Abstract
Stem cell technology in regenerative medicine has the potential to provide an unlimited supply of cells for drug testing, medical transplantation and academic research. In order to engineer a realistic tissue model using stem cells as an alternative to human tissue, it is essential to create artificial stem cell microenvironment or niches. Three-dimensional (3D) bioprinting is a promising tissue engineering field that offers new opportunities to precisely place stem cells within their niches layer-by-layer. This review covers bioprinting technologies, the current development of ‘bio-inks’ and how bioprinting has already been applied to stem-cell culture, as well as their applications for human regenerative medicine. The key considerations for bioink properties such as stiffness, stability and biodegradation, biocompatibility and printability are highlighted. Bioprinting of both adult and pluriopotent stem cells for various types of artificial tissues from liver to brain has been reviewed. 3D bioprinting of stem-cell derived tissues for human regenerative medicine is an exciting emerging area that represents opportunities for new research, industries and products as well as future challenges in clinical translation.
This article is part of the theme issue ‘Designer human tissue: coming to a lab near you’.
Keywords: three dimensional, bioprinting, stem cells, regenerative medicine
1. Introduction
Drug testing, medical transplantation and academic research all require human tissue that is often in short supply so a renewable alternative to human tissue is often desired. Immortalized cell lines from various tissues have been used in both drug testing as well as various academic pursuits, such as disease modelling. Despite these cell lines representing an unlimited supply of cells, the intrinsic abnormalities and mutations they possess limit their use as a model of their relative tissue source. This, in turn, has pushed researchers to use endogenous stem cells as a source for renewable human tissue production. Embryonic, fetal and adult stem cells have been used to generate somatic cells from various tissue types that are more biomimetic than immortalized cell lines. However, stem cells in tissue reside within a niche, which when lost can cause spontaneous differentiation of the stem cells, or cell death [1]. In order to create artificial stem-cell niches, researchers have looked to the field of tissue engineering, and recently, three-dimensional (3D) bioprinting.
Tissue engineering is the combination of biomaterials, cells and biochemical factors to support or replace endogenous tissues [2]. In the case of engineering stem cells, the aim is to reproduce a suitable niche for stem-cell expansion and differentiation, and subsequent formation of artificial tissue. 3D bioprinting is a tissue-engineering technique that allows precise deposition of ‘bio-inks’, combinations of supporting biomaterials and desired cells [3,4]. By combining multiple bio-inks, complex multi-cellular and multi-material structures can be formed that are analogous to human tissue for drug testing and medical transplantations [5–8]. 3D bioprinting has advanced rapidly since the first cell printing with a modified HP ink-jet printer in 2005 [9]. This review will cover the various methods of bioprinting and the development of ‘bio-inks’, as well as how bioprinting has already been applied to stem-cell culture and the outcomes of such research. We will also cover the technical and regulatory limitations that 3D bioprinting currently face.
2. Bioprinting techniques
Bioprinting can be defined as robotically automated, layer by layer, additive biofabrication using both living cells and biomaterials, directed by a digital model [3,4]. By layering deposited biological materials on top of each other, it is possible for a bioprinted structure's architecture to be defined not only in 2D, as in patterned cells, but in 3D. This, along with the ability to deposit various biomaterials and cell types at once, gives the potential to generate complex, tissue-like structures from the ground up. There are various methods of bioprinting; the most prevalent ones are detailed in table 1 and visualized in figure 1.
Table 1.
Bioprinting techniques summary and examples of use.
| bioprinting technology | summary of technology | examples of tissue structures produced | benefits | drawbacks | average resolution |
|---|---|---|---|---|---|
| ink-jet | thermal, electromagnetic or piezo-electric force propels droplets of bio-ink to defined area | vascular [10] skin [10] osteochondral [11,12] ovarian [13] liver [14] neural [15] |
high resolution | potential for high cellular stress during deposition; nozzle blockage common; expensive technology | 20+ µm; picolitres (pl) deposition; single/few cells possible per deposition |
| valve-based | pressure based deposition of bio-ink droplet through valve | embryonic [16] liver [17] |
gentler than ink-jet; high resolution available; high droplet deposition (1000 s−1) |
need for constant external pressure supply; nozzle blockage common | 40+ µm; nanolitres (nl) deposition; single/few cells per deposition |
| stereolithography | photocuring of photo-sensitive polymer by UV light produces solid structure from bath of acellular material or cellular bio-ink | vascular [18] liver [19] |
very high resolution; high-throughput and fast printing possible using digital mirror device technology | excess bio-ink needed to create curing bath; UV radiation, photo-initiator and resultant free radicals are toxic to cells; expensive technology | 150+ nm (higher with cell-laden ink); specific cell deposition not possible |
| laser-assisted | laser is fired to push cell from pool of bio-ink onto target surface | fibroblast [20] vascular [21] |
highest resolution of bioprinting possible; flexibility of printing material viscosity | excess bio-ink needed to create pool; potential damage to cells; expensive technology | single-cell resolution possible |
| extrusion | deposition of materials through motor-driven extruder | neural [22] liver [23] vascular [24,25] pancreatic [26] embryonic [27,28] osteochondral [29] |
flexibility with printing material viscosity; cell-friendly printing process; coaxial or multiple nozzles allow printing various bio-inks; affordable technology | low resolution; slow print times | 50 µm; cell aggregate or spheroid deposition only |
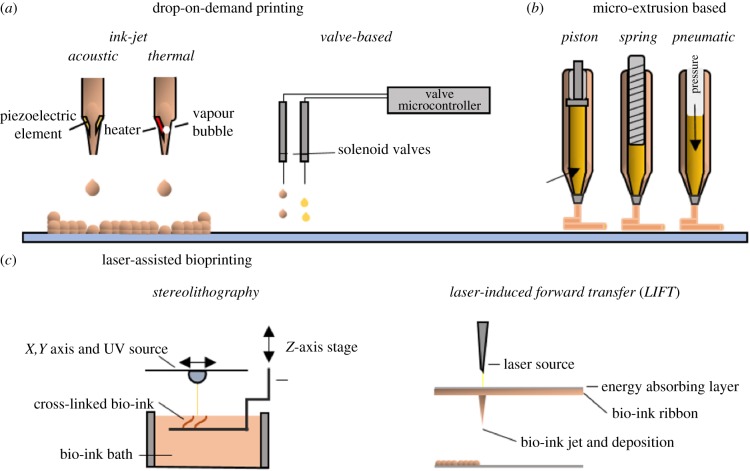
Figure 1.
The three primary bioprinting methods. (a) Drop-on-demand (DoD) bioprinting, comprising: ink-jet and valve-jet bioprinting. (b) Micro-extrusion bioprinting: pressure, spring or piston forces the bio-ink to extrude through a nozzle. (c) Laser-assisted bioprinting: stereolithography and laser-induced forward transfer (LIFT). (Online version in colour.)
(a). Drop-on-demand printing
Drop-on-demand (DoD) printing uses controlled deposition of bio-ink droplets to generate artificial tissue in a high-throughput manner. The two methods of DoD printing are ink-jet and valve-jet bioprinting (figure 1a).
