Abstract
Hepatic stress and injury from drugs continues to be a major concern within the pharmaceutical industry, leading to preclinical and clinical attrition precautionary warnings and post-market withdrawal of drugs. There is a requirement for more predictive and mechanistically accurate models to aid risk assessment. Primary human hepatocytes, subject to isolation stress, cryopreservation, donor-to-donor variation and a relatively short period of functional capability in two-dimensional cultures, are not suitable for high-throughput screening procedures. There are two areas within the drug discovery pipeline that the generation of a stable, metabolically functional hepatocyte-like cell with unlimited supply would have major impact. First, in routine, cell health risk-assessment assays where hepatic cell lines are typically deployed. Second, at later stages of the drug discovery pipeline approaching candidate nomination where bespoke/investigational studies refining and understanding the risk to patients use patient-derived induced pluripotent stem cell (iPSC) hepatocytes retaining characteristics from the patient, e.g. HLA susceptibility alleles, iPSC hepatocytes with defined disease phenotypes or genetic characteristics that have the potential to make the hepatocyte more sensitive to a particular stress mechanism. Functionality of patient-centric hepatocyte-like cells is likely to be enhanced when coupled with emerging culture systems, such as three-dimensional spheroids or microphysiological systems. Ultimately, the aspiration to confidently use human-relevant in vitro models to predict human-specific hepatic toxicity depends on the integration of promising emerging technologies.
This article is part of the theme issue ‘Designer human tissue: coming to a lab near you’.
Keywords: hepatocyte, pharmaceutical industry, risk assessment, drug safety
1. Introduction
Adverse drug reactions (ADRs) are significant health problems that contribute to illness and fatalities within populations receiving medication. Additionally, they are a financial burden on healthcare systems [1]. ADRs are a major issue for the pharmaceutical industry leading to attrition of drugs in development and the post-licensing withdrawal of drugs [1,2]. There are many different types of ADRs, affecting every organ system in the body. However, drug-induced liver injury (DILI) is the most frequent reason for the withdrawal of an approved drug from the market, and it also accounts for more than 50% of cases of acute liver failure. More than 600 drugs have been associated with hepatotoxicity [3]. The clinical disease phenotype is diverse, multidimensional and multifaceted, even for the same drug when given to different patients. The manifestations range from mild, asymptomatic changes in serum transaminases, which occur at a relatively high frequency with a number of drugs, to fulminant hepatic failure, which although rare, is potentially life-threatening and may necessitate a liver transplant. Most drug-induced hepatic injuries that occur in humans are unpredictable and poorly understood. Although the asymptomatic rises in transaminases are common, the more severe forms of liver damage are fortunately rare, generally occurring with a frequency between 1 in 1000 and 1 in 10 000 [3]. Acetaminophen (paracetamol; APAP) is responsible for 80% of drug-associated cases of liver failure [4]. APAP-induced hepatotoxicity is generally predictable from our understanding of its metabolism; however, many other drugs cause idiosyncratic DILI, which, although rare and unpredictable, can cause significant morbidity and mortality. Studies with model compounds and drugs—such as APAP—have helped to define the roles that chemical stress and drug bioactivation have in the various biological outcomes that may be triggered by chemically reactive metabolites. These include effects on transcription factors and/or signalling protein-adaptation (cell defence), apoptosis, necrosis, inflammation and activation of the innate and adaptive immune systems. Adverse drug reactions affecting the liver may be classified into two main types:
— Dose-dependent DILI, which can be replicated in various animal species and is evident during preclinical safety testing (provided the target or off-target is adequately expressed in the animal model).
— Human-specific DILI, which cannot be predicted in animal models. Includes idiosyncratic drug reactions which are unpredictable, occur only in certain susceptible patients and have a complex dose-dependent relationship.
Drug-induced liver toxicity mimics natural disease and, therefore, lessons learned from the study of drug-induced hepatotoxicity should not only enhance drug safety but also provide new pharmacological strategies for the treatment of liver disease. The major advances in molecular toxicology over the past decade have provided a conceptual framework for the mechanism of action of model hepatotoxins at the chemical, molecular, biochemical and cellular levels. In particular, we now have a better understanding of the events that link drug metabolism and the formation of toxic metabolites to changes in liver function and the evolution of liver pathology [2,3,5]. With respect to dose-dependent DILI we now understand that the analgesic acetaminophen, at therapeutic doses, is metabolized predominantly by the phase II metabolic pathways of glucuronidation