Abstract
The capacity to culture stem cells in a controllable, robust and scalable manner is necessary in order to develop successful strategies for the generation of cellular and tissue platforms for drug screening, toxicity testing, tissue engineering and regenerative medicine. Creating substrates that support the expansion, maintenance or directional differentiation of stem cells would greatly aid these efforts. Optimally, the substrates used should be chemically defined and synthetically scalable, allowing growth under defined, serum-free culture conditions. To achieve this, the chemical and physical attributes of the substrates should mimic the natural tissue environment and allow control of their biological properties. Herein, recent advances in the development of materials to study/manipulate stem cells, both in vitro and in vivo, are described with a focus on the novelty of the substrates’ properties, and on application of substrates to direct stem cells.
This article is part of the theme issue ‘Designer human tissue: coming to a lab near you’.
Keywords: biomaterials, stem cells, substrates, peptides, high-throughput
1. Introduction
Stem cells offer great opportunities as tools for screening in drug discovery, as surrogates for primary hepatocytes for toxicity assays as well as for potential use in regenerative medicine owing to their differentiation capacity [1]. To realize these potentials, however, it is necessary to develop defined, scalable and reproducible systems for the in vitro expansion and controlled differentiation of pluripotent stem cells (PSCs).
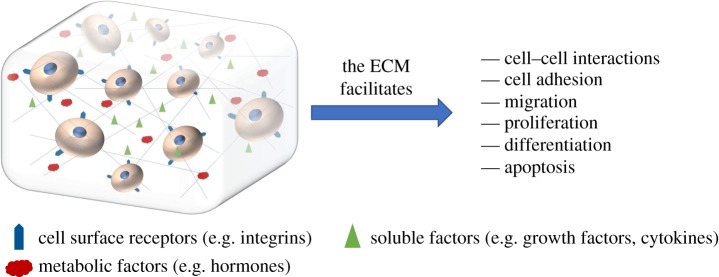
In vivo, cells reside within and are part of a complex and dynamic three-dimensional (3D) environment—the so-called extracellular matrix (ECM) (figure 1). This is composed of many components, including numerous proteins, peptides (including growth factors), polysaccharides and proteoglycans. The ECM not only provides cells with structural cues but also facilitates cell–cell interactions and cell adhesion, and allows cell migration. Furthermore, the soluble factors within the ECM, such a growth factors and cytokines, can also control cell proliferation, differentiation and apoptosis [2,3].
Figure 1.
Representation of the ECM and its functions, which in combination with cell signalling direct stem cell fate.
Creating an artificial ECM, for example via the use of feeder layers (e.g. MEFs) or by applying ECM-mimicking substrates (e.g. Matrigel®), for in vitro stem cell culture has seen great developments over the past decades, but the use of animal-derived materials imposes possible immunogenic responses and innate batch-to-batch variability that could curtail clinical use [4]. Therefore, the development of defined, synthetic materials that can support stem cell renewal, maintenance and differentiation is of key importance. Furthermore, the production of phenotypically stable stem cells which potentially could be achieved by mimicking the stem cell niche using synthetic substrates, is of great importance [5].
Conventional 2D cell culture, although facile, is limited by its capacity to mimic the natural 3D environment, and culture in 2D can result in altered gene expression, changes in cell metabolism, signalling and morphology compared with cells grown in 3D [6]. It should also be considered that in vivo stem cells are subjected to multiple biophysical, chemical and mechanical cues that help direct cell fate [1,7,8] and it is important to not only understand these cues but also apply them to the material being designed.
Cell surface receptors, such as integrins, are critical in cell–matrix interactions. The tripeptide sequence RGD, found in the ECM protein fibronectin, binds to integrins [9] and has therefore become a common addition to materials to facilitate cell attachment. Other ECM-derived peptide sequences based on, for example laminins and collagen I [10,11], have also been used to enhance cell attachment. In addition, the biocompatibility of synthetic substrates can be promoted by the incorporation of other biologically relevant moieties, including proteins and growth factors [12]. Degradation of the material might also be necessary to allow for cell propagation/movement, and this can be achieved by the use of protease-responsive materials that can be broken down by enzymes secreted by the cells, thus allowing migration within the material [13].
Other factors to consider are the mechanical properties of materials, because in vivo the ECM composition and mechanical properties vary and this plays a role in controlling tissue development [14–16]. For example, the ECM of tendon consists largely of collagen I [17], resulting in a high elastic modulus; by contrast, the ECM of the inner eye is mainly composed of hyaluronic acid and consequently has a low elastic modulus [18]. Thus, by controlling the mechanical properties of synthetic materials, a certain level of cell response can be afforded.
The interaction between the ECM and integrins occurs at the nanoscale and the surface- and nanotopography of a substrate can also be used to modulate cell response [19]. Substrate alone, however, is not enough to fully mimic the complex in vivo environment. More complex aspects to materials are needed, such as the introduction of gradients (e.g. oxygen, growth factors) [20] and substrate vascularization [21,22].
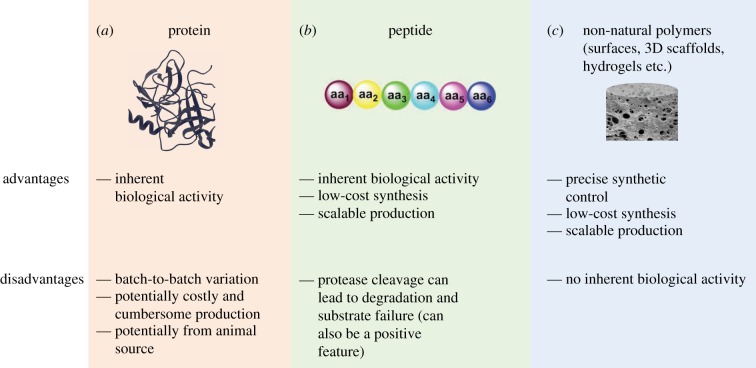
This review will focus on advances over the past 5 years in three classes of materials that have been developed for application within the area of stem cell biology, namely protein, peptide and synthetic polymer-based substrates (figure 2 and table 1), with specific regard to substrate composition, properties and fabrication, as well as their advancements over previously reported materials.