In an ink-jet printer, a droplet is dispensed by generating a discrete thermal, piezo-electric or electromagnetic effect. Indeed, some of the earliest bioprinting was done by using commercial ink-jet printers to pattern proteins [30], and then a patterned culture of hamster ovarian and motor neuron cells [9]. The ability to deposit droplets in the range of picolitres means the resolution available to print live cells is very high and single-cell printing is possible [21]. However, due to the potential for high shear and thermal stresses, cell viability is a concern, although while transient pores were visible following ink-jet printing of mammalian cells, these quickly sealed and were actually used to successfully transfect cells with plasmids following printing [31]. Print nozzle clogging and need for low-viscosity bio-inks also constrained the use for creating larger printed structures (in the scale of cubic centimetres).
Valve-jet bioprinting controls dispensing through solenoid microvalves with bio-inks driven by pneumatic pressure [32]. Very high spatial precision, small deposition volumes (in the nanolitre range), high cell viability [33] and high-throughput generation (1000 droplets per second) make valve-jet bioprinting very appealing for generating human tissue. But like ink-jet, valve-jet bioprinting is still a nozzle-based printing technology where nozzle blockage can be an issue with high viscosity and cell-density bio-inks.
(b). Extrusion-based bioprinting
Extrusion-based bioprinting is currently the most accessible and widespread bioprinting technique [38]. Originally developed for printing plastics as fused deposition modelling (FDM) technology, deposition of biomaterials is often through a motor-driven or pneumatic extruder (figure 1b). Unlike ink-jet printing limited by low viscosity and LAB limited by scalability, extrusion-based bioprinting can print large, scalable constructs with a wide range of viscosities.
However, extrusion is not without its own caveats. Resolution of printed products is lower than other forms of bioprinting, in the range of 30–100 µm for their finest details. Cells also experience shear stress while being deposited with highly viscous materials, therefore material selection is crucial for extrusion printing as the printed structure must maintain its shape while gelled. This means low-viscosity bio-inks are often unusable, although this is being overcome by printing into baths [39,40].
(c). Laser-assisted bioprinting
Laser-assisted bioprinting (LAB) encompasses two forms of bioprinting: laser-induced forward transfer (LIFT) technique, whereby a laser beam pushes a biological sample from a pool of bio-ink to the target substrate; and stereolithography, which involves curing of a photo-sensitive polymer using a laser. Use of a laser allows very high resolution, with single-cell placement being possible [20,21]. As there are no nozzles used in printing, clogging is not an issue, so high viscosity fluids can be printed. Cell viability is high in printed human stem cells [34]. Bearing perhaps the best resolution possible for in situ bioprinting, LIFT theoretically has the closest possibility of printing a completely biomimetic tissue structure. In reality, limited scalability makes this far from feasible for clinical translation. Like stereolithography, a large excess of bio-ink is required to print a structure and the technology is expensive. Constructing the bio-ink ribbon necessary for LIFT is also time-consuming and difficult, especially for larger structures.
Stereolithography is most often used in the production of acellular scaffolds. The capability of a laser-based system allows extremely high resolution, with printed structures having features in the nanometre range [35,36]. The need for excess materials to be present during the curation step represents a limitation for the bioprinting of large structures employing one-step stereolithographic printing. The presence of toxic photo-initiators and the resultant free radicals is also an ongoing concern for cell viability during and after printing [37].
3. Bio-inks
A bio-ink is the biological equivalent of ink for ink printers, but instead of dyes it employs biological materials to generate the 3D structures. Bio-inks are typically composed of structural supporting materials, live cells and can also include bioactive molecules such as growth factors, either encapsulated or covalently tethered to the supporting material [41,42].
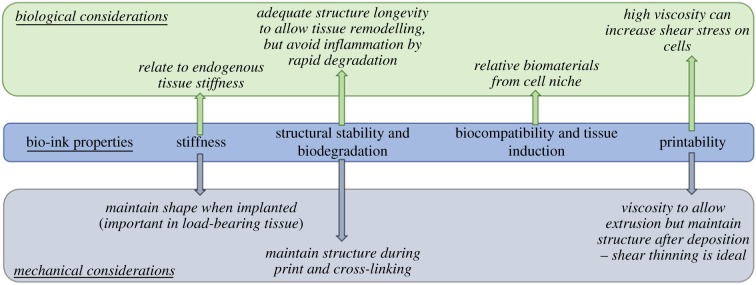
Hydrogels, high water-content polymers that can be cross-linked to form a gel [43], mimicking extracellular matrices (ECM), represent the main component in the bio-ink. Choice of material to form the basis of the bio-ink is crucial for successful printing and tissue formation. The desirable bio-ink should fulfil a range of properties including: (i) mechanical stiffness; (ii) structural stability and biodegradability; (iii) biocompatibility and tissue induction; and importantly for bioprinting (iv) printability, all of which are summarized in figure 2.
Figure 2.
The primary properties of bio-ink material have biological and mechanical effects in the engineered tissue. (Online version in colour.)
(a). Mechanical stiffness
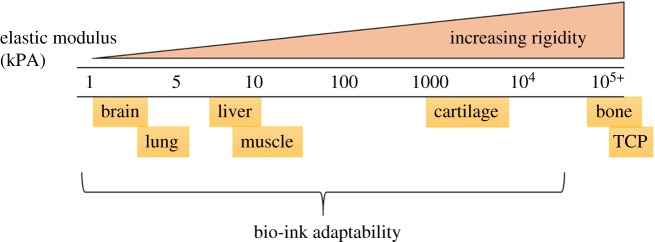
Measured using the shear elastic modulus given in Pascals (Pa) or kilopascals (kPa), the stiffness of a desired tissue is a key biological characteristic often overlooked in in vitro cell culture. Tissue stiffness varies between tissues, from less than 1 kPa for neuronal tissue to greater than 100 kPa in bone (figure 3) [44]. When compared with the stiffness of common tissue culture plastics (TCPs; 1 GPa or 1 000 000 kPa), it is unsurprising that in vitro culture alters cell biology, particularly of cells from low stiffness tissues such as liver or brain. With a wealth of research showing the effects of increased tissue stiffness on tissues such as the liver [45,46], design of the bio-ink must be carefully tailored to match endogenous, healthy tissue. This can be achieved by material selection, modification and cross-linking parameters. Table 2 below details some of the commonly used materials in bio-inks. To alter stiffness, the molecular weight of bio-ink monomers can be altered, as well as the polymer components, and the cross-linking methods used.
Figure 3.
Different endogenous tissue types display varying rigidity. Brain, lung, liver and muscle, for example, all reside in relatively soft tissues, whereas common tissue culture plastic (TCP) is several orders of magnitude more rigid. This can lead to changes in cell viability, function and phenotype when culturing cells in vitro. However, the characteristics of bio-inks can be tuned for the bespoke need of the desired tissue. (Online version in colour.)
Table 2.