and sulfation. There is a species- and strain-dependent proportion of APAP which is metabolized by the phase I cytochrome P450 metabolic pathway to the reactive metabolite NAPQI (N-acetyl-p-benzoquinone imine), which is subsequently detoxified by conjugation with glutathione (GSH) [6–8]. In higher-dose situations, the sulfation pathway becomes saturated with depletion of the sulfate pool, the cofactors of glucuronidation become depleted and hence a larger portion of APAP is metabolized through the phase I pathway. GSH levels are limited and once depleted below a critical level, NAPQI is free to react with cellular macromolecules. After formation of significant quantities of NAPQI, the pathways culminating in cellular injury have been extensively investigated, but their contributions to the actual pathogenesis are more tentative [6–8]. Based on the weight of evidence, NAPQI binds to various proteins and disrupts their function, leading to altered cellular function. However, there are likely to be other direct or indirect effects of NAPQI leading to cell death, such as alteration of cellular redox status or disruption of signalling pathways. Despite the multitude of cellular pathways that have been shown to play a role in APAP-induced hepatotoxicity, disruption of mitochondrial function is one of the key outcomes [6–8]. After covalent binding and GSH depletion occur, APAP induces the mitochondrial permeability transition (MPT), which allows the leakage of mitochondrial constituents into the cytosol. After activation of the MPT, mitochondria swell, lose membrane potential, and exhibit decreased oxidative phosphorylation and ATP depletion. Finally, depending upon the species, strain and fed/fasting status, there may be a window where some hepatic apoptosis occurs, which rapidly degenerates into necrosis [6–8]. Regarding the molecular mechanisms underlying human-specific DILI, a good example is that of fialuridine. Fialuridine was developed as an antiviral therapy for hepatitis B infection. In a phase II study, fialuridine caused severe toxicity: irreversible acute hepatic failure in 7 out of 15 patients, myopathy, myoglobinuria, severe lactic acidosis and neuropathy after 9–13 weeks of treatment [9]. Five out of seven participants with severe hepatotoxicity died and two survived after liver transplantation. Preclinical toxicology studies in mice, rats, dogs and primates did not provide any indication that fialuridine would be hepatotoxic in humans. Histological analysis of human liver tissue showed prominent accumulation of microvesicular fat, with chronic active hepatitis and variable degrees of macrovesicular steatosis, but little hepatocellular necrosis, which is consistent with the absence of substantial elevations in serum aminotransferase levels during treatment [10]. Electron microscopy showed markedly swollen mitochondria, with loss of cristae, matrix dissolution and scattered vesicular inclusions. In studies of fialuridine in a human hepatoma cell line (Hep G2), the drug was incorporated into both nuclear and mitochondrial DNA, but at a much higher rate in the latter [11]. Morphologic changes in mitochondria, microsteatosis, macrosteatosis and increased lactic acid production were also observed. The integration of nucleoside analogues into nuclear DNA represents an alternative but potentially delayed pathway to cytotoxicity and cell apoptosis. Expression of a nucleoside transporter hENT1 in human (but not in mouse) mitochondria, which facilitates entry of fialuridine into mitochondria, may be responsible for the human-specific mitochondrial toxicity caused by fialuridine [12]. Recently, it has been shown that chimeric mice could be used as a model for fialuridine toxicity. The clinical features, laboratory abnormalities, liver histology and ultrastructural changes observed were the same as in humans, and these abnormalities developed in the regions of the livers that contained human hepatocytes but not in regions that contained mouse hepatocytes [13].
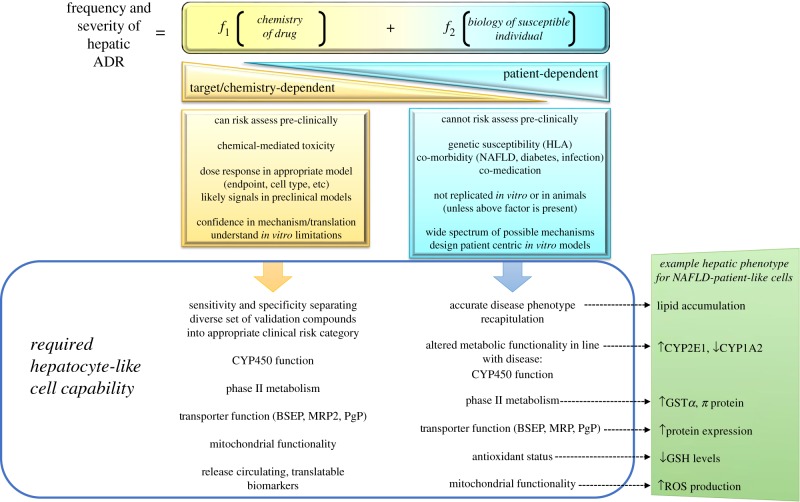
Patients present with a pattern of liver injury that is broadly consistent for each drug and may, therefore, be termed idiosyncratic, a term that does not imply any particular mechanism. The frequency and severity of idiosyncratic DILI is currently thought to arise due to two main influencing factors, the chemical hazard inherent within the structural make-up of the drug and the molecular initiating events leading to liver toxicity within the susceptible individual [14] (figure 1).
Figure 1.
Figure outlining the main contributing functions to severity and frequency of drug-induced hepatotoxicity. In the simplest form, hazard either arises due to the chemistry of the drug or is predominantly dependent on the unusual biology within a susceptible individual. Hazards from the chemistry of the drug should be detected in appropriate in vitro models or animal models in the form of the dose–response curve with relevant hepatic safety endpoints—and in general is predictable. When combining a mild hazard from the drug, where the majority of patients do not experience any adverse effects, with the hepatocyte biology within the susceptible patient—there are currently no in vitro or animal models that are able to be used as predictive of potential hepatotoxicity. HLA, human leucocyte antigen; NAFLD, non-alcoholic fatty liver disease).
