Abstract
Highly contagious classical swine fever (CSF) remains a major trade and health problem in the pig industry, resulting in large economic losses worldwide. In CSF-endemic countries, attenuated CSF virus (CSFV) vaccines have been routinely used to control the disease. However, eradication of CSFV in a geographical area would require permanent reduction to zero presence of the virus. It is therefore of paramount importance to develop a safe, potent, and non-infectious CSF vaccine. We have previously reported on a cost-effective CSF E2 subunit vaccine, KNB-E2, which can protect against CSF symptoms in a single dose containing 75 µg of recombinant CSFV glycoprotein E2. In this study, we report on a series of animal studies undertaken to elucidate further the efficacy of KNB-E2. We found that pigs vaccinated with a single KNB-E2 dose containing 25 µg of recombinant CSFV glycoprotein E2 were protected from clinical symptoms of CSF. In addition, KNB-E2-mediated reduction of CSF symptoms was observed at two weeks post-vaccination and the vaccinated pigs continued to exhibit reduced CSF clinical signs when virus challenged at two months and four months post-vaccination. These results suggest that KNB-E2 effectively reduces CSF clinical signs, indicating the potential of this vaccine for safely minimizing CSF-related losses.
Keywords: adjuvants, classical swine virus, subunit, vaccines
Introduction
Though first described in the early 19th century, classical swine fever (CSF) or hog cholera is a highly contagious pig disease that continues to produce economic losses and agrosecurity threats worldwide. The causative agent, CSF virus (CSFV) is a Pestivirus that belongs to the family Flaviviridae [27]. CSF causes lymphoid depletion by targeting immune cells, which makes the swine more susceptible to other infections [16,38]. Clinical signs include high fever, huddling, weakness, drowsiness, anorexia, ataxia (loss of full control of bodily movements), hemorrhage, purple discoloration or cyanosis of the skin, conjunctivitis, and constipation followed by diarrhea [23]. CSF in its most virulent form causes almost 100% morbidity and mortality [40].
Due to the substantial economic impact of a CSF outbreak, research efforts have focused on the development of CSF vaccines that are safe, highly efficacious, fast-acting, cost-efficient, long-lasting, and can differentiate vaccine-induced immunity from field infection. To date, the most frequently used CSF vaccines were derived from the CSFV C strain [17], an attenuated strain from the virulent CSFV Shimen strain [5,28]. However, modified live virus (MLV) vaccines such as the CSFV C strain vaccine present the challenge of differentiating the MLV-vaccinated pigs from the field-infected pigs. With this constraint, research was directed toward the development of marker CSF vaccines [11]. CSF subunit vaccines are designed to meet the differentiation of infected from vaccinated animals requirement for vaccination [11]. Previous studies have established that the CSFV glycoprotein E2 is the most immunodominant protein in protecting against CSF disease [22,25] with multiple identified epitopes [21]. Therefore, E2 subunit vaccines continue to be the focus of CSF subunit vaccine research [6,7].
Research on CSF E2 subunit vaccines has produced reports of incomplete protection against CSF disease and has shown that inducing E2-specific antibodies is insufficient for conferring protection [3,9]. This is in contrast to in vitro and in vivo studies that have adequately demonstrated that antibodies against E2 can neutralize and protect against CSF [6,25,26]. Current knowledge of the immune system indicates that there are several, sometimes redundant, pathways by which protective immune responses against a particular pathogen can be induced [12,32,33,37]. In subunit vaccine formulations, the immunological component that enhances immune responses, activates multiple immune regulatory pathways, and improves vaccine efficacy are mainly mediated by adjuvants [2,35]. Hence, adjuvants with the ‘right’ formulation can have the ability to enhance both humoral and cell-mediated immune responses, depending on the mechanism needed to protect against a specific disease [10]. Thus, there is no universal adjuvant that will work in every vaccine. The design or formulation of adjuvants must take into consideration several factors such as antigen, target species, type of immune response, route of inoculation, and safety [18]. Hence, it is possible that formulating the appropriate adjuvant or combination of adjuvants for the immunodominant CSF E2 (antigen) is key to having a highly effective CSF subunit vaccine. We have recently reported on a promising novel E2 CSF subunit vaccine, KNB-E2, which is formulated in an oil-in-water-based adjuvant [29]. KNB-E2 contains insect cell-produced recombinant E2 in a natural conformation, and, with a single dose of KNB E2, vaccinated pigs were clinically protected from CSFV symptoms and they survived virus challenge [29]. KNB-E2-vaccinated pigs can also be easily differentiated from naturally infected pigs by an Erns-specific enzyme-linked immunosorbent assay (ELISA). Upon CSFV challenge, KNB-E2-vaccinated pigs do not produce high levels of Erns antibodies in contrast to the levels in mock-vaccinated pigs [29]. In this paper, we expand on our studies on the CSF subunit vaccine KNB-E2 by determining the effective minimum dose of the E2 antigen, as well as determining the onset and duration of KNB-E2-conferred clinical protection against CSF disease.
Materials and Methods
Animals
Conventional large white-Duroc crossbred weaned specific-pathogen-free male and/or female piglets (3 weeks of age) were purchased from a commercial vendor. Animal care and use protocols were approved by the Institutional Animal Care and Use Committee (IACUC No. 3436) at Kansas State University (KSU). The pigs were fed a standard commercial diet and kept under laboratory biosafety level II conditions at the KSU Large Animal Research Center during the vaccination phase, and under laboratory biosafety level III Agriculture conditions at the KSU Biosecurity Research Institute during the CSF challenge phase. The pigs were randomly allotted into two general groups: KNB-E2-vaccinated groups (n = 5 in each group) and phosphate-buffered saline (PBS; Gibco, USA) vaccinated control groups (n = 5 or 2). The control groups were divided further into PBS-vaccinated, non-challenged (–/–) and PBS-vaccinated, CSFV-challenged (–/+) groups.
CSFV used to infect pigs
The CSF Alfort (HCV Alfort C-718 28) isolate was kindly provided by Dr. Sabrina Swenson from the Animal and Plant Health Inspection Service, United States Department of Agriculture (USDA). The CSFV isolate was passaged seven times in swine testicle (ST) cells (American Type Culture Collection [ATCC], USA) cultured in Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% fetal bovine serum (Atlanta Biologicals, USA) and 1% penicillin-streptomycin solution (Gibco).
Preparation of KNB-E2 and immunization
Expression and purification of recombinant CSFV E2 protein were performed as described previously [29]. The CSFV E2 subunit vaccine, KNB-E2, was prepared by simple hand mixing of purified CSFV E2 with an oil-in-water emulsion adjuvant [15]. This adjuvant containing mineral oil and low-cost plant-based emulsifier is stable for prolonged periods of time at temperatures ranging from 4℃ to 40℃ [15]. Injection sites were monitored and no adverse reactions were observed. One dose of the KNB-E2 prepared for these experiments contained 25 to 75 µg of purified E2 protein per pig, depending on the experiment. All vaccinated pigs were immunized once intramuscularly (neck area) with 2 mL of KNB-E2. The non-vaccinated, non-challenged (–/–) and non-vaccinated, CSFV-challenged (–/+) control group pigs were injected intramuscularly with 2 mL of PBS.
CSFV challenge pig studies
We have previously reported that pigs given one dose of KNB-E2 vaccine containing 75 µg of purified E2 were clinically protected and survived CSFV challenge at 35 days post-vaccination (DPV) [29]. To characterize further the efficacy of the KNB-E2 vaccine, three swine experiments were performed.