Characteristics of the most commonly used bio-ink constituents.
| type | cross-linker | pros | cons | references | |
|---|---|---|---|---|---|
| agarose | natural | thermoresponsive | cheap, good printability | non-adherent and bioinert | [47] |
| alginate | natural | ionic, (Ca2+, Sr2+, Ba2+) | good printability, tunable characteristics, fast cross-linking | difficult to control shrinking during cross-linking, bioinert | [48–51] |
| chitosan | natural | pH neutralization | pH changes required | [47] | |
| collagen | natural | thermoresponsive, pH | biologically relevant, adherent, reasonable printability | pH changes required or cold bed for thermal gelling, characteristic of fibrosis, must be sourced from humans for clinical use | [52] |
| fibrin | natural | enzymatic (thrombin) | biologically relevant and adherent | poor printability | [24,53] |
| gelatin | natural | thermoresponsive, or UV if methacryloyl | cheap, good printability, adherent and bioactive | cold bed or UV exposure required to cross-link, must be sourced from humans for clinical use, poorly defined | [13,54] |
| gellan gum | natural | ionic (Ca2+) | cheap, reasonable printability, tuneable with peptide motifs | low mechanical properties, non-adherent and bioinert | [22,55] |
| hyaluronic acid | natural | dependent on modification | reasonable to print, biologically active and relevant | low mechanical properties, must be human sourced for clinical use, cross-linker can be harmful (H2O2) | [18,56] |
| pluronic | synthetic | thermoresponsive | good printability, highly tunable viscosity, sacrificial | mainly used for sacrificial inks, requires cold printing bed to maintain structure | [24] |
| poly(ethylene glycol) (PEG) | synthetic | UV exposure | good printability, tunable, well-defined polymer | potentially harmful UV and photo-initiator exposure | [57] |
| poly(caprolactone) (PCL) | synthetic | thermoresponsive (high temp) | mechanically strong, bioinert | not suitable for cell printing due to high melting point | [24,58] |
(b). Structural stability and biodegradability
The stability of the resulting structure is crucial for the printing process. When depositing low-viscosity bio-inks, definition of the printed structure will suffer owing to spreading of the ink [59]. Structure resolution can also suffer as a result of imperfect cross-linking parameters: lack of cross-linking can cause spreading of the structure, whereas over-cross-linking can result in lamination and a failure of the entire structure to coalesce [40,60]. Certain cross-linking methods, such as ionic with alginate, can also cause shrinkage that will affect the intended structure's shape [61]. Bearing this in mind, an ideal bio-ink would have sufficient viscosity not to spread upon deposition, and its cross-linking method would be tightly controlled. The bio-ink must also have mechanical strength sufficient for initial printed layers to support subsequent deposition without collapse or impairment of the structure.
The ideal fate of most transplanted bioprinted tissue is the degradation of its scaffold, in tandem with integration of the encapsulated cells and regeneration of the target tissue. Biodegradation properties of bio-inks are a key characteristic when considering implantation. There is a balance to strike between too rapid degradation, resulting in loss of cells and potential inflammation, and lack of degradation that can hamper tissue regeneration. Often biodegradability can be tuned by altering the properties of the bio-ink components and their cross-linking procedures.
(c). Biocompatibility and tissue induction
The biological activity of the bio-ink is a key aspect to make it suitable for cell viability and tissue formation. Certain bio-inks, such as alginate or poly ethylene glycol (PEG)-based hydrogels, are used as they are bioinert and non-adherent. This simplifies the process as the bio-ink needs only conform to mechanical suitability, and the biological effect can be disregarded, or biosupportive molecules can be added to the ink. However, other bio-inks make use of biologically active molecules such as gelatin, collagen, hyaluronic acid and fibrin. This becomes a double-edged sword, as while in theory adherent and bioactive matrices should support cell attachment and viability, when constructing a stem-cell niche it is crucial to consider cell–matrix interactions. For example, collagen, while naturally adherent and a large part of endogenous extracellular matrix, is also largely associated with fibrosis of tissue [62]. Stem-cell niches have specific ECM architecture, which likely needs to be mimicked to support stem-cell culture and tissue development.
(d). Printability
Printability of a bio-ink, or bioprintability, is determined by the rheological properties of the bio-ink, comprising its dispensability and ability to maintain structural integrity after bioprinting [59,63,64]. Successful and efficient deposition of the ink depends largely on its viscosity, as well as homogeneity of the solution. An ink that is too viscous will force high shear stresses upon the cells being deposited, and often lead to clogging of the printer nozzle and cell damage. Conversely, low-viscosity inks will result in poor definition of the print due to flowing of the ink after printing. It can also lead to less homogeneous ink and nozzle clogging, as cells will likely sediment in the bio-ink chamber throughout the print. One of the most desirable characteristics for a bio-ink is that it displays shear thinning [65]. This is the behaviour of fluids where, under shear strain (i.e. while being pushed through the printer nozzle), the viscosity of the fluid will temporarily decrease. This allows easy printing with low shear stress on the cells, as well as maintaining the resolution of the printed product.
4. Developments in bioprinting stem cells
Bioprinting research has been important in the study of various tissues, including neural, liver, vascular, pancreatic, bone, cartilage, retinal and ovarian (table 1; figure 4), with the list growing rapidly. Stem-cell bioprinting, while still in its infancy, has also developed immensely in recent years, delivering solutions for expanded and differentiating mesenchymal, neural and pluripotent stem cells (PSC).
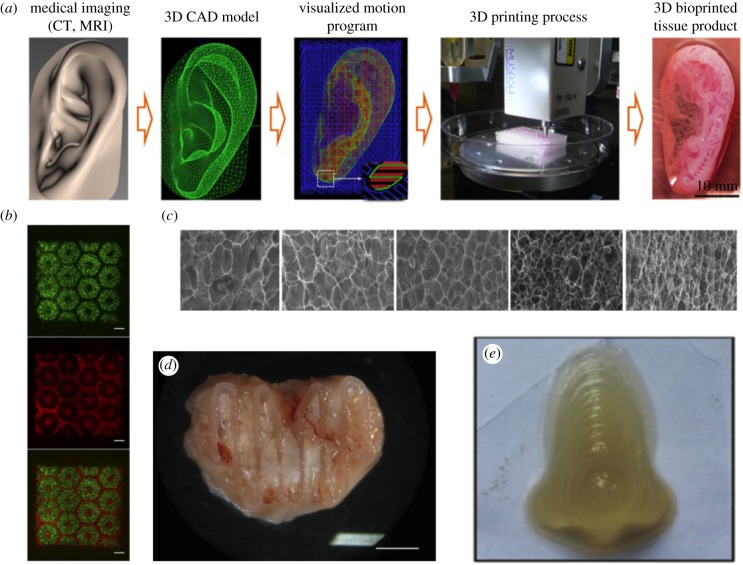
Figure 4.