Figure 2.
The substrates covered in this review for stem cell growth and regulation and some of their pros and cons. (Online version in colour.)
Table 1.
Recent advances in material development for stem cell applications.
| material source | stem cell application |
|---|---|
| protein | |
| albumin [23] | MSC osteogenesis |
| alginate [24] | hPSC switch from self-renewal to differentiation |
| collagen [25] | MSC chondrogenesis |
| collagen nanovibrational bioreactor [26] | MSC osteogenesis |
| collagen-RGD [27] | MSC articular cartilage formation |
| elastin with citric acid [28] | MSC osteogenesis |
| ELP, ELP-HA [29,30] | ADSC, MSC |
| fibrin-IKVAV [31] | neural SC differentiation |
| fibronectin-Au [32] | MSC endothelialization |
| heparin [33] | hPSC expansion |
| hyaluronic acid [34] | MSC chondrogenesis |
| laminin 521 and laminin 111 [35] | hESC to hepatocyte |
| recombinant protein hydrogels [36–39] | hiPSC-EC, ADSC, |
| peptide | |
| Au-SAM with RGD [40] | MSC differentiation capacity |
| EAK [41] | MSC proliferation |
| Fmoc-F2/S [42] | pericyte chondrogenesis |
| RADA-PRG (modified PuraMatrix®) [43,44] | mouse neonatal epidermal cells, rMSC neurogenesis |
| RGD on decellularized pig heart valves [45] | EPC proliferation |
| Synthemax® [46] | iPSC expansion and differentiation |
| polymer | |
| enzymatically responsive PEG [47–52] | iPSC, hESC, mESC, MSC, organoids, mouse pancreatic progenitor |
| gellan gum [53] | hPSC 3D spheres |
| methylcellulose [54] | MSC chondrogenesis |
| nanotopographically imprinted PCL [55,56] | MSC maintenance and osteogenesis |
| NO-releasing chitosan [57] | hP-MSC angiogenic potential |
| P(PEGMEMA-r-GMA-r-VDM) [58] | MSC proliferation |
| PEG with RGD and MMP tethering [59,60] | MSC migration |
| photodegradable PEG [61,62] | MSC ‘mechanical memory’ |
| polyacrylate/acrylamide thermoresponsive hydrogel [63–65] | hESC, MSC, mESC |
| polyacrylate/polyurethane [66–68] | hESC, MSC, C6 rat GSC |
| polypyrrole [69] | MSC osteogenesis |
| polystyrene TopoChip [70] | iPSC proliferation |
| polyurethane [71–74] | hESC-derived HE, HPC, iPSC-derived hepatocytes, H9 |
| ternary polymer blends [75,76] | STRO-1+ skeletal SC, fetal skeletal SC |
2. Protein-based substrates for stem cell control
Protein-based substrates (figure 2a) inherently present multiple cellular signalling molecules that can promote and control cell growth and may result in fewer adverse cell reactions compared with fully synthetic substrates. A fully defined protocol for the differentiation of human embryonic stem cells (hESCs) into hepatocytes was reported using recombinant laminin-coated surfaces [35]. hESCs were plated on either pure laminin 521 (lam521) or on a mixture of lam111 and lam521 (referred to as lam111) and differentiated to hepatocytes using an 11-day, serum-free protocol [77,78]. Both culture substrates produced similar numbers of hepatocytes, but metabolic activity of CYP3A and CYP1A2 was increased on both lam521 and lam111 compared with Matrigel®. Hepatocytes derived on lam111 and lam521 had more organized networks than on Matrigel® and cell phenotype resembling more that of adult hepatocytes, which also was confirmed by whole-genome analysis.
Two recombinant proteins, dubbed C7 and P9, a seven-repeat unit of the CC43 WW domain and a nine-repeat unit of a proline-rich peptide, respectively, generate hydrogels as a mixture through specific recognition-binding and have been used in several stem cell applications [36–39]. The hydrogels were able to encapsulate adipose-derived stem cells (ADSCs) that showed good cell viability in the gels [36]. ADSC-loaded hydrogel injection into nude mice resulted in good cell retention and ECM deposition at the injection site 14 days post-transplantion, in contrast with cells injected without gel encapsulation. In a further study, an eight-arm star-shaped polyethylene glycol (PEG) was conjugated to one repeat unit of the P9 protein (P1). Hydrogels were formed by cross-linking the PEG-P1 conjugate to the C7 protein followed by encapsulation of human-induced pluripotent endothelial stem cells (hiPSC-ECs) and injection into mice [37]. The gels, in combination with vascular endothelial growth factor (VEGF) encapsulation, showed reduced inflammation and demonstrated muscle tissue regeneration. In another study, poly(N-isopropylacrylamide) (PNIPAM) was conjugated to the PEG-P1 unit, creating an eight-arm PEG-PNIPAM-P1 component with seven arms conjugated to P1 and one arm to PNIPAM. This component was cross-linked with C7 to form a double-network hydrogel due to the cross-linking between P1 and C7 and the thermal phase transition of PNIPAM [38]. The enhanced mechanical properties of the double network gave higher cell retention at the injection site compared with single hydrogel networks.
Endothelialization of human mesenchymal stem cells (hMSCs) was observed on microcomposites (FN-Au) made from fibronectin (FN)-modified gold nanoparticles (AuNPs) [32]. Varying the amount of AuNPs in FN-Au composites resulted in different hMSC proliferation rates. Moreover, expression of MMP9 allowed cell migration, while the endothelial marker CD31 was higher in hMSCs grown on FN-Au compared with just FN or tissue culture polystyrene (TCPS). The FN-Au was, therefore, proposed as an alternative coating to increase the biocompatibility of medical devices that encounter blood, e.g. vascular grafts.
