Abstract
Stem cell-derived hepatocyte-like cells (HLCs) offer great opportunities for studies of host–pathogen interactions and tissue regeneration, as well as hepatotoxicity. To reliably predict the outcome of infection or to enhance graft survival, a finely tuned innate immune system is essential. Hepatocytes have long been considered solely metabolic and their critical innate immune potential is only recently gaining attention. Viral infection studies show that pathogen detection by cytosolic receptors leads to interferon (IFN) induction in primary hepatocytes and HLCs. IFN expression in HLCs is characterized by strong expression of type III IFN and low expression of type I IFN which is also a characteristic of primary hepatocytes. The response to IFN differs in HLCs with lower interferon-stimulated gene (ISG)-expression levels than in primary hepatocytes. Tumour necrosis factor-alpha (TNF-α) signalling is less studied in HLCs, but appears to be functional. Expression of toll-like receptors (TLR) 2–5, 7 and 9 has been reported in primary hepatocytes but has been poorly studied in HLCs. In summary, although they retain some immature features, HLCs are in many ways superior to hepatoma cell lines for cell-based modelling. In this review, we will provide an overview of innate immune signalling in HLCs and how this compares with primary hepatocytes.
This article is part of the themed issue ‘Designer human tissue: coming to a lab near you’.
Keywords: stem cells, hepatocytes, innate immunity
1. Introduction
Stem cell-derived hepatocyte-like cells (HLCs) are of interest as alternatives to primary cells for the study of host–pathogen interactions, tissue regeneration and drug development. The discovery of somatic cell reprogramming in 2006, which allows the generation of pluripotent stem cells, has revolutionized the field of stem cell research. The so-called induced pluripotent stem cells (iPSCs) can be produced from the desired genetic background providing diverse stem cell banks [1]. By contrast, human embryonic stem cells (hESCs) are derived from the inner cell mass of preimplantation embryos that are not suitable for human implantation, with the first hESC lines described in 1998 [2]. Currently, both hESCs and iPSCs are used in stem cell research and for differentiation into somatic cells.
The immunogenicity of pluripotent stem cell-derived HLCs and other somatic cells has been studied extensively, while the ability of in vitro differentiated HLCs to mount an immune response by sensing and reacting to pathogens has only recently gained attention [3–13]. As the major metabolic cell type of the liver, hepatocytes are constantly exposed to diverse metabolic products and antigens including the products of digestion as well as hepatotrophic pathogens. In this microenvironment, the hepatocyte has to be immune tolerant to food- and commensal-derived antigens while staying alert to invading pathogens. To manage the dichotomy between immune tolerance and immune activation, the hepatic innate immune system is tightly regulated. In vitro differentiated HLCs that closely mimic primary hepatocytes would be of significant value in identifying these regulatory mechanisms.
Studies of host–pathogen interactions have long relied on hepatoma cell lines where innate immune responses are impaired and intracellular signalling is not representative of healthy hepatocytes [3,4,14–16]. HLCs more closely mimic the in vivo situation. Moreover, as they possess the genetic background of their donor, HLCs can be used to study donor-specific influences on hepatocyte function [17]. Hence, HLCs are a promising tool for understanding cell-based immune responses to pathogens and provide a platform for identification of the mechanisms that underlie inter-individual differences in susceptibility to hepatotrophic pathogens.
Discovering host genetic factors that determine the outcome of hepatotrophic infections and response to antiviral therapy will also lay the basis for the development of new and better therapies with fewer side effects. Host factors important for viral entry and replication, viral sensing mechanisms and the induction of innate immune responses in infected cells all together determine the outcome of infection. For viruses that establish chronic infection such as hepatitis B (HBV) and hepatitis C virus (HCV), the effectiveness of the innate immune response mounted by the host cell determines whether infection becomes chronic or is cleared successfully [18–21]. To enhance the innate immune response of the host and thereby increase the chances of clearing the virus, immune-based therapies are of continued interest for the treatment of HBV and HCV. At the same time, these therapies are of benefit for the treatment of viruses with high genetic variation such as HCV [22].
With regard to regenerative medicine, functional innate immune responses in stem cell-derived somatic cells to be used for transplantation will improve graft survival and allow the cells to react to and fight off invading pathogens. A tightly regulated immune response is particularly important in the liver, an organ that is constantly exposed to a wide variety of antigens including both, food-derived antigens and pathogens [23].
This review will provide an overview of our current knowledge of the innate immune machinery of stem cell-derived HLCs and how it compares with primary hepatocytes. Expression of viral sensing molecules, downstream signalling pathways and generation of antiviral and pro-inflammatory cytokines will be discussed in relation to the in vivo situation with the aim of better understanding the immune phenotype of in vitro differentiated HLCs.
2. Innate immune pathways in stem cell-derived hepatocytes
Protein-based innate immune responses, such as virus sensing by pattern recognition receptors (PRR) and the interferon response, are not detectable in pluripotent cells and are only acquired during maturation [5,24,25]. Innate immunity in pluripotent stem cells was, therefore, long thought to be severely attenuated. However, more recent studies of defence strategies of pluripotent stem cells suggest RNAi as a major antiviral mechanism [5,24,25]. By contrast, innate immune strategies in differentiated cells comprise detection of pathogen-associated molecular patterns (PAMPs), which are conserved structures within a pathogen, by PRRs followed by expression of IFN and interferon-stimulated genes (ISGs) and intrinsic antiviral immunity.
