Abstract
Bone has many functions. It is responsible for protecting the underlying soft organs, it allows locomotion, houses the bone marrow and stores minerals such as calcium and phosphate. Upon damage, bone tissue can efficiently repair itself. However, healing is hampered if the defect exceeds a critical size and/or is in compromised conditions. The isolation or generation of bone-forming progenitors has applicability to skeletal repair and may be used in tissue engineering approaches. Traditionally, bone engineering uses osteochondrogenic stem cells, which are combined with scaffold materials and growth factors. Despite promising preclinical data, limited translation towards the clinic has been observed to date. There may be several reasons for this including the lack of robust cell populations with favourable proliferative and differentiation capacities. However, perhaps the most pertinent reason is the failure to produce an implant that can replicate the developmental programme that is observed during skeletal repair. Pluripotent stem cells (PSCs) can potentially offer a solution for bone tissue engineering by providing unlimited cell sources at various stages of differentiation. In this review, we summarize key embryonic signalling pathways in bone formation coupled with PSC differentiation strategies for the derivation of bone-forming progenitors.
This article is part of the theme issue ‘Designer human tissue: coming to a lab near you’.
Keywords: pluripotent stem cell, mesenchymal stem cell, osteoblast, chondrocyte, bone tissue engineering, bone formation
1. Introduction
The skeleton is predominantly composed of bone tissue, which is a highly specialized form of connective tissue that helps regulate systemic levels of calcium and phosphorus ions. Furthermore, it provides mechanical support during joint movement, protects the inner organs and accommodates haematopoiesis.
Despite the structural integrity of bone, millions of patients suffer yearly from bone fractures. This number is likely to increase owing to an ageing population and high prevalence of low bone mass disorders (i.e. osteoporosis). Fortunately, bone has a remarkable intrinsic capacity to heal; however, fracture healing can be impaired if mechanical or biological environments are not permissive of repair. It is estimated that a total of 10% of annual tibial fractures either heal poorly (delayed-union) or do not heal at all (non-union) [1]. As a result, many strategies have been proposed and developed to enhance bone healing. One such example is tissue engineering, which is defined as an interdisciplinary field that combines the principles of engineering and life sciences towards the development of biological substitutes to restore, maintain or improve bone tissue development and regeneration [2]. Classically, bone tissue engineering combines skeletogenic cells with biocompatible materials and growth factors to create tissue intermediates that upon implantation will form bone tissue [3,4]. However, these strategies are hampered by the lack of robust and reproducible cell populations which display favourable proliferative and differentiation properties for advanced cell-based therapies.
Most tissue engineering efforts have focused on the use of adult stem cells with the major cell type of choice being the bone marrow mesenchymal stem cell (MSC). However, despite apparent in vitro osteogenic differentiation [5], in vivo bone formation has been limited in most cases. It is believed that genetic variations, limited proliferative ability, senescence and poor engraftment may all lead to a poor clinical outcome [6]. Indeed, it has been reported that proliferative capacity is significantly decreased as a function of donor age [7], and in conjunction with this, in vitro expanded MSCs have also been shown to lose their differentiation and proliferative ability [8]. As an in vitro cell expansion step appears to be of importance in tissue engineering strategies, these limiting factors currently hinder the clinical use of adult stem cells for bone augmentation.
Pluripotent stem cells (PSCs) have the potential to differentiate into any adult cell type and can proliferate indefinitely [9,10]. As a result, these stem cells can be used as an alternative source for target cell isolation. However, despite these more favourable characteristics, controlled differentiation and subsequent full maturation of the derived cell types remains an important bottleneck [11]. Complete differentiation of PSCs into terminal cell types relies on the precise recapitulation of embryonic signalling pathways and developmental events [12]. In this review, we provide a summary of embryonic bone formation pathways and an overview of different PSC-based strategies for the derivation of bone-forming progenitor cells.
2. Embryonic bone formation; a blueprint for PSC differentiation
(a). Intramembranous bone formation
Most of the flat bones are formed through a process called intramembranous ossification (direct bone formation). Shortly after gastrulation, the ectoderm and mesoderm give rise to migratory mesenchymal cell populations. Once these cells are committed towards the osteogenic lineage, they subsequently activate key osteogenic transcription factors such as Runt-related transcription factor 2 (Runx2/Cbfa1) and Osterix (Osx/Sp7) that regulate the expression of downstream proteins, including collagen type 1 (Col1) and osteocalcin, and aid differentiation into active osteoblasts [13–15]. These cells are responsible for the synthesis of osteoid, the organic component of the bone matrix. Osteoid predominantly consists of Col1 that upon its deposition quickly mineralizes through association of calcium phosphate (hydroxyapatite)-rich matrix vesicles [16]. Col1 is necessary for the elastic properties of the bone matrix, while the association of Col1 with hydroxyapatite allows bone to increase compressive strength and durability.
The continuous matrix deposition and calcification separate osteoblasts and these cells become either apoptotic, bone lining cells or are entrapped in the bone matrix. Entrapped osteoblasts mature into osteocytes and branch cytoplasmatic extensions (processes) to neighbouring cells, allowing cell–cell signalling. Osteocytes are mechanosensitive cells that allow adaption of the bone mass to the biomechanical needs through secretion of bone synthesis/resorption regulatory signals (e.g. sclerostin and RANKL) [17]. Furthermore, osteocytes regulate systemic levels of phosphorus, through interactions with the kidney, and thus display endocrine functions [18].
A subset of undifferentiated mesenchymal cells remain at the bone periphery and form the periosteum, a fine membrane that covers the bone tissue. This membrane is enriched in skeletal progenitors and is crucial for appositional bone growth and fracture repair. For additional information on periosteal skeletal progenitors, we refer to our recently published review [19].
(b). Endochondral ossification
The appendicular skeleton is formed through the process of endochondral ossification, during which a transient cartilage template precedes bone formation. During chondrogenesis, committed mesenchymal cells condense into compact nodules and differentiate into sex-determining region Y-box9 (Sox9)-positive chondrocytes (cartilage cells) [20]. Chondrocytes secrete an extracellular matrix that is rich in collagen type II (Col2) and proteoglycans such as aggrecan (Acan). As development progresses, these cells proliferate rapidly leading to the growth of the cartilage anlage (cartilage model). Proliferative chondrocytes organize into columnar stacks and start to enlarge and mature into Col2−Col10+ hypertrophic chondrocytes. These cells secrete matrix vesicles, which act as nucleation centres that allow the cartilage template to calcify. As calcification continues, diffusion of nutrients through the calcified matrix decreases, leading to secretion of pro-angiogenic and osteogenic growth factors into the extracellular matrix. The ingrowth of blood vessels together with cartilage resorbing chondroclasts (modified osteoclasts) and perichondrial/periosteal osteoprogenitors ultimately leads to the replacement of cartilage by bone tissue [21,22].
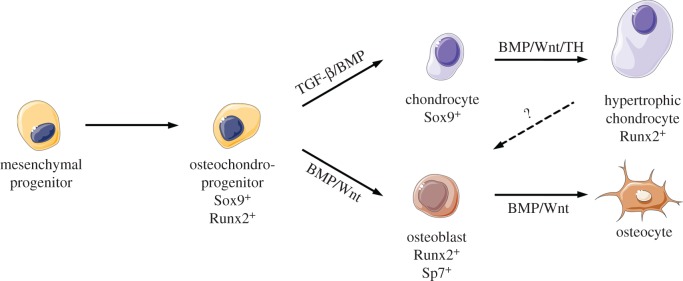
The remaining chondrocytic cell population within the cartilage anlage organizes into the ‘growth plate', a functional cellular unit containing resting, proliferative, prehypertrophic and hypertrophic chondrocytes [23]. The growth plate functions as the main driver for regulating longitudinal bone growth and length through continuous supply of hypertrophic chondrocytes until skeletal maturity. An overview of osteogenic and chondrogenic differentiation is detailed in figure 1.
Figure 1.
Signalling pathways implicated in skeletal cell specification. Mesenchymal progenitor cells are specified to bipotent osteochondroprogenitor cells prior to chondrogenic or osteogenic differentiation. BMP, TGF-β, Wnt and TH signalling pathways regulate specific processes during skeletal cell specification. This figure was created using Servier Medical ART (SMART) Servier Medical ART (SMART) licensed under a Creative Commons Attribution 3.0 unported licence (https://creativecommons.org/licenses/by/3.0/). (Online version in colour.)
