ABSTRACT
Alphaviruses are transmitted to humans via bites of infected mosquitoes. Although alphaviruses have caused a wide range of outbreaks and crippling disease, the availability of licensed vaccines or antiviral therapies remains limited. Mosquito vectors such as Aedes and Culex are the main culprits in the transmission of alphaviruses. This review explores how mosquito saliva may promote alphavirus infection. Identifying the roles of mosquito-derived factors in alphavirus pathogenesis will generate novel tools to circumvent and control mosquito-borne alphavirus infections in humans.
KEYWORDS: alphavirus, disruption of innate immunity, subversion of adaptive immunity
INTRODUCTION
Mosquito-borne diseases are major health threats to the public worldwide (1). Nonetheless, these diseases continue to emerge and reemerge, as demonstrated by the recent autochthonous transmission of chikungunya virus (CHIKV) in France in August 2017 (2). CHIKV, one of the most prominent alphaviruses of modern times, can severely cause musculoskeletal inflammatory disease characterized by fever, polyarthralgia, and skin rashes in humans. Other alphaviruses in the Togaviridae family include Semliki Forest virus (SFV), Ross River virus (RRV), O'nyong-nyong virus (ONNV), Venezuelan equine encephalitis virus (VEEV), and Sindbis virus (SINV). These viruses are typically transmitted human-to-human via bites of infected female mosquitoes (3). A number of mosquito species act as vectors in transmission of alphaviruses. While ONNV is transmitted by anopheline mosquitoes (4), the others are mainly transmitted by culicine mosquitoes (5).
Blood feeding on human hosts by infected female mosquitoes results in simultaneous introduction of virus and mosquito saliva into the skin. It has been shown that mosquito bites enhance infection with dengue virus (DENV) (6), West Nile virus (WNV) (7), and SFV (8). Although mosquito saliva has been demonstrated to have an important influence on virus infection and disease, very little is known about the molecular mechanisms governing the facilitation of virus transmission. Thus, there is a need to understand the determinants present in mosquito saliva that facilitate transmission and how they modulate virus infection in the human host. In this review, the focus is on recent advancements in understanding the roles of arthropod saliva in arthropod-borne infections and on evaluating the potential modulatory impact of mosquito salivary proteins on alphavirus pathogenesis. In addition, the potential use of mosquito salivary proteins as therapeutic or vaccine targets for controlling mosquito-borne alphavirus diseases is highlighted.
CUTANEOUS IMMUNE RESPONSE TO ALPHAVIRUS INFECTION
Infection is initiated when an infected female mosquito probing for blood releases viruses below the skin of the human host. Consequently, the initial cells to contact the viruses are cells residing in the skin, followed by those in the draining lymph node (9). Infection at the skin is a critical stage of alphavirus pathogenesis as the virus must replicate and disseminate before antiviral immune responses are activated.
The human skin is composed of multiple cell types organized in three layers: outermost epidermis, dermis, and deepest hypodermis (Fig. 1). Following virus inoculation, viruses first infect keratinocytes and Langerhans cells in the epidermis layer and then infect stromal cells such as fibroblasts, as well as dendritic cells and macrophages that reside in the skin dermis layer (Fig. 1). The Langerhans cells and dermal dendritic cells subsequently mature following antigen stimulation and migrate rapidly along lymphatic vessels to the skin draining lymph nodes and prime the adaptive immune responses (10). During infection, resident macrophages can be activated into either classically or alternatively activated macrophages depending on the stimuli from the local microenvironments (11). Importantly, infection also triggers the recruitment of peripheral neutrophils and monocytes to the site of infection (12, 13).
FIG 1.
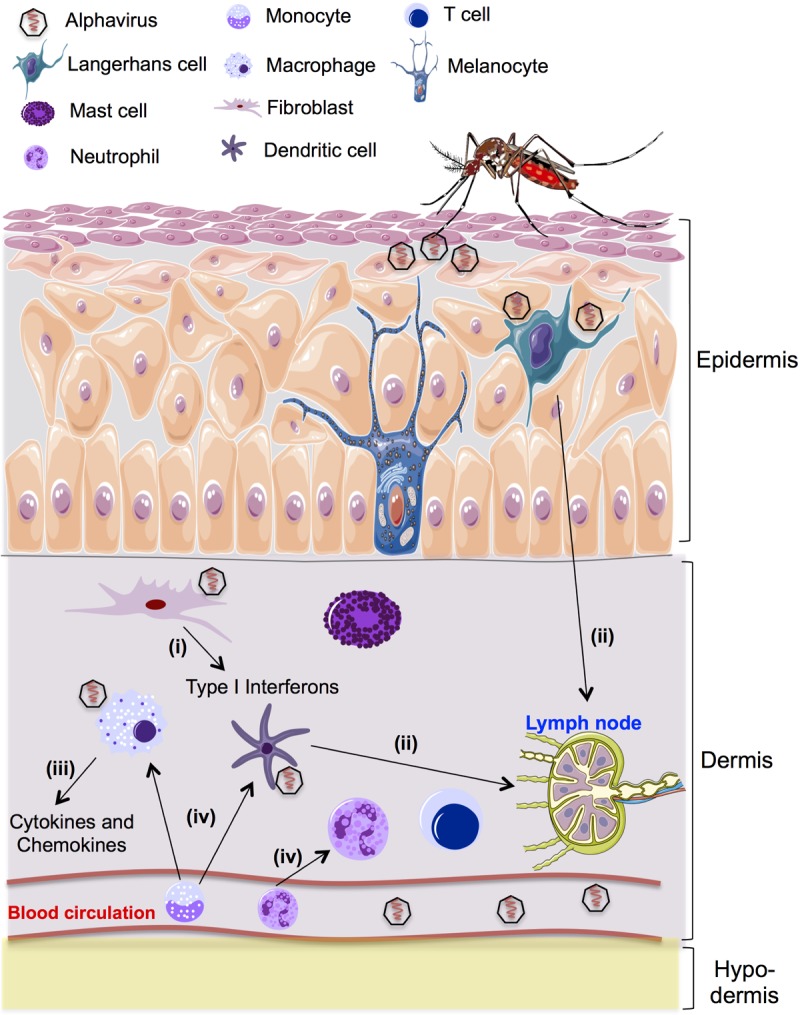
Schematic diagram showing skin immune sentinels and cutaneous immune response following alphavirus inoculation. The epidermis is composed of the outermost layers of cells in the skin. Specialized cells in the epidermis include keratinocytes, melanocytes, and Langerhans cells. The dermis is anatomically composed of many immune cells, including dermal dendritic cells, resident macrophages, fibroblasts, mast cells, and some T cell subsets. Below the dermis is the subcutaneous layer, which is a layer of tissue composed of adipose tissue. Following a mosquito bite, (i) alphaviruses infect and replicate in skin fibroblasts. Infection rapidly triggers an innate immune response that leads to increased expression of type I interferon in skin fibroblasts. (ii) Skin resident dendritic cells become mature following antigen stimulation and rapidly migrate along lymphatic vessels to the skin draining lymph nodes to prime adaptive responses. Aside from their role in priming the adaptive immune response, activated dendritic cells also secrete the cytokines and chemokines that are important for the innate immune response. (iii) Resident macrophages secrete cytokines and chemokines upon activation. Type I interferons produced following alphavirus infection activate resident macrophages to the classical phenotype, with enhanced microbicidal ability and production of proinflammatory cytokines and mediators. (iv) Alphavirus infection also induces inflammation that leads to recruitment of neutrophils to the site of infection, which then secrete additional attractants to recruit monocytes. The newly recruited monocytes differentiate into either inflammatory dendritic cells or macrophages that can become targets for a second wave of alphavirus infection.
