Abstract
Survival and growth of monarch larvae, Danaus plexippus (L.), after exposure to either Cry1Ab-expressing pollen from three Bacillus thuringiensis (Bt) corn (Zea mays L.) events differing in toxin expression or to the insecticide, λ-cyhalothrin, were examined in field studies. First instars exposed to low doses (≈22 grains per cm2) of event-176 pollen gained 18% less weight than those exposed to Bt11 or Mon810 pollen after a 5-day exposure period. Larvae exposed to 67 pollen grains per cm2 on milkweed leaves from within an event-176 field exhibited 60% lower survivorship and 42% less weight gain compared with those exposed to leaves from outside the field. In contrast, Bt11 pollen had no effect on growth to adulthood or survival of first or third instars exposed for 5 days to ≈55 and 97 pollen grains per cm2, respectively. Similarly, no differences in larval survivorship were observed after a 4-day exposure period to leaves with 504–586 (within fields) or 18–22 (outside the field) pollen grains per cm2 collected from Bt11 and non-Bt sweet-corn fields. However, survivorship and weight gain were drastically reduced in non-Bt fields treated with λ-cyhalothrin. The effects of Bt11 and Mon810 pollen on the survivorship of larvae feeding 14 to 22 days on milkweeds in fields were negligible. Further studies should examine the lifetime and reproductive impact of Bt11 and Mon810 pollen on monarchs after long-term exposure to naturally deposited pollen.
Corn (Zea mays L.) has been transformed with a gene from the bacterium Bacillus thuringiensis (Bt) to express the insecticidal 1 epidopteran-active crystalline protein (Cry1Ab) endotoxin (1). The Cry1Ab toxin is specifically active on the lepidopteran species so the impact on nontarget organisms has been considered negligible (2). However, most commercial Bt corn hybrids express the toxin in the pollen (3) that may be deposited on host plants of nontarget species. In particular, the monarch butterfly, Danaus plexippus (L.) may be at risk (4, 5), because monarch larvae feed on the common milkweed, Asclepias syriaca (6), in and near cornfields. About half of the overwintering monarch population originates from the region known as the Corn Belt (7), so a portion of the migratory monarch population may be exposed to Bt pollen. However, the exposure of monarch larvae to the Cry1Ab toxin varies for different Bt corn events. Pollen from event-176 Bt hybrids expresses the highest level of Bt toxin (up to 7.1 μg/g of pollen; http://www.epa.gov/pesticides/biopesticides/factsheets/fs006458t.htm) and has been demonstrated to have adverse effects on first-instar monarch caterpillars (5, 8). The registration of hybrids derived from event 176 will terminate in 2001 (http://www.epa.gov/scipoly/sap/2000/october/brad1_execsum_overview.pdf). Bt11 and Mon810 hybrids express <0.09 μg of Cry1Ab per g of pollen (http://www.epa.gov/scipoly/sap/2000/october/), so the potential negative impacts of these hybrids may be lower than that of event-176 hybrids.
Reported herein are five independent field studies conducted in Iowa, Maryland, Ontario (Canada), and New York to determine the impact of Bt pollen on the survival and growth of D. plexippus. Each study varied in experimental design and treatments tested; however, the general approach was to expose larvae to milkweed leaves containing natural deposits of Bt and non-Bt pollen that accounted for pollen accumulation, natural degradation of toxin within pollen of various ages under different environmental conditions, and the possible ingestion of Bt-expressing plant material other than pollen. In the Maryland study, the responses of larvae to pollen were compared with responses to an insecticide used to control Ostrinia nubilalis.
Materials and Methods
Iowa Study I.
A split-split plot experiment was conducted on two Iowa State University farms planted on May 24, 2000 with six Bt hybrids: NK3030Bt, NK7070Bt (Syngenta Seeds, Golden Valley, MN; Bt11 event), 38G17, 34R07 (Pioneer Hi-Bred, Des Moines, IA; Mon810 event), Maximizer 21 (Syngenta Seeds; event 176), and 2249 (Mycogen Seeds, Indianapolis, IN; event 176). The non-Bt hybrids NK3030, NK7070 (Syngenta), 3489, and 3893 (Pioneer Hi-Bred) were included as controls. The transformation events were randomly assigned as main-plot treatments to four complete blocks on each farm. Plots measured 4.6 by 4.6 m and were separated by ≥18.3 m of soybeans to minimize pollen drift among plots (9). Subplots were potted A. syriaca plants placed at two locations per plot ≈2 days before corn anthesis, with two in the middle and two at the edge of the plot. When 50–75% of the corn plants had shed pollen, three first-instar monarch larvae (<24-h old) were transferred to leaves on the upper half of each milkweed plant. One plant from each subplot was caged with fine-mesh screening to deter predation. Before larval infestation, a leaf was selected randomly from the upper half of each plant and brought back to the laboratory where pollen grains were counted in five randomly chosen 0.25-cm2 sections of the leaf by using an ocular grid and a stereo microscope. Five days after infestation, larval survival and weight were recorded. Each leaf then was removed from the upper half of each plant for a second determination of pollen density.