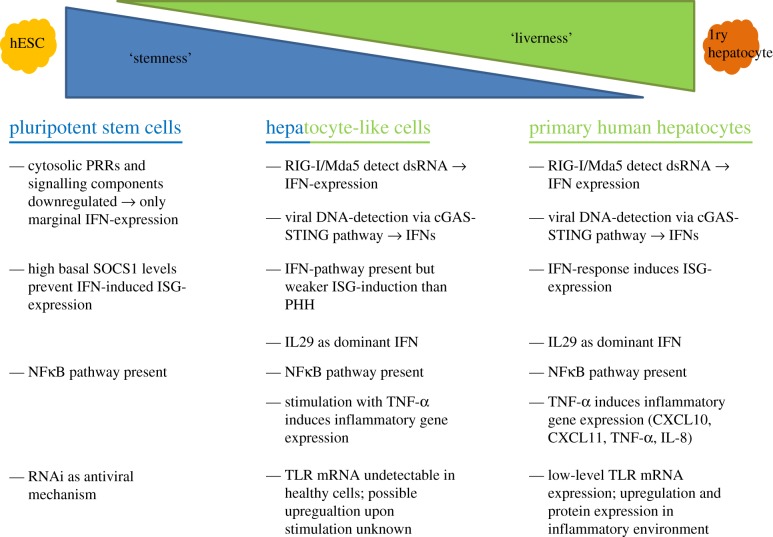
Despite the progress made in differentiation procedures, HLCs retain an immature phenotype, still expressing markers of stem cells and fetal hepatocytes [26,27]. It is, therefore, not farfetched to assume that innate immune pathways might also be of an immature phenotype in the in vitro differentiated cells. From the few studies that have investigated innate immunity in stem cell-derived somatic cells, there is some evidence that the IFN-based arm of innate immunity and also toll-like receptor (TLR)-responses remain downregulated (figure 2). However, research of these mechanisms in HLCs is still limited, requiring further studies into the antiviral response. In the following section, we will describe hepatic innate immune mechanisms and their presence or absence in HLCs.
Figure 2.
Comparison of innate immune pathways in pluripotent stem cells, stem cell-derived hepatocytes and primary hepatocytes. Pluripotent stem cells and primary hepatocytes employ fundamentally different mechanisms to defend themselves against invading pathogens. Innate immune pathways in HLCs resemble primary hepatocytes; however, reduced levels of ISG-expression and possible absence of TLR-responses represent immaturity of immune signalling pathways. Abbreviations: HLC, hepatocyte-like cells; ISG, interferon-stimulated genes; PRR, pattern recognition receptor; SOCS, suppressor of cytokine signalling; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; RIG, retinoic acid inducible gene; Mda, melanoma differentiation-associated gene; IFN, interferon; ISG, interferon-stimulated genes; IL, interleukin; TNF-α, tumour necrosis factor-alpha; CXCL, C-X-C motif chemokine; TLR, toll-like receptor. (Online version in colour.)
(a). Viral sensing by cytosolic pattern recognition receptors
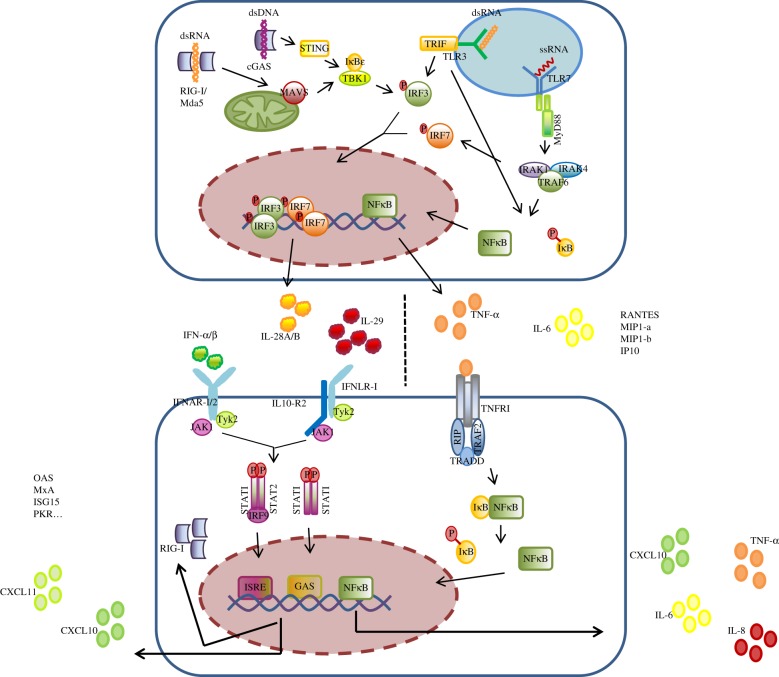
Hepatocytes are equipped with a variety of cytosolic PRRs that allow them to detect PAMPs from DNA- and RNA- viruses, intracellular bacteria and damage-associated molecular patterns, released from damaged cells. Retinoic acid inducible gene-I (RIG-I) and melanoma differentiation-associated gene 5 (Mda-5) are sensors of viral dsRNA. Ligand-binding triggers a signalling cascade via mitochondrial antiviral signalling protein (MAVS) and interferon regulatory factor 3 (IRF3), leading to the induction of IFNs. While RIG-I senses short dsRNA motifs, long dsRNA is sensed by Mda-5. Ligand recognition of RIG-I or Mda-5 results in receptor recruitment to the mitochondrial surface where binding to MAVS via the respective CARD domains occurs. The complex of RIG-I/MAVS and Mda-5/MAVS respectively activates TANK-binding kinase 1 (TBK-1) and IkB kinase-ɛ of the IkB kinase complex which phosphorylates IRF3. Phosphorylated IRF3 translocates to the nucleus and induces the expression of type I and type III IFNs (figure 1) [28].