MOSQUITO SALIVA
The saliva of blood-sucking arthropods contains bioactive compounds that counteract host immune responses (14, 15). Arthropod saliva has been extensively studied in ticks and sand flies (16, 17). Ticks secrete a complex and sophisticated pharmacological armamentarium that consists of bioactive lipids and proteins to assist blood feeding (18). Mosquitoes use their saliva similarly to facilitate blood feeding, as their saliva contains a complex and diverse mixture of antihemostatic, anti-inflammatory, and immunomodulatory compounds that can counteract the host's hemostatic responses. The mechanisms behind this include the following: delaying the coagulation process by the activity of salivary anticoagulant factors (19), inhibition of platelet aggregation by salivary apyrase (20) or inhibition of collagen-induced platelet aggregation (21), thrombin activity (22), and, lastly, vasodilation of host blood vessels (23). With the emergence of state-of-the-art technologies, studies on the transcriptomes and proteomes of the mosquito salivary gland have revealed the presence of hypothetical proteins with unknown functions (24, 25).
MOSQUITO SALIVA-INDUCED MODULATION OF VIRAL PATHOGENESIS
Beyond just assisting in blood feeding, mosquito saliva can also enhance the infectivity of mosquito-transmitted pathogens (6, 26). As alphaviruses are among the most medically important pathogens, there is considerable interest in the role of mosquito saliva during infection. A study utilizing an in vivo mouse model reported that infectivity of SFV was enhanced by mosquito bites (8) and that this enhancement was due to the presence of the mosquito saliva. Although the mechanisms behind this remain poorly understood, possible immune-modulating pathways mediated by the mosquito saliva in alphavirus pathogenesis (Fig. 2) are discussed in the following subsections.
FIG 2.
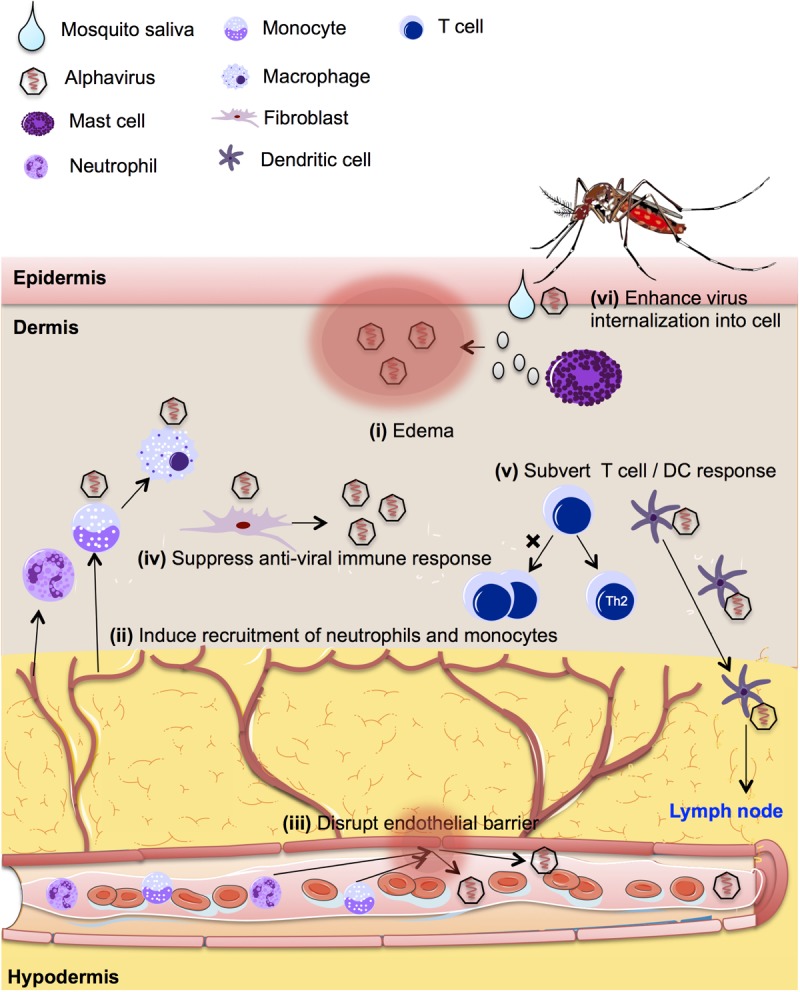
Schematic diagram showing the potential modulatory impact of mosquito salivary proteins on alphavirus pathogenesis, including (i) extensive cutaneous edema to retain viruses at the mosquito bite site, (ii) inflammation and recruitment of virus-susceptible immune cells to the site of infection, (iii) increased endothelial permeability and thus facilitation of virus dissemination and transmigration of cells, (iv) suppression of host innate immune response to benefit virus survival and replication, (v) subversion of the host adaptive immune response, and (vi) interaction with virus and host to enhance internalization of virus into the host cell.
(i) Mosquito saliva promotes extensive cutaneous edema that leads to retention of viruses at the feeding site.