Ontario Study.
The study was conducted in six commercial field-corn sites [4.9–17.4 hectares (ha)] planted within Wellington County, Ontario, Canada in May 2000. Three Bt11 fields (two N2555 and one N27 M3, Syngenta Seeds) and three non-Bt fields (Pride 177, Pioneer Hi-Bred; Hyland 2240, Hyland Seeds, Blenheim, ON, Canada; and 4064NK, Syngenta Seeds) were selected based on whether they were >150 m from other cornfields and had naturally occurring milkweed in the following locations: ≈1 m inside the field, <1 m from the field edge, and ≈5 m from the field edge, along eight transects perpendicular to the field edge. As controls, eight naturally occurring milkweed plants were selected in each of three natural areas situated >150 m from any cornfields.
The eight transects were alternately assigned to one of two bioassays that began on day 6 and day 11 of anthesis, respectively. For each bioassay, a cohort of five larvae, reared on A. syriaca in the laboratory, was weighed, randomly assigned to a plant, and placed on the upper third of the plant. The whole plant was then enclosed in a fine mesh cage to deter predation. Larvae for the first bioassay were first instars (12- to 36-h old), whereas those used in the second bioassay were third instars (<24 h post second molt). After a 5-day exposure period, the number and instar of larvae were recorded. Larval cohorts were brought back to the laboratory where they were weighed and reared to adulthood on clean A. syriaca at 24°C and a 16-h light/8-h dark cycle. Additional data were recorded on days to pupation, pupal weight, percentage of pupation and emergence, and adult weight and wing length. Leaves with feeding damage were collected from plants of the first bioassay and viewed with a video imaging system (XC-75CE, Cosmicar/Pentax 16-mm camera with NORTHERN EXPOSURE V.2.9E software, Empix Imaging, Mississauga, ON, Canada) to estimate the area of consumption. Pollen densities were determined for leaves collected from the tops, middles, and bottoms of plants. To minimize the loss of pollen, all sampled leaves were sandwiched between strips of contact paper (ConTact7 Brand; Decora Manufacturing, North Ridgeville, OH). Pollen counts within five 1-cm2 areas on the tops and bottoms of leaves were added to corresponding counts in the contact paper to obtain density estimates. Grains adhering to the paper were stained with acid fuchsin (Sigma) to facilitate counting.
Maryland Study.
Field-corn experiment.
The study was conducted in an 8-ha cornfield at the University of Maryland Research and Education Center at Beltsville, MD. The field was planted on May 3, 2000 with the hybrid TM5114 (Mycogen Seeds; event 176). Milkweed plants were propagated from rhizomes in 20-cm pots and grown outdoors until they were 50–60 cm in height. Two transects of milkweeds treated as blocks were established along each side of the field before anthesis. At each transect, plants were transplanted within and outside the field at −10, −5, −3, −1 m, and 1, 3, 5, and 10 m from the field edge, respectively. Separate bioassays with field-collected leaves from each plant position were conducted at 3, 6, 9, and 14 days after the onset of anthesis. At each time, pollen densities were estimated by counting the number of grains in a 0.34-cm2 viewing area on a single leaf that was removed from the upper one-third of each milkweed plant. Each leaf then was infested with a cohort of 10 first instars (≈24-h old) for 4 days. Survival and weights of larval cohorts were assessed both initially and after 4 days.
Sweet-corn experiment.
The study was conducted at the University of Maryland Research and Education Center at Upper Marlboro, MD. The plot was planted on May 12, 2000 and consisted of three hybrid treatments: untreated Attribute GSS0937 (Bt), untreated Prime Plus (non-Bt isoline), and treated Prime Plus, replicated four times in a randomized block design. The Attribute hybrid (Syngenta Seeds) was developed by crossing with the Cry1Ab (Bt11 event) field corn and then backcrossing to produce a pure inbred of Bt sweet corn. The treated non-Bt plots received three applications of λ-cyhalothrin (Warrior 1E, Syngenta Crop Protection) delivered at the 94.6-ml formulation rate by an air-blast sprayer in 536 liters of spray volume per ha. Each of the four replicate blocks were planted with 4-m buffers between hybrid treatments, and each block was surrounded by a 9-m buffer zone of bare ground to minimize drift effects. Plants were propagated and transplanted as described above. Two plants were transplanted at 3 m from the inside edge (between rows 4 and 5) and the outside edge of each hybrid plot, along transects evenly spaced along the field edge. The air-blast sprayer ran between the edge of the plot and the outside milkweeds, so these plants were not exposed to the directed spray. Separate bioassays were conducted at 3, 6, and 9 days after the onset of anthesis, as described above. Leaves were removed within 1 h after insecticide treatments that coincided with the three bioassays.