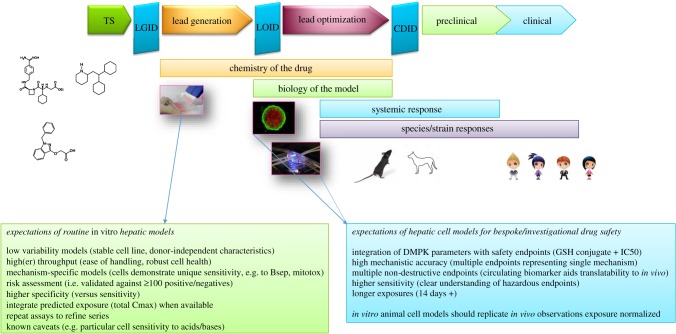
Attrition of chemical series in drug discovery as a consequence of unacceptable hepatic safety is a major barrier to drug research and development productivity [1,2]. Although the causes are broad and multifactorial, the need for more predictive toxicology models and earlier testing to increase future clinical success is crucial. In addition to modelling healthy cellular function, patient-centric hepatic models that address the translational assumptions from bench to bedside are also now emerging. These models are emerging as a potential area for refinement of risk assessment, i.e. hepatic safety risk assessment in the patient versus the healthy volunteer. These refined risk assessment tools are probably more useful at later stages of discovery safety. Discovery-phase toxicology goals are to identify the most promising drug candidates and eliminate those with unacceptable toxicity as early as possible. However, it is not practical to generate an exhaustive hazard and translational risk profile during the early stages of the drug discovery process, nor is it possible to accurately predict risks for all potential clinical hepatotoxicity phenotypes. Consequently, drug developers take a tiered approach to balance the screening volume needs with high translational confidence to select small numbers of potential candidates. Thus, throughput and rapid data generation that can influence early-stage drug design are key considerations for any hepatic in vitro model deployed at this stage. Evaluating drug target liabilities (primary and secondary pharmacology) involves a multilayered cascade of screens and assays, generally starting with simple high-throughput screens, leading into secondary pharmacology and organ-specific risk assessment with validated assays, through to hazard assessment within more complex multicell models (figure 2). To generate this level of understanding, scientists are more frequently deploying complex human and animal three-dimensional models involving microfluidics and stem cells to assess organ-specific and patient-specific toxicity profiles.
Figure 2.
Positioning and performance expectations of hepatic safety assays. Earlier routine risk assessment assays have generally been validated with +100 compounds, which either cause or are clean for hepatotoxicity. These assays are designed to remove the worst offending chemistry, cover the main mechanisms responsible for hepatotoxicity and have defined sensitivity and specificity characteristics. At later stages of the pipeline, there is potential for refining risk assessment to patients by engineering susceptibility characteristics into hepatocytes such that functionality is altered, which may affect sensitivity to certain compounds. TS, target selection; LOID, lead optimization investment decision; CDID, candidate drug investment decision; DMPK, drug metabolism pharmacokinetics.
For the purpose of this opinion piece, the focus will be on cell models that are likely to be a step-change in hepatotoxicity risk assessment: in vitro hepatocyte-like models containing either a patient disease phenotype or a susceptible-patient phenotype. It is recognized that improvements in stem cell culture techniques will improve drug metabolism capability, reproducibility and availability. The deployment of simple cell lines, such as HepG2 and HepaRG, coupled with appropriate use of primary human hepatocytes has enabled the screening out of chemistry that demonstrates overt cytotoxicity towards hepatocytes [15]. The development of increasingly sensitive in vitro models may facilitate discarding potentially useful compounds. The fine balance has to be weighed in light of the risk-to-benefit ratio including the phenotype of the target patient population, duration of treatment, anticipated daily dose, whether the prognosis is disabling or life-threatening and whether the medicine would be first in class. Idiosyncratic liver toxicity is not generally taken into consideration at this early stage because there are no validated models, either in vitro or in vivo, with which to assess this. Many in vitro hepatic safety assays have been validated by using a panel of positive and negative clinical DILI-causing compounds [16]. In general, overt DILI-positive compounds tend to have a number of common features such as high dose, high cLogP, predominant hepatic metabolism component and chronic duration of therapy, and in vitro assays should be expected to separate these compounds from the DILI-negative compounds [17]. Compounds classed as moderate clinical hepatotoxins contain compounds which cause idiosyncratic hepatotoxicity in anywhere from 1 : 1000 to 1 : 10 000 patients. Obviously, the susceptible patient's biology is contributing a significant component to the toxicity when the majority of patients do not experience hepatic injury. Understanding where the susceptibility arises can be a major clue in not only understanding the mechanism of injury but also helping design an appropriate in vitro model for screening purposes [18,19].
2. Induced pluripotent stem cell hepatocytes for drug screening and modelling drug metabolism
Hepatocytes derived from human induced pluripotent stem cells (iPSCs) hold great promise as an in vitro liver model by virtue of their unlimited long-term supply, stability and consistency in functionality, and affordability of donor diversity. However, the suitability of iPSC-derived hepatocytes for toxicology studies has not been fully validated. Hepatocytes undertake anabolic and catabolic activities of nutrients, metabolites, hormones, drugs and other compounds for storage and excretory purposes [20]. The liver cytochrome p450 (CYP450) enzyme system and membrane transporters are critical for drug metabolism and clearance. To validate the maturation and functionality of hepatocytes derived from pluripotent stem cells, induction of CYP enzymes upon drug treatment (e.g. rifampicin and phenobarbital), characterization of drug metabolites generated by the CYP system and transporter activities have been investigated. The CYP450 system comprises phase I and phase II enzymes that are involved in oxidation–reduction and conjugation reactions of xenobiotics [20]. Generally, regarding phase I enzymes, immature hepatocytes express CYP1A1 and CYP3A7, and mature hepatocytes produce CYP3A4, CYP2B6, CYP2C19, CYP2C9, CYP1A2 and CYP2D6, although the generation of an ideal mature human hepatocyte from iPSCs, has not yet been realized. The ideal situation of producing iPSCs from clinically identified donors with defined HLA types [21], drug sensitivities and metabolizer phenotypes, and accessing hepatocytes better representative of a more diverse population which have not been subject to excessive stress through isolation techniques, is yet to emerge for drug toxicity testing. The development of hepatocyte-like cells with hepatotoxicity susceptibility factors either already present or engineered in is an ongoing area of research [22]. These cell models, at this time, are better retrospectively employed, for example when a development candidate demonstrates hepatic stress in a particular population; however, to date such approaches have not been successful [22]. By identifying the genetic factors and in vivo mechanisms that contribute to idiosyncratic DILI, the drug development process can become more refined and responds more effectively, thereby avoiding such negative clinical outcomes. This approach could greatly impact the cost of drug development, which currently is influenced by the attrition rate of tested compounds; for every drug that reaches the marketplace, 7500–10 000 molecules are tested in a preclinical setting. More broadly, it is recognized that genetic variation greatly impacts the individual responses to drug treatment. iPSC-derived hepatocytes would allow for the identification of the patient population subsets most likely to respond to various drug therapies in advance of actual drug treatment. Efforts to stratify patients based on genetic profiling are being used in developing precision medicine for cancer therapy.