Most insect bites result in localized itching and swelling that may persist for up to 72 h (27). It is believed that pharmacologically active substances in the mosquito saliva trigger mast cell activation and histamine release (28), resulting in fluid leakage from the nearby blood capillaries (29), thus explaining the manifestation of cutaneous edema.
It was not expected that this localized response could actually benefit virus retention. The enhancing role of skin edema for virus retention was first demonstrated in mouse models of SFV infection, where edema at the mosquito bite site resulted in retention of SFV in the skin and facilitated infection of cutaneous cells (8). This led to inhibition of virus dissemination into the draining lymph node and delayed the activation of type I interferon (IFN) and transcriptional impediment of antiviral genes. Alphaviruses such as CHIKV can infect and replicate efficiently in endothelial cells, fibroblasts, and macrophages present in the skin (30). Therefore, it is conceivable that virus retention at the skin facilitated by the mosquito saliva could benefit infection and replication of alphaviruses at the primary inoculation site (Fig. 2).
(ii) Mosquito saliva hijacks immune cells and facilitates viral spread from the bite site.
Inoculation of viruses into the human host triggers an influx of immune cells to the site of infection to clear off foreign invaders, heal injuries, and mop up debris (31). However, recent studies in mice brought out the ugly side of the recruitment of these inflammatory immune cells. Instead of clearing the viruses, these cells became infected and subsequently supported virus replication and spreading. In the in vivo infection model of SFV, mosquito bites induced expression of chemokine (C-X-C motif) ligand 2 (CXCL2) and interleukin-1 beta (IL-1β), which triggered an influx of neutrophils to the site of infection (8). This not only resulted in extensive infiltration of macrophages (32) but also provided an additional cellular target for virus infection. In another study, DENV infection led to modest recruitment of inflammatory neutrophils and monocytes to the dermis of infected mice, and the influx was further enhanced by the presence of Aedes aegypti salivary gland extract (SGE) (33). Viruses then targeted the de novo recruited monocyte-derived dendritic cells and transformed them into cellular reservoirs for virus replication (34).
Monocytes, which represent another innate immune cell type found in the blood, are typically recruited to the site of virus inoculation following mosquito bites. Blood monocytes were previously demonstrated to be one of the main targets of CHIKV infection during the acute disease phase (35). Thus, enhanced recruitment of monocytes to the site of infection by mosquito saliva provides an additional cellular target for CHIKV infection. The infected blood monocytes may behave as a “Trojan horse” and may disseminate the virus to other sites, resulting in a more severe disease outcome (36), as demonstrated by the manifestation of polyarthralgia following CHIKV infection in humans (37, 38).
(iii) Mosquito saliva disrupts endothelial barrier function to enable blood feeding, which facilitates virus dissemination and cell migration.
The endothelial barrier of blood capillaries separates the skin from systemic blood circulation and thus acts as a physical defense against virus invasion into the human host (39). Mosquitoes produce bioactive molecules in their saliva that are able to disrupt this protective barrier. Anopheles stephensi mosquito bites induce dermal mast cell activation, leading to the release of vasoactive amines that further enhance vascular permeability in the mouse skin (40). Aedes aegypti SGE was found to decrease the transendothelial electrical resistance of endothelial cell monolayers in vitro and to induce vascular leakage in the skin following intradermal inoculation into the mouse ear (33). The mechanism lies in the serine proteases present in SGE breaking down the extracellular matrix of fibroblasts (41), which subsequently causes actin cytoskeleton disruption leading to increased endothelial permeability.
Mosquito saliva-mediated disruption of the endothelial barrier enhances systemic virus dissemination and also augments virus access to circulating enhancing antibodies from primary infection with a different dengue virus serotype, exacerbating pathogenesis during antibody-dependent enhancement in DENV infection (33). Interestingly, Aedes mosquito SGE was also shown to significantly increase viral titers in the brain of Rift Valley fever virus-infected mice, indicating a role of mosquito saliva in modulating the permeability of the blood-brain barrier and triggering virus dissemination to the central nervous system (42). The disruption of endothelial barrier function in the dermis also enhances transmigration of monocytes and neutrophils through blood vessels to enter the dermis (33). All of these findings highlight the possibility that mosquito saliva facilitates alphavirus infection through disruption of the protective endothelial barrier, leading to enhanced virus dissemination and immune cell transmigration through the blood vessels following injury (Fig. 2).
(iv) Mosquito saliva suppresses the innate immune response of human host cells.
Innate immune responses represent the first line of immune defense of the host against virus infection and play crucial roles in controlling viral replication and pathogenesis during early stages of infection. Robust antiviral immune responses and expression of type I interferon triggered following alphavirus infection induce the expression of hundreds of interferon-stimulated genes that restrict virus replication or modulate immune response by inducing the recruitment and maturation of leukocytes (43). Thus, to effectively infect and replicate in the target cells, alphaviruses must delay their early detection and clearance by the host innate immune system, potentially with the help of the mosquito saliva. Mosquito saliva can play a role in controlling the abundant expression of antiviral genes through targeting components of the type I interferon pathway, allowing more-efficient virus replication in infected cells (44).
The modification of host immune responses by salivary components of blood-feeding arthropods has been extensively studied in ticks and sand flies. Tick saliva creates an immunosuppressive niche to facilitate the transmission of tick-borne pathogens (45–47). Saliva of the Ixodes ricinus tick interferes with activation of the pathway that mediates interferon beta (IFN-β) induction, enhancing Borrelia afzelii infection in dendritic cells (48). Mosquitoes also secrete saliva to suppress antiviral innate immune responses and to modulate early viral replication in human host cells. Proteomic analysis of DENV-infected Aedes aegypti mosquitoes demonstrated high levels of a 34-kDa protein in the salivary glands that enhanced DENV replication in human keratinocytes. This was due to the suppression of interferon regulatory factor 3 (IRF-3) and IRF-7 mediated by the 34-kDa protein that subsequently resulted in reduced expression of both type I interferon and IFN-γ in the cell at the earliest stages of infection (44). Interestingly, the expression of this Aedes-specific protein was also upregulated in the salivary glands of CHIKV-infected Aedes aegypti female mosquitoes (49). Thus, it is highly plausible that alphaviruses utilize the same immune escape strategy to enhance virus replication in permissive skin fibroblast cells by suppressing innate immune responses in the target cells. It will therefore be of significant interest to determine how this 34-kDa protein downregulates the expression of IR3 and IR7 and what cellular pathways and host factors are involved in its function.