Antigen titration (25 µg, 50 µg, and 75 µg of E2 antigen)
Two groups (–/–) and (–/+) of control pigs (n = 5 in each group) were injected intramuscularly with 2 mL PBS and three groups of pigs (n = 5 in each group) were immunized with KNB-E2 formulations (2 mL, intramuscularly) containing 25 µg, 50 µg, or 75 µg of E2 antigen, respectively. Four weeks after vaccination, the pigs were challenged with a 1 × 105 50% tissue culture infective dose (TCID50) of CSFV Alfort (1 mL, intramuscularly).
Onset of immunity (2, 3, and 4 weeks post-vaccination)
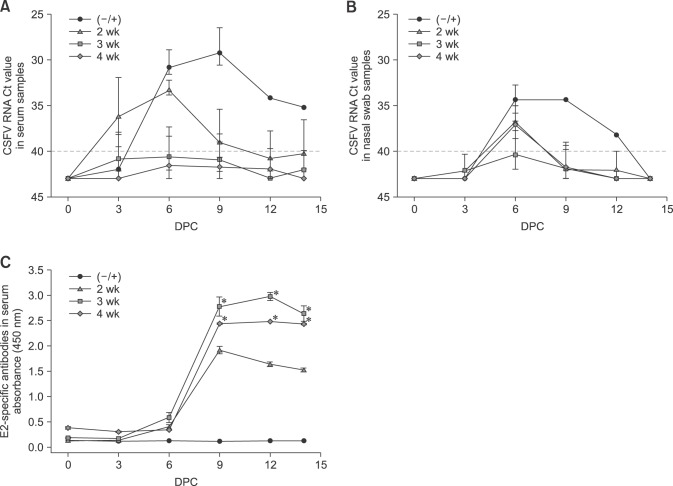
Three groups of pigs (n = 5 in each group) were immunized with KNB-E2 vaccine (2 mL, intramuscularly) containing 40 µg of E2 antigen, whereas three groups of (–/+) control pigs (n = 2 in each group) were injected with PBS (2 mL, intramuscularly). Two weeks after vaccination, one group of KNB-E2-vaccinated pigs and one group of (–/+) pigs were challenged with 1 × 105 TCID50 of CSFV Alfort (1 mL, intramuscularly). At 3 weeks post-vaccination, another group of KNB-E2-vaccinated pigs and a (–/+) control group of pigs were challenged with 1 × 105 TCID50 of CSFV Alfort (1 mL, intramuscularly). The third (final) group of KNB-E2-vaccinated and the final (–/+) control group pigs were challenged at 4 weeks post-vaccination with 1 × 105 TCID50 of CSFV Alfort (1 mL, intramuscularly).
Duration of immunity (2 and 4 months post-vaccination)
Two groups of pigs (n = 5 for each group) were immunized with KNB-E2 vaccine (2 mL, intramuscularly) containing 50 µg E2 antigen and two groups of (–/+) control pigs (n = 2 for each group) were injected with PBS (2 mL, intramuscularly). Two months after vaccination, one group of KNB-E2-vaccinated pigs and one group of (–/+) control pigs were challenged with 1 × 105 TCID50 of CSFV Alfort (1 mL, intramuscularly). The second group of KNB E2-vaccinated and the second (–/+) control group pigs were challenged at 4 months post-vaccination with 1 × 105 TCID50 of CSFV Alfort (1 mL, intramuscularly).
Pigs were monitored daily for clinical signs and rectal temperatures after CSFV challenge. Whole blood, serum, and nasal swabs were collected on the day of challenge (0 days post-challenge [DPC]) and every 3 days during the challenge period. All pigs were euthanatized at 14 or 15 DPC.
Anti-E2 specific antibodies were determined by using ELISA as described previously [29]. Briefly, 62.5 ng/mL of purified E2 was used as a coating antigen on 96-well flat-bottomed microtiter plates (Corning, USA). After three washes with PBS containing 0.05% Tween 20 (PBST), diluted sera were added to plates and incubated for 1 h at room temperature. Then, the ELISA plates were washed three times with PBST before detection of the E2-specific antibodies by using horseradish peroxidase-conjugated goat anti-porcine IgG (Southern Biotech, USA). Tetramethylbenzidine (Novex, USA) was used to develop the ELISA plates, followed by the addition of 2 N sulfuric acid (Ricca Chemical Company, USA) to stop the reactions. Optical spectrophotometer readings at 450 nm were measured by using a SpectraMAX microplate reader (Molecular Devices, USA) to determine the relative antibody concentrations in serum samples.
Isolation of RNA and real-time reverse transcriptase polymerase chain reaction for CSFV quantification
Viral RNA from serum samples and nasal swabs were isolated by using a commercial kit (IBI Scientific, USA). Real-time reverse transcriptase polymerase chain reaction (PCR) and analyses were performed by using a StepOnePlus Real-Time PCR System with StepOne software (ver. 2.3; Applied Biosystems, USA) as described previously [19,29]. Samples with threshold cycle values equal to or less than 40 were considered positive for CSFV. For virus quantification, passage 7 CSFV Alfort stock (108 TCID50) was serially diluted (108 – 102) before RNA isolation and used to generate a standard curve.
Serum virus neutralization assay
An indirect fluorescent antibody (IFA) assay was performed to determine the anti-CSFV-neutralizing titers in serum samples of CSFV-challenged pigs. Serum samples were serially diluted (from 5-fold to 10,240-fold) and incubated together with 100 TCID50 of CSFV Alfort for 1 h at 37℃. Residual virus infectivity was determined by adding the serum-virus mixture to 1 × 104 ST cells per well (96-well plate) and incubated for 72 h at 37℃. The cells were then subjected to indirect immunofluorescence staining with CSFV E2-specific murine monoclonal antibody WH303 (APHA Scientific, UK) followed by Alexa-Fluor 488-labeled anti-mouse IgG (Life Technologies, USA). Neutralizing antibody titers in serum samples were expressed as the reciprocal of the highest dilution that causes 50% neutralization.
Blood cell counts
White blood cells (WBC) were enumerated in EDTA-stabilized samples by using a semi-automated animal blood cell counter (VetScan HM5 Hematology system; Abaxis, USA). To ensure instrument quality control, a normal control sample purchased from the supplier was used to calibrate the whole blood analyzer before every use. All samples were analyzed on the day of acquisition.
Statistical analysis
The variations between groups were analyzed by applying the t-test and one-way analysis of variance (ANOVA) followed by post hoc Dunnett's method and using SigmaPlot 11 software (Systat, USA). Differences were considered statistically significant when p < 0.05.