3. Peripheral blood-derived hepatocyte-like cells
There is evidence that monocytes and monocyte-derived cells are linked to liver injury and regeneration: in the setting of APAP toxicity the course of liver injury is influenced by recruitment of monocytes/macrophages to the liver and there is also evidence that these cells may acquire hepatocyte characteristics [23]. A frequent problem in assessing causality in patients with polypharmacy who have experienced DILI is which of the drugs (especially if some of them are known to be potential hepatotoxins) was causative in the given patient. Benesic et al. [23] developed a standardized method to generate monocyte-derived hepatocyte-like (MH) cells to assess individual compound DILI liability in a polypharmacy situation. The MH cells exhibit several donor-specific hepatocyte characteristics: MH cells are derived from peripheral monocytes and retain several of their characteristics (e.g. low-level expression of CD14). Additionally, MH cells show inducible activities of CYP450 enzymes 1A2, 2C9, 3A4, reflecting the activities in primary human hepatocytes of the same donor. Additional data on phase II metabolism as uridine diphosphate-glucuronosyltransferase activity as well as expression of several sulfotransferases and glutathione-S-transferases have been obtained. MH cells also express transporter proteins involved in excretion of xenobiotics. To investigate novel methods to diagnose idiosyncratic DILI and assign causality by the reactions of patient-derived cells, the group investigated whether hepatocyte-like cells derived from patient monocytes are capable of reflecting clinical idiosyncratic DILI in vitro [23]. MH cells from patients with idiosyncratic DILI were found to be more susceptible to toxicity induced by the causative drug than MH cells from patients with non-drug liver injury or healthy donors. MH cells showed high sensitivity and specificity for the diagnosis and exclusion of idiosyncratic DILI, and outperform the Roussel Uclaf Causality Assessment Method (RUCAM) score in identifying the causative drug in cases of polypharmacy. These cells may, furthermore, be useful in assessing the role of drug–drug interactions in the onset of idiosyncratic DILI. The identification of true positives among patients with multiple co-medications could help to develop more specific biomarkers that identify patients at risk of progressing to more severe DILI [23]. As yet, the mechanism underlying the increased sensitivity of MH cells is unknown. Whether this could simply be a consequence of altered drug metabolism is an easily testable hypothesis, but is unlikely to be the sole reason.
In a similar approach, Choudhury et al. [24] generated iPSCs from blood-isolated lymphocytes of patients who suffered hepatotoxic side effects from pazopanib (PZ) treatment clinically, and differentiated them into functional hepatocyte-like cells (HLCs) [24]. Equivalent HLCs were derived from patients who also received PZ but did not have any hepatotoxic side effects to serve as real-world controls. Comparison of in vitro effects of PZ on the two groups of HLCs confirmed greater sensitivity of patient-derived cells to PZ toxicity compared to volunteer-derived cells. Toxicity was seen for all HLCs with the paradigm hepatotoxic drug, acetaminophen. Given the ability to expand HLCs from patient-specific iPSCs, transcriptomics analysis was performed and demonstrated that oxidative stress is a potential mechanism by which PZ induces damage in these HLCs [24]. These results strengthen the case for iPSC-derived HLCs as a platform for modelling idiosyncratic hepatotoxicity that allows the interrogation of the toxicity mechanism in the appropriate background of patient-specific features, with multiple causative factors at play. This approach has considerable pharmaceutical industry interest, and with the establishment of patient-specific HLCs with known clinical phenotypes, comprising cell banks, with varied genetic backgrounds and range of drug sensitivities, can aid in population-level drug testing.
4. Induced pluripotent stem cells mimicking human disease
iPSCs generated from donors with a specific single nucleotide polymorphism have proved useful, particularly in developing new therapies for that disease. For identification of new therapeutic compounds, the hepatocytes of iPSC lines derived from patients with α1-antitrypsin (A1AT) deficiency have been used. Five clinical drugs were identified through a high-throughput screening approach, which were shown to reduce the defective A1AT protein accumulation in the cytoplasm. This landmark study demonstrated the utilization of iPSC hepatocytes for drug development towards inherited metabolic disorders [25]. There is a huge potential for modelling drug interactions and pharmacokinetic properties using a library of iPSC hepatocytes generated from various genetic backgrounds. Other diseases where this approach has proved fruitful are familial hypercholesterolaemia [26], glycogen storage disease [26–28], Gaucher's disease [29], Crigler–Najjar Type 1 [26], hereditary tyrosinaemia [26], progressive familial hereditary cholestasis [27] and defective mitochondrial respiratory chain complex disorder [30]. In each situation, it was demonstrated that iHLCs recapitulate the disease phenotypes and represent an opportunity to study liver disease phenotypes in vitro, thereby enabling disease study and drug development [25]. A major issue present in all of these studies is that each patient-derived iPSC cell line has a variety of genetic variants and/or mutations, outside of the evaluated and studied mutation, that may modify or impact disease phenotype. Strategies are required to improve the current models to limit the mutations to only study those related to the field of interest. One method would be to evaluate the disease-specific iPSC hepatocyte in context with tool compounds, for example pairing cells with a mitochondrial respiratory chain complex disorder with a mitochondrial toxin, and similarly cells with cholestatic disorders with BSERP/MRP2 inhibitors. Reproducible differences in disease phenotypes may, therefore, be due to these genetic modifiers rather than primarily due to the disease mutation. Identifying correct controls is required to evaluate observed phenotypes and employ gene repair processes to produce these internal controls [25].
