy or stem-cell differentiation techniques, have great potential in the future of disease modelling, drug discovery and personalized medicine. They have been shown to have stable phenotype, are relatively simple to create and manipulate while at the same time able to be maintained in long-term culture. However, current organoid derivation and culture techniques do not allow for the assembly of complex multi-cell-type organoids that can model the complete complexity of a multifactoral disease such as IBD. Furthermore, as our need to modify and manipulate organoids and organoid culture conditions advances, the practice of culturing intestinal organoids may pose technical limitations on what is achievable. Developing our understanding of the initiation and development of complex intestinal disease, such as IBD and CRC, will require continued investment and research into the development of more complex intestinal organoid platforms.
Data accessibility
This article has no additional data.
Authors' contributions
N.R.F.H. and K.L.F. planned and co-wrote the manuscript. J.C. created all figures. Images used for figures 1, 2 and 3 copyright and produced by J.C. © Jennifer Colquhoun 2018. For more information please visit www.jennifercolquhoun.com.
Competing interests
We declare we have no competing interests.
Funding
N.R.F.H. is funded by the Wellcome Trust (204267/Z/16/Z). K.L.F. is funded by the Wellcome Trust (204267/Z/16/Z) and a BBSRC iCASE studentship.
References
- 1.Wells JM, Melton DA. 1999. Vertebrate endoderm development. Annu. Rev. Cell Dev. Biol. 15, 393–410. ( 10.1146/annurev.cellbio.15.1.393) [DOI] [PubMed] [Google Scholar]
- 2.Karasov WH, Douglas AE. 2013. Comparative digestive physiology. Compr. Physiol 3, 741–783. ( 10.1002/cphy.c110054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw D, Gohil K, Basson MD. 2012. Intestinal mucosal atrophy and adaptation. World J. Gastroenterol. 18, 6357–6375. ( 10.3748/wjg.v18.i44.6357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gjorevski N, Ordonez-Moran P. 2017. Intestinal stem cell niche insights gathered from both in vivo and novel in vitro models. Stem Cells Int. 2017, 8387297 ( 10.1155/2017/8387297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Es JH, et al. 2012. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat. Cell Biol. 14, 1099–1104. ( 10.1038/ncb2581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abe T, Ujiie A, Taguchi Y, Satoh S, Shibuya T, Jun Y, Isogai S, Satoh YI. 2017. Anomalous inferior mesenteric artery supplying the ascending, transverse, descending, and sigmoid colons. Anat. Sci. Int. 93, 144–148. ( 10.1007/s12565-017-0401-2) [DOI] [PubMed] [Google Scholar]
- 7.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Reddy DN. 2015. Role of the normal gut microbiota. World J. Gastroenterol. 21, 8787–8803. ( 10.3748/wjg.v21.i29.8787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker N, et al. 2007. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007. ( 10.1038/nature06196) [DOI] [PubMed] [Google Scholar]
- 9.Andersson-Rolf A, Zilbauer M, Koo BK, Clevers H. 2017. Stem cells in repair of gastrointestinal epithelia. Physiology (Bethesda) 32, 278–289. ( 10.1152/physiol.00005.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YS, Ho SB. 2010. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr. Gastroenterol. Rep. 12, 319–330. ( 10.1007/s11894-010-0131-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lievin-Le Moal V, Servin AL. 2006. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin. Microbiol. Rev. 19, 315–337. ( 10.1128/CMR.19.2.315-337.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornick S, Tawiah A, Chadee K. 2015. Roles and regulation of the mucus barrier in the gut. Tissue Barriers 3, e982426 ( 10.4161/21688370.2014.982426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mowat AM, Agace WW. 2014. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 14, 667–685. ( 10.1038/nri3738) [DOI] [PubMed] [Google Scholar]
- 14.Dharmani P, Srivastava V, Kissoon-Singh V, Chadee K. 2009. Role of intestinal mucins in innate host defense mechanisms against pathogens. J. Innate. Immun. 1, 123–135. ( 10.1159/000163037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato T, et al. 2009. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265. ( 10.1038/nature07935) [DOI] [PubMed] [Google Scholar]
- 16.Umar S. 2010. Intestinal stem cells. Curr. Gastroenterol. Rep. 12, 340–348. ( 10.1007/s11894-010-0130-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richmond CA, Breault DT. 2010. Regulation of gene expression in the intestinal epithelium. Prog. Mol. Biol. Transl. Sci. 96, 207–229. ( 10.1016/B978-0-12-381280-3.00009-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato T, et al. 2011. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418. ( 10.1038/nature09637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasaki N, et al. 2016. Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proc. Natl Acad. Sci. USA 113, E5399–E5407. ( 10.1073/pnas.1607327113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farin HF, et al. 2014. Paneth cell extrusion and release of antimicrobial products is directly controlled by immune cell-derived IFN-γ. J. Exp. Med. 211, 1393–1405. ( 10.1084/jem.20130753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clevers H. 2006. Wnt/β-catenin signaling in development and disease. Cell 127, 469–480. ( 10.1016/j.cell.2006.10.018) [DOI] [PubMed] [Google Scholar]
- 22.Chia LA, Kuo CJ. 2010. The intestinal stem cell. Prog. Mol. Biol. Transl. Sci. 96, 157–173. ( 10.1016/B978-0-12-381280-3.00007-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farin HF, et al. 2016. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature 530, 340–343. ( 10.1038/nature16937) [DOI] [PubMed] [Google Scholar]