Figure 1.
Innate immune signalling pathways in hepatocytes. Hepatocytes possess sensors for DNA and RNA viruses in the cytoplasm and in endosomes which, upon activation, induce the expression of IFNs and inflammatory cytokines. IFNs act in an autocrine and paracrine manner turning the cells into an antiviral state. Upon receptor binding of TNF-α signalling via NFκB leads to expression of inflammatory cytokines and chemokines. Abbreviations: ds, double stranded; RIG, retinoic acid inducible gene; Mda, melanoma differentiation-associated gene; MAVS, mitochondrial antiviral signalling protein; TBK, tank-binding kinase; IRF, interferon regulatory factor; TLR, toll-like receptor; IRAK, interleukin 1 receptor-associated kinase; TRAF, TNF-α receptor-associated factor; IFN, interferon; STAT, signal transducer and activator of transcription protein; ISRE, IFN-stimulated response element; TNF-α, tumour necrosis factor-alpha; TNFR, tumour necrosis factor receptor; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; IL, interleukin; CXCL10, C-X-C motif chemokine. (Online version in colour.)
Viral DNA is detected by the cGAS–STING signalling pathway [29,30]. cGAS detects dsDNA and induces STING to recruit TBK-1 and IRF3 to its C-terminus. The close proximity between TBK-1 and IRF3 allows TBK-1 to phosphorylate IRF3 which ultimately leads to IFN-release.
Infection studies with HCV and HBV revealed that cytoplasmic PRRs in HLCs, including RIG-I and cGAS, sense infection and mount an innate immune response against both RNA and DNA viruses. The use of polyI:C to mimic dsRNA revealed that HLCs detect HCV-infection through RIG-I, resulting in strong upregulation of type III and weaker upregulation of type I IFNs [3,6]. Late and low-level induction of type I IFNs is also a characteristic of primary human hepatocytes. IL29 was found to be the major IFN expressed by stem cell-derived hepatocytes upon HCV-infection and polyI:C stimulation, closely mimicking the in vivo situation [3,31]. Viral detection and subsequent IFN-responses further upregulate PRR expression, such as RIG-I, cGAS and STING, in HLCs [3,7].
Genetic variation of IL28B, another type III IFN, is associated with variation in response to therapy for HCV-infection [32]. Expression of IL28 by HLCs upon HCV-infection implicates the potential of stem cell-derived hepatocytes to study the influence of host genetics on viral infection. Markedly, type III IFNs are not expressed by HCV-infected Huh7 cells, showing the superiority of HLCs for studies of host–pathogen interaction [3].
(b). Response to IFN and the Janus kinase/signal transducer and activator of transcription protein pathway
Type I and III IFNs are released by infected hepatocytes and demonstrate autocrine and paracrine activity, inducing an antiviral state in the local tissue. IFN-receptors are heterodimers of transmembrane proteins that contain an extracellular ligand-binding domain and a cytoplasmic tail. Hepatocytes express receptors for type I and III IFNs; IFNAR1/IFNAR2 bind type I IFNs (IFN-α and IFN-β) and IFNLR1/IL10-R2 bind type III IFNs (IFN-λ1/IL29, IFN-λ2/IL28A and IFN-λ3/IL28B). Upon binding of IFNs to their receptor, the cytoplasmic receptor-associated tyrosine kinases Janus kinase 1 (JAK1) and Tyrosine kinase 2 (TYK2) become auto-phosphorylated. Phosphorylated JAK1 and TYK2 recruit and phosphorylate signal transducer and activator of transcription protein 1 (STAT1) and STAT2 which promotes their association with IRF9. The resulting complex of STAT1, STAT2 and IRF9 translocates to the nucleus where it binds to IFN-stimulated response elements (ISRE) on the DNA and induces expression of hundreds of ISGs (figure 1) [33]. JAK/STAT signalling is negatively regulated by suppressor of cytokine signalling (SOCS1) which ubiquinates phosphorylated STAT1 and mediates its proteasomal degradation. In differentiated cells, SOCS1 is expressed at low basal levels but as an ISG itself becomes rapidly upregulated upon induction of the JAK/STAT pathway.
hESCs, iPSCs and derived HLCs express all components of the IFN-signalling pathway (IFNAR1,2, STAT1,2 IRF9) [24]. However, hESCs and iPSCs lack the ability to respond to IFN-α treatment owing to high basal SOCS1 levels [24]. Responsiveness to IFN-α develops early on during differentiation, correlates with downregulation of basal SOCS1 levels and increases over time. D5-definitive endoderm, d15-hepatoblasts and d21-HLCs are all able to respond to IFN-α treatment with phosphorylation of STAT1 and expression of ISGs, but mature HLCs show the strongest ISG-induction levels [8,10]. The antiviral effect of the ISGs expressed by HLCs was confirmed in studies of HCV and HBV infection where IFN-α treatment of HLCs prior to virus infection significantly reduced viral replication and persistence [8,10].