Non-alcoholic fatty liver disease (NAFLD) is a widespread disease in the Western hemisphere. Owing to a high-fat diet and a lack of exercise, hepatocytes of NAFLD patients accumulate fat in the form of lipid droplets [31]. This is often associated with type 2 diabetes and considered part of the metabolic syndrome. Insulin resistance and obesity-associated chronic inflammation of adipose tissue are critical factors for the development and progression of NAFLD [32]. This is often seen as a first hit manifesting in the rather benign accumulation of lipid droplets, called steatosis. A second hit, frequently due to an increase of reactive oxygen species-mediated stress, induces the progression towards non-alcoholic steatohepatitis (NASH), which is accompanied by liver inflammation and fibrosis. Approximately, 29% of patients with NASH develop cirrhosis [32]. Up to 27% of these further develop hepatocellular carcinoma. The driver for this progression is not fully understood; it is not known how NAFLD and its early progression affects the distribution and abundance of lipids in the liver, producing lipotoxicity and inflammation. It is thought that phospholipid zonation may lead to an intra-hepatic pro-inflammatory phenotype and contribute to the hepatic progression from healthy to NAFLD to NASH phenotype, leading to loss of lipid zonation. Cell culture models have been established for deciphering the molecular basis underlying early steps of lipid droplet accumulation in NAFLD, based on hepatocyte-like cells derived from iPSCs [32]. It was demonstrated that both embryonic stem cells and iPSCs differentiate into hepatocyte-like cells that express hepatocyte-specific proteins and have characteristic hepatocyte-like biochemical functions. The cells could be induced to accumulate fat in lipid droplets by incubating them with oleic acid. Global gene expression analysis after oleic acid induction revealed upregulation of many Gene Ontology (GO) categories associated with lipid or glucose metabolism. This effect was qualitatively similar between embryonic and iPSC-derived hepatocyte-like cells, but differed quantitatively due to the different genetic backgrounds of the two cell lines [32]. Appropriate deployment of these patient-specific hepatocyte-like cells has yet to be considered and validated by Pharma. One action AstraZeneca is taking is the development of in vitro models of NAFLD, assessment of altered function and validation of enhanced hepatocyte sensitivity using compounds which have been observed to demonstrate a more severe hepatic injury outcome in NAFLD patients in the clinic.
5. Microphysiological systems for the application of iPSCs for drug metabolism and disease modelling
(a). Three-dimensional hepatic models and co-cultures
Hepatocyte spheroids have shown improved functions and sensitivity (61%)/specificity (85%) for 110 drugs over conventional two-dimensional pure hepatocyte monolayers (sensitivity (33%)/specificity (85%)) for drug-screening applications [33], probably due to the establishment of homotypic cell–cell interactions and the presence of extracellular matrix within and around the spheroids [20]. Cell lines, primary hepatocytes and iPSCs cultured in spheroids have all shown utility for drug toxicity screening [34]. Particularly, culture of iPSCs in spheroidal configurations, such as in collagen matrices, can also improve their functions relative to monolayer controls. One interesting study developed a novel multicellular spheroid-based hepatic differentiation protocol starting from embryoid bodies of hiPSCs for robust mass production of human hepatocyte-like cells using two novel inhibitors of the Wnt pathway [35]. The resultant hepatocyte-like cells expressed liver-specific genes, secreted hepatic proteins such as albumin, α-fetoprotein and fibrinogen, metabolized ammonia, and displayed cytochrome P450 activities and functional activities typical of mature primary hepatocytes, such as LDL storage and uptake, ICG uptake and release and glycogen storage [35]. Cell transplantation of the hepatocyte-like cells in a rat model of acute liver failure (by intra-peritoneal injection of 950 mg kg–1 of sterile D-galactosamine) significantly prolonged the mean survival time and resolved the liver injury when compared with the no-transplantation control animals [35]. The transplanted hepatocyte-like cells secreted human albumin into the host plasma throughout the examination period (2 weeks). Transplantation successfully bridged the animals through the critical period for survival after acute liver failure, providing promising clues of integration and full in vivo functionality of these cells after treatment with WIF-1 and DKK-1 [35].
Other promising stem cell co-culture models have been reported in the literature [36,37], demonstrating promising metabolic function and other hepatic capabilities. The Pharma industry is paying close attention to developments in this area; however, a combination of mechanistic capability, good sensitivity/specificity and throughput of the model is required over a diverse compound set (+100 compounds). Although ATP IC50 generation is a simple and sensitive method of determining cell health, this is not a translatable biomarker that can be assessed alongside endpoints from in vivo studies, and other circulating biomarkers such as miR122, keratin-18 and high-mobility group box-1 are increasingly expected from in vitro models [38].
(b). Zonation
Homeostatic function of the liver requires multiple parallel metabolic pathways, minimizing futile cycles, achieved through specialization of hepatocytes along the sinusoid, known for approximately 40 years as zonation. Regulatory pathways involved include those driven by the sinusoidal oxygen gradient, Wnt/β-catenin, hedgehog pathways and to a lesser extent reactive oxygen species and hormonal regulation. Genome-wide reconstruction of this spatial division of labour in the murine liver was reported in Nature [39], demonstrating 50% of liver genes are significantly zonated and show abundant non-monotonic profiles that peak at the mid-lobule layers [39].