Iowa Study II.
A replicate field site subdivided into paired 0.2-ha plots of N4640Bt corn (Syngenta Seeds; Bt11 event) and non-Bt N4640 corn (Syngenta Seeds) was planted in late April 2000 at each of three Iowa State University farms. Milkweed plants were transplanted to the field sites during May and June. Six plants (5–50 cm tall) were established at each of three locations: 4.6 m inside the plot, at the field edge, and 2 m from the plot edge. Five first instars were placed on each plant ≈7 days after the start of anthesis. The number and life stages of larvae were recorded every 24 h for the first 7 days, and then every 48 h for another 7 days. Leaves were collected and pollen densities were estimated at 3–8 days after larval introduction by counting the number of grains on one 0.5-cm2 leaf disk excised with a #6 cork borer from a middle leaf of each plant (5).
New York Study.
One 0.25-ha field near Ithaca, NY was planted in two 36-row sections, one with the Bt hybrid 36-G32Bt (Pioneer HiBred, Mon810 event) and the other with a non-Bt corn hybrid (3752). Ten milkweed plants were transplanted at three locations in each section: along the edge, and at 6.6 and 32.7 m inside the field. At ≈2 days from the onset of pollen shed, five first instars were placed on each plant. The number and life stages of larvae were recorded every 24 h for 22 days. The pollen density on each plant was measured 6 days after larval introduction according to the same methods used in the Iowa II study.
Data Analysis.
In the Maryland study, variances of data not meeting the assumptions of ANOVA were grouped before analysis (10). In the Ontario and Iowa I studies, log or arcsine transformations were applied as required. In all three experiments, the following variables for each bioassay were analyzed by ANOVA (PROC MIXED; ref. 11): pollen deposition, percentage of survival and weight gain, and in the Ontario study, development, consumption, days to pupation, pupal and adult weights, percent emergence, and adult wing length. In all studies, corn hybrid treatment and milkweed position were treated as fixed effects, whereas block or field was treated as a random effect. For the Iowa I data, bioassay type (caged or uncaged) was treated as a fixed subplot factor. In addition, orthogonal contrasts were conducted to test for differences between each Bt11 and Mon810 hybrid and their isolines, and between the event-176 hybrids vs. the other two Bt hybrids combined. Means were separated with Tukey's studentized range test (P < 0.05). For the Iowa II and New York studies, the number of larvae alive over time was analyzed by ANOVA (PROC GLM). Survival curves for larvae were analyzed separately for each hybrid with LIFETEST (SAS Institute; ref. 11).
Results
Iowa Study I.
Average pollen densities ranged from 21 to 23 grains per cm2 across hybrid treatments, which were not significantly different. These levels were relatively low compared with published reports of pollen deposition (12) that could be a small-plot effect. Only the plant location effect was significant (P = 0.027), showing higher pollen levels in the middle of the plot (26 grains per cm2) compared with the plot edge (18 grains per cm2). For caged tests, average weights ranged from 2.8 to 3.3 mg per larva, and all main and interaction effects were nonsignificant. For the uncaged tests, no significant effects also were indicated by using the general linear model; however, the contrast test showed that the average weights of larvae (1.7 mg) that fed on leaves with event-176 pollen were significantly less than weights of larvae (2.0 mg) on the other Bt hybrids combined (P = 0.037). For percent survival, the hybrid treatment and all interaction effects with hybrid were not statistically significant. However, both the plant location (P = 0.002) and bioassay-type (caged or uncaged; P < 0.001) effects were significant. Survival was significantly higher at the plot edge (53.6%) and in caged cohorts (61.2%), compared with the middle of the plot (38.1%) and uncaged cohorts (30.5%), respectively. Predatory insects may have used the plots as a refuge to escape the prevailing hot-dry conditions in Iowa, resulting in higher predation in the middle of the field compared with the soy buffers surrounding the field. Also, lower predation seemed to occur in caged cohorts.
Ontario Study.