A recent advance in mechanotransduction-induced MSC osteogenesis was demonstrated using an in-house-built six-well-plate nanovibrational bioreactor that allowed nanoscale displacements (1000 Hz) of piezoceramics [26]. MSCs were cultured in well plates coated with a collagen gel that had an elastic modulus a magnitude lower than normally needed for in vitro osteogenesis. When applying nanovibrations, MSC osteogenesis was observed without the need for osteospecific media. Quantitative polymerase chain reaction (qPCR) analysis of cells cultured within the nanostimulated gels showed increased levels of several osteogenic markers, including Runt-related transcription factor 2 (RUNX2), collagen I and osteocalcin, compared with non-vibrated control cultures.
3. Peptide-based substrates—adaptable materials for cellular modulation
Rather than using full-length proteins, the use of short peptide fragments (figure 2b) that support stem cell growth is an attractive alternative owing to their scalability, their ease of GMP production, and synthetic control and affordability. They are also biologically relevant and potentially can provide cells with the cues they need for attachment, signalling, migration, proliferation and differentiation.
Hair follicle regeneration was achieved by encapsulation of stem cells within a self-assembling peptide [43]. The peptide Ac-(RADA)4-NH2 (marketed as PuraMatrix®) self-assembles into nanofibres that subsequently give rise to hydrogels. PuraMatrix® was coupled to an RGD sequence, thereby enhancing cell attachment. Mouse neonatal epidermal cells and tissue-derived multipotent skin-derived precursors were mixed into the hydrogel and implanted into nude mice to promote hair follicle regeneration. After three weeks, hair growth was abundant and densely populated hair follicles were observed with higher hair growth compared with cell encapsulation in Matrigel®. Another study with RGD-functionalized PuraMatrix® demonstrated neuronal differentiation of human bone marrow MSCs (rMSC) [44]. Human brain-derived neurotrophic factor, known to induce neuron differentiation, was engineered into the rMSCs. Cell growth, proliferation and levels of neuron-specific elastase and glial fibrillary acidic protein were increased in rMSCs encapsulated in RGD-functionalized PuraMatrix® compared with non-modified hydrogels.
An Fmoc-protected tripeptide hydrogel (Fmoc-F2/S) was developed and studied for the effect on cell phenotype in chondrogenesis [42]. qPCR indicated high levels of chondrogenic markers RUNX2, SOX9 and type II collagen in human adipose-derived pericytes encapsulated in the hydrogels for 7 days, while immunostaining after 28 days showed production of chondrogenic proteins collagen II and aggrecan within the hydrogels. Pericytes were then encapsulated into the hydrogels for 35 days and cultured with and without the presence of chondrogenic induction media. Although the metabolic profile of cells encapsulated within the two sets of hydrogels was broadly similar, some subtle changes were observed, which could account for the resulting difference in cell phenotype. Thus, it is clear that care must be taken in choosing materials and peptides used for stem cell differentiation to control the desired cell phenotype. It should also be noted that the Fmoc group has inherent lability to basic pH, which could perhaps be used to enable cell harvesting, but might also limit longer-term cell culture.
In another study, a peptide hydrogel based on the self-assembling sequence EAK was investigated for hMSC proliferation capacity. The EAK sequence was conjugated to three different molecules [41]: (i) a 25-amino acid peptide for cell adhesion based on repeating RGD units ((GRGDSP)4-K); (ii) an h-vitronectin-based peptide (FRHRNRKGK-NH2); (iii) the protein insulin-like growth factor 1 (IGF-1). The N-termini of the RGD unit, h-vitronectin and IGF-1 were converted to aldehydes, then conjugated to a hydroxylamine-bearing EAK peptide, affording oxime cross-linked hydrogels. hMSC adhesion and proliferation were assessed after gel encapsulation (8 days) and it was shown that these modified EAK hydrogels increased cell adhesion, proliferation, spreading and elongation compared with the non-conjugated gels.
Peptides have also been incorporated into decellularized tissue scaffolds to improve cell attachment and proliferation. Pig heart valves were decellularized and covalently coupled to an RGD peptide and/or VEGF via a PEG spacer [45]. Endothelial progenitor cells (EPCs) were seeded onto the valves and cell adhesion examined. After 8 days of culture, the valves conjugated with both RGD and VEGF demonstrated the highest cell numbers and proliferation rates as determined by a thymidine incorporation assay and qPCR.
4. Synthetic polymer-based substrates for supporting stem cell growth
The use of fully synthetic polymeric materials (figure 2c) can offer many advantages because exact control of composition and properties are afforded, while starting materials tend to be inexpensive, making synthesis robust, scalable and affordable. A drawback is the lack of inherent biological activity of synthetic polymers, but by decorating the polymer chains with bioactive molecules such as peptides and growth factors, or even incorporating biologically relevant functional groups, this can be overcome.
Hydrogels with switchable stiffness have been developed by inclusion of a photodegradable unit into PEG hydrogels [61]. Upon irradiation (365 nm), the hydrogel's elastic modulus changes from 10 to 2 kPa. hMSCs were initially cultured on very stiff TCPS (3 GPa) or stiff PEG hydrogels (10 kPa), before changing to culturing on softer 2 kPa hydrogels. The activation levels of transcription co-activators YAP/TAZ (regulates cell behaviour in response to mechanical stimulus) and osteogenic marker RUNX2 were dependent on the initial culturing time on the stiffer substrates. This demonstrated that the initial stiff culture conditions had a role in dictating subsequent stem cell response, even after moving to softer substrates, thus indicating stem cells possessed a so-called ‘mechanical memory’.
In a further study of how material mechanical properties affect cell response, spatially controlled PEG hydrogels were created by precisely controlling photodegradation of a stiff (9.6 kPa) hydrogel [62]. This gave regularly patterned hydrogels with mechanically softer and stiffer regions. When hMSCs were grown on hydrogels with higher levels of stiffer regions, greater cell spreading and YAP activation were observed along with increased levels of the osteogenic marker alkaline phosphatase (ALP). Changing from a regular to randomly patterned hydrogel, however, resulted in a more rounded cellular morphology and lower YAP activation, while ALP levels decreased and expression of the stem cell marker CD105 increased, indicating lower levels of differentiation. Thus, this gives clues to how spatially controlling the mechanical properties of biomaterials can aid in directing stem cell fate.