Even though hepatocytes in the liver are protected from flow-induced shear stress by the endothelial fenestrae, flow can cause gradients of oxygen, nutrients and hormones, which have been shown to lead to zonation or differential functions in hepatocytes across the length of the sinusoid [40]. DILI can thus manifest itself with a zonal pattern dependent on the mechanism of action of the drug and its metabolism by specific isoenzymes in the hepatocytes [34]. A parallel-plate bioreactor with oxygen gradients has been used to induce a zonal pattern of CYP450s in rat hepatocytes, which led to a zonal pattern in acetaminophen toxicity, particularly in low-oxygen regions where CYP450 enzymes were expressed at higher levels than in high-oxygen regions [41,42]. In addition to inducing zonal hepatic functions, several investigators have postulated that flow can allow better nutrient exchange and removal of waste products, which can lead to higher hepatic functions than in static cultures. In addition to perfusion of culture medium, microfluidic devices can also be used to control the spatial arrangement of cells to yield the type of architecture (i.e. control over cell–cell interactions) that has been shown to improve liver functions [34]. Microfluidic devices are inherently low-throughput for testing a large panel of drugs and are more difficult to set up and handle relative to industry-standard multiwell plates. Therefore, incorporation of real-time monitoring of toxicity biomarkers in microfluidic devices can not only aid in ease of usability, but also provide more rapid assessment of drug effects than is possible with conventional assays [34].
Recently, technologies for hepatocyte differentiation from human pluripotent stem cells have been greatly improved [43]. Hepatocyte-like cells differentiated from human pluripotent stem cells have hepatic characteristics that are very similar to those of human hepatocytes, such as their capacity for urea synthesis, drug metabolism, lipid synthesis and albumin secretion. However, few HLCs with zone-specific hepatic properties have been generated. The liver is known to consist of various cell types, including hepatocytes, cholangiocytes, liver sinusoidal endothelial cells, stellate cells and Kupffer cells. Among these, hepatocytes and cholangiocytes are well aligned along the sinusoidal spaces and periportal tracts, respectively. Therefore, it has been hypothesized that HLCs with zone-specific hepatic properties could be generated by using parenchymal cell- or non-parenchymal cell-conditioned medium [43]. In one study, HLCs were cultured with parenchymal or non-parenchymal cell-conditioned medium to generate zone-specific HLCs [43]. Mitani et al. identified that the canonical WNT ligands WNT7B and WNT8B, secreted from hepatocytes and cholangiocytes, play important roles in achieving perivenous zone-specific characteristics, such as the enhancement of glutamine secretion, citric acid cycle, cytochrome P450 (CYP) 1A2 metabolism and CYP1A2 induction capacities. They also found that WNT inhibitory factor (WIF-1) secreted from cholangiocytes was necessary for achieving periportal zone-specific characteristics, such as the enhancement of urea secretion and gluconeogenesis capacities; therefore, WNT signal modulators secreted from hepatocytes or cholangiocytes conferred zone-specific hepatic properties onto HLCs [43].
One model system (Hemoshear), currently employs primary human hepatocytes (not iPSCs) to model human fatty liver disease with lipotoxic stress [44]. Primary human hepatocytes were co-cultured with primary human hepatic stellate cells (HSCs) and macrophages (MΦs) and perfused with media containing higher levels of NASH-associated risk factors (glucose, insulin and FFA). Activation of liver-resident MΦs (i.e. Kupffer cells) and HSCs significantly contributes to the pathogenesis of NASH by promoting inflammation and fibrosis locally in the liver and systemically via the secretome [44]. The group demonstrated that lipotoxic stress similar to that seen in NASH patients was recapitulated, and thus were able to measure its impact on hepatocyte morphology and function. These changes were accompanied by increased inflammatory analyte secretion (e.g. IL-6, IL-8, alanine aminotransferase). Critically, one of the take-home messages from this paper is that the better the in vitro culture environment recapitulates the in vivo scenario, the more replicative the model is. Proof of concept examples should use primary human hepatocytes; however, studies repeating the observations using iPSC/hepatocyte-like cells should not be far behind, providing a stable, limitless cell supply required by Pharma for improved safety evaluation. Combination of more functional hepatocyte-like cells or patient-derived hepatocyte-like cells with novel systems providing the physiologically appropriate hepatic-engineered environment will be of intense research interest.
6. Summary
Creation of many different hepatic iPSC lines from different individuals, with greater susceptibility or containing susceptibility factors influencing liver toxicity-specific drugs, may ultimately be necessary to fully understand inter-individual variations in DILI outcomes due to genetic make-up. iPSC hepatocyte cultures containing different diseased backgrounds (i.e. hepatitis B/C viral infection, steatosis and inflammation) could help provide clues as to differences between response in healthy volunteers versus patients versus susceptible DILI patients. However, iPSC hepatocytes need improving and validating with DILI-positive and -negative compounds to approximate primary hepatocyte function and sensitivity before these cell types would be reliably employed within the pharmaceutical industry. Applying iPSCs from patients into novel microphysiological systems will hopefully allow increased functionality for the models to be of use within a safety setting.
Acknowledgements
I would like to acknowledge Kazuko Izura-Williams.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
Funding in-kind time to write and prepare the manuscript was from AstraZeneca plc.