Examples of 3D bioprinting. (a) Workflow of bioprinting large-scale human tissue from Kang et al. [24]. Medical imaging is first used to generate a computer-aided design (CAD) file that the bioprinter can then optimize for printing. The tissue was then printed in the integrated tissue-organ printer (ITOP) bioprinter to form the artificial tissue. (b) Biomimetic patterning of stem-cell derived hepatocytes from Ma et al. [54]. The hexagonal lobule of the liver was used as a template for printing iPSC-derived hepatocytes and supporting endothelial and mesenchymal cells. (c) Fine-tuning of bio-ink characteristics. By adding varying levels of carboxymethylcellulose, Gu et al. [66] were able to control porosity in their bio-ink, optimizing encapsulation of human neural stem cells. (d) Post-implantation, anatomically designed bioprinted bone tissue from Daly et al. [67]. A soft bio-ink with MSCs was reinforced with PCL fibres for mechanical stiffness prior to implantation. Twelve weeks following implantation, the structure was shown to be vascularized. (e) Bioprinting full-scale nose using hybrid bio-ink to optimize printability and porosity for cell viability [68]. This ink was used to encapsulate mesenchymal stem cells and differentiate them to functional chondrogenic and osteogenic cells. (Online version in colour.)
(a). Mesenchymal stem-cell printing
To date, multipotent mesenchymal stromal cells (MSCs) are likely the most used stem cells in bioprinting, possibly owing to their ease of culture and expansion, multipotency and resilience compared to other stem-cell types. MSCs can be sourced from various tissues, including bone marrow and adipose tissue, and have been used to form various tissues [69–71].
Osteochondral engineering has seen the greatest contribution from human mesenchymal stem cell (hMSC) bioprinting. Using the ability to print defined architecture of material and cells, Daly et al. [67] bioprinted a developmentally inspired template for bone growth, which allowed formation of a vascularized bone organ. The artificial tissue comprised MSCs, an alginate-based bio-ink, arginylglycylaspartic (RGD) acid peptide motifs for cell adherence, and supportive poly-caprolactone (PCL) struts to increase the compressive modulus of the structure, akin to endogenous bone. The artificial bone precursor tissue was then implanted, and 12 weeks later was shown to have successfully vascularized and mineralized (figure 4d). Another study in bone engineering used ink-jet printing and bioactive ceramic nanoparticles to recapitulate the bone niche [72]. Encapsulated in a PEG-based bio-ink, MSCs were printed simultaneously with hydroxyapatite and bioactive glass nanoparticles. Hydroxyapatite-laden structures displayed the highest cell viability, as well as compressive modulus and collagen deposition, characteristic of bone development. Other works have shown that deposition with acrylated peptides [57] and bone morphogenetic protein 2 (BMP2) tethered microfibres [73] can improve bone tissue generation from bioprinted MSCs. MSCs have also been used as examples to display the effects of shear stress on stem-cell viability during printing [74] and precise cell patterning to study cell migration during vascularization [75], as well as cardiac differentiation controlled by focal adhesion [76]. Bio-ink derived from the endogenous, decellularized ECM of various tissues has been used to encapsulate MSCs, which produced tissue-specific responses after encapsulation and printing [77].
(b). Neural stem cells
Neurodegenerative diseases and challenging glioma tumours make bioprinted neural tissue from neural stem cells (NSCs) an attractive prospect for regenerative medicine and disease modelling. Encapsulation of NSCs seems to better mimic their endogenous phenotype when compared to culturing on TCPs, likely due to the similarities in stiffness of soft hydrogels to neural tissue. One study showed that murine NSCs encapsulated in a water-based polyurethane gel showed increased expression of neurotrophic genes such as glial cell line-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) compared with TCPs [78]. A zebrafish model of traumatic brain injury also showed significant recovery over untreated control when the bioprinted NSCs were implanted, giving hope to neural regeneration. In another example, human NSCs were encapsulated and printed in an agarose, alginate and carboxymethylcellulose-based bio-ink. Cells showed high viability post-printing, as well as being functionally active following a defined differentiation protocol [66]. Glioma tumours are challenging to treat owing to their malignancy, recurrence and resistance to drugs. However, this drug-resistance is not well recapitulated in standard culture conditions. To develop a more robust disease model and drug testing platform, researchers used a bio-ink that better mimicked the endogenous tumour micro-environment and embedded glioma stem cells therein [79]. Glioma cells maintained their phenotype and showed resistance to anti-cancer drugs, which they do not in standard 2D cultures, showing that bioprinted glioma tissue was a more robust glioma model to study the biology, and the drug treatment, of the cancer.
(c). Pluripotent stem cells
PSC are cells of near unlimited potential owing to their pluripotent nature and theoretical unlimited self-renewal. Human embryonic stem cells (hESC) and the more recently described human-induced pluripotent stem cells (hiPSC) represent the two major sources of PSC [80–83]. In such a light, they seem ideal candidates for generating renewable human tissue. Compared with MSCs, hESCs are more fragile and prone to spontaneous differentiation. To overcome this, a novel valve-based printing method was developed to print hESCs for the first time [16]. This technology was then used to print and differentiate hiPSC into hepatocytes [17]. Since then, the use of the Rho-kinase inhibitor Y-27632 to improve pluripotent cell viability [84], their use to form spheroids and organoids [85], and the possession of optimized printing and bio-ink parameters have allowed more diverse pluripotent cell printing. While still debatably in its infancy, bioprinting pluripotent cells has already been used to control the size of embryoid body formation [86], produce hepatocyte [54,87], neural [28] and pancreatic cells [26], as well as exploring their multilineage differentiation to all three germ lines [28,88].
Liver tissue is formed in characteristic hexagonal lobules, and bioprinting has been used to produce these shapes with hepatocytes and supporting cells in defined regions. By patterning matrix materials and then depositing different bio-inks, tissue-like architecture was achieved that supported hepatocytes matured from iPSCs [54]. Vascularizing artificial tissue is one of tissue engineering's largest hurdles, and pre-vascularized bioprinted liver tissue has been produced to combat this. By first differentiating both endothelial and hepatocytes from iPSCs, multi-fibred tissue structures were produced [89]. When transplanted, host vasculature also penetrated the structure, as seen by human albumin in the host animal. Treatment of degenerative diseases, such as diabetes, is an appealing translation of bioprinting research. As such, researchers have shown that by printing naive ESCs in alginate and then differentiating them to pancreatic islet-like cells, they produced strong markers characteristic of pancreatic islet cells [26]. The encapsulation of these artificial islets also makes them amenable to transplantation, as the alginate shell would provide immune-protection from the host. Bioprinting of naive pluripotent cells has also been shown with their directed differentiation towards neural lineages [28]. This resulted in a population of mature, mixed phenotypes. Notably, functional GABAergic neurons were present, with supporting neuroglia.
5. Opportunities and future challenges
Bioprinted tissues have already found commercial success. The company Organovo is one of the few providing bioprinted human tissue for drug testing or research needs (Functional Stability of exVive3D™ Liver, Bioprinted Human Tissues). Both liver and kidney tissue are available, composed of parenchymal and supporting cells, in suitable bio-ink. These are not derived from PSC, however. Censo Biotechnologies (formerly Roslin Cells) also offers bioprinting as part of their custom cell culture service [17].