Results
KNB-E2 vaccine with 25 µg E2 antigen resulted in decreased CSF symptoms upon virus challenge
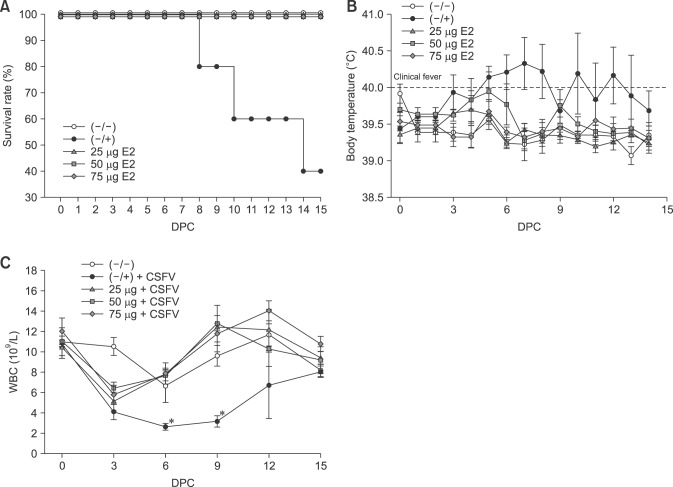
In order to establish the amount of antigen needed in a single vaccine dose, three groups of pigs were immunized with KNB-E2 containing 25 µg, 50 µg, or 75 µg of CSFV E2 antigen. Pigs were then challenged with 1 × 105 TCID50 of CSFV Alfort at 4 weeks post-vaccination (28 DPV). All KNB-E2-vaccinated pigs (vaccine containing 25 µg, 50 µg or 75 µg of E2 antigen) remained apparently healthy and did not develop fever throughout the challenge phase (panels A and B in Fig. 1). No clinical signs that are typical of CSF infection, such as anorexia (low food intake) or depression were observed in the KNB-E2-vaccinated pigs. In contrast, 3 of the 5 (60.0%) pigs in the (–/+) control group had to be euthanatized before the end of study (on 8 DPC, 10 DPC, and 14 DPC) (panel A in Fig. 1) due to severe clinical CSF symptoms, including high fever (panel B in Fig. 1), loss of body weight (data not shown), seizures, diarrhea, and severe leukopenia (panel C in Fig. 1). All (–/+) control pigs became viremic after challenge, exhibiting up to 107 TCID50 CSFV detected in blood (panel A in Fig. 2) and nasal cavity (panel B in Fig. 2). In contrast, a few of the KNB-E2-vaccinated pigs, notably even some in the 25 µg group, had low transient levels of CSFV, which were eventually cleared by the KNB-E2-vaccinated pigs within the study duration (panels A and B in Fig. 2).
Fig. 1. Pigs vaccinated with KNB-E2 containing 25 µg E2 antigen per pig exhibited decreased classical swine fever virus (CSFV) symptoms. Pigs were immunized with KNB-E2 containing 75 µg, 50 µg, or 25 µg of E2 antigen on day 0. Four weeks after vaccination (28 days post-vaccination), pigs were challenged with 1 × 105 50% tissue culture infective dose (TCID50) of CSFV Alfort. (A) All KNB-E2-immunized pigs appeared healthy and survived the CSFV challenge. (B) KNB-E2-vaccinated pigs did not have body temperatures higher than 40.5℃. (C) PBS-vaccinated CSFV-challenged (−/+) control pigs developed a marked reduction of white blood cells (WBC) in the blood with the greatest reduction at 6 days post-challenge (DPC). Lower levels of WBC were transiently observed in KNB-E2-vaccinated pigs at 3 DPC, but the level quickly recovered. Data are presented as mean ± SEM values for five pigs per group. *p < 0.05.
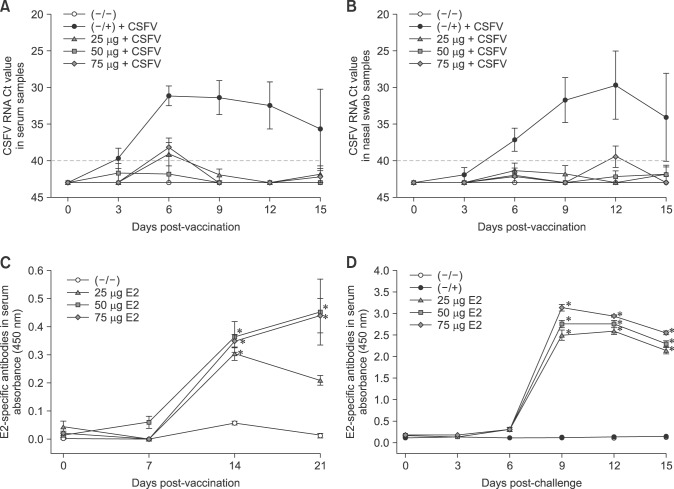
Fig. 2. Decreased viremia and high levels of E2-specific antibodies were detected in pigs immunized with KNB-E2 containing 25 µg, 50 µg, or 75 µg E2 antigen. Classical swine fever virus (CSFV) RNA was detected in serum (A) and nasal cavity (B) by real-time reverse transcriptase polymerase chain reaction analyses in which threshold cycle values equal to or less than 40 were considered CSFV positive. KNB-E2-vaccinated pigs exhibited transient viremia only at 6 days post-challenge compared with the marked viremia in the phosphate-buffered saline–vaccinated CSFV-challenged (−/+) control pigs. E2-specific antibodies were determined in the serum during the vaccination phase (dilution 1/1,000) (C) and the challenge phase (dilution 1/10,000) (D) by enzyme-linked immunosorbent assay. KNB-E2-vaccinated pigs produced significantly high levels of E2-specific antibodies after CSFV challenge. Data are presented as mean ± SEM values for five pigs per group. *p < 0.05.
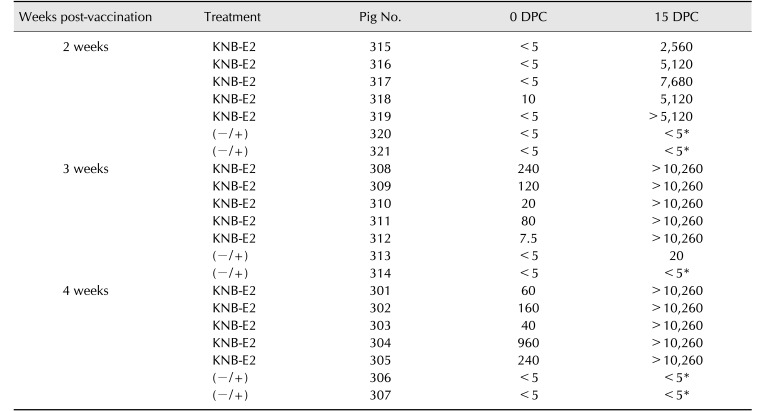
Comparable levels of CSFV E2-specific antibodies were detected in the serum (dilution 1/1,000) by ELISA in all three KNB-E2-vaccinated groups within 14 DPV (panel C in Fig. 2). However, a lower level of E2-specific antibodies was observed in pigs vaccinated with 25 µg of E2 antigen at 21 DPV (panel C in Fig. 2). After CSFV challenge, all three KNB-E2-vaccinated groups displayed an anamnestic response characterized by a rapid increase of E2-specific antibodies in the serum (dilution 1/10,000; panel D in Fig. 2) starting at 6 DPC. Similarly, the serum anti-CSFV-neutralizing antibody titers (VNA) detected by IFA in pigs vaccinated with 25 µg of E2 antigen were slightly lower at 28 DPV/0 DPC (i.e., before challenge), compared to that in pigs vaccinated with 50 µg or 75 µg of E2 antigen (Table 1). However, by the end of the study period (15 DPC) all KNB-E2 vaccinated pigs developed very high (≥ 10,240) VNA titers. These data indicate that the levels of E2 antibodies and VNA titers in KNB-E2 pigs vaccinated with 25 µg of E2 antigen were sufficient for clinical protection against CSF disease.
Table 1. KNB-E2 pigs vaccinated with 75 µg, 50 µg, and 25 µg of E2 antigen developed high titers of anti-CSFV-neutralizing antibodies (ND50) after CSFV challenge.