Pollen densities on leaves did not differ between hybrid types (Bt or non-Bt) but decreased significantly with increasing distance from the field on day 6 (P < 0.0001) and day 11 (P < 0.0001) of anthesis. On day 6, the ranges of pollen densities at each distance were 2–309, 0–176, and 0–75 grains per cm2 for plants found at −1 m within the field, <1 m outside the field, and 5 m outside the field, respectively. On day 11, the range of densities calculated on leaf samples taken from −1, <1, and 5 m were 3–429, <1–320, and <1–50, respectively. Control plants contained ≈1 grain per cm2 on both days, probably resulting from contamination during leaf sampling. Average accumulated rainfall during the first 6 and 11 days of pollen shed (before placing cages on plants) was ≈10 and 13 mm, respectively, and may have washed pollen off of the plants (12).
The comparison of responses to Bt and non-Bt pollen is presented in Table 1. Neither hybrid type nor plant position affected survivorship, developmental rate, or weight gain of first- or third-instar monarchs during the exposure period in the field or in later developmental stages (but see % emergence for first instars in Table 1). No significant differences in survivorship, weight gain, or development to adulthood were observed between larvae in cages within the fields and those in control cages >150 m from any cornfield in either bioassay (P ≥ 0.15 for all variables).
Table 1.
Comparison of survivorship and growth for Monarch larvae exposed as first or third instars to Bt11 and non-Bt pollen on caged milkweed (A. syriaca) plants in Ontario
| Response variable | First instars mean (SE)
|
Third instars mean (SE)
|
||||
|---|---|---|---|---|---|---|
| Bt11 | Non-Bt | P | Bt11 | Non-Bt | P | |
| Survival (in field), % | 84.4 (3.8) | 80.6 (3.4) | 0.47 | 91.7 (2.0) | 87.8 (2.8) | 0.46 |
| Leaf feeding/larva, cm2 | 1.7 (0.3) | 1.0 (0.1) | 0.32 | – | – | 0.25 |
| Development* | 2.4 (0.1) | 2.1 (0.1) | 0.40 | 4.2 (0.1) | 4.0 (0.04) | 0.34 |
| Weight gain/larva† | 20.4 (1.1) | 15.6 (1.8) | 0.37 | 15.1 (0.9) | 10.8 (1.5) | 0.55 |
| Survival (to pupation), % | 59.4 (5.1) | 57.4 (6.2) | 0.70 | 73.3 (3.4) | 63.3 (6.2) | 0.67 |
| Days to pupation‡ | 16.1 (0.3) | 16.3 (0.3) | 0.40 | 11.6 (0.2) | 12.1 (0.4) | 0.75 |
| Pupal weight, mg | 1280 (24.5) | 1246 (37.1) | 0.65 | 1201 (24.7) | 1147 (34.6) | 0.97 |
| Emergence (adults), % | 80.0 (6.0) | 95.2 (2.3) | 0.07 | 80.2 (3.7) | 76.2 (6.1) | 0.86 |
| Adult weight, mg | 534.1 (12.2) | 522.2 (13.2) | 0.33 | 474.9 (11.1) | 467.8 (15.4) | 0.33 |
| Adult wing length, mm | 52.7 (0.4) | 52.0 (0.6) | 0.67 | 50.9 (0.4) | 49.8 (0.5) | 0.67 |
Day 5 avg instar/day 0 avg instar.
Day 6 avg wt/day 0 avg wt.
Days to pupation from the beginning of the exposure period.
Maryland Study.
Field-corn experiment.
The average pollen densities on milkweed leaves in the field on days 3, 6, 9, and 14 of anthesis were 8.2, 63.7, 161.2, and 27.8 grains per cm2, respectively. Average levels outside the field peaked at 18.5 grains per cm2 on day 9 and significantly dropped off along positions extending away from the field edge. The relative differences in pollen deposition across transect positions on each date were not the same (P = 0.0015). During anthesis, seven rain events (11–25 mm per day) occurred that may have washed pollen off of leaves (12).