(c). Transcriptional and molecular control of skeletogenesis
Skeletogenesis is tightly regulated by cell- and site-specific transcription factors and morphogens. Numerous signalling pathways, including bone morphogenetic proteins (BMPs), parathyroid hormone related protein (PTHrP), Indian hedgehog (Ihh), transforming growth factors (TGFs), vascular endothelial growth factor (VEGF) and fibroblast growth factors (FGFs) interact with each other to regulate diverse processes in skeletogenesis including mesenchymal condensation, chondro- and osteogenic differentiation [23–25]. In contrast to endochondral (indirect) bone formation, which involves these aforementioned processes, intramembranous ossification (direct bone formation) does not rely on chondrocyte signalling and is regulated by the cells of the osteoblast/osteocyte lineage alone. The molecular mechanisms underlying endochondral and intramembranous ossification are discussed below.
(d). Mesenchymal commitment towards chondrogenesis
Chondrogenesis is an essential process during embryonic development and is of crucial importance for the formation of ‘stable' and ‘transient' cartilage. Whereas stable cartilage remains cartilage throughout life and constitutes the cartilaginous skeleton (e.g. the nasal cartilage and the articular cartilage of synovial joints), transient cartilage is used as the template for the formation of embryonic bone and during postnatal fracture repair. Chondrogenesis initiates when limb pair-related homeobox-1 (PRX1)+ mesenchymal progenitor cells become committed to the chondrogenic lineage [20]. Members of the TGF/BMP family are responsible for the expression of several cell adhesion molecules including N-cadherin and N-cam, which are of crucial importance for the initial mesenchymal condensation resulting in chondrogenesis [26]. Following condensation, the mesenchymal progenitors begin expressing the master chondrogenic transcription factor Sox9. The role of Sox9 in chondrogenic differentiation was originally identified in a human genetic disease called campomelic dysplasia in which skeletal elements are malformed [27]. Studies have shown that the expression of Sox9 is activated upon TGF/BMP stimulation [28,29]. Importantly, Sox9 is indispensable for cartilage formation. Indeed, chimeric experiments in which Sox9−/− embryonic stem cells were injected in wild-type blastocysts have shown that mutant cells were unable to participate in precartilaginous condensations and subsequent differentiation despite being intermingled with wild-type cells within the cartilage differentiation sites [30]. Furthermore, molecular studies have shown that Sox9 can directly activate the expression of several cartilage extracellular matrix proteins including Col2 [31]. The transition of PRX1+ Sox9− limb precursor cells towards Sox9+ phenotype marks the onset of skeletal commitment of these cells into chondroblasts/prechondrocytes.
(e). Transitioning towards chondroblasts and chondrocytes
The transition of committed Sox9+ precursor cells into early matrix synthesizing chondroblasts requires the expression of Sox5 and Sox6. Lineage tracing studies using the Sox9 promoter have demonstrated that Sox9+ cells contribute to limb bone, tendon and synovium [20]. However, the co-expression of Sox9, Sox5 and Sox6 (commonly referred to as the Sox trio) was only detected in the developing cartilage anlage cells [32–34]. Indeed, despite the lack of DNA transactivating domains in Sox5 and Sox6, these three proteins were found to be necessary for the enhanced expression of cartilaginous genes and thus subsequent synthesis of the extracellular matrix proteins by skeletal precursor cells. Genome-wide molecular analyses further strengthened this hypothesis and revealed the close interaction of the Sox trio proteins in chondrocyte specification. The Sox trio was shown to interact predominantly with super-enhancers, genomic sites with multiple enhancers that can be collectively bound by (master) transcription factors to determine cell fate and identity. This interaction regulates the expression of cartilage-specific genes, such as extracellular matrix proteins (e.g. Col2 and Acan), the Sox trio and proteins that regulate their activity. The close interaction of Sox5, Sox6 and Sox9 results in the formation of a chondrocyte-specific enhanceosome that regulates the onset of cartilage synthesis [35,36]. These results demonstrate that the transition of Sox9+ skeletal precursor cells into early chondroblasts requires the timely activation of Sox5 and Sox6.
(f). Maturation of chondrocytes into hypertrophic chondrocytes
The longitudinal growth of developing bones is mediated by the transition of randomly located proliferative chondroblasts into organized columnar stacks within the growth plate. This organization is, in part, regulated by a PTHrP/Ihh negative feedback loop. During early skeletal development, the expression of PTHrP is dependent on Ihh and is confined to periarticular chondrocytes and perichondrial precursors. PTHrP negatively regulates the transition of proliferative chondrocytes into growth-arrested hypertrophic chondrocytes. However, upon growth and elongation of the cartilage anlage, PTHrP gradients gradually decrease along the growth plate axis leading to progressive maturation of proliferative chondrocytes towards hypertrophy. These cells gradually become Sox9 negative and start to express Runx2 that stimulates the secretion of Ihh. The tight feedback loop between Ihh and PTHrP is crucial for the formation of the growth plate and subsequent bone elongation [23].
In addition to PTHrP/Ihh, many other signalling pathways have been implicated in the regulation of chondrocyte hypertrophy and growth plate development. These include members of the FGF, BMP, Wnt and thyroid hormone (TH) families. The role of FGF in growth plate development was previously identified through gain-of-function mutations in the FGF receptor type 3 (FGFR3) gene. These mutations cause many human skeletal dysplasias including achondroplasia (dwarfism) that result from accelerated chondrocyte maturation [37]. Furthermore, genetic studies in which the activities of BMPs [38–40], Wnts [41,42] or TH [43,44] were reduced led to a decrease in chondrocyte hypertrophy. These results demonstrate that the chondrocyte maturation programme is dependent on a pleiotropic set of autocrine, paracrine and endocrine signalling molecules.
Runx2 is a key driver of the chondrocyte maturation programme [45]. Despite the mutual inhibitory activities between Sox9 and Runx2, the gradual loss in PTHrP-mediated phosphorylation of Sox9 across the growth plate layers is sufficient to allow the transcriptional activity of Runx2 to occur. Molecular studies have demonstrated that Runx2 is able to directly bind to the promoter regions of Col10A1, Ihh and VEGF [45–47]. All of these are commonly used as markers for hypertrophic chondrocytes. However, despite the role of Runx2 in mediating chondrocyte hypertrophy, some skeletal elements are still able to undergo hypertrophy in Runx2 knockout mice [48,49]. This prompted a search for additional transcription factors regulating chondrocyte maturation. Novel single-cell sequencing experiments have demonstrated that the regulation of chondrocyte hypertrophy is a far more complex process than previously thought. Indeed Li et al. have shown that FosB, Fos, Nr4a1, ATF3, Sox11, KLF13 in addition to members of the FoxA and distal homeobox proteins (Dlx) were able to directly activate the expression of Col10A1 in chondrogenic precursor cells [50–52]. The transition of proliferative chondroblasts into terminally differentiated hypertrophic chondrocytes is therefore marked by a loss of Sox9 and Col2 expression and by a subsequent increase in Runx2 and Col10.
(g). Transdifferentiation of chondrocytes into osteoprogenitors
It was previously hypothesized that all chondrocytes undergoing hypertrophic differentiation also underwent apoptosis to allow void spaces to be formed for subsequent vascularization and bone formation. However, recent lineage tracing studies have demonstrated that a portion of these cells transdifferentiate into osterix (Osx/Sp7)+ osteoprogenitors [53,54]. These cells contribute to both endosteal and trabecular bone formation within the primary spongiosa. Flow cytometry assisted cell sorting (FACS) further confirmed the metabolic activity and bone formation capacities of these cells, indicating that hypertrophic chondrocytes actively contribute to bone formation. Indeed, up to 60% of the total mature osteoblasts found in endochondral bones of one-month-old mice appear to originate from ‘terminally' differentiated hypertrophic chondrocytes [53]. The molecular programme of how hypertrophic chondrocytes transdifferentiate into active osteoblasts is not yet fully understood and remains to be elucidated.