A chitosan-bearing polymer with nitric oxide (NO)-releasing capacity was developed to increase the angiogenic potential of MSC-derived exosomes [57,79]. The polymer contained a β-galactose caged NO donor that in response to β-galactosidase released NO. When human placenta-derived MSCs (hP-MSCs) and human umbilical vein endothelial cells (HUVECs) were co-incubated with the NO-releasing polymer, the angiogenesis of HUVECs increased as measured by the tube formation assay. Furthermore, an ischaemic murine model showed increased VEGF and miR-126 expression in exosomes released from the hP-MSCs by NO stimulus. This was proposed as a novel mechanism for increased angiogenesis, demonstrating the potential of small molecule-releasing biomaterials in stem cell applications.
A scalable alternative to adhesion culture for stem cells was achieved by addition of a gellan gum polymer to mTeSR1 media at low concentrations, which inhibited sedimentation of 3D spheres of hPSCs (without gellan gum the spheres sedimented) [53]. The suspension culture was scaled up into gas-permeable membrane bags giving the equivalent number of cells as would have been obtained from 17 100 mm dishes.
The capacity of substrate nanotopography to direct stem cell fate has been demonstrated using a polycaprolactone (PCL), imprinted with the surface topography of the marine shell component nacre [55]. Nacre is known to induce bone formation in vertebrates, and the mimicking of its nanotopography on PCL was sufficient to induce MSC osteogenesis in comparison with planar PCL scaffolds.
High-throughput screening of materials is important for the efficient identification of an appropriate substrate for a specific cellular application. The Bradley group, along with others, have been instrumental in the development of polymer microarray technology to rapidly identify polymer biomaterials [80–83]. Pioneered by Bradley, ink-jet printing has allowed the synthesis of 100–1000s of cross-linked polymers on a single glass slide (figure 3). The synthesis and screening of some 600 thermally responsive polyacrylate and polyacrylamide-based polymers identified a family of polymers, based on the monomers 2-(acryloyloxyethyl) trimethylammonium chloride (AEtMA-Cl) and 2-(diethylamino)ethyl acrylate (DEAEA) in varying ratios, that supported the long-term growth of hESCs (more than 30 passages) and maintenance of the pluripotency markers Oct3/4, Nanog and Sox2 [63]. Importantly, thermal detachment of the hESCs was possible by lowering the temperature to 15°C, enabling passaging without the need of enzymes or chemicals. A similar high-throughput approach identified polymers that supported the long-term culture of primary hMSCs [64] and of mouse embryonic stem cells (mESCs) [65].
Figure 3.
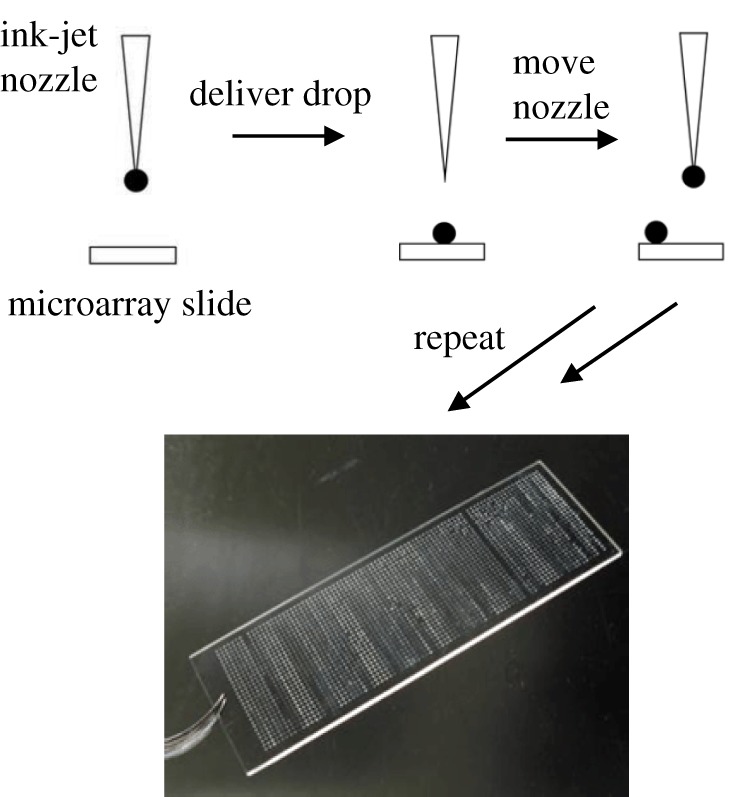
Principle of ink-jet printing for the formation of polymer microarrays.
Another screen of some 7000 different polyacrylates (using ink-jet printing) enabled the identification of a defined substrate able to support the growth and maintenance of hESCs [66]. Hit polymers were scaled up for 35-day incubation and multiple passaging of RH1 cells. Nanog and Oct4 staining showed maintenance of pluripotency on the polymers, which was confirmed by flow cytometry, qPCR and embryoid body differentiation. Similarly, a novel polyurethane was identified via microarray screening and shown to support the growth and self-renewal of the cancer stem cell phenotype of C6 rat glioma stem cells [67].
A step towards high-throughput 3D printing has been the microarray production of PEG hydrogels printed together with mESCs expressing Oct4-GFP [47]. By changing the stiffness of the PEG hydrogels, adding in MMP sequences, ECM components, cell–cell interaction mediating molecules and soluble factors, plus varying the cell seeding density, over 1000 different microenvironments were created in a 1536-well-plate format. Recently, similar PEG hydrogels have been used for iPSC reprogramming [57], intestinal organoid maintenance [79], ESC neural tube formation [53] and pancreatic progenitor cells [48–51].