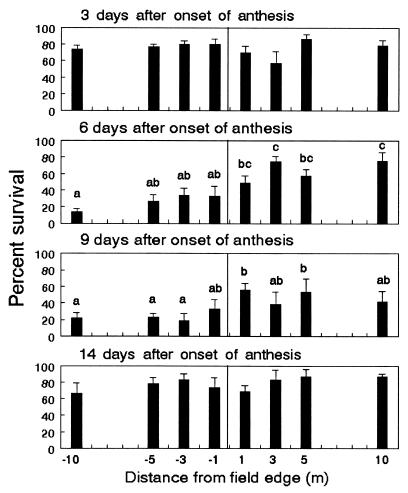
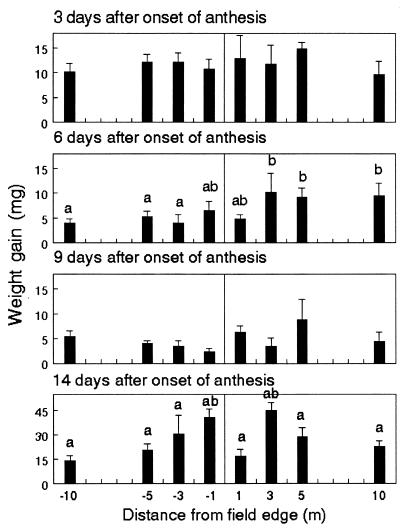
Survival of monarch larvae ranged from 57.5 to 86.3% on day 3 of anthesis (Fig. 1). Survivors, which initially weighed an average of 0.9 mg, gained 9.9 to 12.8 mg after 4 days (Fig. 2). On day 6, the plant position had a significant effect on larval survival (P < 0.0001) and weight gain (P = 0.0081). Sixty-three percent of the larvae survived and gained 8.3 mg each after feeding on leaves outside the field, compared with 25.1% survival and weight gains of 4.8 mg inside. On day 9, larval survival inside the field was reduced by 51% compared with survival of milkweeds located outside (P = 0.0215). Larvae also gained less weight inside the field, but differences across plant positions were not significant. On day 14, weight gain, but not survival, was significantly affected by plant position (P = 0.0418); however, differences across plant positions were not consistent with levels of pollen. This last bioassay used a combination of late-first and early-second instars with an initial weight of 5.6 mg per larva and leaves from only four transects of plants. Thus, weight gains were significantly higher compared with previous bioassays and exhibited greater variability.
Figure 1.
Survival of first-instar monarch larvae feeding on milkweeds placed at 1, 3, 5, and 10 m inside (negative values) and outside the field edge of event-176 field corn in Maryland. Means ± SE are based on separate bioassays conducted on days 3, 6, 9, and 14 of anthesis. Within each graph, columns with the same letters are not significantly different (P < 0.05; Tukey's test).
Figure 2.
Weight gain of first-instar monarch larvae feeding on milkweeds placed at 1, 3, 5, and 10 m inside (negative value) and outside the field edge of event-176 field corn in Maryland. Means ± SE are based on separate bioassays conducted on days 3, 6, 9, and 14 of anthesis. Within each graph, columns with the same letters are not significantly different (P < 0.05; Tukey's test).
Sweet-corn experiment.
Maximum levels of pollen from both Bt and non-Bt hybrids were deposited on milkweeds at 6 to 9 days after the onset of anthesis. Peak levels of pollen on milkweeds situated 3 m inside plots averaged 504–586 grains per cm2 and were significantly higher than levels on plants outside plots that peaked at 18–22 grains per cm2 (P < 0.0001). The overall level of pollen produced by both hybrids over time was statistically the same. Rain events, recorded on day 5 (2 mm) and day 9 (36 mm) of anthesis, may have removed some pollen.
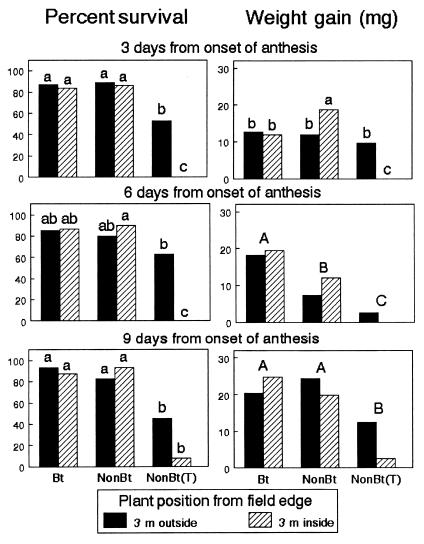
Data on larval survival and weight gain were analyzed separately for each bioassay date and are summarized in Fig. 3. On all dates, the treatment by position-interaction effect on percentage of survival was significant (day 3, P < 0.0001; day 6, P < 0.0001; day 9, P = 0.0027). Survival of monarch larvae feeding on milkweed leaves either inside or outside Bt and non-Bt (unsprayed) plots ranged from 79.9 to 93.2% and were not statistically different at any date. Survival of larvae feeding on leaves collected inside and outside non-Bt (sprayed) plots was significantly reduced by 91–100% and 21–45%, respectively. Most larvae died within 1 h of exposure to sprayed leaves. Initial weights during days 3, 6, and 9 averaged 2.2, 2.2, and 3.5 mg per larva, respectively. On day 3, the weight gain of surviving larvae was significantly influenced by the treatment by position interaction (P = 0.0003). Larvae fed milkweed leaves collected from inside unsprayed non-Bt plots exhibited the greatest weight gain, whereas those larvae exposed to λ-cyhalothrin on leaves inside sprayed plots did not survive and, thus, showed no weight gain. For bioassays conducted on days 6 and 9 of anthesis, the treatment by position interaction and main effect for position were not significant. On day 6, only the treatment effect was significant (P = 0.0045); average weight gains of larvae feeding on milkweeds from Bt plots were significantly higher than those of larvae feeding on plants from non-Bt plots. The insecticide significantly reduced the weight gains of surviving larvae feeding on plants from both inside and outside the sprayed non-Bt plots. On day 9, overall weight gains of larvae after 4 days of feeding were higher than the previous bioassays because of higher initial weights. A significant treatment effect (P = 0.0148) indicated that weight gains for the Bt and non-Bt (unsprayed) treatments were the same but significantly higher than average weights of larvae feeding on milkweed leaves from both inside and outside the sprayed non-Bt plots.