CSFV, classical swine fever virus; DPV, days post-vaccination; DPC, days post-challenge; (−/+), PBS-vaccinated, CSFV-challenged; (−/−), PBSvaccinated, non-challenged; PBS, phosphate-buffered saline. *Pig 166 euthanatized at 10 DPC; pig 167 euthanatized at 8 DPC; pig 169 euthanatized at 14 DPC.
In addition, 2 of the 5 pigs in the (–/+) control group (pigs 168 and 170) survived the CSFV challenge. These two pigs had > 40℃ fever at 3 DPC to 6 DPC along with loss of appetite and depression. The viremia detected in pigs 168 and 170 peaked at 6 DPC and was notably lower at 9 DPC; however, CSFV was still detected in these pigs at the end of the study (panels A and B in Fig. 2). Pigs 168 and 170 had no detectable VNA titers before challenge (0 DPC; Table 1); hence, there was no pre-existing immunity against CSFV. Interestingly, these (–/+) control pigs both developed low detectable VNA titers at 15 DPC but to a much lower degree than that in the KNB-E2-vaccinated pigs (Table 1). Collectively, our data suggest that KNB E2 vaccine containing as little as 25 µg of E2 antigen can confer clinical protection against CSFV infection in swine.
KNB-E2 immunized pigs were clinically protected from CSF as early as 2 weeks post-vaccination
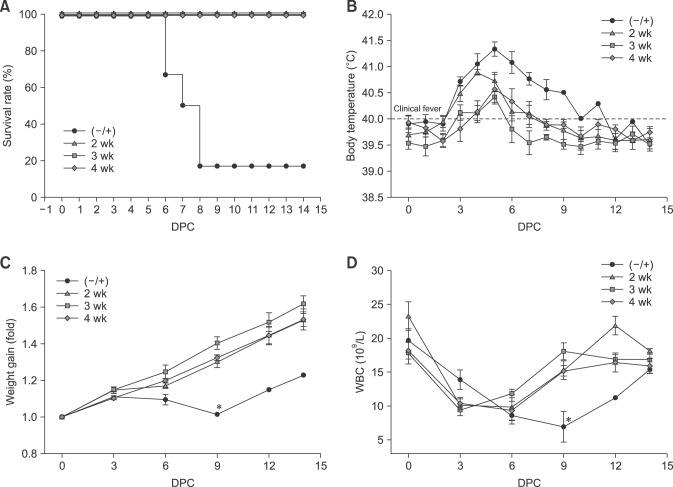
Vaccination was carried out in three groups of pigs with the KNB-E2 formulation containing 40 µg of CSFV E2 antigen. The first group of KNB-E2-vaccinated pigs was challenged with CSFV at 2 weeks post-vaccination. The remaining groups of KNB-E2-vaccinated pigs were challenged at 3 or 4 weeks post-vaccination. Age-matched (–/+) PBS-vaccinated pigs were also challenged at 2 weeks, 3 weeks, and 4 weeks post-vaccination as control groups. All KNB-E2-vaccinated pigs at 2 weeks, 3 weeks, and 4 weeks post-vaccination remained apparently healthy after they were challenged (panel A in Fig. 3). The 2-week KNB-E2-vaccinated group developed fever at 3 DPC similar to that in the (–/+) control group (panel B in Fig. 3). The 3-week and 4-week KNB-E2-vaccinated pig groups developed transient low fevers at 5 DPC. However, from 8 DPC onwards, all KNB E2-challenged pigs recovered and had slight or no fever (panel B in Fig. 3); moreover, they did not further develop other clinical signs of CSF. In contrast, the (–/+) challenged control animals developed typical clinical signs of CSF; and 5 of 6 (83.3%) of the (–/+) control pigs had to be euthanatized between 6 DPC to 8 DPC (panel A in Fig. 3).
Fig. 3. KNB-E2-immunized pigs challenged as early as two weeks post-vaccination have decreased classical swine fever virus (CSFV) symptoms. Pigs were immunized with KNB-E2 containing 40 µg of E2 antigen on day 0. Pigs were challenged with 1 × 105 50% tissue culture infective dose (TCID50) of CSFV Alfort at 2-, 3-, and 4 weeks post-vaccination. (A) All KNB-E2-immunized pigs appeared healthy and survived the CSFV challenge. (B) The CSFV-challenged pigs developed fever starting at 3 days post-challenge (DPC) with the phosphate-buffered saline–vaccinated CSFV-challenged (−/+) control pigs exhibiting high fever. Transient fever was observed in KNB-E2-vaccinated pigs, but no other CSF symptoms were observed. (C) All KNB-E2-vaccinated pigs continued to gain weight after CSFV challenge. Fold values for total body weight gain during the study were calculated by considering the weight of the pig on 0 DPC as 1. At 8 DPC, only 1 of the 6 (−/+) control pigs remained alive and this pig exhibited recovery from CSFV challenge with a normal body temperature (B) and weight gain (C). (D) White blood cell (WBC) numbers of CSFV-challenged pigs decreased after CSFV challenge. The WBC numbers in KNB-E2-vaccinated pigs decreased in the first six days post-challenge and recovered by 9 DPC. Data are presented as mean ± SEM values for five pigs per group. *p < 0.05.
All of the KNB-E2-vaccinated, CSFV E2-challenged pigs (2-week, 3-week, and 4-week groups) steadily gained weight throughout the 14-day challenge period with only a minimal decrease in weight gain observed in the 2 week pig group between 3 DPC and 6 DPC (panel C in Fig. 3). In contrast, weight loss was observed in the (–/+) challenged control group starting at 3 DPC (panel C in Fig. 3). At 8 DPC, only one (–/+) control pig remained (pig 313) and this pig appeared to have recovered from CSF (panels B and C in Fig. 3). Since all pigs were CSF-free, the steady weight gain of pig 313 toward the end of the study suggested the presence of an innate resistance or a conversion into a subacute or chronic disease. Similarly, a decrease in leukopenia was observed in pig 313 starting at 9 DPC (panel D in Fig. 3) and continuing until the end of study period, suggesting recovery from CSFV challenge. On the other hand, slight decreases in WBC numbers were observed in all KNB-E2-vaccinated pigs (2-week, 3-week, and 4-week groups) at 3 DPC and 6 DPC; but these numbers were recovered by 9 DPC (panel D in Fig. 3).
CSFV RNA was detected early in the 2-week KNB-E2-vaccinated pigs but viremia cleared by 12 DPC (panel A in Fig. 4). During the challenge period, CSFV RNA was detected in 2 of 5 pigs in the 2-week KNB-E2-vaccinated group; 1 out of 5 in the 3-week KNB-E2-vaccinated group; and none in the 4-week KNB-E2-vaccinated group at the end of the study period (panel A in Fig. 4). On the other hand, CSFV RNA was not detected in the nasal cavities of any of the KNB-E2-vaccinated pigs (2-week, 3-week, and 4-week groups) at 9 DPC (panel B in Fig. 4). At 9 DPC, in which only pig 313 remained, CSFV RNA was still detected in this pig. Pig 313 remained CSFV RNA positive throughout the challenge period. However, this pig appeared to be recovering from CSF by the end of the study (panel B in Fig. 4).