(h). Formation of embryonic bone by osteoprogenitors
The last step of endochondral ossification requires the gradual replacement of the cartilaginous matrix into bone tissue. Differentiation of perichondrial–periosteal osteoprogenitors to active osteoblasts requires BMP- and Wnt-mediated activation of both Runx2 and Osx [55,56]. These transcription factors allow the subsequent expression of osteogenic genes such as Col1 and osteocalcin (OCN) [56,57]. Once primed, osteoprogenitor cells migrate along with invading blood vessels into the cartilaginous template for bone formation [58]. Gradual cartilage tissue replacement with subsequent ingrowth of blood vessels and osteoprogenitors marks the onset of functional bone formation within the cartilage anlage. The process of endochondral ossification is completed once the cartilaginous matrix template has been replaced by bone.
3. Derivation of bone-forming progenitor cells from PSCs
As detailed above, many cell types have the capacity to form bone in both embryonic and postnatal settings. In the following section, we present an overview of different PSC-based strategies, based on elements of embryonic development and postnatal bone formation, for the derivation of progenitor cell types that are either capable of forming bone (osteochondroprogenitors and osteoblast-like cell types) or recruiting bone-forming progenitors (hypertrophic chondrocytes).
(a). Derivation of osteochondroprogenitors from PSCs
The discovery of bone marrow-derived fibroblast colonies with in vitro and in vivo bone-forming potential in the early to mid-1970s (later defined as bone marrow mesenchymal stem/stromal cells (MSCs)) promoted the development of the bone tissue engineering strategy [59–62]. Since their discovery in bone marrow, cells with MSC properties have been identified in various adult tissues including the periosteum, where they appear to exist as skeletal reparative cells [19]. Interestingly, the term ‘bone marrow-derived MSCs' has recently been challenged. It has now been proposed that ‘skeletal stem cell (SSC)' is a more appropriate terminology as the aforementioned population of MSCs in the bone marrow contains stem cells for the skeletal tissues only. While (in vitro) multipotency has been demonstrated in multiple ‘MSC' cell populations (e.g. muscle, placenta and adipose tissues), in vivo skeletal tissue formation capacity can only be detected with bone marrow- and periosteum-derived cell populations. Other ‘MSCs' do not contribute to embryonic and postnatal bone formation and furthermore require the addition of exogenous growth factors, such as BMPs, to induce in vivo bone formation [63,64]. With the ambiguity over terminology in mind, and for ease of comparison within this review and with the existing literature, we have used the term MSCs to describe SSCs and cells referred to within the literature as MSCs/stromal cells.
Despite the ability to isolate MSCs from postnatal human tissues, long-term in vitro culture is generally associated with a progressive decline in stem cell characteristics [65–67]. MSCs are defined by their rapid adherence to plastic, fibroblast-like morphology and the expression of specific cell surface markers such as CD73, CD90 and CD105. MSCs are multipotent stem cells and can differentiate into adipocytes, osteoblasts and chondrocytes [68]. While many biological activities including immunomodulatory properties have been associated with MSCs, the isolation and expansion of these cells is an intensive, invasive and expensive procedure. Combined with the phenotypic and genetic changes in MSCs following ageing and long-term in vitro culture, this makes the possibility of producing MSCs from PSCs an attractive approach that could potentially represent an unlimited MSC source.
Initial efforts for deriving MSCs from human PSCs (hPSCs) used embryoid bodies (EBs) to trigger differentiation and primitive streak (PS) formation [69–71]. By culturing the outgrowing cells in MSC maintenance media (basal media supplemented with fetal bovine serum), these cells quickly adopted a fibroblastic morphology. Subsequent flow cytometry and in vitro differentiation analysis revealed the expression of specific cell surface markers (CD29, CD44, CD49e, CD73, CD90, CD105 and CD166) and bone-forming potential of these cells. Surprisingly, the chondrogenic differentiation capacity of these MSC-like cells was limited [72]. Despite the relative ease of using EBs for cell isolation, these methodologies often result in downstream contamination with other cell types due to heterogeneous differentiation. Coupled with the PSC supportive characteristics of MSCs and teratoma-forming potential of residual PSCs, an extensive MSC purification step would be necessary if this approach were considered for use in clinical therapy [73]. Nevertheless, MSCs isolated through EBs contributed to de novo bone formation in a murine calvaria defect model [74].
In an attempt to robustly control PSC differentiation, co-culture with feeder cell lines has been proposed for germ layer specification [75–77]. The murine bone marrow stromal cell line OP9 has traditionally been used for the derivation of haematopoietic, vascular and cardiac cells. Co-culture of OP9 cells with hPSCs led to the rapid induction of PS and lateral mesoderm markers such as brachyury, MIXL1, EOMES, FoxF1 and Hand1. When the differentiated cells were subsequently dispersed and grown into semisolid media, the cells gave rise to compact mesenchymal (MS) colonies or blast (BS) colonies. Phenotypic analysis of the MS colonies revealed uniform expression of cell surface markers such as CD140a, CD90, CD56, CD166 and CD146, which are all commonly associated with MSCs. However, the expression of CD73 and CD105 was either not detected or expressed at low levels. When the resulting MS colonies were grown in MSC maintenance media, rapid expansion and outgrowth of fibroblast-like cells was detected. The resulting cell lines appeared to be multipotent and acquired the expression of CD73 and CD105, reminiscent of the typical adult bone marrow MSC phenotype. Interestingly, when MS colonies were isolated and maintained on a Matrigel substrate, these cells differentiated into endothelial cells and formed extensive tubular structures. The authors concluded that MS colonies contained progenitors for both endothelial and mesenchymal progenitor cells and labelled these as ‘mesenchymoangioblasts'. Of note, the BS colonies contained progenitors for haematopoietic cells [77]. The co-induction of both BS and MS colonies in PSCs further established the intimate relationship between haematopoietic and mesenchymal progenitor cells. Indeed, MSCs are also known to contribute to the supportive stromal cells for haematopoietic precursors, as observed within the bone marrow [78,79].
Increased understanding in the origin of MSCs and embryonic specification pathways has led to further refinement of differentiation protocols. During embryonic development, the paraxial mesoderm is known to give rise to the somites, a transient embryonic tissue that develops into the axial and limb muscles, axial skeleton, endothelial cells and fat tissue. Shortly after the formation of the three germ layers (gastrulation), the early mesoderm is subdivided into different regions. Depending on the location, the downstream differentiation potential of the cells contained within each region is restricted. The paraxial mesoderm, which lies next to the developing neural tube, gives rise to the somites. Wnt, FGF and Notch signalling are known to regulate somitogenesis [80,81]. Once thresholds have been reached, the paraxial mesoderm specifies into somites that rapidly differentiate into the dermomyotome and sclerotome. The dermomyotome gives rise to muscles and axial dermis, while the sclerotome differentiates into the axial skeleton. Considering these developmental events, it is not surprising that PSC differentiation strategies have been shifting towards a paraxial mesoderm induction approach. Upon culture of murine (mPSCs) and hPSCs in GSK3-β (Wnt-signalling) and BMP-modulator containing media, PSCs rapidly upregulate the paraxial-somitic mesoderm markers TBX6, Meox1 and Myf5. Subsequently, when these cells were stimulated with osteogenic supplements an increase in osteogenic gene expression and extracellular matrix mineralization was detected. Interestingly, the obtained sclerotomic cells could also differentiate into chondrocytes. Although no detailed characterization of these cells has been carried out, we can assume that these progenitors could be reminiscent of MSC-like cell populations [82]. This hypothesis is further strengthened by the discovery of paraxial mesoderm-derived MSCs (Mesp1+-lineage tracing) in the bone marrow [83].
Despite the paraxial mesoderm induction protocols, general mesoderm-based MSC derivation approaches have also been used. Culture of hPSCs in endothelial media induced the formation of a mesodermal epithelium (BMP4+, Runx1+, GATA4+) that upon passaging, underwent epithelial–mesenchymal transition (EMT). The resulting fibroblastic cells appeared to be multipotent and expressed typical markers that are associated with MSCs [84].