5. Conclusion
Stem cells hold great potential for a range of purposes, from increasing the value of functional cell-based assays and in vitro pharmaceutical testing to revolutionizing regenerative medicine by providing novel treatments for a plethora of diseases and conditions. As highlighted here, the rapid development of substrates to support stem cell growth, maintenance and differentiation can greatly aid in these pursuits. As the understanding of stem cell biology expands, the design and engineering of substrates will be enhanced, particularly with regard to including control over topography, chemical and mechanical properties, biological factor inclusion and modulation of degradation/remodelling properties. In addition, transitioning from 2D to 3D substrates will create better in vivo mimics, although with the added hurdle of more complicated analysis. However, advances in bio-fabrication and the use of high-throughput analysis systems are sure to alleviate this problem. Finally, these efforts require knowledge and skills from multiple disciplines, covering biology, medicine, chemistry, engineering and physics to name but a few. It is only via collaborative efforts that regenerative medicine will ever be able to truly deliver and enable the translation of stem cells and accompanying partner materials into clinical applications.
Data accessibility
This article has no additional data.
Authors' contributions
All the authors contributed equally to the manuscript preparation.
Competing interests
We have no competing interests.
Funding
Funding was received from the European Research Council (Advanced grant no., ADREEM ERC-2013-340469).
References
- 1.McMurray RJ, Dalby MJ, Tsimbouri PM. 2015. Using biomaterials to study stem cell mechanotransduction, growth and differentiation. J. Tissue Eng. Regen. Med. 9, 528–539. ( 10.1002/term.1957) [DOI] [PubMed] [Google Scholar]
- 2.Kharkar PM, Kiick KL, Kloxin AM. 2013. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 42, 7335–7372. ( 10.1039/C3CS60040H) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H, Heilshorn SC. 2015. Adaptable hydrogel networks with reversible linkages for tissue engineering. Adv. Mater. 27, 3717–3736. ( 10.1002/adma.201501558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llames S, García-Pérez E, Meana Á, Larcher F, del Río M. 2015. Feeder layer cell actions and applications. Tissue Eng. B Rev. 21, 345–353. ( 10.1089/ten.teb.2014.0547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ursu A, Schöler HR, Waldmann H. 2018. Small-molecule phenotypic screening with stem cells. Nat. Chem. Biol. 13, 560–563. ( 10.1038/nchembio.2383) [DOI] [PubMed] [Google Scholar]
- 6.Ruedinger F, Lavrentieva A, Blume C, Pepelanova I, Scheper T. 2015. Hydrogels for 3D mammalian cell culture: a starting guide for laboratory practice. Appl. Microbiol. Biotechnol. 99, 623–636. ( 10.1007/s00253-014-6253-y) [DOI] [PubMed] [Google Scholar]
- 7.Ireland RG, Simmons CA. 2015. Human pluripotent stem cell mechanobiology: manipulating the biophysical microenvironment for regenerative medicine and tissue engineering applications. Stem Cells 33, 3187–3196. ( 10.1002/stem.2105) [DOI] [PubMed] [Google Scholar]
- 8.Steward AJ, Kelly DJ. 2015. Mechanical regulation of mesenchymal stem cell differentiation. J. Anat. 227, 717–731. ( 10.1111/joa.12243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruoslahti E. 1996. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12, 697–715. ( 10.1146/annurev.cellbio.12.1.697) [DOI] [PubMed] [Google Scholar]
- 10.Weber LM, Hayda KN, Haskins K, Anseth KS. 2007. The effects of cell–matrix interactions on encapsulated β-cell function within hydrogels functionalized with matrix-derived adhesive peptides. Biomaterials 28, 3004–3011. ( 10.1016/j.biomaterials.2007.03.005) [DOI] [PubMed] [Google Scholar]
- 11.Maeda T, Titani K, Sekiguchi K. 1994. Cell-adhesive activity and receptor-binding specificity of the laminin-derived YIGSR sequence grafted onto staphylococcal protein A. J. Biochem. 115, 182–189. ( 10.1093/oxfordjournals.jbchem.a124315) [DOI] [PubMed] [Google Scholar]
- 12.Kraehenbuehl TP, Langer R, Ferreira LS. 2011. Three-dimensional biomaterials for the study of human pluripotent stem cells. Nat. Methods 8, 731–736. ( 10.1038/nmeth.1671) [DOI] [PubMed] [Google Scholar]
- 13.Page-McCaw A, Ewald AJ, Werb Z. 2007. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 8, 221–233. ( 10.1038/nrm2125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wozniak MA, Chen CS. 2009. Mechanotransduction in development: a growing role for contractility. Nat. Rev. Mol. Cell Biol. 10, 34–43. ( 10.1038/nrm2592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, Chen CS, Fu J. 2012. Forcing stem cells to behave: a biophysical perspective of the cellular microenvironment. Annu. Rev. Biophys. 41, 519–542. ( 10.1146/annurev-biophys-042910-155306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mammoto T, Mammoto A, Ingber DE. 2013. Mechanobiology and developmental control. Annu. Rev. Cell Dev. Biol. 29, 27–61. ( 10.1146/annurev-cellbio-101512-122340) [DOI] [PubMed] [Google Scholar]
- 17.Screen HRC, Berk DE, Kadler KE, Ramirez F, Young MF. 2015. Tendon functional extracellular matrix. J. Orthop. Res. 33, 793–799. ( 10.1002/jor.22818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bishop P. 1996. The biochemical structure of mammalian vitreous. Eye 10, 664–670. ( 10.1038/eye.1996.159) [DOI] [PubMed] [Google Scholar]
- 19.Dalby MJ, Gadegaard N, Oreffo ROC. 2014. Harnessing nanotopography and integrin–matrix interactions to influence stem cell fate. Nat. Mater. 13, 558–569. ( 10.1038/nmat3980) [DOI] [PubMed] [Google Scholar]