- 24.Zachos NC, Kovbasnjuk O, Foulke-Abel J, In J, Blutt SE, De Jonge HR, Estes MK, Donowitz M. 2016. Human enteroids/colonoids and intestinal organoids functionally recapitulate normal intestinal physiology and pathophysiology. J. Biol. Chem. 291, 3759–3766. ( 10.1074/jbc.R114.635995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broutier L, Andersson-Rolf A, Hindley CJ, Boj SF, Clevers H, Koo B-K, Huch M. 2016. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 11, 1724–1743. ( 10.1038/nprot.2016.097) [DOI] [PubMed] [Google Scholar]
- 26.Sampaziotis F, et al. 2015. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat. Biotechnol. 33, 845–852. ( 10.1038/nbt.3275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sampaziotis F, De Brito MC, Geti I, Bertero A, Hannan NRF, Vallier L. 2017. Directed differentiation of human induced pluripotent stem cells into functional cholangiocyte-like cells. Nat. Protoc. 12, 814–827. ( 10.1038/nprot.2017.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sampaziotis F, et al. 2017. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat. Med. 23, 954–963. ( 10.1038/nm.4360) [DOI] [PubMed] [Google Scholar]
- 29.Takasato M, et al. 2015. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568. ( 10.1038/nature15695) [DOI] [PubMed] [Google Scholar]
- 30.Brawner AT, Xu R, Liu D, Jiang P. 2017. Generating CNS organoids from human induced pluripotent stem cells for modeling neurological disorders. Int. J. Physiol. Pathophysiol. Pharmacol. 9, 101–111. [PMC free article] [PubMed] [Google Scholar]
- 31.Forbester JL, Goulding D, Vallier L, Hannan N, Hale C, Pickard D, Mukhopadhyay S, Dougan G. et al. 2015. The interaction of Salmonella enterica Serovar typhimurium with intestinal organoids derived from human induced pluripotent stem cells. Infect. Immun. 83, 2926–2934. ( 10.1128/IAI.00161-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fordham RP, et al. 2013. Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell 13, 734–744. ( 10.1016/j.stem.2013.09.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hannan NR, Fordham RP, Syed YA, Moignard V, Berry A, Bautista R, Hanley NA, Jensen KB, Vallier L. 2013. Generation of multipotent foregut stem cells from human pluripotent stem cells. Stem Cell Rep. 1, 293–306. ( 10.1016/j.stemcr.2013.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fatehullah A, Tan SH, Barker N. 2016. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 18, 246–254. ( 10.1038/ncb3312) [DOI] [PubMed] [Google Scholar]
- 35.Leslie JL, Huang S, Opp JS, Nagy MS, Kobayashi M, Young VB, Spence JR. 2015. Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function. Infect. Immun. 83, 138–145. ( 10.1128/IAI.02561-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spence JR, et al. 2011. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109. ( 10.1038/nature09691) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung P, et al. 2011. Isolation and in vitro expansion of human colonic stem cells. Nat. Med. 17, 1225–1227. ( 10.1038/nm.2470) [DOI] [PubMed] [Google Scholar]
- 38.Zietek T, Rath E, Haller D, Daniel H. 2015. Intestinal organoids for assessing nutrient transport, sensing and incretin secretion. Sci. Rep. 5, 16831 ( 10.1038/srep16831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill DR, Huang S, Tsai Y-H, Spence JR, Young VB. 2017. Real-time measurement of epithelial barrier permeability in human intestinal organoids. J. Vis. Exp. 130, 56960 ( 10.3791/56960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwank G, et al. 2013. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13, 653–658. ( 10.1016/j.stem.2013.11.002) [DOI] [PubMed] [Google Scholar]
- 41.de Souza HS, Fiocchi C. 2016. Immunopathogenesis of IBD: current state of the art. Nat. Rev. Gastroenterol. Hepatol. 13, 13–27. ( 10.1038/nrgastro.2015.186) [DOI] [PubMed] [Google Scholar]
- 42.Alves MM, et al. 2016. Genetic screening of congenital short bowel syndrome patients confirms CLMP as the major gene involved in the recessive form of this disorder. Eur. J. Hum. Genet. 24, 1627–1629. ( 10.1038/ejhg.2016.58) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piscaglia AC. 2014. Intestinal stem cells and celiac disease. World J. Stem Cells 6, 213–229. ( 10.4252/wjsc.v6.i2.213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dekkers JF, et al. 2013. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med. 19, 939–945. ( 10.1038/nm.3201) [DOI] [PubMed] [Google Scholar]
- 45.Dekkers JF, et al. 2016. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci. Transl. Med. 8, 344ra84 ( 10.1126/scitranslmed.aad8278) [DOI] [PubMed] [Google Scholar]
- 46.Fumagalli A, et al. 2017. Genetic dissection of colorectal cancer progression by orthotopic transplantation of engineered cancer organoids. Proc. Natl Acad. Sci. USA 114, E2357–E2364. ( 10.1073/pnas.1701219114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stelzner M, et al. 2012. A nomenclature for intestinal in vitro cultures. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G1359–G1363. ( 10.1152/ajpgi.00493.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y, et al. 2015. Robust bioengineered 3D functional human intestinal epithelium. Sci. Rep. 5, 13708 ( 10.1038/srep13708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watson CL, et al. 2014. An in vivo model of human small intestine using pluripotent stem cells. Nat. Med. 20, 1310–1314. ( 10.1038/nm.3737) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.