Innate immune responses frequently include complement cascades that can be activated on the surface of a pathogen through three pathways: the classical pathway, an alternative pathway, and the mannan-binding lectin pathway (50). The primary function of the complement system is to protect the host from invading infectious pathogens by recruiting and enhancing phagocytosis of target cells by innate immune cells (50). Despite the complex setup of the complement system, blood-sucking arthropods could still transmit infectious pathogens into human hosts during blood feeding.
The sand fly Lutzomyia longipalpis, vector of Leishmania spp., has saliva that is capable of inhibiting both the classical and alternative complement pathways (51), whereas the tick Ixodes scapularis has a salivary protein, Salp20, that acts only on the alternative complement activation pathway by binding properdin and dissociating C3bBbP, the active C3 convertase (52). The saliva of DENV-infected Aedes aegypti was also able to interrupt the mannose-lectin pathway of complement activation, and, surprisingly, the interruption was due to the presence of soluble DENV nonstructural protein 1 in the saliva (53). Once again, these findings illustrate that blood-sucking arthropods have evolved to produce salivary components that are capable of subverting host innate immune responses. It is plausible that in mosquito-borne alphavirus infections, the viruses benefit from the suppression of host innate immune responses triggered by mosquito saliva to infect and replicate in target cells more effectively (Fig. 2).
(v) Mosquito saliva subverts host adaptive immune responses to alphavirus.
Adaptive immune responses also play an important role in controlling virus infection in human hosts (10). Alterations in the function of antigen-presenting cells, such as macrophages or dendritic cells, can greatly impair the development of an appropriate adaptive immune response. It has been shown that tick saliva contains bioactive molecules that are able to inhibit dendritic cell maturation, function, and migration, thereby altering the outcome of dendritic cell-CD4+ T cell interactions (54). Mosquito saliva, like tick saliva, has immunomodulation effects on dendritic cells, but instead of retarding dendritic cell migration, Aedes aegypti SGE boosted migration of activated classical dendritic cells from the skin to the draining lymph node in the presence of enhancing antibodies during DENV infection (33). Dendritic cells are crucial for priming adaptive T and B cell responses following alphavirus infection (55). The resident dendritic cell population in the skin matures and migrates to local draining lymph nodes following infection with alphaviruses (55) and further stimulates antibody- and cell-mediated responses. Thus, the boosted migration of dendritic cells may augment alphavirus pathogenesis by facilitating virus dissemination or skewing host immune responses.
Another antigen-presenting cell that is important in linking innate and adaptive immune responses is the macrophage. Tick saliva has been shown to modulate macrophage cytokine production (56) and costimulatory molecule expression (57). Further characterization of the tick salivary constituents revealed that the vasodilator prostaglandin E2, abundantly present in tick saliva, was able to alter the migratory activity and cytokine profile of macrophages (58). These findings further demonstrate the complex nature of arthropod saliva and highlight the potential redundancy in the mechanisms utilized to regulate host responses. Mosquito saliva also contains pharmacologically active compounds with vasodilatory or anticoagulation properties. Aside from their well-known functions, these compounds might also modulate host immune responses that eventually benefit virus replication and infection. Therefore, it is of interest to explore potential host immunomodulatory roles of these compounds in alphavirus infection.
Blood-feeding mosquitoes have also developed strategies to suppress host adaptive immune responses through regulation of lymphocytes. Mosquito SGE significantly suppressed T cell proliferation in treated splenocytes from naive C3H/HeJ mice (59) at lower concentrations and enhanced apoptosis of both T cells and B cells in the spleen when present at high concentrations (60). Mosquito saliva also significantly reduced T lymphocyte populations in the mouse ear skin after mosquito feeding in WNV-infected mice (61).
Experiments with mosquito saliva or SGE have shown polarization of host cutaneous immune responses from Th1 to Th2 responses (59, 62). Notably, CHIKV-infected mice inoculated via mosquito bites showed a Th2 profile with an upregulation of Th2 IL-4 concomitant with prolonged suppression of Th1 IFN-γ and IL-2 release compared to needle-inoculated virus-infected mice (63). Alterations in cytokine production by T cells could presumably alter cell influx and result in a shift to a predominantly Th2 response, leading to alteration of inflammatory responses and benefitting virus survival and replication.
The chronic musculoskeletal pathology in CHIKV infection is caused by persistent virus infection and controlled by adaptive immune responses (64). It is conceivable that the mosquito saliva delays or disrupts the host adaptive immune response, resulting in prolonged virus infection which subsequently promotes chronic disease in CHIKV infection (Fig. 2). Identifying mosquito-derived factors that are responsible for the suppression of adaptive immune responses will help provide a therapeutic target to stimulate virus clearance and limit the progression of chronic symptoms in alphavirus infection.
(vi) Mosquito saliva contains factors that interact with virus and host to facilitate virus infection.
Studies have been conducted to define the factors within mosquito saliva that modulate arbovirus infection. In most of the studies, the enhancement of arbovirus infection was attributed to the modulation of host cells and immune responses by mosquito salivary components (6, 8, 26). Interestingly, mosquito saliva also contains molecules that could enhance virus attachment and entry into mammalian cells. A 45-kDa sialylated saliva glycoprotein, belonging to the D7 protein family in the SGE of Aedes aegypti, forms a complex with DENV and further enhances DENV internalization into mammalian cells (65). Sialylated glycoproteins have previously been shown to modulate many important biological processes, including cellular recognition, trafficking, and signaling, in human cells (66). All of these findings suggest the hypothesis that mosquito saliva contains sialylated molecules that interact with both virus and host to facilitate successful virus-host tissue transmission, which might be also applicable in mosquito-borne alphavirus infections (Fig. 2).
FUTURE CHALLENGES
Mosquito saliva plays pivotal roles in pathogenesis and transmission of mosquito-borne viruses in human hosts. The various scenarios synergistically create a beneficial microenvironment that facilitates virus replication at the earliest stage of infection (Fig. 2).