With emerging evidence of the importance of IL29 in hepatocyte immunity, research focused on the expression of and response to type III IFNs in HLCs. Indeed, HLCs respond to IL29 treatment with phosphorylation of STAT1 and expression of ISGs (IFIT1, Mx1, OAS1, ISG15, CXCL10, CXCL11, IRF9, IRF7, IRF1, IRF2) [3,6]. These findings show that pluripotent stem cell-derived hepatocytes possess functional IFN-signalling pathways which allow them to mount an innate immune response against invading pathogens that are representative of primary hepatocytes.
However, virus-induced expression of IFNs and ISGs is still reduced when compared with that of primary hepatocytes. One study examined the presence of HBV covalently closed circular DNA (cccDNA), a characteristic of persistent HBV-infection, in HLCs and primary hepatocytes in the presence and absence of an inhibitor of the JAK/STAT pathway (JAKi). While cccDNA in primary hepatocytes was almost exclusively found in cells treated with JAKi, treated and also untreated HLCs harboured cccDNA. Examination of the innate immune response in HLCs revealed that although ISG-expression reflected ISG-expression in primary hepatocytes, gene expression was considerably lower in HLCs, which explains why HBV cccDNA was detectable in HLCs despite an antiviral response of the cells. IL28B, IL29, IRF3, IRF9 ISG15, ISG20 and Mx2 in d20 HLCs were only slightly upregulated compared with HBV-resistant d7 cells [7]. This observation may be representative of an immature immune response in HLCs, although, more studies are needed to better understand this finding.
Studies of chronic viral infection might also benefit from HLCs having immature immune pathways. In HCV-infection for example, only a minority of HCV-positive hepatocytes are found in the in vivo situation (7–20%) and the infected cells seem to be of an immature phenotype (AFP+, EpCAM+) that appear in regenerating areas of the liver. While regenerating progenitor cells represent only a small proportion of cells in the liver, they contain high levels of HCV and it is assumed that they serve as a reservoir for HCV [34,35]. Hence, to understand how hepatotrophic viruses like HCV develop chronic infection, HLCs with reduced PRR-expression and IFN-responses could be critical.
HLCs can also be used to study immune evasion of hepatotrophic viruses. Upon dengue virus (DENV) infection, ISG-expression in infected HLCs was reduced when compared with surrounding uninfected HLCs. This shows that the virus counteracts innate immune responses to evade the immune mechanisms of the infected cells [13].
(c). Intrinsic anti-viral mechanisms
Intrinsic immunity describes destruction of the invading pathogen performed directly by the sensing molecule. Examples of intrinsic immunity are dsRNA-activated protein kinase R (PKR) and ribonuclease L (RNaseL). Although their expression is further upregulated by IFNs, which classifies them as ISGs, they are already highly expressed in uninfected cells and upon infection can become immediately activated. PKR and RNaseL are both detectable in pluripotent stem cells, although PKR has been shown to be unresponsive to polyI:C and responsiveness develops in the course of differentiation [36].
(d). TNF-α pathway
Tumour necrosis factor-alpha (TNF-α) is a key player in liver homeostasis that regulates proliferation, survival and cell death. TNF-α signals through NFκB and C-Jun N-terminal kinase (JNK) and cell signalling is tightly regulated as an imbalance of these pathways results in liver diseases such as cancer development or hepatic failure [37]. TNF-α has been shown to play a prominent role in the pathogenesis of chronic HCV and HBV infection [37].
TNF-α signalling through NFκB promotes cell survival and induces expression of various pro-inflammatory cytokines, while JNK-signalling induces apoptosis [14]. Binding of soluble TNF-α to its receptor TNFR1 on the cell surface induces homotrimer formation of TNFR1. Adaptor protein TNFR-associated death domain (TRADD) is recruited and binds to the cytosolic region of the receptor. Upon receptor binding, TRADD interacts with the downstream signalling molecules TNF-α receptor-associated factor 2 (TRAF2) and receptor-interacting protein kinase (RIP). The complex of TRADD, TRAF2 and RIP can activate both NFκB- and JNK-signalling, leading to either cell survival or death. NFκB is a heterodimer of the two subunits p65 and p50. In the quiescent state, IκB binds to NFκB, thereby inhibiting NFκB and preventing its nuclear localization. TNF-α signalling activates the IκB-kinase (IKK) complex, consisting of the kinase subunits IKKα and IKKβ and the regulatory subunit IKKγ. Phosphorylation of IKKβ activates the complex and induces IκBα degradation via Ser 32 and 36 phosphorylation which marks the protein for ubiquitination on Lys 21 and 22 and subsequent degradation by the proteasome 26S complex [38]. Released from their association with IκB, NFκB heterodimers translocate to the nucleus where they transactivate pro-inflammatory cytokine expression, such as TNF-α, CXCL10, CXCL11 and IL-8 as well as pro-survival genes such as caspase-8 inhibitor c-FLIP and Gadd35β.