Fig. 4. Decreased viremia and high levels of E2-specific antibodies were detected in pigs challenged with classical swine fever virus (CSFV) at 2-week, 3-week, and 4-week post KNB-E2 vaccination. CSFV RNA was detected in the serum (A) and nasal cavity (B) by real-time reverse transcriptase polymerase chain reaction analyses in which threshold cycle values equal to or less than 40 were considered CSFV positive. The KNB-E2-vaccinated pigs challenged at 3 weeks and 4 weeks post-vaccination had little or no CSFV RNA detected. CSFV RNA was detected in KNB-E2-vaccinated pigs challenged at 2 weeks post-vaccination but the level had recovered by 12 days post-challenge (DPC). E2-specific antibodies were determined in the serum during the challenge phase (dilution 1/10,000) (C) by enzyme-linked immunosorbent assay. KNB-E2-vaccinated pigs produced significantly high levels of E2-specific antibodies after CSFV challenge. At 3 weeks and 4 weeks post-vaccination, KNB-E2 pigs produced higher E2-specific antibodies than the pigs challenged at 2 weeks post-vaccination. Data are presented as mean ± SEM values for five pigs per group. *p < 0.05.
The 2-week KNB-E2-vaccinated pig group demonstrated detectable but lower levels of E2-specific antibodies in serum (dilution 1/10,000) compared to the levels in the 3-week and 4-week KNB-E2-vaccinated groups (panel C in Fig. 4); interestingly, no other CSF clinical signs were observed in the 2-week KNB-E2-vaccinated CSFV-challenged pigs. Likewise, the 2-week KNB-E2-vaccinated group had no or little VNA titers on the day of challenge, but the pigs developed sufficient protective titers of anti-CSFV-neutralizing antibodies by the end of the experimental period (Table 2). Collectively, our results suggest that even though some vaccinated pigs were positive for CSFV RNA and had transient fever, the KNB-E2 vaccine conferred clinical protection against CSF disease at 2 weeks post-vaccination.
Table 2. As early as two weeks after KNB-E2 vaccination, pigs developed protective titers of anti-CSFV-neutralizing antibodies after virus challenge (14 DPC).
CSFV, classical swine fever virus; DPC, days post-challenge; (−/+), phosphate-buffered saline–vaccinated, CSFV-challenged. *Pigs 320 and 321 euthanatized at 8 DPC; pig 314 euthanatized at 7 DPC; pigs 306 and 307 euthanatized at 6 DPC.
KNB-E2-immunized pigs were clinically protected from CSF disease at 2 months and 4 months post-vaccination
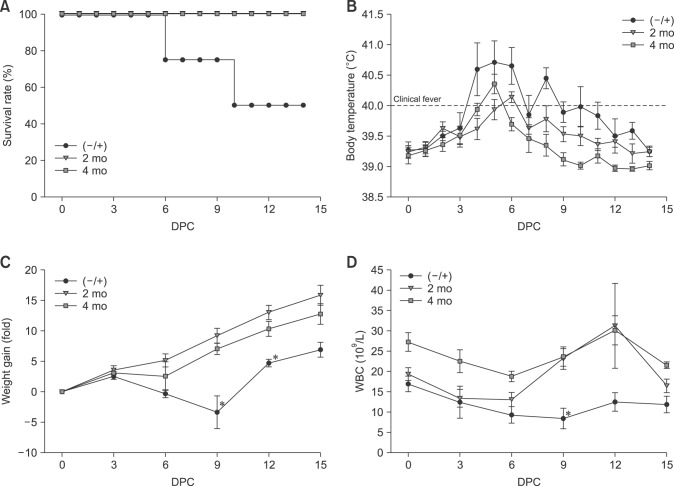
The duration of KNB-E2 vaccine efficacy was determined by challenging KNB-E2-vaccinated pigs with CSFV at 2 months and 4 months post-vaccination. In this pig study, one pig out of the 5 pigs in the 2-month KNB-E2 group had to be euthanatized during the vaccination phase due to a health problem unrelated to CSF. As with the previous pig experiments, at 2 months and 4 months post-vaccination, all KNB-E2-vaccinated pigs survived CSFV challenge (panel A in Fig. 5). In contrast, only 50% of the (–/+) PBS-vaccinated control pigs survived the CSFV challenge (panel A in Fig. 5). The 2-month and 4-month KNB-E2-vaccinated pig groups developed transient one-day fever at 5 DPC and 6 DPC, respectively (panel B in Fig. 5). The challenged (–/+) control pigs had elevated temperatures of up to 41.5℃. Interestingly, the (–/+) control pigs that survived the CSFV challenge exhibited higher fevers than those of the (–/+) control pigs that had to be euthanatized (data not shown).
Fig. 5. KNB-E2-immunized pigs challenged at 2 months and 4 months post-vaccination have decreased classical swine fever virus (CSFV) symptoms. Pigs were immunized with KNB-E2 containing 50 µg of E2 antigen on day 0. Pigs were challenged with 1 × 105 50% tissue culture infective dose (TCID50) of CSFV Alfort at 2 months and 4 months post-vaccination. (A) All KNB-E2-immunized pigs appeared healthy and survived the CSFV challenge. (B) The older CSFV-challenged pigs developed fever starting at 4 days post-challenge (DPC). The KNB-E2-vaccinated pigs developed a one-day fever but quickly recovered and exhibited normal temperatures throughout the remainder of the study. (C) All KNB-E2-vaccinated pigs continued to gain weight (kg) after CSFV challenge but exhibited a slight decrease in weight gain at 6 DPC. At 10 DPC, only 2 of the 4 phosphate-buffered saline–vaccinated CSFV-challenged (−/+) control pigs remained alive and these pigs exhibited recovery from CSFV challenge (A–C). (D) A slight decrease in the WBC numbers was observed in KNB-E2-vaccinated pigs after CSFV challenge and the WBC levels had recovered by 9 DPC. Data are presented as mean ± SEM values for five pigs per group. *p < 0.05.
Continued body weight gain was observed in both the 2-month and 4-month KNB-E2-vaccinated pigs (panel C in Fig. 5) after CSFV challenge. At 10 DPC, only two of the (–/+) control pigs remained, and these pigs seemed to have recovered as evidenced by their body weight gain observed starting at 9 DPC and continuing to the end of the study period (panel C in Fig. 5). Interestingly, the 4-month KNB-E2-vaccinated pigs exhibited higher WBC counts than 2-month KNB E2-vaccinated pigs at 0 DPC (panel D in Fig. 5). A slight decrease in WBC numbers was observed in all 2-month and 4-month KNB-E2-vaccinated pigs immediately after CSFV challenge but these numbers were recovered after 6 DPC (panel D in Fig. 5).
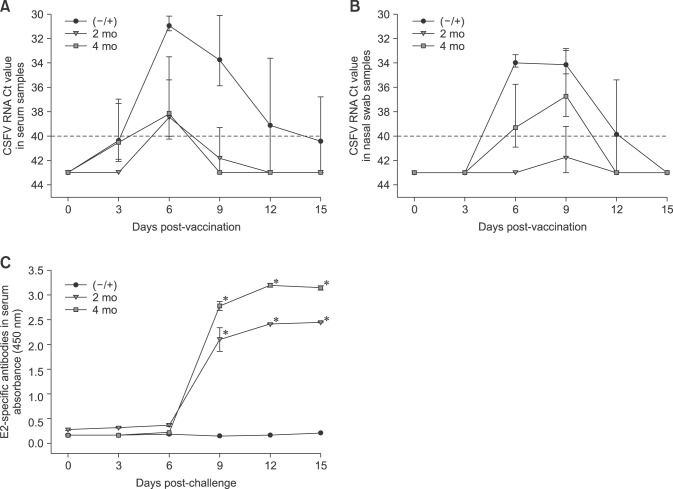
Both the 2-month and 4-month KNB-E2-vaccinated groups had CSFV-positive serum at 6 DPC but both groups cleared the CSFV at 9 DPC (panel A in Fig. 6). In the nasal swab samples, the 4-month KNB-E2-vaccinated group had a higher level of CSFV detected than that in the 2-month KNB-E2 group but was clear of the virus at 12 DPC (panel B in Fig. 6). One pig in the 2-month KNB-E2-vaccinated group and two pigs in the 4-month KNB-E2-vaccinated group did not develop indications of viremia throughout the challenge phase (data not shown). Together with the observed higher WBC counts in the 4-month KNB-E2-vaccinated pigs (panel D in Fig. 5), the results suggest an age-related factor in the immune responses against CSFV.