Figure 3.
Survival and weight gain of first-instar monarch larvae feeding on milkweeds placed at 3 m inside and outside the edge of plots consisting of Bt11, non-Bt (untreated), and non-Bt (λ-cyhalothrin-treated) sweet corn in Maryland. Data are based on separate bioassays conducted at 3, 6, and 9 days of anthesis. Within each graph, columns with the same lowercase letters are not significantly different for the interaction effect; pairs of columns with the same uppercase letters are not significantly different for the treatment effect (P < 0.05; Tukey's test).
Iowa II and New York Studies.
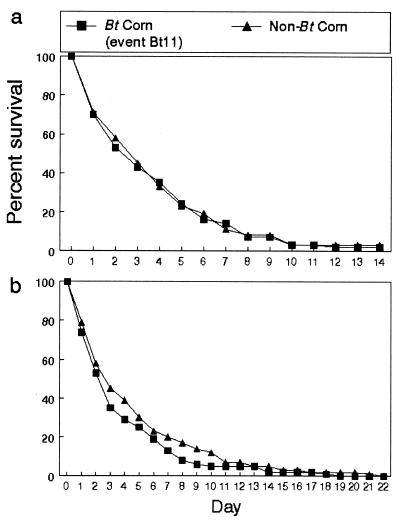
Pollen densities among the three sites in Iowa were similar between hybrids and ranged from 154 to 367, 11 to 116, and 5 to 36 grains per cm2 within, at the edge, and 2 m outside the field, respectively. There were no significant differences in the numbers of larvae surviving and in the survival curves among the three field sites. The number of larvae alive over the 14 days in the Bt and non-Bt corn plots was statistically the same. The survival curves of larvae pooled over the three Bt corn sites were not significantly different from those in non-Bt (Fig. 4a). In New York, trends in survivorship were also statistically the same for cohorts of larvae feeding for 22 days on milkweeds in Bt and non-Bt fields (Fig. 4b). Pollen counts on milkweeds in the field averaged 127 grains per cm2 and were not significantly different between hybrids. The early-instar larvae may have experienced higher pollen levels because counts were made 6 days after larvae were introduced and <24 h after significant precipitation that may have reduced pollen levels (12). For data pooled over hybrids, the survivorship curves in both studies indicated significantly lower survival on milkweeds at the edge of the field compared with survival at the other locations (Iowa: log-rank test, P < 0.09; Wilcoxon test, P < 0.04; New York: log-rank test, P < 0.009; Wilcoxon test, P < 0.04). The same result has been observed in other studies for non-Bt fields and is probably explained by the fact that cornfields are typically less diverse in flora and fauna than field edges, and thus larvae may experience less predation within cornfields (13).
Figure 4.
Survival curves for monarch larvae placed in and near Bt and non-Bt corn fields. (a) Iowa. (b) New York.
Discussion
These studies provide evidence that the amount of pollen deposited on milkweed leaves and the Cry1Ab expression in pollen largely predict impacts of pollen on monarch larvae feeding on milkweed associated with Bt corn during anthesis. In the Iowa I study, low doses of event-176 pollen averaging 23 grains per cm2 had a significant effect on weights of first instars, although survival differences were not statistically significant. Results from the field-corn experiment conducted in Maryland show that the survival and growth of first instars were significantly affected when event-176 pollen levels reached ≈67 grains per cm2 of leaf area. Survival of larvae feeding on milkweeds within the field during peak anthesis was reduced by as much as 60%, and weight gain was also reduced by as much as 42% compared with larvae feeding on milkweeds outside of the field. These results are consistent with those of Jesse and Obrycki (5), who observed a decrease in the survival of larvae exposed to event-176 pollen on leaves from within a field (80–217 pollen grains per cm2) compared with those fed leaves from outside of the field. The results are also consistent with laboratory studies that have shown reductions in weight gain at doses as low as 5–10 grains per cm2 after a 4-day exposure period (8).