References
- 1.Cook D, Brown D, Alexander R, March R, Morgan P, Satterthwaite G, Pangalos MN. 2014. Lessons learned from the fate of AstraZeneca's drug pipeline: a five-dimensional framework. Nat. Rev. Drug Discov. 13, 419–431. ( 10.1038/nrd4309) [DOI] [PubMed] [Google Scholar]
- 2.Park BK, et al. 2011. Managing the challenge of chemically reactive metabolites in drug development. Nat. Rev. Drug Discov. 10, 292–306. ( 10.1038/nrd3408) [DOI] [PubMed] [Google Scholar]
- 3.Park K, Williams DP, Naisbitt DJ, Kitteringham NR, Pirmohamed M. 2005. Investigation of toxic metabolites during drug development. Toxicol. Appl. Pharmacol. 207(Suppl. 2), 425–434. ( 10.1016/j.taap.2005.02.029) [DOI] [PubMed] [Google Scholar]
- 4.Iorga A, Dara L, Kaplowitz N. 2017. Drug-induced liver injury: cascade of events leading to cell death, apoptosis or necrosis. Int. J. Mol. Sci. 18, E1018 ( 10.3390/ijms18051018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antoine DJ, Williams DP, Park BK. 2008. Understanding the role of reactive metabolites in drug-induced hepatotoxicity: state of the science. Expert Opin. Drug Metab. Toxicol. 4, 1415–1427. ( 10.1517/17425255.4.11.1415) [DOI] [PubMed] [Google Scholar]
- 6.Bessems JG, Vermeulen NP. 2001. Paracetamol (acetaminophen)-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit. Rev. Toxicol. 31, 55–138. ( 10.1080/20014091111677) [DOI] [PubMed] [Google Scholar]
- 7.Hinson JA, Roberts DW, James LP. 2010. Mechanisms of acetaminophen-induced liver necrosis. Handb. Exp. Pharmacol. 196, 369–405. ( 10.1007/978-3-642-00663-0_12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaeschke H, McGill MR, Ramachandran A. 2012. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab. Rev. 44, 88–106. ( 10.3109/03602532.2011.602688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKenzie R, et al. 1995. Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B. New Engl. J. Med. 333, 1099–1105. ( 10.1056/NEJM199510263331702) [DOI] [PubMed] [Google Scholar]
- 10.Kleiner DE, et al. 1997. Histopathologic changes associated with fialuridine hepatotoxicity. Mod. Pathol. 10, 192–199. [PubMed] [Google Scholar]
- 11.Cui L, Yoon S, Schinazi RF, Sommadossi JP. 1995. Cellular and molecular events leading to mitochondrial toxicity of 1-(2-deoxy-2-fluoro-1-beta-D-arabinofuranosyl)-5-iodouracil in human liver cells. J. Clin. Invest. 95, 555–563. ( 10.1172/JCI117698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee EW, Lai Y, Zhang H, Unadkat JD. 2006. Identification of the mitochondrial targeting signal of the human equilibrative nucleoside transporter 1 (hENT1): implications for interspecies differences in mitochondrial toxicity of fialuridine. J. Biol. Chem. 281, 16 700–16 706. ( 10.1074/jbc.M513825200) [DOI] [PubMed] [Google Scholar]
- 13.Xu D, et al. 2014. Fialuridine induces acute liver failure in chimeric TK-NOG mice: a model for detecting hepatic drug toxicity prior to human testing. PLoS Med. 11, e1001628 ( 10.1371/journal.pmed.1001628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams DP, Park BK. 2003. Idiosyncratic toxicity: the role of toxicophores and bioactivation. Drug Discov. Today 8, 1044–1050. ( 10.1016/S1359-6446(03)02888-5) [DOI] [PubMed] [Google Scholar]
- 15.Goldring C, et al. 2017. Stem cell-derived models to improve mechanistic understanding and prediction of human drug-induced liver injury. Hepatology 65, 710–721. ( 10.1002/hep.28886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen M, Borlak J, Tong W. 2016. A Model to predict severity of drug-induced liver injury in humans. Hepatology 64, 931–940. ( 10.1002/hep.28678) [DOI] [PubMed] [Google Scholar]
- 17.Shah F, Leung L, Barton HA, Will Y, Rodrigues AD, Greene N, Aleo MD. 2015. Setting clinical exposure levels of concern for drug-induced liver injury (DILI) using mechanistic in vitro assays. Toxicol. Sci. 147, 500–514. ( 10.1093/toxsci/kfv152) [DOI] [PubMed] [Google Scholar]
- 18.Gibson A, et al. 2017. The effect of inhibitory signals on the priming of drug hapten-specific T cells that express distinct Vβ receptors. J. Immunol. 199, 1223–1237. ( 10.4049/jimmunol.1602029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogese MO, et al. 2017. Characterization of drug-specific signaling between primary human hepatocytes and immune cells. Toxicol. Sci. 158, 76–89. ( 10.1093/toxsci/kfx069) [DOI] [PubMed] [Google Scholar]
- 20.Godoy P, et al. 2013. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch. Toxicol. 87, 1315–1530. ( 10.1007/s00204-013-1078-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor CJ, Peacock S, Chaudhry AN, Bradley JA, Bolton EM. 2012. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell 11, 147–152. ( 10.1016/j.stem.2012.07.014) [DOI] [PubMed] [Google Scholar]
- 22.Lundgren H, Martinsson K, Cederbrant K, Jirholt J, Mucs D, Madeyski-Bengtson K, Havarinasab S, Hultman P, Strnad P. 2017. HLA-DR7 and HLA-DQ2: Transgenic mouse strains tested as a model system for ximelagatran hepatotoxicity. PLoS ONE 12, e0184744 ( 10.1371/journal.pone.0184744) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benesic A, Leitl A, Gerbes AL. 2016. Monocyte-derived hepatocyte-like cells for causality assessment of idiosyncratic drug-induced liver injury. Gut 65, 1555–1563. ( 10.1136/gutjnl-2015-309528) [DOI] [PubMed] [Google Scholar]