Fig. 6. Decreased viremia and high levels of E2-specific antibodies were detected in pigs challenged with classical swine fever virus (CSFV) at 2 months and 4 months post-vaccination. CSFV RNA was detected in the serum (A) and nasal cavity (B) by real-time reverse transcriptase polymerase chain reaction analyses in which threshold cycle values equal to or less than 40 were considered CSFV positive. Both 2 months and 4 months post-vaccination KNB E2-vaccinated pig groups exhibited decreased viremia and produced a significantly higher level of E2-specific antibodies post-CSFV challenge (dilution 1/10,000) (C) compared with the level in the phosphate-buffered saline–vaccinated CSFV-challenged (−/+) control group. Data are presented as mean ± SEM values for five pigs per group. *p < 0.05.
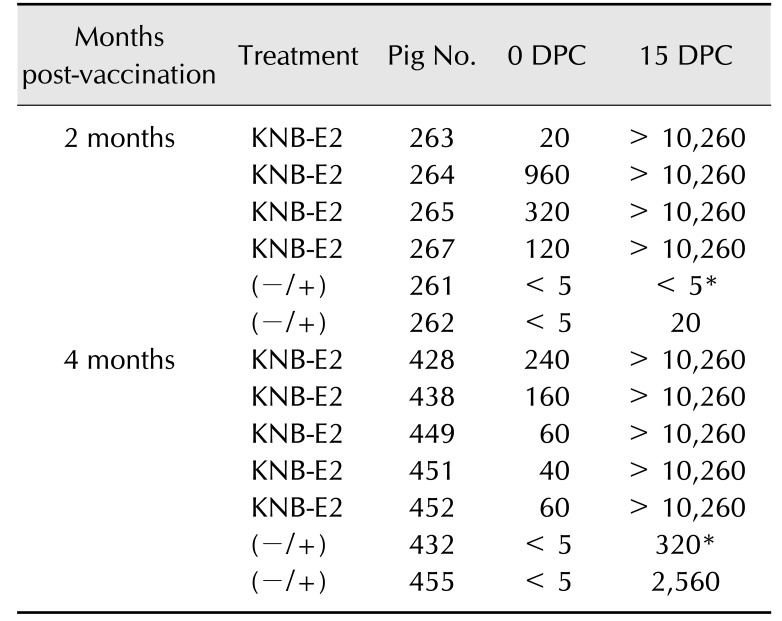
At 0 DPC, the 4-month KNB-E2-vaccinated pigs demonstrated lower levels of E2-specific antibodies than that in the 2-month KNB-E2-vaccinated pigs and a level comparable to that in the (–/+) control pigs from 0 DPC to 6 DPC (panel C in Fig. 6). Interestingly, by 9 DPC, the 4-month KNB-E2-vaccinated group produced higher amounts of E2-specific antibodies than the 2-month KNB-E2-vaccinated group (panel C in Fig. 6). Similarly, the 4-month KNB-E2-vaccinated pigs demonstrated lower levels of VNA titers at 0 DPC than those in the 2-month KNB-E2-vaccinated pigs (Table 3). However, by the end of the challenge period (15 DPC), both 2-month and 4-month KNBE2-vaccinated pig groups developed very high titers (≥ 10,240) of VNA. Taken together, our data suggest that KNB-E2-immunized pigs are clinically protected against CSF disease for at least 4 months post-vaccination.
Table 3. Anti-CSFV neutralizing antibody titers in pigs vaccinated with KNB-E2.
CSFV, classical swine fever virus; DPC, days post-challenge; (−/+), phosphate-buffered saline–vaccinated, CSFV-challenged. *Pig 261 euthanatized at 7 DPC; pig 432 euthanatized at 10 DPC.
The 2-month (–/+) PBS-vaccinated control pig (pig 262) that survived the CSFV challenge developed low VNA titers (Table 3). On the other hand, the older two pigs in the 4-month (–/+) group (pig 432 euthanatized at 10 DPC and pig 455 that survived the CSFV challenge) both exhibited detectable levels of VNA (Table 3) after CSFV challenge. Unlike younger pigs, the 4-month (–/+) control pigs started to develop VNA within 6 DPC and 9 DPC (< 5 anti-CSFV neutralizing bodies that neutralized 50% [ND50] of virus at 6 DPC and 30 ND50 at 9 DPC). In contrast, no E2-specific antibodies were detected by ELISA in these two 4-month (–/+) control group pigs throughout the study period (panel C in Fig. 6). These results suggest an age-related factor that may have an effect on CSF survival.
Discussion
We have demonstrated that KNB-E2, composed of a recombinant E2 protein expressed by insect cells and an oil-in-water emulsion-based adjuvant, is safe to administer and can effectively stimulate protective immunity against CSF with a single dose. Traditional subunit vaccines generally require multiple doses to be effective. For CSF, only two licensed subunit vaccines are commercially available and only in a few countries (Porcilis pesti [MSD Animal Health, USA] and Advasure, formerly Bayovac CSF E2 [Pfizer, USA]) [4,5]. These CSF subunit marker vaccines effectively induce CSF protection [1], but two doses are required followed by single-dose re-vaccination every six months. The efficacy displayed by the KNB-E2 vaccine illustrates the importance of having a potent adjuvant and an optimal interaction between vaccine components [14]. In the present study, antigen titration of the recombinant CSFV E2 glycoprotein showed the development of high CSFV-neutralizing titers and protection from CSF clinical symptoms with a single KNB-E2 containing 25 µg of E2. This antigen amount is less than the minimum 32 µg of E2 glycoprotein per dose in Advasure. During the vaccination phase, the 25 µg KNB-E2-vaccinated pigs had lower E2-specific antibodies and VNA compared to those in pigs vaccinated with 50 µg and 75 µg of KNB-E2. However, the level of protection conferred by the 25 µg KNB-E2 was sufficient to clinically protect against CSF disease as demonstrated by the absence of typical CSF pathology and the high E2-specific antibody neutralizing titers detected at the end of study period.