In contrast, Mon810 and Bt11 hybrids do not seem to have direct adverse effects on larval survival. The Iowa I study demonstrates that low doses of Mon810 pollen (26 grains per cm2) do not affect survivorship or weight gain of first instars after a 5-day exposure period. The New York study also shows that survival of developing larvae on milkweeds located within the field was not significantly affected after exposure to Mon810 pollen for 22 days. These results are not surprising, because Mon810 pollen expresses far less toxin than event-176 pollen. None of the studies show an effect of Bt11 pollen on survivorship or weight gain either outside or inside the field, even where pollen densities as high as 586 grains per cm2 of leaf area were observed. In the Ontario study, neither first nor third instars were detrimentally affected by pollen from event-Bt11 hybrids after exposure to pollen densities of 55 and 97 grains per cm2, respectively. The survival, growth, and development of later-life stages were also unaffected at these doses; larvae caged in Bt11 cornfields, non-Bt cornfields, or control areas developed into pupae and adults of similar weight and size within similar developmental times. However, the percent of emergence may have been affected by Bt11 pollen and warrants further investigation. In the Iowa II study, no effects on survival were observed for larval cohorts feeding for 14 days on milkweed plants within Bt11 corn plots with average pollen levels of 241 grains per cm2. Results from the sweet-corn experiment with the Bt11 event show no effects on monarch survival or growth after 4 days at the three bioassay times during anthesis, compared with larvae in and near non-Bt plots that were not treated with insecticide. These field studies are consistent with those of laboratory studies that suggest that exposure to Bt11 pollen at doses of less than 1,000 grains per cm2 do not detrimentally affect first-instar monarchs after a 4-day exposure period (8).
Most of the studies reported herein did not examine exposure in the middle of cornfields where pollen densities may be higher than at the plot edge (14). However, in the sweet-corn experiment, unaffected larvae fed on milkweed leaves with >3 times the pollen densities typically found in field-corn plots. Bt sweet corn can be viewed as a worst-case scenario corn type for testing nontarget effects because it produces more pollen per plant than field corn and is heavily treated with insecticides (http://www.epa.gov/scipoly/sap/2000/october/). Furthermore, Cry1Ab expression in the pollen of Bt sweet corn is comparable to the expression in field-corn hybrids based on the Bt11 event. Thus, results of nontarget effects in sweet corn can be extrapolated to risk scenarios for field corn.
The sweet-corn experiment also demonstrates the importance of assessing the risks of Bt corn to monarch populations in terms of the relative risks of other agricultural practices. Monarch larvae were adversely affected by treatments of λ-cyhalothrin applied to non-Bt plots of sweet corn. Most larvae died within hours after feeding on milkweed leaves collected from plants exposed within plots to the spray application. Survival and growth of larvae feeding on milkweeds outside of the sprayed plots also was reduced because of insecticide drift. These results were not surprising because λ-cyhalothrin is very effective at controlling lepidopteran pests, and many reports have documented nontarget effects of conventional insecticides (14). Recent data suggest that the use of Bt hybrids has the potential to significantly reduce the number of insecticide treatments that are typically applied to sweet corn (http://www.epa.gov/scipoly/sap/2000/october/).
Differences in overall results of the Iowa I, Ontario, and Maryland bioassays are clearly attributable to the protein expression level in pollen that is regulated by the transformation event. Corn hybrids based on event 176 may be hazardous to susceptible stages of monarch larvae that are present on their host plants within cornfields during anthesis. However, the exposure dose of Bt11 pollen present at peak anthesis and the Cry1Ab concentration in pollen ingested over the 4- or 5-day feeding period was not high enough to significantly affect larval survival. These findings are probably true for Mon810 as well, although it was not vigorously tested, because Cry1Ab expression in Mon810 is similar to that in Bt11 pollen. However, further research is required to better understand the impact of Bt pollen with relatively low toxicity on monarch populations. For example, in the natural setting, larvae hatching at the onset of anthesis may be exposed to biologically active Cry1Ab in pollen for a longer period than 4 or 5 days; thus, exposure duration needs to be considered along with exposure concentration as a determinant of environmental dose. The Iowa II and New York studies recorded the fate of experimental cohorts of larvae for 14 to 22 days and show no apparent effect of Bt11 or Mon810 pollen on survival, although the presence of other mortality factors contributed to high levels of variability in mortality rates; thus, subtle effects of prolonged exposure to Bt toxin cannot be determined. Further, potential sublethal effects in response to long-term exposure, exposure of neonate larvae, and the potential impact of Bt pollen on reproductive fitness and migration abilities could not be determined in these studies. Finally, because the detection of very subtle effects is difficult in field studies because of the relatively small sample sizes, the results of these studies must be considered with the results of much higher doses and more rigorous laboratory studies (8). In addition, the implications of these studies must be understood in the context of the environmental doses anticipated throughout pollen dispersal (12) and the likelihood that monarch larvae will be exposed to toxic doses of pollen (13).