When considering osteochondroprogenitors in other skeletal sites, a subset of bone marrow-derived MSCs (Sox1/P0-lineage tracing) originate from a migratory cell population derived from the ectoderm called the neural crest (NC) during craniofacial skeleton development [85,86]. The NC develops at the intersection of both neural and non-neural ectoderm and contains a migratory multipotent progenitor cell population that gives rise to most of the cranial bones, smooth muscles and pigmented cells within the craniofacial region, in addition to neurons and glial cells of the peripheral nervous system. By culturing hPSCs in media containing inhibitors for activin and GSK3-β, rapid differentiation into neural crest cells (NCCs) (p75+, TFAP2A+, Sox10+, Twist+) has been reported. Subsequent maintenance of these cells in MSC media led to the acquisition of a MSC phenotype with osteogenic differentiation capacities [87].
It is currently unclear whether PSCs can be differentiated directly into committed osteochondroprogenitor cells without going through an MSC-like state. Indeed, current MSC cultures are heterogeneous cell populations and contain committed progenitor cells that preferentially differentiate into skeletal tissues [88,89]. The differentiation of PSCs into committed osteochondroprogenitor cells might ultimately alleviate the need for an MSC step.
In summary, multiple approaches have been taken to isolate MSC-like cell populations from PSCs. While initial attempts focused on spontaneous differentiation, later protocols were more specific leading to increased cell derivation efficiencies. Nevertheless, these approaches clearly show the potential of PSCs for MSC isolation.
(b). Derivation of osteoblast-like cells from PSCs
The derivation of osteoblasts from PSCs has been investigated by culturing these stem cells with osteogenic supplements. Ascorbic acid, β-glycerolphosphate and dexamethasone are common osteogenic supplements that are used for growing and differentiating osteoprogenitors and MSCs. When mPSCs and hPSCs are differentiated in EBs and subsequently grown in osteogenic media, a rapid induction of osteogenic genes, such as Runx2, OCN, alkaline phosphatase and osteopontin, could be detected [90–92]. In vitro bone nodule formation with matrix mineralization was observed after 21–28 days. Interestingly, when the hPSC-derived cell populations were seeded onto scaffolds and subsequently implanted in immunocompromised mice, mineralized tissue formation could be observed after 35 days [93]. However, despite the induction of osteogenic genes, recent reports have indicated that in vivo bone formation associated with this methodology appears to be limited. Moreover, teratoma-like tissues have been detected despite the loss of pluripotency genes and long-term in vitro culture [94]. Nevertheless, these studies indicate that currently used osteogenic supplements could potentially be used for the induction of osteoblastic cell populations from PSCs.
Co-culture of hPSCs with primary bone-derived cell types has also been used for the induction of osteogenic differentiation [95]. This approach appears to be more efficient and rapid compared with the above-described strategy. These observations can be partly attributed to the expression of BMPs by the primary cells. Indeed, Ahn et al. reported that primary osteoblasts were secreting BMP2 and BMP4 which are known to induce the expression of Runx2 in MSCs [56,95,96].
All these approaches use media containing serum to induce osteogenic differentiation. Recently, Kanke et al. described a stepwise differentiation protocol that allows the induction of osteoblasts from both mPSCs and hPSCs. Through the use of small molecules, Kanke could steer the differentiation of PSCs to mesodermal progenitors that upon activation of hedgehog and BMP signalling underwent osteogenic differentiation. However, no in vivo tissue formation analysis was performed [97].
In conclusion, these reports are supportive for the osteogenic capacity of PSCs. Nevertheless, further refinement of induction protocols will be necessary to increase the differentiation efficiency and subsequent in vivo tissue formation capacity.
(c). Derivation of transient chondrocytes from PSCs
Scaffolds containing MSCs or osteoblasts have a limited capacity to induce intramembranous bone formation. This can be partly explained by the quick and fibrotic encapsulation of these scaffolds following implantation, leading to inhibition of host vasculature ingrowth and subsequent donor cell necrosis [98,99]. While different strategies have been investigated to increase cell survival [100–102], recent efforts have been shifting towards the induction of a provisional cartilage template that upon implantation would be replaced by bone tissue. This approach, which largely mimics postnatal fracture repair and embryonic endochondral bone formation, has recently been put forward by our laboratory as a novel tissue engineering strategy known as ‘developmental engineering' [103–105].
The induction of a provisional cartilage template for bone augmentation has numerous advantages when compared with traditional strategies: (1) while bone tissue is highly vascularised, cartilage is avascular and chondrocytes can survive in hypoxic conditions. Furthermore, these cells are able to secrete (2) osteogenic and (3) angiogenic growth factors to recruit progenitor cells from the local environment for bone formation. While a number of studies have delivered proof of principle for this approach using adult progenitor cells [106–108], PSCs can also be differentiated towards chondrocyte hypertrophy.
Indeed, Jukes et al. developed an endochondral bone induction protocol using mPSCs. By culturing these stem cells in serum-free media containing TGF-β, chondrogenic differentiation could be detected through the expression of Col2. Interestingly, when these cartilaginous aggregates were implanted in immunocompromised mice, endochondral bone formation with cartilage remodelling was observed after three weeks. However, in addition to the induction of bone formation, teratoma formation was also reported. Moreover, when the authors attempted to translate this protocol to human settings, no chondrogenic or endochondral bone formation could be detected. Nevertheless, this study is among the first to demonstrate that PSCs could be used for the induction of in vivo endochondral bone formation [109].
A series of studies have used mPSCs and hPSCs for the induction of chondrocytic cell populations for articular cartilage repair, which are likely to inform on endochondral differentiation strategies due to similar pathways governing the initial stages of specification. Through the use of Wnt agonists (GSK3-β inhibitors) and BMP inhibitors (noggin, dorsomorphin), researchers have successfully guided the differentiation of PSCs towards paraxial mesodermal progenitors that upon culture in chondrogenic media differentiated into Sox9+Col2+ chondrocytes [110–113]. Surprisingly, endochondral bone formation was observed following implantation, while limited hypertrophic differentiation was detected. Despite the differential in vivo behaviour of stable and transient chondrocytes, the genetic networks governing both cell states are still a matter of debate. Craft et al. recently developed a protocol in which the differentiation of hPSCs could be specifically steered to either stable or transient chondrocytes. By culturing paraxial mesoderm progenitors in media containing TGF-β, stable chondrocytes expressing Sox9 and Col2 could be obtained. Furthermore, activation of BMP signalling through exposure to exogenous BMP4 induced the formation of transient chondrocytes expressing Runx2 and Col10A1, which initiated the endochondral ossification process in vivo [113].
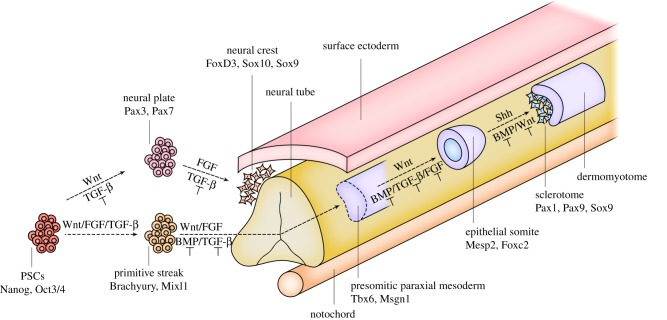
Similarly, transient chondrocytes have also been derived from ectomesenchymal progenitor cells. By culturing hPSCs in media containing TGF-β inhibitors, Umeda et al. could differentiate these cells into Sox9+-chondroprogenitor cells. Upon chondrogenic differentiation, in vitro cartilage formation and in vivo endochondral bone remodelling was observed. Interestingly, human-specific mitochondria labelling has shown that the implanted chondrocytes could transdifferentiate into Col1+-osteoblasts and contributed to de novo bone formation [72]. An overview of paraxial mesoderm and NC differentiation of PSCs can be found in figure 2.
Figure 2.
Skeletal cell specification strategies using PSCs. PSCs are commonly differentiated into neural crest—ectomesenchymal cells (upper branch) or paraxial mesodermal progenitor cells (lower branch) prior to inducing endochondral bone formation. Integration of signalling pathway activation or inhibition (T) is needed to steer PSC differentiation towards bone-forming progenitors. (Online version in colour.)
Transient chondrocytes have also been created through direct cell reprogramming, using Sox9, cMYC and KLF4. Indeed, we have recently reported that upon hypertrophic maturation of induced chondrogenic cell populations, in vivo endochondral bone formation was observed. In contrast to the reports above, the derived bone cells appeared to be host-derived, while no donor cell contribution was detected. This could potentially be attributed to the prolonged expression of Sox9. Indeed, induced chondrocytes with constitutive expression of Sox9 failed to form bone, while cells carrying doxycycline inducible expression vectors could trigger endochondral bone formation [114].