Modulation by mosquito saliva for recruitment of more immune cells to the biting site is likely to generate a permissive environment for the establishment of virus infection with possible repercussions on the pathogenesis. A pertinent issue remains with respect to whether suppressing inflammation at the mosquito bite site controls the spread of viruses from the site of inoculation. Strategies could start with repurposing clinically approved anti-inflammatory drugs to treat inflammation following mosquito bites before the onset of disease symptoms. However, such applications must be looked at carefully, bearing in mind the pleiotropic roles of inflammatory immune cells in defending human hosts against invading viruses.
Due to the complexity of mosquito saliva, there are many more mosquito-derived factors with roles in modulating host responses that have yet to be discovered. High-throughput proteomic and transcriptomic approaches have resulted in the discovery of genes and proteins not previously reported in the mosquito salivary gland. The current challenge is to characterize the biological activities of these salivary molecules, the structures and biological functions of which are largely unknown today.
It is important to understand the molecular properties and host modulatory effects of the components in mosquito saliva, because the factors responsible for facilitation of virus transmission in human hosts can be exploited to develop effective drugs against alphavirus infections. A small-molecule antagonist designed to target mosquito salivary components, or a host receptor of a salivary component, would be able to block mosquito saliva-host interactions at the bite site. Such a mosquito saliva-targeting antiviral would be able to be used simultaneously with a virus-targeting antiviral to effectively treat alphavirus infection. Alternatively, it would also be able to be used as a prophylactic antiviral drug to prevent mosquito to human transmission of viruses in populations at high risk of exposure to alphavirus-infected mosquitoes, with the aim of stopping virus infection at the bite site.
Substantial understanding of the structure and biological function of mosquito salivary components also permits development of vector-based vaccines that target mosquito salivary proteins. Since the vaccine targets the mosquito vector, it has the potential to protect against a wide range of the diseases caused by mosquito-borne pathogens. Currently, a universal vaccine against mosquito-borne disease, AGS-v, which consists of four synthetic proteins from the mosquito salivary gland, is being investigated in a phase I clinical trial (67). This test vaccine is designed to trigger an immune response to mosquito saliva rather than to a specific virus, with the hope of triggering a modified allergic response that can prevent infection when a person is bitten by virus-carrying mosquitoes.
Discovery of how the components in mosquito saliva modulate human hemostasis and immune responses for successful blood feeding could also yield a future cure for other life-threatening diseases. The immunomodulatory, anti-inflammatory, antiplatelet, and anticlotting properties of mosquito saliva could facilitate creation of a lifesaving therapy for treatment of heart diseases and immune-driven diseases. There are several reports addressing the experimental evidence supporting the use of tick saliva components as potential alternative treatments for inflammation-related diseases (68) and even cancer (69, 70). We can make use of yeast surface display technology that expresses hundreds of proteins in yeast cells to allow high-throughput screening of pharmacological or immunomodulatory properties (71) to identify potential components of mosquito saliva that can be translated into clinical therapy.
Multiple studies have shown that needle inoculation of arboviruses does not recapitulate the actual characteristics of mosquito transmission of the virus and elicits contrasting forms of immune activation in response to viruses. This highlights the importance of mosquito saliva in the earliest events of virus infection. In addition, many mosquito-borne alphaviruses such as SINV (72), CHIKV (73), and ONNV (74), have been shown to struggle to replicate and disseminate within wild-type mice after infection by needle inoculation of viruses. Accordingly, immunocompromised mice are often used as an alternative model, which precludes the experimental study of many aspects of host immune responses to these infections. Future studies on mosquito-borne alphaviruses should consider integration of actual mosquito transmission of the virus rather than the use of needle inoculation of virus alone. The use of an integrated host-virus-mosquito model, mimicking natural virus infection through mosquito bites, will lead to a better understanding of mechanisms involved in alphavirus pathology in human hosts. Identification and characterization of the molecules in mosquito saliva that are responsible for immune modulation and enhancement of viral replication will facilitate the development of novel curative or preventive strategies for alphavirus control.
ACKNOWLEDGMENTS
We thank Fok-Moon Lum and Kai-Er Eng for critical comments on the manuscript.
REFERENCES
- 1.World Health Organization. 2017. Vector-borne diseases fact sheet. http://www.who.int/mediacentre/factsheets/fs387/en/ Accessed 12 February 2018.
- 2.European Centre for Disease Prevention and Control. 2017. Rapid risk assessment: cluster of autochthonous chikungunya cases in France (23 August 2017). https://ecdc.europa.eu/sites/portal/files/documents/RRA-Chikungunya-France-revised-Aug-2017.pdf Accessed 1 February 2018.
- 3.Korsman SNJ, van Zyl GU, Nutt L, Andersson MI, Preiser W. 2012. Togaviruses. Churchill Livingstone, London, United Kingdom. doi: 10.1016/B978-0-443-07367-0.00042-2. [DOI] [Google Scholar]
- 4.Corbet PS, Williams MC, Gillett JD. 1961. O'Nyong-Nyong fever: an epidemic virus disease in East Africa. IV. Vector studies at epidemic sites. Trans R Soc Trop Med Hyg 55:463–480. doi: 10.1016/0035-9203(61)90095-5. [DOI] [PubMed] [Google Scholar]