Bioprinter platforms are gaining traction commercially including, among others, RegenHU, GemSIM and Cellink. The BioPen from the University of Wollongong introduces a handheld device for use during surgery for deposition of a chondrocyte-laden bio-ink to treat cartilage defects [29]. Cartilage is one of the favoured candidates for tissue engineering, in general, owing to its minimal vasculature.
To combat the issue of scale in bioprinting, the integrated tissue-organ printer (ITOP) [24] was constructed by researchers at the Wake Forest Institute to generate large, human-sized tissue structures. The printer uses multiple nozzles to deposit multiple types of bio-inks at once, to generate tissue. Some of these bio-inks are ‘sacrificial’, so following printing these inks can be removed from the structure, generating micro-channels for nutrient delivery or vascularization. The design for the printed tissue is sourced from clinical imaging data.
Bioprinting has yet to break through to clinical translation, likely due to regulatory issues, limitations of the size of structures and insufficient good manufacturing practice (GMP) infrastructure to support it. However, research has pushed bioprinting closer to the clinical stage, such as the BioPen and ITOP printer as mentioned previously.
For drug testing on truly biomimetic tissue, or transplantation of bioengineered organs to become a reality, significant hurdles must be overcome [5]. The source of the cells and materials used to construct the tissue must be both renewable and cost-effective. In the case of stem cells, renewability is less of an issue, but control of the cells becomes more important. Stem cells, particularly PSC, are fragile and prone to spontaneous differentiation, so well-defined printing and encapsulation methods must be in place for reproducibility. This is necessary to eliminate any spontaneous differentiation or cancer formation after implantation. Material selection for bio-ink composition is equally crucial. Not only must the material support the biological function in the tissue, ideally by mimicking endogenous stem-niche biomatrix, the material must be amenable to the bioprinting process itself. Different bioprinting technologies are limited to material of specific viscosities: too low and the structure will lose resolution or not form; too high and the nozzle will clog and shear stress will destroy cells during deposition.
Tissue is a complex arrangement of cells and materials, and this is likely the crucial characteristic that is most often lost in in vitro cultures. Selection of cells and materials must be followed by their careful and precise positioning during fabrication to mimic endogenous tissue. Bioprinting is a ‘bottom-up’ approach to biofabrication, where both material and cell deposition can be controlled in three dimensions. This gives bioprinting more flexibility in fabrication than other methods, but also has caveats dependent on the printing technology used. A balance must always be reached as higher resolution typically results in slower print times, poorer cell viability and increased price.
The format in which the cells are deposited also plays a role in tissue fabrication, with research showing various compositions of cells to be successfully bioprinted. Living cells have been successfully printed in suspension [17], encapsulated in hydrogel [90], preformed into spheroids before encapsulation [91], as well as formed into whole-cell fibres by bioprinting [92,93]. The malleability of this technique shows that it can be tailored to the formation of various, differently structured, tissue types.
After well-researched design and proper technology choice, the next limiting factor of bioprinted tissue is size. As the desired construct becomes larger the subsequent printing time becomes longer, which can lead to decreased cell viability and structural integrity. As structures grow in size, diffusion of nutrients also becomes increasingly hampered. In endogenous tissue, this is overcome through the vasculature supplying blood and nutrients throughout. Lack of this in large bioprinted tissue results in the development of necrotic regions that have received inadequate nutrition. Introducing or including an artificial vasculature is an area of avid research in bioprinting [18,24,94], and is seen in many ways as the ‘holy grail’ needed to push construction of larger, organ-like structures.
A boon of stem-cell derived artificial tissue is the ability to generate autologous grafts from the patient's own stem cells. While in theory this has fantastic potential, it is met by the need for appropriate GMP grade handling facilities where a patient's own stem cells can be cultured, expanded, differentiated and bioprinted, all while under strictly-defined conditions and free from contamination from other patients' cells. The infrastructure for such an endeavour is not yet in place, and, while in some ways the ultimate future of personalized medicine, it is still some way in the future.
6. Conclusion
Bioprinting is an area of avid research, bringing together fields of cell biology, mechanical engineering, material science and many others. Stem-cell bioprinting shows potential as a source for renewable human tissue. Various types of artificial tissues have been formed using bioprinted stem cells, from liver to brain. Printing adult stem cells seems to be closer to translation to the clinic or industry, as they are often more controllable and defined, whereas PSC have problems pertaining to viability and spontaneous differentiation.
At the moment, bioprinting is rapidly reaching a point where its main hurdles for translational are in the need for intrinsic vasculature in large bioprinted structures, and the controlled differentiation of stem cells in situ in 3D. Vasculature is necessary to support nutrient and gas exchange in larger engineered structures to prevent the formation of necrotic areas. The step from 2D to 3D cell culture also requires new optimisation and developments in the protocols required to control cell fate decisions for controlled differentiation. If both can be tackled, production of allogenic and autologous tissue replacement willl move from the realm of science fiction to reality. If both can be tackled, production of allogenic and autologous tissue replacement moves from the realm of science fiction to reality.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
G.S. acknowledges the funding support from Baillie Gifford & Co. and Heriot–Watt University for the PhD Scholarship.