The aforementioned approaches indicate that PSCs can be used for the derivation of transient chondrocyte-like cell populations that upon implantation will give rise to endochondral bone formation.
4. Conclusion and future perspectives
Stem cell therapy offers great potential for bone tissue engineering. From the reports above, it is clear that PSCs can be used for the derivation of bone-forming progenitors. However, it also sets the stage for future research and clinical translation. We believe that while substantial achievements have been made, a continued intense effort will be necessary to translate ongoing research to the clinical setting. Indeed, several key aspects require addressing. Importantly, the purity of PSC-derived cell populations must be systematically assessed to avoid residual stem cell contamination which can lead to teratoma formation. While some cell surface markers have been identified and used for routine cell purification, we believe that additional PSC elimination strategies will be necessary. Recently, selective PSC toxicity agents, such as PluriSin and D-3, have been used for removing PSCs from differentiating cultures. The use of these strategies may result in an increase in PSC-associated cell therapies [115,116].
Additionally, while a number of osteochondrogenic cell types have been successfully derived from PSCs, it remains to be investigated which of these cell populations are most relevant to specific stages of in vivo bone formation. Indeed, currently used in vitro chondrogenic and osteogenic differentiation markers appear insufficient to evaluate the in vivo bone tissue formation and cell behaviour. We believe that more in-depth molecular screening techniques, such as transcriptome and secretome analysis, will offer increased sensitivity compared with currently used assays. Ideally, one may develop ‘bone tissue formation scorecards' that can be used for predicting in vivo behaviour. Similar scorecards have already been developed in which the differentiation capacity of PSCs can be assessed [117]. This approach will ultimately increase the robustness of bone tissue engineering strategies.
Finally, current differentiation and cell isolation studies generally aim at the induction of paraxial mesoderm from which the vertebral column develops. Despite the relative homogeneity and in vivo skeletogenic differentiation capacity of these cells, it remains to be elucidated to what extent these cells could contribute to appendicular skeletal repair. Ideally, differentiation strategies that aim at deriving skeletal progenitors from the embryonic limb may replicate long-bone differentiation programmes and tissue formation more accurately. By using specific cell reporters such as PRX1 and TBX5 [118], we hope that novel skeletal progenitors can be identified for disease modelling and cellular therapies. Ultimately, it is the authors' belief that PSCs offer great potential in skeletal tissue engineering and we look forward to further exciting research and developments in this field.
Acknowledgements
The authors thank Dr Cesar Libanati, Dr Gill Holdsworth and UCB Pharma colleagues for reviewing this manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
W.L.T., F.P.L. and S.J.R. conceptualized the review. W.L.T. drafted the review, F.P.L. and S.J.R. revised the review. The review was approved for submission by W.L.T., F.P.L. and S.J.R.
Competing interests
S.J.R. is an employee of UCB Pharma and may hold UCB Pharma shares and/or stock options.
Funding
This work was funded, in part, by the ERC Advanced Grant REJOIND (294191-REJOIND), the BOF-KU Leuven GOA project (3M120209) and the Stem Cell Institute of Leuven-KU Leuven (PF/10/019). The work is part of Prometheus, the Leuven Research and Development Division of Skeletal Tissue Engineering of KU Leuven: http://www.kuleuven.be/Prometheus.
References
- 1.Einhorn TA, Gerstenfeld LC. 2014. Fracture healing: mechanisms and interventions. Nat. Rev. Rheumatol. 11, 45–54. ( 10.1038/nrrheum.2014.164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langer R, Vacanti JP. 1993. Tissue engineering. Science 260, 920–926. ( 10.1126/science.8493529) [DOI] [PubMed] [Google Scholar]
- 3.Daher RJ, Chahine NO, Greenberg AS, Sgaglione NA, Grande DA. 2009. New methods to diagnose and treat cartilage degeneration. Nat. Rev. Rheumatol. 5, 599–607. ( 10.1038/nrrheum.2009.204) [DOI] [PubMed] [Google Scholar]
- 4.Carvalho JL, de Carvalho PH, Gomes DA, de Goes AM. 2013. Innovative strategies for tissue engineering. In Advances in Biomaterials Science and Biomedical Applications (ed. Pignatello R.), pp. 3–13. London: InTech. [Google Scholar]
- 5.Bellows CG, Heersche JNM, Aubin JE. 1990. Determination of the capacity for proliferation and differentiation of osteoprogenitor cells in the presence and absence of dexamethasone. Dev. Biol. 140, 132–138. ( 10.1016/0012-1606(90)90060-V) [DOI] [PubMed] [Google Scholar]
- 6.Bruder SP, Jaiswal N, Haynesworth SE. 1997. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J. Cell. Biochem. 64, 278–294. ( 10.1002/(SICI)1097-4644(199702)64:2%3C278::AID-JCB11%3E3.0.CO;2-F) [DOI] [PubMed] [Google Scholar]
- 7.Stenderup K, Justesen J, Clausen C, Kassem M. 2003. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 33, 919–926. ( 10.1016/j.bone.2003.07.005) [DOI] [PubMed] [Google Scholar]
- 8.Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. 2000. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp. Hematol. 28, 707–715. ( 10.1016/S0301-472X(00)00160-0) [DOI] [PubMed] [Google Scholar]
- 9.Thomson JA. 1998. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147. ( 10.1126/science.282.5391.1145) [DOI] [PubMed] [Google Scholar]
- 10.Evans MJ, Kaufman MH. 1981. Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156. ( 10.1038/292154a0) [DOI] [PubMed] [Google Scholar]
- 11.Tabar V, Studer L. 2014. Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat. Rev. Genet. 15, 82–92. ( 10.1038/nrg3563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keller G. 2005. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 19, 1129–1155. ( 10.1101/gad.1303605) [DOI] [PubMed] [Google Scholar]
- 13.Takarada T, Nakazato R, Tsuchikane A, Fujikawa K, Iezaki T, Yoneda Y, Hinoi E. 2016. Genetic analysis of Runx2 function during intramembranous ossification. Development 143, 211–218. ( 10.1242/dev.128793) [DOI] [PubMed] [Google Scholar]
- 14.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, De Crombrugghe B. 2002. The novel zinc finger-containing transcription factor Osterix is required for osteoblast differentiation and bone formation. Cell 108, 17–29. ( 10.1016/S0092-8674(01)00622-5) [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Cho K, Huang Y, Lyons JP, Zhou X, Sinha K, McCrea PD, de Crombrugghe B. 2008. Inhibition of Wnt signaling by the osteoblast-specific transcription factor Osterix. Proc. Natl Acad. Sci. USA 105, 6936–6941. ( 10.1073/pnas.0710831105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui L, Houston DA, Farquharson C, MacRae VE. 2016. Characterisation of matrix vesicles in skeletal and soft tissue mineralisation. Bone 87, 147–158. ( 10.1016/j.bone.2016.04.007) [DOI] [PubMed] [Google Scholar]
- 17.Winkler DG, et al. 2003. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 22, 6267–6276. ( 10.1093/emboj/cdg599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Razzaque MS. 2009. The FGF23–Klotho axis: endocrine regulation of phosphate homeostasis. Nat. Rev. Endocrinol. 5, 611–619. ( 10.1038/nrendo.2009.196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts SJ, van Gastel N, Carmeliet G, Luyten FP. 2015. Uncovering the periosteum for skeletal regeneration: the stem cell that lies beneath. Bone 70, 10–18. ( 10.1016/j.bone.2014.08.007) [DOI] [PubMed] [Google Scholar]