HLCs release TNF-α in response to infection and respond to stimulation with recombinant TNF-α. Hence, they represent a valuable opportunity to study TNF-α signalling in infection, proliferation and carcinogenesis. This will also help to better understand the mechanisms that regulate the interplay between TNF-α-induced NFκB- and JNK-signalling and thereby the regulation of cell survival and cell death. Studies in this field had been hampered by the lack of an appropriate cell system. Although hepatoma cell lines such as Huh7 cells greatly contributed to our current understanding of cell cycle regulation, these cell lines represent cancer tissue. Deregulated cell death/survival pathways are the main feature of hepatoma cell lines, making these cells an unreliable model. The high survival capacity of Huh7 cells, for example, is promoted by high basal NFκB levels. Understanding the mechanisms that regulate cell death and survival would be of therapeutic value and could lead to the identification of therapies that prevent cell death in the context of liver injury or suppress carcinogenesis and HLCs could be of great value in these studies. Viral proteins such as DENV NS1 can directly activate NFκB-signalling for TNF-α production in HLCs [13]. DENV infection of hESC- and iPSC-HLC activates NFκB-signalling as determined by phosphorylation of the NFκB subunits p65/p50. As phosphorylation of p65/p50 was not limited to infected cells, NFκB activation does not only seem to be a result of direct virus-sensing but is also induced by the paracrine mechanism of TNF-α released from infected cells. NFκB-activation resulted in the synthesis and release of pro-inflammatory cytokines, such as CXCL10, CXCL11, IL6 and to a smaller extent IL-8 and TNF-α, and downregulation of normal hepatocyte-genes such as albumin, E-cadherin, coagulation factor V and proteins of the complement system such as F5. Significant downregulation of albumin and F5 is also seen in DENV-infected primary hepatocytes [13].
(e). TLR expression and downstream signalling pathways
Toll-like receptors (TLR) are central in pathogen sensing and activation of immune responses. The TLR response in hepatocytes is tightly regulated in vivo which is crucial in an organ that is constantly exposed to various antigens, including pathogens coming from the gut. In this microenvironment, the hepatocyte has to stay alert to invading pathogens and initiate appropriate immune responses, while TLR over-activation is associated with pathogenesis of chronic inflammation and disease. In healthy hepatocytes, mRNA for all TLRs is only marginally expressed but upon exposure to certain stimuli, specific TLRs can be upregulated [39]. Furthermore, TLR-signalling and deregulation influence carcinogenesis. It is, therefore, not surprising that TLR-expression in hepatoma cell lines is modified. TLR3 and 7 appear to be underexpressed in hepatoma cell lines which makes these cells unsuitable for studies of TLR-responses to viruses and might explain why the role of TLRs in hepatocytes is often underestimated [40].
PAMP recognition by a specific TLR induces signalling through NFκB, leading to the expression of numerous host defence genes. Nine functional TLRs are known in humans (TLR1–9). TLR3 (which detects dsRNA), TLR7 (which detects ssRNA) and TLR9 (which detects non-methylated DNA CpG-motifs) are located within the endosomes of hepatocytes, while TLR2, TLR4 and TLR5 are localized on the cell membrane. Binding of a ligand to its receptor triggers signalling via MyD88 for TLR2, TLR4, TLR5, TLR7/8 and TLR9 and TRIF for TLR3. MyD88 is anchored to the cytoplasmic TIR-domain of the TLR by its own TIR-domain. Activated MyD88 recruits interleukin 1 receptor-associated kinase (IRAK), leading to IRAK auto-phosphorylation. Phosphorylated IRAK detaches from MyD88 and activates TRAF6 which then activates the IKK-complex resulting in NFκB translocation to the nucleus (see TNF-signalling) and expression of pro-inflammatory cytokines such as TNF-α, CXCL10, CXCL11 and IL-8 [41]. While in professional immune cells such as plasmacytoid dentritic cells, TLR7/8 and TLR9 also signal via IRF7, leading to IFN-α and IL29 expression; data as to whether hepatocytes possess the IRF7 arm of TLR-signalling are conflicting. A study in HepG2 cells reported absence of IRF7 signalling upon TLR7 stimulation [42]. However, as mentioned before, hepatoma cell lines are of limited value in the study of TLR-responses. HLCs would be of great benefit if expression of TLR-proteins and components of the signalling pathways are expressed at the end of the differentiation procedure.
TLR-expression by pluripotent stem cell-derived somatic cells has been studied but, with the exception of Sakurai and colleagues who detected TLR3 mRNA expression in iPSC-derived hepatocytes, none of these examined HLCs [6]. Földes and colleagues investigated responsiveness of hESC, hESC-derived endothelial cells (hESC-EC) and primary human endothelial cells to ligands for TLR1–9 [43]. In contrast to primary cells, neither hESCs nor hESC-ECs responded to TLR-agonists, with the exception of TLR5 which was already expressed in hESC. This lack was attributed to the absence of TLR-protein expression as stimulants that likewise signal through NFκB but act through different receptors (TNF-α, IL-1β, IFN-γ) induced IL-8 expression in all three cell lines. qPCR-analysis revealed very low expression of TLR mRNA in hESC-ECs and especially hESCs. Amounts of NFκB protein were comparable between hESCs, hES-ECs and primary endothelial cells and increased only marginally in the course of differentiation. NFκB-signalling could be induced upon TNF-α, IL-1β and IFN-γ stimulation. This finding shows that hESC-derived ECs do not express TLRs although inflammatory signalling pathways are present and functional in hESCs and hESC-ECs. Markedly, in vivo conditioning of hESC-ECs by injection into nude mice for 21 days did not establish TLR-expression [44].
By contrast, two studies on hESC-derived fibroblasts and keratinocytes reported expression of TLR2 and 4 and responsiveness to Gram-negative bacteria [45,46]. However, both TLRs were already expressed in hESC and responsiveness to bacterial challenge in hESC-fibroblasts appeared to be less specific compared with primary cells [45].