The onset of protection of the KNB-E2 vaccine was determined to be at approximately 2 weeks post-vaccination, based on the protection against mortality and the reduction of CSF clinical signs. Although the 2-week KNB-E2-vaccinated pigs had very low antibody titers at the time of challenge and showed mild signs of the disease (transient elevated temperatures), these pigs had developed sufficient immunity to adequately respond to a CSFV challenge. The dynamics of the anti-CSFV-neutralizing antibody produced within 2 weeks post-vaccination is consistent with results that were previously reported [6,31]. The differential efficacy of KNB-E2 at 2 weeks, 3 weeks, and 4 weeks post-vaccination may be due to the insufficient maturation of the humoral immune response in young pigs. The cellular immune responses that play an important role to control CSFV [17,39] were not addressed in this paper; however, evidence suggests that, with optimal antigen and adjuvant formulations, subunit vaccines can induce both humoral and cellular-mediated immune responses [10,35]. A representative oil-in-water adjuvant is MF59 from Novartis (Switzerland). Its proposed mechanisms for immune enhancement include efficient antigen delivery, immune potentiation at the injection site resulting in early leukocyte recruitment, and activation and maturation of antigen-presenting cells that result in antigen presentation through MHC molecules that subsequently allow T-cell recognition and B-cell activation. In addition, MF59 is reported to be more potent than aluminum-based adjuvants in terms of both antibody and T-cell responses [34]. Future studies will be conducted to investigate the role of cellular immunity in KNB E2 mediated protection against CSFV.
An important marker of an effective CSF vaccine is the period during which pigs are protected after vaccination. Earlier investigations demonstrated that CSF subunit vaccines can induce protective immunity that lasts for up to several months [8,20]. The results presented in our study clearly demonstrate that KNB-E2 can confer protection against CSFV at least 4 months post-vaccination. With the high levels of E2-specific and CSFV-neutralizing antibodies induced, we speculate that pigs vaccinated with KNB-E2 would be clinically protected against the disease for more than 4 months. Even though a low VNA was detected in the 4-month KNB-E2-vaccinated pigs before challenge, interestingly, we observed higher anti-CSFV-neutralizing antibodies in the 4-month KNB-E2 pigs than that in the 2-month KNB-E2 pigs after CSFV challenge. It appears that the CSFV challenge induced an anamnestic antibody response and, therefore, provided clinical protection against CSF disease.
Of interest in this study are the PBS-vaccinated, CSF-free pigs that survived the CSFV challenge. The apparent development of low VNA titers in these pigs toward the end of the challenge study, suggests that the innate immune system may have a critical role in the CSFV challenge. Similar to humans, pigs have been reported to exhibit substantial genetic variation in immunity [13,30]. Hence, the differences in anti-CSFV immunity observed in the present study may be attributed to genetic diversity in the selected pigs.
On the other hand, age-dependent variation in porcine immune responses to viral infection has been reported [24,36]. Therefore, the restriction of CSFV growth observed in the pigs challenged at 4 months post-vaccination may be attributed to an age-related innate immune resistance. Previous studies have reported stronger cell-mediated responses in older pigs; in contrast, induced humoral responses were unrelated to animal age [24]. In this study, we observed that older pigs may have the capacity to quickly mount an anti-CSFV-specific VNA response starting at approximately 6 DPC. Since no E2-specific antibodies were detected in the older pigs throughout the study, these results suggest that anti-CSFV protection could be conferred by the more potent innate immune mechanisms in mature pigs.
Protection against transmission was not investigated in this study, but single-dose KNB-E2-vaccinated pigs did transiently test positive for CSFV RNA. Hence, there remains a certain risk of vertical transmission. Nevertheless, our results suggest that KNB-E2 reduces clinical CSF signs and might be valuable in reducing CSF related losses. Another advantage of using this vaccine is the capacity to discriminate between vaccinated and CSF-infected pigs [29]. However, to ensure complete protection against CSF, a second KNB-E2 dose might be beneficial. An improved, more potent adjuvant or incorporation of multiple immunogenic antigens could also be considered.
Our results have demonstrated that CSF subunit vaccine KNB-E2-mediated protection is dose dependent. A minimal amount of E2 antigen is required for this subunit vaccine to confer rapid and long-lasting clinical protection against CSF. Further evaluation of the efficacy of KNB-E2 against current strains of CSFV will shed light on the potential role of KNB-E2 in reducing CSF related losses.
Acknowledgments
We thank Dr. Brooke Bloomberg and the Comparative Medicine Group staff at Kansas State University for their technical help. We thank the Biosecurity Research Institute husbandry staff for providing the quality high-level animal care. This research was supported by an award from the National Bio and Agro-Defense Facility Transition Fund and USDA ARS Specific Cooperative Agreement (59-5430-001-23S, P-103).
Footnotes
Part of this work was shared in oral presentations at the 2017 CRWAD conference in Chicago, IL, USA and the 2017 Vaccine R±D in Washington, DC, USA.
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Ahrens U, Kaden V, Drexler C, Visser N. Efficacy of the classical swine fever (CSF) marker vaccine Porcilis® Pesti in pregnant sows. Vet Microbiol. 2000;77:83–97. doi: 10.1016/s0378-1135(00)00265-0. [DOI] [PubMed] [Google Scholar]
- 2.Aucouturier J, Dupuis L, Ganne V. Adjuvants designed for veterinary and human vaccines. Vaccine. 2001;19:2666–2672. doi: 10.1016/s0264-410x(00)00498-9. [DOI] [PubMed] [Google Scholar]
- 3.Beer M, Reimann I, Hoffmann B, Depner K. Novel marker vaccines against classical swine fever. Vaccine. 2007;25:5665–5670. doi: 10.1016/j.vaccine.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 4.Blome S, Meindl-Böhmer A, Loeffen W, Thuer B, Moennig V. Assessment of classical swine fever diagnostics and vaccine performance. Rev Sci Tech. 2006;25:1025–1038. [PubMed] [Google Scholar]
- 5.Blome S, Moß C, Reimann I, König P, Beer M. Classical swine fever vaccines: state-of-the-art. Vet Microbiol. 2017;206:10–20. doi: 10.1016/j.vetmic.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Bouma A, De Smit AJ, De Jong MC, De Kluijver EP, Moormann RJ. Determination of the onset of the herd-immunity induced by the E2 sub-unit vaccine against classical swine fever virus. Vaccine. 2000;18:1374–1381. doi: 10.1016/s0264-410x(99)00398-9. [DOI] [PubMed] [Google Scholar]
- 7.Bouma A, de Smit AJ, de Kluijver EP, Terpstra C, Moormann RJ. Efficacy and stability of a subunit vaccine based on glycoprotein E2 of classical swine fever virus. Vet Microbiol. 1999;66:101–114. doi: 10.1016/s0378-1135(99)00003-6. [DOI] [PubMed] [Google Scholar]
- 8.de Smit AJ, Bouma A, de Kluijver EP, Terpstra C, Moormann RJ. Duration of the protection of an E2 subunit marker vaccine against classical swine fever after a single vaccination. Vet Microbiol. 2001;78:307–317. doi: 10.1016/s0378-1135(00)00306-0. [DOI] [PubMed] [Google Scholar]