Acknowledgments
We thank Laura Timms, Pat Beaupre, Matt Van Ast, Bryan Muscat, Chad Harvey, Eric Olson, Terry Patton, Jeff Miner, Jessica Nelson, Keith Bidne, Randy Ritland, Jim Robbins, Colothdian Tate, Patricia Anderson, Denny Bruck, Stacy van Loon, Kate Kronback, Jaleen Bruner, Karen Douchette, Kerry Gillooly, Erin Roe, Tegwin Taylor, Maureen Carter, Lee Macomber, Jeremiah Depue, Christine Cappadora, Jeffrey Fuchsberg, and Christa Hoffman for their assistance in conducting field trials. We also thank Fred Gould, George Kennedy, Kevin Steffey, Anthony Shelton, Jeffrey Wolt, and Eric Sachs for their critical reviews and Orley Taylor (Monarch Watch, University of Kansas) for providing monarch larvae. This research was supported by a pooled grant provided by the United States Department of Agriculture, Agricultural Research Service, and the Agricultural Biotechnology Stewardship Technical Committee (ABSTC), and by funding from the Canadian Food Inspection Agency, Environment Canada, the Ontario Corn Growers' Association, the Maryland Agricultural Experiment Station, and the Leopold Center for Sustainable Agriculture (Ames, IA). Members of ABSTC are Aventis CropScience USA LP, Dow AgroSciences LLC, E. I. du Pont de Nemours and Company, Monsanto Company, and Syngenta Seeds, Inc. L.C.H.J. was supported by an Environmental Protection Agency STAR Fellowship.
Abbreviations
- Bt (Bacillus thuringiensis)
Cry1Ab, 1 epidopteran-active crystalline protein
- ha
hectares
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Koziel M G, Beland G L, Bowman C, Carozzi N B, Crenshaw R, Crossland L, Dawson J, Desai N, Hill M, Kadwell S, et al. Biotechnol. 1993;11:194–200. [Google Scholar]
- 2.Schuler T H, Poppy G M, Kerry B R, Denholm I. Trends Biotechnol. 1999;17:210–216. doi: 10.1016/S0167-7799(98)01298-0. [DOI] [PubMed] [Google Scholar]
- 3.Fearing P L, Brown D, Vlachos D, Meghji M, Privalle L. Mol Breeding. 1997;3:169–176. [Google Scholar]
- 4.Losey J E, Rayor L S, Carter M E. Nature (London) 1999;399:214. doi: 10.1038/20338. [DOI] [PubMed] [Google Scholar]
- 5.Jesse L C H, Obrycki J J. Oecologia. 2000;125:241–248. doi: 10.1007/s004420000502. [DOI] [PubMed] [Google Scholar]
- 6.Malcolm S B, Brower L P. Experientia. 1989;45:284–294. [Google Scholar]
- 7.Wassenaar L I, Hobson K A. Proc Natl Acad Sci USA. 1998;95:15436–15439. doi: 10.1073/pnas.95.26.15436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellmich R L, Siegfried B, Sears M K, Stanley-Horn D E, Daniels M J, Mattila H R, Spencer T, Bidne K G, Lewis L. Proc Natl Acad Sci USA. 2001;98:11925–11930. doi: 10.1073/pnas.211297698. . (First Published September 14, 2001; 10.1073/pnas.211297698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raynor G S, Ogden E C, Hayes J V. Agron J. 1972;64:420–427. [Google Scholar]
- 10.Russek-Cohen E, Douglas L W. Mixed Model Short Course Manual. Beltsville: University of Maryland; 1999. pp. 89–103. [Google Scholar]
- 11.SAS Institute. SAS/STAT User's Guide, Version 6.0. Cary, NC: SAS Inst.; 1990. [Google Scholar]
- 12.Pleasants J M, Hellmich R L, Dively G, Sears M K, Stanley-Horn D E, Mattila H R, Foster J E, Clark P L, Jones G D. Proc Natl Acad Sci USA. 2001;98:11919–11924. doi: 10.1073/pnas.211287498. . (First Published September 14, 2001; 10.1073/pnas.211287498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oberhauser K S, Prysby M, Mattila H R, Stanley-Horn D E, Sears M K, Dively G, Olson E, Pleasants J M, Lam W-K F, Hellmich R L. Proc Natl Acad Sci USA. 2001;98:11913–11918. doi: 10.1073/pnas.211234298. . (First Published September 14, 2001; 10.1073/pnas.211234298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jepson P C. Pesticides and Non-Target Invertebrates. Andover Hants, U.K.: Intercept; 1989. [Google Scholar]