- 5.Powers AM, Logue CH. 2007. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol 88:2363–2377. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 6.Cox J, Mota J, Sukupolvi-Petty S, Diamond MS, Rico-Hesse R. 2012. Mosquito bite delivery of dengue virus enhances immunogenicity and pathogenesis in humanized mice. J Virol 86:7637–7649. doi: 10.1128/JVI.00534-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moser LA, Lim PY, Styer LM, Kramer LD, Bernard KA. 2016. Parameters of mosquito-enhanced West Nile virus infection. J Virol 90:292–299. doi: 10.1128/JVI.02280-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pingen M, Bryden SR, Pondeville E, Schnettler E, Kohl A, Merits A, Fazakerley JK, Graham GJ, McKimmie CS. 2016. Host inflammatory response to mosquito bites enhances the severity of arbovirus infection. Immunity 44:1455–1469. doi: 10.1016/j.immuni.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puiprom O, Morales Vargas RE, Potiwat R, Chaichana P, Ikuta K, Ramasoota P, Okabayashi T. 2013. Characterization of chikungunya virus infection of a human keratinocyte cell line: role of mosquito salivary gland protein in suppressing the host immune response. Infect Genet Evol 17:210–215. doi: 10.1016/j.meegid.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Nestle FO, Filgueira L, Nickoloff BJ, Burg G. 1998. Human dermal dendritic cells process and present soluble protein antigens. J Investig Dermatol 110:762–766. doi: 10.1046/j.1523-1747.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- 11.Martinez FO, Gordon S. 2014. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison TE, Oko L, Montgomery SA, Whitmore AC, Lotstein AR, Gunn BM, Elmore SA, Heise MT. 2011. A mouse model of chikungunya virus-induced musculoskeletal inflammatory disease: evidence of arthritis, tenosynovitis, myositis, and persistence. Am J Pathol 178:32–40. doi: 10.1016/j.ajpath.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner J, Anraku I, Le TT, Larcher T, Major L, Roques P, Schroder WA, Higgs S, Suhrbier A. 2010. Chikungunya virus arthritis in adult wild-type mice. J Virol 84:8021–8032. doi: 10.1128/JVI.02603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mejri N, Rutti B, Brossard M. 2002. Immunosuppressive effects of ixodes ricinus tick saliva or salivary gland extracts on innate and acquired immune response of BALB/c mice. Parasitol Res 88:192–197. doi: 10.1007/s00436-001-0515-1. [DOI] [PubMed] [Google Scholar]
- 15.Gomes R, Oliveira F. 2012. The immune response to sand fly salivary proteins and its influence on leishmania immunity. Front Immunol 3:110. doi: 10.3389/fimmu.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohousová I, Volf P. 2006. Sand fly saliva: effects on host immune response and Leishmania transmission. Folia Parasitol (Praha) 53:161–171. doi: 10.14411/fp.2006.022. [DOI] [PubMed] [Google Scholar]
- 17.Kazimírová M, Štibrániová I. 2013. Tick salivary compounds: their role in modulation of host defences and pathogen transmission. Front Cell Infect Microbiol 3:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francischetti IM, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JM. 2009. The role of saliva in tick feeding. Front Biosci (Landmark Ed) 14:2051–2088. doi: 10.2741/3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stark KR, James AA. 1996. Salivary gland anticoagulants in culicine and anopheline mosquitoes (Diptera:Culicidae). J Med Entomol 33:645–650. doi: 10.1093/jmedent/33.4.645. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro JM, Rossignol PA, Spielman A. 1984. Role of mosquito saliva in blood vessel location. J Exp Biol 108:1–7. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida S, Sudo T, Niimi M, Tao L, Sun B, Kambayashi J, Watanabe H, Luo E, Matsuoka H. 2008. Inhibition of collagen-induced platelet aggregation by anopheline antiplatelet protein, a saliva protein from a malaria vector mosquito. Blood 111:2007–2014. doi: 10.1182/blood-2007-06-097824. [DOI] [PubMed] [Google Scholar]
- 22.Francischetti IM, Valenzuela JG, Ribeiro JM. 1999. Anophelin: kinetics and mechanism of thrombin inhibition. Biochemistry 38:16678–16685. doi: 10.1021/bi991231p. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro JM, Nussenzveig RH, Tortorella G. 1994. Salivary vasodilators of Aedes triseriatus and Anopheles gambiae (Diptera: Culicidae). J Med Entomol 31:747–753. doi: 10.1093/jmedent/31.5.747. [DOI] [PubMed] [Google Scholar]
- 24.Chisenhall DM, Londono BL, Christofferson RC, McCracken MK, Mores CN. 2014. Effect of dengue-2 virus infection on protein expression in the salivary glands of Aedes aegypti mosquitoes. Am J Trop Med Hyg 90:431–437. doi: 10.4269/ajtmh.13-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhawan R, Kumar M, Mohanty AK, Dey G, Advani J, Prasad TS, Kumar A. 2017. Mosquito-borne diseases and omics: salivary gland proteome of the female Aedes aegypti mosquito. OMICS 21:45–54. doi: 10.1089/omi.2016.0160. [DOI] [PubMed] [Google Scholar]
- 26.Vaughan JA, Scheller LF, Wirtz RA, Azad AF. 1999. Infectivity of Plasmodium berghei sporozoites delivered by intravenous inoculation versus mosquito bite: implications for sporozoite vaccine trials. Infect Immun 67:4285–4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rockwell EM, Johnson P. 1952. The insect bite reaction. II. Evaluation of the allergic reaction. J Investig Dermatol 19:137–155. doi: 10.1038/jid.1952.78. [DOI] [PubMed] [Google Scholar]
- 28.Ohtsuka E, Kawai S, Ichikawa T, Nojima H, Kitagawa K, Shirai Y, Kamimura K, Kuraishi Y. 2001. Roles of mast cells and histamine in mosquito bite-induced allergic itch-associated responses in mice. Jpn J Pharmacol 86:97–105. doi: 10.1254/jjp.86.97. [DOI] [PubMed] [Google Scholar]
- 29.Dietzel W, Massion WH, Hinshaw LB. 1969. The mechanism of histamine-induced transcapillary fluid movement. Pflugers Arch 309:99–106. doi: 10.1007/BF00592285. [DOI] [PubMed] [Google Scholar]