The discrepancies among published data emphasize the importance of studying TLR-expression in stem cell-derived somatic cells more closely. Inducibility of TLR-expression and signalling in HLCs is of particular interest as tightly regulated hepatic TLR-responses in vivo are critical to maintain the balance between immune activation and tolerance in the liver. In primary hepatocytes, virus-induced downregulation of TLR7 has been reported, further emphasizing the need to study TLR-responses in HLCs [47].
We are currently examining the expression and inducibility of endosomal TLRs in HLCs from different backgrounds. As TLR responses develop late in the embryo and are again repressed after birth, deriving hepatocytes with functional TLR-responses from hESCs can be challenging. iPSCs might be of advantage here, considering their somatic cell origin. Indeed, the expression and responsiveness of TLR2 and 4 have been detected in iPSC-derived endothelial cells, while the absence of TLR-expression in hESC-ECs was confirmed in this study [44].
(f). Acute phase protein production
The acute phase response describes a systemic immune response against sepsis where hepatocytes secrete defensive proteins into the circulation. The acute phase is initiated by inflammatory cytokines, including IL-1β, TNF-α and IL-6, which become upregulated upon inflammation. IL-6 induces secretion of C-reactive protein, α1-antichymotrypsin, serum amyloid A and fibrinogen, while secretion of albumin, transferrin and fibronectin becomes downregulated.
However, APP production by pluripotent stem cell-derived hepatocytes has not been studied extensively. Irudayam and colleagues analysed gene expression of acute-phase proteins during hepatic differentiation of hESCs and found a 10- to 1000-fold upregulation of genes coding for acute phase proteins, coagulation factors and several members of the acute phase response, such as serum amyloid A1, α1-antitrypsin and α2-macroglobulin in HLCs, compared with definitive endoderm [9]. However, only basal expression of acute phase proteins was examined and the results were not compared with that of primary hepatocytes. LPS- or IL6-induction of the acute phase response in HLCs has not been tested. It is, therefore, difficult to determine whether the acute phase response seen in HLCs is fully representative of the in vivo situation meriting further investigation.
3. Conclusion
In vitro differentiated cells, such as HLCs, endothelial cells, cardiomyocytes, smooth muscle cells and osteoblasts, differ from their in vivo counterparts in numerous ways. This includes the innate immune response. This immature phenotype is characterized by reduced levels of ISG-expression upon infection or stimulation with TLR-ligands. Production of IFNs upon infection and ISG-expression are the major innate immune mechanisms in hepatocytes. These pathways have been shown to be expressed in HLCs. However, other pathways also play important roles in the clearance of infection. Studies on HBV-infection of primary hepatocytes from different donors reported huge variation in virus susceptibility and in some cases even suppression of the IFN-based immune responses could not reconstitute virus replication [7]. This shows that other pathways of the innate immune response play a role in susceptibility to disease, highlighting that IFN pathways in HLCs alone are not sufficient to study inter-individual differences in virus susceptibility. This finding also demonstrates that it is necessary to improve differentiation protocols to deliver intact innate immune pathways in HLCs, to allow further mechanistic studies. One strategy to improve maturation of innate immune pathways could be the addition of immunostimulatory cytokines to the differentiation medium as is standard for the differentiation of immune cells such as natural killer cells and dentritic cells. Innate immune pathways in these cells are more developed than in tissue cells.
The lack of certain pathways of the innate immune response such as TLR-signalling might have important implications for tissue regeneration, drug-testing and host–pathogen studies. For studies of host–pathogen interaction, mature immune signalling will be essential, while for regenerative medicine, immature immune signalling can be beneficial in certain cases, including transplant acceptance. Pathogens are generally detected by more than one sensing molecule; for example TLR4 and the cytoplasmic receptor NOD-I both sense Gram-negative bacteria. In contrast to NOD-I, TLR4 is also associated with vascular inflammation in endothelial cells and hESC-ECs that do not express TLR4 sense Gram-negative bacteria by NOD-I and mount comparable levels of antiviral cytokines. hESC-ECs that lack TLR-responses might, therefore, be a promising target for transplantation into patients with atherosclerosis [44]. The same would apply to situations where tissue or organ damage is a result of excessive immune responses.
In conclusion, pluripotent stem cell-derived HLCs are superior to hepatoma cell lines for mimicking hepatic innate immunity. However, innate immunity is compromised in HLCs when compared with primary hepatocytes (figure 2), necessitating future improvements in cellular differentiation protocols from PSCs.
Data accessibility
This article has no additional data.
Competing Interests
Prof. D.C.H. is a founder, director and shareholder in Stemnovate Limited.
Funding
Prof. D.C.H. was supported by an award from the Chief Scientist's Office, grant reference CSO TCS/16/37. Prof. C.O.F. was supported by an SFI Investigator Award, grant reference 12/IA/1667.