- 20.Bracaglia LG, Smith BT, Watson E, Arumugasaamy N, Mikos AG, Fisher JP. 2017. 3D printing for the design and fabrication of polymer-based gradient scaffolds. Acta Biomater. 56, 3–13. ( 10.1016/j.actbio.2017.03.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon JJ, West JL. 2008. Vascularization of engineered tissues: approaches to promote angio-genesis in biomaterials. Curr. Top. Med. Chem. 8, 300 ( 10.2174/156802608783790983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarker M, Chen XB, Schreyer DJ. 2015. Experimental approaches to vascularisation within tissue engineering constructs. J. Biomater. Sci. Polym. Ed. 26, 683–734. ( 10.1080/09205063.2015.1059018) [DOI] [PubMed] [Google Scholar]
- 23.Li P-S, Liang Lee I, Yu W-L, Sun J-S, Jane W-N, Shen H-H. 2015. A novel albumin-based tissue scaffold for autogenic tissue engineering applications. Sci. Rep. 4, 5600 ( 10.1038/srep05600) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixon JE, Shah DA, Rogers C, Hall S, Weston N, Parmenter CDJ, McNally D, Denning C, Shakesheff KM. 2014. Combined hydrogels that switch human pluripotent stem cells from self-renewal to differentiation. Proc. Natl Acad. Sci. USA 111, 5580–5585. ( 10.1073/pnas.1319685111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parmar PA, Skaalure SC, Chow LW, St-Pierre J-P, Stoichevska V, Peng YY, Werkmeister JA, Ramshaw JAM, Stevens MM. 2016. Temporally degradable collagen-mimetic hydrogels tuned to chondrogenesis of human mesenchymal stem cells. Biomaterials 99, 56–71. ( 10.1016/j.biomaterials.2016.05.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsimbouri PM, et al. 2017. Stimulation of 3D osteogenesis by mesenchymal stem cells using a nanovibrational bioreactor. Nat. Biomed. Eng. 1, 758–770. ( 10.1038/s41551-017-0127-4) [DOI] [PubMed] [Google Scholar]
- 27.Parmar PA, St-Pierre J-P, Chow LW, Spicer CD, Stoichevska V, Peng YY, Werkmeister JA, Ramshaw JAM, Stevens MM. 2017. Enhanced articular cartilage by human mesenchymal stem cells in enzymatically mediated transiently RGDS-functionalized collagen-mimetic hydrogels. Acta Biomater. 51, 75–88. ( 10.1016/j.actbio.2017.01.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sánchez-Ferrero A, Mata Á, Mateos-Timoneda MA, Rodríguez-Cabello JC, Alonso M, Planell J, Engel E. 2015. Development of tailored and self-mineralizing citric acid-crosslinked hydrogels for in situ bone regeneration. Biomaterials 68, 42–53. ( 10.1016/j.biomaterials.2015.07.062) [DOI] [PubMed] [Google Scholar]
- 29.Paul A, Stührenberg M, Chen S, Rhee D, Lee W-K, Odom TW, Heilshorn SC, Enejder A. 2017. Micro- and nano-patterned elastin-like polypeptide hydrogels for stem cell culture. Soft Matter 13, 5665–5675. ( 10.1039/C7SM00487G) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Zhu D, Paul A, Cai L, Enejder A, Yang F, Heilshorn SC. 2017. Covalently adaptable elastin-like protein–hyaluronic acid (ELP–HA) hybrid hydrogels with secondary thermoresponsive crosslinking for injectable stem cell delivery. Adv. Funct. Mater. 27, 1605609 ( 10.1002/adfm.201605609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun W, Incitti T, Migliaresi C, Quattrone A, Casarosa S, Motta A. 2017. Viability and neuronal differentiation of neural stem cells encapsulated in silk fibroin hydrogel functionalized with an IKVAV peptide. J. Tissue Eng. Regen. Med. 11, 1532–1541. ( 10.1002/term.2053) [DOI] [PubMed] [Google Scholar]
- 32.Hung HS, et al. 2013. Biocompatibility and favorable response of mesenchymal stem cells on fibronectin-gold nanocomposites. PLoS ONE 8, e65738 ( 10.1371/journal.pone.0065738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang CW, Hwang Y, Brafman D, Hagan T, Phung C, Varghese S. 2013. Engineering cell-material interfaces for long-term expansion of human pluripotent stem cells. Biomaterials 34, 912–921. ( 10.1016/j.biomaterials.2012.10.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bian L, Guvendiren M, Mauck RL, Burdick JA. 2013. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc. Natl Acad. Sci. USA 110, 10 117–10 122. ( 10.1073/pnas.1214100110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cameron K, et al. 2015. Recombinant laminins drive the differentiation and self-organization of hESC-derived hepatocytes. Stem Cell Rep. 5, 1250–1262. ( 10.1016/j.stemcr.2015.10.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parisi-Amon A, Mulyasasmita W, Chung C, Heilshorn SC. 2013. Protein-engineered injectable hydrogel to improve retention of transplanted adipose-derived stem cells. Adv. Healthc. Mater. 2, 428–432. ( 10.1002/adhm.201200293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulyasasmita W, Cai L, Dewi RE, Jha A, Ullmann SD, Luong RH, Huang NF, Heilshorn SC. 2014. Avidity-controlled hydrogels for injectable co-delivery of induced pluripotent stem cell-derived endothelial cells and growth factors. J. Control Release 191, 71–81. ( 10.1016/j.jconrel.2014.05.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai L, Dewi RE, Heilshorn SC. 2015. Injectable hydrogels with in situ double network formation enhance retention of transplanted stem cells. Adv. Funct. Mater. 25, 1344–1351. ( 10.1002/adfm.201403631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai L, Dewi RE, Goldstone AB, Cohen JE, Steele AN, Woo YJ, Heilshorn SC. 2016. Regulating stem cell secretome using injectable hydrogels with in situ network formation. Adv. Healthc. Mater. 