- 24.Choudhury Y, et al. 2017. Patient-specific hepatocyte-like cells derived from induced pluripotent stem cells model pazopanib-mediated hepatotoxicity. Sci. Rep. 7, 41238 ( 10.1038/srep41238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz RE, Bram Y, Frankel A. 2016. Pluripotent stem cell-derived hepatocyte-like cells: a tool to study infectious disease. Curr. Pathobiol. Rep. 4, 147–156. ( 10.1007/s40139-016-0113-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rashid ST, et al. 2010. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J. Clin. Invest. 120, 3127–3136. ( 10.1172/JCI43122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghodsizadeh A, et al. 2010. Generation of liver disease-specific induced pluripotent stem cells along with efficient differentiation to functional hepatocyte-like cells. Stem Cell Rev. 6, 622–632. ( 10.1007/s12015-010-9189-3) [DOI] [PubMed] [Google Scholar]
- 28.Satoh D, et al. 2013. Establishment and directed differentiation of induced pluripotent stem cells from glycogen storage disease type Ib patient. Genes Cells 18, 1053–1069. ( 10.1111/gtc.12101) [DOI] [PubMed] [Google Scholar]
- 29.Park IH, et al. 2008. Disease-specific induced pluripotent stem cells. Cell 134, 877–886. ( 10.1016/j.cell.2008.07.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Im I, Jang MJ, Park SJ, Lee SH, Choi JH, Yoo HW, Kim S, Han Y-M. 2015. Mitochondrial respiratory defect causes dysfunctional lactate turnover via AMP-activated protein kinase activation in human-induced pluripotent stem cell-derived hepatocytes. J. Biol. Chem. 290, 29 493–29 505. ( 10.1074/jbc.M115.670364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vuppalanchi R, Chalasani N. 2009. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: selected practical issues in their evaluation and management. Hepatology 49, 306–317. ( 10.1002/hep.22603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graffmann N, Ring S, Kawala MA, Wruck W, Ncube A, Trompeter HI, Adjaye J. 2016. Modeling nonalcoholic fatty liver disease with human pluripotent stem cell-derived immature hepatocyte-like cells reveals activation of PLIN2 and confirms regulatory functions of peroxisome proliferator-activated receptor alpha. Stem Cells Dev. 25, 1119–1133. ( 10.1089/scd.2015.0383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Proctor WR, et al. 2017. Utility of spherical human liver microtissues for prediction of clinical drug-induced liver injury. Arch. Toxicol. 91, 2849–2863. ( 10.1007/s00204-017-2002-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin C, Khetani SR. 2016. Advances in engineered liver models for investigating drug-induced liver injury. BioMed Res. Int. 2016, 1829148 ( 10.1155/2016/1829148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pettinato G, Ramanathan R, Fisher RA, Mangino MJ, Zhang N, Wen X. 2016. Scalable differentiation of human iPSCs in a multicellular spheroid-based 3D culture into hepatocyte-like cells through direct Wnt/beta-catenin pathway inhibition. Sci. Rep. 6, 32888 ( 10.1038/srep32888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szkolnicka D, Farnworth SL, Lucendo-Villarin B, Storck C, Zhou W, Iredale JP, Flint O, Hay DC. 2014. Accurate prediction of drug-induced liver injury using stem cell-derived populations. Stem Cells Transl. Med. 3, 141–148. ( 10.5966/sctm.2013-0146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ware BR, Berger DR, Khetani SR. 2015. Prediction of drug-induced liver injury in micropatterned co-cultures containing iPSC-derived human hepatocytes. Toxicol. Sci. 145, 252–262. ( 10.1093/toxsci/kfv048) [DOI] [PubMed] [Google Scholar]
- 38.Ewart L, et al. 2018. Application of microphysiological systems to enhance safety assessment in drug discovery. Annu. Rev. Pharmacol. Toxicol. 58, 65–82. ( 10.1146/annurev-pharmtox-010617-052722) [DOI] [PubMed] [Google Scholar]
- 39.Halpern KB, et al. 2017. Single-cell spatial reconstruction reveals global division of labour in the mammalian liver. Nature 542, 352–356. ( 10.1038/nature21065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jungermann K, Kietzmann T. 1996. Zonation of parenchymal and nonparenchymal metabolism in liver. Annu. Rev. Nutr. 16, 179–203. ( 10.1146/annurev.nu.16.070196.001143) [DOI] [PubMed] [Google Scholar]
- 41.Allen JW, Bhatia SN. 2003. Formation of steady-state oxygen gradients in vitro: application to liver zonation. Biotechnol. Bioeng. 82, 253–262. ( 10.1002/bit.10569) [DOI] [PubMed] [Google Scholar]
- 42.Allen JW, Khetani SR, Bhatia SN. 2005. In vitro zonation and toxicity in a hepatocyte bioreactor. Toxicol. Sci. 84, 110–119. ( 10.1093/toxsci/kfi052) [DOI] [PubMed] [Google Scholar]
- 43.Mitani S, Takayama K, Nagamoto Y, Imagawa K, Sakurai F, Tachibana M, Sumazaki R, Mizuguchi H. 2017. Human ESC/iPSC-derived hepatocyte-like cells achieve zone-specific hepatic properties by modulation of WNT signaling. Mol. Ther. 25, 1420–1433. ( 10.1016/j.ymthe.2017.04.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feaver RE, et al. 2016. Development of an in vitro human liver system for interrogating nonalcoholic steatohepatitis. JCI Insight 1, e90954 ( 10.1172/jci.insight.90954) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.