References
- 1.Morrison SJ, Spradling AC. 2008. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611. ( 10.1016/j.cell.2008.01.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khademhosseini A, Langer R. 2016. A decade of progress in tissue engineering. Nat. Protoc. 11, 1775–1781. ( 10.1038/nprot.2016.123) [DOI] [PubMed] [Google Scholar]
- 3.Moroni L, et al. 2018. Biofabrication: a guide to technology and terminology. Trends Biotechnol. 36, 384–402. ( 10.1016/j.tibtech.2017.10.015) [DOI] [PubMed] [Google Scholar]
- 4.Groll J, et al. 2016. Biofabrication: reappraising the definition of an evolving field. Biofabrication 8, 13001 ( 10.1088/1758-5090/8/1/013001) [DOI] [PubMed] [Google Scholar]
- 5.Holmes AM, Charlton A, Derby B, Ewart L, Scott A, Shu W. 2017. Rising to the challenge: applying biofabrication approaches for better drug and chemical product development. Biofabrication 9, 33001 ( 10.1088/1758-5090/aa7bbd) [DOI] [PubMed] [Google Scholar]
- 6.Murphy SV, Atala A. 2014. 3D bioprinting of tissues and organs. Nat. Biotechnol. 32, 773–785. ( 10.1038/nbt.2958) [DOI] [PubMed] [Google Scholar]
- 7.Cornelissen D-J, Faulkner-Jones A, Shu W. 2017. Current developments in 3D bioprinting for tissue engineering. Curr. Opin. Biomed. Eng. 2, 76–82. ( 10.1016/j.cobme.2017.05.004) [DOI] [Google Scholar]
- 8.Vermeulen N, Haddow G, Seymour T, Faulkner-Jones A, Shu W. 2017. 3D bioprint me: a socioethical view of bioprinting human organs and tissues. J. Med. Ethics 43, 618–624. ( 10.1136/medethics-2015-103347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu T, Jin J, Gregory C, Hickman JJ, Boland T. 2005. Inkjet printing of viable mammalian cells. Biomaterials 26, 93–99. ( 10.1016/j.biomaterials.2004.04.011) [DOI] [PubMed] [Google Scholar]
- 10.Yanez M, Rincon J, Dones A, De Maria C, Gonzales R, Boland T. 2015. In vivo assessment of printed microvasculature in a bilayer skin graft to treat full-thickness wounds. Tissue Eng. Part A 21, 224–233. ( 10.1089/ten.tea.2013.0561) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui X, Gao G, Yonezawa T, Dai G. 2014. Human cartilage tissue fabrication using three-dimensional inkjet printing technology. J. Vis. Exp. 10, e51294 ( 10.3791/51294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao G, Schilling AF, Hubbell K, Yonezawa T, Truong D, Hong Y, Dai G, Cui X. 2015. Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol. Lett. 37, 2349–2355. ( 10.1007/s10529-015-1921-2) [DOI] [PubMed] [Google Scholar]
- 13.Laronda M, Rutz AL, Xiao S, Whelan KA, Duncan FE, Roth EW, Woodruff TK, Shah RN. 2017. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat. Commun. 8, 15261 ( 10.1038/ncomms15261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsusaki M, Sakaue K, Kadowaki K, Akashi M. 2013. Three-dimensional human tissue chips fabricated by rapid and automatic inkjet cell printing. Adv. Healthc. Mater. 2, 534–539. ( 10.1002/adhm.201200299) [DOI] [PubMed] [Google Scholar]
- 15.Lorber B, Hsiao W-K, Hutchings IM, Martin KR. 2013. Adult rat retinal ganglion cells and glia can be printed by piezoelectric inkjet printing. Biofabrication 6, 15001 ( 10.1088/1758-5082/6/1/015001) [DOI] [PubMed] [Google Scholar]
- 16.Faulkner-Jones A, Greenhough S, King JA, Gardner J, Courtney A, Shu W. 2013. Development of a valve-based cell printer for the formation of human embryonic stem cell spheroid aggregates. Biofabrication 5, 15013 ( 10.1088/1758-5082/5/1/015013) [DOI] [PubMed] [Google Scholar]
- 17.Faulkner-Jones A, Fyfe C, Cornelissen D-J, Gardner J, King J, Courtney A, Shu W. 2015. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication 7, 44102 ( 10.1088/1758-5090/7/4/044102) [DOI] [PubMed] [Google Scholar]
- 18.Zhu W, et al. 2017. Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials 124, 106–115. ( 10.1016/j.biomaterials.2017.01.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H, et al. 2016. One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab. Chip 16, 2618–2625. ( 10.1039/C6LC00450D) [DOI] [PubMed] [Google Scholar]
- 20.Guillemot F, et al. 2010. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. 6, 2494–2500. ( 10.1016/j.actbio.2009.09.029) [DOI] [PubMed] [Google Scholar]
- 21.Guillotin B, et al. 2010. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 31, 7250–7256. ( 10.1016/j.biomaterials.2010.05.055) [DOI] [PubMed] [Google Scholar]
- 22.Lozano R, et al. 2015. 3D printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials 67, 264–273. ( 10.1016/j.biomaterials.2015.07.022) [DOI] [PubMed] [Google Scholar]
- 23.Jitraruch S, et al. 2014. Alginate microencapsulated hepatocytes optimised for transplantation in acute liver failure. PLoS ONE 9, e113609 ( 10.1371/journal.pone.0113609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. 2016. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 34, 312–319. ( 10.1038/nbt.3413) [DOI] [PubMed] [Google Scholar]
- 25.Byambaa B, et al. 2017. Bioprinted osteogenic and vasculogenic patterns for engineering 3D bone tissue. Adv. Healthc. Mater. 6, 1700015 ( 10.1002/adhm.201700015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson T, Kumta PN, Banerjee I. 2014. Alginate encapsulation of human embryonic stem cells to enhance directed differentiation to pancreatic islet-like cells. Tissue Eng. Part A 20, 3198–3211. ( 10.1089/ten.tea.2013.0659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouyang L, Yao R, Mao S, Chen X, Na J, Sun W. 2015. Three-dimensional bioprinting of embryonic stem cells directs highly uniform embryoid body formation. Biofabrication 7, 44101 ( 10.1088/1758-5090/7/4/044101) [DOI] [PubMed] [Google Scholar]
- 28.Gu Q, Tomaskovic-Crook E, Wallace GG, Crook JM. 2017. 3D bioprinting human induced pluripotent stem cell constructs for in situ cell proliferation and successive multilineage differentiation. Adv. Healthc. Mater. 6, 1700175 ( 10.1002/adhm.201700175) [DOI] [PubMed] [Google Scholar]
- 29.O'Connell CD, et al. 2016. Development of the Biopen: a handheld device for surgical printing of adipose stem cells at a chondral wound site. Biofabrication 8, 15019 ( 10.1088/1758-5090/8/1/015019) [DOI] [PubMed] [Google Scholar]
- 30.Roth EA, Xu T, Das M, Gregory C, Hickman JJ, Boland T. 2004. Inkjet printing for high-throughput cell patterning. Biomaterials 25, 3707–3715. ( 10.1016/j.biomaterials.2003.10.052) [DOI] [PubMed] [Google Scholar]
- 31.Cui X, Dean D, Ruggeri ZM, Boland T. 2010. Cell damage evaluation of thermal inkjet printed Chinese hamster ovary cells. Biotechnol. Bioeng. 106, 963–969. ( 10.1002/bit.22762) [DOI] [PubMed] [Google Scholar]
- 32.Ng WL, Lee JM, Yeong WY, Naing MW. 2017. Microvalve-based bioprinting – process, bio-inks and applications. Biomater. Sci. 5, 632–647. ( 10.1039/C6BM00861E) [DOI] [PubMed] [Google Scholar]