References
- 1.Takahashi K, Yamanaka S. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. ( 10.1016/j.cell.2006.07.024) [DOI] [PubMed] [Google Scholar]
- 2.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. 1998. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147. ( 10.1126/science.282.5391.1145) [DOI] [PubMed] [Google Scholar]
- 3.Zhou X, Sun P, Lucendo-Villarin B, Angus AGN, Szkolnicka D, Cameron K, Farnworth S, Patel AH, Hay DC. 2014. Modulating innate immunity improves hepatitis C virus infection and replication in stem cell-derived hepatocytes. Stem Cell Rep. 3, 204–214. ( 10.1016/j.stemcr.2014.04.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu X, Robotham JM, Lee E, Dalton S, Kneteman NM, Gilbert DM, Tang H. 2012. Productive hepatitis C virus infection of stem cell-derived hepatocytes reveals a critical transition to viral permissiveness during differentiation. PLoS Pathog. 8, e1002617 ( 10.1371/journal.ppat.1002617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pare JM, Sullivan CS. 2014. Distinct antiviral responses in pluripotent versus differentiated cells. PLoS Pathog. 10, e1003865 ( 10.1371/journal.ppat.1003865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakurai F, Kunito T, Takayama K, Hashimoto R, Tachibana M, Sakamoto N, Wakita T, Mizuguchi H. 2017. Hepatitis C virus-induced innate immune responses in human iPS cell derived hepatocyte-like cells. Virus Res. 242, 7–15. ( 10.1016/j.virusres.2017.09.004) [DOI] [PubMed] [Google Scholar]
- 7.Sholmai A, Schwartz RE, Ramanan V, Bhatta A, de Jong YP, Bhatia SN, Rice CM. 2014. Modeling host interactions with hepatitis B virus using primary and induced pluripotent stem cell-derived hepatocellular systems. Proc. Natl Acad. Sci. USA 111, 12 193–12 198. ( 10.1073/pnas.1412631111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaneko S, et al. 2016. Human induced pluripotent stem cell-derived hepatic cell lines as a new model for host interaction with hepatitis B virus. Nat. Sci. Rep. 6, 29358 ( 10.1038/srep29358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irudayam JI, et al. 2015. Profile of inflammation-associated genes during hepatic differentiation of human pluripotent stem cells. Data Brief. 5, 871–878. ( 10.1016/j.dib.2015.10.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irudayam JI, et al. 2015. Characterisation of type I interferon pathway during hepatic differentiation of human pluripotent stem cells and hepatitis C virus infection. Stem Cell Res. 15, 354–364. ( 10.1016/j.scr.2015.08.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan F, et al. 2017. Human embryonic stem cell-derived hepatoblasts are an optimal lineage stage for hepatitis C virus infection . Hepatology 66, 717–735. ( 10.1002/hep.29134) [DOI] [PubMed] [Google Scholar]
- 12.Helsen N, et al. 2016. Stem cell-derived hepatocytes: A novel model for hepatitis E virus replication. J. Hepatol. 64, 565–573. ( 10.1016/j.jhep.2015.11.013) [DOI] [PubMed] [Google Scholar]
- 13.Lang J, Vera D, Cheng Y, Tang H. 2016. Modeling dengue virus-hepatic cell interactions using human pluripotent stem cell-derived hepatocyte-like cells. Stem Cell Rep. 7, 341–354. ( 10.1016/j.stemcr.2016.07.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papa S, Bubici C, Zazzeroni F, Franzoso G. 2009. Mechanisms of liver disease: cross-talk between NF-κB and JNK pathways. Biol. Chem. 390, 965–976. ( 10.1515/BC.2009.111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sumpter R, Loo Y-M, Foy E, Li K, Yoneyama M, Fujita T, Lemon SM, Gale M. 2005. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 79, 2689–2699. ( 10.1128/JVI.79.5.2689-2699.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li K, Chen Z, Kato N, Gale M, Lemon SM. 2005. Distinct poly(I-C) and virus-activated signaling pathways leading to interferon-β production in hepatocytes. J. Biol. Chem. 280, 16 739–16 747. ( 10.1074/jbc.M414139200) [DOI] [PubMed] [Google Scholar]
- 17.Lucendo-Villarin B, et al. 2017. Modelling foetal exposure to maternal smoking using hepatoblasts from pluripotent stem cells. Arch. Toxicol. 91, 3633–3643. ( 10.1007/s00204-017-1983-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horner SM, Gale M Jr. 2013. Regulation of hepatic innate immunity by hepatitis C virus. Nat. Med. 19, 879–888. ( 10.1038/nm.3253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourke NM, O'Neill MT, Sarwar S, Norris S, Stewart S, Hegarty JE, Stevenson NJ, O'Farrelly C. 2014. In vitro blood cell responsiveness to IFN-α predicts clinical response independently of IL28B in hepatitis C virus genotype 1 infected patients. J. Transl. Med. 12, 206 ( 10.1186/1479-5876-12-206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer J, et al. 2017. Sex-specific effects of TLR9 promoter variants on spontaneous clearance of HCV infection. Gut 66, 1829–1837. ( 10.1136/gutjnl-2015-310239) [DOI] [PubMed] [Google Scholar]