5, 2758–2764. ( 10.1002/adhm.201600497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kilian KA, Mrksich M. 2012. Directing stem cell fate by controlling the affinity and density of ligand–receptor interactions at the biomaterials interface. Angew. Chemie Int. Edn. 51, 4891–4895. ( 10.1002/anie.201108746) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zamuner A, Cavo M, Scaglione S, Messina GML, Russo T, Gloria A, Marletta G, Dettin M. 2016. Design of decorated self-assembling peptide hydrogels as architecture for mesenchymal stem cells. Materials (Basel) 9, 727 ( 10.3390/ma9090727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alakpa EV, Jayawarna V, Burgess KEV, West CC, Péault B, Ulijn RV, Dalby MJ. 2017. Improving cartilage phenotype from differentiated pericytes in tunable peptide hydrogels. Sci. Rep. 7, 6895 ( 10.1038/s41598-017-07255-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Wang X, Liu J, Tredget EE, Wu Y. 2016. Self-assembling peptide hydrogel scaffolds support stem cell-based hair follicle regeneration. Nanomed. Nanotechnol. Biol. Med. 12, 2115–2125. ( 10.1016/j.nano.2016.05.021) [DOI] [PubMed] [Google Scholar]
- 44.Luo H, et al. In press Neural differentiation of bone marrow mesenchymal stem cells with human brain-derived neurotrophic factor gene-modified in functionalized self-assembling peptide hydrogel in vitro. J. Cell. Biochem. ( 10.1002/jcb.26408) [DOI] [PubMed] [Google Scholar]
- 45.Zhou J, Ding J, Nie B, Hu S, Zhu Z, Chen J, Xu J, Shi J, Dong N. 2016. Promotion of adhesion and proliferation of endothelial progenitor cells on decellularized valves by covalent incorporation of RGD peptide and VEGF. J. Mater. Sci. Mater. Med. 27, 142 ( 10.1007/s10856-016-5750-1) [DOI] [PubMed] [Google Scholar]
- 46.Jin S, Yao H, Weber JL, Melkoumian ZK, K Ye. 2012. A synthetic xeno-free peptide surface for expansion and directed differentiation of human induced pluripotent stem cells. PLoS ONE 7, e50880 ( 10.1371/journal.pone.0050880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ranga A, Gobaa S, Okawa Y, Mosiewicz K, Negro A, Lutolf MP. 2014. 3D niche microarrays for systems-level analyses of cell fate. Nat. Commun. 5, 4324 ( 10.1038/ncomms5324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caiazzo M, Okawa Y, Ranga A, Piersigilli A, Tabata Y, Lutolf MP. 2016. Defined three-dimensional microenvironments boost induction of pluripotency. Nat. Mater. 15, 344–352. ( 10.1038/nmat4536) [DOI] [PubMed] [Google Scholar]
- 49.Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordóñez-Morán P, Clevers H, Lutolf MP. 2016. Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560–564. ( 10.1038/nature20168) [DOI] [PubMed] [Google Scholar]
- 50.Meinhardt A, et al. 2014. 3D reconstitution of the patterned neural tube from embryonic stem cells. Stem Cell Rep. 3, 987–999. ( 10.1016/j.stemcr.2014.09.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greggio C, De Franceschi F, Figueiredo-Larsen M, Gobaa S, Ranga A, Semb H, Lutolf M, Grapin-Botton A. 2013. Artificial three-dimensional niches deconstruct pancreas development in vitro. Development 140, 4452–4462. ( 10.1242/dev.096628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lienemann PS, et al. 2015. Locally controlling mesenchymal stem cell morphogenesis by 3D PDGF-BB gradients towards the establishment of an in vitro perivascular niche. Integr. Biol. 7, 101–111. ( 10.1039/C4IB00152D) [DOI] [PubMed] [Google Scholar]
- 53.Otsuji TG, et al. 2014. A 3D sphere culture system containing functional polymers for large-scale human pluripotent stem cell production. Stem Cell Rep. 2, 734–745. ( 10.1016/j.stemcr.2014.03.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cochis A, Grad S, Stoddart MJ, Farè S, Altomare L, Azzimonti B, Alini M, Rimondini L. 2017. Bioreactor mechanically guided 3D mesenchymal stem cell chondrogenesis using a biocompatible novel thermo-reversible methylcellulose-based hydrogel. Sci. Rep. 7, 45018 ( 10.1038/srep45018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alakpa EV, Burgess KEV, Chung P, Riehle MO, Gadegaard N, Dalby MJ, Cusack M. 2017. Nacre topography produces higher crystallinity in bone than chemically induced osteogenesis. ACS Nano 11, 6717–6727. ( 10.1021/acsnano.7b01044) [DOI] [PubMed] [Google Scholar]
- 56.McMurray RJ, et al. 2011. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat. Mater. 10, 637–644. ( 10.1038/nmat3058) [DOI] [PubMed] [Google Scholar]
- 57.Du W, et al. 2017. Enhanced proangiogenic potential of mesenchymal stem cell-derived exosomes stimulated by a nitric oxide releasing polymer. Biomaterials 133, 70–81. ( 10.1016/j.biomaterials.2017.04.030) [DOI] [PubMed] [Google Scholar]
- 58.Schmitt SK, Xie AW, Ghassemi RM, Trebatoski DJ, Murphy WL, Gopalan P. 2015. Polyethylene glycol coatings on plastic substrates for chemically defined stem cell culture. Adv. Healthc. Mater. 4, 1555–1564. ( 10.1002/adhm.201500191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kyburz KA, Anseth KS. 2013. Three-dimensional hMSC motility within peptide-functionalized PEG-based hydrogels of varying adhesivity and crosslinking density. Acta Biomater. 9, 6381–6392. ( 10.1016/j.actbio.2013.01.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schultz KM, Kyburz KA, Anseth KS. 2015. Measuring dynamic cell–material interactions and remodeling during 3D human mesenchymal stem cell migration in hydrogels. Proc. Natl Acad. Sci. USA 112, E3757–E3764. ( 10.1073/pnas.1511304112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang C, Tibbitt MW, Basta L, Anseth KS. 2014. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 13, 645–652. ( 10.1038/nmat3889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang C, DelRio FW, Ma H, Killaars AR, Basta LP, Kyburz KA, Anseth KS. 2016. Spatially patterned matrix elasticity directs stem cell fate. Proc. Natl Acad. Sci. USA 113, E4439–E4445. ( 10.1073/pnas.1609731113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang R, et al. 2013. A thermoresponsive and chemically defined hydrogel for long-term culture of human embryonic stem cells. Nat. Commun. 4, 1335 ( 10.1038/ncomms2341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duffy CRE, Zhang R, How S-E, Lilienkampf A, De Sousa PA, Bradley M. 2014. Long term mesenchymal stem cell culture on a defined synthetic substrate with enzyme free passaging. Biomaterials 35, 5998–6005. ( 10.1016/j.biomaterials.2014.04.013) [DOI] [PubMed] [Google Scholar]