- 20.Akiyama H, et al. 2005. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc. Natl Acad. Sci. USA 102, 14 665–14 670. ( 10.1073/pnas.0504750102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. 2008. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. 40, 46–62. ( 10.1016/j.biocel.2007.06.009) [DOI] [PubMed] [Google Scholar]
- 22.Karsenty G, Wagner EF. 2002. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell. 2, 389–406. ( 10.1016/S1534-5807(02)00157-0) [DOI] [PubMed] [Google Scholar]
- 23.Kronenberg HM. 2003. Developmental regulation of the growth plate. Nature 423, 332–336. ( 10.1038/nature01657) [DOI] [PubMed] [Google Scholar]
- 24.Tsumaki N, Yoshikawa H. 2005. The role of bone morphogenetic proteins in endochondral bone formation. Cytokine Growth Factor Rev. 16, 279–285. ( 10.1016/j.cytogfr.2005.04.001) [DOI] [PubMed] [Google Scholar]
- 25.Murata M, Yudoh K, Masuko K. 2008. The potential role of vascular endothelial growth factor (VEGF) in cartilage. How the angiogenic factor could be involved in the pathogenesis of osteoarthritis? Osteoarthr. Cartil. 16, 279–286. ( 10.1016/j.joca.2007.09.003) [DOI] [PubMed] [Google Scholar]
- 26.Lim J, Tu X, Choi K, Akiyama H, Mishina Y, Long F. 2015. BMP-Smad4 signaling is required for precartilaginous mesenchymal condensation independent of Sox9 in the mouse. Dev. Biol. 400, 132–138. ( 10.1016/j.ydbio.2015.01.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwok C, et al. 1995. Mutations in SOX9, the gene responsible for Campomelic dysplasia and autosomal sex reversal. Am. J. Hum. Genet. 57, 1028–1036. [PMC free article] [PubMed] [Google Scholar]
- 28.Furumatsu T, Ozaki T, Asahara H. 2009. Smad3 activates the Sox9-dependent transcription on chromatin. Int. J. Biochem. Cell Biol. 41, 1198–1204. ( 10.1016/j.biocel.2008.10.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan Q, Yu Y, Chen Q, Li C, Wu H, Wan Y, Ma J, Sun F. 2008. Sox9, a key transcription factor of bone morphogenetic protein-2-induced chondrogenesis, is activated through BMP pathway and a CCAAT box in the proximal promoter. J. Cell. Physiol. 217, 228–241. ( 10.1002/jcp.21496) [DOI] [PubMed] [Google Scholar]
- 30.Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. 1999. Sox9 is required for cartilage formation. Nat. Genet. 22, 85–89. ( 10.1038/8792) [DOI] [PubMed] [Google Scholar]
- 31.Bell DM, et al. 1997. SOX9 directly regulates the type-II collagen gene. Nat. Genet. 16, 174–178. ( 10.1038/ng0697-174) [DOI] [PubMed] [Google Scholar]
- 32.Lefebvre V, Li P, De Crombrugghe B. 1998. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 17, 5718–5733. ( 10.1093/emboj/17.19.5718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lefebvre V, Behringer RR, De Crombrugghe B. 2001. L-Sox5, Sox6 and SOx9 control essential steps of the chondrocyte differentiation pathway. Osteoarthr. Cartil. 9, S69–S75. ( 10.1053/joca.2001.0447) [DOI] [PubMed] [Google Scholar]
- 34.Smits P, Li P, Mandel J, Zhang Z, Deng JM, Behringer RR, De Crombrugghe B, Lefebvre V. 2001. The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev. Cell 1, 277–290. ( 10.1016/S1534-5807(01)00003-X) [DOI] [PubMed] [Google Scholar]
- 35.Liu CF, Lefebvre V. 2015. The transcription factors SOX9 and SOX5/SOX6 cooperate genome-wide through super-enhancers to drive chondrogenesis. Nucleic Acids Res. 43, 8183–8203. ( 10.1093/nar/gkv688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lefebvre V, Dvir-Ginzberg M. 2017. SOX9 and the many facets of its regulation in the chondrocyte lineage. Connect. Tissue Res. 58, 2–14. ( 10.1080/03008207.2016.1183667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilkin DJ, et al. 1998. Mutations in fibroblast growth-factor receptor 3 in sporadic cases of achondroplasia occur exclusively on the paternally derived chromosome. Am. J. Hum. Genet. 63, 711–716. ( 10.1086/302000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Tan X, Li W, Wang Y, Wang J, Cheng X, Yang X. 2005. Smad4 is required for the normal organization of the cartilage growth plate. Dev. Biol. 284, 311–322. ( 10.1016/j.ydbio.2005.05.036) [DOI] [PubMed] [Google Scholar]
- 39.Perry MJ, McDougall KE, Hou SC, Tobias JH. 2008. Impaired growth plate function in bmp-6 null mice. Bone 42, 216–225. ( 10.1016/j.bone.2007.09.053) [DOI] [PubMed] [Google Scholar]
- 40.Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM. 2005. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc. Natl Acad. Sci. USA 102, 5062–5067. ( 10.1073/pnas.0500031102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akiyama H, et al. 2004. Interactions between Sox9 and β-catenin control chondrocyte differentiation. Genes Dev. 18, 1072–1087. ( 10.1101/gad.1171104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Day TF, Guo X, Garrett-Beal L, Yang Y. 2005. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell 8, 739–750. ( 10.1016/j.devcel.2005.03.016) [DOI] [PubMed] [Google Scholar]
- 43.Fraichard A, et al. 1997. The T3Rα gene encoding a thyroid hormone receptor is essential for post-natal development and thyroid hormone production. EMBO J. 16, 4412–4420. ( 10.1093/emboj/16.14.4412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robson H, Siebler T, Stevens DA, Shalet SM, Williams GR. 2000. Thyroid hormone acts directly on growth plate chondrocytes to promote hypertrophic differentiation and inhibit clonal expansion and cell proliferation. Endocrinology 141, 3887–3897. ( 10.1210/endo.141.10.7733) [DOI] [PubMed] [Google Scholar]
- 45.Yoshida CA, et al. 2004. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev. 18, 952–963. ( 10.1101/gad.1174704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li F, et al. 2011. Runx2 contributes to murine Col10a1 gene regulation through direct interaction with its cis-enhancer. J. Bone Miner. Res. 26, 2899–2910. ( 10.1002/jbmr.504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zelzer E, Glotzer DJ, Hartmann C, Thomas D, Fukai N, Soker S, Olsen BR. 2001. Tissue specific regulation of VEGF expression during bone development requires Cbfa1/Runx2. Mech. Dev. 106, 97–106. ( 10.1016/S0925-4773(01)00428-2) [DOI] [PubMed] [Google Scholar]
- 48.Inada M, et al. 1999. Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev. Dyn. 214, 279–290. ( 10.1002/(SICI)1097-0177(199904)214:4%3C279::AID-AJA1%3E3.0.CO;2-W) [DOI] [PubMed] [Google Scholar]
- 49.Kim IS, Otto F, Zabel B, Mundlos S. 1999. Regulation of chondrocyte differentiation by Cbfa1. Mech. Dev. 80, 159–170. ( 10.1016/S0925-4773(98)00210-X) [DOI] [PubMed] [Google Scholar]
- 50.Li J, et al. 2016. Systematic reconstruction of molecular cascades regulating GP development using single-cell RNA-Seq. Cell Rep. 15, 1467–1480. ( 10.1016/j.celrep.2016.04.043) [DOI] [PubMed] [Google Scholar]
- 51.Ionescu A, Kozhemyakina E, Nicolae C, Kaestner KH, Olsen BR, Lassar AB. 2012. FoxA family members are crucial regulators of the hypertrophic chondrocyte differentiation program. Dev. Cell 22, 927–939. ( 10.1016/j.devcel.2012.03.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu H, Bendall AJ. 2009. Dlx5 is a cell autonomous regulator of chondrocyte hypertrophy in mice and functionally substitutes for Dlx6 during endochondral ossification. PLoS ONE 4, e8097 ( 10.1371/journal.pone.0008097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou X, von der Mark K, Henry S, Norton W, Adams H, de Crombrugghe B. 2014. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 10, e1004820 ( 10.1371/journal.pgen.1004820) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang L, Tsang KY, Tang HC, Chan D, Cheah KSE. 2016. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. World Rev. Nutr. Diet. 114, 13 ( 10.1159/000441808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaur T, et al. 2005. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 280, 33 132–33 140. ( 10.1074/jbc.M500608200) [DOI] [PubMed] [Google Scholar]