- 30.Sourisseau M, Schilte C, Casartelli N, Trouillet C, Guivel-Benhassine F, Rudnicka D, Sol-Foulon N, Le Roux K, Prevost MC, Fsihi H, Frenkiel MP, Blanchet F, Afonso PV, Ceccaldi PE, Ozden S, Gessain A, Schuffenecker I, Verhasselt B, Zamborlini A, Saib A, Rey FA, Arenzana-Seisdedos F, Despres P, Michault A, Albert ML, Schwartz O. 2007. Characterization of reemerging chikungunya virus. PLoS Pathog 3:e89. doi: 10.1371/journal.ppat.0030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spiering MJ. 2015. Primer on the immune system. Alcohol Res 37:171–175. [PMC free article] [PubMed] [Google Scholar]
- 32.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. 2000. The neutrophil as a cellular source of chemokines. Immunol Rev 177:195–203. doi: 10.1034/j.1600-065X.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 33.Schmid MA, Glasner DR, Shah S, Michlmayr D, Kramer LD, Harris E. 2016. Mosquito saliva increases endothelial permeability in the skin, immune cell migration, and dengue pathogenesis during antibody-dependent enhancement. PLoS Pathog 12:e1005676. doi: 10.1371/journal.ppat.1005676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marovich M, Grouard-Vogel G, Louder M, Eller M, Sun W, Wu SJ, Putvatana R, Murphy G, Tassaneetrithep B, Burgess T, Birx D, Hayes C, Schlesinger-Frankel S, Mascola J. 2001. Human dendritic cells as targets of dengue virus infection. J Investig Dermatol Symp Proc 6:219–224. doi: 10.1046/j.0022-202x.2001.00037.x. [DOI] [PubMed] [Google Scholar]
- 35.Her Z, Malleret B, Chan M, Ong EK, Wong SC, Kwek DJ, Tolou H, Lin RT, Tambyah PA, Renia L, Ng LF. 2010. Active infection of human blood monocytes by chikungunya virus triggers an innate immune response. J Immunol 184:5903–5913. doi: 10.4049/jimmunol.0904181. [DOI] [PubMed] [Google Scholar]
- 36.Kim WK, Corey S, Alvarez X, Williams K. 2003. Monocyte/macrophage traffic in HIV and SIV encephalitis. J Leukoc Biol 74:650–656. doi: 10.1189/jlb.0503207. [DOI] [PubMed] [Google Scholar]
- 37.Ali Ou Alla S, Combe B. 2011. Arthritis after infection with chikungunya virus. Best Pract Res Clin Rheumatol 25:337–346. doi: 10.1016/j.berh.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Foissac M, Javelle E, Ray S, Guerin B, Simon F. 2015. Post-chikungunya rheumatoid arthritis, Saint Martin. Emerg Infect Dis 21:530–532. doi: 10.3201/eid2103.141397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dianzani F, Baron S. 1996. Nonspecific defenses, chapter 49 In Baron S. (ed), Medical microbiology, 4th ed University of Texas Medical Branch, Galveston, Texas. [PubMed] [Google Scholar]
- 40.Demeure CE, Brahimi K, Hacini F, Marchand F, Peronet R, Huerre M, St-Mezard P, Nicolas JF, Brey P, Delespesse G, Mecheri S. 2005. Anopheles mosquito bites activate cutaneous mast cells leading to a local inflammatory response and lymph node hyperplasia. J Immunol 174:3932–3940. doi: 10.4049/jimmunol.174.7.3932. [DOI] [PubMed] [Google Scholar]
- 41.Conway MJ, Watson AM, Colpitts TM, Dragovic SM, Li Z, Wang P, Feitosa F, Shepherd DT, Ryman KD, Klimstra WB, Anderson JF, Fikrig E. 2014. Mosquito saliva serine protease enhances dissemination of dengue virus into the mammalian host. J Virol 88:164–175. doi: 10.1128/JVI.02235-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Coupanec A, Babin D, Fiette L, Jouvion G, Ave P, Misse D, Bouloy M, Choumet V. 2013. Aedes mosquito saliva modulates Rift Valley fever virus pathogenicity. PLoS Negl Trop Dis 7:e2237. doi: 10.1371/journal.pntd.0002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diamond MS, Farzan M. 2013. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat Rev Immunol 13:46–57. doi: 10.1038/nri3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Surasombatpattana P, Hamel R, Patramool S, Luplertlop N, Thomas F, Despres P, Briant L, Yssel H, Misse D. 2011. Dengue virus replication in infected human keratinocytes leads to activation of antiviral innate immune responses. Infect Genet Evol 11:1664–1673. doi: 10.1016/j.meegid.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Schuijt TJ, Coumou J, Narasimhan S, Dai J, Deponte K, Wouters D, Brouwer M, Oei A, Roelofs JJ, van Dam AP, van der Poll T, Van't Veer C, Hovius JW, Fikrig E. 2011. A tick mannose-binding lectin inhibitor interferes with the vertebrate complement cascade to enhance transmission of the Lyme disease agent. Cell Host Microbe 10:136–146. doi: 10.1016/j.chom.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tyson K, Elkins C, Patterson H, Fikrig E, de Silva A. 2007. Biochemical and functional characterization of Salp20, an Ixodes scapularis tick salivary protein that inhibits the complement pathway. Insect Mol Biol 16:469–479. doi: 10.1111/j.1365-2583.2007.00742.x. [DOI] [PubMed] [Google Scholar]
- 47.Tyson KR, Elkins C, de Silva AM. 2008. A novel mechanism of complement inhibition unmasked by a tick salivary protein that binds to properdin. J Immunol 180:3964–3968. doi: 10.4049/jimmunol.180.6.3964. [DOI] [PubMed] [Google Scholar]
- 48.Lieskovská J, Kopecký J. 2012. Tick saliva suppresses IFN signalling in dendritic cells upon Borrelia afzelii infection. Parasite Immunol 34:32–39. doi: 10.1111/j.1365-3024.2011.01345.x. [DOI] [PubMed] [Google Scholar]
- 49.Tchankouo-Nguetcheu S, Bourguet E, Lenormand P, Rousselle JC, Namane A, Choumet V. 2012. Infection by chikungunya virus modulates the expression of several proteins in Aedes aegypti salivary glands. Parasit Vectors 5:264. doi: 10.1186/1756-3305-5-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nesargikar PN, Spiller B, Chavez R. 2012. The complement system: history, pathways, cascade and inhibitors. Eur J Microbiol Immunol 2:103–111. doi: 10.1556/EuJMI.2.2012.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cavalcante RR, Pereira MH, Gontijo NF. 2003. Anti-complement activity in the saliva of phlebotomine sand flies and other haematophagous insects. Parasitology 127:87–93. doi: 10.1017/S0031182003003329. [DOI] [PubMed] [Google Scholar]