- 65.Mangani C, Lilienkampf A, Roy M, de Sousa PA, Bradley M. 2015. Thermoresponsive hydrogel maintains the mouse embryonic stem cell ‘naïve’ pluripotency phenotype. Biomater. Sci. 3, 1371–1375. ( 10.1039/C5BM00121H) [DOI] [PubMed] [Google Scholar]
- 66.Hansen A, Mjoseng HK, Zhang R, Kalloudis M, Koutsos V, de Sousa PA, Bradley M. 2014. High-density polymer microarrays: identifying synthetic polymers that control human embryonic stem cell growth. Adv. Healthc. Mater. 3, 848–853. ( 10.1002/adhm.201300489) [DOI] [PubMed] [Google Scholar]
- 67.Tabu K, et al. 2016. A synthetic polymer scaffold reveals the self-maintenance strategies of rat glioma stem cells by organization of the advantageous niche. Stem Cells 34, 1151–1162. ( 10.1002/stem.2299) [DOI] [PubMed] [Google Scholar]
- 68.Duffy CRE, Zhang R, How S-E, Lilienkampf A, Tourniaire G, Hu W, West CC, de Sousa P, Bradley M. 2014. A high-throughput polymer microarray approach for identifying defined substrates for mesenchymal stem cells. Biomater. Sci. 2, 1683–1692. ( 10.1039/C4BM00112E) [DOI] [PubMed] [Google Scholar]
- 69.Wei Y, et al. 2017. Directing stem cell differentiation via electrochemical reversible switching between nanotubes and nanotips of polypyrrole array. ACS Nano 11, 5915–5924. ( 10.1021/acsnano.7b01661) [DOI] [PubMed] [Google Scholar]
- 70.Reimer A, Vasilevich A, Hulshof F, Viswanathan P, van Blitterswijk CA, de Boer J, Watt FM. 2016. Scalable topographies to support proliferation and Oct4 expression by human induced pluripotent stem cells. Sci. Rep. 6, 18948 ( 10.1038/srep18948) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hay DC, et al. 2011. Unbiased screening of polymer libraries to define novel substrates for functional hepatocytes with inducible drug metabolism. Stem Cell Res. 6, 92–102. ( 10.1016/j.scr.2010.12.002) [DOI] [PubMed] [Google Scholar]
- 72.Lucendo-Villarin B, Khan F, Pernagallo S, Bradley M, Iredale JP, Hay DC. 2012. Maintaining hepatic stem cell gene expression on biological and synthetic substrata. Biores. Open Access 1, 50–53. ( 10.1089/biores.2012.0206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Medine CN, et al. 2013. Developing high-fidelity hepatotoxicity models from pluripotent stem cells. Stem Cells Transl. Med. 2, 505–509. ( 10.5966/sctm.2012-0138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Villarin BL, et al. 2015. Polymer supported directed differentiation reveals a unique gene signature predicting stable hepatocyte performance. Adv. Healthc. Mater. 4, 1820–1825. ( 10.1002/adhm.201500391) [DOI] [PubMed] [Google Scholar]
- 75.Khan F, Smith JO, Kanczler JM, Tare RS, Oreffo ROC, Bradley M. 2013. Discovery and evaluation of a functional ternary polymer blend for bone repair: translation from a microarray to a clinical model. Adv. Funct. Mater. 23, 2850–2862. ( 10.1002/adfm.201202710) [DOI] [Google Scholar]
- 76.Smith JO, Tayton ER, Khan F, Aarvold A, Cook RB, Goodship A, Bradley M, Oreffo ROC. 2017. Large animal in vivo evaluation of a binary blend polymer scaffold for skeletal tissue-engineering strategies; translational issues. J. Tissue Eng. Regen. Med. 11, 1065–1076. ( 10.1002/term.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cameron K, Lucendo-Villarin B, Szkolnicka D, Hay DC. 2015. Serum-free directed differentiation of human embryonic stem cells to hepatocytes. In Protocols in in vitro hepatocyte research (eds M Vinken, V Rogiers). Methods in molecular biology (methods and protocols), vol. 1250, pp. 105–111. New York, NY: Humana Press; ( 10.1007/978-1-4939-2074-7_7). [DOI] [PubMed] [Google Scholar]
- 78.Szkolnicka D, Farnworth SL, Lucendo-Villarin B, Hay DC. 2014. Deriving functional hepatocytes from pluripotent stem cells. Curr. Protoc. Stem Cell Biol. 30, 1G.5.1–1G.5.12. ( 10.1002/9780470151808.sc01g05s30) [DOI] [PubMed] [Google Scholar]
- 79.Yao X, et al. 2015. Nitric oxide releasing hydrogel enhances the therapeutic efficacy of mesenchymal stem cells for myocardial infarction. Biomaterials 60, 130–140. ( 10.1016/j.biomaterials.2015.04.046) [DOI] [PubMed] [Google Scholar]
- 80.Anderson DG, Levenberg S, Langer R. 2004. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat. Biotechnol. 22, 863–866. ( 10.1038/nbt981) [DOI] [PubMed] [Google Scholar]
- 81.Anderson DG, Putnam D, Lavik EB, Mahmood TA, Langer R. 2005. Biomaterial microarrays: rapid, microscale screening of polymer–cell interaction. Biomaterials 26, 4892–4897. ( 10.1016/j.biomaterials.2004.11.052) [DOI] [PubMed] [Google Scholar]
- 82.Zhang R, Liberski A, Khan F, Diaz-Mochon JJ, Bradley M. 2008. Inkjet fabrication of hydrogel microarrays using in situ nanolitre-scale polymerisation. Chem. Commun. 2008, 1317–1319. ( 10.1039/b717932d) [DOI] [PubMed] [Google Scholar]
- 83.Tare RS, Khan F, Tourniaire G, Morgan SM, Bradley M, Oreffo ROC. 2009. A microarray approach to the identification of polyurethanes for the isolation of human skeletal progenitor cells and augmentation of skeletal cell growth. Biomaterials 30, 1045–1055. ( 10.1016/j.biomaterials.2008.10.038) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.