- 56.Jang WG, Kim EJ, Kim DK, Ryoo HM, Lee KB, Kim SH, Choi HS, Koh JT. 2012. BMP2 protein regulates osteocalcin expression via Runx2-mediated Atf6 gene transcription. J. Biol. Chem. 287, 905–915. ( 10.1074/jbc.M111.253187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ortuño MJ, Susperregui ARG, Artigas N, Rosa JL, Ventura F. 2013. Osterix induces Col1a1 gene expression through binding to Sp1 sites in the bone enhancer and proximal promoter regions. Bone 52, 548–556. ( 10.1016/j.bone.2012.11.007) [DOI] [PubMed] [Google Scholar]
- 58.Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM. 2010. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 19, 329–344. ( 10.1016/j.devcel.2010.07.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friedenstein A, Chailakhjan R, Lalykina K. 1970. The development of fibroblast colonies in monolayer cultures of guinea pig bone marrow and spleen cells. Cell Tissue Kinet. 3, 393–403. ( 10.1111/j.1365-2184.1970.tb00347.x) [DOI] [PubMed] [Google Scholar]
- 60.Friedenstein AJ, Kuralesova AI. 1971. Osteogenic precursor cells of bone marrow in radiation chimeras. Transplantation 12, 99–108. ( 10.1097/00007890-197108000-00001) [DOI] [PubMed] [Google Scholar]
- 61.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. 1974. Stromal cells responsible for transferring the microenvironment of the hematopoietic tissues: cloning in vitro and retransplantation in vivo. Transplantation 17, 331–340. ( 10.1097/00007890-197404000-00001) [DOI] [PubMed] [Google Scholar]
- 62.Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luriá EA, Ruadkow IA. 1974. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp. Hematol. 2, 83–92. ( 10.1016/j.exphem.2005.10.008) [DOI] [PubMed] [Google Scholar]
- 63.Bianco P, Robey PG. 2015. Skeletal stem cells. Development 142, 1023–1027. ( 10.1242/dev.102210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sacchetti B, et al. 2016. No identical ‘mesenchymal stem cells' at different times and sites: Human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Rep. 6, 897–913. ( 10.1016/j.stemcr.2016.05.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaim M, Karaman S, Cetin G, Isik S. 2012. Donor age and long-term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann. Hematol. 91, 1175–1186. ( 10.1007/s00277-012-1438-x) [DOI] [PubMed] [Google Scholar]
- 66.Gu Y, Li T, Ding Y, Sun L, Tu T, Zhu W, Hu J, Sun X. 2016. Changes in mesenchymal stem cells following long-term culture in vitro. Mol. Med. Rep. 13, 5207–5215. ( 10.3892/mmr.2016.5169) [DOI] [PubMed] [Google Scholar]
- 67.Wagner W. 2012. Implications of long-term culture for mesenchymal stem cells: genetic defects or epigenetic regulation? Stem Cell Res. Ther. 3, 54 ( 10.1186/scrt145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dominici M, et al. 2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The International society for cellular therapy position statement. Cytotherapy 8, 315–317. ( 10.1080/14653240600855905) [DOI] [PubMed] [Google Scholar]
- 69.Brown SE, Tong W, Krebsbach PH. 2009. The derivation of mesenchymal stem cells from human embryonic stem cells. Cells Tissues Organs 189, 256–260. ( 10.1159/000151746) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee EJ, et al. 2010. Novel embryoid body-based method to derive mesenchymal stem cells from human embryonic stem cells. Tissue Eng. A 16, 705–715. ( 10.1089/ten.tea.2008.0596) [DOI] [PubMed] [Google Scholar]
- 71.Arpornmaeklong P, Brown SE, Wang Z, Krebsbach PH. 2009. Phenotypic characterization, osteoblastic differentiation, and bone regeneration capacity of human embryonic stem cell–derived mesenchymal stem cells. Stem Cells Dev. 18, 955–968. ( 10.1089/scd.2008.0310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Umeda K, Oda H, Yan Q, Matthias N, Zhao J, Davis BR, Nakayama N. 2015. Long-term expandable SOX9+ chondrogenic ectomesenchymal cells from human pluripotent stem cells. Stem Cell Rep. 4, 712–726. ( 10.1016/j.stemcr.2015.02.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zou Q, Wu M, Zhong L, Fan Z, Zhang B, Chen Q, Ma F. 2016. Development of a xeno-free feeder-layer system from human umbilical cord mesenchymal stem cells for prolonged expansion of human induced pluripotent stem cells in culture. PLoS ONE 11, e0149023 ( 10.1371/journal.pone.0149023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Villa-Diaz LG, Brown SE, Liu Y, Ross A, Lahann J, Parent JM, Krebsbach PH. 2012. Derivation of functional mesenchymal stem cells from human induced pluripotent stem cells culture on synthetic polymer substrates. Stem Cells 30, 1174–1181. ( 10.1002/stem.1084.Derivation) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barberi T, Willis LM, Socci ND, Studer L. 2005. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2, 0554–0560. ( 10.1371/journal.pmed.0020161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trivedi P, Hematti P. 2007. Simultaneous generation of CD34+ primitive hematopoietic cells and CD73+ mesenchymal stem cells from human embryonic stem cells cocultured with murine OP9 stromal cells. Exp. Hematol. 35, 146–154. ( 10.1016/j.exphem.2006.09.003) [DOI] [PubMed] [Google Scholar]
- 77.Vodyanik MA, Yu J, Zhang X, Tian S, Stewart R, Thomson JA, Slukvin II. 2010. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell 7, 718–729. ( 10.1016/j.stem.2010.11.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blazsek I, Chagraoui J, Péault B. 2000. Ontogenic emergence of the hematon, a morphogenetic stromal unit that supports multipotential hematopoietic progenitors in mouse bone marrow. Blood 96, 3763–3771. [PubMed] [Google Scholar]
- 79.Crane GM, Jeffery E, Morrison SJ. 2017. Adult haematopoietic stem cell niches. Nat. Rev. Immunol. 17, 573–590. ( 10.1038/nri.2017.53) [DOI] [PubMed] [Google Scholar]
- 80.Xi H, Fujiwara W, Gonzalez K, Jan M, Liebscher S, Van Handel B, Schenke-Layland K, Pyle AD. 2017. In vivo human somitogenesis guides somite development from hPSCs. Cell Rep. 18, 1573–1585. ( 10.1016/j.celrep.2017.01.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loh KMM, et al. 2016. Mapping the pairwise choices leading from pluripotency to human bone, heart, and other mesoderm cell types. Cell 166, 451–468. ( 10.1016/j.cell.2016.06.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sakurai H, Sakaguchi Y, Shoji E, Nishino T, Maki I, Sakai H, Hanaoka K, Kakizuka A, Sehara-Fujisawa A. 2012. In vitro modeling of paraxial mesodermal progenitors derived from induced pluripotent stem cells. PLoS ONE 7, e47078 ( 10.1371/journal.pone.0047078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Niibe K, Morikawa S, Mabuchi Y, Nakagawa T, Okano H. 2011. Mesp1+ early paraxial mesodermal cells supply initial bone marrow mesenchymal stem cells capable of differentiating into neural crest lineage cells. Inflamm. Regen. 31, 116–124. ( 10.2492/inflammregen.31.116) [DOI] [Google Scholar]
- 84.Boyd NL, Robbins KR, Dhara SK, West FD, Stice SL. 2009. Human embryonic stem cell-derived mesoderm-like epithelium transitions to mesenchymal progenitor cells. Tissue Eng. A 15, 1897–1907. ( 10.1089/ten.tea.2008.0351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takashima Y, Era T, Nakao K, Kondo S, Kasuga M, Smith AG, Nishikawa SI. 2007. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell 129, 1377–1388. ( 10.1016/j.cell.2007.04.028) [DOI] [PubMed] [Google Scholar]
- 86.Morikawa S, et al. 2009. Development of mesenchymal stem cells partially originate from the neural crest. Biochem. Biophys. Res. Commun. 379, 1114–1119. ( 10.1016/j.bbrc.2009.01.031) [DOI] [PubMed] [Google Scholar]
- 87.Fukuta M, et al. 2014. Derivation of mesenchymal stromal cells from pluripotent stem cells through a neural crest lineage using small molecule compounds with defined media. PLoS ONE 9, e112291 ( 10.1371/journal.pone.0112291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG. 2003. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J. Cell. Biochem. 89, 1235–1249. ( 10.1002/jcb.10594) [DOI] [PubMed] [Google Scholar]