- 21.Dring MM, et al. 2011. Innate immune genes synergize to predict increased risk of chronic disease in hepatitis C virus infection. Proc. Natl Acad. Sci. USA 108, 5736–5741. ( 10.1073/pnas.1016358108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funk E, Kottili S, Gilliam B, Talwani R. 2014. Tickling the TLR7 to cure viral hepatitis. J. Transl. Med. 12, 129 ( 10.1186/1479-5876-12-129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamoto N, Kanai T. 2014. Role of Toll-like receptors in immune activation and tolerance in the liver. Front. Immunol. 5, 1–8. ( 10.3389/fimmu.2014.00221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong XX, Carmichael GG. 2013. Innate immunity in pluripotent human cells: attenuated response to interferon-β . J. Biol. Chem. 288, 16 196–16 205. ( 10.1074/jbc.M112.435461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen LL, Yang L, Carmichael GG. 2010. Molecular basis for an attenuated cytoplasmic dsRNA response in human embryonic stem cells. Cell Cycle 9, 3552–3564. ( 10.4161/cc.9.17.12792) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godoy P, et al. 2015. Gene networks and transcription factor motifs defining the differentiation of stem cells into hepatocyte-like cells. J. Hepatol. 63, 934–942. ( 10.1016/j.jhep.2015.05.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cameron K, et al. 2015. Recombinant laminins drive the differentiation and self-organization of hESC-derived hepatocytes. Stem Cell Rep. 5, 1250–1262. ( 10.1016/j.stemcr.2015.10.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Q, Wang Q, Scott MJ, Billiar TR. 2016. Immune activation in the liver by nucleic acids. J. Clin. Transl. Hepatol. 4, 151–157. ( 10.14218/JCTH.2016.00003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gürtler C, Bowie AG. 2014. Innate immune detection of microbial nucleic acids. Trends Microbiol. 21, 413–420. ( 10.1016/j.tim.2013.04.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun L, Wu J, Du F, Chen X, Chen ZJ. 2013. Cyclic GMP-AMP synthase is a cytosolic DANN sensor that activates the type I interferon pathway. Science 15339, 786–791. ( 10.1126/science.1232458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park H, Serti E, Eke O, Muchmore B, Prokunina-Olsson L, Capone S, Folgori A, Rehermann B. 2012. IL-29 is the dominant type III interferon produced by hepatocytes during acute hepatitis C virus infection . Hepatology 56, 2060–2070. ( 10.1002/hep.25897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ge D, et al. 2009. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance . Nature 461, 399–401. ( 10.1038/nature08309) [DOI] [PubMed] [Google Scholar]
- 33.Wack A, Terczynska-Dyla E, Hartman R. 2015. Guarding the frontiers: the biology of type III interferons . Nat. Immunol. 16, 802–809. ( 10.1038/ni.3212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang Y, Shilagard T, Xiao SY, Snyder N, Lau D, Cicalese L, Weiss H, Vargas G, Lemon SM. 2009. Visualizing hepatitis C virus infections in human liver by two-photon microscopy. Gastroenterology 137, 1448–1458. ( 10.1053/j.gastro.2009.07.050) [DOI] [PubMed] [Google Scholar]
- 35.Franchitto A, Onori P, Renzi A, Carpino G, Mancinelli R, Alvaro D, Gaudio E. 2013. Expression of vascular endothelial growth factors and their receptors by hepatic progenitor cells in human liver diseases. Hepatobiliary Surg. Nut. 2, 68–77. ( 10.3978/j.issn.2304-3881.2012.10.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo Y-L. 2017. Utilization of different anti-viral mechanisms by mammalian embryonic stem cells and differentiated cells. Immul. Cell Biol. 95, 17–23. ( 10.1038/icb.2016.70) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun B, Karin M. 2008. NF-κB signalling, liver disease and hepatoprotective agents. Oncogene 25, 6228–6244. ( 10.1038/onc.2008.300) [DOI] [PubMed] [Google Scholar]
- 38.Hay DC, Kemp GD, Dargemont C, Hay RT. 2001. Interaction between hnRNPA1 and IκBα is required for maximal activation of NF-κB-dependent transcription. Mol. Cell. Biol. 21, 3482–3490. ( 10.1128/MCB.21.10.3482-3490.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seki E, Brennder D. 2008. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology 48, 322–335. ( 10.1002/hep.22306) [DOI] [PubMed] [Google Scholar]
- 40.Crispe IN. 2015. Hepatocytes as immunological agents. J. Immunol. 196, 17–21. ( 10.4049/jimmunol.1501668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imler J-L, Hoffmann JA. 2001. Toll receptors in innate immunity. Trends Cell Biol. 11, 304–311. ( 10.1016/S0962-8924(01)02004-9) [DOI] [PubMed] [Google Scholar]
- 42.Lee J, Hayashi M, Lo J-F, Fearns C, Chu W-M, Luo Y, Xiang R, Chuang T-H. 2009. Nuclear factor κB (NF-κB) activation primes cells to a pro-inflammatory polarized response to a Toll-like receptor 7 (TLR7) agonist. Biochem. 421, 301–310. ( 10.1042/BJ20090013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Földes G, et al. 2010. Innate immunity in human embryonic stem cells: comparison with adult human endothelial cells. PLoS ONE 5, e10501 ( 10.1371/journal.pone.0010501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reed DM, et al. 2014. Pathogen sensing pathways in human embryonic stem cell-derived endothelial cells: Role of NOD1 receptors. PLoS ONE. 9, e91119 ( 10.1371/journal.pone.0091119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sriram G, Natu VP, Islam I, Fu X, Seneviratne CJ, Tan KS, Cao T. 2016. Innate immune response of human embryonic stem cell-derived fibroblasts and mesenchymal stem cells to periodontopathogens. Stem Cells Int. 2016, 8905365 ( 10.1155/2016/8905365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kidwai FK, Jokhum CS, Movahednia MM, Yeo JF, Tan KS, Cao T. 2013. Human embryonic stem cells derived keratinocyte as an in vitro research model for the study of immune response. Oral Pathol. Med. 42, 627–634. ( 10.1111/jop.12054) [DOI] [PubMed] [Google Scholar]
- 47.Chang S, Kodys K, Szabo G. 2009. Impaired expression and function of toll-like receptor7 in hepatitis C virus infection in human hepatoma cells. Hepatology 51, 35–42. ( 10.1002/hep.23256) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.