- 9.Dewulf J, Laevens H, Koenen F, Mintiens K, de Kruif A. An E2 sub-unit marker vaccine does not prevent horizontal or vertical transmission of classical swine fever virus. Vaccine. 2001;20:86–91. doi: 10.1016/s0264-410x(01)00320-6. [DOI] [PubMed] [Google Scholar]
- 10.Di Pasquale A, Preiss S, Tavares Da Silva F, Garçon N. Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines (Basel) 2015;3:320–343. doi: 10.3390/vaccines3020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong XN, Chen YH. Marker vaccine strategies and candidate CSFV marker vaccines. Vaccine. 2007;25:205–230. doi: 10.1016/j.vaccine.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 12.Dowling JK, Mansell A. Toll-like receptors: the Swiss Army knife of immunity and vaccine development. Clin Transl Immunology. 2016;5:e85. doi: 10.1038/cti.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flori L, Gao Y, Laloë D, Lemonnier G, Leplat JJ, Teillaud A, Cossalter AM, Laffitte J, Pinton P, de Vaureix C, Bouffaud M, Mercat MJ, Lefèvre F, Oswald IP, Bidanel JP, Rogel-Gaillard C. Immunity traits in pigs: substantial genetic variation and limited covariation. PLoS One. 2011;6:e22717. doi: 10.1371/journal.pone.0022717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox CB, Kramer RM, Barnes VL, Dowling QM, Vedvick TS. Working together: interactions between vaccine antigens and adjuvants. Ther Adv Vaccines. 2013;1:7–20. doi: 10.1177/2051013613480144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galliher-Beckley A, Pappan LK, Madera R, Burakova Y, Waters A, Nickles M, Li X, Nietfeld J, Schlup JR, Zhong Q, McVey S, Dritz SS, Shi J. Characterization of a novel oil-in-water emulsion adjuvant for swine influenza virus and Mycoplasma hyopneumoniae vaccines. Vaccine. 2015;33:2903–2908. doi: 10.1016/j.vaccine.2015.04.065. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Villamandos JC, Salguero FJ, Ruiz-Villamor E, Sánchez-Cordón PJ, Bautista MJ, Sierra MA. Classical swine fever: pathology of bone marrow. Vet Pathol. 2003;40:157–163. doi: 10.1354/vp.40-2-157. [DOI] [PubMed] [Google Scholar]
- 17.Graham SP, Everett HE, Haines FJ, Johns HL, Sosan OA, Salguero FJ, Clifford DJ, Steinbach F, Drew TW, Crooke HR. Challenge of pigs with classical swine fever viruses after C-strain vaccination reveals remarkably rapid protection and insights into early immunity. PLoS One. 2012;7:e29310. doi: 10.1371/journal.pone.0029310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5:505–517. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann B, Beer M, Schelp C, Schirrmeier H, Depner K. Validation of a real-time RT-PCR assay for sensitive and specific detection of classical swine fever. J Virol Methods. 2005;130:36–44. doi: 10.1016/j.jviromet.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 20.Hua RH, Huo H, Li YN, Xue Y, Wang XL, Guo LP, Zhou B, Song Y, Bu ZG. Generation and efficacy evaluation of recombinant classical swine fever virus E2 glycoprotein expressed in stable transgenic mammalian cell line. PLoS One. 2014;9:e106891. doi: 10.1371/journal.pone.0106891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang YL, Deng MC, Wang FI, Huang CC, Chang CY. The challenges of classical swine fever control: modified live and E2 subunit vaccines. Virus Res. 2014;179:1–11. doi: 10.1016/j.virusres.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 22.Hulst MM, Moormann RJ. Inhibition of pestivirus infection in cell culture by envelope proteins E(rns) and E2 of classical swine fever virus: E(rns) and E2 interact with different receptors. J Gen Virol. 1997;78:2779–2787. doi: 10.1099/0022-1317-78-11-2779. [DOI] [PubMed] [Google Scholar]
- 23.Kahn CM. The Merck Veterinary Manual. 9th ed. Whitehouse Station and Great Britain: Merck & Co.; 2005. [Google Scholar]
- 24.Klinge KL, Vaughn EM, Roof MB, Bautista EM, Murtaugh MP. Age-dependent resistance to Porcine reproductive and respiratory syndrome virus replication in swine. Virol J. 2009;6:177. doi: 10.1186/1743-422X-6-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.König M, Lengsfeld T, Pauly T, Stark R, Thiel HJ. Classical swine fever virus: independent induction of protective immunity by two structural glycoproteins. J Virol. 1995;69:6479–6486. doi: 10.1128/jvi.69.10.6479-6486.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin GJ, Deng MC, Chen ZW, Liu TY, Wu CW, Cheng CY, Chien MS, Huang C. Yeast expressed classical swine fever E2 subunit vaccine candidate provides complete protection against lethal challenge infection and prevents horizontal virus transmission. Vaccine. 2012;30:2336–2341. doi: 10.1016/j.vaccine.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 27.Lindenbach BD, Murray CL, Thiel HJ, Rice CM. Flaviviridae. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott Williams and Wilkins; 2013. pp. 712–747. [Google Scholar]
- 28.Luo Y, Li S, Sun Y, Qiu HJ. Classical swine fever in China: a minireview. Vet Microbiol. 2014;172:1–6. doi: 10.1016/j.vetmic.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Madera R, Gong W, Wang L, Burakova Y, Lleellish K, Galliher-Beckley A, Nietfeld J, Henningson J, Jia K, Li P, Bai J, Schlup J, McVey S, Tu C, Shi J. Pigs immunized with a novel E2 subunit vaccine are protected from subgenotype heterologous classical swine fever virus challenge. BMC Vet Res. 2016;12:197. doi: 10.1186/s12917-016-0823-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mallard BA, Wilkie BN. Phenotypic, genetic and epigenetic variation of immune response and disease resistance traits of pigs. Adv Pork Prod. 2007;18:139–146. [Google Scholar]
- 31.Moormann RJ, Bouma A, Kramps JA, Terpstra C, De Smit HJ. Development of a classical swine fever subunit marker vaccine and companion diagnostic test. Vet Microbiol. 2000;73:209–219. doi: 10.1016/s0378-1135(00)00146-2. [DOI] [PubMed] [Google Scholar]
- 32.Moser M, Leo O. Key concepts in immunology. Vaccine. 2010;28(Suppl 3):C2–C13. doi: 10.1016/j.vaccine.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Murphy K, Travers P, Walport M, Janeway C. Janeway's Immunobiology. 8th ed. New York: Garland Science; 2012. [Google Scholar]
- 34.O'Hagan DT. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev Vaccines. 2007;6:699–710. doi: 10.1586/14760584.6.5.699. [DOI] [PubMed] [Google Scholar]
- 35.Perrie Y, Mohammed AR, Kirby DJ, McNeil SE, Bramwell VW. Vaccine adjuvant systems: enhancing the efficacy of sub-unit protein antigens. Int J Pharm. 2008;364:272–280. doi: 10.1016/j.ijpharm.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 36.Post J, Weesendorp E, Montoya M, Loeffen WL. Influence of age and dose of African swine fever virus infections on clinical outcome and blood parameters in pigs. Viral Immunol. 2017;30:58–69. doi: 10.1089/vim.2016.0121. [DOI] [PubMed] [Google Scholar]
- 37.Rivera A, Siracusa MC, Yap GS, Gause WC. Innate cell communication kick-starts pathogen-specific immunity. Nat Immunol. 2016;17:356–363. doi: 10.1038/ni.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Summerfield A, Ruggli N. Immune responses against classical swine fever virus: between ignorance and lunacy. Front Vet Sci. 2015;2:10. doi: 10.3389/fvets.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suradhat S, Intrakamhaeng M, Damrongwatanapokin S. The correlation of virus-specific interferon-gamma production and protection against classical swine fever virus infection. Vet Immunol Immunopathol. 2001;83:177–189. doi: 10.1016/s0165-2427(01)00389-0. [DOI] [PubMed] [Google Scholar]
- 40.van Oirschot JT. Classical swine fever. In: Straw BE, editor. Disease of Swine. Iowa City: Iowa State University Press; 1999. pp. 159–172. [Google Scholar]