- 52.Hourcade DE, Akk AM, Mitchell LM, Zhou HF, Hauhart R, Pham CT. 2016. Anti-complement activity of the Ixodes scapularis salivary protein Salp20. Mol Immunol 69:62–69. doi: 10.1016/j.molimm.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thiemmeca S, Tamdet C, Punyadee N, Prommool T, Songjaeng A, Noisakran S, Puttikhunt C, Atkinson JP, Diamond MS, Ponlawat A, Avirutnan P. 2016. Secreted NS1 protects dengue virus from mannose-binding lectin-mediated neutralization. J Immunol 197:4053–4065. doi: 10.4049/jimmunol.1600323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skallová A, Iezzi G, Ampenberger F, Kopf M, Kopecky J. 2008. Tick saliva inhibits dendritic cell migration, maturation, and function while promoting development of Th2 responses. J Immunol 180:6186–6192. doi: 10.4049/jimmunol.180.9.6186. [DOI] [PubMed] [Google Scholar]
- 55.Johnston LJ, Halliday GM, King NJ. 2000. Langerhans cells migrate to local lymph nodes following cutaneous infection with an arbovirus. J Investig Dermatol 114:560–568. doi: 10.1046/j.1523-1747.2000.00904.x. [DOI] [PubMed] [Google Scholar]
- 56.Chen G, Severo MS, Sohail M, Sakhon OS, Wikel SK, Kotsyfakis M, Pedra JH. 2012. Ixodes scapularis saliva mitigates inflammatory cytokine secretion during Anaplasma phagocytophilum stimulation of immune cells. Parasit Vectors 5:229. doi: 10.1186/1756-3305-5-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brake DK, Wikel SK, Tidwell JP, Perez de Leon AA. 2010. Rhipicephalus microplus salivary gland molecules induce differential CD86 expression in murine macrophages. Parasit Vectors 3:103. doi: 10.1186/1756-3305-3-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poole NM, Mamidanna G, Smith RA, Coons LB, Cole JA. 2013. Prostaglandin E(2) in tick saliva regulates macrophage cell migration and cytokine profile. Parasit Vectors 6:261. doi: 10.1186/1756-3305-6-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wanasen N, Nussenzveig RH, Champagne DE, Soong L, Higgs S. 2004. Differential modulation of murine host immune response by salivary gland extracts from the mosquitoes Aedes aegypti and Culex quinquefasciatus. Med Vet Entomol 18:191–199. doi: 10.1111/j.1365-2915.2004.00498.x. [DOI] [PubMed] [Google Scholar]
- 60.Bizzarro B, Barros MS, Maciel C, Gueroni DI, Lino CN, Campopiano J, Kotsyfakis M, Amarante-Mendes GP, Calvo E, Capurro ML, Sa-Nunes A. 2013. Effects of Aedes aegypti salivary components on dendritic cell and lymphocyte biology. Parasit Vectors 6:329. doi: 10.1186/1756-3305-6-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schneider BS, Soong L, Coffey LL, Stevenson HL, McGee CE, Higgs S. 2010. Aedes aegypti saliva alters leukocyte recruitment and cytokine signaling by antigen-presenting cells during West Nile virus infection. PLoS One 5:e11704. doi: 10.1371/journal.pone.0011704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schneider BS, Soong L, Zeidner NS, Higgs S. 2004. Aedes aegypti salivary gland extracts modulate anti-viral and TH1/TH2 cytokine responses to Sindbis virus infection. Viral Immunol 17:565–573. doi: 10.1089/vim.2004.17.565. [DOI] [PubMed] [Google Scholar]
- 63.Thangamani S, Higgs S, Ziegler S, Vanlandingham D, Tesh R, Wikel S. 2010. Host immune response to mosquito-transmitted chikungunya virus differs from that elicited by needle inoculated virus. PLoS One 5:e12137. doi: 10.1371/journal.pone.0012137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hawman DW, Stoermer KA, Montgomery SA, Pal P, Oko L, Diamond MS, Morrison TE. 2013. Chronic joint disease caused by persistent chikungunya virus infection is controlled by the adaptive immune response. J Virol 87:13878–13888. doi: 10.1128/JVI.02666-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cime-Castillo J, Delannoy P, Mendoza-Hernández G, Monroy-Martínez V, Harduin-Lepers A, Lanz-Mendoza H, Hernández-Hernández FDL, Zenteno E, Cabello-Gutiérrez C, Ruiz-Ordaz BH. 2015. Sialic acid expression in the mosquito Aedes aegypti and its possible role in dengue virus-vector interactions. Biomed Res Int 2015:504187. doi: 10.1155/2015/504187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Varki A. 2007. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature 446:1023–1029. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- 67.U.S. National Institutes of Health, Department of Health and Human Services. 2017. NIH begins study of vaccine to protect against mosquito-borne diseases. https://www.nih.gov/news-events/news-releases/nih-begins-study-vaccine-protect-against-mosquito-borne-diseases Accessed 16 March 2018.
- 68.Singh K, Davies G, Alenazi Y, Eaton JRO, Kawamura A, Bhattacharya S. 2017. Yeast surface display identifies a family of evasins from ticks with novel polyvalent CC chemokine-binding activities. Sci Rep 7:4267. doi: 10.1038/s41598-017-04378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carneiro-Lobo TC, Konig S, Machado DE, Nasciutti LE, Forni MF, Francischetti IM, Sogayar MC, Monteiro RQ. 2009. Ixolaris, a tissue factor inhibitor, blocks primary tumor growth and angiogenesis in a glioblastoma model. J Thromb Haemost 7:1855–1864. doi: 10.1111/j.1538-7836.2009.03553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Oliveira Ada S, Lima LG, Mariano-Oliveira A, Machado DE, Nasciutti LE, Andersen JF, Petersen LC, Francischetti IM, Monteiro RQ. 2012. Inhibition of tissue factor by ixolaris reduces primary tumor growth and experimental metastasis in a murine model of melanoma. Thromb Res 130:e163–e170. doi: 10.1016/j.thromres.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bidlingmaier S, Liu B. 2011. Construction of yeast surface-displayed cDNA libraries. Methods Mol Biol 729:199–210. doi: 10.1007/978-1-61779-065-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ryman KD, Klimstra WB, Nguyen KB, Biron CA, Johnston RE. 2000. Alpha/beta interferon protects adult mice from fatal Sindbis virus infection and is an important determinant of cell and tissue tropism. J Virol 74:3366–3378. doi: 10.1128/JVI.74.7.3366-3378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Teo TH, Lum FM, Lee WW, Ng LF. 2012. Mouse models for chikungunya virus: deciphering immune mechanisms responsible for disease and pathology. Immunol Res 53:136–147. doi: 10.1007/s12026-012-8266-x. [DOI] [PubMed] [Google Scholar]
- 74.Seymour RL, Rossi SL, Bergren NA, Plante KS, Weaver SC. 2013. The role of innate versus adaptive immune responses in a mouse model of O'nyong-nyong virus infection. Am J Trop Med Hyg 88:1170–1179. doi: 10.4269/ajtmh.12-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]