- 33.Horváth L, Umehara Y, Jud C, Blank F, Petri-Fink A, Rothen-Rutishauser B. 2015. Engineering an in vitro air-blood barrier by 3D bioprinting. Sci. Rep. 5, 7974 ( 10.1038/srep07974) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koch L, et al. 2010. Laser printing of skin cells and human stem cells. Tissue Eng. Part C. Methods 16, 847–854. ( 10.1089/ten.tec.2009.0397) [DOI] [PubMed] [Google Scholar]
- 35.Zhang AP, Qu X, Soman P, Hribar KC, Lee JW, Chen S, He S. 2012. Rapid fabrication of complex 3D extracellular microenvironments by dynamic optical projection stereolithography. Adv. Mater. 24, 4266–4270. ( 10.1002/adma.201202024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ouyang X, Zhang K, Wu J, Wong DS-H, Feng Q, Bian L, Zhang AP. 2017. Optical µ-printing of cellular-scale microscaffold arrays for 3D cell culture. Sci. Rep. 7, 997 ( 10.1038/s41598-017-08598-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikehata H, Ono T. 2011. The mechanisms of UV mutagenesis. J. Radiat. Res. 52, 115–125. ( 10.1269/jrr.10175) [DOI] [PubMed] [Google Scholar]
- 38.Pati F, Jang J, Lee JW, Cho DW. 2015. Extrusion bioprinting. In Essentials of 3D biofabrication and translation (ed. JJ Yo), pp. 123–152 Boston, MA: Academic: ( 10.1016/B978-0-12-800972-7.00007-4) [DOI] [Google Scholar]
- 39.Hinton TJ, et al. 2015. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 1, e1500758 ( 10.1126/sciadv.1500758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghanizadeh A, Mills CG, Mullins JJ, Davies JA, Shu W. 2017. Rapid fabrication of cell-laden alginate hydrogel 3D structures by micro dip-coating. Front. Bioeng. Biotechnol. 5, 13 ( 10.3389/fbioe.2017.00013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Underhill GH, Chen AA, Albrecht DR, Bhatia SN. 2007. Assessment of hepatocellular function within PEG hydrogels. Biomaterials 28, 256–270. ( 10.1016/j.biomaterials.2006.08.043) [DOI] [PubMed] [Google Scholar]
- 42.Lee W, Debasitis JC, Lee VK, Lee J-H, Fischer K, Edminster K, Park J-K, Yoo S-S. 2009. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials 30, 1587–1595. ( 10.1016/j.biomaterials.2008.12.009) [DOI] [PubMed] [Google Scholar]
- 43.Hospodiuk M, Dey M, Sosnoski D, Ozbolat IT. 2017. The bioink: a comprehensive review on bioprintable materials. Biotechnol. Adv. 35, 217–239. ( 10.1016/j.biotechadv.2016.12.006) [DOI] [PubMed] [Google Scholar]
- 44.Liu J, Zheng H, Poh PSP, Machens HG, Schilling AF. 2015. Hydrogels for engineering of perfusable vascular networks. Int. J. Mol. Sci. 16, 15 997–16 016. ( 10.3390/ijms160715997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wells RG. 2008. The role of matrix stiffness in regulating cell behavior. Hepatology 47, 1394–1400. ( 10.1002/hep.22193) [DOI] [PubMed] [Google Scholar]
- 46.Wells RG. 2013. Tissue mechanics and fibrosis. Biochim. Biophys. Acta 1832, 884–890. ( 10.1016/j.bbadis.2013.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duarte CDF, Blaeser A, Korsten A, Neuss S, Jäkel J, Vogt M, Fischer H. 2015. The stiffness and structure of three-dimensional printed hydrogels direct the differentiation of mesenchymal stromal cells toward adipogenic and osteogenic lineages. Tissue Eng. Part A 21, 740–756. ( 10.1089/ten.tea.2014.0231) [DOI] [PubMed] [Google Scholar]
- 48.Carrow JK, Kerativitayanan P, Jaiswal MK, Lokhande G, Gaharwar AK. 2015. Polymers for bioprinting. Essen. 3D Biofabr. Transl. 1, 229–248. ( 10.1016/B978-0-12-800972-7.00013-X) [DOI] [Google Scholar]
- 49.Mørch ÝA, Donati I, Strand BL, Skjåk-Bræk G. 2003. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 7, 1471–1480, 2006. ( 10.1021/bm060010d) [DOI] [PubMed] [Google Scholar]
- 50.Jia J, et al. 2014. Engineering alginate as bioink for bioprinting. Acta Biomater. 10, 4323–4331. ( 10.1016/j.actbio.2014.06.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee KY, Mooney DJ. 2013. Alginate: properties and biomedical applications. Prog. Polym. Sci. 37, 106–126. ( 10.1016/j.progpolymsci.2011.06.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Z, Su X, Xu Y, Kong B, Sun W, Mi S. 2016. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci. Rep. 6, 24474 ( 10.1038/srep24474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kober J, Gugerell A, Schmid M, Kamolz LP, Keck M. 2015. Generation of a fibrin based three-layered skin substitute. Biomed Res. Int. 2015, 170427 ( 10.1155/2015/170427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma X, et al. 2016. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl Acad. Sci. USA 113, 2206–2211. ( 10.1073/pnas.1524510113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferris CJ, Gilmore KJ, Wallace GG, In M, Panhuis H. 2013. Modified gellan gum hydrogels for tissue engineering applications. Soft Matter 9, 3705–3711. ( 10.1039/c3sm27389j) [DOI] [Google Scholar]
- 56.Lambrichta L, et al. 2014. The type and composition of alginate and hyaluronic-based hydrogels influence the viability of stem cells of the apical papilla. Dent. Mater. 30, e349–e361. ( 10.1016/j.dental.2014.08.369) [DOI] [PubMed] [Google Scholar]
- 57.Gao G, Yonezawa T, Hubbell K, Dai G, Cui X. 2015. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol. J. 10, 1568–1577. ( 10.1002/biot.201400635) [DOI] [PubMed] [Google Scholar]
- 58.Zhang K, Fu Q, Yoo J, Chen X, Chandra P, Mo X, Song L, Atala A, Zhao W. 2016. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: an in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 50, 154–164. ( 10.1016/j.actbio.2016.12.008) [DOI] [PubMed] [Google Scholar]
- 59.Hölzl K, Lin S, Tytgat L, Van Vlierberghe S, Gu L, Ovsianikov A. 2016. Bioink properties before, during and after 3D bioprinting. Biofabrication 8, 32002 ( 10.1088/1758-5090/8/3/032002) [DOI] [PubMed] [Google Scholar]
- 60.Tabriz AG, Hermida MA, Leslie NR, Shu W. 2015. Three-dimensional bioprinting of complex cell laden alginate hydrogel structures. Biofabrication 7, 45012 ( 10.1088/1758-5090/7/4/045012) [DOI] [PubMed] [Google Scholar]
- 61.Huang S-L, Lin Y-S. 2017. The size stability of alginate beads by different ionic crosslinkers. Adv. Mater. Sci. Eng. 2017, 1–7. ( 10.1155/2017/9304592) [DOI] [Google Scholar]
- 62.Wynn TA. 2008. Cellular and molecular mechanisms of fibrosis. J. Pathol. 214, 199–210. ( 10.1002/path.2277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paxton N, Smolan W, Böck T, Melchels F, Groll J, Jungst T. 2017. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 9, 44107 ( 10.1088/1758-5090/aa8dd8) [DOI] [PubMed] [Google Scholar]
- 64.He Y, Yang F, Zhao H, Gao Q, Xia B, Fu J. 2016. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 6, 921 ( 10.1038/srep29977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Merceron TK, Murphy SV. 2015. Hydrogels for 3D bioprinting applications. In Essentials of 3D biofabrication and translation (ed. JJ Yo), pp. 249–270 Boston, MA: Academic: ( 10.1016/B978-0-12-800972-7.00014-1) [DOI] [Google Scholar]