- 89.Muraglia A, Cancedda R, Quarto R. 2000. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J. Cell Sci. 113(Pt 7), 1161–1166. ( 10.1006/excr.1999.4592) [DOI] [PubMed] [Google Scholar]
- 90.Buttery LD, Bourne S, Xynos JD, Wood H, Hughes FJ, Hughes SP, Episkopou V, Polak JM. 2001. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Eng. 7, 89–99. ( 10.1089/107632700300003323) [DOI] [PubMed] [Google Scholar]
- 91.Bourne S, Polak JM, Hughes SPF, Buttery LDK. 2004. Osteogenic differentiation of mouse embryonic stem cells: differential gene expression analysis by cDNA microarray and purification of osteoblasts by cadherin-11 magnetically activated cell sorting. Tissue Eng. 10, 796–806. ( 10.1089/1076327041348293) [DOI] [PubMed] [Google Scholar]
- 92.Sottile V, Thomson A, McWhir J. 2003. In vitro osteogenic differentiation of human ES cells. Cloning Stem Cells 5, 149–155. ( 10.1089/153623003322234759) [DOI] [PubMed] [Google Scholar]
- 93.Bielby RC, Boccaccini AR, Polak JM, Buttery LD. 2004. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Eng. 10, 1518–1525. ( 10.1089/ten.2004.10.1518) [DOI] [PubMed] [Google Scholar]
- 94.Kuznetsov SA, Cherman N, Robey PG. 2011. In vivo bone formation by progeny of human embryonic stem cells. Stem Cells Dev. 20, 269–287. ( 10.1089/scd.2009.0501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ahn SE, et al. 2006. Primary bone-derived cells induce osteogenic differentiation without exogenous factors in human embryonic stem cells. Biochem. Biophys. Res. Commun. 340, 403–408. ( 10.1016/j.bbrc.2005.12.020) [DOI] [PubMed] [Google Scholar]
- 96.Phimphilai M, Zhao Z, Boules H, Roca H, Franceschi RT. 2006. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J. Bone Miner. Res. 21, 637–646. ( 10.1359/jbmr.060109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kanke K, et al. 2014. Stepwise differentiation of pluripotent stem cells into osteoblasts using four small molecules under serum-free and feeder-free conditions. Stem Cell Rep. 2, 751–760. ( 10.1016/j.stemcr.2014.04.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brien FJO. 2011. Biomaterials and scaffolds for tissue engineering. Mater. Today 14, 88–95. ( 10.1016/S1369-7021(11)70058-X) [DOI] [Google Scholar]
- 99.Amini AR, Laurencin CT, Nukavarapu SP. 2012. Bone tissue engineering: recent advances and challenges. Crit. Rev. Biomed. Eng. 40, 363–408. ( 10.1615/CritRevBiomedEng.v40.i5.10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stegen S, et al. 2016. HIF-1α promotes glutamine-mediated redox homeostasis and glycogen-dependent bioenergetics to support postimplantation bone cell survival. Cell Metab. 23, 265–279. ( 10.1016/j.cmet.2016.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rouwkema J, Westerweel PE, de Boer J, Verhaar MC, van Blitterswijk CA. 2009. The use of endothelial progenitor cells for prevascularized bone tissue engineering. Tissue Eng. A 15, 2015–2027. ( 10.1089/ten.tea.2008.0318) [DOI] [PubMed] [Google Scholar]
- 102.Amini AR, Nukavarapu SP. 2014. Oxygen-tension controlled matrices for enhanced osteogenic cell survival and performance. Ann. Biomed. Eng. 42, 1261–1270. ( 10.1007/s10439-014-0990-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lenas P, Moos M, Luyten FP. 2009. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part II: from genes to networks: tissue engineering from the viewpoint of systems biology and network science. Tissue Eng. B 15, 395–422. ( 10.1089/ten.TEB.2009.0461) [DOI] [PubMed] [Google Scholar]
- 104.Lenas P, Moos M, Luyten FP. 2009. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part I: from three-dimensional cell growth to biomimetics of in vivo development. Tissue Eng. B Rev. 15, 381–394. ( 10.1089/ten.TEB.2008.0575) [DOI] [PubMed] [Google Scholar]
- 105.Lenas P, Luyten FP. 2011. An emerging paradigm in tissue engineering: from chemical engineering to developmental engineering for bioartificial tissue formation through a series of unit operations that simulate the in vivo successive developmental stages. Ind. Eng. Chem. Res. 50, 482–522. ( 10.1021/ie100314b) [DOI] [Google Scholar]
- 106.Scotti C, et al. 2010. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc. Natl Acad. Sci. USA 107, 7251–7256. ( 10.1073/pnas.1000302107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scotti C, Piccinini E, Takizawa H, Todorov A, Bourgine P, Papadimitropoulos A, Barbero A, Manz MG, Martin I. 2013. Engineering of a functional bone organ through endochondral ossification. Proc. Natl Acad. Sci. USA 110, 3997–4002. ( 10.1073/pnas.1220108110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bolander J, Ji W, Leijten J, Teixeira LM, Bloemen V, Lambrechts D, Chaklader M, Luyten FP. 2017. Healing of a large long-bone defect through serum-free in vitro priming of human periosteum-derived cells. Stem Cell Rep. 8, 758–772. ( 10.1016/j.stemcr.2017.01.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jukes JM, Both SK, Leusink A, Sterk LMT, van Blitterswijk CA, de Boer J. 2008. Endochondral bone tissue engineering using embryonic stem cells. Proc. Natl Acad. Sci. USA 105, 6840–6845. ( 10.1073/pnas.0711662105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Umeda K, Zhao J, Simmons P, Stanley E, Elefanty A, Nakayama N. 2012. Human chondrogenic paraxial mesoderm, directed specification and prospective isolation from pluripotent stem cells. Sci. Rep. 2, 455 ( 10.1038/srep00455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao J, Li S, Trilok S, Tanaka M, Jokubaitis-Jameson V, Wang B, Niwa H, Nakayama N. 2014. Small molecule-directed specification of sclerotome-like chondroprogenitors and induction of a somitic chondrogenesis program from embryonic stem cells. Development 141, 3848–3858. ( 10.1242/dev.105981) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Craft AM, Ahmed N, Rockel JS, Baht GS, Alman BA, Kandel RA, Grigoriadis AE, Keller GM. 2013. Specification of chondrocytes and cartilage tissues from embryonic stem cells. Development 140, 2597–2610. ( 10.1242/dev.087890) [DOI] [PubMed] [Google Scholar]
- 113.Craft AM, Rockel JS, Nartiss Y, Kandel RA, Alman BA, Keller GM. 2015. Generation of articular chondrocytes from human pluripotent stem cells. Nat. Biotechnol. 33, 638–645. ( 10.1038/nbt.3210) [DOI] [PubMed] [Google Scholar]
- 114.Tam WL, O DF, Hiramatsu K, Tsumaki N, Luyten FP, Roberts SJ. 2014. Sox9 reprogrammed dermal fibroblasts undergo hypertrophic differentiation in vitro and trigger endochondral ossification in vivo. Cell. Reprogram. 16, 29–39. ( 10.1089/cell.2013.0060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cheng A, Kapacee Z, Peng J, Lu S, Lucas RJ, Hardingham TE, Kimber SJ. 2014. Cartilage repair using human embryonic stem cell-derived chondroprogenitors. Stem Cells Transl. Med. 3, 1287–1294. ( 10.5966/sctm.2014-0101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kuang Y, et al. 2017. Efficient, selective removal of human pluripotent stem cells via ecto-alkaline phosphatase-mediated aggregation of synthetic peptides. Cell Chem. Biol. 24, 685–694.e4. ( 10.1016/j.chembiol.2017.04.010) [DOI] [PubMed] [Google Scholar]
- 117.Tsankov AM, Akopian V, Pop R, Chetty S, Gifford CA, Daheron L, Tsankova NM, Meissner A. 2015. A qPCR ScoreCard quantifies the differentiation potential of human pluripotent stem cells. Nat. Biotechnol. 33, 1182–1192. ( 10.1038/nbt.3387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Minguillon C, Nishimoto S, Wood S, Vendrell E, Gibson-Brown JJ, Logan MPO. 2012. Hox genes regulate the onset of Tbx5 expression in the forelimb. Development 139, 3180–3188. ( 10.1242/dev.084814) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.