- 66.Gu Q, Tomaskovic-Crook E, Lozano R, Chen Y, Kapsa RM, Zhou Q, Wallace GG, Crook JM. 2016. Functional 3D neural mini-tissues from printed gel-based bioink and human neural stem cells. Adv. Healthc. Mater. 5, 1429–1438. ( 10.1002/adhm.201600095) [DOI] [PubMed] [Google Scholar]
- 67.Daly AC, Cunniffe GM, Sathy BN, Jeon O, Alsberg E, Kelly DJ. 2016. 3D bioprinting of developmentally inspired templates for whole bone organ engineering. Adv. Healthc. Mater. 5, 2353–2362. ( 10.1002/adhm.201600182) [DOI] [PubMed] [Google Scholar]
- 68.Armstrong JPK, Burke M, Carter BM, Davis SA, Perriman AW. 2016. 3D bioprinting using a templated porous bioink. Adv. Healthc. Mater. 5, 1724–1730. ( 10.1002/adhm.201600022) [DOI] [PubMed] [Google Scholar]
- 69.Ullah I, Subbarao RB, Rho GJ. 2015. Human mesenchymal stem cells - current trends and future prospective. Biosci. Rep. 35, e00191 ( 10.1042/BSR20150025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pati F, Ha D-H, Jang J, Han HH, Rhie J-W, Cho D-W. 2015. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials 62, 164–175. ( 10.1016/j.biomaterials.2015.05.043) [DOI] [PubMed] [Google Scholar]
- 71.Xu M, Wang X, Yan Y, Yao R, Ge Y. 2010. An cell-assembly derived physiological 3D model of the metabolic syndrome, based on adipose-derived stromal cells and a gelatin/alginate/fibrinogen matrix. Biomaterials 31, 3868–3877. ( 10.1016/j.biomaterials.2010.01.111) [DOI] [PubMed] [Google Scholar]
- 72.Gao G, Schilling AF, Yonezawa T, Wang J, Dai G, Cui X. 2014. Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol. J. 9, 1304–1311. ( 10.1002/biot.201400305) [DOI] [PubMed] [Google Scholar]
- 73.Du M, et al. 2015. 3D bioprinting of BMSC-laden methacrylamide gelatin scaffolds with CBD-BMP2-collagen microfibers. Biofabrication 7, 44104 ( 10.1088/1758-5090/7/4/044104) [DOI] [PubMed] [Google Scholar]
- 74.Blaeser A, Duarte Campos DF, Puster U, Richtering W, Stevens MM, Fischer H. 2016. Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv. Healthc. Mater. 5, 326–333. ( 10.1002/adhm.201500677) [DOI] [PubMed] [Google Scholar]
- 75.Bourget J-M, et al. 2016. Patterning of endothelial cells and mesenchymal stem cells by laser-assisted bioprinting to study cell migration. Biomed Res. Int. 2016, 3569843 ( 10.1155/2016/3569843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu H, Tay CY, Pal M, Leong WS, Li H, Li H, Wen F, Leong DT, Tan LP. 2013. A bio-inspired platform to modulate myogenic differentiation of human mesenchymal stem cells through focal adhesion regulation. Adv. Healthc. Mater. 2, 442–449. ( 10.1002/adhm.201200142) [DOI] [PubMed] [Google Scholar]
- 77.Pati F, et al. 2014. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 5, 3935 ( 10.1038/ncomms4935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hsieh F-Y, Lin H-H, Hsu S-H. 2015. 3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials 71, 48–57. ( 10.1016/j.biomaterials.2015.08.028) [DOI] [PubMed] [Google Scholar]
- 79.Dai X, Ma C, Lan Q, Xu T. 2016. 3D bioprinted glioma stem cells for brain tumor model and applications of drug susceptibility. Biofabrication 8, 45005 ( 10.1088/1758-5090/8/4/045005) [DOI] [PubMed] [Google Scholar]
- 80.Cai J, et al. 2006. Assessing self-renewal and differentiation in human embryonic stem cell lines. Stem Cells 24, 516–530. ( 10.1634/stemcells.2005-0143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoffman LM, Carpenter MK. 2005. Characterization and culture of human embryonic stem cells. Nat. Biotechnol. 23, 699–708. ( 10.1038/nbt1102) [DOI] [PubMed] [Google Scholar]
- 82.Vazin T, Freed WJ. 2010. Human embryonic stem cells: derivation, culture, and differentiation: a review. Restor. Neurol. Neurosci. 28, 589–603. ( 10.3233/RNN-2010-0543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takahashi K, et al. 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. ( 10.1016/j.cell.2007.11.019) [DOI] [PubMed] [Google Scholar]
- 84.Claassen DA, Desler MM, Rizzino A. 2009. ROCK inhibition enhances the recovery and growth of cryopreserved human embryonic stem cells and human induced pluripotent stem cells. Mol. Reprod. Dev. 76, 722–732. ( 10.1002/mrd.21021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sampaziotis F, et al. 2017. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat. Med. 41, 1407–1963. ( 10.1038/nm.4360) [DOI] [PubMed] [Google Scholar]
- 86.Dias AD, Unser AM, Xie Y, Chrisey DB, Corr DT. 2014. Generating size-controlled embryoid bodies using laser direct-write. Biofabrication 6, 025007 ( 10.1088/1758-5082/6/2/025007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pettinato G, Ramanathan R, Fisher RA, Mangino MJ, Zhang N, Wen X. 2016. Scalable differentiation of human iPSCs in a multicellular spheroid-based 3d culture into hepatocyte-like cells through direct Wnt/β-catenin pathway inhibition. Sci. Rep. 6, 32888 ( 10.1038/srep32888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sidhu K, Kim J, Chayosumrit M, Dean S, Sachdev P. 2012. Alginate microcapsule as a 3D platform for propagation and differentiation of human embryonic stem cells (hESC) to different lineages. J. Vis. Exp. 9, 3608 ( 10.3791/3608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Du C, Narayanan K, Leong MF, Wan ACA. 2014. Induced pluripotent stem cell-derived hepatocytes and endothelial cells in multi-component hydrogel fibers for liver tissue engineering. Biomaterials 35, 6006–6014. ( 10.1016/j.biomaterials.2014.04.011) [DOI] [PubMed] [Google Scholar]
- 90.Li C, et al. 2015. Rapid formation of a supramolecular polypeptide-DNA hydrogel for in situ three-dimensional multilayer bioprinting. Angew. Chem. Int. Ed. 54, 3957–3961. ( 10.1002/anie.201411383) [DOI] [PubMed] [Google Scholar]
- 91.Mironov V, Kasyanov V, Markwald RR. 2011. Organ printing: from bioprinter to organ biofabrication line. Curr. Opin. Biotechnol. 22, 667–673. ( 10.1016/j.copbio.2011.02.006) [DOI] [PubMed] [Google Scholar]
- 92.Onoe H, et al. 2013. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater. 12, 584–590. ( 10.1038/nmat3606) [DOI] [PubMed] [Google Scholar]
- 93.Yu Y, Moncal KK, Li J, Peng W, Rivero I, Martin JA, Ozbolat IT. 2016. Three-dimensional bioprinting using self-assembling scalable scaffold-free tissue strands as a new bioink. Sci. Rep. 6, 28714 ( 10.1038/srep28714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gao Q, He Y, Fu J-Z, Liu A, Ma L. 2015. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials 61, 203–215. ( 10.1016/j.biomaterials.2015.05.031) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.